Abstract
SARS-CoV-2 is causing a pandemic resulting in high morbidity and mortality. COVID-19 patients suffering from acute respiratory distress syndrome (ARDS) are often critically ill and show lung injury and hemolysis. Heme is a prosthetic moiety crucial for the function of a wide variety of heme-proteins, including hemoglobin and cytochromes. However, injury-derived free heme promotes adhesion molecule expression, leukocyte recruitment, vascular permeabilization, platelet activation, complement activation, thrombosis, and fibrosis. Heme can be degraded by the anti-inflammatory enzyme heme oxygenase (HO) generating biliverdin/bilirubin, iron/ferritin, and carbon monoxide. We therefore postulate that free heme contributes to many of the inflammatory phenomena witnessed in critically ill COVID-19 patients, whilst induction of HO-1 or harnessing heme may provide protection. HO-activity not only degrades injurious heme, but its effector molecules possess also potent salutary anti-oxidative and anti-inflammatory properties. Until a vaccine against SARS-CoV-2 becomes available, we need to explore novel strategies to attenuate the pro-inflammatory, pro-thrombotic, and pro-fibrotic consequences of SARS-CoV-2 leading to morbidity and mortality. The heme-HO system represents an interesting target for novel “proof of concept” studies in the context of COVID-19.
Keywords: heme, heme oxygenase, SARS-CoV-2, inflammation, COVID-19
1. COVID-19 Infection May Result in Severe Inflammatory Complications
SARS-CoV-2 is causing a pandemic health issue, affecting millions of people and resulting in high morbidity and mortality. This virus is thought to specifically enter cells expressing the angiotensin-converting enzyme-2 (ACE2) receptors at their cell surface, such as cells in the nose, lungs, intestines, and kidneys [1,2,3]. Major common symptoms include fever, a dry cough, dyspnea, fatigue, and myalgia [4]. Minor common symptoms include expectoration, anorexia, chest tightness, nausea and vomiting, headache, pharyngalgia, shivering, and rhinorrhea [4].
Shortly following invasion of SARS-CoV-2 into the lungs, excessive pulmonary edema occurs as a consequence of vascular leakage. Disruption of the alveolar–epithelial barrier hampers optimal gas exchange [5], resulting in dyspnea. The majority of hospitalized Covid-19 patients also experience acute respiratory distress syndrome (ARDS), which appears to have various clinical features different from typical ARDS [6,7]. This is accompanied by other problems, including vascular inflammation, leukocyte recruitment, tissue injury, and the increased expression of interleukin-6, C-reactive protein, ferritin, and tissue factor [8]. In addition to these inflammatory insults, fibrin formation, microthrombi, and angiopathy develop that may result in vascular obstruction and fibrosis [9,10]. In addition to pulmonary damage, other organs with ACE2-positive cells may be affected, causing gastro-intestinal problems, multi-organ damage, and can ultimately even result in death.
In the absence of a vaccine against SARS-CoV-2, treatment is mainly supportive. In the meantime, we should develop hypotheses and perform “proof of principle” studies aimed at preventing or attenuating the complications leading to severe morbidity and death.
2. Can Injury-Derived Free Heme Contribute to COVID-19 Pathogenesis by Promoting Inflammation, Vascular Permeabilization and Thrombosis?
Heme is the functional group of a variety of heme–proteins, including cytochromes and hemoglobin (Hb), and is therefore crucial for many different cellular processes [11]. Excess of free heme has been shown to exacerbate and contribute to the pathogenesis of a wide variety of inflammatory diseases and conditions, such as sepsis, malaria, sickle cell disease, kidney disease, and multi-organ failure [11,12,13,14,15]. Additionally, within the lungs, free Hb and heme may be detrimental [16]. Chronic obstructive pulmonary disease (COPD) patients show increased cell-free Hb, correlating with disease severity [17]. In addition, ARDS patients exhibit alveolar hemorrhage and high levels of erythrocytes, hemolysis, and Hb in their pulmonary edema fluid [16,18,19,20,21]. Unfortunately, it is still not well understood how these erythrocytes enter the alveolar space [16]. This could occur via active transport, by increased endothelial and epithelial permeability, or by pronounced local vascular injury [16].
COVID-19 patients suffering from ARDS are critically ill and show also signs of hemolysis [22,23]. SARS-CoV-2 causes lung injury, resulting in death of inflammatory cells and sloughing of epithelial alveolar cells, the pulmonary vasculature, and hemolysis. Other COVID-19 patients display additional signs of tissue injury such as hemoptysis [24] or rhabdomyolysis [25], which may cause further cellular damage and release of heme-proteins and accumulation of free heme.
This made us to postulate that hemolysis-derived heme could initiate or contribute to many of the inflammatory phenomena witnessed in critically ill COVID-19 patients.
We and others previously demonstrated that excess free heme promotes oxidative and inflammatory stress [26,27,28,29], activates the vascular endothelium, and increases adhesion molecules and leukocyte recruitment [28,30]. Using radiolabeled liposomes, we further demonstrated that free heme causes vascular permeabilization resulting in edema [28]. Accumulated free heme and Hb may thus act as pathophysiologic mechanisms mediating pulmonary permeability and inflammation [31,32]. Alveolar fluid clearance is hampered in ARDS and also likely in hospitalized COVID-19 patients. Intratracheal administration of heme causes alveolar-capillary barrier dysfunction, and increases alveolar permeability, contributing to acute lung injury in mice [19]. Heme was shown to increase pulmonary edema by inhibiting amiloride-sensitive epithelial Na+ channel (ENaC)-activity, which plays a crucial role in sodium transport and fluid reabsorption in the lung [33].
