Abstract
Bak-ri-hyang (Thymus quinquecostatus Celak.) is an important medicinal and aromatic plant in Korea. T. quinquecostatus population and is always mixed with other thyme cultivars during cultivation and marketing. Hence, this study aimed to determine the genetic variability and the essential oil composition of three Korean native thyme, T. quinquecostatus cultivars collected from the Wolchul, Jiri, and Odae mountains, in comparison with six commercial thyme cultivars (T. vulgaris), to distinguish Bak-ri-hyang from other thyme cultivars. The composition of essential oils obtained from nine individuals was analyzed by gas chromatography–mass spectrometry (GC–MS). The random amplified polymorphic DNA (RAPD) analysis was accomplished using 16 different primers. The GC–MS analysis revealed that Wolchul, creeping, golden, and orange cultivars belong to the geraniol chemotype. Whereas the Odae, lemon, and silver cultivars belong to the thymol chemotype. Further, linalool was the most abundant component in carpet and Jiri cultivars. The RAPD analysis demonstrated that all thyme cultivars showed characteristic RAPD patterns that allowed their identification. In total, 133 bands were obtained using 16 primers, and 124 bands were polymorphic, corresponding to 93.2% polymorphism. Cluster analysis of RAPD markers established the presence of clear separation from nine thyme cultivars. The highest dissimilarity and similarity coefficient of the RAPD markers were 0.58 and 0.98, respectively. According to the RAPD patterns, the nine thyme cultivars could be divided into two major clusters. Among three Korean cultivars, the Wolchul and Odae cultivars were placed into the same cluster, but they did not show identical clustering with their essential oil compositions. The findings of the present study suggest that RAPD analysis can be a useful tool for marker-assisted identification of T. quinquecostatus from other Thymus species.
Keywords: essential oil, genetic, RAPD, thyme, Thymus quinquecostatus, Thymus vulgaris
1. Introduction
The genus Thymus (Lamiaceae) consists of approximately 300 species of herbaceous perennials and sub-shrubs, distributed throughout the world and predominantly found in the Mediterranean basin [1,2]. They are widely used as spices, herbal tea, and insecticide in addition to flavor and fragrance materials. Among these, Thymus quinquecostatus (Bak-ri-hyang) is a scrubby subshrub and an important aromatic plant in Korea. Two varieties of T. quinquecostatus such as T. quinquecostatus Celak and T. quinquecostatus var. japonica are found in Korea [3]. In traditional systems of medicine, T. quinquecostatus is used for the treatment of cough, inflammation, preventing excessive intestinal gas, and diaphoresis [3,4,5]. Recent scientific studies reported that T. quinquecostatus has antioxidant, antimicrobial, insecticidal, immunological, antidiabetic, and antitumor properties [6,7,8]. The essential oil of T. quinquecostatus is extensively used in cosmetic industries for fragrance purposes. Owing to its medicinal and aromatic properties, it is also broadly used in pharmaceutical and food industries [9,10,11]. The most abundant components in T. quinquecostatus essential oil are thymol, γ-terpinene, and p-cymene [8].
Currently, thyme seeds are commercially available in the market. However, most thyme cultivars are not yet chemically or genetically characterized. In particular, T. quinquecostatus cultivar is often mixed with other thyme cultivars during cultivation and in nature. Therefore, it is important to validate the methods for the identification of the Korean native thyme cultivar, T. quinquecostatus. The essential oil components were commonly used to examine variations between populations [12,13,14]. It was reported that the genetic constitution and environmental conditions highly influenced the yield and essential oil composition of various plant species [1]. The chemical composition of essential oils might be further altered due to cross hybridization, morphogenesis, polyploidization, extraction methods, drying conditions, stages of harvesting, etc. According to the type, the major components represent 60–95% of the total essential oil. The essential oil composition might vary with the cultivar type. In T. vulgaris, seven different chemotypes, such as thymol, carvacrol, geraniol, linalool, thujanol-4, terpinen-4-ol, and 1,8-cineole are described [15,16]. However, the identification of the factors responsible for the chemical polymorphism registered within species is the most challenging aspect of the essential oil analysis.
In addition, the morphological similarity and anatomical features of thyme cultivars create a problem for the correct identification. The genetic variation of plants was also affected by evolution in both inter- and intra-species. Since the distinctness of Thymus species from another is always challenging to identify, several characters might need to be considered. DNA-based molecular markers were used for the successful detection of this genetic variation, in the process of evolution, gene flow, and population diversity in many plant species. In recent decades, a number of molecular techniques were used to assess genetic diversity in plants. Among them, the PCR-based random amplified polymorphic DNAs (RAPD) were used for the identification of cultivar and genetic relationships, among and within plant species. Similarity banding pattern was scored for calculation of genetic relatedness [13]. Furthermore, RAPD analysis did not need any previous knowledge regarding the target sequence on the genome of the species [15]. When compared with other molecular markers, RAPD markers can produce a high percentage of polymorphism in plants with very similar genetic characteristics. RAPD analysis has various advantages, including easiness, rapidity, requiring a small amount of genomic DNA as a template, and the possibility for detecting dissimilarity in the coding and noncoding areas of the genome [17,18,19]. Previously, several authors have reported the use of RAPD markers to study the genetic diversity, phylogenetic relationship, and its combination with the analysis of essential oil composition in various Thymus species [1,13,15,20,21]. RAPD data are also important to solve the taxonomic issues within and among plant species. Furthermore, the physiological and morphological variations, essential oil composition, ploidy level, and the relationship between the chemical and genetic evaluations of the Thymus species were assessed [15].
In recent times, several companies have commercialized thyme seeds. Hence, Korean native thyme, T. quinquecostatus (Bak-ri-hyang) is always mingled with commercial thyme species during cultivation and marketing. In addition, there are no studies on the RAPD evaluation of T. quinquecostatus and most studies focus on its essential oil composition. In this context, the present study aimed to evaluate the chemical and genetic variations among T. quinquecostatus cultivars from Korea and six commercial T. vulgaris cultivars, by using different RAPD markers and essential oil profiles, in order to distinguish Korean native thyme, Bak-ri-hyang from commercial thyme cultivars.
2. Methods
2.1. Plant Materials
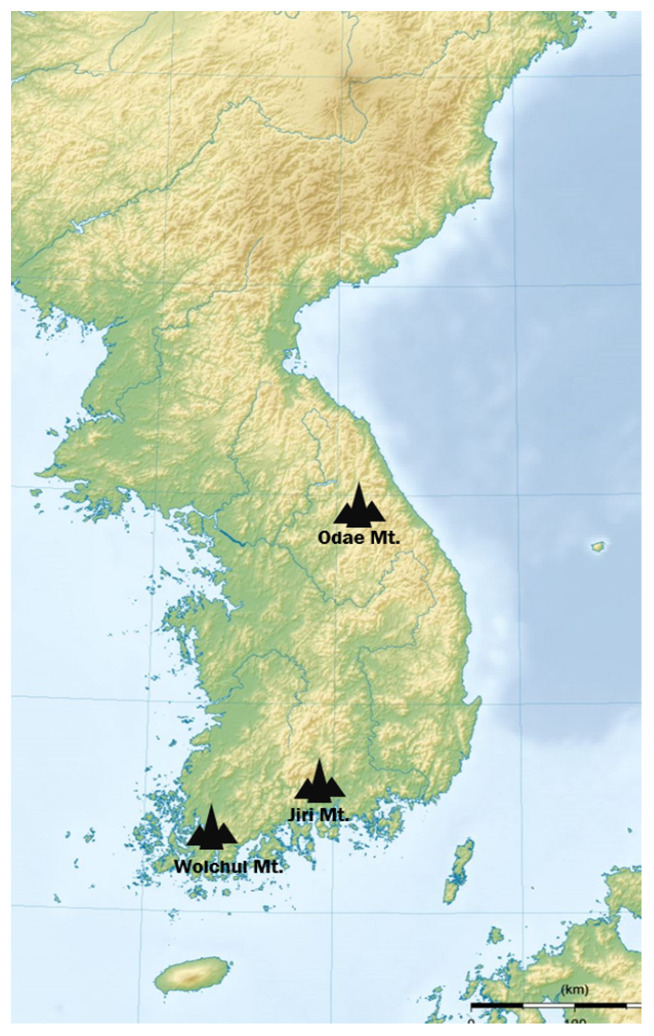
First, a survey of the native thyme species (T. quinquecostatus) grown in Korea was carried out. We also interviewed experts who had ethnobotanical knowledge on Korean native thyme. According to their ethnobotanical information, we collected T. quinquecostatus from three accessions, such as Odae Mt, Wolchul Mt, and Jiri Mt in Korea, during April 2018 (Figure 1 and Figure 2). In addition, fresh plants of six T. vulgaris cultivars (lemon, golden lemon (golden), carpet, orange, silver, and creeping) were purchased from Daerim Horticulture, Gwachon, Happy Horticulture, Goyang and Nature Horticulture, Yangju, Republic of Korea (Figure 2).
