Abstract
Objective:
Children with the temperament of Behavioral Inhibition (BI) face increased risk for developing an anxiety disorder later in life. However, not all children with BI manifest anxiety symptoms, and cognitive-control-strategy use may moderate the pathway between BI and anxiety. Individuals vary widely in the strategy used to instantiate control; the present study examined whether a more planful style of cognitive control (i.e. proactive control) or a more impulsive strategy of control (i.e. reactive control) moderates the association between early BI and later anxiety symptoms.
Method:
Participants were part of a longitudinal study examining the relations between BI (measured at 2-3 years) and later anxiety symptoms (measured at 13 years). Cognitive control strategy use was assessed at age 13 using the AX variant of the Continuous Performance Task (AX-CPT).
Results:
BI in toddlerhood significantly predicted increased use of a more reactive cognitive control style in adolescence. Additionally, cognitive control strategy moderated the relation between BI and anxious symptoms, such that reliance on a more reactive strategy predicted higher levels of anxiety for children high in BI.
Conclusion:
The present study is the first to identify the specific control strategy that increases risk for anxiety. Thus, is it not cognitive control per se, but the specific control strategy children adopt that may increase risk for anxiety later in life. These findings have important implications for future evidence-based interventions given that they suggest an emphasis reducing reactive cognitive control and increasing proactive cognitive control may reduce anxious cognition.
Keywords: Behavioral Inhibition, Cognitive Control, Proactive Control, Reactive Control, Anxiety
Introduction
Behavioral inhibition (BI) is characterized in toddlerhood by heightened reactivity and negative affect in response to novel people and situations.1 BI in toddlerhood increases the likelihood of developing an anxiety disorder in adolescence.2,3 However, not all inhibited children go on to display anxious symptoms in adolescence,4 and a key issue is understanding what factors further increase or decrease risk for anxiety. The current study examines the degree to which individual differences in cognitive control moderates the relations between early BI and later anxiety symptoms.
The flexible nature of human cognition allows for a prioritization of task demands in ways that optimize goal attainment through a processes known as cognitive control.5,6 One prevailing theory, the Dual Mechanisms of Control theory (DMC),7,8 postulates the existence of two chronometrically, or temporally, distinct cognitive control profiles: proactive and reactive control.6–8 Proactive control involves the early selection and maintenance of goal-relevant information; this effectively biases attention in a goal-driven manner, but creates risk for distraction and interruption. Conversely, reactive control is recruited on an as-needed basis, often in response to conflict. Theory and data suggest that typically developing children transition from a preferential reliance on reactive to proactive control during the first decade of life, and that proactive strategy use becomes more efficient during adolescence and young adulthood.9–12 DMC theory also suggests that anxious cognition may influence both proactive and reactive control.7,13
Recent theoretical work examining the relations between anxiety and the chronometry of cognitive control postulates that anxious cognition is associated with reductions in planful (proactive) control and an increased reliance on more instantaneous (reactive) control7,14; a number of empirical studies lend support for this theoretical approach. A study by Krug and Carter15 demonstrated that in situations of high-conflict, high trait anxiety was related to a more reactive-like style of cognitive control. Moreover, high trait anxious individuals exhibited less recruitment of neural regions associated with control when compared to their low anxious peers. In a separate study investigating working memory performance, high trait anxious individuals were shown to exhibit increases in transient activation within regions associated with cognitive control, indicative of a reactive control strategy, as opposed to the sustained neural activity one would expect if a proactive control strategy were used.16 Finally, a training study has recently demonstrated that when high anxious individuals are trained to utilize a proactive control strategy, they actually exhibit lower heart rate and feelings of anxiety when faced with a subsequent stressor.17 Altogether, both theoretical and empirical data suggest that a hallmark of anxiety may be an increased reliance on more transient, reactive control processes; such reliance on reactive control may or may not be accompanied by deficits in more sustained, proactive control processes. While these data are important for understanding anxious cognition in adulthood, we are not aware of any work examining how perturbations in cognitive control relate to anxiety during childhood. As such, it is important to examine developmental populations at-risk for anxiety in order to better understand whether cognitive control deficits develop concurrently with anxious cognition or whether they may precede anxiety symptoms.
