Abstract
High-fat diets are linked with obesity and changes in dopamine neurotransmission. Mounting evidence shows that saturated fat impacts dopamine neurons and their terminal fields, but little is known about the effect a diet high in unsaturated fat has on the dopamine system. This study sought to determine whether fat type, saturated vs. unsaturated, differentially affected body weight, blood glucose regulation, locomotor behavior, and control of dopamine release and uptake at dopamine neuron terminals in the nucleus accumbens (NAc). C57BL/6 mice were fed a control diet or a nutrient-matched diet high in saturated fat (SF), unsaturated flaxseed oil (Flax) or a blend of the two fats. After 6-weeks, mice from each high-fat diet group gained significantly more weight than Controls, but the group fed Flax gained less weight than the SF group and had fasting blood glucose levels similar to Controls. Ex-vivo fast scan cyclic voltammetry revealed the SF group also had significantly slower synaptic dopamine clearance and a reduced capacity for phasic dopamine release in the nucleus accumbens (NAc), but the Flax and Blend groups resembled Controls. These data show that different types of dietary fat have substantially different effects on metabolic phenotype and influence how dopamine terminals in the NAc regulate dopamine neurotransmission. Our data also suggests that a diet high in unsaturated fat may preserve normal metabolic and behavioral parameters as well as dopamine signaling in the NAc.
Keywords: Saturated fat, unsaturated fat, dopamine, voltammetry
Introduction
The prevalence of diet-induced obesity has progressed to historically high levels with greater than 70% of the adult population in the United States having a BMI classification of overweight or obese [1]. Consumption of a highly-palatable, calorie-dense Western Diet, which is high in processed carbohydrates and saturated fat, has been generally implicated as a primary driver of the obesity epidemic. However, results from the National Health and Nutrition Survey revealed that intake of saturated fat is specifically associated with increased BMI [2]. Diets high in saturated fat have also been linked to pathological changes in dopamine neurochemistry in pre-clinical studies which are supported by clinical reports of altered dopaminergic responses to food cues and the perceived reward of palatable food receipt [3–6]. This suggests that saturated fat may promote obesity by altering the dopamine neurochemistry in reward-related areas of the brain that promote overconsumption of food.
Emerging patterns observed with diet-induced obesity include changes in the expression and activity of dopamine transporter (DAT) and dopamine D2 receptors; these changes are associated with pathological behavioral selection and anhedonia, resulting in enhanced seeking/consumption of rewards [3,4,6,7]. The impact of dopamine on food intake behaviors is highlighted by imaging studies that report a correlation between BMI and increased “craving” anticipatory response evidenced by increased striatal dopamine release elicited by images of highly-palatable foods. However, BMI was inversely correlated with striatal activation in response to reward receipt when highly-palatable foods were consumed [3,8]. These studies suggest increased dopamine release is elicited by palatable food cues, but decreased dopaminergic responses occur when the food is consumed. This shift in dopamine signaling could promote food seeking, but less of a dopaminergic response upon the receipt of the palatable food likely promotes overconsumption [5,7,8]. Other imaging studies reported that dopamine D2 receptor expression and function is significantly decreased in obese subjects and negatively correlated with BMI [3,9,10]. Pre-clinical studies investigating the effects of diet and obesity on dopamine neurotransmission consistently use diets high in saturated fat. Since diet can be controlled and better quantified, these studies provide a better understanding of the underlying changes to dopamine neurochemistry. A key finding from preclinical research is that saturated fat is associated with reduced DAT function [11–14]. This has significant implications since reduced DAT function results in delayed termination of phasic burst firing, enhanced tonic signaling, and an overall increase in synaptic dopamine [15]. Enhanced synaptic dopamine that results from reduced DAT function likely contributes to the compensatory reduction in post-synaptic dopamine D2 receptors observed with obesity [3,10]. Together, dysregulated synaptic dopamine may contribute to overconsumption of palatable foods in a manner similar to elevated synaptic dopamine increasing the consumption of other salient rewards [14,16,17]. This has been demonstrated in rodent studies that report an association between saturated-fat-induced obesity and reduced expression of D2 receptors as well as decreased D2 receptor binding compared to control [10]. In addition to changes in post-synaptic D2 receptor function, changes in pre-synaptic D2 auto-receptors are also observed with high fat diet-induced obesity. The effects of saturated fat on dopamine auto-receptors is significant since they serve key feedback roles in modulating dopamine transmission by decreasing dopamine neuron excitability as well as regulating dopamine synthesis and release [18–20].
Peripheral signals that regulate food intake, like insulin and leptin, also modulate dopamine release and uptake [6,21]. Saturated fat is well known to cause insulin and leptin resistance, due in part to saturated fat promoting low-grade inflammation [22,23]. Neuronal insulin resistance also occurs with saturated-fat-induced obesity, which impacts dopamine neurotransmission since insulin increases DAT activity and fine-tunes dopamine neuron firing [24–26]. Interestingly, it has been reported that consuming a diet high in anti-inflammatory mono-unsaturated fat (MUFA) does not impact behavioral response to amphetamine or DAT and dopamine receptor D1 protein levels in the same manner as saturated fat [4]. Similarly, polyunsaturated fatty acids (PUFAs) prevent enhanced behavioral effects of cocaine and D2 agonists caused by saturated fat [27,28]. Collectively, this suggests that the type of dietary fat may differentially impact dopamine signaling with saturated fat reducing but MUFA and PUFA anti-inflammatory fats preserving or restoring dopamine neurotransmission.
A growing body of evidence links diets high in saturated fat to pathological changes in dopamine neurotransmission, with notable effects on synaptic regulation by reducing DAT-mediated dopamine clearance. The question remains, however, whether these changes at dopamine terminals are driven by the high amount of dietary fat or the type of dietary fat. The purpose of this study was to examine whether the type of dietary fat influences synaptic regulation of dopamine in the nucleus accumbens (NAc). Specifically, we examined whether substituting a high-saturated-fat diet with the same amount of polyunsaturated-rich flax seed oil changed dopamine release and uptake kinetics at NAc terminals. Diets high in unsaturated fatty acids are well known to promote brain health and influence dopaminergic behaviors, but little is known on exactly how or if these unsaturated fats change synaptic control of dopamine. We hypothesized that a diet high in PUFA-rich flax would not impair dopamine terminals in the same manner as saturated fat, consistent with reports that MUFA and PUFA-rich diets preserve dopaminergic behaviors that can be altered by saturated fat [29,30]. Here we show a potential mechanism for preserved dopamine neurotransmission with unsaturated fat. In the context of maladaptive eating behaviors, understanding how different dietary fats alter NAc dopamine provides insight to whether satiety circuits that project from the NAc to the hypothalamus could be altered by fat type [31]. Additionally, these data may reveal potential therapeutic targets for the prevention and treatment of obesity.
Methods
Animals and Diet
Six-week-old male C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME), housed three per cage, and maintained on a 12 h light/dark cycle with free access to water and either a control normal fat diet (Control; 10% kcals from fat; D12450J, Research Diets, n=6) or one of three nutrient matched experimental diets containing 60% kcals from fat: either high in saturated fat (SF; D12492, Research Diets, n=9), saturated fat combined with flaxseed oil (Blend; 1:1 ratio of saturated fats to n3 polyunsaturated fats, Research Diets, n=6) or high in unsaturated fat from flaxseed oil (Flax; 3:7 ratio of saturated fats to n3 polyunsaturated fats, n=6). Custom flax diets were micronutrient matched to the high-fat and control diets, and calorically matched to the high saturated-fat diet. Food intake was measured on Mondays, Wednesdays, and Fridays by weighing the amount of diet consumed and calculating caloric intake based on food disappearance. Body weights were collected upon arrival and weekly thereafter. Mice remained on respective diets for 6-weeks prior to experimental tests. All experiments were approved by the UNC Greensboro Animal Care and Use Committee and conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
Open Field Locomotor Behavior
Locomotor and exploratory activities were assessed using an open field test measuring total distance travelled and entries into the center of the open field during a 10-minute habituation period and the total session duration of 60 minutes. Behavioral experiments were conducted during the first four hours of the light cycle using plexiglass chambers (30 cm × 30 cm × 60 cm). Videos were analyzed using TopScanLite (Version 2.0, CleverSys Inc), based on previous reports [32].
Intraperitoneal Glucose Tolerance Test
At the end of the 6-week dietary protocol we measured fasting blood glucose and glucose clearance using an intraperitoneal glucose tolerance test (IPGTT). Mice were fasted for 9 h, then fasting blood glucose was measured from the tail vein using a TRUEtrack glucometer and blood glucose test strips (Rite Aid Pharmacy, Camp Hill, PA). Next, an i.p. bolus of glucose (2 g/kg in 20% w/v saline) was delivered and repeat blood glucose measurements were performed after 15, 30, 60, and 120 minutes to assess glucose clearance.
