Abstract
The coronavirus-disease 2019 (COVID-19) was announced as a global pandemic by the World Health Organization. Challenges arise concerning how to optimally support the immune system in the general population, especially under self-confinement. An optimal immune response depends on an adequate diet and nutrition in order to keep infection at bay. For example, sufficient protein intake is crucial for optimal antibody production. Low micronutrient status, such as of vitamin A or zinc, has been associated with increased infection risk. Frequently, poor nutrient status is associated with inflammation and oxidative stress, which in turn can impact the immune system. Dietary constituents with especially high anti-inflammatory and antioxidant capacity include vitamin C, vitamin E, and phytochemicals such as carotenoids and polyphenols. Several of these can interact with transcription factors such as NF-kB and Nrf-2, related to anti-inflammatory and antioxidant effects, respectively. Vitamin D in particular may perturb viral cellular infection via interacting with cell entry receptors (angiotensin converting enzyme 2), ACE2. Dietary fiber, fermented by the gut microbiota into short-chain fatty acids, has also been shown to produce anti-inflammatory effects. In this review, we highlight the importance of an optimal status of relevant nutrients to effectively reduce inflammation and oxidative stress, thereby strengthening the immune system during the COVID-19 crisis.
Keywords: macronutrients, trace elements, nutrient, protein intake, innate immune system, cytokines, reactive oxygen species, transcription factors, nuclear factors, infection, coronavirus
1. Introduction
A cluster of pneumonia cases caused by a previously unknown virus were noted in December 2019, in Wuhan City, China [1]. This virus is now well-known as the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), resulting in the development of the coronavirus disease 2019 (COVID-19) [2]. The disease has spread worldwide and has been classified by the World Health Organization (WHO) as a global pandemic [2,3]. SARS-CoV-2 manifestation might be asymptomatic or moderate to severe with coughing, fever, and shortness of breath [3]. In more severe cases, complications can include acute respiratory distress syndrome, acute cardiac complications, multiple organ dysfunction syndrome, septic shock, and death [4,5,6,7]. These complications are believed to be related to what has been described as the cytokine storm, in which viral replication triggers an abnormally strong release of cytokines and other immune-related stimuli, resulting in hyper-inflammation [8].
The outbreak of this emerging infectious disease has been evolving rapidly. Strict national policies to control the disease have been implemented, including policies to practice social distancing and encouraging or even forcing people to stay at home. Especially during this self-confinement, often perceived as stressful, individuals are frequently at a loss regarding optimal dietary patterns and adequate nutrient status in order to stay healthy. In order to prevent infection, a healthy functional immune system is paramount, and an important foundation for an optimal immune response is an adequate and balanced diet [9,10,11,12,13,14].
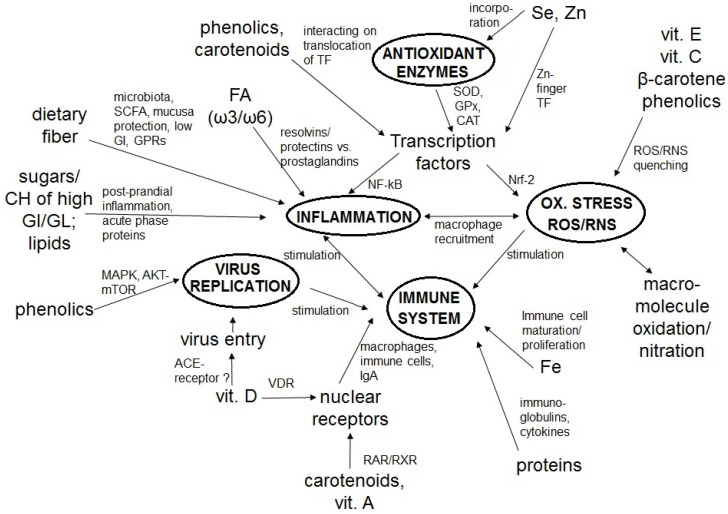
It is well acknowledged that a low protein status can increase the risk of infection, related to, for example, low antibody production [15]. An optimal nutritional status is also fundamental to modulate inflammatory and oxidative stress processes, which are all interrelated with the immune system [16]. The important notion of the relationship between dietary constituents, nutrition, inflammation, and oxidative stress is well-regarded, and has been emphasized, for example, in the development of the anti-inflammatory dietary index [17]. Dietary and nutritional constituents known to exert anti-inflammatory and antioxidant properties include omega-3 fatty acids [18], vitamin A [19], vitamin C [20], as well as a variety of phytochemicals, such as polyphenols [21] and carotenoids [22] that are widely present in plant-based foods. Also, the dietary fiber present in plant-based food items has been associated with various health benefits including anti-inflammatory properties [11], through fermentation by the gut microbiota and consequent formation of metabolic compounds, especially short chain fatty acids (SCFA). Such anti-inflammatory active compounds could be important in the overall homeostasis of inflammation and oxidative stress, both before and/or during acute infection. In fact, dietary fiber [23] and a variety of phytochemicals such as polyphenols [24] have been proposed to influence the gut microbiota, having prebiotic effects such as fostering the growth of bacteria that are associated with health benefits, such as of Bifidobacterium spp., and reducing potential pathogenic ones such as Clostridium spp. Such aspects are of interest as gastro-intestinal complications such as diarrhea have been reported following SARS-CoV-2 infection [25]. In addition to the interrelation of nutrients and infections via inflammation and oxidative stress, additional pathways may play a role. While the vitamin A metabolite retinoic acid interacts with the transcription factor RAR (retinoic acid receptor), which may play a role in immunity, vitamin D has been proposed to interact with its own transcription factors (vitamin D receptor) or the cellular receptor important for viral entry, i.e., ACE2 (angiotensin converting enzyme 2), inhibiting virus particles from entering the cell [26].
In this review, we highlight the importance of an optimal nutrient status to strengthen the immune system during the COVID-19 crisis, focusing on the most relevant constituents that reduce inflammation and oxidative stress.
2. The Immune System, COVID-19, Inflammation, and Oxidative Stress
The immune response is strongly modulated by oxidative stress and inflammatory processes [27]. The non-specific or innate natural defense mechanism is derived from cells of the myelocytic line, and provides an immediate response [28]. If pathogens (i.e., viruses, bacteria) invade the body, the innate response together with the specific or adaptive defense mechanism derived from cells of the lymphocyte line, adapt their response by secreting proteins directed towards intra- and extra-cellular pathogens, including several cytokines and chemokines released by macrophages, triggering inflammation to enhance the response [29]. Inflammation and oxidative stress also contribute to the normal functioning of the human body. In particular, oxidative stress plays an essential role in mitochondrial processes [30,31,32].
Oxidative stress is predominated by an imbalance of reactive oxygen (ROS) and reactive nitrogen species (RNS), including singlet oxygen, lipid peroxides, nitric oxide, versus a decreased function of antioxidant activity or compounds, such as endogenous antioxidants (e.g., albumin, urea, reduced glutathione), exogenous antioxidants (i.e., vitamin E, vitamin C, polyphenols, carotenoids), and endogenous enzymes (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx)), among others [33]. The role of oxidative stress during infection is not fully elucidated, but free radicals have been shown to protect against invading microorganisms [34]. Chronically elevated oxidative stress occurs in long-lasting viral infections, for example, with the Epstein–Barr virus (EBV) and the human immunodeficiency virus (HIV), among others [35,36], and has been associated with impaired immune responses [27,37,38,39]. An association between ROS, such as NO, the superoxide-radical (O2−), and peroxynitrites, and endothelial damage and inflammation has been reported [40]. Both endothelial damage and inflammation appear to play a vital role in COVID-19 [41].
It should be noted that a close relationship between inflammation and oxidative stress exists. High production of free radicals at the site of infection by immune cells, especially macrophages, triggers oxidative stress. Excessive extracellular ROS/RNS, characterized by malondialdehyde (MDA), 8-hydroxy-2′-deoxyguanosine (8-OHdG), and isoprostane accumulation [31,42], can either oxidize biomolecules including RNA/DNA, lipids, or proteins, or can structurally modify proteins and genes to trigger signaling cascades that can lead to the onset of the inflammatory response. The recognition of detrimental stimuli is initiated by pathogen-associated molecular patterns (PAMPs) [43], in immune cells and non-immune cells, via triggering of germline-encoded pattern-recognition receptors (PRRs), [44,45]. Inflammatory stimuli can trigger the activation of intracellular signaling pathways involved in the expression of inflammatory mediators. Primary inflammatory stimuli cause the release of microbial products and cytokines, including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α). These mediate inflammation through the activation of receptors, for instance toll-like receptors (TLRs), IL-1, IL-6 receptors, and the TNF-receptor [46]. As a result, intracellular signaling pathways are stimulated, including the mitogen-activated protein kinase (MAPK), nuclear factor kappa-B (NF-κB), Janus kinase (JAK)-signal transducer, and activator of transcription (STAT) pathways [47,48,49].
Overall, at first exposure to pathogens, a strong response of the innate defense system occurs at the beginning of the infection phase [50]. As such, a certain level of inflammation is physiological and required for optimal triggering of the immune response. However, low-grade systemic inflammation is common in several conditions, including cardiovascular disease (CVD), inflammatory bowel diseases (IBD), diabetes type 2 (T2D), arthritis, cancer, and obesity [51].
Individuals with low-grade chronic inflammation present a dysregulated innate immune system, resulting in an increased risk of infection [52]. Several issues may arise during extreme conditions as those triggered by COVID-19 [53]. In fact, complications related to the severe acute respiratory syndrome caused by SARS-CoV or SARS-CoV-2 are mainly due to pronounced inflammation caused by viral replication [54]. The infection causes increased secretion of IL-1β, IL-4, IL-10, interferon gamma (IFN-γ), IP-10, and monocyte chemoattractant protein 1 (MCP-1) [55]. In addition, it has been noted that human bronchial epithelial cells respond to SARS-CoV infection by producing several NF-κB-mediated cytokines, including IL-6 and IL-8 [56]. Also, patients with severe COVID-19 in intensive care units have shown elevated plasma levels of several cytokines (IL-2, IL-7, IL-10, TNF-α), MCP1, granulocyte-colony stimulating factor (GCSF), IFN-γ-induced protein 10 (IP-10), and macrophage inflammatory proteins (MIP-1A), suggesting a cytokine storm, resulting in hyper-inflammation and excessive reactions, such as hyperpyrexia and organ failure associated with disease severity, which can be life-threatening [53]. In fact, the severe COVID-19 associated cytokine profile (described also as macrophage activation syndrome (MAS) or secondary hemophagocytic lymphohistiocytosis (sHLH)), is characterized by hyper-inflammation together with multiorgan failure [57,58]. Furthermore, the cytokine storm response has been associated with an increased mortality risk, mainly due to respiratory failure from acute respiratory distress syndrome [59].
In addition to the activation of the inflammatory response and promotion of oxidative stress that are intimately related with the immune system [60,61], the immune system is interrelated with multiple aspects of physiological regulation such as hormonal regulation [62,63], metabolic regulation [64,65,66], circadian rhythms [67,68], as well as nutrient utilization [69]. Overall, malnutrition can compromise the immune response [70], altering cell regeneration and function and making individuals more prone to infection [71]. For example, it is well known that in economically poor areas with protein-malnutrition [72], the risk of infection is elevated, though additional factors such as tropical diseases may also be a factor [73,74]. In addition, inflammation related to poor dietary habits has reached alarming proportions [75], especially with regard to numerous cardio-metabolic diseases related to low-grade chronic inflammation such as overweight/obesity, T2D, metabolic syndrome, and auto-immune and cardiovascular diseases. An increasing body of evidence highlights that dietary modifications and nutritional factors can strongly impinge on chronic low-grade inflammation [76,77] and viral infection risk and symptoms (Table 1). These can serve best via the prevention of inflammation and oxidative stress and strengthening the immune system, but may also aid in the management of disease and infections as an adjuvant strategy. Consideration of dietary constituents and nutritional factors during the COVID-19 crisis can serve to strengthen the immune system for infection prevention, as well as to promote overall health in times of ongoing national restrictions. The following sections review the roles of various dietary constituents in infection, inflammation, and oxidative stress.
