Abstract
Through diverse mechanisms, obesity contributes to worsened cardiometabolic health and increases rates of cardiovascular events. Effective treatment of obesity is necessary to reduce the associated burdens of diabetes, cardiovascular disease, and death. Despite increasing cardiovascular outcome data on obesity interventions, only a small fraction of the population with obesity are optimally treated. This is a primary impetus for this article in which we describe the typical weight loss, as well as the associated impact on both traditional and novel cardiovascular disease risk factors, provided by the four primary modalities for obtaining weight loss in obesity – dietary modification, increasing physical activity, pharmacotherapy, and surgery. We also attempt to highlight instances where changes in metabolic risk are relatively specific to particular interventions and appear at least somewhat independent of weight loss. Finally, we suggest important areas for further research to reduce and prevent adverse cardiovascular consequences due to obesity.
Keywords: obesity, risk factors, lifestyle, treatment, weight loss medication, bariatric surgery
Weight loss has been studied in millions of people with results consistently suggesting that the severity of all common obesity-related metabolic comorbidities can be lessened to some extent with different interventions. However, evidence of these changes translating to improved cardiovascular outcomes has been elusive. Prominently, the Look AHEAD trial, which compared intensive lifestyle intervention to diabetes support and education in overweight and obese patients, failed to reduce cardiovascular events over nearly 10 years follow-up. Recently, however, the hard cardiovascular benefits of substantial weight loss have come to bear with several groups demonstrating improved outcomes following bariatric surgery,1–4 although these data are observational and not from randomized controlled trials. Further, GLP-1 agonist medications – agents that are now approved for treatment of overweight and obesity absent diabetes – have repeatedly improved cardiovascular outcomes in diabetic populations.5
These emerging data are reflected in treatment guidelines produced by the American Heart Association, American College of Cardiology, The Obesity Society and the Endocrine Society which recommend augmenting a foundation of lifestyle intervention and individualized support with pharmacotherapy or bariatric surgery focused on goals of ≥10% body weight loss.6,7 Even with ideal support and improvements in lifestyle, achieving and maintaining substantial weight loss, such as ≥10% of starting weight, is uncommon, and most individuals find it difficult. Yet there is reason to think that many individuals with obesity could benefit from weight loss in this range. For instance, while the overall Look AHEAD trial was negative, post-hoc analysis demonstrated that subjects who lost ≥10% of their pre-study body weight had a >20% reduction in risk for cardiovascular events.8 Additionally, based on strong-to-intermediate evidence, American Association of Clinical Endocrinology guidelines encourage ≥10% body weight loss for the specific prevention of diabetes in obesity.9 Despite guideline recommendations and growing numbers and varieties of approaches to treating obesity and mitigating its associated risk (Figure 1), the epidemic continues largely unabated with only approximately 1% of eligible patients undergoing bariatric surgery or filling an antiobesity medication prescription annually.10,11 These are motivating factors behind this comprehensive review.
Figure 1. Typical changes in body weight and traditional cardiovascular disease risk factors via weight loss in obesity.
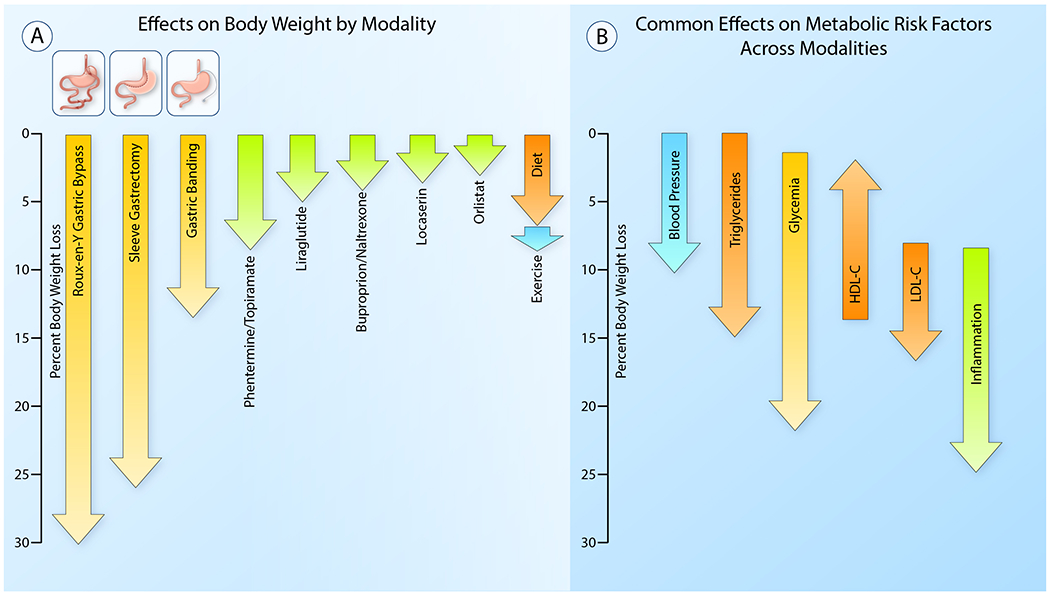
(A) This diagram represents average observed weight loss in clinical studies with lifestyle interventions (dietary modification and increased physical activity), pharmacotherapy and metabolic surgery.
(B) This figure demonstrates the range of weight loss over which changes in traditional cardiovascular risk factors typically occur. Improvements in blood pressure, triglycerides and glycemia become noticeable with modest weight loss. Reductions in LDL-cholesterol and inflammatory markers require more substantial weight loss. Reductions in triglycerides, glycemia and inflammation particularly, continue to improve as weight loss increases and have the potential to be significantly impacted with substantial weight loss, whereas LDL-C and HDL-C generally improve only modestly. (Illustration Credit: Ben Smith)
In this article, we discuss the effects of the four primary modalities for obtaining weight loss in obesity (dietary modification, increasing physical activity, pharmacotherapy, and surgery). We focus first on the typical amount of weight loss achieved via each intervention and then on the effects on both traditional and novel cardiovascular disease risk factors. We have attempted to highlight changes in metabolic risk that appear to be modality-independent, relating primarily to weight loss itself, and changes that appear to be modality-dependent and relatively independent of weight change.
Energy Restriction and Dietary Modification
Effects on metabolic risk via energy restriction
In obesity, many markers of cardiovascular risk tend to improve in response to dietary weight reduction from energy restriction, some earlier or more impressively than others. Studies to date frequently have involved restricting fat, in addition to total, calories, and many interventions have also often included exercise.6
Systolic and diastolic blood pressure (SBP/DBP) tend to show the most sensitive response to weight changes. For example, an observational study showed a 0.43mm Hg rise or fall in 24-hour SBP associated with every 1% change in body weight in overweight individuals instructed to follow a “weight-reducing diet.”12 Meta-analyses have supported similar relationships in various contexts,13,14 with one finding a 0.36mmHg/kg association between SBP and weight.14 Flow-mediated dilation (FMD) also improves with diet-induced weight loss, although more (~10 kg) is required for a notable effect.15
Dysglycemia has tended to improve fairly early in the course of lifestyle-based weight loss in large landmark studies including the Diabetes Prevention Program and Look AHEAD,16,17 with further benefit seen with additional weight loss. Even when HbA1c, a relatively crude measure of dysglycemia, was examined in a small study of individuals with obesity and prediabetes, a dose-response was detected between lifestyle-based weight loss and HbA1c decrease.18 There is evidence from a recent, elegant study using radiolabeled tracers in obese humans that glycemic improvement can be attributed to improved insulin sensitivity at multiple target organs as well as improved beta cell function.19 The results of this study suggest that liver and adipose insulin sensitivity show maximal improvement at 5% weight loss, while muscle insulin sensitivity continues to improve with additional weight loss. A composite measure of beta cell function also continued to improve as weight loss increased, up to the maximum of 16% evaluated in this study.
Favorable changes in lipid profile result from weight loss by lifestyle modification in individuals with BMI >25kg/m2. These alterations suggest partial resolution of pathology within the “adiposopathic dyslipidemia” framework, which posits that insufficient lipoprotein lipase (LPL) activity and adipogenesis at peripheral subcutaneous adipose tissue during positive energy balance results in increased visceral adiposity and increased free fatty acid (FFA) delivery to non-adipose tissues, notably the liver. This results in increased production of VLDL and a trend toward smaller and denser LDL (which holds greater atherogenic potential) and HDL (which is more rapidly cleared, resulting in decreased total HDL). Excess FFA delivery to the liver further contributes to hepatosteatosis, insulin insensitivity, and additional downstream pathophysiologic changes.20
Based on a comprehensive evidence review, the most recent American consensus guidelines on obesity management conclude that substantial triglyceride reduction (≥15mg/dL) is seen with relatively modest weight loss (~3 kg), while larger amounts of weight loss (5-8 kg) are needed to see consistent improvements in LDL-C (~5 mg/dL) and HDL-C (~2-3 mg/dL).21 The only major meta-analysis to specifically focus on changes in lipoproteins after dietary weight loss was published in 1992.22 While including only small studies and <2000 subjects total, this analysis estimated that each kg of weight loss is associated with: LDL-C, −0.8mg/dL; HDL-C,−0.3mg/dL during active weight loss, but +0.3mg/dL once weight stabilizes; triglycerides, −1.3 mg/dL.22 These changes appear driven by increased apoB100 clearance without an increase in production, in addition to increased rates of VLDL to LDL conversion.23 Further, while apoA1 production decreases, it is offset by decreased clearance, at least early on.
As reviewed elsewhere in this Compendium, obesity is associated with multiple inflammatory and immune changes in adipose tissue, which appear to contribute to cardiometabolic risk. These changes, and their reversibility by weight loss, appear to depend on individual context, particularly baseline inflammatory status. In one study of low-fat diet-based weight loss, clinically well individuals with severe obesity and moderately elevated levels of C-reactive protein (CRP) (median ~3.65mg/L) required loss of 16% of starting weight to significantly reduce CRP.19 Contrastingly, in another study, when individuals with psoriatic arthritis and BMI >25 lost weight mostly via low-calorie/fat/sugar high-fiber diet, decrease in CRP was detected when only 5-10% of starting weight had been lost, which also correlated with an improved clinical response to TNF-α blockade.24
In mice, adipose tissue macrophage content has shown an intriguing temporal response to caloric restriction, increasing at first but later decreasing to below the pre-weight-loss levels, in synchrony with markers of adipose tissue lipolysis.25 This has also been reported in humans, specifically in the subcutaneous adipose tissue of a small group of women with obesity who undertook very-low-calorie diets to achieve >10% weight loss.26 Their adipose tissue macrophage markers peaked at the end of the initial 4-week weight loss period, but fell below baseline levels following a period of stability at the reduced weight. Studies in mice and humans find that insulin insensitivity improves in association with reduced inflammatory cell content of adipose with weight loss.27 However, whether reduced adipose inflammation contributes to improved insulin sensitivity or the inverse, is unclear.28 Progression of adipose tissue pathology to fibrosis has been associated with hepatic fibrosis and diabetes, but also to poor weight loss response.29
Effects on metabolic risk from altered dietary composition
Patient-specific factors (genetics, gut microbiota, activity/exercise patterns, comorbidities, etc.), probably interact in important ways with dietary content in affecting cardiometabolic risk, so one single dietary pattern recommendation is unlikely to be optimal for all individuals. Nonetheless, dietary patterns can mediate cardiometabolic risk beyond their caloric content. The clearest examples perhaps are the low sodium and Dietary Approaches to Stop Hypertension (DASH) dietary patterns, which lowered SBP and DBP in a clinical trial while participants maintained stable weights.30 More complex effects of dietary patterns are plausible – for instance, degree of adherence to a “Mediterranean” pattern as assessed by determination of nutrient indices from diet diaries,31 or by a scoring system,32 has been associated with lower levels of multiple markers of inflammation and with improved endothelial function, even after adjusting for body weight or body mass index and other likely confounders – but these effects require further delineation and corroboration.
At the level of defined macronutrients as dietary components, there is a reasonable consensus that saturated fatty acids confer cardiometabolic risk out of proportion to their caloric content,33,34 and that lower-carbohydrate weight-reducing diets tend to reduce triglycerides, increase HDL-C, and transiently increase LDL-C, compared to higher-carbohydrate ones.20
Sugar-sweetened beverages, and perhaps “free” sugars generally, appear to confer cardiometabolic risk beyond their caloric content, perhaps in part because of relatively high fructose content.33 One small human study compared isocaloric high glucose and fructose consumption finding that while glucose increased post-prandial glucose and insulin, fructose led to greater visceral adipose gain in the context of greater de novo lipogenesis, decreased fat oxidation, and increased triglycerides, apoB, small dense LDL, and oxidized LDL.35,36 A plausible basis for these differences is that hepatic uptake of glucose, but not fructose, is regulated by hepatic energy status. Moreover, glucose and fructose consumed together may synergize, raising LDL-C and apoB further than either alone.37 As studies consistently associate consumption of whole fruits, which are high in fructose, with improved cardiovascular outcomes and all-cause mortality even when controlled for BMI and other confounders, monosaccharide content alone is not the only factor mediating the metabolic impact of the food.
