Abstract
Human lung mast cells (HLMCs) express the high-affinity receptor FcεRI for IgE and are involved in chronic pulmonary diseases occurring at high frequency among HIV-infected individuals. Immunoglobulin superantigens bind to the variable regions of either the heavy or light chain of immunoglobulins (Igs). Glycoprotein 120 (gp120) of HIV-1 is a typical immunoglobulin superantigen interacting with the heavy chain, variable 3 (VH3) region of human Igs. The present study investigated whether immunoglobulin superantigen gp120 caused the release of different classes of proinflammatory and immunoregulatory mediators from HLMCs. The results show that gp120 from different clades induced the rapid (30 min) release of preformed mediators (histamine and tryptase) from HLMCs. gp120 also caused the de novo synthesis of cysteinyl leukotriene C4 (LTC4) and prostaglandin D2 (PGD2) from HLMCs. Incubation (6 h) of HLMC with gp120 induced the release of angiogenic (VEGF-A) and lymphangiogenic (VEGF-C) factors from HLMCs. The activating property of gp120 was mediated through the interaction with IgE VH3+ bound to FcεRI. Our data indicate that HIV gp120 is a viral superantigen, which induces the release of different proinflammatory, angiogenic, and lymphangiogenic factors from HLMCs. These observations could contribute to understanding, at least in part, the pathophysiology of chronic pulmonary diseases in HIV-infected individuals.
Keywords: angiogenesis, histamine, HIV, gp120, IgE, leukotriene C4, lymphangiogenesis, mast cells, prostaglandin D2, superantigen
1. Introduction
The human immunodeficiency virus (HIV-1) affects more than 36 million people worldwide [Unaids. UNAIDS Data 2018 (2018)]. Although the combined antiretroviral therapy (ART) can successfully suppress HIV viremia and delays the progression of disease [1], the chronic infection requires lifetime treatment due to the viral persistence in latent reservoirs [2,3,4]. Importantly, a significant percentage of HIV-infected individuals has hepatitis C virus (HCV) co-infection [5] resulting in increased HIV reservoir size [6]. The advent of ART has improved survival of HIV-infected adults and children leading to chronic illnesses such as different pulmonary diseases [7,8]. For instance, chronic obstructive pulmonary disease (COPD), asthma, pulmonary hypertension, lung cancer, and asthma are prevalent among HIV patients [7,9,10,11,12,13].
Mast cells are immune cells localized in murine [14,15,16] and human lung [17,18,19,20]. Mast cells are critical sentinels in immunity [21,22] and were canonically considered key effectors of allergic responses [18,23,24,25,26]. However, increasing evidences indicate that these cells are involved in bacterial and viral infections [27,28,29,30], pulmonary diseases [22,31,32], angiogenesis [33,34,35,36,37], lymphangiogenesis [38,39], autoimmune disorders [40,41,42], and cancer [43,44,45,46]. There is compelling evidence that human mast cell progenitors can be infected by HIV and retain the virus with maturation in vitro [47,48,49,50]. Importantly, HIV-1 can replicate in latently infected human mast cells [51] and these cells are an inducible reservoir of persistent infection [51,52,53].
Human mast cells display the high-affinity receptor (FcεRI) for immunoglobulin E (IgE) and cross-linking of the IgE-FcεRI network causes the release of preformed (e.g., histamine, tryptase) and de novo synthesized lipid mediators (e.g., prostaglandin D2: PGD2 and cysteinyl leukotriene C4: LTC4). Human lung mast cells [33], like macrophages [54,55], basophils [56], and neutrophils [57], also release angiogenic (e.g., vascular endothelial growth factor A: VEGF-A) and/or lymphangiogenic factors (e.g., vascular endothelial growth factor C: VEGF-C) [23,33,55]. Human mast cells isolated from various organs [58,59] are heterogeneous with respect to the mediators produced [19].
Different bacteria and viruses synthesize a variety of proteins, termed “superantigens” (SAgs) that activate T and B cells [60,61,62,63,64]. T cell SAgs bind to the MHC class II molecules and to the Vβ domain of the T cell receptor (TCR) and bypass the conventional presentation of antigens by antigen-presenting cells (APCs) [65,66,67,68]. B cell SAgs are endowed with immunoglobulin (Ig)-binding capacity and bind to either the heavy (H)- or light (L)-chain of Igs [69,70,71]. HIV glycoprotein 120 (gp120) is a viral immunoglobulin SAg, because it binds to Igs VH3+ [72,73,74,75,76,77], the largest of human Ig germline VH family. Therefore, gp120 can stimulate a large percentage of Ig-bearing immune cells, including mast cells.
Chronic lung diseases, such as chronic obstructive pulmonary disease (COPD), lung cancer, pulmonary hypertension, and asthma, are currently a leading concern for patients with HIV infection [7,8,9,10,11,12,13]. There is compelling evidence that mast cells and their proinflammatory and angiogenic mediators are involved in these disorders [26,32,33,45,78]. In this study we evaluated whether viral gp120 superantigen can induce the release of proinflammatory, angiogenic, and lymphangiogenic factors from primary mast cells isolated from human lung parenchyma.
2. Materials and Methods
2.1. Reagents
The following were purchased: bovine serum albumin (BSA), Pipes [piperazine-N,N′-bis (2-ethanesulfonic acid)], L-glutamine, antibiotic-antimycotic solution (10,000 IU penicillin, 10 mg/mL streptomycin, and 25 μg/mL amphotericin B), LTC4, and PGD2 (Sigma-Aldrich, St. Louis, MO, USA), collagenase (Worthington Biochemical Co., Freehold, NJ, USA), Fetal calf serum (FCS) (GIBCO, Grand Island, NY, USA), and pronase (Calbiochem, La Jolla, CA, USA), RPMI 1640 with 25 mM HEPES buffer, Eagle’s minimum essential medium (Flow Laboratories, Irvine, UK), Percoll (Pharmacia Fine Chemicals, Uppsala, Sweden), (3H)-LTC4 and (3H)-PGD2 (New England Nuclear, Boston, MA, USA), CD117 MicroBead (Miltenyi Biotech, Bologna, Italy). The rabbit anti-LTC4 and anti-PGD2 antibodies and the monoclonal antibody anti-FcεRI were a gift of Dr. Lawrence M. Lichtenstein (The Johns Hopkins University, Baltimore, MD, USA).
2.2. Recombinant HIV gp120 Proteins
Recombinant gp120MN, gp120SF2, gp120LAV, and gp120CM were obtained through the AIDS Research and Reference Reagent Program (National Institute of Allergy and Infectious Diseases, USA) [79,80]. Their characteristics are summarized in Table 1.
Table 1.
Recombinant HIV gp120 used in this study.
| Recombinant Envelope Protein | gp120 Isolate | Clade | Geographic Origin | Expression System |
|---|---|---|---|---|
| gp120MN | MN | B | USA | Insect Cells |
| gp120SF2 | SF2 | B | USA | CHO Cells |
| gp120LAV | LAV | B | France | Insect Cells |
| gp120CM | CM | E | Thailand | Insect Cells |
2.3. Human IgG Anti-IgE
Human IgG anti-IgE (H-aIgE) was purified from the serum of a patient with severe atopic dermatitis as previously described [81,82]. The specificity and activity of IgG anti-IgE were tested as described elsewhere [81].
2.4. Human Monoclonal IgM and Human Polyclonal IgG
Monoclonal IgM were purified from the serum of patients with Waldenström’s macroglobulinemia as described elsewhere [83]. Variable regions of these monoclonal IgM were determined using a panel of primary sequence-dependent VH family specific reagents that identify framework regions [84]. Human polyclonal IgG were purified from the serum of healthy donors [85].
2.5. Isolation of HLMCs
The study was approved by the Ethics Committee of the University of Naples Federico II (Protocol: Human MC No 7/19, 16/01/2019). The lung tissue was obtained from patients seronegative for HIV-1, HCV, and HBV undergoing thoracic surgery, mostly for lung cancer. HLMC were purified from human lung tissue by a modification of the method previously described [33]. The enzymatic dispersion tissue yields ≈ 5 × 105 mast cells per gram of lung tissue. The purity of these populations ranged from 3% to 18%. HLMCs were partially purified by flotation through a discontinuous Percoll gradient [83]. Mast cell purity using this technique ranged from 47% to 79% and was assessed by alcian blue staining.
2.6. Assays of Histamine, LTC4, and PGD2
HLMCs (≈3 × 104 mast cells per tube) were resuspended in Pipes buffer containing, in addition to Pipes (25 mM), CaCl2 (2 mM) and dextrose (1 g/L) and 0.3 mL of the cell suspensions were placed in 12 × 75 mm polyethylene tubes [86]. 0.2 mL of each prewarmed releasing stimulus was added, and incubation was continued at 37 °C for 30 min [87]. Histamine was measured in duplicate determinations with an automated fluorometric technique [88]. LTC4 and PGD2 were measured in duplicate determinations by radioimmunoassay [87,89]. The anti-LTC4 and anti-PGD2 antibodies have less than 1% cross-reactivity to other eicosanoids [87,89].
2.7. VEGF-A and VEGF-C Release
HLMCs (≈8 × 104 mast cells/per tube) were incubated (37 °C, 6 h) in RPMI 1640 containing 5% FCS, 2 mM L-glutamine, and 1% antibiotic-antimycotic solution, and activated with various concentrations of gp120. At the end of incubation, cells were centrifuged (1000× g, 4 °C, 5 min) and the supernatants were stored at −80 °C for subsequent assay of mediator release. VEGF-A and VEGF-C were measured in duplicate determinations using ELISA kits (R&D System, Minneapolis, MN, USA [90]. The ELISA sensitivity is 31.1–2000 pg/mL for VEGF-A and 62–4000 pg/mL for VEGF-C.
2.8. Statistical Analysis
Values were expressed as means ± SEM (standard error of the mean). The one-way repeated measures analysis of variance (ANOVA) with Greenhouse–Geisser corrections was used to examine the variations of continuous variables at different experimental conditions. Results were analyzed with GraphPad Prism software (version 8.01; GraphPad Software, La Jolla, CA, USA), and p values of less than 0.05 were considered significant.
