Abstract
Objectives
Poor worldwide rate of blood pressure control is largely due to poor adherence to antihypertensive (AHT) drug treatment. The question of whether sex affects adherence has long been debated but conflicting findings have been reported on this issue. Our objective was to evaluate sex differences in the adherence to AHT therapy.
Research design and methods
Studies were identified through a systematic search of PubMed, CINAHL, PsycINFO, Web of Science and Google Scholar (through January 2020) and manual handsearching of relevant articles. Observational studies reporting adherence to AHT drugs measured by self-report or pharmacy refill prescription-based methods among men and women were included. Summarised estimates of ORs with 95% CIs were calculated using random-effects model and meta-regression models.
Results
From 12 849 potentially relevant publications, 82 studies (15 517 457 men and 18 537 599 women) were included. No significant between-sex differences in adherence to AHT were observed, whether all study-specific estimates were summarised (ORs 1.04, 95% CI 1.00 to 1.09, p=0.07), nor estimates were pooled according to the method for measuring adherence. Among patients aged 65 years or older, lower self-reported adherence was observed in women (ORs 0.84, 95% CI 0.72 to 0.97, p=0.02), while the main result remained unchanged according to other subgroup analyses.
Conclusions
Definitive evidence of sex differences in adherence to AHT therapy cannot be drawn. Our little knowledge about factors affecting adherence, in particular of sex effect among elderly, urgently requires high-quality studies investigating these issues.
Keywords: hypertension, clinical pharmacology, epidemiology
Strengths and limitations of this study.
We systematically selected and collected the available literature on the role of sex in adherence to antihypertensives.
Potential interaction between sex and other variables was explored by means of various analyses.
Although the systematic revision focused on two metrics for measuring adherence to antihypertensives (ie, self-report and pharmacy refill metric), more technological and recent methods for the adherence evaluation were not included in this investigation.
Introduction
Randomised clinical trials have shown that hypertension is a reversible risk factor, that is, that a reduction in elevated blood pressure (BP) values by treatment reduces the risk of fatal and non-fatal cardiovascular (CV) events.1 However, effective BP reductions are rare in patients with hypertension who are thus characterised by a high prevalence of uncontrolled BP2–4 and an increased incidence of CV events,5 keeping hypertension as one of the major risk factors for CV disease, which is leading cause of death.6
Although several factors are involved,7 a consensus exists that the poor worldwide rate of BP control is largely due to poor adherence to the treatment regimen.8–17 In general, adherence may be defined as the extent to which patients follow treatment prescribed by their healthcare providers.18 Adherence to antihypertensive (AHT) medications is an imperative issue which can be directly linked with the management of chronic diseases, such as hypertension.19 In particular, adherence to AHT drug therapy, considered an important factor to control BP, 1 year after initiation is typically reported at <50%.20 Indeed, non-adherence is an additional risk factor of fatal CV events in real-life setting.21
Many factors have been shown to affect adherence to AHT treatment recommendations22–24: (1) demographic aspects, such as age,25–27 ethnicity, marital status, educational level, socioeconomic status28; (2) clinical factors, like cognitive problems, depression, complicated therapeutic regimens28 (eg, number of doses, concurrent medications and changes in AHT treatment)29 30; (3) knowledge of patient about hypertension and AHT treatment,31 perception of the health risk related to the disease32–35 and the relationship between patient and healthcare provider.36
Among these, the question of whether sex may be considered a predictor of adherence has long been debated. In fact, differences between men and women in attitudes, beliefs and motivation towards health issues37 38 might possibly influence adherence to health recommendations, particularly to dispensed drug therapies. Notwithstanding the wide range of published literature on this issue, conflicting findings have been reported about adherence to AHT and sex.39 40 Several studies have found that women have higher levels of hypertension awareness than men,41 42 which tend to increase with age.43 Thus, women may be more motivated to adhere because they understand the risk of non-adherence44 and get better use of healthcare services.45 In addition, women may receive less aggressive treatment after the occurrence of a CV event,46 47 which could promote their better adherence to medication. Finally, it has been reported that women had better adherence to other chronic drug therapies, such as those for treatment of depression48–50 and diabetes mellitus.51 Inconsistently, however, a recent meta-analysis reported higher refill rate of statins in men than women.52
Although there are several self-report instruments to assess drug adherence (eg, Hill-Bone Compliance Scale,53 the Medication adherence rating scale54 and the Hypertension Self-Care Activity Level Effects55), the Morisky Medication Adherence Scale (MMAS)56 is the most applied. MMAS is an adherence-screening tool based on the complexity of assessing adherence in hypertension. The validated questionnaire is composed of four or eight items57 about past use of AHTs with a cut-off value of MMAS mean score of respectively three or six for labelling patients as adherent or not.
To the best of our knowledge, there is only one systematic review focused on this research topic that reported better adherence to AHT therapy in women than men.58 However, because these findings were generated by assembling studies that investigated adherence by means of the MMAS questionnaire, some caution should be adopted due to the questionable between sex reproducibility of answers to medication-taking questions.59
Therefore, we decided to extend the systematic review conducted by Abegaz et al58 to investigations that studied adherence by prescription-refill data, that is, the most used data source for assessing the adherence of large population. Two common measures could be used to quantify adherence by means of prescription refill data: the medication possession ratio (MPR) and the proportion of days covered (PDC).60 61 These two measurements are essentially defined by the number of doses dispensed respect to the observation time and patients with MPR or PDC greater than 80% are classified as adherent.62
With these premises, we performed a systematic review and meta-analysis of available observational studies comparing adherence to AHT medication in men and women, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement63 (online supplementary table S1). Because pre-existing data do not allow of making an initial hypothesis on the possible direction of the sex-adherence association, our synthesis of current knowledge about the issue must be seen as exploratory rather than hypothesis testing.
bmjopen-2019-036418supp001.pdf (283.9KB, pdf)
Materials and methods
Search strategy and study selection
We performed a PubMed, CINAHL, PsycINFO, Web of Science and Google Scholar search for observational studies published up to January 2020 that reported data on adherence to AHT drugs in men and women. Studies were included in our review if they assessed treatment adherence in clinical practice and by means of self-reported or pharmacy refill methods. In the main analysis, no inclusion/exclusion criterion was applied regarding the length of follow-up in which drug adherence was assessed. Search strategy included keywords and/or corresponding MeSH terms related to adherence, AHT medication and sex. Full details on strategy adopted are reported in the online supplementary table S2.
The search was limited to studies published in English language and articles were included if they reported quantitative data on AHT adherence in men and women. When data were published more than once, the most recent and complete paper was selected. Papers, which did not report original findings (ie, letters, case report, systematic review and meta-analysis) or selected a population taking AHT drugs for conditions different from hypertension (eg, myocardial infarction or heart failure) were excluded. Moreover, a hand-checking search was performed in order to identify additional relevant studies. The search was designated by GC and validated by all the authors, whereas extraction of articles was performed by one of the authors (AB) and independently verified by a second author (FR) to determine the eligibility of each article for inclusion. Discrepancies between readers were resolved in conference.
Data collection
For each included study, we extracted details on publication year, country where the study was conducted, characteristics of the investigated persons (eg, mean age, number of women and men), employed AHT agents, adjustment and stratification variables, adherence in men and women, and OR, or other association measures, with 95% CI or p value, for the association between sex and adherence. Moreover, we evaluated the quality of the eligible studies according to the Newcastle Ottawa scale (online supplementary table S3)64 and more than five points identified high-quality studies. In addition, information about the metric adopted for measuring adherence was also recorded. In particular, studies were classified according to whether self-report or pharmacy refill prescription-based methods were adopted. The former ones were based on 4-item or 8-item MMAS (MMAS-4 and MMAS-8, respectively), while the latter ones concerned the MPR or the PDC.65
Statistical analysis
The measure of interest was the summary OR (ORs) that evaluated the association between AHT adherence and sex, using men as reference. Unless otherwise specified,66 a patient with MMAS-4 ≥3, MMAS-8 ≥667 68 or MPR/PDC ≥80% was considered to be on good adherence. Where possible, we pooled adjusted estimates from the original studies; raw data and computed unadjusted ORs were used otherwise. Estimates were summarised if at least three studies reported the association of interest.
Heterogeneity between study-specific estimates was tested using X2 statistics69 and measured with the I2 index (a measure of the percentage variation across the studies caused by heterogeneity).70 To take into account differences in sample characteristics, measurement and other factors, we pooled the original estimates by fitting the DerSimonian and Laird random-effects model.71 Influence analysis was conducted by omitting one study at a time in order to identify to what extent the results were influenced by a single study.
Other than classical meta-analysis, meta-regression models were performed for estimating the effect of above-reported covariates (ie, method for collecting adherence data, incident/prevalent users, adjusted/unadjusted estimates, geographical area) on the log (ORs). The regression models were fitted including one covariate at a time.
To explore the interaction between sex and other variables on the propensity of being adherent, subgroup analyses were carried out. Studies were stratified according to known determinants of adherence, that is, age, prevention status (primary vs secondary) and drug users (incident vs prevalent users). Medication therapy was considered for primary prevention if patients with a pre-existing CV disease were excluded from the study; conversely, the drug use was considered for secondary prevention. In addition, patients were classified as incident users if long-term medication takers were excluded from the analysis; otherwise, the study was considered to be performed among prevalent users.
Furthermore, subgroup analyses were performed according to the length of follow-up, the geographical area where the study was carried out, and whether the estimates were adjusted or not.
All tests were considered statistically significant for p values less than 0.05. The analyses and the correspondent graphical visualisation of forest and funnel plots were respectively performed by using RevMan V.5.3 (Nordic Cochrane Center) and STATA Software Program V.13.1 (STATA).
Patient and public involvement
No patients were involved in the development of the research question, outcome measures, design, study implementation, dissemination of the results of the research to the study participants or interpretation of the results.
