Abstract
The goal of this study was to develop a potential druggable target for lung injury after SABR through the small animal model. Utilising the model, a radiation dose of 70 Gy or 90 Gy was focally (small volume) delivered to the left lung of mice. The highly expressed phosphorylation form of C-Raf was discovered through a protein array experiment, with the protein being extracted from the area of radiated mouse lung tissue, and was confirmed by IHC and western blot. C-Raf activation, along with morphological change and EMT (Epithelial to Mesenchymal Transition) marker expression, was observed after radiation to the mouse type II alveolar cell line MLE-12. C-Raf inhibitor GW5074 was able to reverse the EMT in cells effectively, and was found to be dependent on Twist1 expression. In the animal experiment, pretreatment of GW5074 alleviated EMT and lung injury after 70 Gy radiation was focally delivered to the lung of mice. Conclusively, these results demonstrate that C-Raf inhibitor GW5074 inhibits high-dose small-volume radiation-induced EMT via the C-Raf/Twist1 signalling pathway in mice. Therefore, pharmacological C-Raf inhibitors may be used effectively as inhibitors of SABR-induced lung fibrosis.
Subject terms: Cell biology, Chemical biology
Introduction
Stereotactic ablative radiotherapy (SABR) is a newly emerging radiotherapy treatment that, compared with conventionally fractionated radiation therapy (CFRT), allows an ablative dose of radiation to be delivered to a confined area around a tumour1. Unfortunately, during CFRT or SABR, lung complications such as pneumonitis and fibrosis can cause significant morbidity in cancer survivors. Radiation-induced pulmonary fibrosis (RIPF) develops 1 year to several years after lung radiation, and is characterised by fibroblast proliferation with excessive extracellular matrix (ECM) deposition2. Meanwhile, recent studies have concentrated on epithelial cells that are able to transverse themselves into myofibroblasts through an approach of “epithelial–mesenchymal transition (EMT)”, which has been demonstrated in RIPF3,4. During EMT, an epithelial cell gradually gains mesenchymal characteristics and loses its polarity with increased migratory feasibility5. The exact molecular mechanisms leading to the development of SABR-induced pulmonary fibrosis have yet to be fully identified. In a previous study6, we established an experimental model and an image-guided animal radiation system in order to study high-dose-per-fraction radiation such as SABR at volumes analogous to those used in human beings. With this animal model we observed that the lung complications induced by SABR are remarkably different from CFRT7. In this study, we tried to explore the possible target of inhibiting SABR-induced EMT; thus, experiments were conducted under the condition of high-dose small-volume (HDSV) radiation, which represents the SABR system.
C-Raf (or Raf1) is a member of the Raf serine/threonine kinase family that can be activated by a variety of extracellular stimuli, including TGFβ1 (transforming growth factor beta 1)8,9, fibroblast growth factor (FGF)10, etc. Early studies indicated that C-Raf can partially mediate fibrosis in several organs through a network of signalling and transcriptional events11,12. It was recently shown that EMT occurring in peritoneal, kidney and lung fibrosis, as well as in breast cancer stem cells, was associated with increased p–C-Raf expression13–15, whereby suggesting a rationale for the development of C-Raf inhibitors in fibrosis treatment. Besides C-Raf, Twist proteins are highly conserved basic helix-loop-helix (bHLH) transcription factors that have important regulatory functions during EMT16. Exogenous overexpression of Twist1 increases the invasive and metastatic abilities of human cancer cells by promoting the downregulation of E-cadherin and the induction of epithelial–mesenchymal transition (EMT)17–19. However, as far as we know, the role of Twist1 or C-Raf in radiation (especially HDSV)-induced lung fibrosis is unknown.
The compound GW5074 (5-Iodo-3-[(3,5-dibromo-4-hydroxyphenyl)methy-lene]-2-indolinone) is a synthetic drug and C-Raf kinase inhibitor (IC50 for cRaf-1 = 9 nM). Furthermore, GW5074 is a highly specific inhibitor of C-Raf kinase and has minimal effects on other kinases dysregulated in neurodegenerative conditions such as Jun kinase (JNK), cyclin-dependent kinases (CDKs), MEK, and glycogen synthase kinase 3 (GSK3) α/β kinases. GW5074 is neuroprotective in vitro and does not show toxic effects in mice20. We hypothesised that as a fibrosis modulator, C-Raf may play an important role in SABR-induced lung fibrosis. In the present study, we tried to (i) understand the role of C-Raf in SABR-induced EMT in mice, and (ii) analyse cell signalling events involved in the inhibitory effect of GW5074 on high-dose-induced EMT.
