Abstract
Introduction
Osteoporosis is one of the serious adverse effects associated with glucocorticoid therapy. Although bisphosphonates have been used for glucocorticoid-induced osteoporosis (GIO), some patients have shown an inadequate response. In such cases, denosumab or teriparatide are used. However, there is no consensus on which of these two drugs is superior. We prospectively compared denosumab's and teriparatide's effects on the bone mineral density (BMD) in GIO patients with prior bisphosphonate treatment.
Materials and methods
After receiving oral bisphosphonates for ≥2 years, GIO patients with low T-score BMD (<−2.5) were switched from bisphosphonates to denosumab (n = 20) or daily teriparatide (n = 21). We measured the BMD (lumbar spine, femoral neck, and total hip) in both groups every 6 months for 24 months.
Results
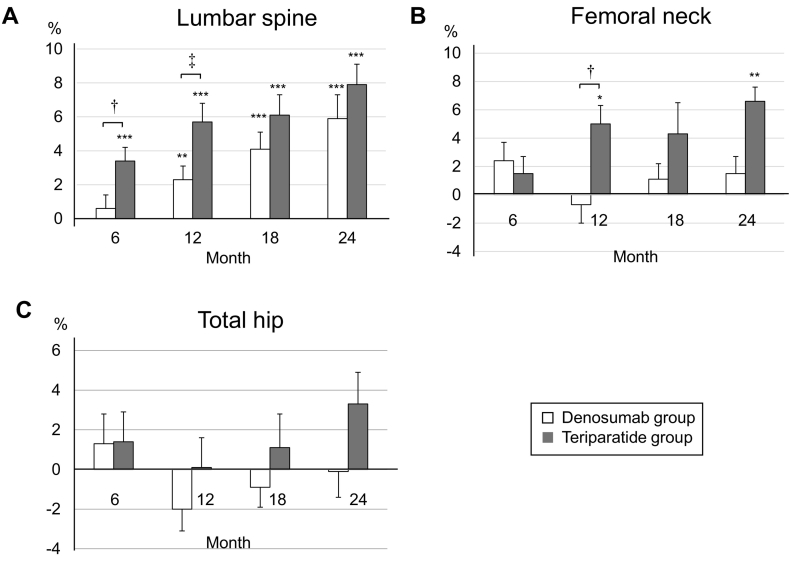
At 24 months of treatment, the lumbar spine BMD increased significantly from baseline in both the denosumab and teriparatide groups (baseline vs. denosumab and teriparatide; 5.9 ± 5.6%, P < 0.001 and 7.9 ± 5.4%, P < 0.001). A significant increase in femoral neck BMD from baseline occurred only in the teriparatide group (6.6 ± 10.8%, P < 0.05); denosumab (1.5 ± 5.0%). No significant changes occurred in the total hip BMD from baseline in either group (−0.1 ± 5.6% and 3.3 ± 7.5%, respectively). There was no significant difference between the denosumab and teriparatide groups at 24 months in lumbar spine and femoral neck BMD, but was significantly higher in the teriparatide group at 12 months (P < 0.01 and P < 0.05 in the lumbar spine and femoral neck, respectively).
Conclusion
Teriparatide might have some advantages over denosumab and be a good alternative for treating GIO patients with prior bisphosphonate treatment.
Keywords: Glucocorticoid-induced osteoporosis, Bisphosphonate, Denosumab, Teriparatide, Bone mineral density
Highlights
-
•
We compared the effects of denosumab and teriparatide on BMD in GIO patients with prior bisphosphonate treatment.
-
•
At 24 months, teriparatide increased lumbar spine and femoral neck BMD, whereas denosumab increased lumbar spine BMD only.
-
•
At 12 months, teriparatide increased lumbar spine and femoral neck BMD more than denosumab.
-
•
Teriparatide might have some advantages over denosumab in GIO patients with prior bisphosphonate treatment.
1. Introduction
Glucocorticoids have potent anti-inflammatory and immunosuppressive effects and are used for many inflammatory or autoimmune diseases. Glucocorticoid-induced osteoporosis (GIO) is the most common and serious adverse effect associated with glucocorticoid use. Glucocorticoids impair osteoblast function and induce the apoptosis of both osteoblasts and osteocytes, leading to a suppression of bone formation (Weinstein, 2011; Angeli et al., 2006). Fragility fracture occurs in 30%–50% of patients receiving long-term glucocorticoid therapy. Bone loss occurs rapidly within the first 3–6 months of glucocorticoid therapy (Laan et al., 1993; Buckley et al., 2017). There are strong associations between exposure to glucocorticoids and the risk of fractures. There is a dose-dependent risk of fracture for both the hips and the vertebrae (Steinbuch et al., 2004).
