Abstract
In this review, we present current state-of-the-art developments and challenges in the areas of thermal therapy, ultrasound tomography, image-guided therapies, ocular drug delivery, and robotic devices in neurorehabilitation. Additionally, intellectual property and regulatory aspects pertaining to therapeutic systems and technologies are addressed.
Keywords: Therapeutic systems and technologies, thermal therapy, ultrasound tomography, image-guided therapy, neurorehabilitation, intellectual property
I. Introduction
THERAPY is defined as “the manipulation, disruption, and/or destruction of biological tissue with the goal of correcting a percieved deficiency or presence of disease.” With this in mind, therapuetic systems contribute to numerous aspects of therapy; however, the emerging trend is for therapeutic systems to address all aspects of therapy in an integrative manner. We would argue that there are three critical building blocks of therapeutic systems – understanding the deficit/disease, understanding the physics of sensing and energy transfer, and devising the system-level engineering to develop the interventional device and workflow. Accordingly, the building blocks of therapeutic systems are the technologies discussed herein.
In this article, we will discuss the recent developments and present challenges in therapeutic systems and technologies (TST). The paper is organized as follows. Section II expands on the concept of a therapeutic system and identifies some of the key technological building blocks of a therapeutic system. Section III focuses on the delivery component of a therapeutic system – describing different techniques for manipulating, distrupting, and disturbing a biological system. In contrast, Section IV will discuss the necessary sensing aspects of a therapeutic system – ranging from pre-therapy, during therapy, and even post-therapy. Finally, Section V highlights some of the critical aspects of deploying therapeutic systems – intellectual property issues and regulatory aspects.
II. Therapeutic Systems
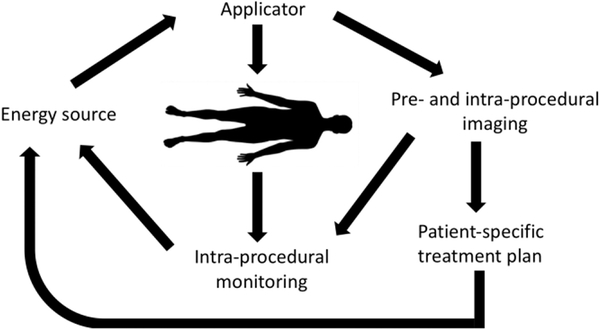
Therapy systems aim to deliver controlled and localized doses of energy (particle radiation, electromagnetic, optical, acoustic) for therapeutic interventions. Figure 1 provides a systems level overview of a generic therapy system. In this simplified model of a therapy system, one can consider two broad “tasks” in treating patients. The first and primary task of a therapy system is to intervene on the patient. This is generally accomplished by deploying energy into the patient (whether it be mechanical, electrical, or chemical). While not the primary purpose of a therapy system, the second “task” of sensing is equally important. This task, involves monitoring the status of the patient, the therapy delivery system, and the physical interaction of the two.
Fig. 1.
Components of a generic image-guided therapy system.
Within the intervening components, energy from an external source is coupled to the targeted region of the body via a minimally-invasive or non-invasive applicator. During the course of energy delivery, physical changes in tissue are induced. Some of these tissue changes may also serve as a surrogate for therapeutic response, and thus provide a means to monitor therapeutic effect. Imaging modalities such as ultrasound, X-ray, computed tomography (CT), fluoroscopy, and/or magnetic resonance imaging (MRI) are typically used to guide the placement of the applicator. These techniques ensure the energy is coupled to the intended target region and enable patient-specific adjustment of energy delivery profiles. Examples of therapeutic applications employing such a framework include: cardiac catheter ablation for treatment of cardiac arrhythmias [1]–[3]; MRI-guided laser interstitial thermal therapy of brain tumors [4]; ultrasound-triggered delivery of therapeutic agents [5].
In several of these applications, various treatment delivery parameters need to be determined on a patient-specific basis in response to variations in patient anatomy, differences in biophysical properties of tissue, and instrumentation setup. Computational models of tissue–energy interactions strive to determine the profile of energy delivered to the targeted anatomy, and surrounding regions, with the ultimate goal of predicting treatment effect. Such models have long been utilized to help design and develop new technologies and treatment delivery strategies. More recently, there have been growing efforts towards the use of modeling approaches to quantify the range of energy delivery parameters and treatment settings for individual patients.
III. Therapy Delivery
An understanding of energy transduction is critical to the concept of therapy delivery. The fundamental forms of energy (and thus energy transduction) are electro-magnetic, mechanical (including kinetic), chemical (and nuclear), and gravitational. The earliest evidence of energy-based treatment is thermal treatment in Neolithic skulls as described by Major [6], in the Edwin Smith Papyrus (ca. 3,000 BC) [7], and others. Over the centuries, our understanding of human structure and function have led to several energy-based therapies. Jean Jallabert, for example, studied the field of electrical stimulation as it applies to patients with paralysis (). Other reserch into electrical currents resulted in the development of cauterizing technology, described by Planta [8]. Soon thereafter, modern day ablation was described. Other technologies such as radiation therapy and therapeutic ultrasound were soon to follow. In the interest of brevity, we highlight several specific examples of modern-day therapy delivery.
A. Thermal Therapy
Thermal therapies are an important aspect of clinical treatment: Kelly and Ward [9] mention that Nagelschmidt applied diathermy as a treatment for articular and circulatory disease as early as 1897. Thermal therapies remain an important aspect of current clinical treatment strategies from diathermy for pain relief to ablation and hyperthermia for cancer therapy to surgical cutting and coagulation. It is a field rich with opportunities for device development and alternate strategies to achieve clinical goals.
Energy sources for thermal therapy include lasers [10], [11], harmonic scalpels, external and internal applicators for radio frequency (RF) [12]–[16] and microwave (MW) fields [17] — including nanoparticles and plasma field approaches [18] — lasers and focused ultrasound [19].Currently, new developments in tumor ablation (by almost all of these energy sources) are increasingly successful as first-line therapy approaches to cancer treatment [20]. Many other uses for thermal therapy range from cosmetic surgery, in which collagen shrinkage by RF heating is used for skin wrinkles and alleviation of varicose veins, to effective sealing of large vessels with RF current [21]–[23]. Laser sources are used theranostically in vascular disease and optical treatment [24]–[27], to name just two such applications.
Thermal therapies can be separated into two categories based on the intended temperature range: (1) hyperthermia (temperatures of 42–45 °C), and (2) thermal ablation (temperatures >50 °C). At hyperthermic temperatures, various local physiologic changes are induced including increased blood flow, reduced hypoxia, and enhanced permeability of vascular and cellular structures, among others. Hyperthermia has been studied for several decades, primarily for cancer treatment. It is currently in clinical use as adjuvant therapy, since hyperthermia is a potent radio- and chemo-sensitizer [28].
During ablation therapy (>50 °C) the goal is direct cell death by heat, which occurs after 1–2 min at 50 °C and within seconds above 60 °C [29]. Thermal ablation was first used in patients for treatment of cardiac arrhythmia starting in the 1980s. This therapy is usually based on radiofrequency (RF) heating, and is termed cardiac RF catheter ablation [30]. During treatment, a RF catheter is advanced into the heart through the vascular system using typically X-ray imaging. Once at the target site, RF energy is applied to the catheter tip to create a small thermal lesion (a few mm in diameter), to kill the tissue region responsible for arrhythmia.
In the 1990s, RF ablation started to be used as cancer therapy. Here, a needle-based heating applicator is advanced into the tumor using ultrasound, CT, or MRI to guide the applicator. The goal is to expose the whole tumor to temperatures that cause cell death (i.e., > 50 °C). Tumor ablation is currently in clinical use for treatment of cancers in the liver, lung, bone, kidney, and other locations. In addition to RF, other heating methodologies have been employed for tumor ablation.
Systems employing microwave power delivered to the targeted tissue via interstitial antennas are in clinical use for thermal ablation of tumors in the liver and other sites [31]. In this context, microwave refers to frequencies in excess of ~300 MHz, while the term RF ablation is restricted to systems operating at frequencies below ~10MHz. In contrast to RF ablation which requires current flow between two or more electrodes in contact with the body, microwave devices are designed to operate as antennas radiating microwave power that is absorbed into the adjacent tissue and causes heating. Most systems in clinical use operate at 915 MHz or 2.45 GHz; nevertheless, systems operating at other frequencies are under investigation [32]. Microwave ablation is particularly well suited for the creation of large volume ablation zones in vascular tissue [33], [34]. Recent technical developments in the field have focused on improving spatial control of ablation zones [35], [36], with the overall goal to reduce variability in ablation outcome [37]. In addition to tumor ablation with interstitial applicators, applicators in other form factors have been developed for treating other conditions including benign prostate hyperplasia [38], hyperhidrosis [39], heavy menstrual bleeding [40], and hypertension [41].
A more recent technology employed for both ablation and hyperthermia is high-intensity focused ultrasound (HIFU) [42]. This method non-invasively focuses ultrasound into deep tissue regions to cause localized tissue heating. Often, HIFU is combined with MRI since tissue temperature can be monitored non-invasively in real-time via MR thermometry. HIFU allows highly localized tissue heating at mm accuracy, and is currently in clinical use for treatment of uterine fibroids, prostate cancer, palliative treatment of bone metastases, and essential tremor. Applications for several other indications are currently under investigation.
Rigid and flexible catheter-based ultrasound devices have also been developed for hyperthermia and thermal ablation of tissue in a range of disease sites [43]. These devices consist of piezoelectric transducers (~5–20 mm in length) typically operating in the range ~5–9 MHz. At these frequencies, the acoustic wavelength in tissue is short (< 1 mm), and the energy radiated from devices is well collimated to the transducer length. Transducers are typically mounted in a linear arrangement, and are independently powered. As a result, a considerable strength of these devices is their ability to tailor energy deposition along the applicator axis by adjusting power applied to each transducer [44]. Angular control of power deposition is realized by appropriate selection of transducer geometry, with the most popular ones consisting of planar, lightly curved, and tubular transducers [45]. A transurethral ultrasound ablation system integrated with MRI thermometry has been clinically applied for treatment of benign prostatic hyperplasia [46]. Interstitial devices are under development for treatment of targets in the brain [47] and liver [47] and deployable applicators are under investigation for targeting pancreatic tumors via an endogastric approach [48].
B. Low-Intensity Ultrasound Devices in Therapy
The use of ultrasound for diagnosis is well known. However, it is not the case for ultrasound therapy. In fact, the exploration of ultrasound therapy can be dated back to 1930s [49]. The early treatment relied on thermal effects (usually correlated to high-intensity ultrasound) [50], while the recent focus has been shifted to the use of its non-thermal effects (or related to low-intensity ultrasound) [51]. Here, low-intensity refers to the spatial average temporal average (SATA) output of ultrasound within a range of 0.02 to 1 W/cm2. Among the low-intensity ultrasound techniques, pulsed ultrasound for therapy is commonly used.
Low-intensity pulsed ultrasound (LIPUS) was initially used for accelerating the healing of the bone fractures, which was approved by the FDA (U.S. Food and Drug Administration) in 1994 [52]. LIPUS’ usage for regenerating soft-tissue, such as tendon, cartilage and ligaments, was also reported [53]–[56]. The general belief that LIPUS can achieve its therapeutic effects is founded on the premise that a high-frequency periodic acoustic wave can cause acoustic streaming, molecular vibrations, and the other mechanical stimulations through its transmission through the medium [57]. Consequently, the intervention between LIPUS and tissue was shown to accelerate the healing of both normal and osteoporotic bones [58], and the formation of dental tissues [59].
Inhibition of inflammation is another important application of LIPUS technology. Before the 1990s, people thought that it was a placebo effect. However, recent double-blinded and randomized controlled trials showed that the LIPUS can treat inflammation [60], [61]. Harris’ group observed that the low-intensity ultrasound is more effective in treating facial swelling and trismus than the high-intensity ultrasound [62].
By using a focusing ultrasound transducer, studies suggested that LIPUS can stimulate neuron cells and brain circuits [63]. The use of LIPUS for treating mental health diseases holds great potential. It is more desirable over chemical treatment (which impacts the body’s metabolic system), electrical treatment (which requires electrode implantation) [64], transcranial magnetic stimulation (TMS) (which has a low spatial resolution of 1 cm) [65], and optical neuronal excitation (which requires genetic alternation) [66]. Focused ultrasound can achieve reversible, non-invasive stimulation with the spatial resolution at the millimeter scale [67].
Although LIPUS has shown great potential applications for different biomedical applications, only very few applications were approved by the FDA. Further research and development is still needed to explore new therapeutic modalities and advances for its clinical use. Regarding engineering designs, it is still very challenging to design LIPUS devices as battery-powered wearable devices. Controlling ultrasound emission intensity is also critical, and thus a LIPUS sensor is required. Such a sensor can detect output intensities. Ideally, a LIPUS generator and LIPUS sensor can form a closed-loop and automatically adjust LIPUS output intensities. A thermoacoustic sensor was proposed [68], but it is far from perfect.