Table 1.
Selected studies associating dietary constituents with viral or other infection risk and symptoms.
| Constituent | Study Design | Description | Main Findings | Ref |
|---|---|---|---|---|
| Proteins | Human cross-sectional study | 23 elderly patients subjected to influenza vaccination and measurement of their nutrient status. | Total protein status (determined by questionnaire) was slightly lower (p < 0.05) in influenza vaccine non-responders vs. responders (66 vs. 69 g/L). Similar results were found for iron, proposing that immune-response was compromised by poor nutrient status in this elderly population. |
Fulop et al., 1999 [78] |
| Animal study (mice) | Group receiving a diet adequate in protein (AP; 18% of energy) vs. group receiving very low protein (VLP; 2%) for 3 weeks. Both groups were subjected to influenza infection. |
Higher mortality in the VLP group (p < 0.001) vs. AP, 25 d post infection (p.i.). The AP vs. the VLP group showed a decreased virus titer by day 9 (p < 0.001) and an efficient clearance within 12 d (p < 0.001). %age of NK cells in lungs were reduced (p < 0.01) in VLP vs. AP group with higher (p < 0.001) neutrophil proportions in response to infection with influenza virus in each group, respectively. The VLP group had less influenza NP-specific CD8+ T cells at days 8 (p < 0.05), 15 (p < 0.05), and 30 (p = 0.001). Switching VLP to AP diet improved CD4+ and CD8+ T cell subset levels on days 8 (p < 0.01), 15 (p < 0.01), and 30 (p < 0.01) and increased IFN-γ (p < 0.001). |
Taylor et al., 2013 [79] | |
| Animal study (mice) | Mycobacteria-infected mice fed 2% protein diet vs. control group receiving 20% protein diet for up to 30 days. | 100% of malnourished mice (fed 2% protein diet) succumbed to M. tuberculosis infection within 66 d p.i. Malnourished mice had a reduced expression of IFN-γ, TNF-α, and iNOS in the lungs. A mortal infection of M. tuberculosis in malnourished animals was reversed upon re-feeding with the 20% protein diet. |
Chan et al., 1996 [80] | |
| Lipids | Animal study (mice) | Mice infected with H5N1 virus treated with omega-3 polyunsaturated fatty acid-derived lipid mediator protectin D1 (PD1), given 3 times i.v. | H5N1 virus pathogenicity decreased with higher levels of PD1. PD1 inhibited virus replication (p < 0.001) via influenza virus nucleoprotein mRNA expression at day 2 p.i. PD1 treatment within 12 h improved the survival (p < 0.05) and pathology of severe influenza (p < 0.001). |
Morita et al., 2013 [81] |
| Animal study (mice) | Influenza A virus (IAV) infected mice fed with a high-fat (HF, 40% of energy) vs. low-fat (LF, 12% of energy) diet for 10 weeks. | HF mice were more susceptible to respiratory disease after IAV infection than were LF mice, with lower blood oxygen saturation (p < 0.05) and an increase in pulmonary viral load (p < 0.05). Decreased pro-inflammatory response to IAV in the serum of HF mice vs. LF for IL-6, IFN-γ, IFN-α, and IP-10 (p < 0.05). Antiviral response in the heart was reduced in HF mice after IAV infection, where higher viral loads were detected in the hearts of HF vs. LF mice (p < 0.05). Correlation between IAV-infected HF mice and viral infection in the heart, left ventricular mass, and thickening of the left ventricular wall, characterized by increased HIF-1α compared to LF group (p < 0.05). |
Siegers et al., 2020 [82] | |
| Animal study (mice) | High-fat diet (HFD) 60% or regular-fat diet (RFD) 5% fat, administered to 4-week old mice for 10 weeks. Influenza vaccination was conducted after 10 weeks. |
Functionality of macrophages was diminished after diet-induced obesity (p < 0.001) via lower CD86-expressing macrophages, lower release of IL-6 and TNF-α, increased Th1 cell subpopulation, and reduced proportion of Treg cells. Vaccination-induced antibody production was decreased in animals receiving HFD vs. RFD (p < 0.001) |
Cho et al., 2016 [83] | |
| Lipids, carbo-hydrates | Animal study (mice) | Feeding mice with ketogenic, i.e., low carbohydrate diet (KD, 90% fat) vs. standard high-fat (60% fats, 20% lipids) diet (HFD) for 7 d before influenza A virus (IAV) infection. | KD protected mice from lethal IAV infection and disease (p < 0.05) compared to HFD-fed mice. KD resulted in an expansion of T-cells (p < 0.001), compared with the HFD group. KD-fed mice had better blood O2 saturation (p < 0.001). KD diet was significantly related to improved antiviral resistance (p < 0.001). |
Goldberg et al., 2019 [84] |
| Fiber | Prospective human cohort study | Study evaluating dietary fiber intake versus health outcomes. n = 219,123 men and 168,999 women, aged 50–71 y. 9 y follow-up. | Consumption of dietary fiber correlated with lowered mortality from infectious and respiratory diseases. Per 10 g/d increase in dietary fiber, the multivariate RRs for infectious and respiratory diseases were 0.66 (CI: 0.52–0.84) and 0.82 (CI: 0.74–0.93) in men and 0.61 (CI: 0.44–0.85) and 0.66 (CI: 0.56–0.78) in women, respectively. |
Park et al., 2011 [85] |
| Animal study (mice) | High-fiber diet (HFD)-fed mice vs. control group, subjected to viral influenza infection | Intake of dietary fiber improved influenza by prolonged survival (p < 0.05) and ameliorated clinical scores (p < 0.001). After 7 d, HFD-fed mice with high-dose infection had better lung function as shown by reduced pulmonary resistance (p > 0.01) and enhanced compliance in response to methacholine (p > 0.01). In HFD-fed mice, the excessive neutrophil influx into the airways was inhibited by blunted levels of CXCL1, produced by lung monocytes and macrophages (p < 0.001) vs. controls. Increased antiviral immunity by dietary fiber through CD8+ T cell activation (p < 0.01) vs. controls. (HFD)-fed mice showed enhanced adaptive immunity by changed CD8+ T cell metabolism (p < 0.05). |
Trompette et al., 2018 [86] | |
| Animal study (mice) | Fiber-free diet group (LFD) vs. control group for up to 40 d, subjected to infection with mucosal pathogen Citrobacter rodentium | Low fiber intake resulting in increases in mucus-degrading microbiota and enhanced lethal colitis cases (p < 0.05). | Desai et al., 2017 [87] | |
| Vitamin A | Meta-analysis of RCTs. | Effects of vitamin A supplementation on acute lower respiratory tract infections (LRTI). 10 studies (n = 33,179 children). | Though some individual studies demonstrated a positive effect of vitamin A supplementation on LRTI, in pooled analyses, there was no effect of vitamin A supplementation on acute LRTI incidence or prevalence of symptoms. | Chen et al., 2008 [88] |
| Meta-analysis of RCTS. | Assessment of vitamin A supplementation on acute respiratory infection. 5 studies (n = 2177 children (1067 children under intervention, 1110 control). | Faster recovery from infection symptoms due to vitamin A, no differences in the placebo group: fever: OR: 0.03, CI: −0.10–0.17; oxygen requirement: OR: −0.08, CI: −0.31–0.16; increased respiratory rate: OR: −0.09, CI: −0.38 –0.19; hospital stay duration: OR: −0.06, CI: −0.52–0.40. | Brown and Roberts 2004 [89] | |
| Vitamin D | Retrospective human study | Study determining mortality patterns of COVID-19 and associated factors: Special focus on vitamin D status. 2 cohorts of 780 cases with confirmed infection of SARS-CoV-2 in Indonesia. | Vitamin D status is strongly associated with COVID-19 mortality (adjusted for age, sex, and comorbidity) (p < 0.001). Individuals with insufficient vitamin D status were ca. 12.6 as likely to die (OR 12.55). | Reharusun et al., 2020 [90] |
| Meta-analysis of RCTs | Assessment of vitamin D supplementation on respiratory tract infections. 5 clinical trials (n = 964 participants). | Significantly fewer respiratory tract infections were observed following a vitamin D supplementation. (OR: 0.58, CI: 0.417–0.812). In clinical trials there were beneficial effects on events of infections due to vitamin D supplementation in children (OR: 0.58, CI: 0.416–0.805) and adults (OR: 0.65, CI: 0.472–0.904). | Charan et al., 2012 [91] | |
| Meta-analysis of RCTs | Assessment of vitamin D supplementation on respiratory tract infection (RTI). 11 placebo-controlled studies (RTCs) (n = 5660 patients). | Vitamin D had protective effects against RTI (OR: 0.64; CI, 0.49- 0.84). This was more pronounced by individual daily dosing compared to bolus doses (OR = 0.51 vs. OR = 0.86, p = 0.01). | Bergman et al., 2013 [92] | |
| Vitamin E | Humans, RCT | Assessment of vitamin E supplementation and community acquired pneumonia. n = 7469 men 50–69 y. |
Lower incidence of pneumonia in individuals receiving vitamin E supplements (RR: 0.28; CI: 0.11–0.69). | Hemila, 2016 [93] |
| Vitamin C | Meta-analysis of RCTs | Supplementation trials with vitamin C and observation of cold symptoms. 9 randomized controlled trials (n = 5500) in children (3 months–18 y of age). |
Daily supplementation in vitamin C with extra doses reduced the time of having a common cold (mean difference = −0.56, 95% confidence interval (CI) (−1.03, −0.10)), fever (mean difference = −0.45, 95% CI (−0.78, −0.11)) and chest pain (mean difference = −0.40, 95% CI (−0.77, −0.03)). | Ran et al., 2018 [94] |
| B-vitamins | Human cross-sectional study | Observation of inflammation markers and nutrient status. HIV infected participants (n = 180 men, 134 women; 18–60 y). |
Serum CRP concentrations were inversely associated with increased vitamin B intake including niacin, pyridoxine, and cobalamin (p for trend p < 0.01, p < 0.05 and p = 0.037, respectively) in men. Trends were observed in women. | Poudel-Tandukar et al., 2016 [95] |
| Zinc | Human double-blinded RCT | Patients in the zinc group (n = 50) received lozenges (13.3 mg of zinc gluconate) as long as they showed cold symptoms. Patients in the placebo group (n = 50) received 5% calcium lactate pentahydrate. | A faster decrease of the cold symptoms (median, 4.4 d vs. 7.6 d; p < 0.001), e.g., fewer days with coughing (median, 2 d compared with 4.5 d; p < 0.05), hoarseness (2 and 3 d; p < 0.05), headache (2 and 3 d; p < 0.05), nasal congestion (4 and 6 d; p < 0.01), and sore throat (1 and 3 d; p < 0.001) were found in the intervention group, supplemented with zinc, in comparison with the placebo group. | Mossad et al., 1996 [96] |
| Iron | Animal trial (Wistar rats) | Administration of low iron diet (4–5 mg powder), medium iron diet (15 mg), control group (35 mg) and normal iron intake diet group. At week 4, rats received injection of inactivated porcine influenza vaccine (HswIN1). | Following immunization, anemic rats exhibited decreased (p < 0.05) antibody titer vs. controls. Antibody synthesis was preserved in moderate iron deficiency, but was hampered by severe anemia. | Dhur et al., 1990 [97] |
| Selenium | Human randomized, double-blinded RCT | Evaluation of response to influenza vaccine. 12-weeks follow up. n = 119 (50–64y) 6 intervention groups: 50, 100, or 200 mgSe/day, meals with Se-enriched onions (50 mg se/day), unenriched onions and placebo. |
SEPS1 mRNA (marker of inflammation) increased (p < 0.05) after one week of vaccine administration, being dependent on the dose of Se per each intervention arm. | Goldson 2011 [98] |
| Polyphenols | Animal study (mice) | Evaluation of effect of polyphenol extract from Cistus Incanus on avian influenza Aviurs (H7N7). Inbred female Balb/c and C57Bl/6 mice at the age of 6–8 weeks. |
The polyphenol extract helped mice to not contract avian influenza, and to not alter bronchiole epithelial cells, as well as to keep constant the body temperature and the gross motor activity. | Droebner et al., 2007 [99] |
| Carotenoids | Longitudinal study with infants | Observation of β-carotene in plasma. 194 HIV-infected infants. |
β-Carotene was related to increased risk of death during HIV infection (OR: 3.16, CI: 1.38 to 7.21; p < 0.01). | Melikian et al., 2001 [100] |
Abbreviations: AP: adequate protein; Balb/c: albino mouse strain; CD-86: cluster of differentiation 86; CRP: C-reactive protein; CXCL1: The chemokine (C-X-C motif) ligand 1; H5N1- influenza A virus subtype H5N1; H7N7: influenza A virus subtype H7N7; HF: high fat; HFD: high-fat diet; HFD: high-fiber diet; HswIN1: swine influenza virus; IAV: influenza A virus; IFN-α/γ: interferon α/γ; IL6: interleukin 6; LF: low-fat; LRTI: lower respiratory tract infections; iNOS: inducible nitric oxide synthase; KD: ketogenic diet; NK: natural killer cells; P1- protectin D1; RCT: randomized controlled trial; RFD: regular-fat diet; RTI: respiratory tract infection; SEPS1: selenoprotein S; TNF-α: Tumor necrosis factor alpha; VLP: very-low protein.