Figure 1.
The map showing the collection sites of three accessions (Wolchul, Odae, and Jiri mountains) of Thymus quinquecostatus in South Korea.
Figure 2.
The morphology of six commercial Thymus vulgaris cultivars and three Korean native Thymus quinquecostatus cultivars. (1) Lemon; (2) golden; (3) carpet; (4) orange; (5) silver; (6) creeping; (7) Odae Mt.; (8) Wolchul Mt.; and (9) Jiri Mt.
The plants were authenticated and deposited in the Herbarium, Daejin University, Pocheon, Gyeonggi-do, Republic of Korea, with voucher numbers: lemon—DJU20180713, golden—DJU20180712, carpet—DJU20180717, orange—DJU20180715, silver—DJU20180714, creeping—DJU20180716, Odae Mt.—DJU20180718, Wolchul Mt.—DJU20180719, and Jiri Mt.—DJU20180720. The collected samples were kept at –20 °C for the essential oil analysis and –80 °C for the molecular analysis.
2.2. Morphological Characteristics
The morphological parameters such as stem type, stem branch, stem color, leaf shape, number of auxiliary leaves, and trichome position were observed for the six commercial and three Korean native thyme cultivars.
2.3. Essential Oil Extraction
The essential oil from nine thyme samples was isolated by steam distillation, using a Clevenger-type apparatus. The steam distillation was performed at 100 °C for 90 min. The essential oil isolation was carried out in triplicates and the yield (%) was calculated as volume (mL) of the isolated oil per 100 g of the fresh plant material. The isolated essential oil was dried using anhydrous sodium sulfate and stored at 4 °C, until tested. The color of essential oils obtained from the three Korean native T. quinquecostatus cultivars was measured, using the Chromameter CT-300 (Mintola Camera Co. Ltd., Japan). The intensity of the color was expressed in terms of L* lightness, a* greenness, and b* yellowness. The color values of L*, a*, and b* were taken in triplicates for each sample.
2.4. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
The identification of the essential oil components from different thyme cultivars was performed using a Varian CP3800 gas chromatograph coupled with a Varian 1200 L mass detector (Varian, CA, USA). The GC–MS was equipped with a VF-5MS polydimethylsiloxane capillary column (30 m × 0.25 mm × 0.25 μm). The oven temperature was programmed from 50 °C to 250 °C, at a rate of 5 °C/min. The injector temperature was 250 °C and the ionization detector temperature was 200 °C. Helium was the carrier gas (1 mL/min) and the injected volume of the sample was 2 μL, with a split ratio of 10:1. For mass spectra, an electron ionization system with ionization energy of 70 eV was used. The mass range was 50–500 m/z. The determination of the percentage composition of each component was based on the normalization of the GC peak areas. The identification of the essential oil components was based on the comparison of their retention indices (RIs), relative to a homologous series of n-alkanes (C8–C22) and mass spectra from the National Institute of Standards and Technology (NIST, 3.0) library and literature data [22].
2.5. DNA Extraction
The total genomic DNA was isolated from one gram of young leaves of plants, according to the CTAB (cetyl trimethylammonium bromide) extraction method [15]. DNA pellets were dissolved in TE (Tris–EDTA) buffer and RNA was removed by digestion with DNase-free RNase A. The purified total DNA was quantified and its quality was verified using a spectrophotometer, and a diluted solution with the same concentration (10 ng/μL) was prepared by adding TE buffer and was stored at 4 °C.
2.6. Randomly Amplified Polymorphic DNA (RAPD) Analysis
A total of 16 primers (OPA-09, OPA-10, OPA-11, OPA-12, OPA-13, OPA-14, OPA-15, OPA-16, OPA-17, OPA-18, OPA-19, OPA-20, OPB-01, OPB-02, OPB-03, and OPB-04) were used for the RAPD analysis (Table 1). The selection of primers was based on high polymorphisms and good reproducibility of the fragments generated. RAPD amplification was performed in a volume of 25 µL containing 10 ng total DNA, 1× PCR buffer, 3.0 mM MgCl2, 200 µM deoxynucleotide triphosphates (dNTPs), 1 μM primer, 1 µg/mL (w/v) Bovine Serum Albumin (BSA), and 1 unit Taq DNA polymerase (Invitrogen). The amplification reactions were performed in a thermocycler and consisted of an initial 5 min denaturation step at 95 °C, followed by 40 cycles of 20 s at 95 °C, 40 s at 35 °C, and 60 s at 72 °C. A final extension of 5 min at 72 °C completed the amplification. The PCR products were separated in 1.2% agarose gels 1× TAE buffer (Tris–Acetate). The gels were stained with ethidium bromide, visualized with a UV transilluminator.
Table 1.
The names and sequences of the primers used for random amplified polymorphic DNA (RAPD) analysis.
| S. No. | Primer | Sequence |
|---|---|---|
| 1 | OPA-09 | GGGTAACGCC |
| 2 | OPA-10 | GTGATCGCAG |
| 3 | OPA-11 | CAATCGCCGT |
| 4 | OPA-12 | TCGGCGATAG |
| 5 | OPA-13 | CAGCACCCAC |
| 6 | OPA-14 | TCTGTGCTGG |
| 7 | OPA-15 | TTCCGAACCC |
| 8 | OPA-16 | AGCCAGCGAA |
| 9 | OPA-17 | GACCGCTTGT |
| 10 | OPA-18 | AGGTGACCGT |
| 11 | OPA-19 | CAAACGTCGG |
| 12 | OPA-20 | GTTGCGATCC |
| 13 | OPB-01 | GTTTCGCTCC |
| 14 | OPB-02 | TGATCCCTGG |
| 15 | OPB-03 | CATCCCCCTG |
| 16 | OPB-04 | GGACTGGAGT |
2.7. Statistical Analysis
To calculate RAPD polymorphism, the RAPD markers were scored for the presence (1) or absence (0) of amplified bands for 9 thyme cultivars. Genetic similarity was estimated using the Jaccard’s coefficients. Cluster analysis was performed using the unweighted pair group method with an arithmetic mean (UPGMA), and dendrograms were drawn using NTSYS software version 2.02.
3. Results
3.1. Morphological Characteristics of the Thyme Cultivars
The morphological characteristics of six commercial and three Korean native thyme cultivars are presented in Table 2. In these, all three T. quinquecostatus cultivars had a creeping type of stem. On the other hand, lemon, golden, orange, and silver cultivars possessed an erect stem type. The length of the stem branch varied among different cultivars. In the creeping stem type, the length of the stem branch ranged from 2 to 8 cm, whereas the length of the stem branch in the erect type ranged from 3 to 11 cm. Carpet cultivar possessed a higher number of stem branches than the other cultivars. The shape of the leaves was mainly oval, followed by oblanceolate. In the case of the Bak-ri-hyang cultivars, the Odae and Jiri cultivars had an oval shape of leaves. The leaf shape of the Wolchul cultivar was oblanceolate. Furthermore, the Bak-ri-hyang cultivars possessed a higher number of auxiliary leaves when compared with the commercial thyme cultivars. The trichome position was mainly observed at the leaf petiole. In the Wolchul cultivar, the trichome position was observed at the leaf margin.
Table 2.
