Abstract
Allergic diseases are immune disorders that are a global health problem, affecting a large portion of the world's population. Allergic asthma is a heterogeneous disease that alters the biology of the airway. A substantial portion of patients with asthma do not respond to conventional therapies; thus, new and effective therapeutics are needed. Dendritic cells (DCs), antigen presenting cells that regulate helper T cell differentiation, are key drivers of allergic inflammation but are not the target of current therapies. Here we review the role of dendritic cells in allergic conditions and propose a disease‐modifying strategy for treating allergic asthma: cAMP‐mediated inhibition of dendritic cells to blunt allergic inflammation. This approach contrasts with current treatments that focus on treating clinical manifestations of airway inflammation. Disease‐modifying agents that target cAMP and its signalling pathway in dendritic cells may provide a novel means to treat asthma and other allergic diseases.
Abbreviations
- AKAP
A‐kinase anchoring protein
- DC
dendritic cell
- Epac
exchange protein directly activated by cAMP; Rap guanine nucleotide exchange factor
- ICS
inhaled corticosteroid
- LABA
long‐acting β2 agonist
- LAMA
long‐acting muscarinic receptor antagonist
- MHC
major histocompatibility complex
- RGS
regulator of G protein signalling
- SABA
short‐acting β2 agonist
- Th2
type 2 helper T cell
- Th17
T helper 17 cell
- Tregs
regulatory T cells
1. INTRODUCTION
Allergic diseases are disorders in which the immune system reacts to environmental stimuli that are typically considered harmless. Common allergic diseases include allergic asthma, allergic rhinitis, atopic dermatitis, and food allergies. Estimates of the prevalence of allergic conditions vary widely (between 10% and 40%) and this prevalence has increased in recent years (Pawankar, Canonica, Holgate, Lockey, & Blaiss, 2013; Simpson, Newton, Hippisley‐Cox, & Sheikh, 2008). In a substantial proportion of asthma patients, the disease is not well‐controlled by current therapies, thus identifying a need to find new and effective treatments. Dendritic cells (DCs), which are antigen presenting cells, are critical in initiating immune responses and thus are important but are not the focus of current treatments for asthma. Here, we review the role of dendritic cells and cAMP in asthma and propose their potential as disease‐modifying therapies for asthma and perhaps other allergic disorders.
2. ASTHMA
Asthma is characterized by chronic airway inflammation and hyperresponsiveness, which is increased sensitivity to bronchoconstrictor agonists (Global Initiative for Asthma, 2019). These features result in increased constriction of bronchial smooth muscle and increased mucus production, which together lead to reversible narrowing of the airway. Characteristic asthmatic symptoms include shortness of breath, chest tightness, wheezing, and coughing. Asthma is defined as having variable expiratory airflow limitation, with symptoms and signs varying in intensity over time (Global Initiative for Asthma, 2019). Diagnosis is based on a patient's (subjective) history and documented (objective) evidence of variable lung function. Pulmonary function tests are measured by a spirometer, usually in response to an inhaled challenge such as a bronchoconstrictor (e.g. the muscarinic agonist methacholine as asthmatics are hyperresponsive to bronchoconstrictors), a bronchodilator (e.g. a short‐acting β2‐adrenoceptor agonist (SABA)) to demonstrate reversibility, and/or an exercise test as some types of asthma are induced by exercise (Global Initiative for Asthma, 2019).
2.1. Asthma prevalence
The word asthma is derived from the Greek aazein (“to pant”). Records of asthma (by Aretaeus of Cappadocia) occur as early as the first century A.D. (Jackson, 2008; Marketos & Ballas, 1982). The prevalence of asthma dramatically increased in the mid‐to‐late 1970s. Surveys in the United Kingdom estimated that its prevalence among children more than doubled between the 1970s and late 1990s (Anderson, Gupta, Strachan, & Limb, 2007; Vollmer, Osborne, & Sonia Buist, 1998). The prevalence of asthma has increased more slowly in the 2000s, reaching a plateau since 2009 (Akinbami, Simon, & Rossen, 2016). The hygiene hypothesis posited that increased cleanliness and decreased microbial exposure might explain the rise in asthma prevalence in the late 20th century. However recent data show that this hypothesis does not fully explain the observed findings (Brooks, Pearce, & Douwes, 2013; Liu, 2015; Weber et al., 2015). Rather, microbial exposure unaffected by personal hygiene has been implicated as a key factor in the increased prevalence of asthma.
The prevalence of asthma varies among countries, from as low as 1.0% (in Vietnam) to 21.5% (in Australia), with an overall global prevalence of 4.5%, affecting an estimated 235 million people worldwide (Global Initiative for Asthma, 2019; To et al., 2012; World Health Organization, 2017). Asthma is the most common non‐communicable disease in children (World Health Organization, 2017). The increase in prevalence of allergic diseases is a public health challenge in all countries independent of a country's developmental status (Nunes, Pereira, & Morais‐Almeida, 2017; World Health Organization, 2017). The economic costs associated with asthma are amongst the highest compared to other chronic diseases, exceeding those of HIV/AIDS and tuberculosis combined (Bahadori et al., 2009; Nunes et al., 2017).
3. PATHOPHYSIOLOGY OF ASTHMA
Asthma is a heterogeneous disease and can be considered a syndrome characterized by symptoms and variable airflow limitation. These clinical manifestations encompass different phenotypes (i.e. disease characteristics independent of molecular mechanism) and endotypes (i.e. distinct disease mechanisms that give rise to that phenotype) (Lötvall et al., 2011; Ozdemir, Kucuksezer, Akdis, & Akdis, 2018). Such phenotypes include allergic (the most prevalent phenotype), non‐allergic, late‐onset, and obesity‐associated asthma, in addition to asthma with persistent airflow limitation (Global Initiative for Asthma, 2019; Ozdemir et al., 2018).
It has been proposed that asthma endotypes rather than asthma phenotypes should direct therapy (Svenningsen & Nair, 2017; Wenzel, 2012; Wenzel et al., 1999). One such classification of endotypes is based on the identity of the predominant cellular inflammatory mediator, as determined by sputum cytology and/or the analysis of peripheral blood cells: eosinophilic, neutrophilic, mixed‐granulocytic (with both eosinophilic and neutrophilic features), and paucigranulocytic (not related to eosinophilic nor neutrophilic) asthma (Green, Brightling, & Bradding, 2007; Ozdemir et al., 2018). An alternative classification system that is gaining increased acceptance divides asthma into type 2 (type 2 helper T cell [Th2]‐high) or non‐type 2 (Th2‐low) asthma (Esteban‐Gorgojo, Antolín‐Amérigo, Domínguez‐Ortega, & Quirce, 2018; Fajt & Wenzel, 2015; Woodruff et al., 2009). Th2‐high asthma is strongly associated with allergic asthma and increased eosinophilic inflammation. An overlap exists between patients classified as having Th2‐high asthma and those with eosinophilic asthma, but they are not the same as the criteria for each is different (Tran et al., 2016).
3.1. Dendritic cells
Dendritic cells originate from macrophage‐dendritic cell progenitors in the bone marrow (Merad, Sathe, Helft, Miller, & Mortha, 2013; Puhr, Lee, Zvezdova, Zhou, & Liu, 2015). Conventional dendritic cells are professional antigen presenting cells that induce immune responses and form the bridge between the innate and adaptive immune responses (Banchereau et al., 2000; Lipscomb & Masten, 2002; Steinman, 2012; Steinman & Cohn, 1973, 1974). Dendritic cells reside in peripheral (non‐lymphoid) tissues where they internalize self‐ and non‐self‐antigens. Processing of these antigens results in the generation of peptide fragments of the internalized antigen. These peptide fragments interact with major histocompatibility complex (MHC) class II molecules; the MHC–peptide complexes are expressed (“presented”) on the cell surface, a process termed antigen presentation. Dendritic cells are inefficient at antigen presentation until they undergo maturation after encountering a second (“danger”) signal (Guermonprez, Valladeau, Zitvogel, Théry, & Amigorena, 2002). Antigen presentation then becomes more efficient as a result of changes in the formation and transport of MHC–peptide complexes. Dendritic cells are the most efficacious antigen presenting cells, for example more so than macrophages (Guermonprez et al., 2002; Helft et al., 2015). In parallel, dendritic cells migrate from the peripheral tissues where they reside to secondary lymphoid organs in which T cells and B cells are located. Adaptive immune responses are initiated and activated when T cells and B cells bind to their cognate antigen presented by dendritic cells on a MHC class II molecule (Figure 1). Dendritic cells are critical for the initiation of immune responses and thus represent targets for modulating diseases influenced by aberrant immune cell activation.
FIGURE 1.
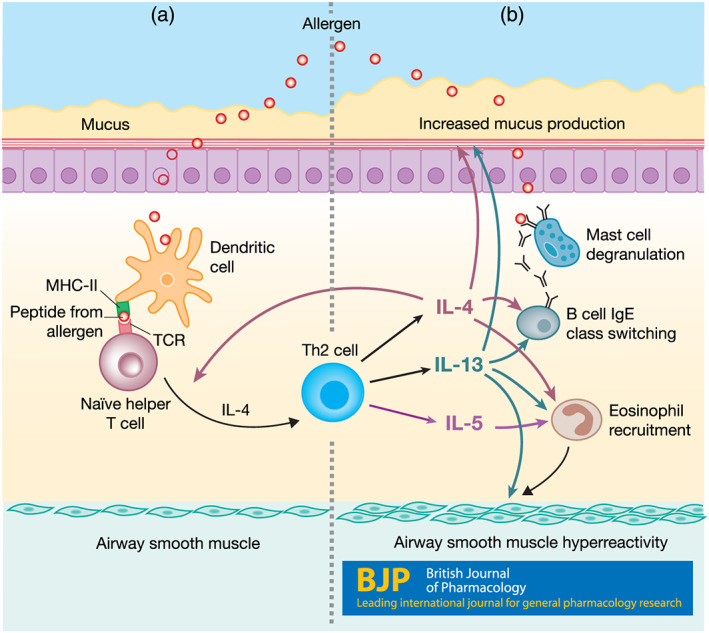
Dendritic cell induction of Th2 differentiation and contribution of Th2 cytokines to asthma pathology. (a) Dendritic cells process and present antigens (allergens) on MHC class II molecules (MHC‐II) to naïve helper T cells. When naïve helper T cells bind to their cognate antigen presented on MHC‐II, they become activated and can differentiate into Th2 cells in the presence of IL‐4. (b) Activated Th2 cells secrete IL‐4, IL‐5, and IL‐13 which promote eosinophil migration and survival, increased mucus production, airway hyperresponsiveness, and IgE isotype class switching in B cells. IgE class switching leads to IgE production and results in mast cell degranulation and release of histamine
3.2. Helper T cells
Naïve helper T cells are driven to differentiate into Th2 cells via the presence of the cytokine IL‐4 during stimulation by their cognate antigen and activation of the transcription factor GATA3 and the signal transducer STAT6 (Sinigalia & D'Ambrosio, 2000; Zhu, Yamane, & Paul, 2010). Th2 cells are a central mediator of inflammation through their secretion of the inflammatory cytokines IL‐4, IL‐5, and IL‐13. In this way, Th2 differentiation works as a positive feedback loop with Th2 cells secreting IL‐4 which in turn promotes other naïve helper T cells to differentiate into Th2 cells. Atopic and asthmatic patients have increased levels of IL‐4 in their serum and bronchoalveolar lavage fluid (Daher et al., 1995; Walker et al., 1994). Allergic disease is thought to be mediated by an overactive Th2 arm of the immune system.
