Abstract
Subclinical hypothyroid men characterized by a rise in only thyroid stimulating hormone (TSH) levels and normal thyroid hormone levels showed a fall in their serum progesterone and testosterone levels. This suggested a role of TSH in regulating Leydig cell steroidogenesis. Therefore, we investigated the direct role of TSH on steroid production and secretion using a mouse Leydig tumour cell line, MLTC-1. MLTC-1 cells were treated with different doses of TSH isolated from porcine pituitary as well as recombinant TSH. Steroid secretion was measured by radioimmunoassay. The mRNA levels of steroidogenic enzymes were quantitated by real time PCR whereas the corresponding protein levels were determined by Western blot. In MLTC-1 cells, pituitary TSH as well as recombinant TSH inhibited progesterone and testosterone secretion in a dose dependent manner. The inhibitory action of TSH on steroid secretion was unique and not mimicked by other anterior pituitary hormones including FSH and ACTH. Recombinant TSH showed no effect on StAR and CYP11A1, the enzymes catalysing the non-steroidogenic and steroidogenic rate-limiting steps of steroid synthesis respectively. Recombinant TSH was shown to inhibit steroidogenesis in MLTC-1 cells by inhibiting the 3β hydroxy steroid dehydrogenase mRNA and protein levels, the enzyme that catalyses the conversion of pregnenolone to progesterone. This inhibitory effect of TSH is probably direct as both mRNA and protein of the TSH receptor were shown to be present in the MLTC-1 cells.
Keywords: Leydig cells, steroidogenesis, TSH/TSH receptor
Summary sentence:
TSH inhibits sex steroid secretion in rat Leydig cell line through its action on TSH receptors in a dose dependent fashion by reducing 3β hydroxy steroid dehydrogenase mRNA and protein levels.
INTRODUCTION
The thyroid hormone 3, 5, 3’-L-triiodothyronine (T3) was shown to increase basal and 3’, 5’ cyclic adenosine monophosphate (cAMP) stimulated steroid production in primary Leydig cells and their derived lines (1-3). Overt hypothyroid men showed a significant reduction in their serum progesterone and testosterone levels (4). This reduction in serum steroid levels could be due to reduced thyroid hormone levels or increased thyroid stimulating hormone (TSH) levels or both. Although animal studies have consistently showed the direct effects of T3 on Leydig cell function proliferation and steroidogenesis, the exact effects and mechanisms of action remain controversial. (5, 6) Inconsistencies in the findings of existing literature about the impact of hypothyroidism on Testosterone and gonadotropin production raise questions about the role of TSH invivo. (7, 8) Studies in subclinical hypothyroid men, characterized by a rise in only TSH levels with normal thyroid hormone levels, also showed a similar reduction in serum progesterone and testosterone levels (9). As only TSH was raised in these subclinical hypothyroid men, this furthers the suggestion that a direct inhibitory action of TSH occurs on steroid production. Interestingly, studies have demonstrated the presence of TRH receptors on Leydig cells, which is unique to them among all testicular cell types. (10) This also raises the possibility of other thyroid hormones such as TSH, acting on Leydig cells through their receptors. To date, no studies have been conducted to see the direct modulation of steroid production and secretion by TSH in Leydig cells.
Therefore, we investigated the role of TSH on steroid production and secretion using a mouse Leydig tumour cell line, MLTC-1. We also investigated the effect of TSH on steroidogenic enzymes to dissect out the molecular mechanism(s) used by TSH for regulating steroid secretion.
MATERIALS AND METHODS
Ethics Clearance
The study protocol was approved by the Institute Ethics committee of AIIMS.
Hormones and Chemicals
Pituitary-derived TSH, Luteinizing hormone (LH), Follicle stimulating hormone (FSH) and Adrenocorticotropic hormone (ACTH) were purchased from Sigma–Aldrich (St. Louis, USA). Recombinant TSH was purchased from R&D systems (Minneapolis, USA). Tritium labelled radioactive progesterone and testosterone were purchased from Perkin Elmer (Waltham, USA). Both the mouse monoclonal testosterone antibody (clone 4E1G2) and rabbit polyclonal progesterone antibody were purchased from Bio-Rad (Hercules, USA). Primers were purchased from Sigma–Aldrich (St. Louis, USA). Rabbit anti-StAR antibody and rabbit anti-CYP11A1 antibody were purchased from Cell Signalling Technologies (Boston, USA). Rabbit anti-3β-hydroxy steroid dehydrogenase-1 (3BHSD-1) antibody and rabbit anti-TSH receptor antibody were purchased from Abcam (Cambridge, UK). Anti-rabbit HRP labelled secondary antibody was purchased from Cell Signalling Technologies (Boston, USA).