3. Dietary Constituents as Key Factors of a Strong Immune System and Low Infection Risk
3.1. Macronutrients
3.1.1. Proteins
Low protein status due to low protein intake, i.e., below the recommended 0.8 g/kg body weight as proposed by the recommended dietary allowance (RDA), [101], such as in economically challenged countries with low protein availability, has been well recognized to increase the risk of infection [15]. It is believed that the low pool of available proteins also results in a decreased amount of functional active immunoglobulins and gut-associated lymphoid tissue (GALT), which play a role in gut-mucosal defense against infection [102], among others. Very low protein intake (2% of energy) increased the severity of influenza infection in mice, for example, through a low antibody response, and enhanced virus persistence in the lungs, related to hyper-inflammation and associated mortality [79]. Even if the prevalence of protein energy malnutrition (PEM) is low in Westernized countries, several protein sources, especially from food items such as processed meats, and cheese, are high in calories and saturated fats and can aggravate post-prandial effects, favoring lipogenesis and elevated inflammation [103,104]. In this respect, the rather pro-inflammatory aspects of proteins from animal sources and anti-inflammatory properties of plant derived proteins have been acknowledged [105]. For example, meat-protein-rich diets increase colonic monocytes [106], though it can be assumed that other matrix components such as saturated fats do play a role as might the absence of fiber and further phytochemicals.
Thus, protein intake of high biological value and from healthy dietary choices, such as from eggs, fish, lean meat (e.g., poultry), and whey protein (or other non-fat dairy protein), when eaten together with meals, may lower post-prandial lipogenesis and inflammation [107]. It is also known that proteins of high biological value, i.e., containing the essential amino acids in required amounts, can reduce post-meal glycemic response and improve satiety due to their effect on prolonged gastric-retention and gastro-intestinal transit time [108,109]. Therefore, high quality proteins are an essential component of an anti-inflammatory diet [110]. The consumption of a certain amount of proteins of high biological value is known to be crucial for optimal production of antibodies [111]. Branched-chain amino acids may maintain villous morphology and increase intestinal immunoglobulin levels, thereby enhancing the gut barrier and response [112]. Some amino acids modulate the metabolism and immune functions [113,114]. For instance, arginine supplementation increased the response of T-lymphocytes and T-helper cell numbers, and rapidly returned to normal T-cell function after operations, compared to control subjects [114], suggesting a role in prolonged or repeated infection.
Glutamine is required for the expression of a variety of genes of the immune system [113,115,116,117]. Glutamine is an energy substrate for macrophages, neutrophils, and lymphocytes, needed for pathogen-identification through the proliferation of immune cells and the repair of tissues [118]. For instance, in the immune system, glutamine plays a key role in controlling the proliferation of cells such as lymphocytes, neutrophils, and macrophages [113,116], activating proteins partaking in signal transduction, e.g., ERK and JNK kinases. Both can activate a number of transcription factors, including JNK and AP-1, eventually promoting the transcription of genes participating in cell proliferation [113,119,120]. In addition, a sufficient glutamine level is important for expressing key lymphocyte cell surface markers and also various cytokines, for instance IL-6, IFN-γ and TNF-α [116,117,121,122].
From observations in humans and from experiments in animals, it is known that a diet with a very low content of protein can be detrimental to fighting off infection. For example, mice fed a 2% versus a 20% protein diet, rapidly died following exposure to M. tuberculosis [80]. This was related to a reduced expression of IF-γ, TNF-α, and iNOS [80]. Interestingly, these effects were reversed rapidly within 2 weeks of changing the diet [123]. In humans, protein malnutrition and increased susceptibility to Zika and influenza viruses is related to cell mediated immunity and decreased bactericidal function of neutrophils, the complement system, and IgA as well as antibody response [124]. Low protein status, characterized by low albumin or pre-albumin levels, but also low iron and vitamin E correlated with lower responses to influenza vaccination in the elderly, thereby highlighting the interrelation between various nutrients and the immune response [78].
3.1.2. Lipids
Fatty acids (FAs) may significantly alter immune responses, including changes in the organization of cellular lipids and interactions with nuclear receptors [125]. FAs in particular have been shown to affect the homeostasis and immune cell functioning in mice, e.g., epithelial cells, macrophages, dendritic cells, innate lymphoid cells, neutrophils, and T- and B cells [126]. In general, increased fibrinogen and high-sensitivity C-reactive protein (hs-CRP), an acute phase protein of hepatic origin, have been linked to saturated FAs consumption, while lower hs-CRP levels have been linked with polyunsaturated FA [127]. In particular, omega-3 FAs appear to have the most potent anti-inflammatory capability, though not all omega-3 FAs are anti-inflammatory [18]. Trans-fatty acid intake, especially from processed foods such as fries and chips, has also been described as pro-inflammatory, being associated with increased TNF-α, IL-6, and hs-CRP levels [12,128].
The two essential FA classes, omega-6 and omega-3, need to be consumed within the diet as the human body is unable to produce them. The intake of omega-3 FAs from fish and seafood has been shown to trigger anti-inflammatory reactions via oxygenated metabolites (oxylipins), including resolvins and protectins [129,130]. Omega-3 FAs include α-linolenic acid (ALA) consumed from various plant sources (Table 2) and eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) consumed especially from fish and seafood sources, such as salmon, mackerel, and tuna. Omega-6 FAs such as arachidonic acid are primarily pro-inflammatory [75,131,132], constituting precursors of several pro-inflammatory mediators including eicosanoids (a subset of oxylipins) such as prostaglandins and leukotrienes derived from cyclooxygenase (COX) and lipoxygenase (LOX) enzymes, respectively [129]. Eicosanoid signaling resembles that of cytokine signaling and is part of the innate immune response [133]. Their signaling is influenced by phospholipase A2 activity, which regulates the different phases of the inflammatory response by releasing eicosanoids. Furthermore, their regulation includes class switching in which pro-inflammatory eicosanoid synthesis (and accompanying TLR4 activation of NF-κB induces pro-interleukin-1B) switches to anti-inflammatory (eicosanoid and docosanoid) synthesis. Many omega-3-derived oxylipins are specialized pro-resolving mediators, capable of enhancing bacterial clearance together with a downregulation of pro-inflammatory cytokines and enhanced removal of apoptotic neutrophils [132]. This is believed to contribute to the positive health effects of omega-3 consumption.
Table 2.
Examples of dietary sources.
| Constituent | Major Food Sources | Quantity |
|---|---|---|
| Protein (g/100 g or mL) | Meat products: | |
| Beef | 25.3 | |
| Chicken | 19.3 | |
| Egg white | 11 | |
| Dairy products: | ||
| Yogurt | 3.5 | |
| Milk | 3.1 | |
| Cereals, roots, and tubers: | ||
| Potatoes | 2.4 | |
| Quinoa | 4.4 | |
| Legumes: | ||
| Soybeans (raw) | 25.9 | |
| Lipids (mg/100 g) | Fruits and vegetables: | |
| (High in omega-3) | Chia seeds | 1783 |
| Edamame | 361 | |
| Avocado | 111 | |
| Animal source: | ||
| Salmon | 2314 | |
| Tuna Fish | 1337 | |
| Whole grain food: | 18 | |
| Oatmeal | ||
| Carbohydrates (g/100 g) | Fruits and vegetables: | |
| Blueberries | 14.5 | |
| Figs | 19.2 | |
| Summer squash | 3.8 | |
| Whole grain food: | ||
| Oatmeal | 12 | |
| Whole-wheat bread | 42.7 | |
| Legumes: | ||
| Black beans | 23.7 | |
| Fiber (g/100 g) | Fruits and vegetables: | |
| Chia seeds | 34.4 | |
| Soybeans | 1.1 | |
| Orange | 2.4 | |
| Brussel Sprouts | 3.8 | |
| Legumes: | ||
| Lentils | 7.9 | |
| Chickpeas | 7.6 | |
| Vitamin A (µg/100 g) | Fruits and vegetables: | |
| Carrots (raw) | 835 | |
| Cantaloupe | 169 | |
| Mango | 54 | |
| Animal source: | ||
| Salmon | 13 | |
| Eggs | 160 | |
| Vitamin D (µg/100 g) | Vegetables: | |
| Portabella mushrooms | 0.33 | |
| Animal source: | ||
| Salmon | 14.4 | |
| Chicken | 0.14 | |
| Egg (whole, raw) | 2.1 | |
| Low fat yogurt | 0.03 | |
| Vitamin E (mg/100 g) | Fruits and vegetables: | |
| Sunflower seeds | 35.2 | |
| Nuts, almonds | 25.6 | |
| Blueberries | 0.6 | |
| Kiwi | 1.5 | |
| Broccoli | 0.8 | |
| Vitamin C (mg/100 g) | Fruits and vegetables: | |
| Oranges | 53.2 | |
| Broccoli | 89.2 | |
| Brussel sprouts | 85 | |
| Lemon | 53 | |
| Cauliflower | 48.2 | |
| Vitamins B6 (mg/100 g) | Plant source: | |
| Peanuts | 0.5 | |
| Lentils | 0.2 | |
| Animal source: | 1 | |
| Tuna fish | 0.4 | |
| Mollusks (raw) | ||
| Vitamin B12 (µg/100 g) | Animal source: | |
| Mollusks (raw) | 14.1 | |
| Plain yogurt | 0.4 | |
| Chicken breast | 0.2 | |
| Zinc (mg/100 g) | Plant source: | |
| Pumpkin and squash seeds | 7 | |
| Nuts | 3.1 | |
| Soybeans | 1.2 | |
| Animal source: | ||
| Beef | 7.4 | |
| Mollusks (raw) | 16.6 | |
| Lamb | 4.9 | |
| Iron (mg/100 g) | Fruits and vegetables: | |
| Apricots (dehydrated) | 2.7 | |
| Tomatoes (cherry) | 0.3 | |
| Peas | 1.5 | |
| Sunflower seeds | 5.3 | |
| Animal Source: | ||
| Mollusks | 5.1 | |
| Egg | 1.8 | |
| Veal (ground) | 1.4 | |
| Copper (mg/100 g) | Vegetables: | |
| Cashew nuts | 2.2 | |
| Tofu | 0.4 | |
| Mushrooms | 0.3 | |
| Animal Source: | ||
| Beef | 0.2 | |
| Oyster | 1.6 | |
| Cereals, roots, and tubers: | ||
| Sweet potato | 0.3 | |
| Quinoa | 0.2 | |
| Selenium (µg/100 g) | Plant source: | |
| Sunflower seeds | 53 | |
| Coconut meat | 17 | |
| Animal Source: | ||
| Mollusks | 77 | |
| Salmon | 47 | |
| Turkey, ham | 37 | |
| Polyphenols | Flavanone | |
| (mg/100 g) | Oranges (raw) | 42.6 |
| Grapefruit juice | 31.2 | |
| Anthocyanidin | ||
| Blueberries (raw) | 163.5 | |
| Strawberries (raw) | 33.6 | |
| Flavan-3-ol | ||
| Black tea | 115.3 | |
| Apple juice | 6 | |
| Carotenoids (mg/100 g) | α-Carotene | |
| Mixed frozen vegetables | 1.4 | |
| Tomatoes | 0.08 | |
| Tangerines | 0.08 | |
| β-Carotene | ||
| Spinach | 10.8 | |
| Kale | 9 | |
| Cantaloupe | 3 |
Source: United States Department of Agriculture.
