Abstract
To investigate changes in cytokine (IL-1β, TNF-α and IL-4) levels before and after chemotherapy and their correlation with cognitive impairment in early-stage breast cancer (BC) patients, the 190 BC patients enrolled in this study were divided into two groups: a before chemotherapy group (BCG) and an after chemotherapy group (ACG). The BCG was also divided into two subgroups according to the cognitive assessment results: one group with normal cognition (CNG) and one group with impaired cognition (CIG). Plasma cytokine levels (IL-1β, TNF-α, and IL-4) were evaluated in all patients. The mini-mental state examination (MMSE), prospective and retrospective memory questionnaire (PRMQ), and functional assessment of cancer therapy-cognitive function version 3 (FACT-Cog, version 3) were used to evaluate patients’ self-perceived cognitive impairments. Furthermore, their quality of life (QOL) was evaluated. Plasma IL-1β, TNF-α and IL-4 levels were higher in the ACG than in the BCG (Z = -3.089, -2.458 and -1.987; P = 0.002, 0.014, and 0.047; respectively). Moreover, plasma IL-1β, TNF-α and IL-4 levels were higher in the CIG than in the CNG (Z = -4.353, -3.383 and -2.522; P = 0.000, 0.001 and 0.012; respectively). Furthermore, a correlation was noted between cognition (MMSE, retrospective memory (RM), prospective memory (PM), and FACT-Cog scores) and QOL in BC patients (r = -0.790, 0.852, 0.847 and 0.937, respectively), and a correlation was observed between cognition and cytokine levels (IL-1β, TNF-α, and IL-4) in BC patients (r = -0.681, -0.572 and -0.626; respectively). The present results indicated that changes in cytokine levels may occur in BC patients with chemotherapy-related cognitive impairment (CRCI). We also found that CRCI was associated with QOL after chemotherapy in BC patients. This study provides a theoretical basis for the improvement of QOL in BC patients.
Keywords: Breast cancer, chemotherapy, cognitive impairment, cytokines, quality of life
Introduction
Breast cancer (BC) is the most common cancer threat to women’s health and the second leading cause of cancer death after lung cancer [1]. With the development of new chemotherapy treatments and advances in medical technology, the recurrence and metastasis rates of cancer have been significantly reduced, especially in BC patients. Compared to a yearly increase of approximately 0.3% in the incidence of BC in the past 5 years, the overall mortality rate decreased by approximately 40% from 1989 to 2017 [1]. Higher survival rates have raised concerns about the chronic side effects of BC treatment. Chemotherapy-related cognitive impairment (CRCI), first reported by van Dam in 1998 [2], refers to impairments in learning, memory, attention, executive function and processing speed in cancer survivors [3]. In the literature, it has been reported that between 35% and 75% of BC patients have impaired cognition during or after treatment [4]. The goal of active anti-tumor therapy is not only to achieve long-term survival but also to regain a socially and professionally acceptable lifestyle after treatment. However, even mild cognitive impairment can have a significant impact on a patient’s quality of life (QOL), including slow reactions, impairment in multitasking, and diminished memory, and thus, they lose the opportunity to rejoin society [3,5].
Although many drugs cannot directly pass through the blood-brain barrier (BBB) due to their molecular weight, the effects of chemotherapy in the brain could be mediated, in part, through neuroinflammatory mechanisms. In addition to playing an important role in the regulation of inflammation, cytokines impact the central nervous system through the regulation of neurons, glial cells, neuronal regeneration, neurodegeneration, and cholinergic and dopaminergic pathways [6]. According to these actions, cytokines can be associated with cognition. Cytokines cause local inflammatory responses in the hippocampus and brain tissue rich in cytokine receptors through oxidation and nitrosation [7]. The increase in cytokine levels that accompanies chemotherapy has been corroborated by studies with several animal models [8-10]. For instance, the levels of several cytokines, including IL-2, IL-4, IL-6, L-selectin, MIP-1α, and TNF-α, were shown to increase by >1.5-fold after adriamycin and cyclophosphamide (AC) chemotherapy treatment [11]. Understanding the potential relationship between cognition and cytokines is therefore crucial.
Further studies are needed to determine the exact pathogenesis of CRCI; to date, several candidate mechanisms, including changes in BBB integrity, DNA damage, telomere length, cytokine regulation, neural repair, and neurotransmitters [12], that may cause cognitive impairment have been identified. Although a large amount of research has been conducted on CRCI, little is known about the exact mechanism of CRCI. Traditionally, studies have suggested that cytokines are related to cognition, but whether they are related to CRCI remains unclear.
In this study, we investigated 190 patients with early-stage BC, and all participants were administered a battery of neuropsychological tests. Furthermore, the levels of cytokines (IL-1β, TNF-α and IL-4) were assessed in all participants to determine whether IL-1β, TNF-α and IL-4 levels were related to the occurrence of CRCI.
Materials and methods
Participants
A total of 190 hospitalized BC patients, before and after chemotherapy, were enrolled in this study in the Department of Oncology of the Affiliated Second Hospital of Anhui Medical University from August 2018 to August 2019. The study was approved by the Research Ethics Committee of the Affiliated Second Hospital of Anhui Medical University, and all subjects provided informed consent.
