Abstract
Mast cell (MC) is an important player in the development of skin diseases, including atopic dermatitis, psoriasis, and urticaria. It is reported that MC infiltration and activation are observed around various types of tumors and speculated that MCs play key roles in their pathogenesis. As MCs in human seborrheic keratosis (SK) have not been well investigated, here we focused on the MCs in SK. The number of c-Kit and tryptase-positive MCs was significantly increased around the SK compared with the marginal lesion. Degranulated MCs were also increased around the tumors. Furthermore, MC growth factor, stem cell factor (SCF), expression within the SK was significantly upregulated compared with the marginal lesion. Interestingly, one of the cognitive regulators of SCF expression, cannabinoid receptor type 1 (CB1) immunoreactivity was downregulated within the SK. Our results suggest that MCs play important roles in the pathogenesis of SK and that SCF can be also deeply involved in the development of SKs. Our current results highlight the CB1–SCF–MC interaction as a novel mechanism of SK development and this also will be utilized for developing a novel treatment.
Keywords: CB1, endocannabinoid, mast cell, seborrheic keratosis, stem cell factor
Introduction
Skin is the largest organ in our body and often causes various kinds of dermatoses including skin tumors. Even if the vast majority of skin tumors are benign,1 some of them (e.g., malignant melanoma and squamous cell carcinoma) are malignant and need to be treated because those may metastasize and be life-threatening. Management of benign skin tumors is usually for cosmetic reasons, and many patients undergo excision.1 Although tumor excision is one of the most effective treatments, surgery by itself might cause aesthetic problems, including color change, or hypertrophic or atrophic scars. Therefore, new options for treatment except surgery have been expected. Thus, it is important to investigate the pathogenetic mechanism in the development of these tumors.
Seborrheic keratosis (SK) is a benign skin tumor that arises from the skin epidermis. Although the detailed mechanism of the development of SK has not been fully investigated, UV irradiation, aging, and somatic mutations in FGFR3 and in a few other genes expressed in human keratinocytes may be involved.2,3 Saeed recently reported that the number of mast cells (MCs) is significantly increased around SK in a mouse model compared with control mice and suggested that MCs could play a role in SK development.4
MCs play important roles not only in the immune system, including host defense,5 but also in wound healing,6 fibrosis,7 angiogenesis,8 and hair growth.9 In addition, MCs are closely related to various types of cutaneous neoplasms, including basal cell carcinoma, squamous cell carcinoma, intradermal nevus, malignant melanoma, hemangioma, Kaposi’s sarcoma, angiosarcoma, dermatofibroma, atypical fibroxanthoma, dermatofibrosarcoma protuberans, and Merkel cell carcinoma.10–14
MCs secrete various types of chemical mediators, growth factors, and cytokines,6,15,16 and these mediators may be involved in the development of cutaneous neoplasms. The effects of MC mediators on the development of cutaneous neoplasms are likely to be mediated through multiple pathways, including immunosuppression, enhanced angiogenesis, disruption of the extracellular matrix, and promotion of tumor cell mitosis.11 Surgical dissection is still selected as a first-line therapy, but chemotherapy, radiation, or biologics could be added depending on the stage and type of tumor. However, these treatments have several side effects, including rash, pancytopenia, and allergic reactions, and more effective and easily manageable therapies are needed. Considering that MCs are deeply involved in the development of these tumors, MC-related compounds could be potential new candidates. Therefore, it is important to determine the mechanism by which MCs play a role in the pathology of these neoplasms.
We have previously shown that cannabinoid receptor type 1 (CB1) signaling regulates excessive MC degranulation and maturation via interactions on MCs, as well as inducing stem cell factor (SCF) production by cells within the epithelium.17 Controlling CB1 signaling could be a new option for inhibiting excessive MC activity.17 Although MCs have been suggested to play important roles in the development of skin tumors,10–14 the role of MCs in human SK has not been reported.
Interestingly, SK is regarded as asymptomatic, but patients with SK often have itching in clinical practice.18 Histamines are also secreted from MCs in response to various types of stimulants, including IgE-FcεRI crosslinking,19 and induce itching via histamine receptors.20 This may also suggest the involvement of MCs in SK pathology. Here, we focused on MC biology (number, degranulation) and investigated MCs around SK.
Materials and Methods
Samples were obtained from nine SK patients (average age, 68.2 years; range, 51–80 years; three males and six females; Table 1) who visited Osaka City University Hospital (Department of Dermatology). Human tissue collection and handling was performed according to the guidelines of the Declaration of Helsinki with approval from the institutional research ethics committee (Osaka City University), and written informed consent was obtained from all patients. The samples were fixed in buffered formalin and embedded in paraffin. Sections (4 μm) were cut from the paraffin blocks. For toluidine blue histochemistry, deparaffinized sections were stained with 1% toluidine blue (pH 8.9; Merck, Darmstadt, Germany) for 3 min at room temperature (RT).21
Table 1.
