Abstract
In social insects, cuticular hydrocarbons function in nest-mate recognition and also provide a waxy barrier against desiccation, but basic evolutionary features, including the heritability of hydrocarbon profiles and how they are shaped by natural selection are largely unknown. We used a new pharaoh ant (Monomorium pharaonis) laboratory mapping population to estimate the heritability of individual cuticular hydrocarbons, genetic correlations between hydrocarbons, and fitness consequences of phenotypic variation in the hydrocarbons. Individual hydrocarbons had low to moderate estimated heritability, indicating that some compounds provide more information about genetic relatedness and can also better respond to natural selection. Strong genetic correlations between compounds are likely to constrain independent evolutionary trajectories, which is expected, given that many hydrocarbons share biosynthetic pathways. Variation in cuticular hydrocarbons was associated with variation in colony productivity, with some hydrocarbons experiencing strong directional selection. Altogether, this study builds on our knowledge of the genetic architecture of the social insect hydrocarbon profile and indicates that hydrocarbon variation is shaped by natural selection.
Keywords: cuticular hydrocarbons, heritability, genetic correlations, selection, nest-mate recognition
1. Introduction
The ability to discriminate between kin and non-kin promotes the evolution and maintenance of sociality because it allows altruistic behaviours to be directed towards related individuals [1]. Nest-mate recognition, the process by which social insects recognize individuals belonging to their colony, is encoded by cuticular hydrocarbons, apolar lipids found on the cuticle of the majority of insect taxa that primarily function as a waxy barrier that prevents desiccation [2–6]. Within a social insect species, individuals have qualitatively similar hydrocarbon profiles, but the profile differs in relative proportions depending on the colony of origin. Cuticular hydrocarbons are homogenized throughout a colony via trophallaxis (the exchange of regurgitated liquid) and allogrooming between colony members [6–9], creating a gestalt colony odour [10]. In addition to nest-mate recognition and desiccation prevention, social insects also rely on hydrocarbons to encode information about an individual's reproductive and dominance status and task within the colony [11–13].
Despite the central role of cuticular hydrocarbons in insect societies, we still know very little about fundamental genetic and evolutionary features shaping them, including the relative contribution of genetic and environmental factors to phenotypic variation in hydrocarbon profiles within and between colonies [14,15], and how natural selection acts on this variation. Numerous studies have demonstrated that the social insect hydrocarbon profile is influenced by genotype, by tracking familial lines [7,16–18], using cross-fostering designs [8], or demonstrating an association between hydrocarbon diversity and within-colony genetic variation [19,20]. However, very few studies have examined the underlying genetic architecture of social insect hydrocarbons within a formal quantitative genetic framework [21]. Traditional quantitative genetic crossing and pedigree-based mapping populations provide a powerful means to elucidate the contribution of genetic and environmental factors to variation in hydrocarbon profiles [22–26].
The genetic architecture of social insect cuticular hydrocarbons is expected to be more complex than solitary insect cuticular hydrocarbons because it depends on the collective genetic makeup of the colony [27,28]. In social insects, the hydrocarbon profile of each individual can be made up of compounds synthesized directly by the individual itself, as well as compounds synthesized by nest-mates and socially transferred to the individual [6–9,28]. More generally, in social organisms such as social insects, an individual's own traits can be influenced directly by its own genotype (i.e. direct genetic effects) but also indirectly via the genotype of social partners (i.e. indirect genetic effects; [27,28]).
To fully understand the hydrocarbon profile's potential evolutionary response to natural selection, in addition to understanding quantitative genetic parameters such as heritability and genetic correlations, we must also understand the fitness consequences of phenotypic variation in the hydrocarbon profile. Knowledge of the fitness consequences of trait variation is necessary to characterize the type (e.g. directional, stabilizing or disruptive) and strength of natural selection acting on a trait [29–32]. Variation in the social insect hydrocarbon profile may affect individual survival and colony productivity by affecting desiccation resistance [33–36], or by influencing chemical communication among nest-mates and the collective behaviour of the colony [11–13,34].
Hydrocarbon structural classes (i.e. alkenes, linear alkanes, monomethyl alkanes and dimethyl alkanes) have distinct functional properties that are likely to influence the roles they play in insect societies and how they are shaped by natural selection [14,20,37,38]. Linear alkanes provide the best desiccation resistance because these molecules tightly aggregate [37,39]. On the other hand, alkenes and monomethyl and dimethyl alkanes are expected to play a larger role in chemical communication because they can be distinguished by the position of their double bond or of the methyl group(s), while linear alkanes can only be distinguished based on their chain length [40,41]. This increased complexity allows alkenes and monomethyl and dimethyl alkanes to encode more information. There is evidence that linear alkanes are less heritable and not transferred between workers as much as monomethyl and dimethyl alkanes, suggesting that linear alkanes are less informative for nest-mate recognition [8].
Here, we use a genetically highly variable laboratory population of pharaoh ant (Monomorium pharaonis) [42,43]. We extracted hydrocarbons from pools of workers and used the pedigree information of the colonies to estimate the heritability of and the genetic correlations between hydrocarbons. Additionally, we used a random forest analysis to identify hydrocarbons that best discriminate between our M. pharaonis colony genotypes. Finally, we estimated the strength and pattern of natural selection putatively acting on hydrocarbons in the laboratory.
2. Methods
(a). Experimental design and colony maintenance
Monomorium pharaonis colonies primarily live in association with humans both in the tropics in their presumed native range and in introduced temperate regions [44]. Colonies contain multiple queens and produce new colonies by budding [45,46]. We used 48 laboratory-reared M. pharaonis colonies (hereafter ‘colony genotypes’) of known pedigree from our heterogeneous stock mapping population, which was created from eight initial laboratory stock colonies that were systematically intercrossed for nine generations (electronic supplementary material, figures S1 and S2; see [42,43] for details). We split each colony genotype into three equally sized replicates (hereafter ‘colony replicates’) that initially consisted of four queens, 400 ± 40 workers, 60 ± 6 eggs, 50 ± 5 first instar larvae, 20 ± 2 second instar larvae, 70 ± 7 third instar larvae, 20 ± 2 prepupae and 60 ± 6 worker pupae (electronic supplementary material, figure S3). These colony demographics represent a typical distribution found in a relatively small M. pharaonis colony [46,47]. We set up these colonies in separate blocks (usually consisting of 15–18 replicate colonies) between May and November 2016.
We maintained all colony replicates on a 12 : 12 h light : dark cycle and at 27 ± 1°C and 50% relative humidity. We fed each colony replicate twice per week with an agar-based synthetic diet [48] and mealworms. Water was provided ad libitum via a glass tube plugged with cotton. Colony replicates nested between two glass slides (5 cm × 10 cm) housed in a plastic container (18.5 cm × 10.5 cm × 10.5 cm) lined with FluonⓇ.