3.3. Effects of cytokines in Th2‐high asthma
IL‐4 and IL‐13 promote immunoglobulin class switching in B cells, thereby producing antibodies of the IgE class (Figure 1). IgE antibodies bind to the high‐affinity IgE receptor FcɛRI on mast cells, which sensitizes them to respond in an antigen‐specific manner when the host is again exposed to the allergen (Galli & Tsai, 2012; Galli, Tsai, & Piliponsky, 2008; Gould & Sutton, 2008). Cross‐linking of IgE‐FcɛRI triggers mast cell degranulation leading to the release of histamine and leukotrienes, which can result in bronchoconstriction, increased mucus production, vasodilation, and recruitment of other inflammatory cells (Galli et al., 2008). IL‐13 causes airway hyperresponsiveness, and both IL‐4 and IL‐13 increase mucus production (Kuperman et al., 2002; McBrien & Menzies‐Gow, 2017).
IL‐5 regulates eosinophil differentiation, activation, and survival and is a strong chemoattractant for eosinophils (Hamid & Tulic, 2009). IL‐4 and IL‐13 further promote recruitment of eosinophils by up‐regulating vascular cell adhesion molecule‐1 expression (Hamid & Tulic, 2009; Schleimer et al., 1992). Eosinophils have large specific granules (i.e. secretory vesicles in granulocytes that are termed secondary granules) that contain inflammatory mediators including proteins, cytokines, chemokines, and enzymes (Carr, Zeki, & Kraft, 2018; McBrien & Menzies‐Gow, 2017). Major basic protein, a predominant entity in secondary granules, produces airway hyperreactivity in primates and rats (Coyle, Ackerman, Burch, Proud, & Irvin, 1995; Gundel, Letts, & Gleich, 1991). Eosinophil‐secreted Th2 cytokines further the inflammatory cycle and produce TGF‐β which contributes to airway remodelling (McBrien & Menzies‐Gow, 2017). Chronic airway inflammation can also lead to airway remodelling which includes airway smooth muscle cell hypertrophy and hyperplasia, increased collagen and fibronectin deposition, and goblet cell hyperplasia. Together, these increase airway obstruction due to airway thickening and increased mucus production (Cohn, Elias, & Chupp, 2004; Keglowich & Borger, 2015).
3.4. Th2‐low asthma
Th2‐low asthma encompasses diverse asthma pathophysiologies, including neutrophilic, paucigranulocytic, obesity‐associated, and asthma related to environmental exposure (Carr et al., 2018). Gene expression analyses have suggested that the Th2‐low endotype can be divided into T helper 17 cell (Th17)‐high and Th2/Th17‐low patterns (Choy et al., 2015). Th17 cells induce the recruitment of polymorphonuclear neutrophils through the secretion of the cytokines IL‐17A and IL‐17F. Increased levels of IL‐17A in sputum are associated with increased recruitment of neutrophils (Hirose, Iwata, Tamachi, & Nakajima, 2017; Liang et al., 2007; McKinley et al., 2008). Corticosteroids (glucocorticoids) are one of the cornerstones of asthma therapy; airway neutrophilia is associated with asthma severity and correlates with corticosteroid resistance (Carr et al., 2018; Choy et al., 2015; Ray & Kolls, 2017).
4. ASTHMA THERAPIES
4.1. Current therapies
The mainstays of current therapies for asthma are inhaled corticosteroids (ICS) and β2‐adrenoceptor agonists (Global Initiative for Asthma, 2019; Table 1). Short‐acting β2 agonists are used as reliever (rescue) medications for quick relief and intermittent asthmatic symptoms. The current guidelines for treatment of mild asthma focus on low‐dose inhaled corticosteroids with the addition of a long‐acting β2 agonist (LABA) if asthma is not controlled by inhaled corticosteroid treatment alone. Inhaled corticosteroids and long‐acting β2 agonists are used together as long‐term “controller” medications to reduce symptoms and airway inflammation. Leukotriene receptor antagonists are an alternative to low‐dose inhaled corticosteroid and long‐acting β2 agonist combination treatment. Medium‐dose inhaled corticosteroids are used in patients whose asthma is uncontrolled by low‐dose inhaled corticosteroids. Asthma treatment is considered successful when a reliever medication is no longer needed to control asthmatic symptoms. Patients with severe, uncontrolled asthma typically use the combination of a high‐dose inhaled corticosteroid and a long‐acting β2 agonist as a controller medication along with one or more additional therapeutic agents/approaches. The latter agents include long‐acting muscarinic receptor antagonists (LAMA), anti‐IgE treatment (omalizumab), antibiotics (azithromycin), anti‐IL‐5/5R treatment (mepolizumab), anti‐IL‐4Rα (dupilumab), and bronchial thermoplasty (Global Initiative for Asthma, 2019).
TABLE 1.
FDA‐approved drugs for asthma
| FDA‐approved drugs for asthma | Abbreviation | Examples | Recommended first‐line therapy | Disease‐modifying agent |
|---|---|---|---|---|
| Inhaled corticosteroids | ICS | Fluticasone and budesonide | ✓ | |
| Short‐acting β2 agonist | SABA | Salbutamol (albuterol) | ✓ | |
| Long‐acting β2 agonists | LABA | Formoterol and salmeterol | ✓ | |
| Antibiotics | Azithromycin | |||
| Anti‐IgE | Omalizumab | ✓ | ||
| Anti‐IL‐4Rα | Dupilumab and pitrakinra | ✓ | ||
| Anti‐IL‐5/5Rα | Mepolizumab, reslizumab, and benralizumab | ✓ | ||
| Leukotriene receptor antagonists | LTRA | Montelukast and zafirlukast | ||
| Long‐acting muscarinic antagonists | LAMA | Tiotropium | ||
| Methylxanthines | Theophylline |
4.2. Disease‐modifying therapies
It is currently estimated that ~50% of adults and ~40% of children have uncontrolled asthma with poor symptom control and/or frequent exacerbations (Centers for Disease Control and Prevention, 2014). These patients have a decreased quality of life (Global Initiative for Asthma, 2019). Among asthma patients, 2.5%–5% of all asthma patients have severe, uncontrolled disease, but this population accounts for ~38% of asthma‐related direct costs, representing an unmet medical need (Hankin, Bronstone, Wang, Small, & Buck, 2013; Yaghoubi, Adibi, Safari, FitzGerald, & Sadatsafavi, 2019).
Asthma is defined by its symptoms and signs (breathlessness, chronic airway inflammation, and airway hyperresponsiveness), and not by its aetiology. Current therapies for asthma focus on symptomatic treatment (e.g. inhaled corticosteroids to reduce airway inflammation and β2 agonists to relax constricted airways). As a syndrome, asthma encompasses similar clinical manifestations that result from multiple pathophysiological mechanisms. These multiple mechanisms result in patients having different asthma endotypes, thus inferring a need for precision/personalized therapeutic approaches.
For patients with a defined asthma endotype, disease‐modifying therapies directed at underlying pathogenic mechanisms (rather than at clinical manifestations) are a potentially promising therapeutic approach. Disease‐modifying therapies have been used successfully in a variety of other immune/inflammatory conditions (Finkelsztejn, 2014; Kemper, Van Mater, Coeytaux, Williams, & Sanders, 2012; Ruderman, 2012; Torkildsen, Myhr, & Bø, 2016). Currently, no disease‐modifying agents are first‐line treatments for asthma, but such agents are sometimes used in patients with severe, uncontrolled asthma (Table 1). The high number of patients with poorly controlled disease reveals a need to identify new therapies for asthma. Disease‐modifying agents are a possible alternative. Moreover, disease‐modifying agents offer the potential to improve the care of asthmatic patients beyond just those with poorly controlled disease.
One might include corticosteroids/glucocorticoids as disease‐modifying agents based on their ability to decrease transcription of pro‐inflammatory genes and increase transcription of anti‐inflammatory genes (Barnes, 2011). By reducing expression of adhesion molecules and multiple chemokines, corticosteroids decrease the number and activity of inflammatory cells in the airway. In this review, we consider corticosteroids as separate from disease‐modifying agents.
Current disease‐modifying therapies for type 2 asthma include those that target IgE, IL‐4Rα, and IL‐5/5Rα. Omalizumab is a humanized monoclonal antibody that reduces allergic reactions via its ability to bind circulating IgE, thereby inhibiting its binding to FcεRI, the high‐affinity IgE receptor expressed on mast cells and basophils (Hu et al., 2018; Samitas, Delimpoura, Zervas, & Gaga, 2015). Omalizumab is unable to bind to IgE that is bound to FcεRI since its epitope is masked, thus avoiding cross‐linking which could activate mast cells. Omalizumab treatment decreases circulating IgE levels and over time reduces the expression of FcεRI on basophils, mast cells, and dendritic cells (Beck, Marcotte, MacGlashan, Togias, & Saini, 2004; Djukanović et al., 2004; MacGlashan et al., 1997; Prussin et al., 2003). Of note, dendritic cells from atopic, asthmatic children have elevated levels of FcεRI expression (Leffler et al., 2019). Omalizumab treatment reduces the number of eosinophils in sputum and decreases T cells, B cells, and IL‐4‐staining submucosal cells in bronchial biopsies (Djukanović et al., 2004; Hoshino & Ohtawa, 2012; Tajiri et al., 2014; Takaku et al., 2013). This antibody can also decrease airway wall thickening, perhaps by interrupting the allergic inflammation process (Hoshino & Ohtawa, 2012; Tajiri et al., 2014). Treatment with omalizumab is associated with decreased Th2 differentiation and decreased Th2 cytokine release, identifying it as a disease‐modifying agent (Schroeder et al., 2010; Takaku et al., 2013).
Other disease‐modifying agents for type 2 asthma focus on inhibiting the actions of Th2 cytokines. Both IL‐4 and IL‐13 activate IL‐4Rα and promote IgE secretion from B cells. Antibodies directed against IL‐4 and IL‐13 have been investigated as disease‐modifying agents. Results from clinical trials with anti‐IL‐13 antibodies have been mixed, but promising results have been noted with anti‐IL‐4 treatments. Such agents include pitrakinra, an IL‐4Rα antagonist, and dupilumab, a monoclonal antibody against IL‐4Rα, which reduce the frequency of asthma exacerbations (Bagnasco, Ferrando, Varricchi, Passalacqua, & Canonica, 2016; Castro et al., 2018; Slager et al., 2012; Wenzel et al., 2013). Mepolizumab, an anti‐IL‐5 monoclonal antibody, is approved for severe eosinophilic asthma as a treatment that reduces the number of eosinophils in blood and sputum and decreases asthma exacerbations (Emma, Morjaria, Fuochi, Polosa, & Caruso, 2018; Walsh, 2015). Other anti‐IL‐5/5Rα monoclonal antibodies have also been developed (Table 1).
A number of these disease‐modifying agents have drawbacks that limit widespread use. One limitation is that as biologics, most are administered subcutaneously (e.g. omalizumab, mepolizumab, and dupilumab) and all have adverse side effects. Notably, omalizumab has a U.S. FDA “black box” warning due to anaphylactic reactions (Jackson & Bahna, 2020; Mavissakalian & Brady, 2020). Dupilumab treatment increases the risk of conjunctivitis and keratosis. All these disease‐modifying agents are expensive and thus generally only used for patients with severe, refractory asthma. In the United States, the annual wholesale acquisition cost of omalizumab is ~$39,000 and mepolizumab and dupilumab are similarly priced (Anderson & Szefler, 2019). Overall, new and effective (and less expensive) disease‐modifying therapies that target underlying mechanisms of type 2 asthma represent an unmet medical need.
5. THE ROLE OF cAMP IN ALLERGIC INFLAMMATION
5.1. The cAMP signalling pathway
cAMP, the first identified second messenger, is a signalling molecule in organisms across all three domains of cellular life: archaea, bacteria, and eukarya (Rall & Sutherland, 1958; Sutherland & Rall, 1958). cAMP can regulate a wide variety of cellular processes, such as proliferation, metabolism, and cell death in numerous cell types, including immune cells, neural cells, and smooth muscle cells. Agonists of Gs‐coupled G protein‐coupled receptors (GPCRs) on the cell surface increase cellular cAMP levels via activation of adenylyl cyclases (ACs). Agonists of Gi‐coupled GPCRs have the opposite effect, inhibiting AC and reducing cellular cAMP levels (Figure 2).