Results
Selection of differentially expressed proteins in mouse lung tissues after high-dose small-volume lung radiation
In order to explore potential druggable targets of HDSV-radiation-induced lung fibrosis, the changes in protein expression were first identified. Proteins were subtracted from mouse lung tissue with or without HDSV radiation (70 Gy) and a protein antibody assay was performed. As shown in Fig. 1, there were noticeable changes in protein expression between control and radiated lung tissues (the expression of a predominant number of proteins was downregulated or upregulated). Interestingly, the protein level of phosphorylated C-Raf scored highly at both time points in log 2 (fold change), which constituted 2.028 (in 2 weeks) and 1.325 (in 4 weeks) compared to the non-radiated control mice. It is suggested that C-Raf might play an important role in HDSV-radiation-induced lung injury. C-Raf phosphorylation was further confirmed by a western blotting experiment, with the protein being extracted from mice lung tissues of the radiated area (Fig. 2A). We can observe that the phosphorylated form of C-Raf can be detected significantly high in comparison to the control at 2 weeks and 4 weeks after radiation. Collectively, these findings suggest that constitutive activation of C-Raf is associated with HDSV-radiation-induced lung injury.
Figure 1.
Protein expressions in mouse lung tissues after high-dose radiation (70 Gy). Protein expression patterns in focal high-dose radiation (70 Gy) at indicated time points. The expression ratio colour scale ranges from red (high) to blue (low), as indicated by the scale bar with log 2 units. (A) List of top 20 candidate proteins that experienced upregulation 2 weeks after radiation. (B) List of top 20 candidate proteins that experienced downregulation 2 weeks after radiation. (C) List of top 20 candidate proteins that experienced upregulation 4 weeks after radiation. (D) List of top 20 candidate proteins that experienced downregulation 4 weeks after radiation.
Figure 2.
C-Raf phosphorylation was further confirmed by IHC and western blot experiments at different time points. (A) Expression of C-Raf and phosphor-C-Raf proteins was confirmed by western blotting, using three individual mouse lung tissue samples after 70 Gy radiation. *P < 0.05 vs. control-2w and control-4w. Full unedited gels were shown in Supplementary Fig. S1. (B) Representative images of immunohistochemical staining for phosphor-C-Raf in radiated lung tissue at each indicated time point after 90 Gy radiation (magnification 200 × 3). Average percentage of p–C-Raf positive cells per microscopic. For quantification, the number of all cells in each microscopic was counted. Difference was evaluated by two- tailed student’s t-test. *P < 0.05 vs. non-radiated control.
According to our previous work, the lung injury induced by HDSV radiation (90 Gy) occurs within 2 weeks, which is much earlier than that induced by CFRT7, 21. Therefore, in order to investigate the sequential alteration of the C-Raf activation level at earlier time points, lung tissues were prepared 1, 3, 5, 7, 9, 11 and 14 days after 90 Gy radiation, and an IHC analysis was conducted using an antibody against phosphor-C-Raf. As shown in Fig. 2B, phosphor-C-Raf was detected significantly high after 7 days, and increased gradually thereafter until day 14. Collectively, these findings suggest the physiologically important role of C-Raf in early and relatively late time periods after HDSV radiation.
Radiation caused MLE-12 cells to transform into a mesenchymal phenotype
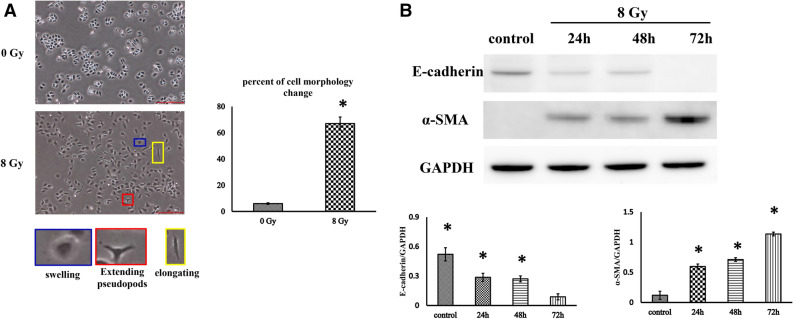
Before further investigating the possible role of C-Raf in EMT, radiation-induced alveolar epithelial–mesenchymal transition should be demonstrated. MLE-12 cells (mouse type II alveolar epithelial) were radiated with a single dose of an 8 Gy X-ray, and cell morphology was observed at 72 h post-radiation. It was found that the morphology of 75% of cells transformed from that of a cuboidal appearance into a turgid or prolonged one with outspread pseudopods after radiation (Fig. 3A). To monitor whether these alterations were in accordance with EMT-related proteins, a western blot was used for detecting the expression of E-cadherin (epithelial marker) and α-SMA (mesenchymal marker). As shown in Fig. 3B, a reduction in the level of E-cadherin and an enhancement in α-SMA were detected in cells 24, 48 and 72 h after radiation in comparison with the non-radiated control. Altogether, these results indicate that radiation causes EMT in MLE-12 cells after radiation with an X-ray dose of 8 Gy.
Figure 3.
