Abstract
The prevalence of suicidal thoughts and behaviors increases dramatically across the transition to puberty, particularly among adolescent girls. Yet we know little about why adolescent girls are at heightened risk, or when girls may be most likely to consider or engage in suicidal behavior. In this article, we outline evidence supporting a role for the menstrual cycle in the onset of and fluctuations in adolescent girls’ suicide risk. This emerging framework outlines developmental (i.e., biological, social, and cognitive) characteristics that might place certain girls at higher risk (e.g., between-subjects factors), as well as potential mechanisms that occur during the perimenstrual phase of the menstrual cycle (i.e., within-subjects factors) that increase adolescent females’ increased risk for suicide.
Keywords: menstrual cycle, puberty, suicide
Suicide is the second leading cause of death among adolescents in the United States. According to epidemiological studies, suicidal thoughts and behaviors rise exponentially during adolescence (Nock et al., 2013). Although more adolescent boys than girls die by suicide, these thoughts and behaviors are more prevalent among adolescent girls than among adolescent boys (Nock et al., 2013). Approximately 22% of U.S. adolescent girls report suicidal ideation (compared to almost 12% of adolescent boys), and 9.3% attempt suicide each year (compared to 5.1% of adolescent boys; Kann et al., 2018). We have little data to understand why suicidal thoughts and behaviors increase during adolescence, why girls are at greater risk, and when girls are most likely to experience these thoughts and behaviors.
In this article, we posit that suicidal thoughts and behaviors among females are associated with neurobiological sensitivity to hormone changes across the menstrual cycle. Specifically, we describe a framework that hypothesizes that exposure to normal hormone flux during the weeks before and during menses (i.e., the perimenstrual weeks) is associated with a rapid co-escalation of physical symptoms, negative affect, deficits in inhibitory control, and perhaps especially important, painful and disruptive social experiences; these hormone-related symptoms may individually or together increase acute risk for suicidal thoughts and behaviors in at-risk girls. Our framework explains why girls are at greater risk for these thoughts and behaviors during adolescence than boys, which girls may be at especially high risk (i.e., between-subjects factors), and what mechanisms may explain an association between the perimenstrual phase (i.e., within-subjects factors) and adolescent females’ increased risk for suicide.
THE MENSTRUAL CYCLE AND SUICIDE
The onset of menses and cycling hormones represent a promising but understudied risk factor for suicidal thoughts and behaviors in adolescents. For most girls, adolescence is characterized by the onset of menses and associated fluctuations in estrogen and progesterone across the menstrual cycle (see Figure 1). However, not all females experience the menstrual cycle similarly; decades of observational and experimental studies have demonstrated staggering (and poorly understood) individual differences in cyclical hormone sensitivity, defined as the extent to which females experience emotional, behavioral, and physical symptoms across the menstrual cycle (reviewed in Wei, Schiller, Schmidt, & Rubinow, 2018).
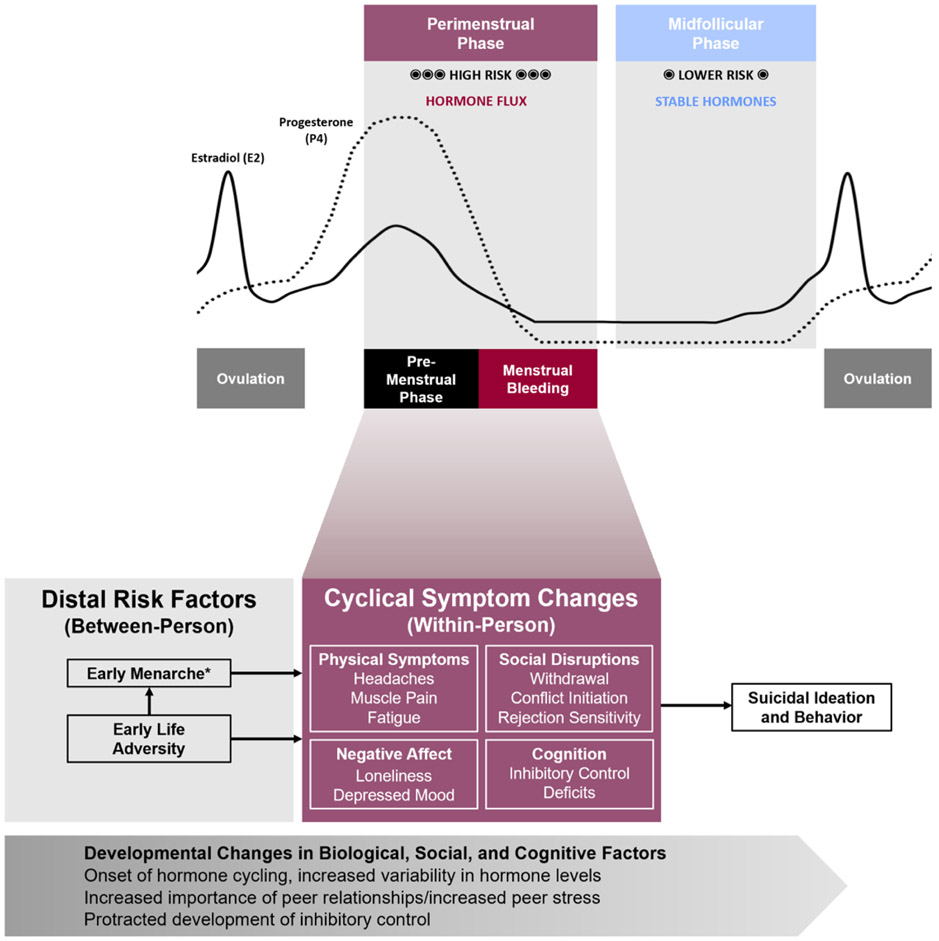
Figure 1.