FIGURE 2.
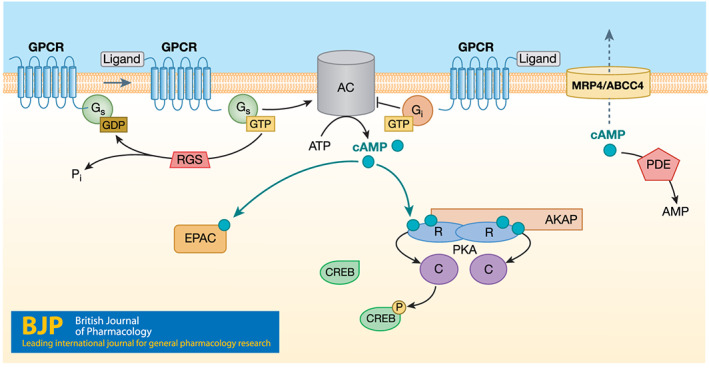
The cAMP signalling pathway. Upon ligand binding to Gs‐coupled GPCRs, the Gαs protein activates adenylyl cyclases (AC) to catalyse the conversion of ATP to cAMP. The GαI protein inhibits AC, resulting in lower cAMP levels. cAMP primarily mediates its effects via PKA and/or Epac. PKA phosphorylates CREB which binds to CRE sites in the promoter of CREB‐responsive genes. A‐kinase anchoring proteins (AKAPs) localize enzymes to their signalling substrates in subcellular locations. cAMP is removed via degradation by phosphodiesterases (PDEs) or effluxed out by the transporter MRP4/ABCC4. Regulators of G protein signalling (RGS) proteins accelerate the hydrolysis of GTP to GDP of heterotrimeric G proteins and thus help terminate G protein activation. Numerous RGS proteins have been identified (O'Brien, Wilkinson, & Roman, 2019). The figure hypothesizes an as‐yet not definitively identified RGS protein for Gαs. Multiple transcription factors, including IRF4, are downstream of and regulated by the cAMP pathway
cAMP mediates its effects via PKA (cAMP‐dependent protein kinase), Epac (exchange protein directly activated by cAMP; Rap guanine nucleotide exchange factor) and cyclic nucleotide‐gated channels (Cheng, Ji, Tsalkova, & Mei, 2008). PKA is a tetramer consisting of two regulatory (R) and two catalytic (C) subunits. Each R subunit has two cAMP binding domains. When four cAMP molecules bind to the two R subunits, the R subunits undergo a conformational change, releasing the active C subunits (Taylor et al., 2004). The C subunits phosphorylate substrates with the consensus sequence Arg‐Arg‐X‐Ser/Thr (Shabb, 2001). Epac has a cAMP binding domain that is homologous to those of the PKA R subunits. cAMP binding to Epac activates the GTPases Rap1 and Rap2, members of the Ras superfamily (Schmidt, Dekker, & Maarsingh, 2013). Cyclic nucleotide‐gated channels can be activated by cAMP and cGMP binding and regulate function in retinal photoreceptors as well as particular types of neurons, certain of which can regulate airway smooth muscle tone (McGovern et al., 2014).
Cells remove cAMP by degradation via cyclic nucleotide phosphoidesterases (PDEs) and cellular efflux via the cyclic nucleotide transporter MRP4/ABCC4 (Copsel et al., 2011; Russel, Koenderink, & Masereeuw, 2008; Wen et al., 2015). PDEs hydrolyse the 3′ cyclic phosphate bond of cAMP and cGMP, thereby regulating their cellular levels, subcellular signalling localization, and duration of response (Bender & Beavo, 2006). There are 11 families of PDEs, certain of which preferentially degrade cAMP or cGMP. PDEs and PKA subunits can localize to distinct subcellular regions, at least in part through their interactions with A‐kinase anchoring proteins (AKAPs), thereby regulating local/regional cAMP signal transduction.
5.2. cAMP in immune cell‐mediated allergic inflammation
cAMP regulates pro‐ and anti‐inflammatory events in innate and adaptive immune cells (Raker, Becker, & Steinbrink, 2016). Effects of cAMP on immune cells are complex with conflicting data in the literature, but in general, increases in cAMP tend to produce anti‐inflammatory effects and immune suppression (Table 2).
TABLE 2.
Effects of altered cAMP concentrations on immune cells
| Cell type | Effect of cAMP on inflammatory function | References |
|---|---|---|
| Dendritic cells |
▪ Increased cAMP, via PKA, inhibits release of pro‐inflammatory cytokines (IL‐12 and TNF‐α) ▪ PGE2 (via Gs‐linked GPCRs) induces dendritic cell migration ▪ Increased cAMP via PKA promotes Th2 differentiation ▪ Regulatory T cells (Tregs) suppress dendritic cell function by increasing cAMP which decreases co‐stimulatory molecule expression and suppresses actin polymerization at the immunological synapse between dendritic cells and T cells |
Galgani et al., 2004; Lee et al., 2015; Legler, Krause, Scandella, Singer, & Groettrup, 2006; Rueda, Jackson, & Chougnet, 2016 |
| Macrophages |
▪ Increased cAMP, via PKA, suppresses production of pro‐inflammatory cytokines (TNF‐α, MIP‐1α, and LTB4) and increases production of anti‐inflammatory cytokines (IL‐10) ▪ Increased cAMP, via Epac, inhibits phagocytosis ▪ Increased cAMP inhibits microbial killing ▪ During differentiation, cAMP increases expression of pro‐inflammatory CXCL and CCL chemokines via Epac ▪ In microglial cells, cAMP acts synergistically with the Th2 cytokine IL‐4 to promote the phenotypic conversion of M1 to M2 |
Aronoff, Canetti, Serezani, Luo, & Peters‐Golden, 2005; Ghosh, Xu, & Pearse, 2016; Hertz et al., 2009; Peters‐Golden, 2009 |
| T cells |
▪ Increased cAMP via PKA inhibits antigen‐specific T cell proliferation and cytokine production ▪ Increased cAMP inhibits T cell chemotaxis ▪ Tregs suppress the function of other T cells and dendritic cells by transferring cAMP through gap junctions and also by converting ATP into adenosine, which acts on the cells primarily via adenosine A2A receptors to activate Gαs and increase cAMP ▪ Tregs suppress T cell responses by nuclear accumulation of the transcription factors ICER/CREM (inducible cAMP early repressor/cAMP responsive element modulator) |
Aandahl et al., 2002; Bodor et al., 2012; Bopp et al., 2007; Hidi et al., 2000; Rueda et al., 2016; Vaeth et al., 2011 |
| B cells | ▪ Increased cAMP, via PKA, inhibits antigen‐stimulated B cell proliferation | Levy et al., 1996; Whisler, Beiqing, Grants, & Newhouse, 1992 |
5.3. cAMP as a target of asthma therapeutics
Peripheral blood leukocytes and airway smooth muscle from asthmatic patients produce less cAMP in response to the non‐selective β agonist isoprenaline (isoproterenol) than do cells from normal healthy controls (Makino, Ikemori, Kashima, & Fukuda, 1977; Morris, Rusnak, & Barzens, 1977; Parker & Smith, 1973; Trian et al., 2011). This response of airway smooth muscle is mediated by increased expression of the phosphodiesterase PDE4D which selectively degrades cAMP (Trian et al., 2011).
Treatments for asthma target cAMP in a variety of ways. Therapy is largely directed at the reversible bronchoconstriction generated by airway smooth muscle cells. Contraction of airway smooth muscle cells is mediated by Ca2+ signalling that is activated by ion channels and intracellular Ca2+ release, especially via agonists of Gq‐linked GPCRs. Increased Ca2+ activates Ca2+/calmodulin‐dependent myosin light chain kinase, which phosphorylates myosin light chain, thereby triggering constriction (Janssen & Killian, 2006). β2 adrenoceptors, Gs‐linked GPCRs, are widely targeted in asthma therapy (by long‐acting and short‐acting β2 agonists [LABA, SABA; Table 1] that increase cellular cAMP concentrations). The increase in cAMP results in activation of PKA and Epac and leads to decreased myosin light chain phosphorylation, thereby reducing airway smooth muscle contraction (Barnes, 2011; Billington, Ojo, Penn, & Ito, 2013). β2 agonists thus function as bronchodilators that can effectively treat asthma. β2 agonist‐induced airway smooth muscle relaxation involves multiple mechanisms, including PKA‐mediated decrease in Ca2+ mobilization and up‐regulation of the Gαq‐selective regulator of G protein signalling 2 (RGS2) which blunts increases in intracellular Ca2+by Gq‐linked GPCRs (Hoiting et al., 1996; Holden et al., 2011). cAMP‐elevating agents also stimulate ciliary motility and inhibit the increased proliferation and migration of airway smooth muscle cells that contribute to airway remodelling in asthma patients (Billington et al., 2013; Goncharova et al., 2003).
Theophylline (1,3‐dimethylxanthine) has been used to treat asthma since the 1930s and based on its low cost, is still commonly used in developing countries (Barnes, 2011). The mechanism of action of theophylline is not fully understood. Its weak inhibition of PDEs, primarily of PDE3, in airway smooth muscle cells increases cAMP levels and promotes bronchodilation (Barnes, 2011; Tanaka, 2015). However, frequent side effects resulting from its inhibition of other PDEs and antagonism of adenosine receptors have led to a decline in the usage of theophylline in developed countries.
Selective PDE4 inhibitors have been investigated, in particular because PDE4 is highly expressed in the inflammatory cells involved in asthma pathogenesis (Beghè, Rabe, & Fabbri, 2013; Li, Zuo, & Tang, 2018; Lipworth, 2005; Luo et al., 2018). The four PDE4 family members (encoded by the Pde4a, Pde4b, Pde4c, and Pde4d genes) selectively degrade cAMP. Each gene has multiple variants that differ in their N termini, which possess phosphorylation sites and regulatory domains (Bender & Beavo, 2006). PDE4 inhibition, by raising cAMP concentrations, can suppress airway inflammation and relax airway smooth muscle. Although the PDE4 inhibitor roflumilast is approved for treatment of chronic obstructive pulmonary disease, the many side effects of PDE4 inhibitors (in particular nausea and diarrhoea) have limited their utility for asthma therapy (Beghè et al., 2013; Spina, 2008). However, interest remains regarding the potential of PDE4 inhibitors for treatment of asthma (Beghè et al., 2013; Lipworth, 2005; Luo et al., 2018).