C. Ocular Drug Delivery
The eye’s anatomy and physiology pose challenges to the development of effective drug delivery systems for the treatment of ocular diseases [69] since its various defense mechanisms make it difficult to achieve drug penetration into the eye at therapeutic levels [70]. Most topically administrated drugs are washed away by tears and blinking [69], [71], [72] and often enter the systemic circulatory system through conjunctival blood capillaries and lymphatics before reaching diseased intraocular tissues [71]–[73]. Topical administration of drugs into the eye is convenient, common, and well accepted by patients [72]. The cornea is the main pathway for topical application of drugs [70]. However, cornea barrier properties limit effective drug delivery into the eye [72]. This is especially true for therapeutic macromolecules such as proteins, bioactive carbohydrates, and DNA [74], [75]. Currently approved macromolecular therapies for the eye involve the use of anti-VEGF agents pegaptanib, ranibizumab, and aflibercept, and off-label use of bevacizumab (Avastin) for treatment of wet age-related macular degeneration (AMD) and diabetic retinopathy. These compounds have also been used off-label with success for treatment of other diseases such as corneal neovascularization, and neovascular glaucoma [75]. In addition, peptide drugs such as cyclosporine, growth factors, interferons and interleukins have been demonstrated to be useful in the treatment of uveitis, corneal wound healing, corneal herpes simplex infections, and in modification of ocular immune response.
Limited diffusion across the cornea results in a low bioavailability of 1–5% for most approved drugs and much lower bioavailability for macromolecules [71], [72]. Challenges that need to be addressed in ocular delivery of macromolecules include reducing treatment frequency, while increasing drug targeting to the diseased site to increase effectiveness and safety, and increasing the bioavailability of extraocular delivery methods [70]–[75]. Various mechanisms have been investigated to improve the delivery of drugs and especially macromolecules into the eye. These include intravitreal injections, hydrogel-contact loaded drugs that prolong drug exposure time, different viscosity enhancing polymers, usage of electric currents via iontophoresis, nanoparticles, and microneedles [71], [76], [77], [78], [79]. Biodegradable implants in the form of rods, plugs, discs or sheets have also been researched for ocular drug delivery [80] without much success in the clinic so far. As such, most currently existing methods suffer from various side effects and limitations. For example, drug-filled contact lenses may cause blurring of vision and local toxicity [81], [82], and ocular iontophoresis requires specialized drug formulations [83], [84]. Intravitreal injections are tolerable and safe for most patients, but they do come with potentially severe complications including retinal detachment, retinal thinning, endophthalmitis, and vitreous hemorrhage [85], [86]. For example, the prevalence of endophthalmitis is estimated to be 0.3% per intravitreal injection and 0.9% per eye and although repeated injections increase the rate of complications, it is often necessary to have these injections once a month [87].
Ultrasound has been used in ophthalmology for decades but mostly as a diagnostic imaging tool [88], [89]. Therapeutic ultrasound also has a potential for clinical applications in ophthalmology [70]. Ultrasound may offer several advantages including non-invasive application, short exposure times, flexibility in adjusting delivery parameters, ability to be applied with standard ophthalmic drugs, and versatility to easily combined with other drug delivery methods [70], [90], [91]. The first studies on the application of ultrasound to deliver compounds to the eye via corneal route were done in Russia in 1970s. In these studies, ultrasound was shown to have a positive effect on the clinical outcome of diseases at the anterior of the eye such as keratitis and corneal opacities [92]–[96]. Ultrasound application was also shown to result in faster healing of corneal ulcers and wounds, and faster resolving of corneal inflammation in patients [94], [95], [97], [98]. Zderic et al. also showed that ultrasound has the potential to be used as a safe and effective tool for transcorneal drug delivery with up to 10 times increase in drug delivery [91], [99], [100], [101], [102]. Specifically, an in vitro study showed that exposure of the cornea to 880 kHz ultrasound for 5 min at intensities of 0.19, 0.34 and 0.56 W/cm2 resulted in 2.1, 2.5, and 4.2 times increase, respectively, in the corneal permeability for a hydrophilic dye sodium fluorescein [99]. The permeability increase in vivo for sodium fluorescein using the same ultrasound parameters was 2 times at 0.19 W/cm2, 4 times at 0.34 W/cm2, and 10.6 times at 0.56 W/cm2 [100]. In a follow up study, ultrasound application in vitro at frequencies of 400–1000 KHz and intensities of 0.3 to 1 W/cm2 with exposure duration of 5 min, resulted in 32–109% of increase in the corneal permeability for a steroid ophthalmic drug dexamethasone [91]. The in vivo increase in the aqueous humor concentration for the same ophthalmic drug was 2.8 times and 2.4 times (p<0.05) with 0.8 W/cm2, 5 min ultrasound application at 400 kHz and 600 kHz, respectively [101]. Further, 880 kHz ultrasound application at 1 W/cm2 for 6 min was shown to enhance penetration of topically applied lipophilic small compound riboflavin into the corneal stroma with minimal epithelial damage for potential treatment of keratoconus [103].
In addition to improving the treatment of corneal disease, another potential application of ultrasound is for non-invasive delivery of vascular endothelial growth factor (VEGF) inhibitors (such as bevacizumab and ranibizumab) for treatment of back of the eye diseases such as wet AMD and diabetic retinopathies. Studies by Cheung et al. indicated that ultrasound-mediated delivery using 30 s of 1 MHz continuous wave ultrasound at 0.05 W/cm2 was effective with up to 1.6 fold increase in delivering macromolecules into the posterior segment of the rabbit eye via transscleral route [90]–[104]. No adverse effects were observedforupto2weeksafterultrasoundapplicationintheexposed eye tissues. Another study by Lafon et al. also indicated that ultrasound (applied at a frequency of 1.1 MHz, acoustic powers of 0.5–5.4 W, with duty cycle of 2.5% for two different pulse repetition frequencies of 100 and 1000 Hz) can enhance delivery of sodium fluorescein by up to 10 times via scleral route with no significant alteration observed in the eye tissues [105]. In this study, inertial cavitation was shown to be the mechanism responsible for this delivery enhancement. Sonoda et al. used ultrasound (frequency of 1 MHz, intensity of 2 W/cm2, exposure duration of 15–120 s, and duty cycle of 20–100%) in conjunction with commercially available microbubbles to enhance gene delivery into the back of the eye of New Zealand albino rabbits with no ocular tissue damage observed and with 1.5 to 2 times increase in the delivery efficiency [106]. Finally, the application of therapeutic ultrasound has also been reported as effective in moderately disrupting the integrity of blood-retinal barrier increasing penetration of systemically administered drugs into the retina [107]. In this study, the application of therapeutic ultrasound at a frequency of 690 kHz was effective in moderately disrupting the integrity of blood-retinal barrier when applied as 10 ms bursts of 1 Hz for 60 s at pressures of up to 1.1 MPa and was shown to increase penetration of systemically administered drugs into the retina.
D. Robotic Device in Neurorehabilitation
Central to the delivery of therapy is the mechanical manipulation of the therapeutic device. While manipulation is often manual (i.e., direct manipulation of a device by an operator), the benefits of robotic manipulation have been clearly demonstrated in the literature. It has been 26 years since the introduction of the first robotic device appeared in the proceedings of IEEE Workshop on Robot and Human Communication that perhaps precipitated the field of rehabilitation robotics [108]. Robotic devices are deployed in the clinical and research settings as both evaluation and intervention tools with a focus on arm function [109] and gait [110], [111] in populations with neurological impairment such as stroke [112]. They can be passive or actuated, instrumented, and portable or stationary with all devices fitting into two categories, based upon their design, referred to as end effectors or exoskeletons. Robotic devices undoubtedly offer incredible potential to advance neurorehabilitation due to their quantitative evaluation of movement and delivery of intervention. However, following three decades of development and inquiry the field remains challenged to reach its potential and advance mainstream neurorehabilitation.
The first challenge to rehabilitation robotics is access. Robotic devices have entered the clinical arena primarily at large academic medical centers, specifically those that have invested heavily in the integration of clinical research endeavors with conventional clinical care. Presence in neighborhood clinics and hospitals is exiguous and therefore the potential to advance neurorehabilitation is greatly restricted. Many device developers have addressed this challenge head-on through the development of portable devices hoping to access clinical settings with more modest budgets. However, cost-optimized versions of full-scale devices stand to lose some of their most clinically valuable and game-changing attributes, namely sensors capable of providing high resolution measurements of movement dysfunction. With the field of neurorehabilitation moving toward consensus on motor control diagnoses [113], the field of rehabilitation robotics must retain high resolution measurement and position it as a critical attribute for evaluating movement dysfunction to avoid missing widespread adoption in an evolving neurorehabilitation environment. Increasing access of devices without compromising its greatest strengths remains a challenge.
The second challenge to the field of rehabilitation robotics is the most daunting because it involves a paradigm shift. The field emerged and has come to rest squarely upon the foundation of increasing efficiency of care or the ability for a clinician to deliver more therapy to more patients in a given period of time [114], [115]. The rationale is sound in that the population is aging and the incidence of stroke is increasing, and that increased intervention often leads to improved outcomes. However, large-scale high-quality clinical trials have demonstrated unequivocally that in comparison to conventional care [116], [117] robotics may not be reaching the expected potential to advance neurorehabilitation. Device developers will need to revisit the rationale driving design of devices and the subsequent methods for application in both evaluation and intervention. Perhaps if devices weren’t designed to emulate conventional care, such as through the practice of functional tasks in a virtual environment, but instead attempted to target key movement impairments, they might leverage their advantage of quantitative control. For example, a device can be designed to specifically target key movement impairments such as upper extremity flexion synergy or shoulder and elbow weakness and be employed to systematically progress an intervention based upon restoration of the impairment [118]. This paradigm shift in the application of rehabilitation robotics may serve to address a looming challenge in conventional stroke rehabilitation that postulates functional improvements to result from enhanced compensatory strategies in lieu of restoration of impairment [119]. In regards to evaluation and diagnosis, this paradigm shift would also position the field of rehabilitation robotics to be more in line with the evolving field of neurorehabilitation that seeks to link explicit movement observations during task analysis to the particular stage of movement contributing to the overall motor system diagnosis [113].
While the field of rehabilitation robotics has come a long way in the last three decades, there is still much work to be done. The strength of quantitative evaluation and intervention progression is powerful but can come at a steep price. Granted developers retain this attribute and increase access, the field will need to revisit the objective of rehabilitation robotics perhaps moving away from the replication of conventional care with greater efficiency and moving toward the integration of new scientific knowledge emerging in the movement science literature into new design and application strategies.
IV. Therapy Sensing
The sensing aspect of a therapeutic system is critical to the success of a procedure. While sensing is often focused on the image-guidance component of a therapeutic system, the process of sensing the patient and disease begins earlier. Pre-therapy or vastly referred to as pre-operative imaging provides the necessary information to plan a procedure for a specific patient. Distinctly different from a diagnsostic task, pre-procedural planning takes into account many factors including the patient characteristics, clinical diagnosis, and available therapeutic devices. Once a therapy is planned, the sensing task moves into the intra-operative domain. During an intervention, the imaging may provide guidance for or feedback from the therapy. Due to the diversity of requirements for sensing (modality, response time, etc), a wide range of sensing technologies may be used. While imaging is common, mechanical, chemical, and electrical sensing are also important. As therapy is applied, feedback is provided in the context of the interaction between the patient and therapeutic device. This feedback loop may occur in real-time or post-procedure. Several specific examples of sensing in therapeutic systems are highlighted below.
A. Image-Based Modelling
One area where patient-specific modeling has already been integrated into clinical practice is image-guided radiotherapy. Here, the computer simulates radiation exposure to ensure adequate radiation dose is delivered to the tumor while limiting exposure to other tissues, with the goal to limit any side effects. One reason that contributed to the rapid integration of computer models into such radiotherapy systems is the complexity of the system (multiple energy sources are rotating around the patient), which makes any direct control by a human operator unfeasible. But there are many other areas where similar approaches may provide patient benefit and/or enhance cost effectiveness.
For example, when conducting a liver tumor ablation procedure with a radiofrequency based system, the clinical objective is to deliver a therapeutic thermal dose to the targeted tumor and a margin of surrounding tissue, while limiting thermal damage to surrounding healthy structures. Computational models may be used to determine a range of energy delivery parameters outlined below with the objective of yielding an optimal, or alternatively, an acceptably good treatment [120].
The number of RF electrodes inserted into the target
The optimal path for guiding the electrodes from the skin surface to the tumor
Power applied to each active electrode
Duration of tissue heating
For this application, computational models may be employed to solve for the electric fields in tissue, resistive heating, and bioheat transfer. Model outputs, such as the transient temperature profile may be used to assess the likelihood of observing specific biophysical outcomes [121], [122]. Differential equation-based models have been extensively investigated for patient-specific modeling. A challenge with physics-based models is the requirement for knowledge of variable tissue biophysical properties. This variability is due to inter-patient differences, tissue/disease state, as well as non-linear changes in tissue physical properties induced by the applied energy. Thus, there is a need to develop techniques for estimating tissue biophysical properties from pre-procedural imaging data that can be readily integrated within the therapeutic procedure workflow. Techniques for quantifying the uncertainty in model outputs are under development [123].