Bisphosphonates are the most commonly used drugs in the treatment of GIO (Compston, 2018). Bisphosphonates are synthetic analogs of pyrophosphate, and they exert antiresorptive effects by being incorporated into osteoclasts at the bone surface (Drake et al., 2008). Bisphosphonates were also effective in preventing apoptosis of osteoblast and osteocyte apoptosis induced by glucocorticoids (Plotkin et al., 2011). In several randomized controlled trials, the bisphosphonates alendronate and risedronate showed an ability to inhibit the loss of bone mineral density (BMD) in the lumbar spine and femur, and they were also shown to significantly reduce the rate of vertebral fractures (Saag et al., 1998; Cohen et al., 1999). Zoledronic acid was shown to increase BMD in the lumbar spine and femur more than risedronate in GIO patients (Reid et al., 2009). Although bisphosphonates have been used to treat patients with GIO, some patients do not improve BMD. BMD reduction alone should not be considered a failure of treatment with bisphosphonates (Diez-Perez et al., 2012). However, BMD is one of the indicators in considering whether the treatment should be changed. In GIO patients who show an inadequate response to bisphosphonates, denosumab or teriparatide could be alternatives to bisphosphonates.
Denosumab is a monoclonal human IgG2 antibody that binds to receptor activator of NF-κB ligand (RANKL), which is a cytokine essential for osteoclast differentiation and activation. Denosumab inhibits RANKL from binding to its receptor RANK, and it suppresses bone resorption by controlling osteoclast differentiation (Lacey et al., 2012). Teriparatide [recombinant human parathyroid hormone, rhPTH (1–34)] is a bone anabolic agent used to treat osteoporosis. Intermittent teriparatide administration promotes osteoblast differentiation while suppressing osteoblast apoptosis (Jilka, 2007; Weinstein et al., 2010).
Denosumab and teriparatide are drugs that are expected to increase the BMD of women with postmenopausal osteoporosis more than bisphosphonates (Kendler et al., 2010; Finkelstein et al., 2010). The effectiveness of denosumab and teriparatide has also been shown for GIO. Several studies indicated that in GIO patients, denosumab and teriparatide increased the lumbar and hip BMD compared to bisphosphonates (Saag et al., 2007; Saag et al., 2009; Saag et al., 2019). However, the evidence of the effectiveness of denosumab and teriparatide in GIO patients who present inadequate response to bisphosphonates is insufficient, and there are no data comparing the effectiveness of these two drugs in such GIO patients. As it stands, it is unclear which of these two drugs has a better therapeutic effect. We conducted the present study to prospectively compare the effects of denosumab and teriparatide on the BMD of GIO patients with prior bisphosphonate treatment.
2. Methods
2.1. Study design
This was a 24-month, prospective, open-label, non-randomized clinical trial conducted from 2014 to 2018 at a single center, Kindai University Hospital (Osaka, Japan). Patients with connective tissue disease and GIO who received bisphosphonates according to Japanese Society for Bone Mineral Research guidelines were enrolled (Suzuki et al., 2014). The inclusion criteria were as follows: (1) ≥20 years old, (2) treated with ≥5.0 mg/day oral prednisolone (PSL) therapy before enrollment, (3) with a low T-score BMD (<−2.5) at the lumbar spine or femoral neck despite having been treated with one or more oral bisphosphonates for ≥2 years. The exclusion criteria were (1) pregnant and breast feeding women, and (2) patients who had been pretreated with denosumab or teriparatide.
Forty-seven patients with GIO were enrolled. Patients who chose daily subcutaneous injection and were judged by their physician to be capable of self-injection were assigned to the teriparatide group (n = 23), and other patients were assigned to the denosumab group (n = 24). In the denosumab group, patients were switched from the bisphosphonate to denosumab (60 mg subcutaneous injection, 1×/6 months). In the teriparatide group, patients were switched from the bisphosphonate to teriparatide (20 μg subcutaneous injection, 1×/day). During the study, the denosumab group also received elemental calcium or vitamin D, although not necessary for the teriparatide group.
The study was conducted according to the principles expressed in the Declaration of Helsinki of 1983, and it was approved by the Research Ethics Committee of Kindai University of Medicine. Written informed consent to participate and have their data published was obtained from all patients.
2.2. Assessments
The demographic characteristics recorded at baseline included the patient's age, sex, body mass index (BMI), and daily dose of PSL. At baseline and at months 6, 12, 18, and 24 of treatment with denosumab or teriparatide, the BMD of each patient's lumbar spine (L1–L4) and femoral neck and total hip of the non-dominant leg were measured by dual-energy X-ray absorptiometry (Discovery A, Hologic, Marlborough, MA, USA). A marker of bone resorption, i.e., tartrate-resistant acid phosphatase 5b (TRACP5b), and a marker of bone formation, i.e., procollagen type 1 N-terminal propeptide (P1NP) were similarly assessed at baseline and at months 6, 12, 18, and 24. The primary endpoint of the study was the percent change in the lumbar spine BMD from baseline to 24 months. The secondary endpoints were the percent changes in the femoral neck BMD, total hip BMD and the bone turnover markers TRACP5b and P1NP from baseline to 24 months.
