Abstract
Background
Identification of effective treatments in severe cases of COVID-19 requiring mechanical ventilation represents an unmet medical need. Our aim was to determine whether the administration of adipose-tissue derived mesenchymal stromal cells (AT-MSC) is safe and potentially useful in these patients.
Methods
Thirteen COVID-19 adult patients under invasive mechanical ventilation who had received previous antiviral and/or anti-inflammatory treatments (including steroids, lopinavir/ritonavir, hydroxychloroquine and/or tocilizumab, among others) were treated with allogeneic AT-MSC. Ten patients received two doses, with the second dose administered a median of 3 days (interquartile range-IQR- 1 day) after the first one. Two patients received a single dose and another patient received 3 doses. Median number of cells per dose was 0.98 × 106 (IQR 0.50 × 106) AT-MSC/kg of recipient's body weight. Potential adverse effects related to cell infusion and clinical outcome were assessed. Additional parameters analyzed included changes in imaging, analytical and inflammatory parameters.
Findings
First dose of AT-MSC was administered at a median of 7 days (IQR 12 days) after mechanical ventilation. No adverse events were related to cell therapy. With a median follow-up of 16 days (IQR 9 days) after the first dose, clinical improvement was observed in nine patients (70%). Seven patients were extubated and discharged from ICU while four patients remained intubated (two with an improvement in their ventilatory and radiological parameters and two in stable condition). Two patients died (one due to massive gastrointestinal bleeding unrelated to MSC therapy). Treatment with AT-MSC was followed by a decrease in inflammatory parameters (reduction in C-reactive protein, IL-6, ferritin, LDH and d-dimer) as well as an increase in lymphocytes, particularly in those patients with clinical improvement.
Interpretation
Treatment with intravenous administration of AT-MSC in 13 severe COVID-19 pneumonia under mechanical ventilation in a small case series did not induce significant adverse events and was followed by clinical and biological improvement in most subjects.
Funding
None.
Keywords: COVID-19, SARS-CoV-2, Pneumonia, Mechanical ventilation, Mesenchymal stromal cells, Cellular therapy, Case series
Research in context.
Evidence before this study
Advanced therapy medical products based on allogeneic mesenchymal stromal cells (MSC) are approved for some inflammatory diseases, as Crohn's disease and graft-versus-host disease after a allogeneic hematopoietic transplantation in some countries, based on their anti-inflammatory and immunomodulatory effects. There is pre-clinical evidence that intravenous administration of MSC in respiratory virus infection models might reduce lung damage. In a PubMed database search using the terms “COVID-19″ OR “SARS-CoV-2″ AND “mesenchymal”, only one severe patient with COVID-19 pneumonia requiring mechanical ventilation has been reported to date, with favorable outcome. A number of clinical trials are already registered in different databases, but no series of patients have been published so far.
Added value of this study
In this first case series of 13 critically ill COVID-19 patients under mechanical ventilation, treatment with AT-MSC was associated with clinical improvement in nine patients, with seven patients being extubated at a median follow-up of 16 days. No adverse events were reported. Improvement in ventilatory, radiological and biological parameters was associated with clinical response.
Implications of all the available evidence
These results are a proof of concept showing that intravenous infusion of AT-MSC does not induce relevant therapy-related adverse events and could be associated with clinical and biological improvement in some patients with severe COVID-19 pneumonia requiring mechanical ventilation. These results support conducting a phase 2 randomized controlled trial already under way.
Alt-text: Unlabelled box
1. Introduction
The current SARS-CoV-2 pandemic has stretched the capacity of the health systems in many of the affected countries to the limit, conditioned by the need for intensive care in many of these patients [1]. Patients admitted to the Intensive Care Unit (ICU) requiring mechanical ventilation show significant mortality that ranges between 30 and 60% [2], [3], [4]. Factors that have been associated with a worse prognosis in patients with severe SARS-CoV-2 pneumonia include age over 65 years, the existence of comorbidities as diabetes, male gender, and elevated levels of C-reactive protein or lactate dehydrogenase (LDH), among others [5], [6], [7], [8]. Even, in those patients with a favorable outcome, an additional problem that contributes to the ICU saturation, is the long average stay under invasive mechanical ventilation [9]. Therefore, any adjuvant treatment that contributes to accelerate the recovery will represent a major step forward.
One of the most striking facts of the physiopathology of pneumonia in COVID-19 disease [10] is the development of a massive inflammatory phase [11,12], with elevation of numerous acute phase reactants and cytokines (e.g. ferritin, C-reactive protein, fibrinogen, LDH or IL-6), leading to acute respiratory distress syndrome (ARDS) and macrophage activation syndrome (MAS)-like disease [13,14]. In addition, the presence of a progressive endothelial thrombo-inflammatory syndrome (with elevated d-dimer) not described in other viral infections adds differential features and aggravates the disease's prognosis [15], [16], [17]. This inflammatory reaction underlies the rationale for the development of clinical trials evaluating the role of drugs with anti-inflammatory activity, such as tocilizumab, anakinra, siltuximab, and others [18], [19], [20].
Among the potential therapeutic options to reduce this clinical and biological picture of massive inflammation the use of mesenchymal stromal cells (MSCs) is generating increasing interest. Nevertheless, to date, information published on critically ill patients undergoing mechanical ventilation treated with MSC is reduced to a single reported case [21]. However, more than 17 clinical trials are registered in ClinicalTrials.gov to evaluate the role of mesenchymal cells from different sources [22]. MSC have been approved for the treatment of Crohn's disease or graft-versus-host disease after hematopoietic transplantation [23] based in their anti-inflammatory and immunomodulatory effects thus suggesting that adipose tissue-derived MSCs (AT-MSCs) could be an attractive therapeutic option for the treatment of severe SARS-CoV-2 pneumonia [24].