Eligible patients met the following criteria: (1) a postoperative immunohistochemical and pathological diagnosis of BC before and after chemotherapy; (2) normal general cognitive function and daily living ability, with an eastern cooperative oncology group score of 0-1; (3) a primary school education or higher with sufficient audio-visual skills to complete the necessary examinations; and (4) sufficient baseline bone marrow and organ function reserve: specifically, an absolute neutrophil count ≥1500/mm3, a platelet count ≥100,000/mm3, a hemoglobin concentration ≥8 g/dL; serum muscle ≤1.5 times the upper limit of normal; aspartate aminotransferase and alanine aminotransferase ≤2.5 times the upper limit of normal; bilirubin ≤1.5 times the upper limit of normal; and left ventricular ejection fraction (LVEF) ≥50%. The exclusion criteria were as follows: (1) primary or secondary brain tumors; (2) history of neurological or mental illness; (3) distant metastasis or advanced cachexia; (4) hormone or radiation therapy; (5) severe anxiety, depression or other abnormal emotions; and (6) other diseases that can lead to impaired cognitive function.
Neuropsychological tests
Patients were given a series of neuropsychological tests before and after chemotherapy to assess their cognition and QOL at baseline and after treatment.
The mini-mental state examination (MMSE) primarily measures general cognition, including memory, orientation in time and place, working memory, visuospatial skills, object naming, writing, reading, and complex motor operations, with an overall score ranging from 1 to 30, and a higher score indicates better cognition [13]. An MMSE score of 26 or lower is considered cognitive impairment, with 27 to 30 being in the normal range [14].
The functional assessment of cancer therapy-cognitive function version 3 (FACT-Cog, version 3) consists of a 37-item questionnaire that tests mental acuity, attention and concentration, memory, verbal fluency, functional interference and multitasking ability and has been widely used. The item scores range from 0 (“never”) to 4 (“several times a day”). Summing all item scores yields an overall score, and a higher score denotes worse cognitive impairment [15,16].
The prospective and retrospective memory questionnaire (PRMQ) evaluates memory failures in daily life with a 4-point scale, with 1-4 points representing never, occasionally, often, and always [17]. It consists of 16 self-reported items that separately evaluate prospective memory (PM) and retrospective memory (RM). A higher scores indicates more severe memory impairment.
The QOL scale is a reliable and effective scale to measure patients’ health-related QOL. It mainly evaluates their physical, social/family, emotional and functional conditions and includes 27 items to be answered and 9 attachment concerns. The physical and emotional areas are scored on a scale of 1 to 5, representing never, a little, some, comparable, and very; the social/family and functional areas are scored on opposite scales. All of the scores are summed for a final total score. Higher scores predict poorer QOL.
Cytokine measurements
A 2-mL blood sample was drawn from each patient and placed in an ethylene diamine tetraacetic acid (EDTA) tube. All samples were centrifuged for 10 minutes at speeds of 2500 RPM and 4000 RPM. Finally, the plasma part of the blood sample was separated after centrifugation. At least 600 μL of each sample was reserved for use and stored aseptically at -80°C until analysis. Three plasma cytokines (IL-1β, TNF-α, and IL-4) were detected by enzyme-linked immunosorbent assays (ELISAs).
Study procedure
We selected 190 patients with BC before (before chemotherapy group, BCG, n = 68) and after (after chemotherapy group, ACG, n = 122) chemotherapy; blood samples were collected, and neuropsychological tests were performed on all subjects. All participants in the ACG had received 4-6 cycles of chemotherapy based on taxanes and anthracyclines, and there were no intolerable side effects. According to the MMSE score, the ACG was further divided into two additional groups: a cognitive impairment group (CIG, n = 44; MMSE score ≤26) and a cognitive normal group (CNG, n = 78; MMSE score >27).
Statistical analyses
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 23. An independent samples nonparametric test was used to analyze the differences in cytokine levels between groups. The questionnaire scores were compared between groups using an independent samples Student’s t-test. In addition, the chi-square test was used to analyze qualitative data, such as pathological type and tumor stage. All assays were two-tailed with the significance level set to 0.05.
Results
Demographic characteristics and clinical information
There were no significant differences between the groups in age, education, karnofsky performance status (KPS) score, pathological pattern or tumor stage (P>0.05), as shown in Table 1.
Table 1.
Comparison of the demographic characteristics and clinical information of the patients (N = 190)
| BCG (n = 68) | ACG (n = 122) | t/χ2 | P | ||
|---|---|---|---|---|---|
|
| |||||
| CNG (n = 44) | CIG (n = 78) | ||||
| Age (years) | 51.55±7.69 | 50.44±6.85 | 1.030 | 0.304 | |
| 50.15±7.15 | 50.60±6.71 | -0.342 | 0.733 | ||
| Education (years) | 8.67±2.07 | 8.84±2.13 | -0.524 | 0.601 | |
| 8.77±2.11 | 8.88±2.16 | -0.277 | 0.782 | ||
| KPS | 87.20±4.52 | 87.78±4.16 | -0.893 | 0.373 | |
| 87.50±4.38 | 87.94±4.06 | -0.569 | 0.570 | ||
| menstruation | |||||
| premenopausal | 30 | 44 | 1.191 | 0.275 | |
| 18 | 26 | 3.173 | 0.075 | ||
| menopausal | 38 | 78 | |||
| 45 | 33 | ||||
| Pathological pattern (cases) | |||||
| Infiltrative non-specific cancer | 34 | 58 | 0.106 | 0.745 | |
| 21 | 37 | 0.001 | 0.975 | ||
| Invasive ductal carcinoma | 34 | 64 | |||
| 23 | 41 | ||||
| Stage (cases) | |||||
| I | 10 | 14 | 0.556 | 0.757 | |
| 5 | 9 | 0.073 | 0.964 | ||
| II | 34 | 60 | |||
| 21 | 39 | ||||
| III | 24 | 48 | |||
| 18 | 30 | ||||
Notes: Data are presented as the mean ± SD. Abbreviations: SD, standard deviation; KPS, karnofsky performance status; BCG, before chemotherapy group; ACG, after chemotherapy group; CNG, cognitive normal group; CIG, cognitive impairment group.