Results
Study selection and characteristics
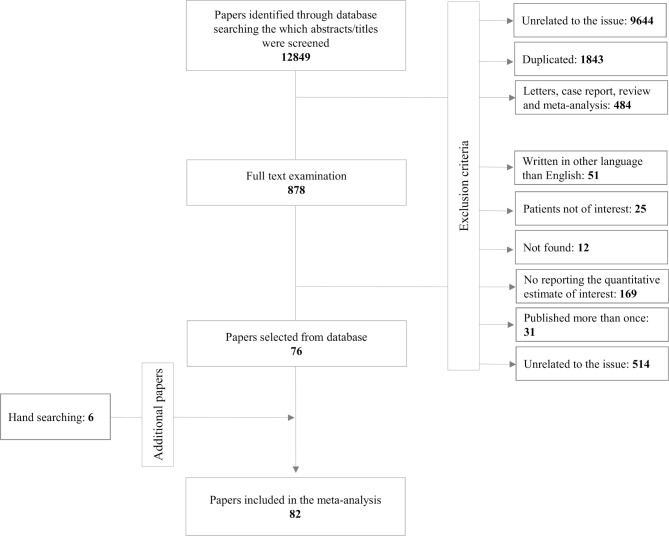
As shown in figure 1, 12 849 papers were first identified. After screening their abstracts and titles, 11 971 articles were excluded mainly because they were (1) no related to the issue, (2) duplicates, (3) letters, case report, review or meta-analysis. Among the remaining 878 articles which were assessed for full-text review, 802 were excluded because not written in English language (n=51), analysed patients not of interest (25), not found (12), not reporting quantitative estimates of interest (169), data were published more than once (31), unrelated to the issue (514). Other than the 76 papers thus selected,28 39 46 66 72–143 six additional papers were found through hand searching of relevant papers.40 144–148
Figure 1.
Flow diagram of the selection of studies regarding self-reported and refill rates used to measure adherence to AHT. AHT, antihypertensive.
Information about the main characteristics of the 82 papers agreeing with the inclusion criteria and included in the current meta-analysis are shown in table 1. Adherence to AHT was measured with MPR and PDC metrics from 16 and 17 studies respectively, while 49 papers applied the MMAS-4 or MMAS-8 scales. Overall, 34 670 674 hypertensive patients (15 517 457 men and 18 537 599 women) were included into these studies. For the most part of them, adherence was measured with MPR (more than 30 million), less with PDC (about 2 million), while MMAS-4 and MMAS-8 scales were used for 27 160 and 12 062 patients, respectively. Moreover, two articles were assigned to the low-quality category86 114 although there was variability among the assigned quality scores.
Table 1.
Characteristics of the studies comparing adherence to AHT drugs between men and women
| First author publication year, country (reference) | Age range | Sample size m/f | Exposure | OR (95% CI) | Controlled variables/notes | Follow-up | Quality |
Adherence to AHT in
|
|||||||
| Alfian 2019, the Netherlands130 | ≥40 | 5468 3068/2400 |
AHT (diuretic, BB, CCB, agent acting on the renin-angiotensin system) | 1.10 (0.93 to 1.31) | Unadjusted estimates | 1 year | High |
| Calderón-Larrañaga 2016, Spain100 | ≥18 | 113 397 50242/63155 |
AHT (ACEi, ARB, BB, CCB, thiazide diuretics) | 0.89 (0.87 to 0.92) | Age, nationality, residence location, blood pressure level, mental comorbidity, health status, CV risk factors, polypharmacy, visit to GP, different specialties visited | 1 year | High |
| Friedman 2010, America101 | ≥66 | 207 473 86308/121165 |
AHT (ACEi, ARB, BB, CCB, thiazide and thiazide-like diuretics, and combination agent) | 1.12 (1.06 to 1.18) | Age, calendar year, therapeutic class, illness severity, socioeconomic status, residence location, medical service type | 2 years | High |
| Holmes 2012, America72 | ≥66 | 168 522 51580/116942 |
AHT (ACEi, alpha-blockers, ARB, BB, CCB, diuretics, vasodilators) | 1.00 (0.94 to 1.02) | Age, ethnicity, socioeconomic status, residence location, education, comorbidities, concomitant comedications | 1 year | High |
| Inkster 2006, Scotland102 | 40–79 | 511 242/269 |
AHT | 0.87 (0.53 to 1.44) | n.a. | 2 years | High |
| Ishisaka 2012, America103 | ≥18 | 51 772 22397/29375 |
AHT (ACEi, alpha one adrenergic antagonists, alpha two adrenergic agonists, ARB, AHT combinations, BB, CCB, other AHT medication (hydralazine, reserpine, minoxidil), thiazide diuretics, and diuretic combinations) | 1.00 (0.97 to 1.04) | Age, ethnicity, CDS | 3 years | High |
| Lee 2013, Taiwan128 | ≥30 | 78 558 39047/39511 |
AHT (alpha-blockers, ACEi, ARB, BB, CCB, other) | 0.92 (0.89 to 0.95) | Age, socioeconomic status, CCI, medical service type, concomitant comedications, public assistance | 1 year | High |
| Manteuffel 2014, America104 | ≥18 | 29 470 455 13458395/16012060 |
AHT | 0.989746 (0.988274 to 0.991221) | Unadjusted estimates | 1 year | High |
| Morris 2006, America28 | ≥18 | 492 132/360 |
AHT (ACEi, alpha receptor antagonists, angiotensin II receptor antagonists, beta adrenergic receptor antagonists, clonidine, diuretics, vasodilators) | 0.77 (0.50 to 1.18) | Unadjusted estimates | 1 year | High |
| Muntner 2013, America145 | ≥65 | 1391 553/838 |
AHT (ACEi, ARB, BB, CCB, diuretics) | 1.00 (0.79 to 1.25) | Unadjusted estimates | 1 year | High |
| Park 2008, South Korea73 | ≥20 | 2455193 1028724/1426469 | AHT | 0.97 (0.95 to 0.99) | Age, disability, comorbidities, treatment duration, socioeconomic status, residence location, concomitant comedications, medical service type | 1 year | High |
| Shah 2007, America142 | ≥18 | 708 378/330 |
AHT | 0.96 (0.71 to 1.29) | Unadjusted estimates | 1 year | High |
| Taira 2007, Hawaii105 | ≥18 | 28 395 13346/15049 |
AHT (ACEi, ARB, BB, CCB, thiazide type diuretics) | 1.00 (0.96 to 1.05) | Age, illness severity, type of medical programme, therapeutic class, comorbidities, sociodemographic characteristics, education, physician characteristics | 1 year | High |
| van Dijk 2007, the Netherlands106 | n.a. | 12 110 5156/6954 |
AHT (ACEi, Angiotensin II receptor antagonists, BB, diuretics, other) | 0.93 (0.81 to 1.05) | Sociodemographic characteristics, concomitant comedications, comorbidities, health status | 1 year | High |
| Van Wijk 2006, the Netherlands99 | Mean age 60.22±14.19 | 1232 595/637 |
AHT (ACEi, Angiotensin II receptor antagonists, BB, CCB, diuretic, other) | 0.97 (0.71 to 1.34) | Unadjusted estimates | 1 year | High |
| Wong 2010, China107 | ≥18 | 83 884 35902/47982 |
AHT (BB, CCB, drugs acting on RAS and others (including alfa blockers, potassium sparing and other diuretics, vasodilators and combination treatement), thiazide diuretics) | 1.19 (1.13 to 1.25) | Age, sociodemographic characteristics, socioeconomic status, medical service type, residence location, different specialties visited, Visit to GP, comorbidities, AHT drug class | 3 years | High |
| PDC | |||||||
| Chang 2019, America131 | ≥18 | 2927 1452/1476 |
(ACEi, ARB, renin-angiotensin system antagonists, BB, CCB, diuretics, other AHTs) | 0.87 (0.74 to 1.02) | Unadjusted estimates | 1 year | High |
| Couto 2014, America108 | ≥18 | 659 553 369372/290181 |
AHT (ACEi, direct renin inhibitors and angiotensin II-receptor antagonists, or any combination product including one or more of these classes) | 0.85 (0.83 to 0.86) | Age, nationality, socioeconomic status | 1 year | High |
| Cyrus 2019, America132 | 22–64 | 1573 829/744 |
AHT (diuretics, BB, ACEi, angiotensin II receptor blockers, CCB, alpha blockers, alpha-2 receptor agonists, central agonists, peripheral adrenergic inhibitors, vasodilators, and renin inhibitors) | 1.11 (0.89 to 1.39) | Age, CCI, comorbidities, concomitant comedications, ethnicity, residence, Visit to GP | 1 year | High |
| Degli Esposti 2010, Italy109 | ≥18 | 94 947 40771/54176 |
AHT (ACEi, ARB, BB, CCB, diuretics) | 1.35 (1.31 to 1.39) | Age, calendar year, prior medications, concomitant comedications | 1 year | High |
| Di Martino 2008, Italy66 | ≥18 | 7626 3222/4404 |
AHT | 1.45 (1.30 to 1.62) | Age, start of treatment, diabetes, hypertension/renal disease, concomitant comedications | 1 year | High |
| Hedna 2015, Sweden46 | n.a. | 867 412/455 |
AHT (ACEi, combination ACEi and diuretics, ARB, combination ARB and diuretics, anti-adrenergic, BB, CCB, diuretics) | 1.02 (0.74 to 1.40) | AHT drug class, age, education, socioeconomic status, Diagnosis Related Group weight, CV risk factors | 2 years | High |
| Iyengar 2014, America147 | ≥65 | 615 618 n.a. |
AHT | 1.06 (1.05 to 1.07) | n.a. | 1 year | High |
| Williams 2018, America74 | ≥65 | 2122 866/1256 |
AHT | 0.93 (0.77 to 1.13) | Unadjusted estimates | 1 year | High |
| Lauffenburger 2017, America110 | ≥18 | 462 227 222912/239315 |
AHT (ACEi, ARB, BB, CCB, diuretics, thiazide, other) | RR 0.89 (0.88 to 0.90) |
Age, residence location, comorbidities, diabetes, Prior hospitalisation, public assistance | 1 year | High |
| Mazzaglia 2009, Italy144 | ≥35 | 18 806 7835/10971 |
AHT | 1.13 (1.07 to 1.21) | Unadjusted estimates | 6 months | High |
| Nguyen 2017, Vietnam75 | 35–64 | 315 171/144 |
AHT | 1.53 (0.96 to 2.45) | Age, ethnicity, CV risk factors | 1 year | High |
| Perseguer-Torregrosa 2014, Spain76 | ≥50 | 419 184/235 |