Conceptual framework for the role of perimenstrual hormone withdrawal in suicide risk.
*May also interact with within-person symptoms to predict risk.
Hormone sensitivity may manifest in several ways. The most severe manifestation is exemplified in Premenstrual Dysphoric Disorder (PMDD), a psychiatric diagnosis introduced in the Diagnostic and Statistical Manual of Mental Disorders–5th ed. (American Psychiatric Association, 2013), characterized by distressing or impairing emotional, behavioral, and physical symptoms only in the luteal phase (i.e., 2 weeks before menses), with symptoms remitting almost entirely in the follicular phase (i.e., the time period from menses through ovulation). While 3–8% of women suffer from PMDD, 20% may experience some clinically significant symptoms (Halbreich, Borenstein, Pearlstein, & Kahn, 2003). Prevalence rates may be comparable or slightly higher among adolescents; in one study, 8% of adolescents met diagnostic criteria for PMDD, whereas an additional 42% experienced clinically significant symptoms (Czajkowska, Drosdzol-Cop, Gałązka, Naworska, & Skrzypulec-Plinta, 2015). Far more women may experience perimenstrual exacerbation (PME) of underlying disorders in which chronic symptoms worsen in the premenstrual or menstrual weeks; for example, 60% of women with depressive disorders show PME in daily ratings (Hartlage, Brandenburg, & Kravitz, 2004). Few studies have examined either type of hormone sensitivity in adolescence, despite compelling evidence that hormone cycling can increase numerous risk factors for suicidal thoughts and behaviors among adolescent females.
For instance, research strongly supports the association between the perimenstrual phase and acute risk for suicidal thoughts and behaviors. In adults, these occur most frequently during the perimenstrual phase; in 17 of 26 cross-sectional studies, suicide attempts were more likely in the perimenstrual phases of the cycle than in other phases (Owens & Eisenlohr-Moul, 2018; Saunders & Hawton, 2006). These studies primarily used self-reports of menstrual bleeding dates before and after psychiatric hospitalization. Results are similar for occurrence of suicidal ideation: Studies of calls to suicide hotlines have identified a positive association between ideation and the perimenstrual period (e.g., Wetzel, Reich, & McClure, 1971).
Several emerging lines of work have begun to demonstrate these links among adolescent girls. First, dramatic increases in suicidal thoughts and behaviors occur among adolescent females during the same period of time when most experience menarche (Nock et al., 2013). The prevalence of these thoughts and behaviors in females declines again after menopause, when hormone cycling ends (Mendle, Eisenlohr-Moul, & Kiesner, 2016). Second, perimenstrual mood changes may be even more prevalent among adolescent girls than in adults, and women reporting severe perimenstrual changes generally report that symptoms began during adolescence (reviewed in Rapkin & Mikacich, 2013). Finally, risk factors associated with adolescents’ suicidal thoughts and behaviors are linked with the menstrual cycle (which we address later).
A DEVELOPMENTAL CONTEXT
Several developmental (i.e., biological, social, cognitive) characteristics may explain why adolescence marks the onset of heightened risk for suicidal thoughts and behaviors, particularly among females. First, adolescents may experience frequent cyclical hormone fluctuations, occurring over the course of the day, the menstrual cycle, and with pubertal development (Janfaza, Sherman, Larmore, Brown-Dawson, & Klein, 2006). Although more work is needed to understand more fully the immature adolescent cycle, frequent fluctuation in hormone levels may be related to more acute changes in the risk for suicide, since hormone changes trigger suicide-relevant symptoms in adults with PMDD (e.g., Schmidt et al., 2017).
