Abstract
Cadmium (Cd) is a ubiquitous environmental metal that is reported to be a “metalloestrogen.” Uterine leiomyomas (fibroids) are estrogen-responsive gynecologic neoplasms that can be the target of xenoestrogens. Previous epidemiology studies have suggested Cd may be associated with fibroids. We have shown that Cd can stimulate proliferation of human uterine leiomyoma (ht-UtLM) cells, but not through classical estrogen receptor (ER) binding. Whether nongenomic ER pathways are involved in Cd-induced proliferation is unknown. In the present study, by evaluating G protein-coupled estrogen receptor (GPER), ERα36 and phospho-epidermal growth factor receptor (EGFR) expression in human tissues, we found GPER, ERα36 and phospho-EGFR were highly expressed in fibroids compared to patient-matched myometrial tissues. In ht-UtLM cells, cell proliferation was increased by low doses of Cd (0.1 μM and 10 μM), and this effect could be inhibited by GPER-specific antagonist (G15) pretreatment, or silencing (si) GPER, but not by siERα36. Cd-activated MAPK was dependent on GPER/EGFR transactivation, through significantly increased phospho-Src, matrix metalloproteinase-2 (MMP2) and MMP9, and heparin-binding EGF-like growth factor (HB-EGF) expression/activation. Also, phospho-Src could interact directly to phosphorylate EGFR. Overall, Cd-induced proliferation of human fibroid cells was through a nongenomic GPER/p- src/EGFR/MAPK signaling pathway that did not directly involve ERα36. This suggests that Cd may be a risk factor for uterine fibroids through crosstalk between hormone and growth factor pathways.
Keywords: cadmium, uterine leiomyomas, GPER, EGFR, ERα36
Introduction
Metalloestrogens are metals, such as cadmium (Cd), lead, and mercury (Byrne et al. 2013), that possess estrogenic properties and can activate estrogen receptors (ER) (Safe 2003; Silva et al. 2012). Cd is a toxic heavy metal that is ubiquitous in the environment. For the general population, major sources of exposure to Cd result from occupational inhalation, food, in particular seafood and rice, drinking water, as well as cigarette smoking (Pollack et al. 2014). Cd exposure affects human populations worldwide, such as Spain, Bangladesh and China, with an estimated dietary intake above the tolerable weekly intake (TWI), which is set at 2.5 μg/kg body weight by the European Food Safety Authority (EFSA) and at 5.8 μg/kg body weight by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (Ba et al. 2017). Cd levels have also been reported to be elevated in pregnant women living in certain counties in Durham, North Carolina (King et al. 2015). Also, elevated Cd concentrations have been found in coal combustion residues effluents, discharged from coal-fired power plants into receiving waters in North Carolina, at 0.8 μg/L, much more than the 0.25 μg/L standard (Ruhl et al. 2012). Cd has been shown to function as a potent metalloestrogen (Byrne et al. 2013; Safe 2003; Silva et al. 2012), associating with an increased incidence of endometrial, breast, and prostate cancers (Cho et al. 2013).
Uterine leiomyomas (fibroids) are highly prevalent gynecologic neoplasms (Commandeur et al. 2015), but the etiology and mechanism(s) involved in their growth remain largely unknown. Fibroids are known as an estrogen-responsive disease, which can make them a target for disruption by environmental xenoestrogens (Hunter et al. 2000). Several studies have found a positive association between fibroids and xenoestrogens, such as DES, phthalates and bisphenol A (Mahalingaiah et al. 2014; Styer and Rueda 2016; Yu et al. 2019).
There is reasonable evidence suggesting that Cd may be associated with the pathogenesis of uterine leiomyoma. In an operative cohort study in the US, an increased odds ratio of a postoperative fibroid diagnosis in women was associated with higher blood concentrations of Cd (Johnstone et al. 2014). One study from Korea also found a positive correlation between blood Cd concentrations and fibroid volume in women with fibroids (Ye et al. 2017). Moreover, Cd concentration in leiomyoma tissues is twice as high as that in surrounding myometrium, and a significant correlation between ER expression and Cd concentration in both leiomyoma and myometrium are reported (Nasiadek et al. 2011). We have shown that Cd can stimulate proliferation of human uterine leiomyoma (ht-UtLM) cells, but not through classical ERα or ERβ binding (Gao et al. 2015). Whether nonclassical ERs signaling pathways are involved in Cd-induced ht-UtLM cell proliferation is unknown.
G protein-coupled estrogen receptor (GPER), a seven transmembrane receptor belonging to the G protein-coupled receptors (GPCRs) family, has been identified as a novel ER mediating rapid nongenomic activity with high affinity to estrogen and functions alongside the classical ER (Prossnitz and Barton 2011). Much evidence has shown that Cd has the ability to induce cell proliferation through activating GPER in addition to ERα and ERβ, in breast, thyroid, and other cancer cells (Huff et al. 2016; Yu et al. 2010; Zhu et al. 2017). GPER is abundantly expressed in human myometrium (Maiti et al. 2011) and uterine leiomyomas (Tian et al. 2013), and its gene polymorphisms are associated with uterine leiomyoma risk (Kasap et al. 2016). Except for GPER, ERα36, a truncated isoform of the full-length 66kD ERα, is another ER that can mediate rapid nongenomic signaling (Nakareangrit et al. 2016; Wang and Yin 2015; Xiao et al. 2014).
The present study aims to investigate the effect of Cd on the ht-UtLM cells and whether nongenomic ERs (GPER or ERα36) signaling pathways are involved in Cd’s effects.
Methods
Cells, human tissues and chemicals
The immortalized UtLM-hTERT (ht-UtLM) cells were established in our laboratory and maintained in supplemented medium as previously described (Carney et al. 2002). Human uterine leiomyoma and patient-matched unaffected myometrial tissues were collected from women at the age of 41–49 years. All uterine samples were confirmed by histological evaluation. The menstrual cycle phase was determined as proliferative based on recorded menstrual cycle clinical history.
Cadmium chloride (CdCl2; 99.999%, catalog no. 439800, Sigma-Aldrich; CAS 10108–64-2) was dissolved in double distilled water. G1 (agonist of GPER, ChemDiv, Inc. San Diego, CA) and G15 (antagonist of GPER, catalog no. 14673, Cayman) were dissolved in DMSO, respectively. E2 (17β-Estradiol, catalog no. E2257, Sigma-Aldrich) was dissolved in ethanol. Based on the previous data in our lab (Gao et al. 2015), cells were treated with 0.1 μM and 10 μM Cd.
Immunohistochemistry
Human uterine leiomyoma and myometrial tissue samples were embedded in paraffin and stained for GPER by immunohistochemistry. The detailed protocols are described in the Supplementary Information.
Real Time-PCR analysis
Ht-UtLM cells treated with Cd were collected at 24 h, 48 h and 72 h for real time-PCR analysis. The procedures are provided in detail in the Supplementary Information.
Receptor Knock-down with siGPER or siERα36
To identity the involvement of GPER or ERα36 in Cd-induced cell proliferation, an interference RNA technique was used to knock down GPER or ERα36 gene expression. The GPER or ERα36 oligonucleotide selective siRNA fragments were predesigned and produced by Qiagen (siGPER, catalog no. 4390824; siERa36, catalog no. 4399666). The transfection of targeting siRNA and a control scrambled siRNA (siScr, catalog no. 4390843) into cells was done using Lipofectamine (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol.
Cell Counting Kit 8 (CCK8) Assay for Cell Proliferation
Cells were seeded into 96-well plates and followed by 0.1 μM and 10 μM Cd, 100 nM G1 and 10 nM E2 treatments, with or without 100 nM G15 preincubating for 30 min, or siGPER or siERa36. Cells were collected after 24 h, 48 h, and 72 h. CCK8 (catalog no. 96992, Sigma-Aldrich) was used to measure the absorbance at 450 nm using a microplate reader.
DNA Analysis by Flow Cytometry
After treatment with Cd for 48 h, ht-UtLM cells were fixed in cold 70% ethanol overnight, and then resuspended in 1 ml of Propidium Iodide (PI) staining solution for 20 min in the dark. Then the samples were examined using a Becton Dickson Fluorescence-Activated Cell Sorting (FACS) Flow Cytometer (Franklin Lakes, NY, USA) with CellQuest software.
