Abstract
Short-, medium-, and long-chain chlorinated paraffins (SCCPs, MCCPs, and LCCPs) were analyzed in human milk from the Yangtze River Delta (YRD) and Scandinavia. Individual samples were collected from Shanghai, Jiaxing, and Shaoxing (China), Stockholm (Sweden), and Bodø (Norway) between 2010 and 2016. Mean concentrations (range) of SCCPs, MCCPs, and LCCPs in samples from the YRD were 124 [<limit of detection (LOD)-676], 146 (<LOD-1260), and 19.1 (<LOD-184) ng g–1 fat, respectively, all of which were significantly (p < 0.05) higher than 15.9 (<LOD-120), 45.0 (<LOD-311), and 5.50 (<LOD-29.0) ng g–1 fat, respectively, in samples from Scandinavia. MCCPs predominate in most samples, and LCCP concentrations exceed reported for polybrominated diphenyl ethers in human milk from the same regions. This study is the first to confirm LCCP exposure via breastfeeding. Principal component analysis showed that the YRD samples were more influenced by SCCPs than the Scandinavian samples, which mirror different exposures to CPs between the regions. Because of a large variation in concentrations among individuals, SCCP intake via breastfeeding indicated a potential health concern in the 90th percentile among Chinese infants. Further, CP concentrations in the YRD samples from first-time mothers were on average three times higher than from second-time mothers. In order to limit the worldwide CP contamination, the inclusion of SCCPs as persistent organic pollutants in the Stockholm Convention needs to be followed up, with the inclusion of MCCPs and LCCPs as well.
Introduction
Chlorinated paraffins (CPs) are well-known industrial chemicals with an extensive global use, for instance, as metal-cutting fluids, flame retardants, plasticizers, and sealants.1 The global production volumes of CPs are extremely high and have exceeded one million metric tons per year.2 CPs are complex mixtures of polychlorinated n-alkanes resulting from radical reactions between chlorine and predefined homologue series of alkane hydrocarbons. Commercial CP products are traditionally divided into short-chain CPs (SCCPs, C10–13), medium-chain CPs (MCCPs, C14–17), and long-chain CPs (LCCPs, C18–30) based on the carbon chain lengths of the alkanes in the products.3 Hence, the carbon chain length defines the “C-homologues,” whereas the number of chlorine substituents implies a second series of “Cl-homologues” for each carbon chain length. The large range of isomers include everything from one chlorine substituent for each carbon chain length up to one chlorine bound to each of the carbons in the carbon chain in question.4 Calculated theoretical numbers of chlorinated alkane isomers per carbon in the chain are presented in Table S1. The total theoretical number of congeners for SCCPs, MCCPs, and LCCPs are approximately 7800, 120 000, and 1 100 000 000, respectively, by assuming no more than one chlorine atom bound to any carbon atom.4
CPs, independent of the carbon chain length and chlorination degree, are chemically stable under general environmental5−7 and ambient8−10 conditions. However, CPs are thermolabile.11−14 They are highly lipophilic (log KOW 4–12)15 and bioaccumulative in humans16,17 and wildlife.18,19 These data confirm the bioavailability and retention of CPs and, accordingly, the bioaccumulativity of this class of chemicals. The bioaccumulative properties of SCCPs and MCCPs were observed early.3 In contrast, LCCPs were only recently found to be bioaccumulative. LCCPs have been shown to bioaccumulate in Daphnia magna, similar to SCCPs and MCCPs.20 LCCPs have also been detected together with SCCPs and MCCPs, as reported in human blood21 and in various wildlife species.22 CPs undergo long-range transport,23 which is supported by CP concentrations in the Arctic as recently reviewed by Vorkamp and co-workers.24 Further, they are also distributed globally in materials and goods, from which the CPs can be mobilized into indoor air and adsorbed onto particulates (dust).25
The mammalian and environmental toxicities of CPs were reviewed in 2010 by El-Sayed Ali and Legler.26 Already from the beginning of CP toxicity research, focus was on SCCPs and far less on MCCPs and LCCPs. Nevertheless, MCCPs appeared to be more toxic than SCCPs based on the lowest observed effect levels (LOELs) in rats.26 However, in the 2019 draft risk assessment of CPs, the European Food Safety Authority (EFSA) CONTAM Panel proposed that adverse toxicity in rats was observed at lower SCCP exposure levels than of MCCPs.27 In mammals, the liver, kidney, thyroid, and parathyroid glands are target organs for CP toxicity, with developmental toxicity such as MCCP-induced adverse effects on rat offspring body weight (BW) and hematological parameters being the most sensitive endpoints.26 SCCPs have been identified as possibly carcinogenic to humans,28 being mutagenic when highly chlorinated.29 They were included as persistent organic pollutants (POPs) in 2017 according to the Stockholm Convention (Annex A).30 A toxicological interaction between SCCPs and other POPs has been reported in rats, where the SCCPs and the polybrominated diphenyl ether (PBDE-47) synergistically induce metabolic activity in the liver (EROD) and decrease the serum levels of free thyroxin, a thyroid hormone.31 Recent zebrafish larva tests showed pronounced neurotoxic effects of highly chlorinated SCCPs.32 SCCPs can also act as immunomodulators in mice.33 Furthermore, Glüge and co-workers state that the MCCPs meet the toxicity threshold defined by REACH and should be considered toxic to the environment.34
As a consequence of CPs identified as potential hazardous chemicals already in the early 1980s, occurrence data for CPs in the environment, wildlife, and humans have been published.16,35 However, the complexity of the CPs has been an obstacle for the chemical instrumental quantitative analysis.36 Recently, advances in analytical methods have been made,37 for instance, by the development of the atmospheric-pressure chemical ionization quadrupole time-of-flight high-resolution mass spectrometry (APCI-QTOF-HRMS) method.38 This method allows for measurements of CP congener groups with chain lengths of C9–C30. The recent methodological achievements have led to an avalanche of occurrence reports. However, the reporting is heavily focused on SCCPs, far less on MCCPs, and rarely on LCCPs. The reported elevated levels of CPs in wildlife18,22,39 and their occurrence in household dust,9,10 foodstuff,40,41 and other environmental matrices5,42 have sparked our interest to perform an in-depth and up-to-date study on CPs in human milk.
CPs have been produced since the mid-1930s, and their POP characteristics have been known for a long time30 The human biomonitoring is still limited with just a few reports on SCCPs and MCCPs in human milk and on infant exposure to these CPs via breastfeeding from UK,43 Sweden,44 China,45 Korea, and Japan.46 It should be noted that the analytical method for the CP analysis would cause greater uncertainty than other legacy POP analyses, hindering the spatial comparison from different areas of the world across several studies.47−49 The concentration of CPs in human milk reported from China varies up to 2 orders of magnitude among studies.45,46 This might be due to regional differences in CP contamination, but problems of comparing results because of use of different analytical methods must not be underestimated. Thus, using the same method for CP determination is essential for spatial comparisons.
The current study performed comprehensive chemical analyses of SCCPs, MCCPs, and LCCPs in human milk samples from three cities in China, one in Sweden, and one in Norway. The objectives were (i) to assess the potential bioaccumulation of SCCPs, MCCPs, and LCCPs in human milk, (ii) to analyze the data for CP pattern differences between sampling countries, and (iii) to estimate CP intakes of infants from breastfeeding exposure and to compare the estimated CP intakes with the lowest exposure levels associated with adverse effects in animal studies (i.e., risk characterization). Human milk samples from two Chinese cities were collected just after modification of the Chinese “birth control policy.” The timing of the collection enabled sampling from both first-time and second-time mothers, which thus opens up the possibility to also investigate the difference in the concentration of CPs depending on parity.
Materials and Methods
Human Milk Samples
Samples were collected from Shanghai, Jiaxing, and Shaoxing, located in the Yangtze River Delta (YRD), China (Figure S1). The cities represent one mega city and two intermediate sized cities with populations of approximately 25, 5, and 1.5 million inhabitants, respectively. The other two sampling cities are located in Scandinavia, Stockholm (Sweden) and Bodø (Norway), the former with a population of 1.5 million and the latter with approximately 50 thousand inhabitants.
Sample selection and collection were based on the World Health Organization guidelines for human milk monitoring;50 the samples were not pooled, and human milk from second-time mothers were also collected in two cities from China. Detailed information on the individual samples is provided in Table S2. Human milk samples from China were collected by the Yangtze Environmental Specimen Bank (YESB) at the Tongji University. A total of 36 human milk samples were collected from donors from Shanghai (n = 10, 2015–2016), Jiaxing (n = 13, 2015–2016), and Shaoxing (n = 13, 2010). The samples from Shaoxing were all from first-time mothers (20- to 25-year old), whereas the samples from Shanghai and Jiaxing included both first- and second-time mothers (mean age: 29 and 33 years, respectively). Human milk samples from Sweden were provided by the Swedish Museum of Natural History. The samples were collected from first-time mothers (mean age: 29 years) in 2011 (n = 10) and 2016 (n = 9) at the Mother’s Milk Center (MMC) at a Stockholm hospital, Södersjukhuset. Human milk from Norway (n = 8) was kindly provided by first-time mothers (mean age: 35 years) at Odland Health and Environment AS, Bodø, Norway based on individual informed consent.
