Abstract
Objectives:
Mortality rates of 5–10% after pneumonectomy have remained constant during the last decade. To understand the patterns of outcomes after pneumonectomy, we investigated the time-varying risks of readmission and death during the first postoperative year and examined the contributions of specific causes to these patterns over time.
Methods:
We retrospectively reviewed all pneumonectomies for lung cancer at our institution from 2000–2018. The time-varying instantaneous risk of all-cause readmission and mortality up to 1 year after pneumonectomy was estimated using parametric analyses, and was repeated for each primary cause of readmission (oncologic, infectious, pulmonary, cardiac, or other) and death (oncologic or nononcologic).
Results:
In our cohort of 355 pneumonectomy patients, risk of readmission was highest immediately after discharge and was halved by 14 days. This risk reached a nadir and remained constant from 4–8 months, after which it gradually increased. Pulmonary causes accounted for most readmissions within 90 days, after which oncologic causes predominated. Similarly, the overall risk of death was highest immediately after surgery, was halved by 7 days, reached a nadir at 90 days, then increased throughout the remainder of the first year. All deaths during the first 90 days after surgery were attributable to nononcologic causes.
Conclusions:
Nononcologic causes of readmission and death predominate in the first 90 days after pneumonectomy, after which oncologic causes prevail. We also identify specific causes that pose the highest risk of readmission immediately after discharge. Efforts are warranted to define the effects of specific causes of readmission on overall mortality after pneumonectomy.
Central Message:
The risk of readmission and death varies during the first year after pneumonectomy. Nononcologic causes predominate in the first 90 days, after which oncologic causes prevail.
Graphical Abstract
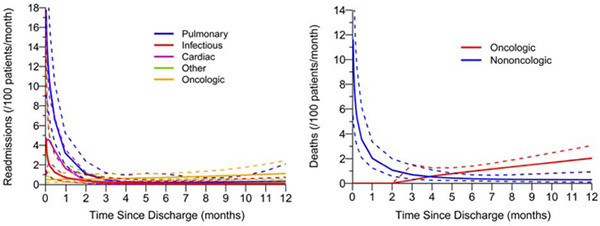
Central Picture: Instantaneous risk of readmission and death. Cause, solid line; 95% CI, dashed line.
INTRODUCTION
Of all operations for primary lung cancer, pneumonectomy has the highest associated mortality, with 30-day rates of 5% to 10% for simple pneumonectomy and 7% to 13% for extrapleural pneumonectomy.1, 2 Although these rates are dramatically lower than those from the 1980s and 76 1990s, mortality after pneumonectomy has not substantially decreased during the last decade. National database studies have reported a 30-day mortality of 5.6% in 2007 and 5.9% in 2014.3,4
Operative mortality is traditionally defined as deaths in or out of the hospital, regardless of cause, within 30 days of surgery.5 This 30-day window is thought to encompass the majority of postoperative complications that lead to death for most cancers (i.e., esophageal, colon/rectal, breast, and renal).6 However, operations for lung cancer (as well as gastric and bladder cancers) are associated with additional mortality of at least 3% between 31 and 90 days postsurgery.6–8 Schneider et al. reported a 90-day postdischarge mortality after pneumonectomy of nearly 1.5 times the in-hospital rate (6.4% vs. 4.4%), with the highest proportional mortality in the 60- to 90-day window.9 Similarly, Kim and Boffa reported 90-day mortality nearly double the rate at 30 days (12% vs. 7%).10 These and other studies have proposed extending the definition of operative mortality to include time points past the traditional 30-day mark, especially for high-risk operations such as pneumonectomy.
Readmission rates after pulmonary resection are also highest in patients who underwent pneumonectomy, with a recent study of the National Cancer Database reporting a 30-day readmission rate of 6.2%, compared with 4.3% after lobectomy and 3.8% after sublobar resection.11 Moreover, an association between 30-day readmission and 90-day mortality has been described, with an up to 6-fold increase in 90-day mortality in those patients who experienced readmission.12, 13 Unlike mortality, however, few studies have assessed readmissions beyond 30 days in pneumonectomy patients.
While prior publications have assessed the time-varying risk of readmission and death After cardiac surgery,14–16 this has not been replicated in thoracic surgery—such an investigation may help identify changes in the rates of these outcomes in the months after pneumonectomy. We therefore performed an in-depth analysis of the timing and patterns of readmission and death during the first year after pneumonectomy using a novel parametric hazard decomposition approach and examined the contributions of specific causes to these patterns over time.
METHODS
Patients
After approval from our institutional review board, we performed a retrospective review of our prospectively maintained database to identify all patients who underwent pneumonectomy for lung cancer at our institution between 2000 and 2018. Patients who underwent extrapleural pneumonectomy, underwent pneumonectomy for benign disease, had an extrapulmonary primary tumor with metastasis to the lung, or had no available follow-up data after hospital discharge were excluded (Supplementary Figure 1). Preoperative comorbidities were classified in accordance with the American Society of Anesthesiologists (ASA) Physical Status Classification System.
