Abstract
Mosquito-borne and sexual transmission of Zika virus (ZIKV), a TORCH pathogen, recently initiated a series of large epidemics throughout the Tropics. Animal models are necessary to determine transmission risk and study pathogenesis, as well screen antivirals and vaccine candidates. In this study, we modeled mosquito and sexual transmission of ZIKV in the African green monkey (AGM). Following subcutaneous, intravaginal or intrarectal inoculation of AGMs with ZIKV, we determined the transmission potential and infection dynamics of the virus. AGMs inoculated by all three transmission routes exhibited viremia and viral shedding followed by strong virus neutralizing antibody responses, in the absence of clinical illness. All four of the subcutaneously inoculated AGMs became infected (mean peak viremia: 2.9 log10 PFU/mL, mean duration: 4.3 days) and vRNA was detected in their oral swabs, with infectious virus being detected in a subset of these specimens. Although all four of the intravaginally inoculated AGMs developed virus neutralizing antibody responses, only three had detectable viremia (mean peak viremia: 4.0 log10 PFU/mL, mean duration: 3.0 days). These three AGMs also had vRNA and infectious virus detected in both oral and vaginal swabs. Two of the four intrarectally inoculated AGMs became infected (mean peak viremia: 3.8 log10 PFU/mL, mean duration: 3.5 days). vRNA was detected in oral swabs collected from both of these infected AGMs, and infectious virus was detected in an oral swab from one of these AGMs. Notably, vRNA and infectious virus were detected in vaginal swabs collected from the infected female AGM (peak viral load: 7.5 log10 copies/mL, peak titer: 3.8 log10 PFU/mL, range of detection: 5–21 days post infection). Abnormal clinical chemistry and hematology results were detected and acute lymphadenopathy was observed in some AGMs. Infection dynamics in all three AGM ZIKV models are similar to those reported in the majority of human ZIKV infections. Our results indicate that the AGM can be used as a surrogate to model mosquito or sexual ZIKV transmission and infection. Furthermore, our results suggest that AGMs are likely involved in the enzootic maintenance and amplification cycle of ZIKV.
Author summary
Zika virus (ZIKV) is primarily maintained in an enzootic cycle involving nonhuman primates and mosquitoes, with epizootics and epidemics occurring when the virus is introduced into naïve populations of nonhuman primates or humans, respectively. While, the primary transmission mechanism of the virus is by the bite on an infected mosquito, ZIKV can also be sexually transmitted. In an effort to develop novel animal models to study ZIKV disease, and to better understand the role of nonhuman primates as amplification and maintenance hosts of ZIKV in nature, we modeled mosquito-borne and sexual transmission of ZIKV in the enzootic host, the African green monkey (AGM). Infection dynamics and neutralizing antibody responses in all three AGM ZIKV models (subcutaneous, intravaginal and intrarectal) in the absence of clinical illness–recapitulated reported generalized human disease course. Furthermore, we detected prolonged shedding with high viral loads and infectious virus in the vaginal swabs collected from an infected female AGM inoculated intrarectally. Notably, these results support limited human clinical evidence that ZIKV transmission can occur during female-to-male vaginal sexual acts, and furthermore indicate the existence of ZIKV super-spreaders. Finally, our results indicate sexual transmission of ZIKV could occur among infected nonhuman primates (e.g. Chlorocebus spp.) in Africa and may serve as a secondary transmission and maintenance mechanism in the absence of mosquito-to-nonhuman primate transmission.
Introduction
Zika virus (ZIKV; family Flaviviridae, genus Flavivirus), a TORCH pathogen, recently initiated a series of large epidemics in virus-naïve tropical regions that were perpetuated by mosquito-to-human and sexual transmission [1, 2] (Fig 1). The majority of human ZIKV infections are asymptomatic (≥80.0%) [1, 3–5], thus resulting case reports severely underestimate the true burden of disease. Symptomatic cases generally display a self-limiting febrile illness with common signs and symptoms including rash, fever, arthralgia, myalgia, headache, conjunctivitis, retro-orbital pain, edema, pruritus and/or fatigue [3–5]. A subset of both asymptomatic and symptomatic infections result in severe clinical manifestations including congenital birth defects (i.e. ZIKV congenital syndrome) or Guillian-Barré syndrome [1–10]. ZIKV strains comprise at least two phylogenetic lineages, African and Asian, constituting a single serotype [11–15]. While only virus strains from the Asian lineage have been reported to be teratogenic, experimental evidence is mounting that infection with strains from either lineage can result in neurological involvement following in utero transmission [16–18]. It is therefore possible that the lack of reported congenital birth defects in Africa may be a result of misdiagnosis, underreporting, and/or ZIKV exposure prior to puberty leading to subsequent protective immunity during a woman’s reproductive years [10]. Viremia in immunocompetent adults is generally transient, with only a fraction of cases displaying vRNA in the blood for an extended period of time [19–21]. As ZIKV has been detected and/or isolated from a variety of bodily fluids including semen, vaginal secretions and saliva [20–36], these specimen types are often used in the diagnosis of active ZIKV infection.
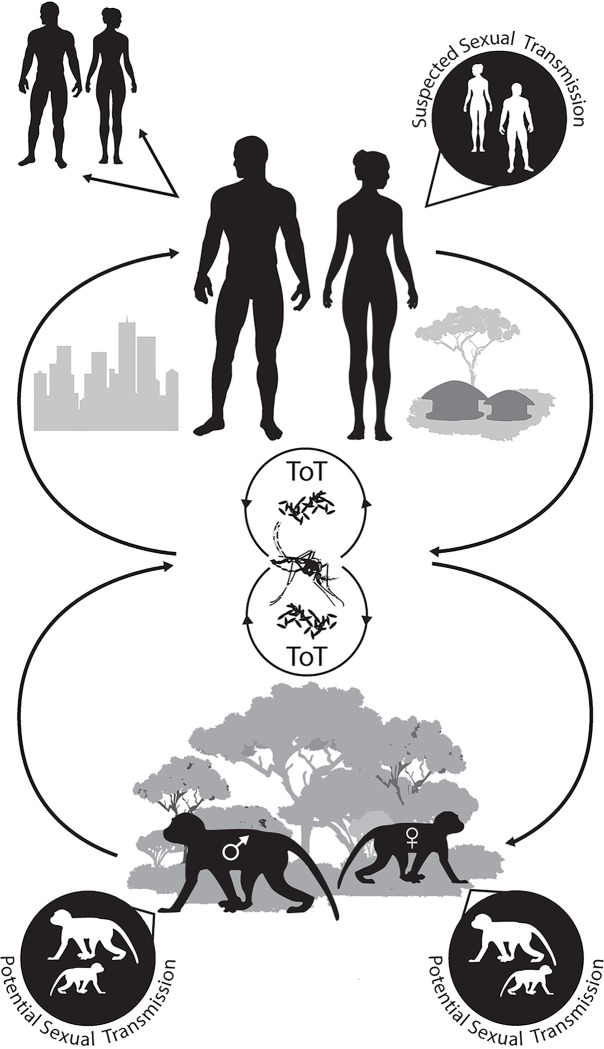
Fig 1. Zika virus transmission and maintenance cycles.
Although ZIKV is primarily transmitted through the bite of an infective mosquito, the virus is unique among flaviviruses, as it can also be sexually transmitted [37]. High vRNA loads (7.5–8.6 log10 copies/mL) have been reported in the semen of some patients [31, 36, 38–40], with virus isolations being made up to 69 days post-illness [34]. While, reported cases involving male-to-partner sexual transmission have occurred within 41 days post-illness onset [36], vRNA has been detected in the semen of previously infected males up to 9 months post-illness onset) [32, 33, 36]. Hence, prolonged viral shedding in the semen of previously infected males’ points to the potential for long-duration super-shedders. Though the majority of sexually transmitted ZIKV cases described in case reports have involve male-to-female or male-to-male transmission [37, 40–46], ZIKV has also been isolated from vaginal secretions (≥14 days post-illness) [22, 27] and vRNA has been detected for up to 180 days post-symptom onset [47], indicating the potential existence of female long-duration super-shedders. The isolation of infectious virus in vaginal secretions coupled with epidemiological case reports suggest ZIKV could potentially be transmitted from infectious females to their sexual partners [22, 27, 48]. Thus, male-to-female, male-to-male, female-to-male or female-to-female sexual transmission could result in virus transmission following the resolution of clinical illness. Nevertheless, in regions with active virus circulation this mode of transmission is generally masked by the primary transmission mode, mosquito-to-human transmission [37, 44–46]. As such, case reports involving sexual transmission primarily involve the female or male sexual partners of males who were infected with ZIKV while traveling to regions with active virus circulation [37, 40–46].