2.3. Safety
The treating physicians performed the physical examinations and laboratory tests (hematological, blood chemistry, and urinalysis). All adverse events were recorded.
2.4. Statistical analyses
We used GraphPad Prism software (GraphPad Software, San Diego, CA) for all statistical analyses. The baseline characteristics of the denosumab and teriparatide groups were compared using the Mann-Whitney U test (the ratio of females was tested using Fisher's exact test). Similarly, the changes in the BMD and bone turnover markers were compared between the two patient groups by the Mann-Whitney U test. Within-group changes (between baseline and months 6, 12, 18 and 24) of the BMD and bone turnover markers were assessed by paired t-test. The relationship between the baseline patient age, BMI, PSL dose, BMD and bone turnover markers, the average dose of PSL during study period, and the percent change in BMD were evaluated by a Spearman rank correlation analysis. The relationship between the percent changes in bone turnover markers and the percent changes in BMD were also evaluated by a Spearman rank correlation analysis. P-values <0.05 were considered significant.
3. Results
3.1. Baseline characteristics and patient disposition
The reason patients switched their treatment from bisphosphonates to denosumab or teriparatide was lack of BMD response, not intolerance or new fracture under bisphosphonate treatment. Of the 47 patients (24 patients in the denosumab and 23 patients in the teriparatide groups), four patients in the denosumab group and two patients in the teriparatide group were discontinued. In the denosumab group, the reasons were: withdrawal due to worsening of the underlying disease (n = 2), moving away at the patient's request (n = 1), withdrawal due to the patient's request (n = 1). In the teriparatide group, the reasons were: moving away at the patient's request (n = 1) and withdrawal due to worsening of the underlying disease (n = 1). A final total of 41 patients was analyzed (denosumab group, n = 20; teriparatide group, n = 21). The patients' underlying connective tissue diseases are listed in Table 1. In the denosumab group, the number of patients who had taken alendronate as their prior treatment was 13 (54.2%); nine patients (37.5%) had taken risedronate, and two (8.3%) had taken minodronate. Similarly, in the teriparatide group 11 patients (47.8%) had taken alendronate, eight (34.8%) had taken risedronate, and four (17.4%) had taken minodronate.
Table 1.
The patients' underlying connective tissue diseases.
| Denosumab group |
Teriparatide group |
|
|---|---|---|
| Disease | n = 24 | n = 23 |
| Polymyositis, dermatomyositis | 3 | 8 |
| Systemic lupus erythematosus | 7 | 3 |
| Rheumatoid arthritis | 5 | 2 |
| Polymyalgia rheumatica | 3 | 1 |
| ANCA-associated vasculitis | 3 | 1 |
| Systemic sclerosis | 1 | 3 |
| Overlap syndrome | 1 | 0 |
| RS3PE syndrome | 1 | 0 |
| Sjögren syndrome | 0 | 1 |
| Mixed connective tissue disease | 0 | 1 |
| Takayasu arteritis | 0 | 1 |
| Polyarteritis nodosa | 0 | 1 |
| Spondyloarthritis | 0 | 1 |
Antineutrophil cytoplasmic antibody; ANCA.
RS3PE; Remitting Seronegative Symmetrical Synovitis with Pitting Edema.
The baseline characteristics of patients are summarized in Table 2. There were no significant differences in the background between the two groups in age, sex, BMI, PSL dose, duration of PSL and bisphosphonate treatment, BMD, or bone turnover markers at baseline. No significant difference was found in the daily average dose of PSL during study period between the two groups: 5.1 ± 3.1 mg/day, denosumab group; 5.3 ± 2.7 mg/day, teriparatide group.
Table 2.
Clinical characteristics at baseline.
| Denosumab group |
Teriparatide group |
P-value | |
|---|---|---|---|
| Characteristics | n = 20 | n = 21 | |
| Age, years | 66.7 ± 10.7 | 61.1 ± 11.7 | 0.07 |
| Female, % | 95 | 100 | 0.49 |
| BMI, kg/m2 | 21.3 ± 3.3 | 19.4 ± 2.9 | 0.35 |
| Duration of prednisolone treatment, months | 177.7 ± 99.6 | 185.5 ± 116.1 | 0.96 |
| Dose of prednisolone at entry, mg | 6.3 ± 4.7 | 5.7 ± 3.5 | 0.78 |
| Duration of bisphosphonate treatment, months | 138.7 ± 88.7 | 134.7 ± 75.7 | 0.97 |
| BMD, g/cm2 | |||
| Lumbar spine | 0.74 ± 0.11 | 0.73 ± 0.11 | 0.65 |
| T score | −2.59 ± 1.02 | −2.77 ± 1.08 | 0.51 |
| Femoral neck | 0.50 ± 0.08 | 0.50 ± 0.06 | 0.57 |
| T score | −2.70 ± 0.63 | −2.61 ± 0.52 | 0.49 |
| Total hip | 0.63 ± 0.09 | 0.64 ± 0.09 | 0.74 |
| T score | −2.13 ± 0.69 | −2.02 ± 0.87 | 0.70 |
| Bone turnover markers | |||
| Serum TRACP-5b, mU/dL | 314.7 ± 134.4 | 269.5 ± 138.4 | 0.27 |
| Serum P1NP, μg/L | 30.5 ± 20.6 | 26.4 ± 19.7 | 0.26 |
Data are mean ± SD. BMI, body mass index; BMD, bone mineral density; TRACP-5b, tartrate-resistant acid phosphatase 5b; P1NP, procollagen type 1 N-terminal propeptide.