An imbalance of FA, such as saturated/unsaturated FAs, and omega-6/omega-3 FAs has important implications for immune system homeostasis, which can foster the onset of allergic, autoimmune, and metabolic conditions [126,134,135,136,137]. As omega-3 and omega-6 FAs may compete for the same enzymes, elevated omega-6 FA concentrations can hamper the omega-3 FA metabolism [138]. Thus, it is recommended to keep a healthy balance between omega-6 and omega-3, with a ratio of 1:1–4:1 [139,140]. Unfortunately, the intake-ratio of omega-6 to omega-3 FAs has been reported to be in the range of 10:1 in individuals consuming Westernized diets, thereby possibly fostering pro-inflammatory responses [141]. For example, in patients with rheumatic diseases, characterized by strong chronic inflammation, omega-3 fatty acid administration resulted in improvements of eicosanoid synthesis toward a less inflammatory profile and reduced pro-inflammatory cytokine production [142]. Responses following 4-month supplementation of omega-3 fatty acids of 2.5 g/d and 1.25 g/d vs. placebo were compared in a randomized controlled trial (RCT) study in healthy adults. A significant decrease in serum IL-6 by 10% and 12% in the low and high dose omega-3 FAs groups, respectively, was seen, compared to a 36% increase in the placebo group [143]. Likewise, another RCT reported that omega-3 FA supplementation resulted in reduced IL-6 production, while decreasing omega-6/omega-3 ratios reduced the production of stimulated IL-6 and TNF-α [144].
These anti-inflammatory effects are expected to contribute to a better immune-system. Indeed, in a study with mice, the omega-3 FA-derived mediator protectin D1 reduced viral replication, and improved survival and symptoms following influenza infection [81]. On the other hand, care has to be taken; in an earlier study, mice fed fish oil for 2 weeks had a reduced inflammatory state, even in the lungs, that resulted in increased morbidity and mortality, the latter by 40% [145]. This was accompanied with reduced CD8+ T cell populations and decreased mRNA expression of protein1-α, TNF-α, and IL-6. Thus, while reducing inflammation during hyper-inflammation and cytokine storm conditions is likely beneficial, a general reduction of inflammatory state during infection may be a double-edged sword. In another study with mice, consumption of 6 weeks of omega-3 FA-rich diets had no effect on vaccinia virus infection of the respiratory tract [146]. It can be hypothesized that prior FA status, amount, time of omega-3 FA intake, and state of infection need to be considered. Unfortunately, well-designed human studies on this are missing.
In addition to omega-3 FA, the amount of lipid-intake has been discussed as playing a role in viral infections. In mice, diets rich in lipids/fat seem to play a crucial role in both respiratory and extra-respiratory complications of influenza A virus infection, related to an increase in viral load in the lungs and heart. This deficient antiviral response has been associated with signaling defects in the inflammatory response in mice, leading to high lung inflammation and damage, as well as increased heart inflammation and damage, i.e., increased left ventricular thickness and mass [82]. A high-fat diet administration in mice has also been associated with an efficacy reduction in influenza vaccine through a reduced antibody response, due to macrophage dysfunction in fatty environments [83,147].
3.1.3. Carbohydrates and Dietary Fiber
High glycemic index-induced acute hyperglycemia and acute insulin response, due to high consumption of processed carbohydrates (white flour, refined sugar), lead to an overload of the mitochondrial capacity and an increase of the production of free radicals [110]. Even a single high glycemic index meal has been associated with an immediate increase of inflammatory cytokines and C-reactive protein [148,149]. Increased levels of TNF-α and IL-6 have also been correlated with a higher glycemic index/glycemic load GI/GL [150]. Even if isocaloric, choosing higher-quality carbohydrates can improve postprandial glycemia and lower inflammatory responses [151]. In contrast, less processed, low-GL foods, such as vegetables, fruit, nuts, seeds, and whole grains, do not trigger such adverse post-prandial inflammatory effects [152]; this is attributed to more complex food matrices slowing down the digestion and absorption of carbohydrates. Interestingly, the administration of a ketogenic diet, i.e., a high fat but low-carbohydrate diet (<10% energy), seemed to protect mice from the severity of influenza A virus infection in terms of morbidity and mortality through the expansion of gamma delta T cells in the lungs. These cells play an essential role of host defense against the influenza A virus infection [84].
Dietary fibers are mostly complex carbohydrates and are an important factor regarding the influence of carbohydrates on inflammation [153,154]. A significant reduction in hs-CRP concentrations has been observed with increased fiber (i.e., 3.3 g/MJ or approximately 30 g/d) consumption [13]. In general, while the intake of 25 g and 38 g of fiber for women and men, respectively, is recommended, true intake is generally lower (around 15–20 g/d), at least in Westernized countries [155]. Another advantage of whole-grain intake is also a more favorable gut microbiome composition, which lowers both gut and systemic inflammation, and even small increases of only 5 g additional fiber per day can be beneficial [156,157]. Increased whole-grain intake (again with amounts of fiber even below 5 g/d) has been associated with decreased hs-CRP, IL-6, and TNF-α and increased SCFA [11,158,159,160], markedly decreasing inflammation-mediated disease risk [161,162], such as CVD, T2D, cancer, and obesity.
Different types of dietary fiber are not the sole influencers of gut health, but they do have a major effect. SCFA production, influencing gut microbiota species toward a composition perceived as healthier and strengthening gut mucosal integrity may be the most important aspects in this regard. SCFA levels are regulated by the diet, and given their importance in their immunomodulatory functions, their intake has been very much discussed in relation to inflammatory diseases in Westernized countries [163,164]. SCFAs such as acetate, propionate, and butyrate constitute important fatty acids that are produced by the gut microbiota during dietary fiber fermentation [165]. In cancer, intestinal homeostasis has in particular been attributed to the SCFA-related histone deacetylase inhibition, involved in enhancing the inflammatory response through gene regulation of cell proliferation and differentiation [166,167,168,169,170]. Anti-inflammatory signaling cascades are also activated through the SCFA-related G-protein-coupled receptor (GPRs) activation, e.g., GPR109A, GPR41, and GPR43 [171,172,173,174,175,176]. Indeed, SCFAs exert anti-inflammatory effects via IL-12 inhibition and upregulated IL-10 production in monocytes [177], repressing pro-inflammatory molecules release such as TNFα, IL-1, and NO [178], and reducing NF-ĸB expression [178,179]. SCFAs might be key regulators of inflammatory diseases by tightly controlling the migration of immune cells toward inflammatory sites as well as by modulating their activation state, enabling accelerated pathogen clearance through ROS activation [163,164].
In addition to SCFA, dietary fiber has been reported to increase the diversity of gut microbiota and promote health-associated bacteria such as Bifidobacterium spp. and Lactobaccillus spp., which have been related to, among others, mucosal inflammation [180]. Such species could contribute to reducing the growth of health-detrimental pathogens, including Clostridium spp. [181]. A healthy gut microbiota rich in Bifidobacterium spp., Faecalibacterium spp., Ruminococcus spp., and Prevotella spp. has been associated in a systematic review with lower systemic inflammation, characterized by reduced hs-CRP and IL-6 [182], even though probiotic interventions with bacteria have not always shown systematic benefits [183]. Furthermore, dietary fiber may, as has been shown in a mouse model, also enhance mucosa thickness. This may prevent bacteria from degrading this important barrier, through which allergens and other microbes could otherwise infiltrate the human host [87]. For further interactions between the gut microbiota, diet, and health-related aspects, the reader is referred to more comprehensive reviews [184,185].
It is important to highlight the emerging role of the microbiota, its modulation by nutrition, and its influence on responses to viral infection, though human studies linking diet, gut microbiota, and infection are scarce. While mainly the gut-microbiota has been studied in relation to the immune system, the nasopharyngeal microbiota may be involved in the etiology of respiratory infections [186]. An interplay between respiratory tract infections and the gut microbiota has been emphasized. Although viral infections can change the microbiome, the latter also is involved in adaptive immune responses against respiratory pathogens [187,188], triggering immune reactions of the innate system [189]. For instance, responses of macrophages to respiratory viruses are linked to the presence of distinct gut microbes. The importance of the gut microbiota for functioning innate immune responses has been demonstrated in animal models. Antibiotic treatment of animals resulted in defective macrophage responses to IFNs, resulting in hampered effects to control viral replication [190].
Health promoting effects of intestinal microbiota against viral infections, including influenza, have been known for more than a decade [191,192]. It has become clear that these effects depend on immune-regulatory cells. In particular, elevated mRNA levels, i.e., IL-1β, IL-2, IL-21, IL-18, IL-12, and IL-15, circulating through the lymphatic and circulatory systems (gut-lung axis) have been observed in mice pre-treated with probiotics. Also, studies employing mouse models indicated the microbiota contributed to establish a defense-system against pathogens, such as blocking cell internalization [193], binding to and destabilizing virion morphology [194], inhibiting further influenza virus infections [195], but also suppressing other viral replication, i.e., of herpes simplex virus (HSV)-2 [196]. It is obvious that the gut microbiota forms a dynamic environment that can be disturbed by virus infection, but can be positively modulated by dietary constituents. COVID-19 has been associated with both respiratory and gastroenteritis symptoms [197]; the latter can affect the diversity of the gut microbiota and increase the risk of contracting secondary bacterial infections.
Interestingly, dietary fiber consumption in adult Americans has been inversely linked to the risk of death from respiratory and infectious diseases [85]. In this study, for each 10 g increase in dietary fiber per day, the mortality-relative risk from infectious and respiratory diseases decreased by 34% and 18% in men and 39% and 34% in women, respectively. In another observational study with 11,897 US men [198], dietary fiber intake was associated with reduced risk of chronic obstructive pulmonary disease (COPD, not necessarily related to infection). Highest versus lowest quintiles showed an odds ratio (OR) of 0.85 for total fiber, 0.83 for cereal fiber, and 0.72 for fruit fiber, all of which were significant. Of note, T-cells from the lungs may migrate to the gut to produce type I IFNs. This may then result in Th17 cell activation, increasing IL-15 and IL-17 secretion, damaging epithelial cells in the gut [199].
The importance of both prebiotics and probiotics for infection risk prevention have just recently been highlighted [200]. In a recent systematic review including 11 RCTs with almost 2500 children, it was highlighted that probiotic treatment reduced respiratory infections [201]. As dietary fiber can influence gut microbiota and have prebiotic functions, it may be hypothesized that a similar effect can be found also with dietary fiber, though direct evidence for humans is, to our knowledge, lacking. Additional evidence has emerged from animal models. Recently, it was shown that mice fed dietary fiber exhibited increased survival rates to influenza virus infection [86] by increasing macrophages with a reduced production of the chemokine CXCL1 (causing neutrophil recruitment to the lung), as well as improving CD8+ T cell function. In another study, mice on a fiber-free diet were more susceptible to Citrobacter rodentium, a mucosal pathogen, likely due to the mucosa erosion also observed [87].
How to modulate the gut microbiome in the context of the COVID-19 crisis through dietary fiber and other dietary constituents corresponds to an important research aspect to be considered in future studies.
3.2. Micronutrients
Micronutrients encompass 12 vitamins, as well as several macro-minerals and trace elements, all of which are defined as essential. By contrast, the roles of ultra-trace elements, such as nickel or boron, are unclear. A decreased status of micronutrients often causes more or less specific deficiency symptoms. However, the cut-off points for deficiency may be unclear, and also depend on individual variability. Furthermore, it is often debatable how best to define micronutrient status, though concentrations in blood generally serve as the best available proxy marker [202], with a few exceptions such as for iron (measured by hemoglobin). However, plasma and serum are often a fast-exchanging pool, and levels in tissues would be a superior marker for status determination.