All Chinese participants were told the objective of this study and signed the participant information and consent form. The study was approved by the Ethics Committee of China National Center for Food Safety Risk Assessment. The Norwegian sampling was part of the MISA Study, with ethical approval from REK North (2009, 1959). The Swedish milk was purchased from MMC which collects human milk to use as nutrition for prematurely born babies. MMC provided anonymous samples, and according to the Regional Ethics Board in Stockholm, ethics approval was thus not required for this part of the study.
Sample Analysis
Sample extraction and pretreatment followed the procedures of previous studies51,52 with some modification. CP congener groups from C9H17Cl3 to C31H52Cl14 (C9–31, Cl2–14) were measured using direct injection dichloromethane-enhanced APCI-QTOF-HRMS (QTOF Premier, Waters, UK). SCCPs, MCCPs, and LCCPs were quantified, respectively, with 5 SCCP, 6 MCCP, and 5 LCCP reference standards (Table S3) using a deconvolution algorithm.38 Detailed information on sample storage and preparation, instrumental analysis, and quantification is presented in the Supporting Information.
Quality Control and Quality Assurance
CP quantification of all samples fulfilled the criterion of R2 ≥ 0.50 (Table S4).38,53 Limit of detection (LOD) and limit of quantification (LOQ) of CPs were defined as mean value plus 3 times and 10 times the standard deviation (SD) of procedural blanks, respectively. LOQs of SCCPs, MCCPs, and LCCPs were 25, 32, and 3.8 ng g–1 fat (lipid weight), respectively. The mean recovery of the internal standard was 92 ± 7% (range: 74–104%), and the results were recovery corrected.
Statistical Analysis
Statistical analysis was performed with SPSS (PASW statistics 18) and OriginPro 9.0. Data below LOD was replaced with zero. So far, there is a lack of standardized method for the concentrations of CPs between LODs and LOQs. Because the instrumental intensities of CPs in the samples with concentrations lower than LOQs were on average 10 times higher than the blank (Figure S2), the recorded data between LOD and LOQ were included as measured. In order to avoid effects from extreme values, the Kruskal–Wallis test was used to test differences in congener group compositions in human milk from the different regions sampled. Because as many as 220 CP congener groups were assessed and they represent a larger number than the number of samples (n = 63), the Kruskal–Wallis test was also applied to select the most discriminating CP congener groups for a principal component analysis (PCA); that is, congener groups with no significant differences (p > 0.05) among groups were excluded (Table S5). PCA was applied to explore the pollution pattern of samples from the different regions sampled. Pearson correlation was used to compare the congener pattern of some of the samples in this study with those in the local commercial product for source identification. The significant level for all tests were set to 5% (α = 0.05).
Estimated Daily Intake via Human Milk
A margin of exposure (MOE) approach was applied to assess the health risks of CP exposure of infants via breast milk. MOE was calculated by the use of the estimated daily intake (EDI, ng kg–1 BW day–1) and the LOEL in animal studies or lower-bound 95th confidence interval benchmark dose for a 10% change in the critical effect (BMDL10)
| 1 |
| 2 |
where C is the concentration of SCCPs, MCCPs, and LCCPs in human milk (ng g–1 fat), M is the daily intake from breast milk (g kg BW–1 day–1), and R is the lipid content of the sample (%/100). The daily human milk intake was assumed to be 750 mL d–1 for an infant BW of 6 kg.54 Assuming a specific weight of human milk of 1 g mL–1, the average daily intake of human milk by an infant weighing 6 kg was estimated to be 125 g kg BW–1 day–1. The median lipid content in the human milk samples from each country in the current study was used for country-specific intake calculations.
Results and Discussion
CP Concentrations in Chinese and Scandinavian Human Milk
The maximum concentration of SCCPs and MCCPs across all human milk samples was 676 and 1260 ng g–1 fat, respectively, as detected in the milk from Shanghai. LCCPs were quantified in human milk for the first time, with a maximum concentration of 184 ng g–1 fat (Shaoxing) and a median concentration of 6.58 ng g–1 fat across all samples (Table 1). Median concentrations of SCCPs, MCCPs, and LCCPs in the human milk (35.0, 78.8, and 8.80 ng g–1 fat, respectively) from the three Chinese cities were all significantly higher than those in samples from the Scandinavian cities (median 14.0, 29.6, and 4.17 ng g–1 fat, respectively) (p < 0.05, Figure 1). SCCP and LCCP concentrations were comparable in samples from the two Scandinavian cities, but MCCP concentrations in the Norwegian samples (median 43.1 ng g–1 fat) were higher than in the Swedish samples (median 27.4 ng g–1 fat) (p > 0.05). Stockholm human milk samples originated from the years 2011 and 2016. SCCP, MCCP, and LCCP concentrations in 2016 were on average 52, 43, and 30% lower than those in 2011, respectively (Table 1), but not statistically significant. Concentrations reported for the SCCPs in human milk from Uppsala, Sweden in human milk sampled in 1996–2010 indicate higher levels (107 ng g–1 fat)44 than the present samples from Stockholm in 2011 and 2016, which seem to be consistent with the decreasing trends of CPs found in sediments and wildlife from Sweden.6,22
Table 1. Comparison of Concentration (ng g–1 fat, Median with Range) of SCCPs, MCCPs, and LCCPs in Human Milk from the Present Study with the Literature Data.
| lipid | chlorine | SCCP | MCCP | LCCP | ||||
|---|---|---|---|---|---|---|---|---|
| sampling site | country | year | (%) | (%)a | (ng g–1 fat)b | (ng g–1 fat)b | (ng g–1 fat)b | references |
| Shanghai | China | 2015–2016 | 3.29 | 53.3 | 35.0(<LOD-676) | 70.1(38–1260) | 10.5 (<LOD-56.9) | this study |
| Jiaxing | China | 2015–2016 | 3.85 | 54.1 | 28.6(<LOD-642) | 63.0(<LOD-547) | 5.23(<LOD-18.4) | this study |
| Shaoxing | China | 2010 | 3.79 | 53.4 | 37.9(<LOD-124) | 121(38–187) | 14.6(4.90–184) | this study |
| 12 provinces (urban) | China | 2007 | 3.5 | 681 (170–6150) | 60.4 (18.7–350) | Xia et al.45 | ||
| 16 provinces (urban) | China | 2011 | 3.7 | 733 (131–16100) | 64.3 (22.3–1501) | Xia et al.45 | ||
| 8 provinces (rural) | China | 2007 | 3.7 | 303 (68–1580) | 35.7 (9–139) | Xia et al.54 | ||
| 16 provinces (rural) | China | 2011 | 3.7 | 360 (66–2310) | 45.4 (10–146) | Xia et al.54 | ||
| Beijing | China | 2007–2009 | (<20–54) | Cao et al.46 | ||||
| Hebei | China | 2014–2015 | 2.2 | 1.46 (0.21–16.2) | Yang et al.75 | |||
| Stockholm | Sweden | 2011 | 3.85 | 52.1 | 15.6(<LOD-24.1) | 37.3 (<LOD-77.7) | 4.28(<LOD-21.4) | this study |
| Stockholm | Sweden | 2016 | 4.56 | 53.0 | <LOD (<LOD-27.8) | 21.0(<LOD-60.0) | 2.80(<LOD-9.34) | this study |
| Bodø | Norway | 2014 | 4.74 | 52.6 | 10.2c(<LOD-120) | 43.1(<LOD-311) | 4.26(<LOD-29.0) | this study |
| Quebec | Canada | (11.0–17.0) | Tomy76 | |||||
| Baden-Wurttemberg | Germany | 2004–2005 | 120 (49–275)d | Reth et al.77 | ||||
| Lancaster | UK | 2001–2002 | 180 (49–820) | 21 (6.2–320) | Thomas et al.43 | |||
| Uppsala | Sweden | 1996–2010 | 3.1 | 112 (45–157) | 15 (1.1–30) | Darnerud et al.44 | ||
| Seoul | Korea | 2007–2010 | <20 | Cao et al.46 | ||||
| Busan | Korea | 2008–2009 | <20 | Cao et al.46 | ||||
| Kyoto | Japan | 2009–2010 | <20 | Cao et al.46 |
Mean value.
LOD of SCCPs, MCCPs, and LCCPs are 12, 16, and 1.1 ng g–1 fat in this study, respectively.
Average value of <LOD and 20.4 ng g–1 fat.
Sum of SCCPs and MCCPs. For concentrations of individual samples, see Table S2.
Figure 1.