Alternatively, patient-specific models can be used to assess the quality of interventions when there is no practicable method for imaging treatment outcome. Examples of such interventions include deep-brain stimulation for treatment of Parkinson’s disease and tumor-treating fields for glioblastomas [124]. In both these interventions, the therapeutic effect is due to the spatial profile of electric fields induced in the brain by implanted or external electrodes. Since there are few practical means for visualizing electric fields in vivo, computational models of tissue-energy interactions capturing the spatial electric field profiles provide a valuable tool for assessing treatment response. And as inter-patient variations in anatomy and tissue biophysical properties may yield substantial differences in electric field profiles for the same applied energy levels, such models provide an added source of information to clinicians for interpreting treatment response.
1). Ultrasound Tomography
Ultrasound Tomography (UT) provides a means to non-invasively image a region of interest (ROI) and it constitutes a promising tool for various cancer detection applications [125]. In addition, it offers clear improvement over the acoustic mapping produced by B-mode imaging, which produces a qualitative mapping of the ROI’s acoustic properties by measuring the echoes produced by the scattered incident field in inhomogeneous medium [126]. Measurement of changes in acoustic impedance allows for the identification of boundaries between media. While B-mode is capable of producing images of reasonable resolution, it only utilizes a subset of the information encapsulated within the measured scattered field. Accordingly, techniques, like UT that are capable of recovering additional information from the scattered wave such as the speed of sound, acoustic attenuation, and density of the tissue within the ROI are currently employed for use in practical applications. The tomography problem can be summarized as relating the measurements of the scattered waves recorded by the transducers to the properties of the inhomogeneous ROI through the wave equation [127]–[129]. The application of UT is a far safer alternative to the ionizing radiation subjects are exposed to in a computer tomography (CT) scan.
During ultrasound tomography, an array of transducers is used to collect information about the scattered field resulting from the excitation of an individual transducer to a region of interest. The process of exciting a unique transducer element while the rest of the array collects measurements is repeated until a predetermined number of elements have been excited [130]–[132]. To implement this in hardware, the collected data, usually on the order of several GBs, is transferred to a PC and then processed with custom reconstruction algorithms [133].
The main limitations of UT lie within its difficulty to discretize the spatial Fourier transform to solve the nonlinear, ill-posed inverse problem with massive amount of data, long computational time and the electronic hardware restrictions. Thus, it is necessary to use an appropriate data acquisition techniques to model the forward problem and solve the inverse problem. In fact, the real-time hardware needed for UT is more challenging than a traditional pulse-echo imaging systems. However, with new advances in computing, UT is becoming a more viable option against conventional imaging methods for detecting cancer and other diseases. On the other hand, ultrasound has limitations on passing noticeable amount of energy through the skull. This is due to the high acoustic attenuation and contrast properties of the skull which result in phase aberration and low signal strength.
Several well characterized modes of operation exist and are used for UT. These include refraction corrected time-of-flight tomography [134], full wave inversion [128], contrast source inversion [135], Born inversion and distorted Born inversion methods [130], [131]. Regardless of the method selected, the objective of each algorithm is to estimate the size and properties of an inhomogeneity within the ROI. This can be achieved by measuring the speed of sound and the acoustic attenuation in various media. However, the inverse problem is ill-posed and requires the use of regularization techniques.
In terms of wave model, there are two main mathematical approaches to solve the inverse problem in UT. The first one uses the Greens functions, which can reduce the problem to the nonlinear Fredholm integral equations [136]. Efficient procedures have been demonstrated to solve the linear and the nonlinear ill-posed inverse problems [136], [137]. Several valid linear approximations to the nonlinear problem of UT have been developed but it will be restricted to the proximity of the required solution [138]–[140].
The second approach to deal with the inverse problems of UT is by wave inversion which solve for the inverse of the hyperbolic partial differential wave equation. A gradient based iterative approach, known as the propagation back projection method, was used to solve the wave inversion problem by minimizing the residual function instead of using the integral representation with the Greens function [141]. An adaptive finite element method was introduced in [142], [143] to solve for the coefficients of the wave inversion problem. It was also shown in [144] that this method could be parallelized to result in a more computationally efficient implementation of the problem than solutions based on the Greens function.
Another method of determining these properties is the Distorted Born Iterative (DBI) method [130], [131], [136] which involves interactively solving the well-posed forward scattering problem followed by solving of the ill-posed inverse scattering problem to gain information about the total field within the ROI and the scattering function, respectively [131], [132]. This method relies upon the Born approximation, which becomes increasingly inaccurate when using high frequencies or imaging strongly scattering medium. While the DBI method is capable of producing high resolution images, it remains limited by the fact that the inverse scattering problem is ill-posed.
Accordingly, it is necessary to use regularization techniques as means of circumventing the ill-posed problem. Many techniques for utilizing regularization in conjunction with the DBI method have been previously proposed. For example, a novel approach that found the regularization parameter necessary to stabilize the inverse problem using the Rayleigh quotient iteration was explored in [145]. The method was able to stabilize both full and partial receiver angular coverage while sacrificing only a minimal amount of spatial resolution. Another method utilizes a multiple frequency iterative process similar to the one presented in [130]. In addition, [146] utilized the Tikhonov regularization to solve the inverse problem. However, these regularization techniques only consider noise in the measurements and neglects the contamination of the coefficient matrices by factors such as round off error. To address this, [147] explored the use of Truncated Total Least-Squares (TTLS) regularization. It was found that TTLS algorithm provides better results for the reconstructed images. In addition, [131], [132] explored different approaches of the regularized TLS and the conjugate gradient method, respectively.
In order to validate the DBI method for reconstructing images with real data, Lavarello and Oelze [146] built a prototype with two transducers. The first transducer acts as a fixed source and the second transducer acts as a receiver that rotates in a circle around the object of interest. The experiment showed a reconstructed image of a balloon phantom achieving moderate contrast in speed of sound measurements.
Another research group was able to build a device for clinical practice [148]. The image reconstructions were still performed by traditional CPUs. In recent years, field programmable gate array (FPGA) based systems have gained attentions over the time for its unmatched data processing performance, and parallel computing capability [149]. Signal pre-processing is possible and the total data processing time is greatly reduced using FPGA based devices [150]. Even more, by combining both advanced FPGAs and graphical processing units GPUs, the research group at Karlsruhe Institute of Technology (KIT) has been able to achieve a speed-up by a factor of 47 [151]. In 2004, Karmanos Cancer Institute at Detroit, Michigan started to develop its ultrasound tomography device, later named computed ultrasound risk evaluation (CURE) [152]. They have built in their prototype a 20-cm ring immersed in water with 256 transducers, each transducer implemented with a dedicated data acquisition channel.
Although current clinical applications are mostly focused on breast tomography, transcranial and brain tomography using ultrasound isalsounder investigation [153]. The strong ultrasound scattering and attenuation caused by the skull could be alleviated by propagating into sinuses using a shear mode conversion instead of longitudinal mode. Such system could also be built by existed technology for performance evaluation [154].
B. Multimodal Sensing, Data Integration and Visualization
Multimodal sensing entails the integration of several multidimensional signals and images from different sources, including 2D, 3D or 4D images, physiological signals (temperature, electrical activation, etc.), surgical tracking and localization information, as well as the patient into a common environment that the clinician uses to deliver therapy in a minimally invasive fashion.
The move toward personalized medicine, in concert with the recent advances in computing, data acquisition, processing and interpretation, is transforming diagnostic and interventional medicine from a traditional artisanal craft based on clinicians’ experience into a discipline that relies on objective decision-making based on the integration of multi-dimension and multimodal data from heterogeneous sources [155]–[157].
Computer-integrated therapeutic systems and technology encompass the processing, analysis, and interpretation of images and signals and their integration with robot-assisted manipulators and surgical trackers to improve the quality of a therapeutic goal. Improvements result from helping clinicians better plan, deliver and monitor therapy, as well as advance training and simulation.
The emergence of medical imaging and robotic assistance have reshaped diagnostic and interventional medicine enabling more precise diagnosis and less invasive therapy. Moreover, some focus has shifted toward the development of specialized infrastructure such as specialized Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) systems specifically designed for intra-operative use. However, these approaches require access to dedicated technology compatible with the high-end imaging equipment, rendering such facilities too costly and decreasing the availability of affordable healthcare. Moreover, the use of non-standard interventional imaging equipment also imposes a highly invasive technology “footprint” on the clinical workflow, requiring significant modifications and potential retraining of the clinical staff. Hence, despite their envisioned benefits, such complex and workflow-intrusive infrastructure is unlikely to become standard-of-care, but rather specialized technology would be available only to centers that can afford it.
To mitigate the reliance on specialized infrastructure, a large body of work has been dedicated to the development of medical image processing algorithms for biomarker quantification, computational physiological models to study organ function and predict tissue response to therapy, and visualization paradigms to facilitate diagnosis and interventional navigation.
Nonetheless, several limitations have hampered the clinical translation and adoption of these tools: their performance, which encompasses accuracy/precision, computational efficiency, robustness and reliability; their obtrusiveness into the standard of care diagnostic and interventional workflow; and, the level of specialized training they require from the user in order to successfully accomplish the task at hand.
The development and evaluation of most computer-integrated interventional tools has focused exhaustively on their accuracy and precision, and is often accompanied by quests for accuracy improvements at the sub-millimeter level. A clear example is the evolution of medical image registration during the past two decades. Two review papers by Viergever and Maintz almost 20 years apart [158], [159] have concluded that the plethora of research in medical image registration during 1998–2016 has resulted in more and more complex algorithms that render incremental accuracy improvements over previous techniques, but yet led to minimal use of image registration in clinical practice beyond very rudimentary landmark-based rigid registration methods.
Similar trends are characteristic of other tools besides medical image registration, leading to the overall observation that wide-spread integration of computer-aided tools into the routine interventional workflow has been slow. This delay has been attributed to the limited availability of diagnostic and interventional data science techniques that can robustly handle the size, diversity and dimensionality of the acquired data that must be manipulated, often in real time [156], [157].
The accuracy and precision of computer-integrated tools for diagnosis and therapy are important metrics. However, their robustness guarantees their performance across a wide variability of biomedical data, including highly variable patient populations, diseases, normal and abnormal anatomy, as well as inhomogeneous biomedical signal and imaging data acquired using different modalities and systems. Similarly, their reliability ensures that the results are realistic and clinically relevant, and not artificial. Lastly, computational performance is a critical criterion for time-sensitive applications such as therapy guidance and monitoring. These applications require near real time performance with minimal delay in visualization and display that does not interfere with the clinical workflow or compromise therapy outcome.
V. Deployment Issues
While the development of new therapeutic technologies can be a very intellectually stimulating task, the reality of deployment cannot be overlooked. There are several pathways to deployment ranging from licensing to contract engineering to in-house development. There are many aspects of technology deployment to be considered, however, two of the most challenging areas are securing intellectual property and addressing regulatory issues.
A. Intellectual Property
Over the past few years, the US Patent and Trademark Office has implemented new rules and offered new fast-track facilities. This change implies that a patent may be issued to whoever filed first, even if someone else invented first. As such filing speed is important, but there may trade-offs to consider between speed and the broadness of a patent application claims. Given that many medical-device start-ups first launch or use their products outside the US, it is imperative to understand how international patent filings work (e.g., Patent Cooperation Treaty (PCT)). It is important to understand differences between European (EU) and US patent laws. For example, certain kinds of method claims are patentable in the US but not in the EU. The EMBS TST-TC can help students, post-graduates, faculty, BMEs from academia and from the industry get a better understanding about elements of patent applications; provide workshops on writing claims, with emphasis on US and EU patent rules; and discuss examples/strategy ideas about how to do effective prior art search. Members of EMBS TST-TC can also teach others how to use available search tools and how to find official patent office examination information on existing prior art of interest. Interacting with TST-TC members may offer a chance to write the outline of your own patent application. Networking with licensed patent attorneys may also be a benefit of approaching EMBS TST-TC members. In what follows, please find some basic information about patents and filings strategies.
Patents are not scientific articles
It is important to understand differences between patents and scientific articles. Original articles present empirical studies and describe the results of research work. They explain scientific methods in detail, cover novel results, discuss the statistical significance of the results. Contrary to articles, patents are property. They carry financial value, can be sold, purchased and can make you money (e.g., royalties, licenses). Patents block others, for a limited time, to use your discovery (e.g., others have to license your discovery or risk being sued by you in a Court of Law). The inventor’s rights are secured by claims, not by the technical description. During prosecution, the claims are negotiated with examiners from patent offices, not the patent application technical description.
Broad claims are essential
Let’s assume you want to patent a soft-drink straw. That is a good attempt because, if allowed, it covers any other variations of straws. But, examiners from patent offices may reject your claim for a general straw as being anticipated by others (e.g., Coca Cola). You could then negotiate a narrower claim. You may claim a straw that comprises at least one preshaped bend. While not the broadest, it may still represent a lucrative claim for you. The broader the claim, more financially beneficial the patent is!
Sections of a patent: Below are the main sections of a patent
Abstract
Figures
Field of invention
Background
This section discusses prior art and disadvantages of prior art. It emphasizes the unmet need addressed by your patent application.