To date, no empirical studies have concurrently examined the relations between behavioral inhibition, control strategy (proactive/reactive), and anxiety, within a developmental population. Nonetheless, a review by Henderson and colleagues13 postulates that a disrupted time course of control likely moderates relations between anxiety risk (e.g. as evidenced by high BI) and later development of anxious cognition. Additionally, mounting evidence suggests that improved performance on inhibitory control tasks (Troller-Renfree et al., unpublished, 2018)18 and exaggerated responses to errors or conflict moderate relations between BI and anxiety.19–24 Performance on inhibitory control tasks, or exaggerated responses to conflict and errors, can be thought of as an indirect measure of reactive control, given that these processes occur rapidly, and only after control is needed (and not before). Therefore, prior work showing that children high in BI exhibit increased responses to errors/conflict (or improved performance on inhibitory control tasks) may reflect indirect support for the notion that children high in BI children rely more heavily on a reactive control strategy. However, a direct test of this hypothesis remains absent from the literature and the role of proactive control has not been specifically tested.
The present study is the first, to our knowledge, to explicitly examine whether proactive or reactive control moderates the relation between BI and anxiety symptoms in a longitudinal sample. The aims of the present study are twofold. First, we ask whether behavioral inhibition predicts an individual’s propensity to enact a more reactive or proactive strategy on an ‘AX’ version of a continuous performance task (AX-CPT). We hypothesize that individuals high in BI in toddlerhood will utilize a more reactive style of responding in early adolescence. Second, we examine whether individual differences in cognitive control strategy use moderate the relations between BI and anxiety symptoms. Consistent with prior research, we expect a more reactive style of responding to increase the risk for anxious cognition in children characterized as high BI.
Method
Participants
Participants were part of a longitudinal study examining infant fearful temperament and its relation to the emergence of anxiety. At four months of age, 779 infants completed an in-lab temperament screening, during which emotional and motor reactivity to novel visual and auditory stimuli were observed.25–27 Subsequently, 291 infants (134 male) were selected to continue in the study based on their temperamental classification, which are as follows: high negative/high motor reactive (n = 105), high positive/high motor reactive (n = 103), and control group (n = 83). These children continued to participate in assessments of socioemotional development throughout childhood and early adolescence. Informed consent and assent (when appropriate) was obtained at each assessment and each visit protocol was approved by the University of Maryland Institutional Review Board.
Behavioral Inhibition
Behavioral inhibition (BI) was assessed at 24 and 36 months of age using both behavioral coding of laboratory assessments and parental report. Laboratory assessments were coded for episodes in which children were presented with unfamiliar persons and objects.25,26 Maternal report of social fear was collected using the Toddler Behavior Assessment Questionnaire (TBAQ). Behavioral coding and parental reports of BI were significantly associated (r(123)=.479, p<.001) As in previous work, measures were standardized and averaged to create a BI composite based on the assumption that combining data from different contexts, informants, and ages better reflects the child’s temperament.20,21,28 The distribution of the BI composite measure was inspected and determined to be normal (Skewness = .126, Kurtosis = .037).
AX-CPT Task
Participants completed a standard behavioral AX-CPT 7,29,30 to generate distinct indices of proactive and reactive control.7 The AX-CPT is presented as a continuous series of letter pairs is comprised of 4 trial types – AX, AY, BX, BY (see Figure 1 for task schematic). AX trials are the target trial for this task and will have a different response (e.g. ‘2’ following the first stimuli called the cue, ‘3’ following the second stimuli called the probe) than the other three trial types (‘2’ following the cue, ‘2’ following the probe). Consistent with past studies using the AX-CPT, AX trials were presented 70% of the time while each other trial type (AY, BX, BY) was presented 10% of the time. Participants completed a total of 150 trials presented in a pseudorandom order. Letter stimuli were presented in a bolded Courier New font with a point size of 60. The cue for each letter pair was presented in cyan and the probe was presented in white. All stimuli were presented on a black background. Each trial began with a centrally located fixation asterisk (200ms) followed by the presentation of the cue stimulus (500ms) with a 1000ms response window (central fixation asterisk). Following the response window, a 3900ms delay was displayed before the presentation of the probe stimulus (500ms) and the following response window (1000ms). The AX-CPT was administered on a Dell Latitude laptop with a 15.5-inch screen using E-prime 2.0 Professional.31
Figure 1.