Morphological characteristics of six commercial Thymus vulgaris cultivars and three Korean native Thymus quinquecostatus cultivars.
| Thymus Sp. | Variety | Stem Type | Stem Branch | Stem Color | Leaf Shape | Number of Auxiliary Leaves | Trichome Position | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Length (cm) | Number | Purple | Pale Purple | Pale Green | Leaf Petiole | Leaf Margin | Leaf Surface | |||||
| Thymus vulgaris | Lemon | Erect | >3 | 2 | - | - | √ | Oval | 0 | - | - | - |
| Golden | Erect | 0~8 | 2 | - | √ | √ | Elliptical | 2 | √ | - | - | |
| Carpet | Creeping | 0~4 | 4 | - | √ | - | Oval | 0 | √ | √ | √ | |
| Orange | Erect | 0~11 | 0e | - | - | √ | Oblanceolate /Oblong |
0~2 | √ | - | - | |
| Silver | Erect | 0~5 | 0 | - | √ | - | Oblong | 0~2 | - | - | - | |
| Creeping | Creeping | 0~2 | 2 | √ | - | - | Oval | 0~2 | √ | - | - | |
| Bak-ri-hyang (Thymus quinquecostatus) |
Odae Mt. | Creeping | >2 | 0 | √ | - | - | Oval | 2~6 | √ | - | - |
| Wolchul Mt. | Creeping | 0~8 | 2 | - | √ | √ | Oblanceolate | 2~8 | - | √ | - | |
| Jiri Mt. | Creeping | >2 | 0 | √ | - | - | Oval | 2~6 | √ | - | - | |
3.2. The Chemical Composition of Essential Oils
The yield and chemical composition of essentials oils obtained from the nine thyme cultivars are presented in Table 3 and Table 4. The essential oil components and their concentration produced by thyme cultivars were very diverse. The essential oil yields ranged between 0.12% and 0.43% (v/w) for the T. quinquecostatus cultivars. The highest yield was obtained from the Odae cultivar (0.43%). For commercial cultivars of T. vulgaris, the essential oil yields ranged from 0.23% to 0.33%. In these, the highest yields were obtained from the lemon and silver cultivars (0.34% and 0.33%, respectively), and the lowest yield was obtained from the carpet cultivar (0.23%). The color profile of the essential oils obtained from the T. quinquecostatus cultivars was measured. The L* value of the essential oils of the Wolchul, Odae, and Jiri cultivars was 92.48, 92.54, and 92.39, respectively. Wolchul and Jiri cultivars possessed similar a* value (0.18). With regards to the b* value, the Wolchul cultivar showed the highest value (2.29) and the Odae cultivar showed the lowest value (1.89). The total number of components in the analyzed essential oils ranged between 32 (creeping cultivar) and 43 (lemon cultivar). In these nine samples, twelve compounds were detected in all essential oil samples and these oils were dominated by monoterpenes, accounting for 79.95–92.16% with 0.03–46.47% of monoterpene hydrocarbons and 43.86–88.46% of oxygenated monoterpenes. Whereas sesquiterpenes achieved 6.50–29.17% with 5.83–15.07% of sesquiterpene hydrocarbons and 0.32%–17.23% of oxygenated sesquiterpenes.
Table 3.
The yield and color of the essential oils isolated from the six commercial Thymus vulgaris cultivars and the three Korean native Thymus quinquecostatus cultivars.
| Thymus Sp. | Cultivar | Essential Oil Yield (%) | Essential Oil Color |
|---|---|---|---|
| Thymus vulgaris | Lemon | 0.34 ± 0.013 | Yellow |
| Golden | 0.29 ± 0.020 | Yellow | |
| Carpet | 0.23 ± 0.015 | Pale yellow | |
| Orange | 0.29 ± 0.033 | Pale yellow | |
| Silver | 0.33 ± 0.042 | Yellow | |
| Creeping | 0.24 ± 0.021 | Yellow | |
| Thymus quinquecostatus (Bakrihyang) | Odae Mt. | 0.43 ± 0.067 | Yellow |
| Wolchul Mt. | 0.28 ± 0.017 | White | |
| Jiri Mt. | 0.12 ± 0.014 | Yellow |
Table 4.
The chemical composition of essential oils isolated from the six commercial Thymus vulgaris cultivars and the three Korean native Thymus quinquecostatus cultivars.
| S. No. | Compound Name | RI Lit | RI Cal | Area (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lemon | Golden | Carpet | Orange | Silver | Creeping | Odae | Wolchul | Jiri | ||||
| 1 | Tricyclene | 926 | 923 | - | - | - | - | - | - | 0.05 ± 0.01 | 0.01 ± 0.00 | - |
| 2 | α-Thujene | 930 | 926 | 0.17 ± 0.01 | - | - | - | 0.04 ± 0.00 | - | 1.91 ± 0.50 | 0.01 ± 0.00 | 0.11 ± 0.02 |
| 3 | α-Pinene | 939 | 934 | 0.09 ± 0.01 | - | - | - | 0.02 ± 0.00 | - | 1.32 ± 0.32 | 0.26 ± 0.01 | 0.09 ± 0.02 |
| 4 | Camphene | 954 | 950 | 0.11 ± 0.01 | - | - | 0.01 ± 0.00 | 0.02 ± 0.00 | - | 1.69 ± 0.42 | 0.67 ± 0.01 | 0.18 ± 0.03 |
| 5 | β-Pinene | 974 | 978 | 0.24 ± 0.33 | - | - | - | 0.19 ± 0.02 | - | 1.89 ± 0.47 | 0.17 ± 0.01 | 0.30 ± 0.03 |
| 6 | 1-Octen-3-ol | 979 | 985 | 3.61 ± 0.04 | 0.70 ± 0.06 | 0.57 ± 0.06 | 0.39 ± 0.02 | 1.46 ± 0.05 | 0.44 ± 0.03 | 2.81 ± 0.54 | 2.47 ± 0.02 | 3.29 ± 0.30 |
| 7 | 3-Octanone | 983 | 989 | 0.76 ± 0.03 | 0.34 ± 0.02 | - | 0.01 ± 0.00 | 0.17 ± 0.01 | 0.0 8 ± 0.00 | 0.07 ± 0.00 | 0.82 ± 0.02 | 1.27 ± 0.20 |
| 8 | 3-Octanol | 991 | 1000 | 0.38 ± 0.01 | 0.36 ± 0.02 | - | 0.06 ±0.01 | 0.23 ±0.02 | 0.15 ± 0.01 | 0.08 ± 0.02 | 0.70 ± 0.01 | 1.21 ± 0.19 |
| 9 | α-Phellandrene | 1002 | 1007 | 0.11 ± 0.01 | - | - | 0.04 ± 0.00 | 0.31 ± 0.09 | - | 0.05 ± 0.01 | ||
| 10 | 3-Carene | 1011 | 1009 | 0.02 ± 0.01 | - | - | 0.08 ± 0.02 | - | 0.02 ± 0.01 | |||