In the current report we describe the outcome of a group of 13 patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation, who had not responded to previous antiviral and anti-inflammatory treatments (including in some cases tocilizumab, steroids, anakinra and/or siltuximab), and who were treated with AT-MSCs on a compassionate-use basis. These results represent the preliminary experience of four academic centers of the Spanish National Cell Therapy Network (TerCel) [25] and establishes the basis for the current phase 2 randomized controlled clinical trial (BALMYS-19 (“BAttLe against CO using MesenchYmal Stromal cells; EudraCT: 2020-001266-11; clinicaltrials.gov identifier NCT04348461) already in progress.
2. Methods
2.1. Patients
Patients with SARS-CoV-2 infection confirmed by reverse-transcriptase-polymerase-chain-reaction assay and COVID-associated pneumonia diagnosed by either chest X-ray or computed tomography and requiring mechanical ventilation in the ICU and in accordance with any one of the following criteria were included in the study: 1) tachypnea (RR ≥ 30 times / min), 2) finger oxygen saturation ≤ 93% in resting state, 3) arterial oxygen partial pressure (PaO2) / fraction of inspired oxygen (FiO2) ≤ 300 mmHg, 4) pulmonary imaging with evidence of progression > 50% in 24–48 h and a Sequential Organ Failure Assessment (SOFA) score [26] >3 points, World Health Organization (WHO) Ordinal Scale for Clinical Improvement [27] level 6 and no evidence of multiorgan failure. Additional inclusion criteria included age older than 18 years, a creatinine clearance above 30 ml per minute and serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) less than five times the upper limit of the normal range (40 units/liter). Patients with active neoplasms or with systemic immunosuppressive treatments prior to admission were excluded from this program. All patients were treated at four different Spanish academic hospitals that are members of the Spanish Cell Therapy Network (TerCel) [25] and had the experience and infrastructure to administer allogeneic MSC: Hospital Universitario de Salamanca, Fundación Jiménez Diaz, Hospital Universitario Gregorio Marañón and Clínica Universidad de Navarra. Treatment with AT-MSC was approved by The Spanish Medicines Agency (AEMPS, https://www.aemps.gob.es/) as part of a compassionate use program after informed consent from the patients' relatives was obtained.
2.2. Cell treatment
AT-MSC were obtained by liposuction from voluntary donors performed by surgeons from the Plastic Surgery Departments of the participating hospitals. In all cases, donors were young (less than 50 years) with no comorbidities or concomitant treatments. The analytical and serological studies required by the legislation on cell and tissue donation in the European Union and in Spain were obtained in each case, as well as specific informed consent to use the cells for clinical application.
Cell production was carried out in the Good Manufacturing Practices (GMP) Facilities of the Hospital Universitario de Salamanca, Clínica Universidad de Navarra and Hospital Gregorio Marañón in accordance with the investigational Medicinal Product Dossier (IMPD) with reference code PEI-15–103, approved by the Spanish Medicines Agency (AEMPS). Briefly, 100 ml of lipoaspirate (corresponding to 50 g of fat tissue) were obtained from each donor. Samples were digested with collagenase and further expanded in the GMP facilities in standard conditions, as previously reported [28,29]. Cells were cryopreserved in bags containing from 50 to 75 × 106 MSC. In all cases, less than 20 population doublings and less than 2 passages were performed before administration. Characterization of the final product included the International Society for Cellular Therapy (ISCT) definition criteria with morphology, immunophenotypic profile, multi-lineage differentiation ability [30]. In addition, comparative genomic hybridization arrays to ensure genomic stability and a potency test evaluating the inhibition of the proliferation of activated T-lymphocytes were performed.
Cell were cryopreserved and stored until use. Cell viability was tested only before cryopreservation. Cryopreserved cells were administered immediately after thawing in a medium containing AB serum and 10% dimethyl sulfoxide without washing at the Hospital Universitario de Salamanca and Clinica Universidad de Navarra, while the remaining centers used "refreshed" cells that were seeded back onto plastic surface for a period of less than 72 h and then trypsinized and resuspended in a solution of Ringer's lactate with 1% albumin before administration, that in all cases were infused in less than 24 h from harvesting. In the first case, steroids and dexchlorpheniramine were administered prior to cell infusion. In every case, administration was performed intravenously by personnel experienced in the use of AT-MSCs using a standard 200-micron transfusion filter at a target dose of 1 × 106 AT-MSCs/kg of recipient's body weight. Treated subjects received cells from five different donors, although each individual patient received cells from the same cell product batch. In this compassionate use program, patients received a first dose of AT-MSCs (day 1) and were scheduled for a second dose if deemed clinically appropriate by the treating physician, between 48 and 96 h later. This was based on previous literature, clinical course and on the putative mechanism of action [31], [32], [33]. Eventually, a third dose was allowed to be administered. Supportive therapy included, in addition to mechanical ventilation and sedation, the use of vasopressor or inotropic drugs, enteral or parenteral nutrition, antibiotics and/or diuretics, among other standard procedures, and was provided at the discretion of the clinicians until discharge from ICU or death.