Coagulation abnormalities, complement activation, endotheliitis, and thrombosis occur frequently in COVID-19 patients [34,35,36]. Interestingly, heme also promotes platelet activation, complement activation, vasculitis, and thrombosis [28,37,38,39]. Heme was recognized to act as a danger signal, damage-associated molecular pattern (DAMP), or alarmin [11,30,40,41,42] and was shown to activate Toll-like receptor 4 (TLR4) signaling [43]. Free heme promotes also oxidative stress by catalyzing the Fenton reaction [44,45], scavenges nitric oxide (NO) [46], and activates the inflammasome via TLR4 and NLR family pyrin domain containing 3 (NLPR3) [47,48]. In addition to heme promoting the expression of inflammatory cytokines [49], it potentiates tumor necrosis factor (TNF)-alpha induced inflammatory events and apoptosis [50].
The deleterious actions of heme could explain many of the observed manifestations during SARS-CoV-2 infection, including the increased capillary leakage resulting in pulmonary edema, vasculitis, leukocyte recruitment, and thrombus formation.
3. Protective Mechanisms against Free Heme
In order to attenuate these heme-induced pro-inflammatory, pro-oxidative, and pro-thrombotic actions, our body is normally equipped with different defense mechanisms. Following hemolysis, Hb is released that can be scavenged by serum haptoglobin (Hp). Free Hb outside the erythrocyte will turn into methemoglobin, which readily liberates its heme group [51]. Normally, this free heme gets scavenged by hemopexin (hpx) to prevent its injurious actions [52,53]. In case of severe hemolysis, or in blood clots, the hemoglobin and heme scavengers may be overwhelmed, exhausted, or physically not able to interact and neutralize free Hb and heme.
Alternative protective mechanisms against free heme are then pivotal for cellular survival. When heme enters the cell, it can modify proteins, DNA, and lipids [16,54,55]. Heme can also be transported out of the cell by Breast Cancer Resistance Protein (BCRP) that increases its chance of survival [56]. Finally, heme can be intracellularly degraded by heme oxygenase (HO) into biliverdin, iron, and carbon monoxide (CO). Biliverdin is then directly converted into the antioxidant bilirubin by biliverdin reductase (BVR), whilst iron gets scavenged by co-induced ferritin [11]. Heme oxygenase activity causes resolution of inflammation. We and others previously demonstrated that HO-activity is pivotal for reducing heme-induced vascular adhesion molecule expression and leukocyte extravasation, whereas inhibition of HO-activity further increases adhesion molecules and leukocyte influx [14,28,30,57,58,59].
The HO-effector molecules biliverdin/bilirubin, CO, and ferritin have each shown to be beneficial. Bilirubin signaling mediates protection against a variety of inflammatory diseases [60]. Increasing bilirubin has also been shown to increase the antioxidant capacity of the serum [61,62] and may protect against the oxidative properties of heme in a similar fashion as ascorbic acid possibly improves the condition of some COVID-19 patients [22].
Carbon monoxide signaling has been shown to protect the lung from inflammatory and oxidative insults and modulates autophagy, mitochondrial biogenesis, apoptosis, and cellular proliferation. [63]. CO downregulates both innate and cell mediated immunity, and approaches with CO-releasing molecules have been shown highly effective in animal models of T-cell mediated autoimmune diseases [64,65]. Genetic polymorphisms determining the protective response against hemoglobin/heme may contribute to differential disease outcomes. For example, a polymorphism in the HO-1 promoter determines the level of HO-1 expression following stress [66,67,68]. Individuals with longer (GT)n repeats have a lower transcriptional activity when compared to individuals with shorter (GT)n repeats, and have lower HO-1 levels and less protection against inflammatory insults [67]. It would be interesting to determine whether this differential protection by the antioxidant enzyme HO-1 has impact on the clinical outcome of SARS-CoV-2 infection. In addition, haptoglobin polymorphisms may result in more or less potent hemoglobin scavengers [69]. A decreased antioxidant capacity associated with the Hp 2-2 isoform, results in an increased risk of heme-induced inflammatory complications [70].
4. How Can We Protect against the Injurious Actions of Free Heme During SARS-CoV-2 Infection?
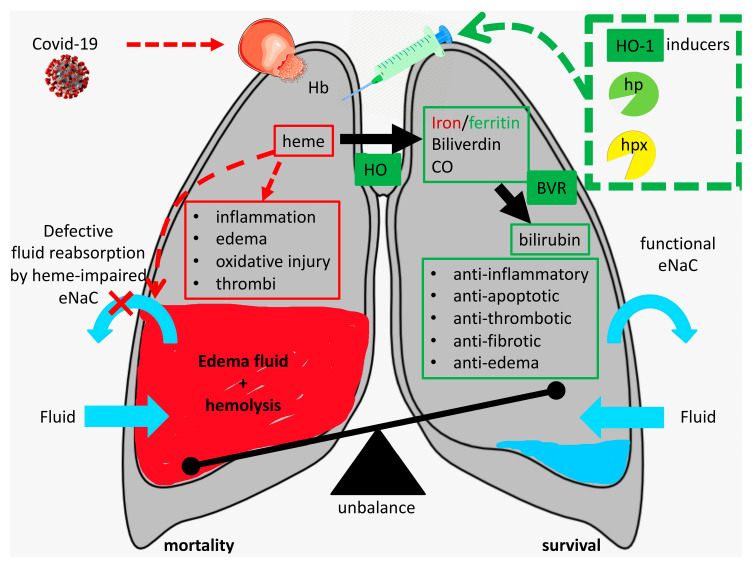
Critically ill COVID-19 patients will often have excess of heme–proteins and heme in their alveoli thereby fueling exudate formation, platelet activation, inflammation, and fibrosis (see Figure 1). How can we prevent or attenuate these pulmonary complications?
Figure 1.