Connections between dietary composition and the innate immune system have recently been reported. In one recent study, non-obese mice were fed a high-sucrose/saturated fat diet for four weeks, but were returned to a control diet before weight gain was noted. Multiple inflammatory markers increased on the experimental diet and reverted on return to control diet, but enhanced toll-like receptor responses also developed and did not revert, indicative of a phenotype of epigenetically trained innate immunity phenotype in monocytes, expected to persist for months.38
A more dramatic change in macronutrient balance, currently the subject of substantial scientific and popular interest, involves very-low carbohydrate consumption, sometimes designed to promote ketosis. Such diets could plausibly have cardiometabolic effects separate from those of the associated weight change, particularly if prolonged ketosis is achieved, but data is lacking in humans in the presence of an appropriate control diet. A National Lipid Association scientific statement recently comprehensively reviewed effects of very low carbohydrate diets on energy metabolism, weight loss, cardiometabolic risk, as well as safety concerns, concluding that “there is a physiological basis for potential metabolic benefits of carbohydrate-restriction compared with dietary strategies with a higher carbohydrate content in some individuals” and that multiple risk markers improve in the short term on these diets, but that “by approximately 2 years, there are no differences for most cardiometabolic risk markers.”39
Regulating the timing of caloric intake has also been of recent interest, generally with the hypothesis that frequency and duration of exposure to fasting can be clinically significant. These approaches can involve avoiding, or markedly limiting, energy intake over times ranging from about half a day to one or more days.40 At long enough fasting durations intermittent fasting probably has some physiologic overlap with ketogenic diets. Multiple cardiovascular benefits have been reported, including on BP, lipid profile, glycemic response, and inflammation, as has been reviewed,40 but whether these differ from the effects seen with equivalent energy restriction from a non-restricted feeding pattern, is unclear. One notable study of a small group of men with BMI >25 succeeded in maintaining approximately stable weights between a 5-week period of early time-restricted feeding and a 5-week period of control feeding schedule.41 Insulin levels and insulin responsiveness improved, with large reductions in SBP and DBP, but fasting triglycerides worsened (+57±13 mg/dL) and no change was noted in inflammatory markers.
Looking forward, personalizing dietary recommendations based on objective as well as subjective data seems likely to become increasingly possible and powerful. For instance, there has been some evidence that baseline glycemic status helps predict successful weight loss with low-carbohydrate/high-fat diets.42 A wide variety of mechanisms may link the microbiome, particularly gut microbiota, to cardiometabolic risk, through effects on energy balance as well as through weight-independent effects. Moreover, the composition of the microbiome responds, sometimes rapidly, to changes in diet.43 Manipulation of the microbiome, or compensating for metabolite deficiencies related to it, through dietary optimization, probiotic/prebiotic supplementation, or perhaps other means, could potentially have a role in preventing or mitigating obesity or its cardiometabolic risks. Some specific considerations with respect to changes in energy intake have been reviewed.43
Exercise
Exercise represents volitional energy expenditure, and as discussed below, improvements in cardiometabolic risk markers appear similar when equivalent weight loss is achieved by increased sustained aerobic activity or dietary modification. Exercise is a valuable adjunct to weight loss (and especially weight maintenance44,45) efforts and also offers myriad cardiovascular benefits independent of weight loss as will be discussed below.
Effects on body weight
The American College of Sports Medicine (ACSM) has suggested that 225-420 minutes of aerobic training per week is needed for 5-7.5 kg weight loss45 – modest weight loss for many individuals with obesity – whereas just over half of all Americans achieve even 150 minutes per week of moderate-intensity physical activity.46 Thus, the American Heart Association statement that “physical activity is not an effective approach for achieving initial weight loss”47 is understandable. An analysis by Shaw and colleagues suggested that combining exercise with diet led to an additional 1.1kg body weight loss vs diet alone, with high-intensity exercise associated with 1.5kg greater weight loss than lower-intensity exercise.48 Further, incorporation of exercise into a weight loss plan promotes beneficial changes in body composition – preservation of lean body mass and greater fat loss.49–52
Effects on metabolic risk in obesity
Several large studies and a meta-analysis suggest that weight loss, whether due to diet or exercise, has comparably beneficial effects on apoB concentration,52,53 triglycerides,53 HbA1c,54 and BP.54 Although greater weight loss offers greater benefit in most situations, there is evidence that cardiometabolic risk in obesity can improve with modest, or even no weight loss, in conjunction with exercise.45,55,56 Data suggest that while increasing BMI is associated with worse outcomes, excess risk is attenuated in obesity individuals who are non-sedentary or cardiovascularly fit.57 In part, this is probably due to improved vascular health resulting from exercise. For example, BP was one of the first measures noted to improve with exercise independent of weight loss.58 Moreover, experiments in obese rodents demonstrate improved endothelium-dependent and -independent dilatation in response to aerobic training,59 as well as increased endothelial prostacyclin production. With respect to exercise-induced improvements in endothelial function, certainly reductions in circulating glucose, cholesterol, and triglycerides contribute, but FMD has been shown to improve in obese humans following either aerobic or resistance training in the absence of change in body weight, lipids, or glucose.60,61 These vasodilatory effects are understood to be largely mediated by shear stress-induced increases in nitric oxide synthase expression occurring with exercise (Figure 2).62 This is mediated through KLF2 signaling in endothelial cells, which also affects adhesion molecule expression, improving endothelial function in subjects with obesity, diabetes and the metabolic syndrome.63,64
Figure 2. Weight loss-independent effects of exercise on cardiovascular disease risk in obesity.
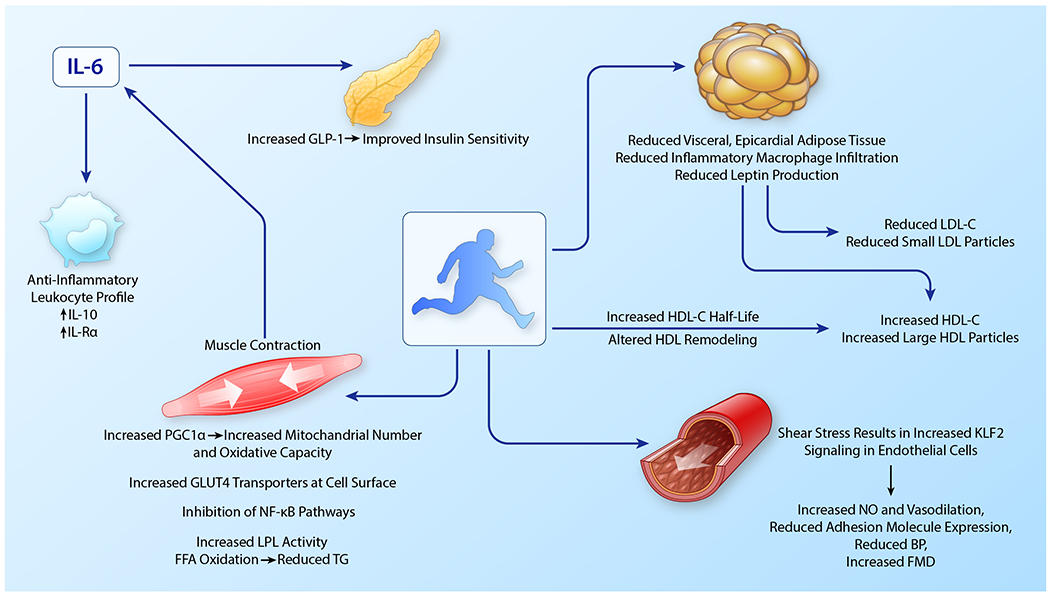
Exercise improves multiple aspects of metabolic risk in obesity. Many of these happen at the level of the skeletal muscle and are mediated with skeletal muscle contraction which increases GLUT4 transporters at the cell surface, PGC1α expression, LPL activity, and induces IL-6 release. IL-6 increases circulating GLP-1 and has anti-inflammatory effects. Increased shear stress in the vasculature with exercise improves endothelial function and reduces blood pressure. Exercise is associated with reductions in visceral and epicardial adipose and adipose inflammation independent of weight loss. Loss of adipose tissue and reduced adipose inflammation contributes to a less atherogenic lipid profile characterized by increased HDL-C and larger HDL particles and reduced LDL-C and small LDL particles. Exercise-mediated changes in the activity of enzymes necessarily for HDL metabolism also play roles in increasing HDL size and HDL-C half-life in the circulation. (Illustration credit: Ben Smith)
Aerobic exercise training may modestly reduce LDL-C in obesity.65 In the STRRIDE study, Kraus and colleagues reported reduced LDL-C with training, but further observed that aerobic exercise specifically reduced the concentrations of small LDL particles in those performing large amounts of high-intensity exercise.66 The subjects in the high amount and intensity group exhibited a degree of reduction in visceral adipose tissue not seen in the other groups67 – in line with resolution of adiposopathic dyslipidemia discussed above. Although limited, some data suggest that resistance training may produce greater reductions in LDL-C than aerobic training.34
A single bout of exercise may be adequate to observe reduced post-prandial triglyceridemia. This is seen after endurance exercise,68 but high intensity interval training (HIIT), an exercise protocol consisting of several repeated episodes of brief, maximal sprint-type exercises followed by a slightly longer recovery periods,69 seems particularly effective at acutely improving postprandial lipemia via increased LPL activity and reduced VLDL synthesis.70 Chronic exercise-induced reductions in triglycerides probably result from improved insulin sensitivity,65, 66 increased LPL activity, and increases in muscle mitochondrial number and activity that result in greater FFA oxidation.71 Increased skeletal muscle mitochrondria and fat oxidation capacity do not occur with weight loss by other means72,73 - results of aerobic exercise’s activity in stimulation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) activity.74 Exercise-mediated increases in skeletal muscle PGC1α also improve glucose and lipid homeostasis,75,76 may act to reduce inflammation, and induce skeletal muscle angiogenesis (Figure 2).77,78
Aerobic exercise may increase HDL-C, and specifically large HDL particles,66 in a dose-dependent manner.79 However, how duration, frequency, or intensity relate to these changes remains unclear.71,80,81 Moreover, this response to exercise exhibits particularly large inter-individual variation,79 is impaired in obesity,80,82 and is even more uncertain in the setting of active weight loss.22,53 Additional data suggest exercise may improve HDL function. Similar to observations regarding HDL-C, it appears that large amounts of high intensity exercise may be required to improve HDL cholesterol efflux capacity in obesity.83
Improvements in insulin sensitivity are consistently seen in studies of aerobic exercise despite weight loss of <3%.84,85 Exercise alone translated to a 46% reduction in incident diabetes over six years in individuals with impaired glucose tolerance in the Da Qing IGT and Diabetes Study.86 Further, some data support additive effects of diet and exercise on insulin sensitivity.87 Acutely, exercise induces an improvement in insulin sensitivity88,89 which, impressively, persists for up to 2 days.90 Aerobic exercise increases skeletal muscle GLUT4 protein expression.91 Skeletal muscle contractions cause translocation of GLUT4 transporters to the muscle cell surface independent of insulin, improving glucose transport and insulin sensitivity.92,52,93 Inhibition of the NF-κB pathway with exercise may further contribute to improved insulin sensitivity in obesity.94,95 Finally, while some evidence suggests HIIT may produce greater improvements in insulin sensitivity than continuous aerobic training,96 even just interrupting prolonged sitting may improve insulin sensitivity in obese children97 and adults.98
Aerobic-exercise-induced visceral adipose tissue reduction, in the absence of weight loss, is associated with reduced circulating inflammatory cytokines.99,100 Exercise in obese mice is adequate to normalize visceral adipose inflammation as indicated by number of macrophages, amount of crown-like structures, and TNFα and IL-6 expression.85,101,102 Exercise also appears to reduce hepatic and skeletal muscle fat, although the findings are less consistent than with visceral adipose tissue.103–105 Endurance exercise in the absence of body weight loss has also been shown to reduce epicardial fat mass.106,107
Exercise has anti-inflammatory effects separate from reduced adipose tissue mass in obesity. Muscle contraction provokes the release of numerous myokines.108 IL-6 release, specifically, occurs in response to acute muscle contraction in a dose-response manner and is felt to be beneficial through its induction of anti-inflammatory cytokines, including IL-10, and IL-1Rα, a competitive inhibitor of IL-1 signaling.90,99,109 Muscle-released IL-6 also stimulates adipocyte lipolysis110 and may increase GLP-1 production,111 further improving glycemia as well as other metabolic risk factors, as discussed below (Figure 3). In fact, administration of an IL-6 receptor antibody has been reported to suppress the epicardial fat loss observed with exercise in obese humans.112
Figure 3.