2.3. Study assessments
In each patient, toxicity and potential adverse events related to cell administration were recorded. All intubated patients in the ICU were continuously monitored with electrocardiography, continuous invasive blood pressure, pulse oximetry and capnography to immediately detect any potential adverse reaction during the infusion of cells and afterwards. Ventilatory, radiological and analytical parameters including complete blood counts (CBC) and basic biochemistry, coagulation (including fibrinogen and d-dimer) and acute phase reactants (C-reactive protein, ferritin, LDH and/or IL-6) were obtained before the first dose of AT-MSCs (routinely in the morning of the same day), and daily for 5 days and at regular intervals thereafter depending on clinical or institutional criteria. Specifically, most centers performed CBC, biochemistry and coagulation daily in ICU, and chest-X rays, ferritin or IL-6 every 5–10 days after the first 5–7 days, and when clinically appropriate. Chest X-rays were evaluated by two independent observers according to the score proposed by Wong et al. [34]. Although there were no pre-specified end points for this compassionate-use program, we registered and quantified prospectively the incidence of potential adverse events, as previously indicated, through continuous monitoring while in ICU. In addition, we evaluated the proportion of patients with clinical improvement, as defined by extubation, discharge from ICU, radiographic improvement or at least one-point decrease in the World Health Organization (WHO and R&D Blueprint Group Ordinal Scale for Clinical Improvement) [27]. The appearance of respiratory or other concomitant infections was also collected, as well as any concomitant medications administered from date of hospital admission. Main lymphocyte subpopulations in peripheral blood (B cells, CD4+ T cells, CD8+ T cells, NK cells) were quantified before and 10 days after AT-MSCs administration. The length of time from admission to the start of mechanical ventilation, the time between mechanical ventilation and the first AT-MSC administration, and the time between the latter and extubation or death were also analyzed.
2.4. Statistical analysis
All data were stored in and Excel file (Microsoft, Redmond, Washington) and then imported into the SPSS.v25 (IBM, Armonk, New York) statistical package. Tables and Fig. 1 were performed with Excel (Microsoft) and GraphPad.v8 (GraphPad software, San Diego, California) was used to create the graphic that compose Fig. 2. Median and interquartile ranges (IQRs) were calculated for quantitative variables.
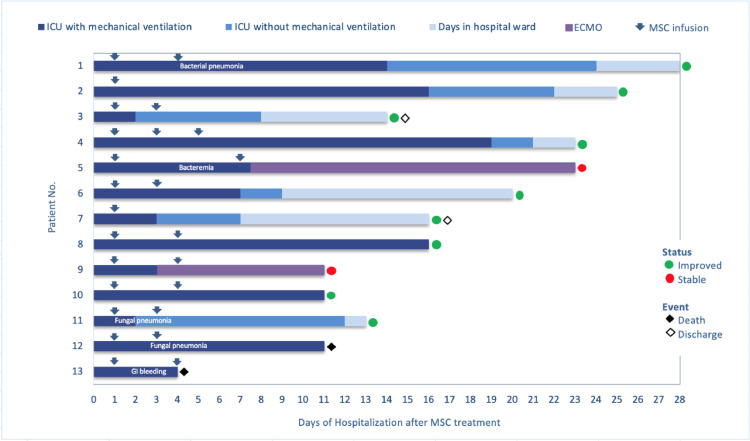
Fig. 1.
Patient disposition and outcome.
Patient evolution is indicated in separate rows, with the same number and order shown in Table 1. Mesenchymal stromal cell (MSC) doses and timing are represented in arrows. In the X axis, days from the first MSC dose are specified. Type of ventilation support is graded in colors through each row. Main complications (infectious complications or bleeding) are included in each row when appropriate. Green and red circles designate those patients with favorable or stable evolution at last day of follow-up. Finally, black or white diamonds denote final outcome, dead or hospital discharge, respectively. Abbreviations. MSC: mesenchymal stromal cells; ICU: Intensive care unit; ECMO: extracorporeal membrane oxygenation; GI: gastrointestinal.
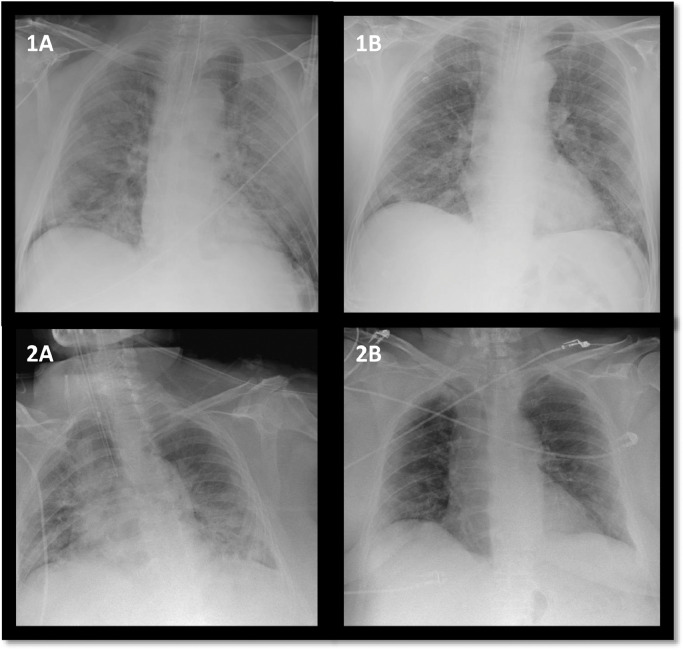
Fig. 2.
Chest X-ray changes of two representative patients improving after AT-MSC administration.
A (before) and B (48 h after) cell infusion.