Outcomes
Readmissions and associated causes (oncologic or nononcologic) were reported at 30 days, 90 days, and 1 year after discharge from initial hospitalization. Nononcologic causes were further subdivided into pulmonary, cardiac, infectious, or other causes (mutually exclusive). If multiple nononcologic causes of readmission were present, the cause with the highest Clavien-Dindo grade was assigned as the “primary cause” for the purpose of analysis.17 For patients who experienced multiple readmissions in the first year after discharge, only the index readmission was included in the statistical analysis. Readmissions at other health-care facilities, when noted in the electronic medical record, were captured. Deaths during initial hospitalization and at 30 days, 90 days, and 1 year postsurgery were also reported. Causes of death were reported for all patients who died within 1 year of surgery and were divided into oncologic or nononcologic causes. Subcategories of nononcologic deaths were also reported but were not included in the statistical analysis due to the low number of events in each subcategory. If the cause of death was not available in the electronic medical record, the patient’s emergency contact, relative, or previous treating physician was contacted. All causes of death during the first year of surgery were able to be verified.
Statistical Analysis
Patient demographic and clinicopathologic characteristics were summarized using frequency (percentage) or median (interquartile range [IQR]). Time to readmission and time to death within 1 year were generated from the time of hospital discharge and time of surgery, respectively. Nonparametric survival estimates were generated using the Kaplan-Meier approach. Patients were otherwise censored at the time of last follow-up or at the 1-year mark, whichever occurred first. Median follow-up was calculated using the reverse Kaplan-Meier method.18
To investigate the timing and pattern of readmission and death during the first year after pneumonectomy, we applied a nonlinear parametric hazard decomposition approach, first introduced by Blackstone et al.19 This method partitions instantaneous risk of an event (hazard function), such as readmission or death, into three phases (early, constant, late). In contrast to conventional, nonparametric (i.e., Kaplan-Meier) methods with step functions, parametric survival models do not require the proportional hazards assumption and can be used to estimate the smoothed hazard functions necessary for the decomposition approach. This also enables the identification of causes of readmission and death that were predominant in the early or late phases or causes that were constant throughout the follow-up period. In this method, all events are assessed in a time-related fashion, in contrast to methods, such as logistic regression, that ignore the timing of events. This is of particular relevance for operations such as pneumonectomy, in which procedural risk appears to extend farther in time than for other, lower-risk operations.
The time-varying instantaneous risk of all-cause readmission up to 1 year after hospital discharge was estimated as described above.19, 20 This estimation procedure was repeated for readmissions by each primary cause (oncologic, infectious, pulmonary, cardiac, or other). Only patients who were discharged alive from the index hospitalization were included in readmission analyses. To investigate the timing and pattern of death after surgery, the time-varying instantaneous risk of all-cause death up to 1 year postsurgery was assessed in the same fashion. The hazard function for death was further investigated by cause of death (oncologic vs nononcologic) using a competing risk approach.
Analyses were conducted using Stata 13.1 (Stata Corp, College Station, TX) and SAS 9.4 (SAS Institute, Cary, NC). A publicly available algorithm20 was adapted to conduct nonlinear parametric temporal decomposition using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics and Outcomes
In total, 355 patients underwent pneumonectomy for lung cancer between 2000 and 2018, with an equal proportion of men and women (Table 1). Median follow-up was 7.0 years (IQR, 3.2–12.1). The majority of patients (n=303; 85%) had a history of tobacco use, with a median of 31.3 pack-years. Nearly half of patients (n=174; 49%) underwent induction therapy. Only 38% (n=135) had ASA class I or II comorbidities. Seventeen percent of operations (n=59) were completion pneumonectomies. Median operative time was 3.4 h, with 16% of patients (n=57) requiring transfusion during the operation. On final pathology, there were nearly equal proportions of squamous cell carcinoma (n=134; 38%) and adenocarcinoma (n=142; 40%), while 11 patients (3.1%) had no viable tumor, indicating a complete pathologic response to induction therapy. Approximately half of tumors (n=170; 48%) were pathologic stage III (American Joint Committee on Cancer 8th Edition), and all but 22 operations (6.2%) were R0 resections. Sixty-one percent of patients (n=215) underwent adjuvant therapy.
Table 1.