3. Results
3.1. Effect of Human IgG Anti-IgE on Mediator Release from HLMCs
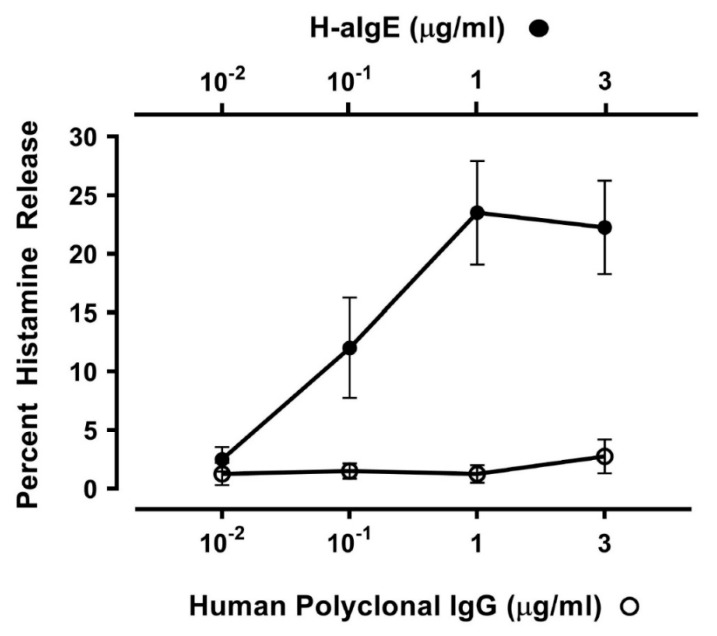
IgG anti-IgE (H-aIgE), purified from a small percentage of atopic dermatitis patients, induces histamine and LTC4 release from human basophils and mast cells [81]. The activating property of H-aIgE is mediated by the interaction with IgE on basophils and mast cells [82,91]. We used this human autoantibody to activate HLMCs in vitro. H-aIgE (10−2 to 3 μg/mL) caused a concentration-dependent histamine secretion from four different preparations of HLMCs isolated from HIV-1-negative subjects (Figure 1). As a control, we used IgG (10−2 to 3 μg/mL) purified from four normal donors which did not induce histamine release from HLMCs. These results indicate that mast cells purified from human lung have FcεRI-bound IgE.
Figure 1.
Effects of increasing concentrations of human IgG anti-IgE (H-aIgE) [81] and four preparations of human polyclonal IgG purified from normal donors on histamine release from HLMCs obtained from four donors negative for HIV-1 antibodies. HLMCs were incubated (30 min at 37 °C) with the indicated concentrations of H-aIgE or polyclonal IgG. Each point shows the mean ± SEM obtained from four different experiments.
3.2. Effects of gp120 from Divergent HIV Isolates from Different Clades on Mediator Release from HLMCs
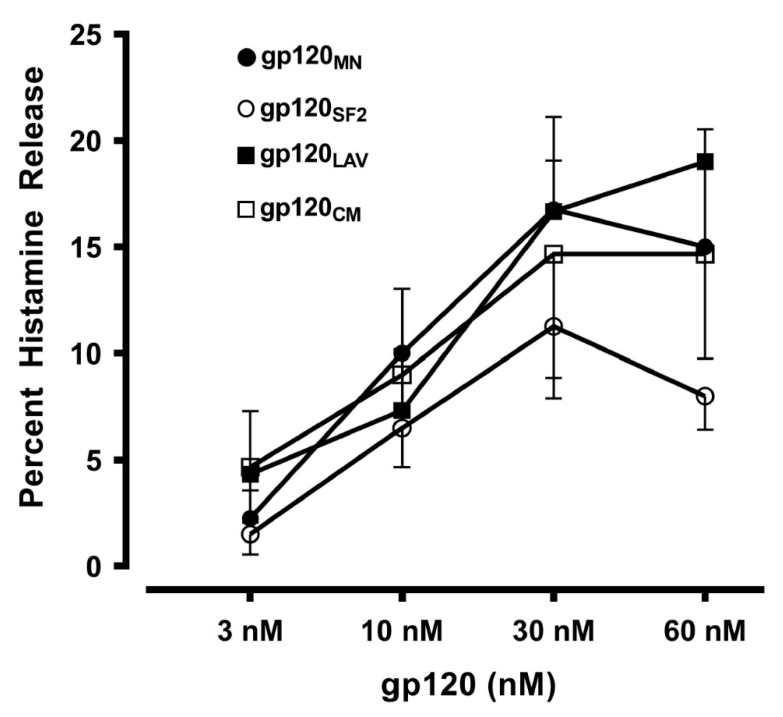
In a group of experiments we compared the effects of four recombinant gp120 (gp120MN, gp120SF2, gp120LAV, and gp120CM) derived from divergent HIV-1 isolates from different viral clades (B and E) of various geographical origins (United States, France, and Thailand) [74] (Table 1) on mediator release from HLMCs. These divergent samples of gp120 concentration-dependently (3 to 60 nM) induced histamine release from HLMCs (Figure 2). These results imply that the capacity to induce mediator release from HLMCs is a general feature of gp120 which has maintained throughout the evolution of the virus.
Figure 2.
Effects of increasing concentrations of gp120 from four different isolates (gp120MN, gp120SF2, gp120LAV, gp120CM) on histamine secretion from HLMCs obtained from four different preparations of HLMCs obtained from donors negative for HIV-1 antibodies. HLMCs were incubated (30 min at 37 °C) with the indicated concentrations of gp120. Each point shows the mean ± SEM obtained from four different experiments.
3.3. Correlation between Histamine and Tryptase Release Induced by gp120 from HLMCs
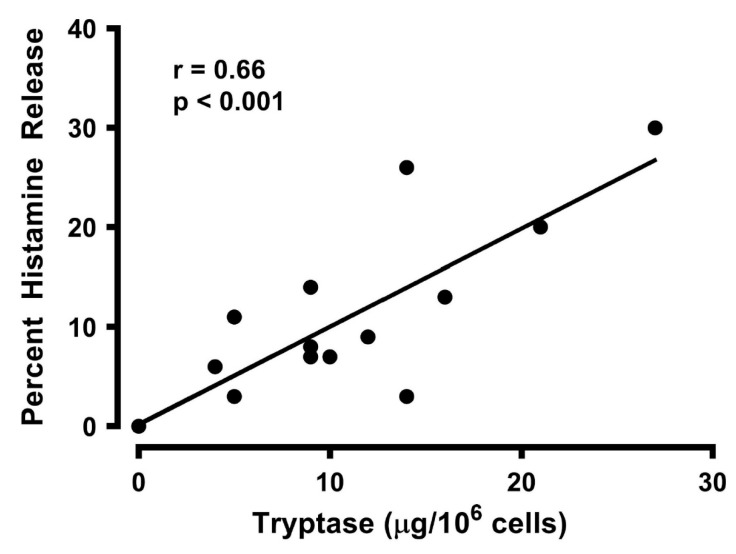
Tryptase is a neutral protease that is a selective marker for mast cells [92,93]. Large quantities of tryptase reside in the secretory granules of all mature human mast cells [92,94]. Activation of HLMCs with gp120 caused the release of tryptase as well as of histamine. Figure 3 shows that there was a positive correlation between the percentage of histamine and tryptase release induced by gp120 (r = 0.66; p < 0.001). These data demonstrate that tryptase, contained in secretory granules of mast cells, is released in parallel with histamine, implying that these cells are the source of both mediators found in supernatants of gp120-activated HLMCs.
Figure 3.
Correlation between the percent histamine release and tryptase secretion caused by gp120 from HLMCs. Each point represents the mean of duplicate determinations from separate experiments. r = 0.66; p < 0.001.
3.4. Effect of Lactic Acid on gp120-Induced Histamine Release from HLMCs
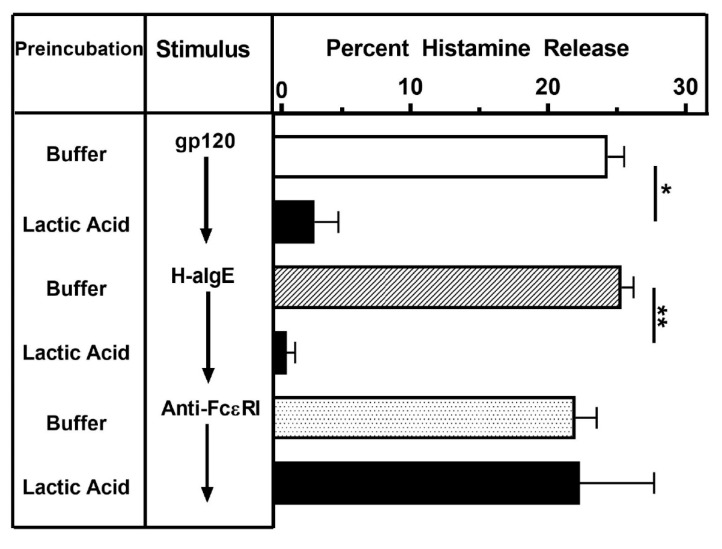
Brief exposure to lactic acid removes IgE bound on FcεRI+ cells, thus inhibiting the activating properties of IgE-mediated stimuli [95]. Lactic acid exposure completely blocked the activating effect of both gp120 and by H-aIgE on histamine release from HLMCs (Figure 4). By contrast, the activating property of the mAb cross-linking the α-chain of FcεRI [84] was not modified by this treatment. These results suggest that gp120 induces histamine release from HLMCs through the interaction with IgE bound on mast cells.
Figure 4.
Effects of lactic acid on histamine release from HLMCs induced by gp120, H-aIgE or anti-FcεRI. HLMCs were either treated with buffer or lactic acid (0.01 M, pH 3.9, 5 min at 22 °C) and washed twice. HLMCs were then challenged (30 min at 37 °C) with gp120 (10 nM), H-aIgE (1 μg/mL), or anti-FcεRI (1 μg/mL). Each bar represents the mean ± SEM of histamine release from triplicate incubations. * p < 0.05 when compared to cells treated with buffer; ** p < 0.01 when compared to cells treated with buffer.