Summary of invention
This section describes briefly the embodiments to be claimed later. It is a very important section as it parallels the structure of claims, but with more technical detail.
Detailed description of invention
This section provides the detailed technical description. It is required to provide sufficient detail to enable another person of skill in the art to ‘build’ the invention.
Claims
Perhaps the most important section of a patent, it presents the exact elements or steps of the invention for which the inventor desires to receive legal protection rights.
Tips for patent strategy
Make sure your idea is indeed novel: Perform relevant Internet and literature searches.
Don’t publish prematurely
In most countries, you have only 12 months to file for patent protection after your idea has been published;
Prepare at least a draft of what you want to claim
Remember that claims establish how valuable the patent is after it is allowed. Therefore, knowing what claims to pursue may tailor the technical description to desired claims.
Consider filing for a provisional patent application, especially if funds for full patent are not available
Filing a provisional patent application is far less expensive than the cost of a non-provisional application. A provisional patent application does not require claims. Hence, it may provide more time to ‘target’ your claims towards achieving specific goals, such as getting a company started. However, the provisional filing must be converted to a full patent application within 12 months. Otherwise, the priority dates may be lost or the coverage may go abandoned.
Consider filing for PCT coverage
A PCT filing initiates the ‘Search’ process. Therefore, it may provide relevant prior art in a non-binding modality. It is very helpful in terms providing information about what to expect when filing nationally, in specific countries. Also, initially, it is somewhat less expensive than filing a full patent application.
Typical IP costs
IP attorneys’ charge between $250–$750/h. For example, it may cost $2000–$10,000 in attorney fees to write, prepare and file one US patent application. Prosecution of US patent application may cost $2000–$8000 in attorney fees (2–3 year process, strong patents take the longest to issue). The total cost for one US patent application (filing + prosecution + maintenance) may reach $15,000–$30,000.
As a result, many companies aggressively file applications in order to protect and defend their technologies and products. Filing patents takes significant time and money. Applications can be under Patent Office examination for more than 2–3 years before issuance. Once issued, maintenance costs can be very expensive. However, litigation costs, or lost market share to competitors copying unprotected products, may be much more expensive.
B. Regulatory Issues
The ultimate goal is the deployment of new technologies is into clinical practice. The benefits of new technologies can seem obvious to a scientist, however, the true benefit must be demonstrated and regulated to ensure that the technology is beneficial to patients. In the United States, the FDA is the regulatory body which approves and provides guidance on (and oversight of) the use of technology in the care of patients. In Canada, the Health Canada–Veterinary Drugs Directorate has a similar responsibility. In the European Union, approval for clinical use of medical devices and technologies is provided by Notified Bodies. Such organizations are set up according to principles laid down in European Union Decision 768/2008/EC (https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32008D0768). In China, regulations are provided by China Food and Drug Administration (http://samr.saic.gov.cn/). Most other countries have their own regulatory requirements for clinical use of medical devices and technologies.
It was the Fair Packaging and Labeling Act of 1967 that required the FDA to regulate medical devices. At the time, the focus was on preventing deception in labelling (https://www.ftc.gov/enforcement/rules/rulemaking-regulatory-reform-proceedings/fair-packaging-labeling-act). It was not until 1976 that safety and effectiveness were added to the list of consideration of medical devices (94th U.S. Congress (December 11, 1975). “H.R.11124: Medical Device Amendments”. U.S. House of Representative Bill Summary & Status. Library of Congress THOMAS. Retrieved February 9, 2013) for patient care. Since that time, processes and procedures have been put in place to properly evaluate the utility and safety of a new technology in patient and subsequent regulation of that technology.
When considering the regulatory pathway to approval and subsequent use in patient care, one must consider the Device Classification of the new therapeutic technology. The FDA Center for Devices and Radiologic Health has identified three broad classes of devices – Class I, II, and III. The FDA provides an online database of device classifications to identify the class of a given device (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm). The classification is based on the risk level associated with the medical device or technology. Most therapeutic technologies and devices are Class II or III devices, representing moderate and high level of risk. Items such as elastic bandages, examination gloves, and hand-held surgical instruments are considered Class I, representing a low level of risk.
Both Class II and III devices have a higher level of scrutiny due to the highest potential for patient harm. When submitting to the FDA for review, there are three types of possible submission applications. The level of effort for each of the three submissions varies greatly, but in all cases, the application must contain extensive data on the purpose, safety, and effectiveness of the device. The first category isa510(k) submission. The purpose of a 510(k) submission is to document that a device is “substantially equivalent” to an existing FDA-approved device (i.e., predicate device). The device must show a similar safety and efficacy profile and must be indicated for the same condition as the existing product on the market. Accordingly, documentation for the 510(k) submission is largely based on comparative data between the proposed device and existing products.
In contrast to the 510(k) submission, the pre-market approval (PMA) submission is for new devices and new indications. Because there is no prior data available to assess the safety and efficacy of the device, pivotal clinical trials may be required. In a pivotal clinical trial, the new device is tested against other established therapies (or possibly control arms). Such trials are statistically powered to show therapeutic equivalency or superiority as a primary endpoint. Secondary endpoints may address safety, quality of life, economics, and other patient reported outcomes. To ensure a valid outcome, the study must be properly designed to avoid bias and confounding factors whenever possible. This includes attention to recruiting, blinding, and statistical testing. While PMA submissions are a substantial investment of time and money, they offer a significant reward when completed. In many cases, the applicant is able to show the unique and superior performance over existing therapies. By doing so, they may secure a significant sector of the marketplace.
The third submission is for a De Novo classification (https://www.fda.gov/training/cdrhlearn/ucm426000.htm). Such submission process is used for devices and technologies which lack a predicate device. Devices which do not have a predicate, are automatically considered Class III by the FDA. However, some devices may present only moderate level of clinical risk. As such, they should be classified as Class II or I. Via the De Novo process, the FDA allows sponsors to provide the necessary information to down-classify devices to Class I or II, even though they may lack a predicate device.
Sponsors may also consider FDA exemptions to get their devices or technologies into clinical use. Two of the most common exceptions are the Investigational Device Exemption (IDE) and humanitarian use exemptions. IDE submissions are a request to use a new device in a small population of patients to obtain preliminary safety and efficacy data. This type of exemption is the most effective way to collect preliminary data for the PMA submission. In contrast, the humanitarian use exemption allows a provider to use an unapproved device on a patient when there are no other viable therapies available to a patient. Generally, the effectiveness of a humanitarian use device (HUD) does not have tobe known, but the technology must hold specific promise to treat the patient. Additionally, humanitarian use exemptions are generally limited to patients with rare diseases (thus, on the order of 4000 patients annually).
Due to the challenges of developing a submission packet for the FDA, the FDA is often willing to meet with the applicant prior to submission. During a pre-submission meeting, the sponsor will provide all preliminary data and, in the case of a PMA, the strategy for the proposed clinical trial. The FDA can provide guidance on the pre-submission plan.
VI. Conclusion
This article serves as a brief and timely tour de force on the state-of-the-art, challenges and opportunities in the design, development, validation and clinical integration of therapeutic systems and technologies, ranging from initial concept development to intellectual property protection. We hope this article will inspire and motivate researchers to join in and contribute to this exciting field and to incorporate advanced technologies (i.e., Nanotechnology, Artificial intelligence, 3D-priting) in therapeutic systems.
Fig. 2.
The hybrid interventional suite of the future equipped with computer-integrated technology for data acquisition, analysis, integration, and interpretation capable to provide the right assistance at the right time. Adapted from Maier-Hein et al. 2017 [155].
Acknowledgments
This work was supported in part by the National Institute of General Medical Sciences of the National Institutes of Health under Award No. R35GM128877 and in part by the Office of Advanced Cyber Infrastructure of the National Science Foundation under Award No. 1808530.
Biography
 Mohamed Almekkawy received the B.S. degree in electrical engineering from Ain Shams University, Cairo, Egypt, in 1998, the M.S. degrees in electrical engineering from Cairo University, Giza, Egypt, and the University of Minnesota, Twin Cities, MN, USA, in 2006 and 2010, respectively, and the Ph.D. degree in electrical engineering from the University of Minnesota, Twin Cities, MN, USA, in 2014. Since 2015, he has been with the School of Electrical Engineering and Computer Science, Pennsylvania State University, University Park, PA, USA. His current research interests include signal processing, ultrasound tomography, elasticity imaging, numerical modeling of focused ultrasound, and optimization in biomedical acoustics and medical devices applications.
Mohamed Almekkawy received the B.S. degree in electrical engineering from Ain Shams University, Cairo, Egypt, in 1998, the M.S. degrees in electrical engineering from Cairo University, Giza, Egypt, and the University of Minnesota, Twin Cities, MN, USA, in 2006 and 2010, respectively, and the Ph.D. degree in electrical engineering from the University of Minnesota, Twin Cities, MN, USA, in 2014. Since 2015, he has been with the School of Electrical Engineering and Computer Science, Pennsylvania State University, University Park, PA, USA. His current research interests include signal processing, ultrasound tomography, elasticity imaging, numerical modeling of focused ultrasound, and optimization in biomedical acoustics and medical devices applications.
 Jie Chen (S’95–M’99–SM’04–F’16) received the Ph.D. degree in electrical and computer engineering from the University of Maryland, College Park, MD, USA. He is currently a Professor with the Electrical and Computer Engineering and an Adjunct Professor with the Biomedical Engineering Department, University of Alberta, Edmonton, AB, Canada. He has coauthored 2 books, more than 190 journal articles and conference proceeding papers (i10-index 68). His current research focuses include MEMS/NEMS SSpoint-of-care colorimetric/impedance devices for precision health and di-electrophoresis device for single cell sequencing, integrated mix-signal biomedical circuits and systems design, artificial intelligence methods to diagnose mental health diseases (depression, anxiety, and bipolar disorders), and invoke clinical intervention if needed. He is a Fellow of the Canadian Academy of Engineering. He was the recipient of the Killam Annual Professorship Award 2015–2016 (one of the highest honors to a professor in Canadian Universities for outstanding contributions in teaching, research, and community services). His supervised students have received the Best Student Paper Award at the IEEE/National Institutes of Health (NIH) Life Science Systems & Applications Workshop 2007. His co-supervised student also received the Best Poster Award by the International Union of Crystallography at the Conference of Biology and Synchrotron Radiation (BSR), Hamburg, Germany, 2013. He helped establish two Bell-Lab spin-off companies. One company focused on developing the fourth-generation wireless network and was acquired by QUALCOMM, San Diego, 2005. The other produces digital HD-radios installed in most brands of cars worldwide and sold in most retail stores.
Jie Chen (S’95–M’99–SM’04–F’16) received the Ph.D. degree in electrical and computer engineering from the University of Maryland, College Park, MD, USA. He is currently a Professor with the Electrical and Computer Engineering and an Adjunct Professor with the Biomedical Engineering Department, University of Alberta, Edmonton, AB, Canada. He has coauthored 2 books, more than 190 journal articles and conference proceeding papers (i10-index 68). His current research focuses include MEMS/NEMS SSpoint-of-care colorimetric/impedance devices for precision health and di-electrophoresis device for single cell sequencing, integrated mix-signal biomedical circuits and systems design, artificial intelligence methods to diagnose mental health diseases (depression, anxiety, and bipolar disorders), and invoke clinical intervention if needed. He is a Fellow of the Canadian Academy of Engineering. He was the recipient of the Killam Annual Professorship Award 2015–2016 (one of the highest honors to a professor in Canadian Universities for outstanding contributions in teaching, research, and community services). His supervised students have received the Best Student Paper Award at the IEEE/National Institutes of Health (NIH) Life Science Systems & Applications Workshop 2007. His co-supervised student also received the Best Poster Award by the International Union of Crystallography at the Conference of Biology and Synchrotron Radiation (BSR), Hamburg, Germany, 2013. He helped establish two Bell-Lab spin-off companies. One company focused on developing the fourth-generation wireless network and was acquired by QUALCOMM, San Diego, 2005. The other produces digital HD-radios installed in most brands of cars worldwide and sold in most retail stores.
 Michael D. Ellis received the Bachelor of Science degree with Honors in exercise science from the University of Iowa, Iowa City, IA, USA, in 1997, and the Master of Physical Therapy and Doctor of Physical Therapy degrees from Emory University Atlanta, Atlanta, GA, USA, in 2000 and 2003, respectively. He is an Associate Professor of Physical Therapy with the Department of Physical Therapy & Human Movement Sciences, Northwestern University, Evanston, IL, USA, and is the Director/PI of the Investigational Technologies in Stroke Recovery Laboratory. He has 12 years of experience in inpatient rehabilitation in a level-1 trauma hospital and 17 years of experience as a Clinical Scientist with Northwestern University. Over the last 15 years, he was a co-investigator on multiple NIH and NIDRR/NIDILRR grants studying discoordination in individuals with stroke and the development of novel therapeutic interventions. He has been the PD/PI of a National Institute on Disability, Independent Living, and Rehabilitation Research grant investigating two robotic interventions targeting upper extremity flexion synergy and weakness impairments in adults with severe stroke-related hemiparesis. His movement science and rehabilitation research interests continue to focus on the elucidation of the neurological underpinnings responsible for movement discoordination and the subsequent development of quantitative clinical evaluation tools and effective rehabilitation therapies for individuals with functionally debilitating movement impairments.