Cell Culture
MLTC-1 cells were obtained from the American Type Culture Collection (Manassas, USA). MLTC-1 cells express the human chorionic gonadotropin (hCG) receptor. They respond to LH/hCG with stimulation of steroid production (11). The MLTC-1 cells were routinely maintained in Waymouth MB 752/1 medium (Sigma Aldrich, St. Louis, USA) supplemented with 10% heat-inactivated fetal bovine serum (Sigma Aldrich, St. Louis, USA) at 37°C in 5% CO2 in a humidified incubator. The MLTC-1 cells used for all the experiments had a passage number less than 30.
Cell Treatments
MLTC-1 cells were seeded at a density of 2 X 105 cells/ ml/well for measuring steroid secretion and at 1 X 106 cells /well for total RNA extraction and Western blot. MLTC-1 cells were pre-incubated separately for 2 hours with different doses of pituitary or recombinant TSH, or FSH or ACTH in serum free media. Thereafter, the cells were incubated with 100 mIU/ml LH or 1mM 8-Br-cAMP. The total period of incubation including the time of pre-incubation was 6 hours as MLTC-1 cells begin proliferation by 9 hours. Viability of the cells after treatment were tested using [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] (MTT, Sigma Aldrich, St. Louis, USA).
Steroid Production
Steroid secretion was measured in cell supernatant by Radioimmunoassay (RIA) according to the WHO guidelines. Both the mouse monoclonal testosterone antibody (clone 4E1G2) and rabbit polyclonal progesterone antibody were used at a dilution of 1:500. The progesterone antibody used for RIA was highly specific and showed no cross-reactivity for pregnenolone as well as for testosterone. However, the testosterone antibody also recognizes 5α-dihydrotestosterone (DHT, 1%) and androstenedione (1%). The intra-assay variation was less than 5% and the inter-assay variation was less than 10%.
RNA Extraction and Reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was extracted using Ribozol RNA extraction reagent (Amresco, Solon, USA) as per manufacturer’s instructions. Total RNA isolated from the cells was reverse transcribed using oligo dT primers and RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, USA). The cDNA synthesized was amplified using gene specific primers (Sigma Aldrich, St. Louis, USA).The sequences of steroidogenic acute regulatory protein (StAR) primers were Forward 5’ GACCTTGAAAGGCTCAGGAAGAAC 3’ and Reverse 5’ TAGCTGAAGATGGACAGACTTGC 3’ (1). The sequences of CYP11A1 primers were Forward 5’ CAACATCACAGAGATGCTGGCAGG3’ and Reverse 5’ CTCAGGCATCAGGATGAGGTTGAA 3’ (12). The sequences of 3BHSD-I primers were Forward 5’ GACAGGAGCAGGAGGGTTTGTGG 3’ and Reverse 5’ CTCCTTCTAACATTGTCACCTTGGCCT 3’ (13). The sequences of TSH receptor (TSHR) primers were Forward 5’ TTC CAG CCG CTG CAG AGT TGC3’ and Reverse 5’ GAG TGT GCG TCT CCA CCC TG 3’ (14). The sequences of GAPDH primers were Forward 5’ ACGGGAAGCTTGTCATCAAT 3’ and Reverse 5’TGGACTCCACGACGTCGTACTCA 3’ (15). The steroidogenic enzymes were amplified by quantitative real time PCR. The PCR conditions used were initial denaturation at 95°C for 5 minutes followed by 40 cycles of 95°C for 20 seconds, 56°C for 20 seconds and 72°C for 40 seconds. Ct values obtained during the real time PCR reaction were used to calculate the relative mRNA expression using the formula: Relative mRNA expressionStAR/CYP11A1/3β HSD= 2−ΔΔCt where ΔCt = CtStAR/CYP11A1/3β HSD– CtGAPDH and ΔΔCt = ΔCtBasal – ΔCtTreated. The TSHR gene was amplified by semi-quantitative RT-PCR. The PCR conditions used were initial denaturation at 95°C for 3 minutes followed by 40 cycles of 95°C for 30 seconds, 60°C for 20 seconds and 72°C for 45 seconds. The amplified products were size-fractionated in a 2% (w/v) agarose gel and visualized by staining with ethidium bromide (0.2ng/ml).