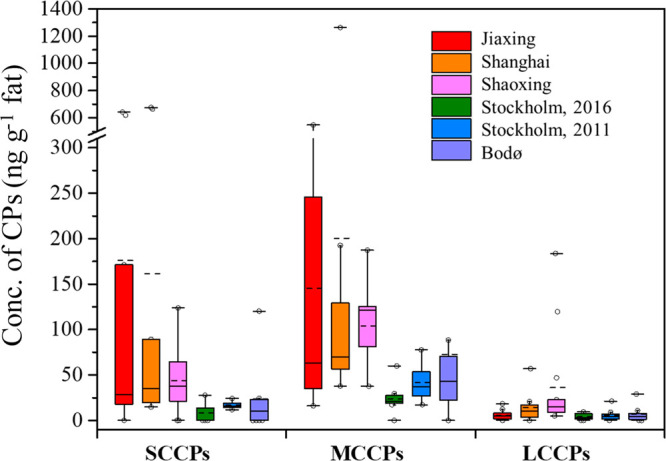
Box and whisker plot (extended to 10th and 90th percentile) of SCCP, MCCP, and LCCP concentrations (ng g–1 fat) in human milk from Jiaxing (n = 13, 2015–2019), Shanghai (n = 10, 2015–2019), and Shaoxing (n = 13, 2010) in China, Stockholm (n = 10 in 2011 and n = 9 in 2016) in Sweden, and Bodø (n = 8, 2014) in Norway. The dash line represents the mean concentration.
Notably, LCCPs were detected in 86% of the human milk samples analyzed. The highest concentrations of LCCPs were detected in Shaoxing with a median concentration of 14.6 (range: 4.90–184) ng g–1 fat, followed by Shanghai, 10.5 (range: <LOD-56.9) ng g–1 fat. The LCCPs contributed to 1.9–20% of the ΣCPs in human milk analyzed in the current study (Figure S3). In a recent study, LCCPs were also detected in human whole blood from Shenzhen, China, with a mean concentration of 150 (range: 20–520) ng g–1 fat,21 indicating that LCCPs are taken up by humans. LCCPs have also recently been reported in wildlife from the YRD and from Scandinavia at concentrations of 14–10 000 and 6.0–1200 ng g–1 fat, respectively.18,22
MCCP concentrations in Chinese milk in the current study (<LOD-1260 ng g–1 fat) are comparable to concentrations reported in previous studies in urban areas, sampled between 2007 and 2011, with median concentrations of 60.4 (range: 18.7–350) and 64.3 (range: 22.3–1500) ng g–1 fat, respectively (Table 1).45 In contrast, they are higher than the concentrations reported in the milk from the UK (median: 21, range: 6.2–320 ng g–1 fat),43 Sweden (median: 15, range: 1.1–30 ng g–1 fat),44 and rural areas in China in 2011 (median: 45.4, range: 10–150 ng g–1 fat) (Table 1).54 In the current study, MCCPs contributed 48–57 and 65–71% to the total CP concentration in the Chinese cities and in Stockholm/Bodø, respectively. MCCPs were in general the most abundant CP groups except in Jiaxing, where the proportion of SCCPs (50%) was slightly higher than MCCPs (48%) (Figure S3). A recent study also found that MCCPs were the dominant CPs in human serum from Australia and that there was an increase in MCCP levels from 2004 to 2015.55
The concentration of SCCPs measured in Chinese human milk in the present work is 1 or 2 orders of magnitude lower than previously reported with median concentrations of 733 and 360 ng g–1 fat in human milk in urban and rural areas from China in 2011, respectively.45,54 However, it is higher than in human milk from Beijing sampled in 2007–2009 (Table 1).46 As visualized in Table 1, the mean level of SCCPs in China was comparable to concentrations reported in human milk from the UK that had been sampled in 2001–200243 and from Uppsala (Sweden) on samples taken between 1996 and 2010.44 Differences in the sampling site, the sampling year, and the parity of mothers may partly explain the concentration differences among studies. For example, the concentrations of SCCPs in pooled human milk were 1 to 2 orders of magnitude different among Chinese provinces.45 The bias due to the analytical technique should also be appreciated and considered when comparison of data is done, given that recent interlaboratory studies on the analysis of SCCPs showed considerably high variation between the different methods applied.37
In comparison to other POPs reported in human milk after 2000,56 CP concentrations detected in our study (Table 1) are lower than the median values of the DDT metabolite p,p′-DDE in human milk reported from China and Sweden (range of medians: 100–3100 and 50–110 ng g–1 fat, respectively). The levels of CPs in our study are roughly comparable or slightly lower than reported concentrations of polychlorinated biphenyls (PCBs). For example, median concentrations of ΣPCBs in human milk reported from China, Sweden, and Norway were in the range of 10–210, 80–140, and 100–200 ng g–1 fat, respectively. However, average CP levels in human milk in our study were significantly higher than the ΣPBDEs. The median levels of PBDEs reported from China, Sweden, and Norway were in the range of 2.2–7.1, 1.9–3.2, and 1.9–3.2 ng g–1 fat, respectively, whereas the SCCP, MCCP, and LCCP median and range concentrations are as shown in Table 1. The CP levels in human milk mirror not only high production volumes of the CPs in China but also general exposures to these pollutants present in food and ambient environments, such as air and indoor dust.25
Assessment of Intake of CPs via Human Milk
Using the observed concentrations of CPs in human milk, the EDIs (eq 1) were calculated and then compared with the LOELs (eq 2). The EFSA CONTAM Panel reported a lower 95% confidence interval benchmark dose for a 10% change in the critical adverse effects (BMDL10) of 2.3 and 36 mg kg BW–1 day–1 for SCCPs and MCCPs, respectively, based on modelling of kidney effects in rodent studies.27 In parallel, a report from the Environment and Climate Change Canada (ECCC) suggested LOELs of 6 and 71 mg kg BW–1 day–1 for MCCPs and LCCPs, respectively, also based on effects on BW (MCCPs) and inflammatory effects (LCCPs) in rodent studies.57 In the current study, we applied both the BMDL10 from EFSA and the LOEL from ECCC for risk characterization of infant CP exposure. The 50th and 90th percentiles of SCCP, MCCP, and LCCP concentrations in human milk from each country (China, Sweden, and Norway) were considered, and the results are presented in Table 2.
Table 2. EDI [ng (kg BW)−1 day–1] of SCCPs, MCCPs, and LCCPs via Breastfeeding of Infants and MOE (Dimensionless) between the Lowest Doses Reported To Cause Adverse Toxicological Effects in Animals [LOEL, ng (kg BW)−1 day–1] and EDIsa.
| China | Sweden | Norway | ||
|---|---|---|---|---|
| n = 36 | n = 20 | n = 8 | ||
| lipid content (%) | 3.81 | 4.07 | 4.74 | |
| Conc (ng g−1 fat) | ||||
| SCCPs | 90th | 543 | 21.6 | 24.3 |
| 50th | 35.0 | 12.8 | 10.2 | |
| MCCPs | 90th | 254 | 56.8 | 88.3 |
| 50th | 78.8 | 33.3 | 43.1 | |
| LCCPs | 90th | 34.9 | 8.62 | 10.8 |
| 50th | 8.80 | 4.89 | 4.26 | |
| EDI [ng (kg BW)–1day–1] | ||||
| SCCPs | 90th | 2580 | 111 | 143 |
| 50th | 166 | 65 | 60 | |
| MCCPs | 90th | 374 | 291 | 519 |
| 50th | 121 | 170 | 253 | |
| LCCPs | 90th | 166 | 44 | 64 |
| 50th | 42 | 25 | 25 | |
| MOE (EU) | ||||
| SCCPs | 90th | 891 | 20 700 | 16 100 |
| MCCPs | 96 300 | 124 000 | 69 400 | |
| MOE (EU) | ||||
| SCCPs | 50th | 13 900 | 35 400 | 38 300 |
| MCCPs | 298 000 | 212 000 | 142 000 | |
| MOE (Canada) | ||||
| MCCPs | 90th | 4960 | 20 600 | 11 600 |
| LCCPs | 428 000 | 1 610 000 | 1 120 000 | |
| MOE (Canada) | ||||
| MCCPs | 50th | 16 000 | 35 200 | 23 700 |
| LCCPs | 1 680 000 | 2 830 000 | 2 830 000 | |
The median EDI of CPs via human milk for Chinese, Swedish, and Norwegian infants, respectively, was 166, 65, and 60 ng kg BW–1 day–1 for SCCPs, 374, 170, and 253 ng kg BW–1 day–1 for MCCPs, and 42, 25, and 25 ng kg BW–1 day–1 for LCCPs. The EDI for MCCPs was comparable to previous data in China in 2007 and 2011 (median: 212 and 152 ng kg BW–1 day–1, respectively).54 EFSA reported the EDIs of SCCPs and MCCPs in pooled human milk from 11 European countries, with a range of 60–445 and <25–514 ng kg BW–1 day–1, respectively, for average milk consumption.27 For comparison, we further calculated the EDIs (Table S6) using mean concentrations of CPs in our study and the same parameters given in EFSA (2019):27 infant BW of 6.1 kg, average daily milk consumption of about 800 mL, high consumption of 1 200 mL, and milk’s fat content of 3.5%. The ranges of SCCP and MCCP intake (average milk consumption) in our study were 58.7–570 and 153–669 ng kg BW–1 day–1, respectively (Table S6), which were generally similar to the EFSA calculation. This is due to the similar range in average concentrations of CPs used in the EFSA and our calculation, with Sweden in the lower range and China in the upper range in our study. The use of pooled samples or mean CP concentrations does not take into account the variation in concentrations within the population. Notably, for infants from China in our study, the 90th EDI of SCCP (2580 ng kg BW–1 day–1) was about 15 times higher than the median EDI in this group of infants. Such variation of CP intake among individuals highlights the necessity for dietary assessment using individual human milk samples instead of pooled samples in order to get a more comprehensive picture of breastfeeding CP intake in the studied population, which, however, requires more organizing efforts for large-scale assessments.