Heme-induced pulmonary complications following SARS-CoV-2 infection. Strategies to harness hemolysis-derived alveolar heme include administration of inducers of heme oxygenase (HO)-1 and hemoglobin (Hb)/heme scavengers (see green dashed box). “Proof of principle” studies should be performed to assess whether targeting heme by induction of HO-1 or administration of scavengers haptoglobin (hp)/hemopexin (hpx) can indeed attenuate or prevent complications in critically ill COVID-19 patients (see text for details).
In a mouse model of ARDS, heme-induced pulmonary edema, endoplasmic reticulum stress, and fibrosis could be attenuated by intramuscular administration of the heme-scavenger hemopexin while lung function improved [17,33,71]. In addition, induction of HO-1 was shown to decrease heme-induced edema and inflammation in this and other models [11,28,30,57,71,72]. Hb and heme scavengers (hp and hpx, respectively), HO-1 induction, and HO-effector molecules have already demonstrated to mediate potent protection against heme-induced inflammation, thrombosis, and fibrosis in diverse diseases [13,14,53,73,74,75,76,77,78,79,80,81], whereas inhibition of HO-activity aggravates disease [28,82,83,84]. Administration of Hb/heme scavengers, induction of HO-1, or HO-effector molecules could thus be beneficial to prevent or treat the injurious actions of heme (see Figure 1). The cytoprotective enzyme HO-1 can be induced by a wide spectrum of agents, including aspirin, statins, probucol, valsartan, niacin, resveratrol, and curcumin that could be safely used in humans [11,73,83,85].
Alternatively, Nrf2 is a transcriptional factor that induces several antioxidant protective target genes, among which is HO-1. Dimethyl fumarate (DMF) is a clinically used Nrf2 activator [86] that could possibly be used to prevent the many heme-induced complications during SARS-CoV-2 infection, such as edema, inflammation, and thrombosis and fibrosis by induction of the versatile HO-1 enzyme. Intriguingly, HO-1 induction and its effector molecules, CO and biliverdin/bilirubin, not only protect against inflammation, but have also potent antiviral properties that may be beneficial for fighting COVID-19 [87,88,89,90,91,92,93,94,95]. Preclinical in vitro and in vivo studies to eventual antiviral effects of HO1 inducers, bilirubin, or CO on Sars-CoV2 are thus warranted to better understand the possible translation of these concepts to the clinical setting.
Obesity, diabetes, chronic kidney disease, cardiovascular diseases, COPD, male sex, and aging form risk factors for developing severe complications when infected with SARS-CoV-2 [96,97,98,99]. These predisposing conditions, and inflammation in general, downregulate HO-1 expression and activity [67,74,100,101,102,103,104,105,106], further supporting that this compromised protection and diminished tolerance against inflammatory and oxidative stress promotes adverse clinical outcome in COVID-19 patients.
Recently, controversial and conflicting reports on possible associations between smoking and COVID-19 survival were reported [107]. Initially, increased risk was reported [107], which could be easily explained by the many well-established adverse health effects of smoking in, for instance, the lungs and vasculature. Surprisingly, recent (prepublished reports) studies, however, suggest that smokers may be under-represented among the COVID-19 patients with more severe disease. The smoking prevalence was lower than expected among hospitalized patients in diverse countries, suggesting that, counterintuitively, smoking could protect from severe complications [97,108,109,110]. This resulted in immediate investigations to the putative protective effects of nicotine against COVID-19 [111]. Although interesting, another intriguing possibility is glooming: not nicotine but the increased vascular carbon monoxide (CO) levels within smokers could possibly be the protective component. CO interacts with hemoglobin to form carboxyhemoglobin, which is thereby protected from the release of debilitating heme as previously also demonstrated for malaria [12].
Recently, the glucocorticoid dexamethasone was found to save many severely ill COVID-19 patients [112]. Since dexamethasone reduces hemolysis and induces HO-1 in macrophages [113], it is tempting to speculate that this increased protection against free heme attenuates the severity of disease in COVID-19 patients.
5. Conclusions
Until a vaccine against SARS-CoV-2 becomes available, we need to explore novel strategies to attenuate the pro-inflammatory, pro-thrombotic, and pro-fibrotic consequences of SARS-CoV-2 leading to morbidity and mortality. With this manuscript, we aimed to stress that heme is a likely culprit that may play a relevant role in initiating and contributing to the pulmonary complications displayed in critically ill COVID-19 patients. Inducing HO-1 expression or administration of Hb/heme scavengers or HO-effector molecules may prevent SARS-CoV-2-induced pulmonary complications by its antiviral, anti-inflammatory, antithrombotic, and antifibrotic activities. The heme-HO system represents an interesting target for novel “proof of concept” studies in the context of COVID-19.
Author Contributions
F.A.D.T.G.W. and N.G.A. designed the study. F.A.D.T.G.W., P.P., S.J.P., S.I., and N.G.A. wrote and critically revised the manuscript. All authors read and approved the published version of the manuscript.