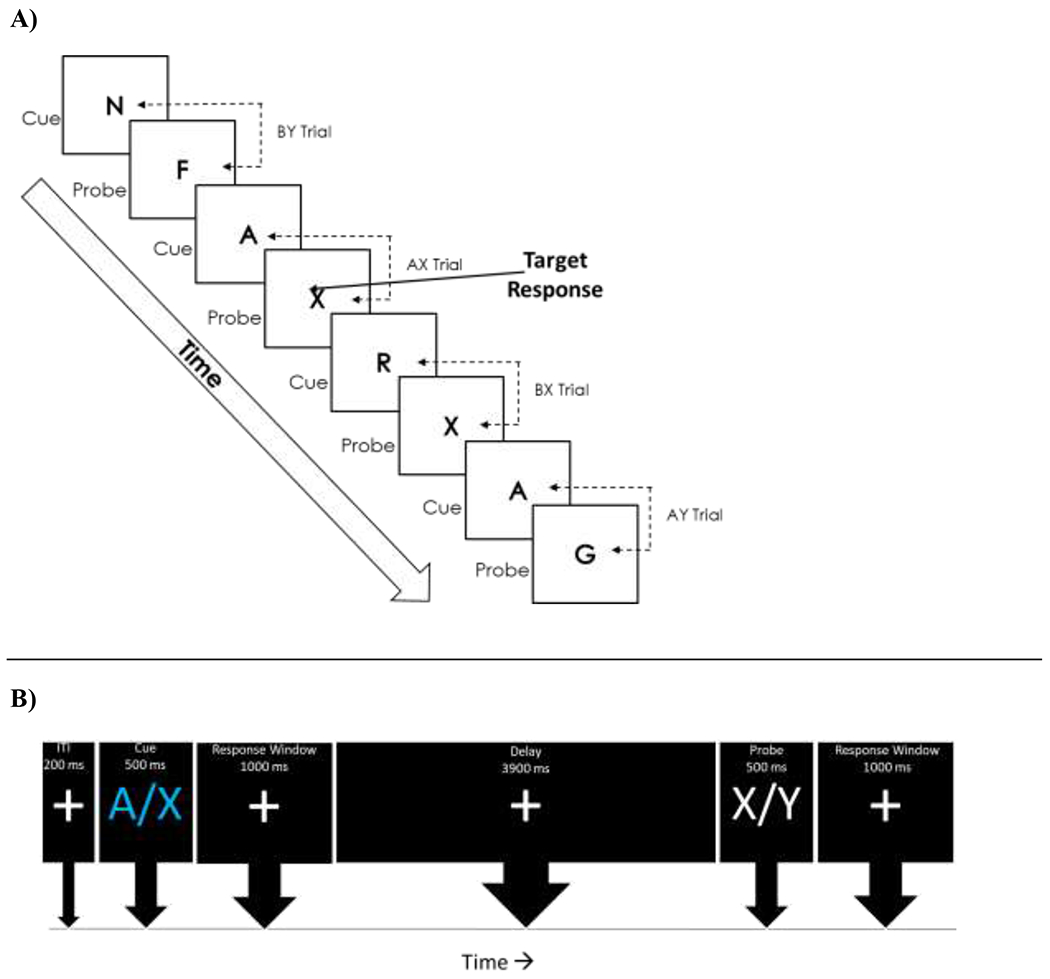
AX-CPT (Continuous Performance Task) Task schematic (A) and AX-CPT Trial Schematic (B)
Consistent with other AX-CPT studies, data were trimmed based on reaction times (RTs). RTs more than 3 SDs from each participants’ mean reaction time on correct trials were removed, resulting in the exclusion of less than 3% (M=2.57, SD=.03) of all trials. After trimming the data, accuracy and mean reaction times were computed for each trial type. Additionally, two commonly used indices hailing from signal detection theory – d’ context and A-cue bias – were computed.30,32 The first measure, d’ context, provides a measure of the sensitivity to the differences between target and non-target trials while controlling for individual differences in response biases.33 In other words, d’ context is a measure of the maintenance of the ‘A’ versus ‘B’ cue prior to the presentation of an ‘X’ probe. d’ context was computed by comparing correct responses on AX trials (hits) relative to incorrect responses on BX trials (false alarms). A correction was applied to cases in which there was a hit rate of 1 (hit rate = 2−(1/N); N = target trials) or a false alarm rate of 0 (false alarm = 1-2−(1/N); N = number of non-target trials), which has been shown to allow for an unbiased estimation of d’.34 The second measure, A-cue bias, provides a measure of the tendency to differentially prepare for target responses based on the presentation of an A cue.35,36 The A-cue index was calculated by computing a criterion score using hits on AX trials and false alarms on AY trials. The distribution of the d’ context measure (Skewness = −.516, Kurtosis = .222) and A-cue bias (Skewness = −.880, Kurtosis = 2.191) were inspected and determined to be normal. For the purposes of this paper, and consistent with a broader literature, higher d’ context scores will indicate a more proactive style of cognitive control since the participant was sensitive to cue information and use it to inform future responses, while lower d’ context scores indicate that the participant used a more reactive style of cognitive control since the cue information was not as motivationally salient to the participant.