Western blotting
A total of 50–100 mg of frozen leiomyoma and patient-matched myometrial tissue samples was collected and protein was extracted from 19 patients with uterine leiomyomas. For the in vitro studies, whole cell lysates were harvested at 10 min, 24 h, 48 h and 72 h. Proteins were loaded on a 4–12% Bis-Tris gel and then transferred to a PVDF membrane. The primary antibodies were used as shown in Table S1. The density of the respective bands was quantitated using a densitometer with AlphaView Software for FluorChem Systems (ProteinSimple™).
Confocal Immunofluorescence Staining
Ht-UtLM cells treated with Cd were fixed and incubated with primary antibodies to GPER, phospho-p44/42 MAPK at 4°C overnight, followed by incubation with Alexa Fluor®-IgG secondary antibodies at room temperature for 1 h. After counterstaining with DAPI (catalog no. 1306, Molecular Probes) for 30 min, slides were examined under a Zeiss LSM710-UV meta confocal microscope. Fluorescence intensities and percent of positive cells were measured by Metamorph image analysis software (v.7.8.60, Molecular Devices, LLC).
Zymography
Enzymatic activities of MMP2 and MMP9 were estimated by gelatin zymography. Samples from treated cells were not heated and were loaded onto a Novex™ 10% Zymogram plus (Gelatin) protein gel (catalog no. ZY00100, Life technology). After electrophoresis, the gel was renatured for 30 mins and then developed overnight for maximum sensitivity. The gel was stained with SimplyBlue™ safestain (catalog no. LC6060, Invitrogen), followed by destaining. Imaging was done and data were analyzed through quantity software.
Coimmunoprecipitation
The coimmunoprecipation of phospho-Src and phospho-EGFR was evaluated by Dynabeads™ Protein G Immunoprecipitation Kit (catalog no. 10007D, Invitrogen) following the manufacturer’s protocol. Anti-phospho-Src antibody (25 μg/ml, catalog no. 05–677, Millipore Sigma) was bound to Dynabeads™ magnetic beads and incubated with rotation for 30 min at room temperature. The supernatant was removed and the magnetic bead-Ab complexes were washed. A total of 300 μg of whole cell lysate was added to the bead-Ab complexes and incubated with rotation for 2 h at room temperature to allow antigen to bind to the magnetic bead-Ab complexes. Target antigen was eluted from magnetic bead-Ab-Ag, denatured by heating at 70 °C for 10 min, followed by western blotting. The primary antibodies were anti-phospho-Src antibody and anti-phospho-EGFR antibody (Y845) (1:400, catalog no. AF3394, R&D). The density of the respective bands was quantitated using a densitometer with AlphaView Software for FluorChem Systems (ProteinSimple™).
Statistical analysis
All experiments were performed at least three times. Results are expressed as mean ± SE. The statistical significance of the differences was determined by t test and one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test using SPSS 20.0 (SPSS, Inc., Chicago, IL, USA). P < 0.05 were considered statistically significant.
Results
GPER, ERα36 and phospho-EGFR expression in human uterine leiomyoma and myometrial tissues
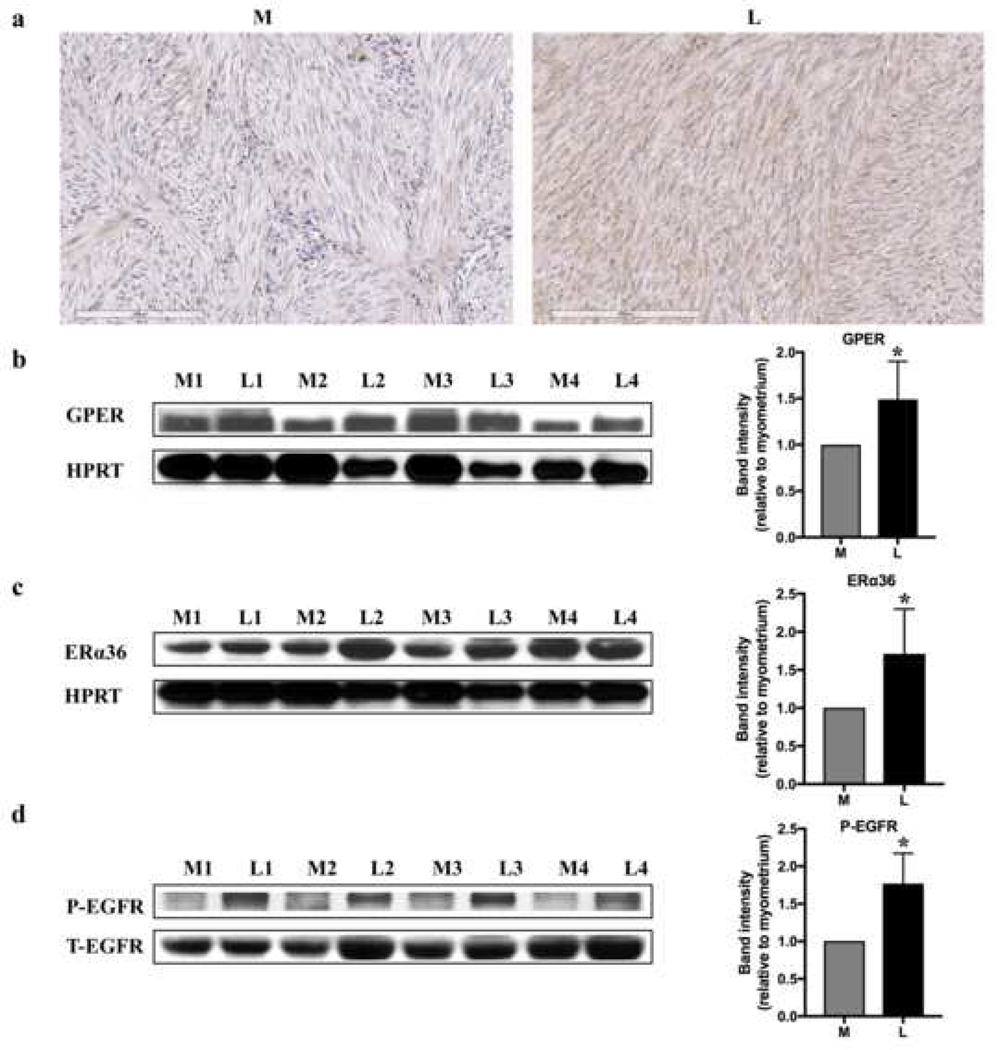
We investigated the expression of GPER, ERα36 and phospho-EGFR in human uterine leiomyomas and patient-matched myometrial tissues. The immunohistochemistry data showed that GPER was mainly located in the cytoplasm and cell membrane in uterine leiomyoma and myometrial tissue, and immunohistochemical staining was more intense in the leiomyoma tissue (Fig. 1a). GPER, ERα36 and phospho-EGFR were all increased in leiomyomas compared to normal myometrial tissues by western blotting analysis (p <0.05) (Fig. 1b, c, d, and Fig. S1), implying the association with fibroids.
Fig. 1.
Expressions of GPER and phospho-EGFR (P-EGFR) in patient-matched myometrial (M) and uterine leiomyoma (L) tissues. (a) Immunohistochemistry for GPER. GPER mainly located in the cytoplasm and cell membrane. (b) GPER, (c) ERa36 and (d) phospho-EGFR representative bands (n=4 patients) and quantification of band intensity (n=19 patients; all protein bands shown in Fig. S1) by western blotting. Increased expression of GPER, ERa36 and phospho-EGFR was observed in the leiomyomas compared to the myometrial tissue samples.*P <0.05. Hypoxanthine phosphoribosyltransferase (HPRT) = loading control. T-EGFR = Total EGFR.
Effects of Cd on GPER expression in ht-UtLM cells
Based on a previous study in our lab, a dose response curve of Cd at concentrations ranging from 10−4 μM to 200 μM showed that Cd at 0.1 μM to 10 μM could significantly increase ht-UtLM cell proliferation at 24h, 48h, and 72h (Gao et al. 2015). In addition, data from the National Health and Nutrition Examination Survey (NHANES 2011) showed that > 60% of the U.S. population has detectable blood Cd levels (range=1.25−77.14 nmol/L) (Verougstraete et al. 2003), with the upper range close to the low dose of 0.1 μM Cd used in our studies. Therefore, we chose to use the lowest 0.1 μM Cd concentration (a more human-relevant exposure concentration) and the highest concentration of Cd (10 μM) where we observed a proliferative response in our earlier studies.
