Abstract
Background
People with human immunodeficiency virus (PWH) are at risk for accelerated development of physical function impairment and frailty; both associated with increased risk of falls, hospitalizations, and death. Identifying factors associated with physical function impairment and frailty can help target interventions.
Methods
The REPRIEVE trial enrolled participants 40–75 years of age, receiving stable antiretroviral therapy with CD4+ T-cell count >100 cells/mm3, and with low to moderate cardiovascular disease risk. We conducted a cross-sectional analysis of those concurrently enrolled in the ancillary study PREPARE at enrollment.
Results
Among the 266 participants, the median age was 51 years; 81% were male, and 45% were black, and 28% had hypertension. Body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) was 25 to <30 in 38% and ≥30 in 30%, 33% had a high waist circumference, 89% were physically inactive, 37% (95% confidence interval, 31%, 43%) had physical function impairment (Short Physical Performance Battery score ≤10), and 6% (4%, 9%) were frail and 42% prefrail. In the adjusted analyses, older age, black race, greater BMI, and physical inactivity were associated with physical function impairment; depression and hypertension were associated with frailty or prefrailty.
Conclusions
Physical function impairment was common among middle-aged PWH; greater BMI and physical inactivity are important modifiable factors that may prevent further decline in physical function with aging.
Clinical Trials Registration
Keywords: HIV, Aging, Physical function, Frailty, SPPB
With combination antiretroviral therapy (ART), longer life expectancy in people with human immunodeficiency virus (HIV) (PWH) is changing the demographics of the HIV epidemic. Nearly half of PWH in the United States are now ≥50 years [1]. However, even with long-term effective ART, physical function impairment and frailty occur at an earlier age, are more common than among people without HIV, and have been associated with increased risk of falls, hospitalizations, and death [2–4]. Furthermore, the combination of both HIV infection and impaired physical function is associated with a greater mortality risk than the presence of HIV infection or impaired function alone [5, 6]. Consequently, the aging PWH population is an emerging risk group for accelerated development of mobility disability, presenting an urgent need for ways to improve physical function and prevent its early decline.
Validated tools have been developed to characterize physical impairment among older populations in which impairment has significant health consequences in the short term [7]. Older adults with physical impairment are particularly vulnerable to additional stressors [8], and they are frequently unable to access the resources and rehabilitation needed to return to a higher level of functioning, often resulting in frailty [9]. Identifying those at highest risk for physical function decline before frailty and disability develop would allow for prevention and early, more effective interventions [10–12].
Our aim was to evaluate the prevalence and degree of physical function impairment and frailty at enrollment among PWH in the AIDS Clinical Trials Group (ACTG) study A5361s (Pitavastatin to REduce Physical Function Impairment and FRailty in HIV [PREPARE]; NCT03070223), an ancillary study of REPRIEVE (Randomized Trial to Prevent Vascular Events in HIV; NCT02344290) [13]. As the clinical relevance of frailty among middle-aged individuals is not well understood, we also explored the associations between objective measures of physical function using (1) a standard index validated in geriatric populations (the Short Physical Performance Battery [SPPB]) [7], (2) a modified version of the SPPB (mSPPB) that has been implemented in healthier, higher functioning cohorts [14], and (3) the frailty phenotype [15]. Finally, we investigated associations of demographic, clinical, and behavioral characteristics with physical function impairment, evaluated using both standard and modified tools, and frailty.
METHODS
Study Participants
The analysis included REPRIEVE participants enrolled at US ACTG sites who concurrently enrolled into the physical function ancillary study PREPARE. REPRIEVE enrolled men and women living with HIV, 40–75 years of age, receiving stable combination ART, with CD4+ T-cell count >100 cells/mm3 and with low-to-moderate traditional cardiovascular disease (CVD) risk, as represented by cardiac risk score [13]. Participants were randomized to pitavastatin calcium or placebo. Key exclusion criteria included current diabetes mellitus if low-density lipoprotein cholesterol levels were ≥70 mg/dL, chronic active hepatitis B or C infection unless fibrosis score indicated no significant liver fibrosis (FIB-4 ≤3.25) [16], and myositis or myopathy within 180 days. PREPARE prioritized recruitment from the REPRIEVE Mechanistic Substudy, which will evaluate the effect of statins on coronary artery disease and inflammatory biomarkers. The Mechanistic Substudy participants were subject to additional eligibility criteria, including body mass index (BMI) <40 (calculated as weight in kilograms divided by height in meters squared) [17].
Physical Function and Frailty Measures
Evaluations were conducted at each site by trained personnel. Physical function was evaluated by the SPPB [7] and mSPPB [14], with evaluation consisting of (1) a balance test, testing the ability to hold 4 positions of increasing difficulty for 10 and 30 seconds (side-by-side, semitandem, tandem, and 1-leg stands); (2) a 4-m walk at usual pace, measured twice; and (3) 10 repeated chair stands, with split times obtained after 5 and 10 stands.
The SPPB score (ordinal from 0 [worst] to 12 [best]) was calculated as described elsewhere [7, 18]. To further differentiate gradations of physical performance, the mSPPB score (ranging from 0 to 3 [maximal performance]) [14] was calculated as a sum of the following 3 components, each divided by the maximal possible performance to derive a ratio between 0 to 1: (1) chair rise rate (stands per second), based on 10 chair stands divided by maximal performance of 1 stand per second; (2) proportion of total standing balance time, calculated as the total time each of the semitandem, tandem, and 1-leg stand positions were held (maximum 30 seconds each), divided by 90 seconds; and (3) gait speed (meters per second), based on an average of the two 4-m walk results, divided by maximal performance (2 m/s). The following cutoff points were used to define impairment: SPPB score ≤10 [5], >16.7 seconds to complete 5 chair stands [18], and gait speed <1 m/s [19, 20].
