Abstract
This study was a retrospective, cross-sectional analysis of exercise performance and left ventricular (LV) morphology in 70 women to examine whether women who have performed regular, lifelong endurance exercise acquire the same beneficial adaptations in cardiovascular structure and function and exercise performance that have been reported previously in men. Three groups of women were examined: 1) 35 older (>60 years) untrained women (older untrained, OU), 2) 13 older women who had consistently performed 4 or more endurance exercise sessions weekly for at least 25 years (older trained, OT) , and 3) 22 middle-aged (range 35-59 years) untrained women (middle-age untrained, MU) as a reference control for the appropriate age-related changes. Oxygen uptake (V̇O2) and cardiovascular function [cardiac output (Q̇); stroke volume (SV)] (acetylene rebreathing) were examined at rest, steady-state submaximal exercise, and maximal exercise (maximal oxygen uptake, V̇O2max). Blood volume (CO rebreathing) and LV mass (cardiac MRI), plus invasive measures of static and dynamic chamber compliance were also examined. V̇O2max (p <0.001) and maximal exercise Q̇ and SV were larger in older trained women compared to the two untrained groups (~17% and ~27% for Q̇ and SV respectively versus MU; ~40% and ~38% versus OU, all p <0.001). Blood volume (ml.kg−1) and LV mass index (g.m2) were larger in OT versus OU (~11% and ~16% respectively, both p ≤ 0.015) Static LV chamber compliance was greater in OT compared to both untrained groups (median (25 - 75%): MU: 0.065(0.049 - 0.080); OU: 0.085(0.061 - 0.138); OT: 0.047(0.031 - 0.054), p ≤ 0.053). Collectively, these findings indicate that lifetime endurance exercise appears to be extremely effective at preserving or even enhancing cardiovascular structure and function with advanced age in women.
Keywords: women, aging, lifelong exercise, exercise capacity, left ventricular function, cardiovascular function
INTRODUCTION
Healthy aging is associated with a progressive decline in maximal exercise capacity as indicated by maximal oxygen uptake (V̇O2max) (Astrand et al., 1973; Fleg et al., 2005). There is considerable epidemiological evidence that demonstrates that a low V̇O2max increases the risk of functional disability and loss of independence, as well as cardiovascular-related and all-cause mortality in men and women (Blair et al., 1989; Paterson et al., 2004); thus, exercise capacity represents an important health-related risk factor with aging (Ross et al., 2016).
Our laboratory (Carrick-Ranson et al., 2014) and several others (Ogawa et al., 1992; Seals et al., 1994; Schulman et al., 1996; Hagberg et al., 1998; McCole et al., 1999; Gates et al., 2003; Dogra et al., 2012) have reported a substantially higher V̇O2max in late middle-aged and older (>50 years) adults who have performed vigorous and sustained endurance exercise compared to age-similar untrained controls. This higher exercise capacity results from a larger maximal cardiac output (Q̇) due primarily to a larger stroke volume (Ogawa et al., 1992; Seals et al., 1994; Schulman et al., 1996; McCole et al., 1999, 2000; Dogra et al., 2012; Carrick-Ranson et al., 2014). The primary mechanism that underpins the superior exercise stroke volume in trained older adults is enhanced left ventricular (LV) diastolic filling (Seals et al., 1994; Schulman et al., 1996; Hagberg et al., 1998; Wiebe et al., 1999). Increased cardiac size and eccentric remodeling, a larger blood volume and greater LV chamber compliance are important training-related adaptations that collectively enhance the recruitment of the Frank-Starling mechanism during exercise in endurance trained individuals (Levine, 1993).
The majority of the beneficial effects of lifelong endurance exercise on cardiovascular function has largely been established in men (Hagberg et al., 1985; Fleg et al., 1994; Seals et al., 1994; Schulman et al., 1996; Hagberg et al., 1998) or mixed cohorts of men and women (Arbab-Zadeh et al., 2004; Shibata et al., 2008; Shibata & Levine, 2012b; Bhella et al., 2014; Carrick-Ranson et al., 2014; Hieda et al., 2018; Shibata et al., 2018), with relatively fewer studies focused on women only (Stevenson et al., 1994; McCole et al., 1999; Wiebe et al., 1999; McCole et al., 2000; Dogra et al., 2012). Given the evidence to suggest distinct sex-related differences in the type and magnitude of functional and structural cardiovascular adaptations to endurance exercise training (Spina et al., 1993; Spina et al., 1996; Howden et al., 2015), there is a need for a comprehensive description of the cardiovascular adaptations in women who have performed lifelong endurance exercise.
Accordingly, the purpose of the current study was to examine in women the effect of lifelong endurance training on LV structure and function at rest and during upright exercise. We hypothesized that V̇O2max, exercise stroke volume, LV mass and blood volume would be significantly larger and LV chamber compliance more “youthful” in women who had performed lifelong exercise compared to older untrained women and would reflect those observed in the middle-aged untrained women.
METHODS
Ethical Approval
All participants signed an informed consent approved by the institutional review boards of the University of Texas Southwestern and Texas Health Resources Presbyterian Hospital of Dallas and performed in accordance with the Declaration of Helsinki.
Participants Recruitment and Screening
This current study is a retrospective, cross-sectional analysis of 70 women previously recruited for several studies examining the effect of healthy aging and endurance exercise training on LV compliance in our laboratory including baseline data from a randomized control trial (http://www.clinicaltrials.gov identifier: NCT01014572)(Fujimoto et al., 2012; Fujimoto et al., 2013; Bhella et al., 2014). Participants were recruited from three primary sources; the Dallas Heart Study (Victor et al., 2004), the Cooper Center Longitudinal Study (Chen et al., 2010), and a random sample of employees of Texas Health Resources, the third largest employer in the Dallas-Fort Worth metroplex and a diverse health care company.
These women included: 1) 22 early-to-late middle-aged (35-59 years) untrained women (middle-age untrained), 2) 35 older (>60 years) untrained women (Older untrained), and 3) 13 older women who had performed at least 4 weekly endurance exercise sessions (for at least 30 minutes per session) for most of their adult lives (>25 years) (Older trained). Untrained middle-aged and older women were performing ≤ 3 exercise session weekly. Eight of the older trained women had performed near daily exercise and were competitive Masters athletes, while the remaining five were “committed” (4-5 sessions per week) but not competitive lifelong exercisers (Bhella et al., 2014; Carrick-Ranson et al., 2014). The middle-aged untrained group were included to demonstrate the “healthy” age-related changes in exercise capacity and cardiovascular variables, while the selection of the lifelong exercise cohort was based on our previous works that demonstrated that 4-to-7 exercise sessions over 25 years resulted in a significant improvement in exercise capacity, cardiovascular function during exercise and LV chamber compliance in a mixed group of men and women (Bhella et al., 2014; Carrick-Ranson et al., 2014). The recruitment process for both untrained and trained women have been described in previous reports (Arbab-Zadeh et al., 2004; Fujimoto et al., 2012; Fujimoto et al., 2013; Bhella et al., 2014).
All participants were rigorously screened for comorbidities and were excluded for any of the following: obesity (BMI ≤ 30 kg/m2), taking cardiovascular medications, chronic lung disease, regular cigarette smoking within the previous 10 years, untreated thyroid disorders, diabetes mellitus, systemic arterial hypertension (24-hour blood pressure >140/90 mmHg), ECG changes suggestive of ischemic coronary artery disease or left bundle-branch block, atrial flutter/fibrillation, atrioventricular block greater than first degree, or structural heart disease by an exercise stress test and echocardiogram.
Assessment of 24-hour blood pressures
24-hour blood pressures were measured with a clinical ambulatory blood pressure monitor that uses a microphone over the brachial artery to detect Korotkoff sounds gated to the ECG to minimize noise (Accutracker II or Oscar 2, Suntech Medical Instruments, Morrisville, NC). These devices have been validated according to the international protocols for the validation of blood pressures measuring devices (Taylor et al., 1993; Jones et al., 2004). For 24-hour monitoring, each participant chose their bedtime and wake time, and measurements were made every 30 minutes during the scheduled wake period and every 60 minutes during the scheduled sleep period. Actual bedtime and wake times were confirmed prior to analysis.
Assessment of Exercise LV Function
Oxygen uptake (V̇O2), hemodynamics, and blood pressures were assessed at the following standardized treadmill conditions as previously reported (Carrick-Ranson et al., 2014; Carrick-Ranson et al., 2016): 1) quiet rest, 2) low-intensity (~30-45% of V̇O2max) steady-state submaximal exercise, 3) moderate-intensity (~60-75% of V̇O2max) steady-state submaximal exercise, and 4) maximal exercise. Two older trained participants were tested on an upright cycle because of orthopedic concerns. Gas fractions were analyzed by mass spectrometry and ventilatory volumes by a Tissot spirometer, as previously reported (Arbab-Zadeh et al., 2004). V̇O2max was defined as the highest V̇O2 at exhaustive exercise measured from at least a 30-second Douglas bag. Q̇ was measured during exercise with the acetylene rebreathing method (Triebwasser et al., 1977), which has been validated previously in this laboratory (Jarvis et al., 2007). Heart rate was measured via a 12-lead ECG, and stroke volume was calculated as Q̇/heart rate, while systemic arteriovenous oxygen content difference (a-vDO2) was calculated from the Fick equation [a-vDO2 = V̇O2/Q̇]. As body size and composition influences the size of cardiovascular variables (Carrick-Ranson et al., 2012), stroke volume was scaled relative to body surface area (BSA) (ml.m2) and fat-free mass (FFM; SV-FFM). We have previously reported typical error of measurement expressed as a coefficient of variation (%) for test-retest reproducibility in our laboratory for V̇O2max and maximal Q̇, stroke volume and heart rate are 4.4%, 11.1%, 11.9% and 3.5% respectively (Carrick-Ranson et al., 2014). Maximal cardiac power (CPO), a global index of ventricular pump function, was calculated using the formula: CPO = Q̇ x mean arterial pressure (MAP) x K, in which K is the conversion factor into watts (Cooke et al., 1998).
Resting and exercise blood pressures were measured on the left arm by ECG gated electrosphygmomanometry (Tango, SunTech Medical, NC, United States of America). Pre-exercise standing resting BP was collected with the participant’s left arm hanging relaxed. During exercise, the arm was allowed to swing freely during measurement. All participants were instructed not to hang onto and/or tightly grip the treadmill handrail during the measurement of exercise blood pressure.