Funding
FW is supported by a grant from the Osteology Foundation 19-054.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao L., Sakagami H., Miwa N. ACE2: The key Molecule for Understanding the Pathophysiology of Severe and Critical Conditions of COVID-19: Demon or Angel? Viruses. 2020;12:491. doi: 10.3390/v12050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albini A., Di Guardo G., Noonan D.M., Lombardo M. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: Implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies. Intern. Emerg. Med. 2020 doi: 10.1007/s11739-020-02364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J., Zhong Z., Ji P., Li H., Li B., Pang J., Zhang J., Zhao C. Clinicopathological characteristics of 8697 patients with COVID-19 in China: A meta-analysis. Fam. Med. Community Health. 2020;8 doi: 10.1136/fmch-2020-000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthay M.A., Folkesson H.G., Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol. Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 6.Leismann D.E., Clifford C.S., Legrand M. Facing COVID-19 in the ICU: Vascular Dysfunction, Thrombosis, and Dysregulated Inflammation. Intensive. Care Med. 2020;28:1–4. doi: 10.1007/s00134-020-06059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jose R.J., Manuel A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benhamou D., Keita H., Bouthors A.S., CARO working group Coagulation changes and thromboembolic risk in COVID-19 pregnant patients. Anaesth Crit. Care Pain Med. 2020 doi: 10.1016/j.accpm.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagener F.A., Volk H.D., Willis D., Abraham N.G., Soares M.P., Adema G.J., Figdor C.G. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol. Rev. 2003;55:551–571. doi: 10.1124/pr.55.3.5. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira A., Balla J., Jeney V., Balla G., Soares M.P. A central role for free heme in the pathogenesis of severe malaria: The missing link? J. Mol. Med. (Berl.) 2008;86:1097–1111. doi: 10.1007/s00109-008-0368-5. [DOI] [PubMed] [Google Scholar]
- 13.Larsen R., Gozzelino R., Jeney V., Tokaji L., Bozza F.A., Japiassu A.M., Bonaparte D., Cavalcante M.M., Chora A., Ferreira A., et al. A central role for free heme in the pathogenesis of severe sepsis. Sci. Transl. Med. 2010;2:51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 14.Wagener F.A., Abraham N.G., van Kooyk Y., de Witte T., Figdor C.G. Heme-induced cell adhesion in the pathogenesis of sickle-cell disease and inflammation. Trends Pharmacol. Sci. 2001;22:52–54. doi: 10.1016/S0165-6147(00)01609-6. [DOI] [PubMed] [Google Scholar]
- 15.Nath K.A., Balla J., Croatt A.J., Vercellotti G.M. Heme protein-mediated renal injury: A protective role for 21-aminosteroids in vitro and in vivo. Kidney Int. 1995;47:592–602. doi: 10.1038/ki.1995.75. [DOI] [PubMed] [Google Scholar]
- 16.Gaggar A., Patel R.P. There is blood in the water: Hemolysis, hemoglobin, and heme in acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;311:L714–L718. doi: 10.1152/ajplung.00312.2016. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal S., Ahmad I., Lam A., Carlisle M.A., Li C., Wells J.M., Raju S.V., Athar M., Rowe S.M., Dransfield M.T., et al. Heme scavenging reduces pulmonary endoplasmic reticulum stress, fibrosis, and emphysema. JCI Insight. 2018;3 doi: 10.1172/jci.insight.120694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janz D.R., Ware L.B. The role of red blood cells and cell-free hemoglobin in the pathogenesis of ARDS. J. Intensive Care. 2015;3:20. doi: 10.1186/s40560-015-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaver C.M., Upchurch C.P., Janz D.R., Grove B.S., Putz N.D., Wickersham N.E., Dikalov S.I., Ware L.B., Bastarache J.A. Cell-free hemoglobin: A novel mediator of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;310:L532–L541. doi: 10.1152/ajplung.00155.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashbaugh D.G., Bigelow D.B., Petty T.L., Levine B.E. Acute respiratory distress in adults. Lancet. 1967;2:319–323. doi: 10.1016/S0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 21.Bastarache J.A., Sebag S.C., Clune J.K., Grove B.S., Lawson W.E., Janz D.R., Roberts L.J., 2nd, Dworski R., Mackman N., Ware L.B. Low levels of tissue factor lead to alveolar haemorrhage, potentiating murine acute lung injury and oxidative stress. Thorax. 2012;67:1032–1039. doi: 10.1136/thoraxjnl-2012-201781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loh D. Covid-19, ARDS & Cell-Free Hemoglobin-The Ascorbic Acid Connection. [(accessed on 24 March 2020)]; Available online: https://www.townsendletter.com/article/online-covid-19-ards-cell-free-hemoglobin-ascorbic-acid-connection/
- 23.Presser L. A Medical Worker Describes Terrifying Lung Failure From COVID-19 — Even in His Young Patients. [(accessed on 21 March 2020)]; Available online: https://www.propublica.org/article/a-medical-worker-describes--terrifying-lung-failure-from-covid19-even-in-his-young-patients.
- 24.Casey K., Iteen A., Nicolini R., Auten J. COVID-19 pneumonia with hemoptysis: Acute segmental pulmonary emboli associated with novel coronavirus infection. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan K.H., Farouji I., Abu Hanoud A., Slim J. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID-19) Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagener F.A., Feldman E., de Witte T., Abraham N.G. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1, and E selectin in vascular endothelial cells. Proc. Soc. Exp. Biol. Med. 1997;216:456–463. doi: 10.3181/00379727-216-44197. [DOI] [PubMed] [Google Scholar]