6. INCREASES IN cAMP LEVELS IN DENDRITIC CELLS: A NOVEL DISEASE‐MODIFYING APPROACH TO TREAT ASTHMA
We propose that approaches designed to selectively increase cAMP in dendritic cells may provide a new way to treat allergic asthma. Increasing cAMP levels in dendritic cells via PDE inhibitors or AC activation decreases the release of pro‐inflammatory cytokines (e.g. TNF‐α) and increases production of the anti‐inflammatory cytokine IL‐10 (Heystek, Thierry, Soulard, & Moulon, 2003; Ross, Lavelle, Mills, & Boyd, 2004). Co‐culturing dendritic cells with regulatory T cells (Tregs) also increases cAMP concentration in dendritic cells, leading to decreased expression of the co‐stimulatory molecules CD80 and CD86 and increased expression of B7‐H3 (CD276), an immune checkpoint molecule (Fassbender et al., 2010). Furthermore, mice with a selective knockout in dendritic cells of the gene Gnas (which encodes Gαs) provide a unique mouse model of allergic asthma (Lee et al., 2015). In this model, Gnas was deleted under the CD11c promoter, yielding CD11cΔGnas (or ΔGnas) mice with selective knockout in CD11c‐expressing cells. CD11c is highly expressed by dendritic cells, which as noted above, are superior antigen presenting cells compared to other cell types that express CD11c (e.g. macrophages) (Cabanas & Sanchez‐Madrid, 1999; Helft et al., 2015). Dendritic cells from wild‐type mice do not induce naïve CD4+ T cells to differentiate into Th2 cells (as measured by IL‐4 secretion and GATA3 mRNA expression); by contrast, dendritic cells from ΔGnas mice induce Th2 differentiation (Lee et al., 2015; Figure 3). Importantly, this increase in Th2 differentiation can be suppressed by treating dendritic cells from ΔGnas mice with cAMP analogues that activate PKA but not ones that activate Epac. Treatment of dendritic cells from control floxed/floxed (fl/fl) littermate mice with the PKA inhibitor H89 also causes them to induce Th2 differentiation, thus implicating a lack of cAMP acting via PKA as the mechanism that promotes the Th2 phenotype, even in control mice (it should be noted that in addition to acting as a PKA inhibitor, H89 also acts as a β adrenoceptor antagonist [Penn, Parent, Pronin, Panettieri, & Benovic, 1999]). Moreover, compared to control mice, ΔGnas mice have an enhanced susceptibility to develop allergic asthma in vivo in response to immunization with ovalbumin, as demonstrated by increased IgE serum levels, airway inflammation, and airway hyperresponsiveness. Of note, unlike in other animal models of asthma, ΔGnas mice do not require immunization and will spontaneously develop asthma at age 6 months. Thus, these mice are an attractive experimental model of allergic asthma that mimics the spontaneous development of asthma in humans. Most importantly, this mouse model shows the key role for cAMP/PKA in dendritic cells in regulating the Th2 state and allergic asthma.
FIGURE 3.
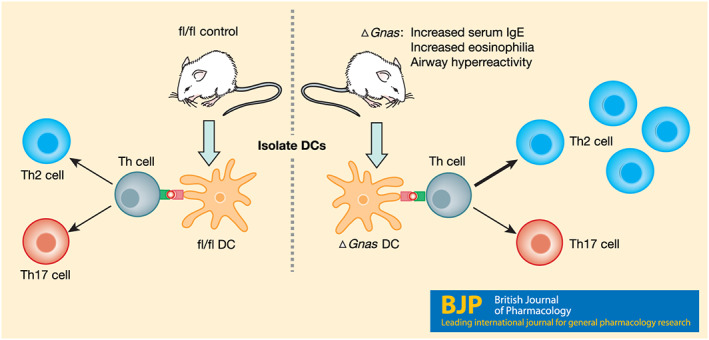
Contribution of cAMP concentrations in dendritic cells to Th2 differentiation. ΔGnas mice, which have lower cAMP levels in their dendritic cells (DCs), have increased serum IgE, increased eosinophils present in bronchoalveolar lavage fluid, and airway hyperreactivity. Isolated dendritic cells from ΔGnas mice also preferentially induce naïve helper T cells (Th cells) to differentiate into Th2 cells. Dendritic cells from control (fl/fl) mice do not preferentially induce naïve helper T cells to differentiate into a particular T cell subset (Lee et al., 2015)
These findings support the idea that raising the cAMP concentration in dendritic cells may be a disease‐modifying approach for asthma that will reduce dendritic cell‐induced inflammation. As antigen presenting cells, dendritic cells initiate the cascade that leads to allergic inflammation and are critical in propagating inflammation via helper T cell differentiation (Figure 1). We propose that drugs or biologics that increase cAMP levels or perhaps the expression and/or activity of post‐cAMP components in dendritic cells have the potential to be disease‐modifying agents that could prevent or reduce allergic inflammation in the context of asthma.
6.1. Approaches to increase cAMP levels in dendritic cells
Selectively targeting dendritic cells without affecting other cell types requires careful identification and quantification of the cAMP pathway components expressed on dendritic cells compared to other cells, especially ones in close proximity to dendritic cells. Since major components of the cAMP signalling pathway are often expressed in many cell types, such proteins (e.g. G proteins, ACs, and PKA) are likely not suitable drug targets, unless perhaps one could selectively target dendritic cells via novel drug delivery approaches.
An exception with respect to ubiquitously expressed cAMP signalling components may be PDEs because cell types can differentially express various PDEs. Dendritic cells express high levels of PDE4 but T cells do as well (Beltejar, Lau, Golkowski, Ong, & Beavo, 2017; Heystek et al., 2003). Defining precise PDE4 isoform subtypes expressed by dendritic cells compared to airway smooth muscle, epithelial, and other immune cells might identify a PDE that could be selectively targeted in dendritic cells. Data are currently lacking with respect to whether one or more such PDEs are expressed and might be targeted in dendritic cells, and if side effects from actions in other cell types might outweigh potential beneficial effects in dendritic cells.
A potentially more fruitful approach to selectively raise cAMP in dendritic cells is to target GPCRs, which not only regulate a wide variety of cellular functions but are also the targets of ~35% of approved drugs (Insel et al., 2019; Sriram & Insel, 2018). The tissue‐specific expression and extracellular accessibility of GPCRs contribute to their utility as drug targets. Proteomic analyses to detect GPCRs are challenging due to the low expression of many/most GPCRs, but transcriptomic (“GPCRomics”) approaches can define cellular GPCR expression (Insel et al., 2015; Insel et al., 2019). Profiling and comparing GPCR expression of dendritic cells and neighbouring cell types (e.g. airway smooth muscle and epithelial cells) may identify GPCRs selectively expressed by dendritic cells. Agonists of Gs‐coupled GPCRs and antagonists of Gi‐coupled GPCRs might be drugs to increase cAMP levels in dendritic cells.
Even with careful selection of a target that is dendritic cell‐selective, other immune cells (e.g. macrophages) may share in expression of certain proteins based on their similar roles as antigen presenting cells. Increasing cAMP concentrations in conjunction with the Th2 cytokine IL‐4 has been shown to promote M1 to M2 phenotypic conversion in macrophages and microglial cells which could lead to off‐target effects (Ghosh et al., 2016; Negreiros‐Lima et al., 2020). As opposed to giving a drug systemically, in the case of asthma, it may be advantageous to administer a drug that raises cAMP levels in dendritic cells through an inhaled route, thereby selectively treating dendritic cells in the airways. Numerous drugs used to treat asthma, including bronchodilators and inhaled corticosteroids, are administered via this route to selectively target airway smooth muscle and airway epithelial cells. By choosing a target expressed on both dendritic cells and airway smooth muscle cells, it may be possible to increase cAMP in both these cell types, thereby having multiple beneficial effects but avoiding side effects from other cell types.
6.2. Alternatives to directly raising intracellular cAMP concentrations
Recent findings have demonstrated that treating murine dendritic cells with the cAMP analogue 8‐(4‐chlorophenylthio)‐cAMP (CPT) ex vivo decreases IL‐4 secretion and GATA3 expression, but it also increases IL‐17A secretion and RORC/Nr1f3 expression (Lee et al., 2020). These effects occur via repression of the transcription factor IRF4. Importantly, ex vivo CPT treatment of dendritic cells that were then transferred into wild‐type mice increased IL‐17A production in the lung in vivo (indicative of Th17 differentiation). In addition, the number of neutrophils present in bronchoalveolar lavage fluid increased and gene expression markers of eosinophil recruitment decreased. These results demonstrate that increasing cAMP concentrations in dendritic cells can decrease Th2 differentiation. However, such a decrease may be associated with an increase in Th17 differentiation and neutrophilic asthma, emphasizing the necessity to optimize the dose for the desired response: a decrease in Th2 differentiation while minimizing Th17 differentiation.
Raising cAMP concentration in particular target cells is a treatment approach used in diseases other than asthma, for example in various endocrine and gastrointestinal disorders (Pierre, Eschenhagen, Geisslinger, & Scholich, 2009; Turalba, Leite‐Morris, & Kaplan, 2004). The ability to successfully alter cAMP concentrations to treat diverse diseases supports the rationale and the potential feasibility for targeting cAMP in asthma. The recent findings noted just above suggest that one might direct therapy at a target downstream of cAMP, for example the transcription factor IRF4. However, therapeutic approaches targeted at transcription factors have been challenging (De Bastiani, Pfaffenseller, & Klamt, 2018; Lambert, Jambon, Depauw, & David‐Cordonnier, 2018; Lee et al., 2020; Papavassiliou & Papavassiliou, 2016). Another approach might be to target genes and proteins regulated by such transcription factors and that link the increase in cAMP to functional activities in dendritic cells.
Proteins downstream of cAMP and PKA may be alternative targets to increase selectivity. Increased cAMP concentrations in dendritic cells result in decreased Th2 differentiation by PKA but not Epac (Lee et al., 2015; 2020). Perhaps a target whose transcription or action is regulated by PKA‐phosphorylated substrates might be druggable.
In addition, dendritic cell subsets (e.g. plasmacytoid dendritic cells and conventional dendritic cells [cDC1 and cDC2]) possess distinct functions and can be defined by cellular markers and expression of transcription factors (Collin & Bigley, 2018; Mildner & Jung, 2014). cDC2s preferentially prime naïve T cells to differentiate into Th2 and Th17 cells and have been shown to be involved in allergic lung inflammation (Moon et al., 2018). Furthermore, cDC2s express IRF4, which may enhance selectivity of response.
Considering the challenges associated with targeting transcription factors, cell surface receptors (e.g. GPCRs) or downstream proteins that mediate cAMP actions in dendritic cells likely have the best potential as successful therapies for asthma and other allergic disorders.
A concern for identifying therapeutic targets is that most data regarding the role of cAMP concentration in dendritic cells on Th2 immunity have been obtained with murine cells. Since differences have been identified between mouse and human dendritic cells, future studies need to be conducted in primary human dendritic cells (Shortman & Liu, 2002).
Currently, the only therapy that specifically targets dendritic cells is Sipuleucel‐T, a personalized dendritic cell vaccine, which is approved to treat prostate cancer (Anassi & Ndefo, 2011). In brief, a patient's antigen presenting cells are collected and then incubated ex vivo with GM‐CSF (a cytokine that promotes dendritic cell maturation) and Sipuleucel‐T, a recombinant protein that contains prostatic acid phosphatase, an antigen present in many prostate cancer cells. The dendritic cells are subsequently infused into the patient. Other dendritic cell vaccines have been generated and investigated in clinical trials, but small molecule and biologics targeted to dendritic cells have not yet been explored (Hackstein & Thomson, 2004; Mastelic‐Gavillet, Balint, Boudousquie, Gannon, & Kandalaft, 2019; Tesfaye, Gudjonsson, Bogen, & Fossum, 2019).
7. SUMMARY AND CONCLUSIONS
Asthma, an inflammatory disorder in the lungs, is characterized by airway hyperresponsiveness and reduced airflow. Asthma is very common, affecting 235 million people globally. Current therapy for asthma focuses on inhaled corticosteroids and β2 agonists, agents that treat the clinical features but not the underlying disease mechanisms. These therapies are unable to control asthmatic symptoms in ~50% of adult patients, necessitating the search for new, effective, and safe therapies for asthma and other allergic disorders.
Certain therapeutics currently approved for the treatment of asthma increase levels of cAMP in the airway. cAMP levels regulate pro‐ and anti‐inflammatory effects on immune cells; increased cAMP concentrations are generally associated with reduced inflammation. Dendritic cells represent important targets for modulating allergic disease due to their role in inducing Th2 differentiation. While current asthma treatments focus on raising cAMP in airway smooth muscle cells to promote bronchodilation (a manifestation of asthma), we propose that drugs designed to selectively raise cAMP levels in dendritic cells could be a novel approach for disease‐modifying therapies for allergic asthma by targeting a key underlying cause of its pathobiology. We discuss various means to develop dendritic cell‐targeted therapeutics to accomplish this. Experiments investigating the translatability of data in murine dendritic cells to human cells need to be undertaken, as well as studies to determine how best to increase cAMP concentrations in dendritic cells without increasing Th17 differentiation, a potentially undesirable side effect. The proposed targeting of dendritic cells in asthma and other allergic disorders would, we believe, be a novel disease‐modifying approach. The current dearth of knowledge regarding potentially druggable targets in the cAMP pathway in dendritic cells creates new opportunities for preclinical and clinical studies, and ultimately could yield new therapeutics to improve the care of asthmatic patients.