Systemic vascular resistance (SVR) was calculated as: MAP/Q̇ × 80, where 80 is a conversion factor to dyn·s·cm−5 (Hagberg et al., 1983). Effective arterial elastance (Ea), an index of total arterial load on the LV, was estimated as ESP/stroke volume, in which ESP represents end-systolic blood pressure, by multiplying brachial systolic blood pressure by 0.9 (Chen et al., 2001). Ea was scaled relative to BSA, as previously reported (Redfield et al., 2005), while systemic arterial compliance (SAC) was estimated by SI/pulse pressure (Chemla et al., 1998).
Invasive Assessment of LV Function and Compliance
The assessment of LV chamber compliance in these participants has been previously described (Arbab-Zadeh et al., 2004; Fujimoto et al., 2012; Fujimoto et al., 2013; Bhella et al., 2014). Briefly, a 6Fr balloon-tipped fluid-filled catheter (Edwards Lifesciences, California) was placed using fluoroscopic guidance through an antecubital vein into the pulmonary artery. The catheter was connected to a pressure transducer with the zero-reference point set 5.0 cm below the sternal angle. Resting supine measures (baseline 1) were collected, and then LV filling was decreased using lower body negative pressure (LBNP) of −15 mmHg and −30 mmHg. Mean pulmonary capillary wedge pressure (PCWP) and LV end-diastolic volume (LVEDV) were collected after 5 minutes of each level of LBNP. After release of LBNP and confirmed return to hemodynamic baseline (baseline 2), LV filling was then increased through a rapid infusion of warm (37°C) isotonic saline solution at 200 ml per minute to achieve total volume infusion of 10-15 ml/kg and 20-30 ml/kg.
Echocardiographic images were digitally acquired using an iE33 (Philips, Netherlands) and ATL HDI5000 (Advanced Technology Laboratories, Bothell, Washington) and were measured offline in Xcelera cardiovascular image management system (Philips, Netherlands). LVEDV was determined using a modified Simpson’s method (Arbab-Zadeh et al., 2004) and scaled relative to BSA (LVEDVI). As LV volumes by echocardiography have been reported to underestimate those by MRI (Jenkins et al., 2007), a correction factor was determined as the ratio of LVEDV by echocardiography at baseline 1 to that by cardiac MRI (described below) for each participant. For those participants in which an MRI assessment was not performed, a mean group correction factor was used. This individual correction factor was used to correct LV volumes by echocardiography during loading and unloading conditions to limit errors due to foreshortening or suboptimal echo images.
Frank-Starling (stroke volume/PCWP) and the preload-recruitable stroke work (PRSW = [stroke volume × MAP]/LVEDV) relationships were constructed for the assessment of global systolic LV function. For LV diastolic function assessment, the pressure-volume (PCWP/LVEDVI) relationship was constructed. For the present study, we characterized two different but related properties of the heart during diastole: 1) static stiffness or overall chamber stiffness (or its inverse compliance) referred to as the stiffness constant, S, of the exponential equation describing the pressure-volume curve (see below); and 2) dynamic compliance is defined as the instantaneous change in LV filling pressure relative to the change in LVEDVI (∆mmHg/∆ml/m2). To characterize the LV pressure-volume relationship, we modelled each individual in the present experiment according to the equation (Mirsky, 1984): P = P∞ (expa(V−V0)− 1), where P is PCWP, P∞ is pressure asymptote of the curve, V is LVEDV index and V0 is equilibrium volume or the volume at which P= 0 mmHg pressure as previously cited in our laboratory (Fujimoto et al., 2012; Fujimoto et al., 2013; Bhella et al., 2014). The averages of the individual LV stiffness constants for all participants within each group are reported. In addition, myocardial pressure-volume curves were also calculated using the difference between PCWP and right atrial (RA) pressure (PCWP - RA) as an index of transmural filling pressure (Belenkie et al., 2002) to assess the contribution of pericardial constraint.
Assessment of LV Mass
Cardiac MRI was performed on a 1.5-T Philips NT MRI scanner. Short-axis, gradient-echo, cine MRI sequences were obtained to calculate LV mass (LVM), which was computed as the difference between epicardial and endocardial areas multiplied by the density of heart muscle, 1.05 g/ml (Arbab-Zadeh et al., 2004). For LV volume determination, the endocardial border of each slice was identified manually at end-diastole and end-systole respectively. LV volumes were calculated by use of the Simpson rule technique as previously described (Peshock et al., 1996). LV mass and LVEDV were scaled to BSA and FFM as previously reported (Whalley et al., 2004; Bhella et al., 2014) and the LV mass-to-volume ratio was used as a global indication of LV remodeling. Cardiac MRI was not performed in two middle-aged untrained and an older trained woman due to claustrophobia.
Assessment of Body Composition
Body density and composition were determined by underwater weighing with correction for residual lung volume (Wilmore & Behnke, 1969). Each participant performed at least three adequate measurements defined as a definite plateau in underwater weight, and the mean value was calculated. In a small number of participants (middle-age untrained women (n=1) and trained older women (n=2)) who could not tolerate being underwater, a seven-site skinfold measurement was used to determine body fat percentage.
Assessment of Blood Volume and Plasma Volume
Total blood volume (BV) was determined using a modified carbon monoxide rebreathing method (Burge & Skinner, 1995) as previously described (Carrick-Ranson et al., 2014). We have previously reported a typical error of measurement expressed as a coefficient of variation (%) for test-retest reproducibility for hemoglobin mass is ~3% for repeated measures (Gore et al., 2006). BV was not assessed in six trained older women (all master athletes) as this method was not routinely performed at the time of study recruitment. BV and plasma volume were scaled to total body mass and FFM as previously described by our laboratory and others (Davy & Seals, 1994; Stevenson et al., 1994; Carrick-Ranson et al., 2012).
Statistical Analysis
A 1-way analysis of variance (ANOVA) was used to determine group differences in participant characteristics, LV structure and function and BV variables and for parametric data Bonferroni’s t-test post hoc testing was used when the ANOVA resulted in a p <0.05 result. Non-parametric data were analyzed via Kruskal-Wallis ANOVA on ranks, with Dunn’s post hoc testing. Repeated-measures (RM) ANOVAs were used to determine group differences in exercise metabolic and hemodynamics variables. When the main group effect achieved p < 0.05, Bonferroni post hoc tests were performed to examine group differences at the different experimental conditions. All statistical analyses were performed using SigmaStat (Systat Software) and p <0.05 was considered statistically significant. Data are presented as means ± SD or median (25% - 75%) in Tables 1–5 and means ± SD in Figs 1-4.
Table 1.
Participants characteristics
| Middle-age untrained | Older untrained | Older trained | ANOVA p value | |
|---|---|---|---|---|
| Number of participants | 22 | 35 | 13 | |
| Age, years | 47.0 (41.8 - 54.4) | 66.0 (64.0 - 68.9)* | 67.0 (65.1 - 68.8)* | <0.001 |
| Height, cm | 164.1 ± 7.1 | 163.5 ± 5.0 | 161.5 ± 5.7 | 0.448 |
| Weight, kg | 64.4 (56.5 - 69.7) | 66.7 (59.2 - 76.7) | 54.1 (49.7 - 58.9)*† | 0.001 |
| BSA, m2 | 1.71 ± 0.2 | 1.74 ± 0.1 | 1.58 ± 0.1*† | 0.006 |
| Body fat‡, % | 33.4 (28.7 - 40.5) (21) | 39.9 (34.3 - 42.1) | 27.7 (15.5 - 35.7)† (12) | 0.001 |
| Fat free mass, kg | 41.6 ± 6.0 (21) | 41.3 ± 5.0 | 40.0 ± 6.2 (12) | 0.717 |
| 24-hour SBP, mmHg | 117.7 ± 9.5 | 123.7 ± 8.8 | 118.3 ± 12.5 (12) | 0.057 |
| 24-hour DBP, mmHg | 69.5 ± 5.6 | 69.2 ± 5.5 | 70.9 ± 6.9 (12) | 0.673 |
| Awake SBP, mmHg | 121.5 (114.0 - 129.5) | 127.0 (122.0 - 135.0) | 121.5 (110.8 - 134.0) (12) | 0.095 |
| Awake DBP, mmHg | 72.7 ± 6.1 | 72.3 ± 5.6 | 73.2 ± 7.5 (12) | 0.895 |
| Sleep SBP, mmHg | 105.0 ± 10.9 | 111.8 ± 11.9 | 105.5 ± 11.8 (12) | 0.066 |
| Sleep DBP, mmHg | 59.4 ± 8.5 | 59.5 ± 6.8 | 60.3 ± 6.5 (12) | 0.945 |
Parametric and nonparametric sampling distributions are shown as mean ± SD or median (25% - 75%) respectively. Parametric data was analysed by 1-way ANOVA with post hoc analysis (Bonferroni t-tests method) and nonparametric data by Kruskal–Wallis ANOVA on Ranks with post hoc analysis (Dunn’s method). The single number in parentheses represents the number of observations if different from the stated n.
BSA, body surface area; 24-hour SBP, 24-hour systolic blood pressure; 24-hour DBP, 24-hour diastolic blood pressure
p <0.05 older trained versus middle-age untrained
p <0.05 older trained versus older untrained
includes skin fold measurements for middle-age untrained n=1 and older trained n=2
Table 5.