3.2.1. Vitamins
Vitamin A
Vitamin A deficiency has traditionally been associated with increased risk of infection [203,204]. In fact, it is among the most abundant micronutrient deficiencies worldwide, especially in countries with low protein and meat intake [205,206]. However, vitamin A can also be formed out of pro-vitamin A carotenoids such as α- or β-carotene, being the major vitamin A source in individuals with low meat intake [207,208]. Vitamin A is important for the morphology of the epithelium, playing a role in its keratinization, stratification, differentiation, and functional maturation [209], constituting a front line of defense against pathogens. Vitamin A is involved in the formation of healthy mucus layers, such as those of the respiratory tract and the intestine, required for mucin secretion and enhancing antigen non-specific immunity functions [209,210].
Retinal, retinol, and retinoic acid are active forms of vitamin A, and the latter acts as a ligand, activating the nuclear retinoic acid receptor (RAR) and unknown metabolites may activate the retinoid X receptor (RXR) [211]. Therefore, (all-trans and 9-cis) retinoic acids play crucial roles in the regulation of the differentiation, maturation, and function of the innate immune system and cells, e.g., macrophages [212] and neutrophils [213]. Retinoic acid promotes an immediate response to pathogen invasion by phagocytosis and activation of natural killer (NK) T-cells, which relate immune-regulatory functions through cytotoxic activity [214,215]. Retinoic acid can also alter the differentiation of dendritic cell precursors [216,217,218]. These are specialized sentinels of the immune system, orchestrating innate and adaptive immune responses [219]. It is thus not surprising that low vitamin A status (measured typically as serum retinol) has been shown to correlate with hampered function of neutrophils, macrophages, as well as T-and B-cells [220]. A critical role in the etiology of influenza, together with vitamin D, has been also proposed [219]. Furthermore, individuals with low vitamin A status exhibit histopathological alterations to the pulmonary epithelial lignin and lung parenchyma, resulting in increased risk of lung dysfunction and respiratory disease [221]. This is particularly relevant considering the effects that COVID-19 has on lung function [222].
It appears that direct clinical evidence linking vitamin A administration to enhanced resistance to infection or proxy markers such as cytokine production or lymphocyte activation is lacking [223]. Furthermore, a lack of association between plasma β-carotene, retinal (as well as α-tocopherol and zinc), and response to the influence of a vaccine was shown in the elderly, suggesting that the immune response was not significantly influenced by the differences of these micronutrients in this population [224]. This may be because a low status of vitamin A in Westernized countries is rather uncommon. Of note, vitamin A supplementation did not affect the risk of lower respiratory diseases (LRD) and symptoms in a systematic review in children [88], suggesting that vitamin A supplementation should not generally be recommended for LRD prevention. Similar results were found in an earlier meta-analysis, where risk of infection of acute respiratory diseases in developing countries was non-significantly higher in the supplemented group [89]. In a recent review, it has been highlighted that vitamin A losses occur in infection via impaired vitamin A absorption and urinary losses [19]. In general, studies investigating the efficacy of vitamin A supplementation on improvement of immune responses to vaccines have produced conflicting results [225]. However, it is suspected that the influence of pre-existing vitamin A stores and extra-nuclear activities of their hormone receptors play crucial roles in resulting responses. More well-controlled studies are warranted in order to sufficiently investigate this topic.
Vitamin D
Vitamin D can be taken up from the diet via fish, eggs, fortified milk, and mushrooms, but it can also be synthesized under the skin in the presence of UV-light from cholesterol. The active form of vitamin D, calcitriol (1,25 dihydroxyvitamin D), formed following kidney and liver hydroxylations, is most renowned for its regulating role in calcium homeostasis and thus bone health, but it has also been shown to regulate the immune system [226]. In fact, T-cell functioning is closely related to vitamin D [227]. T-cells express the CYP27B1 gene, responsible for processing the pre-hormone vitamer of vitamin D (25-hydroxyvitamin D), calcidiol into the active hormone (calcitriol). Only after binding to calcitriol can T-cells can carry out their physiological functions [228]. Other immune cells are involved in CYP27B1 expression, e.g., macrophages and dendritic cells, enabling vitamin D activation [227,228]. As for vitamin A with RAR/retinoid X receptor (RXR), the activated vitamin D form of calcitriol can bind to a specific nuclear receptor (vitamin D receptor, VDR). While this receptor is especially known for its role in regulating phosphorus and calcium levels and thus bone metabolism, its role for both the innate and adaptive immune systems has been highlighted [229]. Vitamin D has been controversially discussed for its role in influenza prevention and therapy [230], and the WHO has discussed its role in the prevention of respiratory diseases in children [91].
Regarding human trials, mixed effects have been reported. In a study in China, protective effects of vitamin D administration were reported regarding incidence and severity of influenza [231] between low- and high-dosed vitamin D children, though low vitamin D status at study onset could have played a role. In a recent review discussing intervention trials, mixed results were observed [230]. Vitamin D therapy, in a meta-analysis, improved conditions in individuals with chronic obstructive pulmonary disease (COPD), though this was not caused only by infection [232], similar to another meta-analysis [232]. Another review reported on a reduced risk of influenza and COVID-19 infections and mortality [233], mostly due to related inflammatory status and anti-microbial peptides such as cathelicidin and defensins and by modulating adaptive immunity, such as reducing Th1 helper cell responses. This is corroborated by a meta-analysis of RCTs, finding protective effects of vitamin D against respiratory tract infection, with daily dosing appearing to be the most effective strategy [92]. Other, more direct effects on virus-receptor binding could also play a role. Interestingly, vitamin D supplementation promoted binding of the SARS-CoV-2 cell entry receptor ACE2 (angiotensin converting enzyme 2) to AGTR1 (angiotensin II receptor type 1), reducing the number of virus particles that could attach to ACE2 and enter the cell [26].
A recent retrospective study including 780 confirmed cases of SARS-CoV-2 infection determined mortality and associated factors, with a special focus on vitamin D status. Older and male cases with pre-existing conditions and below normal vitamin D levels were strongly associated with increasing odds of death, those with insufficient vitamin D status were almost 13 times as likely to succumb [90].
Moreover, recent observational studies investigating COVID-19 infection and mortality globally have observed a latitude effect with most Southern Hemisphere countries (except for Brazil), reporting less cases/mortality [234]. Interestingly, at the same time, the Southern Hemisphere is entering autumn and will have the highest vitamin D blood levels at this season. Conversely, Northern Hemisphere countries are entering Spring and have their lowest blood vitamin D levels after winter. In Europe, COVID-related mortality appears blunted with increased latitude. For instance, Nordic countries such as Finland and Norway that have either mandatory vitamin D fortification or higher vitamin D intakes have among the highest vitamin D levels in Europe but also lower mortality. Conversely, despite the high sun exposure, older populations in Italy and Spain show a much lower vitamin D status and higher COVID-mortality rates [235]. Surely, correlations are not causation (as infection protocols, testing, population isolation, healthcare, etc. all modify infection rates), but it provides an interesting research hypothesis to confirm with RCTs.
Vitamin E
Vitamin E exists in the major forms of tocopherols and tocotrienols, with most research focused on the effects of the former. Tocopherols are present in high amounts in nuts and vegetable oils while tocotrienols are found predominantly in some seeds and grains. Although deficiencies in vitamin E are uncommon in humans, secondary deficiencies might occur, for example, following an intestinal malabsorptive disorder. Of note, in order to replicate its antioxidant effects, vitamin E works synergistically together with vitamin C, by which its tocopheroxyl radical is reduced by vitamin C [236].
Vitamin E has also been shown to regulate the maturation and functions of dendritic cells [237], which are important for intertwining innate and adaptive immune systems to orchestrate immune response [238,239,240]. In addition to increasing NK cells activity, by modulating NO levels [241], vitamin E administration enforces humoral (B cells) as well as antibody responses, both in animals and humans [242,243]. Vitamin E has been shown to improve naïve T-cells immune synapse formation and initiate T-cell activation signals [243,244,245,246].
The role of vitamin E in the prevention of infections such as influenza has been discussed [247,248,249], but well controlled studies in humans are lacking. In a study with mice, vitamin E administration (60 mg/kg per day for up to 7 d) was superior in reducing the elevated oxidative stress resulting from influenza infection [248] compared to vitamin C (80 mg/kg), but the combination of both was most successful in reducing lipid peroxidation. Following influenza infection in mice, vitamin E supplementation decreased lung related-pathology and mortality through the improvement of the T-helper 1-type cytokines response, which produces pro-inflammatory responses against intracellular parasites [250].
Supplementation in humans with vitamin E seems to restore IL-2 production, improving T-cell proliferation and immune system functioning [251,252]. A recent study conducted in Malaysian adults in which volunteers received either tocopherol or tocotrienol supplementation showed increased expression of a variety of genes associated with the immune response [253]. Interestingly, the specific genes altered were different between the two groups. Furthermore, vitamin E supplementation, after a first hospitalization in elderly with pneumonia, was associated with 63% reduced re-hospitalization within 90 days [254]. In an RCT, elderly, healthy participants receiving 200 mg/d capsules of vitamin E for 4 months had a 65% increase delayed-type hypersensitivity skin response (p = 0.04) compared to placebo, and also improved antibody titers of hepatitis B and tetanus vaccines, highlighting implications in T-cell mediated functions [243]. In another study conducted among 2216 smokers receiving 50 mg/d of vitamin E for 5–8 years, it was shown that vitamin E supplementation reduced pneumonia incidence by 69% in elderly men [93].
Vitamin C
Vitamin C is often perceived as a classical antioxidant, directly quenching free radicals in the aqueous layer while being oxidized itself to dehydro-ascorbic acid. In addition, an increased dietary ascorbic acid intake has been related to lower concentrations of C-reactive protein and tissue plasminogen activator [20]. However, vitamin C also acts as a cofactor for a number of biosynthetic and gene regulatory monooxygenase and dioxygenase enzymes, suggesting immune-modulating effects [255,256,257,258,259]. Several in vitro (cell-culture) and pre-clinical studies have highlighted the barrier-enhancing effects of vitamin C, especially regarding lipid synthesis, via influencing signaling and biosynthetic pathways [260,261,262,263,264]. In addition, ascorbic acid can alter gene expression in dermal fibroblasts, enhancing their proliferation and migration that play paramount roles for tissue remodeling, important in wound healing, for example [265,266]. Vitamin C has been shown to stimulate the migration of neutrophils to the infection site, stimulating phagocytosis and ROS generation [267,268,269].
In parallel, vitamin C can also stimulate neutrophil apoptosis, protecting host tissue from strong damage [270], and it further aids in macrophage removal [268]. Finally, ascorbic acid plays a role in the differentiation and maturation of T-cells [271,272]. Similar maturation has been observed with immature NK cells, as well as proliferative and differentiation effects with mature NK cells [273].
Low vitamin C status has been discussed as an adjuvant measure to aid in individuals with the common cold and also pneumonia [274], and positive effects were found in some intervention trials such as shortening the duration of colds [274]. In a study with mice exposed to restraint stress and virus-induced pneumonia (H1N1) [275], vitamin C administration (125 and 250 mg/kg) was shown to reduce mitochondrial antiviral signaling (MAVS) and interferon regulatory factor 3 (IRF3) and to increase NF-κB expression, while reducing steroid hydroxylating enzymes. As reviewed earlier [276], a few controlled studies found significant benefits for supplementing vitamin C in subjects with pneumonia. For example, in a double-blinded controlled trial with elderly participants, 200 mg/d of ascorbic acid for 4 weeks improved respiratory condition [277]. In a recent meta-analysis of nine RCTs, extra doses (0.7 to 8 g/d) of vitamin C against common cold virus infections reduced duration of infection, shortened time of indoor confinement, and relieved symptoms [94]. In another meta-analysis of eight RCTs in 3135 children, supplementation of vitamin C with 0.5–2 g/day did not prevent infection of upper respiratory tract diseases, but reduced the duration of infection by 1.6 days [278].