Demographic and clinicopathologic characteristics (N=355)
| No. (%) or Median (IQR) | |
|---|---|
| Age at surgery, years | 63 (54–70) |
| Sex | |
| Female | 176 (50) |
| Male | 179 (50) |
| BMI (n=339) | 27.2 (23.7–30.5) |
| Smoking status | |
| Never | 52 (15) |
| Ever | 303 (85) |
| Pack-years (n=353) | 31.3 (15.0–50.0) |
| Tumor size on CT, cm (n=330) | 4.4 (3.0–6.0) |
| Primary tumor SUVmax (n=275) | 12.5 (8.0–17.2) |
| Preoperative histologic diagnosis (n=330) | |
| Adenocarcinoma | 119 (36) |
| Squamous cell carcinoma | 120 (36) |
| Other | 91 (28) |
| FEV1, % (n=353) | 82.0 (70.0–91.0) |
| DLCO, % (n=350) | 76.0 (63.0–90.0) |
| Induction therapy | |
| None | 181 (51) |
| Chemotherapy only | 151 (43) |
| Chemoradiotherapy | 23 (6.5) |
| ASA class | |
| I or II | 135 (38) |
| III or IV | 220 (62) |
| Total intravenous fluids, L | 1.2 (0.9–1.7) |
| Estimated blood loss, L | 0.6 (0.4–1.0) |
| Transfusion required in OR | |
| No | 298 (84) |
| Yes | 57 (16) |
| Surgery time, h | 3.4 (2.7–4.6) |
| Completion pneumonectomy | |
| No | 296 (83) |
| Yes | 59 (17) |
| Tumor location | |
| Left | 199 (56) |
| Right | 156 (44) |
| Final pathologic diagnosis | |
| Adenocarcinoma | 142 (40) |
| Squamous cell carcinoma | 134 (38) |
| Other | 68 (19) |
| No viable tumor | 11 (3.1) |
| Pathologic stage (AJCC 8th Edition) | |
| 0 | 11 (3.1) |
| I | 49 (14) |
| II | 119 (34) |
| III | 170 (48) |
| IV | 6 (1.7) |
| Extent of resection | |
| R0 | 333 (94) |
| R1 | 19 (5.4) |
| R2 | 3 (0.8) |
| Adjuvant therapy | |
| None | 140 (39) |
| Chemotherapy only | 110 (31) |
| PORT only | 75 (21) |
| Chemotherapy + PORT | 30 (8.5) |
AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiologists; BMI, body mass index; CT, computed tomography; DLCO, diffusion capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in 1 second; OR, operating room; PORT, postoperative radiotherapy; SUVmax, maximum standardized uptake value.
Readmission Within 1 Year of Index Hospital Discharge
Among the 342 patients who were discharged alive from the hospital, the 30-day readmission rate was 13% (43/342), and the 90-day readmission rate was 19% (64/342) (Table 2). Thirteen patients (3.8%) experienced readmission within both 0–30 days and 31–90 days after initial hospital discharge. The causes of readmission during the first year after discharge are listed in Supplementary Table 1. Readmission-free survival during the first year after index discharge is shown in Figure 1A. The parametric versus nonparametric (i.e., Kaplan-Meier) readmission-free survival curves are presented in Supplementary Figure 2. Based on Kaplan-Meier estimation, 1-year readmission-free survival was 71% (95% CI, 66%−76%). The instantaneous risk of readmission for any cause within 1 year of index discharge was composed of an early decreasing phase and a late increasing phase (Figure 1B). The risk of readmission was highest immediately after discharge (32 readmissions/100 patients per month; 95% CI, 20–52) and decreased to less than half the initial value by 14 days (13 readmissions/100 patients per month; 95% CI, 9–17). After 4 months, the risk of readmission for any cause remained low and was relatively constant (1.2 readmission/100 patients per month; 95% CI, 0.7–2.0), until months 8 to 12, when a slight increase was noted (12 months, 2.3 readmissions/100 patients per month; 95% CI, 1.1–4.6).
Table 2.
Distribution of readmission and death during the first year after discharge and surgery, respectively
| No. (%) | |
|---|---|
| Readmission | |
| Within 30 days of discharge (n=342) | 43 (13) |
| From 31 to 90 days after discharge (n=339) | 34 (10) |
| From 91 days to 1 year after discharge (n=331) | 45 (14) |
| Death | |
| Within 30 days of surgery (N=355) | 16 (4.5) |
| From 31 to 90 days after surgery (n=339) | 8 (2.4) |
| From 91 days to 1 year after surgery (n=331) | 41 (12) |
Figure 1.