(b). Behavioural and colony productivity data collection
As reported in [43], we surveyed each colony replicate for five collective behaviours and two measures of colony productivity (electronic supplementary material, figure S3): (i) foraging, (ii) aggression, (iii) exploratory rate, (iv) group exploration, and (v) colony exploration (see [43] for details). After we completed the behavioural assays, the queens from each colony replicate were removed to trigger the production of new queens and males [49,50]. We conducted weekly surveys of the number of worker, gyne (i.e. virgin queens) and male pupae produced, until all brood matured. We quantified: (i) the total number of sexual pupae (i.e. gynes plus males), and (ii) the total number of worker pupae as measures of colony productivity. Many of the collective behaviours were phenotypically correlated with each other and foraging and exploratory rate were associated with colony productivity (see [43] for full details).
(c). Collection, extraction and analysis of cuticular hydrocarbon samples
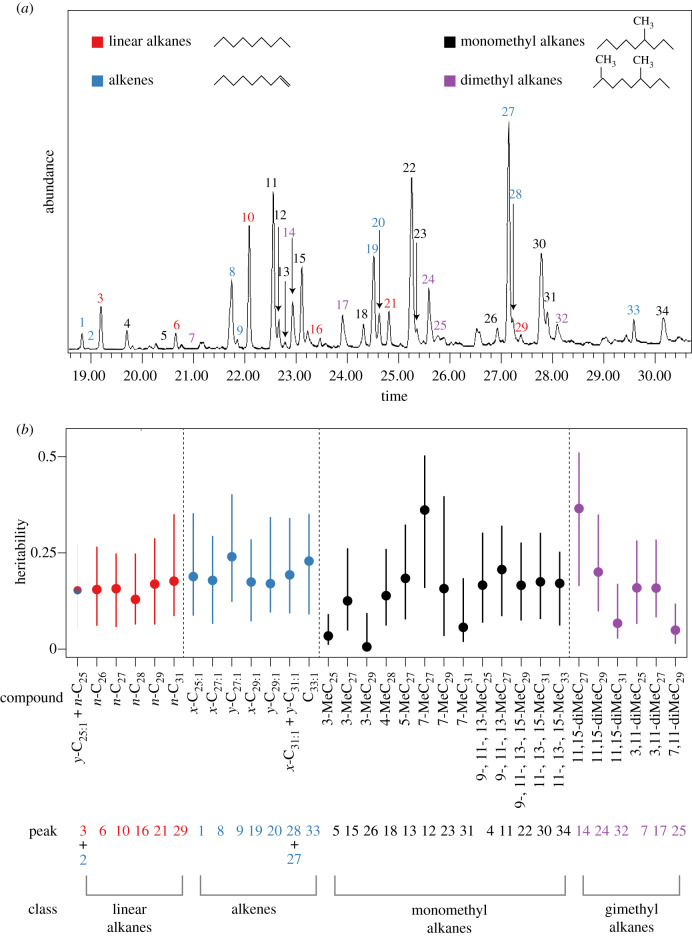
Upon completion of behavioural and productivity surveys, from each colony replicate, we collected three samples, each consisting of 15 workers, for the extraction and analysis of cuticular hydrocarbons (electronic supplementary material, figure S3). To extract the mean cuticular hydrocarbon profile of each replicate colony, we transferred each group of workers into a clean 2 ml glass vial and rinsed in 200 µl of high performance liquid chromatography grade (99%) pentane for 10 min. We injected 3 µl of each extract into an Agilent 6890 N gas chromatograph (GC) coupled with a 5375 Agilent mass spectrometer (MS). We identified 34 chemical compounds (figure 1a) by their retention times, fragmentation patterns and comparison with published results [51,52]. We integrated the area under each peak using MSD Chemstation. As they had similar retention times, some compounds co-eluted into the same peak (peak 2: y-C25:1 with peak 3: n-C25; peak 27: x-C31:1 with peak 28: y-C31:1; figure 1a). We combined the areas of each co-eluting pair, leaving 32 peaks available for statistical analysis. For full details, see the electronic supplementary material, file S1.
Figure 1.
(a) GC-MS spectrum of M. pharaonis cuticular hydrocarbons. All 34 identified peaks are numbered and colour-coded by structural class. Unidentified peaks were either contaminants or unidentifiable compounds. (b) Caterpillar plot showing heritability estimates of individual hydrocarbon compounds, with associated 95% confidence intervals, obtained from univariate animal models. Peak numbers shown in (a) are reported below the corresponding compound(s). Compounds in the plot are grouped and colour-coded by structural class and ordered by chain length (linear alkanes and alkenes) or by chain length and methyl position (mono- and dimethyl alkanes). (Online version in colour.)
We discarded 175 out of 432 cuticular hydrocarbon samples due to contamination and other technical failures, leaving 257 samples from 111 colony replicates (48 colony genotypes) for statistical analysis. The number of used cuticular hydrocarbon samples per colony replicate is reported in the electronic supplementary material, table S1.
(d). Statistical analyses
We standardized the raw hydrocarbon peak areas of each sample using the log-ratio transformation [53], as has commonly been used in the analysis of hydrocarbon data. As in our dataset, some samples had zero area values for certain peaks (electronic supplementary material, table S1), we added a small constant value (0.001) to each peak prior applying the transformation [54,55]. We also separately used a multiplicative simple replacement method [56] to deal with the zeros to verify our results (see Results section).
For each colony replicate, we had a single measure for each of the five collective behaviours and for colony productivity but as many as three measures for hydrocarbon peak values. Therefore, we used the mean hydrocarbon replicate value when estimating phenotypic correlations and selection gradients (see below).
We performed all statistical analyses in R v. 3.4.1 [57]. A detailed R markdown file including the R scripts, as well as a detailed explanation of each analysis, is included as electronic supplementary material, file S1.
(i). Heritability and genetic correlations estimates of cuticular hydrocarbon compounds
To assess narrow sense heritability (h2; defined from 0 to 1) and genetic correlations (rG; defined from −1 to 1) of cuticular hydrocarbon compounds, we analysed our data using the ‘animal model’ approach [58] with the R package ‘MCMCglmm’ [59]. This mixed-effect model uses a Bayesian Markov chain Monte Carlo approach to decompose phenotypic variance into its genetic and environmental components, allowing the estimation of quantitative genetic parameters. In an animal model, the pedigree of many individuals is used to make inferences about expected patterns of genetic relatedness, and together with observed patterns of phenotypic resemblance among individuals, heritability for measured traits and genetic correlations between traits are estimated. We treated our replicate colonies as ‘individuals’ in an animal model, and the pedigree of each colony traced the parents of the worker offspring through the mapping population. While the hydrocarbon profile of each individual worker is expected to depend both directly on its own genotype (direct genetic effects) and indirectly on the genotypes of its nest-mates (indirect genetic effects; [8,27]), our approach does not enable us to estimate the separate contributions of these direct and indirect genetic effects to the observed composite hydrocarbon profile of our replicate worker groups. That is, we cannot quantify the degree to which the hydrocarbon profile of each individual depends on compounds synthesized by that individual, as opposed to compounds synthesized by social partners, but we can quantify the degree to which phenotypic variation in the hydrocarbon profile of groups of workers is predicted by the genotypic makeup of those workers. Similarly, previous animal breeding studies have shown that the total contribution of direct and indirect genetic effects to total genetic variance and total heritability can be estimated by quantifying phenotypic variation among groups of individuals (e.g. [60]), although it is not possible to empirically tease apart the separate contribution of direct and indirect genetic effects.