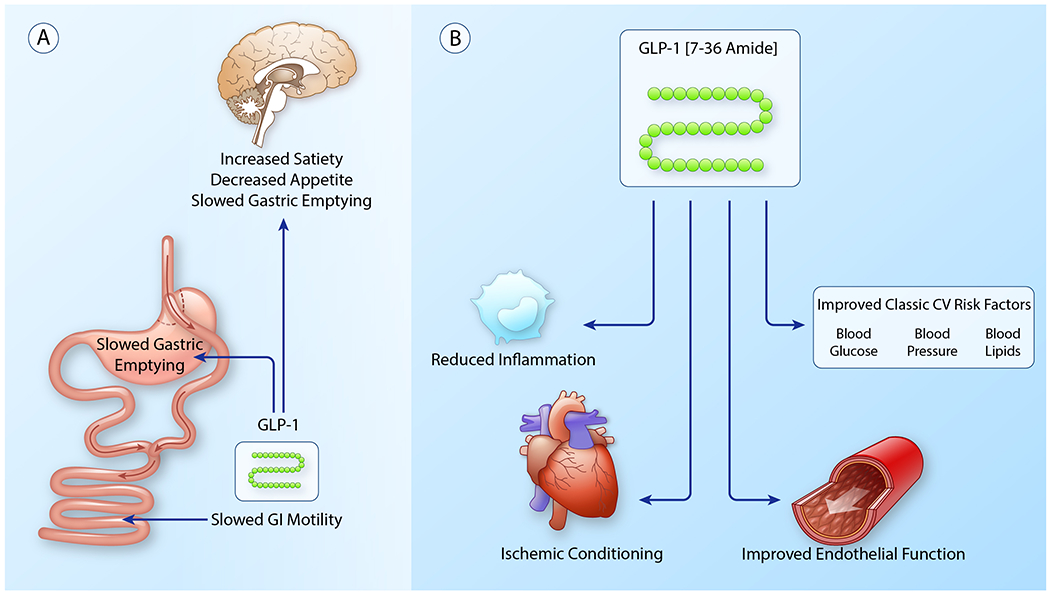
(A) Pathways mediating weight loss via either pharmacologic- or metabolic surgery-induced increases in GLP-1. Metabolic surgery and GLP-1 agonists increase signaling through central and peripheral GLP-1 receptors enhancing satiety and facilitating weight loss. Central receptor activation in the hyppothalamus increases satiety, decreases appetite and slows gastric emptying. Peripheral receptors slow gastric emptying and GI motility, enhancing satiety.
(B) Weight loss-independent activity of GLP-1 in reduction of cardiovascular risk in obesity. As described in detail in the text, increased GLP-1 signaling improves traditional cardiovascular risk factors, reduces systemic inflammation, extensively benefits the vasculature and stimulates myocardial ischemic conditioning. (Illustration credit: Ben Smith)
Aerobic exercise also elicits an intensity-dependent increase in skeletal muscle extracellular vesicle release, and obesity may influence vesicle characteristics.113 These microparticles and exosomes contain proteins, lipids, mRNA and non-coding RNAs,114 the last of which have been reported to differ by exercise type and amount.63 For example, circulating amounts of MiR 27a, 27b and 126 are reduced in obesity, but increase in response to exercise.115 Their relevance to cardiometabolic risk in obesity has yet to be adequately described.
As described in detail in this compendium,116 obesity is characterized by increased numbers of leukocytes, as well as pro-inflammatory leukocyte phenotype. Exercise absent changes in body weight may increase regulatory T cells117 and reduce numbers of inflammatory monocytes,118 with a dose-response to increasing intensity of exercise.99,119 One group recently demonstrated that visceral adipose-derived leptin regulates bone marrow precursor proliferation and contributes to leukocytosis.120 They further showed that aerobic exercise prevents leukocytosis via a reduction in leptin production in non-obese mice. Both chronic and acute exercise seem to increase specialized pro-resolving mediators in mice, and accordingly, improve macrophage efferocytosis activity in in vitro assays, although the effects in humans and in obesity are unclear.121–123
Finally, changes are observed in the intestinal microbiota with exercise in obesity, but given the diversity of studies and interventions it is not currently possible to describe a generalized effect and any impact on metabolic risk remains speculative.124–126 Further, exercise-induced changes in the microbiome appear to not be durable with the cessation of exercise, and possibly impaired in obesity.126
Pharmacotherapy
Major guidelines recommend pharmacotherapy for weight loss as an adjunct to diet, exercise, and behavioral modification in patients with BMI >27kg/m² with obesity related comorbidities or those with BMI >30kg/m².6,7 Five* medications are currently approved by the US Food and Drug Administration (FDA) for long-term use for obesity. They act through both peripheral and central mechanisms, with effects on satiety, energy expenditure, reward pathways, and caloric absorption. While all are efficacious to some degree for weight loss, one single medication class, the GLP-1 agonists, offers particularly notable metabolic risk reduction that appears independent of the associated weight loss.
* This article was completed and accepted prior to the recommended removal of locaserin from the US market by the Food and Drug Administration on February 13, 2020.
Pancreatic lipase inhibitors:
Orlistat is unique among current agents in that it acts completely through a peripheral mechanism of action – reversibly inhibiting lipases in the lumen of the stomach and small intestine, inhibiting dietary fat hydrolysis and thus absorption. The resulting caloric deficit of unabsorbed dietary fat facilitates weight loss. At its recommended therapeutic dose, orlistat inhibits dietary fat absorption by 30%.127
Effects on body weight
Trials over 1-2 years demonstrate average weight loss of ~3% with orlistat relative to placebo.128,129
Effects on metabolic risk in obesity
Multiple studies have demonstrated modest improvement in metabolic parameters with orlistat use, including reductions in SBP, DBP, and HbA1c, comparable to those seen with caloric restriction.12,19 Orlistat reduces LDL-C more than expected with similar weight loss via caloric restriction, likely through the particular reduction in fat absorption.130,131 Orlistat has also been reported to increase levels of adiponectin, thus potentially reducing inflammation and improving insulin sensitivity.132
Phentermine/extended-release topiramate:
Phentermine, a sympathomimetic amine pharmacologically similar to amphetamines, functions as a central nervous system stimulant. Its exact mechanism of action for weight loss remains uncertain, but it is believed to stimulate catecholamine release in the hypothalamus, inhibiting norepinephrine reuptake and decreasing appetite and food consumption.133,134 Topiramate is a traditional anti-epileptic agent. Its mechanism of action on weight loss is also uncertain, but may be due to appetite suppression and satiety enhancement, induced by a combination of effects including: increased activity of the neurotransmitter gamma-aminobutyrate (GABA), inhibition of AMPA/kainite excitatory glutamate receptors, modulation of voltage-gated ion channels, and inhibition of carbonic anhydrase.133 It may also alter neuropeptide Y levels, which may affect satiety.134
Effects on body weight
Placebo-adjusted weight loss from several phase 3 clinical trials of phentermine/topiramate was 7.5-9.3% at the target dose.135–137
Effects on metabolic risk in obesity
In the above trials, phentermine/topiramate improved glycemic control, increased HDL-C, and decreased triglycerides, with a neutral effect on LDL-C, similar to expected changes with weight loss from calorie restriction. SBP generally decreased with phentermine/topiramate, but less than would be expected from a similar amount of weight loss through other means, and DBP changes were neutral. Topiramate has demonstrated inhibition of fat deposition while reducing LPL activity in adipose tissue, but increasing LPL activity in skeletal muscle.138 Given the sympathomimetic action of phentermine, there is concern for potential adverse cardiovascular outcomes. While a single retrospective cohort study examining a database of users suggested no excess cardiovascular risk,139 no prospective trials powered for cardiovascular endpoints have been performed.
Bupropion-naltrexone:
Bupropion inhibits neuronal reuptake of dopamine and norepinephrine, while naltrexone is an opioid antagonist. It is believed that bupropion-naltrexone affects two separate areas of the brain involved in food intake: the mesolimbic dopamine circuit, which is involved in reward pathways, and the hypothalamus, which regulates satiety.140
Effects on body weight
In multiple phase 3 trials, bupropion-naltrexone demonstrated placebo-adjusted weight loss of 2.5-5.2% at target doses.141–143
Effects on metabolic risk in obesity
In the same trials, bupropion/naltrexone was associated with small increases in HDL-C and decreases in LDL-C and triglycerides. Changes in HbA1c ranged from neutral to slightly favorable dependent on weight loss. The effect of bupropion-naltrexone on BP appears to be unfavorable, with increases in SBP during phase 3 testing.141–143 The initial phase 3 trials did not demonstrate any significant change in cardiovascular events, but they were not powered to assess cardiovascular outcomes. Finally, bupropion/naltrexone may have additional considerations for use given its impact on addiction-reward pathways. Of note, bupropion/naltrexone has helped with tobacco cessation and mitigated some of the resulting weight gain typically seen with smoking cessation.144
Serotonin agonists*:
Lorcaserin selectively activates 5-HT2c receptors on anorexigenic pro-opiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus, promoting satiety.45
Effects on body weight
Several trials have demonstrated a placebo-subtracted weight loss of 3-3.6% with lorcaserin at 1 year.145,146
Effects on metabolic risk in obesity
In the CAMELLIA-TIMI 61 study, lorcaserin demonstrated slight improvements in SBP, DBP, triglycerides, LDL-C, and non-HDL-C consistent with improvements expected with the same amount of weight loss via other methods.147 Over a median of 3.3 years, lorcaserin reduced the risk of incident diabetes by 19% in patients with prediabetes and by 23% in patients without prediabetes.148 Improvement in glycemia has been greater than expected for the associated weight loss.145,146 Post-hoc analysis of the BLOOM-DM trial demonstrated improvement in fasting plasma glucose within 2 weeks of lorcaserin initiation, and subjects that achieved <5% weight loss on lorcaserin still demonstrated significant improvement in HbA1c and HOMA-IR.149 The early improvement in fasting glucose suggests improvement in peripheral insulin sensitivity. However it is unclear if these early benefits are from caloric restriction via increased satiety or some other mechanism, in particular, related to lorcaserin’s main effect, 5-HT2c receptor activation. 5-HT2c receptor knockout mice exhibit insulin insensitivity which can be improved with reactivation of receptor function in POMC neurons.150 In POMC neurons, 5-HT2c receptor activation causes second-order signaling via melanocortin 4 (MC4) receptors.151,152 MC4 activation may improve insulin sensitivity, reduce hepatic glucose production and increase GLUT4 expression.153–155
GLP-1 agonists:
Liraglutide, an acylated human glucagon-like peptide-1 (GLP-1) receptor agonist, is a subcutaneously administered medication originally developed for type 2 diabetes, now FDA-approved for treatment of obesity at an increased 3.0 mg daily dose.156 GLP-1 is an incretin hormone secreted by intestinal epithelial L-cells in response to meals. Liraglutide has 97% amino acid sequence homology to endogenous human GLP-1, but liraglutide has a longer half-life because it is stable against metabolic degradation by dipeptidyl-peptidase 4 (DPP-4) and neutral endopeptidase. Liraglutide binds to GLP-1 receptors on pancreatic beta and alpha cells, increasing their sensitivity to glucose, and suppressing glucagon production. GLP-1 agonists also reduce hepatic gluconeogenesis and slow gastric transit, promoting satiety and reducing energy intake (Figure 3A). In addition, GLP-1 receptors are present in several areas of the brain involved in appetite regulation including mesolimbic neurons and the arcuate nucleus of the hypothalamus, suggesting a possible central role (Figure 3A).157 Semaglutide, a newer GLP-1 agonist, has demonstrated even greater weight loss compared to liraglutide, and an oral formulation of semaglutide was recently approved by the FDA.158,159 Semaglutide is not currently FDA-approved specifically for weight loss, but trials are underway for this indication.160
Effects on body weight
Liraglutide has demonstrated efficacy in both weight loss and weight maintenance. In clinical trials, liraglutide has demonstrated a 4.0-5.4% placebo-subtracted weight loss at 1 year.161,162 The SCALE trials, which included obese or overweight subjects with hypertension or dyslipidemia, but excluded patients with diabetes, demonstrated an additional 6.2% weight reduction when liraglutide was administered to obese subjects who had already achieved an average of 6% weight loss on a low-calorie diet.163 The weight loss seen with liraglutide is mediated by reduced energy intake, with no effect on 24-hour energy expenditure.156
Effects on metabolic risk in obesity
In addition to weight loss, the GLP-1 agonists have demonstrated improvement in many traditional cardiovascular risk factors, including SBP, LDL-C, HDL-C, triglycerides and glycemic control, with liraglutide and semaglutide demonstrating the largest reduction in HbA1c.164–167 As expected, given the mechanism of action, the improvement in glycemic control and triglyceride reduction are greater than typically seen in weight loss due to lifestyle alone.168 Importantly, liraglutide and semaglutide use in patients with diabetes has been shown to reduce adverse cardiovascular events and mortality166,167 – effects notably absent from any prior trials of glucose-lowering medications.169
There is evidence to suggest that GLP-1 agonists benefit vascular function – enhancing endothelium-dependent relaxation,170 increasing nitric oxide synthase activity,171,172 improving coronary flow velocity reserve,171 and decreasing production of soluble ICAM-1 and VCAM-1.171 Inhibitor experiments suggested that the actions of exenatide (another GLP-1 agonist) on endothelial function may be mediated by AMPK and PI3K/serine-threonine kinase (Akt)/eNOS pathways in a GLP-1R/cAMP-dependent manner.171 Another study demonstrated that liraglutide decreased endothelin-1, a vasoconstriction-inducing protein produced by endothelial cells, via GLP-1 inhibition of NFκB signaling.172
The anti-inflammatory potential of GLP-1 has also been studied. In humans, the GLP-1 agonist, exenatide, reduced NFkB signaling in monocytes, and decreased the production of TNFα, IL-6, IL-1β, and CRP independent of any weight loss.173,174 Invariant natural killer T (iNKT) cells also express GLP-1 receptors, and in at least one study, a GLP-1 agonist inhibited iNKT cell cytokine secretion.175
Multiple studies have demonstrated improvement in post-myocardial infarction necrotic area176,177 and left ventricular ejection fraction178 in mice treated with GLP-1 agonists, suggesting activity in ischemic conditioning. Rat models have demonstrated GLP-1-mediated improvements in myocardial glucose uptake,179 which may help reduce oxygen demand during ischemia. Additionally, liraglutide has been associated with better systolic function in human patients with myocardial infarction.177As mentioned above, GLP-1 promotes activation of multiple protein kinases, such as PI3K, Akt, mitogen-activated protein kinase (MEK1/2), and extracellular signal–regulated kinase (ERK1/2).180 PI3K increases nitric oxide production and inhibits the proapoptotic protein Bcl-xL/Bcl-2-associated death promoter.181 Furthermore, activation of these signaling pathways stimulates opening of the mitochondrial KATP channels181, 182 and prevents the opening of mitochondrial permeability transition pores,181 which are critical steps in both apoptosis and necrosis.181 These factors may all contribute to the cardioprotective effects of GLP-1 and are summarized in Figure 3B.