Radiation induces changes in cell morphology and EMT-associated protein expression in MLE-12 cells. (A) Representative images of cell morphology (photographed 48 h after 8 Gy radiation or non-radiation). Cells suffering morphological changes were counted in random microscope fields according to whether the cells became swollen (red outline), elongated (green outline) or exhibited extended pseudopodia (blue outline) compared with a cuboidal appearance, and the percentage was calculated. Scale bar represents 100 μm. The data are presented as the mean ± SEM (n = 10). *P < 0.05 vs. non-radiated control. (B) Cells were radiated with a single dose of 8 Gy X-rays, and cell morphology and EMT-associated protein markers were observed 24, 48 and 72 h post-radiation. Representative western blots and densitometric quantification of E-cadherin and α-SMA protein levels. GAPDH was used as the loading control. Full unedited gels were shown in Supplementary Fig. S2. The data are presented as the mean ± SEM (n = 3). *P < 0.05 vs. non-radiated control.
GW5074 alleviated EMT in radiated mouse alveolar epithelial cells
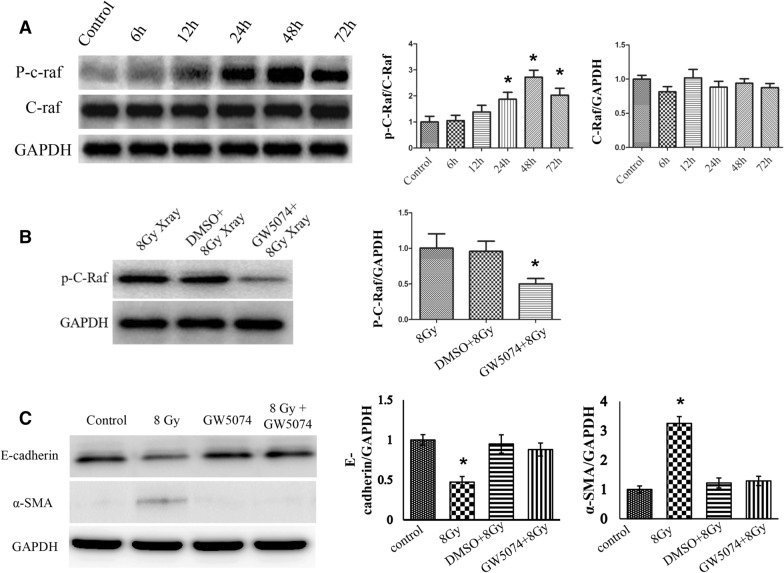
To investigate the role of C-Raf in EMT in MLE-12 cells, we first determined the radiation-responsive property of C-Raf. We subjected MLE-12 cells to a single dose of 8 Gy and found a time-dependent increase in the phospho-C-Raf protein level, with the highest time point being 48 h (Fig. 4A). Thereafter, we confirmed whether C-Raf inhibitor GW5074 has a C-Raf inactivation effect in MLE-12 cells. As shown in Fig. 4B, 100 nM GW5074 dramatically reduced the phosphorylation form of C-Raf compared to control and DMSO (solvent)-treated groups.
Figure 4.
GW5074 can reverse EMT after 8 Gy radiation in MLE-12 cells. (A) Representative western blots and densitometric quantification of phosphor-C-Raf protein levels at different times and after 8 Gy radiation (*P < 0.05 vs. non-radiated control). (B) The inhibitory efficiency of GW5074 was evaluated by western blots with the p–C-Raf expression level (*P < 0.05 vs. 8 Gy and DMSO + 8 Gy). (C) The protein levels of E-cadherin and α-SMA were determined using western blot analysis at 72 h post-treatment with radiation and/or GW5074. Full unedited gels were shown in Supplementary Figs. S3–S5. The data are presented as the mean ± SEM (n = 3). *P < 0.05 vs. non-radiated control and GW5074 alone and 8 Gy + GW5074.
We then investigated the role of C-Raf in the protein expression level of epithelial marker E-cadherin and mesenchymal marker α-SMA in MLE-12 cells. The western blot was performed on the protein extracted from cells harvested 72 h after radiation. Densitometric analysis of the western blotting results obtained from three independent experiments (Fig. 4C) showed that 8 Gy of radiation markedly reduced the protein expression level of E-cadherin and enhanced α-SMA compared to the control. GW5074 treatment alone (without radiation) induced little changes in the protein of E-cadherin and α-SMA when compared to those from the untreated control cells. However, in comparison with the cells radiated by 8 Gy, pretreatment of GW5074 recovered the level of E-cadherin up to 85% of the control and the α-SMA level to near the control. Thus, morphological observations (together with alteration in the epithelial and mesenchymal markers) suggested that GW5074 treatment effectively inhibited the epithelial cells to undergo a transition into a mesenchymal phenotype when exposed to radiation at a dose of 8 Gy.