| 11 | p-Cymene | 1024 | 1026 | 2.84 ± 0.10 | 0.41 ± 0.03 | - | 0.01 ± 0.00 | 2.22 ± 0.04 | 11.13 ± 0.62 | 0.05 ± 0.00 | 2.44 ± 0.15 | |
| 12 | D-Limonene | 1029 | 1031 | 0.17 ± 0.01 | 0.01 ± 0.00 | - | 0.03 ± 0.01 | 0.53 ± 0.05 | 0.11 ± 0.01 | 0.10 ± 0.02 | ||
| 13 | Eucalyptol | 1032 | 1034 | 0.29 ± 0.01 | 0.09 ± 0.01 | - | 0.02 ± 0.01 | 0.41 ± 0.03 | 0.02 ± 0.01 | 0.44 ± 0.01 | - | |
| 14 | β-Ocimene | 1050 | 1049 | 0.25 ± 0.01 | - | - | 0.09 ± 0.00 | 0.04 ± 0.00 | 0.12 ± 0.02 | |||
| 15 | γ-Terpinene | 1059 | 1060 | 8.44 ± 0.01 | 0.88 ± 0.06 | 0.05 ± 0.00 | 0.04 ± 0.00 | 3.85 ± 0.04 | 0.03 ± 0.00 | 23.92 ± 3.30 | 0.12 ± 0.00 | 3.43 ± 0.24 |
| 16 | Sabinene hydrate | 1070 | 1073 | 0.87 ± 0.03 | 0.05 ±0.01 | - | 0.15 ± 0.01 | 0.74 ± 0.06 | 3.02 ± 0.51 | - | - | |
| 17 | 1-Nonen-3-ol | 1078 | 1083 | - | - | - | 0.18 ± 0.01 | 0.30 ± 0.02 | - | - | - | - |
| 18 | Terpinolene | 1088 | 1087 | 0.93 ± 0.04 | 0.06 ± 0.01 | - | - | - | - | 3.55 ± 0.87 | 0.88 ± 0.02 | 0.56 ± 0.05 |
| 19 | Nonanone | 1090 | 1088 | - | - | - | - | 0.09 ± 0.01 | - | |||
| 20 | Linalool | 1096 | 1103 | 2.60 ± 0.12 | 0.47 ± 0.04 | 48.16 ± 0.67 | 0.36 ± 0.02 | 2.08 ± 0.11 | 3.86 ± 0.09 | 0.11 ± 0.03 | 1.49 ± 0.01 | 47.89 ± 3.11 |
| 21 | Nonanal | 1100 | 1108 | - | - | - | - | - | - | - | 0.07 ± 0.00 | - |
| 22 | 1-Octen-3-yl-acetate | 1112 | 1109 | - | - | - | - | - | - | - | 0.02 ± 0.00 | - |
| 23 | Chrysanthemal | 1124 | 1120 | - | - | - | - | - | 0.43 ± 0.01 | - | - | - |
| 24 | Verbenol | 1141 | 1145 | - | 0.27 ± 0.01 | - | - | - | 0.85 ± 0.17 | - | 0.03 ± 0.00 | - |
| 25 | Camphor | 1146 | 1151 | 0.23 ± 0.03 | - | 0.04 ± 0.01 | 0.03 ± 0.00 | 0.62 ± 0.04 | - | 2.47 ± 0.45 | - | 0.10 ± 0.01 |
| 26 | β-Pinene oxide | 1159 | 1166 | 0.14 ± 0.02 | 0.02 ± 0.01 | - | - | - | 0.09 ± 0.02 | - | - | - |
| 27 | Borneol | 1169 | 1180 | 2.07 ± 0.07 | 0.41 ± 0.03 | 1.57 ± 0.16 | 3.05 ± 0.21 | 1.11 ± 0.11 | 0.73 ± 0.02 | 2.17 ± 0.49 | 5.91 ± 0.03 | 2.17 ± 0.34 |
| 28 | Terpinen-4-ol | 1177 | 1185 | 0.40 ± 0.01 | - | 0.15 ± 0.03 | 0.17 ± 0.01 | 0.37 ± 0.05 | 0.05 ± 0.00 | 1.49 ± 0.33 | 0.63 ± 0.03 | 0.17 ± 0.01 |
| 29 | α-Terpineol | 1188 | 1200 | 0.11 ± 0.03 | 0.05 ± 0.00 | 0.67 ± 0.01 | 0.04 ± 0.00 | 0.17 ± 0.04 | 0.03 ± 0.02 | 0.21 ± 0.06 | 0.09 ± 0.01 | 0.05 ± 0.01 |
| 30 | Dihydrocarvone | 1192 | 1202 | - | - | - | 0.04 ± 0.01 | 0.06 ± 0.02 | - | - | 0.13 ± 0.01 | 0.02 ± 0.01 |
| 31 | Decanal | 1201 | 1211 | 0.05 ± 0.01 | - | 0.06 ± 0.02 | 0.02 ± 0.00 | - | - | 0.07 ± 0.03 | 0.10 ± 0.01 | 0.05 ± 0.02 |
| 32 | Nerol | 1229 | 1233 | 2.66 ± 0.12 | 1.16 ± 0.05 | 0.17 ± 0.02 | 0.47 ± 0.03 | 1.99 ± 0.05 | 4.70 ± 0.05 | - | 1.34 ± 0.06 | - |
| 33 | Thymol methyl ether | 1235 | 1235 | 3.00 ± 0.09 | 0.26 ± 0.03 | 0.09 ± 0.03 | - | 2.17 ± 0.10 | - | - | - | 2.27 ± 0.16 |
| 34 | Neral | 1238 | 1243 | 0.99 ± 0.06 | 3.39 ± 0.21 | - | 0.26 ± 0.02 | 0.18 ± 0.05 | 11.75 ± 0.12 | - | 0.85 ± 0.03 | - |
| 35 | 2-Isopropyl-4-methylanisole | 1244 | 1242 | - | - | - | - | - | - | - | - | 1.27 ± 0.18 |
| 36 | Geraniol | 1252 | 1262 | 6.03 ± 0.14 | 65.99 ± 2.30 | 0.73 ± 0.47 | 44.70 ± 0.67 | 3.02 ± 0.26 | 29.57 ± 0.65 | 0.35 ± 0.12 | 42.94 ± 0.32 | 0.03 ± 0.01 |
| 37 | Geranial | 1276 | 1264 | 1.49 ± 0.02 | 5.42 ± 0.18 | 0.03 ± 0.01 | - | 0.31 ± 0.04 | 18.21 ± 0.83 | - | - | - |
| 38 | Cyclodecane | 1271 | 1281 | - | - | - | - | - | - | - | - | 0.08 ± 0.03 |
| 39 | 1-Decanol | 1269 | 1283 | - | - | - | - | - | - | - | 0.18 ± 0.01 | - |
| 40 | Bornyl acetate | 1285 | 1292 | - | - | 0.09 ± 0.00 | 0.19 ± 0.01 | - | 0.04 ± 0.01 | - | 0.53 ± 0.02 | - |
| 41 | Thymol | 1290 | 1295 | 43.91 ± 1.64 | 2.70 ± 0.09 | 13.36 ± 0.31 | 8.05 ± 0.03 | 66.24 ± 1.66 | 2.17 ± 0.08 | 30.54 ± 4.37 | 0.44 ± 0.02 | 15.98 ± 0.18 |
| 42 | Carvacrol | 1299 | 1305 | - | - | 0.78 ± 0.01 | - | 2.62 ± 0.03 | - | - | - | 0.27 ± 0.00 |
| 43 | Methyl geranate | 1324 | 1324 | - | 0.11 ± 0.01 | - | - | - | 0.03 ± 0.00 | - | - | - |
| 44 | Thymol acetate | 1352 | 1349 | 0.25 ± 0.01 | - | - | 0.57 ± 0.03 | 0.42 ± 0.03 | - | 0.29 ± 0.08 | - | 0.10 ± 0.02 |
| 45 | Geranyl acetate | 1381 | 1386 | 2.34 ± 0.11 | 2.89 ± 0.22 | - | 29.86 ± 0.84 | 1.09 ± 0.09 | 6.75 ± 0.08 | 0.20 ± 0.08 | 26.49 ± 0.05 | - |
| 46 | β-Bourbonene | 1388 | 1387 | 0.04 ± 0.00 | 0.13 ± 0.02 | 0.10 ± 0.01 | - | - | 0.15 ± 0.01 | - | - | 0.14 ± 0.02 |
| 47 | β-Cubebene | 1388 | 1390 | 2.61 ± 0.14 | 2.20 ± 0.17 | 5.07 ± 0.02 | 0.40 ± 0.04 | 0.81 ± 0.10 | 2.44 ± 0.16 | 0.19 ± 0.08 | - | 3.47 ± 0.27 |
| 48 | β-Elemene | 1390 | 1394 | 0.06 ± 0.00 | 0.07 ± 0.01 | 0.19 ± 0.02 | 1.02 ± 0.09 | 0.08 ± 0.01 | 0.10 ± 0.02 | 0.65 ± 0.22 | - | 0.67 ± 0.06 |
| 49 | Caryophyllene | 1419 | 1428 | 3.41 ± 0.16 | 4.56 ± 0.18 | 2.62 ± 0.01 | 4.74 ± 0.66 | 4.48 ± 0.27 | 2.87 ± 0.09 | 4.74 ± 1.04 | 4.73 ± 0.02 | 7.02 ± 0.39 |
| 50 | Neryl propionate | 1432 | 1439 | - | 0.46 ± 0.22 | - | - | - | 1.34 ± 0.17 | - | - | - |
| 51 | α-Bergamotene | 1434 | 1438 | - | - | - | - | - | - | - | 0.04 ± 0.01 | - |