AHT | 1.46 (0.95 to 1.97) | Age, CV risk factors, history of hypertension, AHT drug class, concomitant comedications, BMI, diabetes, dyslipidaemia, quality of life survey | <2 months | High |
| Rea 2020, Italy133 | 40–80 | 60 526 30860/29666 |
AHT (diuretics, ACEIs, ARBs, BB, CCB, alpha-blockers) | 0.88 (0.32 to 2.47) | Age, comorbidities, concomitant comedications, multisource comorbidity score, start of treatment | 1 year | High |
| Simon-Tuval 2016, Israel111 | Mean age 64.58±8.94 | 1582 1086/496 |
AHT (ACEI, ARB, BB, CCB) | 1.27 (1.03 to 1.58) | Unadjusted estimates | 4 years | High |
| Walsh 2019, Ireland134 | ≥50 | 1431 645/786 |
AHT (diuretics, BB, CCB, Agents acting on the renin angiotensin system) | 1.08 (0.85 to 1.36) | Unadjusted estimates | 1 year | High |
| Wang 2019, America148 | ≥65 | 10 836 5836/5000 |
AHT | 0.77 (0.70 to 0.85) | Age, start of treatment, nationality, comorbidities, diabetes, prior hospitalisation, type of medical programme, previous use of AHT | 1 year | High |
| Wong 2015, China148 | Mean age 58.65±17.32 | 203 258 89725/113533 |
AHT (ACEi, alfa blockers, BB, CCB, thiazide diuretics) | 0.87 (0.85 to 0.89) | Age, public assistance, medical service type, start of treatment, residence location, treatment duration | 1 year | High |
| 4-item Morisky Medication Adherence Scale | |||||||
| Alhaddad 2016, Lebanon and Jordan77 | >21 | 1470 842/628 |
AHT | 1.04 (0.84 to 1.29) | Unadjusted estimates | High | |
| Ambaw 2012, Ethiopia113 | ≥18 | 384 142/242 |
AHT | 2.08 (1.22 to 3.57) | Residence location, marital status, religion, education, socioeconomic status, comorbidities, blood pressure level, distance from the hospital, dosing frequency, sociodemographic characteristics, AHT drug class, GP characteristics | High | |
| Arshad 2015, Pakistan114 | Mean age 58.81±12.26 | 106 53/53 |
AHT | 0.91 (0.40 to 2.11) | Unadjusted estimates | Low | |
| Bader 2015, Northern United Arab Emirates115 | ≥18 | 250 134/116 |
AHT | 1.91 (1.15 to 3.18) | Unadjusted estimates | High | |
| Cuffee 2013, America116 | ≥19 | 780 314/466 |
AHT | 0.72 (0.52 to 0.98) | Age, sex, education, socioeconomic, Hall Trust Scale | High | |
| Demoner 2012, America117 | >18 | 150 48/102 |
AHT | 1.81 (0.86 to 3.83) | Unadjusted estimates | High | |
| Dosse 2009, America118 | Mean age 61.01±9.46 | 68 24/44 |
AHT | 1.11 (0.25 to 4.88) | Unadjusted estimates | High | |
| Grégoire 2006, America78 | ≥18 | 509 225/284 |
AHT (ACEi, ARB, CCB) | 0.81 (0.53 to 1.22) | Unadjusted estimates | High | |
| Hashmi 2007, Pakistan79 | ≥18 | 438 199/239 |
AHT | 0.93 (0.60 to 1.46) | Unadjusted estimates | High | |
| Khan 2014, America80 | 18–60 | 200 77/123 |
AHT | 0.49 (0.23 to 1.05) | Unadjusted estimates | High | |
| Li 2006, America81 | ≥18 | 200 100/100 |
AHT | 1.45 (0.76 to 2.75) | Unadjusted estimates | High | |
| Lo 2016, China119 | ≥65 | 195 40/155 |
AHT | 0.96 (0.47 to 1.92) | Unadjusted estimates | High | |
| Lulebo 2015, Democratic Republic of Congo82 | >18 | 395 95/300 |
AHT | 0.80 (0.50 to 1.30) | Unadjusted estimates | High | |
| Morrison 2015, Europe83 | ≥18 | 2595 1334/1261 |
AHT | 1.22 (1.01 to 1.47) | Age, education, marital status, socioeconomic status, concomitant comedications, dosing frequency, illness consequences | High | |
| Park 2013, South Korea84 | ≥65 | 241 144/97 |
AHT | 0.67 (0.40 to 1.14) | Unadjusted estimates | High | |
| Stavropoulou 2012, Greece85 | Mean age 61 | 735 294/441 |
AHT | 1.08 (0.83 to 1.39) | Age, education, socioeconomic status, illness consequences | High | |
| Tibebu 2017, Ethiopia40 | ≥18 | 404 210/194 |
AHT | 2.18 (1.33 to 3.58) | Age, marital status, education, socioeconomic, concomitant comedications, sociodemographic characteristics | High | |
| Turner 2009, America86 | >70 | 202 69/133 |
AHT | 1.26 (0.63 to 2.50) | Unadjusted estimates | Low | |
| Usman 2019, Nigeria135 | ≥18 | 237 76/161 |
AHT | 0.32 (0.18 to 0.56) | Unadjusted estimates | High | |
| Wagner 2012, America97 | ≥18 | 16 474 8402/8072 |
AHT | 1.97 (1.85 to 2.11) | Unadjusted estimates | High | |
| Wang 2014, Australia96 | ≥65 | 382 185/197 |
AHT | 0.99 (0.60 to 1.63) | Age, marital status, education, comorbidities, previous use of AHT, public assistance | High | |
| Yang 2016, China120 | ≥18 | 745 345/400 |
AHT | 0.75 (0.56 to 1.01) | Unadjusted estimates | High | |
| 8-items Morisky Medication Adherence Scale | |||||||
| Adidja 2018, Cameroon87 | ≥21 | 183 65/118 |
AHT | 1.10 (0.40 to 2.60) | Age, socioeconomic status, illness consequences, history of hypertension, previous use of AHT | High | |
| Al-Ramahi Rowa’ 2015, Palestine88 | ≥18 | 450 197/253 |
AHT | 1.01 (0.69 to 1.46) | Unadjusted estimates | High | |
| Alkhamis 2019, Saudi Arabia136 | ≥18 | 372 231/141 |
AHT | 1.49 (0.97 to 2.27) | Unadjusted estimates | High | |
| Hacıhasanoğlu Aşılar 2014, Turkey121 | ≥18 | 196 77/119 |
AHT | 1.18 (0.65 to 2.11) | Unadjusted estimates | High | |
| Behnood-Rod 2016, Iran122 | Mean age 60.3±10 | 280 118/162 |
AHT | 1.03 (0.64 to 1.65) | Unadjusted estimates | High | |
| Berhe 2017, Ethiopia123 | ≥18 | 925 355/570 |
AHT | 1.04 (0.81 to 1.36) | Unadjusted estimates | High | |
| Cummings 2016, America124 | Mean age 57.3±12.8 | 495 161/334 |
AHT | 0.96 (0.65 to 1.40) | Unadjusted estimates | High | |
| Esmaeili 2016, Iran94 | Mean age 65.02±8.88 | 422 123/299 |
AHT | 1.44 (0.93 to 2.23) | Unadjusted estimates | High | |
| Fortuna 2018, America89 | ≥18 | 2128 860/1268 |
AHT | 0.99 (0.80 to 1.20) | Age, ethnicity, public assistance, information about treatment | High | |
| Gavrilova 2019, Latvia137 | ≥18 | 171 43/128 |
AHT (beta adrenoceptor blockers, ARB, aldosterone antagonists, CCB, ACEi, diuretics) | 1.90 (0.95 to 3.83) | Unadjusted estimates | High | |
| Gowda 2019, India138 | ≥29 | 150 96/54 |
AHT | 0.41 (0.14 to 1.18) | Unadjusted estimates | High | |
| Han 2015, Myanmar98 | ≥30 | 216 89/127 |
AHT (ACEi, ARB, BB, CCB, other) | 0.54 (0.30 to 0.99) | Age, education, socioeconomic status, comorbidities, history of hypertension, illness consequences, sociodemographic characteristics | High | |
| Hyre 2007, America125 | ≥18 | 295 195/100 |
AHT | 1.29 (0.70 to 2.36) | Unadjusted estimates | High | |
| Holt 2013, America90 | ≥65 | 2194 911/1283 |
AHT | 0.81 (0.67 to 0.98) | Unadjusted estimates | High | |
| Hou 2016, China143 | ≥60 | 585 353/232 |
AHT | 0.93 (0.65 to 1.32) | Unadjusted estimates | High | |
| Mahmood 2020, Pakistan139 | ≥18 | 741 389/352 |
AHT | 0.88 (0.24 to 3.26) | Unadjusted estimates | High | |
| Kang 2015, China91 | ≥18 | 2445 1074/1371 |
AHT | 0.84 (0.70 to 1.02) | Age, education, socioeconomic status, marital status, sociodemographic characteristics, illness consequences, concomitant comedications, comorbidities | High | |
| Kumar 2014, India129 | >18 | 120 76/44 |
AHT | 0.77 (0.36 to 1.62) | Unadjusted estimates | High | |
| Nabi 2019, Bangladesh140 | n.a. | 100 57/43 |
AHT | 3.27 (1.42 to 7.50) | Unadjusted estimates | High | |
| Okeke 2019, Nigeria141 | n.a. | 421 210/211 |
AHT | 1.42 (0.82 to 2.48) | Unadjusted estimates | High | |
| Okello 2016, Uganda95 | n.a. | 329 101/228 |
AHT | 1.21 (0.41 to 1.59) | Age, education, marital status, distance from the clinic, concomitant comedications | High | |
| Jankowska-Polanska 2017, Poland126 | >18 | 620 287/333 |
AHT | 1.47 (1.04 to 2.07) | Unadjusted estimates | High | |
| Rahmawati 2018, Indonesia92 | ≥45 | 203 61/142 |
AHT | 0.95 (0.45 to 1.98) | Unadjusted estimates | High | |
| Saarti 2016, Beirut39 | ≥18 | 117 59/58 |
AHT | 0.50 (0.22 to 1.13) | Unadjusted estimates | High | |
| Korb-Savoldelli 2012, France127 | ≥18 | 199 114/85 |
AHT | 0.86 (0.41 to 1.80) | Unadjusted estimates | High | |
| Sutar 2017, India146 | ≥18 | 213 96/117 |
AHT | 0.80 (0.22 to 2.94) | Unadjusted estimates | High | |
| Yue 2015, China93 | Mean age 64.15±10.81 | 232 110/122 |
AHT | 0.99 (0.59 to 1.66) | Unadjusted estimates | High | |
ACEi, ACE inhibitor; AHT, antihypertensive; ARB, angiotensin II receptor blocker; BB, beta-blocker; BMI, body mass index; CCB, calcium channel blocker; CDS, chronic disease score; CV, cardiovascular; GP, general practitioner; MPR, Medication Possession Ratio; n.a, not available; PDC, Proportion of Days Covered.