Finally, in addition to their effect on glycemic control, the GLP-1 agonists have been shown to reduce post-prandial increases in apoB-48 and apoCIII, independent of weight loss.183 Rat models infused with recombinant GLP-1 demonstrated decreased intestinal lymph flow, decreased triglyceride absorption and decreased production of apoB and apoAIV, potentially due to effects on microcirculation and gastric lipase inhibition.184 Epicardial fat in patients with diabetes was found to be reduced as early as 3 months after initiating treatment with liraglutide.185
Metabolic surgery
Metabolic surgery was one of the first interventions demonstrated to reduce cardiovascular morbidity and mortality.186 However, the partial ileal bypass procedure employed in the POSCH trial was not a treatment for obesity, but rather for hyperlipidemia in the pre-statin era. While lipid management is now nearly wholly a pharmacologic enterprise, metabolic/bariatric surgery has proven to be the most effective way to achieve significant durable weight loss in obesity.187 Long-term data now support that the improvements in metabolic risk in association with weight loss following bariatric surgery translate to reductions in adverse cardiovascular outcomes and mortality.1–4 Consideration of bariatric surgery is recommended for patients with BMI ≥40kg/m2; or ≥35kg/m2; with obesity related comorbidities.6
The varying metabolic benefits of bariatric surgery were originally postulated to be related to a procedure’s restrictive and/or malabsorptive effects.188 However, enhanced incretin and bile acid signaling (and possibly an altered intestinal microbiota) are now recognized to contribute to both weight loss and cardiometabolic risk improvement with some procedures (Figure 4).189 However, the mechanisms underlying metabolic improvements and marked weight loss following metabolic surgery remain far from thoroughly understood and require much more investigation. As a case in point, despite the performance of hundreds of thousands of procedures worldwide annually, the absence of well-controlled studies in this area allowed for the inclusion of data from <1500 subjects in a 2014 Cochrane review.190
Figure 4. Weight loss-independent mechanisms of cardiovascular risk reduction in metabolic surgery.
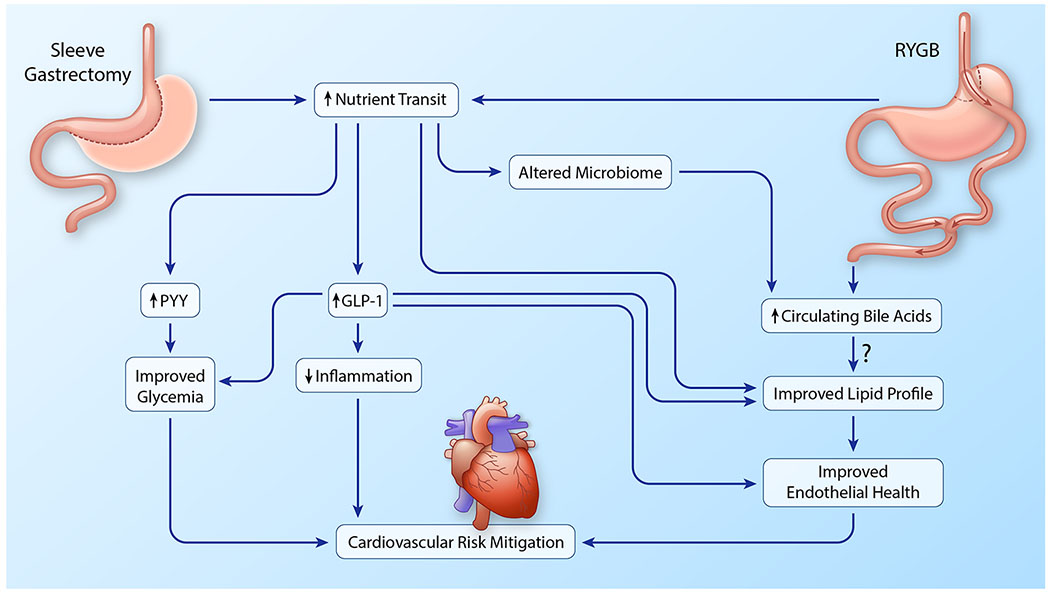
Both RYGB and sleeve gastrectomy increase nutrient transit which results in increases in circulating PYY and GLP-1 which, in turn, improve glycemia, reduce inflammation and improve endothelial function. These activities contribute to cardiovascular risk mitigation following metabolic surgery. RYGB further increases levels of circulating bile acids which further stimulates GLP-1 release and may have additional positive effects on lipid profile (predominantly triglyceride lowering). Changes in the gut microbiome may also contribute to increased circulating bile acids after RYGB. (Illustration credit: Ben Smith)
Gastric Banding
Laparoscopic adjustable gastric banding (LAGB) involves placing an adjustable ring or band at the pylorus. The band is connected to a subcutaneous port at which saline can be injected or removed to adjust pyloric pressure. LAGB is intrinsically a restrictive procedure that limits the volume of food entering the pylorus with any given meal. Beyond restriction of the gastric pouch size, the band pressure increases peristaltic activity of the lower esophagus and upper stomach, leading to vagal nerve stimulation and enhanced satiety with smaller meals.191, 192
Effects on body weight
LAGB, in combination with a calorie-restricted diet and exercise intervention, generally produces ~15% weight loss from baseline at 52 weeks.193,194
Effects on metabolic risk in obesity
The effects of LAGB on cardiovascular risk factors are largely comparable to equivalent weight loss via caloric restriction.19 For example, in patients with grade 1 obesity, we observed significant reductions in SBP, DBP, and triglycerides along with increased HDL-C.195 Larger studies and a meta-analysis demonstrate improvements in glycemia196,197 and modest reductions in LDL-C,198 respectively. Longitudinal data suggest that improvements in dyslipidemia may be more durable than reductions in BP and blood glucose.194 The procedure has largely fallen out of favor despite its potential in specific populations, such as adolescents, 199 that would benefit from reversible procedures.
Sleeve Gastrectomy
Sleeve gastrectomy has recently become the most commonly performed metabolic surgical procedure in both the United States and South America.200 This procedure evolved as a hybrid of other procedures and consists of removal of the fundal and antral portion of the stomach through a plication procedure, leaving the patient with a remnant stomach “sleeve” through which nutrients flow into the small intestine.
Effects on body weight
Sleeve gastrectomy leads to 25-30% mean weight loss from baseline at 1 year, with weight loss being quite durable at 5 years.201,202 The degree of weight loss achieved with sleeve gastrectomy is larger than can be attributed to the restrictive and malabsorptive effects of removal of 75% stomach volume alone.203 Accordingly, it is felt that other mechanisms mediate additional satiety and decreased caloric consumption following sleeve gastrectomy, and may further contribute to reduced metabolic risk. The probable mediators of such hypocaloric effects include the anorectic hormones GLP-1 and Peptide-YY (PYY),204,192,205 as well as ghrelin, an orexogenic hormone that decreases with removal of ghrelin-secreting P-D1 cells in the gastric fundus.192 Sleeve gastrectomy also perturbs the gut microbiota in ways that may facilitate additional weight loss through reduced energy absorption.206
Effects on metabolic risk in obesity
In concert with the substantial weight loss that occurs with sleeve gastrectomy come greater improvements in metabolic risk than any intervention thus far discussed within this review.198,201,202,207 These include sizable reductions in BP, HbA1c, triglycerides, and inflammatory markers, with significant increases in HDL-C. Notably, sleeve gastrectomy has not consistently demonstrated reductions in LDL-C, even in cohorts not taking lipid-lowering medications prior to surgery.208 Notably, increases in GLP-1 and PYY at least partly mediate improvements in glycemia beyond that seen with equivalent non-surgical weight loss.209 Other effects on metabolic risk of this relatively new, yet dominant, procedure are uncertain.
Roux-en-Y Gastric Bypass
Roux-en-Y gastric bypass (RYGB) has the longest history of bariatric procedures and the best evidence for sustained maintenance of weight loss and improvement in cardiovascular risk.207,210,211 This procedure involves connecting a small gastric pouch to the proximal jejunum allowing for transit of nutrients directly to the small intestine, while a biliary limb connects the bypassed stomach and duodenum to the more distal jejunum.
Effects on body weight
Roux-en-Y bariatric procedures have been simplified over the years to be performed laparoscopically or with a single anastomosis, and lead to ≥30% mean weight loss from baseline at 1 year with weight loss largely durable at ≥5 years.1,194,207,212,213 The substantial weight loss is due to a combination of superior reductions in appetite and caloric intake mediated through GLP-1 and PYY action, increased nutrient transit, and gut microenvironment changes, resulting from the unique gastrointestinal tract rearrangement.192
Effects on metabolic risk in obesity
RYGB produces the greatest improvements in BP, glycemia and dyslipidemia of any weight loss modality. Similar to the procedure’s effects on body weight, the associated improvements in metabolic risk are largely durable.1,194,207,212,213 Further, weight loss-independent cardiometabolic improvements with RYGB have long been recognized – the procedure being renowned for improving diabetes while patients are still recovering in the hospital. Altered nutrient transit is one mechanism underlying the benefits. Rapid carbohydrate transit from the pylorus to the small intestine augments GLP-1 secretion beyond that seen with sleeve gastrectomy. Greater increases in post-prandial GLP-1 (possibly in addition to reduced glucose-dependent insulinotropic peptide (GIP) unique to RYGB) contribute to the superior improvements in insulin resistance, beta-cell function, and glycemic variation seen with RYGB.214,215 The absence of nutrient contact with the duodenum specifically, has been additionally implicated in improved glucose control, independent of weight loss,216 although the mechanism has not been elucidated.
Exposure of the small intestine to undigested proteins and lipids that are not as readily absorbed contributes to greater reductions in LDL-C198 than expected with equivalent weight loss through other means. In addition to improvements in triglycerides and HDL are durable and superior to other weight loss methods,194,198 beneficial HDL remodeling217,218 has been reported following RYGB. Increases in GLP-1 following RYGB are believed to mediate cardiovascular benefit similarly to GLP-1 receptor agonists, as outlined in Figure 3. Specifically in the context of RYGB, increases in GLP-1 have also been implicated in improved HDL function, independent of weight loss.219 However, data on the effects on HDL cholesterol efflux following RYGB are mixed.208,218
The rapid transit of less digested nutrients perturbs the intestinal microbiome following RYGB, similar to sleeve gastrectomy. Some work suggests that increased microbial diversity post-RYGB results in reduced bacterial energy harvest from food, decreasing caloric absorption and facilitating weight loss.220–222 The altered microbiota, combined with augmented rates of absorption secondary to small bowel rearrangement, contributes to increased circulating bile acid concentrations following RYGB.223 As farnesoid-X receptor agonists, bile acids act to reduce triglycerides,224 and as TGR5 agonists, they further stimulate GLP-1 secretion.225 Interestingly, a gall bladder-to-ileum anastomosis (which greatly increases circulating bile acids), without intervention on the stomach or small bowel, was able to reproduce many of the metabolic improvements of RYGB, in murine diet-induced obesity.226 While some data suggest a cost of worsening lipoprotein profiles from increased circulating bile acids,227 these appear to be more than mitigated by overall weight loss and competing pathways stimulated by RYGB outlined above.198
As mentioned earlier, bariatric surgery is the only weight loss modality demonstrated to improve cardiovascular outcomes in obesity. This is likely due to a combination of the substantial weight loss induced by procedures, in addition to mechanisms by which metabolic surgery, specifically sleeve gastrectomy and RYGB, mitigate cardiovascular risk beyond that of matched, non-surgical weight loss. We outline these mechanisms in Figure 4. The mechanisms highlighted in this figure have largely been uncovered over the past decade and remain under intense investigation. We are confident that this figure is incomplete and that additional basic and translational research will further elucidate the mechanisms and identify potentially novel outcomes affected by these procedures. Should these mechanisms be extricated from the procedures themselves, this could provide new therapeutic strategies in the treatment of obesity, diabetes, and consequent cardiovascular disease.
