Abstract
目的
分析miR155异常表达在多发性骨髓瘤(MM)化疗耐药机制中的作用,探讨靶向抑制miR155表达对耐阿霉素MM细胞株RPMI8226/DOX耐药性的影响,进一步分析其作用机制。
方法
通过浓度梯度递增法建立耐阿霉素MM细胞系RPMI8226/DOX;采用实时荧光定量PCR法检测RPMI8226/DOX细胞和MM敏感细胞株RPMI8226/S miR155基因表达,Western blot检测FOXO3a、BCL-2蛋白表达。在RPMI8226/DOX细胞中分别转染miR155抑制物和模拟物,通过实时荧光定量PCR法检测miR155抑制物和模拟物的转染效率,应用CCK-8法检测转染后细胞对阿霉素的敏感性。在靶向抑制物干预MM细胞后,Western blot分析FOXO3a、BCL-2通路蛋白表达的变化。
结果
①RPMI8226/DOX细胞miR155相对表达量为RPMI8226/S细胞的(26.860±2.340)倍,BCL-2蛋白表达上调,FOXO3a蛋白表达下调。②靶向抑制miR155表达72 h后,转染抑制率为64.57%,miR155基因表达下调,FOXO3a蛋白表达上调,BCL-2蛋白表达下调;RPMI8226/DOX细胞部分恢复了对阿霉素的敏感性,逆转耐药倍数为2.518。
结论
miR155异常表达与MM的化疗耐药形成相关,靶向抑制miR155表达可以通过影响FOXO3a蛋白表达恢复MM耐药细胞对化疗药物的敏感性。
Keywords: 多发性骨髓瘤, 抗药性, 肿瘤, 微RNAs, FOXO3a
Abstract
Objective
To explore the mechanism of abnormal expression of microRNA155 (miR155) in myeloma drug-resistance to probe the possibility of inhibiting miR155 expression to restore chemotherapy sensitivity and its molecular mechanism in drug-resistant myeloma cells.
Methods
Drug-resistant myeloma cell-line RPMI8226/DOX was established by culturing RPMI8226 cells with continuous low concentration and intermittent gradually increasing concentration of doxorubicin in vitro; The levels of miR155 mRNA were measured by qRT-PCR, and both proteins FOXO3a and BCL-2 expressions were detected by Western blot in cell-lines RPMI8226/S and RPMI8226/Dox. RPMI8226/DOX cells were transfected by miR155 inhibitor and mimic using gene transfer method, and then CCK-8 was used to measure proliferation and inhibition ratio, the changes of miR155 expression were detected by RT-PCR. Proteins FOXO3a and BCL-2 were detected by Western blot.
Results
Comparing with RPMI8226 cells, the level of miR155 mRNA was obviously up-regulated with the relative expression of 26.860±2.340, together with increased expression of Bcl-2 protein but decreased expression of FOXO3a in RPMI8226/DOX cells. After 72 h treatment with miR155 inhibitor, the inhibition rate of transfection was 64.57%, miR155 expression decreased sharply, the level of FOXO3a expression was upregulated while BCL-2 expression decreased, chemotherapy sensitivity was restored on cell-line RPMI8226/DOX with reversed drug-resistance ratio of 2.518.
Conclusion
The abnormal expression of miR155 was closely associated with myeloma drug-resistance, targeting inhibition of miR155 expression could restore chemotherapy sensitivity by increasing FOXO3a expression in drug-resistant myeloma cells.