3.2. Changes in BMD
Fig. 1 illustrates the percent changes in BMD of the lumbar spine, femoral neck, and total hip over the 24-month treatment period. At 24 months, the lumbar spine BMD had increased significantly from baseline in both groups (Fig. 1A). The percent changes in the lumbar spine BMD from baseline to 24 months were 5.9 ± 5.6% (P < 0.001) and 7.9 ± 5.4% (P < 0.001) for the denosumab and teriparatide groups, respectively. At 24 months, there was no significant difference in the lumbar spine BMD between the two groups, but at 6 and 12 months, the changes were significantly greater in the teriparatide group compared to the denosumab group (P < 0.05 and P < 0.01, respectively). In the femoral neck, the percent changes in BMD from baseline to 24 months were 1.5 ± 5.0% (P = 0.21) and 6.6 ± 10.8% (P < 0.05) for the denosumab and teriparatide groups, respectively (Fig. 1B). The femoral neck BMD at 24 months had increased significantly from baseline in only the teriparatide group. No significant increase was revealed in the femoral neck BMD in the denosumab group. As in the lumbar spine, there was no significant difference between the two groups at 24 months in the femoral neck, but at 12 months, greater increase was found in the teriparatide group (P < 0.05). The percent changes in the total hip BMD from baseline to 24 months were −0.1 ± 5.6% and 3.3 ± 7.5% for the denosumab and teriparatide group, respectively (Fig. 1C). The total hip BMD decreased after 12 months of treatment in both groups, but this decline was reversed at 24 months in the teriparatide group. No significant change from baseline in the total hip BMD was observed in either group, but there was a trend for improvement in the teriparatide group (P = 0.06). There were no significant differences in the total hip BMD between the denosumab and teriparatide groups through the observatory period (6, 12, 18, and 24 months). During the study period, clinical vertebral fractures occurred in 2 patients in the denosumab group.
Fig. 1.
Mean percent changes in BMD from baseline to 24 months in the lumbar spine (A), femoral neck (B), and total hip (C). Error bars: SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. baseline. †P < 0.05, ‡P < 0.01, Denosumab group vs. Teriparatide group.
3.3. Changes in the bone turnover markers
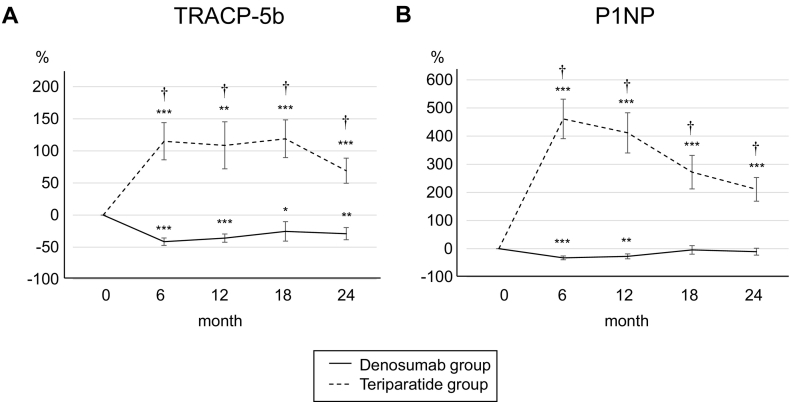
The changes in bone turnover markers are shown as a percentage change from baseline (Fig. 2). In the denosumab group, the patients' serum TRACP-5b levels were decreased significantly from baseline at 6, 12, 18 and 24 months. In the teriparatide group, the serum TRACP-5b levels were increased significantly at each of the treatment time points (Fig. 2A). Similarly, the serum P1NP levels increased significantly at each time point in the teriparatide group but decreased significantly at 6 and 12 months in the denosumab group (Fig. 2B).
Fig. 2.
Percent changes in serum TRACP-5b (A) and P1NP (B) from baseline to 24 months. Error bars: SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. baseline. †P < 0.001, Denosumab group vs. Teriparatide group. TRACP-5b, tartrate-resistant acid phosphatase 5b; P1NP, procollagen type 1 N-terminal propeptide.