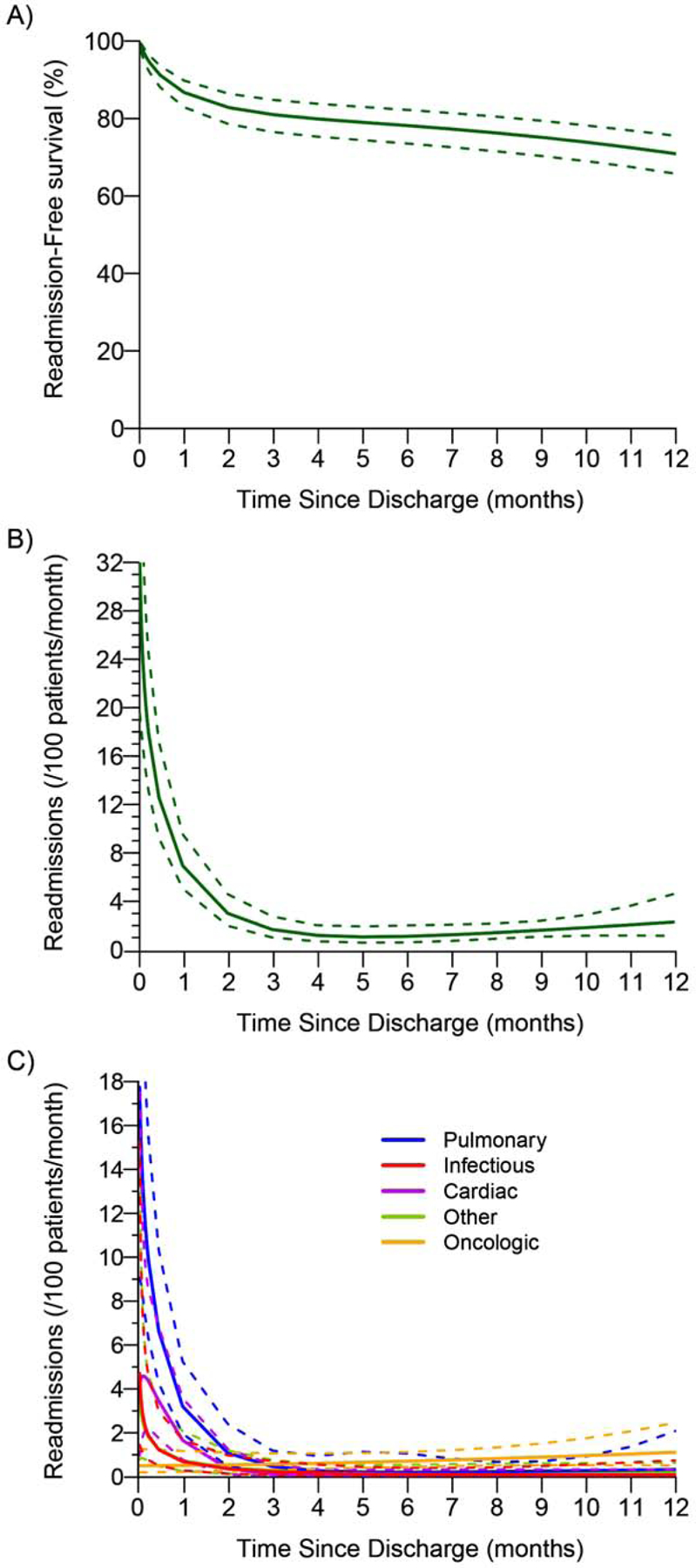
Readmission-free survival and risk of readmission within 1 year of index hospital discharge after pneumonectomy. (A) Readmission-free survival: the solid line is the parametric estimate of readmission-free survival, along with the 95% confidence band. (B) Instantaneous risk of readmission: parametric estimates (solid line) with corresponding 95% confidence bands (dashed line). (C) Instantaneous risk (hazard function) of readmission within 1 year of index hospital discharge by cause (solid lines). The dashed lines correspond to the 95% confidence bands.
The time-varying instantaneous risk of readmission by cause was also investigated (Figure 1C). The risk of readmission for all nononcologic causes was highest in the postdischarge period and declined during the first year after index discharge; the risk of readmission for oncologic causes slowly increased during the first year. More specifically, the risk of readmission due to infectious causes (4.7 readmissions/100 patients per month; 95% CI, 1.4–15) or “other” causes (3.5 readmissions/100 patients per month; 95% CI, 0.8–16) was highest immediately after discharge then sharply declined, reaching negligible values by 60 days. Pulmonary causes were the greatest contributor to overall readmissions immediately after discharge (18 readmissions/100 patients per month; 95% CI, 9–34). The risk of readmission for pulmonary causes followed a similar trend as above and had the slowest decline, with risk persisting until 4 months after discharge. Interestingly, the risk of readmission for cardiac causes was highest 4 days after discharge (4.6 readmissions/100 patients per month; 95% CI, 2.0–10) and persisted until 60 days after discharge. Seventy-five percent (71/95) of all nononcologic readmissions in the first year after index discharge occurred before 90 days. The hazard phases that compose each cause of readmission are shown in Supplementary Figures 3 and 4.