To compare the calculated EDI with the LOEL value obtained from the toxicological studies, as determined by EFSA and ECCC,27,57 a total safety factor of 1000 was applied, using a factor of 10 for intraspecies variation, 10 for interspecies variation, and 10 for lack of data on carcinogenicity and only data from subchronic exposure.57 A health concern was assumed if the ratio of LOEL/EDI, that is, MOE (eq 2), was below the safety factor (1000). In Table 2, it is shown that for the majority of scenarios, the MOE (90th percentile) was 1–2 orders of magnitude greater than 1000, indicating that there are no appreciable health concerns regarding SCCP, MCCP, and LCCP exposure via breastfeeding. However, the MOE (90th percentile) of SCCPs for Chinese infants (891) was lower than 1000, indicating a health concern for SCCP exposure. For Chinese infants from some other provinces, the MOE of SCCPs was as low as 143 based on previous studies45,54 using pooled human milk samples.
It should be noted that the daily human milk intake was set to 750 mL day–1, and an infant BW of 6 kg was assumed for all three countries in the EDI calculations.54 Using average values for milk intake and BW does not take into account the variability in the infant population, thus not giving the full picture of the EDI variability. The LOELs applied included other exposure windows than lactational exposure, which adds to the uncertainty of the comparisons between EDIs and LOELs. For instance, in certain cases, LOELs were based on exposure of adult rodents, and since exposure of contaminants during infancy may be a more sensitive window of exposure, such LOELs may underestimate the toxicity. Finally, possible additive effects due to coexposures of SCCPs, MCCPs, and LCCPs have not been considered, which is a likely scenario based on the structural similarities of the CPs. The CP exposure also adds to the total POP exposures from human milk,56 but assessing possible health risks with cumulative exposure to the total POP mixture in human milk is beyond the possibilities in the current study because POPs other than CPs were not included in the present study. In the risk assessment of cumulative exposure to chemicals, substances may be grouped based on, for instance, common mechanisms of action/toxicity pathways or common toxic effects.58 The critical toxic effects of CPs in the risk assessments of EFSA27 and ECCC57 [kidney effects, growth retardation, and immunotoxic response (inflammation)] are shared with several other POPs, such as perfluoroalkyl acids, polychlorinated dibenzo-p-dioxins and furans, and PCBs.59,60 Consequently, studies where the whole range of POPs are measured in human milk samples from different human populations around the world are needed in order to enable risk assessment of cumulative POP exposure among different populations of breast-fed infants. The results of the cumulative POP exposure risk assessment should then be weighed against the well-known beneficial health effects of breastfeeding61 in a full health risk-benefit assessment of breastfeeding.
Parity Difference
Breastfeeding is considered to be an important route of elimination of POPs, and therefore it is expected that the body burden and also the concentration in the milk will decrease with parity.62−64 To examine whether this is the case for CPs, collection of human milk samples of first- and second-time mothers from Shanghai and Jiaxing, China was conducted, which was possible because the government abolished the “birth control policy” and imposed the “two-child policy.” Concentrations of CPs in human milk from Shanghai and Jiaxing between 2015 and 2016 are presented in Table 3. SCCP, MCCP, and LCCP concentrations in human milk from first-time mothers were generally higher than those from second-time mothers, except for LCCPs from Jiaxing, which could be due to their concentrations being close to background levels. Caution should be applied in the interpretation of these results, as each mother group includes only four to eight individual human milk samples, and thus the statistical power is not high enough to detect significant differences. Several other factors such as BMI and weight gain during pregnancy could also influence the results. Finally, firm evidence for lower levels of CPs in human milk after giving birth to a second child can only be obtained with paired samples, that is, the same mothers donate milk as they nurse both their first and second child.
Table 3. Difference of CP Concentrations between First- and Second-Time Mothers in Shanghai and Jiaxing, China (2015–2016).
| Shanghai |
Jiaxing |
||||
|---|---|---|---|---|---|
| category | parity | average ± SD | 1st/2nd | average ± SD | 1st/2nd |
| SCCPs | first-time mother | 238 ± 167 | 5.16 | 352 ± 157 | 5.29a |
| second-time mother | 46.2 ± 14.5 | 66.4 ± 34.6 | |||
| MCCPs | first-time mother | 283 ± 241 | 3.65 | 181 ± 70.3 | 1.48 |
| second-time mother | 77.4 ± 19.1 | 123 ± 88.2 | |||
| LCCPs | first-time mother | 16.3 ± 10.6 | 1.43 | 2.54 ± 1.66 | 0.33 |
| second-time mother | 11.4 ± 3.36 | 7.60 ± 2.84 | |||
| CPs | first-time mother | 537 ± 387 | 3.98 | 536 ± 227 | 2.72 |
| second-time mother | 135 ± 33.9 | 197 ± 115 | |||
p < 0.05. Shanghai: no. of first-time mothers = 6 and no. of second-time mothers = 4; Jiaxing: no. of first-time mothers = 5 and no. of second-time mothers = 8; Unit: average ± SD (ng g–1 fat), first/second (dimensionless).
Carbon and Chlorine Profiles
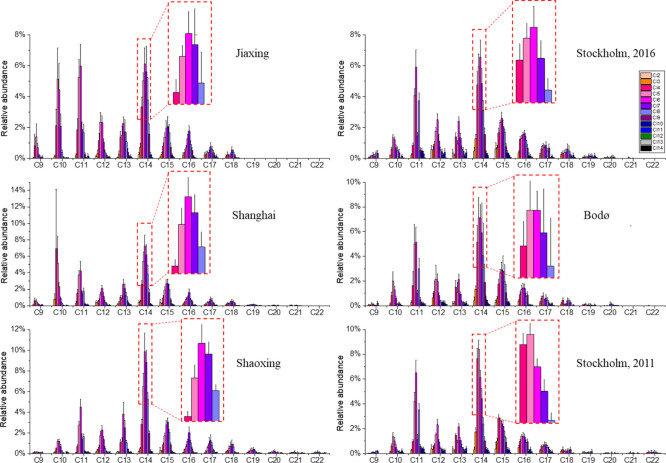
CPs with carbon chain length as long as C27 were observed in the current study, although C>22 CPs were less abundant than C9–22 CPs. Mean relative abundances of C-homologue profiles (C9–27) of human milk from the five cities are shown in Figure S4, and their congener group patterns (C9–22) are shown in Figure 2. In general, C14 was the most abundant C homologue among all CPs detected, followed by C11 and C15, and the most abundant C homologue of LCCPs was C18.
Figure 2.
Average CP congener group patterns, carbon chain length C9–22, and chlorine number Cl2–14 in human milk from Jiaxing (2015–2016), Shanghai (2015–2016), Shaoxing (2010) (China), Stockholm (2011 and 2016) (Sweden), and Bodø (2014) (Norway).
C11 and C14 were the most abundant C-homologues of SCCPs and MCCPs, respectively, in human milk from the two Scandinavian cities in the present work. Similar carbon profiles were also found in milk from the UK.43 The milk samples from Shanghai and Jiaxing showed a relative abundance of C14 comparable to C10 and C11. The CP homologues, C10, C11, and C14, have been shown to be the most abundant C-homologues of SCCPs and MCCPs in other previous studies43,45 on human milk. In a study by Xia et al.,54 for example, SCCPs were dominated by C10 CPs and MCCPs by C14 CPs.