2.5. Role of funding
Funding source: none. Fermin Sanchez-Guijo had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Patient and baseline characteristics
Thirteen patients were treated with AT-MSC between April 3rd and April 22nd, 2020. In two cases, a single dose was administered, one patient received 3 doses and the remaining ten patients received 2 doses, the second administered at a median of 3 days (IQR 1 day) after the first one. More specifically, the two patients that received a single dose did improve significantly after administration of the AT-MSC and no need for additional doses was deemed necessary. On the other hand, in one patient, although improvement was observed after the first 2 doses, worsening of his condition and availability of an additional cell dose was considered as a reason for an additional administration of cells. Median number of AT-MSCs per dose was 0.98 (IQR 0.5) x 106 /kg. In 7 patients, cells were reseeded and refreshed for 72 h while in the remaining 6 patients AT-MSCs were directly thawed and immediately infused intravenously. Baseline and treatment characteristics of the patients are summarized in Table 1. Median age was 60 years (IQR 11 years). Twelve of the 13 patients were male. All patients were under invasive mechanical ventilation at baseline (before the first MSC administration). Median time from Hospital admission to mechanical ventilation was 4 days (IQR 3 days) and the median duration of invasive mechanical ventilation before the first dose of AT-MSC was 7 days (IQR 12 days). All patients received corticosteroids, prophylactic antibiotics (mainly ceftriaxone) and low-molecular weight heparin. Eleven of 13 patients (85%) had received hydroxychloroquine (7 in combination with azithromycin), and the same percentage had received tocilizumab. Anakinra was given in 2 patients (15%), one after tocilizumab, whereas siltuximab was additionally administered after tocilizumab and anakinra in one patient. Finally, lopinavir/ritonavir was also administered in 11 patients (85%). At the time of cell administration only steroids were administered concomitantly. Most patients received supportive treatment during their ICU stay. This included, in addition to mechanical ventilation and sedation, the use of vasopressor or inotropic drugs, enteral or parenteral nutrition, antibiotics and/or diuretics, among other standard procedures.
Table 1.
Patient baseline characteristics and treatments.
| Patients |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Age (years) | 55 | 64 | 68 | 55 | 47 | 62 | 63 | 62 | 50 | 59 | 55 | 71 | 73 |
| Gender | M | M | F | M | M | M | M | M | M | M | M | M | M |
| Ethnicity | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian |
| BMI | 24.49 | 30.86 | 35.16 | 25.83 | 26.12 | 25.83 | 25.06 | 27.55 | 25.95 | 25.95 | 28.29 | 26.73 | 24.49 |
| Days from diagnosis (PCR SARS-CoV positive) | 8 | 22 | 3 | 14 | 14 | 8 | 9 | 5 | 19 | 24 | 19 | 18 | 4 |
| Comorbidities | HBV | None | Diabetes Hypertension | COPD Hyperthyroi-dism | None | Hypertension Behçet syndrome | Hypertension | None | None | Hypothyroidism | Hypertension | Hypertension COPD | Hypertension |
| Smoking | No | No | No | Ex- smoker | No | Ex -smoker | Ex -smoker | No | No | No | Ex -smoker | Ex -smoker | No |
| Laboratory | |||||||||||||
| IL-6 | 1000 | 238 | 87.2 | 7.8 | 82.3 | 92.8 | 209 | NT | NT | NT | 195.4 | 206.6 | 13.7 |
| CRP | 2.21 | 1.90 | 3.67 | 10.80 | 0.50 | 0.70 | 13.10 | 9.10 | 26.80 | 34.00 | 3.71 | 0.60 | 3.60 |
| Ferritin | 1062 | 3194 | 401 | 3592 | 1095 | 3603 | 2400 | NT | 2155 | 1602 | 1401 | 531 | 5428 |
| D-dimer | 25,000 | 1865 | 970 | 2980 | 1821 | 7500 | 6352 | 6400 | 3456 | 852 | 1690 | 1750 | 1766 |
| Fibrinogen | 213 | 176 | 699 | 788 | 287 | 90 | 630 | 640 | 896 | 793 | 426 | 226 | 622 |
| Hb | 14.6 | 11 | 9.9 | 12.8 | 11.4 | 12.8 | 14.9 | 13.7 | 9.2 | 9.4 | 8.9 | 10.2 | 14.7 |
| Platelets | 215 | 210 | 429 | 221 | 134 | 131 | 297 | 449 | 224 | 158 | 170 | 138 | 349 |
| Lymphocytes | 1.98 | 0.5 | 1.16 | 0.46 | 0.50 | 0.46 | 0.30 | 0.40 | 0.40 | 0.40 | 0.70 | 0.71 | 0.30 |
| LDH | 519 | 308 | 543 | 774 | 299 | 1453 | 530 | 677 | 239 | 187 | 305 | 316 | 777 |
| SOFA score before AT-MSC infusion | 6 | 5 | 2 | 3 | 7 | 3 | 2 | 2 | 4 | 2 | 4 | 11 | 2 |
| Previous therapy for COVID | |||||||||||||
| Tocilizumab (doses) | 2 × 400mg | 1 × 400mg | 1 × 600mg | No | 1 × 400mg | 1 × 400mg | 2 × 600mg | 1 × 600mg | 1 × 600mg | No | 1 × 600mg | 1 × 600 mg 1 × 400mg | 1 × 400mg |
| Anakinra | No | No | No | No | No | 1 × 100mg | No | No | No | 1 × 100mg | No | No | No |
| Siltuximab | No | No | No | No | No | 1 × 800mg | No | No | NO | No | No | No | No |
| Hydroxychloroquine | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Lopinavir/Ritonavir | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Steroids | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| AT-MSC, No. doses | 2 | 1 | 2 | 3 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| Days in ICU with mechanical ventilation after AT-MSC infusion | 14 | 16 | 2 | 19 | 23 | 7 | 3 | 16 | 11 | 11 | 2 | 11 | 4 |
| Days in ICU without mechanical ventilation after AT-MSC infusion | 10 | 6 | 6 | 2 | 0 | 2 | 4 | 0 | 0 | 0 | 10 | 0 | 0 |
M: male; F: female; BMI: Body Mass Index (kg/m2); HBV: Hepatitis B virus; COPD: Chronic Obstructive Pulmonary Disease; IL-6: Interleukin-6 (pg/mL); CRP: C-reactive protein (mg/dL); Ferritin (mg/dL), d-dimer: ng/mcL; Fibrinogen (mg/dL), Hb: hemoglobin (g/dL); Platelets: (x103/mcL); Lymphocytes (x103/mcL); LDH: lactate dehydrogenase (U/L). AT-MSC: Adipose-tissue derived mesenchymal stromal cell; ICU: Intensive Care Unit; NT: non tested.