Mortality Within 1 Year of Surgery
Mortality analysis included all 355 patients. In-hospital mortality was 3.7% (13/355), 30-day mortality was 4.5% (16/355), and 90-day mortality was 6.8% (24/355) (Table 2). Of note, total 90-day mortality was lower for patients who received induction therapy (vs. no induction therapy; 5.7% vs. 7.7%) and patients who underwent left pneumonectomy (vs. right pneumonectomy; 5.0% vs. 9.0%). The causes of death during the first year after pneumonectomy are listed in Supplementary Table 2. Overall survival for the first year after surgery is shown in Figure 2A. The parametric versus nonparametric (i.e., Kaplan-Meier) overall survival curves are presented in Supplementary Figure 5. Based on Kaplan-Meier estimation, 1-year overall survival was 81% (95% CI, 77%−85%). The instantaneous risk of death due to any cause during the first year after surgery was again composed of an early decreasing phase followed by a late increasing phase (Figure 2B). The risk of death was highest immediately after surgery (12 deaths/100 patients per month; 95% CI, 5.4–27) and decreased to less than half the initial value by 7 days (5.6 deaths/100 patients per month; 95% CI, 3.1–10). The risk of death from any cause reached its nadir at 90 days (1.1 deaths/100 patients per month; 95% CI, 0.6–2.0) then gradually increased throughout the remainder of the first year (12 months, 2.1 deaths/100 patients per month; 95% CI, 1.3–3.5).
Figure 2.
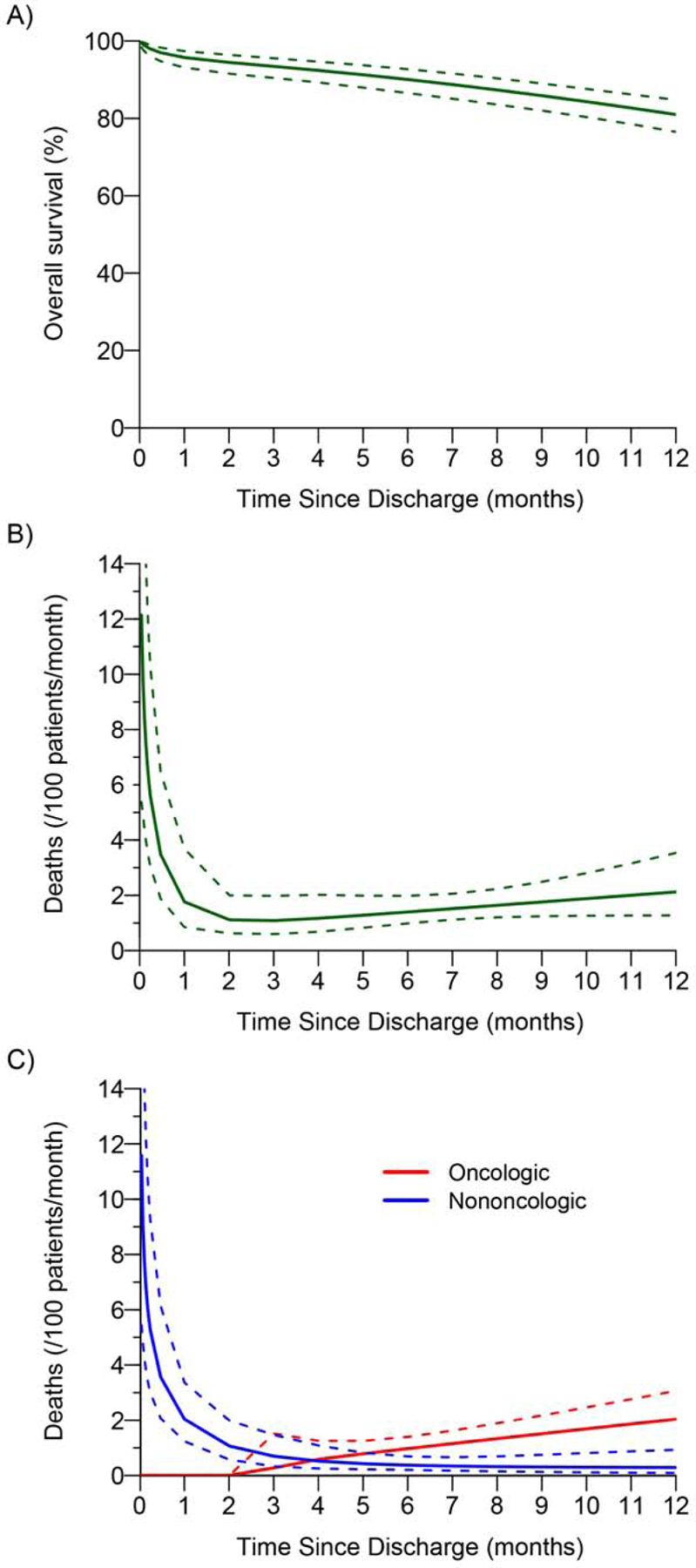
Overall survival and risk of death within 1 year of surgery after pneumonectomy. (A) Overall survival: the solid line is the parametric estimate of overall survival, along with the 95% confidence band. (B) Instantaneous risk of death: parametric estimates (solid line) with corresponding 95% confidence bands (dashed line). (C) Instantaneous risk (hazard function) of death within 1 year of surgery by cause (solid lines). The dashed lines correspond to the 95% confidence bands.