The majority of experimental animal work to date has been carried out in immunosuppressed mice to study specific aspects of transmission or viral pathogenesis [49–54], however these models are generally lethal and do not recapitulate human infection. Thus, immunocompetent nonhuman primates (NHPs) have remained the gold standard for modeling transmission risk and studying viral pathogenesis. Uniform infection is generally achieved following subcutaneous inoculation of macaques using a ZIKV dose similar to that inoculated by a mosquito bite [55–78], whereas two studies reported non-traumatic intravaginal inoculation of macaques using a dose that recapitulates human semen viral loads resulted in considerably less transmission potential [78–80]. We previously modeled ZIKV sexual transmission risk in rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques, however only 50% of the exposed macaques of each species became infected following intravaginal inoculation [79]. Researchers elsewhere investigating intravaginal ZIKV transmission reported similar results in rhesus macaques, but used repeated exposures or medroxyprogesterone, a progesterone-based contraceptive that thins the vaginal epithelium, to initiate successful transmission and subsequent infection in some resistant macaques [80]. Thus, a highly sensitive intravaginal transmission model is needed to study acute pathogenesis. As such, there is an immediate need to investigate infection dynamics in additional NHP species.
We speculated the African green monkey (AGM: Chlorocebus sabaeus) would be more sensitive to infection and display a higher transmission potential than rhesus macaques, as the geographic distribution of the AGM overlaps many regions with enzootic ZIKV circulation in Africa. Furthermore, there are multiple attributes that would make the AGM a valuable alternative to the rhesus macaque for modeling ZIKV transmission and infection. Both wild and captive AGMs are widely utilized in biomedical research, as such their biology is well characterized [81, 82]. Additionally, as Vero cells are derived from AGM kidney cells [83, 84], comparisons between in vitro and in vivo studies are more appropriate than those studies utilizing discordant cell lines and NHP species. In comparison to the rhesus macaque, the AGM is smaller and easier to handle, and their weights are generally sufficient to permit blood draws over multiple days. The gestation of the AGM is similar to that of rhesus macaques (approx. 165 days) [85], making them suitable to explore in utero ZIKV transmission and pathogenesis. Importantly, the AGM is not an endangered species, and is more easily sourced than Indian rhesus macaques.
In this study, we model mosquito and sexual transmission of ZIKV–two principle modes of virus transmission. Herein, we report ZIKV infection dynamics and moderate-duration shedding in AGMs infected by concurrent subcutaneous, intravaginal or intrarectal inoculation; and we describe a highly sensitive non-traumatic intravaginal ZIKV transmission model. We observed viremia and shedding in bodily fluids followed by a strong virus neutralizing antibody response, in the absence of overt clinical illness–infection dynamics similar to those reported in the majority of human infections. These three AGM ZIKV models will be crucial for investigating viral pathogenesis, screening antivirals and vaccine candidates, as well as providing critical data on the role of the AGM as an enzootic amplification host.
Methods and materials
Ethics statement
Research was conducted under an Institutional Animal Care and Use Committee (IACUC)-approved protocol at United States Army Medical Research Institute for Infectious Diseases (USAMRIID). This protocol complied with the Animal Welfare Act, Public Health Service Policy, and other Federal statutes and regulations relating to animals and experiments involving animals. USAMRIID is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council (2011).
Animal procedures
Adult AGMs originated from the Caribbean population located on St. Kitts. All AGMs were prescreened and determined to be negative for ZIKV, Herpes B virus, Simian-T-lymphotropic virus-1, Simian immunodeficiency virus, Simian retrovirus 1/2/3 antibodies, and Mycobacterium tuberculosis, Salmonella spp., Campylobacter spp., Hypermucoid (HVM) Klebsiella pneumoniae, and Shigella spp. infections. AGMs were individually housed throughout the duration of this study.
Study design
The goal of the study was to determine if AGMs were susceptible to ZIKV infection by subcutaneous, intravaginal or intrarectal inoculation. Sample size estimates were based on historic reports of ZIKV experimental infections involving NHPs [55–58], and the results of our recent intravaginal and intrarectal studies in rhesus and cynomolgus macaques [79]. Power analysis with the type I error rate set to 0.05 indicated that a group size of four individuals had an 80% probability to detect infection following subcutaneous, intravaginal or intrarectal inoculation with ZIKV. Experiments were carried out simultaneously to assess the potential for temporal variation in AGM infection dynamics. Inoculation doses were based on the anticipated natural ZIKV dose that would be inoculated via infective mosquito feeding (subcutaneous route), or through sexual transmission via infective semen (intravaginal and intrarectal route). Investigators were not blinded during the course of the study.
We utilized the sylvatic ArD 41525 strain of ZIKV (Genbank Accession: KU955591), which was isolated from a pool of Aedes africanus mosquitoes collected in Eastern Senegal in 1984 (passage history: AP61#1, C6/36#1, Vero #3). This isolate was kindly provided by Drs. Robert Tesh and Scott Weaver at the University of Texas Medical Branch. We selected this strain due to its low passage history, an intact N-linked glycosylation site [12, 86, 87], the results of in vivo and in vitro characterization studies [87, 88], and its ability to initiate systemic infection in rhesus and cynomolgus macaques following intravaginal or intrarectal inoculation [79]. Furthermore, this strain was isolated in a region within the known distribution of AGMs. Before study initiation, the virus challenge stock was confirmed to be pure, free of Mycoplasma spp. and its entire genome was sequenced [86].
Prior to the initiation of experimental work, AGMs were acclimatized to their study environment for a period of 14 days (Table 1). Each inoculation group was comprised of four AGMs. Two females and two males were anesthetized and then subcutaneously inoculated between the scapulas with 4.5 log10 PFU of ZIKV in 1 mL PBS. Four female AGMs were inoculated intravaginally with ZIKV as previously described [79]. Briefly, anesthetized AGMs for intravaginal inoculation were placed in dorsal recumbency with their hips elevated above their torso at a 30˚ angle. A dose of 6.3 log10 PFU of ZIKV in 2 mL of PBS was then administered to the vaginal canal using a lubricated size 7FR infant feeding tube (Mallinckrodt Pharmaceuticals, St. Louis, MO, USA). AGMs remained in dorsal recumbency with hip elevation for 20 minutes. An additional four AGMs (2 females and 2 males) were intrarectally inoculated with ZIKV as previously described [79]. Briefly, anesthetized AGMs for intrarectal inoculation were placed in an inverted Trendelenburg position (25˚ to 30˚ down angle) and a lubricated size 7FR infant feeding tube (Mallinckrodt Pharmaceuticals) was inserted 3 to 5 cm into the rectum. A 10 mL, 0.9% sodium chloride flush was administered to soften impacted fecal material lining the rectum, after which 6.5 log10 PFU of ZIKV in 3 mL of PBS was administered. AGMs stayed in dorsal recumbency with hip elevation for 20 minutes.
Table 1. Timeline of specimens collected according to study phase.
| Time | Study phase | Specimen collection schedule | Specimen type(s)* |
|---|---|---|---|
| Pre-bleed | Prior | -21 DPI | Sera |
| -14 to -1 DPI | Acclimatization | None | None |
| Pre-bleed | Prior | -7 DPI | Sera |
| 0 DPI | Inoculation | None | None |
| 1 to 15 DPI | Early | 1–7, 9, 12 and 15 DPI | Whole blood, sera and oral swabs |
| 1 to 15 DPI | Early | 2, 5, 7, 9 and 15 DPI | Vaginal swabs |
| >15 DPI | Late | 21 and 28 DPI | Whole blood, sera, oral and vaginal swabs |
DPI, days post-inoculation.
*Sera were used for the detection of infectious virus or virus-specific neutralizing antibodies. Whole blood, oral swabs, and vaginal swabs were used for the detection of vRNA. vRNA positive oral and vaginal swabs were screened for infectious virus.
Observations and sample collections
Observations and sample collection techniques have been previously described [79]. Following inoculation, AGMs were evaluated daily for signs of illness. Physical examinations were carried at days -7, 0 to 7, 9, 12, 15, 21 and 28 days post-inoculation (DPI; Table 2). Menstruation was noted during daily observations (days 0–28), but may have occurred on additional days (e.g. light or transient events). Femoral blood and oral swabs were collected daily from 1 to 7 DPI and then on 9, 12, 15, 21 and 28 DPI; and vaginal swabs were collected at 2, 5, 7, 9, 15, 21 and 28 DPI (Table 1).
Table 2. Clinical observations and physical examinations.
| Clinical observations | Physical examinations |
|---|---|
| Presence or absence of rash | Presence or absence of rash |
| Ocular evaluation | Ocular evaluation |
| Appearance of joints | Joint evaluation |
| Motor function | Oral evaluation |
| Presence or absence of blood and source | Presence or absence of lymphadenopathy |
| Presence or absence of cough | Lymph node size |
| Food consumption | Dehydration-skin test time |
| Condition of stool | Capillary refill time |
| Urine output | Presence or absence of blood and source |
| Severity of bleeding, if present | |
| Presence of absence of exudate and source | |
| Severity of exudate, if present | |
| Weight | |
| Rectal temperature |
Clinical observations were made daily from 1–28 days post-inoculation. Physical examinations were made on -7, 0–7, 9, 12, 15, 21 and 28 DPI. Lymphadenopathy was determined via manual palpation, and measured using a ruler.