- 27.Balla J., Vercellotti G.M., Nath K., Yachie A., Nagy E., Eaton J.W., Balla G. Haem, haem oxygenase and ferritin in vascular endothelial cell injury. Nephrol. Dial. Transpl. 2003;18:v8–v12. doi: 10.1093/ndt/gfg1034. [DOI] [PubMed] [Google Scholar]
- 28.Wagener F.A., Eggert A., Boerman O.C., Oyen W.J., Verhofstad A., Abraham N.G., Adema G., van Kooyk Y., de Witte T., Figdor C.G. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98:1802–1811. doi: 10.1182/blood.V98.6.1802. [DOI] [PubMed] [Google Scholar]
- 29.Frimat M., Boudhabhay I., Roumenina L.T. Hemolysis Derived Products Toxicity and Endothelium: Model of the Second Hit. Toxins. 2019;11:660. doi: 10.3390/toxins11110660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagener F.A., van Beurden H.E., von den Hoff J.W., Adema G.J., Figdor C.G. The heme-heme oxygenase system: A molecular switch in wound healing. Blood. 2003;102:521–528. doi: 10.1182/blood-2002-07-2248. [DOI] [PubMed] [Google Scholar]
- 31.Rafikova O., Williams E.R., McBride M.L., Zemskova M., Srivastava A., Nair V., Desai A.A., Langlais P.R., Zemskov E., Simon M., et al. Hemolysis-induced Lung Vascular Leakage Contributes to the Development of Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2018;59:334–345. doi: 10.1165/rcmb.2017-0308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meegan J.E., Shaver C.M., Putz N.D., Jesse J.J., Landstreet S.R., Lee H.N.R., Sidorova T.N., McNeil J.B., Wynn J.L., Cheung-Flynn J., et al. Cell-free hemoglobin increases inflammation, lung apoptosis, and microvascular permeability in murine polymicrobial sepsis. PLoS ONE. 2020;15:e0228727. doi: 10.1371/journal.pone.0228727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aggarwal S., Lazrak A., Ahmad I., Yu Z., Bryant A., Mobley J.A., Ford D.A., Matalon S. Heme impairs alveolar epithelial sodium channels post toxic gas inhalation. BioRxiv. 2020 doi: 10.1101/2020.01.22.909879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(1020)30145-30149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell C.M., Kahwash R. Will Complement Inhibition be the New Target in Treating COVID-19 Related Systemic Thrombosis? Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047419. [DOI] [PubMed] [Google Scholar]
- 36.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourne J.H., Colicchia M., Di Y., Martin E., Slater A., Roumenina L.T., Dimitrov J.D., Watson S.P., Rayes J. Heme induces human and mouse platelet activation through C-type-lectin-like receptor-2. Haematologica. 2020 doi: 10.3324/haematol.2020.246488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merle N.S., Grunenwald A., Rajaratnam H., Gnemmi V., Frimat M., Figueres M.L., Knockaert S., Bouzekri S., Charue D., Noe R., et al. Intravascular hemolysis activates complement via cell-free heme and heme-loaded microvesicles. JCI Insight. 2018;3 doi: 10.1172/jci.insight.96910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neely S.M., Gardner D.V., Green D., Ts’ao C.H. Effect of hematin on endothelial cells and endothelial cell-platelet interactions. Am. J. Pathol. 1984;115:390–396. [PMC free article] [PubMed] [Google Scholar]
- 40.Soares M.P., Bozza M.T. Red alert: Labile heme is an alarmin. Curr. Opin. Immunol. 2016;38:94–100. doi: 10.1016/j.coi.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Mendonca R., Silveira A.A., Conran N. Red cell DAMPs and inflammation. Inflamm. Res. 2016;65:665–678. doi: 10.1007/s00011-016-0955-9. [DOI] [PubMed] [Google Scholar]
- 42.Wegiel B., Hauser C.J., Otterbein L.E. Heme as a danger molecule in pathogen recognition. Free Radic. Biol. Med. 2015;89:651–661. doi: 10.1016/j.freeradbiomed.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Figueiredo R.T., Fernandez P.L., Mourao-Sa D.S., Porto B.N., Dutra F.F., Alves L.S., Oliveira M.F., Oliveira P.L., Graca-Souza A.V., Bozza M.T. Characterization of heme as activator of Toll-like receptor 4. J. Biol. Chem. 2007;282:20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- 44.Sadrzadeh S.M., Graf E., Panter S.S., Hallaway P.E., Eaton J.W. Hemoglobin. A biologic fenton reagent. J. Biol. Chem. 1984;259:14354–14356. [PubMed] [Google Scholar]
- 45.Balla J., Jacob H.S., Balla G., Nath K., Eaton J.W., Vercellotti G.M. Endothelial-cell heme uptake from heme proteins: Induction of sensitization and desensitization to oxidant damage. Proc. Natl. Acad. Sci. USA. 1993;90:9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deem S. Nitric oxide scavenging by hemoglobin regulates hypoxic pulmonary vasoconstriction. Free Radic. Biol. Med. 2004;36:698–706. doi: 10.1016/j.freeradbiomed.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 47.Erdei J., Toth A., Balogh E., Nyakundi B.B., Banyai E., Ryffel B., Paragh G., Cordero M.D., Jeney V. Induction of NLRP3 Inflammasome Activation by Heme in Human Endothelial Cells. Oxid. Med. Cell Longev. 2018;2018:4310816. doi: 10.1155/2018/4310816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dutra F.F., Alves L.S., Rodrigues D., Fernandez P.L., de Oliveira R.B., Golenbock D.T., Zamboni D.S., Bozza M.T. Hemolysis-induced lethality involves inflammasome activation by heme. Proc. Natl. Acad. Sci. USA. 2014;111:E4110–E4118. doi: 10.1073/pnas.1405023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Q., Fu W., Yao J., Ji Z., Wang Y., Zhou Z., Yan J., Li W. Heme induces IL-1beta secretion through activating NLRP3 in kidney inflammation. Cell Biochem. Biophys. 2014;69:495–502. doi: 10.1007/s12013-014-9823-9. [DOI] [PubMed] [Google Scholar]