3.4. The correlations between the BMD response and other factors
In the denosumab group, lower baseline BMD levels were associated with greater increases in the lumbar spine BMD at 24 months (data not shown). No significant relationships were found between the percent change in any BMD site and the patients' baseline age, BMI, PSL dose, and bone turnover markers, or the daily average dose of PSL during study period. There was no significant correlation between the percent change of BMD and the percent change of bone turnover markers.
3.5. Adverse events
A summary of the adverse events is provided in Table 3. There were no significant differences between the denosumab and teriparatide groups in the number of each type of adverse event. Mild asymptomatic hypercalcemia (>11.2 mg/dL) was reported in three patients in the teriparatide group. Since one of them was taking vitamin D, hypercalcemia was resolved by discontinuing vitamin D. The other two patients were observed without any additional treatment. None of the adverse events in either group led to treatment discontinuation.
Table 3.
Adverse events.
| Denosumab group |
Teriparatide group |
P-value | |
|---|---|---|---|
| Characteristics | n = 20 | n = 21 | |
| Any adverse events | 5 (25.0) | 6 (28.6) | 1.00 |
| Bronchitis | 1 (5.0) | 0 | 0.49 |
| Pneumonia | 2 (10.0) | 1 (4.8) | 0.61 |
| Colitis | 0 | 1 (4.8) | 1.00 |
| Rash | 1 (5.0) | 0 | 0.49 |
| Thrombophlebitis | 1 (5.0) | 0 | 0.49 |
| Hypercalcemia | 0 | 3 (14.3) | 0.23 |
Values are the number (%).
4. Discussion
Osteoporosis is the most common and serious adverse effect associated with glucocorticoid therapy. The normal bone turnover depends on the balance between osteoblastic bone formation and osteoclastic bone resorption (Hadjidakis and Androulakis, 2006; Martin and Sims, 2005). Treatment with a glucocorticoid can cause an imbalance of bone formation and bone resorption, which leads to bone loss. GIO is characterized by decreased bone formation due to increased apoptosis of osteoblasts and osteocytes (Weinstein, 2011; Angeli et al., 2006). Besides the decrease in bone formation, there is at least a temporarily increase of bone resorption due to increased both in the number and activity of osteoclasts (Compston, 2018).
Although bisphosphonates are the first-line agents for treating GIO (Compston, 2018), the alternatives are not clear in patients with prior bisphosphonate treatment. The effectiveness of denosumab and teriparatide for GIO has been reported (Saag et al., 2007; Saag et al., 2009; Saag et al., 2019), but most of the reported results were those of bisphosphonate-naïve patients. Obermayer et al. conducted a 24-month randomized controlled trial examining patients with postmenopausal osteoporosis, and they reported that teriparatide treatment was associated with a significant increase in BMD in the patients who had shown an inadequate response to antiresorptive treatment occupying 93.0% with bisphosphonates (Obermayer-Pietsch et al., 2008). Although prior antiresorptive treatment modestly blunted the BMD response to teriparatide, they showed that the patients' BMD values increased significantly from baseline; the mean percent changes were 9.8%, 3.9%, and 2.3% in the lumbar spine, femoral neck, and total hip, respectively. However, in GIO patients, the therapeutic potential of teriparatide remains unclear.
There are a few reports about the effectiveness of denosumab in GIO patients who were treated previously with bisphosphonates. Mok et al. performed a 12-month randomized controlled trial to evaluate the effect of switching bisphosphonates to denosumab on their patients' BMD (Mok et al., 2015), and they observed that compared to the continuation of the bisphosphonates, switching to denosumab was associated with a greater increase in the lumbar spine BMD (+1.5% vs. +3.4%, respectively), but not in the femoral neck or total hip BMD values. In contrast, Suzuki et al. reported that switching bisphosphonates to denosumab did not significantly improve the lumbar spine BMD in their retrospective study (Suzuki et al., 2018). These studies' results suggest that the treatment effect of denosumab might be limited in GIO patients switching from bisphosphonate treatment. Our present findings support this hypothesis; i.e., that the therapeutic effect of denosumab might be limited by prior bisphosphonate treatment. Our findings also clarify the efficacy of teriparatide in GIO patients with prior bisphosphonate treatment.
Our assessment of denosumab's and teriparatide's effects on the BMD of GIO patients with prior bisphosphonate treatment demonstrated that after 24 months, a significant increase occurred in the lumbar spine and femoral neck BMD in the teriparatide group, but only in the lumbar spine BMD in the denosumab group. At 12 months, teriparatide group showed a greater increase in the lumbar spine and femoral neck BMD than denosumab group. Though the difference was not statistically significant, this tendency continued until 24 months. Our data suggest that teriparatide has better therapeutic effects than denosumab in GIO patients receiving bisphosphonates as pretreatment.