The carbon pattern found in human milk from Shanghai in the current study was slightly different from the carbon pattern found in wildlife (e.g., fish and snake) from the same sampling area, where C14 was dominating among the CP congeners, followed by C13 and C15.18 For comparison, C11 and C13 were the major carbon chains in the urban soil from Shanghai.65
The Cl-homologue series of CPs in human milk are presented in Figure S5. CPs with 2–14 chlorine substituents were present (Cl2–14). Similar mean chlorine contents were observed in the human milk samples analyzed, that is, 53.3–54.1% in the Chinese human milk and 52.1–53.0% in the Scandinavian samples. Cl-homologues with Cl4–7 were the most abundant congeners among the SCCPs, regardless of the sampling site and time. For MCCPs, a slight difference was observed between China and the Scandinavian countries; Cl6–7 was the major congener in the Chinese human milk, whereas Cl5–6 was more abundant in the Scandinavian human milk. The chlorine profile in the Chinese human milk is similar to the chlorine profiles observed in previous studies in China, where Cl6–7 and Cl7–8 showed the highest abundance for SCCPs and MCCPs, respectively.45,54 The pattern also resembles the pattern seen in urban soil from the Shanghai area, predominated by Cl5–7 for SCCPs and Cl7–8 for MCCPs.65 The CP congener group pattern found in Scandinavian milk samples is comparable to the profiles reported in wildlife and sediments from the same area.22,66
Regional CP Contamination Patterns
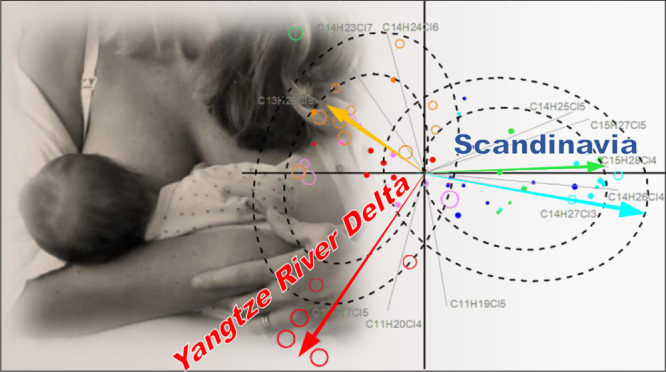
The congener group data presented herein (Figure 2) makes it possible to discuss both regional and some individual human milk contamination patterns. Accordingly, all Chinese mothers had resided in their local area for the most recent 10 years or more. The Swedish mothers had lived in Sweden most of their life as also the mothers from Bodø (Table S2). It is therefore possible to examine the pollution pattern of CPs based on individual results by PCA to examine if there is a difference in the pattern between the regions (Figure 3). Concentrations relative to the total concentration were used to study the differences in the patterns rather than the total concentration. The confidence ellipses were used to test for differences between the Chinese and Scandinavian samples. The first and second principal components contributed to 38 and 33% of total variance, respectively. Based on the PCA plot, the human milk samples can be divided into several groups.
Figure 3.
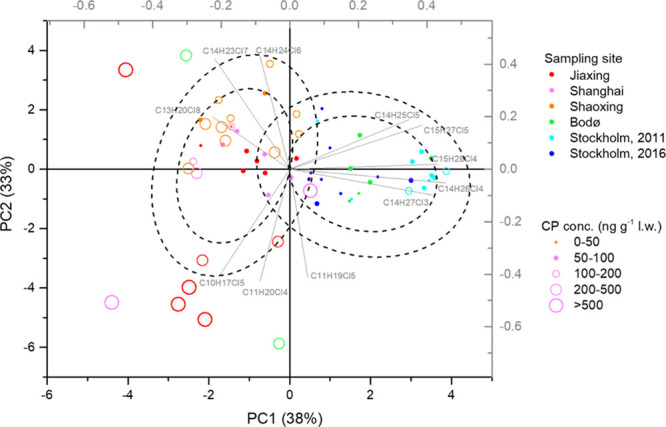
PCA of relative abundances of 11 selected CP congener groups in human milk from Jiaxing (2015–2016), Shanghai (2015–2016), Shaoxing (2010), Stockholm (2011 and 2016), and Bodø (2014). The left dotted circles represent the 75 and 50% confidence intervals of the normal distribution for Scandinavian samples, respectively, as also the right dotted circles for the Chinese samples.
Twenty-five out of 27 samples from the Scandinavian countries were located in an ellipse representing the 50% confidence interval of a bivariate normal distribution of the two first principal components. Total CP concentrations of these 25 samples ranged from <LOD to 120 ng g–1 fat (average 52 ng g–1 fat). The vector loading factors showed that C14–15Cl3–5 contributed the most to the separation seen in the PCA plot. These samples can be considered to represent a general CP exposure level and congener group patterns in the Scandinavian countries.
Samples from Shaoxing and Shanghai and some of the samples from Jiaxing were grouped above the Scandinavian samples in the PCA plot. Thirty out of 36 samples of Chinese origin were located in a circle representing the 50% confidence interval of normal distribution. The vector loading factors showed that the SCCP congener groups (such as C10Cl5, C11Cl4, and C13Cl8) and the MCCP congener groups C14Cl6–7 contribute to the separation seen in the PCA plot. Total CP concentrations of these 30 samples ranged from 22 to 370 ng g–1 fat (mean 142 ng g–1 fat), which on average was approximately 3 times higher than those in the Scandinavian ellipse. These samples were considered as representative for the background CP pollution pattern in the YRD, although a minor difference on loading factors can be observed, in which C14 contributes more to Shaoxing than to Jiaxing or Shanghai samples (cf. Figure S4).
Ellipses of CP patterns in the PCA plot seem to be a mirror of different productions/importations and use of CPs in the YRD and Scandinavian regions. Nowadays, the global production of CPs exceeds 1 000 000 tons/year, and China is the largest CP producer and consumer in the world.2 Many Chinese CP products do not differentiate between SCCPs, MCCPs, or LCCPs and may consist of C10–20 CPs.67 In the European Union (EU), the production and use of SCCPs have been restricted during the last decades,2 leading to smaller manufacturing volumes and import, with SCCPs contributing 1500–2500 tons/year (until 2007)44 and MCCPs and LCCPs contributing 10 000–100 000 tons/year.68 The Norwegian Ministry of the Environment banned the sale and use of SCCPs in 2002.69 The Swedish government has tried to decrease the import and use of CPs since the 1990s. According to a report from the Swedish Chemicals Agency, the use of SCCPs has declined by 95% from 1998 to 2010 in Sweden, and the pattern of CPs shifted to M/LCCPs.70 However, manufactured products from China may contain CPs used for the Chinese market.71 How this is influencing the global trade of CPs is unclear.
The results in the current study show a number of participants with specific CP contamination patterns, which are considered as outliers. Two samples from Norway (sample ID: Bodø-2 and Bodø-6, Table S2) were outside the circle representing the 50% confidence interval of normal distribution for Scandinavian countries. The total concentrations of CPs were 240 and 310 ng g–1 fat for Bodø-2 and Bodø-6, respectively, which were higher than the background concentration in the Scandinavian countries suggested above (mean 52 ng g–1 fat). Similar results were found in six samples from China with high total CP concentrations compared to the background concentration in the Chinese regions (mean 142 ng g–1 fat); Jiaxing-5 (720 ng g–1 fat), Jiaxing-9 (880 ng g–1 fat), Jiaxing-10 (980 ng g–1 fat), Jiaxing-12 (720 ng g–1 fat), Shaoxing-1 (2000 ng g–1 fat), and Shaoxing-6 (870 ng g–1 fat). The loading from the PCA plot in Figure 3 showed that the distribution of these samples is primarily attributed to the congener of C10–11Cl4–6. This indicates that there might be a pollution source for CPs, in particular for SCCPs in Jiaxing, which releases CPs to the environment during production, application, and potential disposal.
The individual CP congener group pattern for Bodø-6 from Norway is presented in Figure S6. For comparison, the congener pattern in one of the commercial products (MP57, Table S3) used in Europe is also presented. A significant correlation (R2 = 0.94, p < 0.01) was observed between MP57 and Bodø-6, strongly suggesting that the exposure of CPs in Bodø-6 is attributed to this particular CP product. This was also the case for Jiaxing-12 and Shaoxing-12 from YRD, China, with a significant correlation with YS CP-52 (a CP commercial product used in YRD, R2 = 0.89, p < 0.01, Figure S7) and MP52 (R2 = 0.91, p < 0.01, Figure S8), respectively. However, information from the questionnaire showed that there is no apparent difference between the mothers regarding diet, occupation, or migration. It is indicated that these samples with extremely high concentrations as well as specific CP congener group patterns, compared to background concentration of both China and Scandinavia, may have specific exposure source(s) of CPs. The results provide a more detailed picture of CP contamination and illustrate the importance and advantages of performing individual analysis on CPs compared to pooled sample analyses.
Regulatory Implications
MCCPs are not yet included as POPs in the Stockholm Convention even though they apparently fulfill the criteria according to the Stockholm Convention. In accordance with what is shown in the present study and elsewhere (cf. above), LCCPs should also be handled as SCCPs and MCCPs and be included as POPs in the Stockholm Convention. The cumulated data on CPs, including LCCPs, show contamination far from production sites (e.g., Sweden and Norway), indicating not only long-range transport but also leakages from materials and goods to indoor and ambient environments. In addition, our data shows human milk concentrations of the LCCPs up to 15 ng g–1 fat (median) in the Chinese samples with individual samples above 100 ng g–1 fat. In the Scandinavian human milk, the concentrations of LCCPs were lower with median values of 2.6–4.3 ng g–1 fat, which are similar to levels reported in human milk for penta-BDEs and octa-BDEs at the time of regulation by the EU in 2003.72 This implies that it is time to act. The Chinese levels of LCCPs were in general higher than the PBDE concentrations at the time when the PBDEs were considered as POPs. For example, the median LCCP level in human milk from Shanghai was 10.5 ng g–1 fat (range: <LOD-56.9 ng g–1 fat, Table 1), whereas the PBDEs’ was 7.8 ng g–1 fat (range: 1.8–26.7 ng g–1 fat, in 2007).73
Finally, the structural resemblance of the CPs, independent of the chain length and chlorination degree, is high enough to handle all CPs as one class of chemicals. Past, more recent, and present data clearly point to prompt action against all CPs by intergovernmental bodies (United Nations Environment Programme through the Stockholm Convention) and EU through REACH. This is also supported by precautionary action according to the EU law.74
Acknowledgments
Yiwei Jiang (YESB, Tongji Jiaxing Research Institution) is acknowledged for helping in the collection of human milk samples from Jiaxing and Shanghai. Henrik Dahlgren (ESB, Swedish Museum of Natural History) is thanked for preparing the Swedish human milk samples. The study was financially supported by the Swedish Research Council (no. 639-2013-6913), the National Natural Science Foundation of China (grant nos. 41401571 and 21876136), and the Fundamental Research Funds for the Central Universities.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.9b06089.