Fast Scan Cyclic Voltammetry (FSCV)
FSCV was used to characterize baseline dopamine release and uptake within the NAc core. FSCV is a useful tool to measure dopamine neurotransmission in the NAc due to its excellent temporal and spatial resolution as well as its sensitivity for dopamine. Importantly, FSCV measures millisecond changes in synaptic dopamine allowing for kinetic measurements of dopamine release by pre-synaptic terminals and dopamine uptake via the DAT. All voltammetry experiments were conducted after the 6-week dietary protocol and within two weeks after IPGTT using a Latin square design. All voltammetry experiments began ~3 h into the light cycle. Experimental procedures were executed and modelled as previously described [33]. Briefly, mice were rendered unconscious using 5% isoflurane, decapitated, and the brain swiftly removed. The brain was hemisected with one hemisphere submerged in ice cold artificial cerebral spinal fluid (aCSF) (in mM: 126 NaCl, 25 NaHCO3, 11 D-glucose, 2.5 KCl, 2.4 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 0.4 L-ascorbic acid, pH adjusted to 7.4) for slicing on a compresstome (Precisionary Instruments; Greenville, NC). Next, 300 μm brain slices containing the NAc (from +1.45 to +0.74 from bregma) were transferred to the voltammetry chamber and allowed to equilibrate for 60 min at 32 °C while bathed in oxygenated (95% O2/5% CO2) aCSF at 100 mL/min. Dopamine was recorded using a triangular waveform applied to a glass capillary-pulled carbon-fiber working microelectrode (70–100 μm length, 7 μm diameter). The working electrode penetrated brain slices within the NAc core ~50 μM in depth, and a bipolar stimulating electrode (Plastics One, Roanoke, VA, 8IMS3033SPCE) was placed on the surface of the slice approximately 100–200 μm away from the working electrode. The working electrode was maintained at a potential of −0.4 V versus an Ag/AgCl reference electrode and subsequently ramped up to +1.2 V and back to −0.4 V at a scan rate of 400 V/s every 100ms. Dopamine release was evoked with a single electrical pulse (20Hz, 4 ms pulse width, 350 μA stimulation amplitude) from the stimulating electrode every 3 min. Next, dopamine release was evoked using 5 pulse stimulations at 5, 10, 20, 40, and 100 Hz frequencies to characterize phasic dopamine release. Dopamine signals were acquired and modeled using Demon Voltammetry Software (Wake Forest School of Medicine; Winston-Salem, NC), based on Michaelis−Menten kinetics using a Km of 160 nM for dopamine [34]. Baseline recordings were obtained from one to two brain slices from each animal. To measure dopamine D2 auto-receptor function at dopamine terminals, quinpirole (Tocris; Minneapolis, MN; Cat. No. 1061) was subsequently washed over the slices with cumulative half-log applications to obtain a dose response curve (1–100 nM) at the same location as baseline collections. Dopamine current was converted to concentration by electrode calibration after each experiment using a flow cell, adding 3 μM of dopamine in combination with the Demon Analysis Software.
Statistical Analysis
Graph Pad Prism v.8 (La Jolla, CA) was used for all statistical analyses. Two-way repeated measures analysis of variance (ANOVA) was used to identify significant differences within and between treatment groups for body weight x IPGTT and voltammetry dose response curves using Tukey’s post hoc analysis to identify significant variations between groups when applicable. One-way ANOVA tests were used to analyze between group differences for food intake, fasting blood glucose, area under the curve data for IPGTT, locomotor activity measurements, dopamine release, and Vmax. Pearson’s correlational analysis was used to identify relationships between body weight or glucose clearance and dopamine release and uptake kinetics. Group data are presented as mean ± SEM; statistical significance was set at p ≤ 0.05.
Results
Flax attenuated weight gain and improved blood glucose regulation.
All high-fat groups consumed significantly more kcals per day than controls (Control 10.0 ± 0.18; SF 13.9 ± 0.18; Flax 13.5 ± 0.31; and Blend 14.1 ± 0.39) (p < 0.0001), no difference in daily kcal intake was observed between the SF, Flax, and Blend groups (Figure 1A). Final body weight was also significantly higher (p < 0.0001) in all high-fat groups (SF 37.7 ± 0.9 g; Flax 32.6 ± 1.1 g; Blend 36.9 ± 1.3 g) compared to Control (25.8 ± 0.9 g); however, the Flax group gained significantly less body weight than the SF (p < 0.001) or Blend (p < 0.01) groups (Figure 1B). A two-way repeated measures ANOVA identified a significant interaction between diet and body weight (F(3, 23) = 20.7; p < 0.0001) with main effects of diet (F(3, 23) = 25.57; p < 0.0001) and time (F(1, 23) = 369.1; p < 0.0001), indicating that dietary fat amount and fat type both impacted body weight.
Figure 1: Food Intake and Body Weight -.
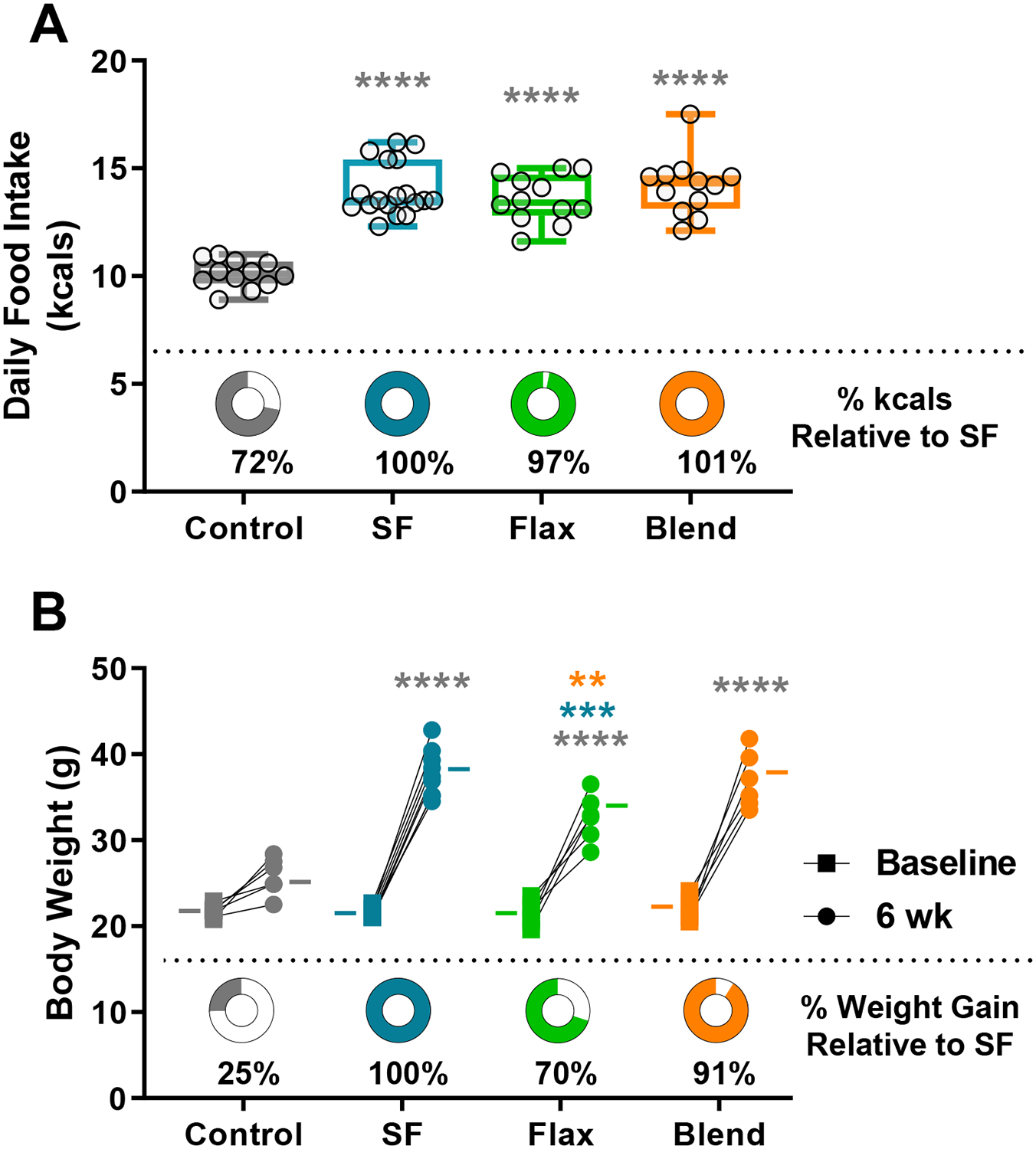
(A) Average daily food intake in kilocalories (kcals) over 6-weeks, with circle graphs indicating the amount of daily kcals consumed relative to the saturated fat (SF) group. Box plot represents the median, 25th and 75th percentiles, with the whiskers indicating the minimum and maximum values. (B) Initial and final body weights in grams (g) connected by a line for each individual mouse grouped by diet. The (−) marks indicate group means, and the circle graphs below show the percent weight gain compared to the SF group. (**, p < 0.01; ***, p < 0.001, ****, p < 0.0001)
To examine the effect of dietary fat on blood glucose regulation, we measured fasting blood glucose and blood glucose clearance with IPGTTs. Fasting blood glucose was not significantly different between the Control (118.3 ± 6.7 mg/dl) and Flax (109.7 ± 10.8 mg/dl) groups (p = 0.9637), but fasting blood glucose was significantly elevated in the SF (184.9 ± 13.1 mg/dl) and Blend (186.7 ± 13.7 mg/dl) groups compared to the Control (p < 0.01, each) and Flax groups (p < 0.001, and p < 0.01; SF and Blend, respectively) (Figure 2A). Similarly, there were main effects of diet group (F(3, 23) = 20.63; p < 0.0001) and time (F(3.259, 74.95) = 225.7; p < 0.0001) on blood glucose clearance after IPGTT, with a diet by time interaction (F(12, 92) = 8.044; p < 0.0001) and post hoc analysis showed delayed glucose clearance in the SF and Blend groups compared to Control and Flax groups at 30, 60, and 120 minutes (Figure 2B). Interestingly, blood glucose clearance was negatively correlated with body weight in the SF group (r = −0.69; p < 0.05) (Figure 2C), indicating that mice with higher body weights were better at clearing blood glucose in the SF group.