Second, while healthy adult menstrual cycles range from 21 to 35 days, cycles in adolescence are more variable, ranging from 21 to 45 days during the first gynecologic year (reviewed in American Academy of Pediatrics Committee on Adolescence, American College of Obstetricians and Gynecologists Committee on Adolescent Health Care, Diaz, Laufer, & Breech, 2006). This is due in part to the greater risk of anovulatory cycles (cycles in which ovulation does not occur), which reduces with advancing gynecologic age (American Academy of Pediatrics Committee on Adolescence et al., 2006). Given that ovulation drives the hormone fluctuations that cause perimenstrual emotional symptoms (e.g., Hammarbäck, Ekholm, & Bäckström, 1991), irregularity in ovulation may contribute to unpredictability in symptoms. This could increase the risk for suicidal thoughts and behaviors in susceptible adolescents, since variability in symptoms of depression predicts suicide attempts (Melhem et al., 2019). Thus, adolescence is characterized not only by the start of menstruation, but also by frequent hormone fluctuation and unpredictability in hormone fluctuation that could confer greater risk.
Adolescence also is characterized by marked changes in social experiences, especially among females, including significant increases in the occurrence of interpersonal stress (Ge, Lorenz, Conger, Elder, & Simons, 1994) and in emotional and physiological responses to interpersonal stress (e.g., Stroud et al., 2009). For some individuals, adolescence may also mark the onset of hormone-driven fluctuations in social stress and social perception over the course of the menstrual cycle (which we explain in greater detail later). These social changes may be especially relevant for understanding the risk for suicidal thoughts and behaviors: Almost all theories developed over than past 50 years to explain suicide highlight interpersonal loss, isolation, or rejection as central if not pre-requisite experiences. Interpersonal stress is a strong predictor of suicidal thoughts and behaviors among adolescent girls, and often precipitates self-injurious behavior (Heilbron & Prinstein, 2010).
Adolescence is also a period of cognitive changes, particularly regarding inhibitory control. Although systems related to reward sensitivity begin maturing early in puberty, regions associated with inhibitory control and executive function mature later in adolescence or in emerging adulthood (Somerville & Casey, 2010). Consequently, adolescents demonstrate impaired inhibitory control under conditions of heightened emotion compared to adults, increasing the likelihood of acting on emotion-related impulses (e.g., engaging in social conflict, acting on urges to attempt suicide) following experiences of stress or perceived social rejection (Somerville & Casey, 2010). After menarche, inhibitory control may also fluctuate over the course of the menstrual cycle for some adolescents (which we also describe in greater detail later). Among adolescents, impaired inhibitory control in the presence of negative emotional stimuli is associated with suicidal thoughts and behaviors (e.g., Cohen-Gilbert et al., 2019).
AN EMERGING FRAMEWORK FOR PERIMENSTRUAL SUICIDE RISK IN ADOLESCENCE
We propose a framework that integrates within-person menstrual cycle fluctuations in suicidal thoughts and behaviors with distal, between-person risk factors in the unique developmental context of adolescence to explain why some adolescent girls are at elevated risk for these thoughts and behaviors and when they are most likely to occur. First, we discuss between-subject factors associated with suicide risk that may be relevant for understanding adolescent hormone sensitivity. Second, we describe within-subject factors that may serve as mechanisms connecting the perimenstrual phase with acute increases in suicidal thoughts and behaviors (see Figure 1). We also offer illustrative examples of potential interactions to guide research, including interactions between developmental contextual factors and the between or within-person risks, or transactions among between-person and within-person factors.
Between-Person Factors
Several factors often linked with increased risk for suicidal thoughts and behaviors are also associated with hormone sensitivity. Early menarche and early adversity are two such factors.
Early Menarche
Early menarche has been associated with both suicidal ideation and behavior (e.g., Roberts, Fraser, Gunnell, Joinson, & Mars, 2019). While the mechanisms of this relationship have not been tested, preliminary data suggest several hypotheses. First, early menarche predicts stronger retrospectively reported perimenstrual symptoms (e.g., PMDD diagnosis; e.g., Cohen et al., 2002). Thus, individuals who experience early menarche may be more likely to experience sensitivity to ovarian hormone fluctuations, which causes perimenstrual elevations in suicidal thoughts and behaviors. Alternatively, both early menarche and perimenstrual symptoms could represent different manifestations of the same intrinsic hormone sensitivity.