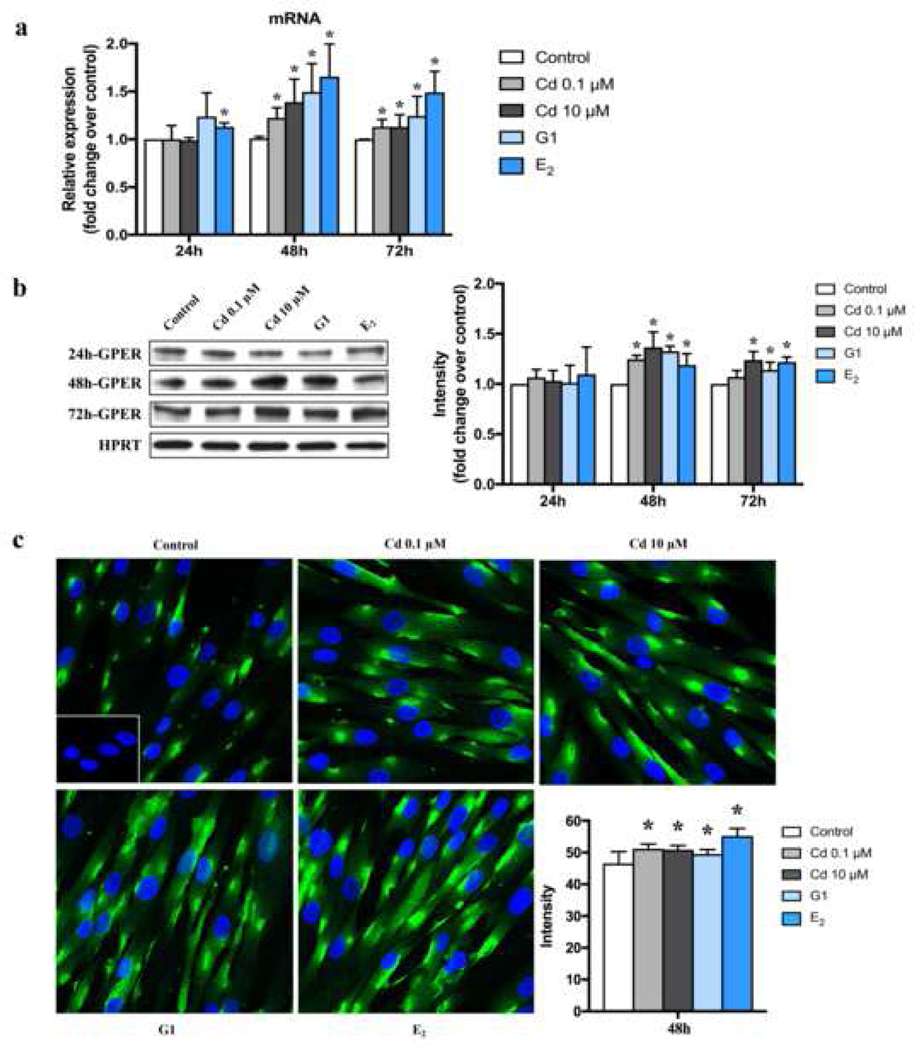
To evaluate the effect of Cd on GPER expression, ht-UtLM cells were treated with 0.1 μM and 10 μM Cd for 24 h, 48 h and 72 h. As G1 has been identified as a selective agonist of GPER (Prossnitz and Barton 2014), we used G1 and E2 as positive controls. Cd, G1 and E2 increased the expression of GPER at both the mRNA and protein levels, especially at 48 h and 72 h (p <0.05) (Fig. 2a, b). As a result of these findings, we chose 48 h for additional experiments. Immunofluorescence staining of ht-UtLM cells showed that GPER was expressed in the cytoplasm mainly concentrated in the perinuclear region and cell membrane (Fig. 2c), consistent with the GPER cytoplasmic location in human uterine tissues (Fig. 1a). In addition, GPER expression was increased after Cd, G1 and E2 treatment, with higher mean cytoplasmic fluorescence intensities in Cd-treated cells compared to control (p <0.05) (Fig. 2c), although the percent of positive cells for GPER didn’t change significantly (Fig. S2).
Fig. 2.
Effects of Cd on the expression of GPER mRNA and protein levels in ht-UtLM cells at 24h, 48h and 72h. (a) GPER mRNA levels by real-time PCR. *P <0.05 compared with respective control. (b) GPER protein levels by western blotting and band intensity levels relative to controls. *P <0.05 compared with respective control. HPRT = loading control. (c) Perinuclear and plasma membrane expression of GPER in ht-UtLM cells treated with Cd for 48h by immunofluorescence. Representative images of GPER expression and average intensity. Inset: Negative control with normal rabbit serum. Green = GPER; Blue = DAPI (x 400, original magnification). *P <0.05, compared with control. All experiments were repeated independently in triplicate.
Role of GPER or ERα36 in Cd-induced cell proliferation
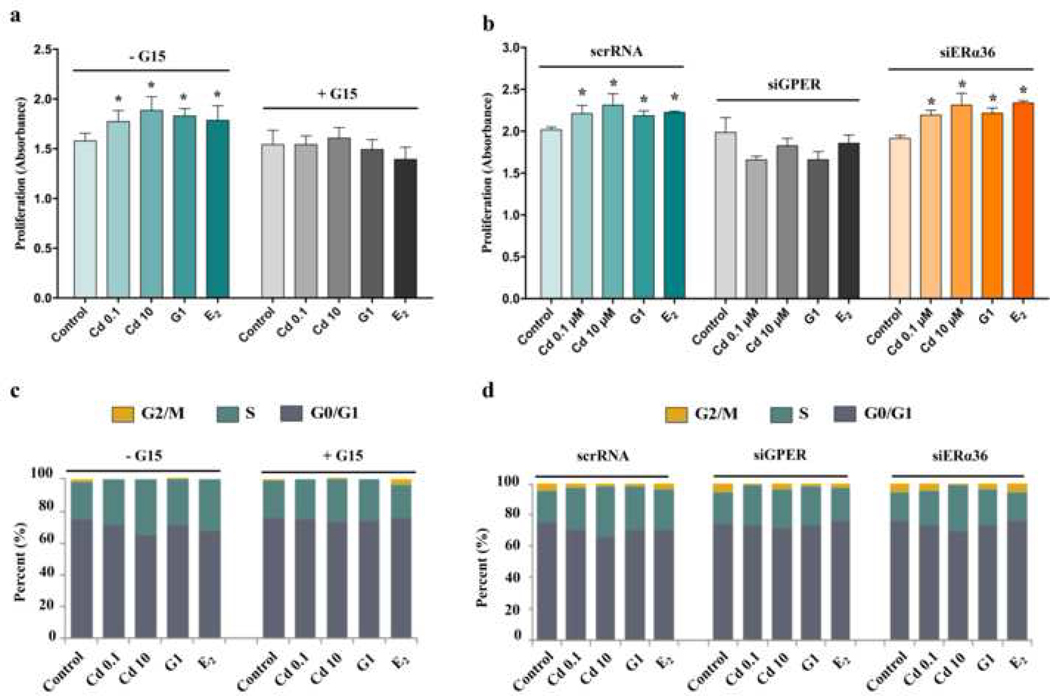
The effect of Cd on cell proliferation was also evaluated after treatment with Cd, G1 or E2 at 24h, 48h, and 72h. Compared to respective controls, Cd, G1 and E2 all increased cell proliferation significantly at each time point (p <0.05) (Fig. S3). To test whether Cd induced cell proliferation was through GPER or ERα36, G15 (a specific antagonist of GPER) (Prossnitz and Barton 2014), siGPER or siERα36 was used to inhibit the function of GPER or ERα36. Ht-UtLM cells in the absence or presence of pretreatment with G15, siGPER or siERα36, were then treated with Cd, G1 or E2 for 48 h, followed by conducting cell proliferation assays and cell cycle analysis. Compared with control, Cd, G1 and E2 increased cell proliferation, while pretreatment with G15 or siGPER inhibited these effects; but there was no change after pretreatment with siERα36 (Fig. 3a, b). The cell cycle analysis data were consistent with these findings. Compared to controls, Cd, G1 and E2 stimulated more cells into S and/or G2/M phases, demonstrating the proliferative status of cells. While upon pretreatment with G15 or siGPER, but not siERα36, this effect was inhibited (Fig. 3c, d), indicating that GPER but not ERα36 was necessary for Cd-induced cell proliferation.