Materials and methods; number of CP positional isomers; sample information and raw data; reference standards; pattern reconstruction results; test statistics of Kruskal–Wallis; EDIs; sampling map; instrumental intensities of CPs in the blank and in the samples; S/M/LCCP profiles, carbon profiles; chlorine profiles; and comparisons of congener group patterns (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- De Boer J.; El-Sayed Ali T.; Fiedler H.; Legler J.; Muir D. C.; Nikiforov V. A.; Tomy G. T.; Tsunemi K.. The Handbook of Environmental Chemistry 10: Chlorinated Paraffins; Springer-Verlag: Berlin, 2010; pp 1–40. [Google Scholar]
- Glüge J.; Wang Z.; Bogdal C.; Scheringer M.; Hungerbühler K. Global production, use, and emission volumes of short-chain chlorinated paraffins–A minimum scenario. Sci. Total Environ. 2016, 573, 1132–1146. 10.1016/j.scitotenv.2016.08.105. [DOI] [PubMed] [Google Scholar]
- van Mourik L. M.; Gaus C.; Leonards P. E. G.; de Boer J. Chlorinated paraffins in the environment: A review on their production, fate, levels and trends between 2010 and 2015. Chemosphere 2016, 155, 415–428. 10.1016/j.chemosphere.2016.04.037. [DOI] [PubMed] [Google Scholar]
- Tomy G. T.; Stern G. A.; Muir D. C. G.; Fisk A. T.; Cymbalisty C. D.; Westmore J. B. Quantifying C-10-C-13 polychloroalkanes in environmental samples by high-resolution gas chromatography electron capture negative ion high resolution mass spectrometry. Anal. Chem. 1997, 69, 2762–2771. 10.1021/ac961244y. [DOI] [Google Scholar]
- Zhang C.; Chang H.; Wang H.; Zhu Y.; Zhao X.; He Y.; Sun F.; Wu F. Spatial and Temporal Distributions of Short-, Medium-, and Long-Chain Chlorinated Paraffins in Sediment Cores from Nine Lakes in China. Environ. Sci. Technol. 2019, 53, 9462–9471. 10.1021/acs.est.8b07296. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Brüchert V.; Sobek A.; de Wit C. A. Temporal Trends of C8–C36 Chlorinated Paraffins in Swedish Coastal Sediment Cores over the Past 80 Years. Environ. Sci. Technol. 2017, 51, 14199–14208. 10.1021/acs.est.7b04523. [DOI] [PubMed] [Google Scholar]
- Bogdal C.; Niggeler N.; Glüge J.; Diefenbacher P. S.; Wächter D.; Hungerbühler K. Temporal trends of chlorinated paraffins and polychlorinated biphenyls in Swiss soils. Environ. Pollut. 2017, 220, 891–899. 10.1016/j.envpol.2016.10.073. [DOI] [PubMed] [Google Scholar]
- Hilger B.; Fromme H.; Völkel W.; Coelhan M. Occurrence of chlorinated paraffins in house dust samples from Bavaria, Germany. Environ. Pollut. 2013, 175, 16–21. 10.1016/j.envpol.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Brits M.; de Boer J.; Rohwer E. R.; De Vos J.; Weiss J. M.; Brandsma S. H. Short-, medium-, and long-chain chlorinated paraffins in South African indoor dust and cat hair. Chemosphere 2020, 238, 124643. 10.1016/j.chemosphere.2019.124643. [DOI] [PubMed] [Google Scholar]
- Wong F.; Suzuki G.; Michinaka C.; Yuan B.; Takigami H.; de Wit C. A. Dioxin-like activities, halogenated flame retardants, organophosphate esters and chlorinated paraffins in dust from Australia, the United Kingdom, Canada, Sweden and China. Chemosphere 2017, 168, 1248–1256. 10.1016/j.chemosphere.2016.10.074. [DOI] [PubMed] [Google Scholar]
- Bergman A.; Hagman A.; Jacobsson S.; Jansson B.; Ahlman M. Thermal degradation of polychlorinated alkanes. Chemosphere 1984, 13, 237–250. 10.1016/0045-6535(84)90130-9. [DOI] [Google Scholar]
- Schinkel L.; Lehner S.; Knobloch M.; Lienemann P.; Bogdal C.; McNeill K.; Heeb N. V. Transformation of chlorinated paraffins to olefins during metal work and thermal exposure – Deconvolution of mass spectra and kinetics. Chemosphere 2018, 194, 803–811. 10.1016/j.chemosphere.2017.11.168. [DOI] [PubMed] [Google Scholar]
- Schinkel L.; Lehner S.; Heeb N. V.; Lienemann P.; McNeill K.; Bogdal C. Deconvolution of Mass Spectral Interferences of Chlorinated Alkanes and Their Thermal Degradation Products: Chlorinated Alkenes. Anal. Chem. 2017, 89, 5923–5931. 10.1021/acs.analchem.7b00331. [DOI] [PubMed] [Google Scholar]
- Xin S.; Gao W.; Wang Y.; Jiang G. Identification of the Released and Transformed Products during the Thermal Decomposition of a Highly Chlorinated Paraffin. Environ. Sci. Technol. 2018, 52, 10153–10162. 10.1021/acs.est.8b01729. [DOI] [PubMed] [Google Scholar]
- Bettina H.; Hermann F.; Wolfgang V.; Mehmet C. Effects of Chain Length, Chlorination Degree, and Structure on the Octanol-Water Partition Coefficients of Polychlorinated n-Alkanes. Environ. Sci. Technol. 2011, 45, 2842–2849. 10.1021/es103098b. [DOI] [PubMed] [Google Scholar]
- Campbell I.; McConnell G. Chlorinated Paraffins and the Environment .1. Environmental Occurrence. Environ. Sci. Technol. 1980, 14, 1209–1214. 10.1021/es60170a001. [DOI] [Google Scholar]
- Qiao L.; Gao L.; Zheng M.; Xia D.; Li J.; Zhang L.; Wu Y.; Wang R.; Cui L.; Xu C. Mass Fractions, Congener Group Patterns, and Placental Transfer of Short-and Medium-Chain Chlorinated Paraffins in Paired Maternal and Cord Serum. Environ. Sci. Technol. 2018, 52, 10097–10103. 10.1021/acs.est.8b02839. [DOI] [PubMed] [Google Scholar]
- Du X.; Yuan B.; Zhou Y.; Benskin J. P.; Qiu Y.; Yin G.; Zhao J. Short-, Medium-, and Long-Chain Chlorinated Paraffins in Wildlife from Paddy Fields in the Yangtze River Delta. Environ. Sci. Technol. 2018, 52, 1072–1080. 10.1021/acs.est.7b05595. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; de Wit C. A.; Yin G.; Du X.; Yuan B. Shorter than short-chain: Very short-chain chlorinated paraffins (vSCCPs) found in wildlife from the Yangtze River Delta. Environ. Int. 2019, 130, 104955. 10.1016/j.envint.2019.104955. [DOI] [PubMed] [Google Scholar]
- Castro M.; Sobek A.; Yuan B.; Breitholtz M. Bioaccumulation Potential of CPs in Aquatic Organisms: Uptake and Depuration in Daphnia magna. Environ. Sci. Technol. 2019, 53, 9533–9541. 10.1021/acs.est.9b01751. [DOI] [PubMed] [Google Scholar]
- Li T.; Wan Y.; Gao S.; Wang B.; Hu J. High-Throughput Determination and Characterization of Short-, Medium-, and Long-Chain Chlorinated Paraffins in Human Blood. Environ. Sci. Technol. 2017, 51, 3346–3354. 10.1021/acs.est.6b05149. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Vorkamp K.; Roos A. M.; Faxneld S.; Sonne C.; Garbus S. E.; Lind Y.; Eulaers I.; Hellström P.; Dietz R.; Persson S.; Bossi R.; de Wit C. A. Accumulation of Short-, Medium-, and Long-Chain Chlorinated Paraffins in Marine and Terrestrial Animals from Scandinavia. Environ. Sci. Technol. 2019, 53, 3526–3537. 10.1021/acs.est.8b06518. [DOI] [PubMed] [Google Scholar]
- Bohlin-Nizzetto P.; Aas W.; Warner N.. Monitoring of Environmental Contaminants in Air and Precipitation: Annual Report 2017; NILU Rapport, 2018.