Second, for girls who experience hormone sensitivity, early menarche may amplify risk by both increasing the frequency of symptomatic cycles and initiating hormone (and symptom) fluctuation during an earlier stage of neural development. Girls who experience menarche earlier have fewer anovulatory cycles than their later-developing peers (American Academy of Pediatrics Committee on Adolescence et al., 2006), which confers greater exposure to the hormone flux that underlies symptoms (Hammarbäck et al., 1991).
In addition, early menarche may interact with broader developmental contextual factors. For instance, since neural systems associated with inhibitory control mature later in development, girls who start menarche early may have less inhibitory control over emotion-driven urges than their older peers, and may therefore experience more functional impairment from cyclical symptoms. This developmental interaction suggests that the relationship between early menarche and suicidal thoughts and behaviors may decline as inhibitory control matures. Consistent with this hypothesis, in one study, the association between early menarche and suicidal behavior dissipated from ninth to 11th grade (Fried, Williams, Cabral, & Hacker, 2013), although in another study, it persisted into young adulthood (Roberts et al., 2019). In yet another study, among girls who experienced early menarche, more time since menarche was associated with girls’ greater use of effective coping strategies (Alcalá-Herrera & Marván, 2014). Taken together, these findings suggest that the effects of early menarche on perimenstrual risk of suicidal thoughts and behaviors may be strongest during early adolescence.
Early Life Adversity
Early life adversity may also affect perimenstrual increases in suicidal thoughts and behaviors by increasing hormone sensitivity. Among women with PMDD, early life adversity has been associated with exaggerated negative mood responses to cyclical hormone changes (Eisenlohr-Moul et al., 2016). Early life adversity may also indirectly affect perimenstrual risk by increasing the likelihood of early menarche; abuse, exposure to parental conflict, maternal mood disorders, and paternal absence all contribute to the early onset of menarche (e.g., Allison & Hyde, 2013). In addition, early adversity may affect hormone sensitivity and perimenstrual risk for suicidal thoughts and behaviors by influencing trait-level cognitive control (e.g., Lambert, King, Monahan, & McLaughlin, 2017), which may contribute to greater perimenstrual increases in impulsive behavior (Roberts, Eisenlohr-Moul, & Martel, 2018) and thus risk for such thoughts and behaviors.
Within-Person Factors
Our framework suggests that the perimenstrual phase is associated with increases in risk for suicidal thoughts and behaviors among girls with hormone sensitivity. Next, we articulate four psychological and physiological mechanisms that may explain how hormone sensitivity may be associated with risk for suicide among adolescent girls. Then we briefly outline several hypothesized transactions among social disruptions, inhibitory control deficits, negative affect, and physical symptoms that may lead to suicidal thoughts and behaviors. Given the centrality of social disruption and inhibitory control deficits in prevailing theories of suicide and the unique milieu of social and cognitive changes during adolescence, we focus on social disruptions and changes in inhibitory control.
Social Disruptions
Impairment in social functioning is one of the most commonly experienced perimenstrual symptoms; among adolescents, the prevalence of retrospectively reported perimenstrual social impairment may be as high as 39% (adolescent gynecology out-patients; Vichnin, Freeman, Lin, Hillman, & Bui, 2006). Interpersonal sensitivity, perceived burdensomeness, felt invalidation, and rejection sensitivity all increase at the onset of menses in hormone-sensitive adults (Eisenlohr-Moul et al., 2018; Pearlstein, Yonkers, Fayyad, & Gillespie, 2005) and have been linked to suicidal thoughts and behaviors. Yet few studies have examined perimenstrual social changes among adolescents, despite the fact that social disruptions are associated longitudinally with suicide among adolescents (Heilbron & Prinstein, 2010). Two prospective studies of adolescents revealed perimenstrual increases in angry outbursts, aggression, or social withdrawal (Buddhabunyakan et al., 2017; Czajkowska et al., 2015), which are suicide risk factors that harm both group and dyadic social relationships.