7.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018) and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos, et al., 2019; Alexander, Fabbro, et al., 2019).
CONFLICT OF INTEREST
The authors have no conflicts of interest.
ACKNOWLEDGEMENTS
This work was supported in part by grants from the National Institutes of Health R56AI110505 and T32GM007752. The authors thank their UCSD colleagues Eyal Raz, Jihung Lee, and Nicholas Webster for many useful discussions regarding this topic.
Chinn AM, Insel PA. Cyclic AMP in dendritic cells: A novel potential target for disease‐modifying agents in asthma and other allergic disorders. Br J Pharmacol. 2020;177:3363–3377. 10.1111/bph.15095
After acceptance of this article, the authors found prior work on this topic, as summarized in a review regarding the ability of certain prostanoids to elevate cAMP and blunt pro‐allergic actions of antigen presenting cells (dendritic cells) in asthma ( Debeuf, N. , Lambrecht, B. N. (2018). Eicosanoid control over antigen presenting cells in asthma. Front Immunol, 9, 2006 10.3389/fimmu.2018.02006 ).
REFERENCES
- Aandahl, E. M. , Moretto, W. J. , Haslett, P. A. , Vang, T. , Bryn, T. , Tasken, K. , & Nixon, D. F. (2002). Inhibition of antigen‐specific T cell proliferation and cytokine production by protein kinase A type I. Journal of Immunology (Baltimore, Md. : 1950), 169(2), 802–808. 10.4049/jimmunol.169.2.802 [DOI] [PubMed] [Google Scholar]
- Akinbami, L. J. , Simon, A. E. , & Rossen, L. M. (2016). Changing trends in asthma prevalence among children. Pediatrics, 137(1). 10.1542/peds.2015-2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … Yao, C. (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(S1), S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … Watts, V. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176(S1), S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anassi, E. , & Ndefo, U. A. (2011). Sipuleucel‐T (Provenge) injection the first immunotherapy agent (Vaccine) for hormone‐refractory prostate cancer. P and T, 36(4), 197–202. [PMC free article] [PubMed] [Google Scholar]
- Anderson, H. R. , Gupta, R. , Strachan, D. P. , & Limb, E. S. (2007). 50 years of asthma: UK trends from 1955 to 2004. Thorax, 62(1), 85–90. 10.1136/thx.2006.066407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, W. C. , & Szefler, S. J. (2019). Cost‐effectiveness and comparative effectiveness of biologic therapy for asthma: To biologic or not to biologic? In Annals of allergy, asthma and immunology (Vol. 122, Issue 4) (pp. 367–372). American College of Allergy, Asthma and Immunology; 10.1016/j.anai.2019.01.018 [DOI] [PubMed] [Google Scholar]
- Aronoff, D. M. , Canetti, C. , Serezani, C. H. , Luo, M. , & Peters‐Golden, M. (2005). Cutting edge: Macrophage inhibition by cyclic AMP (cAMP): Differential roles of protein kinase A and exchange protein directly activated by cAMP‐1. The Journal of Immunology, 174(2), 595–599. 10.4049/jimmunol.174.2.595 [DOI] [PubMed] [Google Scholar]
- Bagnasco, D. , Ferrando, M. , Varricchi, G. , Passalacqua, G. , & Canonica, G. W. (2016). A critical evaluation of anti‐IL‐13 and anti‐IL‐4 strategies in severe asthma. International Archives of Allergy and Immunology, 170(2), 122–131. 10.1159/000447692 [DOI] [PubMed] [Google Scholar]
- Bahadori, K. , Doyle‐Waters, M. M. , Marra, C. , Lynd, L. , Alasaly, K. , Swiston, J. , & FitzGerald, J. M. (2009). Economic burden of asthma: A systematic review. BMC Pulmonary Medicine, 9(1), 24 10.1186/1471-2466-9-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau, J. , Briere, F. , Caux, C. , Davoust, J. , Lebecque, S. , Liu, Y.‐J. , … Palucka, K. (2000). Immunobiology of dendritic cells. Annual Review of Immunology, 18(1), 767–811. 10.1146/annurev.immunol.18.1.767 [DOI] [PubMed] [Google Scholar]
- Barnes, P. J. (2011). Biochemical basis of asthma therapy. The Journal of Biological Chemistry, 286(38), 32,899–32,905. 10.1074/jbc.R110.206466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, L. A. , Marcotte, G. V. , MacGlashan, D. , Togias, A. , & Saini, S. (2004). Omalizumab‐induced reductions in mast cell FcεRI expression and function. Journal of Allergy and Clinical Immunology, 114(3), 527–530. 10.1016/j.jaci.2004.06.032 [DOI] [PubMed] [Google Scholar]
- Beghè, B. , Rabe, K. F. , & Fabbri, L. M. (2013). Phosphodiesterase‐4 inhibitor therapy for lung diseases. American Journal of Respiratory and Critical Care Medicine, 188(3), 271–278. 10.1164/rccm.201301-0021PP [DOI] [PubMed] [Google Scholar]
- Beltejar, M. C. G. , Lau, H. T. , Golkowski, M. G. , Ong, S. E. , & Beavo, J. A. (2017). Analyses of PDE‐regulated phosphoproteomes reveal unique and specific cAMP‐signaling modules in T cells. Proceedings of the National Academy of Sciences of the United States of America, 114(30), E6240–E6249. 10.1073/pnas.1703939114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, A. T. , & Beavo, J. A. (2006). Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacological Reviews, 58(3), 488–520. 10.1124/pr.58.3.5 [DOI] [PubMed] [Google Scholar]
- Billington, C. K. , Ojo, O. O. , Penn, R. B. , & Ito, S. (2013). cAMP regulation of airway smooth muscle function. Pulmonary Pharmacology & Therapeutics, 26(1), 112–120. 10.1016/j.pupt.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor, J. , Bopp, T. , Vaeth, M. , Klein, M. , Serfling, E. , Hünig, T. , … Schmitt, E. (2012). Cyclic AMP underpins suppression by regulatory T cells. European Journal of Immunology, 42(6), 1375–1384. 10.1002/eji.201141578 [DOI] [PubMed] [Google Scholar]
- Bopp, T. , Becker, C. , Klein, M. , Klein‐Hessling, S. , Palmetshofer, A. , Serfling, E. , … Schmitt, E. (2007). Cyclic adenosine monophosphate is a key component of regulatory T cell‐mediated suppression. The Journal of Experimental Medicine, 204(6), 1303–1310. 10.1084/jem.20062129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, C. , Pearce, N. , & Douwes, J. (2013). The hygiene hypothesis in allergy and asthma. Current Opinion in Allergy and Clinical Immunology, 13(1), 70–77. 10.1097/ACI.0b013e32835ad0d2 [DOI] [PubMed] [Google Scholar]
- Cabanas, C. , & Sanchez‐Madrid, F. (1999). CD11c (leukocyte integrin CR4 α subunit). Journal of Biological Regulators and Homeostatic Agents, 13(2), 134–136. [PubMed] [Google Scholar]
- Carr, T. F. , Zeki, A. A. , & Kraft, M. (2018). Eosinophilic and noneosinophilic asthma. American Journal of Respiratory and Critical Care Medicine, 197(1), 22–37. 10.1164/rccm.201611-2232PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, M. , Corren, J. , Pavord, I. D. , Maspero, J. , Wenzel, S. , Rabe, K. F. , … Teper, A. (2018). Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. New England Journal of Medicine, 378(26), 2486–2496. 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . (2014). Uncontrolled asthma among persons with current asthma. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf
- Cheng, X. , Ji, Z. , Tsalkova, T. , & Mei, F. (2008). Epac and PKA: A tale of two intracellular cAMP receptors. Acta Biochimica et Biophysica Sinica, 40(7), 651–662. 10.1111/j.1745-7270.2008.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy, D. F. , Hart, K. M. , Borthwick, L. A. , Shikotra, A. , Nagarkar, D. R. , Siddiqui, S. , … Bradding, P. (2015). TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Science Translational Medicine, 7(301), 301ra129. 10.1126/scitranslmed.aab3142 [DOI] [PubMed] [Google Scholar]
- Cohn, L. , Elias, J. A. , & Chupp, G. L. (2004). Asthma: Mechanisms of disease persistence and progression. Annual Review of Immunology, 22(1), 789–815. 10.1146/annurev.immunol.22.012703.104716 [DOI] [PubMed] [Google Scholar]
- Collin, M. , & Bigley, V. (2018). Human dendritic cell subsets: An update. Immunology, 154(1), 3–20. 10.1111/imm.12888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copsel, S. , Garcia, C. , Diez, F. , Vermeulem, M. , Baldi, A. , Bianciotti, L. G. , … Davio, C. (2011). Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation. The Journal of Biological Chemistry, 286(9), 6979–6988. 10.1074/jbc.M110.166868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle, A. J. , Ackerman, S. J. , Burch, R. , Proud, D. , & Irvin, C. G. (1995). Human eosinophil‐granule major basic protein and synthetic polycations induce airway hyperresponsiveness in vivo dependent on bradykinin generation. Journal of Clinical Investigation, 95(4), 1735–1740. 10.1172/JCI117850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher, S. , Santos, L. M. , Solé, D. , De Lima, M. G. , Naspitz, C. K. , & Musatti, C. C. (1995). Interleukin‐4 and soluble CD23 serum levels in asthmatic atopic children. Journal of Investigational Allergology & Clinical Immunology, 5(5), 251–254. http://www.ncbi.nlm.nih.gov/pubmed/8574430 [PubMed] [Google Scholar]
- De Bastiani, M. A. , Pfaffenseller, B. , & Klamt, F. (2018). Master regulators connectivity map: A transcription factors‐centered approach to drug repositioning. Frontiers in Pharmacology, 9(1), 697 10.3389/fphar.2018.00697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukanović, R. , Wilson, S. J. , Kraft, M. , Jarjour, N. N. , Steel, M. , Chung, K. F. , … Fahy, J. V. (2004). Effects of treatment with anti‐immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. American Journal of Respiratory and Critical Care Medicine, 170(6), 583–593. 10.1164/rccm.200312-1651OC [DOI] [PubMed] [Google Scholar]
- Emma, R. , Morjaria, J. B. , Fuochi, V. , Polosa, R. , & Caruso, M. (2018). Mepolizumab in the management of severe eosinophilic asthma in adults: Current evidence and practical experience In Therapeutic advances in respiratory disease (vol. 12). SAGE Publications Ltd; 10.1177/1753466618808490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban‐Gorgojo, I. , Antolín‐Amérigo, D. , Domínguez‐Ortega, J. , & Quirce, S. (2018). Non‐eosinophilic asthma: Current perspectives. Journal of Asthma and Allergy, 11, 267–281. 10.2147/JAA.S153097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajt, M. L. , & Wenzel, S. E. (2015). Asthma phenotypes and the use of biologic medications in asthma and allergic disease: The next steps toward personalized care. Journal of Allergy and Clinical Immunology, 135(2), 299–310. 10.1016/J.JACI.2014.12.1871 [DOI] [PubMed] [Google Scholar]
- Fassbender, M. , Gerlitzki, B. , Ullrich, N. , Lupp, C. , Klein, M. , Radsak, M. P. , … Schild, H. (2010). Cyclic adenosine monophosphate and IL‐10 coordinately contribute to nTreg cell‐mediated suppression of dendritic cell activation. Cellular Immunology, 265(2), 91–96. 10.1016/j.cellimm.2010.07.007 [DOI] [PubMed] [Google Scholar]