Western Blot
Total protein extracts were separated by SDS-PAGE using 12% resolving gel. The protein bands in the gel were transferred onto a 0.45μm PVDF membrane. PVDF membrane was blocked with 5% bovine serum albumin (BSA) solution made in TBS-T (0.1% Tween-20 in 1X TBS). The membrane was probed with rabbit anti-StAR antibody, rabbit anti-CYP11A1 antibody, rabbit anti-3BHSD-1 antibody, rabbit anti-TSHR antibody or rabbit anti-β-actin antibody. A 1:1000 dilution was used for each of the primary antibody and incubated for overnight at 4°C. Anti-rabbit HRP labelled secondary antibody at 1:3000 dilution was used. Protein bands were visualized using ECL system (Merck Millipore, Billerica, USA). The density of the protein bands were measured using ImageJ software developed at the National Institute of Health (Bethesda, USA).
Statistical Analysis
All the treatments were carried out in triplicates and at least three times. The data was pooled and analysed using GraphPad Prism 4.0 software (GraphPad Prism Software Inc., San Diego, CA, USA). The results were analysed by Student’s t test. p<0.05 was considered significant.
RESULTS
TSH inhibits steroid secretion
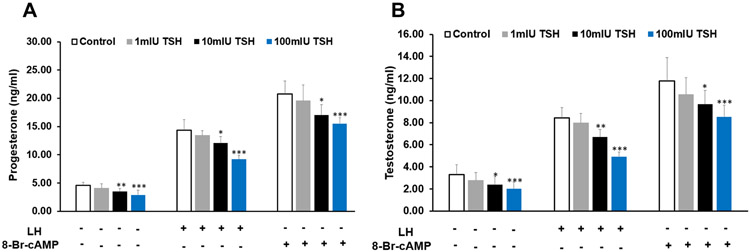
Initially, different doses of pituitary TSH ranging from 1 – 100 mIU/ml were tested for their effect on steroid secretion by MLTC-1 cells. The minimum dose of 1mIU/ml of TSH showed no significant effect on basal, LH or 8-Br-cAMP-stimulated progesterone and testosterone secretion. However, the higher doses of TSH significantly inhibited both progesterone and testosterone secretion in a dose dependent manner (Fig. 1 a, b). 100mIU/ml of TSH inhibited basal progesterone and testosterone secretion by 37% and 39% respectively (Fig. 1 a, b). 100mIU/ml of TSH also inhibited LH and 8-Br-cAMP-stimulated progesterone secretion by 35% and 25% respectively. 100mIU/ml of TSH inhibited LH and 8-Br-cAMP-stimulated testosterone secretion by 42% and 27% respectively (Fig. 1 a, b).
Figure 1.
Effect of pituitary TSH on (a) progesterone (b) testosterone secretion by MLTC-1 cells under basal and treated conditions. LH or 8-Br-cAMP were added after 2 hour pre-incubation with different doses of TSH. Bars represent mean ± SD of three experiments; each experiment was done at least in triplicates (n=10). * indicate p<0.05; ** indicate p<0.01; *** indicate p<0.001 in cells treated without and with TSH in the corresponding groups.
Effect of TSH on steroid secretion is specific
To rule out the possibility that the inhibitory effect of TSH on steroid secretion is non-specific in nature, we used two other pituitary-derived hormones, FSH and ACTH. However, both FSH and ACTH showed no significant effect on basal, LH or 8-Br-cAMP-stimulated progesterone and testosterone secretion (Fig. 2a, b, c, d). This suggested that steroid secretion is specifically inhibited by TSH.
Figure 2.

Effect of pituitary FSH on (a) progesterone (b) testosterone secretion; effect of pituitary ACTH on (c) progesterone and (d) testosterone secretion by MLTC-1 cells under basal and treated conditions. LH or 8-Br-cAMP were added after 2 hour pre-incubation with different doses of FSH or ACTH. Bars represent mean ± SD of three experiments; each experiment was done at least in triplicates (n=10).