Inhibitory Control
Women with PMDD frequently report perimenstrual symptoms related to inhibitory control, such as difficulty concentrating and behavioral impulsivity (Pearlstein et al., 2005). In addition, several studies using behavioral tasks have documented perimenstrual reductions in inhibitory control and associated cortical activity for some hormone-sensitive women (for a review, see Owens & Eisenlohr-Moul, 2018). Studies with younger participants have found similar perimenstrual elevations in self-reported impulsive behavior among those with high trait-level impulsivity (Roberts et al., 2018) or high borderline personality disorder traits (reviewed in Eisenlohr-Moul, 2019), but no studies of adolescents have examined within-person changes in inhibitory control across the cycle using behavioral measures. In addition, deficits of inhibitory control have been identified as a potential risk factor in the transition from suicidal thoughts to suicidal behavior, particularly among adolescents (e.g., Glenn & Nock, 2014).
Negative Affect
The perimenstrual phase is associated with increases in negative affect, including depressed mood, anxiety, mood lability, and anger/irritability among women with PMDD. It is also associated with worsened mood among women with depressive disorders (reviewed in Eisenlohr-Moul, 2019) and hormone-sensitive adolescents (Buddhabunyakan et al., 2017; Czajkowska et al., 2015). Naturally, within-person increases in negative affect are associated strongly with increases in adolescent girls’ suicidal thoughts and behaviors (e.g., Nock, Prinstein, & Sterba, 2010).
Physical Symptoms
Changes in physical symptoms across the menstrual cycle may also confer elevated risk for suicidal thoughts and behaviors in the perimenstrual phase. In prospective studies of adolescents, dysmenorrhea, breast tenderness, headaches, and abdominal bloating were among the most commonly reported perimenstrual symptoms, occurring in 42–75% of individuals (Buddhabunyakan et al., 2017; Czajkowska et al., 2015). These physical symptoms may have important psychological consequences, causing or exacerbating distress across the cycle (Kiesner, 2009). Acute headache pain and chronic pain are also associated with increased risk of attempting suicide (e.g., Breslau, Schultz, Lipton, Peterson, & Welch, 2012).
Potential Transactions Among Within-Person Symptoms
Reciprocal transactions among social disruptions, inhibitory control deficits, negative affect, and physical symptoms may contribute to heightened risk for suicidal thoughts and behaviors, although data are limited. For example, perimenstrual increases in anger and embarrassment have been associated with increases in aggressive behavior in prospective studies of adolescents (Czajkowska et al., 2015), whereas increased depression and decreased self-worth negatively affected social functioning (Czajkowska et al., 2015). Numerous additional potential transactions require further exploration. For example, cyclical increases in sensitivity to rejection (Eisenlohr-Moul et al., 2018) and irritability (Buddhabunyakan et al., 2017; Czajkowska et al., 2015) may increase the likelihood of angry outbursts and interpersonal conflict (Czajkowska et al., 2015) under conditions of reduced inhibitory control (e.g., Pearlstein et al., 2005). These conflicts may in turn lead to increased social withdrawal and negative affect (Buddhabunyakan et al., 2017; Czajkowska et al., 2015), which may be further exacerbated by physical pain and discomfort (Kiesner, 2009). In summary, hormone-driven changes in mood, cognition, physical symptoms, and behavior may interact to increase suicidal thoughts and behaviors through a variety of plausible, mutually reinforcing pathways.
CONCLUSIONS AND LOOKING AHEAD
To improve efforts to predict and prevent suicide among adolescent girls, it is critical to assess not only distal, between-person risk factors, but also acute within-person fluctuations in risk, and how these factors may change throughout development. Understanding how changes in reproductive hormones can trigger social disruptions, cognitive disinhibition, negative affect, and physical symptoms offers a promising avenue for further research on adolescents’ suicidal thoughts and behaviors. Together, these findings suggest that the onset of menstrual cycle hormone fluctuations may help clarify why risk for suicidal behavior increases at puberty and why girls who experience early menarche and early life adversity are at elevated risk. Further development of this framework can help improve prediction and prevention of female adolescent suicide.
Studies that seek to test and refine this framework would benefit by addressing several critical limitations of prior work. First, while the validity of retrospective (cross-sectional) reports of premenstrual symptoms in adolescents remains untested, among adults, retrospective reports appear to be biased by sociocultural stereotypes (Marván & Cortés-Iniestra, 2001) and often have little association with cyclical changes observed in daily ratings of symptoms (e.g., Eisenlohr-Moul et al., 2018). Therefore, longitudinal and experimental studies using daily symptom ratings linked to cycle phase are needed to understand more clearly cyclical changes in symptoms among adolescents, and determine which specific hormonal triggers lead to changes in symptoms (see Schmalenberger, Owens, & Eisenlohr-Moul, 2019, for a methodological review).