Screen for Child Anxiety Related Emotional Disorders (SCARED)
Each participant and their parent completed the revised version of the SCARED questionnaire at the 13-year assessment.37 The parent and child versions of the SCARED are comprised of 41 items presented on a 3-point Likert scale (0=never/hardly ever true, 1=sometimes/somewhat true, 2=very/often true). Parent and Child total anxiety scores were computed individually for all respondents who completed the questionnaire in full. The SCARED and its subscales show good internal consistency (α from .74 to .89), retest reliability (ICCs from 0.9 to 0.7), and discriminant validity between children with anxiety diagnoses and other diagnoses as well as between individual anxiety disorders.37 BI was significantly associated with parent report of anxiety (r = .175, p =.032), but not child report (r = −.038, p =.649). As such, parent report was the focus of the following analyses, however the analogous analyses for child report can be found in the Supplement 1, available online.
Inclusion Criteria
A total of 141 participants were administered the AX-CPT task at the 13-year assessment. Eleven participants did not complete the task due to technical difficulties (n = 2) and participant refusal or stopping responding during the assessment (n = 9). Consistent with other studies with children, participants were excluded from analysis if they had less than 60% accuracy on BY trials (n = 11).10 Together, this left a total of 119 participants with usable data on the AX-CPT task.
Analytic Plan
Data were analyzed in three steps. First, to establish that the excepted condition-level relations were observed, two separate repeated measures ANOVAs were conducted – one for accuracy and one for reaction time. For both models, Trial Type was the within-subject effect. A greenhouse-geisser correction was applied where appropriate. Next, to examine whether there were any associations between BI and cognitive control, a series of linear regressions were conducted with BI predicting accuracy for each trial type, reaction times for each trial type, d’ context, and A-cue bias scores; see Supplement 2 and Table S1, available online, for additional analyses. Finally, moderation analyses were conducted using PROCESS 2.16.338 to assess the influence of cognitive control on the BI-anxiety relation. All predictor variables were centered prior to calculation of interaction terms; given some prior work suggesting that gender may influence the BI-anxiety relation and maternal education was associated with parent-report of anxiety (r=−.232, p=.005), gender and maternal education were controlled for.39
Results
Preliminary Behavioral Analyses
Descriptive statistics are reported in Table 1. Analyses examining accuracy revealed a significant within-subject effect of trial type F(3,354) = 93.28, p < .001; post-hoc tests revealed that accuracy in each trial type was statistically significant from all other trial types (all ps <.02). Specifically, participant accuracy was as follows: BY (M = 88.8%, SE = .9%), AX (M = 82.9%, SE = 1.4%), BX (M = 78.9%, SE = 1.8%), and AY (M = 60.8%, SE = 1.9%).
Table 1.
Sample Descriptives and Task Performance for Participants Who Completed the AX-Continuous Performance Tast (CPT) With a Valid Score at Age 12
| Variable | N | Mean |
|---|---|---|
| Behavioral Inhibition | 118 | .06 (.07) |
| Gender (% Female) | 119 | 57.1% |
| Maternal Education | ||
| High School (% Complete) | 17.9% | |
| College (% Complete) | 47.3% | |
| Graduate School (% Complete) | 34.8% | |
| Age | 118 | 13.25 (.06) |
| AX Accuracy | 119 | .83 (.01) |
| AY Accuracy | 119 | .61 (.02) |
| BX Accuracy | 119 | .79 (.02) |
| BY Accuracy | 119 | .89 (.01) |
| AX Reaction Time | 119 | 472.56 (8.64) |
| AY Reaction Time | 119 | 619.99 (12.57) |
| BX Reaction Time | 119 | 416.51 (12.12) |
| BY Reaction Time | 119 | 420.94 (10.35) |
| d’ context | 119 | 2.00 (1.11) |
| A-cue bias | 119 | .38 (.03) |
| Anxiety (parent report) | 100 | 9.32 (.82) |
Note: Reported as: Mean (standard error).
Analyses examining reaction times by trial type revealed a significant within-subject effect of trial type F(3,354) = 260.497, p < .001; post-hoc tests revealed that reaction times on BX (M = 416.51, SE = 12.12) and BY (M = 420.94, SE = 10.35) trials were significantly faster than AX (M = 472.56, SE = 8.64) and AY (M = 619.99, SE = 12.57) trials (ps < .001). Additionally, participants were slower on AY trials than all other trial types (ps < .001).