3.2. Safety
No adverse events were associated with the infusion of AT-MSC including fever orworsening of respiratory or hemodynamic parameters. One patient developed severe hypotension and tachycardia 24 h after cell administration despite treatment with steroids and dexchlorpheniramine. Subsequently, a severe decrease in hemoglobin was observed. A CT scan and upper gastrointestinal endoscopy were performed showing massive digestive bleeding due to a gastric ulcer, whose location coincided with the position of the nasogastric tube. Despite intensive supportive therapy, the patient died. In light of this information, this early complication was deemed not to be related to cell administration.
No significant changes in physiological parameters measured by continuous monitoring were observed immediately after AT-MSC infusion, nor in the biological parameters (including coagulation, hepatic, cardiac or renal function) performed daily after cell administration (Supplementary Table 1).
3.3. Clinical and radiological improvement
After a median follow-up of 16 days (IQR 9 days) after the first dose of AT-MSCs, 9 patients (70%) had improved clinically and 7 (53%) were extubated (Fig. 1) with a median time from the first MSC dose to extubation of 7 days (IQR 14 days). In two patients, extracorporeal mechanical oxygenation (ECMO) was required but both remain stable at the time of writing this report, 11 and 23 days after the first cell dose. Two patients died, one from massive gastrointestinal bleeding (described before) and another one from secondary fungal pneumonia by Saccharomyces spp. One patient developed a concurrent respiratory pneumonia due to a methicillin-resistant Staphylococcus aureus and one patient developed a fungal infection by Candida spp. However, they subsequently improved after appropriate antibacterial and antifungal therapy according to the respective antibiogram and antifungigram and were then extubated. Radiological improvement in sequential X-rays was confirmed after AT-MSC administration (in the quantitative scale previously described [34]) in 40% of evaluable patients (Fig. 2). For radiological evaluation we considered 10 evaluable patients, excluding the patient who died early 24 h after cell administration and the two patients with concomitant fungal infection at the time of cell infusion.
When we examined the time from intubation to administration of AT-MSC, we observed that patient successfully extubated had received the cells earlier than patients that were not extubated (median 5 days, IQR: 11 days versus 10 days, IQR: 15 days).
3.4. Laboratory response and immune monitoring
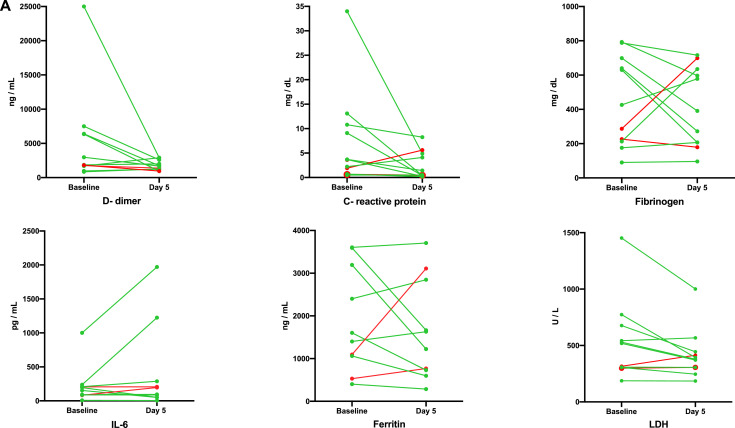
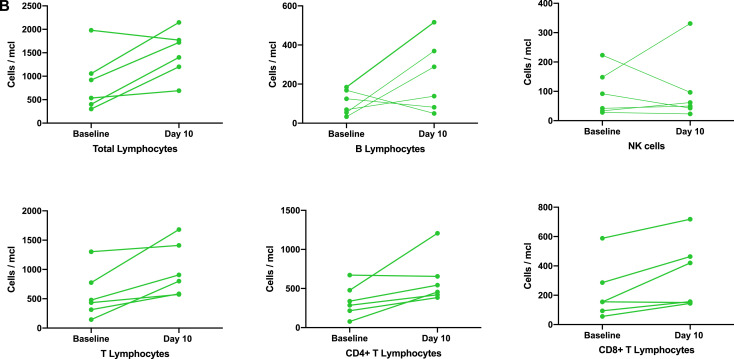
For laboratory evaluation, the patient who died prematurely and the patient who was on ECMO before day +5 (after the first AT-MSC dose) was excluded from the analysis. An additional patient was not evaluable for ferritin and IL-6 as these analyses were not performed on the fifth day after cell infusion. When we analyzed the nine patients that improved clinically (according to Fig. 1), a decrease in inflammatory parameters associated with AT-MSC therapy was observed at day 5 after infusion (Fig. 3A) with a decrease in C-reactive protein in 8 patients (88%), LDH in 9 (100%), and d-dimer and ferritin in 5 of 8 evaluable patients (63%), since one responding patient did not have the results of both parameters at day +5 after the first AT-MSC dose. In six patients in which lymphocyte counts were measured by flow cytometry, an increase in the levels of total lymphocytes was observed in five of them (86%), as well as an increase in B- (67%) and CD4+ and CD8+ (100%) T lymphocytes (Fig. 3B). Lymphocyte subset analysis was available in six patients that improved after MSC therapy, but no information on non-responding as well as in 3 responding patients was available.
Fig. 3.
(A) Changes in acute phase reactants and coagulation parameters after AT-MSC therapy.
Values of each parameter are indicated by dots joined by a color line before adipose tissue derived mesenchymal stromal cell therapy and at day five from the first dose for each individual and evaluable patient. Green or red colors represent patients with favorable or unfavorable evolution (stable or death), according to the color codes of Fig. 1.
(B) Changes in lymphocyte counts and lymphocyte subsets after AT-MSC therapy.