B Vitamins
B vitamins are involved in many energy-related enzymatic processes. Riboflavin (vitamin B2), as it is a photosensitizer, was employed together with ultra-violet radiation to reduce viral load of batches of blood for transfusion, and as such impinged effectively on the titer of the Middle East respiratory syndrome-related coronavirus (MERS-CoV) in human plasma to below the detection limit [279]. However, the effect of vitamin B2 alone is not known. Administration of vitamin B3 for lung injury treatment in mice, through nicotinamide treatment, significantly decreased inflammation and reduced the neutrophil infiltration, although hypoxemia was surprisingly increased [280]. NAD+-dependent enzymes and their role regarding inhibiting effects of nicotinamide deserve further investigation. Low plasma pyridoxal 5′phosphate (PLP), the active coenzyme form of vitamin B6, has been significantly associated with impaired humoral and cell-mediated immunity [281,282,283,284]. In critically ill patients, vitamin B6 supplementation increased plasma PLP concentrations associated with increased total lymphocyte cells, including T-helper and T-suppressor cells [285]. The inverse relation of vitamin B6 intake and inflammation status has been well reviewed previously [286].
In the case of folate (vitamin B9), there have been responses to high-dose folic acid supplementation on altered mRNA expression in cytokines, together with a lowered number of cytotoxicity of NK cells in healthy participants [287]. Individual studies have also shown that cobalamin (vitamin B12) may act as an immunomodulator. For example, vitamin B12-deficient patients have shown decreased levels of CD8+ cells, an abnormally high CD4/CD8 ratio, and subdued activity of NK cells [288]. Methylcobalamin administration to these patients improved the CD4/CD8 ratio and suppressed NK cell activity and CD3-/CD16+ cell increases [288]. Vitamin B forms have also shown to be efficient in lowering inflammation caused by virus infection. In particular, in patients with HIV, high vitamin B3, vitamin B6, and vitamin B12 intake in the form of niacin, pyridoxine, and cobalamin, respectively, have been significantly associated with lower inflammation levels such as decreased CRP [95].
Prolonged periods (6 months) with high-doses (exceeding daily intake recommendations for adults) of B-group multivitamins was carried out within an RCT trial with 32 healthy adults and improved levels of total plasma homocysteine, as a marker of oxidative stress [289]. Homocysteine is associated with oxidative stress and is known to increase during B-vitamin deficiencies, especially folate and vitamin B12. In another long-term (7 years) RCT trial it was tested whether a daily administration of folic acid (2.5 mg), vitamin B6 (50 mg), and vitamin B12 (1 mg), versus placebo, could prevent CVD disease markers in women (n = 300). The combination treatment did not change major biomarkers of vascular inflammation [290]. Trials with a larger number of participants and stronger epidemiological designs would provide more definitive information about the role of B vitamins in the immune system and infection.
3.2.2. Minerals
Reduced macro-minerals and trace elements have been associated with increased risk of infection. For instance, magnesium intake has been inversely associated with the concentrations of hs-CRP, IL-6, and TNF-α in a dose-dependent manner [9]. Magnesium is co-factor for many enzymes, playing a role in energy-metabolism, and a low status could interfere with numerous enzymatic reactions. Similarly, trace-elements such as zinc, copper, and selenium are required as co-factors for a variety of enzymes involved in antioxidant reactions, and have been implicated in boosting the immune system [291,292]. Several mineral deficiencies often occur together, such as low iron together with low zinc status [293].
Zinc
Zinc deficiency is a serious public health problem worldwide [294] and also appears to be prevalent in Westernized countries [295,296]. A low zinc status has been associated with increased risk of viral infections [297]. Zinc has been shown to be important for skin maintenance and mucosal membrane integrity [292], and the un-chelated free form of zinc has been shown to have direct antiviral effects, such as on rhinovirus replication in vitro [291]. Zinc is essential for cellular growth and differentiation of immune cells, having a fast differentiation and turnover rate, and helps to modulate cytokine release and trigger CD8+ T-cell proliferation [298]. Zinc has also been proposed to be crucial for intracellular binding of tyrosine kinase to T-cell receptors, which is needed for the development and activation of T-lymphocytes [299]. Zinc is an important cofactor for over 750 zinc-finger transcription factors, and thus implicated in DNA and RNA synthesis [300], also necessary for the production of immune-related proteins. By stabilizing the tertiary structure or as an essential component of the enzyme’s catalytic site [301], zinc serves as a cofactor for over 200 enzymes involved in antioxidant defense, notably of SOD and anti-inflammatory SMAD proteins [302].
In a recent review, the role of low zinc status in the elderly and its relation to pneumonia has been emphasized [303]. The mortality due to pneumonia has been reported to be twice as high in individuals with low zinc status versus individuals with normal zinc levels [303]. For some time, zinc has been suggested to improve symptoms of the common cold. In a randomized, double-blind, placebo-controlled study, patients (n = 100) with common cold symptoms took 13.3 mg of zinc as long as symptoms were present [96]. Compared to a placebo, zinc significantly reduced the duration of symptoms of the common cold, from 7.6 to 4.4 days.
Iron
Iron deficiency is highly prevalent worldwide [296], and its association with infectious diseases is well-recognized [304,305]. Often, low iron and low vitamin A status go together, as both are absorbed well from foods with a high content of protein such as meat and meat products [306,307]. Vitamin A appears to modulate hematopoiesis and iron metabolism, enhancing immunity to infectious diseases [306,307,308]. Iron appears as an essential component in cell differentiation, growth, and functioning (e.g., DNA synthesis through ribonucleotide reductase). Iron helps to fight off infections by enabling T-lymphocyte immune cell proliferation and maturation as well as regulating cytokines production and action against bacteria, for example, by neutrophil action [291,292].
The role of iron for bacterial infections [309] and viral infections [310], including respiratory infections [311], has been critically reviewed, highlighting that iron homeostasis and levels are tightly controlled. During inflammation, iron absorption is downregulated via hepcidin [309] in order to limit the available pool of iron for proliferating bacteria and virus particles and to limit excessive oxidative stress. However, during prolonged periods of iron deficiency, antibody production is typically reduced, as shown in experimental studies with mice exposed to influenza virus [97]. This has also been shown in elderly adults, relating iron deficiency to disturbed cell-mediated and innate immunity [312]. In a case-control study in 485 hospitalized 2–5 year old children receiving 3 months of iron supplementation, recurrences of acute respiratory tract infections, urinary tract infections, and gastroenteritis were significantly reduced [313].
Copper
Copper has been shown to have a role in the innate immune response to bacterial infections [292] and has been associated with IL-2 production and response. High concentrations of copper may be toxic for invading microbes and appear to be employed by macrophages as a defense strategy [314], which could play a role in secondary infections following viral infection. Copper is further involved in T cell proliferation [315], antibody production, and cellular immunity [298]. A healthy copper status has been related to aiding in the defense against several bacterial infections, including E. coli, Salmonella, and tuberculosis [316]. However, since copper needs are very low (it is often considered an ultra-trace element) and it is ubiquitously distributed, copper deficiency is rather rare.
Selenium
The role of selenium as an adjuvant therapy in viral and bacterial infections has been discussed [317], and its relationships with influenza virus, hepatitis C virus, coxsackie virus, among others, have been reported [317,318]. Low selenium status has been reported in multiple geographical regions including Western countries [319]. Requiring selenium for their synthesis, selenoproteins include several antioxidant enzymes such as GPx, selenoprotein P, and thioredoxin reductase [320,321]. Therefore, one of selenium’s primary roles is its ability as an antioxidant to quench ROS [322]. For example, these selenoproteins play vital roles in the hosts’ antioxidant defense system, influencing leukocyte and NK cell function [315]. It has been reported that selenium is protective against the heart-damaging effects of the cytomegalovirus [291] and is involved in T-lymphocyte proliferation and the humoral system, especially immunoglobulin production [291,315].
Selenium deficiencies have been associated with viral infections such as influenza, influencing adaptive and innate immunity responses and leading to a high level of virus-related pathogenicity. In this context, dietary selenium supplementations were suggested as adjuvant therapies of influenza infection, supporting the immune response [317]. Selenium supplementation seems to act on selenoprotein gene expression, improving the vaccine response [98]. However, supplementation with selenium may be a double-edged sword of low therapeutic width, as supplementation has been discussed in relation to elevated incidence of type 2 diabetes [323].
A prospective study was carried out in 83 patients with respiratory diseases requiring intensive care. Selenium levels in serum at admission were 28% lower in the intensive care unit (ICU) group vs. a general ward group. Poor serum selenium status was associated with decreased numbers of lymphocytes and albumin concentration, a marker of protein status, and correlated with increased CRP [324]. In a recent RCT, critically ill patients with acute respiratory distress syndrome received selenium in the form of sodium selenite (1 mg for 3 days and 1 mg/d for a further 6 days) [325]. Selenium concentrations were linearly associated with serum GPx levels and antioxidant activity, as determined by ferric-reducing antioxidant power. Both IL-1β and IL-6 serum concentrations were inversely associated with selenium serum levels. However, no effects on overall survival, the duration of mechanical ventilation, or ICU stay were apparent, which also raises caution in interpolating findings from sub-clinical markers to harder endpoints.
3.3. Phytochemicals
3.3.1. Polyphenols
Individuals regularly consuming vegetables and fruits have lower rates of inflammatory markers such as CRP, IL-6, and adhesion factors [326,327,328]. This has been attributed to the high fiber content and higher concentrations of certain vitamins and minerals in these food items, together with a lower caloric density as described above, and other protective phytochemicals abundant in a plant-based diet. Adding fruits and vegetables to the diet, such as those rich in flavonoids, have significantly reduced serum inflammatory markers [21,329,330] by improving the microvascular reactivity, reducing CRP values [331,332], improving lipid profiles [333,334,335], and enhancing endothelial function [336,337,338]. A number of flavonoids including quercetin have been studied in vitro, i.e., in cell culture monolayers, to examine their potential antiviral properties, for example, infectivity and replication of herpes simplex virus type 1 (HSV-I), polio-virus type 1, parainfluenza virus type 3 (Pf-3), and respiratory syncytial virus (RSV). Quercetin decreased, dependent on its concentration, viral infectivity, and hampering of intracellular viral replication, when mono-layers were infected and subsequently cultured in quercetin-containing medium [339].
Among the most abundant phytochemicals or secondary plant compounds in the diet are polyphenols, with dietary intakes about 1g/d in Westernized countries [340]. The consumption of high-polyphenol diets, which likely exert numerous antioxidant [341,342] and anti-inflammatory effects [343,344] through inhibition of NF-κB and AP-1 and activation of Nrf2 [345], have improved lipid profiles and reduced dyslipidemia, via improving concentration and function of HDL-cholesterol and reduction in LDL-cholesterol [346,347,348]. In addition to their potential, direct antioxidant effects, several phytochemicals have been proposed to interact with transcription factors, especially NF-kB and Nrf-2 [22]. Polyphenols have also been advertised for their potential pre-biotic effects on the gut [349,350].
The role of polyphenols against influenza viruses, both regarding prevention and treatment, has been reviewed recently [351]. The major important mechanisms highlighted were suppression of neuramidase and hemagglutinin activity, influences on viral replication, viral hemagglutination, adhesion and penetration into the host cell, as well as modifying cellular signaling pathways and transcription factors. A strong anti-influenza virus activity in chicken embryo fibroblast cells and in mice was shown following the administration of an extract of Geranium sanguineum L., rich in polyphenols [352]. Likewise, coumarin derivatives (bis(triazolothiadiazinyl coumarin)) were studied for their anti-influenza activity. Within various cell models, the positive effect of coumarin against viral infections such as of HIV, influenza, enterovirus 71(EV71), and coxsackievirus A16 (CVA16) was shown. This was explained by different mechanisms, including changing protein conformation needed for viral entry, effects on replication and infectivity, and also cellular signaling regulation via AKT-mTOR (mammalian target of rapamycin), NF-κB, and Nrf-2, important for stimulating the body’s own antioxidative system [353]. In addition, theaflavin derivatives (polyphenols from black tea) had a strong inhibitory effect against influenza virus in vitro [354], perhaps due to downregulation of IL-6 expression. Similar anti-viral activities were reported for further polyphenol constituents due to the implications of MAPK kinases involved in the transport of viral ribonucleoprotein complexes in the cytosol and interactions in vital hemagglutinin protein via redox-sensitive pathways [355]. Though human RCTs, to our knowledge do not exist, an intervention trial in mice receiving a polyphenol extract from Cistus Incanus resulted in a lower influenza virus infection rate and reduced mortality of mice receiving the extract [99].