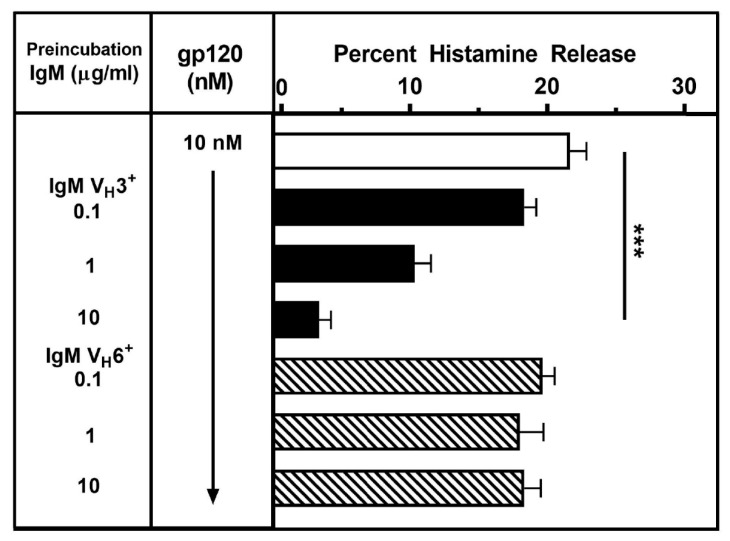
3.5. Effects of Different IgM Myeloma Proteins on gp120-Induced Mediator Release from HLMCs
To evaluate the mechanism whereby gp120 activates HLMCs, gp120 was incubated with monoclonal IgM of different VH families following the procedure previously described [84]. gp120 (10 nM) was preincubated (15 min, 37 °C) with increasing concentrations (0.1 to 10 μg/ml) of a preparation of monoclonal IgM VH3+ or monoclonal IgM VH6+. HLMCs were then added, and the incubation was continued for 30 min at 37 °C. At the end of this incubation, histamine was measured in the supernatants. Preincubation with a preparation of monoclonal IgM which has the VH3 domain, concentration-dependently inhibited the effect of gp120 on histamine release from HLMCs (Figure 5). By contrast, a monoclonal IgM which has a VH6 domain, had no effect. These results are compatible with the hypothesis that binding of gp120 to the VH3 domain of human monoclonal IgM inhibits the interaction with IgE bound to FcεRI on HLMCs.
Figure 5.
Effects of human monoclonal IgMs on the activation of HLMCs induced by gp120. gp120 (10 nM) was preincubated (15 min at 37 °C) with increasing concentrations (1 to 10 μg/mL) of human monoclonal IgM VH3+ or IgM VH6+. HLMCs were then added and incubation continued for 30 min at 37 °C. Each bar shows the mean ± SEM of histamine release from triplicate incubations. *** p < 0.001 when compared to controls.
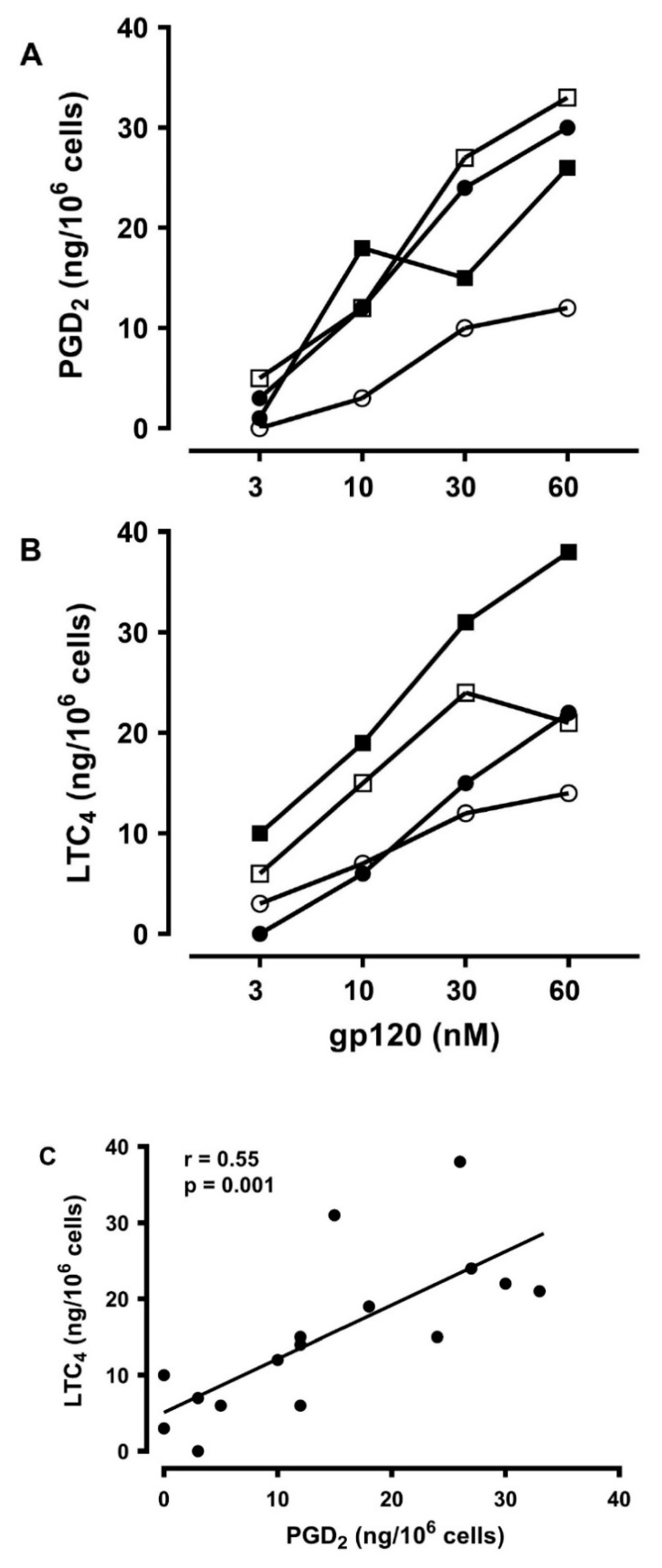
3.6. Effects of gp120 on the De Novo Synthesis of PGD2 and LTC4 from HLMCs
Activated human mast cells can rapidly synthesize several lipid mediators [23,89]. Prostaglandin D2 (PGD2) is the main cyclooxygenase metabolite synthesized de novo by human mast cells [78,96]. Activated human mast cells also synthesize cysteinyl leukotriene C4 (LTC4) through the 5-lipoxygenase pathway [96,97,98]. Both lipid mediators exert a variety of proinflammatory and vasoactive effects [99,100]. In four experiments, we evaluated the production of PGD2 and LTC4 from HLMCs in response to increasing concentrations of gp120. Figure 6 shows that gp120 (3 to 60 nM) caused the de novo synthesis of both PGD2 (Figure 6A) and LTC4 (Figure 6B). Figure 6C shows that there was a significant correlation between the production of PGD2 and of LTC4 caused by gp120 from HLMCs (r =0.55; p < 0.001).
Figure 6.
Effects of increasing concentrations of gp120 on the de novo synthesis of PGD2 (A) and LTC4 (B) from four different preparations of HLMCs. HLMCs were incubated (30 min at 37 °C) with the indicated concentrations of gp120. Each point is the mean of duplicate determinations (C). Correlation between PGD2 and LTC4 production caused by gp120 from HLMCs. r = 0.55; p < 0.001.
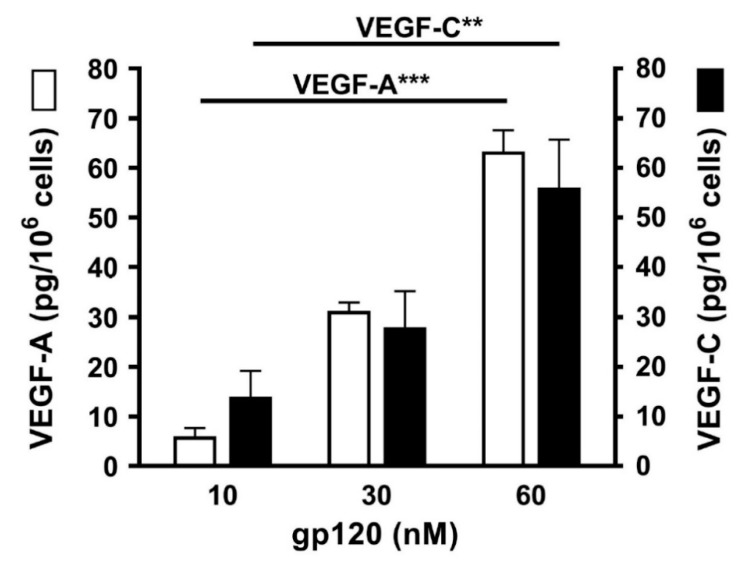
3.7. Effects of gp120 on the Release of Angiogenic and Lymphangiogenic Factors from HLMCs
Vascular endothelial growth factors (VEGFs) are essential regulators of the development and functions of blood and lymphatic vessels [101,102]. VEGFs play a major role in new vessel formation and in pulmonary pathophysiology [33,103]. VEGF-A is the most potent proangiogenic molecule [34,104], whereas VEGF-C plays an essential role in inflammatory and tumor lymphangiogenesis [101,102]. Therefore, we investigated the effects of increasing concentrations (10 to 60 nM) of gp120 on the release of angiogenic (VEGF-A) and lymphangiogenic factors (VEGF-C) from HLMCs. HLMCs were cultured (6 h at 37 °C) with gp120 and, at the end of incubation, the release of VEGF-A and VEGF-C was assayed in the supernatants of mast cells. Figure 7 shows that gp120 concentration-dependently caused the release of VEGF-A and VEGF-C from four different preparations of HLMCs.