Fig. 3.
Effects of GPER and ERa36 on cell proliferation in ht-UtLM cells treated with Cd for 48h. (a) and (b) were determined by CCK-8 analysis: (a) Cells in the absence or presence of pretreatment with a GPER antagonist, G15, for 30 min; (b) GPER and ERα36 were silenced by siRNA before treatment with Cd, G1or E2. (c) and (d) represented DNA analysis by flow cytometry: (c) Cells in the absence or presence of G15 pretreatment for 30 min. (d) GPER and ERa36 were silenced by siRNA before treatment with Cd, G1, or E2. *P < 0.05, compared with respective control. All experiments were repeated independently in triplicate.
GPER/Src/EGFR/MAPK pathway was involved in Cd-induced ht-UtLM cell proliferation.
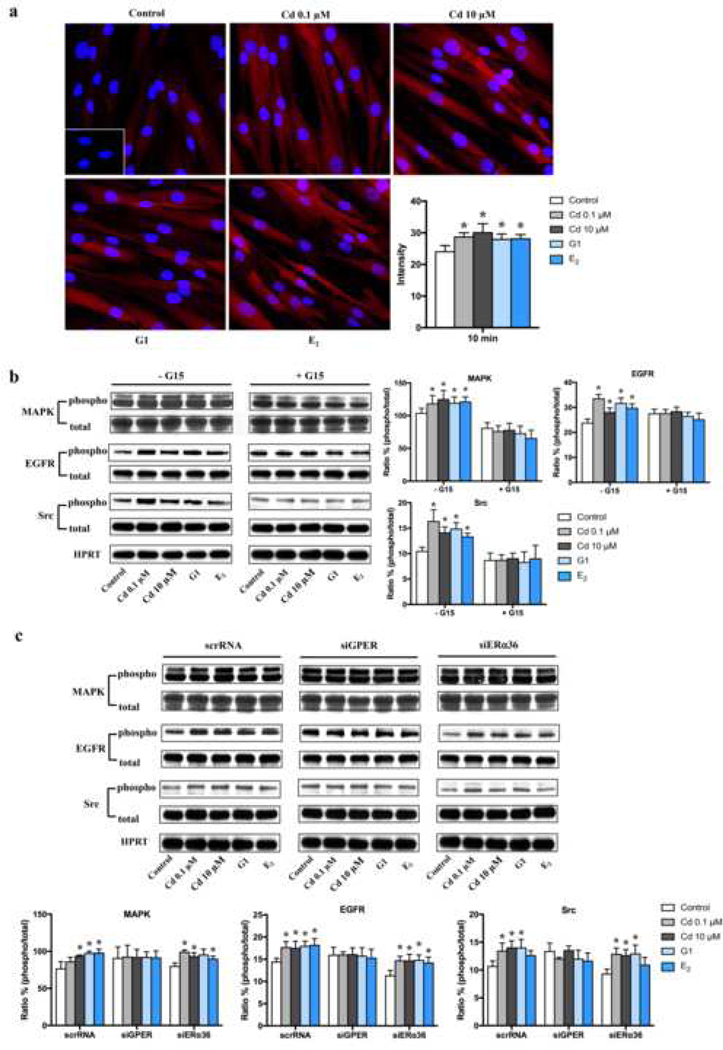
Possible mechanism of Cd-induced cell proliferation is through MAPK pathway, which has been well identified as a critical mediator of cell proliferation (Gao et al. 2015), so the influence of Cd on activation of p44/42 MAPK was investigated. The immunofluorescence data showed that activation of phospho-MAPK was induced in cells treated with Cd, G1 and E2 for 10 min, with higher fluorescence intensities compared to control (p <0.05) (Fig. 4a); there was no significant change in the percent of positive cells for phospho-MAPK among groups except the higher dose of Cd (p <0.05) (Fig. S4). The similar result was found by western blotting. Compared to controls, Cd, G1 and E2 induced an increase of phospho-MAPK in ht-UtLM cells at 10 min (Fig. 4b, c), and this effect was inhibited by pretreatment with G15 (Fig. 4b) or siGPER (Fig. 4c), but not by siERα36 (Fig. 4c). This indicated that GPER, but not siERa36, was directly involved in the Cd-induced activation of MAPK pathway.
Fig. 4.
Expression of phospho-MAPK, phospho-EGFR and phospho-Src in ht-UtLM cells following administration of Cd for 10 min.
(a) Representative images of p-MAPK expression by immunofluorescence and average intensity. Inset: Negative control with normal rabbit serum. Red = p-MAPK; Blue = DAPI (x 400, original magnification). *P <0.05, compared with control. (b) Representative immunoblots and band intensities in the absence or presence of pretreatment with a GPER antagonist, G15, for 30 min. *P <0.05 compared with respective control. (c) Representative immunoblots and band intensities with or without siRNA pretreatment. *P <0.05 compared with respective control. HPRT = loading control. All experiments were repeated independently in triplicate.
As activation of EGFR can trigger the MAPK cascade, so the role of EGFR in Cd-induced proliferation in ht-UtLM cells was further studied. We probed several tyrosine sites on EGFR for increased phosphorylation following Cd, G1, and E2 treatment. It was shown that Y845 was the major phosphotyrosine (Y) residue activated in Cd-treated ht-UtLM cells (Fig. S5), which is the site of Src-mediated phosphorylation in the regulation of EGFR function and tumor progression (Biscardi et al. 1999). And EGFR was activated as early as 10 min following Cd treatment (Fig. 4b, c). Both MAPK activation and EGFR phosphorylation were GPER-dependent, showing that G15 (Fig. 4b) or siGPER (Fig. 4c), but not siERα36 (Fig. 4c) inhibited the increased phosphorylation of EGFR induced by Cd.
A common kinase activated by membrane-associated ER and plays a critical role in EGFR activation is Src, and it has been well-established that Src can phosphorylate EGFR on tyrosine residue Y845 (Sato 2013; Tice et al. 1999). So, we investigated whether Cd could affect Src activation. We found phospho-Src was increased significantly in cells treated with Cd, or positive controls G1 and E2 (Fig. 4b, c), consistent with the activation of MAPK and EGFR. Src activation was also inhibited by G15 (Fig. 4b) or siGPER pretreatment (Fig. 4c), which confirmed the necessity of GPER in the activation of Src. Taken together, these data suggest that Cd activated Src/EGER/MAPK pathway through GPER, but not ERα36.
Effects of Cd on activity and expression of MMP2, MMP9, TIMP1, TIMP2 and HB-EGF
The main mechanism for the transactivation of EGFR by GPER is controlled by the activation of membrane-bound matrix metalloproteases (MMPs), which are able to cleave EGFR ligands such as heparin-binding EGF-like growth factor (HB-EGF) (Wang 2016) (Fig. S6). It has been reported that G-protein-coupled receptors can induce transactivation of EGFR through phospho-Src/MMPs/HB-EGF in different types of cells (Overland and Insel 2015; Prenzel et al. 1999; Thornton et al. 2015).
In order to further investigate if this crosstalk between signaling pathways was involved in GPER-dependent transactivation of EGFR induced by Cd, we conducted zymography and western blotting to separately evaluate the activation and expression of MMPs. As MMP2 and MMP9 are the main metalloproteinases expressed in uterine leiomyoma and myometrium, we evaluated both of them (Bogusiewicz et al. 2007; Wolanska et al. 2004). We found the activities of both MMPs were stimulated by Cd, G1 and E2 compared to the controls (Fig. 5a, b, c, d); whereas pretreatment with G15 or siGPER, resulted in no significant differences in MMP2 or MMP9 activity among the groups (Fig. 5a, b, c, d).