Neuropsychological and QOL assessments between groups
The scale scores, including the MMSE (t = 10.12, P<0.01), RM (t = -10.25, P<0.01), PM (t = -11.26, P<0.01), FACT-Cog (t = -13.91, P<0.01) and QOL (t = -14.06, P<0.01) scores, were significantly different between the BCG and ACG, as shown in Table 2.
Table 2.
Neuropsychological tests and memory performance in the BCG and ACG
| Items | BCG (n = 68) | ACG (n = 122) | t | P |
|---|---|---|---|---|
| MMSE | 27.82±1.14 | 23.81±4.09 | 10.12 | 0.000 |
| RM | 10.33±2.04 | 15.22±4.48 | -10.25 | 0.000 |
| PM | 10.36±2.01 | 16.37±5.24 | -11.26 | 0.000 |
| FACT-Cog | 37.27±3.95 | 65.31±21.62 | -13.91 | 0.000 |
| QOL | 44.73±6.36 | 74.81±22.02 | -14.06 | 0.000 |
Notes: Data are presented as the mean ± SD. Abbreviations: SD, standard deviation; MMSE, mini-mental state examination; RM, retrospective memory; PM, prospective memory; FACT-Cog, functional assessment of cancer therapy-cognitive Function; QOL, quality of life; BCG, before chemotherapy group; ACG, after chemotherapy group.
Compared to the BCG, the CNG showed no significant differences in the MMSE (t = -0.51, P>0.05), RM (t = 1.30, P>0.05), PM (t = 0.12, P>0.05), FACT-Cog (t = -0.75, P>0.05) and QOL (t = -1.53, P>0.05) scores. In contrast, there were significant differences in the MMSE (t = 16.03, P<0.01), RM (t = -23.69, P<0.01), PM (t = -23.74, P<0.01), FACT-Cog (t = -46.09, P<0.01), and QOL (t = -46.67, P<0.01) scores between the BCG and CIG, as shown in Table 3.
Table 3.
Neuropsychological tests and memory performance in the BCG, CNG and CIG
| Items | BCG (n = 68) | CNG (n = 44) | t | P |
|---|---|---|---|---|
| CIG (n = 78) | ||||
| MMSE | 27.82±1.14 | 27.93±0.99 | -0.51 | 0.609 |
| 21.48±3.26 | 16.03 | 0.000 | ||
| RM | 10.33±2.04 | 9.84±1.82 | 1.30 | 0.194 |
| 18.25±1.98 | -23.69 | 0.000 | ||
| PM | 10.36±2.01 | 10.31±2.19 | 0.12 | 0.903 |
| 19.79±2.76 | -23.74 | 0.000 | ||
| FACT-Cog | 37.27±3.95 | 37.86±4.09 | -0.75 | 0.453 |
| 80.80±7.18 | -46.09 | 0.000 | ||
| QOL | 44.73±6.36 | 46.50±5.21 | -1.53 | 0.128 |
| 90.78±5.55 | -46.67 | 0.000 |
Notes: Data are presented as the mean ± SD. Abbreviations: SD, standard deviation; MMSE, mini-mental state examination; RM, retrospective memory; PM, prospective memory; FACT-Cog, functional assessment of cancer therapy-cognitive Function; QOL, quality of life; BCG, before chemotherapy group; CNG, cognitive normal group; CIG, cognitive impairment group.
Cytokines and cognition
The levels of IL-1β (Z = -3.089, P<0.05), TNF-α (Z = -2.458, P<0.05) and IL-4 (Z = -1.987, P<0.05) were significantly increased in the ACG compared to the BCG, as shown in Table 4 and Figure 1.
Table 4.
Comparison of cytokine levels (IL-1β, TNF-α, and IL-4) in the BCG and ACG
| Cytokine levels (pg/mL) | BCG (n = 68) | ACG (n = 122) | Z | P |
|---|---|---|---|---|
| IL-1β | 45.04 (13.51) | 52.22 (17.84) | -3.089 | 0.002 |
| TNF-α | 47.85 (21.50) | 53.97 (21.40) | -2.458 | 0.014 |
| IL-4 | 30.96 (17.88) | 34.87 (16.21) | -1.987 | 0.047 |
Notes: Data are presented as medians (interquartile). Abbreviations: BCG, before chemotherapy group; ACG, after chemotherapy group.
Figure 1.