The frailty phenotype was defined using the following components: (1) weight loss (self-report of unintentional weight loss of ≥10 pounds in the prior year), (2) exhaustion (experiencing at least 3–4 times per week the feeling that “everything I do is an effort” or “sometimes I cannot get going”), (3) low physical activity (being “limited a lot” in response to the Short Form 36 question: “Does your health limit you in vigorous activities such as running, lifting heavy objects, or participating in strenuous sports?”), (4) slow gait by average of two 4-m walk times, and (5) weak grip by average of 3 measurements performed with a handheld Jamar dynamometer. Grip was classified by sex and BMI, using criteria published elsewhere [15]. Gait was classified by sex and height [15], with the cutoffs adjusted for 4-m walk, and low physical activity was modified from the initial criteria [15], as described elsewhere by Erlandson et al [21]. Participants were classified as nonfrail if they had no components present, prefrail with 1 or 2 components present, or frail with ≥3 components present.
Covariates
Baseline demographics evaluated in association with physical function included age, natal sex, race, and ethnicity. Behavioral characteristics included self-reported smoking status, substance use, alcohol use, and physical activity. Report of both “rarely/never” doing <30 minutes of physical activity ≥3 days a week (Rapid Eating Assessment for Patients [REAP] question 26) [22] and “rarely/never” having >2 hours of screen time per day (REAP question 27) was classified as physically active. Cardiovascular and metabolic factors included hypertension diagnosis, BMI, and waist circumference. HIV-related factors included time since HIV diagnosis (subsequently referred to as HIV duration), history of AIDS (AIDS-defining illness or nadir CD4+ T-cell count <200 cells/mm3) [23], CD4+ T-cell count, total cumulative ART use, and exposure to thymidine analogues. Among other comorbid conditions, the history of depression (self-report of ever having been treated for depression with medication) was evaluated. Further information on methods for ascertainment of these REPRIEVE data elements and their presentation is provided in the introductory article and associated material in the current supplement [24].
Ethics Statement
Each clinical research site obtained institutional review board/ethics committee approval and any other applicable regulatory entity approvals. Participants were provided with study information, including discussion of risks and benefits, and were asked to sign the approved declaration of informed consent.
Statistical Analysis
The proportions of participants with physical function impairment and frailty were summarized along with 2-sided 95% confidence intervals (CIs), using the Wilson score method. Spearman correlation tests were used for associations between physical function measures and frailty.
To evaluate associations between physical function and baseline characteristics, the primary analysis used SPPB score ≤10 as the outcome measure. Each covariate was first evaluated in a separate logistic regression model (the unadjusted models). Covariates with P < .05 in the unadjusted models were included in the adjusted model. As a supporting analysis, linear regression was used with mSPPB score (continuous) as the outcome measure, including covariates identified from SPPB or mSPPB unadjusted models. Owing to collinearity between age, HIV duration, ART duration, and thymidine analogue exposure, age and thymidine exposure were prioritized for inclusion in the adjusted model. Similarly, BMI and waist circumference were strongly associated; BMI was included in the adjusted model when either variable had P value <.05 in the unadjusted models, owing to missing data for waist circumference. Although the sample size to assess interactions was limited, key interactions were considered, including age, sex, race, and BMI. Associations with frailty phenotype were also examined, using logistic regression.
Two-sided P values < .05 were considered to indicate statistical significance, with no adjustment for multiple testing in this exploratory analysis. The data analysis was conducted using SAS software, version 9.4 for UNIX (SAS Institute).
RESULTS
Analysis Population Description
A total of 601 eligible participants enrolled into PREPARE. Of those, 335 were enrolled retrospectively after they had started REPRIEVE study treatment. The current analysis focused on the 266 participants who enrolled into REPRIEVE and PREPARE concurrently and underwent physical function evaluation before starting REPRIEVE study treatment (referred to as baseline).
Baseline Characteristics of Analysis Population
The 266 participants with baseline physical function assessments were enrolled across 29 ACTG centers in the United States between March 2017 and February 2018; 62% were coenrolled in the REPRIEVE Mechanistic Substudy. Baseline characteristics are shown in Table 1. Briefly, the median age was 51 years (only 1 participant was >65 years old), 81% were male, and 45% were black or African American (subsequently collectively referred to as black). Thirty percent had a BMI ≥30, including 9% with BMIs ≥35 to <40 and 3% with BMIs ≥40, and 33% had a high waist circumference. Eighty-nine percent were classified as not physically active. Twenty-four percent reported “usually/often” and 30% reported “sometimes” engaging in <30 minutes of physical activity ≥3 days a week (REAP question 26), and 54% reported “usually/often” and 29% “sometimes” having >2 hours of screen time per day (REAP question 27).
Table 1.