Values of each cell population are indicated by dots joined by a color line before adipose tissue derived mesenchymal stromal cell therapy and at day ten from the first dose for each individual and evaluable patient. Green color represents patients with favorable evolution, according to the color codes of Fig. 1.
4. Discussion
The severity of the COVID-19 pandemic and the lack of effective proven therapies represents a formidable challenge and have stimulated multiple research groups on an urgent search for potentially useful therapeutic options [18], [19], [20]. Multiple clinical trials are currently underway with a wide range of drugs (in the https://clinicaltrials.gov/hclinicaltrials.gov website accessed on May 1st are registered up to 600 clinical trials, almost half of which are already under recruitment). Furthermore, due to the therapeutic urgency many treatments are being used off-label through compassionate use programs [35]. Here, we report the first series of COVID-19 patients requiring mechanical ventilation treated with AT-MSC, generating preliminary evidence of the absence of significant adverse events along with improvement in most of these patients. These results warrant conducting a multicenter randomized controlled trial already underway.
The outcome of COVID-19 patients admitted to the ICU is poor. In a recent series of 1581 Italian patients with COVID-19 ARDS admitted to ICU (88% requiring mechanical ventilation), with a median follow up of 9 days, 26% have died and only 16% had been discharged [36]. In another series of patients with longer median follow-up (19 days), one third of them (31%) have been discharged from ICU and 23% have died [37]. In our series, with a median follow-up of 16 days, mortality rate was 15%, while seven patients (53%) had been extubated and discharged from ICU and two additional patients were improving.
One of the most relevant findings of our work is the lack of adverse events associated with cell administration in these extremely critical patients with respiratory insufficiency, massive inflammation and prothrombotic risk. Safety of MSC treatments administered intravenously has been well demonstrated in multiple clinical trials for different conditions (e.g. Crohn's disease, graft-versus-host disease, etc.) as reported in two meta-analyses including almost 3000 patients [38,39]. Pre-clinical evidence of the potential for MSCs in viral lung infections is still scarce and, in some cases, controversial [22,40]. It is true that there are no preclinical studies in animal models of SARS-CoV-2 infection and that most preclinical evidence comes from influenza virus infection models, where the pathophysiology and systemic manifestations are different. Nevertheless, although results of these studies are not uniformly positive, no adverse events related to cell therapy in this setting has been reported [22,40]. Stromal cells have been employed at the clinical level in other instances of severe pulmonary disease induced by viral infection [41]. In this regard, menstrual-blood derived MSCs were administered in 17 Chinese patients H7N9-induced ARDS patients during the 2013–2014 outbreak, again with no associated toxicity and a better survival compared to a control group of 44 patients (82.4% versus 45.4%) [42]. However, cases of secondary concomitant or subsequent bacterial or fungal infection after cell administration merit further comment. Although in patients with COVID-19 under mechanical ventilation the risk of bacterial or fungal secondary infection has been described to be around 8% and its diagnosis is challenging [43], [44], [45], theoretically an anti-inflammatory or immunomodulatory treatment such as MSC can potentially increase this risk and should be monitored and strictly assessed and followed in subsequent clinical trials.
The association between AT-MSC and a decrease in inflammatory parameters also support the hypothesis that cell administration may contribute to generate an inflammatory and immunomodulatory microenvironment [24]. This fact is of particular relevance, since almost all the patients included in our series had received steroids as well as tocilizumab (some also with anakinra and/or siltuximab), without clinical response. Unlike monoclonal antibodies that act only by blocking the effect of a single interleukin (e.g. tocilizumab for IL-6 or anakinra for IL-1) [46], AT-MSCs could act in the inflammatory microenvironment of endothelial and alveolar damage by interacting with various targets, releasing anti-inflammatory and anti-apoptotic molecules in a paracrine fashion, and modulating the action of the hyper-activated immune system, including macrophages, neutrophils and other cell types, and improving endothelial function [24,47,48].
Patients with COVID-19 pneumonia have been described to suffer from a pro-coagulant status and high levels of d-dimer have been associated to poor prognosis [16,17,37]. Remarkably, we observed a reduction of d-dimer 5 days after the first AT-MSC dose in most patients, and none of the patients developed a thromboembolic event, although mesenchymal cells preferentially home to the pulmonary circulation after endovenous administration [49]. Because of these pro-coagulant status patients with COVID-19 are routinely treated with low-molecular weight heparin [50]. It is possible that receiving prophylactic anticoagulant therapy may have contributed to decrease the potential prothrombotic risk that might have been induced by MSCs, but due to the sample size this should be further evaluated in subsequent studies before making a definite recommendation for concomitant administration of low weight heparin in patients with COVID-19 receiving AT-MSC.
Finally, although the sample size does not allow definitive conclusions, our results suggest that treatment with AT-MSC early after mechanical intubation might improve the outcome. This is also a potentially useful fact to take into account for the design of randomized clinical trials such as our BALMYS-19 trial. In addition, we have not found differences in patients treated with thawed versus fresh cells due to the limited number of patients included. This issue, that has been widely debated in the MSC field [51,52], should be also clarified in future trials.
Additional limitations of our study, besides the sample size, are related to the type of study (non-randomized case series) and to the variability inherent in the previous treatments, the different time of cell administration and the non-uniformity in the number of doses. The favorable response in many patients cannot be exclusively attributed to the effect of the cells, since other concomitant treatments were administered a few days before the cell administration and in a varied pattern. This proof-of-concept study, which constitutes the first case series of intubated COVID-19 patients treated with AT-MSC, has been used to design a randomized phase II clinical trial with a control arm that will provide better knowledge of the real scope of the potential of this therapeutic approach in this clinical setting.