Second, sampling considerations may limit the conclusions that can be drawn from studies of the menstrual cycle. To examine cycle effects, samples exclude individuals taking hormonal medications or using hormonal forms of birth control (e.g., oral contraceptives, the pill, hormonal IUDs). It remains unclear how and if these limitations may alter results. Since the use of hormonal birth control increases from early adolescence to adulthood, studies of the menstrual cycle in adolescents may capture broader, more representative samples than studies of adults.
Third, while this framework suggests new lines of inquiry for suicide prevention and intervention, novel studies of treatment targets in adolescents are essential because current treatment recommendations for adults with PMDD and PME of other disorders are untested for hormone-sensitive adolescents. Although the first line of treatment for PMDD are selective serotonin reuptake inhibitors (SSRIs; Eisenlohr-Moul, 2019), SSRIs may increase suicide risk among adolescents (Rapkin & Mikacich, 2013). In addition, while suppressing hormone cycling using oral contraceptives may be recommended for adults with PMDD, this approach is ineffective in alleviating PME of other disorders (Eisenlohr-Moul, 2019) and is prospectively associated with increased risk for suicidal thoughts and behaviors among adolescents generally (e.g., Skovlund, Mørch, Kessing, Lange, & Lidegaard, 2018). In summary, we need large clinical trials to determine the effectiveness of traditional treatment options among adolescents with PMDD and PME, perhaps especially with respect to outcomes related to suicidal thoughts and behaviors (Eisenlohr-Moul, 2019).
Fourth, studies of psychosocial and behavioral mechanisms have been limited by the predominant use of self-report measures. Going forward, studies would benefit from including behavioral measures and dyadic interaction tasks to assess perimenstrual changes in behavior among adolescents. While hormone-sensitive individuals report perimenstrual changes in social functioning and impulsivity, it remains unclear whether these effects are driven by changes in self-perception, changes in perception of others (e.g., perceived rejection), or measurable changes in behavior (e.g., aggression, inhibitory control). Behavioral tasks and dyadic interaction paradigms will help distinguish changes in behavior and perception that may elevate perimenstrual risk.
In summary, the menstrual cycle can cause social disruptions, inhibitory control deficits, negative affect, and physical symptoms in adolescents, and these changes may cause cyclical changes in acute risk for suicide. The onset of hormone fluctuations may mark the start of monthly fluctuations in suicide risk, and thus represents a novel risk factor for both the onset of suicidality in development and the acute exacerbation of suicidality each month. Continued study in this area could help researchers and clinicians identify nuanced mechanisms that lead to death from suicide and that build novel interventions targeting proximal risk.
Acknowledgments
The work reported in this article was supported in part by grants from the National Institute of Mental Health to Sarah A. Owens (F31-MH120965), Tory A. Eisenlohr-Moul (R00-MH109667 and RF1-MH120843), and Mitchell J. Prinstein (R01-MH107479 and 1R01MH122446).
REFERENCES
- Alcalá-Herrera V, & Marván ML (2014). Early menarche, depressive symptoms, and coping strategies. Journal of Adolescence, 37, 905–913. 10.1016/j.adolescence.2014.06.007 [DOI] [PubMed] [Google Scholar]
- Allison CM, & Hyde JS (2013). Early menarche: Confluence of biological and contextual factors. Sex Roles, 68, 55–64. 10.1007/s11199-011-9993-5 [DOI] [Google Scholar]
- American Academy of Pediatrics Committee on Adolescence, American College of Obstetricians and Gynecologists Committee on Adolescent Health Care, Diaz A, Laufer MR, & Breech L (2006). Menstruation in girls and adolescents: Using the menstrual cycle as a vital sign. Pediatrics, 118, 2245–2250. 10.1542/peds.2006-2481 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: Author; 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Breslau N, Schultz L, Lipton R, Peterson E, & Welch KMA (2012). Migraine headaches and suicide attempt. Headache: The Journal of Head and Face Pain, 52, 723–731. 10.1111/j.1526-4610.2012.02117.x [DOI] [PubMed] [Google Scholar]