We ran Bayesian univariate models to estimate the heritability of each hydrocarbon compound, and bivariate models (one for each pairwise combination of hydrocarbon variables) to calculate genetic correlations between compounds. Additionally, to verify the results of our univariate heritability models, we ran bivariate models for all combinations of hydrocarbons and took the average of these to get an additional heritability estimate for each hydrocarbon. Univariate and bivariate models had the same random and fixed effect structure, and differed only in terms of priors specification (electronic supplementary material, file S1). We included in the models individual identity, environmental variance and block (samples were collected at different time points) as random factors. Details of model specification are described in the electronic supplementary material, file S1.
(ii). Linear and quadratic selection gradients estimates
The total number of new reproductives (gynes and males) produced by colonies is a natural measure of colony-level investment in reproduction, and hence a measure of colony-level fitness [61]. However, because M. pharaonis queens cannot form new colonies without workers (i.e. colonies reproduce by budding; [45,46]), we also quantified a second measure of colony-level fitness, the total number of new workers produced. We estimated strength and type of selection (e.g. directional, stabilizing or disruptive) acting on individual hydrocarbons using a regression approach [32]. Briefly, we first estimated the fitness function relating colony productivity to the abundance of a specific hydrocarbon with a generalized additive model (GAM), using the R package ‘mgcv’ [62]. Then, we obtained linear (β) and quadratic (γ) selection gradients from the fitted GAM model using the function gam.gradients in the package ‘gsg’ [63]. Prior to running the model, hydrocarbon variables were mean-centred and variance standardized. Details of model specification are described in the electronic supplementary material, file S1. We adjusted p-values using the false discovery rate (FDR) method.
(iii). Principal component analysis
Our heritability and selection gradient estimates described above are univariate, rather than multivariate, because mixed models can experience issues with model convergence when a large number of traits are included in one model [58,64]. To also consider multivariate models, we first conducted a principal components analysis (PCA) to reduce the dimensionality of our dataset. We included 29 of the 32 hydrocarbons in the PCA, excluding the three hydrocarbons (peaks 23, 26 and 31) that had zero values in the data. We excluded these peaks because PCA is very sensitive to small values, and samples with zeros were clear outliers in a PCA including them (results not shown). We kept the first eight principal components (PCs) which explained approximately 90% of the variation in our dataset (electronic supplementary material, figures S4–S7 and table S2; see the electronic supplementary material, table S3 for PC loadings). We subsequently used the same approaches described above to estimate the heritability and selection gradients of the eight PCs, but included all eight PCs in all models. We did not estimate the genetic correlations between the PCs because, by definition, PCs are orthogonal to each other and, therefore, unlikely to be correlated.
(iv). Correlation between hydrocarbon compounds and collective behaviours
We ran Spearman's rank-order correlations to evaluate the strength and direction of association between cuticular hydrocarbons and collective behaviours. We ran a model between each compound and each of the five collective behaviours (160 models in total; [43]), with adjusted p-values using the FDR method.
(v). Random forest classification analysis
Finally, we used a random forest (RF) classification analysis [65] to determine which cuticular hydrocarbon peaks can best discriminate across the 48 colony genotypes. Although this method does not take into account pedigree relationships, it can provide hints about which hydrocarbons are more variable among colony genotypes, thus highlighting compounds that might be involved in nest-mate recognition. We ran a stratified sampling RF classification model with replacement using the R package ‘randomForest’ [66], and we considered hydrocarbon samples from colony replicates belonging to the same colony genotype as part of one of the 48 colony genotypes classes. We used the mean decrease in model accuracy [67] to interpret hydrocarbons importance in classifying the colony genotypes. We tested whether hydrocarbon structural classes varied in their ability to discriminate between colony genotypes using a linear model. Model details and specifications are included in the electronic supplementary material, file S1.
3. Results
(a). Heritabilities and genetic correlations
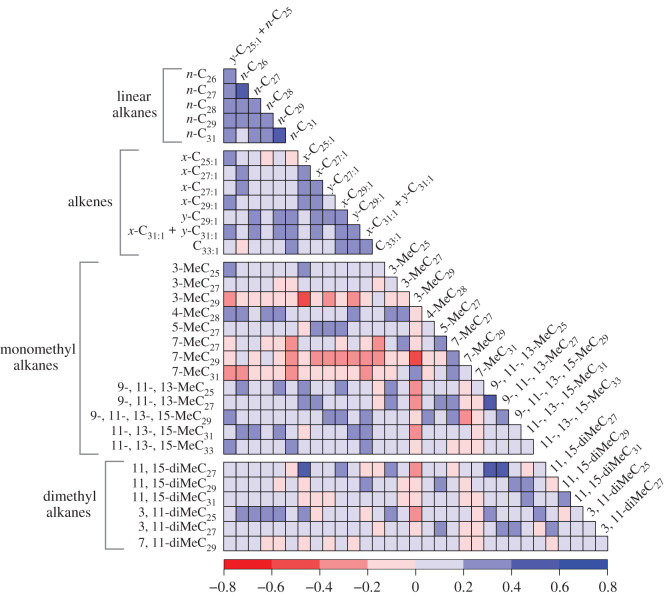
We estimated the heritability of individual hydrocarbons to be between 0.006 and 0.36, with a median estimated heritability of 0.17, in our univariate models (figure 1b). The mean of our bivariate heritability estimates was very similar to our univariate estimates (electronic supplementary material, figure S8). We estimated the heritability of the eight PCs to be between 0.004 and 0.20, with a median estimated heritability of 0.15 (electronic supplementary material, figure S9). Our pairwise genetic correlations estimates indicate that strong genetic correlations are common as 203 out of 503 (40.3%) estimates were greater than 0.2 or less than −0.2 and most of these (167) were positive (figure 2). Strong, positive genetic correlations were especially common between two linear alkanes or between two alkenes (figure 2).
Figure 2.
Heatmap showing genetic correlation estimates among cuticular hydrocarbons obtained from bivariate animal models. Compounds are grouped by structural class and ordered by chain length (linear alkanes and alkenes) or by chain length and methyl position (mono- and dimethyl alkanes). Different colours indicate the magnitude and direction of the correlation. (Online version in colour.)
To ensure the additions of a small constant value did not skew our results, we also tried a multiplicative simple replacement method [56] to deal with the zeros in our data. Using this approach, we re-estimated the heritability of and genetic correlations between a subset of our hydrocarbons, focusing on the three peaks (23, 26 and 31) that contained zeros. The heritability and genetic correlation estimates were extremely similar between the two methods of dealing with zeros (electronic supplementary material, tables S4 and S5).