GW5074 inhibited a radiation-induced mesenchymal transformation via the C-Raf /twist1 signalling pathway
Previous studies have demonstrated that twist1 plays a crucial role in EMT regulation in fibrosis16. Accordingly, we hypothesised that twist1 might be a key downstream regulator in the GW5074-mediated reversion of radiation-induced EMT. To investigate the potential role of Twist1, we first need to test whether sh-Twist1 lentivirus is able to downregulate twist1 expression. As a result, it is verified that sh-Twist1 lentivirus can inhibit the protein expression level of twist1 efficiently (Fig. 5A). Thereafter, GW5074 and sh-Twist1 are applied to radiated MLE-12 cells in order to observe the possible relations between Twist1 and C-Raf. Our results indicate that pretreating MLE-12 cells with GW5074 reduced Twist1 expression and reversed EMT. Meanwhile, pretreatment of sh-Twist1 alone is not able to reverse radiation-induced EMT, which demonstrates that Twist1 possesses an essential downstream of the C-Raf signalling pathway in radiation-induced EMT (Fig. 5B). Therefore, these results reveal that GW5074 can block radiation-induced EMT via the C-Raf/Twist1 signalling pathway.
Figure 5.
Western blotting analysis to evaluate possible signalling pathway in GW5074-mediated reversion of EMT after radiation. (A) Representative western blot and densitometric quantification of Twist1 following infection with Twist1-specific shRNA lentivirus to confirm target suppression. (B) Western blotting analysis and densitometric quantification to evaluate the protein expression level of p–C-Raf, Twist1 and EMT-related markers with or without 8 Gy radiation in MLE-12 cells. Cells were pretreated with or without GW5074 or shTwist1. Full unedited gels were shown in Supplementary Figs. S6 and S7. The data are presented as the mean ± SEM (n = 3) (* or #P < 0.05 vs. indicated groups).
GW5074 alleviated radiation-induced pulmonary injury and fibrosis
To elucidate whether small-molecule GW5074 inhibits radiation-mediated lung fibrosis in mice, we compared the changes in left lung surface morphology in the control and radiation groups. In contrast to the brown-coloured lungs in the control mice, the lungs of the radiated mice exhibited a definite white area, which is correlated with the field of radiation (Fig. 6A, lower panel). An intraperitoneal injection of GW5074 resulted in less injury than was apparent in the radiation-only mice. H&E staining slides confirmed that GW5074-treated mice exhibited reduced tissue damage (Fig. 6A, upper panel). Masson’s trichrome staining revealed a marked increase in collagen deposition in the radiation group in comparison with the control group, which was significantly reversed by GW5074 treatment (Fig. 6B).
Figure 6.
GW5074 can partially reverse EMT in HDSV radiation mouse model. C57BL/6 mice (n = 3/group) were pretreated with GW5074 (2 mg/kg) or a vehicle and the thoracic part of the left lung was radiated (70 Gy) with a 3 mm collimator. After radiation, GW5074 treatment was continued on 3 days per week for 2 weeks, and lung samples were then obtained 6 weeks after radiation. (A) The images show representative gross observation data (top) and H&E staining slides with the injury area indicated by a red circle (down). (B) Evaluation of collagen deposition. Lung sections were stained with Masson’s trichrome stain to visualise collagen deposition. Representative micrographs of stained lung tissue are shown as indicated treatment (magnification 400 × 3). (C) Co-localisation (yellow pixels) of E-cadherin and a-SMA. *P < 0.01 vs. non-radiated control. #P < 0.05 vs. 70 Gy-radiated groups.
GW5074 inhibited EMT in lung tissues after radiation
To examine the role of C-Raf in radiation-induced EMT in vivo, we assessed the expression of EMT-related markers in different groups of mice after radiation (Fig. 6C). Our data shows that local lung radiation resulted in obvious EMT changes such as the downregulation of E-cadherin and the upregulation of α-SMA, and was assessed by the extent of co-localisation of a-SMA and E-cadherin in the alveolar epithelium. Decreased co-staining of E-cadherin/α-SMA after radiation was restored by treatment with GW5074, indicating that the radiation-induced EMT process was blocked by GW5074 treatment.
Discussion
RIPF is a common side effect in lung cancer radiotherapy. Pulmonary fibrosis usually develops within 6 to 24 months after radiotherapy, and stabilises after 2 years. In recent years, high-dose-per-fraction hypofractionated radiotherapy, such as SBRT, has become an effective way to treat various cancers. This new radiotherapy method has been proved to be very effective in the control of various cancers, including early-stage non-small cell lung cancer. Although this technique is highly confirmative and, thus, minimises normal tissue complications, serious complications have appeared nonetheless22. However, there is no effectively therapeutic target has been developed thus far. Therefore, at the beginning of this study, we conducted an exploratory experiment in order to look for an underlying target for SABR-induced lung injury. Fortunately, we found that C-Raf is a potential target according to the protein array experiment at late time points. Furthermore, the phosphorylation of C-Raf was confirmed by a western blot at the same time points, as well as by IHC at earlier time points, indicating that the activation of C-Raf contributes to the pathogenesis of lung fibrosis during the entire process.