Summary of the Characteristics of Each Patient.
| No. | Age (Years) | Sex | Tumor Location | Diameter (mm) | Histological Type | MC Numbera | SCF | CB1 |
|---|---|---|---|---|---|---|---|---|
| 1 | 69 | Female | Thigh | 20 × 15 | Hyperkeratotic | NS | ↑ | ↓ |
| 2 | 80 | Female | Chest | 6 × 9 | Acanthotic | ↑ | ↑ | ↓ |
| 3 | 77 | Female | Lower leg | 5 × 5 | Acanthotic | ↑ | NS | ↓ |
| 4 | 51 | Male | Abdomen | 4 × 3 | Acanthotic | ↑ | ↑ | ↓ |
| 5 | 60 | Female | Lower back | 15 × 15 | Acanthotic | ↑ | ↑ | ↓ |
| 6 | 68 | Female | Lower leg | 8 × 5 | Hyperkeratotic | ↑ | ↑ | ↓ |
| 7 | 73 | Male | Back | 8 × 10 | Acanthotic | ↑ | ↑ | ↓ |
| 8 | 66 | Female | Back | 9 × 8 | Acanthotic | ↑ | ↑ | ↓ |
| 9 | 70 | Male | Face | 9 × 9 | Acanthotic | ↑ | NS | ↓ |
Abbreviations: MC, mast cell; SCF, stem cell factor; CB1, cannabinoid receptor type1; NS, not significant.
MC number counted by toluidine blue histochemistry and tryptase immunohistochemistry.
Reagents
Antibodies, including mouse anti-tryptase antibody, rabbit anti-SCF antibody, rabbit anti-nerve growth factor (NGF) antibody, rabbit anti-chymase antibody, and rabbit anti-Ki-67 antibody were purchased from Abcam (Cambridge, UK). Rabbit anti-CB1 antibody was purchased from Cayman Chemical (Ann Arbor, MI) and rabbit anti-c-Kit (CD117) antibody from Cell Marque (Rocklin, CA).
Immunohistochemistry and Immunofluorescence Microscopy
The sections from each skin sample were used for tryptase, chymase, c-Kit, SCF, CB1, and NGF immunohistochemistry and tryptase/terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) or tryptase/Ki-67 double immunofluorescence. All deparaffinized sections underwent antigen retrieval using an autoclave at 121C for 15 min. After preincubation with 0.5% Triton-X in phosphate-buffered saline (PBS) containing 6% bovine serum albumin at RT for 30 min, sections were incubated overnight at 4C with primary antibodies diluted in PBS: mouse anti-human tryptase (Abcam) at 1:500, rabbit anti-human c-Kit (CD117; Cell Marque) at 1:200, goat anti-human chymase at 1:500, rabbit anti-human SCF (Abcam) at 1:50, rabbit anti-human NGF (Abcam) at 1:50, and rabbit anti-CB1 (Cayman Chemical) at 1:50.
Next, the sections were incubated with goat biotinylated antibodies against rabbit or mouse IgG (Jackson ImmunoResearch Laboratories; West Grove, PA) at 1:200 for 45 min at RT. The sections were then treated with alkaline phosphatase–based avidin–biotin complex (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA), and the expression of these antigens was visualized with Fast Red (Sigma-Aldrich; St. Louis, MO) or Vector Black (Vector). For tryptase and Ki-67 double immunofluorescence, after antigen retrieval, the sections were incubated overnight at 4C with primary antibodies diluted in PBS: rabbit anti-human tryptase (Abcam) at 1:500 and rabbit anti-human Ki-67 antigen (Abcam) at 1:20. This was followed by incubation with goat anti-rabbit IgG Alexa Fluor 594 (Thermo Fisher Scientific, Waltham, MA) for tryptase and Alexa 488 (Thermo Fisher Scientific) for Ki-67 diluted 1:200 in PBS for 45 min at RT. For tryptase and TUNEL double immunofluorescence, after immunostaining for tryptase, the sections were immersed in 1× terminal deoxynucleotidyl transferase (TdT) labeling buffer from the TACS 2 TdT-Flour In Situ Apoptosis Detection Kit (Trevigen; Gaithersburg, MD) for 5 min. Next, the samples were incubated with labeling reaction mix in the presence of TdT (60 min at 37C). After incubation with stop buffer (5 min at RT) and an additional wash with PBS, TUNEL-positive cells were visualized by Step-Fluorescein (20 min at RT). Nuclear labeling was performed using 4′,6-diamidino-2-phenylindole (DAPI) (Thermo Fisher Scientific).
The expression of SCF, NGF, and CB1 within the epidermis of the tumor and marginal lesion was quantified by assessing immunoreactivity in defined reference areas and by quantitative immunohistomorphometry using ImageJ software (National Institutes of Health; Bethesda, MD).