Comprehensive metabolic panels were performed on serum using a Piccolo Xpress Chemistry Analyzer and Piccolo General Chemistry 13 Panel (Abbott Point of Care, Princeton, NJ, USA). Complete blood counts were performed on whole blood using a CELL-DYN 3700 system (Abbott Point of Care). Due to our sampling schedule and the weights of the AGMs, we were only able to collect a limited amount of blood at each time point. Therefore, whole blood was used to attempt detection of vRNA (RT-qPCR), and sera were used to attempt detection of viremia (plaque assay) and virus neutralizing antibodies (PRNT80) (Table 3). Oral and vaginal swabs were used to detect vRNA shedding (RT-qPCR), and positive swabs were screened by plaque assay for infectious virus (Table 3).
Table 3. Specimens and associated Zika virus assays.
*vRNA positive RT-qPCR specimens were screened by plaque assay.
Rectal temperatures were taken during physical examinations and M00 radio telemetry devices (DSI, Saint Paul, MN USA) were used to monitor temperatures in real-time throughout the study [79]. To generate a single data point every 30 seconds, temperature data points were averaged and then statistically filtered to remove noise and signal artifacts (Notocord-hem Evolution software platform, Version 4.3.0.47, Notocord Inc., Newark, NJ, USA).
Infectious virus quantification
As previously described [79], virus titration was performed on confluent Vero cell (CCL-81, ATCC, Manassas, VA, USA) monolayers in six-well plates by plaque assay. Wells were infected with serial 10-fold diluted virus in media comprised of Dulbecco's Modified Eagle Medium (Corning Life Sciences, Tewksbury, MA, USA), supplemented with 50 μg/mL gentamicin (Gibco, Carlsbad, CA, USA), 1.0 mM sodium pyruvate, 1% v/v non-essential amino acids (Sigma Aldrich, St. Louis, MO, USA); media was further supplemented with 0.5 mg/mL of amphotericin B (Gibco), 100 U/ml of penicillin (Sigma), and 100 mg/ml of streptomycin (Sigma) for oral and vaginal swab specimens for infectious virus quantification. Virus or specimens were allowed to absorb for 1 hr at 37°C and cell monolayers were overlayed with 3 mL of 1% w/v Sea-Plaque agarose (Cambrex Bio Science, East Rutherford, NJ, USA) in media. Cells were incubated at 37°C (5% CO2) for 4–5 days and then fixed with 4% formalin (Fisher Scientific, Waltham, MA, USA) in phosphate buffered saline (PBS; Corning Life Sciences) for 24 hrs. The agarose overlay was removed and cell monolayers were fixed and stained with 2% crystal violet (Sigma Aldrich) in 70% methanol (Sigma Aldrich). Excess stain was removed under running water. Results are reported as the number of plaque forming units (PFU)/mL, with a lower limit of detection of 1.0 log10 PFU/mL.
Viral RNA extraction and quantification
As previously described [79], vRNA was extracted from whole blood, saliva and vaginal specimens inactivated in TRIzol LS Reagent (Ambion) using the phenol-chloroform extraction technique. Viral RNA was then quantified using primers and a probe targeting envelope gene bases 1188–1316 [89] in a BioRad CFX96 Touch Real-Time PCR Detection System (BioRad, Hercules, CA, USA). A standard curve was generated against a synthetic oligonucleotide and genome copies were expressed as log10 copies (c)/mL, with a lower limit of detection of 3.0 log10 c/mL.
Serology
Plaque reduction neutralization tests (PRNTs) were used to determine seroconversion in AGMs inoculated with ZIKV as previously described [79]. Serum samples were first heat-inactivated at 56°C (30 min) and subsequently serially diluted 2-fold in PBS, mixed with an equal volume of 3.3 log10 PFU/mL of ZIKV after which they were incubated for 1 h at 37°C (5% CO2). Confluent Vero cell monolayers in 6-well plates were inoculated with a serum/virus mixture in triplicate. Plates were incubated at 37°C (5% CO2) for 5 days and then fixed and stained as described above. PRNT80 titers were calculated and expressed as the reciprocal of serum dilution yielding a >80% reduction in the number of plaques. At -21 DPI, none of the AGMs had titers of 1:20 or higher, indicating these animals had not been previously infected with ZIKV. Post-exposure sera were screened on 7, 15, 21 and 28 DPI for neutralizing antibodies. We considered those AGMs with a four-fold or greater rise in titers to have seroconverted (≥1:160).
Results
General observations
Throughout the course of the study, none of the infected AGMs exhibited rash, ocular abnormalities, joint swelling, decreased motor function, cough, changes in urine output, abnormal stool, decreased food consumption, pyrexia or weight loss (S1, S2, S3, S4 and S5 Figs). However, acute lymphadenopathy was observed in several AGMs (Table 4 and S6 Fig). While the study lacked the statistical power to resolve variations in laboratory values, we observed abnormal values indicative of infection in some AGMs (Table 4; S7, S8 and S9 Figs). Viremia, as measured by plaque assay, was seen in all infected AGMs with the exception of a single female AGM who seroconverted following intravaginal inoculation (Table 5). Viral shedding was observed in the saliva of all AGMs who had detectable vRNA in the whole blood (Fig 2 and Table 6), and virus was isolated in a subset of these vRNA positive specimens (Fig 2). In addition, prolonged viral shedding with high viral loads/infectious virus were detected/isolated in the vaginal swabs of a female AGM inoculated intrarectally (Fig 2 and Table 6). With the exception of the female AGM who seroconverted in the absence of detectable viremia or vRNA, all infected AGMs displayed robust virus neutralizing antibody titers (Table 5). We observed a 1:1 ratio of male to female AGMs infected by the subcutaneous or intrarectal routes, indicating a similar susceptibility to ZIKV infection for both male and female AGMs.
Table 4. Abnormal laboratory results and clinical observations, days 1 to 15 post-inoculation.
| Animal | Route | Sex | Red blood cell parameters* | White blood cell parameters | Glucose† | Electrolytes* | Liver function† | Kidney function† | Pancreatic function† | Acute lymphadenopathy |
|---|---|---|---|---|---|---|---|---|---|---|
| AGM 1 | SC | F | RBC, HBC, HCT | %EOS (high) | – | – | ALP, AST | – | – | Axillary |
| AGM 2 | SC | F | RBC, HBC, HCT | %LYMPH (high) | – | – | AST, ALT | – | – | Axillary |
| AGM 3 | SC | M | – | – | – | CA | AST, ALT, TBIL | – | – | Axillary |
| AGM 4 | SC | M | – | – | – | – | AST, ALT, TBIL | CRE | – | – |
| AGM 5 | IVAG | F | – | %MONO (high) | GLU | CA | AST | – | – | Inguinal |
| AGM 6 | IVAG | F | – | WBC (high) | CA | AST | – | – | Axillary, Inguinal | |
| AGM 7 | IVAG | F | – | %NEUT (low), %LYMPH (high) | GLU | AST | – | AMY | Inguinal | |
| AGM 8 | IVAG | F | RBC, HBC, HCT | GLU | CA | AST, ALT | – | – | – | |
| AGM 9 | IR | F | RBC, HBC, HCT | WBC (high), %LYMPH (high) | GLU | CA | ALP, AST, ALT | – | – | – |
| AGM 10 | IR | F | RBC, HBC, HCT | – | – | – | ALP, AST, ALT | BUN | – | – |
| AGM 11 | IR | M | – | – | – | – | ALP, AST, TBIL | – | – | – |
| AGM 12 | IR | M | – | – | – | CA | ALP, AST, ALT, TBIL | CRE | – | – |
*Low.
†Elevated.
Red blood cell parameters: RBC (Red blood cell count), HGB (Hemoglobin), HCT (Hematocrit)
White blood cell parameters: WBC (White blood cell count), %NEUT (% Neutrophils), %LYMP (% Lympocytes), %MONO (% Monocytes), %BASO (% Basophils), %EOS (% Eosinophils)
Glucose: GLU (Glucose)
Electrolytes: CA (Calcium)
Liver function: ALP (Alkaline phosphatase), ALT (Alanine aminotransferase), AST (Aspartate aminotransferase), TBIL (Total bilirubin), GGT (Gamma glutamyltransferase)
Kidney function: BUN (Blood urea nitrogen), CRE (Creatinine)
Pancreatic function: AMY (Amylase)
No animals exhibited abnormal platelet or protein values
Table 5. Virus neutralizing antibody titers in African green monkeys according to route of Zika virus inoculation and sex.
| Animal | Route | Sex | Serological response† (PRNT80) DPI |
|||
|---|---|---|---|---|---|---|
| 7 | 15 | 21 | 28 | |||
| AGM 1 | SC | F | – | 1:640 | 1:1280 | 1:1280 |
| AGM 2 | SC | F | – | 1:1280 | 1:2560 | 1:2560 |
| AGM 3 | SC | M | – | 1:640 | 1:640 | 1:1280 |
| AGM 4 | SC | M | – | 1:640 | 1:640 | 1:640 |
| AGM 5 | IVAG | F | – | 1:320 | 1:1280 | 1:1280 |
| AGM 6 | IVAG | F | – | 1:80 | 1:640 | 1:1280 |
| AGM 7 | IVAG | F | – | 1:40 | 1:320 | 1:1280 |
| AGM 8 | IVAG | F | – | 1:40 | 1:80 | 1:160 |
| AGM 9 | IR | F | – | – | – | – |
| AGM 10 | IR | F | – | 1:80 | 1:1280 | 1:1280 |
| AGM 11 | IR | M | – | – | – | – |
| AGM 12 | IR | M | – | 1:320 | 1:1280 | 1:1280 |
DPI, Day post-inoculation. Route: SC, subcutaneous; IVAG, intravaginal; IR, intrarectal.