| 52 | γ-Elemene | 1436 | 1442 | - | 0.03 ± 0.01 | - | - | - | - | - | - | - |
| 53 | Aromadendrene | 1441 | 1438 | - | - | 0.12 ± 0.02 | - | - | - | 0.08 ± 0.02 | - | - |
| 54 | α-Humulene | 1454 | 1463 | 3.51 ± 0.15 | - | 0.17 ± 0.01 | - | 0.18 ± 0.02 | - | - | 0.66 ± 0.03 | - |
| 55 | β-Farnesene | 1456 | 1454 | - | - | 0.04 ± 0.00 | 0.02 ± 0.01 | - | - | - | - | - |
| 56 | Germacrene D | 1480 | 1487 | - | - | - | - | - | - | - | 0.53 ± 0.02 | - |
| 57 | β-Selinene | 1490 | 1494 | - | - | 0.03 ± 0.00 | - | - | - | - | - | - |
| 58 | α-Farnesene | 1505 | 1506 | - | - | - | - | - | - | - | - | 0.40 ± 0.07 |
| 59 | β-Bisabolene | 1505 | 1514 | 2.22 ± 0.10 | 3.70 ± 0.26 | 2.47 ± 0.31 | 3.84 ± 0.45 | 0.23 ± 0.03 | 1.81 ± 0.11 | 1.21 ± 0.34 | 3.86 ± 0.03 | 3.00 ± 0.27 |
| 60 | γ-Cadinene | 1513 | 1518 | - | - | - | - | - | - | 0.05 ± 0.01 | - | - |
| 61 | Butylated hydroxytoluene | 1515 | 1518 | 0.14 ± 0.02 | 0.14 ± 0.02 | 0.60 ± 0.09 | 0.06 ± 0.01 | 0.18 ± 0.01 | 0.24 ± 0.04 | - | - | - |
| 62 | δ-Cadinene | 1523 | 1522 | - | - | 0.11 ± 0.01 | - | - | - | 0.12 ± 0.03 | - | - |
| 63 | β-Sesquiphellandrene | 1522 | 1528 | 0.12 ± 0.01 | 0.06 ± 0.01 | 1.02 ± 0.12 | 0.08 ± 0.01 | 0.05 ± 0.01 | 0.15 ± 0.03 | - | 0.10 ± 0.01 | 0.36 ± 0.07 |
| 64 | α-Elemol | 1549 | 1554 | 0.31 ± 0.02 | 0.05 ± 0.01 | 11.62 ± 0.25 | - | 0.04 ± 0.01 | 8.52 ± 0.52 | - | - | - |
| 65 | Geranyl butyrate | 1562 | 1558 | - | 1.08 ± 0.10 | - | - | - | 0.53 ± 0.12 | - | - | - |
| 66 | cis-3-Hexenyl benzoate | 1566 | 1570 | - | - | 0.09 ± 0.01 | - | - | - | - | - | - |
| 67 | Germacrene D-4-ol | 1575 | 1583 | - | - | - | - | - | - | 0.89 ± 0.38 | - | - |
| 68 | Spathulenol | 1578 | 1584 | - | - | - | 0.04 ± 0.00 | - | - | - | 0.03 ± 0.01 | 0.06 ± 0.01 |
| 69 | Caryophyllene oxide | 1583 | 1590 | 0.08 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.02 | 0.20 ± 0.01 | 0.32 ± 0.06 | 0.23 ± 0.04 | 0.22 ± 0.10 | 0.26 ± 0.01 | 0.26 ± 0.07 |
| 70 | Humulene epoxide II | 1608 | 1618 | - | - | - | - | - | - | - | 0.02 ± 0.00 | - |
| 71 | γ-Eudesmol | 1632 | 1639 | - | - | 1.94 ± 0.19 | - | - | 0.09 ± 0.03 | - | - | - |
| 72 | T-Muurolol | 1646 | 1651 | - | - | - | - | - | - | 0.03 ± 0.01 | - | - |
| 73 | β-Eudesmol | 1650 | 1663 | - | - | 2.53 ± 0.26 | - | - | 0.26 ± 0.07 | - | - | - |
| 74 | α-Cadinol | 1654 | 1664 | 0.04 ± 0.01 | 0.02 ± 0.01 | - | 0.02 ± 0.01 | - | - | 0.08 ± 0.03 | - | - |
| 75 | α-Bisabolol | 1685 | 1691 | 0.04 ± 0.01 | - | - | 0.01 ± 0.00 | - | - | - | - | - |
| 76 | Benzyl benzoate | 1760 | 1767 | - | - | 0.30 ± 0.03 | - | - | - | - | - | - |
| 77 | 1-Hexadecanol | 1875 | 1884 | - | - | - | - | 0.12 ± 0.02 | - | - | - | - |
| Total | 98.15 ± 0.51 | 98.67 ± 0.36 | 95.71 ± 1.60 | 99.09 ± 0.30 | 98.66 ± 0.11 | 98.61 ± 0.35 | 98.60 ± 0.56 | 98.32 ± 0.11 | 99.00 ± 0.30 | |||
| Monoterpene hydrocarbons | 13.38 | 1.37 | 0.05 | 0.21 | 7.11 | 0.03 | 46.47 | 2.31 | 7.39 | |||
| Oxygenated monoterpenes | 72.17 | 84.67 | 66.48 | 88.46 | 85.05 | 79.87 | 43.86 | 85.76 | 76.22 | |||
| Sesquiterpene hydrocarbons | 11.98 | 10.74 | 11.94 | 10.09 | 5.83 | 7.51 | 7.04 | 9.93 | 15.07 | |||
| Oxygenated sesquiterpenes | 0.62 | 1.89 | 17.23 | 0.33 | 0.67 | 11.20 | 1.23 | 0.32 | 0.32 | |||
RI Lit, comparison of retention indices with those reported in the literature (Adams, 2007). RI Cal, retention indices relative to n-alkanes (C8–C22) on the VF-5MS column. Values are the mean of the three replicate determinations ± standard deviation.
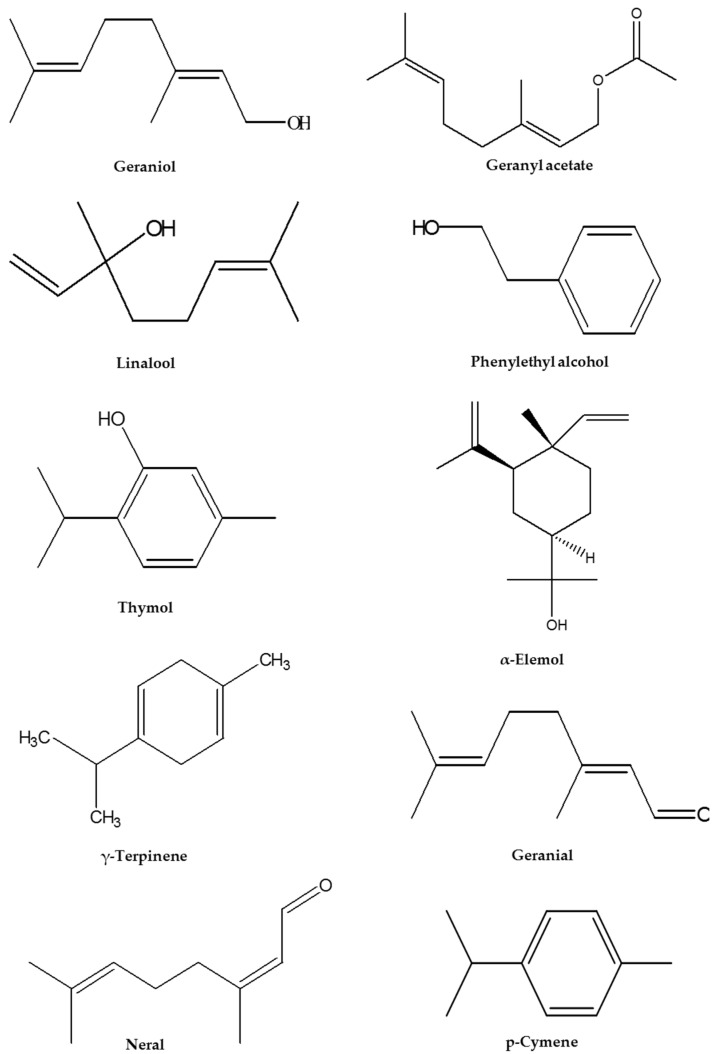
Geraniol, geranyl acetate, linalool, phenylethyl alcohol, γ-terpinene, and thymol were detected as the most abundant components, which comprised more than 20% in at least one essential oil (Figure 3). Results of the essential oil composition revealed that all three T. quinquecostatus cultivars tested belonged to different chemotypes (Supplementary Figure S1). Cultivars of Wolchul, Odae, and Jiri were mainly composed of geraniol (42.94%), thymol (30.54%), and linalool (47.89%), respectively. In the case of the commercial cultivars of T. vulgaris, the lemon and silver cultivars belonged to the thymol chemotype (43.91% and 66.24%, respectively). On the other hand, the creeping, golden, and orange cultivars belonged to the geraniol chemotype (29.57%, 65.99%, and 44.70%, respectively). With regards to the carpet cultivar, linalool (48.16%) was recorded as the most abundant component. Furthermore, geranyl acetate was detected as a major component in the Wolchul (26.49%) and silver (29.86%) cultivars. γ-Terpinene (23.92%) and p-cymene (11.13%) were major components in the Odae cultivar. In the creeping cultivar, neral (11.75%) and geranial (18.21%) were also recorded as major components. Other important compounds detected in all essential oils were caryophyllene (2.87%–7.02%), borneol (0.41%–5.91%), β-bisabolene (0.23%–3.86%), and 1-octen-3-ol (0.39%–3.61%). α-Elemol (11.62% and 8.52%, respectively) was also recorded as a major component in the carpet and creeping cultivars.
Figure 3.