Michael D. Ellis received the Bachelor of Science degree with Honors in exercise science from the University of Iowa, Iowa City, IA, USA, in 1997, and the Master of Physical Therapy and Doctor of Physical Therapy degrees from Emory University Atlanta, Atlanta, GA, USA, in 2000 and 2003, respectively. He is an Associate Professor of Physical Therapy with the Department of Physical Therapy & Human Movement Sciences, Northwestern University, Evanston, IL, USA, and is the Director/PI of the Investigational Technologies in Stroke Recovery Laboratory. He has 12 years of experience in inpatient rehabilitation in a level-1 trauma hospital and 17 years of experience as a Clinical Scientist with Northwestern University. Over the last 15 years, he was a co-investigator on multiple NIH and NIDRR/NIDILRR grants studying discoordination in individuals with stroke and the development of novel therapeutic interventions. He has been the PD/PI of a National Institute on Disability, Independent Living, and Rehabilitation Research grant investigating two robotic interventions targeting upper extremity flexion synergy and weakness impairments in adults with severe stroke-related hemiparesis. His movement science and rehabilitation research interests continue to focus on the elucidation of the neurological underpinnings responsible for movement discoordination and the subsequent development of quantitative clinical evaluation tools and effective rehabilitation therapies for individuals with functionally debilitating movement impairments.
 Dieter Haemmerich received the Ph.D. B.M.E. degree from the University of Wisconsin–Madison, Madison, WI, USA, in 2001. He is currently a Professor of pediatrics with the Medical University of South Carolina, Charleston, SC, USA, and an Adjunct Faculty Member of bioengineering with Clemson University, Clemson, SC, USA. His research interests include thermal ablation, image-guided drug delivery, biomedical instrumentation, measurement of thermal and dielectric tissue properties, and computational modeling of biological heat transfer problems and targeted drug delivery (see ablation.musc.edu). He has coauthored more than 100 peer-reviewed papers. He is a fellow of the Heart Rhythm Society, a past chair of the EMBC Technical Committee on Therapeutic Systems and Technologies, and a past president of the Society for Thermal Medicine.
Dieter Haemmerich received the Ph.D. B.M.E. degree from the University of Wisconsin–Madison, Madison, WI, USA, in 2001. He is currently a Professor of pediatrics with the Medical University of South Carolina, Charleston, SC, USA, and an Adjunct Faculty Member of bioengineering with Clemson University, Clemson, SC, USA. His research interests include thermal ablation, image-guided drug delivery, biomedical instrumentation, measurement of thermal and dielectric tissue properties, and computational modeling of biological heat transfer problems and targeted drug delivery (see ablation.musc.edu). He has coauthored more than 100 peer-reviewed papers. He is a fellow of the Heart Rhythm Society, a past chair of the EMBC Technical Committee on Therapeutic Systems and Technologies, and a past president of the Society for Thermal Medicine.
 David R. Holmes, III (S’00–A’02–M’04–SM’15) received the master’s degree in clinical research, under the NIH Clinical Research Scholars program, and the doctorate degree in biomedical engineering from the Mayo Graduate School, Rochester, MN, USA, in 2002. He received the Postdoctoral Fellowship in biomedical electronics between 2003 and 2005. He is the Director of the Biomedical Imaging Resource Core and the PI of the Biomedical Analytics and Computational Engineering Lab, Mayo Clinic. He is a member of the faculty of the Department of Physiology and Biomedical Engineering, Mayo Clinic. In addition to his research, he is an active member of the teaching faculty of the Mayo Graduate School. His research interests include machine learning and artificial intelligence for biomedical signal analysis. He focuses much of his effort on combining physiologic signals with medical images and outcomes data.
David R. Holmes, III (S’00–A’02–M’04–SM’15) received the master’s degree in clinical research, under the NIH Clinical Research Scholars program, and the doctorate degree in biomedical engineering from the Mayo Graduate School, Rochester, MN, USA, in 2002. He received the Postdoctoral Fellowship in biomedical electronics between 2003 and 2005. He is the Director of the Biomedical Imaging Resource Core and the PI of the Biomedical Analytics and Computational Engineering Lab, Mayo Clinic. He is a member of the faculty of the Department of Physiology and Biomedical Engineering, Mayo Clinic. In addition to his research, he is an active member of the teaching faculty of the Mayo Graduate School. His research interests include machine learning and artificial intelligence for biomedical signal analysis. He focuses much of his effort on combining physiologic signals with medical images and outcomes data.
 Cristian A. Linte (S’06–M’09–SM’17) received the Ph.D. degree in biomedical engineering from the University of Western Ontario, London, ON, Canada, and Robarts Research Institute, London, ON, Canada. He is currently an Assistant Professor in Biomedical engineering and the Chester F. Carlson Center for Imaging Science with Rochester Institute of Technology, Rochester, NY, USA. He has authored more than 80 publications. His research interests include biomedical imaging and image computing, modeling, image-guided navigation and visualization in support of computer-aided diagnosis, and minimally invasive therapy delivery. He is currently the Vice-Chair of the IEEE EMBS Technical Committee on Therapeutic Systems and Technologies, a Chair of the Conference on Image-Guided Procedures, Robotic Interventions and Modeling as part of the SPIE Medical Imaging Symposium, and is the past Program Chair of the Information Processing for Computer-Assisted Interventions Conference.
Cristian A. Linte (S’06–M’09–SM’17) received the Ph.D. degree in biomedical engineering from the University of Western Ontario, London, ON, Canada, and Robarts Research Institute, London, ON, Canada. He is currently an Assistant Professor in Biomedical engineering and the Chester F. Carlson Center for Imaging Science with Rochester Institute of Technology, Rochester, NY, USA. He has authored more than 80 publications. His research interests include biomedical imaging and image computing, modeling, image-guided navigation and visualization in support of computer-aided diagnosis, and minimally invasive therapy delivery. He is currently the Vice-Chair of the IEEE EMBS Technical Committee on Therapeutic Systems and Technologies, a Chair of the Conference on Image-Guided Procedures, Robotic Interventions and Modeling as part of the SPIE Medical Imaging Symposium, and is the past Program Chair of the Information Processing for Computer-Assisted Interventions Conference.
 Dorin Panescu (S’92–M’93–SM’98–F’11) received the M.S. and Ph.D. degrees in electrical and computer engineering from the University of Wisconsin–Madison, Madison, WI, USA. He is a Chief Technical Officer and the Vice President, R&D, with HeartBeam, Inc., Santa Clara, CA, USA. His research interests focus on devices for cardiac ablation, pacing, defibrillation, and diagnosis and therapy delivery. He is an inventor of more than 160 issued U.S. patents. He has coauthored more than 150 technical publications. He held various offices with the IEEE EMBS, such as Chair of the Industry Relations Committee and past Chair of the Therapeutic Systems and Technologies Technical Committee. He was the recipient of the 2002 IEEE EMBS Early Career Achievement Award and of the 2009 IEEE EMBS Professional Career Achievement Award.
Dorin Panescu (S’92–M’93–SM’98–F’11) received the M.S. and Ph.D. degrees in electrical and computer engineering from the University of Wisconsin–Madison, Madison, WI, USA. He is a Chief Technical Officer and the Vice President, R&D, with HeartBeam, Inc., Santa Clara, CA, USA. His research interests focus on devices for cardiac ablation, pacing, defibrillation, and diagnosis and therapy delivery. He is an inventor of more than 160 issued U.S. patents. He has coauthored more than 150 technical publications. He held various offices with the IEEE EMBS, such as Chair of the Industry Relations Committee and past Chair of the Therapeutic Systems and Technologies Technical Committee. He was the recipient of the 2002 IEEE EMBS Early Career Achievement Award and of the 2009 IEEE EMBS Professional Career Achievement Award.
 John Pearce received the B.S. and M.S. degrees in mechanical engineering from Clemson University, Clemson, SC, USA, in 1968 and1971, respectively, and the M.S. and Ph.D. degrees in electrical engineering from the Purdue University, West Lafayette, IN, USA, in 1977 and 1980, respectively. In August 2016, he retired as the Temple Foundation Professor (#3) in electrical and computer engineering with The University of Texas at Austin, where he has taught since 1982.
John Pearce received the B.S. and M.S. degrees in mechanical engineering from Clemson University, Clemson, SC, USA, in 1968 and1971, respectively, and the M.S. and Ph.D. degrees in electrical engineering from the Purdue University, West Lafayette, IN, USA, in 1977 and 1980, respectively. In August 2016, he retired as the Temple Foundation Professor (#3) in electrical and computer engineering with The University of Texas at Austin, where he has taught since 1982.
 Punit Prakash (S’00–M’09–SM’18) received the B.S. degree in electrical and computer engineering from Worcester Polytechnic Institute, Worcester, MA, USA, in 2004, and the Ph.D. degree in biomedical engineering from the University of Wisconsin–Madison, Madison, WI, USA, in 2008. Since 2012, he has been with the Department of Electrical and Computer Engineering, Kansas State University, where he is currently the Paul L. Spainhour Professor in Electrical Engineering, Associate Professor, and Michelle Munson – Serban Simu Keystone Research Faculty Scholar. His research interests include technologies for image-guided thermal ablation, computational modeling of therapeutic devices and systems, and medical instrumentation. He is a past Chair of the EMBS Technical Committee on Therapeutic Systems and Technologies.
Punit Prakash (S’00–M’09–SM’18) received the B.S. degree in electrical and computer engineering from Worcester Polytechnic Institute, Worcester, MA, USA, in 2004, and the Ph.D. degree in biomedical engineering from the University of Wisconsin–Madison, Madison, WI, USA, in 2008. Since 2012, he has been with the Department of Electrical and Computer Engineering, Kansas State University, where he is currently the Paul L. Spainhour Professor in Electrical Engineering, Associate Professor, and Michelle Munson – Serban Simu Keystone Research Faculty Scholar. His research interests include technologies for image-guided thermal ablation, computational modeling of therapeutic devices and systems, and medical instrumentation. He is a past Chair of the EMBS Technical Committee on Therapeutic Systems and Technologies.
 Vesna Zderic (S’00–M’04–SM’14) received the B.S. degree in electrical engineering from the University of Belgrade, Belgrade, Serbia, in 1998, and the Ph.D. degree in bioengineering from the University of Washington, Seattle, WA, USA, in 2004. She is an Associate Professor with the Department of Biomedical Engineering, The George Washington University (GW), Washington, DC, USA. Before coming to GW in 2006, she received the Postdoctoral Fellowship with the National Space Biomedical Research Institute. Her current research interests include the application of ultrasound to enhance drug delivery through different biological barriers, studies of safety of therapeutic ultrasound application, and ultrasound application for functional modification of cells and tissues. During her professional career, she authored or coauthored more than 40 peer-reviewed journal papers, several book chapters, and an edited book. She has also been active in the research community as an Associate Editor and Technical Committee Member for the IEEE EMBS, a member of the Advisory Editorial Board of Ultrasound in Medicine & Biology, and a reviewer for NIH granting programs and several engineering and ultrasound journals.
Vesna Zderic (S’00–M’04–SM’14) received the B.S. degree in electrical engineering from the University of Belgrade, Belgrade, Serbia, in 1998, and the Ph.D. degree in bioengineering from the University of Washington, Seattle, WA, USA, in 2004. She is an Associate Professor with the Department of Biomedical Engineering, The George Washington University (GW), Washington, DC, USA. Before coming to GW in 2006, she received the Postdoctoral Fellowship with the National Space Biomedical Research Institute. Her current research interests include the application of ultrasound to enhance drug delivery through different biological barriers, studies of safety of therapeutic ultrasound application, and ultrasound application for functional modification of cells and tissues. During her professional career, she authored or coauthored more than 40 peer-reviewed journal papers, several book chapters, and an edited book. She has also been active in the research community as an Associate Editor and Technical Committee Member for the IEEE EMBS, a member of the Advisory Editorial Board of Ultrasound in Medicine & Biology, and a reviewer for NIH granting programs and several engineering and ultrasound journals.
Contributor Information
Mohamed Almekkawy, Pennsylvania State University, University Park, PA 16802 USA.
Jie Chen, University of Alberta Edmonton, AB T6G 2R3, Canada.
Michael D. Ellis, Northwestern University, Evanston, IL 60208 USA.
Dieter Haemmerich, University of South Carolina, Columbia, SC 29208 USA.
David R. Holmes, III, Mayo Clinic, Rochester, MN 55905 USA.
Cristian A. Linte, Rochester Institute of Technology, Rochester, NY 14623 USA.
Dorin Panescu, HeartBeam, Inc., Santa Clara, CA 95050-2525 USA.
John Pearce, University of Texas at Austin, Austin, TX 78712 USA.
Punit Prakash, Kansas State University, Manhattan, KS 66506 USA.
Vesna Zderic, George Washington University, Washington, DC 20052 USA.