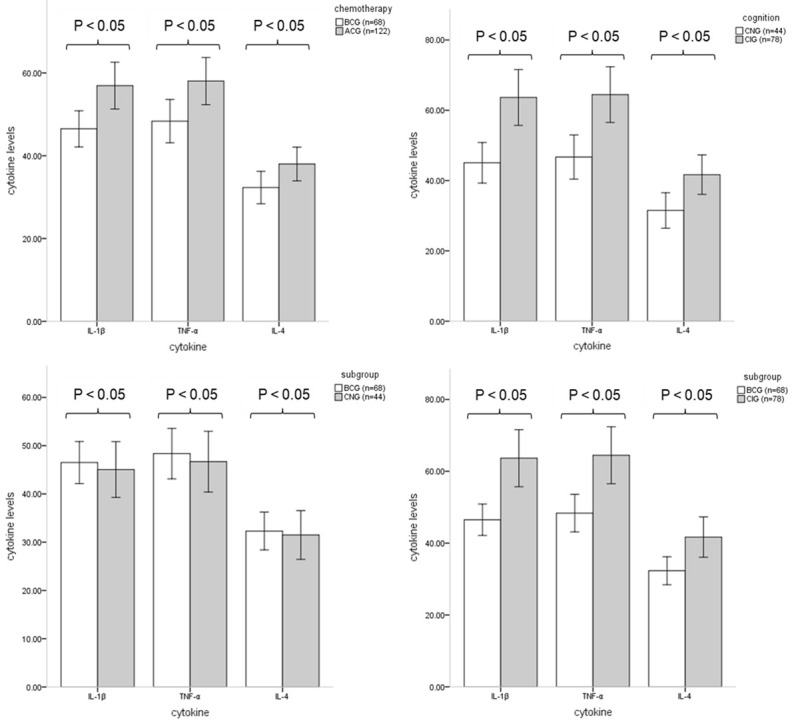
Comparison of cytokine levels between groups. All samples were centrifuged for 10 minutes at speeds of 2500 RPM and 4000 RPM, and the plasma part of the blood sample was separated after centrifugation. The cytokine levels of all samples were determined by ELISAs, and the data are expressed in pg/mL. Significant differences in cytokine levels were observed between the BCG (n = 68) and ACG (n = 122), CNG (n = 44) and CIG (n = 78), and BCG (n = 68) and CIG (n = 78), P<0.05. There were no significant differences in cytokine levels between the BCG (n = 68) and CNG (n = 44), P>0.05.
A comparison of the CNG and CIG showed that IL-1β (Z = -4.353, P<0.05), TNF-α (Z = -3.383, P<0.05) and IL-4 (Z = -2.522, P<0.05) levels were increased in the CIG, as shown in Table 5 and Figure 1.
Table 5.
Comparison of cytokine levels (IL-1β, TNF-α, and IL-4) in the CNG and CIG
| Cytokine levels (pg/mL) | CNG (n = 44) | CIG (n = 78) | Z | P |
|---|---|---|---|---|
| IL-1β | 43.57 (14.57) | 55.84 (16.96) | -4.353 | 0.000 |
| TNF-α | 47.08 (22.41) | 56.76 (22.23) | -3.383 | 0.001 |
| IL-4 | 30.69 (21.36) | 36.28 (15.41) | -2.522 | 0.012 |
Notes: Data are presented as medians (interquartile). Abbreviations: CNG, cognitive normal group; CIG, cognitive impairment group.
There were no significant differences in IL-1β (Z = -0.515, P>0.05), TNF-α (Z = -0.256, P>0.05) and IL-4 (Z = -0.185, P>0.05) levels in the CNG compared to the BCG. In contrast, there were significant differences in IL-1β (Z = -4.743, P<0.01), TNF-α (Z = -3.672, P<0.01) and IL-4 (Z = -2.954, P<0.01) levels between the BCG and CIG, as shown in Table 6 and Figure 1.
Table 6.
Comparison of cytokine levels (IL-1β, TNF-α, and IL-4) in the BCG, CNG and CIG
| Cytokine levels (pg/mL) | BCG (n = 68) | CNG (n = 44) | Z | P |
|---|---|---|---|---|
| CIG (n = 78) | ||||
| IL-1β | 45.04 (13.51) | 43.57 (14.57) | -0.515 | 0.606 |
| 55.84 (16.96) | -4.743 | 0.000 | ||
| TNF-α | 47.85 (21.50) | 47.08 (22.41) | -0.256 | 0.798 |
| 56.76 (22.23) | -3.672 | 0.000 | ||
| IL-4 | 30.96 (17.88) | 30.69 (21.36) | -0.185 | 0.853 |
| 36.28 (15.41) | -2.954 | 0.003 |
Notes: Data are presented as medians (interquartile). Abbreviations: BCG, before chemotherapy group; CNG, cognitive normal group; CIG, cognitive impairment group.
Correlations
Significant negative correlations were observed between cognition and cytokine levels, including IL-1β (r = -0.681; P<0.05), TNF-α (r = -0.572; P<0.05) and IL-4 (r = -0.626; P<0.05) (Figure 2). In addition, QOL was correlated with cognition, as measured by the MMSE (r = -0.779; P<0.05), RM (r = 0.852; P<0.05), PM (r = 0.847; P<0.05), and FACT-Cog (r = 0.936; P<0.05) values (Figure 3).
Figure 2.