Figure 7.
Effects of increasing concentrations of gp120 on the release of VEGF-A and VEGF-C from HLMCs from four different preparations of HLMCs. HLMCs were incubated (6 h at 37 °C) in the presence of the indicated concentrations of gp120. Each bar is the mean ± SEM obtained from four different experiments. ** p < 0.01; *** p < 0.001.
4. Discussion
Primary mast cells isolated from human lung parenchyma of HIV-1 negative subjects can be activated by a human IgG anti-IgE isolated from a patient with atopic dermatitis. These results suggest that HLMCs bind IgE which has a role in allergic diseases [18,105], in pulmonary disorders [22,31] and also in HIV-1 infection [106,107,108,109,110,111]. Viral (gp120) superantigen activates HLMCs to release preformed (histamine and tryptase) and de novo synthesized proinflammatory mediators (LTC4 and PGD2), angiogenic (VEGF-A), and lymphangiogenic (VEGF-C) factors. The activating property of gp120 appears to be mediated by interaction with IgE VH3+ on HLMCs.
Mast cells, present in strategic locations of human lung [19,20,26,32], are involved in several pulmonary diseases [22,31], lung remodeling [112,113], COPD [9,12,32], lung cancer [114,115,116], and asthma [17,19]. In recent years, the widespread use of ART has improved the survival of people with HIV [1], leading to the emergence of chronic inflammatory lung diseases as a noteworthy concern in this population [7,9]. COPD [9,11,12,117], lung cancer [10,118], pulmonary hypertension [13], and bronchial asthma [7,119] occur at high frequency among HIV-infected individuals. HIV infection induces a state of chronic inflammation characterized by persistent immune dysregulation [120], which likely induces the release of proinflammatory cytokines. Although our results were obtained in an experimental model in vitro, the release of several proinflammatory mediators from gp120-activated human lung mast cells may contribute to the pathophysiology of chronic pulmonary diseases in HIV-infected patients.
There is compelling evidence that serum IgE levels are elevated in subjects with HIV infection compared to control [106,107,108,109,110,111], suggesting that mast cells and perhaps other immune cells expressing FcεRI (e.g., dendritic cells, macrophages, basophils) could be involved in various aspects of this infection [27,47,73,91]. Human mast cell progenitors can be infected HIV and retain the virus with their maturation [47,48,49,50]. Moreover, human mast cells are important reservoir of persistent HIV infection [51,52,53]. The involvement of mast cells in HIV infection is not unprecedented. In fact, these cells are involved in several viral infections such as Sendai virus [121], dengue infection [122,123], herpes simplex [124], influenza [125], hantaviruses [126], and cytomegalovirus [127,128].
Angiogenesis [129] plays a role in pulmonary pathophysiology [38,130,131]. VEGF-A is a major mediator of angiogenesis and can be produced by several immune cells [33,55,56,104,132,133,134]. To our knowledge, this is the first evidence that gp120 can induce the release of angiogenic factors from HLMCs raising the possibility that these cells can contribute to angiogenesis, a process of pivotal relevance in lung pathophysiology [38,130,131]. Further studies are needed to comprehensively define the contributive role of lung mast cells to angiogenesis in pulmonary diseases (e.g., COPD, asthma, etc.) [7,9,10,11,12,13] associated with HIV infection.
The mammalian lung is rich of lymphatic vessels [135] which are increased in human lung following infections [136,137,138,139]. We provide the first evidence that a superantigenic viral activation of HLMCs leads to the production of VEGF-C, a major mediator of lymphangiogenesis [140]. Lymphangiogenesis is canonically considered pivotal for the diffusion of metastasis to draining lymph nodes [101,102]. However, recent evidences indicate that VEGF-C can potentially exert protective effects, since inflammation-associated lymphangiogenesis can improve the resolution of inflammation [141,142]. Therefore, the contribution of lung mast cells to lymphangiogenesis during HIV infection commands additional investigations.
5. Conclusions
We have previously shown that gp120 causes cytokines (IL-4 and IL-13) release from human basophils [73]. Together with the data of the present study, it is possible to conclude that gp120 [143] can function as a viral superantigen activating HLMCs and basophils to release proinflammatory mediators (histamine, tryptase, PGD2, LTC4), cytokines (IL-4 and IL-13), and angiogenic/lymphangiogenic factors (VEGF-A and VEGF-C). The latter results could contribute to immune dysfunction in patients with HIV.
The successful rollout of anti-viral therapy ensured that HIV infection is managed as a chronic condition [2,3,4,98]. Persistent inflammation and immune dysregulation associated with HIV lead to accelerated aging and pulmonary diseases [7,8,9,10,11,12,13]. HIV-positive persons are, therefore, exhibiting increasing pulmonary complications. Our results, indicating that gp120 can induce the release of potent proinflammatory mediators (histamine, tryptase, PGD2 and LTC4) [144,145,146,147,148,149,150] from HLMCs might explain, at least in part, how HIV can cause lung damage.
Our study has a limitation that should be pointed out. The in vitro experiments were performed using primary human lung mast cells purified from HIV- patients undergoing thoracic surgery for lung cancer. Several independent investigators have demonstrated that human mast cells can be infected by HIV [47,48,49,52]. Importantly, human mast cells represent a long-lived reservoir of persistent HIV infection even in patients treated with ART [52,53]. There is also some evidence that circulating levels of histamine, a major mast cell mediator [17], are increased in HIV infected patients [151]. Future studies should compare the activating property of gp120 on lung mast cells isolated from HIV- and HIV+ subjects to support the clinical significance of our findings.
In conclusion, our results indicate that immunoglobulin superantigen gp120 can interact with IgE VH3+ bound to FcεRI to induce the release of proinflammatory, angiogenic, and lymphangiogenic mediators from human lung mast cells. Future studies are needed to investigate whether these observations in vitro can help to understand the pathophysiology of chronic lung diseases among HIV patients.
Acknowledgments
We are grateful to all patients for donating their samples.
Abbreviations
| APCs | Antigen-presenting cells |
| BSA | Bovine serum albumin |
| COPD | Chronic obstructive pulmonary disease |
| FcεRI | High-affinity receptor for IgE |
| FCS | Fetal calf serum |
| gp120 | Glycoprotein 120 |
| H | Heavy |
| H-aIgE | Human IgG anti-IgE |
| HIV | Human immunodeficiency virus |
| HLMCs | Human lung mast cells |
| Ig | Immunoglobulin |
| IL | Interleukin |
| L | Light |
| LTC4 | Cysteinyl leukotriene C4 |
| MHC | Major histocompatibility complex |
| PGD2 | Prostaglandin D2 |
| Sag | Superantigen |
| SE | Staphylococcus aureus enterotoxins |
| TCR | T cell receptor |
| V | Variable |
| VEGF | Vascular endothelial growth factor |
Author Contributions
Conceptualization, G.M. (Giancarlo Marone), F.W.R., A.d.P., G.S., G.M. (Gianni Marone); Formal Analysis, A.P.; Investigation, G.C., A.P., G.V.; Data Curation, A.P.; Writing—Original Draft Preparation, G.M. (Giancarlo Marone), G.M. (Gianni Marone), G.V.; Writing—Review and Editing, G.V., G.M. (Gianni Marone), V.P., G.C.; Supervision, G.M. (Gianni Marone), G.S.; Funding Acquisition, G.M. (Gianni Marone), G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by grants from Regione Campania CISI-Lab Project, CRèME Project, and TIMING Project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Liu C., Ma X., Liu B., Chen C., Zhang H. HIV-1 functional cure: Will the dream come true? BMC Med. 2015;13:284. doi: 10.1186/s12916-015-0517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siliciano J.D., Kajdas J., Finzi D., Quinn T.C., Chadwick K., Margolick J.B., Kovacs C., Gange S.J., Siliciano R.F. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 3.Coiras M., Lopez-Huertas M.R., Perez-Olmeda M., Alcami J. Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat. Rev. Microbiol. 2009;7:798–812. doi: 10.1038/nrmicro2223. [DOI] [PubMed] [Google Scholar]