References
- [1].Barnett AS, Bahnson TD, and Piccini JP, “Recent advances in lesion formation for catheter ablation of atrial fibrillation,” Circ.: Arrhythm. Electrophysiol, vol. 9, no. 5, 2016, Art. no. e003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Linte CA et al. , “Accuracy considerations in image-guided cardiac interventions: Experience and lessons learned,” Int. J. Comput. Assisted Radiol. Surg, vol. 7, no. 1, pp. 13–25, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Linte CA et al. , “On mixed reality environments for minimally invasive therapy guidance: Systems architecture, successes and challenges in their implementation from laboratory to clinic,” Comput. Med. Imag. Graph, vol. 37, no. 2, pp. 83–97, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sloan AE et al. , “Results of the neuroblate system first-in-humans phase I clinical trial for recurrent glioblastoma,” J. Neurosurg, vol. 118, no. 6, pp. 1202–1219, 2013. [DOI] [PubMed] [Google Scholar]
- [5].Boissenot T, Bordat A, Fattal E, and Tsapis N, “Ultrasound-triggered drug delivery for cancer treatment using drug delivery systems: From theoretical considerations to practical applications,” J. Control. Release, vol. 241, pp. 144–163, 2016. [DOI] [PubMed] [Google Scholar]
- [6].Major RH, A History of Medicine. Springfield, IL, USA: Charles C. Thomas, 1954. [Google Scholar]
- [7].Breasted JH, The Edwin Smith Surgical Papyrus. Chicago, IL, USA: Univ. Chicago Press, 1930. [Google Scholar]
- [8].Planté G, Recherches sur L’électricité Paris, France: Gauthier-Villars, 1883. [Google Scholar]
- [9].Kelly HA and Ward GE, Electrosurgery. Philadelphia, PA, USA: Saunders, 1932. [Google Scholar]
- [10].Rubio-Ruiz B et al. , “High-precision photothermal ablation using biocompatible palladium nanoparticles and laser scanning microscopy,” ACS Appl. Mater. Interfaces, vol. 10, no. 4, pp. 3341–3348, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schena E, Saccomandi P, and Fong Y, “Laser ablation for cancer: Past, present and future,” J. Funct. Biomater, vol. 8, no. 2, 2017, Art. no. E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen R, Jiang T-A, Lu F, Wang K, and Kong D, “Semiautomatic radiofrequency ablation planning based on constrained clustering process for hepatic tumors,” IEEE Trans. Biomed. Eng, vol. 65, no. 3, pp. 645–657, March 2018. [DOI] [PubMed] [Google Scholar]
- [13].McDaniel GM and Van Hare GF, “Catheter ablation in children and adolescents,” Heart Rhythm, vol. 3, no. 1, pp. 95–101, January 2006. [DOI] [PubMed] [Google Scholar]
- [14].Huang SKS and Wood MA, Catheter Ablation of Cardiac Arrhythmias. Philadelphia, PA, USA: Saunders, 2006. [Google Scholar]
- [15].Ward GE, “An efficient method of hemostasis without suture,” Med. J. Rec, vol. 121, p. 470–1925. [Google Scholar]
- [16].Cushing H and Bovie WT, “Electrosurgery as an aid to the removal of intracranial tumors,” Surg., Gynecol., Obstet, vol. 47, pp. 751–784, 1928. [Google Scholar]
- [17].Liu D and Brace CL, “Numerical simulation of microwave ablation incorporating tissue contraction based on thermal dose,” Phys. Med. Biol, vol. 62, no. 6, pp. 2070–2086, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang Z et al. , “Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer,” Biomaterials, vol. 127, pp. 25–35, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chiu L-C, Wu S-K, Lin W-L, and Chen G-S, “Synergistic effects of nanodrug, ultrasound hyperthermia, and thermal ablation on solid tumors-an animal study,” IEEE Trans. Biomed. Eng, vol. 64, no. 12, pp. 2880–2889, December 2017. [DOI] [PubMed] [Google Scholar]
- [20].Wust P et al. , “Hyperthermia in combined treatment of cancer,” Lancet Oncol., Rev, vol. 3, pp. 487–497, 2002. [DOI] [PubMed] [Google Scholar]
- [21].Pearce JA and Thomsen S, “The effect of vessel architecture on fusion by radio frequency current,” presented at the Surgical Appl. Energy, San Jose, CA, USA, 1998. [Google Scholar]
- [22].Sigel B and Dunn MR, “The mechanism of blood vessel closure by high frequency electrocoagulation,” Surg., Gynecol., Obstet, vol. 121, no. 4, pp. 823–831, 1965. [PubMed] [Google Scholar]
- [23].Kramer EA, Cezo JD, Fankell DP, Taylor KD, Rentschler ME, and Ferguson VL, “Strength and persistence of energy-based vessel seals rely on tissue water and glycosaminoglycan content,” Ann. Biomed. Eng, vol. 44, no. 11, pp. 3421–3431, 2016. [DOI] [PubMed] [Google Scholar]
- [24].Pearce JA, “Mathematical models of laser-induced tissue thermal damage,” Int. J. Hyperthermia, vol. 27, no. 8, pp. 741–750, 2011. [DOI] [PubMed] [Google Scholar]
- [25].Chen WR, Bartels KE, Liu H, and Nordquist RE, “Laser-photothermal effect on skin tissue–Damage and recovery,” J. X-Ray Sci. Technol, vol. 14, no. 3, pp. 207–215, 2006. [Google Scholar]
- [26].Tunnell JW, Stern RA, and Pope K, “RF non-ablative cutaneous thermal therapy,” presented at the Lasers Surg., Adv. Characterization, Therapeutics, Syst. XII, San Jose, CA, USA, 2002. [Google Scholar]
- [27].Hardy LA et al. , “Rapid sealing of porcine renal blood vessels, ex vivo, using a high power, 1470-nm laser, and laparoscopic prototype,” J. Biomed. Opt, vol. 22, no. 5, 2017, Art. no. 58002. [DOI] [PubMed] [Google Scholar]
- [28].Falk M and Issels R, “Hyperthermia in oncology,” Int. J. Hyperthermia, vol. 17, no. 1, pp. 1–18, 2001. [DOI] [PubMed] [Google Scholar]
- [29].He X, “Thermostability of biological systems: Fundamentals, challenges, and quantification,” Open Biomed. Eng. J, vol. 5, pp. 47–73, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zipes DP and Häıssaguerre M, Catheter Ablation of Arrhythmias. New York, NY, USA: Futura, 1994. [Google Scholar]
- [31].Ryan TP and Brace CL, “Interstitial microwave treatment for cancer: Historical basis and current techniques in antenna design and performance,” Int. J. Hyperthermia, vol. 33, no. 1, pp. 3–14, 2017. [DOI] [PubMed] [Google Scholar]
- [32].Sawicki JF, Luyen H, Mohtashami Y, Shea JD, Behdad N, and Hagness SC, “The performance of higher frequency microwave ablation in the presence of perfusion,” IEEE Trans. Biomed. Eng, vol. 66, no. 1, pp. 257–262, January 2019. [DOI] [PubMed] [Google Scholar]
- [33].Wright AS, Lee FT, and Mahvi DM, “Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis,” Ann. Surg. Oncol, vol. 10, no. 3, pp. 275–283, 2003. [DOI] [PubMed] [Google Scholar]
- [34].Wright AS, Sampson LA, Warner TF, Mahvi DM, and Lee FT Jr., “Radiofrequency versus microwave ablation in a hepatic porcine model,” Radiology, vol. 236, no. 1, pp. 132–139, 2005. [DOI] [PubMed] [Google Scholar]
- [35].Sebek J, Curto S, Bortel R, and Prakash P, “Analysis of minimally invasive directional antennas for microwave tissue ablation,” Int. J. Hyperthermia, vol. 33, no. 1, pp. 51–60, 2017. [DOI] [PubMed] [Google Scholar]
- [36].Mohtashami Y, Luyen H, Hagness SC, and Behdad N, “Non-coaxialbased microwave ablation antennas for creating symmetric and asymmetric coagulation zones,” J. Appl. Phys, vol. 123, no. 21, 2018, Art. no. 214903. [Google Scholar]
- [37].Heerink WJ et al. , “The relationship between applied energy and ablation zone volume in patients with hepatocellular carcinoma and colorectal liver metastasis,” Eur. Radiol, vol. 28, pp. 3228–3236, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wagrell L, Schelin T, Bolmsjö M, and Mattiasson A, “Aspects on¨ transurethral microwave thermotherapy of benign prostatic hyperplasia,” vol. 6, no. 4, pp. 251–255, 2000. [PubMed] [Google Scholar]
- [39].Johnson JE, O’shaughnessy KF, and Kim S, “Microwave thermolysis of sweat glands,” Lasers Surg. Med, vol. 44, no. 1, pp. 20–25, 2012. [DOI] [PubMed] [Google Scholar]
- [40].Hodgson DA, Feldberg IB, Sharp N, Cronin N, Evans M, and Hirschowitz L, “Microwave endometrial ablation: Development, clinical trials and outcomes at three years,” Brit. J. Obstet. Gynaecol, vol. 106, no. 7, pp. 684–694, 1999. [DOI] [PubMed] [Google Scholar]
- [41].Qian PC et al. , “Transcatheter non-contact microwave ablation may enable circumferential renal artery denervation while sparing the vessel intima and media,” EuroIntervention, vol. 12, no. 15, pp. e1907–e1915, 2017. [DOI] [PubMed] [Google Scholar]
- [42].Staruch R, Chopra R, and Hynynen K, “MRI-controlled ultrasound thermal therapy,” IEEE Pulse, vol. 2, no. 5, pp. 39–47, Sep./Oct. 2011. [DOI] [PubMed] [Google Scholar]
- [43].Salgaonkar VA and Diederich CJ, “Catheter-based ultrasound technology for image-guided thermal therapy: Current technology and applications,” Int. J. Hyperthermia, vol. 31, no. 2, pp. 203–215, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Deardorff DL and Diederich CJ, “Axial control of thermal coagulation using a multi-element interstitial ultrasound applicator with internal cooling,”IEEETrans.Ultrason.,Ferroelect.,Freq.Control,vol.47,no.1, pp. 170–178, January 2000. [DOI] [PubMed] [Google Scholar]
- [45].Ross AB et al. , “Highly directional transurethral ultrasound applicators with rotational control for MRI-guided prostatic thermal therapy,” Phys. Med. Biol, vol. 49, no. 2, pp. 189–204, 2004. [DOI] [PubMed] [Google Scholar]
- [46].Bonekamp D et al. , “Twelve-month prostate volume reduction after MRI-guided transurethral ultrasound ablation of the prostate,” Eur. Radiol, vol. 29, pp. 299–308, 2019. [DOI] [PubMed] [Google Scholar]
- [47].Boctor EM, Choti MA, Burdette EC, and Webster III RJ, “Three-dimensional ultrasound-guided robotic needle placement: An experimental evaluation,” Int. J. Med. Robot. Comput. Assisted Surg, vol. 4, no. 2, pp. 180–191, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Adams MS, Salgaonkar VA, Scott SJ, Sommer G, and Diederich CJ, “Integration of deployable fluid lenses and reflectors with endoluminal therapeutic ultrasound applicators: Preliminary investigations of enhanced penetration depth and focal gain,” Med. Phys, vol. 44, no. 10, pp. 5339–5356, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wood RW and Loomis AL, “XXXVIII. The physical and biological effects of high-frequency sound-waves of great intensity,” Lond. Edinb., Dubl. Philos. Mag. J. Sci, vol. 4, no. 22, pp. 417–436, 1927. [Google Scholar]
- [50].Watson T, “Ultrasound in contemporary physiotherapy practice,” Ultrasonics, vol. 48, no. 4, pp. 321–329, 2008. [DOI] [PubMed] [Google Scholar]
- [51].Lin G, Reed-Maldonado AB, Lin M, Xin Z, and Lue TF, “Effects and mechanisms of low-intensity pulsed ultrasound for chronic prostatitis and chronic pelvic pain syndrome,” Int. J. Mol. Sci, vol. 17, no. 7, 2016, Art. no. 1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rubin C, Bolander M, Ryaby JP, and Hadjiargyrou M, “The use of low-intensity ultrasound to accelerate the healing of fractures,” J. Bone Joint Surg, vol. 83, no. 2, pp. 259–270, 2001. [DOI] [PubMed] [Google Scholar]
- [53].Júnior SLJ, Camanho GL, Bassit ACF, Forgas A, Ingham SJ, and Abdalla RJ, “Low-intensity pulsed ultrasound accelerates healing in rat calcaneus tendon injuries,” J. Orthop. Sports Phys. Ther, vol. 41, no. 7, pp. 526–531, 2011. [DOI] [PubMed] [Google Scholar]
- [54].Ren L, Yang Z, Song J, Wang Z, Deng F, and Li W, “Involvement of p38 MAPK pathway in low intensity pulsed ultrasound induced osteogenic differentiation of human periodontal ligament cells,” Ultrasonics, vol. 53, no. 3, pp. 686–690, 2013. [DOI] [PubMed] [Google Scholar]