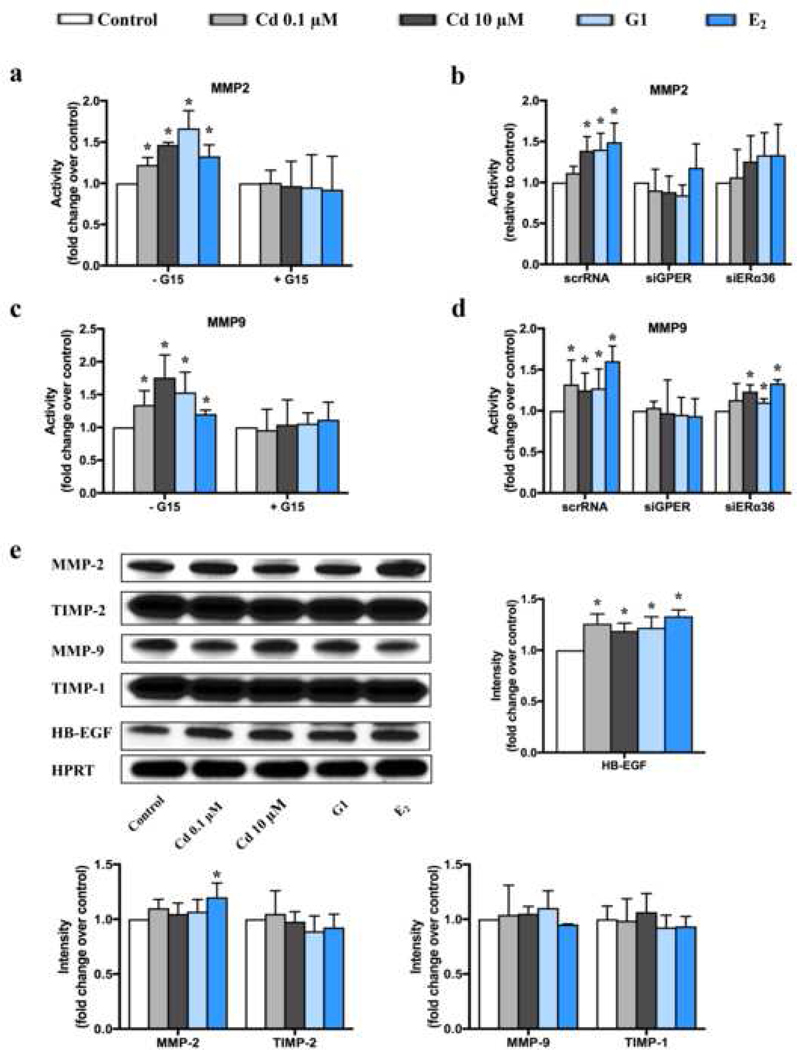
Fig. 5.
Effects of Cd on MMP2, MMP9, TIMP1, TIMP2 and HB-EGF in ht-UtLM cells.
(a-d) The activity of MMPs was tested by zymography following administration of Cd for 10 min. Activity of MMP2 with or without pretreatment by G15 (a) or silencing RNA (b); activity of MMP9 with or without pretreatment by G15 (c) or silencing RNA (d). *P <0.05 compared to respective controls. (e) Expression of MMP2, MMP9, TIMP1, TIMP2 and HB-EGF were evaluated by western blotting following administration of Cd for 48 h. *P <0.05 compared to control. HPRT = loading control. All experiments were repeated independently in triplicate.
We also evaluated the expression of MMP2 and MMP9 as well as their inhibitors (TIMP1 and TIMP2) in ht-UtLM cells after treatment with Cd, G1 or E2 for 48h. As shown in Fig. 5e, there was no change among different groups except only E2 increased MMP2 expression. However, as stated above there was an increase in the activity of MMP2 and MMP9 that was inhibited when GPER was silenced (see Fig. 5a, b, c, d). In order to evaluate if the increased enzymatic activity of MMP2 and MMP9 could result in cleaved EGFR ligand, HB-EGF, we further tested the expression of HB-EGF in ht-UtLM cells, and observed increased expression following Cd, G1 and E2 administration (Fig. 5e). These manifested that Cd could transactivate EGFR indirectly through GPER/phosphor-Src/MMPs/HB-EGF pathway.
Direct interaction of phospho-Src and phospho-EGFR
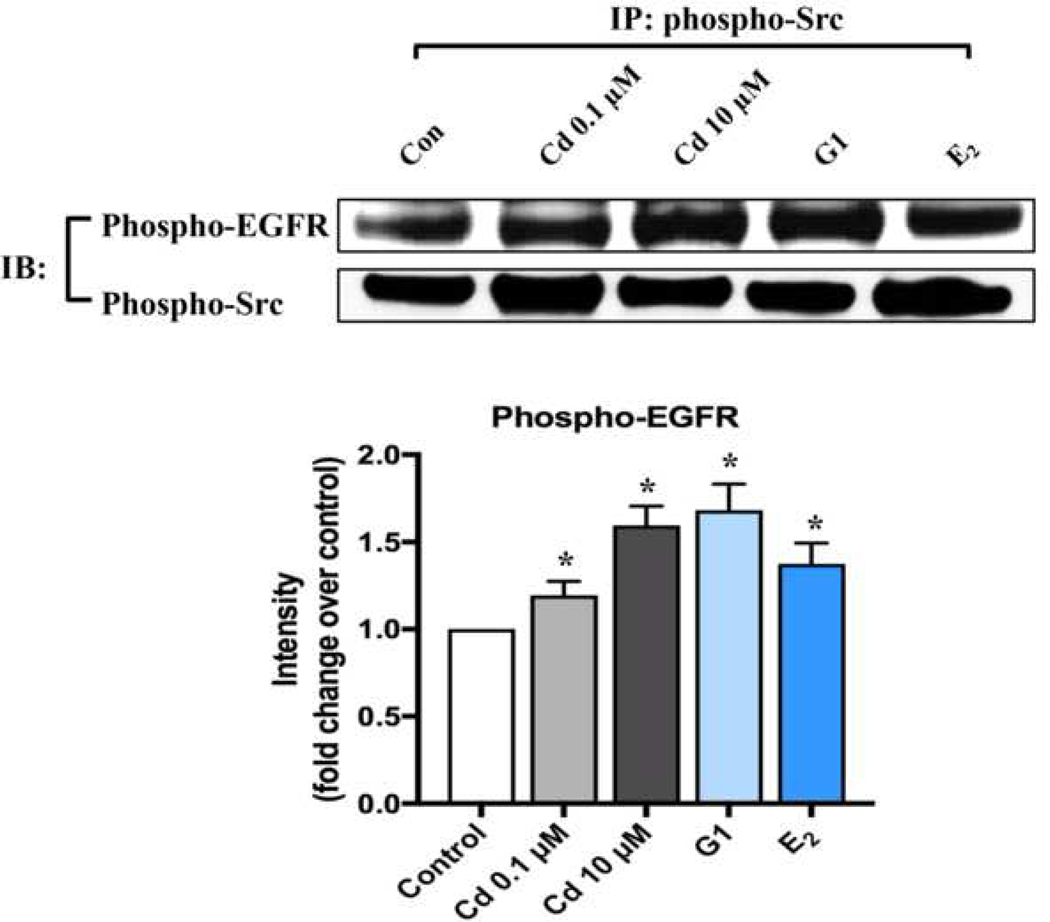
As there has been report that EGFR activation can also occur directly by GPCRs-induced Src phosphorylation (Wang 2016) (Fig. S6), so we evaluated if phospho-Src could interact directly to phosphorylate EGFR. Coimmunoprecipitation studies were performed by using phospho-Src antibody to capture phospho-EGFR. As shown in Fig. 6, both phospho-Src and phospho-EGFR coimmunoprecipitated following Cd treatment, indicating there was direct interaction between phospho-Src and phospho-EGFR that was enhanced by Cd administration.
Fig. 6.
Cd-induced interactions between phospho-EGFR and phospho-Src in ht-UtLM cells. Interactions between phospho-EGFR and phospho-Src was determined by coimmunoprecipitation (IP) as described in Materials and Methods. Representative immunoblots and band intensities of western blotting. *P <0.05 compared to control. Experiments were repeated independently in triplicate.