Figure 2: Blood Glucose Regulation -.
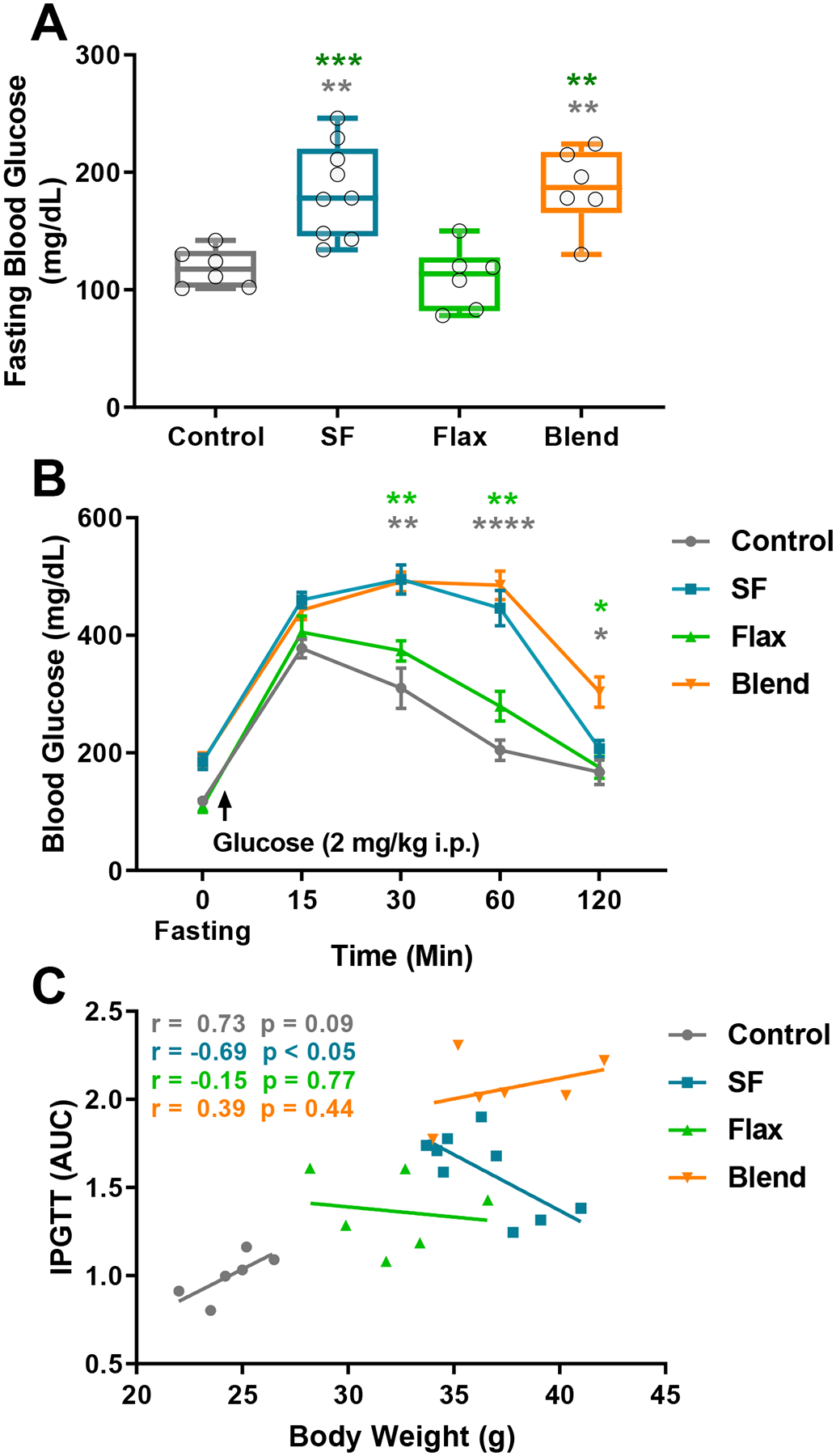
(A) Blood glucose measurements following a 9 hr fast. Box plot represents the median, 25th and 75th percentiles, with the whiskers indicating the minimum and maximum values. (B) Blood glucose values expressed as group mean ± SEM before and after delivery of glucose (2mg/kg i.p.). (C) Correlational analysis of glucose clearance plotted as a function of body weight, with lines representing linear regression analysis within group. Pearson’s r values and corresponding p value are inset in vertical order of treatment group legend. (**, p < 0.01; ***, p < 0.001, ****, p < 0.0001)
Exploratory behaviors were reduced with a high saturated fat diet, but not flax.
Open field analysis was conducted to observe changes in ambulation and exploratory behaviors. Total distance travelled was not significantly different between any diet group after 10 minutes (Figure 3A) or 60 minutes of testing (Data not shown), but there was a significant effect of diet on entries into the center of the open field (F(3, 23) = 3.990; p = 0.02) with post hoc analysis indicating the SF group entered the center of the arena significantly fewer times than the control group (p = 0.034). Because total distance travelled was not different between groups, avoidance of the center of the open field was likely not caused by simply less ambulation in heavier mice from the SF and Blend groups. Decreased center entries have been previously described as an anxiodepressive phenotype associated with pathological changes in dopamine neurotransmission [30]. Representative line traces from video analysis show reduced center exploration in the SF group, but not the Flax or Blend groups (Figure 3C).
Figure 3: Locomotor Behaviors -.