- Buddhabunyakan N, Kaewrudee S, Chongsomchai C, Soontrapa S, Somboonporn W, & Sothornwit J (2017). Premenstrual syndrome (PMS) among high school students. International Journal of Women’s Health, 9, 501–505. 10.2147/IJWH.S140679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Otto MW, Sweeney BH, Liberman RF, & Harlow BL (2002). Prevalence and predictors of premenstrual dysphoric disorder (PMDD) in older premenopausal women: The Harvard Study of Moods and Cycles. Journal of Affective Disorders, 70, 125–132. 10.1016/S0165-0327(01)00458-X [DOI] [PubMed] [Google Scholar]
- Cohen-Gilbert J, Schuttenberg E, Rieselbach M, Stein E, Sarvey D, Feinberg J, … Silveri M (2019). T141. Suicidality and emotional inhibitory control in dually-diagnosed adolescents. Biological Psychiatry, 85, S183–S184. 10.1016/j.biopsych.2019.03.464 [DOI] [Google Scholar]
- Czajkowska M, Drosdzol-Cop A, Gałązka I, Naworska B, & Skrzypulec-Plinta V (2015). Menstrual cycle and the prevalence of premenstrual syndrome/premenstrual dysphoric disorder in adolescent athletes. Journal of Pediatric and Adolescent Gynecology, 28, 492–498. 10.1016/j.jpag.2015.02.113 [DOI] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA (2019). Premenstrual disorders: A primer and research agenda for psychologists. The Clinical Psychologist, 72, 5–17. 10.31234/osf.io/tw4bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, Rubinow DR, Schiller CE, Johnson JL, Leserman J, & Girdler SS (2016). Histories of abuse predict stronger within-person covariation of ovarian steroids and mood symptoms in women with menstrually related mood disorder. Psychoneuroendocrinology, 67, 142–152. 10.1016/j.psyneuen.2016.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, Schmalenberger KM, Owens SA, Peters JR, Dawson DN, & Girdler SS (2018). Perimenstrual exacerbation of symptoms in borderline personality disorder: Evidence from multilevel models and the Carolina Premenstrual Assessment Scoring System. Psychological Medicine, 48, 2085–2095. 10.1017/S0033291718001253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LE, Williams S, Cabral H, & Hacker K (2013). Differences in risk factors for suicide attempts among 9th and 11th grade youth: A longitudinal perspective. The Journal of School Nursing, 29, 113–122. 10.1177/1059840512461010 [DOI] [PubMed] [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, & Simons RL (1994). Trajectories of stressful life events and depressive symptoms during adolescence. Developmental Psychology, 30, 467–483. 10.1037/0012-1649.30.4.467 [DOI] [Google Scholar]
- Glenn CR, & Nock MK (2014). Improving the prediction of suicidal behavior in youth. International Journal of Behavioral and Consultation Therapy, 9, 7–10. 10.1037/h0101633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Borenstein J, Pearlstein T, & Kahn LS (2003). The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology, 28, 1–23. 10.1016/S0306-4530(03)00098-2 [DOI] [PubMed] [Google Scholar]
- Hammarbäck S, Ekholm UB, & Bäckström T (1991). Spontaneous anovulation causing disappearance of cyclical symptoms in women with the premenstrual syndrome. Acta Endocrinologica, 125, 132–137. 10.1530/acta.0.1250132 [DOI] [PubMed] [Google Scholar]
- Hartlage SA, Brandenburg DL, & Kravitz HM (2004). Premenstrual exacerbation of depressive disorders in a community-based sample in the United States. Psychosomatic Medicine, 66, 698–706. 10.1097/01.psy.0000138131.92408.b9 [DOI] [PubMed] [Google Scholar]
- Heilbron N, & Prinstein MJ (2010). Adolescent peer victimization, peer status, suicidal ideation, and nonsuicidal self-injury. Merrill-Palmer Quarterly, 56, 388–419. 10.1353/mpq.0.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janfaza M, Sherman TI, Larmore KA, Brown-Dawson J, & Klein KO (2006). Estradiol levels and secretory dynamics in normal girls and boys as determined by an ultrasensitive bioassay: A 10 year experience. Journal of Pediatric Endocrinology & Metabolism, 19, 901–909. 10.1515/jpem.2006.19.7.901 [DOI] [PubMed] [Google Scholar]
- Kann L, McManus T, Harris WA, Shanklin S, Flint K, Queen B, … Ethier KA (2018). Youth risk behavior surveillance—United States, 2017. MMWR Surveillance Summaries, 67, 1–114. 10.15585/mmwr.ss6708a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesner J (2009). Physical characteristics of the menstrual cycle and premenstrual depressive symptoms. Psychological Science, 20, 763–770. 10.1111/j.1467-9280.2009.02358.x [DOI] [PubMed] [Google Scholar]
- Lambert HK, King KM, Monahan KC, & McLaughlin KA (2017). Differential associations of threat and deprivation with emotion regulation and cognitive control in adolescence. Development and Psychopathology, 29, 929–940. 10.1017/S0954579416000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marván ML, & Cortés-Iniestra S (2001). Women’s beliefs about the prevalence of premenstrual syndrome and biases in recall of premenstrual changes. Health Psychology, 20, 276–280. 10.1037//0278-6133.20.4.276 [DOI] [PubMed] [Google Scholar]