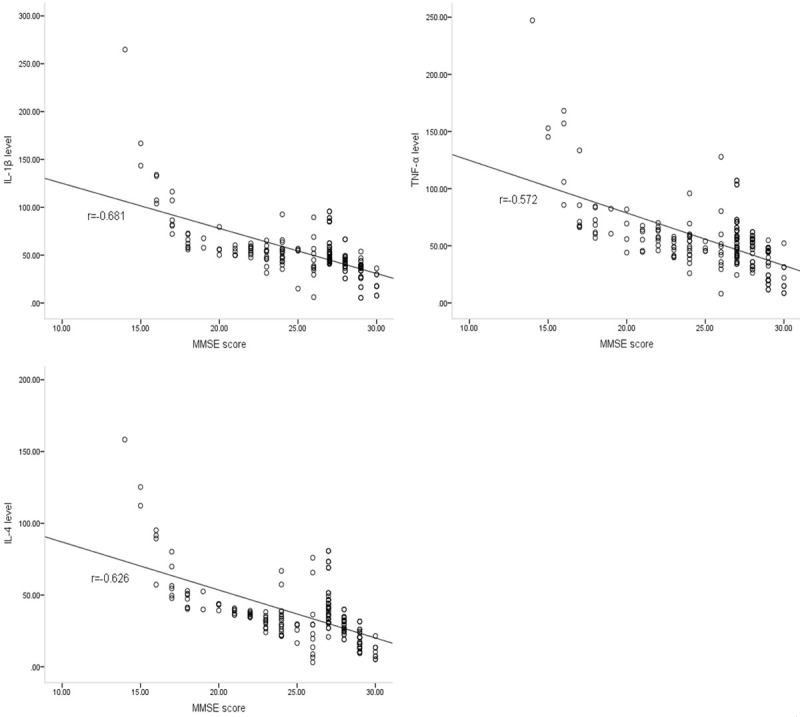
Correlations between cognition and cytokine levels. The MMSE score was used as the basis for cognitive assessment, and a correlation was observed between MMSE scores and cytokine levels, including IL-1β (r = -0.681), TNF-α (r = -0.572) and IL-4 (r = -0.626).
Figure 3.
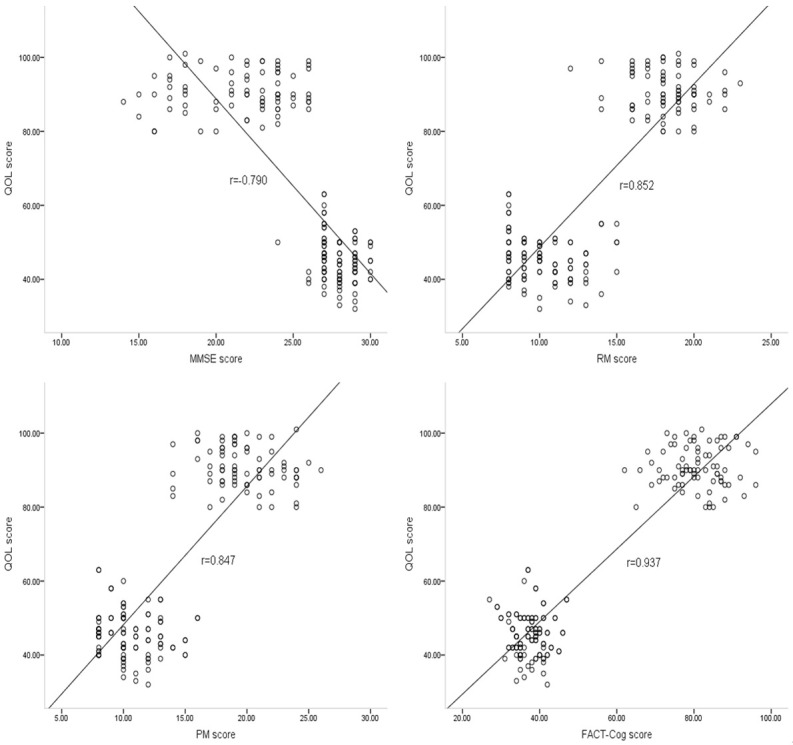
Correlations between cognition and QOL. Correlations were found between QOL and cognition, as measured by the MMSE (r = -0.790), RM (r = 0.852), PM (r = 0.847), and FACT-Cog (r = 0.937).
Discussion
The present results indicated changes in cytokine levels and cognition in BC patients after chemotherapy, and correlations were found between the cytokine levels (IL-1β, TNF-α and IL-4) and CRCI. The relationship between cytokines and cognitive-related diseases, such as Alzheimer’s disease and dementia, has been widely reported in previous studies [18-20].
CRCI objectively exists in BC patients, and the importance of research on this topic is undeniable. Studies on its possible mechanisms, including brain functional imaging [21], electroencephalography [22], and gene polymorphism [23] studies, are common, but studies on the relationship of CRCI with cytokines remain rare. The correlation between cytokine levels and CRCI has been reported in previous studies, but no systematic study on this aspect has been conducted. This study aims to fill the gap in the previous literature.
Higher levels of IL-1β and TNF-α result in poorer cognition. In patients with traumatic brain injury, high levels of IL-1β were associated with a 2-fold increased risk of cognitive decline compared to those with normal IL-1β levels [24]. Excessive IL-1β increases the neuroinflammatory response by activating the NLRP3 inflammasome produced by microglia, eventually leading to increased neuronal cell death and cognitive impairment [25]. A mouse model of mild traumatic brain injury (mTBI) was used to show that TNF-α is a key regulator of a cascade leading to neuron loss and cognitive impairment. According to the signaling pathways, TNF-α exacerbates trauma and oxidative stress in the brain, leading to glutamate release and BBB dysfunction, which further facilitates the transport of large amounts of inflammatory cytokines from the blood into the brain [26,27]. In mice injected with doxorubicin, excessive production of TNF-α, which caused damage to working memory through reactive oxygen species and nitrogen, oxidative stress and mitochondrial damage, was detected in peripheral blood and brain tissues, mainly including the hippocampus and cortex [28]. TNF-α synergistically enhances IL-1β toxicity, and these inflammatory cytokines regulate and induce neuronal inflammation [27].