Perspective
To comprehensively review all proven and promising weight loss/management approaches would be impossible, particularly in the space available. We have only touched on a small subset of the countless diet and exercise variations that have been studied, much less all that could be conceived of. New and novel pharmacologic agents, surgical and minimally invasive techniques continue to be developed as basic and translational research suggest promising targets for achieving weight loss and reduction of metabolic risk.
For example, oxyntomodulin is a peptide hormone secreted in the gut with GLP-1 after nutrient ingestion. It activates both the GLP-1 receptor and the glucagon receptor (GCGR).228,229 Trials of oxyntomodulin have demonstrated weight loss in humans and evidence of both increased satiety and increased energy expenditure.230,231 The impact on metabolic risk in obesity remains to be seen, but a dual GLP-1/GCGR agonist for the treatment of diabetes and obesity is currently in development and a triple GLP-1/GCGR and GIP co-agonist is currently being developed with early promising results in both obesity and hepatosteatosis.232 Further, preliminary studies of novel hormonal multiagonists of existing incretins, including GLP-1-estrogen, GLP-1-triiodothyronine and glucagon-dexamethasone, indicated that they may provide the metabolic benefit of each individual hormone without the adverse effects common in hormonal supplementation.233–235
In addition to research on variations in sleeve gastrectomy and RYGB, there is growing interest in endoscopic bariatric procedures. Endoscopic gastroplasty involves reduction of gastric volume via creation of a mucosa-to-mucosa tissue apposition, similar to a sleeve gastrectomy.236 Early trials of duodenal resurfacing, which involved catheter-based thermal ablation of the duodenal mucosa, has demonstrated durable efficacy in diabetes management in humans, despite minimal weight loss.237 Basic research into the biology underlying the benefits of these procedures may suggest molecules and pathways that can be targeted to further optimize metabolic risk reduction in obesity.
We have also largely omitted the topic of consolidating a healthier weight when it is achieved and preventing weight regain, a frustrating problem to which no intervention is immune. Research into ways that successfully maintain reduced weight and metabolic risk is needed. Given the genetic, molecular and behavior pathways that contribute to obesity, an integrated, personalized approach seems necessary.238
Finally, while we have discussed how diverse obesity treatments can reduce cardiometabolic risk markers, several points warrant emphasis. Only significant weight loss via bariatric surgery has proven sufficient to reduce cardiovascular events. Even in this setting, current approaches may not fully reverse the risk in all individuals, particularly after advanced changes such as adipose tissue fibrosis have set in.29 Further, accumulating data support immune memory as mitigating persistently elevated metabolic risk in obesity despite weight loss.239,240 This is suggestive of a concept of, “once obese, always at risk,” and is an area of intense investigation by many groups, including our own. While these observations suggest that targeting immune cell metabolism may hold benefit for cardiovascular risk reduction in obesity, they should also highlight the importance of prevention. In 2009, the ACSM made the following apropos statement – “the prevention of weight gain may be the easiest way to prevent the development of undesirable changes in cardiovascular disease risk factors.”45 While preventing obesity has proven far from easy, it is likely the most effective way to reduce metabolic risk. Considering this, effectively preventing obesity and its progression are paramount goals at the individual and population level, and deserve substantial research and policy attention.
Acknowledgments
Sources of Funding
Sean P Heffron - K23HL135398
Jay Pendse – T32HL98129
Jose O Aleman – K08DK117064, American Heart Association 17SFRN33490004, Doris Duke Foundation
Abbreviations
- ACSM
American College of Sports Medicine
- BMI
Body Mass Index
- CRP
C-reactive protein
- DASH
Dietary Approaches to Stop Hypertension
- DBP
diastolic blood pressure
- DPP4
dipeptidyl-peptidase 4
- ERK
extracellular signal–regulated kinase
- FDA
Food and Drug Administration
- FFA
free fatty acids
- FMD
flow-mediated dilation
- FXR
farnesyl X receptor
- GABA
gamma-aminobutyrate
- GIP
glucose-dependent insulinotropic peptide
- GLP-1
glucagon-like peptide 1
- GLUT4
glucose transporter type 4
- HbA1c
Hemoglobin A1c
- HIIT
high intensity interval training
- ICAM1
intercellular adhesion molecule 1
- IL-1
interleukin 1
- IL-1Rα
interleukin 1 receptor antagonist
- IL-10
interleukin 10
- iNKT
Invariant natural killer T cells
- KLF2
kruppel-like factor 2
- LAGB
laparoscopic adjustable gastric banding
- LPL
lipoprotein lipase
- MC4
melanocortin 4
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- POMC
pro-opiomelanocortin
- PYY
Peptide-YY
- RYGB
Roux-en-Y Gastric Bypass
- SBP
systolic blood pressure
- TNF-α
Tumor Necrosis factor alpha
- VCAM1
vascular cell adhesion molecule 1
- VLDL
very low density lipoprotein
Footnotes
Disclosures
None
References
- 1.Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. [DOI] [PubMed] [Google Scholar]
- 2.Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PR, Kattan MW, Nissen SE. Association of Metabolic Surgery With Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes and Obesity. JAMA. 2019. doi: 10.1001/jama.2019.14231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundstrom J, Bruze G, Ottosson J, Marcus C, Naslund I, Neovius M. Weight Loss and Heart Failure: A Nationwide Study of Gastric Bypass Surgery Versus Intensive Lifestyle Treatment. Circulation. 2017;135:1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benotti PN, Wood GC, Carey DJ, Mehra VC, Mirshahi T, Lent MR, Petrick AT, Still C, Gerhard GS, Hirsch AG. Gastric Bypass Surgery Produces a Durable Reduction in Cardiovascular Disease Risk Factors and Reduces the Long-Term Risks of Congestive Heart Failure. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Kober L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–785.113. [DOI] [PubMed] [Google Scholar]
- 6.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, Ryan DH, Still CD, Endocrine Society . Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342–62. [DOI] [PubMed] [Google Scholar]
- 8.Gregg EW, Jakicic JM, Blackburn G, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, Nadolsky K, Pessah-Pollack R, Plodkowski R. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY COMPREHENSIVE CLINICAL PRACTICE GUIDELINES FOR MEDICAL CARE OF PATIENTS WITH OBESITY. Endocr Pract. 2016;22 Suppl 3:1–203. [DOI] [PubMed] [Google Scholar]
- 10.Saxon DR, Iwamoto SJ, Mettenbrink CJ, McCormick E, Arterburn D, Daley MF, Oshiro CE, Koebnick C, Horberg M, Young DR, Bessesen DH. Antiobesity Medication Use in 2.2 Million Adults Across Eight Large Health Care Organizations: 2009-2015. Obesity. 2019;27:1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin M, Beekley A, Kjorstad R, Sebesta J. Socioeconomic disparities in eligibility and access to bariatric surgery: a national population-based analysis. Surg Obes Relat Dis. 2010;6:8–15. [DOI] [PubMed] [Google Scholar]
- 12.Schillaci G, Pasqualini L, Vaudo G, Lupattelli G, Pirro M, Gemelli F, De Sio M, Porcellati C, Mannarino E. Effect of body weight changes on 24-hour blood pressure and left ventricular mass in hypertension: a 4-year follow-up. Am J Hypertens. 2003;16:634–9. [DOI] [PubMed] [Google Scholar]
- 13.Semlitsch T, Jeitler K, Berghold A, Horvath K, Posch N, Poggenburg S, Siebenhofer A. Long-term effects of weight-reducing diets in people with hypertension. Cochrane Database Syst Rev. 2016;3:Cd008274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gay HC, Rao SG, Vaccarino V, Ali MK. Effects of Different Dietary Interventions on Blood Pressure: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Hypertension. 2016;67:733–9. [DOI] [PubMed] [Google Scholar]
- 15.Joris PJ, Zeegers MP, Mensink RP. Weight loss improves fasting flow-mediated vasodilation in adults: a meta-analysis of intervention studies. Atherosclerosis. 2015;239:21–30. [DOI] [PubMed] [Google Scholar]
- 16.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauman V, Ariel-Donges AH, Gordon EL, Daniels MJ, Xu D, Ross KM, Limacher MC, Perri MG. Effect of dose of behavioral weight loss treatment on glycemic control in adults with prediabetes. BMJ Open Diabetes Res Care. 2019;7:e000653.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, de Las Fuentes L, He S, Okunade AL, Patterson BW, Klein S. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016;23:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bays HE, Toth PP, Kris-Etherton PM, Abate N, Aronne LJ, Brown WV, Gonzalez-Campoy JM, Jones SR, Kumar R, La Forge R, Samuel VT. Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:304–83. [DOI] [PubMed] [Google Scholar]
- 21.Expert Panel Report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity. 2014;22 Suppl 2:S41–410. [DOI] [PubMed] [Google Scholar]
- 22.Dattilo AM and Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56:320–8. [DOI] [PubMed] [Google Scholar]
- 23.Ng TW, Watts GF, Barrett PH, Rye KA, Chan DC. Effect of weight loss on LDL and HDL kinetics in the metabolic syndrome: associations with changes in plasma retinol-binding protein-4 and adiponectin levels. Diabetes Care. 2007;30:2945–50. [DOI] [PubMed] [Google Scholar]
- 24.Di Minno MN, Peluso R, Iervolino S, Russolillo A, Lupoli R, Scarpa R. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor alpha blockers. Ann Rheum Dis. 2014;73:1157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW Jr. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aleman JO, Iyengar NM, Walker JM, Milne GL, Da Rosa JC, Liang Y, Giri DD, Zhou XK, Pollak MN, Hudis CA, Breslow JL, Holt PR, Dannenberg AJ. Effects of Rapid Weight Loss on Systemic and Adipose Tissue Inflammation and Metabolism in Obese Postmenopausal Women. J Endocr Soc. 2017;1:625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burhans MS, Hagman DK, Kuzma JN, Schmidt KA, Kratz M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr Physiol. 2018;9:1–58.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimobayashi M, Albert V, Woelnerhanssen B, et al. Insulin resistance causes inflammation in adipose tissue. J Clin Invest. 2018;128:1538–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdennour M, Reggio S, Le Naour G, et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: links with diabetes and BMI loss after gastric bypass. J Clin Endocrinol Metab. 2014;99:898–907. [DOI] [PubMed] [Google Scholar]
- 30.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 31.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D’Armiento M, D’Andrea F and Giugliano D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–6. [DOI] [PubMed] [Google Scholar]
- 32.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN and Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J Am Coll Cardiol. 2004;44:152–8. [DOI] [PubMed] [Google Scholar]
- 33.Stanhope KL, Goran MI, Bosy-Westphal A, et al. Pathways and mechanisms linking dietary components to cardiometabolic disease: thinking beyond calories. Obes Rev. 2018;19:1205–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–99. [DOI] [PubMed] [Google Scholar]
- 35.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Havel PJ, Keim NL. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur J Clin Nutr. 2012;66:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanhope KL, Bremer AA, Medici V, Nakajima K, Ito Y, Nakano T, Chen G, Fong TH, Lee V, Menorca RI, Keim NL, Havel PJ. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab. 2011;96:E1596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christ A, Gunther P, Lauterbach MAR, et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell. 2018;172:162–175.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirkpatrick CF, Bolick JP, Kris-Etherton PM, Sikand G, Aspry KE, Soffer DE, Willard KE, Maki KC. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors. J Clin Lipidol. 2019;13:689–711.e1. [DOI] [PubMed] [Google Scholar]