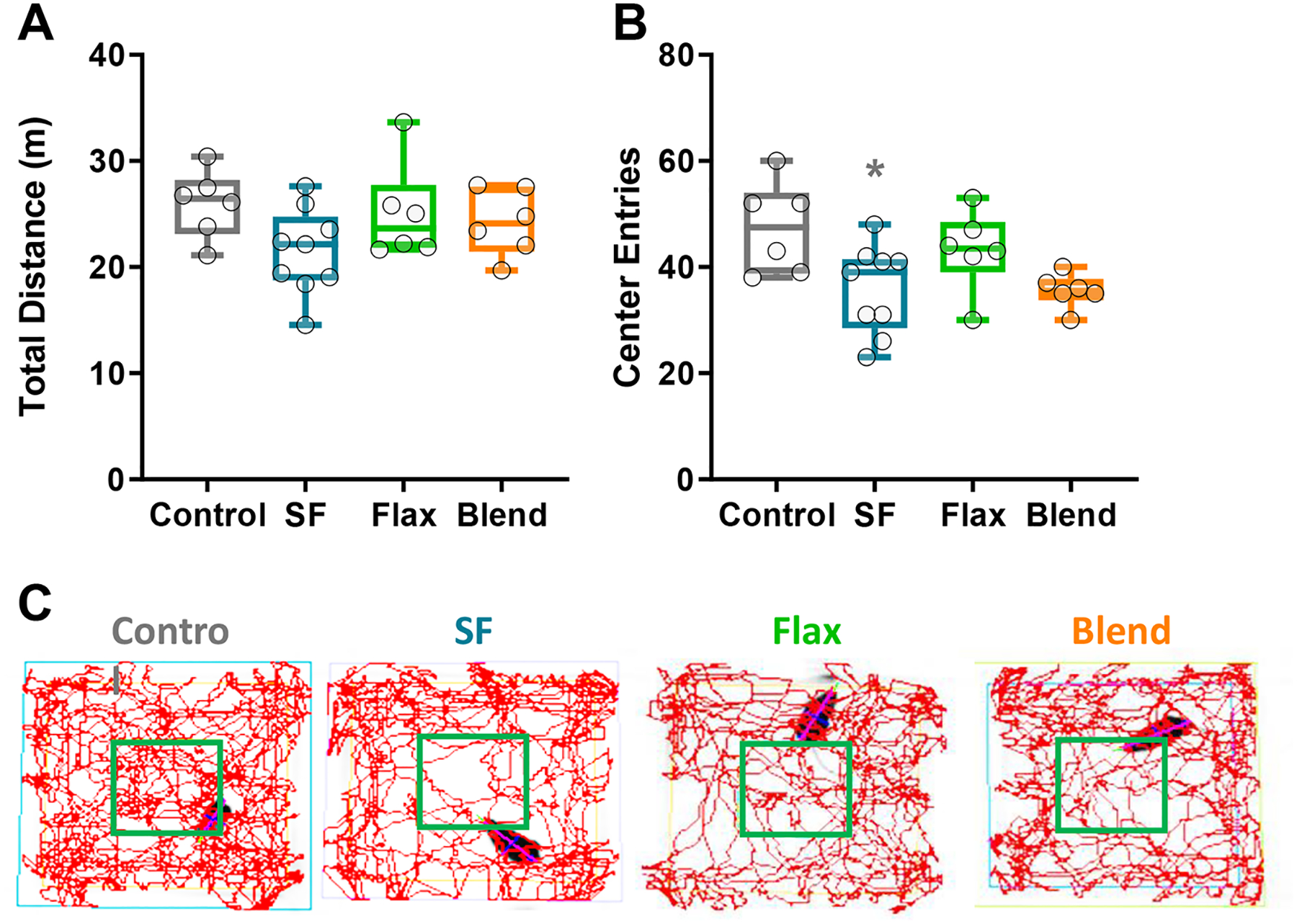
(A) Total distance traveled during 10 minutes in an open field. (B) Number of times each animal entered the center area of the open field during 10-minute exploration. (C) Representative line traces showing spatial location of exploratory behaviors of the open field. Box plot represents the median, 25th and 75th percentiles, with the whiskers indicating the minimum and maximum values. (*, p < 0.05)
Unsaturated fat-rich flax does not slow dopamine clearance or reduce phasic dopamine release in the same manner as a diet high in saturated fat.
Ex-vivo voltammetry was used to characterize dopamine terminal changes in the NAc following six-week exposure to the control and experimental high-fat diets. Dopamine release evoked by a single-pulse stimulation was not affected by any of the high-fat diets (Figure 4A); however, diet significantly altered the maximal rate of dopamine clearance (Vmax) (F(3,73) = 4.351; p = 0.007), and post-hoc analysis revealed dopamine clearance was significantly slower in mice receiving SF (1.87 ± 0.08 μM/s) compared to Control (2.32 ± 0.15 μM/s) and Flax (2.29 ± 0.07 μM/s) groups (p < 0.05) (Figure 4B). Representative cyclic voltammograms show dopamine oxidation signatures at 0.6 volts corresponding with pseudo color plots that show the intensity and duration of electrochemical conductance of dopamine following a single pulse stimulation at 4.9 seconds (Figure 4C).
Figure 4: Dopamine Release and Uptake in the NAc -.
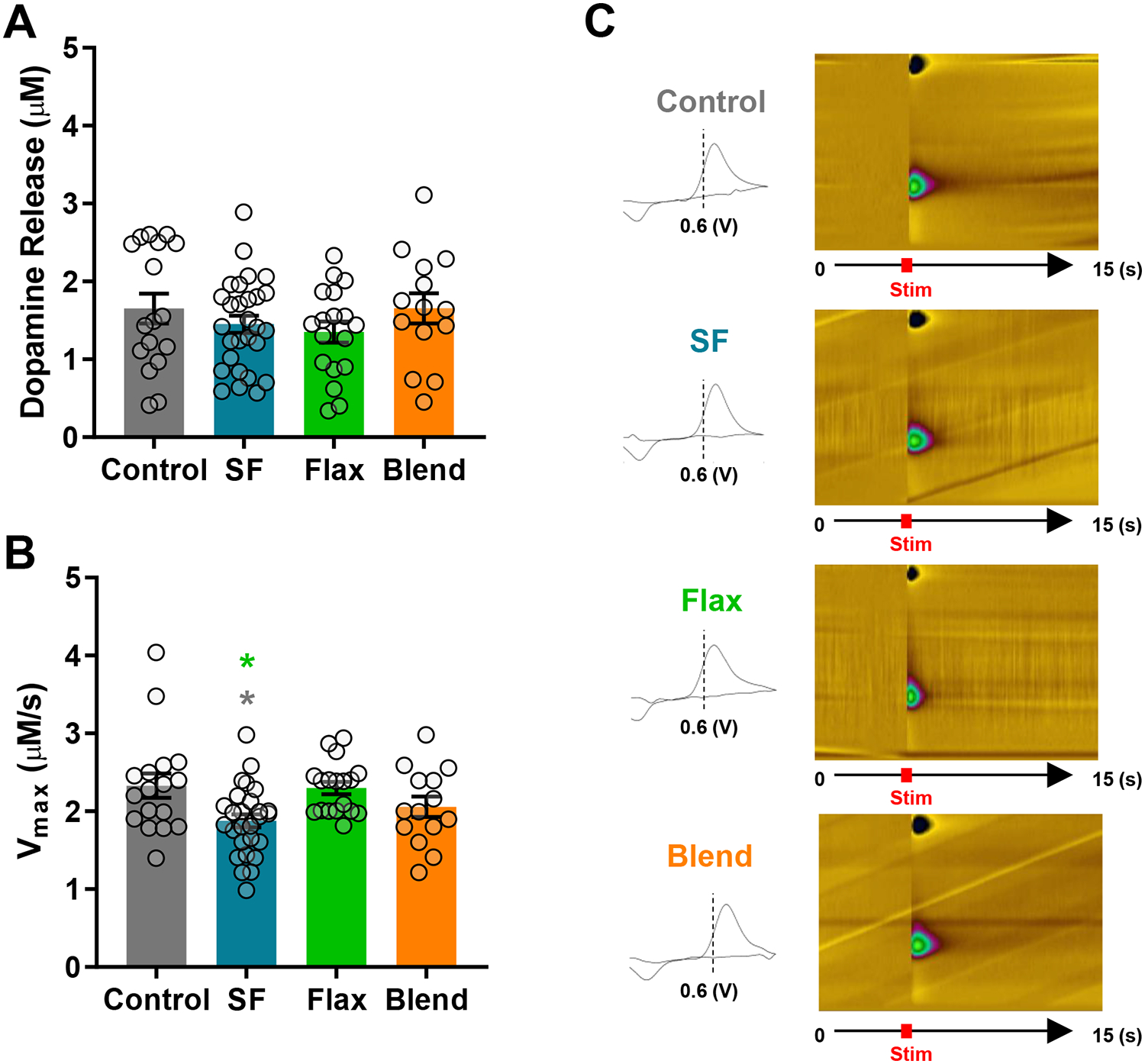
Dopamine release (A) and maximal rate of dopamine uptake (Vmax) (B) evoked by a single pulse stimulation. To account for heterogeneous differences in dopamine release and uptake between micro-domains within dopamine terminal regions of the NAc, at least two baseline recordings were obtained from two to three separate coronal slices containing the NAc, per mouse. The following are the (n) values for mice, slices, and total recordings for each group, respectively: Control n=6 mice, 11 slices, 17 recordings; SF n=9 mice, 18 slices, 28 recordings; Flax n=6 mice, 12 slices, 18 recordings; Blend n=6 mice, 10 slices, 14 recordings. (C) Representative cyclic voltammograms showing the oxidation peak of dopamine at 0.6 volts (V) accompanied by the corresponding pseudo color plot showing the change in current magnitude over time after evoking dopamine release by a single pulse at 4.9 seconds.