Baseline Characteristicsa
| Characteristic | Descriptor | Participants, No. (%) (N = 266) |
|---|---|---|
| Demographic and Behavioral | ||
| Age, y | Median (Q1, Q3) | 51 (46, 55) |
| Natal sex | Female | 50 (19) |
| Male | 216 (81) | |
| Gender identity | Cisgender | 254 (98) |
| Transgender spectrum | 6 (2) | |
| Race | Black or African American | 121 (45) |
| White | 125 (47) | |
| Otherb | 20 (8) | |
| Ethnicity | Hispanic or Latino | 49 (18) |
| Not Hispanic or Latino | 216 (81) | |
| Unknown | 1 (<0.5) | |
| Smoking status | Current | 84 (32) |
| Former | 82 (31) | |
| Never | 100 (38) | |
| Alcohol use | Usually/often | 17 (6) |
| Sometimes | 58 (22) | |
| Never/rarely | 190 (72) | |
| Substance use | Current | 6 (2) |
| Former | 138 (52) | |
| Never | 122 (46) | |
| Physical activity | Active | 28 (11) |
| Not active | 237 (89) | |
| Cardiovascular and Metabolic | ||
| History of hypertension | 74 (28) | |
| History of diabetes | 1 (<0.5) | |
| BMIc | <18.5 | 2 (1) |
| 18.5–24.9 | 83 (31) | |
| 25–29.9 | 101 (38) | |
| ≥30 | 80 (30) | |
| Waist circumference | High | 85 (33) |
| Normal | 171 (67) | |
| HIV-Related Health Status | ||
| Time since HIV diagnosis, y | Median (Q1, Q3) | 15 (9, 22) |
| Nadir CD4+ T-cell count | <200 cells/mm3 | 123 (46) |
| ≥200 cells/mm3 | 130 (49) | |
| Unknown | 13 (5) | |
| History of AIDS diagnosis | 138 (52) | |
| CD4+ T-cell count, cells/mm3 | Median (Q1, Q3) | 610 (437, 840) |
| HIV-1 RNA level | <50 copies/mL | 235 (93) |
| ≥50 copies/mL | 19 (7) | |
| Total ART use, y | Median (Q1, Q3) | 11 (6, 16) |
| Thymidine exposure | 91 (34) | |
| ART regimen class | NRTI + INSTI | 159 (60) |
| NRTI + NNRTI | 42 (16) | |
| NRTI + PI | 31 (12) | |
| Other | 33 (12) | |
| Other Comorbidities | ||
| History of non-AIDS cancer | 14 (5) | |
| History of kidney disease | 5 (2) | |
| History of depression | 133 (50) | |
| Chronic active HBV | 5 (2) | |
| Chronic active HCV | 11 (4) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
aFrequency (%) for categorical measures, median with lower and upper quartiles (Q1, Q3) for continuous measures. All statistics were calculated based on participants with data collected. Data were missing for gender identity in 6 participants, for alcohol use and physical activity in 1 each, for waist circumference in 10, and for HIV-1 RNA level in 12.
bThe “other” category for race includes participants self-identifying as Asian, native or indigenous, of >1 race (with no single race noted as predominant), or of unknown race.
cBMI is calculated as weight in kilograms divided by height in meters squared.
The distributions of key baseline characteristics were similar to those of the overall REPRIEVE US participants enrolling during the same time frame (not shown). More integrase strand transfer inhibitor–containing ART use was observed among PREPARE participants not included in this analysis, likely reflective of the time of enrollment, as discussed by Fichtenbaum et al [25]. Excluded participants also had higher proportion with white race and lower BMI; distributions of other characteristics were similar between the 2 groups (not shown).
Prevalence of Physical Function Impairment and Frailty
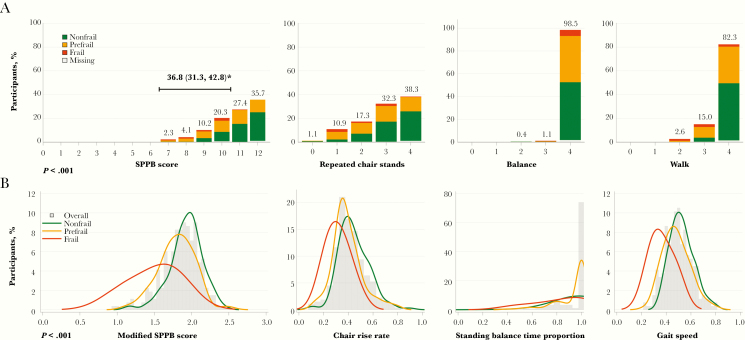
The prevalence of physical function impairment (SPPB score ≤10) was 37% (95% CI, 31%, 43%), with scores ranging from 7 to 12 (Figure 1A). The chair stand component demonstrated the most frequent limitations: 62% of the participants had some evidence of impairment on the 5 chair stands, including 12% with the greatest impairment (requiring >16.7 seconds to complete 5 stands). The median mSPPB score was 1.88 (Q1, Q3, 1.70, 2.03), driven mostly by slower chair rise rate and gait speed (Figure 1B). Twenty-five percent of participants were unable to complete the balance assessment, and 54% had slow gait (<1 m/s).
Figure 1.
A, Short Physical Performance Battery (SPPB) score by frailty phenotype. The SPPB score and its components (repeated chair stands, balance, walk time) are shown with bars stacked by frailty phenotype. SPPB components are scored from 0 to 4, with 4 indicating the highest level of performance. The total SPPB score (sum of the 3 components) ranges from 0–12, with 12 indicating the highest level of performance. P value is for the test of the association between SPPB score (both ordinal from 0 to 12, and classified as ≤10 or >10) and frailty phenotype (Spearman correlation test). *Percentage of participants with SPPB score ≤10, with 2-sided 95% Wilson confidence interval. B,
Modified SPPB (mSPPB) score by frailty phenotype. The mSPPB score and its components (chair rise rate, standing balance time proportion, gait speed) are shown overall (histogram) and by frailty phenotype (density lines). mSPPB components are scored from 0 to 1, with 1 indicating the highest level of performance. The total mSPPB score (sum of the 3 components) ranges from 0 to 3, with 3 indicating the highest level of performance. P value is for the test of the association between mSPPB score and frailty phenotype (Spearman correlation test).