(b). Selection gradients
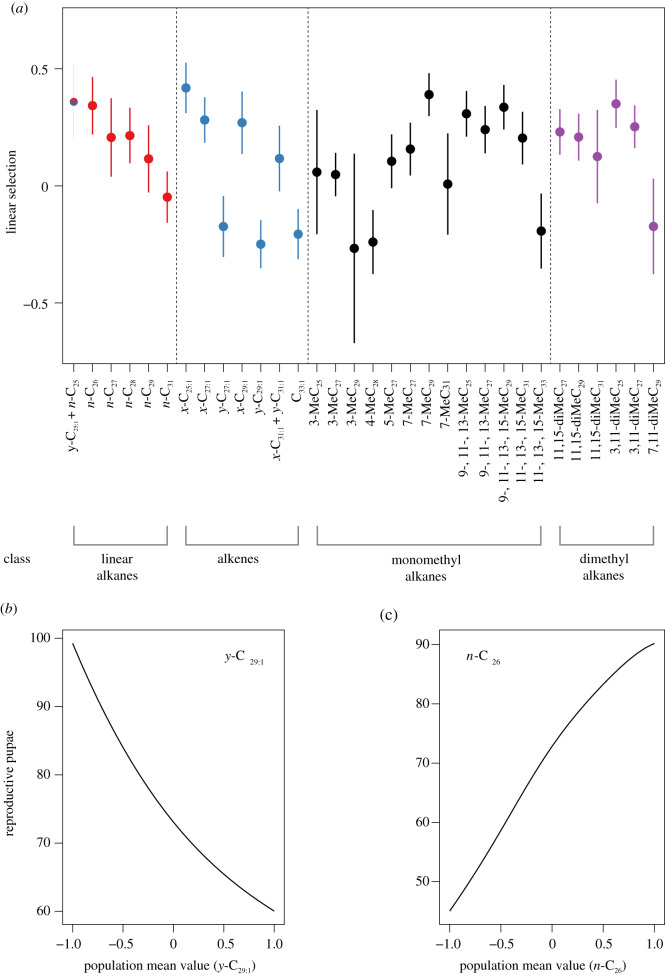
We report productivity data for each replicate colony in the electronic supplementary material, table S1. Our two definitions of fitness (the production of reproductives and workers) were positively correlated (Spearman's rank, ρ = 0.611, p < 0.001). We found evidence for significant positive or negative linear selection for 10 and 6 hydrocarbons when defining fitness as the production of reproductives or workers, respectively (figure 3; see the electronic supplementary material, table S6 for estimates, s.e. and p-values). All quadratic selection estimates were not significant. Additionally, we found evidence for positive linear selection on the first PC when defining fitness as the production of workers (see the electronic supplementary material, table S7 for estimates, non-parametric case-bootstrapped s.e. and p-values).
Figure 3.
(a) Caterpillar plot showing linear selection estimates, with associated case-bootstrapped standard errors, for individual hydrocarbons using sexual pupae production as a measure of colony fitness. Compounds in the plot are grouped and colour-coded by structural class and ordered by chain length (linear alkanes and alkenes), or by chain length and methyl position (mono- and dimethyl alkanes). Compounds showing a statistically significant selection gradient are labelled in bold. (b,c) Representative fitness landscapes for sexual pupae production as a function of population mean phenotype values of y-C29:1 and n-C26, respectively. (Online version in colour.)
(c). Phenotypic correlations
We report behavioural data for each replicate colony in the electronic supplementary material, table S1. We found significant phenotypic correlations between individual hydrocarbons and four of the five collective behaviours (foraging, aggression, colony exploration and group exploration) (electronic supplementary material, figure S10). We found that 20 of the 32 hydrocarbons were correlated with foraging, nine with group exploration, two with aggression and one with colony exploration.
(d). Random forest analysis
The error rate of the RF model was 17.1%, indicating that most of the hydrocarbon samples were assigned to the correct colony genotype (electronic supplementary material, file S1 and table S8). Two compounds, 11,15-diMeC27 and 7-MeC27, showed the best discrimination accuracy (electronic supplementary material, figure S11). Alkenes (t = 2.743, p = 0.011) and monomethyl alkanes (t = 2.261, p = 0.032) showed higher discrimination power than linear alkanes. There were no other differences in discrimination accuracy among pairwise combinations of structural classes.
4. Discussion
In solitary insects, many studies have characterized the heritability (e.g. [22,24]) and patterns of natural and sexual selection shaping the cuticular hydrocarbon profile (e.g. [26,68,69]). By contrast, little is known about the heritability and patterns of natural selection shaping the cuticular hydrocarbon profiles in social insects, despite the well-known role that cuticular hydrocarbon profiles play in nest-mate recognition, intra-colony signalling of task and reproductive and dominance status [8,13,21]. Here, we begin to elucidate the genetic architecture underlying variation in the hydrocarbon profile and to characterize how selection acts on it in a laboratory population of the ant M. pharaonis. We provide evidence that the hydrocarbon profile is heritable, shaped by selection and that many hydrocarbons, especially linear alkanes and alkenes, are genetically correlated with each other.
We estimated the total heritability of individual hydrocarbons to be between 0.006 and 0.36 with a median of 0.17 (figure 1b). Our heritability estimates of PCs were very similar, between 0.004 and 0.20 with a median of 0.14. These estimates are broadly similar to the estimated heritability for collective behaviour body size, caste ratio and sex ratio made with the same population [43], and are also similar to the range of heritability for individual hydrocarbons estimated from solitary and gregarious insect populations (e.g. [22,24,64]). We expected that compounds with high heritability estimates would also be among the best at distinguishing between colony genotypes in the RF analysis. In accordance with this prediction, the two compounds with the highest heritability estimates (11,15-diMeC27 and 7-MeC27) were also two of the top three compounds at distinguishing between colony genotypes in the random forest analysis. Many of the compounds with relatively high heritability in our study were also highly variable in a previous study of variation in hydrocarbon profiles among 36 M. pharaonis colonies collected at sites around the world [70]. Our study, together with this previous study, indicates that heritable variation for many cuticular hydrocarbons is maintained in M. pharaonis, both in our laboratory mapping population and in nature.
As described above, cuticular hydrocarbons play key roles in nest-mate recognition, and hence mediate aggression between colonies. However, the degree to which the necessary variation for genetically based recognition cues is maintained within populations remains broadly unclear [71]. Our results indicate that most compounds making up the cuticular hydrocarbon profile harbour genetic variation that could be informative for genetically based nest-mate recognition or mate choice. Alkenes and monomethyl and dimethyl alkanes are expected to play a larger role in chemical communication (e.g. nest-mate recognition) than linear alkanes because they can be distinguished by the position of the double bond or methyl group(s), while linear alkanes can only be distinguished based on their chain length [40,41]. In support of this prediction, previous work in ants found that monomethyl alkanes were more heritable than linear alkanes, suggesting that monomethyl alkanes are better indicators of colony membership [8]. Our results mostly support this prediction as well. For example, our RF analysis revealed that alkenes and monomethyl alkanes had a higher discrimination power than linear alkanes (electronic supplementary material, figure S11).