Relations between BI and Cognitive Control
Analyses examining the relations between BI and accuracy and reaction times on the AX-CPT revealed that behavioral inhibition was negatively related to BX accuracy, B = −.048, F(1,116) = 4.346, p= .039. BI was unrelated to accuracy and reaction times in all other conditions (ps > .426). Additionally, analyses examining the association between BI and context sensitivity (as indexed by d’ context), revealed that BI was negatively related to d’ context, B = −.278, F(1,116) =4.594, p=.034. Analyses examining the association between BI and response bias (as indexed by A-cue bias), revealed that BI was not related to A-cue bias, B = −.015, F(1,116) =.127, p=.722.
Moderating role of context sensitivity between BI and Anxiety.
Analyses examining the moderating role of context sensitivity on the relation revealed that d’ context moderated the relation between BI and parent-report of anxiety, ΔR2 = .0395, F(1, 88) = 4.3885, p = .0391. Follow-up tests revealed that at low (B = 5.1286, t = 3.6219, p = .0005) and middling (B = 3.0660, t = 2.8609, p = .0053) levels of context sensitivity there was a positive relation between BI and anxiety, whereas when d’ context was high (B = 1.0034, t = .6718, p = .5035) there was no significant association between BI and anxiety (see Figure 2). Analyses examining the moderating role of response bias on the relation revealed that the A-cue bias did not moderate the relation between BI and anxiety, ΔR2 = .0004, F(1, 88) = .0386, p = .8447.
Figure 2.
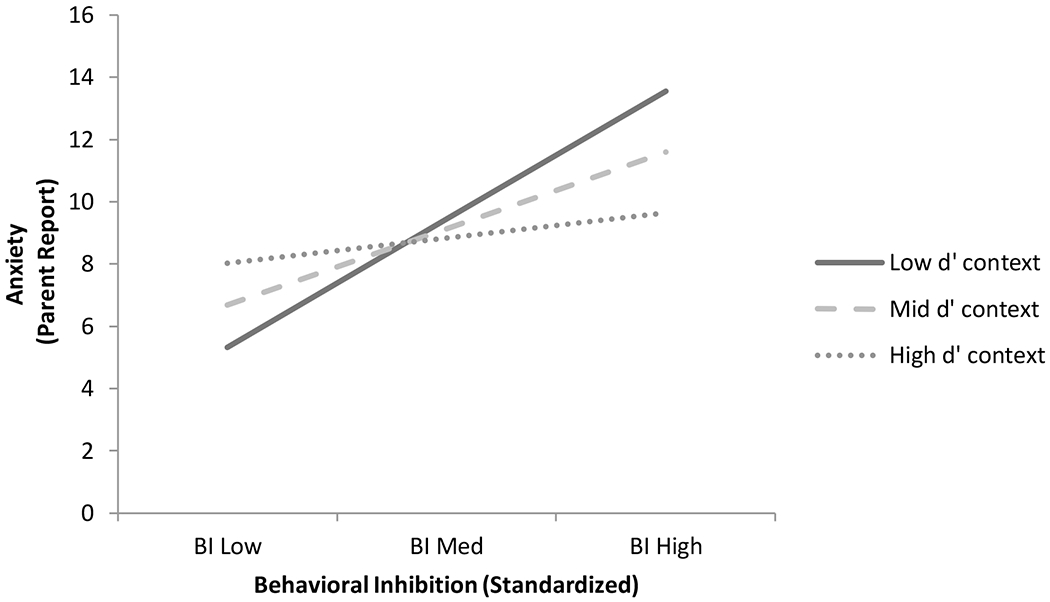
Moderating Influence of Context Sensitivity on the Relations Between Behavioral Inhibition and Anxiety
Discussion
The present study is the first to examine how the chronometry of cognitive control relates to BI and anxiety symptoms within a longitudinal framework. The data suggest that BI assessed in toddlerhood prospectively predicts cognitive control strategy use in adolescence. Specifically, children with high BI in toddlerhood are less likely to utilize meaningful preparatory information (i.e. sustain cue identity) to prepare for future responses. Additionally, we found that the adoption of a more reactive control strategy in adolescence moderated longitudinal relations between BI and parent report of anxiety symptoms, with a more reactive strategy predicting increased anxiety symptoms in children high in BI. Conversely, these data suggest that a more proactive control strategy may protect against anxiety in children high in BI. Critically, BI did not predict variations in response bias, nor did response bias moderate the relations between BI and anxiety, suggesting a degree of selectivity in the how BI, anxiety and cognitive control interact.