For toluidine blue histochemistry and tryptase, c-Kit, and chymase immunohistochemistry, MCs were counted in the upper dermis of both SK and the marginal lesion. We defined the “marginal lesion” as margin part of the samples showing no obvious epidermis changes, including hyperkeratosis, papillomatosis, and acanthosis which are characteristic pathological features of SK.
The results were expressed as the average number of positive cells per 1 mm2.
MCs were classified as “degranulated” when five or more extracellularly located tryptase+ granules were detected by light microscopy (visual field) around a cell at high magnification (200×–400×).17,22,23
Statistical Analysis
Data were analyzed by the Mann–Whitney U-test for unpaired samples using GraphPad Prism 8.0 (GraphPad Software; San Diego, CA). p<0.05 was considered significant. All data were expressed as mean ± SEM (standard error of the mean).
Results
The Number of MCs Increased Around the Tumor
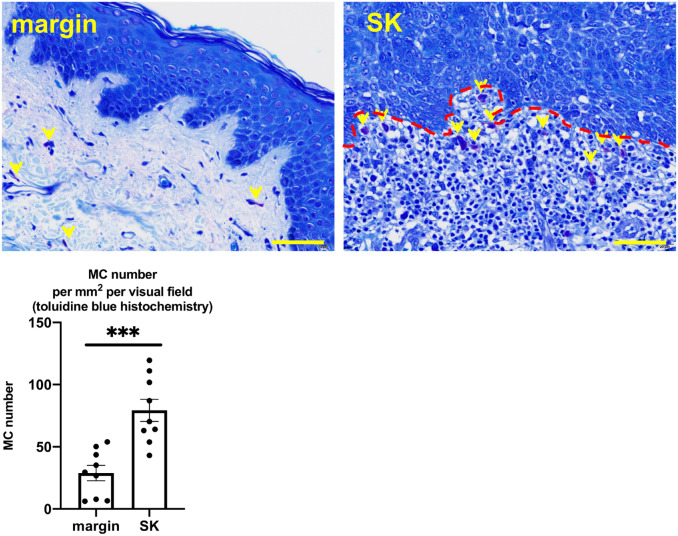
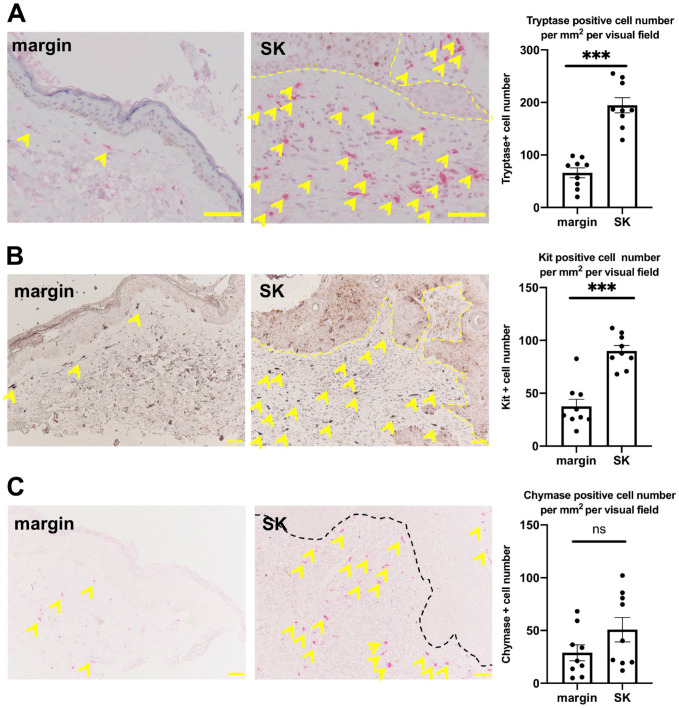
A significantly increased number of MCs has been demonstrated around SK in a mouse model of SK.4 We asked whether this could be also observed in human SKs. In fact, we found a significantly greater number of MCs around SK than the marginal lesion by toluidine blue histochemistry (Fig. 1 and Supplemental Fig. 1). To evaluate the number of MCs more specifically, we performed immunohistochemical staining for detecting different types of MCs in their differentiation stages,17 namely tryptase-, c-Kit-, and chymase-positive MCs. The number of both c-Kit and tryptase-positive MCs was significantly increased around the tumor compared with its marginal lesion (Fig. 2A and B, and Supplemental Figs. 2 and 3). The number of chymase-positive MCs was also increased, but the difference was not significant (Fig. 2C and Supplemental Fig. 4).
Figure 1.
Toluidine blue histochemistry. The mean number of MCs (yellow arrows) significantly increased around SK compared with its marginal lesion. Error bars indicate SEM, n=9. *p<0.05, **p<0.01, ***p<0.001. Scale bar = 50 µm. Abbreviations: MCs, mast cells; SK, seborrheic keratosis; SEM, standard error of the mean.
Figure 2.