Blood volumes and LV mass and volumes
| Middle-age untrained | Older untrained | Older trained | ANOVA p value | |
|---|---|---|---|---|
| Absolute and scaled BV and plasma volume | ||||
| BV, ml | 4044.1 ± 592.0 | 4053.3 ± 475.9 | 4018.0 ± 417.0 (7) | 0.986 |
| BV, ml.kg−1 | 62.3 (56.5 - 69.5) | 60.8 (58.3 - 63.0) | 67.2 (64.5 - 75.6)† (7) | 0.018 |
| BV, ml.kgFFM−1 | 98.5 ± 9.4 (21) | 98.5 ± 8.2 | 109.0 ± 10.2*† (6) | 0.028 |
| PV, ml | 2712.9 ± 394.8 | 2664.6 ± 318.6 | 2662.6 ± 325.3 (7) | 0.868 |
| PV, ml.kg−1 | 43.2 (37.5 - 46.1) | 40.0 (37.5 - 41.4) | 44.9 (40.8 - 49.2)† (7) | 0.010 |
| PV, ml.kgFFM−1 | 66.1 ± 6.4 (21) | 64.8 ± 6.1 | 71.9 ± 7.0† (6) | 0.046 |
| LV mass, absolute and scaled LVEDV and mass-to-volume ratio | ||||
| LVM, g | 77.6 (69.5 - 91.6) (19) | 76.2 (69.8 - 87.8) | 78.8 (74.0 - 85.7) (12) | 0.820 |
| LVMI, g.m2 | 46.5 (41.5 - 48.6) (19) | 44.7 (41.0 - 49.6) | 51.8 (48.5 - 53.9)*† (12) | 0.002 |
| LVM, g.kgFFM−1 | 1.9 (1.7 - 2.1) (18) | 1.9 (1.8 - 2.0) | 2.0 (1.8 - 2.4) (12) | 0.338 |
| LVEDV, ml | 101.2 ± 17.7 (19) | 95.4 ± 16.7 | 98.8 ± 13.0 (12) | 0.454 |
| LVEDVI, ml.m2 | 58.8 ± 7.6 (19) | 54.7 ± 8.3 | 63.7 ± 8.0† (12) | 0.005 |
| LVEDV, ml.kgFFM−1 | 2.5 ± 0.4 (18) | 2.3 ± 0.4 | 2.5 ± 0.4 (12) | 0.218 |
| LV mass-to-volume ratio, g.ml−1 | 0.77 (0.69 - 0.92) (19) | 0.80 (0.75 - 0.89) | 0.78 (0.75 - 0.92) (12) | 0.637 |
Parametric and nonparametric sampling distributions are shown as mean ± SD or median (25% - 75%) respectively. Parametric data was analysed by 1-way ANOVA with post hoc analysis (Bonferroni t-tests method) and nonparametric data by Kruskal–Wallis ANOVA on Ranks with post hoc analysis (Dunn’s method). The single number in parentheses represents the number of observations if different from the stated n in Table 1.
BV, blood volume; PV, plasma volume; LVM, left ventricular mass; LVMI, left ventricular mass index; LVEDV, left ventricular end-diastolic volume; LVEDVI, left ventricular end-diastolic volume index.
p <0.05 older trained versus middle-age untrained
p <0.05 older trained versus older untrained
Figure 1.
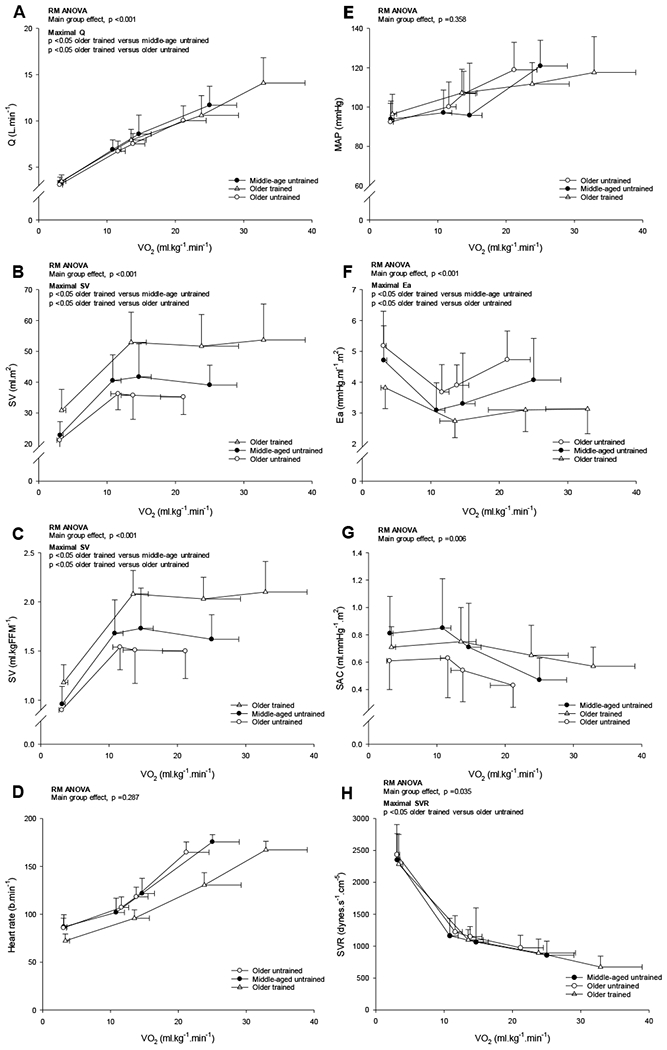
Q̇ (A), stroke volume relative to BSA (B), stroke volume relative to FFM (C), heart rate (D), mean arterial pressure (MAP; E), Ea (F), SAC (G) and SVR (H) as a function of V̇O2 relative to total body mass. Values are means ± SD with only statistically significant results at maximal exercise are indicated. Please refer to text for further details. P-values are derived from repeated measures ANOVA with post hoc analysis (Bonferroni t-tests method).
Number of observations for SV relative to FFM (MA n=21; OU n=35; OT n=12) and for MAP, Ea, SAC and SVR (MA n=21; OU n=35; OT n=13).
RESULTS
Participants Characteristics and Maximal Exercise Capacity
Older trained women were lighter, had a smaller BSA, and were leaner, but had similar FFM. 24-hour systolic blood pressure was higher in the older untrained women, though this result was variable with an ANOVA p value of 0.057 while diastolic blood pressure was not different among groups (ANOVA p =0.673) (Table 1).
Resting and Exercise Metabolic and Hemodynamics Variables
Rest:
V̇O2 and Q̇ in absolute levels were not significantly different among groups (ANOVA p ≥0.169); however, the older trained women had a lower heart rate and a larger absolute and scaled stroke volume (versus middle-aged and older untrained all p ≤ 0.050; ANOVA p ≤ 0.006). CPO was not significantly different among groups (ANOVA p =0.111). Ea was lower in older trained compared to age-similar untrained women (p <0.001), while SAC was higher with sedentary aging (older untrained versus middle-age untrained p =0.008; ANOVA p ≤ 0.006). MAP and SVR were unaltered with aging or lifelong endurance exercise (ANOVA p ≥ 0.406) (Table 2).
Table 2.
Resting upright metabolic and hemodynamic variables
| Middle-age untrained | Older untrained | Older trained | ANOVA p value | |
|---|---|---|---|---|
| V̇O2, ml.kg−1.min−1 | 3.1 (2.8 - 3.5) (21) | 3.0 (2.9 - 3.3) | 3.3 (2.8 - 3.7) | 0.420 |
| V̇O2, ml.kgFFM−1.min−1 | 4.9 (4.5 - 5.0) (20) | 4.8 (4.5 - 5.4) | 4.6 (4.0 - 5.5) (12) | 0.663 |
| Q̇, L.min−1 | 3.3 (2.9 - 3.7) | 3.1 (2.8 - 3.4) | 3.4 (3.0 - 3.8) | 0.169 |
| QI, L.m2.min−1 | 1.90 (1.68 - 2.13) | 1.80 (1.60 - 1.95) | 2.20 (1.95 - 2.45)† | <0.001 |
| Q̇, ml.kgFFM−1.min−2 | 84.3 (67.4 - 88.7) (21) | 76.0 (70.0 - 83.0) | 86.5 (77.3 - 91.8) (12) | 0.048 |
| CPO, watts | 0.68 (0.59 - 0.74) (20) | 0.60 (0.50 - 0.70) | 0.70 (0.60 - 0.80)† | 0.111 |
| Heart rate, b.min−1 | 86.8 ± 12.7 | 85.8 ± 10.1 | 72.2 ± 7.1*† | <0.001 |
| SV, ml | 37.8 (33.6 - 43.3) | 36.2 (31.6 - 41.8) | 46.1 (39.6 - 59.9)*† | 0.006 |
| SV, ml.m2 | 22.0 (19.9 - 25.0) | 21.0 (18.4 - 24.2) | 30.1 (25.7 - 38.2)*† | <0.001 |
| SV, ml.kgFFM−1 | 0.96 ± 0.18 | 0.90 ± 0.18 | 1.18 ± 0.18*† (12) | <0.001 |
| MAP, mmHg | 89.8 (86.4 - 99.7) (20) | 89.0 (84.3 - 101.0) | 93.7 (90.6 - 99.7) | 0.406 |
| Ea, mmHg.ml−1.m2 | 4.7 ± 1.1 (20) | 5.2 ± 1.1 | 3.8 ± 0.7† | <0.001 |
| SAC, ml.mmHg−1.m2 | 0.8 (0.6 - 1.0) (20) | 0.6 (0.4 - 0.7)* | 0.7 (0.6 - 0.9) | 0.006 |
| SVR, dynes.s−1.cm−5 | 2243.2 (1964.7 - 2769.6) (20) | 2353.4 (2054.6 - 2774.0) | 2161.5 (2049.6 - 2592.4) | 0.605 |
| a-vDO2, ml.100 ml−1 | 6.1 ± 1.0 | 6.7 ± 1.2 | 5.5 ± 0.9† | 0.003 |
Parametric and nonparametric sampling distributions are shown as mean ± SD or median (25% - 75%) respectively. Parametric data was analysed by 1-way ANOVA with post hoc analysis (Bonferroni t-tests method) and nonparametric data by Kruskal–Wallis ANOVA on Ranks with post hoc analysis (Dunn’s method). The single number in parentheses represents the number of observations if different from the stated n in Table 1.
V̇O2, oxygen uptake; Q̇, cardiac output; CPO, cardiac power; SV, stroke volume; MAP, mean arterial pressure; Ea, effective arterial elastance; SAC, systemic arterial compliance; SVR, systemic vascular resistance; a-vDO2, arterial-venous oxygen content difference
p <0.05 older trained versus middle-age untrained
p <0.05 older trained versus older untrained
Exercise:
V̇O2max relative to total body mass and FFM was higher in older trained women compared to the untrained older (~58% and ~34% respectively, p <0.001) and middle-age women (~31% and ~19% respectively, p ≤0.015) (ANOVA p <0.001; Table 3). The mean group ∆Q̇ /∆V̇O2 relation during incremental exercise was basically superimposable among groups (Fig. 1A; Middle-aged untrained 5.8±1.0 L.L−1; Older untrained 5.7±0.9 L.L−1; Older trained 6.3±0.9 L.L−1; ANOVA p =0.235).