- Melhem NM, Porta G, Oquendo MA, Zelazny J, Keilp JG, Iyengar S, … Brent DA (2019). Severity and variability of depression symptoms predicting suicide attempt in high-risk individuals. JAMA Psychiatry, 76, 603–613. 10.1001/jamapsychiatry.2018.4513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Eisenlohr-Moul TA, & Kiesner J (2016). From menarche to menopause: Women’s reproductive milestones and risk for psychopathology—An introduction to the special series. Clinical Psychological Science, 4, 859–866. 10.1177/2167702616650424 [DOI] [Google Scholar]
- Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, & Kessler RC (2013). Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: Results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry, 70, 300–310. 10.1001/2013.jamapsychiatry.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Prinstein MJ, & Sterba SK (2010). Revealing the form and function of self-injurious thoughts and behaviors: A real-time ecological assessment study among adolescents and young adults. Psychology of Violence, 1, 36–52. 10.1037/2152-0828.1.S.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens SA, & Eisenlohr-Moul TA (2018). Suicide risk and the menstrual cycle: A review of candidate RDoC mechanisms. Current Psychiatry Reports, 20, 106 10.1007/s11920-018-0962-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein T, Yonkers KA, Fayyad R, & Gillespie JA (2005). Pretreatment pattern of symptom expression in premenstrual dysphoric disorder. Journal of Affective Disorders, 85, 275–282. 10.1016/j.jad.2004.10.004 [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, & Mikacich JA (2013). Premenstrual dysphoric disorder and severe premenstrual syndrome in adolescents. Paediatric Drugs, 15, 191–202. 10.1007/s40272-013-0018-4 [DOI] [PubMed] [Google Scholar]
- Roberts B, Eisenlohr-Moul TA, & Martel MM (2018). Reproductive steroids and ADHD symptoms across the menstrual cycle. Psychoneuroendocrinology, 88, 105–114. 10.1016/j.psyneuen.2017.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E, Fraser A, Gunnell D, Joinson C, & Mars B (2019). Timing of menarche and self-harm in adolescence and adulthood: a population-based cohort study. Psychological Medicine, 1–9. 10.1017/S0033291719002095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders KEA, & Hawton K (2006). Suicidal behaviour and the menstrual cycle. Psychological Medicine, 36, 901–912. 10.1017/S0033291706007392 [DOI] [PubMed] [Google Scholar]
- Schmalenberger KM, Owens SA, & Eisenlohr-Moul TA (2019). Studying the menstrual cycle as an independent variable: Practical recommendations and tools for getting started. Open Science Framework. 10.31219/osf.io/94jua [DOI] [Google Scholar]
- Schmidt PJ, Martinez PE, Nieman LK, Koziol DE, Thompson KD, Schenkel L, … Rubinow DR (2017). Exposure to a change in ovarian steroid levels but not continuous stable levels triggers PMDD symptoms following ovarian suppression. The American Journal of Psychiatry, 174, 980–989. 10.1176/appi.ajp.2017.16101113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovlund CW, Mørch LS, Kessing LV, Lange T, & Lidegaard Ø (2018). Association of hormonal contraception with suicide attempts and suicides. The American Journal of Psychiatry, 175, 336–342. 10.1176/appi.ajp.2017.17060616 [DOI] [PubMed] [Google Scholar]
- Somerville LH, & Casey BJ (2010). Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology, 20, 236–241. 10.1016/j.conb.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, & Niaura R (2009). Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology, 21, 47–68. 10.1017/S0954579409000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichnin M, Freeman EW, Lin H, Hillman J, & Bui S (2006). Premenstrual syndrome (PMS) in adolescents: Severity and impairment. Journal of Pediatric and Adolescent Gynecology, 19, 397–402. 10.1016/j.jpag.2006.06.015 [DOI] [PubMed] [Google Scholar]
- Wei S-M, Schiller CE, Schmidt PJ, & Rubinow DR (2018). The role of ovarian steroids in affective disorders. Current Opinion in Behavioral Sciences, 23, 103–112. 10.1016/j.cobeha.2018.04.013 [DOI] [Google Scholar]
- Wetzel RD, Reich T, & McClure JN (1971). Phase of the menstrual cycle and self-referrals to a suicide prevention service. The British Journal of Psychiatry, 119, 523–524. 10.1192/bjp.119.552.523 [DOI] [PubMed] [Google Scholar]