In a recent RCT, the effectiveness of eight weeks of curcuminoid supplementation, a rather apolar polyphenol, at a daily dose of 1 g, on markers of oxidative stress and inflammation in patients with metabolic syndrome was investigated. Supplementation with curcuminoids significantly improved serum SOD activity and reduced MDA and CRP concentrations compared with placebo [356]. The same study included a meta-analysis of data from all RCTs to study the impact of curcuminoid administration on CRP. Overall, curcuminoids vs. placebo reduced circulating CRP concentrations by about 2.2 mg/L.
3.3.2. Carotenoids
Carotenoids are a group of mostly C-40 tetraterpenoid plant pigments and have been discussed for their antioxidant properties and quenching of ROS such as singlet oxygen and lipid peroxides within the lipid bilayer of the cell membrane [357]. Low levels of α- and β-carotene, lutein/zeaxanthin, as well as total carotenoids have been significantly associated with increased levels of oxidative stress, as recently reviewed [358], and also of inflammation [359]. For instance, low levels of α- and β-carotene, lutein/zeaxanthin, and total carotenoids were significantly more likely to be associated with increased IL-6 levels in women [359]. Another randomized, cross-over study determined the effect of orange and blackcurrant juice (high carotenoid content) supplementation in patients with peripheral arterial disease for four weeks; supplementation resulted in reduced inflammation markers, i.e., CRP and fibrinogen in plasma, while no significant differences regarding IL-6 and endothelial markers compared to placebo group were found [360]. Carotenoids such as lutein, zeaxanthin, and carotene plasma concentrations have also received attention for their potential antiviral roles [361]. A higher risk of mortality has been associated with low concentrations of plasma carotenoids in patients having contracted an HIV infection [100]. Carotenoids may also impact immune function by regulating membrane fluidity and gap-junction communication [362]. Finally, some carotenoids serve as precursors for vitamin A and may thereby exert immune-modulating functions attributed directly to vitamin A status.
A prospective serologic analysis of over 29,000 men revealed that higher serum β-carotene had significantly lower all-cause mortality, by about 19%. In addition, serum β-carotene was significantly associated with reduced risk of death from CVDs, cancer, diabetes, respiratory disease, and diabetes, providing evidence that higher β-carotene biochemical status is associated with a multitude of mortality causes [363]. However, cause and consequence could not be established in this study. In line with this finding are results from a meta-analysis in which one study highlighted that in elderly subjects, higher lutein/zeaxanthin levels were associated with 23% lower respiratory mortality [364]. Care should be taken as supplementation of higher doses of β-carotene (20–30 mg/d for several years) has caused increased cancer rates, though specifically in smokers and asbestos-exposed workers [365].
4. Summary and Future Directions
In summary, this review thoroughly covers nutrients, micronutrients, and phytonutrients known to affect immunity and infection risk, particularly relevant during the COVID-19 crisis. Figure 1 shows a schematic diagram illustrating interactions between selected dietary constituents, the immune system, and viral infection. Evidence indicates that a diet that positively impacts immune function contains adequate amounts of protein, particularly including glutamine, arginine and branched-chain amino acids (BCAAs); high omega-3 versus lower saturated, trans fat, and omega-6 fatty acids, low refined sugars, high fiber content such as whole grains, and micronutrients including vitamin A, vitamin D, vitamin C, vitamin E, B vitamins, zinc, selenium and iron, as well as phytochemicals. Table 2 shows examples of dietary sources. However, as shown well for dietary fiber, an excessive anti-inflammatory response may also reduce the immune response and increase susceptibility to infection. Controversial effects were also found for vitamin A, while carotenoid supplementation may only be detrimental for smokers and asbestos-exposed workers. Selenium supplementation should be considered with care due to the small therapeutic window and possible side-effects, especially in subjects with diabetes. While anti-inflammatory dietary constituents may be beneficial during hyper-inflammation, such as the COVID-19 cytokine storm, care should be taken against high doses of isolated anti-inflammatory compounds and/or antioxidants during healthier conditions in order to not suppress inflammation and the immune system too strongly.
Figure 1.
Schematic diagram showing interactions between selected dietary constituents, the immune system, and viral infection. Abbreviations: CH: carbohydrates; GALT: gut-associated lymphoid tissue; GPRs: G-protein-coupled receptors; FA: fatty acids; GI/GL: glycemic index/load; RAR/RXR: retinoic acid receptor/retinoid X receptor; SCFA: short-chain fatty acids; TF: transcription factors; VDR: vitamin D receptor.
The dietary components reviewed herein highlight the importance of an optimal nutrient status to reduce inflammation and oxidative stress, thereby strengthening the immune system during the COVID-19 crisis. The multiple associations highlight the complexity of the topic, and may also point out to not only individual marker determination, but rather toward the simultaneous determination of a nutrient signature, such as determined by nutrigenomics tools (i.e., metabolomics fingerprinting) [366]. Such an approach may also reveal yet unnoticed relations between nutrients, their metabolites, inflammation, oxidative stress, and the immune system. Furthermore, confounding factors such as medication and environmental pollutants could be also taken into account.
This work has been prepared with the purpose of contextualizing the available evidence to the current context of the COVID-19 pandemic crisis. An important additional aspect to keep in mind is the control of low-grade chronic inflammation related to chronic diseases such as obesity, diabetes, auto-immune, and CVDs. This might be possible through controlling nutritional deficiencies and promoting adequate nutritional status, which might improve immune response in infection phases. Our work was prepared with a focus on nutritional components available in the diet. However, some of these components are also available as supplements. Due to the potential limited therapeutic window of some of the dietary constituents discussed, we encourage obtainment of the beneficial nutritional and dietary constituents through the promotion of healthy dietary habits.
Acknowledgments
We would like to thank Eamon Laird, Trinity College Dublin, for his valuable input on vitamin D deficiency and general aspects. The purpose of this review was primarily to inform scientists. It is advised that the public consult with their physician and dietitian prior to making any significant dietary changes.
Author Contributions
The author responsibilities: All authors conceptualized the study. M.I., A.B., and T.B. interpreted information and were in charge of the original draft preparation. G.D., S.S.F.D.C., H.S., M.R.L.F. and T.B. provided further input to the manuscript. T.B. has final responsibility for all parts of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
A.B. has worked as an independent consultant for DSM Nutritional products, for the Société des Produits Nestlé SA, and for Global Healthcare Focus L.L.C. M.I., G.D., S.S.F.D.C., H.S., M.R.L., and T.B. have no conflict of interest to declare.
References
- 1.Weston S., Frieman M.B. COVID-19: Knowns, Unknowns, and Questions. mSphere. 2020;5 doi: 10.1128/mSphere.00203-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lake M.A. What we know so far: COVID-19 current clinical knowledge and research. Clin. Med. (Lond.) 2020;20:124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab. Syndr. 2020;14:247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singhal T. A Review of Coronavirus Disease-2019 (COVID-19) Indian J. Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochi A.N., Tagliari A.P., Forleo G.B., Fassini G.M., Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J. Cardiovasc. Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie P., Ma W., Tang H., Liu D. Severe COVID-19: A Review of Recent Progress With a Look Toward the Future. Front. Public Health. 2020;8:189. doi: 10.3389/fpubh.2020.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chacko S.A., Song Y., Nathan L., Tinker L., de Boer I.H., Tylavsky F., Wallace R., Liu S. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care. 2010;33:304–310. doi: 10.2337/dc09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George S.M., Neuhouser M.L., Mayne S.T., Irwin M.L., Albanes D., Gail M.H., Alfano C.M., Bernstein L., McTiernan A., Reedy J., et al. Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol. Biomark. Prev. 2010;19:2220–2228. doi: 10.1158/1055-9965.EPI-10-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y., Hebert J.R., Li W., Bertone-Johnson E.R., Olendzki B., Pagoto S.L., Tinker L., Rosal M.C., Ockene I.S., Ockene J.K., et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition. 2008;24:941–949. doi: 10.1016/j.nut.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mozaffarian D., Pischon T., Hankinson S.E., Rifai N., Joshipura K., Willett W.C., Rimm E.B. Dietary intake of trans fatty acids and systemic inflammation in women. Am. J. Clin. Nutr. 2004;79:606–612. doi: 10.1093/ajcn/79.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North C.J., Venter C.S., Jerling J.C. The effects of dietary fibre on C-reactive protein, an inflammation marker predicting cardiovascular disease. Eur. J. Clin. Nutr. 2009;63:921–933. doi: 10.1038/ejcn.2009.8. [DOI] [PubMed] [Google Scholar]
- 14.Jahns L., Conrad Z., Johnson L.K., Whigham L.D., Wu D., Claycombe-Larson K.J. A diet high in carotenoid-rich vegetables and fruits favorably impacts inflammation status by increasing plasma concentrations of IFN-α2 and decreasing MIP-1β and TNF-α in healthy individuals during a controlled feeding trial. Nutr. Res. 2018;52:98–104. doi: 10.1016/j.nutres.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez L., Cervantes E., Ortiz R. Malnutrition and gastrointestinal and respiratory infections in children: A public health problem. Int. J. Environ. Res. Public Health. 2011;8:1174–1205. doi: 10.3390/ijerph8041174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabriele M., Pucci L. Diet Bioactive Compounds: Implications for Oxidative Stress and Inflammation in the Vascular System. Endocr. Metab. Immune Disord. Drug Targets. 2017;17:264–275. doi: 10.2174/1871530317666170921142055. [DOI] [PubMed] [Google Scholar]
- 17.Shivappa N., Steck S.E., Hurley T.G., Hussey J.R., Hebert J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calder P.C. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2:355–374. doi: 10.3390/nu2030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin L.P., Ross A.C., Stephensen C.B., Bohn T., Tanumihardjo S.A. Metabolic effects of inflammation on vitamin A and carotenoids in humans and animal models. Adv. Nutr. 2017;8:197–212. doi: 10.3945/an.116.014167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wannamethee S.G., Lowe G.D., Rumley A., Bruckdorfer K.R., Whincup P.H. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am. J. Clin. Nutr. 2006;83:567–574. doi: 10.1093/ajcn.83.3.567. [DOI] [PubMed] [Google Scholar]
- 21.Khan N., Khymenets O., Urpi-Sarda M., Tulipani S., Garcia-Aloy M., Monagas M., Mora-Cubillos X., Llorach R., Andres-Lacueva C. Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients. 2014;6:844–880. doi: 10.3390/nu6020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaulmann A., Bohn T. Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014;34:907–929. doi: 10.1016/j.nutres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Tao N., Gao Y., Liu Y., Ge F. Carotenoids from the peel of Shatian pummelo (Citrus grandis Osbeck) and its antimicrobial activity. Am. Eur. J. Agric. Environ. Sci. 2010;7:110–115. [Google Scholar]
- 24.Kumar Singh A., Cabral C., Kumar R., Ganguly R., Kumar Rana H., Gupta A., Rosaria Lauro M., Carbone C., Reis F., Pandey A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients. 2019;11:2216. doi: 10.3390/nu11092216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L., Tu L. Implications of gastrointestinal manifestations of COVID-19. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/S2468-1253(20)30132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glaab E., Ostaszewski M. The Role of Spike-ACE2 Interaction in Pulmonary Blood Pressure Regulation. FAIRDOM Hub. [(accessed on 27 May 2020)];2020 Available online: https://fairdomhub.org/models/709.