Next, we evoked phasic dopamine release with 5-pulse stimulation trains from 5–100 Hz, and 2-way ANOVA revealed main effects of diet (F(1,70) = 6.556; p = 0.0126) and stimulation frequency (F(4,70) = 23.23; p < 0.0001) between the Control and SF groups (Figure 5A). Phasic dopamine release across frequencies was not different between the Control, Flax, or Blend diets (Figure 5A). The average AUC of compiled line traces corresponding with dopamine release after 5-pulses at 20 Hz (the physiological burst firing rate of dopamine neurons) was significantly greater than one-pulse for each diet group (p < 0.001) (Figure 5B), and the 5-pulse 20 Hz AUC was only significantly different between the Control and SF groups (p < 0.05). Notably, the difference between 5-pulse to one-pulse AUC shows the SF group had the smallest dynamic range of tonic (1-pulse) to phasic (5-pulse) dopamine release. We then examined D2 auto-receptor function to determine if reduced phasic release in the SF group was associated with increased D2 auto-receptor inhibition of dopamine release. There was a main effect of quinpirole dose on dopamine release (F(2.719,35.34) = 177.8; p < 0.0001), as expected with a D2 agonist, but no differences in quinpirole’s ability to reduce dopamine release was detected between groups (Figure 5C), suggesting reduced dynamic range of 1:5 pulse release in the SF group is not driven by heightened sensitivity of D2 auto-receptors.
Figure 5: Phasic Dopamine Release and D2 Auto-receptor Function -.
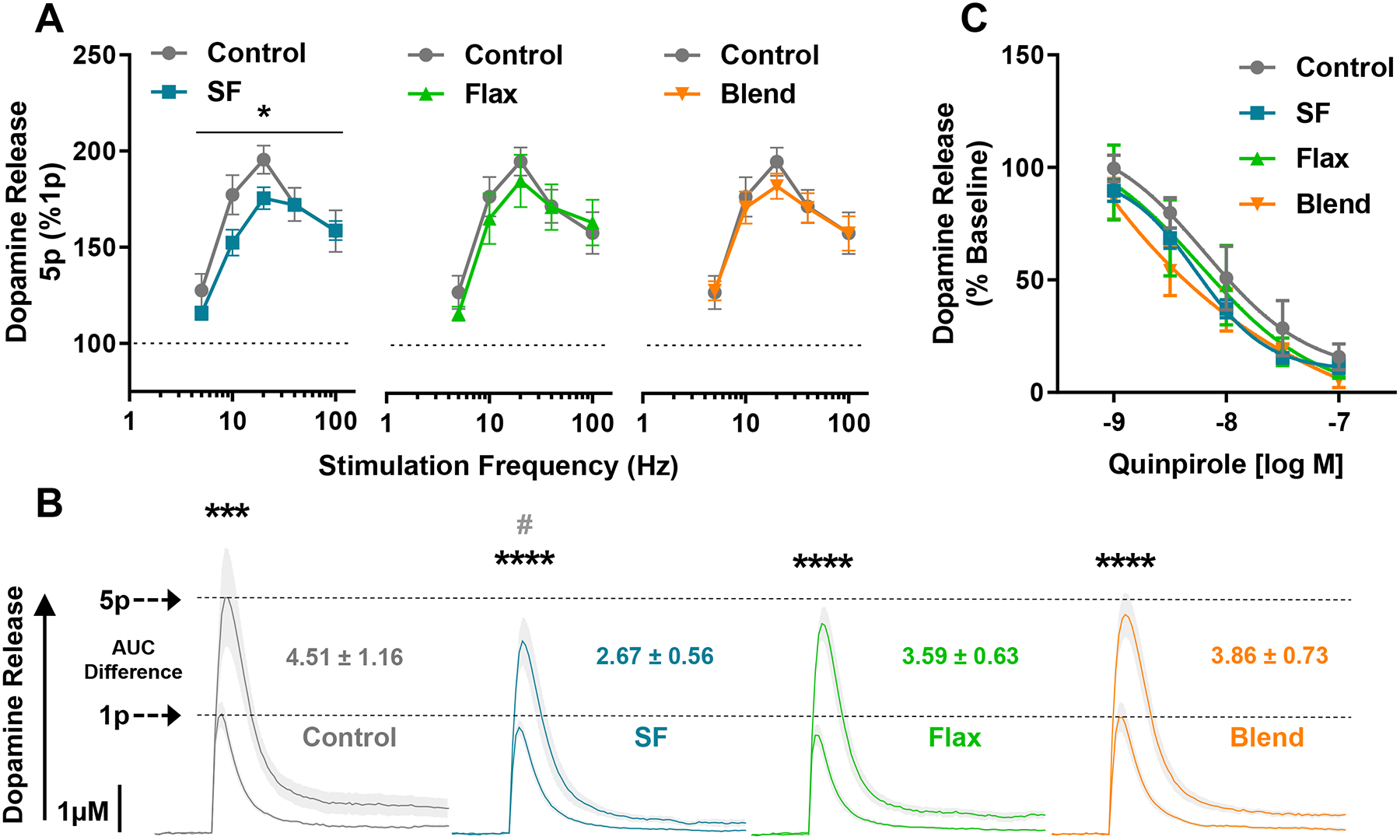
(A) Frequency response curve showing dopamine release evoked by 5-pulse trains (5p) at 5, 10, 20, 40, and 100 Hz. Phasic 5-pulse dopamine release is expressed as a percent of single pulse (1p) dopamine release, and values are mean ± SEM. (B) Aggregated line curves showing dopamine release from 1 and 5 pulses, along with the difference in AUC from 5p to 1p. The shaded area between dotted lines represents SEM, asterisks denote significant difference between 1p and 5p within group, and the pound sign represents a significant reduction in 5p dopamine release in the SF group compared to Control. (C) Dopamine release following a cumulative half-log dose-response curve of the D2 agonist, quinpirole. Changes in dopamine release are expressed as percent stable baseline prior to quinpirole application. (* and #, p < 0.05; ***, p < 0.001, ****, p < 0.0001)
Body weight and glucose clearance differentially predict dopamine uptake rate in the SF group.
Pearson’s correlational analysis was conducted to determine if biometric measurements were related to dopamine release and uptake. Dopamine release was not correlated with body weight (Figure 6A) or IPGTT AUCs (Figure 6B) in any diet group. A significant negative relationship was observed in the SF group between body weight and dopamine uptake rate (Vmax) (r = −0.39; p < 0.05), indicating dopamine clearance slowed as body weight increased (Figure 6C). Interestingly, we observed the opposite relationship between Vmax and blood glucose clearance. A significant positive relationship was observed in the SF group between the AUC for IPGTT and Vmax (r = 4.68; p < 0.05), indicating dopamine clearance increased as blood glucose clearance decreased (Figure 6D). Linear regression in the Control, Flax, and Blend groups showed negative, but not significant, slopes when Vmax was plotted against glucose clearance (Figure 6D) and relatively flat slopes when plotted against body weight (Figure 6C). While increased body weight does not predict reduced glucose clearance (Figure 2C), it selectively predicts slower dopamine clearance rate with high saturated fat intake. Similarly, impaired glucose clearance selectively predicts faster glucose clearance rate in the saturated fat group.
Figure 6: Relationship Between Dopamine Release and Uptake with Body Weight and Glucose Clearance -.