Bone turnover markers have been used as a tool for monitoring patients' responses to treatment for osteoporosis (Eastell et al., 2018). Changes in bone turnover markers are associated with later changes in BMD. The levels of both serum P1NP and TRACP5b are increased with teriparatide treatment and decreased with denosumab treatment, and similarly in our study, the levels of both serum P1NP and TRACP5b increased significantly in the teriparatide group and decreased significantly in the denosumab group at the early phase of treatment. These changes may have brought about an increase in the lumbar spine BMD at 24 months in both patient groups. However, these changes in bone turnover markers are not sufficient to explain the differences in therapeutic effects between our denosumab and teriparatide groups.
Only a few studies compared denosumab and teriparatide (Leder et al., 2014; Ebina et al., 2018; Hattori and Hirano, 2019). In the Denosumab And Teriparatide Administration (DATA) extension study, which described excellent therapeutic effects of a combination of both denosumab and teriparatide for treating postmenopausal osteoporosis, the increases in the lumbar spine, femoral neck, and total hip BMD did not differ significantly between the denosumab monotherapy group and the teriparatide monotherapy group after 24 months of treatment (Leder et al., 2014).
Ebina et al. conducted an observational, non-randomized study switching bisphosphonate to denosumab or teriparatide in osteoporosis patients with rheumatoid arthritis (Ebina et al., 2018). In their study, 56.7 to 70.0% of patients used glucocorticoids. After 18 months, the switch to denosumab and the switch to teriparatide both resulted in higher increases in the lumbar spine, femoral neck, and total hip BMD compared to the values observed in the bisphosphonate continuation patients. The patients who were switched to teriparatide showed a significantly greater increase in lumbar spine BMD compared to the patients switched to denosumab (+9.5% vs. +5.2%).
The evidence of the effectiveness of denosumab and teriparatide in GIO patients who present inadequate response to bisphosphonates is insufficient. The results of our analyses demonstrated that teriparatide may have a higher therapeutic potential than denosumab for GIO patients with prior bisphosphonate treatment. Osteoporotic patients who have been treated with bisphosphonates have already had their bone turnover sufficiently suppressed. Thus, denosumab (which suppresses bone turnover like bisphosphonates do) may not be able to achieve increased bone mineral density. In addition, since GIO is caused mainly by a suppression of bone formation, we consider teriparatide appropriate for the treatment of GIO because of its effect characterized by the promotion of bone formation.
There were no significant differences in adverse events between our denosumab and teriparatide groups. In the teriparatide group, mild asymptomatic hypercalcemia was observed in the cases of three patients, but none resulted in treatment discontinuation.
Our study has some limitations. First, the lack of randomization and blinding may have created a selection bias. Second, our sample size was small (n = 41), and the primary endpoint was the percent changes in BMD, not the incidence of fractures. There was no power calculation performed in which to determine sample size. The patients in the teriparatide group tended to be younger than those in the denosumab group. This might be related to the superiority of teriparatide. Although the age difference between the two groups was not statistically significant, the small sample size may not eliminate a type II error. Thus, the ages of the two groups while not statistically different might be important clinically. The increase in the femoral neck BMD in our study was better in the teriparatide group and worse in the denosumab group than in previous reports (Ebina et al., 2018; Obermayer-Pietsch et al., 2008). Although the exact cause of these discrepancies is unknown, there were differences in baseline characteristics of patients between our study and previous reports in that our study had a longer duration of treatment with corticosteroids and bisphosphonates. In our results of the changes in BMD, there were deviations between the femoral neck and the total hip. These results seem unfamiliar. In our study, already at baseline, there was a difference in BMD between the femoral neck and the total hip, with the femoral neck showing a lower T-score than the total hip. This may be related to the larger increase in BMD in the femoral neck than in the total hip. The most important therapeutic effect of osteoporosis is the prevention of fractures. Since the threshold for BMD causing fractures in GIO may be higher than the BMD threshold in postmenopausal osteoporosis (Van Staa et al., 2003), the increase in BMD may not necessarily be associated with fracture suppression. It may be insufficient to determine the treatment effect solely by measuring the BMD without assessing the incidence of fractures. However, the BMD is an important predictor of fractures, and therefore it is very significant to evaluate the changes in BMD considering fracture prevention.
In recent years, the emergence of molecular targeted agents has advanced the treatment of rheumatic diseases, but the role played by glucocorticoids remains significant. The optimal management of GIO is thus an important problem. It is necessary to properly use drugs such as bisphosphonates, denosumab, and teriparatide when treating GIO patients. Our study provides new findings for considering alternatives for GIO patients with prior bisphosphonate treatment.
5. Conclusions
In our 24-month study, a significant increase was demonstrated in the lumbar spine and femoral neck BMD in the teriparatide group, and in only the lumbar spine BMD in the denosumab group. In an earlier phase, the teriparatide group showed greater increase in the lumbar spine and femoral neck BMD than in the denosumab group. Teriparatide might have some advantages over denosumab and might be a good alternative for treating GIO patients who received bisphosphonates as pretreatment. Further studies of larger patient populations are necessary, with fractures as the primary endpoint.
Conflicts of interest
All authors have no conflicts of interest.
Transparency document
Transparency document.