- [55].Chan Y-S et al. , “Using low-intensity pulsed ultrasound to improve muscle healing after laceration injury: An in vitro and in vivo study,” Ultrasound Med. Biol, vol. 36, no. 5, pp. 743–751, 2010. [DOI] [PubMed] [Google Scholar]
- [56].Loyola-Sánchez A, Richardson J, Beattie KA, Otero-Fuentes C, Adachi JD, and MacIntyre NJ, “Effect of low-intensity pulsed ultrasound on the cartilage repair in people with mild to moderate knee osteoarthritis: A double-blinded, randomized, placebo-controlled pilot study,” Arch. Phys. Med. Rehabil, vol. 93, no. 1, pp. 35–42, 2012. [DOI] [PubMed] [Google Scholar]
- [57].Khanna A, Nelmes RT, Gougoulias N, Maffulli N, and Gray J, “The effects of LIPUS on soft-tissue healing: A review of literature,” Brit. Med. Bull, vol. 89, no. 1, pp. 169–182, 2008. [DOI] [PubMed] [Google Scholar]
- [58].Cheung WH, Chin WC, Qin L, and Leung KS, “Low intensity pulsed ultrasound enhances fracture healing in both ovariectomy-induced osteoporotic and age-matched normal bones,” J. Orthop. Res, vol. 30, no. 1, pp. 129–136, 2012. [DOI] [PubMed] [Google Scholar]
- [59].Ang WT, Scurtescu C, Hoy W, El-Bialy T, Tsui YY, and Chen J, “Design and implementation of therapeutic ultrasound generating circuit for dental tissue formation and tooth-root healing,” IEEE Trans. Biomed. Circuits. Syst, vol. 4, no. 1, pp. 49–61, February 2010. [DOI] [PubMed] [Google Scholar]
- [60].Engelmann J et al. , “Pulsed ultrasound and dimethylsulfoxide gel treatment reduces the expression of pro-inflammatory molecules in an animal model of muscle injury,” Ultrasound Med. Biol, vol. 38, no. 8, pp. 1470–1475, 2012. [DOI] [PubMed] [Google Scholar]
- [61].Victor EG et al. , “Pulsed ultrasound associated with gold nanoparticle gel reduces oxidative stress parameters and expression of pro-inflammatory molecules in an animal model of muscle injury,” J. Nanobiotechnol, vol. 10, no. 1, pp. 11–20, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hashish I, Harvey W, and Harris M, “Anti-inflammatory effects of ultrasound therapy: Evidence for a major placebo effect,” Rheumatology, vol. 25, no. 1, pp. 77–81, 1986. [DOI] [PubMed] [Google Scholar]
- [63].Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, and Majestic C, “Remote excitation of neuronal circuits using low-intensity, low-frequencyultrasound,”PloSOne,vol.3,no.10,2008,Art.no.e3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ressler KJ and Mayberg HS, “Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic,” Nat. Neurosci, vol. 10, no. 9, 2007, Art. no. 1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wagner T,Valero-Cabre A,andA.Pascual-Leone,“Noninvasive human brain stimulation,” Annu. Rev. Biomed. Eng, vol. 9, pp. 527–565, 2007. [DOI] [PubMed] [Google Scholar]
- [66].Szobota S et al. , “Remote control of neuronal activity with a light-gated glutamate receptor,” Neuron, vol. 54, no. 4, pp. 535–545, 2007. [DOI] [PubMed] [Google Scholar]
- [67].Fini M and Tyler WJ, “Transcranial focused ultrasound: A new tool for non-invasive neuromodulation,” Int. Rev. Psychiatry, vol. 29, no. 2, pp. 168–177, 2017. [DOI] [PubMed] [Google Scholar]
- [68].Xing J and Chen J, “Design of a thermoacoustic sensor for low intensity ultrasound measurements based on an artificial neural network,” Sensors, vol. 15, no. 6, pp. 14788–14808, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Eljarrat-Binstock E, Raiskup F, Stepensky D, Domb AJ, and Frucht-Pery J, “Delivery of gentamicin to the rabbit eye by drug-loaded hydrogel iontophoresis,” Invest. Ophthalmol. Vis. Sci, vol. 45, no. 8, pp. 2543–2548, 2004. [DOI] [PubMed] [Google Scholar]
- [70].Aptel F and Lafon C, “Therapeutic applications of ultrasound in ophthalmology,” Int. J. Hyperthermia, vol. 28, no. 4, pp. 405–418, 2012. [DOI] [PubMed] [Google Scholar]
- [71].Gaudana R, Ananthula HK, Parenky A, and Mitra AK, “Ocular drug delivery,” AAPS J, vol. 12, no. 3, pp. 348–360, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Achouri D, Alhanout K, Piccerelle P, and Andrieu V, “Recent advances in ocular drug delivery,” Drug Develop. Ind. Pharm, vol. 39, no. 11, pp. 1599–1617, 2013. [DOI] [PubMed] [Google Scholar]
- [73].Kompella UB, Kadam RS, and Lee VH, “Recent advances in ophthalmic drug delivery,” Ther. Deliv, vol. 1, no. 3, pp. 435–456, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Edwards A and Prausnitz MR, “Predicted permeability of the cornea to topical drugs,” Pharm. Res, vol. 18, no. 11, pp. 1497–1508, 2001. [DOI] [PubMed] [Google Scholar]
- [75].Kim YC, Chiang B, Wu X, and Prausnitz MR, “Ocular delivery of macromolecules,” J. Control. Release, vol. 190, pp. 172–181, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lee JW and Prausnitz MR, “Drug delivery using microneedle patches: Not just for skin,” Expert Opinion Drug Del, vol. 15, pp. 541–543, 2018. [DOI] [PubMed] [Google Scholar]
- [77].Hao J, Li SK, Liu C-Y, and Kao WW, “Electrically assisted delivery of macromolecules into the corneal epithelium,” Exp. Eye Res, vol. 89, no. 6, pp. 934–941, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chopra P, Hao J, and Li SK, “Iontophoretic transport of charged macromolecules across human sclera,” Int. J. Pharm, vol. 388, no. 1–2, pp. 107–113, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Patel SP, Vaishya R, Mishra GP, Tamboli V, Pal D, and Mitra AK, “Tailor-made pentablock copolymer based formulation for sustained ocular delivery of protein therapeutics,” J. Drug Deliv, vol. 2014, 2014, Art. no. 401747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Yasukawa T, Ogura Y, Kimura H, Sakurai E, and Tabata Y, “Drug delivery from ocular implants,” Expert Opin. Drug Deliv, vol. 3, no. 2, pp. 261–273, 2006. [DOI] [PubMed] [Google Scholar]
- [81].Hu X et al. , “Hydrogel contact lens for extended delivery of ophthalmic drugs,” Int. J. Polym. Sci, vol. 2011, 2011, Art. no. 814163. [Google Scholar]
- [82].Mishra GP, Bagui M, Tamboli V, and Mitra AK, “Recent applications of liposomes in ophthalmic drug delivery,” J. Drug Deliv, vol. 2011, 2011, Art. no. 863734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lam TT, Fu J, Chu R, Stojack K, Siew E, and Tso MO, “Intravitreal delivery of ganciclovir in rabbits by transscleral iontophoresis,” J. Ocul. Pharmacol. Ther, vol. 10, no. 3, pp. 571–575, 1994. [DOI] [PubMed] [Google Scholar]
- [84].Cohen AE, Assang C, Patane MA, From S, and Korenfeld M, “Evaluation of dexamethasone phosphate delivered by ocular iontophoresis for treating noninfectious anterior uveitis,” Ophthalmology, vol. 119, no. 1, pp. 66–73, 2012. [DOI] [PubMed] [Google Scholar]
- [85].Ambati J and Adamis AP, “Transscleral drug delivery to the retina and choroid,” Prog. Retinal Eye Res, vol. 21, no. 2, pp. 145–151, 2002. [DOI] [PubMed] [Google Scholar]
- [86].Gibson JM and Gibson SJ, “A safety evaluation of ranibizumab in the treatment of age-related macular degeneration,” Expert Opin. Drug Saf, vol. 13, no. 9, pp. 1259–1270, 2014. [DOI] [PubMed] [Google Scholar]
- [87].Jager RD, Aiello LP, Patel SC, and Cunningham ET Jr., “Risks of intravitreous injection: A comprehensive review,” Retina, vol. 24, no. 5, pp. 676–698, 2004. [DOI] [PubMed] [Google Scholar]
- [88].Mundt GH and Hughes WF, “Ultrasonics in ocular diagnosis,” Amer. J. Ophthalmol, vol. 41, no. 3, pp. 488–498, 1956. [DOI] [PubMed] [Google Scholar]
- [89].Allemann N, Silverman RH, Reinstein DZ, and Coleman DJ, “High-frequency ultrasound imaging and spectral analysis in traumatic hyphema,” Ophthalmology, vol. 100, no. 9, pp. 1351–1357, 1993. [DOI] [PubMed] [Google Scholar]
- [90].Cheung ACY, Yu Y, Tay D, Wong HS, Ellis-Behnke R, and Chau Y, “Ultrasound-enhanced intrascleral delivery of protein,” Int. J. Pharm, vol. 401, no. 1–2, pp. 16–24, 2010. [DOI] [PubMed] [Google Scholar]
- [91].Nabili M, Patel H, Mahesh SP, Liu J, Geist C, and Zderic V, “Ultrasound-enhanced delivery of antibiotics and anti-inflammatory drugs into the eye,” Ultrasound Med. Biol, vol. 39, no. 4, pp. 638–646, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Cherkasov I, Marmur R, Radkovskaia A, and Loskova L, “Phonophoresis of hypotensive agents in the treatment of simple glaucoma,” Oftalmol. Zh, vol. 29, no. 2, pp. 114–118, 1974. [PubMed] [Google Scholar]
- [93].Filippenko V, “The treatment of eye diseases using the gamma-G ultrasonic apparatus,” Voen. Med. Zh, vol. 8, pp. 30–31, 1989. [PubMed] [Google Scholar]
- [94].Marmur R, Moiseeva N, and Korkhov S, “Current state and perspectives of further development of phonophoresis of drugs in ophthalmology (review of the literature),” Oftalmol. Zh, vol. 34, no. 2, pp. 68–73, 1979. [PubMed] [Google Scholar]
- [95].Tsok R, “Phonophoresis in various diseases of the anterior segment of the eye,” Oftalmol. Zh, vol. 34, no. 2, pp. 73–76, 1979. [PubMed] [Google Scholar]
- [96].Tsok R, Gereliuk I, Tsok O, and Kaminskĭ I, “The effect of ultrasonic oscillations of different frequencies on radionuclide accumulation in the eye tissues,” Oftalmol. Zh, no. 1, pp. 46–49, 1990. [PubMed] [Google Scholar]
- [97].Iakimenko S, Chalanova R, and Artemov A, “The use of lekozim phonophoresis for the treatment of eye burns,” Oftalmol. Zh, vol. 8, no. 8, pp. 492–497, 1989. [PubMed] [Google Scholar]
- [98].Egorov E, Kriukova M, Khabush T, and Kriukov A, “Results of intraocular pharmaco-physical therapy in inflammatory diseases of the cornea,” Vestn. Oftalmol, vol. 111, no. 1, pp. 31–34, 1995. [PubMed] [Google Scholar]
- [99].Zderic V, Clark J, Martin R, and Vaezy S, “Transcorneal drug delivery using 880 KHz ultrasound,” Cornea, vol. 23, pp. 804–811, 2004. [DOI] [PubMed] [Google Scholar]
- [100].Zderic V, Clark JI, and Vaezy S, “Drug delivery into the eye with the use of ultrasound,” J. Ultrasound Med, vol. 23, no. 10, pp. 1349–1359, 2004. [DOI] [PubMed] [Google Scholar]
- [101].Nabili M et al. ,“Ultrasound-enhanced ocular delivery of dexamethasone sodium phosphate: an in vivo study,” J. Ther. Ultrasound, vol. 2, no. 1, pp. 6–19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Nabili M, Geist C, and Zderic V, “Thermal safety of ultrasoundenhanced ocular drug delivery: A modeling study,” Med. Phys, vol. 42, no. 10, pp. 5604–5615, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lamy R et al. , “Ultrasound-enhanced penetration of topical riboflavin into the corneal stroma,” Invest. Ophthalmol. Vis. Sci, vol. 54, no. 8, pp. 5908–5912, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Suen W-LL, Wong HS, Yu Y, Lau LCM, Lo AC-Y, and Chau Y, “Ultrasound-mediated transscleral delivery of macromolecules to the posterior segment of rabbit eye in vivo,” Invest. Ophthalmol. Vis. Sci, vol. 54, no. 6, pp. 4358–4365, 2013. [DOI] [PubMed] [Google Scholar]
- [105].Razavi A et al. , “Contribution of inertial cavitation in the enhancement of in vitro transscleral drug delivery,” Ultrasound Med. Biol, vol. 40, no. 6, pp. 1216–1227, 2014. [DOI] [PubMed] [Google Scholar]
- [106].Sonoda S et al. , “Gene transfer to corneal epithelium and keratocytes mediated by ultrasound with microbubbles,” Invest. Ophthalmol. Vis. Sci, vol. 47, no. 2, pp. 558–564, 2006. [DOI] [PubMed] [Google Scholar]
- [107].Park J, Zhang Y, Vykhodtseva N, Akula JD, and McDannold NJ, “Targeted and reversible blood-retinal barrier disruption via focused ultrasound and microbubbles,” PLoS One, vol. 7, no. 8, 2012, Art. no. e42754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hogan N, Krebs HI, Charnnarong J, Srikrishna P, and Sharon A, “MIT-MANUS: A workstation for manual therapy and training. I,” in Proc. IEEE Int. Workshop Robot Human Commun., 1992, pp. 161–165. [Google Scholar]