The majority of the studies considered younger subjects, particularly among the 82 selected studies (1) 4228 39 40 66 73 77–79 81–83 87–89 91 97 100 103–105 107–110 113 115–117 120 121 123 125–127 129 131 135–137 139 142 146 were focused on a younger population, (2) 1176 81 93 99 103 129 131 133 134 139 145 were focused on individuals aged 30 years old or more and (3) 14 papers72 74 76 84 86 90 96 101 119 134 143 145 147 148 selected older subjects. Conversely, 1546 85 93–95 99 106 111 112 114 118 122 124 140 141 studies did not specify the age range of enrolled patients.
Regarding the sample size, a great proportion of the studies involved around or less than 50028 39 40 75 76 78–88 92–94 96 98 102 113–122 124 125 127 129 135–138 140 141 143 146 or 100046 74 77 83 89–91 99 111 123 126 131 132 134 139 142 145 individuals. Just two studies66 130 were based on less than 10 000 subjects, five and four considered, respectively, around or more than 10 00097 105 106 144 148 or 50 000103 107 128 133 participants, three72 100 109 involved about 100 000 subjects and six73 101 107 108 110 147 studies were based on 200 000 or more individuals. Just one study104 involved about 30 million of hypertensive subjects. The majority of the studies conducted with the use of MPR/PDC metric considered a wide list of AHT28 46 72 99–101 103 105–112 128 130–134 145 and adjustments46 66 72 73 75 76 100 101 103 105–110 112 128 132 133 148 while just 378 98 137 and 1140 83 85 87 89 91 95 98 113 116 148 were found among those based on questionnaires. The length of follow-up was accounted for studies based on refill rates by mainly considering 1 year of observation,28 66 72–75 99 100 104–106 108–110 112 128 130–134 142 145 147 148 while the remaining papers considered less than 1,76 144 2o46 101 102 or more than 3 years.103 107 111 Considering geographical area, 26 studies were conducted respectively in America28 72 74 78 80 81 86 89 90 97 101 103 104 108 110 116–118 124 125 131 132 142 145 147 148 and Asia,73 75 79 84 91–94 98 105 107 112 114 115 119–122 128 129 136 138–140 143 146 15 in the Mediterranean countries,39 66 76 77 83 85 88 100 109 111 126 127 133 137 144 8 in Africa,40 82 87 95 113 123 135 141 6 in North Europe46 99 102 106 130 134 and just 1 in Australia.96
Sex–adherence association
As shown in figure 2, no significant between-sex differences in adherence to AHT were observed, whether all study-specific estimates were summarised (ORs 1.04, 95% CI 1.00 to 1.09, p=0.07), or estimates were pooled according to the metric used for measuring adherence (the ORs ranging between 1.00, 95% CI 0.96 to 1.03, and 1.06, 95% CI 0.95 to 1.18). With the exception of summarised estimates based on MMAS-8 metric, significant between-study heterogeneity was observed with I2 values ranging from 90% (MMAS-4) to 99% (PDC). No evidence of influence of any individual study (online supplementary table S4) was observed for any summarised estimate.
Figure 2.
Forest plots of study-specific and summary relative risks for adherence to antihypertensive drugs in women compared with men obtained by the following measurements: PDC, MPR, 4-item and 8-item Morisky Medication Scale. Squares represent study-specific relative risk estimates (size of the square reflects the study-specific statistical weight, ie, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with corresponding 95% CIs; p values are from testing for heterogeneity between study-specific estimates. Different lengths of follow-up are shown for PDC and MPR measurements. MPR, medication possession ratio; PDC, proportion of days covered.
Exploring sources of confounding of sex–adherence association
The effect of selected characteristics of the included studies in modifying the sex–adherence association is shown in online supplementary table S5. There was no statistical evidence that men and women differently adhered to AHT therapy (model 1), not even when the effect of the method for collecting adherence data (model 2), the inclusion of incident or prevalent AHT users (model 3), adjustment of the original estimates (model 4), nor the geographical area where the study was conducted (model 5) were taken into account.
Exploring sources of heterogeneity of sex–adherence association
As shown in figure 3, inconsistent findings were observed among older patients according to the adherence measure: men were more adherent according to the Morisky metric (ORs 0.84, 95% CI 0.72 to 0.97, p=0.02) but this result was not confirmed by the PDC/MPR scale.
Figure 3.
Forest plots of study-specific and summary relative risks for adherence to antihypertensive drugs in women compared with men obtained by MPR and PDC measurements together and Morisky among the elderly population (ie, ≥65 years). Squares represent study-specific relative risk estimates (size of the square reflects the study-specific statistical weight, ie, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with corresponding 95% CIs; p values are from testing for heterogeneity between study-specific estimates. Different lengths of follow-up are shown. MPR, Medication Possession Ratio; PDC, Proportion of Days Covered.
Accordingly, subgroup analyses focusing on patients aged more than 18 years (online supplementary figure S1), 1-year length of follow-up (online supplementary figure S2), geographical area where the study was performed (online supplementary figure S3) and adjusted or unadjusted estimates (online supplementary figure S4), never provided convincing evidence that adherence was different between men and women. Furthermore, sex did not show any effect not even stratifying the analysis for prevention status (primary vs secondary) nor for drug users (incident vs prevalent users).
bmjopen-2019-036418supp002.pdf (70.8KB, pdf)
bmjopen-2019-036418supp003.pdf (47KB, pdf)
bmjopen-2019-036418supp004.pdf (123KB, pdf)
bmjopen-2019-036418supp005.pdf (111.1KB, pdf)
Discussion
The current meta-analysis did not provide convincing evidence that men and women differently adhere to AHT drug therapy. However, although we did not find evidence of influence of any individual study, and almost all the included articles were classified as high-quality studies, inconsistency between studies suggests that sex–adherence association need careful discussion before being judged absent.
Several reasons might explain the between-study heterogeneity for adherence detected by self-report and pharmacy refill metric. A first cause could be due to different methods assessing adherence. Two measurement methods were considered by our meta-analysis, namely self-report and pharmacy refill prescription-based ones. Findings conflicting with the ours were reported by a previous review based on the self-reported 8-item Morisky scale.57 The Morisky scale is a common and validated tool for the adherence screening that has been shown to predict adherence with CV medications.55 149 However, direct questions about the use of medications could cause the overestimation of adherence that is likely due by the willingness of patients to appear adherent150–153; thus, the identification of subjects who forget to take drugs could be difficult. Pharmacy refill metrics (ie, the more diffuse tools for assessing adherence of large population153–155) provide highly accurate and inexpensive information about the prescribed treatment.59 155 However, pharmacy records rarely report data on the prescribed dose. This is an important limitation in our setting since the between-sex difference in drugs dosing is requested according to difference in pharmacokinetics parameters. However, notwithstanding the differences between measurement methods, our meta-analysis did not find that sex affected both self-reported adherence and refill rate.
A second cause of between-study heterogeneity might be due to differences in characteristics of the included patients that may interact with sex and affect drug adherence. To assess if age, prevention status (primary vs secondary), incident/prevalent users and other characteristics could modify the sex–adherence association, stratified analyses were performed. For example, by limiting the analysis to patients older than 65 years, between-study homogeneous estimates were obtained for self-reported based but not for pharmacy-refill based investigations. Moreover, we found that, compared with older women, older men had higher Morisky-based adherence to AHT therapy, while no difference in the refill rate was found. It is possible that the reproducibility of answers to medication-taking questions of the MMAS questionnaire could be different between sex groups among the elderly population, showing better compliance in men and/or worse behaviour among women than what actually is. However, because this remains a speculative and unverified hypothesis, the association between sex and AHT adherence among elderly must be further investigated.
Our meta-analysis did not offer any evidence that men and women from five continents and broad areas (Americas, North Europe, Mediterranean countries, Asia and Africa) differently adhere to AHT drug therapy, thus excluding that between-population cultural differences might explain the observed between-study inconsistency. In addition, we did not find that between-study heterogeneity diminished by limiting the analysis to 1-year adherence, rather than for heterogeneous periods of follow-up, or by stratifying studies on adjusted estimates.
Eligibility and exclusion criteria likely explain between-study heterogeneity. For example, the exclusion of AHT prevalent users (ie, the inclusion of new-user only156) or the setting for AHT treatment (ie, for primary or secondary prevention of CV disease157) most likely contribute to explain between-study inconsistency.
A further explanation for between-study inconsistency might be a difference in methods for reducing confounding. Estimates adjusted for the main known confounders of the association of interest were reported from studies based on pharmacy-refill measurement of adherence, while rough estimates were usually reported from self-reports. Characteristics like the level of education, the presence of diabetes or the socioeconomic status may have influenced the pooled estimate. Although the majority of papers adjusted estimates for sociodemographic and economic factors, concomitant medications and comorbidities, just a few of them considered CV risk factors, medical service type and type of AHT drug as the initial treatment strategy. Under these circumstances, we decided to perform a random-effect model to incorporate the heterogeneity due to the wide range of populations studied in the included investigations. Furthermore, we undertook also meta-regression analyses to identify important determinants of heterogeneity. However, there was no evidence that men and women differently adhered to AHT therapy also when some selected characteristics (eg, the inclusion of incident or prevalent AHT users) were taken into account.