CRediT authorship contribution statement
Yasuaki Hirooka:Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing.Yuji Nozaki:Conceptualization, Formal analysis, Methodology, Writing - review & editing.Asuka Inoue:Formal analysis, Investigation, Writing - review & editing.Jinhai Li:Data curation, Investigation.Toshihiko Shiga:Data curation, Investigation.Kazuya Kishimoto:Data curation, Investigation.Masafumi Sugiyama:Data curation, Investigation.Koji Kinoshita:Conceptualization, Methodology, Supervision.Masanori Funauchi:Conceptualization, Methodology, Supervision.Itaru Matsumura:Conceptualization, Methodology, Supervision.
Acknowledgments
Acknowledgement
We thank the investigators who participated in this study: T. Itami and K. Sakai.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
The Transparency document associated with this article can be found, in online version.
Contributor Information
Yasuaki Hirooka, Email: nagare@med.kindai.ac.jp.
Yuji Nozaki, Email: yuji0516@med.kindai.ac.jp.
Asuka Inoue, Email: asuka@med.kindai.ac.jp.
Jinhai Li, Email: jinhai@med.kindai.ac.jp.
Toshihiko Shiga, Email: sg-t1129@med.kindai.ac.jp.
Kazuya Kishimoto, Email: kazuya-k@med.kindai.ac.jp.
Masafumi Sugiyama, Email: m-sugi@med.kindai.ac.jp.
Koji Kinoshita, Email: kkino@med.kindai.ac.jp.
Masanori Funauchi, Email: mn-funa@med.kindai.ac.jp.
Itaru Matsumura, Email: imatsumura@med.kindai.ac.jp.
References
- Angeli A., Guglielmi G., Dovio A., Capelli G., de Feo D., Giannini S., Giorgino R., Moro L., Giustina A. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39:253–259. doi: 10.1016/j.bone.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Buckley L., Guyatt G., Fink H.A., Cannon M., Grossman J., Hansen K.E., Humphrey M.B., Lane N.E., Magrey M., Miller M., Morrison L., Rao M., Byun Robinson A., Saha S., Wolver S., Bannuru R.R., Vaysbrot E., Osani M., Turgunbaev M., Miller A.S., McAlindon T. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res. 2017;69:1095–1110. doi: 10.1002/acr.23279. [DOI] [PubMed] [Google Scholar]
- Cohen S., Levy R.M., Keller M., Boling E., Emkey R.D., Greenwald M., Zizic T.M., Wallach S., Sewell K.L., Lukert B.P., Axelrod D.W., Chines A.A. Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 1999;42:2309–2318. doi: 10.1002/1529-0131(199911)42:11<2309::AID-ANR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Compston J. Glucocorticoid-induced osteoporosis: an update. Endocrine. 2018;61:7–16. doi: 10.1007/s12020-018-1588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Perez A., Adachi J.D., Agnusdei D., Bilezikian J.P., Compston J.E., Cummings S.R., Eastell R., Eriksen E.F., Gonzalez-Macias J., Liberman U.A., Wahl D.A., Seeman E., Kanis J.A., Cooper C., IOF CSA Inadequate Responders Working Group Treatment failure in osteoporosis. Osteoporos. Int. 2012;23:2769–2774. doi: 10.1007/s00198-012-2093-8. [DOI] [PubMed] [Google Scholar]
- Drake M.T., Clarke B.L., Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastell R., Pigott T., Gossiel F., Naylor K.E., Walsh J.S., Peel N.F.A. Diagnosis of endocrine disease: bone turnover markers: are they clinically useful? Eur. J. Endocrinol. 2018;178:R19–R31. doi: 10.1530/EJE-17-0585. [DOI] [PubMed] [Google Scholar]
- Ebina K., Hirao M., Hashimoto J., Hagihara K., Kashii M., Kitaguchi K., Matsuoka H., Iwahashi T., Chijimatsu R., Yoshikawa H. Assessment of the effects of switching oral bisphosphonates to denosumab or daily teriparatide in patients with rheumatoid arthritis. J. Bone Miner. Metab. 2018;36:478–487. doi: 10.1007/s00774-017-0861-4. [DOI] [PubMed] [Google Scholar]
- Finkelstein J.S., Wyland J.J., Lee H., Neer R.M. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 2010;95:1838–1845. doi: 10.1210/jc.2009-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjidakis D.J., Androulakis I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006;1092:385–396. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- Hattori K., Hirano Y. The comparison of the efficacy of switching bisphosphonates to either denosumab or daily teriparatide osteoporosis in patients with rheumatoid arthritis (in Japanese) Rinsho Riumachi (Clin. Rheumatol. Rel. Res.) 2019;31:33–40. [Google Scholar]
- Jilka R.L. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler D.L., Roux C., Benhamou C.L., Brown J.P., Lillestol M., Siddhanti S., Man H.S., San Martin J., Bone H.G. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J. Bone Miner. Res. 2010;25:72–81. doi: 10.1359/jbmr.090716. [DOI] [PubMed] [Google Scholar]