Table 3.
Maximal exercise variables
| Middle-age untrained | Older untrained | Older trained | ANOVA p value | |
|---|---|---|---|---|
| V̇O2max, L.min−1 | 1.6 ± 0.3 | 1.4 ± 0.2* | 1.8 ± 0.2† | <0.001 |
| V̇O2max, ml.kg−1.min−1 | 25.9 (21.6 - 27.8) | 21.5 (18.5 - 24.3)* | 34.0 (29.2 - 38.9)*† | <0.001 |
| V̇O2max, ml.kgFFM−1.min−1 | 39.1 ± 4.7 (21) | 34.6 ± 5.4* | 46.4 ± 6.5*† (12) | <0.001 |
| Q̇I, L.m−2.min−1 | 6.8 ± 1.0 | 5.8 ± 0.8* | 8.9 ± 1.8*† | |
| Q̇, ml.kgFFM−1.min−2 | 284.1 ± 38.1 (21) | 245.1 ± 39.6* | 349.8 ± 43.2*† (12) | |
| CPO, watts | 3.1 ± 0.5 (21) | 2.6 ± 0.5* | 3.7 ± 0.9*† (12) | |
| Systemic a-vDO2, ml.100 ml | 13.8 ± 1.8 | 14.3 ± 2.0 | 13.2 ± 2.1 |
Parametric and nonparametric sampling distributions are shown as mean ± SD or median (25% - 75%) respectively. Parametric data was analysed by 1-way ANOVA with post hoc analysis (Bonferroni t-tests method) and nonparametric data by Kruskal–Wallis ANOVA on Ranks with post hoc analysis (Dunn’s method). Q̇I, Q̇, CPO, and systemic a-vDO2 results are from repeated measures ANOVA’s with post hoc analysis (Bonferroni t-tests method). The single number in parentheses represents the number of observations if different from the stated n in Table 1.
V̇O2max, maximal oxygen uptake; Q̇ cardiac output; Q̇I, cardiac index; CPO, cardiac power; systemic a-vDO2, systemic arteriovenous oxygen content difference
p <0.05 older trained versus middle-age untrained
p <0.05 older trained versus older untrained
At maximal exercise, Q̇ was larger in the older trained compared to older (~40%, p <0.001) and middle-aged (~17%, p <0.001) untrained women (RM ANOVA group main effect p <0.001; Fig, 1A). Likewise, CPO at maximal exercise was significantly higher in trained older women compared to the untrained groups (p <0.001 versus middle-aged and older untrained; RM ANOVA group main effect p <0.001). Irrespective of unit, stroke volume was shifted upward during all exercise conditions in the trained older women compared to the middle-aged and older untrained women (RM ANOVA group main effect p <0.001; Figs. 1B–C for stroke volume relative to BSA and FFM). Heart rate was shifted rightward and down, reflecting a lower heart rate for any V̇O2 in the older trained women but the overall heart rate response was not different among the groups (RM ANOVA group main effect p =0.287; Fig. 1D).
Ea was shifted downward during submaximal exercise and was significantly lower at maximal exercise in the older trained women compared to both middle-aged and older untrained women (27% and 34% respectively p ≤ 0.012; RM ANOVA group main effect p <0.001; Fig. 1F). There were no significant differences in MAP across the experimental conditions, due in part to the variable exercise responses between groups (RM ANOVA group main effect p =0.358; Fig. 1E). SVR was ~31% lower (p =0.023) in trained versus untrained older women at maximal exercise (RM ANOVA group main effect p =0.035; Fig. 1H). Likewise, SAC was higher at rest and exercise in trained older women compared to their age-similar untrained peers (RM ANOVA group main effect p =0.006); however, due to a variable response this effect did not achieve statistical significance at maximal exercise (p =0.450; Fig. 1G).
Systemic a-vDO2 was significantly higher (p ≤ 0.004 versus middle-age untrained and older trained) at low-intensity, steady-state exercise in the older untrained women but there were no group differences at moderate-intensity, steady-state or maximal exercise (all p ≥ 0.109; RM ANOVA group main effect p =0.011; Table 3 for maximal exercise values).
Invasive Assessment of LV Function and Cardiac Compliance
Group-averaged Frank-Starling relations were not different among groups (ANOVA, p =0.228; Fig. 2A); however, the preload recruitable stroke work relation was less steep in older trained women compared to middle-aged and older untrained women (ANOVA p <0.001; slope of the preload recruitable stroke work relation (ml x mmHg.ml.m2); median (25 - 75%) middle-aged untrained: 149.0 (132.0 - 220.0); older untrained 177.0 (144.0 - 265.0); older trained; 117.0 (98.0 - 139.5); Fig. 2B; p ≤ 0.039 between groups).
Figure 2.
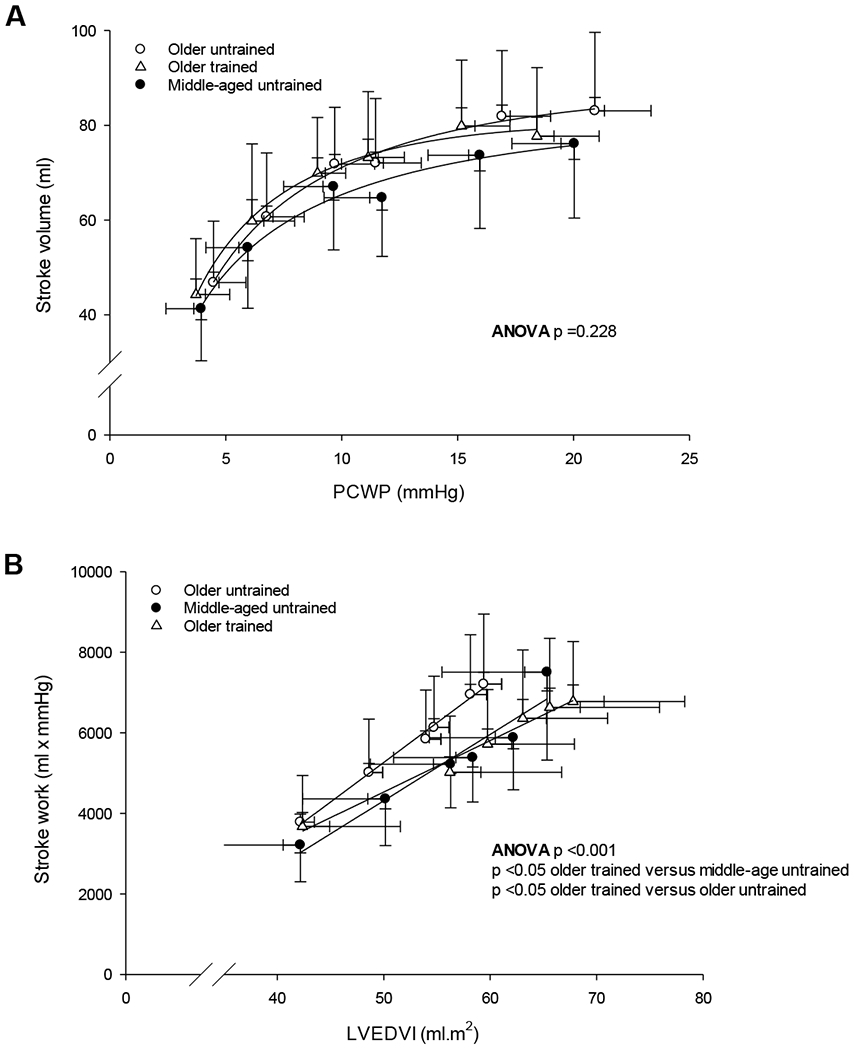
Group-averaged Frank-Starling (A) and preload recruitable stroke work relationship (B). P-values are derived from Kruskal–Wallis ANOVA on Ranks with post hoc analysis (Dunn’s method).
The LV pressure-volume relationship was steeper and shifted upward and left in older untrained compared to middle-age untrained women, indicating a stiffer (less compliant/flexible) ventricle with sedentary aging (p =0.018; Table 4). In contrast, this relationship was less steep and shifted rightward in older trained women, resulting in a significantly greater ventricular chamber compliance compared to older untrained women (p <0.001), while it was likely greater in older trained women compared to middle-aged untrained women (p =0.053; Cohen’s d = 0.899) (Fig. 3A, median (25 - 75%): middle-age untrained: 0.065 (0.049 - 0.080); older untrained: 0.085 (0.061 - 0.138); older trained: 0.047 (0.031 - 0.054)). Dynamic LV compliance during increased LV preload via saline infusion was lower with sedentary aging (middle-aged untrained versus older untrained, p <0.001) but was preserved in older trained women (p =1.000 versus middle-age untrained, ANOVA p <0.001; Table 4).
Table 4.
LV function stiffness during cardiac loading and unloading
| Middle-age untrained | Older untrained | Older trained | ANOVA p value | |
|---|---|---|---|---|
| Individual static LV chamber stiffness constant | 0.065 (0.049 - 0.080) | 0.085 (0.061 - 0.138) | 0.047 (0.031 - 0.054)†# | <0.001 |
| Dynamic operating stiffness during LV unloading, ∆mmHg/∆ml/m2 | 0.47 (0.38 - 0.55) | 0.58 (0.36 - 0.81) | 0.41 (0.30 - 0.44)† | 0.016 |
| Dynamic operating stiffness during LV loading, ∆mmHg/∆ml/m2 | 1.10 (0.78 - 1.55) (20) | 2.28 (1.20 - 3.83) (34)* | 1.08 (0.95 - 1.62)† (12) | <0.001 |
Nonparametric sampling distributions are shown as median (25% - 75%). Data was analysed by Kruskal–Wallis ANOVA on Ranks with post hoc analysis (Dunn’s method). The single number in parentheses represents the number of observations if different from the stated n in Table 1.
LV, left ventricular
p <0.05 older trained versus middle-age untrained
p <0.05 older trained versus older untrained
p =0.053 older trained versus middle-age untrained
Figure 3.