In summary, our preliminary results, indicate that MSC derived from adipose tissue can be safely administered in critically ill patients with COVID-19 pneumonia and that administration of AT-MSC was followed by clinical improvement and changes in inflammatory and immune populations, which suggest a potential biological effect of the cells. These results have served as an initial proof of concept (especially taking into account the health emergency we are experiencing) for the design of a randomized, controlled phase 2 clinical trial of treatment with AT-MSCs in patients with COVID-19 requiring mechanical ventilation (BALMYS-19-“BAttLe against CO using MesenchYmal Stromal cells”-; EudraCT: 2020-001266-11; clinicaltrials.gov identifier NCT04348461). Our trial, as other potential randomized trials with a control arm consisting of standard treatment, will contribute to understanding the real potential of this cell-based therapeutic strategy [35,53].
Declaration of Competing Interest
Dr. Sanchez-Guijo reports grants and personal fees from Novartis, personal fees from BMS, Pfizer, Incyte, Gilead, Roche and Amgen, outside the submitted work; Dr. López-Parra reports personal fees from Gilead, Novartis, BMS and Janssen, outside the submitted work. Dr. JL del Pozo reports grants from Novartis and personal fees from Novartis, Pfizer, Gilead and Roche, outside the submitted work; Dr. Moraleda reports personal fees and other from Gilead, grants and personal fees from Jazz Pharma, and personal fees from Novartis and from Sandoz, outside the submitted work. Dr. García-Olmo has received personal fees from Takeda, outside the submitted work; Dr. Prosper reports personal fees from Oryzon Genomics, and from Janssen, outside the submitted work; Dr. García-Arranz and Dr. García-Olmo have applied for two patents related with this study entitled “Identification and isolation of multipotent cells from nonosteochondral mesenchymal tissue” (WO 2006/057649) and “Use of adipose tissue-derived stromal stem cells in treating fistula” (WO 2006/136244), and both are shareholders of Biosurgery, an educational company providing services to Takeda. All other authors declare no conflict of interest.
Acknowledgement
We would like to acknowledge the Instituto de Salud Carlos III (ISCIII) through the project “RD16/0011: Red de Terapia Celular”, from the sub-program RETICS, integrated in the “Plan Estatal de I+D+I 2013–2016″ and co-financed by the European Regional Development Fund “A way to make Europe”, groups RD16/0011/0001, -/0002, -/005, -/0013, -/0015, -/0029), the Centro en Red de Medicina Regenerativa y Terapia Celular de Castilla y León, Spain and AvanCell-CM (Red de Investigación de Terapia Celular de la Comunidad de Madrid, Spain), for supporting some personnel and networking activities.
In addition, we thank all the staff of the Cellular Production Units for their key effort in conducting this study during the pandemic, and all the staff of the Plastic Surgery Departments of our institutions for performing the lipoaspirates. We are also indebted to Prof. Jesús San Miguel for critically reviewing the manuscript and providing valuable suggestions.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100454.
Contributor Information
Fermín Sánchez-Guijo, Email: ferminsg@usal.es.
Felipe Prósper, Email: fprosper@unav.es.
Appendix. Supplementary materials
References
- 1.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical Characteristics of coronavirus disease 2019 in China. N Eng J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. April 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. February 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically Ill patients in the seattle region - case series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. March 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wynants L., van Calster B., Bonten M.M.J., Collins G.S., Debray T.P.A., de Vos M. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji D., Zhang D., Xu J., Chen Z., Yang T., Zhao P. Prediction for progression risk in patients with COVID-19 Pneumonia: the CALL Score. Clin Infect Dis. 2020;April 9 doi: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du R.-.H., Liang L.-.R., Yang C.-.Q., Wang W., Cao T.-.Z., Li M. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Res J. 2020 doi: 10.1183/13993003.00524-2020. April 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Raya B. Predictors of refractory coronavirus disease (COVID-19) pneumonia. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa409. April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phua J., Weng L., Ling L., Egi M., Lim C.-.M., Divatia J.V. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020 doi: 10.1038/s41577-020-0311-8. April 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore B.J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Diff. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGonagle D., Sharif K., O'Regan A., Bridgewood C. Interleukin-6 use in COVID-19 pneumonia related macrophage activation syndrome. Autoimmun Rev. 2020:2020. doi: 10.1016/j.autrev.2020.102537. April 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciceri F., Beretta L., Scandroglio A.M., Colombo S., Landoni G., Ruggeri A. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020 doi: 10.51893/2020.2.pov2. April 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z. D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J Thromb Haemost. 2020 doi: 10.1111/jth.14859. April 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020 doi: 10.1182/blood.2020006000. April 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCreary E.K., Pogue J.M. Coronavirus disease 2019 treatment: a review of early and emerging options. Open Forum Infect Dis. 2020;7:ofaa105. doi: 10.1093/ofid/ofaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H., Zhou Y., Zhang M., Wang H., Zhao Q., Liu J. Updated approaches against SARS-CoV-2. Antimicrob Agents Chemother. 2020 doi: 10.1128/aac.00483-20. March 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barlow A., Landolf K.M., Barlow B., Yeung S.Y.A., Heavner J.J., Claassen C.W. Review of Emerging Pharmacotherapy for the Treatment of Coronavirus Disease 2019. Pharmacotherapy. 2020 doi: 10.1002/phar.2398. April 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoury M., Cuenca J., Cruz F.F., Figueroa F.E., Rocco P.R.M., Weiss D.J. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. European Respir J. 2020 doi: 10.1183/13993003.00858-2020. April 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galipeau J., Sensébé L., Sensebe L. Mesenchymal Stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.gentile p., sterodimas a. Adipose-derived stromal stem cells (ASCs) as a new regenerative immediate therapy combating Coronavirus (COVID-19)-induced pneumonia. Expert Opin Biol Ther. 2020 doi: 10.1080/14712598.2020.1761322. April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sánchez-Guijo F., García-Olmo D., Prósper F., Martínez S., Zapata A., Fernández-Avilés F. Spanish cell therapy network (TerCel): 15 years of successful collaborative translational research. Cytotherapy. 2020;22:1–5. doi: 10.1016/j.jcyt.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Lambden S., Laterre P.F., Levy M.M., Francois B. The SOFA score - Development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019;23(1) doi: 10.1186/s13054-019-2663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. World Health Organization and R&D Blueprint strategy for COVID-19 n.d. https://www.who.int/teams/blueprint/covid-19 (Accessed May 2, 2020).