- [109].Maciejasz P, Eschweiler J, Gerlach-Hahn K, Jansen-Troy A, and Leonhardt S, “A survey on robotic devices for upper limb rehabilitation,” J. Neuroeng. Rehabil, vol. 11, pp. 3–32, January 9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Louie DR and Eng JJ, “Powered robotic exoskeletons in post-stroke rehabilitation of gait: A scoping review,” J. Neuroeng. Rehabil, vol. 13, no. 1, pp. 53–63, June 8, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Mehrholz J and Pohl M, “Electromechanical-assisted gait training after stroke: A systematic review comparing end-effector and exoskeleton devices,” J. Rehabil. Med, vol. 44, no. 3, pp. 193–199, March 2012. [DOI] [PubMed] [Google Scholar]
- [112].Mehrholz J, Pohl M, Platz T, Kugler J, and Elsner B, “Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke,” Cochrane Database Syst. Rev, no. 11, November 07, 2015, Art. no. CD006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hedman LD et al. , “White paper: Movement system diagnoses in neurologic physical therapy, ”J. Neurol. Phys. Ther, vol. 42, no. 2, pp. 110–117, April 2018. [DOI] [PubMed] [Google Scholar]
- [114].Chua KSG and Kuah CWK, “Innovating with rehabilitation technology in the real world: Promises, potentials, and perspectives,” Amer. J. Phys. Med. Rehabil, vol. 96, pp. S150–S156, October 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Laut J, Porfiri M, and Raghavan P, “The present and future of robotic technology in rehabilitation,” Curr. Phys. Med. Rehabil. Rep, vol. 4, no. 4, pp. 312–319, December 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Klamroth-Marganska V et al. , “Three-dimensional, task-specific robot therapy of the arm after stroke: A multicentre, parallel-group randomised trial,” Lancet Neurol, vol. 13, no. 2, pp. 159–66, February 2014. [DOI] [PubMed] [Google Scholar]
- [117].Lo AC et al. , “Robot-assisted therapy for long-term upper-limb impairment after stroke,” N. Engl. J. Med, vol. 362, no. 19, pp. 1772–83, May 13, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ellis MD, Carmona C, Drogos J, and Dewald JPA, “Progressive abduction loading therapy with horizontal-plane viscous resistance targeting weakness and flexion synergy to treat upper limb function in chronic hemiparetic stroke: A randomized clinical trial,” (in English), Frontiers Neurol., Original Res, vol. 9, no. 71, February 19, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kitago T et al. , “Improvement after constraint-induced movement therapy: Recovery of normal motor control or task-specific compensation?” Neurorehabil. Neural Repair, vol. 27, no. 2, pp. 99–109, February 2013. [DOI] [PubMed] [Google Scholar]
- [120].Schumann C et al. , “Interactive multi-criteria planning for radiofrequency ablation,” Int. J. Comput. Assisted Radiol. Surg, vol. 10, no. 6, pp. 879–889, 2015. [DOI] [PubMed] [Google Scholar]
- [121].Pearce JA, “Models for thermal damage in tissues: Processes and applications,” Crit. Rev. Biomed. Eng, vol. 38, no. 1, pp. 1–20, 2010. [DOI] [PubMed] [Google Scholar]
- [122].Voglreiter P et al. , “RFA guardian: Comprehensive simulation of radiofrequency ablation treatment of liver tumors,” Sci. Rep, vol. 8, no. 1, pp. 787–800, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Sebek J, Albin N, Bortel R, Natarajan B, and Prakash P, “Sensitivity of microwave ablation models to tissue biophysical properties: A first step toward probabilistic modeling and treatment planning,” Med. Phys, vol. 43, no. 5, pp. 2649–2661, 2016. [DOI] [PubMed] [Google Scholar]
- [124].Raman F, Scribner E, Saut O, Wenger C, Colin T, and Fathallah-Shaykh HM, “Computational trials: Unraveling motility phenotypes, progression patterns, and treatment options for glioblastoma multiforme,” PLoS One, vol. 11, no. 1, 2016, Art. no. e0146617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Mueller RK, Kaveh M, and Wade G, “Reconstructive tomography and applications to ultrasonics,” Proc. IEEE, vol. 67, no. 4, pp. 567–587, April 1979. [Google Scholar]
- [126].Schueler CF, Lee H, and Wade G, “Fundamentals of digital ultrasonic processing,” IEEE Trans. Sonics Ultrason, vol. 31, no. 4, pp. 195–217, July 1984. [Google Scholar]
- [127].Bernard S, Monteiller V, Komatitsch D, and Lasaygues P, “Ultrasonic computed tomography based on full-waveform inversion for bone quantitative imaging,” Phys. Med. Biol, vol. 62, no. 17, pp. 7011–7035, 2017. [DOI] [PubMed] [Google Scholar]
- [128].Matthews TP, Wang K, Li C, Duric N, and Anastasio MA, “Regularized dual averaging image reconstruction for full-wave ultrasound computed tomography,” IEEE Trans. Ultrason., Ferroelect., Freq. Control, vol. 64, no. 5, pp. 811–825, May 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Pérez-Liva M, Herraiz J, Udías J, Miller E, Cox B, and Treeby B, “Time domain reconstruction of sound speed and attenuation in ultrasound computed tomography using full wave inversion,” J. Acoust. Soc. Amer, vol. 141, no. 3, pp. 1595–1604, 2017. [DOI] [PubMed] [Google Scholar]
- [130].Haddadin OS and Ebbini ES, “Imaging strongly scattering media using a multiple frequency distorted Born iterative method,” IEEE Trans. Ultrason., Ferroelect., Freq. Control, vol. 45, no. 6, pp. 1485–1496, November 1998. [DOI] [PubMed] [Google Scholar]
- [131].Carević A et al. , “Solving the ultrasound inverse scattering problem of inhomogeneous media using different approaches of total least squares algorithms,” Proc. SPIE, vol. 10580, 2018, Art. no. 105800J. [Google Scholar]
- [132].Yun X, He J, Carevic A, Slapnicar I, Barlow J, and Almekkawy M, “Reconstruction of ultrasound tomography for cancer detection using total least squares and conjugate gradient method,” Proc. SPIE, vol. 10580, 2018, Art. no. 105800K. [Google Scholar]
- [133].Ruiter NV, Zapf M, Hopp T, Dapp R, and Gemmeke H, “Phantom image results of an optimized full 3D USCT,” Proc. SPIE, vol. 8320, 2012, Art. no. 832005. [Google Scholar]
- [134].Huthwaite P, Simonetti F, and Duric N, “Combining time of flight and diffraction tomography for high resolution breast imaging: Initial in vivo results (L),” J. Acoust. Soc. Amer, vol. 132, no. 3, pp. 1249–1252, 2012. [DOI] [PubMed] [Google Scholar]
- [135].Abubakar A, Habashy TM, van den Berg PM, and Gisolf D, “The diagonalized contrast source approach: An inversion method beyond the Born approximation,” Inverse Probl, vol. 21, no. 2, p. 685, 2005. [Google Scholar]
- [136].Bakushinsky A and Goncharsky A, Ill-Posed Problems: Theory and Applications. Berlin, Germany: Springer, 2012. [Google Scholar]
- [137].Tikhonov AN, Goncharsky A, Stepanov V, and Yagola AG, Numerical Methods for the Solution of Ill-Posed Problems. Berlin, Germany: Springer, 2013. [Google Scholar]
- [138].Peterlik I, Jirik R, Ruiter N, and Jan J, “Regularized image reconstruction for ultrasound attenuation transmission tomography,” Radioengineering, vol. 17, no. 2, pp. 125–132, 2008. [Google Scholar]
- [139].Yilmaz Ö,¨ Seismic Data Analysis: Processing, Inversion, and Interpretation of Seismic Data. Tulsa, OK, USA: Society of Exploration Geophysicists, 2001. [Google Scholar]
- [140].Schmidt S, Duric N, Li C, Roy O, and Huang ZF, “Modification of Kirchhoff migration with variable sound speed and attenuation for acoustic imaging of media and application to tomographic imaging of the breast,” Med. Phys, vol. 38, no. 2, pp. 998–1007, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Natterer F, “Incomplete data problems in wave equation imaging,” Inverse Probl. Imag, vol. 4, no. 4, pp. 685–691, 2010. [Google Scholar]
- [142].Beilina L and Clason C, “An adaptive hybrid FEM/FDM method for an inverse scattering problem in scanning acoustic microscopy,” SIAM J. Sci. Comput, vol. 28, no. 1, pp. 382–402, 2006. [Google Scholar]
- [143].Beilina L, “Adaptive hybrid FEM/FDM methods for inverse scattering problems,” 2002. [Google Scholar]
- [144].Goncharskii A and Romanov SY, “Two approaches to the solution of coefficient inverse problems for wave equations,” Comput. Math. Math. Phys, vol. 52, no. 2, pp. 245–251, 2012. [Google Scholar]
- [145].Greenleaf JF, Johnson S, Lee S, Hermant G, and Woo E, “Algebraic reconstruction of spatial distributions of acoustic absorption within tissue from their two-dimensional acoustic projections,” in Acoustical Holography, Boston, MA, USA: Springer, 1974, pp. 591–603. [Google Scholar]
- [146].Lavarello R and Oelze M, “A study on the reconstruction of moderate contrast targets using the distorted Born iterative method,” IEEE Trans. Ultrason., Ferroelect., Freq. Control, vol. 55, no. 1, pp. 112–124, January 2008. [DOI] [PubMed] [Google Scholar]
- [147].Liu C, Wang Y, and Heng PA, “A comparison of truncated total least squares with Tikhonov regularization in imaging by ultrasound inverse scattering,” Phys. Med. Biol, vol. 48, no. 15, pp. 2437–2451, 2003. [DOI] [PubMed] [Google Scholar]
- [148].Gemmeke H and Ruiter N, “3D ultrasound computer tomography for medical imaging,” Nucl. Instrum. Methods Phys. Res. Sect. A, Accel., Spectrometers, Detect. Assoc. Equip, vol. 580, no. 2, pp. 1057–1065, 2007. [Google Scholar]
- [149].Ruiter NV, Gobel G, Berger L, Zapf M, and Gemmeke H, “Realiza-¨ tion of an optimized 3D USCT,” Proc. SPIE, vol. 7968, 2011, Art. no. 796805. [Google Scholar]
- [150].Birk M, Koehler S, Balzer M, Huebner M, Ruiter NV, and Becker J, “FPGA-based embedded signal processing for 3D ultrasound computer tomography,” in Proc. 17th IEEE-NPSS Real Time Conf., 2010, pp. 1–5. [Google Scholar]
- [151].Birk M, Kretzek E, Figuli P, Weber M, Becker J, and Ruiter NV, “High-speed medical imaging in 3D ultrasound computer tomography,” IEEETrans.ParallelDistrib.Syst,vol.27,no.2,pp.455–467,February2016. [Google Scholar]
- [152].Duric N et al. , “Development of ultrasound tomography for breast imaging: Technical assessment,” Med. Phys, vol. 32, no. 5, pp. 1375–1386, 2005. [DOI] [PubMed] [Google Scholar]
- [153].Tang SC and Clement GT, “A computerized tomography system for transcranial ultrasound imaging,” in Proc. Meetings Acoust, 2014, vol. 22, no. 1, Art. no. 020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Tang SC, Clement GT, and Hynynen K, “A computer-controlled ultrasound pulser-receiver system for transskull fluid detection using a shear wave transmission technique,” IEEE Trans. Ultrason., Ferroelect., Freq. Control, vol. 54, no. 9, pp. 1772–1783, September 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Maier-Hein L et al. , “Surgical data science: Enabling next-generation surgery,” 2017, arXiv:1701.06482. [Google Scholar]
- [156].Obermeyer Z and Emanuel EJ, “Predicting the future—big data, machine learning, and clinical medicine,” N. Engl. J. Med, vol. 375, no. 13, pp. 1216–1219, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Porter ME, Larsson S, and Lee TH, “Standardizing patient outcomes measurement,” N. Engl. J. Med, vol. 374, no. 6, pp. 504–506, 2016. [DOI] [PubMed] [Google Scholar]
- [158].Maintz JA and Viergever MA, “A survey of medical image registration,” Med. Image Anal, vol. 2, no. 1, pp. 1–36, 1998. [DOI] [PubMed] [Google Scholar]
- [159].Viergever MA, Maintz JA, Klein S, Murphy K, Staring M, and Pluim JP, “A survey of medical image registration–Under review,” Med. Image Anal, vol. 33, pp. 140–144, 2016. [DOI] [PubMed] [Google Scholar]