Keywords: Myeloma, Drug resistance, Neoplasm, MicroRNAs, FOXO3a
来那度胺、硼替佐米等新型药物的出现显著改善了多发性骨髓瘤(MM)患者的生存,但是联合化疗仍是MM的重要治疗手段,化疗耐药仍是影响疗效的主要问题[1]–[2]。microRNA(miRNA)是一类非编码的小分子RNA,通过抑制目的mRNA翻译或直接降解来实现基因的转录后调控[3]。超过50%的蛋白质编码基因受miRNA调控,且miRNA分子在许多疾病中都出现异常表达[4]。某些miRNA分子在肿瘤中的致病作用被称为癌基因miRNA[5],而对肿瘤有抑制作用的miRNA(如let-7)被称为抑癌基因miRNA[6]。对血液肿瘤的研究发现:miR15/16表达下调与慢性B淋巴细胞白血病发病相关[7],miR21、miR155及miR210表达上调与弥漫大B细胞淋巴瘤(DLBCL)发病相关[8],miR21、miR155等表达异常与MM的发生、演变和耐药有密切关系[9]。为了进一步明确miR155异常表达与MM化疗耐药的关系,我们以对阿霉素耐药的MM细胞株RPMI8226/DOX[10]为研究对象,探讨靶向抑制miR155表达对耐药MM细胞恢复化疗药物敏感性及作用机制。
材料与方法
1.试剂和仪器:阿霉素购自美国Sigma公司,以超纯水配成10 g/L储存液,−20 °C保存。P-170抗体购自美国Santa Cruz公司。GAPDH、BCL-2、FOXO3a抗体购自美国Cell Signaling Technology公司。二抗购自美国Santa Cruz公司。CCK-8试剂盒购自上海七海复泰生物科技有限公司。总RNA提取试剂盒、RevertAid First Strand cDNA Synthesis Kit逆转录试剂盒购自美国Thermo Fisher Scientific公司;AccuPower 2×Greenstar qPCR Master Mix实时荧光定量PCR(qRT-PCR)试剂盒购自韩国Bioneer公司。MiR155 Inhibitor/Mimic购自广州锐博生物科技有限公司。Lipofectamine 2000购自上海Invitrogen公司。DMEM培养液购自美国Gibco公司。酶标仪、核酸蛋白测定仪、普通PCR仪为美国Biorad公司产品。ABI7500实时荧光定量PCR仪为美国ABI公司产品。
2.细胞株:MM细胞株RPMI8226购自温州医学院附属第一医院,由本实验室于37 °C、5%CO2、饱和湿度培养箱内培养、传代。耐阿霉素细胞株RPMI8226/DOX由本实验室用浓度梯度法建立[10]。RPMI8226/S为敏感细胞株。
3.qRT-PCR检测:收集RPMI8226/S、RPMI8226/DOX细胞,用Fermentas试剂盒提取总RNA,根据试剂盒说明逆转录为cDNA作为模板,通过qRT-PCR对miR155、miR21、miR551a、miR551b基因进行检测,以相对定量法分析基因表达量。引物由生工生物工程(上海)股份有限公司设计并合成(表1)。按说明书配制PCR体系。反应条件:95 °C变性10 min,95 °C变性10 s,60 °C退火延伸60 s,共40个循环。基因表达以相对定量法换算为倍数关系(计算公式为F=2−ΔΔCT)后,以F的均数±标准差作图,各组目的基因与内参基因CT值之差,经转换后采用Prism 5.0软件分析。
表1. 荧光定量PCR检测的引物序列.
| 基因 | 序列(5′→3′) | 产物(bp) |
| miR155 | 上游ACACTCCAGCTGGGTTAATGCTAATCGTGA | 30 |
| 下游TGGTGTCGTGGAGTCG | 16 | |
| miR21 | 上游ACACTCCAGCTGGGTAGCTTATCAGACTG | 29 |
| 下游TGGTGTCGTGGAGTCG | 16 | |
| miR551a | 上游TCCCTGCGACCCACTCTT | 18 |
| 下游CAGTGCGTGTCGTGGAGT | 18 | |
| miR551b | 上游TGCCGAAATCAAGCGTGG | 18 |
| 下游CAGTGCGTGTCGTGGAGT | 18 | |