Data from the present study suggest that using a more reactive control strategy may increase the risk for anxiety symptoms in adolescence among children who had exhibited high behavioral inhibition during toddlerhood. Specifically, high BI children exhibited the highest risk for anxiety when they also relied on a more reactive cognitive control strategy in adolescence, as indexed by low context sensitivity. This pattern was not evident when high BI children utilized a more proactive cognitive control strategy. This novel finding fits nicely with existing theory suggesting that the balance between proactivity and reactivity may be perturbed in anxious individuals.7,13,14 This finding also fits nicely with the existing evidence suggesting that rapidly-occurring neural indices of conflict,21,24 response monitoring,20,23,40 and inhibitory control performance18,41 moderate the relations between BI and anxiety. Moreover, these findings fit nicely into a broader neuroimaging literature suggesting that behavioral inhibition is accompanied by altered neurocognitive functioning – particularly in frontal and limbic circuitry – both when at rest42 and when performing cognitive tasks involving emotional stimuli.43–46
Of note, this finding is not merely a demonstration of a concurrent association between anxiety and reactive control, since anxiety showed no significant relations with reactive control in adolescents who exhibited low levels of behavioral inhibition in toddlerhood. While reactive control appeared to place only high BI children at risk, proactive control appeared to be protective for high BI and low BI children alike. Future studies should investigate in greater depth the possible protective nature of more planful proactive strategies given the lack of (and maybe even negative) relation between BI and anxiety when more planful strategies are enacted. If using more planful proactive strategies is protective, this may be an intriguing direction for evidence-based interventions.
While prior work has demonstrated that neural measures of cognitive control moderate relations between early BI and later anxiety, past studies have rarely found a direct relation between BI and behavioral performance on cognitive tasks.22,23 Remarkably, the present study found that BI in toddlerhood predicted a behavioral measure of cognitive control strategy approximately 10 years later. This pattern may have emerged for our behavioral measure of context sensitivity because it takes into account both sustained (ability to maintain cue information) and conflict-related process (the conflictual nature of the target probe). That is, this measure does not treat cognitive control as a unitary construct and instead identifies individual variation in the control strategy used, yielding a more nuanced, and potentially more sensitive measure than traditional measures of cognitive control. However, it should be noted that while measurement of d’ context provides insight into the relative use of a proactive vs. reactive control strategy in terms of how well cue information is maintained, it does not reflect a direct measure of control strategy per se. As such, future studies should aim to use methods such as eye tracking, event-related potentials, or time-frequency EEG approaches to extract more direct measures of proactive and reactive control to better understand how these constructs relate to BI and anxiety. Furthermore, while it appears that individual differences in cognitive control are meaningful in adolescence, future studies should examine when these differences emerge and how early in development they can be detected. Future studies should also examine how cognitive control relates to everyday functioning in children (i.e. regulatory control and social functioning). Finally, while the present study presents exciting, novel findings examining the moderating role of cognitive control on the relations between BI and anxious cognition, it is important to note that cognitive control and anxiety were both measured at age 13, so it is not possible to assess whether differences in cognitive control predict later anxious cognition. Furthermore, the effect of the chronometry of cognitive control on the relations between BI and anxiety were relatively modest in size. As such, future studies should use either longitudinal methods or more robust experimental manipulations to examine the directional relations between cognitive control and anxiety as well as other coincident pathways to anxious cognition. In addition, alternative theories, such as pre-existing differences in prefrontal cortical structure47 or function42,48 leading to both anxiety and reactive cognitive control, should be investigated.
In sum, the present study is the first to examine the relations between the chronometry of cognitive control strategy use, behavioral inhibition, and anxiety symptoms. The major contributions of the present paper are twofold: first, our data suggest that behavioral inhibition in toddlerhood predicts a more reactive style of cognitive control in early adolescence. Second, this more reactive style of responding increases the risk for parent report of anxiety symptoms at age 13, but only for adolescents previously identified as high in BI during toddlerhood. Together, these findings suggest cognitive control strategy use, and more specifically the chronometry of cognitive control, may be a potential risk factor for the emergence of anxiety symptoms in behaviorally inhibited children and an effective target for future evidence-based interventions.
Supplementary Material
Acknowledgements:
This research was supported by grants from the National Institute of Mental Health (U01MH093349 and U01MH093349-S to N.A.F.), the National Science Foundation (NSF; DGE1322106 to S.V.T.), and the NIMH Intramural Research Program (supporting D.S.P.). A special thanks to Brady K. Stevens, BA, New York Institute of Technology, for his help with data collection and all of the families for their time and continued participation.