- 28.Araña M., Mazo M., Aranda P., Pelacho B., Prosper F. Adipose tissue-derived mesenchymal stem cells: isolation, expansion, and characterization. Methods Mol Biol. 2013;1036:47–61. doi: 10.1007/978-1-62703-511-8_4. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Arranz M., Garcia-Olmo D., Herreros M.D., Gracia-Solana J., Guadalajara H., de la Portilla F. Autologous adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistula: a randomized clinical trial with long-term follow-up. Stem Cells Transl Med. 2020;9:295–301. doi: 10.1002/sctm.19-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dominici M., Le B.K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. Int Soc Cell Ther Position Stat Cytother. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 31.Sanz-Baro R., García-Arranz M., Guadalajara H., de la Quintana P., Herreros M.D., García-Olmo D. First-in-human case study: pregnancy in women with Crohn's perianal fistula treated with adipose-derived stem cells: a safety study. Stem Cells Transl Med. 2015;4:598–602. doi: 10.5966/sctm.2014-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 33.Sánchez-Guijo F., Caballero-Velázquez T., López-Villar O., Redondo A., Parody R., Martínez C. Sequential third-party mesenchymal stromal cell therapy for refractory acute graft-versus-host disease. Biol Blood Marrow Transpl. 2014;20:1580–1585. doi: 10.1016/j.bbmt.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Wong H.Y.F., Lam H.Y.S., Fong A.H.T., Leung S.T., Chin T.W.Y., Lo C.S.Y. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. 2020 doi: 10.1148/radiol.2020201160. March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalil A.C. Treating COVID-19 - off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA. 2020 doi: 10.1001/jama.2020.4742. March 24. [DOI] [PubMed] [Google Scholar]
- 36.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zangrillo A., Beretta L., Scandroglio A.M., Monti G., Fominskiy E., Colombo S. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020 doi: 10.1016/S1441-2772(23)00387-3. April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lalu M.M., McIntyre L., Pugliese C., Fergusson D., Winston B.W., Marshall J.C. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS ONE. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson M., Mei S.H.J., Wolfe D., Champagne J., Fergusson D., Stewart D.J. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: an updated systematic review and meta-analysis. EClinicalMedicine. 2020;19 doi: 10.1016/j.eclinm.2019.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yen B.L., Yen M., Wang L., Liu K., Sytwu H. Current status of mesenchymal stem cell therapy for immune/inflammatory lung disorders: gleaning insights for possible use in COVID‐19. Stem Cells Transl Med. 2020 doi: 10.1002/sctm.20-0186. June 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du J., Li H., Lian J., Zhu X., Qiao L., Lin J. Stem cell therapy: a potential approach for treatment of influenza virus and coronavirus-induced acute lung injury. Stem Cell Res Ther. 2020;11:192. doi: 10.1186/s13287-020-01699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J., Hu C., Chen L., Tang L., Zhu Y., Xu X. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: a hint for COVID-19 treatment. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.02.006. February 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rawson T.M., Moore L.S., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa530. May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.François B., Laterre P.-.F., Luyt C.-.E., Chastre J. The challenge of ventilator-associated pneumonia diagnosis in COVID-19 patients. Crit Care. 2020;24:289. doi: 10.1186/s13054-020-03013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rawson T.M., Ming D., Ahmad R., Moore L.S.P., Holmes A.H. Antimicrobial use, drug-resistant infections and COVID-19. Nat Rev Microbiol. 2020 doi: 10.1038/s41579-020-0395-y. June 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maes B., Bosteels C., de Leeuw E., Declercq J., van Damme K., Delporte A. Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:468. doi: 10.1186/s13063-020-04453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golchin A., Seyedjafari E., Ardeshirylajimi A. Mesenchymal Stem Cell Therapy for COVID-19: present or Future. Stem Cell Rev Rep. 2020 doi: 10.1007/s12015-020-09973-w. April 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atluri S., Manchikanti L., Hirsch J.A. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically Ill COVID-19 patients: the case for compassionate use. Pain Phys. 2020;23:E71–E83. [PubMed] [Google Scholar]
- 49.Cardenes N., Aranda-Valderrama P., Carney J.P., Sellares Torres J., Alvarez D., Kocydirim E. Cell therapy for ARDS: efficacy of endobronchial versus intravenous administration and biodistribution of MAPCs in a large animal model. BMJ Open Respir Res. 2019;6 doi: 10.1136/bmjresp-2018-000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14810. March 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oja S., Kaartinen T., Ahti M., Korhonen M., Laitinen A., Nystedt J. The utilization of freezing steps in mesenchymal stromal cell (MSC) manufacturing: potential impact on quality and cell functionality attributes. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.01627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moll G., Geißler S., Catar R., Ignatowicz L., Hoogduijn M.J., Strunk D. Cryopreserved or fresh mesenchymal stromal cells: only a matter of taste or key to unleash the full clinical potential of MSC therapy? Adv Exp Med Biol. 2016;951:77–98. doi: 10.1007/978-3-319-45457-3_7. [DOI] [PubMed] [Google Scholar]
- 53.Oldenburg C.E., Doan T. Rigorous randomized controlled trial implementation in the era of novel coronavirus disease (COVID-19) Am J Trop Med Hyg. 2020 doi: 10.4269/ajtmh.20-0262. April 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.