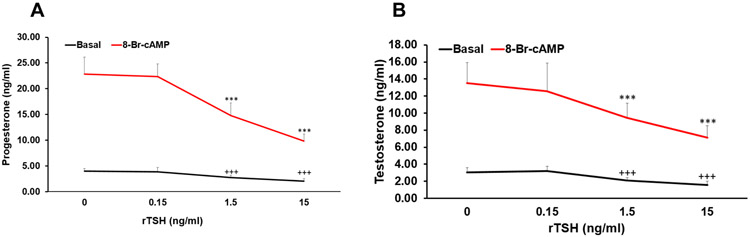
To further confirm that the inhibitory effect of TSH was not due to any other contaminant present in the pituitary extract, we used recombinant TSH (rTSH). The minimum dose of 0.15 ng/ml of rTSH showed no significant effect on basal or 8-Br-cAMP-stimulated progesterone and testosterone secretion. However, both the higher doses of rTSH significantly inhibited both progesterone and testosterone secretion in a dose dependent manner (Fig. 3a, b). While 1.5 ng/ml of rTSH inhibited basal progesterone and testosterone secretion by 32% and 31%, 15 ng/ml of rTSH inhibited those by 61% and 45% respectively (Fig. 3a, b). The inhibitory effect of rTSH on 8-Br-cAMP-stimulated progesterone and testosterone secretion was even more pronounced. Maximal inhibition was observed with 15 ng/ml of rTSH on 8-Br-cAMP-stimulated progesterone and testosterone secretion by 48% and 49% respectively (Fig. 3a, b).
Fig. 3.
Effect of recombinant TSH (rTSH) on (a) progesterone (b) testosterone secretion by MLTC-1 cells under basal and treated conditions. 8-Br-cAMP was added after 2 hour pre-incubation with different doses of TSH. Bars represent mean ± SD of three experiments; each experiment was done atleast in triplicates (n=10). +++ indicate p<0.001in cells treated without and with TSH in the basal group. *** indicate p<0.001in cells treated without and with TSH in the 8-Br-cAMP treated group.
Effect of TSH on expression of steroidogenic enzymes
The molecular mechanism underlying the inhibition of steroid secretion by TSH was worked out by studying the expression of the steroidogenic enzymes after treatment with rTSH. Interestingly, rTSH showed no significant effect on StAR and CYP11A1 mRNA and protein levels in MLTC-1 cells (Fig. 4a, b; 5a, b) but expression of 3BHSD-1 was significantly reduced by both 1.5 ng/ml and 15 ng/ml dose of rTSH (Fig. 4c; 5c). 15 ng/ml of rTSH inhibited basal and 8-Br-cAMP-stimulated 3BHSD-I mRNA levels by 37% and 51% respectively (Fig. 4c) while the protein levels were inhibited by 40% and 53% respectively (Fig. 5c).
Figure 4.

Effect of rTSH on (a) StAR (b) CYP11A1 and (c) 3β-HSD mRNA levels by quantitative real time PCR after normalization with GAPDH. Bars represent mean ± SD of three experiments; each experiment was done in triplicates (n =9). *** indicate p<0.001 in cells treated without and with TSH in the corresponding groups.
Figure 5.
Effect of rTSH on (a) StAR (b) CYP11A1 and (c) 3β-HSD protein levels by Western blot after normalization with β-actin. Bars represent mean ± SD of densitometric measurements of Western blot bands. * indicate p<0.05; ** indicate p<0.01; *** indicate p<0.001 in cells treated without and with TSH in the corresponding groups.
TSHR is expressed by MLTC-1 cells
Next we investigated whether the action of TSH on MLTC-1 cells was mediated through its specific G-protein coupled plasma membrane receptor. We performed RT-PCR using specific primers for TSHR and western blot using anti-TSHR antibody to detect the presence of TSHR mRNA and protein in MLTC-1 cells. RNA or protein isolated from thyroid tissue was used as positive control while Vero cells, a monkey kidney derived cell line, were used as negative control. TSHR mRNA and the corresponding protein were identified in the MLTC-1 cells (Fig. 6).
Figure 6.

Presence of (a) TSHR mRNA and (b) TSHR protein in thyroid tissue and MLTC-1 cells.
DISCUSSION
Primary Leydig cells are available only in small numbers and are often contaminated with other cell types. Therefore, for the present investigation we have used Leydig cell derived cell line, MLTC-1.