(A) Mean number of tryptase-positive MCs (yellow arrows). (B). Mean number of c-Kit-positive MCs (yellow arrows). Both significantly increased around SK compared with its marginal lesion. (C). The mean number of chymase-positive MCs (yellow arrows) had a tendency to increase around SK compared with its marginal lesion. Error bars indicate SEM, n=9. *p<0.05, **p<0.01, ***p<0.001. Scale bar = 50 µm. Abbreviations: MCs, Mast cell; SK, seborrheic keratosis; SEM, standard error of the mean.
MC Degranulation Increased Around the Tumor
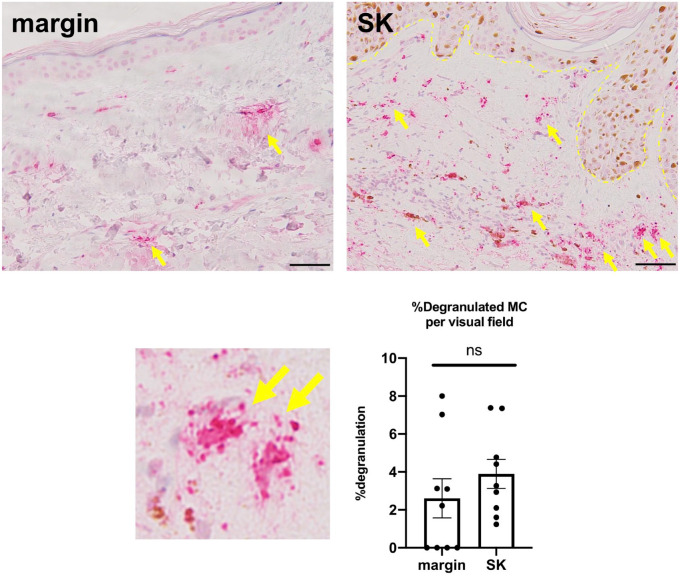
Although the number of MCs has been reported to increase around SK in a mouse model of SK,4 MC degranulation around SK has not been well investigated. Therefore, we next evaluated the percentage of degranulated MCs around the SK compared with its marginal lesion using tryptase immunohistochemistry. As a result, although MC degranulation seemed to be increased around SK, no significant differences were found between the SK and marginal lesion (Fig. 3 and Supplemental Fig. 5).
Figure 3.
Tryptase immunohistochemistry. The mean proportion of degranulated MCs (yellow arrow) had a tendency to increase around SK compared with its marginal lesion. Error bars indicate SEM, n=9. ns, not significant. Scale bar = 50 µm. Abbreviations: MCs, mast cells; SK, seborrheic keratosis; SEM, standard error of the mean.
MC Proliferation and Apoptosis
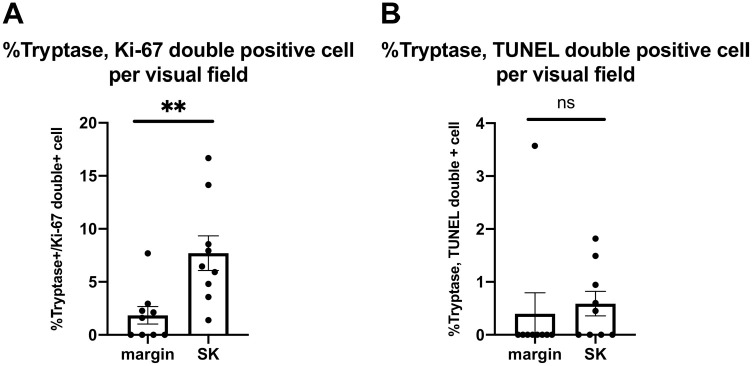
As the number of MCs significantly increased around the tumor (Figs. 1 and 2), we next investigated the proliferation and apoptosis of MCs using tryptase/Ki-67 and tryptase/TUNEL double immunofluorescence. The number of tryptase and Ki-67 double-positive MCs significantly increased around the SK compared with its marginal lesion. In contrast, the number of tryptase and TUNEL double-positive MCs was not affected (Fig. 4). This suggests that the proliferation of MCs is stimulated around the tumor; therefore, the number of MCs increased (Fig. 1).
Figure 4.
Mean proportion of double-positive cells as determined by Ki-67 or TUNEL and tryptase double immunofluorescence. (A). The proportion of tryptase and Ki-67 double-positive cells increased around the tumor compared with its marginal lesion. (B). No significant difference was found in the proportion of tryptase and TUNEL double-positive cells around the tumor compared with the marginal lesion. Data are presented as mean ± SEM, n=9. *p<0.05, **p<0.01, ***p<0.001; ns, not significant. Abbreviations: TUNEL, terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling; SK, seborrheic keratosis; SEM, standard error of the mean.