| U6 | 上游CTCGCTTCGGCAGCAC | 16 |
| 下游AACGCTTCACGAATTTGCGT | 19 | |
| FOXO3a | 上游TCTACGAGTGGATGGTGCGTT | 21 |
| 下游CGACTATGCAGTGACAGGTTGTG | 23 | |
| GAPDH | 上游GAGAAGGCTGGGGCTCATTT | 20 |
| 下游TAAGCAGTTGGTGGTGCAGG | 20 |
4.CCK-8实验:取对数生长期细胞,接种于96孔圆底细胞培养板(每孔3×103细胞),在37 °C、5% CO2、饱和湿度条件下分别培养48、72 h后,每孔加入CCK-8溶液10 µl,于37 °C、5%CO2、饱和湿度条件下避光作用3 h,测定490 nm波长处各孔吸光度(A)值。每个样本设3个复孔,实验重复3次,结果以x±s表示。按以下公式计算细胞增殖抑制率并得出实验组半数抑制浓度IC50。
耐药倍数及逆转倍数按下列公式计算:
5.基因转染:取对数生长期RPMI8226/DOX细胞,按2×105细胞/ml接种到细胞培养板,加入DMEM培养液,置培养箱中培养24 h后,800 r/min(离心半径13.5 cm)离心后弃上清,PBS洗涤2次,加入6 ml DMEM培养液重悬细胞。取2个去酶EP管,分别加入1 ml DMEM培养液,分别加入20 µl Lipofectamine 2000试剂、20 µl模拟物试剂,混匀静置5 min,随后混合放置20 min。将该混合物加入重悬好的细胞,置于CO2培养箱中培养6 h后,更换含13%胎牛血清的RPMI1640培养液进行培养。转染至48、72 h后收获细胞。根据预实验结果,本实验使用模拟物终浓度为50 nmol/L,抑制物终浓度为200 nmol/L。按以下公式计算转染抑制率。
6.Western blot分析:收集RPMI8226/S、RPMI8226/DOX细胞,提取总蛋白。用BCA法测定蛋白质含量。以100 g/L SDS-PAGE电泳分离,湿转法转膜,室温下摇床封闭1 h,加入封闭牛奶稀释的FOXO3a(1∶1 000)、BCL-2(1∶1 000)、GAPDH(1∶1 000),4 °C孵育过夜,TBST充分洗膜后分别加入用TBST稀释(1∶3 000)的辣根过氧化物酶标记的羊抗兔二抗,室温下摇床孵育1 h,TBST洗膜后化学发光法显色,X线底片曝光,显影、定影。
7.统计学处理:采用Prism 5.0软件进行数据分析,组间比较采用多样本均数的单因素方差分析,进一步进行Turkey检验分析组间差异。两组均数比较采用t检验。P<0.05表示差异具有统计学意义。
结果
1.CCK-8法鉴定RPMI8226/DOX细胞系:RPMI8226/S和RPMI8226/DOX组IC50为(0.489±0.078)mg/L、(8.670±0.790)mg/L,RPMI8226/DOX细胞耐药指数(RI)为17.73。
2.qRT-PCR检测RPMI8226/DOX细胞miR155、miR21、miR551a、miR551b表达水平:RPMI8226/DOX组miR155、miR21、miR551a、miR551b相对表达量分别为RPMI8226/S组的(26.860±2.340)倍(t=19.100,P<0.001)、(2.538±0.460)倍(t=57.810,P<0.001)、(2.512±0.150)倍(t=17.100,P<0.001)、(2.156±0.430)倍(t=3.624,P=0.022)。
3.Western blot检测RPMI8226/S、RPMI8226/DOX细胞FOXO3a、BCL-2蛋白表达:与RPMI8226/S细胞比较,RPMI8226/DOX BCL-2蛋白表达上调(1.790±0.052对0.639±0.035,t=50.11,P<0.001),FOXO3a蛋白表达下调(0.665±0.033对1.527±0.065,t=26.15,P<0.001)(图1),提示MM化疗耐药与BCL-2、FOXO3a表达失调有关。
图1. Western blot方法检测多发性骨髓瘤(MM)细胞株FOXO3a、BCL-2蛋白表达.