- 50.Gozzelino R., Soares M.P. Heme sensitization to TNF-mediated programmed cell death. Adv. Exp. Med. Biol. 2011;691:211–219. doi: 10.1007/978-1-4419-6612-4_22. [DOI] [PubMed] [Google Scholar]
- 51.Bunn H.F., Jandl J.H. Exchange of heme among hemoglobins and between hemoglobin and albumin. J. Biol. Chem. 1968;243:465–475. [PubMed] [Google Scholar]
- 52.Muller-Eberhard U. Hemopexin. N. Engl. J. Med. 1970;283:1090–1094. doi: 10.1056/NEJM197011122832007. [DOI] [PubMed] [Google Scholar]
- 53.Immenschuh S., Vijayan V., Janciauskiene S., Gueler F. Heme as a Target for Therapeutic Interventions. Front Pharmacol. 2017;8:146. doi: 10.3389/fphar.2017.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higdon A.N., Benavides G.A., Chacko B.K., Ouyang X., Johnson M.S., Landar A., Zhang J., Darley-Usmar V.M. Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: The protective role of autophagy. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H1394–H1409. doi: 10.1152/ajpheart.00584.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suliman H.B., Carraway M.S., Velsor L.W., Day B.J., Ghio A.J., Piantadosi C.A. Rapid mtDNA deletion by oxidants in rat liver mitochondria after hemin exposure. Free Radic. Biol. Med. 2002;32:246–256. doi: 10.1016/S0891-5849(01)00797-3. [DOI] [PubMed] [Google Scholar]
- 56.Wagener F.A., Dankers A.C., van Summeren F., Scharstuhl A., van den Heuvel J.J., Koenderink J.B., Pennings S.W., Russel F.G., Masereeuw R. Heme Oxygenase-1 and breast cancer resistance protein protect against heme-induced toxicity. Curr. Pharm Des. 2013;19:2698–2707. doi: 10.2174/1381612811319150004. [DOI] [PubMed] [Google Scholar]
- 57.Wagener F.A., da Silva J.L., Farley T., de Witte T., Kappas A., Abraham N.G. Differential effects of heme oxygenase isoforms on heme mediation of endothelial intracellular adhesion molecule 1 expression. J. Pharmacol. Exp. Ther. 1999;291:416–423. [PubMed] [Google Scholar]
- 58.Nader E., Romana M., Connes P. The Red Blood Cell-Inflammation Vicious Circle in Sickle Cell Disease. Front Immunol. 2020;11:454. doi: 10.3389/fimmu.2020.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belcher J.D., Chen C., Nguyen J., Milbauer L., Abdulla F., Alayash A.I., Smith A., Nath K.A., Hebbel R.P., Vercellotti G.M. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123:377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vitek L. Bilirubin as a signaling molecule. Med. Res. Rev. 2020 doi: 10.1002/med.21660. [DOI] [PubMed] [Google Scholar]
- 61.Dekker D., Dorresteijn M.J., Pijnenburg M., Heemskerk S., Rasing-Hoogveld A., Burger D.M., Wagener F.A., Smits P. The bilirubin-increasing drug atazanavir improves endothelial function in patients with type 2 diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2011;31:458–463. doi: 10.1161/ATVBAHA.110.211789. [DOI] [PubMed] [Google Scholar]
- 62.Dekker D., Dorresteijn M.J., Welzen M.E.B., Timman S., Pickkers P., Burger D.M., Smits P., Wagener F., Russel F.G.M. Parenteral bilirubin in healthy volunteers: A reintroduction in translational research. Br. J. Clin. Pharmacol. 2018;84:268–279. doi: 10.1111/bcp.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryter S.W., Ma K.C., Choi A.M.K. Carbon monoxide in lung cell physiology and disease. Am. J. Physiol. Cell Physiol. 2018;314:C211–C227. doi: 10.1152/ajpcell.00022.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nikolic I., Saksida T., Mangano K., Vujicic M., Stojanovic I., Nicoletti F., Stosic-Grujicic S. Pharmacological application of carbon monoxide ameliorates islet-directed autoimmunity in mice via anti-inflammatory and anti-apoptotic effects. Diabetologia. 2014;57:980–990. doi: 10.1007/s00125-014-3170-7. [DOI] [PubMed] [Google Scholar]
- 65.Fagone P., Mangano K., Coco M., Perciavalle V., Garotta G., Romao C.C., Nicoletti F. Therapeutic potential of carbon monoxide in multiple sclerosis. Clin. Exp. Immunol. 2012;167:179–187. doi: 10.1111/j.1365-2249.2011.04491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raval C.M., Lee P.J. Heme oxygenase-1 in lung disease. Curr. Drug Targets. 2010;11:1532–1540. doi: 10.2174/1389450111009011532. [DOI] [PubMed] [Google Scholar]
- 67.Exner M., Minar E., Wagner O., Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic. Biol. Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 68.Wagener F.A., Toonen E.J., Wigman L., Fransen J., Creemers M.C., Radstake T.R., Coenen M.J., Barrera P., van Riel P.L., Russel F.G. HMOX1 promoter polymorphism modulates the relationship between disease activity and joint damage in rheumatoid arthritis. Arthritis Rheum. 2008;58:3388–3393. doi: 10.1002/art.23970. [DOI] [PubMed] [Google Scholar]