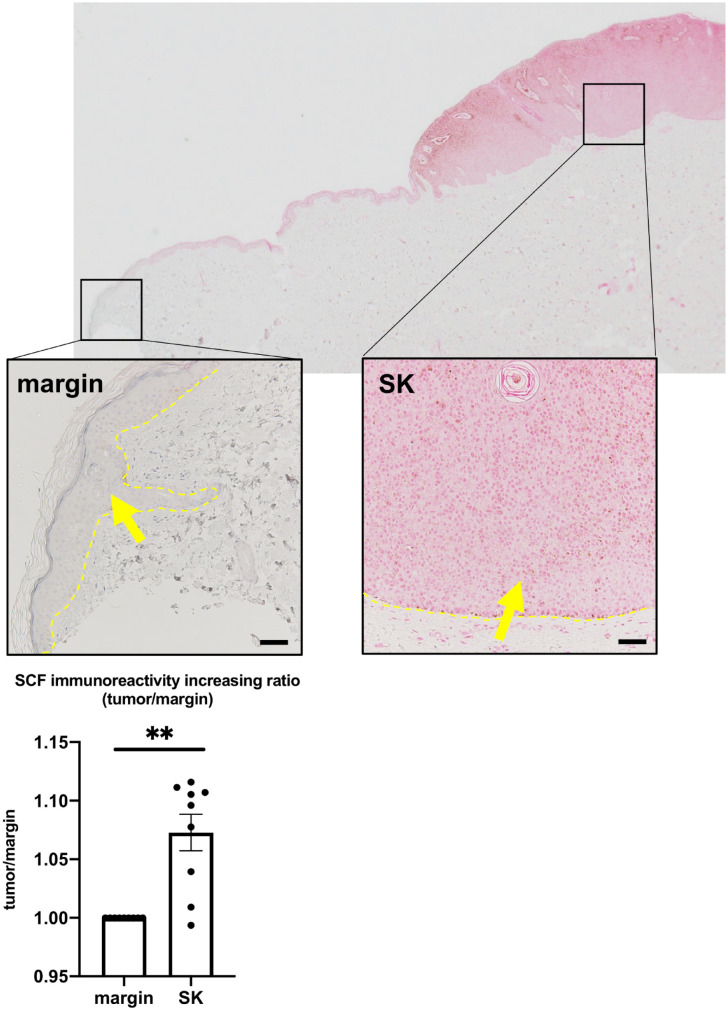
SCF Expression Is Upregulated in SK
The number of MCs in different stages of their differentiation (i.e., c-Kit, tryptase, and chymase) was increased (Figs. 1 and 2). In addition, MC proliferation was also stimulated around SK (Fig. 4). We next asked whether the MC growth factor was affected in SK. As SCF is one of the key growth factors for MCs,24 we investigated the expression of SCF within SK using immunohistochemistry. SCF immunoreactivity was significantly increased within SK compared with its marginal lesion (Fig. 5 and Supplemental Fig. 6). We also investigated the expression of NGF, another MC growth factor,15 but found no significant differences in NGF immunoreactivity between the SK and its marginal lesion (Supplemental Fig. 7).
Figure 5.
Mean SCF expression in the epidermis significantly increased in the tumor compared with its marginal lesion. Error bars indicate SEM, n=9. *p<0.05, **p<0.01, ***p<0.001. Scale bar = 50 µm. Abbreviations: SCF, stem cell factor; SK, seborrheic keratosis; SEM, standard error of the mean.
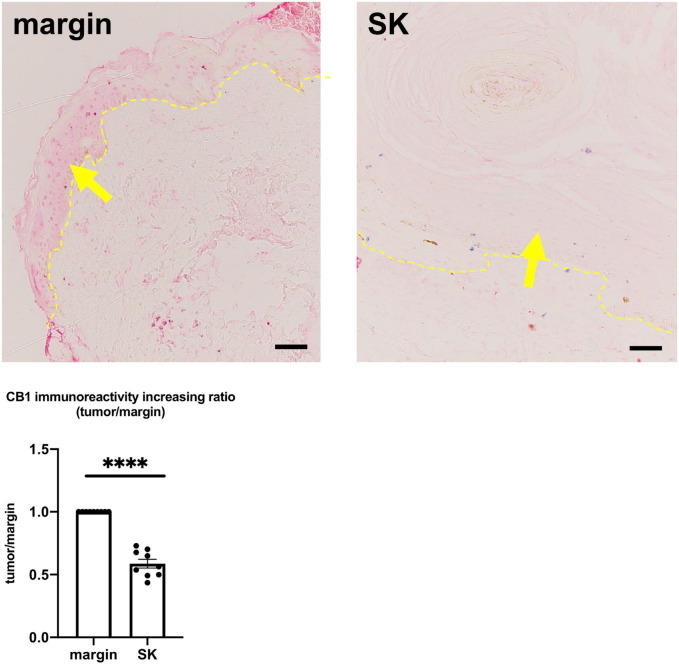
CB1 Expression Is Downregulated in SK
Our results indicate that MC proliferation and differentiation are stimulated around SK, probably due to the upregulation of SCF. This suggests that SCF and MCs play important roles in the development of SK. We have previously reported that CB1 signaling regulates excessive MC degranulation and differentiation in a human hair follicle (HF) organ culture system.17 The inhibitory effect of CB1 signaling on MCs is associated at least in part via controlling the expression of SCF with the epithelium of HF.17 Next, we investigated whether CB1 is involved in the pathomechanism of SK by performing CB1 immunohistochemistry. As a result, CB1 immunoreactivity was significantly downregulated within the SK compared with its marginal lesion (Fig. 6 and Supplemental Fig. 8).