- 40.Malinowski B, Zalewska K, Wesierska A, Sokolowska MM, Socha M, Liczner G, Pawlak-Osinska K, Wicinski M. Intermittent Fasting in Cardiovascular Disorders-An Overview. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018;27:1212–1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hjorth MF, Ritz C, Blaak EE, Saris WH, Langin D, Poulsen SK, Larsen TM, Sorensen TI, Zohar Y, Astrup A. Pretreatment fasting plasma glucose and insulin modify dietary weight loss success: results from 3 randomized clinical trials. Am J Clin Nutr. 2017;106:499–505. [DOI] [PubMed] [Google Scholar]
- 43.Krajmalnik-Brown R, Ilhan ZE, Kang DW, DiBaise JK. Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract. 2012;27:201–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swift DL, McGee JE, Earnest CP, Carlisle E, Nygard M, Johannsen NM. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Prog Cardiovasc Dis. 2018;61:206–213. [DOI] [PubMed] [Google Scholar]
- 45.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–71. [DOI] [PubMed] [Google Scholar]
- 46.National Center for Health Statistics. Participation in leisure-time aerobic and muscle-strengthening activities that meet the federal 2008 Physical Activity Guidelines for Americans among adults aged 18 and over, by selected characteristics: United States, selected years 1998–2016. Hyattsville, MD: National Center for Health Statistics; 2018. [Google Scholar]
- 47.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–67. [DOI] [PubMed] [Google Scholar]
- 48.Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006:Cd003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz RS. The independent effects of dietary weight loss and aerobic training on high density lipoproteins and apolipoprotein A-I concentrations in obese men. Metabolism. 1987;36:165–71. [DOI] [PubMed] [Google Scholar]
- 50.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. [DOI] [PubMed] [Google Scholar]
- 51.Garrow JS and Summerbell CD. Meta-analysis: effect of exercise, with or without dieting, on the body composition of overweight subjects. Eur J Cli Nutr. 1995;49:1–10. [PubMed] [Google Scholar]
- 52.Despres JP, Pouliot MC, Moorjani S, Nadeau A, Tremblay A, Lupien PJ, Theriault G, Bouchard C. Loss of abdominal fat and metabolic response to exercise training in obese women. Am J Physiol. 1991;261:E159–67. [DOI] [PubMed] [Google Scholar]
- 53.Kelley GA, Kelley KS , Vu Tran Z. Aerobic exercise, lipids and lipoproteins in overweight and obese adults: a meta-analysis of randomized controlled trials. Int J Obes. 2005;29:881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balducci S, Zanuso S, Nicolucci A, De Feo P, Cavallo S, Cardelli P, Fallucca S, Alessi E, Fallucca F, Pugliese G. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES). Arch Intern Med. 2010;170:1794–803. [DOI] [PubMed] [Google Scholar]
- 55.Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clin Nutr. 1999;69:198–204. [DOI] [PubMed] [Google Scholar]
- 56.Flechtner-Mors M, Ditschuneit HH, Johnson TD, Suchard MA, Adler G. Metabolic and weight loss effects of long-term dietary intervention in obese patients: four-year results. Obes Res. 2000;8:399–402. [DOI] [PubMed] [Google Scholar]
- 57.Barry VW, Baruth M, Beets MW, Durstine JL, Liu J, Blair SN. Fitness vs. fatness on all-cause mortality: a meta-analysis. Prog Cardiovasc Dis. 2014;56:382–90. [DOI] [PubMed] [Google Scholar]
- 58.Arroll B and Beaglehole R. Does physical activity lower blood pressure: a critical review of the clinical trials. J Clin Epidemiol. 1992;45:439–47. [DOI] [PubMed] [Google Scholar]
- 59.Arvola P, Wu X, Kahonen M, Makynen H, Riutta A, Mucha I, Solakivi T, Kainulainen H, Porsti I. Exercise enhances vasorelaxation in experimental obesity associated hypertension. Cardiovasc Res. 1999;43:992–1002. [DOI] [PubMed] [Google Scholar]
- 60.Pugh CJ, Sprung VS, Jones H, Richardson P, Shojaee-Moradie F, Umpleby AM, Green DJ, Cable NT, Trenell MI, Kemp GJ, Cuthbertson DJ. Exercise-induced improvements in liver fat and endothelial function are not sustained 12 months following cessation of exercise supervision in nonalcoholic fatty liver disease. Int J Obes. 2016;40:1927–1930. [DOI] [PubMed] [Google Scholar]
- 61.Stensvold D, Tjonna AE, Skaug EA, Aspenes S, Stolen T, Wisloff U, Slordahl SA. Strength training versus aerobic interval training to modify risk factors of metabolic syndrome. J Appl Physiol. 2010;108:804–10. [DOI] [PubMed] [Google Scholar]
- 62.Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93:3–30. [DOI] [PubMed] [Google Scholar]
- 63.Krankel N, Bahls M, Van Craenenbroeck EM, Adams V, Serratosa L, Solberg EE, Hansen D, Dorr M, Kemps H. Exercise training to reduce cardiovascular risk in patients with metabolic syndrome and type 2 diabetes mellitus: How does it work? Eur J Prev Cardiol. 2019;26:701–708. [DOI] [PubMed] [Google Scholar]
- 64.Greyling A, Schreuder TH, Landman T, Draijer R, Verheggen RJ, Hopman MT, Thijssen DH. Elevation in blood flow and shear rate prevents hyperglycemia-induced endothelial dysfunction in healthy subjects and those with type 2 diabetes. J Appl Physiol. 2015;118:579–85. [DOI] [PubMed] [Google Scholar]
- 65.Katzel LI, Bleecker ER, Colman EG, Rogus EM, Sorkin JD, Goldberg AP. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. A randomized controlled trial. JAMA. 1995;274:1915–21. [DOI] [PubMed] [Google Scholar]
- 66.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–92. [DOI] [PubMed] [Google Scholar]
- 67.Slentz CA, Aiken LB, Houmard JA, Bales CW, Johnson JL, Tanner CJ, Duscha BD, Kraus WE. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol. 2005;99:1613–8. [DOI] [PubMed] [Google Scholar]
- 68.Magkos F Basal very low-density lipoprotein metabolism in response to exercise: mechanisms of hypotriacylglycerolemia. Prog Lipid Res. 2009;48:171–90. [DOI] [PubMed] [Google Scholar]
- 69.MacInnis MJ and Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. 2017;595:2915–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freese EC, Gist NH, Cureton KJ. Effect of prior exercise on postprandial lipemia: an updated quantitative review. J Appl Physiol. 2014;116:67–75. [DOI] [PubMed] [Google Scholar]
- 71.Kraus WE and Slentz CA. Exercise training, lipid regulation, and insulin action: a tangled web of cause and effect. Obesity. 2009;17 Suppl 3:S21–6. [DOI] [PubMed] [Google Scholar]
- 72.Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J. 1999;13:2051–60. [DOI] [PubMed] [Google Scholar]
- 73.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52:2191–7. [DOI] [PubMed] [Google Scholar]
- 74.Di Meo S, Iossa S, Venditti P. Improvement of obesity-linked skeletal muscle insulin resistance by strength and endurance training. J Endocrinol. 2017;234:R159–r181. [DOI] [PubMed] [Google Scholar]
- 75.Ringseis R, Eder K, Mooren FC, Kruger K. Metabolic signals and innate immune activation in obesity and exercise. Exerc Immunol Rev. 2015;21:58–68. [PubMed] [Google Scholar]
- 76.Wu J, Ruas JL, Estall JL, Rasbach KA, Choi JH, Ye L, Bostrom P, Tyra HM, Crawford RW, Campbell KP, Rutkowski DT, Kaufman RJ, Spiegelman BM. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1alpha/ATF6alpha complex. Cell Metab. 2011;13:160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A. 2009;106:21401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eisele PS, Furrer R, Beer M, Handschin C. The PGC-1 coactivators promote an anti-inflammatory environment in skeletal muscle in vivo. Biochem Biophys Res Commun. 2015;464:692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44:211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, Suzuki E, Shimano H, Yamamoto S, Kondo K, Ohashi Y, Yamada N, Sone H. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med. 2007;167:999–1008. [DOI] [PubMed] [Google Scholar]
- 81.Nicklas BJ, Katzel LI, Busby-Whitehead J, Goldberg AP. Increases in high-density lipoprotein cholesterol with endurance exercise training are blunted in obese compared with lean men. Metabolism. 1997;46:556–61. [DOI] [PubMed] [Google Scholar]
- 82.Durstine JL, Grandjean PW, Davis PG, Ferguson MA, Alderson NL, DuBose KD. Blood lipid and lipoprotein adaptations to exercise: a quantitative analysis. Sports Med. 2001;31:1033–62. [DOI] [PubMed] [Google Scholar]
- 83.Sarzynski MA, Ruiz-Ramie JJ, Barber JL, Slentz CA, Apolzan JW, McGarrah RW, Harris MN, Church TS, Borja MS, He Y, Oda MN, Martin CK, Kraus WE, Rohatgi A. Effects of Increasing Exercise Intensity and Dose on Multiple Measures of HDL (High-Density Lipoprotein) Function. Arterioscler Thromb Vasc Biol. 2018;38:943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ross R and Bradshaw AJ. The future of obesity reduction: beyond weight loss. Nat Rev Endocrinol. 2009;5:319–25. [DOI] [PubMed] [Google Scholar]
- 85.Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295:E586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–44. [DOI] [PubMed] [Google Scholar]
- 87.Dengel DR, Pratley RE, Hagberg JM, Rogus EM, Goldberg AP. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol. 1996;81:318–25. [DOI] [PubMed] [Google Scholar]
- 88.Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–62. [DOI] [PubMed] [Google Scholar]
- 89.Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol. 1988;254:E248–59. [DOI] [PubMed] [Google Scholar]
- 90.Gorgens SW, Eckardt K, Jensen J, Drevon CA, Eckel J. Exercise and Regulation of Adipokine and Myokine Production. Prog Mol Biol Transl Sci. 2015;135:313–36. [DOI] [PubMed] [Google Scholar]
- 91.Hughes VA, Fiatarone MA, Fielding RA, Kahn BB, Ferrara CM, Shepherd P, Fisher EC, Wolfe RR, Elahi D, Evans WJ. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol. 1993;264:E855–62. [DOI] [PubMed] [Google Scholar]
- 92.Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982;69:785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol. 2005;99:338–43. [DOI] [PubMed] [Google Scholar]
- 94.da Luz G, Frederico MJ, da Silva S, Vitto MF, Cesconetto PA, de Pinho RA, Pauli JR, Silva AS, Cintra DE, Ropelle ER, De Souza CT. Endurance exercise training ameliorates insulin resistance and reticulum stress in adipose and hepatic tissue in obese rats. Eur J Appl Physiol. 2011;111:2015–23. [DOI] [PubMed] [Google Scholar]
- 95.Sriwijitkamol A, Christ-Roberts C, Berria R, Eagan P, Pratipanawatr T, DeFronzo RA, Mandarino LJ, Musi N. Reduced skeletal muscle inhibitor of kappaB beta content is associated with insulin resistance in subjects with type 2 diabetes: reversal by exercise training. Diabetes. 2006;55:760–7. [DOI] [PubMed] [Google Scholar]
- 96.Jelleyman C, Yates T, O’Donovan G, Gray LJ, King JA, Khunti K, Davies MJ. The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev. 2015;16:942–61. [DOI] [PubMed] [Google Scholar]
- 97.Broadney MM, Belcher BR, Berrigan DA, et al. Effects of Interrupting Sedentary Behavior With Short Bouts of Moderate Physical Activity on Glucose Tolerance in Children With Overweight and Obesity: A Randomized Crossover Trial. Diabetes Care. 2018;41:2220–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, Shaw JE, Bertovic DA, Zimmet PZ, Salmon J, Owen N. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35:976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–15. [DOI] [PubMed] [Google Scholar]
- 100.Nassis GP, Papantakou K, Skenderi K, Triandafillopoulou M, Kavouras SA, Yannakoulia M, Chrousos GP, Sidossis LS. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism. 2005;54:1472–9. [DOI] [PubMed] [Google Scholar]
- 101.Kawanishi N, Mizokami T, Yano H, Suzuki K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med Sci Sports Exerc. 2013;45:1684–93. [DOI] [PubMed] [Google Scholar]
- 102.Kawanishi N, Niihara H, Mizokami T, Yano H, Suzuki K. Exercise training attenuates adipose tissue fibrosis in diet-induced obese mice. Biochem Biophys Res Commun. 2013;440:774–9. [DOI] [PubMed] [Google Scholar]
- 103.Sabag A, Way KL, Keating SE, Sultana RN, O’Connor HT, Baker MK, Chuter VH, George J, Johnson NA. Exercise and ectopic fat in type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2017;43:195–210. [DOI] [PubMed] [Google Scholar]
- 104.Winn NC, Liu Y, Rector RS, Parks EJ, Ibdah JA, Kanaley JA. Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity - A randomized trial. Metabolism. 2018;78:128–140. [DOI] [PubMed] [Google Scholar]
- 105.Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105–12. [DOI] [PubMed] [Google Scholar]
- 106.Kim MK, Tomita T, Kim MJ, Sasai H, Maeda S, Tanaka K. Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol. 2009;106:5–11. [DOI] [PubMed] [Google Scholar]
- 107.Christensen RH, Wedell-Neergaard AS, Lehrskov LL, et al. Effect of Aerobic and Resistance Exercise on Cardiac Adipose Tissues: Secondary Analyses From a Randomized Clinical Trial. JAMA Cardiol. 2019. doi: 10.1001/jamacardio.2019.2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Raschke S, Eckardt K, Bjorklund Holven K, Jensen J, Eckel J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS One. 2013;8:e62008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433–7. [DOI] [PubMed] [Google Scholar]