- 4.Buzon M.J., Sun H., Li C., Shaw A., Seiss K., Ouyang Z., Martin-Gayo E., Leng J., Henrich T.J., Li J.Z., et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat. Med. 2014;20:139–142. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vazquez-Moron S., Berenguer J., Gonzalez-Garcia J., Jimenez-Sousa M.A., Canorea I., Guardiola J.M., Crespo M., Quereda C., Sanz J., Carrero A., et al. Prevalence of hepatitis E infection in HIV/HCV-coinfected patients in Spain (2012–2014) Sci. Rep. 2019;9:1143. doi: 10.1038/s41598-018-37328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Huertas M.R., Palladino C., Garrido-Arquero M., Esteban-Cartelle B., Sanchez-Carrillo M., Martinez-Roman P., Martin-Carbonero L., Ryan P., Dominguez-Dominguez L., Santos I.L., et al. HCV-coinfection is related to an increased HIV-1 reservoir size in cART-treated HIV patients: A cross-sectional study. Sci. Rep. 2019;9:5606. doi: 10.1038/s41598-019-41788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzpatrick M.E., Kunisaki K.M., Morris A. Pulmonary disease in HIV-infected adults in the era of antiretroviral therapy. AIDS. 2018;32:277–292. doi: 10.1097/QAD.0000000000001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Githinji L.N., Gray D.M., Zar H.J. Lung function in HIV-infected children and adolescents. Pneumonia (Nathan) 2018;10:6. doi: 10.1186/s41479-018-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bigna J.J., Kenne A.M., Asangbeh S.L., Sibetcheu A.T. Prevalence of chronic obstructive pulmonary disease in the global population with HIV: A systematic review and meta-analysis. Lancet Glob. Health. 2018;6:e193–e202. doi: 10.1016/S2214-109X(17)30451-5. [DOI] [PubMed] [Google Scholar]
- 10.Kiderlen T.R., Siehl J., Hentrich M. HIV-Associated Lung Cancer. Oncol. Res. Treat. 2017;40:88–92. doi: 10.1159/000458442. [DOI] [PubMed] [Google Scholar]
- 11.Singhvi D., Bon J., Morris A. Obstructive Lung Disease in HIV-Phenotypes and Pathogenesis. Curr. HIV/Aids Rep. 2019;16:359–369. doi: 10.1007/s11904-019-00456-3. [DOI] [PubMed] [Google Scholar]
- 12.de Miguel-Diez J., Lopez-de-Andres A., Jimenez-Garcia R., Puente-Maestu L., Jimenez-Trujillo I., Hernandez-Barrera V., Resino S., Alvaro-Meca A. Trends in Epidemiology of COPD in HIV-Infected Patients in Spain (1997-2012) PLoS ONE. 2016;11:e0166421. doi: 10.1371/journal.pone.0166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basyal B., Jarrett H., Barnett C.F. Pulmonary Hypertension in HIV. Can. J. Cardiol. 2019;35:288–298. doi: 10.1016/j.cjca.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Rodewald H.R., Feyerabend T.B. Widespread immunological functions of mast cells: Fact or fiction? Immunity. 2012;37:13–24. doi: 10.1016/j.immuni.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Reber L.L., Marichal T., Galli S.J. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012;33:613–625. doi: 10.1016/j.it.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z., Liu S., Xu J., Zhang X., Han D., Liu J., Xia M., Yi L., Shen Q., Xu S., et al. Adult Connective Tissue-Resident Mast Cells Originate from Late Erythro-Myeloid Progenitors. Immunity. 2018;49:640–653e5. doi: 10.1016/j.immuni.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Varricchi G., Rossi F.W., Galdiero M.R., Granata F., Criscuolo G., Spadaro G., de Paulis A., Marone G. Physiological Roles of Mast Cells: Collegium Internationale Allergologicum Update 2019. Int. Arch. Allergy Immunol. 2019;179:247–261. doi: 10.1159/000500088. [DOI] [PubMed] [Google Scholar]
- 18.Borriello F., Granata F., Varricchi G., Genovese A., Triggiani M., Marone G. Immunopharmacological modulation of mast cells. Curr. Opin. Pharm. 2014;17:45–57. doi: 10.1016/j.coph.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Casolaro V., Galeone D., Giacummo A., Sanduzzi A., Melillo G., Marone G. Human basophil/mast cell releasability. V. Functional comparisons of cells obtained from peripheral blood, lung parenchyma, and bronchoalveolar lavage in asthmatics. Am. Rev. Respir. Dis. 1989;139:1375–1382. doi: 10.1164/ajrccm/139.6.1375. [DOI] [PubMed] [Google Scholar]
- 20.Zilionis R., Engblom C., Pfirschke C., Savova V., Zemmour D., Saatcioglu H.D., Krishnan I., Maroni G., Meyerovitz C.V., Kerwin C.M., et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity. 2019;50:1317–1334.e10. doi: 10.1016/j.immuni.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olivera A., Beaven M.A., Metcalfe D.D. Mast cells signal their importance in health and disease. J. Allergy Clin. Immunol. 2018;142:381–393. doi: 10.1016/j.jaci.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 22.Piliponsky A.M., Romani L. The contribution of mast cells to bacterial and fungal infection immunity. Immunol. Rev. 2018;282:188–197. doi: 10.1111/imr.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varricchi G., Raap U., Rivellese F., Marone G., Gibbs B.F. Human mast cells and basophils-How are they similar how are they different? Immunol. Rev. 2018;282:8–34. doi: 10.1111/imr.12627. [DOI] [PubMed] [Google Scholar]
- 24.Mukai K., Tsai M., Saito H., Galli S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018;282:121–150. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galli S.J. The Mast Cell-IgE Paradox: From Homeostasis to Anaphylaxis. Am. J. Pathol. 2016;186:212–224. doi: 10.1016/j.ajpath.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradding P., Arthur G. Mast cells in asthma--state of the art. Clin. Exp. Allergy. 2016;46:194–263. doi: 10.1111/cea.12675. [DOI] [PubMed] [Google Scholar]
- 27.Marone G., Varricchi G., Loffredo S., Galdiero M.R., Rivellese F., de Paulis A. Are Basophils and Mast Cells Masters in HIV Infection? Int. Arch. Allergy Immunol. 2016;171:158–165. doi: 10.1159/000452889. [DOI] [PubMed] [Google Scholar]
- 28.Suurmond J., Rivellese F., Dorjee A.L., Bakker A.M., Rombouts Y.J., Rispens T., Wolbink G., Zaldumbide A., Hoeben R.C., Huizinga T.W., et al. Toll-like receptor triggering augments activation of human mast cells by anti-citrullinated protein antibodies. Ann. Rheum. Dis. 2015;74:1915–1923. doi: 10.1136/annrheumdis-2014-205562. [DOI] [PubMed] [Google Scholar]
- 29.Marshall J.S., Portales-Cervantes L., Leong E. Mast Cell Responses to Viruses and Pathogen Products. Int. J. Mol. Sci. 2019;20:4241. doi: 10.3390/ijms20174241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piliponsky A.M., Acharya M., Shubin N.J. Mast Cells in Viral, Bacterial, and Fungal Infection Immunity. Int. J. Mol. Sci. 2019;20:2851. doi: 10.3390/ijms20122851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voehringer D. Protective and pathological roles of mast cells and basophils. Nat. Rev. Immunol. 2013;13:362–375. doi: 10.1038/nri3427. [DOI] [PubMed] [Google Scholar]
- 32.Mortaz E., Folkerts G., Redegeld F. Mast cells and COPD. Pulm. Pharm. 2011;24:367–372. doi: 10.1016/j.pupt.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Detoraki A., Staiano R.I., Granata F., Giannattasio G., Prevete N., de Paulis A., Ribatti D., Genovese A., Triggiani M., Marone G. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J. Allergy Clin. Immunol. 2009;123:1142–1149. doi: 10.1016/j.jaci.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 34.Varricchi G., Loffredo S., Galdiero M.R., Marone G., Cristinziano L., Granata F. Innate effector cells in angiogenesis and lymphangiogenesis. Curr. Opin. Immunol. 2018;53:152–160. doi: 10.1016/j.coi.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Marone G., Varricchi G., Loffredo S., Granata F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur. J. Pharm. 2016;778:146–151. doi: 10.1016/j.ejphar.2015.03.088. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Majid R.M., Marshall J.S. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J. Immunol. 2004;172:1227–1236. doi: 10.4049/jimmunol.172.2.1227. [DOI] [PubMed] [Google Scholar]
- 37.Theoharides T.C., Zhang B., Kempuraj D., Tagen M., Vasiadi M., Angelidou A., Alysandratos K.D., Kalogeromitros D., Asadi S., Stavrianeas N., et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc. Natl. Acad. Sci. USA. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Detoraki A., Granata F., Staibano S., Rossi F.W., Marone G., Genovese A. Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy. 2010;65:946–958. doi: 10.1111/j.1398-9995.2010.02372.x. [DOI] [PubMed] [Google Scholar]
- 39.Varricchi G., Granata F., Loffredo S., Genovese A., Marone G. Angiogenesis and lymphangiogenesis in inflammatory skin disorders. J. Am. Acad. Derm. 2015;73:144–153. doi: 10.1016/j.jaad.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 40.Rivellese F., Suurmond J., Habets K., Dorjee A.L., Ramamoorthi N., Townsend M.J., de Paulis A., Marone G., Huizinga T.W., Pitzalis C., et al. Ability of Interleukin-33- and Immune Complex-Triggered Activation of Human Mast Cells to Down-Regulate Monocyte-Mediated Immune Responses. Arthritis Rheumatol. 2015;67:2343–2353. doi: 10.1002/art.39192. [DOI] [PubMed] [Google Scholar]
- 41.Rivellese F., Nerviani A., Rossi F.W., Marone G., Matucci-Cerinic M., de Paulis A., Pitzalis C. Mast cells in rheumatoid arthritis: Friends or foes? Autoimmun. Rev. 2017;16:557–563. doi: 10.1016/j.autrev.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Rivellese F., Mauro D., Nerviani A., Pagani S., Fossati-Jimack L., Messemaker T., Kurreeman F.A.S., Toes R.E.M., Ramming A., Rauber S., et al. Mast cells in early rheumatoid arthritis associate with disease severity and support B cell autoantibody production. Ann. Rheum. Dis. 2018;77:1773–1781. doi: 10.1136/annrheumdis-2018-213418. [DOI] [PubMed] [Google Scholar]
- 43.Visciano C., Liotti F., Prevete N., Cali G., Franco R., Collina F., de Paulis A., Marone G., Santoro M., Melillo R.M. Mast cells induce epithelial-to-mesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8-Akt-Slug pathway. Oncogene. 2015;34:5175–5186. doi: 10.1038/onc.2014.441. [DOI] [PubMed] [Google Scholar]