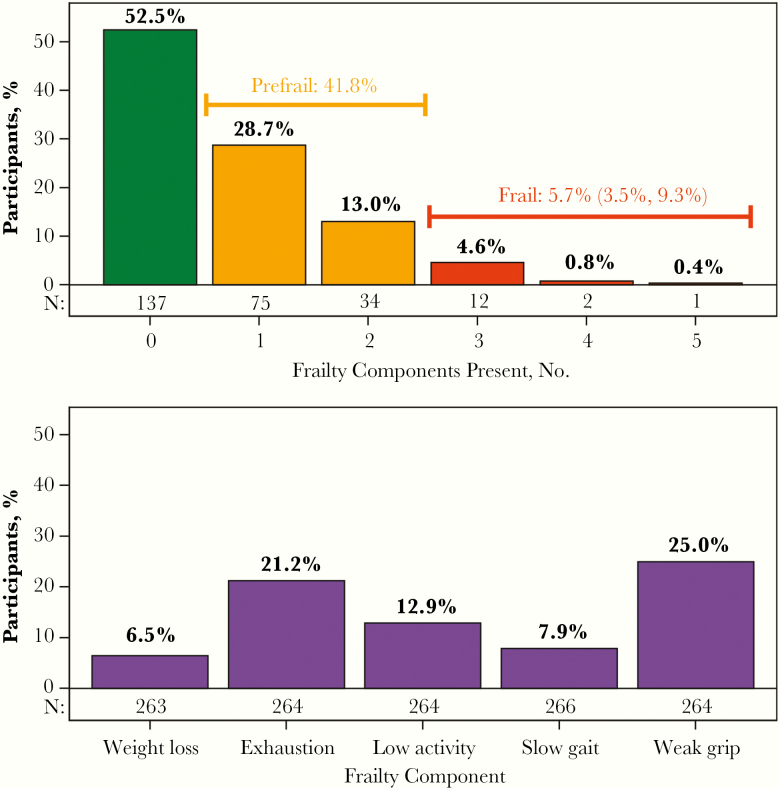
Of the 261 participants with data available, 6% were frail (95% CI, 4%, 9%) and 42% were prefrail (Figure 2). The most prevalent frailty components were weak grip (25%) and exhaustion (21%). Of the 15 participants classified as frail, 12 had 3 of the 5 frailty components present, and only 3 had >3 components. There was a strong association between frailty and SPPB score (Figure 1A): 23% of the nonfrail, 46% of the prefrail, and 93% of the frail participants had an SPPB score ≤10 (P < .001). Similarly, differences by frailty were apparent in mSPPB, with a median score of 1.94 (Q1, Q3, 1.81, 2.05) among the nonfrail, 1.81 (1.64, 1.95) among the prefrail, and 1.56 (1.18, 1.78) among the frail participants (P < .001; Figure 1B).
Figure 2.
Frailty phenotype and prevalence of individual frailty components. Top, Percentage of participants by the number of frailty components present, with bars colored by frailty phenotype. For the percentage of participants who are frail, the 2-sided 95% Wilson confidence interval is provided. Bottom, Percentage of participants with each individual frailty component present.
Associations Between Covariates and Physical Function Impairment
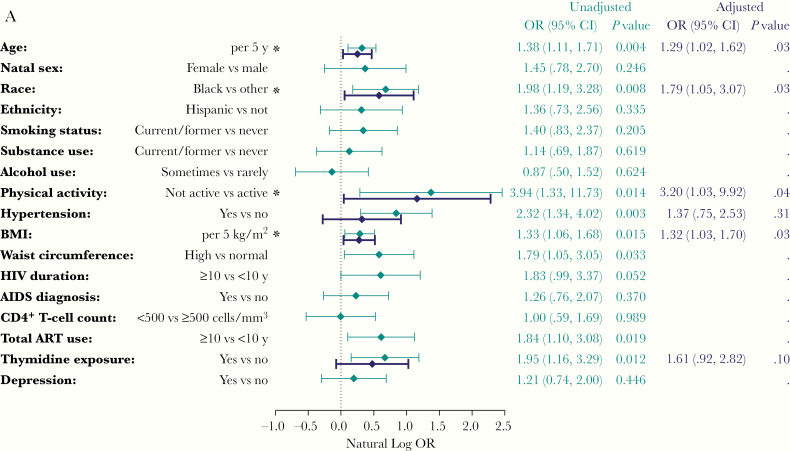
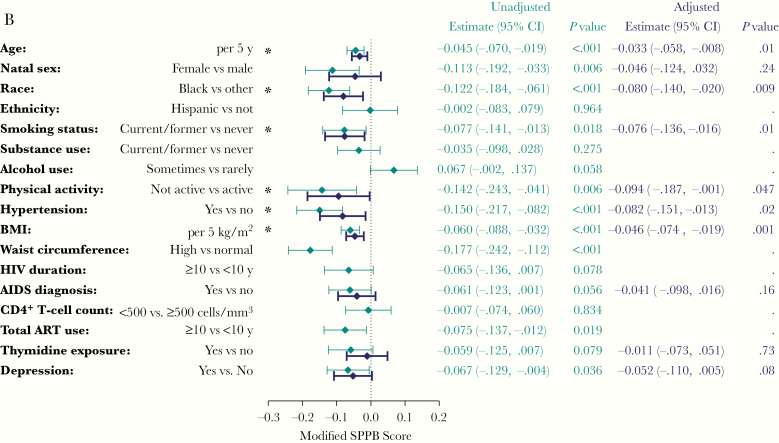
In unadjusted analyses, older age, black race, ≥10 years of ART, thymidine exposure, higher BMI, higher waist circumference, hypertension, and lack of physical activity were associated with physical function impairment (SPPB score ≤10; Figure 3A). In adjusted analysis, older age, black race, higher BMI, and lack of physical activity remained associated with physical function impairment. The effect of thymidine exposure was attenuated by age, which is probably reflective of thymidine exposure among older participants (not shown). Similar associations were observed with the mSPPB score. In addition, hypertension and smoking were associated with lower mSPPB in the adjusted analysis (Figure 3B). The association observed between thymidine exposure and physical function impairment (SPPB score ≤10) was weaker in the unadjusted analyses of mSPPB, and further attenuated by age in the adjusted analysis. For SPPB and mSPPB, we did not find a difference in age or BMI effect by sex or race, nor an interaction between sex and race (not shown).
Figure 3.