The time-varying instantaneous risk of death by cause was also investigated (Figure 2C). The risk of nononcologic death was highest immediately after discharge (12 deaths/100 patients per month; 95% CI, 5.4–25) then steadily declined, reaching values <1 by 90 days. All patients who died within 90 days died of nononcologic causes. In contrast, the risk of oncologic death was negligible until just before 90 days, after which a steady increase was noted throughout the remainder of the first year after surgery (12 months, 2.0 deaths/100 patients per month; 95% CI, 1.4–3.1). The hazard phases for each cause of death are shown in Supplementary Figure 6.
DISCUSSION
In our cohort of 355 pneumonectomy patients, readmission and death occurred in distinct patterns during the first year after pneumonectomy. The use of a novel parametric hazard decomposition approach enabled us to more comprehensively understand the time-varying trends in these outcomes and allowed us to examine the contributions of specific causes to these patterns over time. We found that the overall risk of readmission was highest immediately after index discharge and decreased to less than half the original value by 14 days. This risk reached a nadir and remained constant between 4 and 8 months, after which it gradually increased. The risk of readmission for cardiac causes peaked at 4 days after discharge, whereas the risk of readmission for all other nononcologic causes was highest immediately after discharge. Pulmonary causes were the most common cause of readmission during the first 90 days; after 90 days, oncologic causes were most common. Similarly, the overall risk of death was highest immediately after surgery and fell to half the original value by 7 days. This risk reached a nadir at 90 days, then increased throughout the remainder of the first year after surgery. All deaths were attributable to nononcologic causes until 90 days after surgery, at which point deaths from oncologic causes began to occur.
Selecting the ideal time point to assess morbidity and mortality after pneumonectomy not only has direct implications on patient care, but is also used as a quality metric for health care institutions.21 Due to the magnitude of the operation, 90 days has been increasingly chosen as the time point to encompass “operative” morbidity and mortality after pneumonectomy.9, 10, 13 Despite this, studies assessing readmissions after pneumonectomy are characteristically limited to 30 days, and the causes of readmission are frequently not reported. An analysis of the National Surgical Quality Improvement Program database by Rajaram and colleagues found a 30-day readmission rate for pneumonectomy patients of 11.8%, with pulmonary causes accounting for 44% of readmissions.22 Our 30-day readmission rate of 13% is slightly higher than this rate and rates in other studies,23, 24 though we reiterate that pulmonary causes (responsible for 56% of 30-day readmissions in our study) are the primary contributor to early readmission. Few studies have attempted to establish temporal trends for readmission after pneumonectomy. The Rajaram study noted that half of readmissions during the first 30 days occurred before 10 days.22 Another study by Hu and colleagues found that half of readmissions within the first 90 days occurred before 30 days, although readmissions were noted to persist beyond 90 days.13 Although these and other studies have characterized patterns of readmission, no study to date has examined both the type and timing of readmissions up to 1 year after pneumonectomy. Our study demonstrates that, at approximately 90 days, the predominant cause of readmission shifts from nononcologic (i.e., procedure related) to oncologic. Additionally, 75% (71/95) of all nononcologic readmissions that occurred during the first year after index discharge happened before 90 days.
The 30- and 90-day mortality in our study of 4.5% and 6.8%, respectively, are notably lower than those in other large-volume institution or database studies.3, 4, 24, 25 Mortality between 31 and 90 days was also lower in our study (2.4%) than in other studies (4.8%−5.2%).10, 23, 25 All deaths before 90 days (24/24; 100%) were attributable to nononcologic causes, and these 24 deaths accounted for 73% of all nononcologic deaths (24/33) during the first year after surgery In contrast, death from oncologic causes was absent until just before 90 days, yet by 4 months after surgery, oncologic death was the predominant contributor to overall mortality. Taken together, although a small number of procedure-related events occurred beyond 90 days, our findings provide evidence that 90 days after index discharge (for readmission) or surgery (for death) is an appropriate time point to assess operative morbidity and mortality after pneumonectomy.