- 44.Galdiero M.R., Varricchi G., Marone G. The immune network in thyroid cancer. Oncoimmunology. 2016;5:e1168556. doi: 10.1080/2162402X.2016.1168556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varricchi G., Galdiero M.R., Loffredo S., Marone G., Iannone R., Granata F. Are Mast Cells MASTers in Cancer? Front. Immunol. 2017;8:424. doi: 10.3389/fimmu.2017.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varricchi G., Galdiero M.R., Marone G., Granata F., Borriello F. Controversial role of mast cells in skin cancers. Exp. Derm. 2017;26:11–17. doi: 10.1111/exd.13107. [DOI] [PubMed] [Google Scholar]
- 47.Jiang A.P., Jiang J.F., Wei J.F., Guo M.G., Qin Y., Guo Q.Q., Ma L., Liu B.C., Wang X., Veazey R.S., et al. Human Mucosal Mast Cells Capture HIV-1 and Mediate Viral trans-Infection of CD4+ T Cells. J. Virol. 2015;90:2928–2937. doi: 10.1128/JVI.03008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bannert N., Farzan M., Friend D.S., Ochi H., Price K.S., Sodroski J., Boyce J.A. Human Mast cell progenitors can be infected by macrophagetropic human immunodeficiency virus type 1 and retain virus with maturation in vitro. J. Virol. 2001;75:10808–10814. doi: 10.1128/JVI.75.22.10808-10814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qi J.C., Stevens R.L., Wadley R., Collins A., Cooley M., Naif H.M., Nasr N., Cunningham A., Katsoulotos G., Wanigasek Y., et al. IL-16 regulation of human mast cells/basophils and their susceptibility to HIV-1. J. Immunol. 2002;168:4127–4134. doi: 10.4049/jimmunol.168.8.4127. [DOI] [PubMed] [Google Scholar]
- 50.Taub D.D., Mikovits J.A., Nilsson G., Schaffer E.M., Key M.L., Petrow-Sadowski C., Ruscetti F.W. Alterations in mast cell function and survival following in vitro infection with human immunodeficiency viruses-1 through CXCR4. Cell Immunol. 2004;230:65–80. doi: 10.1016/j.cellimm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Sundstrom J.B., Little D.M., Villinger F., Ellis J.E., Ansari A.A. Signaling through Toll-like receptors triggers HIV-1 replication in latently infected mast cells. J. Immunol. 2004;172:4391–4401. doi: 10.4049/jimmunol.172.7.4391. [DOI] [PubMed] [Google Scholar]
- 52.Sundstrom J.B., Hair G.A., Ansari A.A., Secor W.E., Gilfillan A.M., Metcalfe D.D., Kirshenbaum A.S. IgE-FcepsilonRI interactions determine HIV coreceptor usage and susceptibility to infection during ontogeny of mast cells. J. Immunol. 2009;182:6401–6409. doi: 10.4049/jimmunol.0801481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sundstrom J.B., Ellis J.E., Hair G.A., Kirshenbaum A.S., Metcalfe D.D., Yi H., Cardona A.C., Lindsay M.K., Ansari A.A. Human tissue mast cells are an inducible reservoir of persistent HIV infection. Blood. 2007;109:5293–5300. doi: 10.1182/blood-2006-11-058438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Granata F., Frattini A., Loffredo S., Staiano R.I., Petraroli A., Ribatti D., Oslund R., Gelb M.H., Lambeau G., Marone G., et al. Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. J. Immunol. 2010;184:5232–5241. doi: 10.4049/jimmunol.0902501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Staiano R.I., Loffredo S., Borriello F., Iannotti F.A., Piscitelli F., Orlando P., Secondo A., Granata F., Lepore M.T., Fiorelli A., et al. Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J. Leukoc Biol. 2016;99:531–540. doi: 10.1189/jlb.3HI1214-584R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Paulis A., Prevete N., Fiorentino I., Rossi F.W., Staibano S., Montuori N., Ragno P., Longobardi A., Liccardo B., Genovese A., et al. Expression and functions of the vascular endothelial growth factors and their receptors in human basophils. J. Immunol. 2006;177:7322–7331. doi: 10.4049/jimmunol.177.10.7322. [DOI] [PubMed] [Google Scholar]
- 57.Loffredo S., Borriello F., Iannone R., Ferrara A.L., Galdiero M.R., Gigantino V., Esposito P., Varricchi G., Lambeau G., Cassatella M.A., et al. Group V Secreted Phospholipase A2 Induces the Release of Proangiogenic and Antiangiogenic Factors by Human Neutrophils. Front. Immunol. 2017;8:443. doi: 10.3389/fimmu.2017.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benyon R.C., Lowman M.A., Church M.K. Human skin mast cells: Their dispersion, purification, and secretory characterization. J. Immunol. 1987;138:861–867. [PubMed] [Google Scholar]
- 59.De Paulis A., Marino I., Ciccarelli A., de Crescenzo G., Concardi M., Verga L., Arbustini E., Marone G. Human synovial mast cells. I. Ultrastructural in situ and in vitro immunologic characterization. Arthritis Rheum. 1996;39:1222–1233. doi: 10.1002/art.1780390723. [DOI] [PubMed] [Google Scholar]
- 60.White J., Herman A., Pullen A.M., Kubo R., Kappler J.W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: Stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989;56:27–35. doi: 10.1016/0092-8674(89)90980-X. [DOI] [PubMed] [Google Scholar]
- 61.Kotzin B.L., Leung D.Y., Kappler J., Marrack P. Superantigens and their potential role in human disease. Adv. Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 62.Marone G., Rossi F.W., Detoraki A., Granata F., Genovese A., Spadaro G. Role of superallergens in allergic disorders. Chem. Immunol. Allergy. 2007;93:195–213. doi: 10.1159/000100896000100896. [DOI] [PubMed] [Google Scholar]
- 63.Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:1066. doi: 10.1126/science.2343314. [DOI] [PubMed] [Google Scholar]
- 64.Bouvet J.P., Pires R., Lunel-Fabiani F., Crescenzo-Chaigne B., Maillard P., Valla D., Opolon P., Pillot J., Protein F. A novel F(ab)-binding factor, present in normal liver, and largely released in the digestive tract during hepatitis. J. Immunol. 1990;145:1176–1180. [PubMed] [Google Scholar]
- 65.Fields B.A., Ober B., Malchiodi E.L., Lebedeva M.I., Braden B.C., Ysern X., Kim J.K., Shao X., Ward E.S., Mariuzza R.A. Crystal structure of the V alpha domain of a T cell antigen receptor. Science. 1995;270:1821–1824. doi: 10.1126/science.270.5243.1821. [DOI] [PubMed] [Google Scholar]
- 66.Li H., Llera A., Tsuchiya D., Leder L., Ysern X., Schlievert P.M., Karjalainen K., Mariuzza R.A. Three-dimensional structure of the complex between a T cell receptor beta chain and the superantigen staphylococcal enterotoxin B. Immunity. 1998;9:807–816. doi: 10.1016/S1074-7613(00)80646-9. [DOI] [PubMed] [Google Scholar]
- 67.Malchiodi E.L., Eisenstein E., Fields B.A., Ohlendorf D.H., Schlievert P.M., Karjalainen K., Mariuzza R.A. Superantigen binding to a T cell receptor beta chain of known three-dimensional structure. J. Exp. Med. 1995;182:1833–1845. doi: 10.1084/jem.182.6.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sundberg E.J., Li H., Llera A.S., McCormick J.K., Tormo J., Schlievert P.M., Karjalainen K., Mariuzza R.A. Structures of two streptococcal superantigens bound to TCR beta chains reveal diversity in the architecture of T cell signaling complexes. Structure. 2002;10:687–699. doi: 10.1016/S0969-2126(02)00759-1. [DOI] [PubMed] [Google Scholar]
- 69.Pascual V., Capra J.D. B-cell superantigens? Curr. Biol. 1991;1:315–317. doi: 10.1016/0960-9822(91)90097-G. [DOI] [PubMed] [Google Scholar]
- 70.Silverman G.J., Goodyear C.S. A model B-cell superantigen and the immunobiology of B lymphocytes. Clin. Immunol. 2002;102:117–134. doi: 10.1006/clim.2001.5143. [DOI] [PubMed] [Google Scholar]
- 71.Zouali M. B-cell superantigens: Implications for selection of the human antibody repertoire. Immunol. Today. 1995;16:399–405. doi: 10.1016/0167-5699(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 72.Florio G., Petraroli A., Patella V., Triggiani M., Marone G. The immunoglobulin superantigen-binding site of HIV-1 gp120 activates human basophils. AIDS. 2000;14:931–938. doi: 10.1097/00002030-200005260-00004. [DOI] [PubMed] [Google Scholar]
- 73.Patella V., Florio G., Petraroli A., Marone G. HIV-1 gp120 induces IL-4 and IL-13 release from human Fc epsilon RI+ cells through interaction with the VH3 region of IgE. J. Immunol. 2000;164:589–595. doi: 10.4049/jimmunol.164.2.589. [DOI] [PubMed] [Google Scholar]
- 74.Karray S., Zouali M. Identification of the B cell superantigen-binding site of HIV-1 gp120. Proc. Natl. Acad. Sci. USA. 1997;94:1356–1360. doi: 10.1073/pnas.94.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silverman G.J. B-cell superantigens. Immunol. Today. 1997;18:379–386. doi: 10.1016/S0167-5699(97)01101-8. [DOI] [PubMed] [Google Scholar]
- 76.Zouali M. B cell superantigens subvert innate functions of B cells. Chem. Immunol. Allergy. 2007;93:92–105. doi: 10.1159/000100860000100860. [DOI] [PubMed] [Google Scholar]
- 77.Berberian L., Goodglick L., Kipps T.J., Braun J. Immunoglobulin VH3 gene products: Natural ligands for HIV gp120. Science. 1993;261:1588–1591. doi: 10.1126/science.7690497. [DOI] [PubMed] [Google Scholar]
- 78.Marone G., Galdiero M.R., Pecoraro A., Pucino V., Criscuolo G., Triassi M., Varricchi G. Prostaglandin D2 receptor antagonists in allergic disorders: Safety, efficacy, and future perspectives. Expert Opin. Investig. Drugs. 2019;28:73–84. doi: 10.1080/13543784.2019.1555237. [DOI] [PubMed] [Google Scholar]