- Finkelsztejn, A. (2014). Multiple sclerosis: Overview of disease‐modifying agents. Perspectives in Medicinal Chemistry, 6, 65–72. 10.4137/PMC.S13213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgani, M. , De Rosa, V. , De Simone, S. , Leonardi, A. , D'Oro, U. , Napolitani, G. , … Racioppi, L. (2004). Cyclic AMP modulates the functional plasticity of immature dendritic cells by inhibiting Src‐like kinases through protein kinase A‐mediated signaling. The Journal of Biological Chemistry, 279(31), 32,507–32,514. 10.1074/jbc.M403355200 [DOI] [PubMed] [Google Scholar]
- Galli, S. J. , & Tsai, M. (2012). IgE and mast cells in allergic disease. Nature Medicine, 18(5), 693–704. 10.1038/nm.2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli, S. J. , Tsai, M. , & Piliponsky, A. M. (2008). The development of allergic inflammation. Nature, 454(7203), 445–454. 10.1038/nature07204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, M. , Xu, Y. , & Pearse, D. D. (2016). Cyclic AMP is a key regulator of M1 to M2a phenotypic conversion of microglia in the presence of Th2 cytokines. Journal of Neuroinflammation, 13(1), 9 10.1186/s12974-015-0463-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Initiative for Asthma . (2019). Global strategy for asthma management and prevention. www.ginasthma.org
- Goncharova, E. A. , Billington, C. K. , Irani, C. , Vorotnikov, A. V. , Tkachuk, V. A. , Penn, R. B. , … Panettieri, R. A. (2003). Cyclic AMP‐mobilizing agents and glucocorticoids modulate human smooth muscle cell migration. American Journal of Respiratory Cell and Molecular Biology, 29(1), 19–27. 10.1165/rcmb.2002-0254OC [DOI] [PubMed] [Google Scholar]
- Gould, H. J. , & Sutton, B. J. (2008). IgE in allergy and asthma today. Nature Reviews Immunology, 8(3), 205–217. 10.1038/nri2273 [DOI] [PubMed] [Google Scholar]
- Green, R. H. , Brightling, C. E. , & Bradding, P. (2007). The reclassification of asthma based on subphenotypes. Current Opinion in Allergy and Clinical Immunology, 7(1), 43–50. 10.1097/ACI.0b013e3280118a32 [DOI] [PubMed] [Google Scholar]
- Guermonprez, P. , Valladeau, J. , Zitvogel, L. , Théry, C. , & Amigorena, S. (2002). Antigen presentation and T cell stimulation by dendritic cells. Annual Review of Immunology, 20(1), 621–667. 10.1146/annurev.immunol.20.100301.064828 [DOI] [PubMed] [Google Scholar]
- Gundel, R. H. , Letts, L. G. , & Gleich, G. J. (1991). Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. Journal of Clinical Investigation, 87(4), 1470–1473. 10.1172/JCI115155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstein, H. , & Thomson, A. W. (2004). Dendritic cells: Emerging pharmacological targets of immunosuppressive drugs In Nature reviews immunology (Vol. 4, Issue 1) (pp. 24–34). Nature Publishing Group; 10.1038/nri1256 [DOI] [PubMed] [Google Scholar]
- Hamid, Q. , & Tulic, M. (2009). Immunobiology of asthma. Annual Review of Physiology, 71(1), 489–507. 10.1146/annurev.physiol.010908.163200 [DOI] [PubMed] [Google Scholar]
- Hankin, C. S. , Bronstone, A. , Wang, Z. , Small, M. B. , & Buck, P. (2013). Estimated prevalence and economic burden of severe, uncontrolled asthma in the United States. Journal of Allergy and Clinical Immunology, 131(2), AB126 10.1016/j.jaci.2012.12.1118 [DOI] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helft, J. , Böttcher, J. , Chakravarty, P. , Zelenay, S. , Huotari, J. , Schraml, B. U. , … Reis e Sousa, C. (2015). GM‐CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c+MHCII+ macrophages and dendritic cells. Immunity, 42(6), 1197–1211. 10.1016/J.IMMUNI.2015.05.018 [DOI] [PubMed] [Google Scholar]
- Hertz, A. L. , Bender, A. T. , Smith, K. C. , Gilchrist, M. , Amieux, P. S. , Aderem, A. , & Beavo, J. A. (2009). Elevated cyclic AMP and PDE4 inhibition induce chemokine expression in human monocyte‐derived macrophages. Proceedings of the National Academy of Sciences of the United States of America, 106(51), 21,978–21,983. 10.1073/pnas.0911684106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heystek, H. C. , Thierry, A. C. , Soulard, P. , & Moulon, C. (2003). Phosphodiesterase 4 inhibitors reduce human dendritic cell inflammatory cytokine production and Th 1‐polarizing capacity In International immunology (Vol. 15, Issue 7) (pp. 827–835). Oxford University Press; 10.1093/intimm/dxg079 [DOI] [PubMed] [Google Scholar]
- Hidi, R. , Timmermans, S. , Liu, E. , Schudt, C. , Dent, G. , Holgate, S. T. , & Djukanović, R. (2000). Phosphodiesterase and cyclic adenosine monophosphate‐dependent inhibition of T‐lymphocyte chemotaxis. The European Respiratory Journal, 15(2), 342–349. http://www.ncbi.nlm.nih.gov/pubmed/10706503, 10.1034/j.1399-3003.2000.15b21.x [DOI] [PubMed] [Google Scholar]
- Hirose, K. , Iwata, A. , Tamachi, T. , & Nakajima, H. (2017). Allergic airway inflammation: Key players beyond the Th2 cell pathway. Immunological Reviews, 278(1), 145–161. 10.1111/imr.12540 [DOI] [PubMed] [Google Scholar]
- Hoiting, B. H. , Meurs, H. , Schuiling, M. , Kuipers, R. , Elzinga, C. R. S. , & Zaagsma, J. (1996). Modulation of agonist‐induced phosphoinositide metabolism, Ca2+ signalling and contraction of airway smooth muscle by cyclic AMP‐dependent mechanisms. British Journal of Pharmacology, 117(3), 419–426. 10.1111/j.1476-5381.1996.tb15207.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden, N. S. , Bell, M. J. , Rider, C. F. , King, E. M. , Gaunt, D. D. , Leigh, R. , … Newton, R. (2011). 2‐Adrenoceptor agonist‐induced RGS2 expression is a genomic mechanism of bronchoprotection that is enhanced by glucocorticoids. Proceedings of the National Academy of Sciences, 108(49), 19,713–19,718. 10.1073/pnas.1110226108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino, M. , & Ohtawa, J. (2012). Effects of adding omalizumab, an anti‐immunoglobulin E antibody, on airway wall thickening in asthma. Respiration, 83(6), 520–528. 10.1159/000334701 [DOI] [PubMed] [Google Scholar]
- Hu, J. , Chen, J. , Ye, L. , Cai, Z. , Sun, J. , & Ji, K. (2018). Anti‐IgE therapy for IgE‐mediated allergic diseases: From neutralizing IgE antibodies to eliminating IgE+ B cells. Clinical and Translational Allergy, 8(1), 27 10.1186/s13601-018-0213-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel, P. A. , Wilderman, A. , Zambon, A. C. , Snead, A. N. , Murray, F. , Aroonsakool, N. , … Corriden, R. (2015). G protein‐coupled receptor (GPCR) expression in native cells: “Novel” endoGPCRs as physiologic regulators and therapeutic targets. Molecular Pharmacology, 88(1), 181–187. 10.1124/mol.115.098129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel, P. A. , Sriram, K. , Gorr, M. W. , Wiley, S. Z. , Michkov, A. , Salmerón, C. , & Chinn, A. M. (2019). GPCRomics: An approach to discover GPCR drug targets. Trends in Pharmacological Sciences, 40(6), 378–387. 10.1016/j.tips.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, K. , & Bahna, S. L. (2020). Hypersensitivity and adverse reactions to biologics for asthma and allergic diseases. Expert Review of Clinical Immunology, 16, 311–319. 10.1080/1744666x.2020.1724089 [DOI] [PubMed] [Google Scholar]
- Jackson, M. (2008). Asthma, illness, and identity. Lancet, 372(9643), 1030–1031. 10.1016/s0140-6736(08)61429-4 [DOI] [PubMed] [Google Scholar]
- Janssen, L. J. , & Killian, K. (2006). Airway smooth muscle as a target of asthma therapy: History and new directions. Respiratory Research, 7(1), 123 10.1186/1465-9921-7-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keglowich, L. F. , & Borger, P. (2015). The three A's in asthma—Airway smooth muscle, airway remodeling & angiogenesis. The Open Respiratory Medicine Journal, 9, 70–80. 10.2174/1874306401509010070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper, A. R. , Van Mater, H. A. , Coeytaux, R. R. , Williams, J. W. , & Sanders, G. D. (2012). Systematic review of disease‐modifying antirheumatic drugs for juvenile idiopathic arthritis. BMC Pediatrics, 12, 29 10.1186/1471-2431-12-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman, D. A. , Huang, X. , Koth, L. L. , Chang, G. H. , Dolganov, G. M. , Zhu, Z. , … Erle, D. J. (2002). Direct effects of interleukin‐13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nature Medicine, 8(8), 885–889. 10.1038/nm734 [DOI] [PubMed] [Google Scholar]
- Lambert, M. , Jambon, S. , Depauw, S. , & David‐Cordonnier, M. H. (2018). Targeting transcription factors for cancer treatment In Molecules (Vol. 23, Issue 6). MDPI AG; 10.3390/molecules23061479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Kim, T. H. , Murray, F. , Li, X. , Choi, S. S. , Broide, D. H. , … Raz, E. (2015). Cyclic AMP concentrations in dendritic cells induce and regulate Th2 immunity and allergic asthma. Proceedings of the National Academy of Sciences, 112(5), 1529–1534. 10.1073/pnas.1417972112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Zhang, J. , Chung, Y.‐J. , Kim, J. H. , Kook, C. M. , González‐Navajas, J. M. , … Raz, E. (2020). Inhibition of IRF4 in dendritic cells by PRR‐independent and ‐dependent signals inhibit Th2 and promote Th17 responses. eLife, 9, e49416 10.7554/eLife.49416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler, J. , Read, J. F. , Jones, A. C. , Mok, D. , Hollams, E. M. , Laing, I. A. , … Strickland, D. H. (2019). Progressive increase of FcεRI expression across several PBMC subsets is associated with atopy and atopic asthma within school‐aged children. Pediatric Allergy and Immunology, 30(6), 646–653. 10.1111/pai.13063 [DOI] [PubMed] [Google Scholar]
- Legler, D. F. , Krause, P. , Scandella, E. , Singer, E. , & Groettrup, M. (2006). Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. The Journal of Immunology, 176(2), 966–973. 10.4049/jimmunol.176.2.966 [DOI] [PubMed] [Google Scholar]
- Levy, F. O. , Rasmussen, A.‐M. , Taskén, K. , Skålhegg, B. S. , Huitfeldt, H. S. , Funderud, S. , … Hansson, V. (1996). Cyclic AMP‐dependent protein kinase (cAK) in human B cells: Co‐localization of type I cAK (RIα2C2) with the antigen receptor during anti‐immunoglobulin‐induced B cell activation. European Journal of Immunology, 26(6), 1290–1296. 10.1002/eji.1830260617 [DOI] [PubMed] [Google Scholar]
- Li, H. , Zuo, J. , & Tang, W. (2018). Phosphodiesterase‐4 inhibitors for the treatment of inflammatory diseases. Frontiers in Pharmacology, 9, 1048 10.3389/fphar.2018.01048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, S. C. , Long, A. J. , Bennett, F. , Whitters, M. J. , Karim, R. , Collins, M. , … Fouser, L. A. (2007). An IL‐17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. The Journal of Immunology, 179(11), 7791–7799. 10.4049/jimmunol.179.11.7791 [DOI] [PubMed] [Google Scholar]