Finally, few studies in thoracic surgery have explored the implications of readmission on mortality.11–13 A large study of the Medicare database demonstrated an association between 30-day readmission and 90-day mortality after lung resection, with the greatest risk of death in patients who were readmitted in the first 5 days after discharge.12 Additionally, Kozower and colleagues noted a 6-fold increase in 90-day mortality among patients who had been readmitted.13 Both of these studies, however, included all anatomical lung resection operations We found that 30% of patients (19/64) who experienced readmission within 90 days of index discharge eventually died within 1 year of surgery. Notably, 11 of these 19 deaths (58%) were due to nononcologic causes, and 10 of these 11 patients (91%) were readmitted for nononcologic reasons before 90 days. This suggests a potential link between the pattern of attributed cause of readmission and the cause of death.
This study is the first to characterize the timing and patterns of oncologic and nononcologic causes of readmission and death after pneumonectomy. Additionally, due to a comprehensive and detailed review, we were able to obtain accurate causes of nononcologic readmission in our cohort, enabling us to determine which causes were present at each time point after discharge. Our study also has important limitations. Although we were able to capture readmissions to other institutions (which constitute a large proportion of total readmissions among patients originating from tertiary referral centers13), some readmissions that occurred at other health-care facilities were likely missed. Furthermore, although our cohort is similar to other large, geographically diverse cohorts3, 9, 25 in terms of age, sex, tumor laterality, and tumor histology, our cohort is from a single high-volume institution, and a larger proportion of our patients received induction therapy, which may limit the generalizability of our results. Finally, despite the large size of our cohort, there were few nononcologic deaths, precluding the ability to draw robust conclusions with regard to patterns of nononcologic death after pneumonectomy.
CONCLUSIONS
In this analysis, we describe the time-varying risk of readmission and death during the first year after pneumonectomy. We show that nononcologic (i.e., procedure-related) causes of readmission and death predominate in the first 90 days after pneumonectomy, while oncologic causes are responsible for the majority of readmissions and deaths after this point. We also identify specific causes that pose the highest risk of readmission in the early phase after index discharge. Further efforts are warranted to link these outcomes and define the potential impact of specific causes of readmission on overall risk of death after pneumonectomy.
Supplementary Material
Perspective Statement:
Mortality within 30 days of pneumonectomy has remained constant during the last decade, in the range of 5% to 10%. To better understand the patterns of readmission and death after pneumonectomy, we performed a thorough investigation of the time-varying risk of these outcomes in the first postoperative year, with a focus on the contributions of specific causes to these patterns over time.
Funding
This work was supported, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748.
Glossary of Abbreviations
- ASA
American Society of Anesthesiologists
- IQR
interquartile range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI statement: Bernard J. Park has served as a proctor for Intuitive Surgical and consultant for COTA. Matthew J. Bott serves as a consultant for AstraZeneca. James M. Isbell reports stock ownership in LumaCyte. David R. Jones serves as a senior medical advisor for Diffusion Pharmaceuticals and a consultant for Merck and AstraZeneca. Gaetano Rocco has financial relationships with Baxter, Scanlan, and Medtronic. All other authors have no potential conflicts to disclose.
REFERENCES
- 1.Thomas PA, Berbis J, Baste JM, Le Pimpec-Barthes F, Tronc F, Falcoz PE, et al. Pneumonectomy for lung cancer: contemporary national early morbidity and mortality outcomes. J Thorac Cardiovasc Surg. 2015;149:73–82. [DOI] [PubMed] [Google Scholar]
- 2.Batirel HF. Extrapleural pneumonectomy (EPP) vs. pleurectomy decortication (P/D). Ann Transl Med. 2017;5:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro M, Swanson SJ, Wright CD, Chin C, Sheng S, Wisnivesky J, et al. Predictors of major morbidity and mortality after pneumonectomy utilizing the Society for Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg. 2010;90:927–934; discussion 934–925. [DOI] [PubMed] [Google Scholar]