The genetic correlation estimates between many pairs of hydrocarbons were high, in particular between pairs of linear alkanes or alkenes (figure 2). Similarly, previous studies in fruit flies found many strong positive genetic correlations between individual hydrocarbons [24,72]. Such genetic correlations between hydrocarbons are not surprising, especially between compounds of the same structural class, because the production of different hydrocarbons involves many of the same biosynthetic processes (reviewed by Ginzel & Blomquist [73]). Overall, these genetic correlations mean that the independent evolution of hydrocarbons will be constrained to some degree.
Our study is, to our knowledge, the first social insect study to link variation in cuticular hydrocarbons with variation in colony productivity, although previous social insect studies have linked variation in cuticular hydrocarbons to worker survival [35] or to climatic or biotic variation [14,20,34,35]. We defined fitness in two ways: as the production of either new reproductives or new workers. Interestingly, we found similar linear selection patterns using both definitions, as all significant linear estimates were in the same direction between the two fitness measures (figure 3; electronic supplementary material, table S6). This suggests that the hydrocarbon profile optima are largely aligned for the production of both reproductives and workers in our study population. We note that a study of natural selection in a natural population of the red harvester ant Pogonomyrmex barbatus found no relationship between the number of gynes a colony produced and the number of its daughter colonies that survived at least 1 year [74], calling into question whether the number of reproductives produced by a colony is actually a good measure of fitness. However, this is likely in part because of very high mortality by queens attempting to found colonies independently [75,76], and this high variation in the mortality of new queens can be considered a component of offspring (i.e. new queen) fitness that depends on new queen traits, and not parent fitness that depends on the traits of the parental colony (see [77]). Moreover, we suggest that considering both the number of new reproductives and the number of new workers produced are probably better estimates of colony-level fitness in a species like M. pharaonis that reproduces by budding, where new queens do not found new colonies independently, but are accompanied by multiple other queens and workers.
These selection results beg the question: what is the likely causal link between variation in worker cuticular hydrocarbons and variation in colony productivity in our laboratory study population? Interestingly, in our study population, the relative abundance of many cuticular hydrocarbons was phenotypically correlated with the collective behaviour of replicate colonies (electronic supplementary material, figure S10), in particular the foraging rate, which in turn was also positively associated with colony productivity [43]. This relationship between variation in cuticular hydrocarbon profile, foraging rate and colony productivity could be mediated by effects of hydrocarbons on the desiccation resistance of workers [33,36]. However, our colonies probably experienced relatively low water stress because the colonies were kept in climate-controlled chambers at 50% humidity, and the colonies always had access to water. Alternatively, we speculate that worker hydrocarbon profiles might influence colony-level division of labour or task allocation [11,12,36,78,79], which could in turn influence foraging rate and colony productivity. As described above, in addition to effects on desiccation resistance, ant cuticular hydrocarbons are well known to influence nest-mate recognition and inter-colonial aggression in many ant species, including M. pharaonis [80]. However, colonies in our study were isolated from each other throughout the course of the study, so that differences in the outcome of aggressive encounters between colonies that probably contribute to differences in nature for colony survival and productivity [81] cannot explain the patterns of selection on hydrocarbon profile that we observed in our laboratory population. Similarly, while cuticular hydrocarbon profiles might also mediate mate choice in M. pharaonis, such a mechanism could not explain the association between worker hydrocarbons and colony productivity that we observed.
An interesting complication of the genetic architecture of social insect cuticular hydrocarbon profiles is that the social environment experienced by each individual within a social insect colony strongly influences its hydrocarbon profile, because hydrocarbons are mixed throughout the colony via trophallaxis and allogrooming between colony members [4,6–8]. As a result, the genetic architecture of the hydrocarbon profile, like other socially influenced traits, depends on the collective genetic makeup of colonies [27,28]. Because we quantified the cuticular hydrocarbon profile of groups of workers from each colony, we were not able to distinguish between hydrocarbons that were readily transferred among nest-mates and those that were only produced by a subset of workers and not transferred (see [8]), or to separately estimate the contribution of variation in direct versus indirect genetic effects to estimated total heritability [60,82]. Furthermore, we were not able to consider differences in cuticular hydrocarbons between individual workers based on age, task within the colony, or differences in genotypes within a colony.
We conducted the current study in a laboratory environment, which enabled us to strictly control the colony demography (i.e. queen number, worker number, etc.), diet and environmental conditions experienced by the colonies. Such control in particular is valuable, given the complexity of social insect colonies [27,83] and the sensitivity of the hydrocarbon profile to changes in the environment or diet [7,14,15,84,85]. Because M. pharaonis tends to be found in association with humans, we speculate that the laboratory conditions of our study might be more similar to the natural conditions experienced by M. pharaonis, when compared with other non-synanthropic species. Although future field studies are necessary, in particular to determine how variation in cuticular hydrocarbons affects colony productivity in a natural setting and whether the patterns of selection we observed in the laboratory are consistent in nature, a field study on a similar scale as our study is probably not feasible.
Overall, this study increases our understanding of the genetic architecture of the hydrocarbon profile and demonstrates that the hydrocarbon profile is shaped by natural selection. Although numerous genes underlying variation in the hydrocarbon profile have been identified in Drosophila [25], the hydrocarbon profile performs different functions in social insects and, therefore, future studies should focus on determining whether the same genes are involved in the expression of social insect hydrocarbon profiles, and how variation in these genes affects variation in the hydrocarbon profile. For example, a recent study used a candidate gene approach and found that inotocin, a peptide similar to oxytocin/vasopressin, regulates the production of hydrocarbons in the ant Camponotus fellah [86]. Future studies should use unbiased approaches such as quantitative trait locus mapping in mapping populations (e.g. [87]) such as ours, and association mapping in natural populations (e.g. [88]).
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Chloé Leroy for conducting the GC injections. We thank Michael Warner and Rohini Singh for comments that improved the manuscript.
Data accessibility
The data supporting this paper are included in the electronic supplementary material files.
Authors' contributions
J.W., L.P., P.d.E. and T.A.L.: conceptualization and methodology; J.W., L.P., T.A.L. and P.d.E.: experimental design and writing—review and editing; L.P. and J.W.: data analysis and writing—original draft; P.d.E. and T.A.L.: project supervision; T.A.L.: funding acquisition.
Competing interests
We have no competing interests.
Funding
This work was supported by National Science Foundation grant no. IOS-1452520 awarded to T.A.L.