Our study has three main limitations. First, although the adjusted estimates with the largest number of confounders were included in our meta-analysis, covariates definition and their distribution could be not sufficiently homogeneous among studies and this may have contributed to the observed heterogeneity.147 Second, language, publication and reporting biases may have affected our findings. However, few studies were excluded because written in other languages than English. In addition, if the studies that found no statistically significant differences had been less published or disseminated, the inclusion of them in our analysis should move the (already not significant) summarised estimate towards the null. Third, we decided to evaluate the information obtained by only self-report and prescription refill metrics. In fact, further methods exist to assess drug adherence,153 such as pill counts, electronic monitoring158 159 and measurement of plasma or urinary level.160 However, almost all the studies assessing adherence to AHT drugs in biochemical assays involve a population affected by resistant hypertension. Because the aim of our meta-analysis was to synthesise the evidence regarding the sex differences in the adherence to pharmacological treatment among hypertensive patients, we preferred to exclude studies on specific populations. Nevertheless, future systematic reviews on this topic, above all on studies based on adherence methods whose use has dramatically increased in the last years (eg, electronic monitoring), should address this gap.
Conclusions
Although, our study offers the most updated estimates on this issue, weak and non-definitive evidence for sex differences in drug adherence were obtained. Therefore, there are no reasons to focus the clinical attention to and introduce policies aimed at specific sex strata. Being poor adherence to chronic drug therapies a ubiquitously issue of public health, our little knowledge about factors affecting adherence, urgently requires high-quality studies investigating this issue. Indeed, further researches carried out by a multidisciplinary team of healthcare professionals could shed light on this critical topic and help decision-makers to develop comprehensive programmes of hypertension management.
Supplementary Material
Footnotes
Contributors: GC generated the study idea and wrote the final manuscript. AB and FR contributed to study search and selection; AB carried out the statistical analyses. TI, AF and GM assisted in interpreting the results under clinical prospective. All authors edited the manuscript and approved the final version.
Funding: This study was supported by grants from the Italian Ministry of the Education, University and Research (‘Fondo d’Ateneo per la Ricerca’ portion, year 2017).
Competing interests: GC received research support from the European Community (EC), the Italian Agency of Drug (AIFA) and the Italian Ministry for University and Research (MIUR). He took part to a variety of projects that were funded by pharmaceutical companies (ie, Novartis, GSK, Roche, AMGEN and BMS). He also received honoraria as member of Advisory Board from Roche. GM has received honoraria for participation as speaker/chairman in national/international meetings from Bayer, Boehringer Ingelheim, CVRx, Daiichi Sankyo, Ferrer, Medtronic, Menarini Int., Merck, Novartis, Recordati and Servier.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplementary information.
References
- 1.Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of hypertension (ESH) and of the European Society of cardiology (ESC). J Hypertens 2007;25:1105–87. 10.1097/HJH.0b013e3281fc975a [DOI] [PubMed] [Google Scholar]
- 2.Wolf-Maier K, Cooper RS, Kramer H, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension 2004;43:10–17. 10.1161/01.HYP.0000103630.72812.10 [DOI] [PubMed] [Google Scholar]
- 3.Kearney PM, Whelton M, Reynolds K, et al. Worldwide prevalence of hypertension: a systematic review. J Hypertens 2004;22:11–19. 10.1097/00004872-200401000-00003 [DOI] [PubMed] [Google Scholar]
- 4.Volpe M, Tocci G, Trimarco B, et al. Blood pressure control in Italy: results of recent surveys on hypertension. J Hypertens 2007;25:1491–8. 10.1097/HJH.0b013e3280fa83a6 [DOI] [PubMed] [Google Scholar]
- 5.Benetos A, Thomas F, Bean KE, et al. Why cardiovascular mortality is higher in treated hypertensives versus subjects of the same age, in the general population. J Hypertens 2003;21:1635–40. 10.1097/00004872-200309000-00011 [DOI] [PubMed] [Google Scholar]
- 6.Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. The Lancet 2018;392:2052–90. 10.1016/S0140-6736(18)31694-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banegas JR, Segura J, Ruilope LM, et al. Blood pressure control and physician management of hypertension in hospital hypertension units in Spain. Hypertension 2004;43:1338–44. 10.1161/01.HYP.0000127424.59774.84 [DOI] [PubMed] [Google Scholar]
- 8.Caro JJ, Speckman JL, Salas M, et al. Effect of initial drug choice on persistence with antihypertensive therapy: the importance of actual practice data. CMAJ 1999;160:41–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Degli Esposti E, Sturani A, Di Martino M, et al. Long-term persistence with antihypertensive drugs in new patients. J Hum Hypertens 2002;16:439–44. 10.1038/sj.jhh.1001418 [DOI] [PubMed] [Google Scholar]
- 10.Bourgault C, Sénécal M, Brisson M, et al. Persistence and discontinuation patterns of antihypertensive therapy among newly treated patients: a population-based study. J Hum Hypertens 2005;19:607–13. 10.1038/sj.jhh.1001873 [DOI] [PubMed] [Google Scholar]
- 11.Fitz-Simon N, Bennett K, Feely J. A review of studies of adherence with antihypertensive drugs using prescription databases. Ther Clin Risk Manag 2005;1:93–106. 10.2147/tcrm.1.2.93.62915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzaglia G, Mantovani LG, Sturkenboom MCJM, et al. Patterns of persistence with antihypertensive medications in newly diagnosed hypertensive patients in Italy: a retrospective cohort study in primary care. J Hypertens 2005;23:2093–100. 10.1097/01.hjh.0000186832.41125.8a [DOI] [PubMed] [Google Scholar]
- 13.Van Wijk BL, Klungel OH, Heerdink ER, et al. Rate and determinants of 10-year persistence with antihypertensive drugs. J Hypertens 2005;23:2101–7. 10.1097/01.hjh.0000187261.40190.2e [DOI] [PubMed] [Google Scholar]
- 14.Burke TA, Sturkenboom MC, Lu S-en, et al. Discontinuation of antihypertensive drugs among newly diagnosed hypertensive patients in UK general practice. J Hypertens 2006;24:1193–200. 10.1097/01.hjh.0000226211.95936.f5 [DOI] [PubMed] [Google Scholar]
- 15.Elliott WJ, Plauschinat CA, Skrepnek GH, et al. Persistence, adherence, and risk of discontinuation associated with commonly prescribed antihypertensive drug monotherapies. J Am Board Fam Med 2007;20:72–80. 10.3122/jabfm.2007.01.060094 [DOI] [PubMed] [Google Scholar]
- 16.Corrao G, Zambon A, Parodi A, et al. Discontinuation of and changes in drug therapy for hypertension among newly-treated patients: a population-based study in Italy. J Hypertens 2008;26:819–24. 10.1097/HJH.0b013e3282f4edd7 [DOI] [PubMed] [Google Scholar]
- 17.Vrijens B, Vincze G, Kristanto P, et al. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 2008;336:1114–7. 10.1136/bmj.39553.670231.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization Adherence to long-term therapies: evidence for action, 2003. [Google Scholar]
- 19.Mekonnen HS, Gebrie MH, Eyasu KH, et al. Drug adherence for antihypertensive medications and its determinants among adult hypertensive patients attending in chronic clinics of referral hospitals in Northwest Ethiopia. BMC Pharmacol Toxicol 2017;18:27. 10.1186/s40360-017-0134-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burnier M, Egan BM. Adherence in hypertension. Circ Res 2019;124:1124–40. 10.1161/CIRCRESAHA.118.313220 [DOI] [PubMed] [Google Scholar]
- 21.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009;119:3028–35. 10.1161/CIRCULATIONAHA.108.768986 [DOI] [PubMed] [Google Scholar]
- 22.Balkrishnan R. Predictors of medication adherence in the elderly. Clin Ther 1998;20:764–71. 10.1016/S0149-2918(98)80139-2 [DOI] [PubMed] [Google Scholar]
- 23.Morrow D, Leirer V, Sheikh J. Adherence and medication instructions. review and recommendations. J Am Geriatr Soc 1988;36:1147–60. 10.1111/j.1532-5415.1988.tb04405.x [DOI] [PubMed] [Google Scholar]
- 24.Krousel-Wood M, Thomas S, Muntner P, et al. Medication adherence: a key factor in achieving blood pressure control and good clinical outcomes in hypertensive patients. Curr Opin Cardiol 2004;19:357–62. 10.1097/01.hco.0000126978.03828.9e [DOI] [PubMed] [Google Scholar]
- 25.Coons SJ, Sheahan SL, Martin SS, et al. Predictors of medication noncompliance in a sample of older adults. Clin Ther 1994;16:110–7. [PubMed] [Google Scholar]
- 26.Monane M, Bohn RL, Gurwitz JH, et al. Compliance with antihypertensive therapy among elderly Medicaid enrollees: the roles of age, gender, and race. Am J Public Health 1996;86:1805–8. 10.2105/AJPH.86.12.1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billups SJ, Malone DC, Carter BL. The relationship between drug therapy noncompliance and patient characteristics, health-related quality of life, and health care costs. Pharmacotherapy 2000;20:941–9. 10.1592/phco.20.11.941.35266 [DOI] [PubMed] [Google Scholar]
- 28.Morris AB, Li J, Kroenke K, et al. Factors associated with drug adherence and blood pressure control in patients with hypertension. Pharmacotherapy 2006;26:483–92. 10.1592/phco.26.4.483 [DOI] [PubMed] [Google Scholar]
- 29.Caro JJ, Payne K, Wright JM. Real-World effectiveness of antihypertensive drugs. CMAJ 2000;162:190–1. [PMC free article] [PubMed] [Google Scholar]
- 30.MacLaughlin EJ, Raehl CL, Treadway AK, et al. Assessing medication adherence in the elderly: which tools to use in clinical practice? Drugs Aging 2005;22:231–55. 10.2165/00002512-200522030-00005 [DOI] [PubMed] [Google Scholar]