Figure 6.
Mean CB1 immunoreactivity in the epidermis was significantly downregulated in the tumor compared with its marginal lesion. Error bars indicate SEM, n=9. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Scale bar = 50 µm. Abbreviations: CB1, cannabinoid receptor type1; SK, seborrheic keratosis; SEM, standard error of the mean.
Discussion
We showed that the number of MCs is significantly increased around SK compared with its marginal lesion, the proliferation of MCs is significantly stimulated around the SK, SCF expression in the epidermis is upregulated in SK compared with its marginal lesion, and CB1 expression is downregulated in the epidermis in SK. MCs play pivotal roles in the development of various types of diseases. For example, MCs are crucial cells not only in allergic responses, atopic dermatitis, allergic rhinitis, and bronchial asthma but also in other non-allergic diseases, including psoriasis,25 wound healing,7 ulcerative colitis,26 and alopecia areata.23 Furthermore, MCs are involved in the pathogenesis of different kinds of neoplasms.10–14 MCs promote the angiogenesis of neoplasms by secreting vascular growth factors, including vascular endothelial growth factor (VEGF).11 MCs also contribute to remodeling the surrounding stroma around the tumor via matrix metalloproteinase (MMP) secretion.27 However, to the best of our knowledge, the role of MCs in the pathogenesis of SK has not been reported. Our results of a significant increase in the number of MCs around SK compared with its marginal lesion support the involvement of MCs in the pathogenesis of SK. We also observed that MC degranulation seemed to be activated around the SK; however, there was no significant difference (Fig. 3). In this study, we evaluated MC degranulation around the SK by comparing with that of marginal lesion. However, some skin samples had only smaller marginal area. MC degranulation may be significantly increased if we could obtain additional skin samples of SK with enough volume of marginal area and further evaluate MC degranulation.
MCs are crucial cells for inducing itch by releasing several itch mediators including histamines during degranulation.20 As some SK patients have itch around the SK,28 it would be interesting to perform further additional study about the relationship between SK patients with/without itch and MC degranulation around the SK. The finding of significantly increased number of MCs (Figs. 1 and 2) is in line with Saeed and Salmo’s4 observation obtained from skin samples of an ultraviolet B (UVB)-irradiated SK mouse model. However, the mechanism underlying activated MCs around SK has not been investigated. Here, we focused on key MC growth factors, SCF and NGF.15 Although we found no significant difference in NGF expression between SK and the surrounding epidermis, we found a significant increase in SCF expression within SK (Fig. 5). SCF mediates MC proliferation, differentiation, migration, and survival via c-Kit receptor.29 The number of different types of MCs depends on their stage of differentiation, with c-Kit, tryptase, and chymase17 increased around SK (Fig. 2). Therefore, SCF can also be regarded to play an important role in SK pathogenesis. However, the mechanism by which SCF is induced is not well understood.
Endocannabinoids are increasingly recognized as important neuroendocrine regulators for maintaining the homeostasis of various organs, including skin.30 Endocannabinoids interact with specific cannabinoid receptors,31 including CB1 and CB2.32 It is reported that CB1 is expressed in human keratinocyte.33,34 These receptors, their endogenous ligands, and enzymes responsible for endocannabinoid synthesis and degradation comprise the endocannabinoid system.32 MCs are also controlled via specific cannabinoid receptors.31 We previously reported that MCs in human skin express CB1, and that blockade of this receptor by a selective CB1 antagonist, AM251, or CB1 gene knockdown stimulates MC maturation and degranulation in an HF organ culture system.17 CB1 blockade also significantly increased SCF expression in the epithelium of HFs.17 This suggests that endocannabinoid–CB1 signaling regulates excessive MC degranulation and maturation by controlling SCF expression in human skin. Therefore, we investigated whether CB1 expression also affects SK.
The significant downregulation of CB1 immunoreactivity in the SK compared with its marginal lesion (Fig. 6) indicates that SCF upregulation can be related to the downregulation of CB1. We still need to investigate the detailed mechanism by which CB1 expression is downregulated in SK; our results (Table 1) suggest that CB1 regulates MC activity around the SK directly and/or via inhibiting SCF expression. The regulator of CB1 in keratinocyte has not been well investigated. Further study for investigating the regulators of CB1 is needed.