A, Associations with physical function impairment. Estimates are from logistic regression models with physical function impairment (Short Physical Performance Battery [SPPB] score ≤10) as the dependent variable. Odds ratios (OR) and confidence intervals (CIs) are shown in log scale for visual purposes. Each unadjusted model includes a single covariate. The adjusted model includes all covariates with P values <.05 in the unadjusted models. *P < .05 in the adjusted model. B, Associations with modified SPPB Score (mSPPB). Estimates are from linear regression models, with mSPPB score as the dependent variable. Each unadjusted model includes a single covariate. The adjusted model includes all covariates with P values <.05 in the unadjusted models for mSPPB or for SPPB score ≤10 (A). For alcohol use, in both A and B, note that "Sometimes vs rarely" is shorthand for "Sometimes or usually/often versus rarely or never." *P < .05 in the adjusted model. Abbreviations: ART, antiretroviral therapy; BMI, body mass index.
For evaluation of associations with frailty phenotype, frailty and prefrailty were combined, given the limited number of frail participants (n = 15). Only depression and hypertension were associated with higher odds of frailty or prefrailty in the adjusted analysis (Supplementary Figure 1).
DISCUSSION
With the increasing lifespan of PWH, clinicians are confronted with a growing number of aging PWH with physical function limitations that contribute to excess morbidity rates and reduced quality of life. It remains unclear how health and behavioral factors during early adulthood and middle age contribute to the progressive physical function decline that appears earlier in PWH. In this REPRIEVE ancillary study, we evaluated factors associated with physical function impairment and frailty among individuals receiving stable ART with low-to-moderate CVD risk and no overt CVD, thus without some of the risk factors commonly associated with frailty and functional impairment in older adults. In our population with a median age of 51 years, whereas overt frailty was uncommon, impairment in measures of physical function (most commonly in the ability to rise from a chair) and prefrailty occurred in nearly half of participants. Although impairment, as indicated by the SPPB and mSPPB, and frailty were strongly associated with each other, the ceiling effect of the SPPB and floor effect of frailty may limit the use of these measures to identify early impairment in this relatively young population (ie, compared with populations ≥65 years). Finally, the observed modifiable factors associated with impairment identified important targets to potentially alter the trajectory of impairment and frailty in this population, including interventions to increase physical activity, improve strength, and achieve a healthy body weight.
Physical function impairment in our study appeared mostly in strength (grip) and power (the ability to rise from a chair), important manifestations of skeletal muscle function. Impairments in skeletal muscle are well recognized in PWH. Lean mass, a marker with skeletal muscle quantity, declines over time in PWH [26], with steeper decline than in uninfected controls [27]. PWH may also have poorer skeletal muscle quality, as evidenced by fatty infiltration [28, 29]. The cause of this association in PWH is unknown, but increasing evidence suggests that newer ART agents are associated with clinically significant overall weight gain in some populations [30]. As greater weight gain is associated with greater fatty muscle infiltration, this ART-associated weight gain is expected to further worsen skeletal muscle quality [31]. Other studies have reported that PWH have an atypical skeletal muscle profile with evidence of fibrosis [32, 33]. Furthermore, many ART agents have been linked to mitochondrial toxicity, a key driver of energy production within skeletal muscle [34]. Our observed association between prior thymidine analogue exposure and physical function and frailty, albeit slightly attenuated in the adjusted analyses, may suggest a potential residual effect of older ART agents on mitochondrial function or skeletal muscle itself. Associations between physical function and muscle quality in these participants will be further examined in our future analyses of muscle density measured by computed tomography.
The evidence of existing muscle impairment in our population underlines the importance of this study of statins in HIV. Early evidence of a potential beneficial statin effect on body composition was demonstrated in a placebo-controlled study of pravastatin among men with HIV infection and hypercholesterolemia, the majority of whom also had lipoatrophy. A significant increase in total, limb, and subcutaneous fat was demonstrated in participants randomized to pravastatin versus placebo, suggesting a beneficial statin effect on lipoatrophy [35]. A subsequent large observational cohort study of PWH found a greater increase in thigh circumference among statin users also taking thymidine analogues, suggesting a beneficial statin effect on muscle [36].
In a randomized, placebo-controlled study of rosuvastatin (SATURN-HIV), participants randomized to rosuvastatin experienced gains in total lean body mass and leg lean mass than those randomized to placebo, without differences in total body fat or limb fat [37]. Although the prevalence of myalgias may be as high as 20% in clinical practice [38], the risk of myalgias with statin therapy is similar to placebo in randomized controlled clinical trials [39], and it was only 2% in a clinical trial of PWH receiving pitavastatin [40]. Although data suggest that myalgias are not associated with decreased physical function [41, 42], observational cohort studies have reported lower strength, physical activity, and lean mass among participants using statins [43–45] that may contribute to development of frailty. The presence of frailty may further increase the risk of statin-associated musculoskeletal symptoms [46]. The combination of statins with some antiretrovirals may lead to drug-drug interactions in PWH [47] and PWH may be less responsive to statin therapy than HIV-uninfected persons [48], requiring higher doses of potent therapy associated with greater toxicity. Whether the statin strategy tested in this trial has a beneficial effect on muscle mass, quality, and function over time will be evaluated in our longitudinal assessments.
Our findings suggest that chair stand time may be a useful screening tool in this higher-functioning population. Many tools (eg, SPPB and frailty phenotype) were developed for older populations (ie, ≥65 years), and their ability to detect early impairment in younger populations such as ours has not been validated. In the current study, as in prior work [49], we demonstrated the ceiling and floor effects of the SPPB and frailty, respectively. In contrast, subtle changes in mobility measures involving the lower extremities (eg, chair stand time) may be detectable at an earlier age. In our prior work, in a middle-aged cohort of PWH (aged 45–65 years), 18% had slow gait (an SPPB score of ≤3, corresponding to ≤ 1.2 m/s), and 10% had weak grip, while 33% had evidence of impaired chair stand time (defined as an SPPB score ≤3) [49]. Similarly, chair stand impairment was common in the ALIVE cohort of people who inject drugs, with or without HIV infection (median age, 51 years): 42% of participants had evidence of impaired chair stand time (SPPB component score ≤3) compared with 28% with slow gait (SPPB component score ≤3) [5]. Importantly, lower-extremity strength impairment seems to be a strong predictor of subsequent disability in the general population, as reported for numerous other cohorts of both higher- and lower-functioning older individuals [50–54].