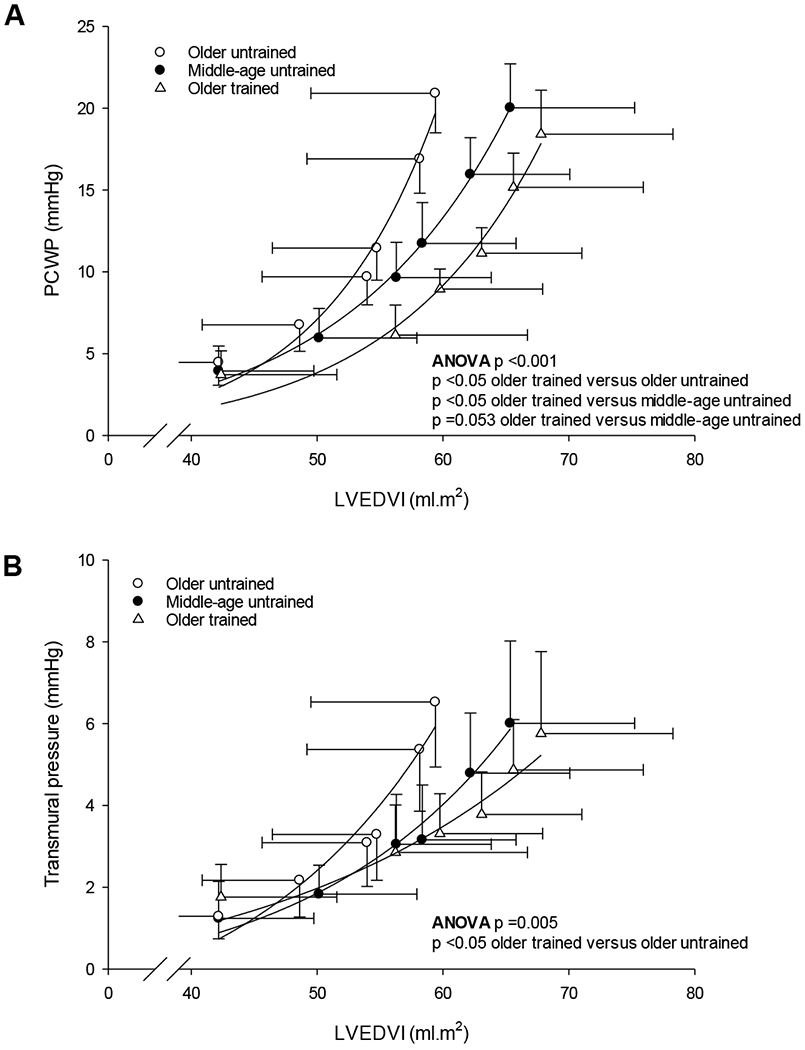
Group-averaged LV pressure-volume relationship (A) and LV transmural pressure-volume relationship (B). P-values are derived from Kruskal–Wallis ANOVA on Ranks with post hoc analysis (Dunn’s method). Note the different scale used for Fig. 3A and B.
When examined by the LV transmural pressure-volume relationship, which is more representative of myocardial compliance without the effects of external constraint, the effect of sedentary aging was less convincing (p =0.106) but remained significantly enhanced in the trained versus untrained older women (p =0.007; ANOVA p =0.005; Fig. 3B).
Blood Volume and MRI measurements
Absolute levels of BV and plasma volume were not different among groups (ANOVA p ≥ 0.868 for both); however, these variables were significantly larger in trained women compared to their age-similar untrained peers when scaled relative to total body mass and FFM (all p ≤ 0.041, ANOVA p ≤ 0.046; Table 5).
Irrespective of age, LVM index (g.m2) was significantly larger in trained women (p ≤ 0.020 versus both untrained groups, ANOVA p =0.002); however, this difference was eliminated when examined in absolute levels or scaled relative to FFM (ANOVA p > 0.338). LVEDVI was significantly different between trained and untrained older women (p =0.004, ANOVA p =0.005), while the LV mass-to-volume ratio was not significantly different among groups (ANOVA p =0.637; Table 5).
DISCUSSION
There are two novel findings from this study. First, vigorous endurance exercise four or more times per week for at least 25 years is associated with a larger maximal exercise capacity, exercise stroke volume, LV size and BV, and greater ventricular chamber compliance compared to age-similar untrained women, and in most instances, compared to untrained women that were on average ~20 years younger. Second, there were also notable favorable effects of lifelong exercise on arterial function (Ea, SAC) and heart rate during exercise. Collectively, these findings highlight lifelong endurance exercise training as an effective strategy for preserving or even enhancing cardiovascular structure and function with aging in women.
Effect of Prolonged or Lifelong Endurance Exercise on V̇O2max in Women
Several investigators have previously reported the beneficial effect of lifelong or sustained endurance exercise training on V̇O2max in middle-aged and older women compared to age-similar controls (Ogawa et al., 1992; Stevenson et al., 1994; Jones et al., 1997; Parker Jones et al., 1999; McCole et al., 2000; Dogra et al., 2012). In this current study, V̇O2max scaled relative to total body mass or FFM in the older trained women was substantially larger compared to untrained older and middle-aged women, reinforcing the powerful effect of being physically active over several decades on reducing the unfavorable effects of aging on cardiovascular structure and function and overall physical function.
Effect of Prolonged or Lifelong Exercise on Hemodynamics and Systemic a-vDO2 in Women
There was a remarkably consistent V̇O2-Q̇ relationship during exercise in the current younger and older cohorts. Similar observations have been reported in endurance trained young and older male and female athletes (Proctor et al., 1998) and sedentary and endurance trained young men and women (Fu & Levine, 2005). These findings suggest that the complex integration of cardiac and non-cardiac regulatory mechanisms that impact central hemodynamics during exercise are remarkably well preserved even after several decades of biological aging irrespective of training status.
The current findings support previous investigations (Ogawa et al., 1992; McCole et al., 2000; Dogra et al., 2012) demonstrating a substantially larger sub-maximal and maximal exercise stroke volume in endurance trained older women compared to age-similar controls. A larger LVEDV and thus greater recruitment of the Frank-Starling mechanism is recognized as the primary factor that facilitates the larger exercise stroke volume in highly trained older men (Fleg et al., 1994; Seals et al., 1994; Schulman et al., 1996; Hagberg et al., 1998). Unfortunately, we did not assess ventricular volumes or dynamic diastolic filling during exercise in these cohorts; however, we speculate that a larger more compliant ventricle coupled with an expanded blood volume observed in the older trained women is a key mechanism to enhance ventricular filling during exercise (Hagberg et al., 1998).
Enhanced ventricular-arterial coupling with increased preload (Shibata et al., 2008; Hieda et al., 2018) and potentially more vigorous ventricular suction with exercise (Popovic et al., 2006) could also contribute to a larger stroke volume response in endurance trained older women. Ea, an index of total arterial load on the LV, was lower at rest and during exercise in the trained older women compared to middle-aged and older untrained women. An observation of a lower Ea during exercise in healthy older adults has been previously reported with lifelong exercise (Carrick-Ranson et al., 2014) and in response to a 12-month endurance training program in previously untrained older men and women (Shibata & Levine, 2012b; Carrick-Ranson et al., 2016). From a physiological and clinical perspective, these findings are important as Ea during exercise is adversely altered with healthy aging (Najjar et al., 2004) and is abnormal in women with cardiovascular disease (systolic arterial hypertension) (Chantler et al., 2008; Park et al., 2008).
Based on the current findings, the lower Ea in middle-aged and older trained women likely reflects primarily a lower SVR and to a smaller degree a higher SAC during submaximal and maximal exercise. Several reports from our laboratory have reported that invasive and non-invasive indices of ventricular-arterial coupling (v-a coupling) during physiologic stress are greater with long-term training in older adults (Shibata et al., 2008; Shibata & Levine, 2012a; Hieda et al., 2018; Shibata et al., 2018). However, Shibata & Levine (Shibata & Levine, 2012b) previously demonstrated that training-related changes in Ea and SAC in previously untrained older men and women were largely reflective of a larger stroke volume, as Ea and SAC were restored to pre-training levels when stroke volume was reduced by lower body negative pressure; highlighting the critical role of stroke volume on training-related changes in these indices of effective elastance and LV afterload.
A larger stroke volume during exercise with lifelong or prolonged training is an important observation (Ogawa et al., 1992; McCole et al., 1999, 2000; Dogra et al., 2012), as stroke volume is reported to be only modestly increased with relatively short- (12 weeks) (Murias et al., 2010) and long-term (9-12 months) (Spina et al., 1993) endurance exercise training in previously sedentary older women. These findings are supported by reports of unaltered LV systolic and diastolic function during various physiological provocations after several months of exercise training in older women (Spina et al., 1996; Spina et al., 2000). Collectively, these findings reinforce the importance of engaging in committed levels of endurance exercise throughout adulthood on cardiovascular function and exercise performance during latter life in women (Bhella et al., 2014; Carrick-Ranson et al., 2014; Hieda et al., 2018; Shibata et al., 2018).
Systemic a-vDO2 increased during exercise in all groups and was unaffected by training status at maximal exercise in the current cohorts. This finding is consistent with some (McCole et al., 2000; Dogra et al., 2012) but not all previous studies (Ogawa et al., 1992) in endurance trained and untrained women, which may reflect measurement techniques or potentially the age or previous training history of the trained participants. The importance of systemic a-vDO2 to exercise performance is particularly pertinent in older women, as this characteristic may represent the principal mechanism for an increased V̇O2max after several months of exercise training in this population (Spina et al., 1993; Murias et al., 2010). Improved redistribution of blood flow to contracting skeletal muscle, increased skeletal muscle capillarization, and proliferation of mitochondrial size and number and well as aerobic metabolism enzymes may contribute to a higher systemic and regional oxygen extraction and utilization after exercise training in older women; however, to date, these factors have rarely been examined with relatively short- or long-term training in women (Coggan et al., 1992).
Effects of Prolonged or Lifelong Exercise on LV Morphology in Women
While the “athletes heart syndrome” has been reported in young male (Scharhag et al., 2002; Utomi et al., 2013) and female endurance athletes (Riley-Hagan et al., 1992), and male endurance Masters athletes (Heath et al., 1981; Seals et al., 1994; Bohm et al., 2016), there is only very limited information on the effect of lifelong endurance exercise on cardiac morphology in Master women athletes. Hagmar and colleagues (Hagmar et al., 2005) reported a larger LV chamber size and LVEDV in former elite endurance athletes who were now recreational endurance athletes compared to controls; however, neither absolute LVM nor LVMI were significantly different between athletes and controls. A extremely large LVMI (mean: 165.1 g/m2) was reported in post-menopausal marathon runners (mean age ~55 years, range 45-69 years); however, no control group was examined, making it difficult to interpret these findings (Knebel et al., 2014).