- 110.van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Moller K, Saltin B, Febbraio MA, Pedersen BK. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003;88:3005–10. [DOI] [PubMed] [Google Scholar]
- 111.Ellingsgaard H, Hauselmann I, Schuler B, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17:1481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Christensen RH, Lehrskov LL, Wedell-Neergaard AS, Legaard GE, Ried-Larsen M, Karstoft K, Krogh-Madsen R, Pedersen BK, Ellingsgaard H, Rosenmeier JB. Aerobic Exercise Induces Cardiac Fat Loss and Alters Cardiac Muscle Mass Through an Interleukin-6 Receptor-Dependent Mechanism: Cardiac Analysis of a Double-Blind Randomized Controlled Clinical Trial in Abdominally Obese Humans. Circulation. 2019;140:1684–1686. [DOI] [PubMed] [Google Scholar]
- 113.Rigamonti AE, Bollati V, Pergoli L, Iodice S, De Col A, Tamini S, Cicolini S, Tringali G, De Micheli R, Cella SG, Sartorio A. Effects of an acute bout of exercise on circulating extracellular vesicles: tissue-, sex-, and BMI-related differences. Int J Obes. 2019. doi: 10.1038/s41366-019-0460-7 [DOI] [PubMed] [Google Scholar]
- 114.Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol. 2016;12:504–17. [DOI] [PubMed] [Google Scholar]
- 115.Improta Caria AC, Nonaka CKV, Pereira CS, Soares MBP, Macambira SG, Souza BSF. Exercise Training-Induced Changes in MicroRNAs: Beneficial Regulatory Effects in Hypertension, Type 2 Diabetes, and Obesity. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arivazhagan L, Ruiz HH, Wilson RA, Manigrasso MB, Gugger PF, Fisher EA, Moore KJ, Ramasamy R, Schmidt AM. An eclectic cast of cellular actors orchestrates innate immune responses in the mechanisms driving obesity and metabolic perturbation. Circ Res. 2020;126:1565–1589. doi: 10.1161/CIRCRESAHA.120.315900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang J, Song H, Tang X, Yang Y, Vieira VJ, Niu Y, Ma Y. Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand J Med Sci Sports. 2012;22:643–52. [DOI] [PubMed] [Google Scholar]
- 118.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol. 2008;84:1271–8. [DOI] [PubMed] [Google Scholar]
- 119.Barry JC, Simtchouk S, Durrer C, Jung ME, Little JP. Short-Term Exercise Training Alters Leukocyte Chemokine Receptors in Obese Adults. Med Sci Sports Exerc. 2017;49:1631–1640. [DOI] [PubMed] [Google Scholar]
- 120.Frodermann V, Rohde D, Courties G, et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med. 2019;25:1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chuong P, Wysoczynski M, Hellmann J. Do Changes in Innate Immunity Underlie the Cardiovascular Benefits of Exercise? Front Cardiovasc Med. 2019;6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fehr HG, Lotzerich H, Michna H. Human macrophage function and physical exercise: phagocytic and histochemical studies. Eur J Appl Physiol Occup Physiol. 1989;58:613–7. [DOI] [PubMed] [Google Scholar]
- 123.Zheng JJ, Pena Calderin E, Hill BG, Bhatnagar A, Hellmann J. Exercise Promotes Resolution of Acute Inflammation by Catecholamine-Mediated Stimulation of Resolvin D1 Biosynthesis. J Immunol. 2019;203:3013–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Denou E, Marcinko K, Surette MG, Steinberg GR, Schertzer JD. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am J Physiol Endocrinol Metab. 2016;310:E982–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Welly RJ, Liu TW, Zidon TM, Rowles JL 3rd, Park YM, Smith TN, Swanson KS, Padilla J, Vieira-Potter VJ. Comparison of Diet versus Exercise on Metabolic Function and Gut Microbiota in Obese Rats. Med Sci Sports Exerc. 2016;48:1688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, Holscher HD, Woods JA. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc. 2018;50:747–757. [DOI] [PubMed] [Google Scholar]
- 127.Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee DC, Earnest CP, Church TS, O’Keefe JH, Milani RV, Blair SN. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 2015;117:207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Leblanc ES, O’Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:434–47. [DOI] [PubMed] [Google Scholar]
- 129.Yanovski SZ and Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tonstad S, Pometta D, Erkelens DW, et al. The effect of the gastrointestinal lipase inhibitor, orlistat, on serum lipids and lipoproteins in patients with primary hyperlipidaemia. Eur J Clin Pharmacol. 1994;46:405–10. [DOI] [PubMed] [Google Scholar]
- 131.Sjostrom L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, Krempf M. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352:167–72. [DOI] [PubMed] [Google Scholar]
- 132.Derosa G, Maffioli P, Sahebkar A. Improvement of plasma adiponectin, leptin and C-reactive protein concentrations by orlistat: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81:819–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. [DOI] [PubMed] [Google Scholar]
- 135.Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, Tam PY, Troupin B, Day WW. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity. 2012;20:330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–52. [DOI] [PubMed] [Google Scholar]
- 137.Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesit. 2013;21:2163–71. [DOI] [PubMed] [Google Scholar]
- 138.Richard D, Ferland J, Lalonde J, Samson P, Deshaies Y. Influence of topiramate in the regulation of energy balance. Nutrition. 2000;16:961–6. [DOI] [PubMed] [Google Scholar]
- 139.Ritchey ME, Harding A, Hunter S, Peterson C, Sager PT, Kowey PR, Nguyen L, Thomas S, Cainzos-Achirica M, Rothman KJ, Andrews EB, Anthony MS. Cardiovascular Safety During and After Use of Phentermine and Topiramate. J Clin Endocrinol Metab. 2019;104:513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Polak J, Klimcakova E, Moro C, Viguerie N, Berlan M, Hejnova J, Richterova B, Kraus I, Langin D, Stich V. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor alpha in obese women. Metabolism. 2006;55:1375–81. [DOI] [PubMed] [Google Scholar]
- 141.Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O’Neil PM, Perri MG, Pi-Sunyer FX, Rock CL, Erickson JS, Maier HN, Kim DD, Dunayevich E. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity. 2013;21:935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. [DOI] [PubMed] [Google Scholar]
- 144.Wilcox CS, Oskooilar N, Erickson JS, Billes SK, Katz BB, Tollefson G, Dunayevich E. An open-label study of naltrexone and bupropion combination therapy for smoking cessation in overweight and obese subjects. Addict Behav. 2010;35:229–34. [DOI] [PubMed] [Google Scholar]
- 145.Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067–77. [DOI] [PubMed] [Google Scholar]
- 146.O’Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J, Raether B, Anderson CM, Shanahan WR. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity. 2012;20:1426–36. [DOI] [PubMed] [Google Scholar]
- 147.Bohula EA, Wiviott SD, McGuire DK, et al. Cardiovascular Safety of Lorcaserin in Overweight or Obese Patients. N Engl J Med. 2018;379:1107–1117. [DOI] [PubMed] [Google Scholar]
- 148.Bohula EA, Scirica BM, Inzucchi SE, et al. Effect of lorcaserin on prevention and remission of type 2 diabetes in overweight and obese patients (CAMELLIA-TIMI 61): a randomised, placebo-controlled trial. Lancet. 2018;392:2269–2279. [DOI] [PubMed] [Google Scholar]
- 149.Magkos F, Nikonova E, Fain R, Zhou S, Ma T, Shanahan W. Effect of lorcaserin on glycemic parameters in patients with type 2 diabetes mellitus. Obesity. 2017;25:842–849. [DOI] [PubMed] [Google Scholar]
- 150.Xu Y, Berglund ED, Sohn JW, Holland WL, Chuang JC, Fukuda M, Rossi J, Williams KW, Jones JE, Zigman JM, Lowell BB, Scherer PE, Elmquist JK. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate insulin sensitivity in liver. Nat Neurosci. 2010;13:1457–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bays HE. Adiposopathy, diabetes mellitus, and primary prevention of atherosclerotic coronary artery disease: treating “sick fat” through improving fat function with antidiabetes therapies. Am J Cardiol. 2012;110:4B–12B. [DOI] [PubMed] [Google Scholar]
- 152.Burke LK and Heisler LK. 5-hydroxytryptamine medications for the treatment of obesity. J Neuroendocrinol. 2015;27:389–98. [DOI] [PubMed] [Google Scholar]
- 153.Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The central melanocortin system can directly regulate serum insulin levels. Endocrinology. 2000;141:3072–9. [DOI] [PubMed] [Google Scholar]
- 154.Morgan DA, McDaniel LN, Yin T, Khan M, Jiang J, Acevedo MR, Walsh SA, Ponto LL, Norris AW, Lutter M, Rahmouni K, Cui H. Regulation of glucose tolerance and sympathetic activity by MC4R signaling in the lateral hypothalamus. Diabetes. 2015;64:1976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fenwick PH, Jeejeebhoy K, Dhaliwal R, Royall D, Brauer P, Tremblay A, Klein D, Mutch DM. Lifestyle genomics and the metabolic syndrome: A review of genetic variants that influence response to diet and exercise interventions. Crit Rev Food Sci Nutr. 2019;59:2028–2039. [DOI] [PubMed] [Google Scholar]
- 157.Vorsanger MH, Subramanyam P, Weintraub HS, Lamm SH, Underberg JA, Gianos E, Goldberg IJ, Schwartzbard AZ. Cardiovascular Effects of the New Weight Loss Agents. J Am Coll Cardiol. 2016;68:849–59. [DOI] [PubMed] [Google Scholar]
- 158.O’Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, Carson CG, Jepsen CH, Kabisch M, Wilding JPH. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392:637–649. [DOI] [PubMed] [Google Scholar]
- 159.Strasser B, Arvandi M, Siebert U. Resistance training, visceral obesity and inflammatory response: a review of the evidence. Obes Rev. 2012;13:578–91. [DOI] [PubMed] [Google Scholar]
- 160.Lee S, Kuk JL, Davidson LE, Hudson R, Kilpatrick K, Graham TE, Ross R. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without Type 2 diabetes. J Appl Physiol. 2005;99:1220–5. [DOI] [PubMed] [Google Scholar]
- 161.Davies MJ, Bergenstal R, Bode B, et al. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA. 2015;314:687–99. [DOI] [PubMed] [Google Scholar]
- 162.Pi-Sunyer X, Astrup A, Fujioka K, et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med. 2015;373:11–22. [DOI] [PubMed] [Google Scholar]
- 163.Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes. 2013;37:1443–51. [DOI] [PubMed] [Google Scholar]
- 164.Sun F, Chai S, Li L, Yu K, Yang Z, Wu S, Zhang Y, Ji L, Zhan S. Effects of glucagon-like peptide-1 receptor agonists on weight loss in patients with type 2 diabetes: a systematic review and network meta-analysis. J Diabetes Res. 2015;2015:157201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fonseca VA, Devries JH, Henry RR, Donsmark M, Thomsen HF, Plutzky J. Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient-level pooled analysis of six randomized clinical trials. J Diabetes Complications. 2014;28:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 168.Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular Actions and Clinical Outcomes With Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors. Circulation. 2017;136:849–870. [DOI] [PubMed] [Google Scholar]
- 169.Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, Erqou S, Sattar N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–72. [DOI] [PubMed] [Google Scholar]
- 170.Richter G, Feddersen O, Wagner U, Barth P, Goke R, Goke B. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am J Physiol. 1993;265:L374–81. [DOI] [PubMed] [Google Scholar]
- 171.Wei R, Ma S, Wang C, Ke J, Yang J, Li W, Liu Y, Hou W, Feng X, Wang G, Hong T. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am J Physiol Endocrinol Metab. 2016;310:E947–57. [DOI] [PubMed] [Google Scholar]
- 172.Dai Y, Mehta JL, Chen M. Glucagon-like peptide-1 receptor agonist liraglutide inhibits endothelin-1 in endothelial cell by repressing nuclear factor-kappa B activation. Cardiovasc Drugs Ther. 2013;27:371–80. [DOI] [PubMed] [Google Scholar]