Discussion
It is well established that E2 or xenoestrogens stimulate genomic ER signaling by binding to ERs to induce ERE-dependent transcriptional activation of genes. However, not all of the activities mediated by ERs are accomplished through a direct effect on gene transcription. In addition to the classical genomic ER signaling, rapid nongenomic ER signaling that involves cytoplasmic signaling proteins, growth factor receptors, and other membrane-initiated signaling pathways has been reported (Prossnitz and Barton 2011; Wang and Yin 2015).
GPER is one of the membrane-bound ERs that mediate rapid, nongenomic responses to estrogens (Prossnitz and Barton 2011). However, its subcellular localization is still a subject of debate, with varying reports showing cell membrane (Cheng et al. 2011; Zimmerman et al. 2016), intracytosolic membrane especially in the perinuclear endoplasmic reticulum region (Revankar et al. 2005; Revankar et al. 2007), and even nuclear localization (Pupo et al. 2013; Tian et al. 2013). Different subcellular locations of GPER may be associated with cell types and tumor stage. In this study, we provide evidence for the expression of GPER in uterine leiomyoma, where it is higher than that in the myometrium, which is consistent with the paper published by Tian et al (Tian et al. 2013), but the location is different. Tian et al showed the nuclear localization of GPER in leiomyoma tissues, while we found GPER predominantly expressed in cytoplasm and cell membrane both in uterine leiomyoma tissues and ht-UtLM cells.
A variety of signaling pathways activated by GPER has been recognized, especially EGFR transactivation (Filardo 2002). Much evidence indicates that in various human cancers, GPER activation causes the release of intracellular MMPs and HB-EGF, which results in transactivation of EGFR and subsequent activation of the MAPK pathway (Filardo and Thomas 2005; Molina et al. 2017). It is important to recognize if Cd can transactivate the EGFR/MAPK pathway through GPER. In human lung adenocarcinoma cells, Cd can activate rapid MAPK/ERK phosphorylation involving Src, EGFR and GPER (Huff et al. 2016). In thyroid cancer cells, GPER/ERK&AKT/NF-kB pathway is involved in Cd-induced cell proliferation, invasion and migration (Zhu et al. 2017). In breast cancer cells, Cd can stimulate cell proliferation by elevating cAMP levels, activating kinases in the ERK pathway through GPER (Yu et al. 2010). The present study also shows that Cd induced EGFR signaling through GPER and Src in ht-UtLM cells, as G15 or siGPER inhibited the phosphorylation of Src and EGFR. Src family kinases have been reported to be associated with GPCRs (Liebmann 2011). However, how Src is activated by GPCRs is unclear. One way may be through the direct interaction between Src SH3 domain and the GPCR cytoplasmic domain that contains consensus proline-rich motifs in its C-terminal tail or third intracellular loop (Wang 2016). Another way may be through the binding to GPCR-associated proteins such as G protein subunits, which can activate Src and then phosphorylate EGFR at its intracellular domain (Liebmann 2011).
The ligand-dependent EGFR transactivation by HB-EGF through an MMPs mechanism has been well defined (Wang 2016). GPER activation stimulates phosphorylation of Src, which activates MMPs. The MMPs in turn cleave pro-HB-EGF, releasing free HB-EGF that can then bind to and activate EGFR. The main MMPs in both myometrium and uterine leiomyoma include MMP1, MMP2 (gelatinase A), MMP3 and MMP9 (gelatinase B), and the amount of MMP2 is significantly higher in comparison to other metalloproteinases, which is responsible for remodeling of the extracellular matrix in the growing fibroid tumors (Wolanska et al. 2004). MMP2 and MMP9 are proteolytic enzymes degrading extracellular matrix proteins, mainly collagen type IV. Our data show that GPER activation increases the activity of MMP2 and MMP9 in ht-UtLM cells treated with Cd, supporting that these proteases may be implicated in the growth of uterine leiomyoma.
In addition to the ligand-dependent EGFR transactivation, there is another possibility that EGFR can be transactivated by GPCR without detectable EGF-like ligands, which is ligand-independent EGFR transactivation (Liebmann 2011; Wang 2016). Several mechanisms have been suggested regarding the pathways of ligand-independent EGFR transactivation. It is mainly through the direct interaction between activation of intracellular protein tyrosine kinases (PTKs) such as Src family proteins and phosphorylation of EGFR (Cattaneo et al. 2014; George et al. 2013). In the present study, we have also confirmed there is an increase in Src activation and an enhanced direct interaction between phospho-Src and phospho-EGFR, indicating that EGFR transactivation by GPER is through phospho-Src by both direct and indirect pathways.
GPER is not the only ER that can initiate nongenomic signaling in estrogen-responsive cells, as ERα36, a truncated isoform of ERα, can also induce rapid signaling (Zimmerman et al. 2016). ERα36 is a novel variant of the full-length 66kD ERα that lacks transcriptional activation domains (AF-1 and AF-2) but maintains the DNA-binding domain and partial dimerization and ligand-binding domains (Wang and Yin 2015). Previously, ERα36 has been reported to activate the MAPK/ERK and PI3K/AKT pathways (Lin et al. 2010) and the PKCδ/ERK signaling pathway (Tong et al. 2010). Therefore, it is likely that xenoestrogens can interact with ERα36, resulting in activation of nongenomic pathways. Our lab has found that ERα36 is predominantly localized in mitochondria of human uterine smooth muscle and leiomyoma cells (Yan et al. 2017). And in this study, ERα36 has been proved highly expressed in leiomyoma tissues than myometrium tissues, implying the possible involvement of ERα36 in effect of xenoestrogens on uterine leiomyomas. Our group has reported that ERα36 mediates bisphenol A-induced cell proliferation in ht-UtLM cells (Yu et al. 2019). However, in the present study, when we silenced ERa36 in ht-UtLM cells following administration with Cd, there is no change in Cd-induced cell proliferation or signaling, indicating that ERα36 does not directly mediate Cd’s nongenomic signaling effects, which appears to occur through GPER signaling.
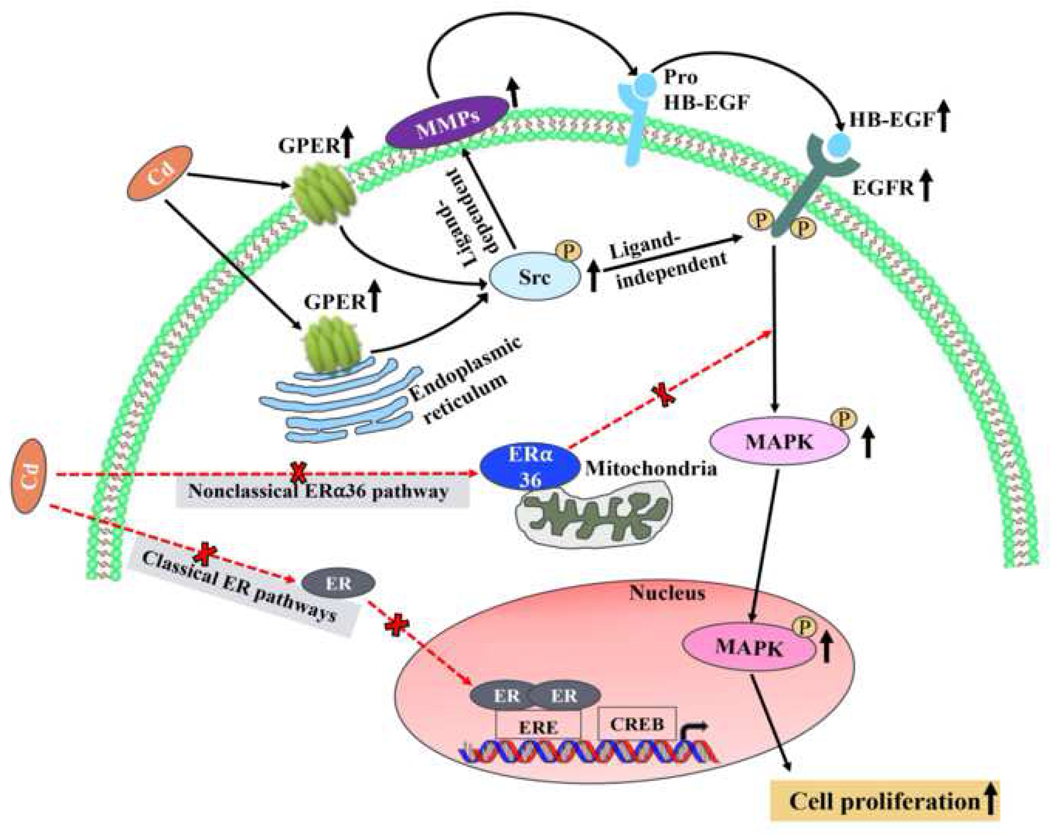
The present study demonstrates that Cd-induced proliferation of human leiomyoma cells is through a nongenomic pathway requiring membrane-associated GPER and EGFR transactivation that did not directly involve the ERα36 nongenomic signaling pathway. The effects of Cd on cell proliferation are mainly through GPER activation and phosphorylation of Src, which then activates MMPs, and they in turn cleave pro-HB-EGF into HB-EGF to activate EGFR; phosphorylated Src can also directly activate EGFR initiating downstream MAPK signaling (Fig. 7).