In this current study, we found a ~16% larger LVM when scaled relative to BSA. Likewise, LVEDVI was larger in trained older women while the LV mass-to-volume ratio was not significantly different between groups. These findings suggest that eccentric and balanced physiologic hypertrophy is the primary mechanism of adaptation to lifelong endurance exercise in women.
Effect of Prolonged or Lifelong Exercise on Invasive Measures of LV Performance and Compliance in Women
We have previously shown that sedentary but otherwise healthy aging is associated with a reduction in LV chamber compliance and distensibility (Arbab-Zadeh et al., 2004; Fujimoto et al., 2012); however, this stiffening was prevented in male and female Masters athletes (Arbab-Zadeh et al., 2004). An important observation of the present study is that habitual and vigorous endurance exercise over several decades enhances LV compliance and distensibility in older women to a degree that is similar or even surpasses that observed in women with a mean age that is ~20 years younger. The enhanced cardiac compliance observed in lifelong exercising women compared to middle-aged sedentary individuals reinforces the powerful effect of lifelong exercise on cardiac compliance with senescence (Arbab-Zadeh et al., 2004; Bhella et al., 2014).
In contrast to the marked effect of lifelong exercise, 1-year of dynamic exercise in previously sedentary older (>60 years) men and women did not improve cardiac compliance (Fujimoto et al., 2010) even when combined with an advanced glycation end-product cross-link breaker (Alagebrium) (Fujimoto et al., 2013). However, a recent investigation showed that sustained (2 years) endurance exercise significantly improved LV chamber compliance in middle-aged adults (Howden et al., 2018). Collectively, these findings suggest that exercise may need to be initiated earlier in life and performed over a longer duration to enhance or preserve cardiac compliance with aging in women as well as men.
The preservation of cardiac compliance with lifelong endurance training may be attributable to changes in the intrinsic viscoelastic properties of the myocardium, cardiac morphology or pericardial constraint. In the present study, compliance remained superior in trained compared to untrained older women after accounting for pericardial constraint suggesting other myocardial factors contribute to the enhanced cardiac compliance in these participants.
Effect of Prolonged or Lifelong Exercise on Blood Volume in Women
Previous cross-sectional studies have reported that absolute and body size and composition relative levels of BV and plasma volume are larger in recreationally active older females (Jones et al., 1997), and middle-aged and older women distance runners (Stevenson et al., 1994) and swimmers (Parker Jones et al., 1999) compared to their untrained peers. In this present study, only BV and plasma volume relative to BSA and FFM were significantly larger in the trained compared to untrained older women. This current finding likely reflects smaller absolute levels but comparable BSA and FFM relative levels of BV and plasma volume to those reported in middle-aged and older women athletes (Stevenson et al., 1994).
These findings of a larger BV in trained older women may be important given that BV does not appear to increase with relatively short-term training (3 months) in previously sedentary older women (Stachenfeld et al., 1998; Katyal et al., 2003). These observations cannot be explained by a suboptimal training stimulus as there was a relatively large increase (14 - 17%) in V̇O2max relative to total body mass post-training in these investigations. The mechanism(s) that underpin this blunted adaptation in BV in older women are not well defined. Based on findings in older men, changes in the regulation of the plasma protein albumin, which causes a fluid shift from the interstitial to intravascular fluid space, is implicated in the diminished adaptation in plasma volume after exercise training in older adults (Zappe et al., 1996; Okazaki et al., 2002). Given the tightly coupled relationship between BV expansion and exercise stroke volume with endurance exercise (Bonne et al., 2014), these findings potentially indicate that a significant period of intensive exercise may be required to elicit favorable blood volume adaptations in women particularly if exercise is initiated later in life.
Effect of Menopausal Status and Menopausal Hormone Therapy on CV Structure and Function during Exercise
Given the board chronological ages of our untrained middle-aged group, this cohort included women who were premenopausal, perimenopausal and postmenopausal. Alterations in autonomic nervous system activity, endothelial function and cardiovascular tissue stiffness with menopause could all negatively impact central and peripheral hemodynamics during exercise (Taddei et al., 1996; Staessen et al., 2001; Barnes et al., 2014). Several recent elegant studies in premenopausal and recently postmenopausal women reported no differences in V̇O2max relative to total body mass (Nyberg et al., 2017) and LV mass and ventricular dimensions (Egelund et al., 2017) prior to commencing a 12-week exercise training program. In premenopausal and postmenopausal women, Q̇ and stroke volume were not significantly different at submaximal exercise workloads (50 watts and 60% of V̇O2max) (Green et al., 2002). Unfortunately, we did not examine menopausal status comprehensively in our middle-aged women at the time of study recruitment; however, there were no significant difference in primary and secondary outcome measures (maximal Q̇ and stroke volume, LV mass and LVEDV, and LV compliance) when the middle-aged women were split into two chronological age ranges; aged 35-49 (mean age: 42.1 years) and 50-59 years (mean age: 55.4 years) (data not shown). Nevertheless, we cannot completely exclude the possibility that menopausal status did not influence our exercise and LV function and compliance results.
We are unable to comment on the potential modulating effects of menopausal hormone replacement therapy (HRT) or hormonal contraceptives on our findings, as these factors were not extensively examined in the current cohorts of women. While limited to only small cohorts of women, cross-sectional investigations have reported that short or long-term HRT did not elicit notable effects on V̇O2, Q̇, stroke volume or blood pressures during submaximal or maximal exercise in asymptomatic untrained or highly-trained women (Fleg et al., 1995; McCole et al., 1999, 2000; Kirwan et al., 2004). In response to exercise training, adaptations in submaximal exercise Q̇ and stroke volume were not significantly influenced by HRT (O’Donnell et al., 2009).
Several pieces of evidence indicate the important role of oestrogens in endothelial-mediated macrovascular function in previously sedentary postmenopausal women (Pierce et al., 2011; Moreau et al., 2013) and more recently for micro- and macrovascular function in response to habitual exercise (Santos-Parker et al., 2017). However, it is unclear whether these adaptations confer an improvement in central circulatory function during large muscle mass exercise (Kirwan et al., 2004). Future research is required to establish the role of HRT and hormonal contraceptives on CV structure and function and how this information could be used in the development of exercise-based strategies to optimize CV health in women.
Study Limitations
There are several limitations that should be acknowledged. Similar to our previous investigation in a larger cohort (Carrick-Ranson et al., 2014), LV volumes during exercise were not collected, which would have allowed for a more comprehensive description of aging and lifelong exercise-related changes in LV filling function and v-a coupling during exercise.
A relatively small sample size combined with the mixture of committed exercisers and Master athletes could be viewed as a limitation. However, comprehensive physiological investigations in trained and untrained older women are rare and therefore the current findings are novel. Nevertheless, the small sample size combined with a reduced number of observations for certain outcomes measures (BV and LVM for example) may have resulted in a type-2 error.
The lack of a younger endurance trained group could be considered a limitation; however, the primary purpose of this analysis was to examine the effects of sedentary aging and lifelong exercise on cardiovascular structure and function and thus we do not believe this addition would have significantly strengthened the current findings.
Finally, we did not examine factors such as genetics, HRT, hormonal contraceptives, menopausal status or other lifestyle factors such as diet that may influence cardiovascular structure and function in the current cohorts; therefore, we cannot exclude the possibility that the improved exercise in our trained older women may be related to factors other than lifelong exercise.
Conclusion
In summary, the current findings suggest that lifelong exercise of four or more exercise sessions weekly is associated with substantial effects on maximal exercise capacity, exercise hemodynamics and heart rate control, LV mass and compliance, and total blood volumes in women. Exercise training should be considered a key strategy to prevent cardiovascular disease with aging in women as well as men.
Supplementary Material
KEY POINTS:
The beneficial effects of sustained or lifelong (>25 years) endurance exercise on cardiovascular structure and exercise function have been largely established in men.
The current findings indicate that committed (≥ 4 weekly exercise sessions) lifelong exercise results in substantial benefits in exercise capacity (V̇O2max), cardiovascular function at submaximal and maximal exercise, left ventricular mass and compliance, and blood volume compared to similarly aged or even younger (middle-age) untrained women.
Endurance exercise training should be considered a key strategy to prevent cardiovascular disease with aging in women as well as men.
Acknowledgments
Funding support: This project was supported by the National Institutes of Health (ref#AG17479).
Footnotes
Disclosures: The authors have no conflicts to disclose.