–<1:20. * Limit of detection 1.0 log10 PFU/mL.
† Limit of detection 1:20.
Fig 2. Viremia and viral RNA detected in bodily fluids by day post-inoculation.
Viremia and viral RNA detected in subcutaneously inoculated African green monkeys (panel A, B, C and D); intravaginally inoculated African green monkeys (panel E, F, G and H); intrarectally inoculated African green monkeys (panel I, J, K and L). Circles and/or dotted lines connecting points represent genome copies in log10 c/mL. Squares and/or solid lines connecting points represent virus titers in log10 PFU/mL. The lower limit of detection for genome copies was 3.0 log10 c/mL. The lower limit of detection for virus titers was 1.0 log10 PFU/mL.
Table 6. Detection of viremia, viral RNA and virus-specific neutralizing antibodies in Zika virus-infected African green monkeys.
| Number infected according to sample type/total number infected (Percent) | ||||||
|---|---|---|---|---|---|---|
| Inoculation group | Number infected/total (Percent) * | Sera (Plaque assay) |
Sera (PRNT80) |
Whole blood (RT-qPCR) |
Oral swabs (RT-qPCR) |
Vaginal swabs (RT-qPCR) |
| All animals | 10/12 (83.3) | 9/10 (90.0) | 9/10 (90.0) | 9/10 (90.0) | 9/10 (90.0) | 4/7 (57.1) |
| Subcutaneous | ||||||
| Males | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2/2 (100) | N/A |
| Females | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2/2 (100) | 0/2 (0.0) |
| Intravaginal | ||||||
| Females | 4/4 (100) | 3/4 (75.0) | 4/4 (100) | 3/4 (75.0) | 3/4 (75.0) | 3/4 (75.0) |
| Intrarectal | ||||||
| Males | 1/2 (50.0) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) | N/A |
| Females | 1/2 (50.0) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) |
*As confirmed by titration of infectious virus by plaque assay, detection of vRNA by RT-qPCR, and/or detection of virus-specific neutralizing antibodies by PRNT80. N/A = Not applicable
The numerical data used to create Fig 2 is included in S1 Data.
Subcutaneously inoculated animals
Viremia, viral shedding, and seroconversion
Viremia (infectious virus) was detected in all AGMs (Fig 2) ranging from 1 to 7 DPI (viremia mean duration: 4.3 d), with a mean peak titer of 2.9 log10 PFU/mL. vRNA was also detected in the blood of all AGMs ranging from 1 to 7 DPI (mean duration 5.0 d) (Fig 2), with a mean peak vRNA load of 6.2 log10 c/mL. AGM3 displayed the highest detectable viremia at 6 DPI (3.3 log10 PFU/mL), while AGM2 displayed the highest detectable vRNA load in the blood at 5 DPI (6.3 log10 c/mL). All four AGMs had vRNA detected in oral swabs (Fig 2), with a mean peak vRNA load of 5.2 log10 c/mL. vRNA was first detected in the oral swab of AGM1 at 3 DPI and was detected up to 15 DPI in AGM3. Infectious virus was isolated from oral swabs in AGM1 at 3 and 4 DPI (1.0 log10 PFU/mL both days), AGM2 at 2 and 7 DPI (1.3 log10 PFU/mL and 1.6 log10 PFU/mL, respectively), AGM3 at 7 DPI (1.0 log10 PFU/mL), and AGM4 at 5 and 7 DPI (1.7 log10 PFU/mL and 1.0 log10 PFU/mL, respectively). vRNA was not detected in any vaginal swab from the two female AGMs. By 15 DPI, all AGMs had seroconverted (Table 5).
Clinical observations and laboratory results
Acute lymphadenopathy involving the left and right axillary lymph nodes was observed in AGM1 and AGM2 at 15 DPI, and AGM3 on 12 DPI (Table 4 and S6 Fig). Potentially clinically significant marked increases or decreases in laboratory values observed in infected animals for at least two days during the study period were: alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, calcium, creatinine, eosinophils, hematocrit, hemoglobin, lymphocytes, neutrophils, red blood cell count, total bilirubin (Table 4 and S7 Fig).
Intravaginally inoculated animals
Viremia, viral shedding, and seroconversion
Viremia (infectious virus) was detected in 75% of AGMs (Fig 2) between 5 and 7 DPI (viremia mean duration: 3.0 d), with a mean peak titer in the sera of 4.0 log10 PFU/mL. vRNA was detected in the blood of all viremic AGMs ranging from 5 to 12 DPI (mean duration 4.3 d) (Fig 2), with a mean peak vRNA load of 6.9 log10 c/mL. AGM5 displayed the highest detectable viremia and vRNA load in the blood at 7 DPI, reaching a titer of 4.4 log10 PFU/mL and a vRNA load of 7.3 log10 c/mL. vRNA was first detected in the oral swab of AGM6 at 6 DPI and was detectable in AGM6 and AGM7 through 12 DPI, while vRNA was detected in oral swabs of AGM5 at 7 and 9 DPI (Fig 2). The mean peak vRNA load in oral swabs was 5.2 log10 c/mL. Infectious virus was detected in oral swabs from AGM5 at 7 and 9 DPI (2.0 log10 PFU/mL and 1.3 log10 PFU/mL, respectively), AGM6 at 7, 9 and 12 DPI (1.0 log10 PFU/mL, all days), AGM7 at 12 DPI (1.3 log10 PFU/mL), and AGM7 at 12 DPI (1.3 log10 PFU/mL). vRNA was detected in the vaginal swabs of AGM5, AGM6 and AGM7 by 2 DPI (Fig 2), with a mean peak vRNA load of 4.7 log10 c/mL. Infectious virus was detected in vaginal swabs from AGM5 at 2 and 5 DPI (1.0 log10 PFU/mL, both days), AGM6 at 2 and 5 DPI (1.5 log10 PFU/mL and 1.0 log10 PFU/mL, respectively), and AGM7 at 2 DPI (1.3 log10 PFU/mL). Although we did not detect viremia or vRNA in AGM8, all AGMs seroconverted by 15 DPI (Table 5).
Clinical observations and laboratory results
Acute lymphadenopathy was observed in viremic AGMs at various times during the study period (Table 4 and S6 Fig). At 4 DPI, 75% of the AGMs (AGM5, AGM6 and AGM7) displayed lymphadenopathy involving the inguinal lymph nodes. This was a transient event in AGM5 and AGM7 lasting a single day. However, AGM6 continued to display lymphadenopathy involving the axillary or inguinal lymph nodes at multiple time points. Menstruation was observed in AGM8 during inoculation, as well as at 3 and 28 DPI; but was not observed in AGM5, AGM6 and AGM7 at inoculation or post-inoculation. Potentially clinically significant marked increases or decreases in laboratory values observed in infected animals for at least two days during the study period were: alanine aminotransferase, amylase, aspartate aminotransferase, calcium, glucose, hematocrit, hemoglobin, lymphocytes, monocytes, neutrophils, red blood cell count, and white blood cell count (Table 4 and S8 Fig).
Intrarectally inoculated animals
Viremia, viral shedding, and seroconversion
Viremia (infectious virus) was detected in 50% of AGMs (Fig 2) ranging from 4 to 7 DPI (viremia mean duration: 3.5 d), with a mean peak titer of 3.8 log10 PFU/mL. vRNA was detected in the blood of viremic AGMs ranging from 4 to 9 DPI (mean duration 4.5 d) (Fig 2), with a mean peak vRNA load of 6.9 log10 c/mL. AGM10 displayed the highest detectable viremia and vRNA load in the blood at 7 DPI, reaching a titer of 4.1 log10 PFU/mL and a vRNA load of 7.2 log10 c/mL. vRNA was detected in the oral swabs from AGM10 at 6 DPI and AGM12 at 7 DPI (Fig 2), with a mean peak vRNA load of 4.5 log10 c/mL. Infectious virus was detected in AGM10s oral swab at 6 DPI (1.3 log10 PFU/mL). AGM10 had prolonged vRNA and infectious virus detected in vaginal swabs from 5 to 21 DPI (Fig 2), with a peak vRNA load of 7.5 log10 c/mL and a titer of 3.8 log10 PFU/mL at 7 DPI. By 15 DPI, all AGMs with detectable viremia seroconverted (Table 5).
Clinical observations and laboratory results
Menstruation was observed in AGM10 at 7, 9, 12, 15, 21 and 28 DPI. Potentially clinically significant marked increases or decreases in laboratory values observed in infected animals for at least two days during the study period were: alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, blood urea nitrogen, creatinine, calcium, hematocrit, hemoglobin, red blood cell count and total bilirubin (Table 4 and S9 Fig).