References
- 1.Hamilton WD. 1964. The genetical evolution of social behaviour. I and II. J. Theor. Biol. 7, 1–16. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 2.Bonavita-Cougourdan A, Clément JL, Lange C. 1987. Nestmate recognition: the role of cuticular hydrocarbons in the ant Camponotus vagus Scop. J. Entomol. Sci. 22, 1–10. ( 10.18474/0749-8004-22.1.1) [DOI] [Google Scholar]
- 3.Lahav S, Soroker V, Hefetz A, Vander Meer RK. 1999. Direct behavioral evidence for hydrocarbons as ant recognition discriminators. Naturwissenschaften 86, 246–249. ( 10.1007/s001140050609) [DOI] [Google Scholar]
- 4.Lenoir A, Fresneau D, Errard C, Hefetz A. 1999. Individuality and colonial identity in ants: the emergence of the social representation concept. In Information processing in social insects (eds Detrain C, Deneubourg JL, Pasteels JM), pp. 219–237. Basel, Switzerland: Birkhäuser Basel. [Google Scholar]
- 5.Greene MJ, Gordon DM. 2007. Structural complexity of chemical recognition cues affects the perception of group membership in the ants Linephithema humile and Aphaenogaster cockerelli. J. Exp. Biol. 210, 897 ( 10.1242/jeb.02706) [DOI] [PubMed] [Google Scholar]
- 6.van Zweden JS, d'Ettorre P. 2010. Nestmate recognition in social insects and the role of hydrocarbons. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Bagnères A-G, Blomquist GJ), pp. 222–243. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 7.van Zweden JS, Dreier S, d'Ettorre P. 2009. Disentangling environmental and heritable nestmate recognition cues in a carpenter ant. J. Insect. Physiol. 55, 158–163. ( 10.1016/j.jinsphys.2008.11.001) [DOI] [PubMed] [Google Scholar]
- 8.van Zweden JS, Brask JB, Christensen JH, Boomsma JJ, Linksvayer TA, d'Ettorre P. 2010. Blending of heritable recognition cues among ant nestmates creates distinct colony gestalt odours but prevents within-colony nepotism. J. Evol. Biol. 23, 1498–1508. ( 10.1111/j.1420-9101.2010.02020.x) [DOI] [PubMed] [Google Scholar]
- 9.Leonhardt SD, Menzel F, Nehring V, Schmitt T. 2016. Ecology and evolution of communication in social insects. Cell 164, 1277–1287. ( 10.1016/j.cell.2016.01.035) [DOI] [PubMed] [Google Scholar]
- 10.Crozier RH, Dix MW. 1979. Analysis of two genetic models for the innate components of colony odor in social Hymenoptera. Behav. Ecol. Sociobiol. 4, 217–224. ( 10.1007/BF00297645) [DOI] [Google Scholar]
- 11.Greene MJ, Gordon DM. 2003. Cuticular hydrocarbons inform task decisions. Nature 423, 32 ( 10.1038/423032a) [DOI] [PubMed] [Google Scholar]
- 12.Martin SJ, Drijfhout FP. 2009. Nestmate and task cues are influenced and encoded differently within ant cuticular hydrocarbon profiles. J. Chem. Ecol. 35, 368–374. ( 10.1007/s10886-009-9612-x) [DOI] [PubMed] [Google Scholar]
- 13.Liebig J. 2010. Hydrocarbon profiles indicate fertility and dominance status in ant, bee, and wasp colonies. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Bagnères A-G, Blomquist GJ), pp. 254–281. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 14.Menzel F, Blaimer BB, Schmitt T. 2017. How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait. Proc. R. Soc. B 284, 20161727 ( 10.1098/rspb.2016.1727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menzel F, Schmitt T, Blaimer BB. 2017. The evolution of a complex trait: cuticular hydrocarbons in ants evolve independent from phylogenetic constraints. J. Evol. Biol. 30, 1372–1385. ( 10.1111/jeb.13115) [DOI] [PubMed] [Google Scholar]
- 16.Nehring V, Evison SE, Santorelli LA, d'Ettorre P, Hughes WO. 2011. Kin-informative recognition cues in ants. Proc. R. Soc. B 278, 1942–1948. ( 10.1098/rspb.2010.2295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin S, Trontti K, Shemilt S, Drijfhout F, Butlin R, Jackson D. 2012. Weak patriline effects are present in the cuticular hydrocarbon profiles of isolated Formica exsecta ants but they disappear in the colony environment. Ecol. Evol. 2, 2333–2346. ( 10.1002/ece3.319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin SJ, Vitikainen E, Shemilt S, Drijfhout FP, Sundström L. 2013. Sources of variation in cuticular hydrocarbons in the ant Formica exsecta. J. Chem. Ecol. 39, 1415–1423. ( 10.1007/s10886-013-0366-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dronnet S, Lohou C, Christides J-P, Bagnères A-G. 2006. Cuticular hydrocarbon composition reflects genetic relationship among colonies of the introduced termite Reticulitermes santonensis Feytaud. J. Chem. Ecol. 32, 1027 ( 10.1007/s10886-006-9043-x) [DOI] [PubMed] [Google Scholar]
- 20.Menzel F, Radke R, Foitzik S. 2016. Odor diversity decreases with inbreeding in the ant Hypoponera opacior. Evolution 70, 2573–2582. ( 10.1111/evo.13068) [DOI] [PubMed] [Google Scholar]
- 21.Boomsma JJ, Nielsen J, Sundström L, Oldham NJ, Tentschert J, Petersen HC, Morgan ED. 2003. Informational constraints on optimal sex allocation in ants. Proc. Natl Acad. Sci. USA 100, 8799–8804. ( 10.1073/pnas.1430283100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas ML, Simmons LW. 2008. Cuticular hydrocarbons are heritable in the cricket Teleogryllus oceanicus. J. Evol. Biol. 21, 801–806. ( 10.1111/j.1420-9101.2008.01514.x) [DOI] [PubMed] [Google Scholar]
- 23.Niehuis O, Büllesbach J, Judson AK, Schmitt T, Gadau J. 2011. Genetics of cuticular hydrocarbon differences between males of the parasitoid wasps Nasonia giraulti and Nasonia vitripennis. Heredity 107, 61–70. ( 10.1038/hdy.2010.157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma MD, Mitchell C, Hunt J, Tregenza T, Hosken DJ. 2012. The genetics of cuticular hydrocarbon profiles in the fruit fly Drosophila simulans. J. Hered. 103, 230–239. ( 10.1093/jhered/esr132) [DOI] [PubMed] [Google Scholar]
- 25.Dembeck LM, Böröczky K, Huang W, Schal C, Anholt RRH, Mackay TFC. 2015. Genetic architecture of natural variation in cuticular hydrocarbon composition in Drosophila melanogaster. eLife 4, e09861 ( 10.7554/eLife.09861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berson Jacob D, Zuk M, Simmons Leigh W. 2019. Natural and sexual selection on cuticular hydrocarbons: a quantitative genetic analysis. Proc. R. Soc. B 286, 20190677 ( 10.1098/rspb.2019.0677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linksvayer TA. 2006. Direct, maternal, and sibsocial genetic effects on individual and colony traits in an ant. Evolution 60, 2552–2561. ( 10.1111/j.0014-3820.2006.tb01889.x) [DOI] [PubMed] [Google Scholar]
- 28.Linksvayer TA. 2015. The molecular and evolutionary genetic implications of being truly social for the social insects. In Genomics, physiology and behaviour of social insects (eds Zayed A, Kent CF), pp. 271–292. London, UK: Academic Press Ltd-Elsevier Science Ltd. [Google Scholar]
- 29.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 30.Arnold SJ, Wade MJ. 1984. On the measurement of natural and sexual selection: theory. Evolution 38, 709–719. ( 10.2307/2408383) [DOI] [PubMed] [Google Scholar]