In the current study, the progress of RIPF in mice induced by two radiation doses (90 or 70 Gy) was explored through the use of a microbeam collimator (3 mm in diameter) to the left lung. Under radiation conditions, it is common that EMT accompanying phenotypic changes occurs during the development of RIPF3,23. Thus, we advise that minimising the EMT may be practical in ameliorating RIPF developing after various radiotherapies. EMT is an approach in which epithelial cells lose their cell-to-cell contact and gradually lose their epithelial constituents and raise the expression of the mesenchymal marker24. According to the morphological analysis, we found that radiation to type II alveolar epithelial cells resulted in the cells losing their cuboidal nature, exhibiting a spindle appearance in respect of their mesenchymal morphology. On the other hand, this transformation was efficiently inhibited by the treatment of GW5074 in radiated alveolar epithelial cells, indicating the prohibitive feasibility of GW5074. Following the morphological investigation, we detected the expression of both E-cadherin and α-SMA in MLE-12 cells and the results indicate that GW5074 could ameliorate EMT efficiently by conserving the expression of E-cadherin and prohibiting α-SMA.
C-Raf occurs in many cellular processes including cell differentiation, cell cycle progression, and proliferation25–27. Moreover, TGFβ1-influenced EMT in normal murine mammary gland epithelial cells was regulated by C-Raf via the activation of ERK28. Nowadays, C-Raf inhibitors draw the attention of many researchers due to their anti-fibrotic and anti-cancer potential, and the availability of C-Raf inhibitors for the treatment of fibrosis has been explored in recent years11,12,29. Raf kinase activity can be prohibited by small-molecule drugs, as well as by disturbance of the Ras–Raf interaction30–32. Chen et al. interpreted that sorafenib, which is a multi-kinase inhibitor with activity against VEGFR, PDGFR and RAF kinases, attenuated bleomycin-induced pulmonary fibrosis via prohibition of fibroblast proliferation and EMT33. Interestingly, with potent tumour-killing properties, bleomycin and radiation have similar mechanisms with respect to killing cancer cells and inducing lung fibrosis as well. Inducing DNA damage is the main theory behind them with regard to killing cancer cells, and both of them cause lung fibrosis through EMT34,35. Such evidence might imply the similar mechanisms of sorafenib and GW5074 in respect of their anti-fibrotic effect. Nevertheless, in their study they did not disclose whether or not the effect of sorafenib was via prohibition of C-Raf. Prohibition of VEGFR/PDGFR has been well known to diminish fibrotic responses via the prohibition of fibrocyte activity36; thus, the anti-fibrotic efficacy of sorafenib might also be caused by the prohibition of receptor tyrosine kinases. GW5074 is a potential and selective inhibitor of C-Raf with IC50 of 9 nM and is invalid for CDKs, p38 MAP kinase, MKK6 and MKK7 in vitro37. On account of the high selectivity for Raf kinases, GW5074 was widely utilised as a C-Raf inhibitor in order to investigate its function38. In the current study, the prohibition of C-Raf by GW5074 revealed a remarkable decrease of C-Raf phosphorylation, which led to prominent attenuation of EMT after HDSV radiation in vitro and in vivo. As far as we know, however, because the toxic dose is slightly higher than the therapeutic dose, GW5074 has not yet been examined in humans.
Twist1 is upregulated in human and murine skin fibrosis and the overexpression of Twist1 is mediated by TGFβ signalling39. In addition to activating fibroblasts, Twist1 has been found to promote epithelial–mesenchymal transition39. During embryonic development, Twist1 proteins play a pivotal role in mesenchymal differentiation40. Furthermore, Twist1 induces EMT on the basis of injury and can mediate mesenchymal tissue responses in an adult41,42. Abnormal Twist1 signalling, nonetheless, may result in pathological EMT and, thus, lead to cancer development and fibrotic disorders23,43,44. Escaping from premature senescence regulated by Twist1/Twist2 is associated with complete EMT in human epithelial cells16. Nonetheless, Twist1 has never been investigated in RIPF and its mechanism has been hardly unveiled. In our study, we have first demonstrated that Twist1 is an important participant in the C-Raf downstream signalling pathway in HDSV-radiation-induced EMT.
Even though currently approved therapies for lung fibrosis, such as Pirfenidone and Nintedanib, are clinically available, an alternate strategy with which to address the unmet therapeutic need for SABR-induced lung fibrosis is needed. Our results, for the first time, demonstrate that C-Raf inhibitor GW5074 inhibits HDSV-radiation-induced EMT via the C-Raf/Twist1 signalling pathway in mice. Therefore, pharmacological C-Raf inhibitors may be used effectively as inhibitors of SABR-induced lung fibrosis.
Materials and methods
Mice and radiation
All procedures were approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Xinxiang Medical University. C57BL/6 mice were housed at the Institute of Experimental Animal Sciences (Xinxiang Medical University) in accordance with guidelines approved by the Institutional Animal Care and Use Committee of Xinxiang Medical University. Radiation was delivered using the X-RAD 225 platform (Precision X-Ray) as described previously6. The left lungs of 8-week-old male mice were radiated using a 3 mm-diameter field and the mice were maintained for certain days and weeks according to the experimental design. GW5074 (MedChemExpress, Monmouth Junction, USA) was dissolved in DMSO, further diluted in saline (0.9% NaCl), and administered i.p. (2 mg/kg).