Disclosure: Dr. Fox has received additional funding from the following granting agencies: NIH (R01MH091363) for The Effects of Early Psychosocial Deprivation on Mental Health in Adolescence; Harvard University (256458-509584) for Assessing the Efficacy of Attention Bias Modification Training; the NIMH (1R01MH107444) for Prospective Determination of Neurobehavioral Risk for the Development of Emotion Disorders; the NICHD (P01HD064653) for Functions and Development of the Mirror Neuron System-Continuation; the NSF (1625495) for Collaborative Research: Action, Learning, and Social Cognition; and the National Institutes of Health (1UG3OD023279-01) for Environmental Influences on Child Health Outcomes in the Northern Plains Safe Passage Study Cohort. Drs. Buzzell, Henderson, Pine and Troller-Renfree report no biomedical financial interests or potential conflicts of interest.
Footnotes
Presentation Information: This study was presented as an abstract at the Society for Research in Child Development Biannual Meeting, Austin, Texas, 2017.
References
- 1.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. [DOI] [PubMed] [Google Scholar]
- 2.Chronis-Tuscano A, Degnan KA, Pine DS, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J Am Acad Child Adolesc Psychiatry. 2009;48(9):928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clauss JA, Blackford JU. Behavioral Inhibition and Risk for Developing Social Anxiety Disorder: A Meta-Analytic Study. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1066–1075.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Dev Psychopathol. 2007;19(03):729. [DOI] [PubMed] [Google Scholar]
- 5.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. [DOI] [PubMed] [Google Scholar]
- 6.Munakata Y, Snyder HR, Chatham CH. Developing cognitive control three key transitions. Curr Dir Psychol Sci. 2012;21(2):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braver TS. The variable nature of cognitive control: A dual mechanisms framework. Trends Cogn Sci. 2012;16(2):106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braver TS, Gray JR, Burgess GC. Explaining the Many Varieties of Working Memory Variation: Dual Mechanisms of Cognitive Control. (Conway A, Jarrold C, Kane M, Miyake A, Towse J, eds.). Oxford: Oxford Univ Press; 2007. [Google Scholar]
- 9.Chevalier N, Martis SB, Curran T, Munakata Y. Metacognitive processes in executive control development: The case of reactive and proactive control. J Cogn Neurosci. 2015;27(6):1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatham CH, Frank MJ, Munakata Y. Pupillometric and behavioral markers of a developmental shift in the temporal dynamics of cognitive control. Proc Natl Acad Sci U S A. 2009;106(14):5529–5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucenet J, Blaye A. Age-related changes in the temporal dynamics of executive control: A study in 5- and 6-year-old children. Front Psychol. 2014;5:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorsbach TC, Reimer JF. Context processing and cognitive control in children and young adults. J Genet Psychol. 2008;169(1):34–50. [DOI] [PubMed] [Google Scholar]
- 13.Henderson HA, Pine DS, Fox NA. Behavioral inhibition and developmental risk: a dual-processing perspective. Neuropsychopharmacology. 2015;40(1):207–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moser JS, Moran TP, Schroder HS, Donnellan MB, Yeung N. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Front Hum Neurosci. 2013;7:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krug MK, Carter CS. Proactive and reactive control during emotional interference and its relationship to trait anxiety. Brain Res. 2012;1481:13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fales CL, Barch DM, Burgess GC, et al. Anxiety and cognitive efficiency: Differential modulation of transient and sustained neural activity during a working memory task. Cogn Affect Behav Neurosci. 2008;8(3):239–253. [DOI] [PubMed] [Google Scholar]
- 17.Birk JL, Rogers AH, Shahane AD, Urry HL. The heart of control: Proactive cognitive control training limits anxious cardiac arousal under stress. Motiv Emot. December 2017:1–15. [Google Scholar]
- 18.White LK, McDermott JM, Degnan KA, Henderson HA, Fox NA. Behavioral inhibition and anxiety: the moderating roles of inhibitory control and attention shifting. J Abnorm Child Psychol. 2011;39(5):735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorell LB, Bohlin G, Rydell A-M. Two types of inhibitory control: Predictive relations to social functioning. Int J Behav Dev. 2004;28(3):193–203. [Google Scholar]