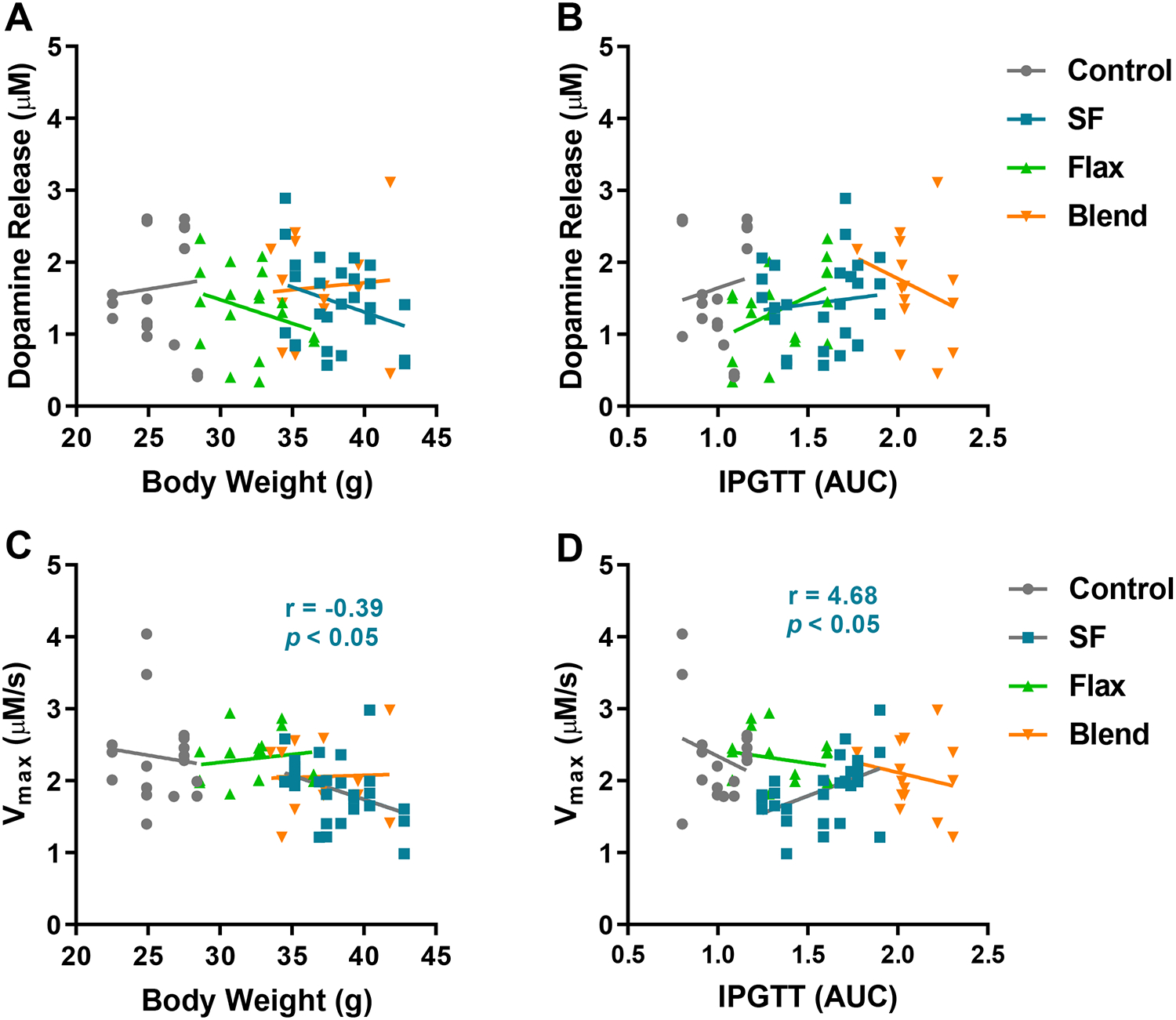
Correlational analysis revealed no association between dopamine release and final body weight (A) or AUC for glucose clearance during the IPGTT (B). In the SF group only, a significant negative correlation was observed between the maximal rate of dopamine uptake (Vmax) and body weight (C), and a significant positive correlation was observed between Vmax and reduced ability to clear blood glucose (D). Significant Pearson’s r values for the SF group are inset.
Discussion
It is well-established that diets high in saturated fat produce a metabolic syndrome-like phenotype characterized by obesogenic weight gain and impaired blood glucose regulation related to insulin-resistance. Consistent with previous studies, we induced a metabolic syndrome-like phenotype in our mice with a diet high in saturated fat and observed that a diet high in monounsaturated and polyunsaturated fat in the form of flaxseed oil did not cause metabolic impairments. Previous studies show that saturated fat-induced metabolic phenotypes are associated with impaired dopamine neurotransmission [13,14,27,33,35]. In contrast to saturated fat, recent studies showed that a diet high in mono- and omega-3 polyunsaturated fat prevented dopamine-related behavioral changes in locomotion, exploration, sensitization to cocaine, and compulsive food seeking and also preserved normal protein expression of DAT and dopamine receptors [27,30]. Findings reported here build on these previous studies by directly measuring presynaptic dopamine release and uptake after mice consume a diet high in saturated fat or unsaturated fat. We show that a diet high in saturated fat decreased phasic dopamine release and dopamine uptake, producing NAc dopamine terminals functionally distinct from controls. Importantly, we also report a novel observation that a diet high in polyunsaturated fat specifically preserved the dynamic range of phasic dopamine release and dopamine uptake rate at dopamine terminals in the NAc, similar to controls. This suggests that the type of dietary fat is distinct from the amount of dietary fat regarding its impact on dopamine neurotransmission.
Our study used an open field test to assess exploratory behaviors associated with an anxiodepressive phenotype, since high fat diets and diet-induced obesity are implicated in dopamine-modulated processes that contribute to both rewarding and aversive salient stimuli. While high fat diets have been associated with compulsive sucrose seeking, decreased exploratory activity and behaviors associated with an anxiodepressive phenotype, recent studies comparing the effects of different lipid species report that the changes associated with a high fat diet are only observed with diets high in saturated fat while mono- and omega-3 poly-unsaturated fats appear to confer a protective effect [30,36–38]. Our examination of dopamine-related behaviors, albeit limited, are in line with other more extensive appraisals of dietary fat on behavioral outcomes We showed that mice fed diets high in saturated fat entered the center of the open field significantly fewer times than the Control, Blend, and Flax groups, even though they travelled the same distance. Future studies employing additional behavioral tests, specifically with dopamine agonists like cocaine or amphetamine, are merited to fully characterize the impact of fatty acid type on dopamine related behaviors linked to DAT dysfunction. We, however, show a behavioral phenotype in our SF group similar to previous reports, and unsaturated Flax appears to attenuate SF-induced effects.
The most significant observation of this study is the differences in dopamine reuptake by the DAT between groups. The maximal rate of dopamine uptake (measured by calculating Vmax, which directly represents DAT-mediated dopamine clearance) was significantly slower in the SF group compared to the Control and Flax groups. In addition, an analysis of the relationship between body weight and rate of dopamine clearance also suggests that different types of dietary fat have different effects on dopamine regulation. We report a negative association between Vmax and body weight unique to mice consuming saturated fat, even though mice fed flax were significantly heavier than controls. Because DAT function was preferentially altered in the SF group, our findings suggest that the type of dietary fat is an additional independent factor that impairs dopamine uptake and DAT activity apart from diet-induced obesity. The effect of impaired DAT activity is significant because the DAT is the key regulator of synaptic dopamine, and it is associated with increased food intake and susceptibility to binge eating behaviors [39].
We also evaluated whether there were changes in pre-synaptic D2 auto-receptor function using quinpirole given pre-synaptic D2 auto-receptors are involved in regulation of dopamine release and reuptake by DAT [19]. Furthermore, it is well established that a decrease in post-synaptic D2 receptor binding is associated with obesity, susceptibility to binge eating behaviors and substance abuse [3,7,9]. In the present study, we did not observe a difference in dopamine release between groups with quinpirole, suggesting that the differences in phasic dopamine release are primarily mediated by mechanisms other than changes to pre-synaptic D2 auto-receptors. Therefore, the downstream impact of impaired synaptic dopamine clearance by DAT may have a more impact on post-synaptic D2 receptors.
Our observation that dampened phasic dopamine release only occurred in the SF group is also noteworthy since phasic burst-release of dopamine specifically occurs in response to salient stimuli, like high-calorie palatable food [11,20]. This suggests that diets high in saturated fat could promote dysregulated feeding by dampening phasic dopamine release, which could reduce the perceived enjoyment of food, leading to compensatory intake to achieve the same reward magnitude [10,16]. In addition, D1 receptors preferentially respond to phasic burst-firing due to their low affinity for dopamine [18,19,21,40]. Since D1 receptors project to the hypothalamus to promote satiety and terminate feeding, reduced phasic dopamine release could further promote dysregulated feeding [31]. This is supported by findings from the Fulton Lab, who reported that a saturated fat diet impaired the D1 receptor/Protein Kinase A (PKA) signaling pathway which is involved with the sensitizing and conditioning of response to reward [4]. In contrast, a diet high in monounsaturated fat in the form of olive oil preserved normal signaling associated with D1 receptors.