Importantly, our results may encourage one to investigate the biology of MCs in other types of cutaneous tumors. For example, actinic keratosis (AK) is another type of skin tumor which resembles SK.35 As SK model in mice is related to UVB irradiation,4 UV is also reported to be deeply involved in the pathogenesis of AK.33 Furthermore, some patients with AK have itch,28 suggesting involvement of MCs in the pathomechanism not only of SK but also of AK. In addition, MCs are reported to promote the angiogenesis of neoplasms by secreting vascular growth factors11 and the remodeling of the surrounding stroma via MMP secretion27; further study for evaluating VEGF and MMP expression around the SK is also interesting. Although the opposing role for MCs has also reported the development and progression of cutaneous malignancies,11 MCs have a vast array of mediators, some of which have inhibitory effects on malignancies.11 In addition, several studies have shown a tumor cytotoxic role for MCs in cutaneous malignancies.11 As Bertolini et al.23 reported the existence of both proinflammatory and immunoinhibitory phenotypes involved in the pathomechanism of alopecia areata, it is possible that tumor-activating and tumor-inhibitory MCs play roles in the development of SK. Although we still do not know about the detailed MC phenotypes in the development of tumors, CB1–SCF signaling may have opposing impacts on proliferation/apoptosis/maturation depending on the MC phenotypes, that is, tumor-activating or tumor-inhibitory.
The current results suggest that controlling MCs may be important for inhibiting SK development. The MC–SCF–CB1 pathway could be a new target for elucidating the pathogenesis of SK and developing optional treatments beyond tumor dissection.
Supplemental Material
Supplemental material, 2020-00066R1_Production_Supplemental_Material_online_supp for The Mast Cell–SCF–CB1 Interaction Is a Key Player in Seborrheic Keratosis by Mika Yamanaka-Takaichi, Koji Sugawara, Rieko Sumitomo and Daisuke Tsuruta in Journal of Histochemistry & Cytochemistry
Acknowledgments
We thank Prof. Ralf Paus, University of Miami Miller School of Medicine (Miami, USA), for providing professional suggestions for this study. We also appreciate the excellent technical support of Ms. Ayano Yonamine, Ms. Yuko Uetani, and Ms. Ayumi Satoji (Dermatology department, Osaka City University Graduate School of Medicine), and Mr. Keisuke Inoue and Ms. Emi Donoue (research support platform of Osaka City University Graduate School of Medicine).
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MY-T prepared skin samples of SK, performed the immunohistochemistry, analyzed the data, and drafted this manuscript. KS and RS performed immunohistochemistry including tryptase and SCF. KS and DT participated in study design and manuscript editing. All authors (MY-T, KS, RS, and DT) contributed substantial effort toward the conception and design of the study and interpretation of the data. All authors critically revised the manuscript and finally approved the manuscript before submission. All authors are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Literature Cited
- 1. Khandpur S, Ramam M. Skin tumours. J Cutan Aesthet Surg. 2012;5(3):159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heidenreich B, Denisova E, Rachakonda S, Sanmartin O, Dereani T, Hosen I, Nagore E, Kumar R. Genetic alterations in seborrheic keratoses. Oncotarget. 2017;8(22):36639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wollina U. Recent advances in managing and understanding seborrheic keratosis. F1000Res. 2019;8:F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saeed AK, Salmo N. Epidermal growth factor receptor expression in mice skin upon ultraviolet B exposure—Seborrheic keratosis as a coincidental and unique finding. Adv Biomed Res. 2012;1:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson-Weaver B, Choi HW, Abraham SN, Staats HF. Mast cell activators as novel immune regulators. Curr Opin Pharmacol. 2018;41:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl. 2):S73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilgus TA, Wulff BC. The importance of mast cells in dermal scarring. Adv Wound Care (New Rochelle). 2014;3(4):356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McHale C, Mohammed Z, Gomez G. Human skin-derived mast cells spontaneously secrete several angiogenesis-related factors. Front Immunol. 2019;10: 1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maurer M, Paus R, Czarnetzki BM. Mast cells as modulators of hair follicle cycling. Exp Dermatol. 1995;4(4 Pt. 2):266–71. [DOI] [PubMed] [Google Scholar]
- 10. Beer TW, Ng LB, Murray K. Mast cells have prognostic value in Merkel cell carcinoma. Am J Dermatopathol. 2008;30(1):27–30. [DOI] [PubMed] [Google Scholar]