In Leydig cell derived MLTC-1 cell line, TSH isolated from porcine pituitary significantly decreased basal progesterone and testosterone secretion in a dose-dependent manner (Fig. 1a, b). TSH also inhibited LH and 8-Br-cAMP-stimulated progesterone and testosterone secretion suggesting that the inhibitory effect of TSH on steroids is distal to adenylate cyclase enzyme effect. Similarly, in primary cultures of rat granulosa cells, TSH was shown to inhibit steroid secretion (16). This finding was also confirmed in monkey and human granulosa cells (17).
To rule out if the inhibitory effect of TSH on steroid secretion is non-specific in nature, we used FSH and ACTH. FSH is structurally related to TSH; both having a common α subunit. FSH is secreted by anterior pituitary gonadotrophs. The inhibitory action of TSH on steroid secretion was not mimicked by FSH (Fig. 2a, b). ACTH, another protein secreted by the anterior pituitary but structurally unrelated to TSH also showed no significant effect on basal or 8-Br-cAMP-stimulated steroid secretion (Fig.2c, d). This suggests that the inhibitory effect of TSH on steroid secretion is unique and a specific property of the TSH hormone.
To work out the molecular mechanism of inhibition we have used recombinant TSH (rTSH) for further experiments. Pituitary preparations of TSH are mostly contaminated with other contaminants. Therefore, to rule out the possibility that the inhibitory effect of TSH on steroids was not due to the contaminating molecules, we used rTSH. rTSH too significantly inhibited progesterone and testosterone secretion from MLTC-1 cells confirming a direct inhibitory effect of TSH on steroid production (Fig. 3a, b).
To investigate the inhibitory mechanism of TSH on steroid synthesis, we studied the effect of rTSH on various steroidogenic enzymes. StAR, a de novo synthesized labile protein, catalyses the inter-mitochondrial cholesterol transport (18-20). The cholesterol in the inner mitochondrial membrane is converted to pregnenolone with the help of CYP11A1 enzyme. StAR and CYP11A1 catalyses the non-steroidogenic and steroidogenic rate-limiting steps of steroid synthesis respectively (21, 22). TSH showed no significant effect on StAR and CYP11A1 mRNA and protein levels in MLTC-1 cells (Fig. 4, 5). This suggests that TSH acts at a site distal to pregnenolone synthesis. However, as TSH inhibits progesterone secretion, therefore its site of action is proximal to progesterone synthesis.
In rodents, steroidogenesis is primarily through the Δ4 pathway where conversion of pregnenolone to progesterone is catalysed by 3BHSD enzyme (23). In mouse, the 3BHSD–1 isoform is primarily expressed in the gonads (24). Therefore, we studied the effect of rTSH on 3BHSD–1 mRNA and protein levels. In MLTC-1 cells TSH significantly inhibited basal and 8-Br-cAMP-treated 3BHSD mRNA and protein levels (Fig. 4, 5). The inhibition of 3BHSD by TSH in this study is similar to that observed in primary cultures of rat granulosa cells (16).
Next we investigated whether the direct action of TSH on MLTC-1 cells was mediated through its specific G-protein coupled plasma membrane receptor. Till date, there is only one additional report showing the presence of TSH receptors on Leydig cells that was published after our findings were presented (25, 26). However, TSH receptors have been identified on the granulosa cells of several species including rat and monkey (17, 27). In MLTC-1 cells like that seen in thyroid tissue, both the TSHR and the corresponding protein were identified suggesting that the inhibitory action of TSH on steroidogenesis could be mediated through its receptor. TSHR was not identified in Vero cells, a monkey kidney derived line but has been identified in rat Leydig cells (25).
However, in human thyroid tissue, when TSH binds to its receptor, it activates two G-proteins – Gs and Gq/11 (28). The active Gs induce the adenylyl cyclase (AC)-cAMP pathway. The active Gq/11 activates the phospholipase C (PLC)-IP3 (inositoltriphosphate)/diacyl glycerol (DAG) pathway. DAG activates the protein kinase C (PKC) (28, 29). In Y1 adrenocortical cells, PKC was shown to inhibit basal steroidogenesis by inhibiting the mRNA expression of two steroidogenic enzymes – P450-11 beta-hydroxylase and 3BHSD (30). In hamster cell lines, PKC has also been shown to influence the genetic expression of CYP11B2 (31). The signalling mechanism by which TSH inhibits the 3BHSD enzyme is yet to be determined in Leydig cells.