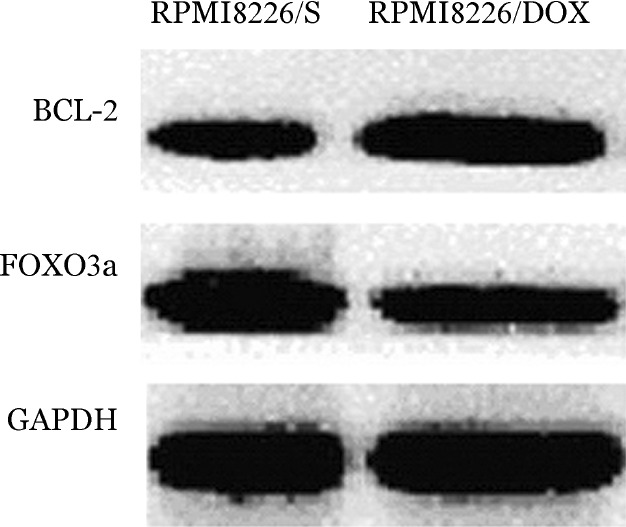
RPMI8226/S:MM敏感细胞株;RPMI8226/DOX:耐阿霉素MM细胞株
4.RPMI8226/DOX细胞转染miR155抑制物/模拟物的效率:用基因转染方法将miR155抑制物/模拟物转入RPMI8226/DOX细胞,qRT-PCR检测转染后miR155的表达。RPMI8226/DOX细胞转染miR155抑制物处理72 h后,抑制物阴性对照组miR155相对表达量高于抑制物处理实验组(1.050±0.330对0.420±0.080,t=4.143,P=0.003),转染抑制率为64.57%。RPMI8226/DOX细胞转染miR155模拟物72 h后,模拟物阴性对照组相对表达量低于模拟物处理实验组(1.010±0.090对94.590±7.160,t=92.540,P<0.001)。
5.CCK-8法检测靶向抑制miR155表达对RPMI8226/DOX细胞阿霉素敏感性的影响:RPMI8226/DOX细胞转染miR155抑制物处理72 h后,抑制物阴性对照组IC50高于抑制物处理组[(8.860±0.081)mg/L对(3.519±0.239)mg/L,t=52.180,P<0.001],转染miR155抑制物后逆转耐药倍数为2.518,提示靶向抑制miR155表达可以部分恢复细胞对阿霉素的敏感性。
RPMI8226/DOX细胞转染miR155模拟物处理72 h后,模拟物处理组IC50高于模拟物阴性对照组[(18.130±0.413)mg/L对(8.906±0.201)mg/L,t=48.750,P<0.001],耐药性升高2.036倍,提示转染miR155模拟物使RPMI8226/DOX细胞的耐药性增强。
6.Western blot检测抑制miR155对RPMI8226/DOX细胞FOXO3a、Bcl-2蛋白表达的影响:RPMI8226/DOX转染miR155抑制物处理72 h后,抑制物处理组BCL-2蛋白表达低于抑制物阴性对照组(0.770±0.150对1.378±0.220,t=6.255,P<0.001),FOXO3a蛋白表达高于抑制物阴性对照组(1.403±0.013对1.176±0.113,t=5.485,P<0.001)(图2),说明转染miR155抑制物能够下调抗凋亡蛋白BCL-2的表达、上调抑癌蛋白FOXO3a表达。RPMI8226/DOX细胞转染miR155模拟物处理72 h后,BCL-2蛋白表达量高于模拟物阴性对照组(1.360±0.026对0.807±0.011,t=53.720,P<0.001)、FOXO3a蛋白表达低于模拟物阴性对照组(0.634±0.005对0.817±0.050,t=9.866,P<0.001)。
图2. 耐阿霉素多发性骨髓瘤细胞RPMI8226/DOX转染miR155抑制物/模拟物72 h后FOXO3a、BCL-2蛋白表达水平.