Discussion
The speed by which ZIKV spread once being introduced into the New World is uncommon among arboviruses, which are generally maintained in transmission cycles involving arthropods and virus amplification hosts in the absence of sexual transmission. The ability of a TORCH pathogen to be transmitted by hematophagous arthropod vectors requires sensitive animal models to study transmission risk and viral pathogenesis, as well as to screen antivirals and vaccine candidates. In this study, we report ZIKV infection dynamics and viral shedding in AGMs infected via subcutaneous, intravaginal or intrarectal inoculation to model mosquito-borne and sexual transmission. Our results indicate that ZIKV infection of AGMs by these routes, strain and doses produce a mild asymptomatic infection characterized by viremia, viral shedding and a strong virus neutralizing antibody response–recapitulating generalized human disease course.
Although infection by all three routes resulted in similar viremia profiles, the onset of viremia was delayed by two to three days in intravaginally and intrarectally inoculated AGMs compared to subcutaneously inoculated AGMs. A similar delay in viremia has been previously reported in ZIKV sexual transmission experiments involving NHPs [78–80]. Of interest, we detected prolonged oral shedding and the presence of infectious virus in subcutaneously and intravaginally inoculated AGMs. While experimental evidence indicates there is less potential for transmission via infectious saliva [63], super-shedders could potentiate this mode of transmission [90, 91]. Our results are similar to the majority of human case reports, which describe a generally asymptomatic infection despite low-level viremias and the detection of vRNA shedding in bodily fluids.
Even though the clinical signs and symptoms associated with ZIKV infection are now well defined, there is little published data on laboratory values of clinical interest. Notwithstanding, a recent well-documented case series involving 18 patients reported elevated liver enzymes [92]. Similar to these findings and previous NHP studies [59, 60, 79, 93], a subset of ZIKV-infected AGMs in this study displayed elevated liver enzymes consistent with acute liver involvement that resolved following the clearance of viremia. While it is also possible repeated anesthesia may have influenced some laboratory values [94], it is important to note that human infection with Spondweni virus, the closest related virus to ZIKV [15], has been reported to result in acute liver injury in some patients [10, 95]. Although reported cases of liver involvement among ZIKV patients maybe uncommon, patients with liver disease or those with comorbidities that impact liver function should be monitored for acute liver injury.
While ZIKV-infected AGMs did not display overt signs of disease, lymphadenopathy was observed in some AGMs during physical examinations. We found that the route of inoculation coincided with external lymphatic drainage, associated with axillary (subcutaneous route) and/or inguinal (intravaginal route) lymphadenopathy. Although we did not observe acute axillary or inguinal lymphadenopathy in AGMs infected via the intrarectal route, we speculate that infection could result in intra-abdominal lymph node changes. However, abdominal palpation would likely be insufficiently sensitive to detect minor-to-moderately enlarged lymph nodes in the abdomen and may require the use of ultrasound imaging. To our knowledge lymphadenopathy has not been previously reported in ZIKV-experimentally infected NHPs, though several studies reported vRNA persistence in lymphoid tissues [62, 65, 76, 78, 80, 96, 97].
Sexual transmission likely accounts for a substantial number of asymptomatic ZIKV cases [44–46], however recent NHP research has primarily focused on modeling mosquito and in utero ZIKV transmission rather than investigating sexual transmission risk and its resulting viral pathogenesis [14, 59, 61, 64, 65, 70–75, 98]. Previous work in macaques demonstrated high rates of infection following intravaginal or intrarectal inoculation [78–80]. In this study, we demonstrated that AGMs are highly sensitive to ZIKV infection following intravaginal inoculation. Infection with ZIKV through vaginal secretions has been identified as a possible transmission mode, and there is at least one well documented case of suspected female-to-male transmission [48]. In this study, a female AGM infected intrarectally displayed high vRNA loads and prolonged shedding of infectious virus in vaginal secretions, supporting clinical and epidemiological evidence of transmission to a sexual partner via this route. While the rate of intrarectal transmission was not as high as that observed in the intravaginally inoculated AGMs, it is important to remember that inoculation was by a one-time, non-traumatic inoculation event; thus repeated exposures, rectal trauma resulting in micro-tears during intercourse and/or the presence of co-infections could increase the chances of viral transmission [79].
The susceptibility of AGMs via all three-transmission routes has implications for zoonotic virus amplification and maintenance. The geographical range of Chlorocebus spp. overlaps with multiple sylvatic ZIKV mosquito vectors [1, 12, 81, 99]. Furthermore, Chlorocebus spp., including the AGM, are known to inhabit locations with reported ZIKV epizootics [56, 99–103]. The observed viremias among all infected AGMs in this study were sustained and likely reached titers needed to infect a portion of the principle sylvatic mosquito vectors [77]. In addition to mosquito transmission, the sensitivity to infection via intravaginal or intrarectal routes indicates the potential for sexual transmission between AGMs. Mating behaviors within AGM populations could exacerbate this potential as dominant males mate with the majority of females in a group and have multiple mating encounters with each female during the mating season [104]. Viral shedding in the vaginal secretions of female AGMs would also increase the chance of mucosal transmission during grooming, male-to-female mating, female-to-female genital investigation or female-to-female rubbing–all of which have been observed in Chlorocebus spp. in the wild [105]. Male-to-male mounting or anal penetration has been reported among various NHP species [105, 106], and may serve as another potential transmission mode.
Our results and recent work demonstrating ZIKV sexual transmission in macaques [79, 80], support the possibility that sexual transmission may be more common than previously suggested among various NHP species involved in ZIKV enzootic cycles. Furthermore, recent work in macaques demonstrated the potential for oropharyngeal ZIKV transmission [63]. It is therefore possible that in nature, grooming or biting by infectious AGMs shedding virus in the saliva may serve as a tertiary transmission mode, as reported in SIV transmission between AGMs [107, 108]. Super-spreaders [109, 110], could further potentiate ZIKV transmission through grooming, biting or sexual transmission. Moreover, dispersion by infected males or troop-to-troop contact could consequently initiate epizootics in virus-naïve areas or groups. Such a cycle, coupled with transovarial transmission [99, 111] and the movement of infected mosquitoes in air currents above canopies [112–116], may partially explain the long periods of increased ZIKV enzootic activity reported in Uganda and in Senegal [99, 102]. Furthermore, the wide range of ecological niches Chlorocebus spp. inhabit, coupled with their broad geographic distribution in Africa would increase the likelihood of virus spillover events into human populations involving terrestrial amplification hosts/mosquito vector species.
Our study has some limitations. This was a pilot study designed to investigate the susceptibility of AGMs to ZIKV infection following subcutaneous, intravaginal or intrarectal inoculation; consequently, the study was not designed with the statistical power to perform statistical analyses of chemistry, hematology or temperature data. Nevertheless, we were able to infer ZIKV-associated transient hepatic involvement based on elevated transaminase levels, similar to studies reported elsewhere [59, 60, 79, 93]. While a single uninfected intrarectally inoculated AGM displayed a substantial increase in transaminases, this AGM had historically displayed liver enzyme levels (alanine aminotransferase, alkaline phosphatase and aspartate aminotransferase) at the high-end of normal or slightly above normal; and gamma-glutamyl transferase levels observed during the study were consistent with this AGMs historic values pre-exposure. Though we were able to detect acute axial and inguinal lymphadenopathy in the majority of viremic AGMs (SC and IVAG), manual abdominal examination may have failed to reveal lymphadenopathy in the abdomen of AGMs. Therefore, future studies should consider the use of ultrasound imaging, which could also be used to detect other transient pathologic changes associated with ZIKV infection. Although infection dynamics and the lack of overt clinical signs among infected AGMs are similar to those reported in the majority of human infections, it is possible that we did not observe the full spectrum of disease presentation (i.e. severe disease) due to the size of our animal cohorts.
In summary, we report the first subcutaneous, intravaginal and intrarectal models of ZIKV infection in AGMs that recapitulates infection dynamics and lymphadenopathy reported in human cases–providing a single, easily-sourced NHP species to model mosquito and sexual ZIKV transmission. These models will be critical for investigating viral pathogenesis, as well as screening antivirals and vaccine candidates. Additionally, our results indicate the AGM is an enzootic amplification host and sexual transmission between AGMs may contribute to the maintenance of ZIKV in nature.