Although frailty was uncommon (6%), prefrailty was quite common (42%). The observed low prevalence of frailty was similar to findings reported in other PWH populations of similar age and HIV or ART duration, reviewed by Piggott et al [55]. Given that frailty and CVD increase with age and have similar underlying pathogenesis (inflammation and immune activation) [55, 56] and modifiable risk factors (inactivity, smoking, poor nutrition, and hypertension), our observed low prevalence of frailty is not surprising. The relevance of prefrailty is uncertain, though data from a cohort of PWH, aged ≥45 years, suggested that prefrailty may be associated with subsequent cardiovascular events [57].
In contrast to our findings with SPPB and mSPPB, frailty was associated with a history of depression. There is substantial overlap between symptoms of depression and components of the frailty phenotype [58]. Indeed, exhaustion, a common symptom of depression, was seen in 21% of participants and may contribute to the low levels of physical activity and slow gait, components of the frailty phenotype. Similarly, depression may suppress appetite and contribute to the weight loss component of frailty. It is not clear whether frailty and prefrailty are describing depressive symptoms in this relatively younger population or are measuring the same physiologic vulnerability to stressors described in older populations. Because the prevalence of depressive symptoms is approximately 3 times greater in PWH [59], the performance of frailty phenotype in this population with frequent comorbid depression may be limited. However, depression is also strongly associated with increased risk for CVD [60]. Thus, future analyses of PREPARE follow-up will examine the independent contribution of frailty (or prefrailty) and depression to cardiac events.
Several limitations should be acknowledged. Our study represents individuals enrolling into a clinical trial and may not be representative of the overall population of PWH. The lack of an HIV-uninfected control group does not allow us to evaluate how these associations differ by HIV serostatus. Similarly, the inclusion and exclusion criteria may have limited the ability to identify important comorbid conditions or risk factors. The number of women was small, limiting the ability to detect effects by sex. Finally, the associations identified in this cross-sectional analysis may not reflect causation or directionality. Regardless of these limitations, the study incorporates a variety of physical function assessments and represents a racially and ethnically diverse population from across the United States, recruited in the modern era of ART.
In summary, in a population of PWH at low-to-moderate CVD risk, physical function impairment, particularly impairment of muscle strength, was common, whereas overt frailty was uncommon. Many factors associated with physical function impairment are modifiable, and early interventions targeting these may have long-lasting effects on health span. Interventions to increase physical activity and reduce BMI, hypertension, and smoking may not only slow development of physical function impairment but may also decrease the risk of CVD, cancer, and numerous other age-associated comorbid conditions [61]. Furthermore, with growing evidence of weight gain with newer ART agents [30], the impact of BMI and physical inactivity is becoming increasingly relevant with worldwide use of these agents. Further research on the impact of routine incorporation of objective assessments of physical function impairment and frailty, together with interventions designed to reduce or delay them, is warranted to improve care and outcomes in PWH.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The study investigators thank the study participants, site staff, and study-associated personnel for their ongoing participation in the trial, as well as the AIDS Clinical Trials Group (ACTG) for clinical site support; ACTG clinical trials specialists for administrative support; the data management center, Frontier Science Foundation, for data support; and the Center for Biostatistics in AIDS Research for statistical support.
Disclaimer. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute on Aging (NIA), the National Institutes of Health (NIH), or the US Department of Health and Human Services.
Financial support. This work was supported by the NIA (grant R01AG 054366), the NHLBI (grants U01 HL123336 and U01 HL123339), and the NIAID (UM1 AI068636 and UM1 AI106701).
Supplement sponsorship. This supplement is sponsored by Massachusetts General Hospital, through NIH funding including U01HL123336.
Potential conflicts of interest. T. U. reports grants from NIH/NIA and NIH/NHLBI during the conduct of the study, as well as grants from Kowa Pharmaceuticals America, outside the submitted work. E. T. O. reports grants from NIH during the conduct of the study; grant support through his institution from ViiV Healthcare, Gilead Sciences, Janssen, and Bavarian Nordic, outside the submitted work; and personal fees from Gilead Sciences, Theratechnologies, ViiV Healthcare, and Merck, outside the submitted work. H. J. R. reports grants from NIH/NHLBI and Kowa Pharmaceuticals America, during the conduct of the study. K. V. F. reports an educational grant from Gilead Sciences, outside the submitted work. P. S. D. reports grant support from Kowa Pharmaceuticals America and Gilead Sciences, for the conduct of the study. S. K. G. reports grant support through his institution from Kowa Pharmaceuticals America, and Gilead Sciences, for the conduct of the study, and from Theratechnologies, Navidea, and ViiV Healthcare, outside the submitted work. S. H. reports serving on the Gilead advisory board during the conduct of the study. B. R. reports grants from NIH during the conduct of the study, as well as honoraria from Gilead Sciences and ViiV Healthcare, outside the submitted work. C. A. B. reports grants from NIH/NIAID during the conduct of the study and serves on Data and Safety Monitoring Board for GlaxoSmithKline, outside the submitted work. K. M. E. reports grant support from NIH/NIA, during the conduct of the study, and grants and personal fees from Gilead Sciences and personal fees from ViiV Healthcare, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Conference on Retroviruses and Opportunistic Infections (CROI), Boston, Massachusetts, 8–11 March 2020. Abstract 0707.