- 31.Sharkness CM, Snow DA. The patient's view of hypertension and compliance. Am J Prev Med 1992;8:141–6. 10.1016/S0749-3797(18)30821-3 [DOI] [PubMed] [Google Scholar]
- 32.McInnes GT. Integrated approaches to management of hypertension: promoting treatment acceptance. Am Heart J 1999;138:S252–5. 10.1016/S0002-8703(99)70318-2 [DOI] [PubMed] [Google Scholar]
- 33.Mosleh SM, Almalik MM. Illness perception and adherence to healthy behaviour in Jordanian coronary heart disease patients. Eur J Cardiovasc Nurs 2016;15:223–30. 10.1177/1474515114563885 [DOI] [PubMed] [Google Scholar]
- 34.Chia LR, Schlenk EA, Dunbar-Jacob J. Effect of personal and cultural beliefs on medication adherence in the elderly. Drugs Aging 2006;23:191–202. 10.2165/00002512-200623030-00002 [DOI] [PubMed] [Google Scholar]
- 35.Marshall IJ, Wolfe CDA, McKevitt C. Lay perspectives on hypertension and drug adherence: systematic review of qualitative research. BMJ 2012;345:e3953. 10.1136/bmj.e3953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson FA, Cox K, Britten N, et al. A systematic review of the research on communication between patients and health care professionals about medicines: the consequences for concordance. Health Expect 2004;7:235–45. 10.1111/j.1369-7625.2004.00281.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Government of Canada CI of HR What a Difference Sex and Gender Make: A Gender, Sex and Health Research Casebook - CIHR [Internet], 2012. Available: http://www.cihr-irsc.gc.ca/e/44734.html
- 38.Haidinger T, Zweimüller M, Stütz L, et al. Effect of gender on awareness of cardiovascular risk factors, preventive action taken, and barriers to cardiovascular health in a group of Austrian subjects. Gend Med 2012;9:94–102. 10.1016/j.genm.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 39.Saarti S, Hajj A, Karam L, et al. Association between adherence, treatment satisfaction and illness perception in hypertensive patients. J Hum Hypertens 2016;30:341–5. 10.1038/jhh.2015.86 [DOI] [PubMed] [Google Scholar]
- 40.Tibebu A, Mengistu D, Bulto LN. Adherence to prescribed antihypertensive medications and associated factors for hypertensive patients attending chronic follow-up units of selected public hospitals in Addis Ababa, Ethiopia. Int J Health Sci 2017;11:47–52. [PMC free article] [PubMed] [Google Scholar]
- 41.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 2010;303:2043–50. 10.1001/jama.2010.650 [DOI] [PubMed] [Google Scholar]
- 42.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA 2003;290:199–206. 10.1001/jama.290.2.199 [DOI] [PubMed] [Google Scholar]
- 43.Burnier M. Treatment of hypertension in the elderly in 2017/2018 - what's new? Expert Opin Pharmacother 2019;20:1869–77. 10.1080/14656566.2019.1638911 [DOI] [PubMed] [Google Scholar]
- 44.Pittman DG, Tao Z, Chen W, et al. Antihypertensive medication adherence and subsequent healthcare utilization and costs. Am J Manag Care 2010;16:568–76. [PubMed] [Google Scholar]
- 45.Hong SH. Potential for physician communication to build favorable medication beliefs among older adults with hypertension: a cross-sectional survey. PLoS One 2019;14:e0210169. 10.1371/journal.pone.0210169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hedna K, Hakkarainen KM, Gyllensten H, et al. Adherence to antihypertensive therapy and elevated blood pressure: should we consider the use of multiple medications? PLoS One 2015;10:e0137451. 10.1371/journal.pone.0137451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. results from the third National health and nutrition examination survey, 1988-1991. Hypertension 1995;25:305–13. 10.1161/01.hyp.25.3.305 [DOI] [PubMed] [Google Scholar]
- 48.Serna MC, Real J, Cruz I, et al. Monitoring patients on chronic treatment with antidepressants between 2003 and 2011: analysis of factors associated with compliance. BMC Public Health 2015;15:1184. 10.1186/s12889-015-2493-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serna MC, Cruz I, Real J, et al. Duration and adherence of antidepressant treatment (2003 to 2007) based on prescription database. Eur Psychiatry 2010;25:206–13. 10.1016/j.eurpsy.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 50.Alekhya P, Sriharsha M, Ramudu RV, et al. Adherence to antidepressant therapy: sociodemographic factor wise distribution. Int J Pharm Clin Res 2015;7:5. [Google Scholar]
- 51.Camus V, Kraehenbühl H, Preisig M, et al. Geriatric depression and vascular diseases: what are the links? J Affect Disord 2004;81:1–16. 10.1016/j.jad.2003.08.003 [DOI] [PubMed] [Google Scholar]
- 52.Lewey J, Shrank WH, Bowry ADK, et al. Gender and racial disparities in adherence to statin therapy: a meta-analysis. Am Heart J 2013;165:665–78. 678.e1. 10.1016/j.ahj.2013.02.011 [DOI] [PubMed] [Google Scholar]
- 53.Kim MT, Hill MN, Bone LR, et al. Development and testing of the Hill-Bone compliance to high blood pressure therapy scale. Prog Cardiovasc Nurs 2000;15:90–6. 10.1111/j.1751-7117.2000.tb00211.x [DOI] [PubMed] [Google Scholar]
- 54.Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new medication adherence rating scale (MARS) for the psychoses. Schizophr Res 2000;42:241–7. 10.1016/S0920-9964(99)00130-9 [DOI] [PubMed] [Google Scholar]
- 55.Warren-Findlow J, Seymour RB. Prevalence rates of hypertension self-care activities among African Americans. J Natl Med Assoc 2011;103:503–12. 10.1016/S0027-9684(15)30365-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67–74. 10.1097/00005650-198601000-00007 [DOI] [PubMed] [Google Scholar]
- 57.Oliveira-Filho AD, Barreto-Filho JA, Neves SJF, et al. Association between the 8-item Morisky medication adherence scale (MMAS-8) and blood pressure control. Arq Bras Cardiol 2012;99:649–58. 10.1590/s0066-782x2012005000053 [DOI] [PubMed] [Google Scholar]
- 58.Abegaz TM, Shehab A, Gebreyohannes EA, et al. Nonadherence to antihypertensive drugs: a systematic review and meta-analysis. Medicine 2017;96:e5641. 10.1097/MD.0000000000005641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinn VW. Sex and gender factors in medical studies: implications for health and clinical practice. JAMA 2003;289:397–400. 10.1001/jama.289.4.397 [DOI] [PubMed] [Google Scholar]
- 60.Hess LM, Raebel MA, Conner DA, et al. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother 2006;40:1280–8. 10.1345/aph.1H018 [DOI] [PubMed] [Google Scholar]
- 61.Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf 2006;15:565–74. 10.1002/pds.1230 [DOI] [PubMed] [Google Scholar]
- 62.Costa FV. Compliance with antihypertensive treatment. Clin Exp Hypertens 1996;18:463–72. 10.3109/10641969609088977 [DOI] [PubMed] [Google Scholar]
- 63.Page MJ, Moher D. Evaluations of the uptake and impact of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and extensions: a scoping review. Syst Rev 2017;6:263. 10.1186/s13643-017-0663-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 65.Forbes CA, Deshpande S, Sorio-Vilela F, et al. A systematic literature review comparing methods for the measurement of patient persistence and adherence. Curr Med Res Opin 2018;34:1613–25. 10.1080/03007995.2018.1477747 [DOI] [PubMed] [Google Scholar]
- 66.Di Martino M, Veronesi C, Degli Esposti L, et al. Adherence to antihypertensive drug treatment and blood pressure control: a real practice analysis in Italy. J Hum Hypertens 2008;22:51–3. 10.1038/sj.jhh.1002253 [DOI] [PubMed] [Google Scholar]
- 67.de Oliveira-Filho AD, Morisky DE, Neves SJF, et al. The 8-item Morisky medication adherence scale: validation of a Brazilian-Portuguese version in hypertensive adults. Res Social Adm Pharm 2014;10:554–61. 10.1016/j.sapharm.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 68.Morisky DE, Ang A, Krousel-Wood M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens 2008;10:348–54. 10.1111/j.1751-7176.2008.07572.x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101–29. 10.2307/3001666 [DOI] [Google Scholar]
- 70.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 72.Holmes HM, Luo R, Hanlon JT, et al. Ethnic disparities in adherence to antihypertensive medications of Medicare Part D beneficiaries. J Am Geriatr Soc 2012;60:1298–303. 10.1111/j.1532-5415.2012.04037.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park J-H, Shin Y, Lee S-Y, et al. Antihypertensive drug medication adherence and its affecting factors in South Korea. Int J Cardiol 2008;128:392–8. 10.1016/j.ijcard.2007.04.114 [DOI] [PubMed] [Google Scholar]
- 74.Williams LG, Peacock E, Joyce C, et al. Risk factors for low pharmacy refill adherence among older hypertensive men and women by race. Am J Med Sci 2018;356:464–75. 10.1016/j.amjms.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nguyen T-P-L, Schuiling-Veninga CCM, Nguyen TBY, et al. Adherence to hypertension medication: quantitative and qualitative investigations in a rural Northern Vietnamese community. PLoS One 2017;12:e0171203. 10.1371/journal.pone.0171203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perseguer-Torregrosa Z, Orozco-Beltrán D, Gil-Guillen VF, et al. Magnitude of pharmacological nonadherence in hypertensive patients taking antihypertensive medication from a community pharmacy in Spain. J Manag Care Spec Pharm 2014;20:1217–25. 10.18553/jmcp.2014.20.12.1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alhaddad IA, Hamoui O, Hammoudeh A, et al. Treatment adherence and quality of life in patients on antihypertensive medications in a middle Eastern population: adherence. Vasc Health Risk Manag 2016;12:407–13. 10.2147/VHRM.S105921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grégoire J, Moisan J, Guibert R, et al. Predictors of self-reported noncompliance with antihypertensive drug treatment: a prospective cohort study. Can J Cardiol 2006;22:323–9. 10.1016/S0828-282X(06)70917-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hashmi SK, Afridi MB, Abbas K, et al. Factors associated with adherence to anti-hypertensive treatment in Pakistan. PLoS One 2007;2:e280. 10.1371/journal.pone.0000280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khan MU, Shah S, Hameed T. Barriers to and determinants of medication adherence among hypertensive patients attended National health service Hospital, Sunderland. J Pharm Bioallied Sci 2014;6:104–8. 10.4103/0975-7406.129175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li W-W, Stewart AL, Stotts N, et al. Cultural factors associated with antihypertensive medication adherence in Chinese immigrants. J Cardiovasc Nurs 2006;21:354–62. 10.1097/00005082-200609000-00005 [DOI] [PubMed] [Google Scholar]