Tissue histology and immunohistochemical staining
Tissue histology and IHC staining were performed as described previously7,21. Briefly, the mice were sacrificed and lung tissues extracted and fixed in 10% (v/v) neutral buffered formalin before the preparation of paraffin sections. Paraffin-embedded sections were deparaffinised and stained with haematoxylin and eosin (H&E; Sigma-Aldrich), or Masson’s trichrome stain kit (Sigma-Aldrich) was utilised in order to detect collagen.
Before immunohistochemistry, deparaffinised sections were boiled in 0.1 mol/L of citrate buffer (pH 6.0) for 30 min and then incubated with 0.3% (v/v) hydrogen peroxide in methanol for 15 min. Sections were blocked in normal horse serum at room temperature for 30 min and immunostained overnight at 4 °C with primary antibodies against phospho-C-Raf (1:100; Abcam, ab60985). The target proteins were visualised using ABC and DAB kits (Vector Laboratories) and counterstained with haematoxylin. For immunofluorescence staining, sections stained with primary antibodies were incubated with appropriate fluorescently labelled secondary antibodies (1:250; Molecular Probes) and counterstained with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI; 3 mmol/L). Images were obtained using a Zeiss microscope.
Hybridisation of protein antibody array
The protein antibody array, i.e. Explorer Antibody Array, was purchased from FullMoon Biosystems, Inc. (Sunnyvale, CA, USA) and was performed according to the protocol suggested by the manufacturer. An Explorer Antibody Array comprising 656 antibodies was used to quantify a range of proteins. Proteins were extracted from the radiated area of mice lung tissues. Three individual mice lung tissues for each experimental group were prepared and used for the antibody array experiments.
Cell culture
Type II alveolar epithelial cell lines (MLE-12) were purchased from ATCC (Manassas, VA, USA) and cultured as described previously7. These were routinely maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% foetal bovine serum, 2 mM of l-glutamine, 100 IU/mL of penicillin, and 100 μg/ML of streptomycin (purchased from Invitrogen, Carlsbad, CA) at 37 °C and with 5% CO2 in the air.
Cell radiation and GW5074 treatment
Once cells reached > 80% confluence, DMEM was replaced by serum-free medium for 24 h prior to radiation. Thereafter, cells were radiated with a single dose of 8 Gy X-rays. (An X-RAD 225 radiator was utilised at a dose rate of 19.7 cGy/s.) All radiation was performed at room temperature. To analyse the effect of GW5074 (MedChemExpress, Monmouth Junction, USA), GW5074 (at a final concentration of 100 nM) was added to the culture and incubated for 2 h prior to radiation at a dose of 8 Gy.
Western blot analysis
Western blot analysis was conducted as described previously45. Briefly, MLE-12 cells were lysed using RIPA buffer containing 1 mM of PMSF, 1 μg/mL of aprotinin, 1 μg/mL of leupeptin, 1 mM of Na3VO4, and 1 mM of NaF, and then stored in aliquots at − 80 °C until further analysis. The lysate (20 μg) was mixed with an equal volume of sample buffer, denatured by boiling, and then separated on 10–15% polyacrylamide mini-gel. The proteins were transferred to nitrocellulose membranes (Amersham, Arlington Heights, IL), blocked with 5% milk, and incubated overnight with E-cadherin (Abcam, Cambridge, MA, ab11512), C-Raf (Abcam, ab137435), phospho-C-Raf (Abcam, ab60985), Twist1 (Abcam, ab49254), and ɑ-SMA (Abcam, ab21027).
Immunofluorescence staining
Immunofluorescence staining was performed as described previously46. Briefly, cells grown on eight-well chamber slides were pretreated with GW5074 (followed by 8 Gy radiation) and then fixed with 4% neutral formalin for 30 min. After being washed three times with 1 × PBS, the cells were blocked with 3% BSA for 1 h. Thereafter, the cells were incubated with the antibody of E-cadherin (Abcam, ab11512) and ɑ-SMA (Abcam, ab21027) at 4˚C overnight. After being washed with PBS, the sections were incubated with the corresponding conjugated second antibody (Vector Laboratories, Burlingame, CA) at room temperature for 30 min. Nuclei were counterstained with 4′-6-diamidino-2-phenylindole (DAPI), and the slides were analysed using a fluorescence microscope.
Statistical analysis
Statistical analysis was conducted using a one-sample Student t-test in order to compare differences between the groups. A p-value of ≤ 0.05 was considered to be significant.
Supplementary information
Acknowledgements
Funding was provided by The First Affiliated Hospital of Xinxiang Medical University (Grant No. xyyfy2014BS-006), Henan Educational Administration (Grant No. 16B320014), National Natural Science Foundation of China (Grant Nos. 81503414, 81503579 and 81874392), Henan Joint International Laboratory of Radiation Injury Translational Medicine (Grant No. [2017]21) and The plateau Subject of Xinxiang Medical University.