- Lipscomb, M. F. , & Masten, B. J. (2002). Dendritic cells: Immune regulators in health and disease. Physiological Reviews, 82(1), 97–130. 10.1152/physrev.00023.2001 [DOI] [PubMed] [Google Scholar]
- Lipworth, B. J. (2005). Phosphodiesterase‐4 inhibitors for asthma and chronic obstructive pulmonary disease. The Lancet, 365(9454), 167–175. 10.1016/S0140-6736(05)17708-3 [DOI] [PubMed] [Google Scholar]
- Liu, A. H. (2015). Revisiting the hygiene hypothesis for allergy and asthma. Journal of Allergy and Clinical Immunology, 136(4), 860–865. 10.1016/j.jaci.2015.08.012 [DOI] [PubMed] [Google Scholar]
- Lötvall, J. , Akdis, C. A. , Bacharier, L. B. , Bjermer, L. , Casale, T. B. , Custovic, A. , … Greenberger, P. A. (2011). Asthma endotypes: A new approach to classification of disease entities within the asthma syndrome. Journal of Allergy and Clinical Immunology, 127(2), 355–360. 10.1016/J.JACI.2010.11.037 [DOI] [PubMed] [Google Scholar]
- Luo, J. , Yang, L. , Yang, J. , Yang, D. , Liu, B.‐C. , Liu, D. , … Liu, C.‐T. (2018). Efficacy and safety of phosphodiesterase 4 inhibitors in patients with asthma: A systematic review and meta‐analysis. Respirology, 23(5), 467–477. 10.1111/resp.13276 [DOI] [PubMed] [Google Scholar]
- MacGlashan, D. W. , Bochner, B. S. , Adelman, D. C. , Jardieu, P. M. , Togias, A. , McKenzie‐White, J. , … Lichtenstein, L. M. (1997). Down‐regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti‐IgE antibody. Journal of Immunology (Baltimore, Md. : 1950), 158(3), 1438–1445. http://www.ncbi.nlm.nih.gov/pubmed/9013989 [PubMed] [Google Scholar]
- Makino, S. , Ikemori, K. , Kashima, T. , & Fukuda, T. (1977). Comparison of cyclic adenosine monophosphate response of lymphocytes in normal and asthmatic subjects to norepinephrine and salbutamol. The Journal of Allergy and Clinical Immunology, 59(5), 348–352. 10.1016/0091-6749(77)90016-1 [DOI] [PubMed] [Google Scholar]
- Marketos, S. G. , & Ballas, C. N. (1982). Bronchial asthma in the medical literature of Greek antiquity. The Journal of Asthma: Official Journal of the Association for the Care of Asthma, 19(4), 263–269. http://www.ncbi.nlm.nih.gov/pubmed/6757243, 10.3109/02770908209104771 [DOI] [PubMed] [Google Scholar]
- Mastelic‐Gavillet, B. , Balint, K. , Boudousquie, C. , Gannon, P. O. , & Kandalaft, L. E. (2019). Personalized dendritic cell vaccines—Recent breakthroughs and encouraging clinical results In Frontiers in immunology (Vol. 10, Issue APR) (p. 766). Frontiers Media S.A; 10.3389/fimmu.2019.00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavissakalian, M. , & Brady, S. (2020). The current state of biologic therapies for treatment of refractory asthma In Clinical reviews in allergy and immunology (pp. 1–13). Springer; 10.1007/s12016-020-08776-8 [DOI] [PubMed] [Google Scholar]
- McBrien, C. N. , & Menzies‐Gow, A. (2017). The biology of eosinophils and their role in asthma. Frontiers in Medicine, 4, 93 10.3389/fmed.2017.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern, A. E. , Robusto, J. , Rakoczy, J. , Simmons, D. G. , Phipps, S. , & Mazzone, S. B. (2014). The effect of hyperpolarization‐activated cyclic nucleotide‐gated ion channel inhibitors on the vagal control of guinea pig airway smooth muscle tone. British Journal of Pharmacology, 171(15), 3633–3650. 10.1111/bph.12745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley, L. , Alcorn, J. F. , Peterson, A. , Dupont, R. B. , Kapadia, S. , Logar, A. , … Kolls, J. K. (2008). TH17 cells mediate steroid‐resistant airway inflammation and airway hyperresponsiveness in mice. Journal of Immunology (Baltimore, Md. : 1950), 181(6), 4089–4097. 10.4049/jimmunol.181.6.4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad, M. , Sathe, P. , Helft, J. , Miller, J. , & Mortha, A. (2013). The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annual Review of Immunology, 31, 563–604. 10.1146/annurev-immunol-020711-074950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner, A. , & Jung, S. (2014). Immunity review development and function of dendritic cell subsets classical dendritic cells (cDCs) form a critical interface between innate and adaptive immunity. 10.1016/j.immuni.2014.04.016 [DOI] [PubMed]
- Moon, H.‐G. , Kim, S.‐j. , Jeong, J. J. , Han, S.‐S. , Jarjour, N. N. , Lee, H. , … Park, G. Y. (2018). Airway epithelial cell‐derived colony stimulating factor‐1 promotes allergen sensitization. Immunity, 49(2), 275–287.e5. 10.1016/j.immuni.2018.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, H. G. , Rusnak, S. A. , & Barzens, K. (1977). Leukocyte cyclic adenosine monophosphate in asthmatic children Effects of adrenergic therapy. Clinical Pharmacology & Therapeutics, 22(3), 352–357. 10.1002/cpt1977223352 [DOI] [PubMed] [Google Scholar]
- Negreiros‐Lima, G. L. , Lima, K. M. , Moreira, I. Z. , Jardim, B. L. O. , Vago, J. P. , Galvão, I. , … Sousa, L. P. (2020). Cyclic AMP regulates key features of macrophages via PKA: Recruitment, reprogramming and efferocytosis. Cell, 9(1), 128 10.3390/cells9010128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes, C. , Pereira, A. M. , & Morais‐Almeida, M. (2017). Asthma costs and social impact. Asthma Research and Practice, 3, 1 10.1186/s40733-016-0029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, J. B. , Wilkinson, J. C. , & Roman, D. L. (2019). Regulator of G‐protein signaling (RGS) proteins as drug targets: Progress and future potentials. Journal of Biological Chemistry, 294(49), 18,571–18,585. 10.1074/jbc.REV119.007060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir, C. , Kucuksezer, U. C. , Akdis, M. , & Akdis, C. A. (2018). The concepts of asthma endotypes and phenotypes to guide current and novel treatment strategies. Expert Review of Respiratory Medicine, 12(9), 733–743. 10.1080/17476348.2018.1505507 [DOI] [PubMed] [Google Scholar]
- Papavassiliou, K. A. , & Papavassiliou, A. G. (2016). Transcription factor drug targets. Journal of Cellular Biochemistry, 117(12), 2693–2696. 10.1002/jcb.25605 [DOI] [PubMed] [Google Scholar]
- Parker, C. W. , & Smith, J. W. (1973). Alterations in cyclic adenosine monophosphate metabolism in human bronchial asthma. I. Leukocyte responsiveness to β‐adrenergic agents. The Journal of Clinical Investigation, 52(1), 48–59. 10.1172/JCI107173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawankar, R. , Canonica, G. W. , Holgate, S. T. , Lockey, F. , & Blaiss, M. (2013). WAO White Book on Allergy 2013 Update WAO White Book on Allergy. https://www.worldallergy.org/UserFiles/file/WhiteBook2-2013-v8.pdf
- Penn, R. B. , Parent, J.‐L. , Pronin, A. N. , Panettieri, R. A. , & Benovic, J. L. (1999). Pharmacological inhibition of protein kinases in intact cells: Antagonism of beta adrenergic receptor ligand binding by H‐89 reveals limitations of usefulness. The Journal of Pharmacology and Experimental Therapeutics, 288(2), 428–437. http://www.jpet.org [PubMed] [Google Scholar]
- Peters‐Golden, M. (2009). Putting on the brakes: Cyclic AMP as a multipronged controller of macrophage function. Science Signaling, 2(75), pe37 10.1126/scisignal.275pe37 [DOI] [PubMed] [Google Scholar]
- Pierre, S. , Eschenhagen, T. , Geisslinger, G. , & Scholich, K. (2009). Capturing adenylyl cyclases as potential drug targets. Nature Reviews Drug Discovery, 8(4), 321–335. 10.1038/nrd2827 [DOI] [PubMed] [Google Scholar]
- Prussin, C. , Griffith, D. T. , Boesel, K. M. , Lin, H. , Foster, B. , & Casale, T. B. (2003). Omalizumab treatment downregulates dendritic cell FcεRI expression. Journal of Allergy and Clinical Immunology, 112(6), 1147–1154. 10.1016/j.jaci.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Puhr, S. , Lee, J. , Zvezdova, E. , Zhou, Y. J. , & Liu, K. (2015). Dendritic cell development—History, advances, and open questions. Seminars in Immunology, 27(6), 388–396. 10.1016/j.smim.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raker, V. K. , Becker, C. , & Steinbrink, K. (2016). The cAMP pathway as therapeutic target in autoimmune and inflammatory diseases. Frontiers in Immunology, 7, 123 10.3389/fimmu.2016.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall, T. W. , & Sutherland, E. W. (1958). Formation of a cyclic adenine ribonucleotide by tissue particles. The Journal of Biological Chemistry, 232(2), 1065–1076. http://www.ncbi.nlm.nih.gov/pubmed/13549487 [PubMed] [Google Scholar]
- Ray, A. , & Kolls, J. K. (2017). Neutrophilic inflammation in asthma and association with disease severity. Trends in Immunology, 38(12), 942–954. 10.1016/j.it.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, P. J. , Lavelle, E. C. , Mills, K. H. G. , & Boyd, A. P. (2004). Adenylate cyclase toxin from Bordetella pertussis synergizes with lipopolysaccharide to promote innate interleukin‐10 production and enhances the induction of Th2 and regulatory T cells. Infection and Immunity, 72(3), 1568–1579. 10.1128/iai.72.3.1568-1579.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman, E. M. (2012). Overview of safety of non‐biologic and biologic DMARDs. Rheumatology, 51(suppl 6), vi37–vi43. 10.1093/rheumatology/kes283 [DOI] [PubMed] [Google Scholar]
- Rueda, C. M. , Jackson, C. M. , & Chougnet, C. A. (2016). Regulatory T‐cell‐mediated suppression of conventional T‐cells and dendritic cells by different cAMP intracellular pathways. Frontiers in Immunology, 7, 216 10.3389/fimmu.2016.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel, F. G. M. , Koenderink, J. B. , & Masereeuw, R. (2008). Multidrug resistance protein 4 (MRP4/ABCC4): A versatile efflux transporter for drugs and signalling molecules. Trends in Pharmacological Sciences, 29(4), 200–207. 10.1016/J.TIPS.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Samitas, K. , Delimpoura, V. , Zervas, E. , & Gaga, M. (2015). Anti‐IgE treatment, airway inflammation and remodelling in severe allergic asthma: Current knowledge and future perspectives In European respiratory review (Vol. 24, Issue 138) (pp. 594–601). European Respiratory Society; 10.1183/16000617.00001715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleimer, R. P. , Sterbinsky, S. A. , Kaiser, J. , Bickel, C. A. , Klunk, D. A. , Tomioka, K. , … McIntyre, B. W. (1992). IL‐4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. Association with expression of VCAM‐1. Journal of Immunology (Baltimore, Md. : 1950), 148(4), 1086–1092. http://www.ncbi.nlm.nih.gov/pubmed/1371130 [PubMed] [Google Scholar]