References
- Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D & Levine BD. (2004). Effect of aging and physical activity on left ventricular compliance. Circulation 110, 1799–1805. [DOI] [PubMed] [Google Scholar]
- Astrand I, Astrand PO, Hallback I & Kilbom A. (1973). Reduction in maximal oxygen uptake with age. J Appl Physiol 35, 649–654. [DOI] [PubMed] [Google Scholar]
- Barnes JN, Hart EC, Curry TB, Nicholson WT, Eisenach JH, Wallin BG, Charkoudian N & Joyner MJ. (2014). Aging enhances autonomic support of blood pressure in women. Hypertension 63, 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenkie I, Kieser TM, Sas R, Smith ER & Tyberg JV. (2002). Evidence for left ventricular constraint during open heart surgery. Can J Cardiol 18, 951–959. [PubMed] [Google Scholar]
- Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick-Ranson G, Palmer MD, Boyd KN, Adams-Huet B & Levine BD. (2014). Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. J Am Coll Cardiol 64, 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SN, Kohl HW 3rd, Paffenbarger RS Jr., Clark DG, Cooper KH & Gibbons LW. (1989). Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262, 2395–2401. [DOI] [PubMed] [Google Scholar]
- Bohm P, Schneider G, Linneweber L, Rentzsch A, Kramer N, Abdul-Khaliq H, Kindermann W, Meyer T & Scharhag J. (2016). Right and Left Ventricular Function and Mass in Male Elite Master Athletes: A Controlled Contrast-Enhanced Cardiovascular Magnetic Resonance Study. Circulation 133, 1927–1935. [DOI] [PubMed] [Google Scholar]
- Bonne TC, Doucende G, Fluck D, Jacobs RA, Nordsborg NB, Robach P, Walther G & Lundby C. (2014). Phlebotomy eliminates the maximal cardiac output response to six weeks of exercise training. Am J Physiol Regul Integr Comp Physiol 306, R752–760. [DOI] [PubMed] [Google Scholar]
- Burge CM & Skinner SL. (1995). Determination of hemoglobin mass and blood volume with CO: evaluation and application of a method. J Appl Physiol (1985) 79, 623–631. [DOI] [PubMed] [Google Scholar]
- Carrick-Ranson G, Fujimoto N, Shafer KM, Hastings JL, Shibata S, Palmer MD, Boyd K & Levine BD. (2016). The effect of 1 year of Alagebrium and moderate-intensity exercise training on left ventricular function during exercise in seniors: a randomized controlled trial. J Appl Physiol (1985) 121, 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrick-Ranson G, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, Boyd K, Livingston S, Dijk E & Levine BD. (2014). The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol (1985) 116, 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrick-Ranson G, Hastings JL, Bhella PS, Shibata S, Fujimoto N, Palmer D, Boyd K & Levine BD. (2012). The Effect of Age-related Differences in Body Size and Composition on Cardiovascular Determinants of VO2max. J Gerontol A Biol Sci Med Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler PD, Melenovsky V, Schulman SP, Gerstenblith G, Becker LC, Ferrucci L, Fleg JL, Lakatta EG & Najjar SS. (2008). The sex-specific impact of systolic hypertension and systolic blood pressure on arterial-ventricular coupling at rest and during exercise. American Journal of Physiology-Heart and Circulatory Physiology 295, H145–H153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P & Lecarpentier Y. (1998). Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol 274, H500–505. [DOI] [PubMed] [Google Scholar]
- Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M & Kass DA. (2001). Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 38, 2028–2034. [DOI] [PubMed] [Google Scholar]
- Chen J, Das S, Barlow CE, Grundy S & Lakoski SG. (2010). Fitness, fatness, and systolic blood pressure: data from the Cooper Center Longitudinal Study. Am Heart J 160, 166–170. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM & Holloszy JO. (1992). Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol (1985) 72, 1780–1786. [DOI] [PubMed] [Google Scholar]
- Cooke GA, Marshall P, al-Timman JK, Wright DJ, Riley R, Hainsworth R & Tan LB. (1998). Physiological cardiac reserve: development of a non-invasive method and first estimates in man. Heart 79, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy KP & Seals DR. (1994). Total blood volume in healthy young and older men. J Appl Physiol (1985) 76, 2059–2062. [DOI] [PubMed] [Google Scholar]
- Dogra S, Spencer MD & Paterson DH. (2012). Higher cardiorespiratory fitness in older trained women is due to preserved stroke volume. J Sport Sci Med 11, 745–750. [PMC free article] [PubMed] [Google Scholar]
- Egelund J, Jorgensen PG, Mandrup CM, Fritz-Hansen T, Stallknecht B, Bangsbo J, Nyberg M & Hellsten Y. (2017). Cardiac Adaptations to High-Intensity Aerobic Training in Premenopausal and Recent Postmenopausal Women: The Copenhagen Women Study. Journal of the American Heart Association 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleg J, Morrell C, Bos A, Brant L, Talbot L, Wright J & Lakatta E. (2005). Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 112, 674–682. [DOI] [PubMed] [Google Scholar]
- Fleg J, O’Connor F, Gerstenblith G, Becker L, Clulow J, Schulman S & Lakatta E. (1995). Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol 78, 890–900. [DOI] [PubMed] [Google Scholar]
- Fleg J, Schulman S, O’Connor F, Gerstenblith G, Becker L, Fortney S, Goldberg A & Lakatta E. (1994). Cardiovascular responses to exhaustive upright cycle exercise in highly trained older men. J Appl Physiol 77, 1500–1506. [DOI] [PubMed] [Google Scholar]
- Fu Q & Levine BD. (2005). Cardiovascular response to exercise in women. Med Sci Sports Exerc 37, 1433–1435. [DOI] [PubMed] [Google Scholar]
- Fujimoto N, Hastings JL, Bhella PS, Shibata S, Gandhi NK, Carrick-Ranson G, Palmer D & Levine BD. (2012). Effect of ageing on left ventricular compliance and distensibility in healthy sedentary humans. J Physiol 590, 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto N, Hastings JL, Carrick-Ranson G, Shafer KM, Shibata S, Bhella PS, Abdullah SM, Barkley KW, Adams-Huet B, Boyd KN, Livingston SA, Palmer D & Levine BD. (2013). Cardiovascular effects of 1 year of alagebrium and endurance exercise training in healthy older individuals. Circ Heart Fail 6, 1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D & Levine BD. (2010). Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation 122, 1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates PE, Tanaka H, Graves J & Seals DR. (2003). Left ventricular structure and diastolic function with human ageing. Relation to habitual exercise and arterial stiffness. Eur Heart J 24, 2213–2220. [DOI] [PubMed] [Google Scholar]
- Gore CJ, Rodriguez FA, Truijens MJ, Townsend NE, Stray-Gundersen J & Levine BD. (2006). Increased serum erythropoietin but not red cell production after 4 wk of intermittent hypobaric hypoxia (4,000–5,500 m). J Appl Physiol (1985) 101, 1386–1393. [DOI] [PubMed] [Google Scholar]
- Green JS, Stanforth PR, Gagnon J, Leon AS, Rao DC, Skinner JS, Bouchard C, Rankinen T & Wilmore JH. (2002). Menopause, estrogen, and training effects on exercise hemodynamics: the HERITAGE study. Med Sci Sports Exerc 34, 74–82. [DOI] [PubMed] [Google Scholar]
- Hagberg J, Allen W, Seals D, Hurley B, Ehsani A & Holloszy J. (1985). A hemodynamic comparison of young and older endurance athletes during exercise. J Appl Physiol 58, 2041–2046. [DOI] [PubMed] [Google Scholar]
- Hagberg J, Goldberg A, Lakatta L, O’Connor F, Becker L, Lakatta E & Fleg J. (1998). Expanded blood volumes contribute to the increased cardiovascular performance of endurance-trained older men. J Appl Physiol 85, 484–489. [DOI] [PubMed] [Google Scholar]
- Hagberg JM, Ehsani AA & Holloszy JO. (1983). Effect of 12 months of intense exercise training on stroke volume in patients with coronary artery disease. Circulation 67, 1194–1199. [DOI] [PubMed] [Google Scholar]
- Hagmar M, Hirschberg AL, Lindholm C, Schenck-Gustafsson K & Eriksson MJ. (2005). Athlete’s heart in postmenopausal former elite endurance female athletes. Clin J Sport Med 15, 257–262. [DOI] [PubMed] [Google Scholar]
- Heath GW, Hagberg JM, Ehsani AA & Holloszy JO. (1981). A physiological comparison of young and older endurance athletes. J Appl Physiol Respir Environ Exerc Physiol 51, 634–640. [DOI] [PubMed] [Google Scholar]
- Hieda M, Howden E, Shibata S, Fujimoto N, Bhella PS, Hastings JL, Tarumi T, Sarma S, Fu Q, Zhang R & Levine BD. (2018). Impact of Lifelong Exercise Training Dose on Ventricular-Arterial Coupling. Circulation 138, 2638–2647. [DOI] [PubMed] [Google Scholar]
- Howden EJ, Perhonen M, Peshock RM, Zhang R, Arbab-Zadeh A, Adams-Huet B & Levine BD. (2015). Females have a blunted cardiovascular response to one year of intensive supervised endurance training. J Appl Physiol (1985) 119, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden EJ, Sarma S, Lawley JS, Opondo M, Cornwell W, Stoller D, Urey MA, Adams-Huet B & Levine BD. (2018). Reversing the Cardiac Effects of Sedentary Aging in Middle Age-A Randomized Controlled Trial: Implications For Heart Failure Prevention. Circulation 137, 1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG & Pawelczyk JA. (2007). Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol (1985) 103, 867–874. [DOI] [PubMed] [Google Scholar]
- Jenkins C, Bricknell K, Chan J, Hanekom L & Marwick TH. (2007). Comparison of two- and three-dimensional echocardiography with sequential magnetic resonance imaging for evaluating left ventricular volume and ejection fraction over time in patients with healed myocardial infarction. Am J Cardiol 99, 300–306. [DOI] [PubMed] [Google Scholar]
- Jones PP, Davy KP, DeSouza CA, van Pelt RE & Seals DR. (1997). Absence of age-related decline in total blood volume in physically active females. Am J Physiol 272, H2534–2540. [DOI] [PubMed] [Google Scholar]
- Jones SC, Bilous M, Winship S, Finn P & Goodwin J. (2004). Validation of the OSCAR 2 oscillometric 24-hour ambulatory blood pressure monitor according to the International Protocol for the validation of blood pressure measuring devices. Blood Press Monit 9, 219–223. [DOI] [PubMed] [Google Scholar]
- Katyal S, Freeman M, Miller JA & Thomas SG. (2003). Short-term aerobic training and circulatory function in women: age and hormone-replacement therapy. Clin Sci (Lond) 104, 267–273. [DOI] [PubMed] [Google Scholar]
- Kirwan LD, MacLusky NJ, Shapiro HM, Abramson BL, Thomas SG & Goodman JM. (2004). Acute and chronic effects of hormone replacement therapy on the cardiovascular system in healthy postmenopausal women. J Clin Endocrinol Metab 89, 1618–1629. [DOI] [PubMed] [Google Scholar]