- 4.Pages PB, Mordant P, Renaud S, Brouchet L, Thomas PA, Dahan M, et al. Sleeve lobectomy may provide better outcomes than pneumonectomy for non-small cell lung cancer. A decade in a nationwide study. J Thorac Cardiovasc Surg. 2017;153:184–195 e183. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs JP, Mavroudis C, Jacobs ML, Maruszewski B, Tchervenkov CI, Lacour-Gayet FG, et al. What is operative mortality? Defining death in a surgical registry database: a report of the STS Congenital Database Taskforce and the Joint EACTS-STS Congenital Database Committee. Ann Thorac Surg. 2006;81:1937–1941. [DOI] [PubMed] [Google Scholar]
- 6.Damhuis RA, Wijnhoven BP, Plaisier PW, Kirkels WJ, Kranse R, van Lanschot JJ. Comparison of 30-day, 90-day and in-hospital postoperative mortality for eight different cancer types. Br J Surg. 2012;99:1149–1154. [DOI] [PubMed] [Google Scholar]
- 7.Pezzi CM, Mallin K, Mendez AS, Greer Gay E, Putnam JB Jr. Ninety-day mortality after resection for lung cancer is nearly double 30-day mortality. J Thorac Cardiovasc Surg. 2014;148:2269–2277. [DOI] [PubMed] [Google Scholar]
- 8.Moore CB, Cox ML, Mulvihill MS, Klapper J, D’Amico TA, Hartwig MG. Challenging 30-day mortality as a site-specific quality metric in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2019; [DOI] [PubMed] [Google Scholar]
- 9.Schneider L, Farrokhyar F, Schieman C, Shargall Y, D’Souza J, Camposilvan I, et al. Pneumonectomy: the burden of death after discharge and predictors of surgical mortality. Ann Thorac Surg. 2014;98:1976–1981; discussion 1981–1972. [DOI] [PubMed] [Google Scholar]
- 10.Kim AW, Boffa DJ, Wang Z, Detterbeck FC. An analysis, systematic review, and meta analysis of the perioperative mortality after neoadjuvant therapy and pneumonectomy for non-small cell lung cancer. J Thorac Cardiovasc Surg. 2012;143:55–63. [DOI] [PubMed] [Google Scholar]
- 11.Puri V, Patel AP, Crabtree TD, Bell JM, Broderick SR, Kreisel D, et al. Unexpected readmission after lung cancer surgery: A benign event? J Thorac Cardiovasc Surg. 2015;150:1496–1504, 1505 e1491–1495; discussion 1504–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez AA, Abdelsattar ZM, Dimick JB, Dev S, Birkmeyer JD, Ghaferi AA. Time-to readmission and mortality after high-risk surgery. Ann Surg. 2015;262:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Y, McMurry TL, Isbell JM, Stukenborg GJ, Kozower BD. Readmission after lung cancer resection is associated with a 6-fold increase in 90-day postoperative mortality. J Thorac Cardiovasc Surg. 2014;148:2261–2267 e2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khot UN, Johnson MJ, Wiggins NB, Lowry AM, Rajeswaran J, Kapadia S, et al. Long term time-varying risk of readmission after acute myocardial infarction. J Am Heart Assoc. 2018;7(21):e009650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackstone EH, Kirklin JW. Death and other time-related events after 360 valve replacement. Circulation. 1985;72:753–767. [DOI] [PubMed] [Google Scholar]
- 16.Robich MP, Sabik JF, Houghtaling PL, Kelava M, Gordon S, Blackstone EH, et al. Prolonged effect of postoperative infectious complications on survival after cardiac surgery. Ann Thorac Surg. 2015;99:1591–1599. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shuster JJ. Median follow-up in clinical trials. J Clin Oncol. 1991;9:191–192. [DOI] [PubMed] [Google Scholar]
- 19.Blackstone EH, Naftel DC, Turner ME. The decomposition of time-varying hazard into phases, each incorporating a separate stream of concomitant information. J Am Stat Assoc. 1986;81:615–624. [Google Scholar]
- 20.Blackstone EH. The Hazard Package (Cleveland Clinic Foundation). https://www.lerner.ccf.org/qhs/software/hazard/. Accessed Jan 3, 2020.
- 21.Numan RC, Berge MT, Burgers JA, Klomp HM, van Sandick JW, Baas P, et al. Peri- and postoperative management of stage I-III non small cell lung cancer: which quality of care indicators are evidence-based? Lung Cancer. 2016;101:129–136. [DOI] [PubMed] [Google Scholar]
- 22.Rajaram R, Ju MH, Bilimoria KY, Ko CY, DeCamp MM. National evaluation of hospital readmission after pulmonary resection. J Thorac Cardiovasc Surg. 2015;150:1508–1514 e1502. [DOI] [PubMed] [Google Scholar]
- 23.Yang CJ, Shah SA, Lin BK, VanDusen KW, Chan DY, Tan WD, et al. Right-sided versus left-sided pneumonectomy after induction therapy for non-small cell lung cancer. Ann Thorac Surg. 2019;107:1074–1081. [DOI] [PubMed] [Google Scholar]
- 24.Abdelsattar ZM, Shen KR, Yendamuri S, Cassivi S, Nichols FC 3rd, Wigle DA, et al. Outcomes after sleeve lung resections versus pneumonectomy in the United States. Ann Thorac Surg. 2017;104:1656–1664. [DOI] [PubMed] [Google Scholar]
- 25.Mansour Z, Kochetkova EA, Santelmo N, Meyer P, Wihlm JM, Quoix E, et al. Risk factors for early mortality and morbidity after pneumonectomy: a reappraisal. Ann Thorac Surg. 2009;88:1737–1743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