Our study is the first of its kind demonstrating a direct role of TSH on Leydig cell line and has demonstrated a molecular basis for its effects. We are also the first to demonstrate the presence of TSH receptors in Leydig cell line. There is a high probability of this physiology in human Leydig cells but further studies are needed on primary human Leydig cell lines to redemonstrate these findings and establish the presence of this phenomenon and these receptors in human Leydig cells.
This study was previously presented as a poster presentation at the Endocrine Society meeting in April, 2017. (26)
Acknowledgments
Funding: This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
SS: receives funding from the National Institutes of Health intramural research program.
Footnotes
DECLARATION OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Manna PR, Kero J, Tena-Sempere M, Pakarinen P, Stocco DM, Huhtaniemi IT. Assessment of mechanisms of thyroid hormone action in mouse Leydig cells: regulation of the steroidogenic acute regulatory protein, steroidogenesis, and luteinizing hormone receptor function. Endocrinology. 2001;142(1):319–31. [DOI] [PubMed] [Google Scholar]
- 2.Manna PR, Tena-Sempere M, Huhtaniemi IT. Molecular mechanisms of thyroid hormone-stimulated steroidogenesis in mouse leydig tumor cells. Involvement of the steroidogenic acute regulatory (StAR) protein. J Biol Chem. 1999;274(9):5909–18. [DOI] [PubMed] [Google Scholar]
- 3.Maran RR, Arunakaran J, Aruldhas MM. T3 directly stimulates basal and modulates LH induced testosterone and oestradiol production by rat Leydig cells in vitro. Endocr J. 2000;47(4):417–28. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Mohanty BP, Rani L. Secretion of testicular steroids and gonadotrophins in hypothyroidism. Andrologia. 2007;39(6):253–60. [DOI] [PubMed] [Google Scholar]
- 5.Teerds KJ, de Rooij DG, de Jong FH, van Haaster LH. Development of the adult-type Leydig cell population in the rat is affected by neonatal thyroid hormone levels. Biology of reproduction. 1998;59(2):344–50. [DOI] [PubMed] [Google Scholar]
- 6.Hardy MP, Sharma RS, Arambepola NK, Sottas CM, Russell LD, Bunick D, et al. Increased proliferation of Leydig cells induced by neonatal hypothyroidism in the rat. Journal of andrology. 1996;17(3):231–8. [PubMed] [Google Scholar]
- 7.Cristovao FC, Bisi H, Mendonca BB, Bianco AC, Bloise W. Severe and mild neonatal hypothyroidism mediate opposite effects on Leydig cells of rats. Thyroid : official journal of the American Thyroid Association. 2002;12(1):13–8. [DOI] [PubMed] [Google Scholar]
- 8.Rao JN, Liang JY, Chakraborti P, Feng P. Effect of thyroid hormone on the development and gene expression of hormone receptors in rat testes in vivo. Journal of endocrinological investigation. 2003;26(5):435–43. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Chaturvedi PK, Mohanty BP. Hypoandrogenaemia is associated with subclinical hypothyroidism in men. Int J Androl. 2007;30(1):14–20. [DOI] [PubMed] [Google Scholar]
- 10.Mendis-Handagama SM, Siril Ariyaratne HB. Leydig cells, thyroid hormones and steroidogenesis. Indian journal of experimental biology. 2005;43(11):939–62. [PubMed] [Google Scholar]
- 11.Rebois RV. Establishment of gonadotropin-responsive murine leydig tumor cell line. J Cell Biol. 1982;94(1):70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cascio C, Prasad VV, Lin YY, Lieberman S, Papadopoulos V. Detection of P450c17-independent pathways for dehydroepiandrosterone (DHEA) biosynthesis in brain glial tumor cells. Proc Natl Acad Sci U S A. 1998;95(6):2862–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Shaughnessy PJ, Morris ID, Baker PJ. Leydig cell re-generation and expression of cell signaling molecules in the germ cell-free testis. Reproduction. 2008;135(6):851–8. [DOI] [PubMed] [Google Scholar]