In addition to PKA, impairments in kinase signaling pathways such as protein kinase C (PKC) might also explain the observed differences in DAT activity between saturated fat and flax-fed groups. PKC, a primary regulator of DAT surface expression, co-localizes with DAT and leads to the internalization of DAT [41,42]. One of the molecules that activates PKC is diacylglycerol (DAG) [43]. Recent research has elucidated that different PKC isoforms have varying sensitivity to DAG depending on whether DAG contains shorter and saturated fatty acids or longer and polyunsaturated fatty acids [44]. Moreover, there is evidence that saturated fatty acids are preferentially converted to DAG compared to PUFA [45]. Therefore, enhanced PKC activation by DAG driven by saturated fat in our SF group could contribute to decreased DAT activity indicated by reduced Vmax. In addition to the putative direct role of PKC and DAG on DAT internalization, extracellular signal regulated kinase (ERK) also regulates DAT activity [42]. This is highly relevant to our study where we observe an insulin-resistant phenotype, as insulin acts on the ERK signaling pathway via PI3K/Akt kinase signaling to regulate DAT activity [46]. Previous studies also show that impaired insulin receptor function on dopamine neurons, associated with an insulin-resistant phenotype, slows dopamine uptake [26,33]. Finally, saturated fat specifically impairs insulin-activated regulation of dopamine uptake in the NAc in a PI3K/Akt-dependent manner by decreasing dopamine transporter expression on the cell surface and reducing dopamine uptake [35,47]. In the present study we indirectly assessed the effects of dietary fat on insulin resistance by monitoring the rate of blood glucose clearance. We previously reported delayed blood glucose clearance in mice fed a high fat diet, and numerous literature reports show that obesity caused by a high fat diet slows blood glucose clearance, consistent with insulin resistance and type II diabetes-induced metabolic dysfunction [33,48–51]. Our study replicates previous findings with respect to the high saturated fat group. In addition, our results are consistent with previous reports that mono- and poly-unsaturated fats (Flax) do not have the same metabolic impact since the Flax group, in contrast to the SF group, had normal blood glucose clearance that was similar to the Control group [52]. While the blood glucose clearance curves followed expected patterns based on previous reports of insulin resistance associated with saturated fat, an unanticipated significant negative correlation between increased body weight and increased blood glucose clearance was observed in the SF group. Even though this was not a strong negative correlation, it is interesting in context of the strong, non-significant positive correlation observed in the LF group. Blood glucose clearance is affected by many factors including the insulin sensitivity and the volume of peripheral tissues present to handle large boluses of glucose; therefore, further characterization of this relationship in future studies will likely need to include analysis of adipose tissue samples may provide insights into these findings. Overall, we suspect that a decrease in insulin sensitivity caused the delayed glucose clearance in the SF and Blend groups, consistent with reports of saturated fat causing insulin resistance.
In addition to glucose regulation and cellular signaling pathways altered by fatty acids, saturated and unsaturated fatty acids have varying effects on membrane lipid raft composition which in turn affect dopamine related membrane bound proteins and kinase signaling pathways [23,42,53,54]. This is relevant in context to our findings because dietary fatty acids influence the fatty acid composition of lipid rafts, which is associated with kinetic changes of transporters or receptors contained therein [42]. Specifically, dopamine receptor membrane-localization and oligomerization, as well as DAT conformation/activity is dependent on lipid raft composition [53]. While direct effects of saturated and unsaturated fats on lipid membranes may regulate dopamine neurotransmission, there is also evidence that these fat species differentially influence the inflammatory process [22,55]. Therefore, the differences we observed could be partially attributed to the well-established effects of different fatty acids on inflammatory response. There is a large body of evidence demonstrating that saturated fats promote peripheral inflammation while unsaturated fats counter these effects [23]. Within the CNS, the effects of saturated and unsaturated fats have similar effects on the glial cells that support and protect neurons. Specifically, astrocytes and microglia undergo morphological changes (e.g. gliosis) and produce inflammatory cytokines during pathophysiological conditions such as injury and infection as well as consumption of a high saturated fat diet [22,55,56]. The implications of astrogliosis on regulation of dopamine signaling could be significant, since astrocytes are not only involved in metabolic support of dopamine neurons but they also modulate the signaling and excitability of neurons in the dopaminergic VTA-NAc circuitry [57,58]. More broadly, astro- or micro-glosis caused by saturated fat could lead to production of inflammatory cytokines that are implicated in dysfunctional dopamine neurotransmission [59]. While a diet high in saturated fat is associated with inflammation and pathophysiological changes in dopamine neurotransmission, there is preliminary evidence that diets high in saturated fat induce inflammatory response and gliosis specifically within the NAc, while a high fat diet primarily composed of unsaturated fat does not elicit the same effects [30]. Since unsaturated fats have well-established, beneficial anti-inflammatory effects, differences in inflammatory status between the SF and Flax groups could provide insight into the observed differences of blood glucose regulation and dopamine terminal changes in the NAc. One of the proposed mechanisms for the development of gliosis and subsequent glial inflammatory response with diets specifically high in saturated fat is associated with the unique properties of saturated fat in activating toll-like receptors such as TLR-4. This would also provide an explanation for the differences observed with the Flax diet since unsaturated fats have been shown to counter these inflammatory responses and inactivate TLR-induced signaling pathways [23]. This also has broader implications as TLR- induced signaling pathways have been shown to disrupt the regulatory kinase pathways that affect dopamine neurotransmission.
Further appraisal of the impact that dietary fat and inflammation mechanisms have on dopamine neurotransmission are merited. However, in the context of the divergent effects of different fat species on dopamine neurotransmission, our findings are an important initial step to elucidate the underlying mechanistic changes associated with specific dietary fat type on dopamine neurotransmission. By directly testing real-time dopamine release and uptake kinetics, we report that a diet high in saturated fat slows dopamine clearance and reduces phasic dopamine release. In contrast, a high-fat diet containing PUFA-rich flax preserved “normal” dopamine terminal function when compared to controls. Since the saturated fat-induced changes in dopamine release and uptake have been linked with pathological behaviors and anhedonia which promote enhanced seeking and consumption of rewards, our findings suggest that saturated fat may contribute to individuals developing maladaptive eating behaviors by disrupting synaptic control of dopamine. Disrupting synaptic dopamine in brain regions like the NAc, which integrates into broader homeostatic control of satiety via the hypothalamus, may contribute to the development of obesity. Additionally, given the fact that our study used 60% kcals of fat, when the average American diet is 30–45% kcals fat, the question remains as to what threshold of saturated fat is necessary to impair dopamine neurotransmission. There is likely a graded effect of saturated fat on the brain with the higher end of the American diet range causing impairments. This specific examination needs to be completed, along with future studies that explore the efficacy of an unsaturated fat (e.g. flax) to reverse or restore deficits in dopamine neurotransmission caused by saturated fat. From a clinical perspective, the strategy of substituting dietary fat type rather than removing or reducing dietary fat amount could improve the compliance of dietary prescription. Specifically, when dietary changes are necessary to address systemic and neurological health concerns caused by a high saturated fat western style diet.
Funding:
R15DK119897, HHS Research Excellence Grant (SCF)
Footnotes
Disclosure: The authors declared no conflicts of interest.