- 11. Ch’ng S, Wallis RA, Yuan L, Davis PF, Tan ST. Mast cells and cutaneous malignancies. Mod Pathol. 2006;19(1):149–59. [DOI] [PubMed] [Google Scholar]
- 12. Biswas A, Richards JE, Massaro J, Mahalingam M. Mast cells in cutaneous tumors: innocent bystander or maestro conductor? Int J Dermatol. 2014;53(7):806–11. [DOI] [PubMed] [Google Scholar]
- 13. Aoki M, Pawankar R, Niimi Y, Kawana S. Mast cells in basal cell carcinoma express VEGF, IL-8 and RANTES. Int Arch Allergy Immunol. 2003;130(3):216–23. [DOI] [PubMed] [Google Scholar]
- 14. Dyduch G, Okon K, Pescarini E. Mast cells in melanocytic skin lesions. An immunohistochemical and quantitative study. Pol J Pathol. 2011;62(3):139–44. [PubMed] [Google Scholar]
- 15. Kritas SK, Caraffa A, Antinolfi P, Saggini A, Pantalone A, Rosati M, Tei M, Speziali A, Saggini R, Pandolfi F, Cerulli G, Conti P. Nerve growth factor interactions with mast cells. Int J Immunopathol Pharmacol. 2014;27(1):15–19. [DOI] [PubMed] [Google Scholar]
- 16. Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10(6):440–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sugawara K, Biro T, Tsuruta D, Toth BI, Kromminga A, Zakany N, Zimmer A, Funk W, Gibbs BF, Zimmer A, Paus R. Endocannabinoids limit excessive mast cell maturation and activation in human skin. J Allergy Clin Immunol. 2012;129(3):726–38e8. [DOI] [PubMed] [Google Scholar]
- 18. Kraigher O. Seborrheic keratoses-induced generalized pruritus. Int J Dermatol. 2014;53(6):e343–4. [DOI] [PubMed] [Google Scholar]
- 19. Woolhiser MR, Brockow K, Metcalfe DD. Activation of human mast cells by aggregated IgG through FcgammaRI: additive effects of C3a. Clin Immunol. 2004;110(2):172–80. [DOI] [PubMed] [Google Scholar]
- 20. Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ito T, Ito N, Saathoff M, Stampachiacchiere B, Bettermann A, Bulfone-Paus S, Takigawa M, Nickoloff BJ, Paus R. Immunology of the human nail apparatus: the nail matrix is a site of relative immune privilege. J Invest Dermatol. 2005;125(6):1139–48. [DOI] [PubMed] [Google Scholar]
- 22. Sugawara K, Zakany N, Hundt T, Emelianov V, Tsuruta D, Schafer C, Kloepper JE, Biro T, Paus R. Cannabinoid receptor 1 controls human mucosal-type mast cell degranulation and maturation in situ. J Allergy Clin Immunol. 2013;132(1):182–93. [DOI] [PubMed] [Google Scholar]
- 23. Bertolini M, Zilio F, Rossi A, Kleditzsch P, Emelianov VE, Gilhar A, Keren A, Meyer KC, Wang E, Funk W, McElwee K, Paus R. Abnormal interactions between perifollicular mast cells and CD8+ T-cells may contribute to the pathogenesis of alopecia areata. PLoS ONE. 2014;9(5):e94260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boyce JA. Mast cells: beyond IgE. J Allergy Clin Immunol. 2003;111(1):24–32; quiz 3. [DOI] [PubMed] [Google Scholar]
- 25. Conti P, Gallenga CE, Ronconi G, Caraffa A, Kritas SK. Activation of mast cells mediates inflammatory response in psoriasis: potential new therapeutic approach with IL-37. Dermatol Ther. 2019;32(4):e12943. [DOI] [PubMed] [Google Scholar]
- 26. Bischoff SC. Mast cells in gastrointestinal disorders. Eur J Pharmacol. 2016;778:139–45. [DOI] [PubMed] [Google Scholar]
- 27. Maltby S, Khazaie K, McNagny KM. Mast cells in tumor growth: angiogenesis, tissue remodelling and immune-modulation. Biochim Biophys Acta. 2009;1796(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee HJ, Kim GW, Kim HS, Ko HC, Kim MB, Kim BS. Pruritus in epithelial tumours of the face. J Eur Acad Dermatol Venereol. 2017;31(11):e496–7. [DOI] [PubMed] [Google Scholar]
- 29. Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34(2):97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Biro T, Toth BI, Hasko G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30(8):411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Filippis D, D’Amico A, Iuvone T. Cannabinomimetic control of mast cell mediator release: new perspective in chronic inflammation. J Neuroendocrinol. 2008;20(Suppl. 1):20–25. [DOI] [PubMed] [Google Scholar]
- 32. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tóth BI, Dobrosi N, Dajnoki A, Czifra G, Oláh A, Szöllosi AG, Juhász I, Sugawara K, Paus R, Bíró T. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J Invest Dermatol. 2011;131(5):1095–104. [DOI] [PubMed] [Google Scholar]
- 34. Casanova ML, Blázquez C, Martínez-Palacio J, Villanueva C, Fernández-Aceñero MJ, Huffman JW, Jorcano JL, Guzmán M. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J Clin Invest. 2003;111(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmitt JV, Miot HA. Actinic keratosis: a clinical and epidemiological revision. An Bras Dermatol. 2012;87(3):425–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 2020-00066R1_Production_Supplemental_Material_online_supp for The Mast Cell–SCF–CB1 Interaction Is a Key Player in Seborrheic Keratosis by Mika Yamanaka-Takaichi, Koji Sugawara, Rieko Sumitomo and Daisuke Tsuruta in Journal of Histochemistry & Cytochemistry