It is worth noting that previous studies on IL-4 have suggested that it has some protective effects on cognition, which contradicts our study. For instance, IL-4 has been shown to play a beneficial role in the ageing hippocampus, particularly in promoting neuroplasticity and counteracting age-related pro-inflammatory responses [29]. IL-4 antagonized the effects of harmful pro-inflammatory cytokines on astrocytes and neurons [30]. The following reasons were mainly considered to contribute to the inconsistent results of our study and previous reports. The time node of the change in cytokine levels caused by chemotherapy was different, and the timing of blood sample extraction directly affects the levels of cytokines. The effects of various cytokines on cognition were different among individuals.
Although CRCI has been extensively studied, the exact mechanism of its occurrence and development has not been explained in the scientific literature. Previous studies have focused on direct cytotoxicity and related inflammatory mechanisms. Many chemotherapeutic drugs, such as alkylating agents and antibiotics, achieve their anti-tumor effects mainly by destroying the DNA of cancer cells and eventually inducing apoptosis [31]. Meanwhile, chemotherapy is often accompanied by oxidative stress and reactive oxygen species/reactive nitrogen species production, which further aggravates DNA damage in tissues. In addition, defects in the OGG1 and APEX1 genes that protect DNA from oxidative damage may increase the risk of cognitive impairment due to neuron loss before and after chemotherapy [3,32,33].
Accelerated shortening of telomere length after chemotherapy is another possible mechanism of CRCI. A telomere is a region with a repeated nucleotide sequence at the end of chromosomes that is gradually shortened with the replication of DNA. When its length reaches a certain critical value, it can cause ageing and apoptosis [34]. Genetic variation, oxidative stress and chemotherapy are the main factors that affect the speed of telomere shortening [12]. Unlike most nerve cells with regard to mitosis, glial cells remain mitotic and are susceptible to telomere length. Chemotherapy has long-term effects on cognitive function by accelerating the ageing process [35].
As a physical and metabolic barrier, the BBB can restrict the transportation between blood and nerve tissue, effectively maintain the stability of the physiological environment of brain tissue, and protect it from harmful substances in circulating blood [36]. However, certain chemotherapy drugs, such as BCNU, paclitaxel, and 5-fluorouracil, can directly or indirectly damage BBB integrity, making it easier for peripheral toxins and inflammatory mediators to enter the brain, ultimately leading to the death of local neurons associated with cognition [37,38].
Cytokines, stimulated by cancer and chemotherapy, enter brain tissue by means of active transport through the integrity-impaired BBB, causing a local immune response and enhancing oxidative stress and mitochondrial damage[39]. In addition to nitric oxide and reactive oxygen species, microglia can also produce pro-inflammatory cytokines [40]. Microglia, activated by surrounding inflammation, produce chemokines that attract monocytes to the brain and further produce pro-inflammatory cytokines. Excessive inflammatory cytokines in the brain increase neurotoxic metabolites that affect the brain through the kynurenine pathway or by direct neurotoxic effects in specific brain regions [41]. Moreover, cytokines may perpetuate this cognitive impairment by causing somatic epigenetic changes [42]. After a careful study of various candidate mechanisms of CRCI in recent years, CRCI was found to be not necessarily caused by a single element but is probably the result of multiple correlated and interacting factors. This paper focuses on the possible correlation between cytokines and CRCI.
In addition, evaluation of the QOL of the BC patients was also one of the highlights of this study. The QOL of patients with cognitive impairment after chemotherapy was significantly lower than that of those with normal cognition. Indeed, a previous study has reported that a better cognitive reserve was significantly associated with a higher QOL, with a β coefficient ranging from 0.16 to 0.21 [43]. This result highlights the need for CRCI research.
One major limitation of the study was the low number of BC patients included in the study. Moreover, this study was only a cross-sectional study, and a long-term follow-up study should be conducted in the future. Thus, subsequent studies on this aspect can appropriately increase the sample size and improve the credibility of the experiment. In addition, it remains to be determined whether cognitive decline will continue over a longer period of time and whether there are differences in the status and meaningful pace of cognitive recovery among different age groups.
Conclusion
Overall, our preliminary study not only shows changes in cytokines and cognition after chemotherapy but also reveals the correlation between cytokines and cognition. In addition, the impact of CRCI on QOL in BC patients should not be ignored. According to the above results, future studies should focus more on cytokine levels in the prevention and treatment of CRCI to provide a theoretical basis for clinical practice.
Acknowledgements
We appreciate all of the breast cancer patients who participated in this study. This research was supported by the National Natural Science Foundation of China (No. 81872504; 81372487).
Disclosure of conflict of interest
None.