- 79.Levy J.A., Hoffman A.D., Kramer S.M., Landis J.A., Shimabukuro J.M., Oshiro L.S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984;225:840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 80.Sanchez-Pescador R., Power M.D., Barr P.J., Steimer K.S., Stempien M.M., Brown-Shimer S.L., Gee W.W., Renard A., Randolph A., Levy J.A., et al. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2) Science. 1985;227:484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- 81.Marone G., Casolaro V., Paganelli R., Quinti I. IgG anti-IgE from atopic dermatitis induces mediator release from basophils and mast cells. J. Investig. Derm. 1989;93:246–252. doi: 10.1111/1523-1747.ep12277582. [DOI] [PubMed] [Google Scholar]
- 82.Marone G., Spadaro G., Palumbo C., Condorelli G. The anti-IgE/anti-FcepsilonRIalpha autoantibody network in allergic and autoimmune diseases. Clin. Exp. Allergy. 1999;29:17–27. doi: 10.1046/j.1365-2222.1999.00441.x. [DOI] [PubMed] [Google Scholar]
- 83.Patella V., Marino I., Arbustini E., Lamparter-Schummert B., Verga L., Adt M., Marone G. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation. 1998;97:971–978. doi: 10.1161/01.CIR.97.10.971. [DOI] [PubMed] [Google Scholar]
- 84.Patella V., Giuliano A., Bouvet J.P., Marone G. Endogenous superallergen protein Fv induces IL-4 secretion from human Fc epsilon RI+ cells through interaction with the VH3 region of IgE. J. Immunol. 1998;161:5647–5655. [PubMed] [Google Scholar]
- 85.Marone G., Tamburini M., Giudizi M.G., Biagiotti R., Almerigogna F., Romagnani S. Mechanism of activation of human basophils by Staphylococcus aureus Cowan 1. Infect. Immun. 1987;55:803–809. doi: 10.1128/IAI.55.3.803-809.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patella V., de Crescenzo G., Marino I., Genovese A., Adt M., Gleich G.J., Marone G. Eosinophil granule proteins activate human heart mast cells. J. Immunol. 1996;157:1219–1225. [PubMed] [Google Scholar]
- 87.Patella V., Casolaro V., Bjorck L., Marone G. Protein L. A bacterial Ig-binding protein that activates human basophils and mast cells. J. Immunol. 1990;145:3054–3061. [PubMed] [Google Scholar]
- 88.Siraganian R.P. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal. Biochem. 1974;57:383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 89.De Paulis A., Cirillo R., Ciccarelli A., de Crescenzo G., Oriente A., Marone G. Characterization of the anti-inflammatory effect of FK-506 on human mast cells. J. Immunol. 1991;147:4278–4285. [PubMed] [Google Scholar]
- 90.Loffredo S., Ferrara A.L., Bova M., Borriello F., Suffritti C., Veszeli N., Petraroli A., Galdiero M.R., Varricchi G., Granata F., et al. Secreted Phospholipases A2 in Hereditary Angioedema With C1-Inhibitor Deficiency. Front. Immunol. 2018;9:1721. doi: 10.3389/fimmu.2018.01721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Varricchi G., Loffredo S., Borriello F., Pecoraro A., Rivellese F., Genovese A., Spadaro G., Marone G. Superantigenic Activation of Human Cardiac Mast Cells. Int. J. Mol. Sci. 2019;20:1828. doi: 10.3390/ijms20081828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Irani A.A., Schechter N.M., Craig S.S., DeBlois G., Schwartz L.B. Two types of human mast cells that have distinct neutral protease compositions. Proc. Natl. Acad. Sci. USA. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pejler G. The emerging role of mast cell proteases in asthma. Eur. Respir. J. 2019;54 doi: 10.1183/13993003.00685-2019. [DOI] [PubMed] [Google Scholar]
- 94.Schwartz L.B., Irani A.M., Roller K., Castells M.C., Schechter N.M. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J. Immunol. 1987;138:2611–2615. [PubMed] [Google Scholar]
- 95.Patella V., Bouvet J.P., Marone G. Protein Fv produced during vital hepatitis is a novel activator of human basophils and mast cells. J. Immunol. 1993;151:5685–5698. [PubMed] [Google Scholar]
- 96.Patella V., de Crescenzo G., Ciccarelli A., Marino I., Adt M., Marone G. Human heart mast cells: A definitive case of mast cell heterogeneity. Int. Arch. Allergy Immunol. 1995;106:386–393. doi: 10.1159/000236871. [DOI] [PubMed] [Google Scholar]
- 97.Kanaoka Y., Maekawa A., Penrose J.F., Austen K.F., Lam B.K. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J. Biol. Chem. 2001;276:22608–22613. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- 98.Liu T., Kanaoka Y., Barrett N.A., Feng C., Garofalo D., Lai J., Buchheit K., Bhattacharya N., Laidlaw T.M., Katz H.R., et al. Aspirin-Exacerbated Respiratory Disease Involves a Cysteinyl Leukotriene-Driven IL-33-Mediated Mast Cell Activation Pathway. J. Immunol. 2015;195:3537–3545. doi: 10.4049/jimmunol.1500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vigorito C., Giordano A., Cirillo R., Genovese A., Rengo F., Marone G. Metabolic and hemodynamic effects of peptide leukotriene C4 and D4 in man. Int. J. Clin. Lab. Res. 1997;27:178–184. doi: 10.1007/BF02912454. [DOI] [PubMed] [Google Scholar]
- 100.Kanaoka Y., Austen K.F. Roles of cysteinyl leukotrienes and their receptors in immune cell-related functions. Adv. Immunol. 2019;142:65–84. doi: 10.1016/bs.ai.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 101.Varricchi G., de Paulis A., Marone G., Galli S.J. Future Needs in Mast Cell Biology. Int. J. Mol. Sci. 2019;20:4397. doi: 10.3390/ijms20184397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng W., Aspelund A., Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J. Clin. Investig. 2014;124:878–887. doi: 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taimeh Z., Loughran J., Birks E.J., Bolli R. Vascular endothelial growth factor in heart failure. Nat. Rev. Cardiol. 2013;10:519–530. doi: 10.1038/nrcardio.2013.94. [DOI] [PubMed] [Google Scholar]
- 104.Albini A., Bruno A., Noonan D.M., Mortara L. Contribution to Tumor Angiogenesis From Innate Immune Cells Within the Tumor Microenvironment: Implications for Immunotherapy. Front. Immunol. 2018;9:527. doi: 10.3389/fimmu.2018.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varricchi G., Harker J., Borriello F., Marone G., Durham S.R., Shamji M.H. T follicular helper (Tfh ) cells in normal immune responses and in allergic disorders. Allergy. 2016;71:1086–1094. doi: 10.1111/all.12878. [DOI] [PubMed] [Google Scholar]
- 106.Lucey D.R., Zajac R.A., Melcher G.P., Butzin C.A., Boswell R.N. Serum IgE levels in 622 persons with human immunodeficiency virus infection: IgE elevation with marked depletion of CD4+ T-cells. Aids Res. Hum. Retrovir. 1990;6:427–429. doi: 10.1089/aid.1990.6.427. [DOI] [PubMed] [Google Scholar]
- 107.Paganelli R., Scala E., Mezzaroma I., Pinter E., D’Offizi G., Fanales-Belasio E., Rosso R.M., Ansotegui I.J., Pandolfi F., Aiuti F. Immunologic aspects of hyperimmunoglobulinemia E-like syndrome in patients with AIDS. J. Allergy Clin. Immunol. 1995;95:995–1003. doi: 10.1016/S0091-6749(95)70100-1. [DOI] [PubMed] [Google Scholar]
- 108.Rancinan C., Morlat P., Chene G., Guez S., Baquey A., Beylot J., Salamon R. IgE serum level: A prognostic marker for AIDS in HIV-infected adults? J. Allergy Clin. Immunol. 1998;102:329–330. doi: 10.1016/S0091-6749(98)70107-1. [DOI] [PubMed] [Google Scholar]
- 109.Secord E.A., Kleiner G.I., Auci D.L., Smith-Norowitz T., Chice S., Finkielstein A., Nowakowski M., Fikrig S., Durkin H.G. IgE against HIV proteins in clinically healthy children with HIV disease. J. Allergy Clin. Immunol. 1996;98:979–984. doi: 10.1016/S0091-6749(96)80015-7. [DOI] [PubMed] [Google Scholar]
- 110.Shor-Posner G., Miguez-Burbano M.J., Lu Y., Feaster D., Fletcher M., Sauberlich H., Baum M.K. Elevated IgE level in relationship to nutritional status and immune parameters in early human immunodeficiency virus-1 disease. J. Allergy Clin. Immunol. 1995;95:886–892. doi: 10.1016/S0091-6749(95)70133-8. [DOI] [PubMed] [Google Scholar]
- 111.Vigano A., Principi N., Crupi L., Onorato J., Vincenzo Z.G., Salvaggio A. Elevation of IgE in HIV-infected children and its correlation with the progression of disease. J. Allergy Clin. Immunol. 1995;95:627–632. doi: 10.1016/S0091-6749(95)70326-8. [DOI] [PubMed] [Google Scholar]
- 112.Maun H.R., Jackman J.K., Choy D.F., Loyet K.M., Staton T.L., Jia G., Dressen A., Hackney J.A., Bremer M., Walters B.T., et al. An Allosteric Anti-tryptase Antibody for the Treatment of Mast Cell-Mediated Severe Asthma. Cell. 2019;179:417–431.e419. doi: 10.1016/j.cell.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wernersson S., Pejler G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014;14:478–494. doi: 10.1038/nri3690. [DOI] [PubMed] [Google Scholar]
- 114.Komi D.E.A., Redegeld F.A. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin. Rev. Allergy Immunol. 2019 doi: 10.1007/s12016-019-08753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salamon P., Mekori Y.A., Shefler I. Lung cancer-derived extracellular vesicles: A possible mediator of mast cell activation in the tumor microenvironment. Cancer Immunol. Immunother. 2020;69:373–381. doi: 10.1007/s00262-019-02459-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Welsh T.J., Green R.H., Richardson D., Waller D.A., O’Byrne K.J., Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J. Clin. Oncol. 2005;23:8959–8967. doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]