Author contributions
Z.-Y.H. and P.L. were responsible for the study design. S.L., X.L., X.-M.L. and X.K. were responsible for the data acquisition, analysis and interpretation. Z.-Y.H., M.-Q.G. and P.L. drafted the manuscript. Z.M., H.N. provided some intellectual content. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1038/s41598-025-06708-0
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zhen-Yu Hong and Sanke Li.
Change history
7/7/2025
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41598-025-06708-0
Contributor Information
Ming-Qing Gao, Email: gmq126@126.com.
Ping Lu, Email: lupingdoctor@163.com.
Supplementary information
is available for this paper at 10.1038/s41598-020-68175-z.
References
- 1.Videtic, G. M. & Stephans, K. L. The role of stereotactic body radiotherapy in the management of non-small cell lung cancer: an emerging standard for the medically inoperable patient?. Curr. Oncol. Rep.12, 235–241. 10.1007/s11912-010-0108-1 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Choi, S. H. et al. A hypoxia-induced vascular endothelial-to-mesenchymal transition in development of radiation-induced pulmonary fibrosis. Clin. Cancer Res.21, 3716–3726. 10.1158/1078-0432.CCR-14-3193 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Farhood, B. et al. Intercellular communications-redox interactions in radiation toxicity; potential targets for radiation mitigation. J. Cell Commun. Signal13, 3–16. 10.1007/s12079-018-0473-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willis, B. C., DuBois, R. M. & Borok, Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc. Am. Thorac. Soc.3, 377–382. 10.1513/pats.200601-004TK (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radisky, D. C. Epithelial-mesenchymal transition. J. Cell Sci.118, 4325–4326. 10.1242/jcs.02552 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Balli, D. et al. Foxm1 transcription factor is required for lung fibrosis and epithelial-to-mesenchymal transition. EMBO J.32, 231–244. 10.1038/emboj.2012.336 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong, Z. Y. et al. Development of a small animal model to simulate clinical stereotactic body radiotherapy-induced central and peripheral lung injuries. J. Radiat. Res.55, 648–657. 10.1093/jrr/rrt234 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong, Z. Y., Song, K. H., Yoon, J. H., Cho, J. & Story, M. D. An experimental model-based exploration of cytokines in ablative radiation-induced lung injury in vivo and in vitro. Lung193, 409–419. 10.1007/s00408-015-9705-y (2015). [DOI] [PubMed] [Google Scholar]
- 9.Reimann, T. et al. Transforming growth factor-beta1 induces activation of Ras, Raf-1, MEK and MAPK in rat hepatic stellate cells. FEBS Lett.403, 57–60. 10.1016/s0014-5793(97)00024-0 (1997). [DOI] [PubMed] [Google Scholar]
- 10.Axmann, A., Seidel, D., Reimann, T., Hempel, U. & Wenzel, K. W. Transforming growth factor-beta1-induced activation of the Raf-MEK-MAPK signaling pathway in rat lung fibroblasts via a PKC-dependent mechanism. Biochem. Biophys. Res. Commun.249, 456–460. 10.1006/bbrc.1998.9188 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Morrison, D. K., Kaplan, D. R., Rapp, U. & Roberts, T. M. Signal transduction from membrane to cytoplasm: growth factors and membrane-bound oncogene products increase Raf-1 phosphorylation and associated protein kinase activity. Proc. Natl. Acad. Sci. USA85, 8855–8859. 10.1073/pnas.85.23.8855 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, Q. et al. Raf kinase inhibitory protein down-expression exacerbates hepatic fibrosis in vivo and in vitro. Cell Physiol. Biochem.40, 49–61. 10.1159/000452524 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Wang, Y. et al. New insights into the antifibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J. Hepatol.53, 132–144. 10.1016/j.jhep.2010.02.027 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Vargha, R. et al. Effects of epithelial-to-mesenchymal transition on acute stress response in human peritoneal mesothelial cells. Nephrol. Dial. Transpl.23, 3494–3500. 10.1093/ndt/gfn353 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Vidyasagar, A., Reese, S., Acun, Z., Hullett, D. & Djamali, A. HSP27 is involved in the pathogenesis of kidney tubulointerstitial fibrosis. Am. J. Physiol. Renal Physiol.295, F707-716. 10.1152/ajprenal.90240.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei, L. et al. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-kappaB. Breast Cancer Res.13, R101. 10.1186/bcr3042 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansieau, S. et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell14, 79–89. 10.1016/j.ccr.2008.06.005 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Yang, J. et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell117, 927–939. 10.1016/j.cell.2004.06.006 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Kwok, W. K. et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res.65, 5153–5162. 10.1158/0008-5472.CAN-04-3785 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Mironchik, Y. et al. Twist overexpression induces in vivo angiogenesis and correlates with chromosomal instability in breast cancer. Cancer Res.65, 10801–10809. 