Supporting information
(XLSX)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(TIF)
(TIF)
(TIF)
Acknowledgments
The authors wish to thank Drs. Sarah Norris and Samuel Washington for their assistance regarding statistical power analysis, and Mr. John Braun for his illustrations. The views expressed in this article are those of the authors and do not reflect the official policy or position of the U.S. Department of Defense or the U.S. Army.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the Defense Advanced Research Projects Agency (DARPA), grant number A718-ISA to MLMP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Aliota MT, Bassit L, Bradrick SS, Cox B, Garcia-Blanco MA, Gavegnano C, et al. Zika in the Americas, year 2: What have we learned? What gaps remain? A report from the Global Virus Network. Antiviral Research. 2017;144:223–46. 10.1016/j.antiviral.2017.06.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coyne CB, Lazear HM. Zika virus—reigniting the TORCH. Nature Reviews Microbiology. 2016;14(11):707–15. 10.1038/nrmicro.2016.125 . [DOI] [PubMed] [Google Scholar]
- 3.Musso D, Gubler DJ. Zika Virus. Clinical Microbiology Reviews. 2016;29(3):487–524. 10.1128/CMR.00072-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. The New England journal of medicine. 2016. 10.1056/NEJMra1602113 . [DOI] [PubMed] [Google Scholar]
- 5.Plourde AR, Bloch EV. A literature review of Zika virus. Emerging Infectious Diseases. 2016;22(7). 10.3201/eid2207.151990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, et al. Zika virus associated with microcephaly. The New England Journal of Medicine. 2016. 10.1056/NEJMoa1600651 . [DOI] [PubMed] [Google Scholar]
- 7.Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Eurosurveillance. 2014;19(9). 10.2807/1560-7917.es2014.19.9.20720 . [DOI] [PubMed] [Google Scholar]
- 8.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016. 10.1016/S0140-6736(16)00562-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Araujo TV, Rodrigues LC, de Alencar Ximenes RA, de Barros Miranda-Filho D, Montarroyos UR, de Melo AP, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. The Lancet Infectious Diseases. 2016. 10.1016/S1473-3099(16)30318-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddow AD, Woodall JP. Distinguishing between Zika and Spondweni viruses. Bulletin of the World Health Organization. 2016;94(10):711-A 10.2471/BLT.16.181503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowd KA, DeMaso CR, Pelc RS, Speer SD, Smith AR, Goo L, et al. Broadly neutralizing activity of Zika virus-immune sera identifies a single viral serotype. Cell Reports. 2016;16(6):1485–91. 10.1016/j.celrep.2016.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Neglected Tropical Diseases. 2012;6(2):e1477 10.1371/journal.pntd.0001477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. The American Journal of Tropical Medicine and Hygiene. 1969;18(3):411–5. 10.4269/ajtmh.1969.18.411 . [DOI] [PubMed] [Google Scholar]
- 14.Aliota MT, Dudley DM, Newman CM, Mohr EL, Gellerup DD, Breitbach ME, et al. Heterologous protection against Asian Zika virus challenge in rhesus macaques. PLoS Neglected Tropical Diseases. 2016;10(12):e0005168 10.1371/journal.pntd.0005168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haddow AD, Nasar F, Guzman H, Ponlawat A, Jarman RG, Tesh RB, et al. Genetic characterization of Spondweni and Zika viruses and susceptibility of geographically distinct strains of Aedes aegypti, Aedes albopictus and Culex quinquefasciatus (Diptera: Culicidae) to Spondweni virus. PLoS Neglected Tropical Diseases. 2016;10(10):e0005083 10.1371/journal.pntd.0005083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenfeld AB, Doobin DJ, Warren AL, Racaniello VR, Vallee RB. Replication of early and recent Zika virus isolates throughout mouse brain development. Proceedings of the National Academy of Sciences of the United States of America; 2017;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaeger AS, Murrieta RA, Goren LR, Crooks CM, Moriarty RV, Weiler AM, et al. Zika viruses of African and Asian lineages cause fetal harm in a mouse model of vertical transmission. PLoS Neglected Tropical Diseases. 2019;13(4):e0007343 Epub 2019/04/18. 10.1371/journal.pntd.0007343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Udenze D, Trus I, Berube N, Gerdts V, Karniychuk U. The African strain of Zika virus causes more severe in utero infection than Asian strain in a porcine fetal transmission model. Emerging Microbes & Infections. 2019;8(1):1098–107. Epub 2019/07/26. 10.1080/22221751.2019.1644967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansuy JM, Mengelle C, Pasquier C, Chapuy-Regaud S, Delobel P, Martin-Blondel G, et al. Zika virus infection and prolonged viremia in whole-blood specimens. Emerging Infectious Diseases. 2017;23(5):863–5. 10.3201/eid2305.161631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lustig Y, Mendelson E, Paran N, Melamed S, Schwartz E. Detection of Zika virus RNA in whole blood of imported Zika virus disease cases up to 2 months after symptom onset, Israel, December 2015 to April 2016. Eurosurveillance. 2016;21(26). 10.2807/1560-7917.ES.2016.21.26.30269 . [DOI] [PubMed] [Google Scholar]
- 21.Rossini G, Gaibani P, Vocale C, Cagarelli R, Landini MP. Comparison of Zika virus (ZIKV) RNA detection in plasma, whole blood and urine—Case series of travel-associated ZIKV infection imported to Italy, 2016. The Journal of Infection. 2017;75(3):242–5. 10.1016/j.jinf.2017.05.021 . [DOI] [PubMed] [Google Scholar]
- 22.Nicastri E, Castilletti C, Balestra P, Galgani S, Ippolito G. Zika virus infection in the central nervous system and female genital tract. Emerging Infectious Diseases. 2016;22(12):2228–30. 10.3201/eid2212.161280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, et al. Zika virus in the female genital tract. The Lancet Infectious Diseases. 2016;16(9):1000–1. 10.1016/S1473-3099(16)30193-1 . [DOI] [PubMed] [Google Scholar]
- 24.Penot P, Brichler S, Guilleminot J, Lascoux-Combe C, Taulera O, Gordien E, et al. Infectious Zika virus in vaginal secretions from an HIV-infected woman, France, August 2016. Eurosurveillance. 2017;22(3). 10.2807/1560-7917.ES.2017.22.3.30444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prisant N, Breurec S, Moriniere C, Bujan L, Joguet G. Zika virus genital tract shedding in infected women of shildbearing age. Clinical Infectious Diseases. 2017;64(1):107–9. 10.1093/cid/ciw669 . [DOI] [PubMed] [Google Scholar]
- 26.Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. Journal of Clinical Virology. 2015;68:53–5. 10.1016/j.jcv.2015.04.021 . [DOI] [PubMed] [Google Scholar]
- 27.Murray KO, Gorchakov R, Carlson AR, Berry R, Lai L, Natrajan M, et al. Prolonged Detection of Zika virus in vaginal secretions and whole blood. Emerging Infectious Diseases. 2017;23(1):99–101. 10.3201/eid2301.161394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barzon L, Pacenti M, Berto A, Sinigaglia A, Franchin E, Lavezzo E, et al. Isolation of infectious Zika virus from saliva and prolonged viral RNA shedding in a traveller returning from the Dominican Republic to Italy, January 2016. Eurosurveillance. 2016;21(10). 10.2807/1560-7917.ES.2016.21.10.30159 . [DOI] [PubMed] [Google Scholar]
- 29.Bonaldo MC, Ribeiro IP, Lima NS, Dos Santos AA, Menezes LS, da Cruz SO, et al. Isolation of infective Zika virus from urine and saliva of patients in Brazil. PLoS Neglected Tropical Diseases. 2016;10(6):e0004816 10.1371/journal.pntd.0004816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, et al. Persistence of Zika virus in body fluids—Preliminary report. The New England Journal of Medicine. 2017. 10.1056/NEJMoa1613108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mansuy JM, Suberbielle E, Chapuy-Regaud S, Mengelle C, Bujan L, Marchou B, et al. Zika virus in semen and spermatozoa. The Lancet Infectious Diseases. 2016;16(10):1106–7. 10.1016/S1473-3099(16)30336-X . [DOI] [PubMed] [Google Scholar]
- 32.Nicastri E, Castilletti C, Liuzzi G, Iannetta M, Capobianchi M, Ippolito G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Eurosurveillance. 2016;21 10.2807/1560-7917.ES.2016.21.32.30314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barzon L, Pacenti M, Franchin E, Lavezzo E, Trevisan M, Sgarabotto D, et al. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy, January 2016. Eurosurveillance. 2016;21 10.2807/1560-7917.ES.2016.21.32.30316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arsuaga M, Bujalance SG, Diaz-Menendez M, Vazquez A, Arribas JR. Probable sexual transmission of Zika virus from a vasectomised man. The Lancet Infectious Diseases. 2016;16(10):1107 10.1016/S1473-3099(16)30320-6 . [DOI] [PubMed] [Google Scholar]
- 35.Gourinat AC, O'Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerging Infectious Diseases. 2015;21(1):84–6. 10.3201/eid2101.140894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mead PS, Duggal NK, Hook SA, Delorey M, Fischer M, Olzenak McGuire D, et al. Zika Virus shedding in semen of symptomatic infected men. The New England Journal of Medicine. 2018;378(15):1377–85. 10.1056/NEJMoa1711038 . [DOI] [PubMed] [Google Scholar]