Structure of the major components identified in the essential oils of commercial Thymus vulgaris cultivars and Korean native Thymus quinquecostatus cultivars. Geraniol, geranyl acetate, linalool, phenylethyl alcohol, γ-terpinene, and thymol were identified as the major components, comprising >20% in at least one of the essential oil obtained from the different cultivars.
3.3. RAPD Analysis
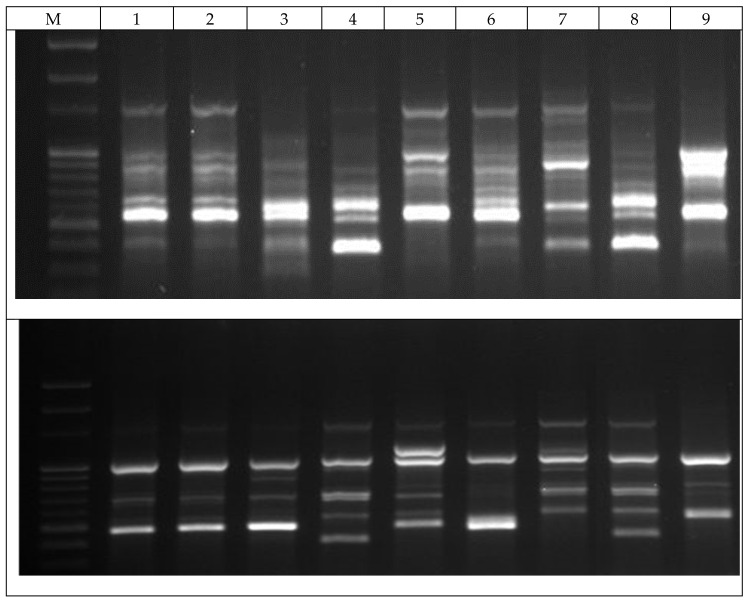
The molecular analysis revealed that the RAPD primers produced clear and reproducible polymorphic bands (Figure 4) among 9 thyme cultivars, and generated a total of 133 amplicons from 16 primers. The number of bands per primer varied from 4 (OPA-12, OPA-14, and OPB-04) to 16 (OPA-19), with an average of 8.31 bands per primer. In these, 124 amplicons were polymorphic, corresponding to 93.23% polymorphism (Table 5). Eight primers gave the highest percentage of polymorphism (100%), while the lowest percentage of polymorphism (75%) was obtained by OPA-12 and OPB-04 primers (Table 5).
Figure 4.
The example of a pattern among the three Korean native Thymus quinquecostatus cultivars and the six commercial Thymus vulgaris cultivars using OPB-01 (top) and OPA-11 (bottom) primers separated in 1.2% agarose gel electrophoresis. M, PCR marker; 1–6, commercial Thymus vulgaris cultivars: (1, lemon; 2, golden; 3, carpet; 4, orange; 5, silver; and 6, creeping); 7–9, Korean native Thymus quinquecostatus cultivars (7, Odae Mt; 8, Wolchul; 9, Jiri).
Table 5.
Bands and polymorphism revealed by the RAPD primers among the 9 Thymus cultivars.
| Primer | Total No. of Bands | No. of Polymorphic Bands | Polymorphism (%) |
|---|---|---|---|
| OPA-09 | 10 | 10 | 100 |
| OPA-10 | 8 | 8 | 100 |
| OPA-11 | 12 | 11 | 91.67 |
| OPA-12 | 4 | 3 | 75 |
| OPA-13 | 10 | 8 | 80 |
| OPA-14 | 4 | 4 | 100 |
| OPA-15 | 8 | 7 | 87.50 |
| OPA-16 | 10 | 9 | 90 |
| OPA-17 | 5 | 5 | 100 |
| OPA-18 | 9 | 9 | 100 |
| OPA-19 | 16 | 16 | 100 |
| OPA-20 | 10 | 9 | 90 |
| OPB-01 | 8 | 8 | 100 |
| OPB-02 | 7 | 7 | 100 |
| OPB-03 | 8 | 7 | 87.5 |
| OPB-04 | 4 | 3 | 75 |
| Total | 133 | 124 | |
| Average | 8.31 | 7.75 | 93.23 |
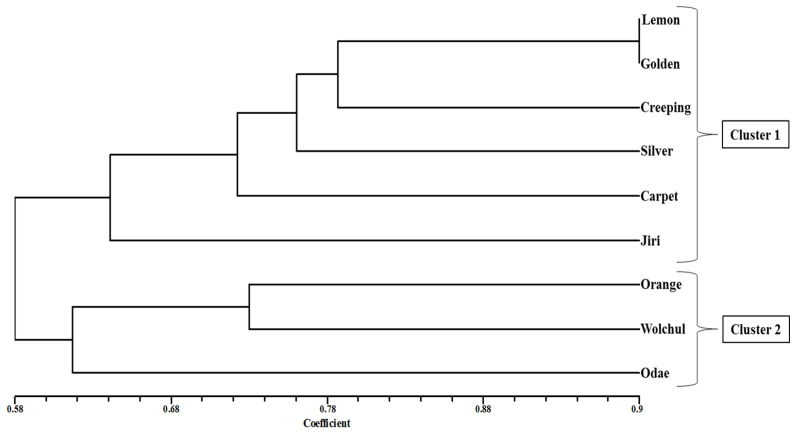
The dendrogram realized from the RAPD markers grouped the 9 thyme cultivars into two major clusters and showed a clear separation (Figure 5). Levels of genetic similarity indices ranged from 0.58 to 0.98. Cluster 1 consisted of lemon, golden, creeping, silver, carpet, and Jiri. Whereas cluster 2 consisted of orange, Wolchul, and Odae.
Figure 5.
Clustering tree of the three Thymus quinquecostatus cultivars and the six commercial Thymus vulgaris cultivars, based on the unweighted pair-group method with the arithmetic average (UPGMA), using 16 RAPD markers.
4. Discussion
The identification of the Thymus species is extremely difficult because of the high levels of diversity within the genus. This genus contains several commercially important aromatic species. For this purpose, the relationship among the chemical composition of essential oils and molecular analysis was carried out for different Thymus species [20,23]. In this context, the essential oil composition and molecular analysis of nine thyme cultivars were investigated in this study, to distinguish between commercial thyme cultivars and Korean native thyme cultivars. In the morphological study, the T. quinquecostatus and T. vulgaris cultivars exhibited a significant level of variability in recorded parameters. In the qualitative traits, a considerable variability was observed in stem type, stem color, length and number of stem branches, leaf shape, and trichome position, among and within T. quinquecostatus and T. vulgaris cultivars.
The present study showed a high chemical diversity among nine thyme cultivars. Results revealed that essential oils from Korean cultivars (T. quinquecostatus) belonged to the geraniol, thymol, and linalool chemotypes. Essential oils from the commercial thyme cultivars (T. vulgaris) such as creeping, golden, and orange belonged to the geraniol chemotype and lemon, and the silver cultivars belonged to the thymol chemotype. Further, carpet cultivar belonged to the linalool chemotype. In particular, these essential oils were dominated by monoterpenes. 1-Octen-3-ol, γ-terpinene, linalool, borneol, α-terpineol, nerol, geraniol, thymol, β-cubebene, β-elemene, caryophyllene, β-bisabolene, butylated hydroxytoluene, β-sesquiphellandrene, and caryophyllene oxide were detected in all six essential oils from the commercial cultivars. With regards to the chemical composition of T. vugaris essential oils, seven different chemotypes such as thymol, carvacrol, linalool, geraniol, thujanol-4, terpineol, and 1,8-cineole were identified [15,16]. In the case of T. quinquecostatus essential oils, Shin and Kim [8] found that thymol (41.70%), γ-terpinene (16.00%), and p-cymene (13.00%) were the most prominent compounds. Similarly, thymol (30.54%), γ-terpinene (23.92%), and p-cymene (11.13%) were the major components in the essential oil obtained from the Odae cultivar. However, the major components in the essential oils obtained from Wolchul and Jiri cultivars of T. quinquecostatus were not identical. In the Wolchul cultivar, geraniol (42.94%) and geranyl acetate (26.49%) were detected as the major components, whereas linalool (47.89%) and thymol (15.98%) were found to be abundant in the Jiri cultivar.