- 31.Janzen FJ, Stern HS. 1998. Logistic regression for empirical studies of multivariate selection. Evolution 52, 1564–1571. ( 10.1111/j.1558-5646.1998.tb02237.x) [DOI] [PubMed] [Google Scholar]
- 32.Morrissey MB, Sakrejda K. 2013. Unification of regression-based methods for the analysis of natural selection. Evolution 67, 2094–2100. ( 10.1111/evo.12077) [DOI] [PubMed] [Google Scholar]
- 33.Gordon DM. 2013. The rewards of restraint in the collective regulation of foraging by harvester ant colonies. Nature 498, 91 ( 10.1038/nature12137) [DOI] [PubMed] [Google Scholar]
- 34.Buellesbach J, Whyte BA, Cash E, Gibson JD, Scheckel KJ, Sandidge R, Tsutsui ND. 2018. Desiccation resistance and micro-climate adaptation: cuticular hydrocarbon signatures of different Argentine ant supercolonies across California. J. Chem. Ecol. 44, 1101–1114. ( 10.1007/s10886-018-1029-y) [DOI] [PubMed] [Google Scholar]
- 35.Sprenger PP, Burkert LH, Abou B, Federle W, Menzel F. 2018. Coping with the climate: cuticular hydrocarbon acclimation of ants under constant and fluctuating conditions. J. Exp. Biol. 221, jeb171488 ( 10.1242/jeb.171488) [DOI] [PubMed] [Google Scholar]
- 36.Friedman DA, Greene MJ, Gordon DM. 2019. The physiology of forager hydration and variation among harvester ant (Pogonomyrmex barbatus) colonies in collective foraging behavior. Sci. Rep. 9, 5126 ( 10.1038/s41598-019-41586-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin S, Drijfhout F. 2009. A review of ant cuticular hydrocarbons. J. Chem. Ecol. 35, 1151 ( 10.1007/s10886-009-9695-4) [DOI] [PubMed] [Google Scholar]
- 38.Menzel F, Morsbach S, Martens JH, Räder P, Hadjaje S, Poizat M, Abou B. 2019. Communication versus waterproofing: the physics of insect cuticular hydrocarbons. J. Exp. Biol. 222, jeb210807 ( 10.1242/jeb.210807) [DOI] [PubMed] [Google Scholar]
- 39.Gibbs AG, Rajpurohit S. 2010. Cuticular lipids and water balance. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Bagnères A-G, Blomquist GJ), pp. 100–120. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 40.Gibbs A, Pomonis JG. 1995. Physical properties of insect cuticular hydrocarbons: the effects of chain length, methyl-branching and unsaturation. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 112, 243–249. ( 10.1016/0305-0491(95)00081-X) [DOI] [Google Scholar]
- 41.Bos N, Dreier S, Jørgensen CG, Nielsen J, Guerrieri FJ, d'Ettorre P. 2012. Learning and perceptual similarity among cuticular hydrocarbons in ants. J. Insect. Physiol. 58, 138–146. ( 10.1016/j.jinsphys.2011.10.010) [DOI] [PubMed] [Google Scholar]
- 42.Pontieri L, Schmidt AM, Singh R, Pedersen JS, Linksvayer TA. 2017. Artificial selection on ant female caste ratio uncovers a link between female-biased sex ratios and infection by Wolbachia endosymbionts. J. Evol. Biol. 30, 225–234. ( 10.1111/jeb.13012) [DOI] [PubMed] [Google Scholar]
- 43.Walsh JT, Garnier S, Linksvayer TA. 2019. Ant collective behavior is heritable and shaped by selection. bioRxiv, 567503 ( 10.1101/567503) [DOI]
- 44.Wetterer J. 2010. Worldwide spread of the pharaoh ant, Monomorium pharaonis (Hymenoptera: Formicidae). Myrmecol. News 13, 115–129. [Google Scholar]
- 45.Passera L. 1994. Characteristics of tramp species. Boulder, CO: Westview Press. [Google Scholar]
- 46.Buczkowski G, Bennett G. 2009. Colony budding and its effects on food allocation in the highly polygynous ant, Monomorium pharaonis. Ethology 115, 1091–1099. ( 10.1111/j.1439-0310.2009.01698.x) [DOI] [Google Scholar]
- 47.Warner MR, Lipponen J, Linksvayer TA. 2018. Pharaoh ant colonies dynamically regulate reproductive allocation based on colony demography. Behav. Ecol. Sociobiol. 72, 31 ( 10.1007/s00265-017-2430-1) [DOI] [Google Scholar]
- 48.Dussutour A, Simpson SJ. 2008. Description of a simple synthetic diet for studying nutritional responses in ants. Insectes Sociaux 55, 329–333. ( 10.1007/s00040-008-1008-3) [DOI] [Google Scholar]
- 49.Edwards JP. 1991. Caste regulation in the pharaoh ant Monomorium pharaonis—recognition and cannibalism of sexual brood by workers. Physiol. Entomol. 16, 263–271. ( 10.1111/j.1365-3032.1991.tb00565.x) [DOI] [Google Scholar]
- 50.Walsh JT, Warner MR, Kase A, Cushing BJ, Linksvayer TA. 2018. Ant nurse workers exhibit behavioural and transcriptomic signatures of specialization on larval stage. Anim. Behav. 141, 161–169. ( 10.1016/j.anbehav.2018.05.015) [DOI] [Google Scholar]
- 51.Schmidt AM, d'Ettorre P, Pedersen JS. 2010. Low levels of nestmate discrimination despite high genetic differentiation in the invasive pharaoh ant. Front. Zool. 7, 20 ( 10.1186/1742-9994-7-20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Zweden JS, Pontieri L, Pedersen JS. 2014. A statistical approach to identify candidate cues for nestmate recognition. Front. Ecol. Evol. 2, 73 ( 10.3389/fevo.2014.00073) [DOI] [Google Scholar]
- 53.Aitchison J. 1982. The statistical analysis of compositional data. J. R. Stat. Soc. Ser. B (Method.) 44, 139–177. [Google Scholar]
- 54.Geiselhardt S, Otte T, Hilker M. 2012. Looking for a similar partner: host plants shape mating preferences of herbivorous insects by altering their contact pheromones. Ecol. Lett. 15, 971–977. ( 10.1111/j.1461-0248.2012.01816.x) [DOI] [PubMed] [Google Scholar]
- 55.Finck J, Berdan EL, Mayer F, Ronacher B, Geiselhardt S. 2016. Divergence of cuticular hydrocarbons in two sympatric grasshopper species and the evolution of fatty acid synthases and elongases across insects. Sci. Rep. 6, 33695 ( 10.1038/srep33695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martín-Fernández JA, Barceló-Vidal C, Pawlowsky-Glahn V. 2003. Dealing with zeros and missing values in compositional data sets using nonparametric imputation. Math. Geol. 35, 253–278. ( 10.1023/A:1023866030544) [DOI] [Google Scholar]
- 57.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 58.Wilson AJ, Réale D, Clements MN, Morrissey MM, Postma E, Walling CA, Kruuk LEB, Nussey DH. 2010. An ecologist's guide to the animal model. J. Anim. Ecol. 79, 13–26. ( 10.1111/j.1365-2656.2009.01639.x) [DOI] [PubMed] [Google Scholar]
- 59.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 60.Peeters K, Ellen ED, Bijma P. 2013. Using pooled data to estimate variance components and breeding values for traits affected by social interactions. Genet. Select. Evol. 45, 27 ( 10.1186/1297-9686-45-27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagner D, Gordon DM. 1999. Colony age, neighborhood density and reproductive potential in harvester ants. Oecologia 119, 175–182. ( 10.1007/s004420050774) [DOI] [PubMed] [Google Scholar]
- 62.Wood SN. 2017. Generalized additive models: an introduction with R. Boca Raton, FL: Chapman and Hall/CRC. [Google Scholar]
- 63.Morrissey M, Sakrejda K. 2014. gsg: calculation of selection coefficients. R package version 2. See https://rdrr.io/cran/gsg/.