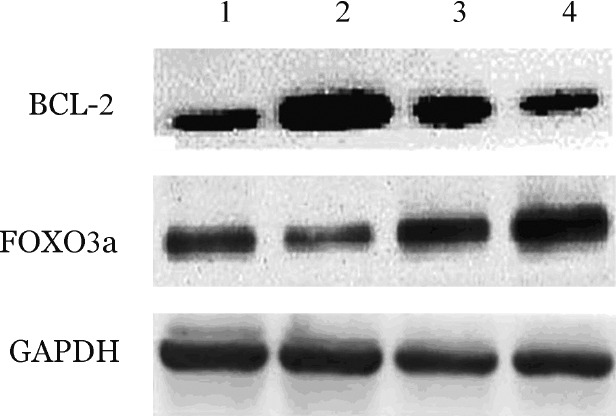
1:模拟物阴性对照组;2:模拟物处理组;3:抑制物阴性对照组;4:抑制物处理组
讨论
目前,在胃癌、结肠癌和肺癌耐药细胞系中均存在多种miRNA的表达异常[11]–[12]。对MM耐药细胞系的研究中存在let7、miR21、miR155表达异常,以miR155的表达异常最为显著[13]。Ling等[14]在神经胶质瘤细胞的研究中发现,miR155表达上调并通过负性调节Akt信号通路,使瘤细胞内FOXO3a表达下调而促进细胞增殖、抑制细胞凋亡、增强神经胶质瘤细胞的迁移和侵袭能力。Gironella等[15]研究发现miR155在胰腺导管癌细胞中的表达显著上调,抑制癌细胞下游TP53INP1靶基因的表达,增强癌细胞侵袭转移能力。这些研究结果初步证明miR155参与多种肿瘤细胞耐药的形成。本研究结果显示,与MM敏感细胞株RPMI8226/S相比,RPMI8226/DOX中存在miR155的过度表达;RPMI8226/DOX转染miR155抑制物后,miR155表达被显著抑制,RPMI8226/DOX细胞对阿霉素的敏感性得到恢复,证明MM细胞化疗耐药机制中同样有miR155表达异常的因素,miR155的表达异常可能作为MM新的化疗耐药的分子标志,以miR155为靶点调节耐药细胞对化疗药物的敏感性可能成为克服耐药的新途径。
转录因子FOXO3a广泛表达于人类各组织器官中[16]。FOXO3a与细胞增殖、分化、肿瘤的发生发展及其血管生成有关[17],其表达水平增高可促进细胞凋亡、抑制细胞增殖力[18]。Le-Niculescu等[19]的研究显示,FOXO3a能与FasL基因启动子结合,诱导基因的表达,并通过结合受体Fas产生一系列凋亡级联效应。FOXO3a也可以通过Fas和BCL-2等多途径活化Caspase系统,诱导肿瘤细胞凋亡。
通过生物信息学分析发现,FOXO3a是miR155的靶基因。功能实验证明,在T细胞和HOZOT细胞中FOXO3a蛋白的表达受miR155调控[20]–[21]。Kong等[22]在乳腺癌细胞株及其肿瘤标本的研究中证实,miR155的持续过度表达可导致FOXO3a蛋白表达下降,从而提高瘤细胞的生存能力并增加瘤细胞对化疗药物的抗药性。而Germani等[23]对结肠癌的研究表明,p38a通过激活FOXO3a表达而促进细胞凋亡、增强结肠癌耐药细胞的敏感性。在本研究中,我们证实了MM细胞miR155作用的靶基因是FOXO3a,miR155通过调控FOXO3a蛋白的表达而影响MM细胞的增殖、凋亡及对阿霉素的敏感性。
对实体肿瘤细胞的研究发现,miRNA通过直接作用于抗凋亡基因BCL-2,从而调节BCL-2蛋白表达,促进细胞发生耐药或恢复药物的敏感性[24]–[25]。我们的研究结果也证实:作为MM耐药细胞株的RPMI8226/DOX细胞miR155表达上调,通过靶向作用于FOXO3a使其表达降低,同时上调抗凋亡基因BCL-2表达,从而促进细胞耐药的形成。转染miR155抑制物处理后,FOXO3a蛋白表达水平明显增加,BCL-2蛋白表达水平下调,使细胞恢复对阿霉素的敏感性。
Funding Statement
基金项目:福建省医学创新课题(2014-CXB-42)
Fund program: Medical Innovation Project of Fujian Province(2014-CXB-42)
References
- 1.Richardson PG, Xie W, Jagannath S, et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma[J] Blood. 2014;123(10):1461–1469. doi: 10.1182/blood-2013-07-517276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jakubowiak AJ, Griffith KA, Reece DE, et al. Lenalidomide, bortezomib, pegylated liposomal doxorubicin, and dexamethasone in newly diagnosed multiple myeloma: a phase 1/2 Multiple Myeloma Research Consortium trial[J] Blood. 2011;118(3):535–543. doi: 10.1182/blood-2011-02-334755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay[J] Nat Rev Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function[J] Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Olive V, Jiang I, He L. mir-17-92, a cluster of miRNAs in the midst of the cancer network[J] Int J Biochem Cell Biol. 2010;42(8):1348–1354. doi: 10.1016/j.biocel.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B, Pan X, Cobb GP, et al. microRNAs as oncogenes and tumor suppressors[J] Dev Biol. 2007;302(1):1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia[J] Proc Natl Acad Sci U S A. 2002;99(24):15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawrie CH, Gal S, Dunlop HM, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma[J] Br J Haematol. 2008;141(5):672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 9.Munker R, Liu CG, Taccioli C, et al. MicroRNA profiles of drug-resistant myeloma cell lines[J] Acta Haematol. 2010;123(4):201–204. doi: 10.1159/000302889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.卢 燕燕, 肖 翠容, 陈 华英, et al. 阿霉素诱导的骨髓瘤细胞化疗耐药的分子机制分析[J] 中国实验血液学杂志. 2014;22(5):1336–1340. doi: 10.7534/j.issn.1009-2137.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Pickard K, Jenei V, et al. miR-153 supports colorectal cancer progression via pleiotropic effects that enhance invasion and chemotherapeutic resistance[J] Cancer Res. 2013;73(21):6435–6447. doi: 10.1158/0008-5472.CAN-12-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu W, Xu H, Zhu D, et al. miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP[J] Cancer Chemother Pharmacol. 2012;69(3):723–731. doi: 10.1007/s00280-011-1752-3. [DOI] [PubMed] [Google Scholar]