Hudaib and Aburjai [24] determined variations in the composition of essential oils from cultivated and wild-growing plants of T. vulgaris grown in Jordan. Higher oil yields were obtained in plants growing wild, when compared to the cultivated plants. Among the four different samples, thymol (0.8–63.8%) and carvacrol (6.9–86.1%) were the most abundant components in the T. vulgaris essential oils. A study indicated that the essential oil composition of T. vulgaris highly varied both qualitatively and quantitatively during the vegetative cycle [25]. The variations in the yield and composition of essential oils could be influenced by various factors, such as the geographical region of the plant, plant’s maturity, cultivation practices, and weather parameters (temperature, humidity, sunlight duration, and rainfall) [26,27,28]. In addition, the genetic constitution of the cultivars also played a considerable role in the essential oil composition [1,25].
According to previous reports, it is difficult to distinguish Thymus species and cultivars by analyzing the essential oil profile alone. Hence, the combined analysis of chemical composition and molecular techniques was used for the correct identification of the different plant species. In recent decades, the correlation between the chemical composition and molecular analysis of different Thymus species were investigated by various researchers [1,13,20,21]. Previous studies showed that both essential oil composition and RAPD analysis could be used to distinguish different thyme cultivars, and especially, to determine their relationships [1]. In addition, RAPD analysis revealed high polymorphisms even when using closely related genotypes. Even though the essential oil composition of plants was different from one another, RAPD analysis clustered these plants together, owing to their similar genetic background [15].
In the present study, 16 primers were used to amplify segments of DNA of the genome of three Korean thyme cultivars and six commercial thyme cultivars, to investigate the genetic variations. A total of 133 bands were obtained and the average percentage of the polymorphic bands was 93.23%. Based on the RAPD data, the similarity of the cultivars, estimated by the Jaccard’s coefficient, is depicted in Figure 5. The nine cultivars of thyme fell into two clusters. Cluster 1 was formed by six cultivars (lemon, golden, creeping, silver, carpet, and Jiri) and cluster 2 by three cultivars (orange, Wolchul, and Odae). This emphasized the obvious variation between the Korean cultivars (except Jiri cultivar) and the commercial cultivars. The dendrogram indicated a clear separation of T. quinquecostatus from T. vulgaris, with the exception of the Jiri cultivar. According to the RAPD similarity matrix, it was observed that the Wolchul and Odae cultivars were closely related. Nevertheless, there was no significant relationship between the essential oil composition and RAPD data. The ability to discriminate all studied cultivars using RAPD bands indicated that RAPD analysis can provide a rapid and inexpensive technique to identify phenotypically similar thyme cultivars.
Based on previous reports, a high correlation between genetic and chemical relationships was attained in several plants. These data indicated that the composition of the essential oil is regulated by a number of genes that are extensively distributed throughout the plant genome [1,29,30]. Khalil et al. [31] used RAPD analysis to determine the genetic relationship between T. vulgaris populations collected in Syria. In their study, 13 individuals were analyzed using 27 primers, which generated 180 polymorphic bands from 198 bands. The authors found a significant correlation between T. vulgaris populations and their geographic areas. The present study also proved that the geographic distribution had a significant influence on genetic variation. Comparing the groups formed by the cluster analysis based on RAPD data (Figure 5) and chemotype, based on essential oil composition, we can observe that the groups formed in both cases were not identical.
In another study, the composition of essential oils and genetic relationships between six commercial cultivars of T. vulgaris were analyzed. A total of 104 were polymorphic RAPD bands (63.8%) were obtained using 15 primers. Among 15 primers, the highest percentage of polymorphism was obtained by the OPA-05 primer (90.9%). Similar to the essential oil composition, the six T. vulgaris cultivars fell into two major clusters, according to the RAPD patterns, with a correlation coefficient of −0.779 [1]. The chemical and genetic variations of 20 taxa from four Hungarian Thymus species (T. glabrescens, T. pannonicus, T. praecox, and T. pulegioides) were studied by Pluhár et al. [23]. In the molecular analysis, 114 polymorphic RAPD bands (80.8%) were obtained using 13 primers. The results revealed that partial correlation was found between the essential oil and RAPD analyses. The essential oil composition and genetic variation in six micropropagated genotypes (in vitro and in vivo) of T. saturejoides were investigated by Nordine et al. [32]. RAPD results and the essential oil composition grouped these six genotypes into three clusters exhibiting significant intraspecific chemical and genetic differences. Furthermore, a significant correlation was observed between RAPD and essential oil composition obtained from the in vitro genotypes.
Similar to our report, several studies also reported that the combined use of RAPD and essential oil analyses were not significantly correlated. For example, the genetic and chemical relationships among 31 individuals of T. caespititius collected from the islands of Pico, Sao Jorge, and Terceira (Azores) were determined. In the RAPD analysis, 187 polymorphic bands were obtained using 17 primers. However, there was no close relationship between the collection site, the essential oil composition, and RAPD analysis [15]. Rustaiee et al. [20] also studied the essential oil composition and genetic variability between some Thymus species such as T. daenensis (two populations), T. fallax, T. fedtschenkoi, T. migricus, and T. vulgaris, using GC-MS and RAPD. Although the RAPD markers allowed a perfect distinction among different Thymus species according to their characteristic genetic background, there was no identical clustering with the essential oil composition. In addition, Masi et al. [33] found that the essential oil compositions did not match with the results achieved from agronomic and genetic analyses in Ocimum basilicum. In another study, there was no correlation between RAPD and the essential oil obtained from the in vivo genotypes of T. saturejoides [32]. Based on the previous and present studies, marker-assisted RAPD technique had a high advantage for the assessment of the genetic differences of plant species without prior molecular knowledge.
Results of the present study revealed that there was a significant correlation between the genetic and geographic distances of the Korean thyme cultivars (Wolchul and Odae cultivars), compared to the commercial thyme cultivars. However, the chemical polymorphism of these thyme cultivars is not well-understood. Hence, other molecular techniques should be investigated in order to understand this question in T. quinquecostatus and other Thymus cultivars.
5. Conclusions
The present study emphasized that RAPD analysis allowed a perfect distinction between the Korean thyme cultivars (Wolchul and Odae) and commercial thyme cultivars, based on their unique genetic background. However, the chemical composition of the Wolchul and Odae cultivars was not identical. Furthermore, there was no significant relationship between the RAPD data and essential oil composition of both T. quinquecostatus and T. vulgaris cultivars. The chemical composition and molecular data obtained in this study delivered a good starting point for future investigations. It could be concluded that the RAPD markers proved to be an effective tool for discriminating different Thymus species. The sample collection must be done from different geographical regions in Korea to understand the genetic and chemical variability of the T. quinquecostatus cultivars.
Acknowledgments
This study was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ014506012020)”, Rural Development Administration, Republic of Korea.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/9/6/289/s1, Figure S1: GC-MS chromatograms of essential oils from three Korean native Thymus quinquecostatus cultivars. A, Odae cultivar; B, Wolchul cultivar; C, Jiri cultivar.