References
- 1. High KP, Brennan-Ing M, Clifford DB, et al. OAR Working Group on HIV and Aging HIV and aging: state of knowledge and areas of critical need for research: a report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012; 60suppl 1:S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Desquilbet L, Jacobson LP, Fried LP, et al. A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. J Gerontol A Biol Sci Med Sci 2011; 66:1030–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erlandson KM, Allshouse AA, Jankowski CM, et al. Risk factors for falls in HIV-infected persons. J Acquir Immune Defic Syndr 2012; 61:484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tassiopoulos K, Abdo M, Wu K, et al. Frailty is strongly associated with increased risk of recurrent falls among older HIV-infected adults. AIDS 2017; 31:2287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greene M, Covinsky K, Astemborski J, et al. The relationship of physical performance with HIV disease and mortality. AIDS 2014; 28:2711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piggott DA, Varadhan R, Mehta SH, et al. Frailty, inflammation, and mortality among persons aging with HIV infection and injection drug use. J Gerontol A Biol Sci Med Sci 2015; 70:1542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49:M85–94. [DOI] [PubMed] [Google Scholar]
- 8. Perracini MR, Mello M, de Oliveira Máximo R, et al. Diagnostic accuracy of the Short Physical Performance Battery for detecting frailty in older people. Phys Ther 2020; 100:90–8. [DOI] [PubMed] [Google Scholar]
- 9. Giné-Garriga M, Roqué-Fíguls M, Coll-Planas L, Sitjà-Rabert M, Salvà A. Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: a systematic review and meta-analysis. Arch Phys Med Rehabil 2014; 95:753–769.e3. [DOI] [PubMed] [Google Scholar]
- 10. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995; 332:556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daniels R, van Rossum E, de Witte L, Kempen GI, van den Heuvel W. Interventions to prevent disability in frail community-dwelling elderly: a systematic review. BMC Health Serv Res 2008; 8:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jadczak AD, Makwana N, Luscombe-Marsh N, Visvanathan R, Schultz TJ. Effectiveness of exercise interventions on physical function in community-dwelling frail older people: an umbrella review of systematic reviews. JBI Database System Rev Implement Rep 2018; 16:752–75. [DOI] [PubMed] [Google Scholar]
- 13. Grinspoon SK, Fitch KV, Overton ET, et al. REPRIEVE Investigators Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am Heart J 2019; 212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simonsick EM, Newman AB, Nevitt MC, et al. Health ABC Study Group Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci 2001; 56:M644–9. [DOI] [PubMed] [Google Scholar]
- 15. Fried LP, Tangen CM, Walston J, et al. Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–56. [DOI] [PubMed] [Google Scholar]
- 16. Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection—comparison with liver biopsy and FibroTest. Hepatology 2007; 46:32–6. [DOI] [PubMed] [Google Scholar]
- 17. Hoffmann U, Lu MT, Olalere D, et al. REPRIEVE Investigators Rationale and design of the Mechanistic Substudy of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE): effects of pitavastatin on coronary artery disease and inflammatory biomarkers. Am Heart J 2019; 212:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Institute on Aging. Short Physical Performance Battery Protocol (SPPB): assessing physical performance in the older patient https://www.nia.nih.gov/research/labs/leps/short-physical-performance-battery-sppb. Accessed 3 February 2020.
- 19. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc 2003; 51:314–22. [DOI] [PubMed] [Google Scholar]
- 20. Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people–results from the health, aging and body composition study. J Am Geriatr Soc 2005; 53:1675–80. [DOI] [PubMed] [Google Scholar]
- 21. Erlandson KM, Wu K, Koletar SL, et al. Association between frailty and components of the frailty phenotype with modifiable risk factors and antiretroviral therapy. J Infect Dis 2017; 215:933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gans KM, Risica PM, Wylie-Rosett J, et al. Development and evaluation of the nutrition component of the Rapid Eating and Activity Assessment for Patients (REAP): a new tool for primary care providers. J Nutr Educ Behav 2006; 38:286–92. [DOI] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention. Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults 1993. https://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.htm. Accessed 3 February 2020.
- 24. Grinspoon SK, Douglas PS, Hoffman U, Ribaudo HJ. Leveraging a landmark trial of primary cardiovascular disease prevention in human immunodeficiency virus: introduction from the REPRIEVE coprincipal investigators. J Infect Dis 2020; 222(Suppl 1):S1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fichtenbaum CJ, Ribaudo HJ, Leon-Cruz J, et al. Patterns of antiretroviral therapy use and immunologic profiles at enrollment in the REPRIEVE trial. J Infect Dis 2020; 222(Suppl 1): S8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Debroy P, Lake JE, Sim M, et al. Lean mass declines consistently over 10 years in people living with HIV on antiretroviral therapy, with patterns differing by sex. Antivir Ther 2019; 24:383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grant PM, Kitch D, McComsey GA, et al. Long-term body composition changes in antiretroviral-treated HIV-infected individuals. AIDS 2016; 30:2805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torriani M, Thomas BJ, Barlow RB, Librizzi J, Dolan S, Grinspoon S. Increased intramyocellular lipid accumulation in HIV-infected women with fat redistribution. J Appl Physiol (1985) 2006; 100:609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Natsag J, Erlandson KM, Sellmeyer DE, et al. HIV infection is associated with increased fatty infiltration of the thigh muscle with aging independent of fat distribution. PLoS One 2017; 12:e0169184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis doi: 10.1093/cid/ciz999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Erlandson KM, Fiorillo S, Masawi F, et al. Antiretroviral initiation is associated with increased skeletal muscle area and fat content. AIDS 2017; 31:1831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tran T, Guardigni V, Pencina KM, et al. Atypical skeletal muscle profiles in human immunodeficiency virus-infected asymptomatic middle-aged adults. Clin Infect Dis 2018; 66:1918–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kusko RL, Banerjee C, Long KK, et al. Premature expression of a muscle fibrosis axis in chronic HIV infection. Skelet Muscle 2012; 2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gardner K, Hall PA, Chinnery PF, Payne BA. HIV treatment and associated mitochondrial pathology: review of 25 years of in vitro, animal, and human studies. Toxicol Pathol 2014; 42:811–22. [DOI] [PubMed] [Google Scholar]