- 64.Lihoreau M, Rivault C, van Zweden JS. 2016. Kin discrimination increases with odor distance in the German cockroach. Behav. Ecol. 27, 1694–1701. ( 10.1093/beheco/arw099) [DOI] [Google Scholar]
- 65.Breiman L. 2001. Random forests. Mach. Learn. 45, 5–32. ( 10.1023/A:1010933404324) [DOI] [Google Scholar]
- 66.Liaw A, Wiener M. 2001. Classification and regression by randomForest. R News 3, 18–22. [Google Scholar]
- 67.Cutler DR, Edwards TC Jr, Beard KH, Cutler A, Hess KT, Gibson J, Lawler JJ. 2007. Random forests for classification in ecology. Ecology 88, 2783–2792. ( 10.1890/07-0539.1) [DOI] [PubMed] [Google Scholar]
- 68.Blows MW. 2002. Interaction between natural and sexual selection during the evolution of mate recognition. Proc. R. Soc. B 269, 1113–1118. ( 10.1098/rspb.2002.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foley B, Chenoweth SF, Nuzhdin SV, Blows MW. 2007. Natural genetic variation in cuticular hydrocarbon expression in male and female Drosophila melanogaster. Genetics 175, 1465 ( 10.1534/genetics.106.065771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmidt AM. 2010. The invasion biology and sociogenetics of pharaoh ants. PhD thesis, University of Copenhagen, København, Denmark. [Google Scholar]
- 71.Rousset F, Roze D. 2007. Constraints on the origin and maintenance of genetic kin recognition. Evolution 61, 2320–2330. ( 10.1111/j.1558-5646.2007.00191.x) [DOI] [PubMed] [Google Scholar]
- 72.Ingleby FC, Hosken DJ, Flowers K, Hawkes MF, Lane SM, Rapkin J, Dworkin I, Hunt J. 2013. Genotype-by-environment interactions for cuticular hydrocarbon expression in Drosophila simulans. J. Evol. Biol. 26, 94–107. ( 10.1111/jeb.12030) [DOI] [PubMed] [Google Scholar]
- 73.Ginzel MD, Blomquist GJ. 2016. Insect hydrocarbons: biochemistry and chemical ecology. In Extracellular composite matrices in arthropods (eds Cohen E, Moussian B), pp. 221–252. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 74.Ingram KK, Pilko A, Heer J, Gordon DM. 2013. Colony life history and lifetime reproductive success of red harvester ant colonies. J. Anim. Ecol. 82, 540–550. ( 10.1111/1365-2656.12036) [DOI] [PubMed] [Google Scholar]
- 75.Wilson EO. 1971. The insect societies Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 76.Marti HE, Carlson AL, Brown BV, Mueller UG. 2015. Foundress queen mortality and early colony growth of the leafcutter ant, Atta texana (Formicidae. Hymenoptera). Insect. Soc. 62, 357–363. ( 10.1007/s00040-015-0413-7) [DOI] [Google Scholar]
- 77.Wolf JB, Wade MJ. 2001. On the assignment of fitness to parents and offspring: whose fitness is it and when does it matter? J. Evol. Biol. 14, 347–356. ( 10.1046/j.1420-9101.2001.00277.x) [DOI] [Google Scholar]
- 78.Wagner D, Brown MJF, Broun P, Cuevas W, Moses LE, Chao DL, Gordon DM. 1998. Task-related differences in the cuticular hydrocarbon composition of harvester ants, Pogonomyrmex barbatus. J. Chem. Ecol. 24, 2021–2037. ( 10.1023/A:1020781508889) [DOI] [Google Scholar]
- 79.Wagner D, Tissot M, Gordon D. 2001. Task-related environment alters the cuticular hydrocarbon composition of harvester ants. J. Chem. Ecol. 27, 1805–1819. ( 10.1023/A:1010408725464) [DOI] [PubMed] [Google Scholar]
- 80.Pontieri L. 2014. Discrimination behavior in the supercolonial pharaoh ant PhD thesis, University of Copenhagen, København, Denkmark.
- 81.Reeve HK, Hölldobler B. 2007. The emergence of a superorganism through intergroup competition. Proc. Natl Acad. Sci. USA 104, 9736–9740. ( 10.1073/pnas.0703466104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brinker T, Raymond B, Bijma P, Vereijken A, Ellen ED. 2017. Estimation of total genetic effects for survival time in crossbred laying hens showing cannibalism, using pedigree or genomic information. J. Anim. Breed. Genet. 134, 60–68. ( 10.1111/jbg.12245) [DOI] [PubMed] [Google Scholar]
- 83.Kronauer DJC, Libbrecht R. 2018. Back to the roots: the importance of using simple insect societies to understand the molecular basis of complex social life. Curr. Opin. Insect Sci. 28, 33–39. ( 10.1016/j.cois.2018.03.009) [DOI] [PubMed] [Google Scholar]
- 84.Liang D, Silverman J. 2000. ‘You are what you eat’: diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften 87, 412–416. ( 10.1007/s001140050752) [DOI] [PubMed] [Google Scholar]
- 85.Buczkowski G, Kumar R, Suib SL, Silverman J. 2005. Diet-related modification of cuticular hydrocarbon profiles of the Argentine ant, Linepithema humile, diminishes intercolony aggression. J. Chem. Ecol. 31, 829–843. ( 10.1007/s10886-005-3547-7) [DOI] [PubMed] [Google Scholar]
- 86.Koto A, Motoyama N, Tahara H, McGregor S, Moriyama M, Okabe T, Miura M, Keller L. 2019. Oxytocin/vasopressin-like peptide inotocin regulates cuticular hydrocarbon synthesis and water balancing in ants. Proc. Natl Acad. Sci. USA 116, 5597–5606. ( 10.1073/pnas.1817788116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Valdar W, et al. 2006. Genome-wide genetic association of complex traits in heterogeneous stock mice. Nat. Genet. 38, 879–887. ( 10.1038/ng1840) [DOI] [PubMed] [Google Scholar]
- 88.Kocher SD, Mallarino R, Rubin BER, Yu DW, Hoekstra HE, Pierce NE. 2018. The genetic basis of a social polymorphism in halictid bees. Nat. Commun. 9, 4338 ( 10.1038/s41467-018-06824-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this paper are included in the electronic supplementary material files.