- 37.Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerging Infectious Diseases. 2011;17(5):880–2. 10.3201/eid1705.101939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? The Lancet Infectious Diseases. 2016;16(4):405 10.1016/S1473-3099(16)00138-9 . [DOI] [PubMed] [Google Scholar]
- 39.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerging Infectious Diseases. 2015;21(2):359–61. 10.3201/eid2102.141363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D'Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, et al. Evidence of sexual transmission of Zika virus. The New England Journal of Medicine. 2016;374(22):2195–8. 10.1056/NEJMc1604449 . [DOI] [PubMed] [Google Scholar]
- 41.Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—Continental United States, 2016. Morbidity and Mortality Weekly Report. 2016;65(8):215–6. 10.15585/mmwr.mm6508e2 . [DOI] [PubMed] [Google Scholar]
- 42.Brooks RB, Carlos MP, Myers RA, White MG, Bobo-Lenoci T, Aplan D, et al. Likely sexual transmission of Zika virus from a man with no symptoms of infection—Maryland, 2016. Morbidity and Mortality Weekly Report. 2016;65:Early Release. [DOI] [PubMed] [Google Scholar]
- 43.Frank C, Cadar D, Schlaphof A, Neddersen N, Gunther S, Schmidt-Chanasit J, et al. Sexual transmission of Zika virus in Germany, April 2016. Eurosurveillance. 2016;21(23). 10.2807/1560-7917.ES.2016.21.23.30252 . [DOI] [PubMed] [Google Scholar]
- 44.Moreira J, Peixoto TM, Siqueira Md, Lamas CC. Sexually acquired Zika virus: a systematic review. Clinical Microbiology and Infection. 2017. 10.1016/j.cmi.2016.12.027 [DOI] [PubMed] [Google Scholar]
- 45.Grischott F, Puhan M, Hatz C, Schlagenhauf P. Non-vector-borne transmission of Zika virus: A systematic review. Travel Medicine and Infectious Disease. 2016;14(4):313–30. 10.1016/j.tmaid.2016.07.002 . [DOI] [PubMed] [Google Scholar]
- 46.Coelho FC, Durovni B, Saraceni V, Lemos C, Codeco CT, Carmargo S, et al. Higher incidence of Zika in adult women than adult men in Rio de Janeiro suggests a significant contribution of sexual transmission from men to women. International Journal of Infectious Diseases. 2016;51:128–32. 10.1016/j.ijid.2016.08.023 [DOI] [PubMed] [Google Scholar]
- 47.Reyes Y, Bowman NM, Becker-Dreps S, Centeno E, Collins MH, Liou GA, et al. Prolonged shedding of Zika virus RNA in vaginal secretions, Nicaragua. Emerging Infectious Diseases. 2019;25(4):808–10. Epub 2019/03/19. 10.3201/eid2504.180977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus—New York City, 2016. Morbidity and Mortality Weekly Report. 2016;65. [DOI] [PubMed] [Google Scholar]
- 49.Morrison TE, Diamond MS. Animal models of Zika virus infection, pathogenesis, and immunity. Journal of Virology. 2017;91(8). 10.1128/JVI.00009-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duggal NK, Ritter JM, Pestorius SE, Zaki SR, Davis BS, Chang GJ, et al. Frequent Zika virus sexual transmission and prolonged viral RNA shedding in an immunodeficient mouse model. Cell Reports. 2017;18(7):1751–60. 10.1016/j.celrep.2017.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016. 10.1016/j.stem.2016.02.016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, et al. Vaginal exposure to Zika virus during pregnancy leads to fetal brain infection. Cell. 2016;166(5):1247–56 e4. 10.1016/j.cell.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDonald EM, Duggal NK, Brault AC. Pathogenesis and sexual transmission of Spondweni and Zika viruses. PLoS Neglected Tropical Diseases. 2017;11(10):e0005990 10.1371/journal.pntd.0005990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osuna CE, Whitney JB. Nonhuman primate models of Zika virus infection, immunity, and therapeutic development. The Journal of Infectious Diseases. 2017;216(suppl_10):S928–S34. 10.1093/infdis/jix540 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dick GW. Zika virus. II. Pathogenicity and physical properties. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1952;46(5):521–34. 10.1016/0035-9203(52)90043-6 . [DOI] [PubMed] [Google Scholar]
- 56.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1952;46(5):509–20. 10.1016/0035-9203(52)90042-4 . [DOI] [PubMed] [Google Scholar]
- 57.Boorman JP, Porterfield JS. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1956;50(3):238–42. 10.1016/0035-9203(56)90029-3 . [DOI] [PubMed] [Google Scholar]
- 58.Henderson BE, Cheshire PP, Kirya GB, Lule M. Immunologic studies with yellow fever and selected African group B arboviruses in rhesus and vervet monkeys. The American Journal of Tropical Medicine and Hygiene. 1970;19(1):110–8. 10.4269/ajtmh.1970.19.110 . [DOI] [PubMed] [Google Scholar]
- 59.Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nature Communications. 2016;7:12204 10.1038/ncomms12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nature Medicine. 2016. 10.1038/nm.4206 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koide F, Goebel S, Snyder B, Walters KB, Gast A, Hagelin K, et al. Development of a Zika virus infection model in cynomolgus macaques. Frontiers in Microbiology. 2016;7:2028 10.3389/fmicb.2016.02028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, et al. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathogens. 2017;13(3):e1006219 10.1371/journal.ppat.1006219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newman CM, Dudley DM, Aliota MT, Weiler AM, Barry GL, Mohns MS, et al. Oropharyngeal mucosal transmission of Zika virus in rhesus macaques. Nature Communications. 2017;8(1):169 10.1038/s41467-017-00246-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Best K, Guedj J, Madelain V, de Lamballerie X, Lim SY, Osuna CE, et al. Zika plasma viral dynamics in nonhuman primates provides insights into early infection and antiviral strategies. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(33):8847–52. 10.1073/pnas.1704011114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aid M, Abbink P, Larocca RA, Boyd M, Nityanandam R, Nanayakkara O, et al. Zika Virus persistence in the central nervous system and lymph nodes of rhesus monkeys. Cell. 2017;169(4):610–20 e14. 10.1016/j.cell.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li XF, Dong HL, Huang XY, Qiu YF, Wang HJ, Deng YQ, et al. Characterization of a 2016 clinical isolate of Zika virus in non-human primates. EBioMedicine. 2016. 10.1016/j.ebiom.2016.09.022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016. 10.1126/science.aah6157 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, et al. Rapid development of a DNA vaccine for Zika virus. Science. 2016;354(6309):237–40. 10.1126/science.aai9137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543(7644):248–51. 10.1038/nature21428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Magnani DM, Rogers TF, Maness NJ, Grubaugh ND, Beutler N, Bailey VK, et al. Fetal demise and failed antibody therapy during Zika virus infection of pregnant macaques. Nature Communications. 2018;9(1):1624 10.1038/s41467-018-04056-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirsch AJ, Roberts VHJ, Grigsby PL, Haese N, Schabel MC, Wang X, et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nature Communications. 2018;9(1):263 10.1038/s41467-017-02499-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dudley DM, Newman CM, Lalli J, Stewart LM, Koenig MR, Weiler AM, et al. Infection via mosquito bite alters Zika virus tissue tropism and replication kinetics in rhesus macaques. Nature Communications. 2017;8(1):2096 10.1038/s41467-017-02222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mohr EL, Block LN, Newman CM, Stewart LM, Koenig M, Semler M, et al. Ocular and uteroplacental pathology in a macaque pregnancy with congenital Zika virus infection. PloS One. 2018;13(1):e0190617 10.1371/journal.pone.0190617 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinot AJ, Abbink P, Afacan O, Prohl AK, Bronson R, Hecht JL, et al. Fetal neuropathology in Zika virus-infected pregnant female rhesus monkeys. Cell. 2018. 10.1016/j.cell.2018.03.019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rayner JO, Kalkeri R, Goebel S, Cai Z, Green B, Lin S, et al. Comparative pathogenesis of Asian and African-lineage Zika virus in Indian rhesus macaque's and development of a non-human primate model suitable for the evaluation of new drugs and vaccines. Viruses. 2018;10(5). 10.3390/v10050229 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abbink P, Larocca RA, Visitsunthorn K, Boyd M, De La Barrera RA, Gromowski GD, et al. Durability and correlates of vaccine protection against Zika virus in rhesus monkeys. Science Translational Medicine. 2017;9(420). 10.1126/scitranslmed.aao4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Azar SR, Rossi SL, Haller SH, Yun R, Huang JH, Plante JA, et al. ZIKV Demonstrates Minimal pathologic effects and mosquito infectivity in viremic cynomolgus macaques. Viruses. 2018;10(11). Epub 2018/11/25. 10.3390/v10110661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woollard SM, Olwenyi OA, Dutta D, Dave RS, Mathews S, Gorantla S, et al. Preliminary studies on immune response and viral pathogenesis of Zika virus in rhesus macaques. Pathogens. 2018;7(3). Epub 2018/08/22. 10.3390/pathogens7030070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haddow AD, Nalca A, Rossi FD, Miller LJ, Wiley MR, Perez-Sautu U, et al. High infection rates for adult macaques after intravaginal or intrarectal inoculation with Zika virus. Emerging Infectious Diseases. 2017;23(8):1274–81. 10.3201/eid2308.170036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carroll T, Lo M, Lanteri M, Dutra J, Zarbock K, Silveira P, et al. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathogens. 2017;13(7):e1006537 10.1371/journal.ppat.1006537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Warren WC, Jasinska AJ, Garcia-Perez R, Svardal H, Tomlinson C, Rocchi M, et al. The genome of the vervet (Chlorocebus aethiops sabaeus). Genome Research. 2015;25(12):1921–33. 10.1101/gr.192922.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carlsson HE, Schapiro SJ, Farah I, Hau J. Use of primates in research: A global overview. American Journal of Primatology. 2004;63:225–37. 10.1002/ajp.20054 [DOI] [PubMed] [Google Scholar]