- 35. Mallon PW, Miller J, Kovacic JC, et al. Effect of pravastatin on body composition and markers of cardiovascular disease in HIV-infected men—a randomized, placebo-controlled study. AIDS 2006; 20:1003–10. [DOI] [PubMed] [Google Scholar]
- 36. Brown TT, Smurzynski M, Wu K, Bosch RJ, McComsey GA. Statin therapy and changes in hip circumference among HIV-infected participants in the ALLRT Cohort. Antivir Ther 2009; 14:853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Erlandson KM, Jiang Y, Debanne SM, McComsey GA. Effects of 96 weeks of rosuvastatin on bone, muscle, and fat in HIV-infected adults on effective antiretroviral therapy. AIDS Res Hum Retroviruses 2016; 32:311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med 2011; 78:393–403. [DOI] [PubMed] [Google Scholar]
- 39. Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 316:2008–24. [DOI] [PubMed] [Google Scholar]
- 40. Aberg JA, Sponseller CA, Ward DJ, Kryzhanovski VA, Campbell SE, Thompson MA. Pitavastatin versus pravastatin in adults with HIV-1 infection and dyslipidaemia (INTREPID): 12 week and 52 week results of a phase 4, multicentre, randomised, double-blind, superiority trial. Lancet HIV 2017; 4:e284–94. [DOI] [PubMed] [Google Scholar]
- 41. Panza GA, Taylor BA, Roman W, Thompson PD. Changes in muscle strength in patients with statin myalgia. Am J Cardiol 2014; 114:1215–6. [DOI] [PubMed] [Google Scholar]
- 42. Mallinson JE, Marimuthu K, Murton A, et al. Statin myalgia is not associated with reduced muscle strength, mass or protein turnover in older male volunteers, but is allied with a slowing of time to peak power output, insulin resistance and differential muscle mRNA expression. J Physiol 2015; 593:1239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scott D, Blizzard L, Fell J, Jones G. Statin therapy, muscle function and falls risk in community-dwelling older adults. QJM 2009; 102:625–33. [DOI] [PubMed] [Google Scholar]
- 44. Lee DS, Markwardt S, Goeres L, et al. Statins and physical activity in older men: the osteoporotic fractures in men study. JAMA Intern Med 2014; 174:1263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dzien A, Winner H, Theurl E, Dzien-Bischinger C, Lechleitner M. Fat-free mass and fasting glucose values in patients with and without statin therapy assigned to age groups between <60 and >75 years. Obes Facts 2013; 6:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ward NC, Watts GF, Eckel RH. Response by Ward et al to letter regarding article, “Statin toxicity: mechanistic insights and clinical implications.” Circ Res 2019; 124:e121–2. [DOI] [PubMed] [Google Scholar]
- 47. Wiggins BS, Lamprecht DG Jr, Page RL 2nd, Saseen JJ. Recommendations for managing drug-drug interactions with statins and HIV medications. Am J Cardiovasc Drugs 2017; 17:375–89. [DOI] [PubMed] [Google Scholar]
- 48. Courlet P, Livio F, Alves Saldanha S, et al. Real-life management of drug-drug interactions between antiretrovirals and statins. J Antimicrob Chemother. 2020; 75:1972–80. [DOI] [PubMed] [Google Scholar]
- 49. Erlandson KM, Allshouse AA, Jankowski CM, et al. Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials 2012; 13:324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc 2016; 64:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ohta J, Seki M, Ao M, et al. Comparison of lower leg muscle strength and grip strength for diagnosing slower gait speed in the elderly. Osteoporos Sarcopenia 2017; 3:128–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pinter D, Ritchie SJ, Gattringer T, et al. Predictors of gait speed and its change over three years in community-dwelling older people. Aging (Albany NY) 2018; 10:144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Glenn JM, Gray M, Binns A. Relationship of sit-to-stand lower-body power with functional fitness measures among older adults with and without sarcopenia. J Geriatr Phys Ther 2017; 40:42–50. [DOI] [PubMed] [Google Scholar]
- 54. Buchner DM, Larson EB, Wagner EH, Koepsell TD, de Lateur BJ. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing 1996; 25:386–91. [DOI] [PubMed] [Google Scholar]
- 55. Piggott DA, Erlandson KM, Yarasheski KE. Frailty in HIV: epidemiology, biology, measurement, interventions, and research needs. Curr HIV/AIDS Rep 2016; 13:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stewart R. Cardiovascular disease and frailty: what are the mechanistic links? Clin Chem 2019; 65:80–6. [DOI] [PubMed] [Google Scholar]
- 57. Kelly SG, Wu K, Tassiopoulos K, Erlandson KM, Koletar SL, Palella FJ. Frailty Is an independent risk factor for mortality, cardiovascular disease, bone disease, and diabetes among aging adults with human immunodeficiency virus. Clin Infect Dis 2019; 69:1370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sutton JL, Gould RL, Coulson MC, et al. Multicomponent frailty assessment tools for older people with psychiatric disorders: a systematic review. J Am Geriatr Soc 2019; 67:1085–95. [DOI] [PubMed] [Google Scholar]
- 59. Do AN, Rosenberg ES, Sullivan PS, et al. Excess burden of depression among HIV-infected persons receiving medical care in the united states: data from the medical monitoring project and the behavioral risk factor surveillance system. PLoS One 2014; 9:e92842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Khambaty T, Stewart JC, Gupta SK, et al. Association between depressive disorders and incident acute myocardial infarction in human immunodeficiency virus-infected adults: veterans aging cohort study. JAMA Cardiol 2016; 1:929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Montoya JL, Jankowski CM, O’Brien KK, et al. Evidence-informed practical recommendations for increasing physical activity among persons living with HIV. AIDS 2019; 33:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.