- 20.Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA, Fox NA. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. J Am Acad Child Adolesc Psychiatry. 2014;53(4):447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamm C, Walker OL, Degnan KA, et al. Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Dev Sci. 2014;17(5):667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biol Psychiatry. 2009;65(5):445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buzzell GA, Troller-Renfree SV, Barker TV, et al. A Neurobehavioral Mechanism Linking Behaviorally Inhibited Temperament and Later Adolescent Social Anxiety. J Am Acad Child Adolesc Psychiatry. 2017;56(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahat A, Walker OL, Lamm C, Degnan KA, Henderson HA, Fox NA. Cognitive Conflict Links Behavioural Inhibition and Social Problem Solving During Social Exclusion in Childhood. Infant Child Dev. 2014;23(3):273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calkins SD, Fox NA, Marshall TR. Behavioral and Physiological Antecedents of Inhibited and Uninhibited Behavior. Child Dev. 1996;67(2):523–540. [PubMed] [Google Scholar]
- 26.Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and Discontinuity of Behavioral Inhibition and Exuberance: Psychophysiological and Behavioral Influences across the First Four Years of Life. Child Dev. 2001;72(1):1–21. [DOI] [PubMed] [Google Scholar]
- 27.Hane AA, Cheah C, Rubin KH, Fox NA. The Role of Maternal Behavior in the Relation between Shyness and Social Reticence in Early Childhood and Social Withdrawal in Middle Childhood. Soc Dev. 2008;17(4):795–811. [Google Scholar]
- 28.Walker OL, Henderson HA, Degnan KA, Penela EC, Fox NA. Associations Between Behavioral Inhibition and Children’s Social Problem-solving Behavior During Social Exclusion. Soc Dev. 2014;23(3):487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35(10):1373–1380. [DOI] [PubMed] [Google Scholar]
- 30.Cohen JD, Barch DM, Carter CS, Servan-Schreiber D. Context-processing deficits in schizophrenia: Converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108(1):120–133. [DOI] [PubMed] [Google Scholar]
- 31.Schneider W, Eschman A, Zuccolotto A. E-Prime: User’s guide. 2002. [Google Scholar]
- 32.Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic Deficits in the Processing of Context: A Test of a Theoretical Model. Arch Gen Psychiatry. 1996;53(12):1105–1112. [DOI] [PubMed] [Google Scholar]
- 33.Braver TS, Satpute AB, Rush BK, Racine CA, Barch DM. Context Processing and Context Maintenance in Healthy Aging and Early Stage Dementia of the Alzheimer’s Type. Psychol Aging. 2005;20(1):33–46. [DOI] [PubMed] [Google Scholar]
- 34.Nuechterlein KH. Vigilance in schizophrenia and related disorders In: Handbook of Schizophrenia: Vol. 5. Neuropsychology, Psychophysiology, and Information Processing. Amsterdam: Elsevier; 1991:397–433. [Google Scholar]
- 35.Richmond LL, Redick TS, Braver TS. Remembering to Prepare: The Benefits (and Costs) of High Working Memory Capacity. Vol 41 NIH Public Access; 2015:1764–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonthier C, Braver TS, Bugg JM. Dissociating proactive and reactive control in the Stroop task. Mem Cognit. February 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale Construction and Psychometric Characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36(4):545–553. [DOI] [PubMed] [Google Scholar]
- 38.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Press; 2013. [Google Scholar]
- 39.Schwartz CE, Snidman N, Kagen J. Adolescent Social Anxiety as an Outcome of Inhibited Temperament in Childhood. J Am Acad Child Adolesc Psychiatry. 1999;38(8):1008–1015. [DOI] [PubMed] [Google Scholar]
- 40.McDermott JM, Troller-Renfree SV, Vanderwert RE, Nelson CA, Zeanah CH, Fox NA. Psychosocial deprivation, executive functions, and the emergence of socio-emotional behavior problems. Front Hum Neurosci. 2013;7:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Troller-Renfree S V, Buzzell GA, Salo V, et al. Development of inhibitory control during childhood and relations to early temperament and later social anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taber-Thomas BC, Morales S, Hillary FG, Pérez-Edgar K. Altered topography of intrinsic functional connectivity in childhood risk for social anxiety. Depress Anxiety. April 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jarcho JM, Fox NA, Pine DS, et al. The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biol Psychol. 2013;92(2):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jarcho JM, Fox NA, Pine DS, et al. Enduring influence of early temperament on neural mechanisms mediating attention-emotion conflict in adults. Depress Anxiety. 2014;31(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clauss JA, Benningfield MM, Rao U, Blackford JU. Altered Prefrontal Cortex Function Marks Heightened Anxiety Risk in Children. J Am Acad Child Adolesc Psychiatry. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu X, Taber-Thomas BC, Pérez-Edgar K. Frontolimbic functioning during threat-related attention: Relations to early behavioral inhibition and anxiety in children. Biol Psychol. 2017;122:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sylvester CM, Barch DM, Harms MP, et al. Early Childhood Behavioral Inhibition Predicts Cortical Thickness in Adulthood. J Am Acad Child Adolesc Psychiatry. 2016;55(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clauss JA, Avery SN, VanDerKlok RM, et al. Neurocircuitry underlying risk and resilience to social anxiety disorder. Depress Anxiety. 2014;31(10):822–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