- 69.Quaye I.K. Haptoglobin, inflammation and disease. Trans. R. Soc. Trop. Med. Hyg. 2008;102:735–742. doi: 10.1016/j.trstmh.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 70.Goldenstein H., Levy N.S., Levy A.P. Haptoglobin genotype and its role in determining heme-iron mediated vascular disease. Pharmacol. Res. 2012;66:1–6. doi: 10.1016/j.phrs.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aggarwal S., Lam A., Bolisetty S., Carlisle M.A., Traylor A., Agarwal A., Matalon S. Heme Attenuation Ameliorates Irritant Gas Inhalation-Induced Acute Lung Injury. Antioxid. Redox. Signal. 2016;24:99–112. doi: 10.1089/ars.2015.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nath K.A., Grande J.P., Belcher J.D., Garovic V.D., Croatt A.J., Hillestad M.L., Barry M.A., Nath M.C., Regan R.F., Vercellotti G.M. Antithrombotic effects of heme-degrading and heme-binding proteins. Am. J. Physiol. Heart Circ. Physiol. 2020;318:H671–H681. doi: 10.1152/ajpheart.00280.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vijayan V., Wagener F., Immenschuh S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018;153:159–167. doi: 10.1016/j.bcp.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 74.van Bon L., Cossu M., Scharstuhl A., Pennings B.W., Vonk M.C., Vreman H.J., Lafyatis R.L., van den Berg W., Wagener F.A., Radstake T.R. Low heme oxygenase-1 levels in patients with systemic sclerosis are associated with an altered Toll-like receptor response: Another role for CXCL4? Rheumatology (Oxford) 2016;55:2066–2073. doi: 10.1093/rheumatology/kew251. [DOI] [PubMed] [Google Scholar]
- 75.van Loon R.L., Bartelds B., Wagener F.A., Affara N., Mohaupt S., Wijnberg H., Pennings S.W., Takens J., Berger R.M. Erythropoietin Attenuates Pulmonary Vascular Remodeling in Experimental Pulmonary Arterial Hypertension through Interplay between Endothelial Progenitor Cells and Heme Oxygenase. Front Pediatr. 2015;3:71. doi: 10.3389/fped.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lundvig D.M., Immenschuh S., Wagener F.A. Heme oxygenase, inflammation, and fibrosis: The good, the bad, and the ugly? Front Pharmacol. 2012;3:81. doi: 10.3389/fphar.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wagener F.A., Scharstuhl A., Tyrrell R.M., Von den Hoff J.W., Jozkowicz A., Dulak J., Russel F.G., Kuijpers-Jagtman A.M. The heme-heme oxygenase system in wound healing; implications for scar formation. Curr. Drug Targets. 2010;11:1571–1585. doi: 10.2174/1389450111009011571. [DOI] [PubMed] [Google Scholar]
- 78.Soares M.P., Lin Y., Anrather J., Csizmadia E., Takigami K., Sato K., Grey S.T., Colvin R.B., Choi A.M., Poss K.D., et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat. Med. 1998;4:1073–1077. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 79.Soares M.P., Brouard S., Smith R.N., Bach F.H. Heme oxygenase-1, a protective gene that prevents the rejection of transplanted organs. Immunol. Rev. 2001;184:275–285. doi: 10.1034/j.1600-065x.2001.1840124.x. [DOI] [PubMed] [Google Scholar]
- 80.Pamplona A., Ferreira A., Balla J., Jeney V., Balla G., Epiphanio S., Chora A., Rodrigues C.D., Gregoire I.P., Cunha-Rodrigues M., et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat. Med. 2007;13:703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 81.Krishnamoorthy S., Pace B., Gupta D., Sturtevant S., Li B., Makala L., Brittain J., Moore N., Vieira B.F., Thullen T., et al. Dimethyl fumarate increases fetal hemoglobin, provides heme detoxification, and corrects anemia in sickle cell disease. JCI Insight. 2017;2 doi: 10.1172/jci.insight.96409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kartikasari A.E., Wagener F.A., Yachie A., Wiegerinck E.T., Kemna E.H., Swinkels D.W. Hepcidin suppression and defective iron recycling account for dysregulation of iron homeostasis in heme oxygenase-1 deficiency. J. Cell Mol. Med. 2009;13:3091–3102. doi: 10.1111/j.1582-4934.2008.00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abraham N.G., Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 84.Fagone P., Patti F., Mangano K., Mammana S., Coco M., Touil-Boukoffa C., Chikovani T., Di Marco R., Nicoletti F. Heme oxygenase-1 expression in peripheral blood mononuclear cells correlates with disease activity in multiple sclerosis. J. Neuroimmunol. 2013;261:82–86. doi: 10.1016/j.jneuroim.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 85.Drummond G.S., Baum J., Greenberg M., Lewis D., Abraham N.G. HO-1 overexpression and underexpression: Clinical implications. Arch. Biochem. Biophys. 2019;673:108073. doi: 10.1016/j.abb.2019.108073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gold R., Arnold D.L., Bar-Or A., Fox R.J., Kappos L., Chen C., Parks B., Miller C. Safety and efficacy of delayed-release dimethyl fumarate in patients with relapsing-remitting multiple sclerosis: 9 years’ follow-up of DEFINE, CONFIRM, and ENDORSE. Ther. Adv. Neurol. Disord. 2020;13:1756286420915005. doi: 10.1177/1756286420915005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Espinoza J.A., Gonzalez P.A., Kalergis A.M. Modulation of Antiviral Immunity by Heme Oxygenase-1. Am. J. Pathol. 2017;187:487–493. doi: 10.1016/j.ajpath.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 88.Kah J., Volz T., Lutgehetmann M., Groth A., Lohse A.W., Tiegs G., Sass G., Dandri M. Haem oxygenase-1 polymorphisms can affect HCV replication and treatment responses with different efficacy in humanized mice. Liver Int. 2017;37:1128–1137. doi: 10.1111/liv.13347. [DOI] [PubMed] [Google Scholar]
- 89.Tseng C.K., Lin C.K., Wu Y.H., Chen Y.H., Chen W.C., Young K.C., Lee J.C. Human heme oxygenase 1 is a potential host cell factor against dengue virus replication. Sci. Rep. 2016;6:32176. doi: 10.1038/srep32176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma Z., Pu F., Zhang X., Yan Y., Zhao L., Zhang A., Li N., Zhou E.M., Xiao S. Carbon monoxide and biliverdin suppress bovine viral diarrhoea virus replication. J. Gen. Virol. 2017;98:2982–2992. doi: 10.1099/jgv.0.000955. [DOI] [PubMed] [Google Scholar]