- Vorkamp K.; Balmer J.; Hung H.; Letcher R. J.; Rigét F. F. A review of chlorinated paraffin contamination in Arctic ecosystems. Emerging Contam. 2019, 5, 219–231. 10.1016/j.emcon.2019.06.001. [DOI] [Google Scholar]
- Gallistl C.; Lok B.; Schlienz A.; Vetter W. Polyhalogenated compounds (chlorinated paraffins, novel and classic flame retardants, POPs) in dishcloths after their regular use in households. Sci. Total Environ. 2017, 595, 303–314. 10.1016/j.scitotenv.2017.03.217. [DOI] [PubMed] [Google Scholar]
- EI-Sayed Ali Y.; Legler J.. Overview of the Mammalian and Environmental Toxicity of Chlorinated Paraffins. The Handbook of Environmental Chemistry 10: Chlorinated Paraffins; Springer-Verlag; Berlin, 2010; pp 135–154. [Google Scholar]
- EFSA . Scientific Opinion on the Risk for Animal and Human Health Related to the Presence of Chlorinated Paraffins in Feed and Food. 2019, https://www.efsa.europa.eu/sites/default/files/consultation/consultation/EFSA_CONTAM_Chlorinated_paraffins.pdf (accessed February 05, 2020).
- IARC . IARC Monographs Volume 48: Chlorinated Paraffins, 1990; Vol. 48, p 55. [PMC free article] [PubMed]
- Myhr B.; McGregor D.; Bowers L.; Riach C.; Brown A. G.; Edwards I.; McBride D.; Martin R.; Caspary W. J. L5178Y mouse lymphoma cell mutation assay results with 41 compounds. Environ. Mol. Mutagen. 1990, 16, 138–167. 10.1002/em.2850160506. [DOI] [PubMed] [Google Scholar]
- UNEP . UNEP/POPS/COP.8/SC-8/11. Listing of Short-Chain Chlorinated Paraffins. 2017, available at http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.8-SC-8-11.English.pdf (accessed February 18, 2019).
- Hallgren S.; Darnerud P. O. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats—testing interactions and mechanisms for thyroid hormone effects. Toxicology 2002, 177, 227–243. 10.1016/s0300-483x(02)00222-6. [DOI] [PubMed] [Google Scholar]
- Yang X.; Zhang B.; Gao Y.; Chen Y.; Yin D.; Xu T. The chlorine contents and chain lengths influence the neurobehavioral effects of commercial chlorinated paraffins on zebrafish larvae. J. Hazard. Mater. 2019, 377, 172–178. 10.1016/j.jhazmat.2019.05.047. [DOI] [PubMed] [Google Scholar]
- Wang X.; Zhu J.; Xue Z.; Jin X.; Jin Y.; Fu Z. The environmental distribution and toxicity of short-chain chlorinated paraffins and underlying mechanisms: Implications for further toxicological investigation. Sci. Total Environ. 2019, 695, 133834. 10.1016/j.scitotenv.2019.133834. [DOI] [PubMed] [Google Scholar]
- Glüge J.; Schinkel L.; Hungerbühler K.; Cariou R.; Bogdal C. Environmental Risks of Medium-Chain Chlorinated Paraffins (MCCPs): A Review. Environ. Sci. Technol. 2018, 52, 6743–6760. 10.1021/acs.est.7b06459. [DOI] [PubMed] [Google Scholar]
- Jansson B.; Andersson R.; Asplund L.; Litzen K.; Nylund K.; Sellströom U.; Uvemo U.-B.; Wahlberg C.; Wideqvist U.; Odsjö T.; Olsson M. Chlorinated and brominated persistent organic compounds in biological samples from the environment. Environ. Toxicol. Chem. 1993, 12, 1163–1174. 10.1002/etc.5620120704. [DOI] [Google Scholar]
- Tomy G. T.Analysis of Chlorinated Paraffins in Environmental Matrices: The Ultimate Challenge for the Analytical Chemist. The Handbook of Environmental Chemistry 10: Chlorinated Paraffins; Springer, 2010; pp 83–106. [Google Scholar]
- van Mourik L. M.; Leonards P. E. G.; Gaus C.; de Boer J. Recent developments in capabilities for analysing chlorinated paraffins in environmental matrices: A review. Chemosphere 2015, 136, 259–272. 10.1016/j.chemosphere.2015.05.045. [DOI] [PubMed] [Google Scholar]
- Bogdal C.; Alsberg T.; Diefenbacher P. S.; MacLeod M.; Berger U. Fast quantification of chlorinated paraffins in environmental samples by direct injection high-resolution mass spectrometry with pattern deconvolution. Anal. Chem. 2015, 87, 2852–2860. 10.1021/ac504444d. [DOI] [PubMed] [Google Scholar]
- Zeng L.; Lam J. C. W.; Wang Y.; Jiang G.; Lam P. K. S. Temporal Trends and Pattern Changes of Short- and Medium-Chain Chlorinated Paraffins in Marine Mammals from the South China Sea over the Past Decade. Environ. Sci. Technol. 2015, 49, 11348–11355. 10.1021/acs.est.5b02473. [DOI] [PubMed] [Google Scholar]
- Sprengel J.; Wieselmann S.; Kröpfl A.; Vetter W. High amounts of chlorinated paraffins in oil-based vitamin E dietary supplements on the German market. Environ. Int. 2019, 128, 438–445. 10.1016/j.envint.2019.04.065. [DOI] [PubMed] [Google Scholar]
- Krätschmer K.; Schächtele A.; Malisch R.; Vetter W. Chlorinated paraffins (CPs) in salmon sold in southern Germany: Concentrations, homologue patterns and relation to other persistent organic pollutants. Chemosphere 2019, 227, 630–637. 10.1016/j.chemosphere.2019.04.016. [DOI] [PubMed] [Google Scholar]
- Li T.; Gao S.; Ben Y.; Zhang H.; Kang Q.; Wan Y. Screening of Chlorinated Paraffins and Unsaturated Analogues in Commercial Mixtures: Confirmation of Their Occurrences in the Atmosphere. Environ. Sci. Technol. 2018, 52, 1862–1870. 10.1021/acs.est.7b04761. [DOI] [PubMed] [Google Scholar]
- Thomas G. O.; Farrar D.; Braekevelt E.; Stern G.; Kalantzi O. I.; Martin F. L.; Jones K. C. Short and medium chain length chlorinated paraffins in UK human milk fat. Environ. Int. 2006, 32, 34–40. 10.1016/j.envint.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Darnerud P. O.; Aune M.; Glynn A.; Borgen A.. Chlorinated Paraffins in Swedish Breast Milk. PM 18/12 (22 pp). 2012, https://www.kemi.se/global/pm/2012/pm-18-12.pdf (accessed September 19, 2019).
- Xia D.; Gao L.; Zheng M.; Li J.; Zhang L.; Wu Y.; Tian Q.; Huang H.; Qiao L. Human Exposure to Short- and Medium-Chain Chlorinated Paraffins via Mothers’ Milk in Chinese Urban Population. Environ. Sci. Technol. 2017, 51, 608–615. 10.1021/acs.est.6b04246. [DOI] [PubMed] [Google Scholar]
- Cao Y.; Harada K. H.; Hitomi T.; Niisoe T.; Wang P.; Shi Y.; Yang H.-R.; Takasuga T.; Koizumi A. Lactational exposure to short-chain chlorinated paraffins in China, Korea, and Japan. Chemosphere 2017, 173, 43–48. 10.1016/j.chemosphere.2016.12.078. [DOI] [PubMed] [Google Scholar]
- Bayen S.; Obbard J. P.; Thomas G. O. Chlorinated paraffins: a review of analysis and environmental occurrence. Environ. Int. 2006, 32, 915–929. 10.1016/j.envint.2006.05.009. [DOI] [PubMed] [Google Scholar]
- van Mourik L. M.; van der Veen I.; Crum S.; de Boer J. Developments and interlaboratory study of the analysis of short-chain chlorinated paraffins. TrAC, Trends Anal. Chem. 2018, 102, 32–40. 10.1016/j.trac.2018.01.004. [DOI] [Google Scholar]
- Krätschmer K.; Schächtele A. Interlaboratory studies on chlorinated paraffins: Evaluation of different methods for food matrices. Chemosphere 2019, 234, 252–259. 10.1016/j.chemosphere.2019.06.022. [DOI] [PubMed] [Google Scholar]
- WHO . Fourth WHO-Coordinated Survey of Human Milk for Persistent Organic Pollutants in Cooperation with UNEP. 2007, available at https://www.who.int/foodsafety/chem/POPprotocol.pdf (accessed September 19, 2019).