Fig. 7.
Cd-induced proliferation of human leiomyoma cells was not through classical ER binding (Gao et al. 2015) or ERa36 nongenomic ER signaling pathways, but through a nongenomic pathway requiring membrane-associated GPER and EGFR transactivation. The effects of Cd on cell proliferation are mainly through GPER/p-Src/MMP2/MMP9/HB-EGF/EGFR/MAPK signaling pathway (ligand-dependent), and also through p-Src-induced EGFR transactivation directly (ligand-independent).
Taken together, our study has proved the “metalloestrogenic” effects of Cd on uterine leiomyoma cells, and the mechanisms involved in Cd-induced cell proliferation of ht-UtLM cells appears to be mediated in part via a GPER/EGFR pathway. Cd may be a risk factor for women with uterine fibroids and its effects are through crosstalk between hormone and growth factor signaling pathways.
Supplementary Material
Acknowledgements
The authors sincerely thank Mr. Charles J. Tucker (Fluorescence Microscopy and Imaging Center), NIEHS for helping setup confocal analysis software and Drs. Jerry J. Liu and Wendy Jefferson for their critical review and comments on the manuscript. J.L. thanks the Natural Science Foundation of China (81500613) and Jiangsu Province (BK20150108) for their support. This work was funded by the Intramural Research Program of the NIH, NIEHS and NTP.
Footnotes
Compliance with ethical standards
The collection of human uterine tissues in the present study was in accordance with the approval of Institutional Review Board (IRB) of the NIEHS. Every patient had signed the informed consent prior to their inclusion in the study.
Conflict of interest
The authors declare no conflict of interest.
References
- Ba Q, Li M, Chen P, et al. (2017) Sex-Dependent Effects of Cadmium Exposure in Early Life on Gut Microbiota and Fat Accumulation in Mice. Environ Health Perspect 125(3):437–446 doi: 10.1289/EHP360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ (1999) c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyrl 101 is associated with modulation of receptor function. J Biol Chem 274(12):8335–43 [DOI] [PubMed] [Google Scholar]
- Bogusiewicz M, Stryjecka-Zimmer M, Postawski K, Jakimiuk AJ, Rechberger T (2007) Activity of matrix metalloproteinase-2 and −9 and contents of their tissue inhibitors in uterine leiomyoma and corresponding myometrium. Gynecol Endocrinol 23(9):541–6 doi: 10.1080/09513590701557416 [DOI] [PubMed] [Google Scholar]
- Byrne C, Divekar SD, Storchan GB, Parodi DA, Martin MB (2013) Metals and breast cancer. J Mammary Gland Biol Neoplasia 18(1):63–73 doi: 10.1007/s10911-013-9273-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney SA, Tahara H, Swartz CD, et al. (2002) Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics. Lab Invest 82(6):719–28 [DOI] [PubMed] [Google Scholar]
- Cattaneo F, Guerra G, Parisi M, et al. (2014) Cell-surface receptors transactivation mediated by g protein-coupled receptors. Int J Mol Sci 15(11):19700–28 doi: 10.3390/ijms151119700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SB, Graeber CT, Quinn JA, Filardo EJ (2011) Retrograde transport of the transmembrane estrogen receptor, G-protein-coupled-receptor-30 (GPR30/GPER) from the plasma membrane towards the nucleus. Steroids 76(9):892–6 doi: 10.1016/j.steroids.2011.02.018 [DOI] [PubMed] [Google Scholar]
- Cho YA, Kim J, Woo HD, Kang M (2013) Dietary cadmium intake and the risk of cancer: a meta-analysis. PLoS One 8(9):e75087 doi: 10.1371/journal.pone.0075087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commandeur AE, Styer AK, Teixeira JM (2015) Epidemiological and genetic clues for molecular mechanisms involved in uterine leiomyoma development and growth. Hum Reprod Update 21(5):593–615 doi: 10.1093/humupd/dmv030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ (2002) Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol 80(2):231–8 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P (2005) GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab 16(8):362–7 doi: 10.1016/j.tem.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Gao X, Yu L, Moore AB, Kissling GE, Waalkes MP, Dixon D (2015) Cadmium and proliferation in human uterine leiomyoma cells: evidence of a role for EGFR/MAPK pathways but not classical estrogen receptor pathways. Environ Health Perspect 123(4):331–6 doi: 10.1289/ehp.1408234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George AJ, Hannan RD, Thomas WG (2013) Unravelling the molecular complexity of GPCR-mediated EGFR transactivation using functional genomics approaches. FEBS J 280(21):5258–68 doi: 10.1111/febs.12509 [DOI] [PubMed] [Google Scholar]
- Huff MO, Todd SL, Smith AL, et al. (2016) Arsenite and Cadmium Activate MAPK/ERK via Membrane Estrogen Receptors and G-Protein Coupled Estrogen Receptor Signaling in Human Lung Adenocarcinoma Cells. Toxicol Sci 152(1):62–71 doi: 10.1093/toxsci/kfw064 [DOI] [PubMed] [Google Scholar]
- Hunter DS, Hodges LC, Eagon PK, et al. (2000) Influence of exogenous estrogen receptor ligands on uterine leiomyoma: evidence from an in vitro/in vivo animal model for uterine fibroids. Environ Health Perspect 108 Suppl 5:829–34 [DOI] [PubMed] [Google Scholar]
- Johnstone EB, Louis GM, Parsons PJ, et al. (2014) Increased urinary cobalt and whole blood concentrations of cadmium and lead in women with uterine leiomyomata: Findings from the ENDO Study. Reprod Toxicol 49:27–32 doi: 10.1016/j.reprotox.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasap B, Ozturk Turhan N, Edgunlu T, Duran M, Akbaba E, Oner G (2016) G-protein-coupled estrogen receptor-30 gene polymorphisms are associated with uterine leiomyoma risk. Bosn J Basic Med Sci 16(1):39–45 doi: 10.17305/bjbms.2016.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KE, Darrah TH, Money E, et al. (2015) Geographic clustering of elevated blood heavy metal levels in pregnant women. BMC Public Health 15:1035 doi: 10.1186/s12889-015-2379-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebmann C (2011) EGF receptor activation by GPCRs: an universal pathway reveals different versions. Mol Cell Endocrinol 331(2):222–31 doi: 10.1016/j.mce.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Lin SL, Yan LY, Zhang XT, et al. (2010) ER-alpha36, a variant of ER-alpha, promotes tamoxifen agonist action in endometrial cancer cells via the MAPK/ERK and PI3K/Akt pathways. PLoS One 5(2):e9013 doi: 10.1371/journal.pone.0009013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingaiah S, Hart JE, Wise LA, Terry KL, Boynton-Jarrett R, Missmer SA (2014) Prenatal diethylstilbestrol exposure and risk of uterine leiomyomata in the Nurses’ Health Study II. Am J Epidemiol 179(2):186–91 doi: 10.1093/aje/kwt250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti K, Paul JW, Read M, et al. (2011) G-1-activated membrane estrogen receptors mediate increased contractility of the human myometrium. Endocrinology 152(6):2448–55 doi: 10.1210/en.2010-0979 [DOI] [PubMed] [Google Scholar]
- Molina L, Figueroa CD, Bhoola KD, Ehrenfeld P (2017) GPER-1/GPR30 a novel estrogen receptor sited in the cell membrane: therapeutic coupling to breast cancer. Expert Opin Ther Targets 21(8):755–766 doi: 10.1080/14728222.2017.1350264 [DOI] [PubMed] [Google Scholar]