- 173.Chaudhuri A, Ghanim H, Vora M, Sia CL, Korzeniewski K, Dhindsa S, Makdissi A, Dandona P. Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab. 2012;97:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bunck MC, Diamant M, Eliasson B, Corner A, Shaginian RM, Heine RJ, Taskinen MR, Yki-Jarvinen H, Smith U. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care. 2010;33:1734–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hogan AE, Tobin AM, Ahern T, Corrigan MA, Gaoatswe G, Jackson R, O’Reilly V, Lynch L, Doherty DG, Moynagh PN, Kirby B, O’Connell J, O’Shea D. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia. 2011;54:2745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen WR, Chen YD, Tian F, Yang N, Cheng LQ, Hu SY, Wang J, Yang JJ, Wang SF, Gu XF. Effects of Liraglutide on Reperfusion Injury in Patients With ST-Segment-Elevation Myocardial Infarction. Circ Cardiovasc Imaging. 2016;9. [DOI] [PubMed] [Google Scholar]
- 177.Woo JS, Kim W, Ha SJ, Kim JB, Kim SJ, Kim WS, Seon HJ, Kim KS. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol. 2013;33:2252–60. [DOI] [PubMed] [Google Scholar]
- 178.Chen WR, Hu SY, Chen YD, Zhang Y, Qian G, Wang J, Yang JJ, Wang ZF, Tian F, Ning QX. Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J. 2015;170:845–54. [DOI] [PubMed] [Google Scholar]
- 179.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–13. [DOI] [PubMed] [Google Scholar]
- 180.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–51. [DOI] [PubMed] [Google Scholar]
- 181.Hausenloy DJ and Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–53. [DOI] [PubMed] [Google Scholar]
- 182.Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–10. [DOI] [PubMed] [Google Scholar]
- 183.Schwartz EA, Koska J, Mullin MP, Syoufi I, Schwenke DC, Reaven PD. Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis. 2010;212:217–22. [DOI] [PubMed] [Google Scholar]
- 184.Qin X, Shen H, Liu M, Yang Q, Zheng S, Sabo M, D’Alessio DA, Tso P. GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am J Physiol Gastrointest Liver Physiol. 2005;288:G943–9. [DOI] [PubMed] [Google Scholar]
- 185.Iacobellis G, Mohseni M, Bianco SD, Banga PK. Liraglutide causes large and rapid epicardial fat reduction. Obesity. 2017;25:311–316. [DOI] [PubMed] [Google Scholar]
- 186.Loy JJ, Youn HA, Schwack B, Kurian M, Ren Fielding C, Fielding GA. Improvement in nonalcoholic fatty liver disease and metabolic syndrome in adolescents undergoing bariatric surgery. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2015;11:442–9. [DOI] [PubMed] [Google Scholar]
- 187.Hubbard VS and Hall WH. Gastrointestinal Surgery for Severe Obesity. Obesity surgery. 1991;1:257–265. [DOI] [PubMed] [Google Scholar]
- 188.Bothe A Jr., Bistrian BR, Greenberg I, Blackburn GL. Energy regulation in morbid obesity by multidisciplinary therapy. Surg Clin North Am. 1979;59:1017–31. [DOI] [PubMed] [Google Scholar]
- 189.Andrew CA, Umashanker D, Aronne LJ, Shukla AP. Intestinal and Gastric Origins for Diabetes Resolution After Bariatric Surgery. Current obesity reports. 2018;7:139–146. [DOI] [PubMed] [Google Scholar]
- 190.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014:Cd003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Burton PR and Brown WA. The mechanism of weight loss with laparoscopic adjustable gastric banding: induction of satiety not restriction. Int J Obes. 2011;35 Suppl 3:S26–30. [DOI] [PubMed] [Google Scholar]
- 192.Miras AD and le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gstroenterol Hepatol. 2013;10:575–84. [DOI] [PubMed] [Google Scholar]
- 193.Courcoulas AP, Christian NJ, O’Rourke RW, et al. Preoperative factors and 3-year weight change in the Longitudinal Assessment of Bariatric Surgery (LABS) consortium. Surg Obes Relat Dis. 2015;11:1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Courcoulas AP, King WC, Belle SH, et al. Seven-Year Weight Trajectories and Health Outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Heffron SP, Singh A, Zagzag J, Youn HA, Underberg JA, Fielding GA, Ren-Fielding CJ . Laparoscopic gastric banding resolves the metabolic syndrome and improves lipid profile over five years in obese patients with body mass index 30-40 kg/m(2.). Atherosclerosis. 2014;237:183–90. [DOI] [PubMed] [Google Scholar]
- 196.Dixon JB OBP, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. [DOI] [PubMed] [Google Scholar]
- 197.O’Brien PE, Dixon JB, Laurie C, Skinner S, Proietto J, McNeil J, Strauss B, Marks S, Schachter L, Chapman L, Anderson M. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Int Med. 2006;144:625–33. [DOI] [PubMed] [Google Scholar]
- 198.Heffron SP, Parikh A, Volodarskiy A, Ren-Fielding C, Schwartzbard A, Nicholson J, Bangalore S. Changes in Lipid Profile of Obese Patients Following Contemporary Bariatric Surgery: A Meta-Analysis. Am J Med. 2016;129:952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Inge TH, Laffel LM, Jenkins TM, et al. Comparison of Surgical and Medical Therapy for Type 2 Diabetes in Severely Obese Adolescents. JAMA Pediatr. 2018;172:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25:1822–32. [DOI] [PubMed] [Google Scholar]
- 201.Peterli R, Wolnerhanssen BK, Peters T, Vetter D, Kroll D, Borbely Y, Schultes B, Beglinger C, Drewe J, Schiesser M, Nett P, Bueter M. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients With Morbid Obesity: The SM-BOSS Randomized Clinical Trial. JAMA. 2018;319:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Salminen P, Helmio M, Ovaska J, Juuti A, Leivonen M, Peromaa-Haavisto P, Hurme S, Soinio M, Nuutila P, Victorzon M. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA. 2018;319:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Huang R, Ding X, Fu H and Cai Q. Potential mechanisms of sleeve gastrectomy for reducing weight and improving metabolism in patients with obesity. Surg Obes Relat Dis. 2019;15:1861–1871. [DOI] [PubMed] [Google Scholar]
- 204.McCarty TR, Jirapinyo P, Thompson CC. Effect of Sleeve Gastrectomy on Ghrelin, GLP-1, PYY, and GIP Gut Hormones: A Systematic Review and Meta-analysis. Ann Surg. 2019. doi: 10.1097/SLA.0000000000003614190. [DOI] [PubMed] [Google Scholar]
- 205.Hutch CR and Sandoval D. The Role of GLP-1 in the Metabolic Success of Bariatric Surgery. Endocrinology. 2017;158:4139–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Damms-Machado A, Mitra S, Schollenberger AE, Kramer KM, Meile T, Konigsrainer A, Huson DH, Bischoff SC. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Bioced Res Int. 2015;2015:806248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. New Engl J Med. 2017;376:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Heffron SP, Lin BX, Parikh M, Scolaro B, Adelman SJ, Collins HL, Berger JS, Fisher EA. Changes in High-Density Lipoprotein Cholesterol Efflux Capacity After Bariatric Surgery Are Procedure Dependent. Arterioscler Thromb Vasc Biol. 2018;38:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang Y, Guo X, Lu X, Mattar S, Kassab G. Mechanisms of Weight Loss After Sleeve Gastrectomy and Adjustable Gastric Banding: Far More Than Just Restriction. Obesity. 2019;27:1776–1783. [DOI] [PubMed] [Google Scholar]
- 210.Sjostrom CD, Peltonen M, Wedel H, Sjostrom L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension. 2000;36:20–5. [DOI] [PubMed] [Google Scholar]
- 211.Maciejewski ML, Arterburn DE, Van Scoyoc L, Smith VA, Yancy WS Jr., Weidenbacher HJ, Livingston EH, Olsen MK. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA surgery. 2016;151:1046–1055.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311:2297–304. [DOI] [PubMed] [Google Scholar]
- 213.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. New Engl J Med. 2014;370:2002–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ferrannini E and Mingrone G. Impact of different bariatric surgical procedures on insulin action and beta-cell function in type 2 diabetes. DiabetesCcare. 2009;32:514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kashyap SR, Bhatt DL, Wolski K, Watanabe RM, Abdul-Ghani M, Abood B, Pothier CE, Brethauer S, Nissen S, Gupta M, Kirwan JP, Schauer PR. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36:2175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ramos AC, Galvao Neto MP, de Souza YM, Galvao M, Murakami AH, Silva AC, Canseco EG, Santamaria R, Zambrano TA . Laparoscopic duodenal-jejunal exclusion in the treatment of type 2 diabetes mellitus in patients with BMI<30 kg/m2 (LBMI). Obes Surg. 2009;19:307–12. [DOI] [PubMed] [Google Scholar]
- 217.Asztalos BF SM, Schaefer EJ, Dallal GE, Horvath KV, Ai M, Stanhope KL, Austrheim-Smith I, Wolfe BM, Ali M, Havel PJ. Effects of weight loss, induced by gastric bypass surgery, on HDL remodeling in obese women. J Lipid Res. 2010;51:2405–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Aron-Wisnewsky J JZ, Poitou C, Bouillot JL, Basdevant A, Chapman MJ, Clement K, Guerin M. Effect of bariatric surgery-induced weight loss on SR-BI-, ABCG1-, and ABCA1-mediated cellular cholesterol efflux in obese women. J Clin Endocrinol Metab. 2011;96:1151–1159. [DOI] [PubMed] [Google Scholar]
- 219.Osto E, Doytcheva P, Corteville C, et al. Rapid and body weight-independent improvement of endothelial and high-density lipoprotein function after Roux-en-Y gastric bypass: role of glucagon-like peptide-1. Circulation. 2015;131:871–81. [DOI] [PubMed] [Google Scholar]
- 220.Liou AP, Paziuk M, Luevano JM Jr., Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. [DOI] [PubMed] [Google Scholar]
- 222.Shen N, Caixas A, Ahlers M, Patel K, Gao Z, Dutia R, Blaser MJ, Clemente JC, Laferrere B. Longitudinal changes of microbiome composition and microbial metabolomics after surgical weight loss in individuals with obesity. Surg Obes Relat Dis. 2019;15:1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang W, Cheng Z, Wang Y, Dai Y, Zhang X, Hu S. Role of Bile Acids in Bariatric Surgery. Front Physiol. 2019;10:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Flynn CR, Albaugh VL, Cai S, Cheung-Flynn J, Williams PE, Brucker RM, Bordenstein SR, Guo Y, Wasserman DH and Abumrad NN. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun. 2015;6:7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Siddiqui MS, Van Natta ML, Connelly MA, Vuppalanchi R, Neuschwander-Tetri BA, Tonascia J, Guy C, Loomba R, Dasarathy S, Wattacheril J, Chalasani N, Sanyal AJ. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. J Hepatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Le Quellec A, Kervran A, Blache P, Ciurana AJ, Bataille D. Oxyntomodulin-like immunoreactivity: diurnal profile of a new potential enterogastrone. J Clin Endocrinol Metab. 1992;74:1405–9. [DOI] [PubMed] [Google Scholar]
- 229.Kieffer TJ and Habener JF. The glucagon-like peptides. Endocrine Rev. 1999;20:876–913. [DOI] [PubMed] [Google Scholar]
- 230.Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA, Bloom SR. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes. 2005;54:2390–5. [DOI] [PubMed] [Google Scholar]
- 231.Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127:546–58. [DOI] [PubMed] [Google Scholar]
- 232.Kim J, Lee JS, Choi J, Jung SY, Lee SH, Choi IY, Kim SJ. Novel Combination of a Long-Acting GLP-1/GIP/Glucagon Triple Agonist (HM15211) and Once-Weekly Basal Insulin (HM12460a) Offers Improved Glucose Lowering and Weight Loss in a Diabetic Animal Model. Diabetes. 2018;67:77–OR. [Google Scholar]
- 233.Finan B, Yang B, Ottaway N, et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat Med. 2012;18:1847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Quarta C, Clemmensen C, Zhu Z, et al. Molecular Integration of Incretin and Glucocorticoid Action Reverses Immunometabolic Dysfunction and Obesity. Cell Metab. 2017;26:620–632.e6. [DOI] [PubMed] [Google Scholar]
- 235.Finan B, Clemmensen C, Zhu Z, et al. Chemical Hybridization of Glucagon and Thyroid Hormone Optimizes Therapeutic Impact for Metabolic Disease. Cell. 2016;167:843–857.e14. [DOI] [PubMed] [Google Scholar]
- 236.Choi HS and Chun HJ. Recent Trends in Endoscopic Bariatric Therapies. Clin Endosc. 2017;50:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.van Baar ACG, Holleman F, Crenier L, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study. Gut. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Severin R, Sabbahi A, Mahmoud AM, Arena R, Phillips SA. Precision Medicine in Weight Loss and Healthy Living. Prog Cardiovasc Dis. 2019;62:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cottam MA, Itani HA, Beasley AA, Hasty AH. Links between Immunologic Memory and Metabolic Cycling. J Immunol. 2018;200:3681–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lee JM, Govindarajah V, Goddard B, Hinge A, Muench DE, Filippi MD, Aronow B, Cancelas JA, Salomonis N, Grimes HL, Reynaud D. Obesity alters the long-term fitness of the hematopoietic stem cell compartment through modulation of Gfi1 expression. J Exp Med. 2018;215:627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]