- 91.Gutierrez-Grobe Y., Vitek L., Tiribelli C., Kobashi-Margain R.A., Uribe M., Mendez-Sanchez N. Biliverdin and heme oxygenase antiviral activity against hepatitis C virus. Ann. Hepatol. 2011;10:105–107. [PubMed] [Google Scholar]
- 92.Santangelo R., Mancuso C., Marchetti S., Di Stasio E., Pani G., Fadda G. Bilirubin: An Endogenous Molecule with Antiviral Activity in vitro. Front Pharmacol. 2012;3:36. doi: 10.3389/fphar.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Korenblat K.M., Berk P.D. Hyperbilirubinemia in the setting of antiviral therapy. Clin. Gastroenterol. Hepatol. 2005;3:303–310. doi: 10.1016/S1542-3565(05)00083-2. [DOI] [PubMed] [Google Scholar]
- 94.Zhang A., Wan B., Jiang D., Wu Y., Ji P., Du Y., Zhang G. The Cytoprotective Enzyme Heme Oxygenase-1 Suppresses Pseudorabies Virus Replication in vitro. Front Microbiol. 2020;11:412. doi: 10.3389/fmicb.2020.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deng X., Yasuda H., Sasaki T., Yamaya M. Low-Dose Carbon Monoxide Inhibits Rhinovirus Replication in Human Alveolar and Airway Epithelial Cells. Tohoku J. Exp. Med. 2019;247:215–222. doi: 10.1620/tjem.247.215. [DOI] [PubMed] [Google Scholar]
- 96.Gargaglioni L.H., Marques D.A. Let’s talk about sex in the context of COVID-19. J. Appl. Physiol. (1985) 2020 doi: 10.1152/japplphysiol.00335.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Lusignan S., Dorward J., Correa A., Jones N., Akinyemi O., Amirthalingam G., Andrews N., Byford R., Dabrera G., Elliot A., et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: A cross-sectional study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Korakas E., Ikonomidis I., Kousathana F., Balampanis K., Kountouri A., Raptis A., Palaiodimou L., Kokkinos A., Lambadiari V. Obesity and COVID-19: Immune and metabolic derangement as a possible link to adverse clinical outcomes. Am. J. Physiol. Endocrinol. Metab. 2020 doi: 10.1152/ajpendo.00198.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- 100.Toth B., Yokoyama Y., Kuebler J.F., Schwacha M.G., Rue L.W., 3rd, Bland K.I., Chaudry I.H. Sex differences in hepatic heme oxygenase expression and activity following trauma and hemorrhagic shock. Arch. Surg. 2003;138:1375–1382. doi: 10.1001/archsurg.138.12.1375. [DOI] [PubMed] [Google Scholar]
- 101.Weir L.R., Schenck E., Meakin J., McClure F., Driver R., Walker S., Lynch A.M. Biophotonic imaging in HO-1.luc transgenic mice: Real-time demonstration of gender-specific chloroform induced renal toxicity. Mutat. Res. 2005;574:67–75. doi: 10.1016/j.mrfmmm.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 102.Dorresteijn M.J., Paine A., Zilian E., Fenten M.G., Frenzel E., Janciauskiene S., Figueiredo C., Eiz-Vesper B., Blasczyk R., Dekker D., et al. Cell-type-specific downregulation of heme oxygenase-1 by lipopolysaccharide via Bach1 in primary human mononuclear cells. Free Radic. Biol. Med. 2015;78:224–232. doi: 10.1016/j.freeradbiomed.2014.10.579. [DOI] [PubMed] [Google Scholar]
- 103.Bloomer S.A., Zhang H.J., Brown K.E., Kregel K.C. Differential regulation of hepatic heme oxygenase-1 protein with aging and heat stress. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:419–425. doi: 10.1093/gerona/gln056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ito Y., Betsuyaku T., Moriyama C., Nasuhara Y., Nishimura M. Aging affects lipopolysaccharide-induced upregulation of heme oxygenase-1 in the lungs and alveolar macrophages. Biogerontology. 2009;10:173–180. doi: 10.1007/s10522-008-9164-4. [DOI] [PubMed] [Google Scholar]
- 105.Sabaawy H.E., Zhang F., Nguyen X., ElHosseiny A., Nasjletti A., Schwartzman M., Dennery P., Kappas A., Abraham N.G. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001;38:210–215. doi: 10.1161/01.HYP.38.2.210. [DOI] [PubMed] [Google Scholar]
- 106.Slebos D.J., Kerstjens H.A., Rutgers S.R., Kauffman H.F., Choi A.M., Postma D.S. Haem oxygenase-1 expression is diminished in alveolar macrophages of patients with COPD. Eur. Respir. J. 2004;23:652–653; author reply 653. doi: 10.1183/09031936.04.00127904. [DOI] [PubMed] [Google Scholar]
- 107.Farsalinos K., Barbouni A., Niaura R. Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: Could nicotine be a therapeutic option? Intern. Emerg. Med. 2020 doi: 10.1007/s11739-020-02355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miyara M. Low incidence of daily active tobacco smoking in patients with symptomatic COVID-19. Qeios. 2020 doi: 10.32388/WPP19W.3. [DOI] [Google Scholar]
- 109.Guan W.J. Clinical characteristics of Coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rentsch C. COVID-19 testing, hospital admission, and intensive care among 2.026.227 United States veterans aged 54-75 years. medRxiv. 2020 doi: 10.1101/2020.04.09.20059964. [DOI] [Google Scholar]
- 111.Tindle H.A., Newhouse P.A., Freiberg M.S. Beyond Smoking Cessation: Investigating Medicinal Nicotine to Prevent and Treat COVID-19. Nicotine Tob. Res. 2020 doi: 10.1093/ntr/ntaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. [(accessed on 28 May 2020)]; Available online: http://www.ox.ac.uk/news/2020-06-16-dexamethasone-reduces-death-hospitalised-patients-severe-respiratory-complications#.
- 113.Vallelian F., Schaer C.A., Kaempfer T., Gehrig P., Duerst E., Schoedon G., Schaer D.J. Glucocorticoid treatment skews human monocyte differentiation into a hemoglobin-clearance phenotype with enhanced heme-iron recycling and antioxidant capacity. Blood. 2010;116:5347–5356. doi: 10.1182/blood-2010-04-277319. [DOI] [PubMed] [Google Scholar]