- Schmidt, M. , Dekker, F. J. , & Maarsingh, H. (2013). Exchange protein directly activated by cAMP (epac): A multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacological Reviews, 65(2), 670–709. 10.1124/pr.110.003707 [DOI] [PubMed] [Google Scholar]
- Schroeder, J. T. , Bieneman, A. P. , Chichester, K. L. , Hamilton, R. G. , Xiao, H. Q. , Saini, S. S. , & Liu, M. C. (2010). Decreases in human dendritic cell‐dependent TH2‐like responses after acute in vivo IgE neutralization. Journal of Allergy and Clinical Immunology, 125(4), 896–901.e6. 10.1016/j.jaci.2009.10.021 [DOI] [PubMed] [Google Scholar]
- Shabb, J. B. (2001). Physiological substrates of cAMP‐dependent protein kinase. Chemical Reviews, 101(8), 2381–2411. 10.1021/cr000236l [DOI] [PubMed] [Google Scholar]
- Shortman, K. , & Liu, Y. J. (2002). Mouse and human dendritic cell subtypes In Nature reviews immunology (Vol. 2, Issue 3) (pp. 151–161). Nature Publishing Group; 10.1038/nri746 [DOI] [PubMed] [Google Scholar]
- Simpson, C. R. , Newton, J. , Hippisley‐Cox, J. , & Sheikh, A. (2008). Incidence and prevalence of multiple allergic disorders recorded in a national primary care database. Journal of the Royal Society of Medicine, 101(11), 558–563. 10.1258/jrsm.2008.080196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigalia, F. , & D'Ambrosio, D. (2000). Regulation of helper T cell differentiation and recruitment in airway inflammation. American Journal of Respiratory and Critical Care Medicine, 162(supplement_3), S157–S160. 10.1164/ajrccm.162.supplement_3.15tac3 [DOI] [PubMed] [Google Scholar]
- Slager, R. E. , Otulana, B. A. , Hawkins, G. A. , Yen, Y. P. , Peters, S. P. , Wenzel, S. E. , … Bleecker, E. R. (2012). IL‐4 receptor polymorphisms predict reduction in asthma exacerbations during response to an anti‐IL‐4 receptor α antagonist. Journal of Allergy and Clinical Immunology, 130(2), 516–522.e4. 10.1016/j.jaci.2012.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina, D. (2008). PDE4 inhibitors: Current status. British Journal of Pharmacology, 155(3), 308–315. 10.1038/bjp.2008.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram, K. , & Insel, P. A. (2018). G protein‐coupled receptors as targets for approved drugs: How many targets and how many drugs? Molecular Pharmacology, 93(4), 251–258. 10.1124/mol.117.111062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman, R. M. , & Cohn, Z. A. (1973). Identification of a novel cell type in peripheral lymphoid organs in mice: I. Morphology, quantitation, tissue distribution. Journal of Experimental Medicine, 137(5), 1142–1162. 10.1084/jem.137.5.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman, R. M. , & Cohn, Z. A. (1974). Identification of a novel cell type in peripheral lymphoid organs in mice: II. Functional properties in vitro. Journal of Experimental Medicine, 139(2), 380–397. 10.1084/jem.139.2.380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman, R. M. (2012). Decisions about dendritic cells: Past, present, and future. Annual Review of Immunology, 30(1), 1–22. 10.1146/annurev-immunol-100311-102839 [DOI] [PubMed] [Google Scholar]
- Sutherland, E. W. , & Rall, T. (1958). Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. The Journal of Biological Chemistry, 232(2), 1077–1091. http://www.ncbi.nlm.nih.gov/pubmed/13549488 [PubMed] [Google Scholar]
- Svenningsen, S. , & Nair, P. (2017). Asthma endotypes and an overview of targeted therapy for asthma. Frontiers in Medicine, 4, 158 10.3389/fmed.2017.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri, T. , Niimi, A. , Matsumoto, H. , Ito, I. , Oguma, T. , Otsuka, K. , … Mishima, M. (2014). Comprehensive efficacy of omalizumab for severe refractory asthma: A time‐series observational study. Annals of Allergy, Asthma & Immunology, 113(4), 470–475.e2. 10.1016/j.anai.2014.06.004 [DOI] [PubMed] [Google Scholar]
- Takaku, Y. , Soma, T. , Nishihara, F. , Nakagome, K. , Kobayashi, T. , Hagiwara, K. , … Nagata, M. (2013). Omalizumab attenuates airway inflammation and interleukin‐5 production by mononuclear cells in patients with severe allergic asthma. International Archives of Allergy and Immunology, 161(s2), 107–117. 10.1159/000350852 [DOI] [PubMed] [Google Scholar]
- Tanaka, A. (2015). Past, present and future therapeutics of asthma: A review. Journal of General and Family Medicine, 16(3), 158–169. 10.14442/jgfm.16.3_158 [DOI] [Google Scholar]
- Taylor, S. S. , Yang, J. , Wu, J. , Haste, N. M. , Radzio‐Andzelm, E. , & Anand, G. (2004). PKA: A portrait of protein kinase dynamics. Biochimica et Biophysica Acta (BBA) ‐ Proteins and Proteomics, 1697(1–2), 259–269. 10.1016/J.BBAPAP.2003.11.029 [DOI] [PubMed] [Google Scholar]
- Tesfaye, D. Y. , Gudjonsson, A. , Bogen, B. , & Fossum, E. (2019). Targeting conventional dendritic cells to fine‐tune antibody responses In Frontiers in immunology (Vol. 10, Issue JULY) (p. 1529). Frontiers Media S.A; 10.3389/fimmu.2019.01529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To, T. , Stanojevic, S. , Moores, G. , Gershon, A. S. , Bateman, E. D. , Cruz, A. A. , & Boulet, L.‐P. (2012). Global asthma prevalence in adults: Findings from the cross‐sectional world health survey. BMC Public Health, 12(1), 204 10.1186/1471-2458-12-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkildsen, O. , Myhr, K. M. , & Bø, L. (2016). Disease‐modifying treatments for multiple sclerosis—A review of approved medications. European Journal of Neurology, 23(Suppl 1), 18–27. 10.1111/ene.12883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, T. N. , Zeiger, R. S. , Peters, S. P. , Colice, G. , Newbold, P. , Goldman, M. , & Chipps, B. E. (2016). Overlap of atopic, eosinophilic, and TH2‐high asthma phenotypes in a general population with current asthma. Annals of Allergy, Asthma & Immunology, 116(1), 37–42. 10.1016/J.ANAI.2015.10.027 [DOI] [PubMed] [Google Scholar]
- Trian, T. , Burgess, J. K. , Niimi, K. , Moir, L. M. , Ge, Q. , Berger, P. , … Oliver, B. G. (2011). β2‐Agonist induced cAMP is decreased in asthmatic airway smooth muscle due to increased PDE4D. PLoS ONE, 6(5), e20000 10.1371/journal.pone.0020000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turalba, A. V. , Leite‐Morris, K. A. , & Kaplan, G. B. (2004). Antipsychotics regulate cyclic AMP‐dependent protein kinase and phosphorylated cyclic AMP response element‐binding protein in striatal and cortical brain regions in mice. Neuroscience Letters, 357(1), 53–57. 10.1016/j.neulet.2003.11.059 [DOI] [PubMed] [Google Scholar]
- Vaeth, M. , Gogishvili, T. , Bopp, T. , Klein, M. , Berberich‐Siebelt, F. , Gattenloehner, S. , … Bodor, J. (2011). Regulatory T cells facilitate the nuclear accumulation of inducible cAMP early repressor (ICER) and suppress nuclear factor of activated T cell c1 (NFATc1). Proceedings of the National Academy of Sciences of the United States of America, 108(6), 2480–2485. 10.1073/pnas.1009463108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer, W. M. , Osborne, M. L. , & Sonia Buist, A. (1998). 20‐year trends in the prevalence of asthma and chronic airflow obstruction in an HMO. American Journal of Respiratory and Critical Care Medicine, 157(4), 1079–1084. 10.1164/ajrccm.157.4.9704140 [DOI] [PubMed] [Google Scholar]
- Walker, C. , Bauer, W. , Braun, R. K. , Menz, G. , Braun, P. , Schwarz, F. , … Villiger, B. (1994). Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. American Journal of Respiratory and Critical Care Medicine, 150(4), 1038–1048. 10.1164/ajrccm.150.4.7921434 [DOI] [PubMed] [Google Scholar]
- Walsh, G. M. (2015). Mepolizumab‐based therapy in asthma: An update In Current opinion in allergy and clinical immunology (Vol. 15, Issue 4) (pp. 392–396). Lippincott Williams and Wilkins; 10.1097/ACI.0000000000000183 [DOI] [PubMed] [Google Scholar]
- Weber, J. , Illi, S. , Nowak, D. , Schierl, R. , Holst, O. , von Mutius, E. , & Ege, M. J. (2015). Asthma and the hygiene hypothesis. Does cleanliness matter? American Journal of Respiratory and Critical Care Medicine, 191(5), 522–529. 10.1164/rccm.201410-1899OC [DOI] [PubMed] [Google Scholar]
- Wen, J. , Luo, J. , Huang, W. , Tang, J. , Zhou, H. , & Zhang, W. (2015). The pharmacological and physiological role of multidrug‐resistant protein 4. The Journal of Pharmacology and Experimental Therapeutics, 354(3), 358–375. 10.1124/jpet.115.225656 [DOI] [PubMed] [Google Scholar]
- Wenzel, S. , Ford, L. , Pearlman, D. , Spector, S. , Sher, L. , Skobieranda, F. , … Pirozzi, G. (2013). Dupilumab in persistent asthma with elevated eosinophil levels. New England Journal of Medicine, 368(26), 2455–2466. 10.1056/NEJMoa1304048 [DOI] [PubMed] [Google Scholar]
- Wenzel, S. E. (2012). Asthma phenotypes: The evolution from clinical to molecular approaches. Nature Medicine, 18(5), 716–725. 10.1038/nm.2678 [DOI] [PubMed] [Google Scholar]
- Wenzel, S. E. , Schwartz, L. B. , Langmack, E. L. , Halliday, J. L. , Trudeau, J. B. , Gibbs, R. L. , & Chu, H. W. (1999). Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. American Journal of Respiratory and Critical Care Medicine, 160(3), 1001–1008. 10.1164/ajrccm.160.3.9812110 [DOI] [PubMed] [Google Scholar]
- Whisler, R. L. , Beiqing, L. , Grants, I. S. , & Newhouse, Y. G. (1992). Cyclic AMP modulation of human B cell proliferative responses: Role of cAMP‐dependent protein kinases in enhancing B cell responses to phorbol diesters and ionomycin. Cellular Immunology, 142(2), 398–415. 10.1016/0008-8749(92)90300-e [DOI] [PubMed] [Google Scholar]
- Woodruff, P. G. , Modrek, B. , Choy, D. F. , Jia, G. , Abbas, A. R. , Ellwanger, A. , … Fahy, J. V. (2009). T‐helper type 2‐driven inflammation defines major subphenotypes of asthma. American Journal of Respiratory and Critical Care Medicine, 180(5), 388–395. 10.1164/rccm.200903-0392OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2017). Asthma. https://www.who.int/news-room/fact-sheets/detail/asthma
- Yaghoubi, M. , Adibi, A. , Safari, A. , FitzGerald, J. M. , & Sadatsafavi, M. (2019). The projected economic and health burden of uncontrolled asthma in the United States. American Journal of Respiratory and Critical Care Medicinerccm.201901‐0016OC, 200, 1102–1112. 10.1164/rccm.201901-0016OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J. , Yamane, H. , & Paul, W. E. (2010). Differentiation of effector CD4 T cell populations. Annual Review of Immunology, 28, 445–489. 10.1146/annurev-immunol-030409-101212 [DOI] [PMC free article] [PubMed] [Google Scholar]