- 14.Marians RC, Ng L, Blair HC, Unger P, Graves PN, Davies TF. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci U S A. 2002;99(24):15776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konieczna A, Szczepanska A, Sawiuk K, Wegrzyn G, Lyzen R. Effects of partial silencing of genes coding for enzymes involved in glycolysis and tricarboxylic acid cycle on the enterance of human fibroblasts to the S phase. BMC Cell Biol. 2015;16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehendale RG, Bruot BC. Thyroid stimulating hormone inhibits rat granulosa cell steroidogenesis in primary culture. Endocrine. 1995;3(3):215–20. [DOI] [PubMed] [Google Scholar]
- 17.Agard JA, Duffy DM, Jacot T, Archer DF. Thyroid stimulating hormone (TSH) receptor on granulosa cells. Fertility and sterility. 2011;96(3):S118. [Google Scholar]
- 18.Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem. 1994;269(45):28314–22. [PubMed] [Google Scholar]
- 19.Meinsohn MC, Smith OE, Bertolin K, Murphy BD. The Orphan Nuclear Receptors Steroidogenic Factor-1 and Liver Receptor Homolog-1: Structure, Regulation, and Essential Roles in Mammalian Reproduction. Physiological reviews. 2019;99(2):1249–79. [DOI] [PubMed] [Google Scholar]
- 20.Zirkin BR, Papadopoulos V. Leydig cells: formation, function, and regulation†. Biology of reproduction. 2018;99(1):101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Zhang Z, Shen WJ, Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab (Lond). 2010;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acconcia F, Marino M. Steroid Hormones: Synthesis, Secretion, and Transport In: Belfiore A, LeRoith D, editors. Principles of Endocrinology and Hormone Action. Cham: Springer International Publishing; 2016. p. 1–31. [Google Scholar]
- 23.Hall PF. Testicular steroid synthesis: organization and regulation In: Knobil E, Neill J, editors. The Physiology of Reproduction. 2nd ed. New York: Raven Press; 1994. p. 1335–62. [Google Scholar]
- 24.Payne AH, Abbaszade IG, Clarke TR, Bain PA, Park CH. The multiple murine 3 beta-hydroxysteroid dehydrogenase isoforms: structure, function, and tissue- and developmentally specific expression. Steroids. 1997;62(1):169–75. [DOI] [PubMed] [Google Scholar]
- 25.Fadlalla MB, Wei Q, Fedail JS, Mehfooz A, Mao D, Shi F. Effects of hyper- and hypothyroidism on the development and proliferation of testicular cells in prepubertal rats. Animal science journal = Nihon chikusan Gakkaiho. 2017;88(12):1943–54. [DOI] [PubMed] [Google Scholar]
- 26.Shekhar SDB, Gupta S, Kumar A. A Unique Anti-Gonadotropic Effect of TSH on Leydig Cell Derived Mltc-1 Line. The Endocrine Society Meeting 2017; 2017, April 3; Orlando USA2017, April 3. [Google Scholar]
- 27.Sun SC, Hsu PJ, Wu FJ, Li SH, Lu CH, Luo CW. Thyrostimulin, but not thyroid-stimulating hormone (TSH), acts as a paracrine regulator to activate the TSH receptor in mammalian ovary. J Biol Chem. 2010;285(6):3758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allgeier A, Offermanns S, Van Sande J, Spicher K, Schultz G, Dumont JE. The human thyrotropin receptor activates G-proteins Gs and Gq/11. J Biol Chem. 1994;269(19):13733–5. [PubMed] [Google Scholar]
- 29.Krude H, Biebermann H. The Thyroid and Its Regulation by the TSHR: Evolution, Development, and Congenital Defects In: Luster M, Duntas LH, Wartofsky L, editors. The Thyroid and Its Diseases: A Comprehensive Guide for the Clinician. Cham: Springer International Publishing; 2019. p. 219–33. [Google Scholar]
- 30.Reyland ME. Protein kinase C is a tonic negative regulator of steroidogenesis and steroid hydroxylase gene expression in Y1 adrenal cells and functions independently of protein kinase A. Mol Endocrinol. 1993;7(8):1021–30. [DOI] [PubMed] [Google Scholar]
- 31.LeHoux JG, Dupuis G, Lefebvre A. Control of CYP11B2 gene expression through differential regulation of its promoter by atypical and conventional protein kinase C isoforms. The Journal of biological chemistry. 2001;276(11):8021–8. [DOI] [PubMed] [Google Scholar]