- 13.Pichiorri F, Suh SS, Ladetto M, et al. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis[J] Proc Natl Acad Sci U S A. 2008;105(35):12885–12890. doi: 10.1073/pnas.0806202105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling N, Gu J, Lei Z, et al. microRNA-155 regulates cell proliferation and invasion by targeting FOXO3a in glioma[J] Oncol Rep. 2013;30(5):2111–2118. doi: 10.3892/or.2013.2685. [DOI] [PubMed] [Google Scholar]
- 15.Gironella M, Seux M, Xie MJ, et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development[J] Proc Natl Acad Sci U S A. 2007;104(41):16170–16175. doi: 10.1073/pnas.0703942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weigel D, Jürgens G, Küttner F, et al. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo[J] Cell. 1989;57(4):645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- 17.Jünger MA, Rintelen F, Stocker H, et al. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling[J] J Biol. 2003;2(3):20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson MJ, Viars CS, Czekay S, et al. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily[J] Genomics. 1998;47(2):187–199. doi: 10.1006/geno.1997.5122. [DOI] [PubMed] [Google Scholar]
- 19.Le-Niculescu H, Bonfoco E, Kasuya Y, et al. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death[J] Mol Cell Biol. 1999;19(1):751–763. doi: 10.1128/mcb.19.1.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babar IA, Czochor J, Steinmetz A, et al. Inhibition of hypoxia-induced miR-155 radiosensitizes hypoxic lung cancer cells[J] Cancer Biol Ther. 2011;12(10):908–914. doi: 10.4161/cbt.12.10.17681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto M, Kondo E, Takeuchi M, et al. miR-155, a modulator of FOXO3a protein expression, is underexpressed and cannot be upregulated by stimulation of HOZOT, a line of multifunctional treg[J] PLoS One. 2011;6(2):e16841. doi: 10.1371/journal.pone.0016841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong W, He L, Coppola M, et al. MicroRNA-155 regulates cell survival, growth, and chemosensitivity by targeting FOXO3a in breast cancer[J] J Biol Chem. 2010;285(23):17869–17879. doi: 10.1074/jbc.M110.101055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Germani A, Matrone A, Grossi V, et al. Targeted therapy against chemoresistant colorectal cancers: inhibition of p38α modulates the effect of cisplatin in vitro and in vivo through the tumor suppressor FoxO3A[J] Cancer Lett. 2014;344(1):110–118. doi: 10.1016/j.canlet.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 24.Qiu T, Zhou L, Wang T, et al. miR-503 regulates the resistance of non-small cell lung cancer cells to cisplatin by targeting Bcl-2[J] Int J Mol Med. 2013;32(3):593–598. doi: 10.3892/ijmm.2013.1439. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Wu J, Li S, et al. The function role of miR-181a in chemosensitivity to adriamycin by targeting Bcl-2 in low-invasive breast cancer cells[J] Cell Physiol Biochem. 2013;32(5):1225–1237. doi: 10.1159/000354521. [DOI] [PubMed] [Google Scholar]