References
- 1.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 2.van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, Rodenhuis S. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90:210–8. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 3.Ren X, Boriero D, Chaiswing L, Bondada S, St Clair DK, Butterfield DA. Plausible biochemical mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”), a condition that significantly impairs the quality of life of many cancer survivors. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1088–1097. doi: 10.1016/j.bbadis.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, LaMonte SJ, Warner E, Lyman GH, Ganz PA. American cancer society/American society of clinical oncology breast cancer survivorship care guideline. J. Clin. Oncol. 2016;34:611–35. doi: 10.1200/JCO.2015.64.3809. [DOI] [PubMed] [Google Scholar]
- 5.Hermelink K. Chemotherapy and cognitive function in breast cancer patients: the so-called chemo brain. J Natl Cancer Inst Monogr. 2015;2015:67–69. doi: 10.1093/jncimonographs/lgv009. [DOI] [PubMed] [Google Scholar]
- 6.Pomykala KL, Ganz PA, Bower JE, Kwan L, Castellon SA, Mallam S, Cheng I, Ahn R, Breen EC, Irwin MR, Silverman DH. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 2013;7:511–23. doi: 10.1007/s11682-013-9243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung YT, Ng T, Shwe M, Ho HK, Foo KM, Cham MT, Lee JA, Fan G, Tan YP, Yong WS, Madhukumar P, Loo SK, Ang SF, Wong M, Chay WY, Ooi WS, Dent RA, Yap YS, Ng R, Chan A. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol. 2015;26:1446–51. doi: 10.1093/annonc/mdv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aluise CD, Miriyala S, Noel T, Sultana R, Jungsuwadee P, Taylor TJ, Cai J, Pierce WM, Vore M, Moscow JA, St Clair DK, Butterfield A. 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-alpha release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radic Biol Med. 2011;50:1630–8. doi: 10.1016/j.freeradbiomed.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Shi DD, Huang YH, Lai CSW, Dong CM, Ho LC, Wu EX, Li Q, Wang XM, Chung SK, Sham PC, Zhang ZJ. Chemotherapy-induced cognitive impairment is associated with cytokine dysregulation and disruptions in neuroplasticity. Mol Neurobiol. 2019;56:2234–2243. doi: 10.1007/s12035-018-1224-4. [DOI] [PubMed] [Google Scholar]
- 10.Ma G, Chen C, Jiang H, Qiu Y, Li Y, Li X, Zhang X, Liu J, Zhu T. Ribonuclease attenuates hepatic ischemia reperfusion induced cognitive impairment through the inhibition of inflammatory cytokines in aged mice. Biomed Pharmacother. 2017;90:62–68. doi: 10.1016/j.biopha.2017.02.094. [DOI] [PubMed] [Google Scholar]
- 11.Bagnall-Moreau C, Chaudhry S, Salas-Ramirez K, Ahles T, Hubbard K. Chemotherapy-Induced cognitive impairment is associated with increased inflammation and oxidative damage in the hippocampus. Mol Neurobiol. 2019;56:7159–7172. doi: 10.1007/s12035-019-1589-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korver S, van de Schraaf SAJ, Geurtsen GJ, Hollak CEM, van Schaik IN, Langeveld M. The mini mental state examination does not accurately screen for objective cognitive impairment in fabry disease. JIMD Rep. 2019;48:53–59. doi: 10.1002/jmd2.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reijneveld JC, Taphoorn MJB, Coens C, Bromberg JEC, Mason WP, Hoang-Xuan K, Ryan G, Hassel MB, Enting RH, Brandes AA, Wick A, Chinot O, Reni M, Kantor G, Thiessen B, Klein M, Verger E, Borchers C, Hau P, Back M, Smits A, Golfinopoulos V, Gorlia T, Bottomley A, Stupp R, Baumert BG. Health-related quality of life in patients with high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17:1533–1542. doi: 10.1016/S1470-2045(16)30305-9. [DOI] [PubMed] [Google Scholar]
- 15.Ng T, Lee YY, Chae JW, Yeo AHL, Shwe M, Gan YX, Ng RCH, Chu PPY, Khor CC, Ho HK, Chan A. Evaluation of plasma brain-derived neurotrophic factor levels and self-perceived cognitive impairment post-chemotherapy: a longitudinal study. BMC Cancer. 2017;17:867. doi: 10.1186/s12885-017-3861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung YT, Foo YL, Shwe M, Tan YP, Fan G, Yong WS, Madhukumar P, Ooi WS, Chay WY, Dent RA, Ang SF, Lo SK, Yap YS, Ng R, Chan A. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: cognitive function (FACT-Cog) in breast cancer patients. J Clin Epidemiol. 2014;67:811–20. doi: 10.1016/j.jclinepi.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Avgan N, Sutherland HG, Lea RA, Spriggens LK, Haupt LM, Shum DHK, Griffiths LR. A creb1 gene polymorphism (rs2253206) is associated with prospective memory in a healthy cohort. Front Behav Neurosci. 2017;11:86. doi: 10.3389/fnbeh.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forlenza OV, Diniz BS, Talib LL, Mendonca VA, Ojopi EB, Gattaz WF, Teixeira AL. Increased serum il-1 beta level in Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis. 2016;52:1479–85. [Google Scholar]
- 19.Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS, Ho RC. IL-1 beta, IL-6, TNF-alpha and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Am J Psychiatry. 2017;174:329–340. doi: 10.1038/s41598-018-30487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang YY, Fan YC, Wang M, Wang D, Li XH. Atorvastatin attenuates the production of IL-1beta, IL-6, and TNF-alpha in the hippocampus of an amyloid beta1-42-induced rat model of Alzheimer’s disease. Clin Interv Aging. 2013;8:103–10. doi: 10.2147/CIA.S40405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Ding K, Zhao J, Chao HH, Li CR, Cheng H. The dorsolateral prefrontal cortex is selectively involved in chemotherapy-related cognitive impairment in breast cancer patients with different hormone receptor expression. Am J Cancer Res. 2019;9:1776–1785. [PMC free article] [PubMed] [Google Scholar]