- 117.Presti R.M., Flores S.C., Palmer B.E., Atkinson J.J., Lesko C.R., Lau B., Fontenot A.P., Roman J., McDyer J.F., Twigg H.L., 3rd Mechanisms Underlying HIV-Associated Noninfectious Lung Disease. Chest. 2017;152:1053–1060. doi: 10.1016/j.chest.2017.04.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Silverberg M.J., Lau B., Achenbach C.J., Jing Y., Althoff K.N., D’Souza G., Engels E.A., Hessol N.A., Brooks J.T., Burchell A.N., et al. Cumulative Incidence of Cancer Among Persons With HIV in North America: A Cohort Study. Ann. Intern. Med. 2015;163:507–518. doi: 10.7326/M14-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gingo M.R., Wenzel S.E., Steele C., Kessinger C.J., Lucht L., Lawther T., Busch M., Hillenbrand M.E., Weinman R., Slivka W.A., et al. Asthma diagnosis and airway bronchodilator response in HIV-infected patients. J. Allergy Clin. Immunol. 2012;129:708–714.e708. doi: 10.1016/j.jaci.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fauci A.S., Pantaleo G., Stanley S., Weissman D. Immunopathogenic mechanisms of HIV infection. Ann. Intern. Med. 1996;124:654–663. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 121.Sugiyama K. Histamine release from rat mast cells induced by Sendai virus. Nature. 1977;270:614–615. doi: 10.1038/270614a0. [DOI] [PubMed] [Google Scholar]
- 122.St John A.L., Rathore A.P., Yap H., Ng M.L., Metcalfe D.D., Vasudevan S.G., Abraham S.N. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc. Natl. Acad. Sci. USA. 2011;108:9190–9195. doi: 10.1073/pnas.1105079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brown M.G., McAlpine S.M., Huang Y.Y., Haidl I.D., Al-Afif A., Marshall J.S., Anderson R. RNA sensors enable human mast cell anti-viral chemokine production and IFN-mediated protection in response to antibody-enhanced dengue virus infection. PLoS ONE. 2012;7:e34055. doi: 10.1371/journal.pone.0034055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aoki R., Kawamura T., Goshima F., Ogawa Y., Nakae S., Nakao A., Moriishi K., Nishiyama Y., Shimada S. Mast cells play a key role in host defense against herpes simplex virus infection through TNF-alpha and IL-6 production. J. Investig. Derm. 2013;133:2170–2179. doi: 10.1038/jid.2013.150. [DOI] [PubMed] [Google Scholar]
- 125.Graham A.C., Temple R.M., Obar J.J. Mast cells and influenza a virus: Association with allergic responses and beyond. Front. Immunol. 2015;6:238. doi: 10.3389/fimmu.2015.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guhl S., Franke R., Schielke A., Johne R., Kruger D.H., Babina M., Rang A. Infection of in vivo differentiated human mast cells with hantaviruses. J. Gen. Virol. 2010;91:1256–1261. doi: 10.1099/vir.0.019505-0. [DOI] [PubMed] [Google Scholar]
- 127.Ebert S., Becker M., Lemmermann N.A., Buttner J.K., Michel A., Taube C., Podlech J., Bohm V., Freitag K., Thomas D., et al. Mast cells expedite control of pulmonary murine cytomegalovirus infection by enhancing the recruitment of protective CD8 T cells to the lungs. PLoS Pathog. 2014;10:e1004100. doi: 10.1371/journal.ppat.1004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Becker M., Lemmermann N.A., Ebert S., Baars P., Renzaho A., Podlech J., Stassen M., Reddehase M.J. Mast cells as rapid innate sensors of cytomegalovirus by TLR3/TRIF signaling-dependent and-independent mechanisms. Cell. Mol. Immunol. 2015;12:192–201. doi: 10.1038/cmi.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marone G., Granata F. Angiogenesis, lymphangiogenesis and clinical implications. Preface. Chem. Immunol. Allergy. 2014;99:XI–XII. doi: 10.1159/000352074000352074. [DOI] [PubMed] [Google Scholar]
- 130.Tatari N., Movassagh H., Shan L., Koussih L., Gounni A.S. Semaphorin 3E Inhibits House Dust Mite-Induced Angiogenesis in a Mouse Model of Allergic Asthma. Am. J. Pathol. 2019;189:762–772. doi: 10.1016/j.ajpath.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 131.Chatterjee S., Heukamp L.C., Siobal M., Schottle J., Wieczorek C., Peifer M., Frasca D., Koker M., Konig K., Meder L., et al. Tumor VEGF:VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. J. Clin. Investig. 2013;123:1732–1740. doi: 10.1172/JCI65385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bosisio D., Ronca R., Salvi V., Presta M., Sozzani S. Dendritic cells in inflammatory angiogenesis and lymphangiogenesis. Curr. Opin. Immunol. 2018;53:180–186. doi: 10.1016/j.coi.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 133.Longo V., Tamma R., Brunetti O., Pisconti S., Argentiero A., Silvestris N., Ribatti D. Mast cells and angiogenesis in pancreatic ductal adenocarcinoma. Clin. Exp. Med. 2018;18:319–323. doi: 10.1007/s10238-018-0493-6. [DOI] [PubMed] [Google Scholar]
- 134.Wilson A.M., Shao Z., Grenier V., Mawambo G., Daudelin J.F., Dejda A., Pilon F., Popovic N., Boulet S., Parinot C., et al. Neuropilin-1 expression in adipose tissue macrophages protects against obesity and metabolic syndrome. Sci. Immunol. 2018;3 doi: 10.1126/sciimmunol.aan4626. [DOI] [PubMed] [Google Scholar]
- 135.Stump B., Cui Y., Kidambi P., Lamattina A.M., El-Chemaly S. Lymphatic Changes in Respiratory Diseases: More than Just Remodeling of the Lung? Am. J. Respir. Cell Mol. Biol. 2017;57:272–279. doi: 10.1165/rcmb.2016-0290TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baluk P., Yao L.C., Feng J., Romano T., Jung S.S., Schreiter J.L., Yan L., Shealy D.J., McDonald D.M. TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J. Clin. Investig. 2009;119:2954–2964. doi: 10.1172/JCI3762637626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yao L.C., Baluk P., Feng J., McDonald D.M. Steroid-resistant lymphatic remodeling in chronically inflamed mouse airways. Am. J. Pathol. 2010;176:1525–1541. doi: 10.2353/ajpath.2010.090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hardavella G., Tzortzaki E.G., Siozopoulou V., Galanis P., Vlachaki E., Avgousti M., Stefanou D., Siafakas N.M. Lymphangiogenesis in COPD: Another link in the pathogenesis of the disease. Respir. Med. 2012;106:687–693. doi: 10.1016/j.rmed.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 139.Mori M., Andersson C.K., Graham G.J., Lofdahl C.G., Erjefalt J.S. Increased number and altered phenotype of lymphatic vessels in peripheral lung compartments of patients with COPD. Respir. Res. 2013;14:65. doi: 10.1186/1465-9921-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aspelund A., Robciuc M.R., Karaman S., Makinen T., Alitalo K. Lymphatic System in Cardiovascular Medicine. Circ. Res. 2016;118:515–530. doi: 10.1161/CIRCRESAHA.115.306544. [DOI] [PubMed] [Google Scholar]
- 141.Brakenhielm E., Alitalo K. Cardiac lymphatics in health and disease. Nat. Rev. Cardiol. 2019;16:56–68. doi: 10.1038/s41569-018-0087-8. [DOI] [PubMed] [Google Scholar]
- 142.Kim H., Kataru R.P., Koh G.Y. Inflammation-associated lymphangiogenesis: A double-edged sword? J. Clin. Investig. 2014;124:936–942. doi: 10.1172/JCI71607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gelderblom H.R., Hausmann E.H., Ozel M., Pauli G., Koch M.A. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987;156:171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 144.Griffin M., Weiss J.W., Leitch A.G., McFadden E.R., Jr., Corey E.J., Austen K.F., Drazen J.M. Effects of leukotriene D on the airways in asthma. N. Engl. J. Med. 1983;308:436–439. doi: 10.1056/NEJM198302243080807. [DOI] [PubMed] [Google Scholar]
- 145.Dahlen S.E., Bjork J., Hedqvist P., Arfors K.E., Hammarstrom S., Lindgren J.A., Samuelsson B. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: In vivo effects with relevance to the acute inflammatory response. Proc. Natl. Acad. Sci. USA. 1981;78:3887–3891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mesquita-Santos F.P., Vieira-de-Abreu A., Calheiros A.S., Figueiredo I.H., Castro-Faria-Neto H.C., Weller P.F., Bozza P.T., Diaz B.L., Bandeira-Melo C. Cutting edge: Prostaglandin D2 enhances leukotriene C4 synthesis by eosinophils during allergic inflammation: Synergistic in vivo role of endogenous eotaxin. J. Immunol. 2006;176:1326–1330. doi: 10.4049/jimmunol.176.3.1326. [DOI] [PubMed] [Google Scholar]
- 147.Fuller R.W., Dixon C.M., Dollery C.T., Barnes P.J. Prostaglandin D2 potentiates airway responsiveness to histamine and methacholine. Am. Rev. Respir. Dis. 1986;133:252–254. doi: 10.1164/arrd.1986.133.2.252. [DOI] [PubMed] [Google Scholar]
- 148.Hardy C.C., Bradding P., Robinson C., Holgate S.T. Bronchoconstrictor and antibronchoconstrictor properties of inhaled prostacyclin in asthma. J. Appl. Physiol. (1985) 1988;64:1567–1574. doi: 10.1152/jappl.1988.64.4.1567. [DOI] [PubMed] [Google Scholar]
- 149.Peters S.P., Kagey-Sobotka A., MacGlashan D.W., Jr., Lichtenstein L.M. Effect of prostaglandin D2 in modulating histamine release from human basophils. J. Pharm. Exp. 1984;228:400–406. [PubMed] [Google Scholar]
- 150.Triggiani M., Gentile M., Secondo A., Granata F., Oriente A., Taglialatela M., Annunziato L., Marone G. Histamine induces exocytosis and IL-6 production from human lung macrophages through interaction with H1 receptors. J. Immunol. 2001;166:4083–4091. doi: 10.4049/jimmunol.166.6.4083. [DOI] [PubMed] [Google Scholar]
- 151.Burtin C., Blanche S., Galoppin L., Merval R., Griscelli C., Scheinmann P. Blood histamine levels in HIV-1-infected infants and children. Int. Arch. Allergy Appl. Immunol. 1990;91:142–144. doi: 10.1159/000235105. [DOI] [PubMed] [Google Scholar]