- Fängström B.; Strid A.; Grandjean P.; Weihe P.; Bergman Å. A retrospective study of PBDEs and PCBs in human milk from the Faroe Islands. Environ. Health: Global Access Sci. Source 2005, 4, 12. 10.1186/1476-069x-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Asplund L.; Yin G.; Athanassiadis I.; Wideqvist U.; Bignert A.; Qiu Y.; Zhu Z.; Zhao J.; Bergman Å. Extensive organohalogen contamination in wildlife from a site in the Yangtze River Delta. Sci. Total Environ. 2016, 554-555, 320–328. 10.1016/j.scitotenv.2016.02.176. [DOI] [PubMed] [Google Scholar]
- Brandsma S. H.; van Mourik L.; O’Brien J. W.; Eaglesham G.; Leonards P. E. G.; de Boer J.; Gallen C.; Mueller J.; Gaus C.; Bogdal C. Medium-Chain Chlorinated Paraffins (CPs) Dominate in Australian Sewage Sludge. Environ. Sci. Technol. 2017, 51, 3364–3372. 10.1021/acs.est.6b05318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia D.; Gao L.-R.; Zheng M.-H.; Li J.-G.; Zhang L.; Wu Y.-N.; Qiao L.; Tian Q.-C.; Huang H.-T.; Liu W.-B.; Su G.-J.; Liu G.-R. Health risks posed to infants in rural China by exposure to short- and medium-chain chlorinated paraffins in breast milk. Environ. Int. 2017, 103, 1–7. 10.1016/j.envint.2017.03.013. [DOI] [PubMed] [Google Scholar]
- van Mourik L. M.; Toms L.-M. L.; He C.; Banks A.; Hobson P.; Leonards P. E. G.; de Boer J.; Mueller J. F. Evaluating age and temporal trends of chlorinated paraffins in pooled serum collected from males in Australia between 2004 and 2015. Chemosphere 2020, 244, 125574. 10.1016/j.chemosphere.2019.125574. [DOI] [PubMed] [Google Scholar]
- Fång J.; Nyberg E.; Winnberg U.; Bignert A.; Bergman Å. Spatial and temporal trends of the Stockholm Convention POPs in mothers’ milk — a global review. Environ. Sci. Pollut. Res. 2015, 22, 8989–9041. 10.1007/s11356-015-4080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environment Canada . Canadian Environmental Protection Act: Priority Substances List Assessment Report Chlorinated Paraffins. 1993, http://www.hc-sc.gc.ca/ewh-semt/alt_formats/hecs-sesc/pdf/pubs/contaminants/psl1-lsp1/paraffins-paraffines/paraffins-paraffines-eng.pdf (accessed February 27, 2019).
- Rotter S.; Beronius A.; Boobis A. R.; Hanberg A.; van Klaveren J.; Luijten M.; Machera K.; Nikolopoulou D.; van der Voet H.; Zilliacus J.; Solecki R. Overview on legislation and scientific approaches for risk assessment of combined exposure to multiple chemicals: the potential EuroMix contribution. Crit. Rev. Toxicol. 2018, 48, 796–814. 10.1080/10408444.2018.1541964. [DOI] [PubMed] [Google Scholar]
- Knutsen H. K.; Alexander J.; Barregård L.; Bignami M.; Brüschweiler B.; Ceccatelli S.; Cottrill B.; Dinovi M.; Edler L.; Grasl-Kraupp B.; Hogstrand C.; Hoogenboom L.; Nebbia C. S.; Oswald I. P.; Petersen A.; Rose M.; Roudot A.-C.; Vleminckx C.; Vollmer G.; Wallace H.; Bodin L.; Cravedi J.-P.; Halldorsson T. I.; Haug L. S.; Johansson N.; van Loveren H.; Gergelova P.; Mackay K.; Levorato S.; van Manen M.; Schwerdtle T. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J. 2018, 16, e05194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen H. K.; Alexander J.; Barregård L.; Bignami M.; Brüschweiler B.; Ceccatelli S.; Cottrill B.; Dinovi M.; Edler L.; Grasl-Kraupp B.; Hogstrand C.; Nebbia C. S.; Oswald I. P.; Petersen A.; Rose M.; Roudot A.-C.; Schwerdtle T.; Vleminckx C.; Vollmer G.; Wallace H.; Fürst P.; Håkansson H.; Halldorsson T.; Lundebye A.-K.; Pohjanvirta R.; Rylander L.; Smith A.; van Loveren H.; Waalkens-Berendsen I.; Zeilmaker M.; Binaglia M.; Gómez Ruiz J. Á.; Horváth Z.; Christoph E.; Ciccolallo L.; Ramos Bordajandi L.; Steinkellner H.; Hoogenboom L. Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA J. 2018, 16, e05333 10.2903/j.efsa.2018.5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M. S.; Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst. Rev. 2012, 15, CD003517. 10.1002/14651858.CD003517.pub2. [DOI] [PubMed] [Google Scholar]
- Albers J. M. C.; Kreis I. A.; Liem A. K. D.; van Zoonen P. Factors that influence the level of contamination of human milk with poly-chlorinated organic compounds. Arch. Environ. Contam. Toxicol. 1996, 30, 285–291. 10.1007/bf00215810. [DOI] [PubMed] [Google Scholar]
- Dillon J.-C.; Martin G. B.; O’Brien H. T. Pesticide residues in human milk. Food Chem. Toxicol. 1981, 19, 437–442. 10.1016/0015-6264(81)90447-8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald E. F.; Hwang S.-A.; Deres D. A.; Bush B.; Cook K.; Worswick P. The association between local fish consumption and DDE, mirex, and HCB concentrations in the breast milk of Mohawk women at Akwesasne. J. Exposure Sci. Environ. Epidemiol. 2001, 11, 381–388. 10.1038/sj.jea.7500180. [DOI] [PubMed] [Google Scholar]
- Wang X.-T.; Wang X.-K.; Zhang Y.; Chen L.; Sun Y.-F.; Li M.; Wu M.-H. Short- and medium-chain chlorinated paraffins in urban soils of Shanghai: Spatial distribution, homologue group patterns and ecological risk assessment. Sci. Total Environ. 2014, 490, 144–152. 10.1016/j.scitotenv.2014.04.121. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Benskin J. P.; Chen C.-E. L.; Bergman Å. Determination of Chlorinated Paraffins by Bromide-Anion Attachment Atmospheric-Pressure Chemical Ionization Mass Spectrometry. Environ. Sci. Technol. Lett. 2018, 5, 348–353. 10.1021/acs.estlett.8b00216. [DOI] [Google Scholar]
- van Mourik L. M.; Lava R.; O’Brien J.; Leonards P. E. G.; de Boer J.; Ricci M. The underlying challenges that arise when analysing short-chain chlorinated paraffins in environmental matrices. J. Chromatogr. A 2019, 1610, 460550. 10.1016/j.chroma.2019.460550. [DOI] [PubMed] [Google Scholar]
- Persson J.; Hagberg J.; Wang T.. A Survey of Organic Flame Retardants and Plasticizers in Building Materials on the Swedish Market and Their Occurrence in Indoor Environments; Örebro, 2018; Vol. 67. [Google Scholar]
- UNEP . Draft Technical Guidelines on the Environmentally Sound Management of Wastes Consisting of, Containing or Contaminated with Short-Chain Chlorinated Paraffins. 2018, available at http://www.basel.int/Portals/4/download.aspx?d=UNEP-CHW-SUBM-POPs-TGs-Comment-SCCP-Canada-201801.English.docx (accessed September 19, 2019).
- KEMI . 2016, available at: https://www.kemi.se/en/statistics/statistics-in-brief/substances-and-substance-groups/chlorinated-paraffins (accessed February 15, 2019).
- Yuan B.; Strid A.; Darnerud P. O.; de Wit C. A.; Nyström J.; Bergman Å. Chlorinated paraffins leaking from hand blenders can lead to significant human exposures. Environ. Int. 2017, 109, 73–80. 10.1016/j.envint.2017.09.014. [DOI] [PubMed] [Google Scholar]
- EUR-Lex . Directive 2002/95/EC of the European Parliament and of the Council of 27 January 2003 on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment. 2003, https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:037:0019:0023:en:PDF (ccessed December 20, 2019).
- Ma S.; Yu Z.; Zhang X.; Ren G.; Peng P. A.; Sheng G.; Fu J. Levels and congener profiles of polybrominated diphenyl ethers (PBDEs) in breast milk from Shanghai: Implication for exposure route of higher brominated BDEs. Environ. Int. 2012, 42, 72–77. 10.1016/j.envint.2011.04.006. [DOI] [PubMed] [Google Scholar]
- EU . Summary of Communication (COM(2000) 1final) on the Precautionary Principle. 2000, https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=LEGISSUM:l32042&from=EN (accessed February 5, 2020).
- Yang L.; Wang Z.; Li J.; Ma Y.; Kong L.; Yang H.; Wang L.; Liu Y.; Lu Y.; Zhang J. Evaluation of short chain chlorinated paraffins in human milk and their intake by infants in Hebei Province, China. Food Addit. Contam., Part A 2018, 35, 2011–2021. 10.1080/19440049.2018.1492155. [DOI] [PubMed] [Google Scholar]
- Tomy G. T.The Mass Spectrometric Characterization of Polychlorinated n-Alkanes and the Methodology for Their Analysis in the Environment. Electronic Dissertation, University of Manitoba, 1997. [Google Scholar]
- Reth M.; Kypke K.; Schächtele J.; Oehme M. Chlorinated paraffins in human milk from Germany analyzed by HRGC-EI-MS/MS. Organohalogen Compd. 2005, 67, 3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.