Author Contributions
M.K. performed the experiment; K.S. compiled the data and wrote the manuscript; J.-C.M. analyzed the data; and S.K. designed the experiment and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Echeverrigaray S., Agostini G., Atti-Serfini L., Paroul N., Pauletti G.F., dos Santos A.C. Correlation between the chemical and genetic relationships among commercial thyme cultivars. J. Agric. Food Chem. 2001;49:4220–4223. doi: 10.1021/jf010289j. [DOI] [PubMed] [Google Scholar]
- 2.Maissa B.J., Walid H. Antifungal activity of chemically different essential oils from wild Tunisian Thymus spp. Nat. Prod. Res. 2015;29:869–873. doi: 10.1080/14786419.2014.984182. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y.S., Lee S.J., Hwang J.W., Kim E.K., Kim S.E., Kim E.H., Moon S.H., Jeon B.T., Park P.J. In vitro protective effects of Thymus quinquecostatus Celak extracts on t-BHP-induced cell damage through antioxidant activity. Food Chem. Toxicol. 2012;50:4191–4198. doi: 10.1016/j.fct.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Jia P., Gao T., Xin H. Changes in structure and histochemistry of glandular trichomes of Thymus quinquecostatus Celak. Sci. World J. 2012;2012:187261. doi: 10.1100/2012/187261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X., He T., Wang X., Shen M., Yan X., Fan S., Wang L., Wang X., Xu X., Sui H., et al. Trditional uses, chemical constituents and biological activities of plants from the genus Thymus. Chem. Biodivers. 2019;17:e1900254. doi: 10.1002/cbdv.201900254. [DOI] [PubMed] [Google Scholar]
- 6.Hyun T.K., Kim H.C., Kim J.S. Antioxidant and antidiabetic activity of Thymus quinquecostatus Celak. Ind. Crops Prod. 2014;52:611–616. doi: 10.1016/j.indcrop.2013.11.039. [DOI] [Google Scholar]
- 7.Jia P., Liu H., Gao T., Xin H. Glandular trichomes and essential oil of Thymus quinquecostatus. Sci. World J. 2013;2013:387952. doi: 10.1155/2013/387952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin S., Kim J.H. Antifungal activities of essential oils from Thymus quinquecostatus and T. magnus. Planta Med. 2004;70:1090–1092. doi: 10.1055/s-2004-832654. [DOI] [PubMed] [Google Scholar]
- 9.Kykkidou S., Giatrakou V., Papavergou A., Kontominas M.G., Savvaidis I.N. Effect of thyme essential oil and packaging treatments on fresh Mediterranean swordfish fillets during storage at 4 °C. Food Chem. 2009;115:169–175. doi: 10.1016/j.foodchem.2008.11.083. [DOI] [Google Scholar]
- 10.Chang Y.L., Shen M., Ren X.Y., He T., Wang L., Fan S.S., Wang X.H., Li X., Wang X.P., Chen X.Y., et al. Multi-response extraction optimization based on anti-oxidative activity and quality evaluation by main indicator ingredients coupled with chemometric analysis on Thymus quinquecostatus Celak. Molecules. 2018;23:957. doi: 10.3390/molecules23040957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomakos N., Govaris A., Koidis P., Botsoglou N. The antimicrobial effect of thyme essential oil, nisin, and their combination against Listeria monocytogenes in minced beef during refrigerated storage. Food Microbiol. 2008;25:120–127. doi: 10.1016/j.fm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Adams R.P., Socorro M., Elizondo G., Elizondo M.G., Slinkman E. A new subalpine variety of Juniperus blancoi Martinez (Cupressaceae) from Durango, Mexico. Biochem. Syst. Ecol. 2006;34:205–211. doi: 10.1016/j.bse.2005.11.004. [DOI] [Google Scholar]
- 13.Younsi F., Rahali N., Mehdi S., Boussaid M., Messaoud C. Relationship between chemotypic and genetic diversity of natural populations of Artemisia herba-alba Asso growing wild in Tunisia. Phytochemistry. 2018;148:48–56. doi: 10.1016/j.phytochem.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Boulila A., Bejaoui A., Messaoud C., Boussaid M. Variation of volatiles in Tunisian populations of Teucrium polium L. (Lamiaceae) Chem. Biodivers. 2008;7:1389–1400. doi: 10.1002/cbdv.200890127. [DOI] [PubMed] [Google Scholar]
- 15.Trindade H., Costa M.M., Sofia B.L.A., Pedro L.G., Figueiredo A.C., Barroso J.G. Genetic diversity and chemical polymorphism of Thymus caespititius from Pico, Sao Jorge and Terceira islands (Azores) Biochem. Syst. Ecol. 2008;36:790–797. doi: 10.1016/j.bse.2008.09.001. [DOI] [Google Scholar]
- 16.Torras J., Dolors Grau M., Lopez J.F., de Las Heras F.X.C. Analysis of essential oils from chemotypes of Thymus vulgaris in Catalonia. J. Sci. Food Agric. 2007;87:2327–2333. doi: 10.1002/jsfa.2995. [DOI] [Google Scholar]
- 17.Gajera B.B., Kumar N., Singh A.S., Punvar B.S., Ravikiran R., Subhash N., Jadeja G.C. Assessment of genetic diversity in castor (Ricinus communis L.) using RAPD and ISSR markers. Ind. Crops Prod. 2010;32:491–498. doi: 10.1016/j.indcrop.2010.06.021. [DOI] [Google Scholar]
- 18.Mahajan V., Chouhan R., Kitchlu S., Bindu K., Koul S., Singh B., Bedi Y.S., Gandhi S.G. Assessment of chemical and genetic variability in Tanacetum gracile accessions collected from cold desert of Western Himalaya. 3 Biotech. 2018;8:284. doi: 10.1007/s13205-018-1299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowdhury T., Mandal A., Roy S.C., Sarker D.D. Diversity of the genus Ocimum (Lamiaceae) through morpho-molecular (RAPD) and chemical (GC–MS) analysis. J. Genet. Eng. Biotechnol. 2017;15:275–286. doi: 10.1016/j.jgeb.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rustaiee A.R., Yavari A., Nazeri V., Shokrpour M., Sefidkon F., Rasouli M. Genetic diversity and chemical polymorphism of some Thymus species. Chem. Biodivers. 2013;10:1088–1098. doi: 10.1002/cbdv.201200020. [DOI] [PubMed] [Google Scholar]
- 21.Trindade H., Costa M.M., Sofia B.L.A., Pedro L.G., Figueiredo A.C., Barroso J.G. A combined approach using RAPD, ISSR and volatile analysis for the characterization of Thymus caespititius from Flores, Corvo and Graciosa islands (Azores, Portugal) Biochem. Syst. Ecol. 2009;37:670–677. doi: 10.1016/j.bse.2009.10.006. [DOI] [Google Scholar]
- 22.Adams R.P. Identification of Essential Oil Components by gAs Chromatography/Mass Spectrometry. Allured Publishing Co.; Carol Stream, IL, USA: 2007. [Google Scholar]
- 23.Pluhár Z., Kocsis M., Kuczmog A., Csete S., Simkó H., Sárosi S., Molnár P., Horváth G. Essential oil composition and preliminary molecular study of four Hungarian Thymus species. Acta Biol. Hung. 2012;63:81–96. doi: 10.1556/ABiol.63.2012.1.7. [DOI] [PubMed] [Google Scholar]
- 24.Hudaib M., Aburjai T. Volatile components of Thymus vulgaris L. from wild-growing and cultivated plants in Jordan. Flavour Frag. J. 2007;22:322–327. doi: 10.1002/ffj.1800. [DOI] [Google Scholar]
- 25.Hudaib M., Speroni E., Di Pietra A.M., Cavrini V. GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J. Pharm. Biomed. Anal. 2002;29:691–700. doi: 10.1016/S0731-7085(02)00119-X. [DOI] [PubMed] [Google Scholar]
- 26.Dhouioui M., Boulila A., Chaabane H., Zina M.S., Casabianca H. Seasonal changes in essential oil composition of Aristolochia longa L. ssp. paucinervis Batt. (Aristolochiaceae) roots and its antimicrobial activity. Ind. Crops Prod. 2016;83:301–306. [Google Scholar]
- 27.Mewes S., Kruger H., Pank F. Physiological, morphological, chemical and genomic diversities of different origins of thyme (Thymus vulgaris L.) Genet. Resour. Crop Evol. 2008;55:1303–1311. doi: 10.1007/s10722-008-9329-7. [DOI] [Google Scholar]
- 28.Rajabi Z., Ebrahimi M., Farajpour M., Mirza M., Ramshini H. Compositions and yield variation of essential oils among and within nine Salvia species from various areas of Iran. Ind. Crops Prod. 2014;61:233–239. doi: 10.1016/j.indcrop.2014.06.038. [DOI] [Google Scholar]
- 29.Vieira R.F., Grayer R.J., Paton A., Simon J.E. Genetic diversity of Ocimum gratissimum L. based on volatile oil constituents, flavonoids and RAPD markers. Biochem. Syst. Ecol. 2001;29:287–304. doi: 10.1016/S0305-1978(00)00062-4. [DOI] [PubMed] [Google Scholar]
- 30.Keskitalo M., Pehu E., Simon J.E. Variation in volatile compounds from tansy (Tanacetum vulgare L.) related to genetic and morphological differences of genotypes. Biochem. Syst. Ecol. 2001;29:267–285. doi: 10.1016/S0305-1978(00)00056-9. [DOI] [PubMed] [Google Scholar]
- 31.Khalil R., Khalil R., Li Z. Determination of genetic variation and relationship in Thymus vulgaris populations in Syria by random RAPD markers. Plant Biosyst. 2012;146:217–225. doi: 10.1080/11263504.2012.727882. [DOI] [Google Scholar]
- 32.Nordine A., Udupa S.M., Iraqi D., Meksem K., Hmamouchi M., ElMeskaoui A. Correlation between the chemical and genetic relationships among Thymus saturejoides genotypes cultured under in vitro and in vivo environments. Chem. Biodivers. 2016;13:387–394. doi: 10.1002/cbdv.201500102. [DOI] [PubMed] [Google Scholar]
- 33.Masi L., Siviero P., Esposito C., Castaldo D., Siano F., Laratta B. Assessment of agronomic, chemical and genetic variability in common basil (Ocimum basilicum L.) Eur. Food Res. Technol. 2006;223:273–281. doi: 10.1007/s00217-005-0201-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.