References:
- [1].Overweight & Obesity Statistics | NIDDK [Internet]. Natl. Inst. Diabetes Dig. Kidney Dis 2017. [cited 2019 Aug 20]. Available from: https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity. [Google Scholar]
- [2].Raatz SK, Conrad Z, Johnson LK, et al. Relationship of the Reported Intakes of Fat and Fatty Acids to Body Weight in US Adults. Nutrients [Internet]. 2017. [cited 2019 Jul 15];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5452168/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Volkow ND, Wang G-J, Tomasi D, et al. The Addictive Dimensionality of Obesity. Biol. Psychiatry 2013;73:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hryhorczuk C, Florea M, Rodaros D, et al. Dampened Mesolimbic Dopamine Function and Signaling by Saturated but not Monounsaturated Dietary Lipids. Neuropsychopharmacology 2016;41:811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stice E, Burger KS, Yokum S. Reward Region Responsivity Predicts Future Weight Gain and Moderating Effects of the TaqIA Allele. J. Neurosci 2015;35:10316–10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Coccurello R, Maccarrone M. Hedonic Eating and the “Delicious Circle”: From Lipid-Derived Mediators to Brain Dopamine and Back. Front. Neurosci. [Internet]. 2018. [cited 2018 Jun 21];12. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5928395/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Volkow ND, Wang G-J, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn. Sci 2011;15:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cosgrove KP, Veldhuizen MG, Sandiego CM, et al. Opposing relationships of BMI with BOLD and dopamine D2/3 receptor binding potential in the dorsal striatum. Synap. N. Y. N 2015;69:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang G-J, Volkow ND, Logan J, et al. Brain dopamine and obesity. The Lancet 2001;357:354–357. [DOI] [PubMed] [Google Scholar]
- [10].Matikainen‐Ankney BA, Kravitz AV. Persistent effects of obesity: a neuroplasticity hypothesis. Ann. N. Y. Acad. Sci 2018;1428:221–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baik J-H. Dopamine Signaling in reward-related behaviors. Front. Neural Circuits [Internet]. 2013. [cited 2018 May 21];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3795306/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cone JJ, Chartoff EH, Potter DN, et al. Prolonged High Fat Diet Reduces Dopamine Reuptake without Altering DAT Gene Expression. PLOS ONE 2013;8:e58251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fordahl SC, Locke JL, Jones SR. High Fat Diet Augments Amphetamine Sensitization in Mice: Role of Feeding Pattern, Obesity, and Dopamine Terminal Changes. Neuropharmacology 2016;109:170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Narayanaswami V, Thompson A, Cassis L, et al. Diet-induced obesity: dopamine transporter function, impulsivity and motivation. Int. J. Obes. 2005 [Internet]. 2013. [cited 2018 Jul 6];37. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3856583/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sulzer D, Cragg SJ, Rice ME. Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia 2016;6:123–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Blum K, Thanos PK, Gold MS. Dopamine and glucose, obesity, and reward deficiency syndrome. Front. Psychol. [Internet]. 2014. [cited 2018 Mar 30];5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4166230/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koob GF. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front. Psychiatry [Internet]. 2013. [cited 2018 Dec 19];4. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3730086/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Beaulieu J-M, Gainetdinov RR. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev 2011;63:182–217. [DOI] [PubMed] [Google Scholar]
- [19].Ford CP. The Role of D2-Autoreceptors in Regulating Dopamine Neuron Activity and Transmission. Neuroscience 2014;282:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Baik J-H. Dopamine signaling in food addiction: role of dopamine D2 receptors. BMB Rep 2013;46:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ferrario CR, Labouèbe G, Liu S, et al. Homeostasis Meets Motivation in the Battle to Control Food Intake. J. Neurosci 2016;36:11469–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jais A, Brüning JC. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Invest 2017;127:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rogero MM, Calder PC. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients [Internet]. 2018. [cited 2018 Nov 11];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5946217/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Daws LC, Avison MJ, Robertson SD, et al. Insulin signaling and addiction. Neuropharmacology 2011;61:1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mebel DM, Wong JCY, Dong YJ, et al. Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur. J. Neurosci 2012;36:2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stouffer MA, Woods CA, Patel JC, et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat. Commun 2015;6:8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Serafine KM, Labay C, France CP. Dietary supplementation with fish oil prevents high fat diet-induced enhancement of sensitivity to the locomotor stimulating effects of cocaine in adolescent female rats. Drug Alcohol Depend 2016;165:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hernandez-Casner C, Ramos J, Serafine KM. Dietary supplementation with fish oil prevents high fat diet-induced enhancement of sensitivity to the behavioral effects of quinpirole. Behav. Pharmacol 2017;28:477–484. [DOI] [PubMed] [Google Scholar]
- [29].Hryhorczuk C, Sheng Z, Décarie-Spain L, et al. Oleic Acid in the Ventral Tegmental Area Inhibits Feeding, Food Reward, and Dopamine Tone. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 2018;43:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Décarie-Spain L, Sharma S, Hryhorczuk C, et al. Nucleus accumbens inflammation mediates anxiodepressive behavior and compulsive sucrose seeking elicited by saturated dietary fat. Mol. Metab 2018;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].O’Connor EC, Kremer Y, Lefort S, et al. Accumbal D1R Neurons Projecting to Lateral Hypothalamus Authorize Feeding. Neuron 2015;88:553–564. [DOI] [PubMed] [Google Scholar]
- [32].Seibenhener ML, Wooten MC. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. J. Vis. Exp. JoVE [Internet]. 2015. [cited 2018 Jul 17]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4354627/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fordahl SC, Jones SR. High-Fat-Diet-Induced Deficits in Dopamine Terminal Function Are Reversed by Restoring Insulin Signaling. ACS Chem. Neurosci 2017;8:290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J. Neurosci. Methods 2011;202:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Speed N, Saunders C, Davis AR, et al. Impaired Striatal Akt Signaling Disrupts Dopamine Homeostasis and Increases Feeding. PLOS ONE 2011;6:e25169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int. J. Obes. 2005 2013;37:382–389. [DOI] [PubMed] [Google Scholar]
- [37].Cheema MAR, Nawaz S, Gul S, et al. Neurochemical and behavioral effects of Nigella sativa and Olea europaea oil in rats. Nutr. Neurosci 2018;21:185–194. [DOI] [PubMed] [Google Scholar]
- [38].Díaz-Gerevini GT, Daín A, Pasqualini ME, et al. Diabetic encephalopathy: beneficial effects of supplementation with fatty acids ω3 and nordihydroguaiaretic acid in a spontaneous diabetes rat model. Lipids Health Dis 2019;18:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shinohara M, Mizushima H, Hirano M, et al. Eating disorders with binge-eating behaviour are associated with the s allele of the 3’-UTR VNTR polymorphism of the dopamine transporter gene. J. Psychiatry Neurosci 2004;29:134–137. [PMC free article] [PubMed] [Google Scholar]
- [40].Burke DA, Rotstein HG, Alvarez VA. Striatal local circuitry: a new framework for lateral inhibition. Neuron 2017;96:267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].O’Malley HA, Park Y, Isom LL, et al. PKCβ co-localizes with the dopamine transporter in mesencephalic neurons. Neurosci. Lett 2010;480:40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vaughan RA, Foster JD. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. [Internet]. 2013. [cited 2017 Dec 24];34. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3831354/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Melis M, Pistis M. Hub and switches: endocannabinoid signalling in midbrain dopamine neurons. Phil Trans R Soc B 2012;367:3276–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kamiya Y, Mizuno S, Komenoi S, et al. Activation of conventional and novel protein kinase C isozymes by different diacylglycerol molecular species. Biochem. Biophys. Rep 2016;7:361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Timmers S, de Vogel-van den Bosch J, de Wit N, et al. Differential effects of saturated versus unsaturated dietary fatty acids on weight gain and myocellular lipid profiles in mice. Nutr. Diabetes 2011;1:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nash AI. Crosstalk between insulin and dopamine signaling: A basis for the metabolic effects of antipsychotic drugs. J. Chem. Neuroanat 2017;83–84:59–68. [DOI] [PubMed] [Google Scholar]
- [47].Patel JC, Stouffer MA, Mancini M, et al. Interactions between insulin and diet on striatal dopamine uptake kinetics in rodent brain slices. Eur. J. Neurosci. [Internet]. 2018. [cited 2018 Nov 9]; Available from: http://onlinelibrary.wiley.com/doi/abs/10.1111/ejn.13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Williams LM, Campbell FM, Drew JE, et al. The Development of Diet-Induced Obesity and Glucose Intolerance in C57Bl/6 Mice on a High-Fat Diet Consists of Distinct Phases. PLoS ONE [Internet]. 2014. [cited 2018 Aug 30];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4149520/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Winzell MS, Ahrén B. The High-Fat Diet–Fed Mouse: A Model for Studying Mechanisms and Treatment of Impaired Glucose Tolerance and Type 2 Diabetes. Diabetes 2004;53:S215–S219. [DOI] [PubMed] [Google Scholar]
- [50].Nagy C, Einwallner E. Study of In Vivo Glucose Metabolism in High-fat Diet-fed Mice Using Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT). J. Vis. Exp. JoVE 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Andrikopoulos S, Blair AR, Deluca N, et al. Evaluating the glucose tolerance test in mice. Am. J. Physiol.-Endocrinol. Metab 2008;295:E1323–E1332. [DOI] [PubMed] [Google Scholar]
- [52].Lalia AZ, Lanza IR. Insulin-Sensitizing Effects of Omega-3 Fatty Acids: Lost in Translation? Nutrients [Internet]. 2016. [cited 2018 Nov 16];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4924170/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Corradi V, Sejdiu BI, Mesa-Galloso H, et al. Emerging Diversity in Lipid–Protein Interactions. Chem. Rev 2019;119:5775–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Huang X, Liu G, Guo J, et al. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci 2018;14:1483–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mendes NF, Kim Y-B, Velloso LA, et al. Hypothalamic Microglial Activation in Obesity: A Mini-Review. Front. Neurosci. [Internet]. 2018. [cited 2019 Aug 10];12. Available from: https://www.frontiersin.org/articles/10.3389/fnins.2018.00846/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Valdearcos M, Robblee MM, Benjamin DI, et al. Microglia Dictate the Impact of Saturated Fat Consumption on Hypothalamic Inflammation and Neuronal Function. Cell Rep 2014;9:2124–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gomez JA, Perkins JM, Beaudoin GM, et al. Ventral tegmental area astrocytes orchestrate avoidance and approach behavior. Nat. Commun. [Internet]. 2019. [cited 2019 Jul 31];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6440962/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kardos J, Dobolyi Á, Szabó Z, et al. Molecular Plasticity of the Nucleus Accumbens Revisited—Astrocytic Waves Shall Rise. Mol. Neurobiol. [Internet]. 2019. [cited 2019 Aug 20]; Available from: 10.1007/s12035-019-1641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Felger JC, Treadway MT. Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology 2017;42:216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]