- Laan R.F., van Riel P.L., van de Putte L.B., van Erning L.J., van’t Hof M.A., Lemmens J.A. Low-dose prednisone induces rapid reversible axial bone loss in patients with rheumatoid arthritis: a randomized, controlled study. Ann. Intern. Med. 1993;119:963–968. doi: 10.7326/0003-4819-119-10-199311150-00001. [DOI] [PubMed] [Google Scholar]
- Lacey D.L., Boyle W.J., Simonet W.S., Kostenuik P.J., Dougall W.C., Sullivan J.K., San Martin J., Dansey R. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Rev. Drug Discov. 2012;11:401–419. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- Leder B.Z., Tsai J.N., Uihlein A.V., Burnett-Bowie S.A., Zhu Y., Foley K., Lee H., Neer R.M. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (the DATA extension study): a randomized controlled trial. J. Clin. Endocrinol. Metab. 2014;99:1694–1700. doi: 10.1210/jc.2013-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T.J., Sims N.A. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol. Med. 2005;11:76–81. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Mok C.C., Ho L.Y., Ma K.M. Switching of oral bisphosphonates to denosumab in chronic glucocorticoid users: a 12-month randomized controlled trial. Bone. 2015;75:222–228. doi: 10.1016/j.bone.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Obermayer-Pietsch B.M., Marin F., McCloskey E.V., Hadji P., Farrerons J., Boonen S., Audran M., Barker C., Anastasilakis A.D., Fraser W.D., Nickelsen T. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J. Bone Miner. Res. 2008;23:1591–1600. doi: 10.1359/jbmr.080506. [DOI] [PubMed] [Google Scholar]
- Plotkin L.I., Bivi N., Bellido T. A bisphosphonate that does not affect osteoclasts prevents osteoblast and osteocyte apoptosis and the loss of bone strength induced by glucocorticoids in mice. Bone. 2011;49:122–127. doi: 10.1016/j.bone.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid D.M., Devogelaer J.P., Saag K., Roux C., Lau C.S., Reginster J.Y., Papanastasiou P., Ferreira A., Hartl F., Fashola T., Mesenbrink P., Sambrook P.N., HORIZON investigators Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2009;373:1253–1263. doi: 10.1016/S0140-6736(09)60250-6. [DOI] [PubMed] [Google Scholar]
- Saag K.G., Emkey R., Schnitzer T.J., Brown J.P., Hawkins F., Goemaere S., Thamsborg G., Liberman U.A., Delmas P.D., Malice M.P., Czachur M., Daifotis A.G. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N. Engl. J. Med. 1998;339:292–299. doi: 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- Saag K.G., Shane E., Boonen S., Marín F., Donley D.W., Taylor K.A., Dalsky G.P., Marcus R. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N. Engl. J. Med. 2007;357:2028–2239. doi: 10.1056/NEJMoa071408. [DOI] [PubMed] [Google Scholar]
- Saag K.G., Zanchetta J.R., Devogelaer J.P., Adler R.A., Eastell R., See K., Krege J.H., Krohn K., Warner M.R. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of randomized, double-blind, controlled trial. Arthritis Rheumatol. 2009;60:3346–3355. doi: 10.1002/art.24879. [DOI] [PubMed] [Google Scholar]
- Saag K.G., Pannacciulli N., Geusens P., Adachi J.D., Messina O.D., Morales-Torres J., Emkey R., Butler P.W., Yin X., Lems W.F. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: final results of a twenty-four-month randomized, double-blind, double-dummy trial. Arthritis Rheumatol. 2019;71:1174–1184. doi: 10.1002/art.40874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuch M., Youket T.E., Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos. Int. 2004;15:323–328. doi: 10.1007/s00198-003-1548-3. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Nawata H., Soen S., Fujiwara S., Nakayama H., Tanaka I., Ozono K., Sagawa A., Takayanagi R., Tanaka H., Miki T., Masunari N., Tanaka Y. Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J. Bone Miner. Metab. 2014;32:337–350. doi: 10.1007/s00774-014-0586-6. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Nakamura Y., Kato H. Significant improvement of bone mineral density by denosumab without bisphosphonate pre-treatment in glucocorticoid-induced osteoporosis. Mod. Rheumatol. 2018;28:885–889. doi: 10.1080/14397595.2017.1416919. [DOI] [PubMed] [Google Scholar]
- Van Staa T.P., Laan R.F., Barton I.P., Cohen S., Reid D.M., Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48:3224–3229. doi: 10.1002/art.11283. [DOI] [PubMed] [Google Scholar]
- Weinstein R.S. Glucocorticoid-induced bone disease. N. Engl. J. Med. 2011;365:62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- Weinstein R.S., Jilka R.L., Almeida M., Roberson P.K., Manolagas S.C. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology. 2010;151:2641–2649. doi: 10.1210/en.2009-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.