- 83.Osada N, Kohara A, Yamaji T, Hirayama N, Kasai F, Sekizuka T, et al. The genome landscape of the African green monkey kidney-derived vero cell line. DNA Research. 2014;21(6):673–83. 10.1093/dnares/dsu029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yasumura Y, Kawakita Y. Studies on SV40 in tissue culture: preliminary step for cancer reserach in vitro. Nihon Rinsho. 1963;21:1201–15. [Google Scholar]
- 85.Cho F, Hiyaoka A, Suzuki MT, Honjo S. Breeding of African green monkeys (Cercopithecus aethiops) under indoor individually-caged conditions. Experimental Animals. 2002;51(4):343–51. 10.1538/expanim.51.343 . [DOI] [PubMed] [Google Scholar]
- 86.Ladner JT, Wiley MR, Prieto K, Yasuda CY, Nagle E, Kasper MR, et al. Complete genome sequences of five Zika virus isolates. Genome Announcements. 2016;4(3). 10.1128/genomeA.00377-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith DR, Sprague TR, Hollidge BS, Valdez SM, Padilla SL, Bellanca SA, et al. African and Asian Zika virus isolates display phenotypic differences both in vitro and in vivo. The American Journal of Tropical Medicine and Hygiene. 2017. 10.4269/ajtmh.17-0685 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller LJ, Nasar F, Schellhase CW, Norris SL, Kimmel AE, Valdez SM, et al. Zika Virus infection in Syrian golden hamsters and strain 13 guinea pigs. The American Journal of Tropical Medicine and Hygiene. 2018. 10.4269/ajtmh.17-0686 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corman VM, Rasche A, Baronti C, Aldabbagh S, Cadar D, Reusken CB, et al. Assay optimization for molecular detection of Zika virus. Bulletin of the World Health Organization. 2016;94(12):880–92. 10.2471/BLT.16.175950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brent C, Dunn A, Savage H, Faraji A, Rubin M, Risk I, et al. Preliminary findings from an investigation of Zika virus infection in a patient with no known risk factors—Utah, 2016. Morbidity and Mortality Weekly Report. 2016;65(36):981–2. 10.15585/mmwr.mm6536e4 . [DOI] [PubMed] [Google Scholar]
- 91.Krow-Lucal ER, Novosad SA, Dunn AC, Brent CR, Savage HM, Faraji A, et al. Zika virus infection in patient with no known risk factors, Utah, USA, 2016. Emerging Infectious Diseases. 2017;23(8):1260–7. 10.3201/eid2308.170479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duijster JW, Goorhuis A, van Genderen PJ, Visser LG, Koopmans MP, Reimerink JH, et al. Zika virus infection in 18 travellers returning from Surinam and the Dominican Republic, The Netherlands, November 2015-March 2016. Infection. 2016;44(6):797–802. 10.1007/s15010-016-0906-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Calvet GA, Filippis AM, Mendonca MC, Sequeira PC, Siqueira AM, Veloso VG, et al. First detection of autochthonous Zika virus transmission in a HIV-infected patient in Rio de Janeiro, Brazil. Journal of Clinical Virology. 2016;74:1–3. 10.1016/j.jcv.2015.11.014 . [DOI] [PubMed] [Google Scholar]
- 94.Lugo-Roman L, Rico PJ, Sturdivant R, Burks R, Settle TL. Effects of serial anesthesia using ketamine or ketamine/medetomidine on hematology and serum biochemistry values in rhesus macaques (Macaca mulatta). Journal of Medical Primatology 2010;39(1):41–9. 10.1111/j.1600-0684.2009.00394.x [DOI] [PubMed] [Google Scholar]
- 95.Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1954;48(2):139–45. 10.1016/0035-9203(54)90006-1 . [DOI] [PubMed] [Google Scholar]
- 96.Li XF, Dong HL, Huang XY, Qiu YF, Wang HJ, Deng YQ, et al. Characterization of a 2016 clinical isolate of Zika virus in non-human primates. EBioMedicine. 2016;12:170–7. 10.1016/j.ebiom.2016.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chiu CY, Sanchez-San Martin C, Bouquet J, Li T, Yagi S, Tamhankar M, et al. Experimental Zika virus inoculation in a New World monkey model reproduces key features of the human infection. Scientific Reports. 2017;7(1):17126 10.1038/s41598-017-17067-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nature Medicine. 2016;22(12):1448–55. 10.1038/nm.4206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Diallo D, Sall AA, Diagne CT, Faye O, Faye O, Ba Y, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PloS One. 2014;9(10):e109442 10.1371/journal.pone.0109442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weinbren MP, Williams MC. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1958;52(3):263–8. 10.1016/0035-9203(58)90085-3 . [DOI] [PubMed] [Google Scholar]
- 101.Haddow AJ, Williams MC, Woodall JP, Simpson DI, Goma LK. Twelve isolations of Zika virus from Aedes (Stegomyia) africanus (Theobald) taken in and above a Uganda forest. Bulletin of the World Health Organization. 1964;31:57–69. [PMC free article] [PubMed] [Google Scholar]
- 102.McCrae AW, Kirya BG. Yellow fever and Zika virus epizootics and enzootics in Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1982;76(4):552–62. 10.1016/0035-9203(82)90161-4 . [DOI] [PubMed] [Google Scholar]
- 103.Kirya BG, Okia NO. A yellow fever epizootic in Zika Forest, Uganda, during 1972: Part 2: Monkey serology. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1977;71(4):300–3. 10.1016/0035-9203(77)90104-3 . [DOI] [PubMed] [Google Scholar]
- 104.Ma D, Jasinska AJ, Feyertag F, Wijewardana V, Kristoff J, He T, et al. Factors associated with siman immunodeficiency virus transmission in a natural African nonhuman primate host in the wild. Journal of Virology. 2014;88(10):5687–705. 10.1128/JVI.03606-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dixson A, editor. Homosexual behaviour in primates. Cambridge, England, UK: Cambridge University Press; 2010. [Google Scholar]
- 106.Erwin J, Maple T. Ambisexual behavior with male-male anal penetration in male rhesus monkeys. Archives of Sexual Behavior. 1976;5(1):9–14. 10.1007/BF01542236 . [DOI] [PubMed] [Google Scholar]
- 107.Jolly C, Phillips-Conroy JE, Turner TR, Broussard S, Allan JS. SIVagm incidence over two decades in a natural population of Ethiopian grivet monkeys (Cercopithecus aethiops aethiops). Journal of Medical Primatology. 1996;25(2):78–83. 10.1111/j.1600-0684.1996.tb00198.x . [DOI] [PubMed] [Google Scholar]
- 108.Phillips-Conroy JE, Jolly CJ, Petros B, Allan JS, Desrosiers RC. Sexual transmission of SIVagm in wild grivet monkeys. Journal of Medical Primatology. 1994;23(1):1–7. 10.1111/j.1600-0684.1994.tb00088.x . [DOI] [PubMed] [Google Scholar]
- 109.Woolhouse ME, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(1):338–42. 10.1073/pnas.94.1.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stein RA. Super-spreaders in infectious diseases. International Journal of Infectious Diseases. 2011;15(8):e510–3. 10.1016/j.ijid.2010.06.020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thangamani S, Huang J, Hart CE, Guzman H, Tesh RB. Vertical transmission of Zika virus in Aedes aegypti mosquitoes. The American Journal of Tropical Medicine and Hygiene. 2016;95(5):1169–73. 10.4269/ajtmh.16-0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haddow AJ. The stratification of biting diptera in tropical forest. Journal of Ecology. 1966;54:282. [Google Scholar]
- 113.Haddow AJ, Corbet PS. Entomological studies from a high tower in Mpanga Forest, Uganda. V. Transactions of the Royal Entomological Society London. 1961;113:284–300. [Google Scholar]
- 114.Corbet PS, Haddow AJ. Diptera swarming high above the forest canopy in Uganda, with special reference to Tabanidae. Transactions of the Royal Entomological Society London. 1962;114:267–84. [Google Scholar]
- 115.Corbet PS. Entomological studies from a high tower in Mpanga Forest, Uganda. VIII. The age-composition of biting mosquito populations according to time and level. Transactions of the Royal Entomological Society London. 1961;113:336–45. [Google Scholar]
- 116.Haddow AJ, Williams MC, Woodall JP, Ssenkubuge Y, Lule M, Simpson DIH, et al. Isolations of Zika virus from Aedes africanus. Report of the East African Virus Research Insitute. 1963. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.