- Knebel F, Spethmann S, Schattke S, Dreger H, Schroeckh S, Schimke I, Hattasch R, Makauskiene R, Kleczka J, Sanad W, Lock J, Brechtel L, Baumann G & Borges AC. (2014). Exercise-induced changes of left ventricular diastolic function in postmenopausal amateur marathon runners: assessment by echocardiography and cardiac biomarkers. Eur J Prev Cardiol 21, 782–790. [DOI] [PubMed] [Google Scholar]
- Levine BD. (1993). Regulation of central blood volume and cardiac filling in endurance athletes: the Frank-Starling mechanism as a determinant of orthostatic tolerance. Med Sci Sports Exerc 25, 727–732. [PubMed] [Google Scholar]
- McCole SD, Brown MD, Moore GE, Zmuda JM, Cwynar JD & Hagberg JM. (1999). Cardiovascular hemodynamics with increasing exercise intensities in postmenopausal women. J Appl Physiol (1985) 87, 2334–2340. [DOI] [PubMed] [Google Scholar]
- McCole SD, Brown MD, Moore GE, Zmuda JM, Cwynar JD & Hagberg JM. (2000). Enhanced cardiovascular hemodynamics in endurance-trained postmenopausal women athletes. Med Sci Sports Exerc 32, 1073–1079. [DOI] [PubMed] [Google Scholar]
- Mirsky I (1984). Assessment of diastolic function: suggested methods and future considerations. Circulation 69, 836–841. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Stauffer BL, Kohrt WM & Seals DR. (2013). Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98, 4507–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias JM, Kowalchuk JM & Paterson DH. (2010). Mechanisms for increases in V O2max with endurance training in older and young women. Med Sci Sports Exerc 42, 1891–1898. [DOI] [PubMed] [Google Scholar]
- Najjar SS, Schulman SP, Gerstenblith G, Fleg JL, Kass DA, O’Connor F, Becker LC & Lakatta EG. (2004). Age and gender affect ventricular-vascular coupling during aerobic exercise. J Am Coll Cardiol 44, 611–617. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Egelund J, Mandrup CM, Andersen CB, Hansen K, Hergel IF, Valbak-Andersen N, Frikke-Schmidt R, Stallknecht B, Bangsbo J & Hellsten Y. (2017). Leg vascular and skeletal muscle mitochondrial adaptations to aerobic high-intensity exercise training are enhanced in the early postmenopausal phase. J Physiol 595, 2969–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell E, Kirwan LD & Goodman JM. (2009). Aerobic exercise training in healthy postmenopausal women: effects of hormone therapy. Menopause-the Journal of the North American Menopause Society 16, 770–776. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Spina R, Martin W, Kohrt W, Schechtman K, Holloszy J & Ehsani A. (1992). Effects of Aging, Sex and Physical Training on Cardiovascular Responses to Exercise. Circulation 86, 494–503. [DOI] [PubMed] [Google Scholar]
- Okazaki K, Kamijo Y, Takeno Y, Okumoto T, Masuki S & Nose H. (2002). Effects of exercise training on thermoregulatory responses and blood volume in older men. J Appl Physiol (1985) 93, 1630–1637. [DOI] [PubMed] [Google Scholar]
- Park S, Ha JW, Shim CY, Choi EY, Kim JM, Ahn JA, Lee SW, Rim SJ & Chung N. (2008). Gender-related difference in arterial elastance during exercise in patients with hypertension. Hypertension 51, 1163–1169. [DOI] [PubMed] [Google Scholar]
- Parker Jones P, Davy KP, Desouza CA & Tanaka H. (1999). Total blood volume in endurance-trained postmenopausal females: relation to exercise mode and maximal aerobic capacity. Acta Physiol Scand 166, 327–333. [DOI] [PubMed] [Google Scholar]
- Paterson DH, Govindasamy D, Vidmar M, Cunningham DA & Koval JJ. (2004). Longitudinal study of determinants of dependence in an elderly population. J Am Geriatr Soc 52, 1632–1638. [DOI] [PubMed] [Google Scholar]
- Peshock RM, Willett DL, Sayad DE, Hundley WG, Chwialkowski MC, Clarke GD & Parkey RW. (1996). Quantitative MR imaging of the heart. Magn Reson Imaging Clin N Am 4, 287–305. [PubMed] [Google Scholar]
- Pierce GL, Eskurza I, Walker AE, Fay TN & Seals DR. (2011). Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 120, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic ZB, Prasad A, Garcia MJ, Arbab-Zadeh A, Borowski A, Dijk E, Greenberg NL, Levine BD & Thomas JD. (2006). Relationship among diastolic intraventricular pressure gradients, relaxation, and preload: impact of age and fitness. Am J Physiol Heart Circ Physiol 290, H1454–1459. [DOI] [PubMed] [Google Scholar]
- Proctor D, Beck K, Shen P, Eickhoff T, Halliwill J & Joyner M. (1998). Influence of age and gender on cardiac output-VO2 relationships during submaximal cycle ergometer. J Appl Physiol 84, 599–605. [DOI] [PubMed] [Google Scholar]
- Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ & Kass DA. (2005). Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112, 2254–2262. [DOI] [PubMed] [Google Scholar]
- Riley-Hagan M, Peshock RM, Straygundersen J, Katz J, Ryschon TW & Mitchell JH. (1992). Left-Ventricular Dimensions and Mass Using Magnetic-Resonance-Imaging in Female Endurance Athletes. Am J Cardiol 69, 1067–1074. [DOI] [PubMed] [Google Scholar]
- Ross R, Blair SN, Arena R, Church TS, Despres JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui XM, Wisloff U, Hlth CLC, Cardiology CC, Prevention CE, Nursing CCS, Genomics CF & Council S. (2016). Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign A Scientific Statement From the American Heart Association. Circulation 134, E653–E699. [DOI] [PubMed] [Google Scholar]
- Santos-Parker JR, Strahler TR, Vorwald VM, Pierce GL & Seals DR. (2017). Habitual aerobic exercise does not protect against micro- or macrovascular endothelial dysfunction in healthy estrogen-deficient postmenopausal women. J Appl Physiol (1985) 122, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B & Kindermann W. (2002). Athlete’s heart: right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol 40, 1856–1863. [DOI] [PubMed] [Google Scholar]
- Schulman SP, Fleg JL, Goldberg AP, Busby-Whitehead J, Hagberg JM, O’Connor FC, Gerstenblith G, Becker LC, Katzel LI, Lakatta LE & Lakatta EG. (1996). Continuum of cardiovascular performance across a broad range of fitness levels in healthy older men. Circulation 94, 359–367. [DOI] [PubMed] [Google Scholar]
- Seals D, Hagberg J, Spina R, Rogers M, Schechtman K & Ehsani A. (1994). Enhanced left ventricular performance in endurance trained older men. Circulation 89, 198–205. [DOI] [PubMed] [Google Scholar]
- Shibata S, Fujimoto N, Hastings JL, Carrick-Ranson G, Bhella PS, Hearon CM & Levine BD. (2018). The effect of lifelong exercise frequency on arterial stiffness. Journal of Physiology-London 596, 2783–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Hastings JL, Prasad A, Fu Q, Okazaki K, Palmer MD, Zhang R & Levine BD. (2008). ‘Dynamic’ Starling mechanism: effects of ageing and physical fitness on ventricular-arterial coupling. J Physiol 586, 1951–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S & Levine BD. (2012a). Biological aortic age derived from the arterial pressure waveform. J Appl Physiol 110, 981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S & Levine BD. (2012b). Effect of exercise training on biologic vascular age in healthy seniors. Am J Physiol Heart Circ Physiol 302, H1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina R, Ogawa T, Kohrt W, Holloszy J & Ehsani A. (1993). Differences in cardiovascular adaptations to endurance exercise training between older men and women. J Appl Physiol 75, 849–855. [DOI] [PubMed] [Google Scholar]
- Spina R, Rashid S, Davila-Roman V & Ehsani A. (2000). Adaptations in beta-adrenergic cardiovascular responses to training in older women. J Appl Physiol 89, 2300–2305. [DOI] [PubMed] [Google Scholar]
- Spina RJ, Miller TR, Bogenhagen WH, Schechtman KB & Ehsani AA. (1996). Gender-related differences in left ventricular filling dynamics in older subjects after endurance exercise training. J Gerontol A Biol Sci Med Sci 51, B232–237. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Mack GW, DiPietro L, Morocco TS, Jozsi AC & Nadel ER. (1998). Regulation of blood volume during training in post-menopausal women. Med Sci Sports Exerc 30, 92–98. [DOI] [PubMed] [Google Scholar]
- Staessen JA, van der Heijden-Spek JJ, Safar ME, Den Hond E, Gasowski J, Fagard RH, Wang JG, Boudier HA & Van Bortel LM. (2001). Menopause and the characteristics of the large arteries in a population study. J Hum Hypertens 15, 511–518. [DOI] [PubMed] [Google Scholar]
- Stevenson ET, Davy KP & Seals DR. (1994). Maximal aerobic capacity and total blood volume in highly trained middle-aged and older female endurance athletes. J Appl Physiol (1985) 77, 1691–1696. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S & Salvetti A. (1996). Menopause is associated with endothelial dysfunction in women. Hypertension 28, 576–582. [DOI] [PubMed] [Google Scholar]
- Taylor R, Chidley K, Goodwin J, Broeders M & Kirby B. (1993). Accutracker-Ii (Version 30/23) Ambulatory Blood-Pressure Monitor - Clinical Validation Using the British-Hypertension-Society and Association-for-the-Advancement-of-Medical-Instrumentation-Standards. J Hypertens 11, 1275–1282. [PubMed] [Google Scholar]
- Triebwasser JH, Johnson RL, Burpo RP, Campbell JC, Reardon WC & Blomqvist CG. (1977). Noninvasive Determination of Cardiac-Output by a Modified Acetylene Rebreathing Procedure Utilizing Mass-Spectrometer Measurements. Aviat Space Environ Med 48, 203–209. [PubMed] [Google Scholar]
- Utomi V, Oxborough D, Whyte GP, Somauroo J, Sharma S, Shave R, Atkinson G & George K. (2013). Systematic review and meta-analysis of training mode, imaging modality and body size influences on the morphology and function of the male athlete’s heart. Heart 99, 1727–1733. [DOI] [PubMed] [Google Scholar]
- Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH & Dallas Heart Study I. (2004). The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 93, 1473–1480. [DOI] [PubMed] [Google Scholar]
- Whalley GA, Doughty RN, Gamble GD, Oxenham HC, Walsh HJ, Reid IR & Baldi JC. (2004). Association of fat-free mass and training status with left ventricular size and mass in endurance-trained athletes. J Am Coll Cardiol 44, 892–896. [DOI] [PubMed] [Google Scholar]
- Wiebe CG, Gledhill N, Jamnik VK & Ferguson S. (1999). Exercise cardiac function in young through elderly endurance trained women. Med Sci Sports Exerc 31, 684–691. [DOI] [PubMed] [Google Scholar]
- Wilmore JH & Behnke AR. (1969). An anthropometric estimation of body density and lean body weight in young men. J Appl Physiol 27, 25–31. [DOI] [PubMed] [Google Scholar]
- Zappe DH, Bell GW, Swartzentruber H, Wideman RF & Kenney WL. (1996). Age and regulation of fluid and electrolyte balance during repeated exercise sessions. Am J Physiol 270, R71–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