10.1158/0008-5472.CAN-05-0712 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess, S. & Echeverria, V. Raf inhibitors as therapeutic agents against neurodegenerative diseases. CNS Neurol. Disord. Drug. Targets9, 120–127 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Hong, Z. Y. et al. A preclinical rodent model of acute radiation-induced lung injury after ablative focal irradiation reflecting clinical stereotactic body radiotherapy. Radiat. Res.182, 83–91. 10.1667/RR13535.1 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Mehta, V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int. J. Radiat. Oncol. Biol. Phys.63, 5–24. 10.1016/j.ijrobp.2005.03.047 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Kang, Y. & Massague, J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell118, 277–279. 10.1016/j.cell.2004.07.011 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Yang, J. & Weinberg, R. A. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell14, 818–829. 10.1016/j.devcel.2008.05.009 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Wellbrock, C., Karasarides, M. & Marais, R. The RAF proteins take centre stage. Nat. Rev. Mol. Cell Biol.5, 875–885. 10.1038/nrm1498 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Fischer, A. et al. Regulation of RAF activity by 14–3-3 proteins: RAF kinases associate functionally with both homo- and heterodimeric forms of 14–3-3 proteins. J. Biol. Chem.284, 3183–3194. 10.1074/jbc.M804795200 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Rapp, U. R., Gotz, R. & Albert, S. BuCy RAFs drive cells into MEK addiction. Cancer Cell9, 9–12. 10.1016/j.ccr.2005.12.022 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Xie, L. et al. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia6, 603–610. 10.1593/neo.04241 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, X. et al. Didymin alleviates hepatic fibrosis through inhibiting ERK and PI3K/Akt pathways via regulation of raf kinase inhibitor protein. Cell Physiol. Biochem.40, 1422–1432. 10.1159/000453194 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Leicht, D. T. et al. Raf kinases: function, regulation and role in human cancer. Biochim. Biophys. Acta1773, 1196–1212. 10.1016/j.bbamcr.2007.05.001 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strumberg, D. & Seeber, S. Raf kinase inhibitors in oncology. Onkologie28, 101–107. 10.1159/000083373 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Sridhar, S. S., Hedley, D. & Siu, L. L. Raf kinase as a target for anticancer therapeutics. Mol. Cancer Ther.4, 677–685. 10.1158/1535-7163.MCT-04-0297 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Chen, Y. L. et al. Sorafenib ameliorates bleomycin-induced pulmonary fibrosis: potential roles in the inhibition of epithelial-mesenchymal transition and fibroblast activation. Cell Death Dis.4, e665. 10.1038/cddis.2013.154 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hay, J., Shahzeidi, S. & Laurent, G. Mechanisms of bleomycin-induced lung damage. Arch. Toxicol.65, 81–94. 10.1007/bf02034932 (1991). [DOI] [PubMed] [Google Scholar]
- 36.Kim, W. et al. Cellular stress responses in radiotherapy. Cells10.3390/cells8091105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato, S. et al. Anti-fibrotic efficacy of nintedanib in pulmonary fibrosis via the inhibition of fibrocyte activity. Respir. Res.18, 172. 10.1186/s12931-017-0654-2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chin, P. C. et al. The c-Raf inhibitor GW5074 provides neuroprotection in vitro and in an animal model of neurodegeneration through a MEK-ERK and Akt-independent mechanism. J. Neurochem.90, 595–608. 10.1111/j.1471-4159.2004.02530.x (2004). [DOI] [PubMed] [Google Scholar]
- 39.Bain, J. et al. The selectivity of protein kinase inhibitors: a further update. Biochem. J.408, 297–315. 10.1042/BJ20070797 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palumbo-Zerr, K. et al. Composition of TWIST1 dimers regulates fibroblast activation and tissue fibrosis. Ann. Rheum. Dis.76, 244–251. 10.1136/annrheumdis-2015-208470 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Murre, C. et al. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell58, 537–544. 10.1016/0092-8674(89)90434-0 (1989). [DOI] [PubMed] [Google Scholar]
- 42.Gort, E. H. et al. The TWIST1 oncogene is a direct target of hypoxia-inducible factor-2alpha. Oncogene27, 1501–1510. 10.1038/sj.onc.1210795 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Yoo, Y. G., Christensen, J., Gu, J. & Huang, L. E. HIF-1alpha mediates tumor hypoxia to confer a perpetual mesenchymal phenotype for malignant progression. Sci. Signal10.1126/scisignal.2002072 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Kida, Y., Asahina, K., Teraoka, H., Gitelman, I. & Sato, T. Twist relates to tubular epithelial-mesenchymal transition and interstitial fibrogenesis in the obstructed kidney. J. Histochem. Cytochem..55, 661–673. 10.1369/jhc.6A7157.2007 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Pozharskaya, V. et al. Twist: a regulator of epithelial-mesenchymal transition in lung fibrosis. PLoS ONE4, e7559. 10.1371/journal.pone.0007559 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong, Z. Y. et al. Inhibition of Akt/FOXO3a signaling by constitutively active FOXO3a suppresses growth of follicular thyroid cancer cell lines. Cancer Lett.314, 34–40. 10.1016/j.canlet.2011.09.010 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.