- 22.Gan C, Lv Y, Zhao J, Chao HH, Li CR, Zhang C, Yu F, Cheng H. Neural correlates of chemotherapy-induced emotion regulation impairment in breast cancer patients. Am J Cancer Res. 2019;9:171–179. [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng H, Li W, Gan C, Zhang B, Jia Q, Wang K. The COMT (rs165599) gene polymorphism contributes to chemotherapy-induced cognitive impairment in breast cancer patients. Am J Transl Res. 2016;8:5087–5097. [PMC free article] [PubMed] [Google Scholar]
- 24.Samatra DPGP, Pratiwi NMD, Widyadharma IPE. High Il-1beta serum as a predictor of decreased cognitive function in mild traumatic brain injury patients. Open Access Maced J Med Sci. 2018;6:1674–1677. doi: 10.3889/oamjms.2018.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding HG, Deng YY, Yang RQ, Wang QS, Jiang WQ, Han YL, Huang LQ, Wen MY, Zhong WH, Li XS, Yang F, Zeng HK. Hypercapnia induces IL-1 beta overproduction via activation of NLRP3 inflammasome: implication in cognitive impairment in hypoxemic adult rats. J Neuroinflammation. 2018;15:4. doi: 10.1186/s12974-017-1051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baratz R, Tweedie D, Wang JY, Rubovitch V, Luo W, Hoffer BJ, Greig NH, Pick CG. Transiently lowering tumor necrosis factor-alpha synthesis ameliorates neuronal cell loss and cognitive impairments induced by minimal traumatic brain injury in mice. J Neuroinflammation. 2015;12:45. doi: 10.1186/s12974-015-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuttolomondo A, Pecoraro R, Pinto A. Studies of selective TNF inhibitors in the treatment of brain injury from stroke and trauma: a review of the evidence to date. Drug Des Devel Ther. 2014;8:2221–38. doi: 10.2147/DDDT.S67655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raffa RB. A proposed mechanism for chemotherapy-related cognitive impairment (‘chemo-fog’) J Clin Pharm Ther. 2011;36:257–9. doi: 10.1111/j.1365-2710.2010.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boccardi V, Westman E, Pelini L, Lindberg O, Muehlboeck JS, Simmons A, Tarducci R, Floridi P, Chiarini P, Soininen H, Kloszewska I, Tsolaki M, Vellas B, Spenger C, Wahlund LO, Lovestone S, Mecocci P, AddNeuroMed P. Differential associations of IL-4 with hippocampal subfields in mild cognitive impairment and Alzheimer’s disease. Front Aging Neurosci. 2019;10:439. doi: 10.3389/fnagi.2018.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–80. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puyo S, Montaudon D, Pourquier P. From old alkylating agents to new minor groove binders. Crit Rev Oncol Hematol. 2014;89:43–61. doi: 10.1016/j.critrevonc.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Sliwinska A, Sitarek P, Toma M, Czarny P, Synowiec E, Krupa R, Wigner P, Bialek K, Kwiatkowski D, Korycinska A, Majsterek I, Szemraj J, Galecki P, Sliwinski T. Decreased expression level of BER genes in Alzheimer’s disease patients is not derivative of their DNA methylation status. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:311–316. doi: 10.1016/j.pnpbp.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Verjat T, Dhenaut A, Radicella JP, Araneda S. Detection of 8-oxoG DNA glycosylase activity and OGG1 transcripts in the rat CNS. Mutat Res. 2000;460:127–38. doi: 10.1016/s0921-8777(00)00022-7. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Z, Pan X, Liu L, Liu N. Telomere length maintenance, shortening, and lengthening. J Cell Physiol. 2014;229:1323–9. doi: 10.1002/jcp.24537. [DOI] [PubMed] [Google Scholar]
- 35.Bolzan AD, Bianchi MS. DNA and chromosome damage induced by bleomycin in mammalian cells: an update. Mutat Res Jan-Mar. 2018;775:51–62. doi: 10.1016/j.mrrev.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Xie J, Shen Z, Anraku Y, Kataoka K, Chen X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials. 2019;224:119491. doi: 10.1016/j.biomaterials.2019.119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabaczar S, Czepas J, Koceva-Chyla A, Kilanczyk E, Piasecka-Zelga J, Gwozdzinski K. The effect of the nitroxide pirolin on oxidative stress induced by doxorubicin and taxanes in the rat brain. J Physiol Pharmacol. 2017;68:295–308. [PubMed] [Google Scholar]
- 38.Wardill HR, Mander KA, Van Sebille YZ, Gibson RJ, Logan RM, Bowen JM, Sonis ST. Cytokine-mediated blood brain barrier disruption as a conduit for cancer/chemotherapy-associated neurotoxicity and cognitive dysfunction. Int J Cancer. 2016;139:2635–2645. doi: 10.1002/ijc.30252. [DOI] [PubMed] [Google Scholar]
- 39.Myers JS. The possible role of cytokines in chemotherapy-induced cognitive deficits. In: Raffa RB, Tallarida RJ, editors. Chemo fog: cancer chemotherapy-related cognitive impariment. 2010. pp. 119–123. [Google Scholar]
- 40.Harry GJ, Kraft AD. Neuroinflammation and microglia: considerations and approaches for neurotoxicity assessment. Expert Opin Drug Metab Toxicol. 2008;4:1265–77. doi: 10.1517/17425255.4.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YK, Won E. The influence of stress on neuroinflammation and alterations in brain structure and function in major depressive disorder. Behav Brain Res. 2017;329:6–11. doi: 10.1016/j.bbr.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 42.Wang XM, Walitt B, Saligan L, Tiwari AF, Cheung CW, Zhang ZJ. Chemobrain: a critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy. Cytokine. 2015;72:86–96. doi: 10.1016/j.cyto.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lara E, Koyanagi A, Caballero F, Domenech-Abella J, Miret M, Olaya B, Rico-Uribe L, Luis Ayuso-Mateos J, Maria Haro J. Cognitive reserve is associated with quality of life: a population-based study. Exp Gerontol. 2017;87:67–73. doi: 10.1016/j.exger.2016.10.012. [DOI] [PubMed] [Google Scholar]