- 82.Lulebo AM, Mutombo PB, Mapatano MA, et al. Predictors of non-adherence to antihypertensive medication in Kinshasa, Democratic Republic of Congo: a cross-sectional study. BMC Res Notes 2015;8:526. 10.1186/s13104-015-1519-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morrison VL, Holmes EAF, Parveen S, et al. Predictors of self-reported adherence to antihypertensive medicines: a multinational, cross-sectional survey. Value Health 2015;18:206–16. 10.1016/j.jval.2014.12.013 [DOI] [PubMed] [Google Scholar]
- 84.Park Y-H, Kim H, Jang S-N, et al. Predictors of adherence to medication in older Korean patients with hypertension. European Journal of Cardiovascular Nursing 2013;12:17–24. 10.1016/j.ejcnurse.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 85.Stavropoulou C. Perceived information needs and non-adherence: evidence from Greek patients with hypertension. Health Expect 2012;15:187–96. 10.1111/j.1369-7625.2011.00679.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Turner BJ, Hollenbeak C, Weiner MG, et al. Barriers to adherence and hypertension control in a racially diverse representative sample of elderly primary care patients. Pharmacoepidemiol Drug Saf 2009;18:672–81. 10.1002/pds.1766 [DOI] [PubMed] [Google Scholar]
- 87.Adidja NM, Agbor VN, Aminde JA, et al. Non-adherence to antihypertensive pharmacotherapy in Buea, Cameroon: a cross-sectional community-based study. BMC Cardiovasc Disord 2018;18:150. 10.1186/s12872-018-0888-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al-Ramahi Rowa', Al-Ramahi R. Adherence to medications and associated factors: a cross-sectional study among Palestinian hypertensive patients. J Epidemiol Glob Health 2015;5:125–32. 10.1016/j.jegh.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fortuna RJ, Nagel AK, Rocco TA, et al. Patient experience with care and its association with adherence to hypertension medications. Am J Hypertens 2018;31:340–5. 10.1093/ajh/hpx200 [DOI] [PubMed] [Google Scholar]
- 90.Holt EW, Joyce C, Dornelles A, et al. Sex differences in barriers to antihypertensive medication adherence: findings from the cohort study of medication adherence among older adults (CoSMO). Circulation 2013;127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kang CD, Tsang PPM, Li WTL, et al. Determinants of medication adherence and blood pressure control among hypertensive patients in Hong Kong: a cross-sectional study. Int J Cardiol 2015;182:250–7. 10.1016/j.ijcard.2014.12.064 [DOI] [PubMed] [Google Scholar]
- 92.Rahmawati R, Bajorek B. Factors affecting self-reported medication adherence and hypertension knowledge: a cross-sectional study in rural villages, Yogyakarta Province, Indonesia. Chronic Illn 2018;14:212–27. 10.1177/1742395317739092 [DOI] [PubMed] [Google Scholar]
- 93.Yue Z, Bin W, Weilin Q, et al. Effect of medication adherence on blood pressure control and risk factors for antihypertensive medication adherence. J Eval Clin Pract 2015;21:166–72. 10.1111/jep.12268 [DOI] [PubMed] [Google Scholar]
- 94.Esmaeili R, Matlabi M, Khajavi A, et al. Factors affecting adherence to antihypertensive medication: results from a rural population study in East of Iran. Glob J Health Sci 2016;9:286 10.5539/gjhs.v9n5p286 [DOI] [Google Scholar]
- 95.Okello S, Nasasira B, Muiru ANW, et al. Validity and reliability of a self-reported measure of antihypertensive medication adherence in Uganda. PLoS One 2016;11:e0158499. 10.1371/journal.pone.0158499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang T-D, Chen Y-H, Huang C-H, et al. Bidirectional adherence changes and associated factors in patients switched from free combinations to equivalent single-pill combinations of antihypertensive drugs. Hypertension 2014;63:958–67. 10.1161/HYPERTENSIONAHA.113.02455 [DOI] [PubMed] [Google Scholar]
- 97.Wagner S, Lau H, Frech-Tamas F, et al. Impact of medication adherence on work productivity in hypertension. Am J Pharm Benefits 2012;4:e88–96. [Google Scholar]
- 98.Han WP, Hong SA, Tiraphat S. Factors related to medication adherence among essential hypertensive patients in tertiary hospitals in Yangon, Myanmar 2015;13:57–70. [Google Scholar]
- 99.Van Wijk BLG, Klungel OH, Heerdink ER, et al. Generic substitution of antihypertensive drugs: does it affect adherence? Ann Pharmacother 2006;40:15–20. 10.1345/aph.1G163 [DOI] [PubMed] [Google Scholar]
- 100.Calderón-Larrañaga A, Diaz E, Poblador-Plou B, et al. Non-adherence to antihypertensive medication: the role of mental and physical comorbidity. Int J Cardiol 2016;207:310–6. 10.1016/j.ijcard.2016.01.069 [DOI] [PubMed] [Google Scholar]
- 101.Friedman O, McAlister FA, Yun L, et al. Antihypertensive drug persistence and compliance among newly treated elderly hypertensives in Ontario. Am J Med 2010;123:173–81. 10.1016/j.amjmed.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 102.Inkster ME, Donnan PT, MacDonald TM, et al. Adherence to antihypertensive medication and association with patient and practice factors. J Hum Hypertens 2006;20:295–7. 10.1038/sj.jhh.1001981 [DOI] [PubMed] [Google Scholar]
- 103.Ishisaka DY, Jukes T, Romanelli RJ, et al. Disparities in adherence to and persistence with antihypertensive regimens: an exploratory analysis from a community-based provider network. J Am Soc Hypertens 2012;6:201–9. 10.1016/j.jash.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 104.Manteuffel M, Williams S, Chen W, et al. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J Womens Health 2014;23:112–9. 10.1089/jwh.2012.3972 [DOI] [PubMed] [Google Scholar]
- 105.Taira DA, Gelber RP, Davis J, et al. Antihypertensive adherence and drug class among Asian Pacific Americans. Ethn Health 2007;12:265–81. 10.1080/13557850701234955 [DOI] [PubMed] [Google Scholar]
- 106.van Dijk L, Heerdink ER, Somai D, et al. Patient risk profiles and practice variation in nonadherence to antidepressants, antihypertensives and oral hypoglycemics. BMC Health Serv Res 2007;7:51. 10.1186/1472-6963-7-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wong MCS, Jiang JY, Griffiths SM. Factors associated with antihypertensive drug compliance in 83,884 Chinese patients: a cohort study. J Epidemiol Community Health 2010;64:895–901. 10.1136/jech.2009.091603 [DOI] [PubMed] [Google Scholar]
- 108.Couto JE, Panchal JM, Lal LS, et al. Geographic variation in medication adherence in commercial and Medicare Part D populations. J Manag Care Spec Pharm 2014;20:834–42. 10.18553/jmcp.2014.20.8.834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Degli Esposti L, Saragoni S, Batacchi P, et al. Antihypertensive therapy among newly treated patients: an analysis of adherence and cost of treatment over years. Clinicoecon Outcomes Res 2010;2:113–20. 10.2147/CEOR.S11933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lauffenburger JC, Shrank WH, Bitton A, et al. Association between patient-centered medical homes and adherence to chronic disease medications: a cohort study. Ann Intern Med 2017;166:81–8. 10.7326/M15-2659 [DOI] [PubMed] [Google Scholar]
- 111.Simon-Tuval T, Triki N, Chodick G, et al. The association between adherence to cardiovascular medications and healthcare utilization. Eur J Health Econ 2016;17:603–10. 10.1007/s10198-015-0703-z [DOI] [PubMed] [Google Scholar]
- 112.Wong MCS, Tam WWS, Wang HHX, et al. Duration of initial antihypertensive prescription and medication adherence: a cohort study among 203,259 newly diagnosed hypertensive patients. Int J Cardiol 2015;182:503–8. 10.1016/j.ijcard.2014.12.058 [DOI] [PubMed] [Google Scholar]
- 113.Ambaw AD, Alemie GA, W/Yohannes SM, et al. Adherence to antihypertensive treatment and associated factors among patients on follow up at University of Gondar Hospital, Northwest Ethiopia. BMC Public Health 2012;12:282. 10.1186/1471-2458-12-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arshad AR. Frequency of poor adherence to antihypertensive treatment and an analysis of clinico-demographic correlates. J Coll Physicians Surg Pak 2015;25:911–3. doi:12.2015/JCPSP.911913 [PubMed] [Google Scholar]
- 115.Bader RJK, Koprulu F, Hassan NAGM, et al. Predictors of adherence to antihypertensive medication in northern United Arab Emirates. East Mediterr Health J 2015;21:309–18. 10.26719/2015.21.5.309 [DOI] [PubMed] [Google Scholar]
- 116.Cuffee YL, Hargraves JL, Rosal M, et al. Reported racial discrimination, trust in physicians, and medication adherence among inner-city African Americans with hypertension. Am J Public Health 2013;103:e55–62. 10.2105/AJPH.2013.301554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Demoner MS, Ramos ERdeP, Pereira ER. Factors associated with adherence to antihypertensive treatment in a primary care unit. Acta paul. enferm. 2012;25:27–34. 10.1590/S0103-21002012000800005 [DOI] [Google Scholar]
- 118.Dosse C, Cesarino CB, Martin JFV, et al. Factors associated to patients' noncompliance with hypertension treatment. Rev Lat Am Enfermagem 2009;17:201–6. 10.1590/S0104-11692009000200010 [DOI] [PubMed] [Google Scholar]