- Nakareangrit W, Thiantanawat A, Visitnonthachai D, Watcharasit P, Satayavivad J (2016) Sodium arsenite inhibited genomic estrogen signaling but induced pERalpha (Ser118) via MAPK pathway in breast cancer cells. Environ Toxicol 31(9):1133–46 doi: 10.1002/tox.22122 [DOI] [PubMed] [Google Scholar]
- Nasiadek M, Swiatkowska E, Nowinska A, Krawczyk T, Wilczynski JR, Sapota A (2011) The effect of cadmium on steroid hormones and their receptors in women with uterine myomas. Arch Environ Contam Toxicol 60(4):734–41 doi: 10.1007/s00244-010-9580-8 [DOI] [PubMed] [Google Scholar]
- Overland AC, Insel PA (2015) Heterotrimeric G proteins directly regulate MMP14/membrane type-1 matrix metalloprotease: a novel mechanism for GPCR-EGFR transactivation. J Biol Chem 290(16):9941–7 doi: 10.1074/jbc.C115.647073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack AZ, Ranasinghe S, Sjaarda LA, Mumford SL (2014) Cadmium and Reproductive Health in Women: A Systematic Review of the Epidemiologic Evidence. Curr Environ Health Rep 1(2):172–184 doi: 10.1007/s40572-014-0013-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, et al. (1999) EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402(6764):884–8 doi: 10.1038/47260 [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M (2011) The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 7(12):715–26 doi: 10.1038/nrendo.2011.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M (2014) Estrogen biology: new insights into GPER function and clinical opportunities. Mol Cell Endocrinol 389(1–2):71–83 doi: 10.1016/j.mce.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupo M, Vivacqua A, Perrotta I, et al. (2013) The nuclear localization signal is required for nuclear GPER translocation and function in breast Cancer-Associated Fibroblasts (CAFs). Mol Cell Endocrinol 376(1– 2):23–32 doi: 10.1016/j.mce.2013.05.023 [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307(5715):1625–30 doi: 10.1126/science.1106943 [DOI] [PubMed] [Google Scholar]
- Revankar CM, Mitchell HD, Field AS, et al. (2007) Synthetic estrogen derivatives demonstrate the functionality of intracellular GPR30. ACS Chem Biol 2(8):536–44 doi: 10.1021/cb700072n [DOI] [PubMed] [Google Scholar]
- Ruhl L, Vengosh A, Dwyer GS, et al. (2012) The impact of coal combustion residue effluent on water resources: a North Carolina example. Environ Sci Technol 46(21):12226–33 doi: 10.1021/es303263x [DOI] [PubMed] [Google Scholar]
- Safe S (2003) Cadmium’s disguise dupes the estrogen receptor. Nat Med 9(8):1000–1 doi: 10.1038/nm0803-1000 [DOI] [PubMed] [Google Scholar]
- Sato K (2013) Cellular functions regulated by phosphorylation of EGFR on Tyr845. Int J Mol Sci 14(6):10761–90 doi: 10.3390/ijms140610761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva N, Peiris-John R, Wickremasinghe R, Senanayake H, Sathiakumar N (2012) Cadmium a metalloestrogen: are we convinced? J Appl Toxicol 32(5):318–32 doi: 10.1002/jat.1771 [DOI] [PubMed] [Google Scholar]
- Styer AK, Rueda BR (2016) The Epidemiology and Genetics of Uterine Leiomyoma. Best Pract Res Clin Obstet Gynaecol 34:3–12 doi: 10.1016/j.bpobgyn.2015.11.018 [DOI] [PubMed] [Google Scholar]
- Thornton KJ, Kamange-Sollo E, White ME, Dayton WR (2015) Role of G protein-coupled receptors (GPCR), matrix metalloproteinases 2 and 9 (MMP2 and MMP9), heparin-binding epidermal growth factor-like growth factor (hbEGF), epidermal growth factor receptor (EGFR), erbB2, and insulin-like growth factor 1 receptor (IGF-1R) in trenbolone acetate-stimulated bovine satellite cell proliferation. J Anim Sci 93(9):4291–301 doi: 10.2527/jas.2015-9191 [DOI] [PubMed] [Google Scholar]
- Tian R, Wang Z, Shi Z, et al. (2013) Differential expression of G-protein-coupled estrogen receptor-30 in human myometrial and uterine leiomyoma smooth muscle. Fertil Steril 99(1):256–63 doi: 10.1016/j.fertnstert.2012.09.011 [DOI] [PubMed] [Google Scholar]
- Tice DA, Biscardi JS, Nickles AL, Parsons SJ (1999) Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci U S A 96(4):1415–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong JS, Zhang QH, Wang ZB, et al. (2010) ER-alpha36, a novel variant of ER-alpha, mediates estrogen-stimulated proliferation of endometrial carcinoma cells via the PKCdelta/ERK pathway. PLoS One 5(11):e15408 doi: 10.1371/journal.pone.0015408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verougstraete V, Lison D, Hotz P (2003) Cadmium, lung and prostate cancer: a systematic review of recent epidemiological data. J Toxicol Environ Health B Crit Rev 6(3):227–55 doi: 10.1080/10937400306465 [DOI] [PubMed] [Google Scholar]
- Wang Z (2016) Transactivation of Epidermal Growth Factor Receptor by G Protein-Coupled Receptors: Recent Progress, Challenges and Future Research. Int J Mol Sci 17(1) doi: 10.3390/ijms17010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Yin L (2015) Estrogen receptor alpha-36 (ER-alpha36): A new player in human breast cancer. Mol Cell Endocrinol 418 Pt 3:193–206 doi: 10.1016/j.mce.2015.04.017 [DOI] [PubMed] [Google Scholar]
- Wolanska M, Sobolewski K, Bankowski E, Jaworski S (2004) Matrix metalloproteinases of human leiomyoma in various stages of tumor growth. Gynecol Obstet Invest 58(1):14–8 doi: 10.1159/000077177 [DOI] [PubMed] [Google Scholar]
- Xiao HH, Gao QG, Zhang Y, et al. (2014) Vanillic acid exerts oestrogen-like activities in osteoblast-like UMR 106 cells through MAP kinase (MEK/ERK)-mediated ER signaling pathway. J Steroid Biochem Mol Biol 144 Pt B:382–91 doi: 10.1016/j.jsbmb.2014.08.002 [DOI] [PubMed] [Google Scholar]
- Yan Y, Yu L, Castro L, Dixon D (2017) ERalpha36, a variant of estrogen receptor alpha, is predominantly localized in mitochondria of human uterine smooth muscle and leiomyoma cells. PLoS One 12(10):e0186078 doi: 10.1371/journal.pone.0186078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Chung HW, Jeong K, et al. (2017) Blood cadmium and volume of uterine fibroids in premenopausal women. Ann Occup Environ Med 29:22 doi: 10.1186/s40557-017-0178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Das P, Vall AJ, et al. (2019) Bisphenol A induces human uterine leiomyoma cell proliferation through membrane-associated ERalpha36 via nongenomic signaling pathways. Mol Cell Endocrinol doi: 10.1016/j.mce.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Filardo EJ, Shaikh ZA (2010) The membrane estrogen receptor GPR30 mediates cadmium-induced proliferation of breast cancer cells. Toxicol Appl Pharmacol 245(1):83–90 doi: 10.1016/j.taap.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Zhu P, Liao LY, Zhao TT, Mo XM, Chen GG, Liu ZM (2017) GPER/ERK&AKT/NF-kappaB pathway is involved in cadmium-induced proliferation, invasion and migration of GPER-positive thyroid cancer cells. Mol Cell Endocrinol 442:68–80 doi: 10.1016/j.mce.2016.12.007 [DOI] [PubMed] [Google Scholar]
- Zimmerman MA, Budish RA, Kashyap S, Lindsey SH (2016) GPER-novel membrane oestrogen receptor. Clin Sci (Lond) 130(12):1005–16 doi: 10.1042/CS20160114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.