- 119.Lo SHS, Chau JPC, Woo J, et al. Adherence to antihypertensive medication in older adults with hypertension. J Cardiovasc Nurs 2016;31:296–303. 10.1097/JCN.0000000000000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang S, He C, Zhang X, et al. Determinants of antihypertensive adherence among patients in Beijing: application of the health belief model. Patient Educ Couns 2016;99:1894–900. 10.1016/j.pec.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 121.Hacıhasanoğlu Aşılar R, Gözüm S, Çapık C, et al. Reliability and validity of the Turkish form of the eight-item Morisky medication adherence scale in hypertensive patients. Anadolu Kardiyol Derg 2014;14:692–700. 10.5152/akd.2014.4982 [DOI] [PubMed] [Google Scholar]
- 122.Behnood-Rod A, Rabbanifar O, Pourzargar P, et al. Adherence to antihypertensive medications in Iranian patients. Int J Hypertens 2016;2016:1508752. 10.1155/2016/1508752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Berhe DF, Taxis K, Haaijer-Ruskamp FM, et al. Impact of adverse drug events and treatment satisfaction on patient adherence with antihypertensive medication - a study in ambulatory patients. Br J Clin Pharmacol 2017;83:2107–17. 10.1111/bcp.13312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cummings DM, Wu J-R, Cene C, et al. Perceived social standing, medication nonadherence, and systolic blood pressure in the rural South. J Rural Health 2016;32:156–63. 10.1111/jrh.12138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hyre AD, Krousel-Wood MA, Muntner P, et al. Prevalence and predictors of poor antihypertensive medication adherence in an urban health clinic setting. J Clin Hypertens 2007;9:179–86. 10.1111/j.1524-6175.2007.06372.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jankowska-Polańska B, Chudiak A, Uchmanowicz I, et al. Selected factors affecting adherence in the pharmacological treatment of arterial hypertension. Patient Prefer Adherence 2017;11:363–71. 10.2147/PPA.S127407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Korb-Savoldelli V, Gillaizeau F, Pouchot J, et al. Validation of a French version of the 8-item Morisky medication adherence scale in hypertensive adults. J Clin Hypertens 2012;14:429–34. 10.1111/j.1751-7176.2012.00634.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee C-Y, Huang C-C, Shih H-C, et al. Factors influencing antihypertensive medication compliance in Taiwan: a nationwide population-based study. Eur J Prev Cardiol 2013;20:930–7. 10.1177/2047487312451252 [DOI] [PubMed] [Google Scholar]
- 129.Kumar N, Unnikrishnan B, Thapar R, et al. Factors associated with adherence to antihypertensive treatment among patients attending a tertiary care hospital in Mangalore, South India. Int J Curr Res Rev 2014;6:77–85. [Google Scholar]
- 130.Alfian SD, Denig P, Coelho A, et al. Pharmacy-based predictors of non-adherence, non-persistence and reinitiation of antihypertensive drugs among patients on oral diabetes drugs in the Netherlands. PLoS One 2019;14:e0225390. 10.1371/journal.pone.0225390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chang TE, Ritchey MD, Park S, et al. National rates of nonadherence to antihypertensive medications among insured adults with hypertension, 2015. Hypertension 2019;74:1324–32. 10.1161/HYPERTENSIONAHA.119.13616 [DOI] [PubMed] [Google Scholar]
- 132.Cyrus AC, Royer J, Carroll DD, et al. Anti-Hypertensive medication use and factors related to adherence among adults with intellectual and developmental disabilities. Am J Intellect Dev Disabil 2019;124:248–62. 10.1352/1944-7558-124.3.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rea F, Mella M, Monzio Compagnoni M, et al. Women discontinue antihypertensive drug therapy more than men. Evidence from an Italian population-based study. J Hypertens 2020;38:142–9. 10.1097/HJH.0000000000002222 [DOI] [PubMed] [Google Scholar]
- 134.Walsh CA, Cahir C, Bennett KE. Association between adherence to antihypertensive medications and health outcomes in middle and older aged community dwelling adults; results from the Irish longitudinal study on ageing. Eur J Clin Pharmacol 2019;75:1283–92. 10.1007/s00228-019-02699-w [DOI] [PubMed] [Google Scholar]
- 135.Usman MN, Umar MD, Idris FA, et al. Medication adherence and its associated factors among hypertensive patients in a tertiary health facility in Minna, North central Nigeria. Arch Clin Hypertens 2019;5:003–7. [Google Scholar]
- 136.Alkhamis AM, Alsalman AJ, Al Khamis M, et al. Prevalence of nonadherence to antihypertensive medications among adults attending primary healthcare clinics in Al-Hasa region: a cross-sectional study. DSAHMJ 2019;1:36–43. 10.2991/dsahmj.k.190516.001 [DOI] [Google Scholar]
- 137.Gavrilova A, Bandere D, Rutkovska I, et al. Knowledge about disease, medication therapy, and related medication adherence levels among patients with hypertension. Medicina 2019;55:715. 10.3390/medicina55110715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gowda CGK, Savitha RBB, Iyengar K, et al. Assessment of adherence to antihypertensive treatment among patients attending a urban health care facility of a medical College, Tumkur. Int J Med Public Health 2019;9:42–5. [Google Scholar]
- 139.Mahmood S, Jalal Z, Hadi MA, et al. Non-Adherence to prescribed antihypertensives in primary, secondary and tertiary healthcare settings in Islamabad, Pakistan: a cross-sectional study. Patient Prefer Adherence 2020;14:73–85. 10.2147/PPA.S235517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nabi MU, Barua M, Kabir M. Revalance and determinants of adherence to antihypertensive medication among hypertensive patients attending a tertiary care hospital of Bangladesh. IJISRT 2019;2:353–62. [Google Scholar]
- 141.Okeke H, Osonwa K, Awhen P. Patients' socio-demographic variables and adherence to antihypertensive therapy in southern Senatorial district of cross river state, Nigeria. J Med Allied Sci 2019;9:83–9. 10.5455/jmas.47510 [DOI] [Google Scholar]
- 142.Shah ND, Steiner ME, Vermeulen LC, et al. The role of medication adherence as a determinant of blood pressure control in a managed care population. Dis Manage Health Outcomes 2007;15:249–56. 10.2165/00115677-200715040-00006 [DOI] [Google Scholar]
- 143.Hou Y, Zhang D, Gu J, et al. The association between self-perceptions of aging and antihypertensive medication adherence in older Chinese adults. Aging Clin Exp Res 2016;28:1113–20. 10.1007/s40520-015-0516-z [DOI] [PubMed] [Google Scholar]
- 144.Mazzaglia G, Ambrosioni E, Alacqua M, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation 2009;120:1598–605. 10.1161/CIRCULATIONAHA.108.830299 [DOI] [PubMed] [Google Scholar]
- 145.Muntner P, Levitan EB, Joyce C, et al. Association between antihypertensive medication adherence and visit-to-visit variability of blood pressure. J Clin Hypertens 2013;15:112–7. 10.1111/jch.12037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sutar P, Shah H. A study of adherence pattern toward antihypertensive therapy (antihypertensive drugs, dietary habits, and physical activity) and certain factors affecting it. Int J Med Sci Public Health 2017;6:1 10.5455/ijmsph.2017.0847930082016 [DOI] [Google Scholar]
- 147.Iyengar RN, Balagere DS, Henderson RR, et al. Association between dispensing channel and medication adherence among Medicare beneficiaries taking medications to treat diabetes, high blood pressure, or high blood cholesterol. J Manag Care Spec Pharm 2014;20:851–61. 10.18553/jmcp.2014.20.8.851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang X, Chen H, Essien E, et al. Medication adherence to antihypertensive Triple-Combination therapy among patients enrolled in a Medicare advantage plan. J Manag Care Spec Pharm 2019;25:678–86. 10.18553/jmcp.2019.25.6.678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shalansky SJ, Levy AR, Ignaszewski AP. Self-reported Morisky score for identifying nonadherence with cardiovascular medications. Ann Pharmacother 2004;38:1363–8. 10.1345/aph.1E071 [DOI] [PubMed] [Google Scholar]
- 150.McDonald-Miszczak L, Maki SA, Gould ON. Self-reported medication adherence and health status in late adulthood: the role of beliefs. Exp Aging Res 2000;26:189–207. 10.1080/036107300404859 [DOI] [PubMed] [Google Scholar]
- 151.Haynes RB, Taylor DW, Sackett DL, et al. Can simple clinical measurements detect patient noncompliance? Hypertension 1980;2:757–64. 10.1161/01.HYP.2.6.757 [DOI] [PubMed] [Google Scholar]
- 152.van der Wal MHL, Jaarsma T, Moser DK, et al. Compliance in heart failure patients: the importance of knowledge and beliefs. Eur Heart J 2006;27:434–40. 10.1093/eurheartj/ehi603 [DOI] [PubMed] [Google Scholar]
- 153.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97. 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 154.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care 1999;37:846–57. 10.1097/00005650-199909000-00002 [DOI] [PubMed] [Google Scholar]
- 155.Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014:CD000011. 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003;158:915–20. 10.1093/aje/kwg231 [DOI] [PubMed] [Google Scholar]
- 157.Prevention of cardiovascular disease: pocket guidelines for assessment and management of cardiovascular risk: (WHO/ISH cardiovascular risk prediction charts for the European Region) [Internet]. Available: https://apps.who.int/iris/handle/10665/43784
- 158.van Onzenoort HAW, Verberk WJ, Kroon AA, et al. Electronic monitoring of adherence, treatment of hypertension, and blood pressure control. Am J Hypertens 2012;25:54–9. 10.1038/ajh.2011.153 [DOI] [PubMed] [Google Scholar]
- 159.van Onzenoort HAW, Verberk WJ, Kessels AGH, et al. Assessing medication adherence simultaneously by electronic monitoring and pill count in patients with mild-to-moderate hypertension. Am J Hypertens 2010;23:149–54. 10.1038/ajh.2009.207 [DOI] [PubMed] [Google Scholar]
- 160.Gupta P, Patel P, Štrauch B, et al. Biochemical screening for nonadherence is associated with blood pressure reduction and improvement in adherence. Hypertension 2017;70:1042–8. 10.1161/HYPERTENSIONAHA.117.09631 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-036418supp001.pdf (283.9KB, pdf)
bmjopen-2019-036418supp002.pdf (70.8KB, pdf)
bmjopen-2019-036418supp003.pdf (47KB, pdf)
bmjopen-2019-036418supp004.pdf (123KB, pdf)
bmjopen-2019-036418supp005.pdf (111.1KB, pdf)