- 27.Lauridsen C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019;98:4240–4246. doi: 10.3382/ps/pey407. [DOI] [PubMed] [Google Scholar]
- 28.Porcelli S.A. Chapter 17-Innate Immunity. In: Firestein G.S., Budd R.C., Gabriel S.E., McInnes I.B., O’Dell J.R., editors. Kelley and Firestein’s Textbook of Rheumatology. 10th ed. Elsevier; Amsterdam, The Netherlands: 2017. pp. 247–287. [DOI] [Google Scholar]
- 29.Spiering M.J. Primer on the Immune System. Alcohol Res. 2015;37:171–175. doi: 10.35946/arcr.v37.2.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J.H., Jung J.Y., Jeong Y.J., Park J.H., Yang K.H., Choi N.K., Kim S.H., Kim W.J. Involvement of both mitochondrial- and death receptor-dependent apoptotic pathways regulated by Bcl-2 family in sodium fluoride-induced apoptosis of the human gingival fibroblasts. Toxicology. 2008;243:340–347. doi: 10.1016/j.tox.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Park J., Min J.S., Kim B., Chae U.B., Yun J.W., Choi M.S., Kong I.K., Chang K.T., Lee D.S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-kappaB pathways. Neurosci. Lett. 2015;584:191–196. doi: 10.1016/j.neulet.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Perez G.I., Acton B.M., Jurisicova A., Perkins G.A., White A., Brown J., Trbovich A.M., Kim M.R., Fissore R., Xu J., et al. Genetic variance modifies apoptosis susceptibility in mature oocytes via alterations in DNA repair capacity and mitochondrial ultrastructure. Cell Death Differ. 2007;14:524–533. doi: 10.1038/sj.cdd.4402050. [DOI] [PubMed] [Google Scholar]
- 33.Bouayed J., Bohn T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev. 2010;3:228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pohanka M. Role of oxidative stress in infectious diseases. A review. Folia Microbiol. 2013;58:503–513. doi: 10.1007/s12223-013-0239-5. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov A.V., Bartosch B., Isaguliants M.G. Oxidative Stress in Infection and Consequent Disease. Oxid. Med. Cell Longev. 2017;2017:3496043. doi: 10.1155/2017/3496043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beck M.A., Handy J., Levander O.A. The role of oxidative stress in viral infections. Ann. N. Y. Acad. Sci. 2000;917:906–912. doi: 10.1111/j.1749-6632.2000.tb05456.x. [DOI] [PubMed] [Google Scholar]
- 37.Rahman I., Adcock I.M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 38.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De la Fuente M., Hernanz A., Vallejo M.C. The immune system in the oxidative stress conditions of aging and hypertension: Favorable effects of antioxidants and physical exercise. Antioxid. Redox Signal. 2005;7:1356–1366. doi: 10.1089/ars.2005.7.1356. [DOI] [PubMed] [Google Scholar]
- 40.Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA. 2018;115:5839–5848. doi: 10.1073/pnas.1804932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopresti A.L., Maker G.L., Hood S.D., Drummond P.D. A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;48:102–111. doi: 10.1016/j.pnpbp.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 43.Janeway C.A., Jr., Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 44.Brusselle G., Bracke K. Targeting immune pathways for therapy in asthma and chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2014;11(Suppl. 5):S322–S328. doi: 10.1513/AnnalsATS.201403-118AW. [DOI] [PubMed] [Google Scholar]
- 45.Gudkov A.V., Komarova E.A. p53 and the Carcinogenicity of Chronic Inflammation. Cold Spring Harb. Perspect Med. 2016;6:a026161. doi: 10.1101/cshperspect.a026161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 47.Girard S., Kadhim H., Roy M., Lavoie K., Brochu M.E., Larouche A., Sebire G. Role of perinatal inflammation in cerebral palsy. Pediatr. Neurol. 2009;40:168–174. doi: 10.1016/j.pediatrneurol.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 48.Hendrayani S.F., Al-Harbi B., Al-Ansari M.M., Silva G., Aboussekhra A. The inflammatory/cancer-related IL-6/STAT3/NF-kappaB positive feedback loop includes AUF1 and maintains the active state of breast myofibroblasts. Oncotarget. 2016;7:41974–41985. doi: 10.18632/oncotarget.9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kyriakis J.M., Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 50.Dudley D.J. 1 The immune system in health and disease. Baillière’s Clin. Obstet. Gynaecol. 1992;6:393–416. doi: 10.1016/S0950-3552(05)80003-3. [DOI] [PubMed] [Google Scholar]
- 51.Libby P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007;65:S140–S146. doi: 10.1301/nr.2007.dec.S140-S146. [DOI] [PubMed] [Google Scholar]
- 52.Goldberg E.L., Shaw A.C., Montgomery R.R. How Inflammation Blunts Innate Immunity in Aging. Interdiscip Top Gerontol. Geriatr. 2020;43:1–17. doi: 10.1159/000504480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol. Sin. 2020 doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I.H., Lit L.C., Hui D.S., Chan M.H., Chung S.S., et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshikawa T., Hill T., Li K., Peters C.J., Tseng C.T. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J. Virol. 2009;83:3039–3048. doi: 10.1128/JVI.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu P., Zhou Q., Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann. Hematol. 2020:1–4. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y., Zhou Z., Min W. Mitochondria, Oxidative Stress and Innate Immunity. Front. Physiol. 2018;9:1487. doi: 10.3389/fphys.2018.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crapo J.D. Oxidative stress as an initiator of cytokine release and cell damage. Eur. Respir. J. 2003;22:4s–6s. doi: 10.1183/09031936.03.00000203a. [DOI] [PubMed] [Google Scholar]
- 62.Veiga-Fernandes H., Mucida D. Neuro-Immune Interactions at Barrier Surfaces. Cell. 2016;165:801–811. doi: 10.1016/j.cell.2016.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wick G., Hu Y., Schwarz S., Kroemer G. Immunoendocrine communication via the hypothalamo-pituitary-adrenal axis in autoimmune diseases. Endocr. Rev. 1993;14:539–563. doi: 10.1210/edrv-14-5-539. [DOI] [PubMed] [Google Scholar]
- 64.Ganeshan K., Chawla A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014;32:609–634. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matarese G., La Cava A. The intricate interface between immune system and metabolism. Trends Immunol. 2004;25:193–200. doi: 10.1016/j.it.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 66.Osborn O., Olefsky J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nature Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 67.Besedovsky L., Lange T., Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Majde J.A., Krueger J.M. Links between the innate immune system and sleep. J. Allergy Clin. Immunol. 2005;116:1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 69.Marcos A., Nova E., Montero A. Changes in the immune system are conditioned by nutrition. Eur. J. Clin. Nutr. 2003;57:S66–S69. doi: 10.1038/sj.ejcn.1601819. [DOI] [PubMed] [Google Scholar]
- 70.Langley-Evans S.C., Carrington L.J. Diet and the developing immune system. Lupus. 2006;15:746–752. doi: 10.1177/0961203306070001. [DOI] [PubMed] [Google Scholar]
- 71.Park J.E., Barbul A. Understanding the role of immune regulation in wound healing. Am. J. Surg. 2004;187:11s–16s. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 72.Stanton J. Listening to the Ga: Cicely Williams’ discovery of kwashiorkor on the Gold Coast. Clio Med. 2001;61:149–171. doi: 10.1163/9789004333390_008. [DOI] [PubMed] [Google Scholar]
- 73.Barbeito-Andres J., Pezzuto P., Higa L.M., Dias A.A., Vasconcelos J.M., Santos T.M.P., Ferreira J., Ferreira R.O., Dutra F.F., Rossi A.D., et al. Congenital Zika syndrome is associated with maternal protein malnutrition. Sci. Adv. 2020;6:eaaw6284. doi: 10.1126/sciadv.aaw6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.França T., Ishikawa L., Zorzella-Pezavento S., Chiuso-Minicucci F., da Cunha M., Sartori A. Impact of malnutrition on immunity and infection. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009;15:374–390. doi: 10.1590/S1678-91992009000300003. [DOI] [Google Scholar]
- 75.Kohatsu W., Karpowicz S. Chapter 88-Antiinflammatory Diet. In: Rakel D., editor. Integrative Medicine. 4th ed. Elsevier; Amsterdam, The Netherlands: 2018. [DOI] [Google Scholar]
- 76.Cavicchia P.P., Steck S.E., Hurley T.G., Hussey J.R., Ma Y., Ockene I.S., Hebert J.R. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 2009;139:2365–2372. doi: 10.3945/jn.109.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giugliano D., Ceriello A., Esposito K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll Cardiol. 2006;48:677–685. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 78.Fulop T., Jr., Wagner J.R., Khalil A., Weber J., Trottier L., Payette H. Relationship between the response to influenza vaccination and the nutritional status in institutionalized elderly subjects. J. Gerontol. A Biol. Sci. Med. Sci. 1999;54:M59–M64. doi: 10.1093/gerona/54.2.M59. [DOI] [PubMed] [Google Scholar]
- 79.Taylor A.K., Cao W., Vora K.P., De La Cruz J., Shieh W.J., Zaki S.R., Katz J.M., Sambhara S., Gangappa S. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J. Infect Dis. 2013;207:501–510. doi: 10.1093/infdis/jis527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chan J., Tian Y., Tanaka K.E., Tsang M.S., Yu K., Salgame P., Carroll D., Kress Y., Teitelbaum R., Bloom B.R. Effects of protein calorie malnutrition on tuberculosis in mice. Proc. Natl. Acad. Sci. USA. 1996;93:14857–14861. doi: 10.1073/pnas.93.25.14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morita M., Kuba K., Ichikawa A., Nakayama M., Katahira J., Iwamoto R., Watanebe T., Sakabe S., Daidoji T., Nakamura S., et al. The Lipid Mediator Protectin D1 Inhibits Influenza Virus Replication and Improves Severe Influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 82.Siegers J.Y., Novakovic B., Hulme K.D., Marshall R., Bloxham C.J., Thomas W.G., Reichelt M.E., Leijten L., van Run P., Knox K., et al. A high fat diet increases influenza A virus-associated cardiovascular damage. J. Infect. Dis. 2020:1537–6613. doi: 10.1093/infdis/jiaa159. [DOI] [PubMed] [Google Scholar]
- 83.Cho W.J., Lee D.K., Lee S.Y., Sohn S.H., Park H.L., Park Y.W., Kim H., Nam J.H. Diet-induced obesity reduces the production of influenza vaccine-induced antibodies via impaired macrophage function. Acta Virol. 2016;60:298–306. doi: 10.4149/av_2016_03_298. [DOI] [PubMed] [Google Scholar]
- 84.Goldberg E.L., Molony R.D., Kudo E., Sidorov S., Kong Y., Dixit V.D., Iwasaki A. Ketogenic diet activates protective gammadelta T cell responses against influenza virus infection. Sci. Immunol. 2019;4:eaav2026. doi: 10.1126/sciimmunol.aav2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park Y., Subar A.F., Hollenbeck A., Schatzkin A. Dietary fiber intake and mortality in the NIH-AARP diet and health study. Arch. Intern. Med. 2011;171:1061–1068. doi: 10.1001/archinternmed.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Trompette A., Gollwitzer E.S., Pattaroni C., Lopez-Mejia I.C., Riva E., Pernot J., Ubags N., Fajas L., Nicod L.P., Marsland B.J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c(-) Patrolling Monocyte Hematopoiesis and CD8(+) T Cell Metabolism. Immunity. 2018;48:992–1005. doi: 10.1016/j.immuni.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 87.Desai M.S., Seekatz A.M., Koropatkin N.M., Kamada N., Hickey C.A., Wolter M., Pudlo N.A., Kitamoto S., Terrapon N., Muller A., et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen H., Zhuo Q., Yuan W., Wang J., Wu T. Vitamin A for preventing acute lower respiratory tract infections in children up to seven years of age. Cochrane Database Syst. Rev. 2008:Cd006090. doi: 10.1002/14651858.CD006090.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown N., Roberts C. Vitamin A for acute respiratory infection in developing countries: A meta-analysis. Acta Paediatr. 2004;93:1437–1442. doi: 10.1111/j.1651-2227.2004.tb02625.x. [DOI] [PubMed] [Google Scholar]
- 90.Raharusun P., Priambada S., Budiarti C., Agung E., Budi C. Patterns of COVID-19 Mortality and Vitamin D: An Indonesian Study. SSRN. 2020 doi: 10.2139/ssrn.3585561. [DOI] [Google Scholar]
- 91.Charan J., Goyal J.P., Saxena D., Yadav P. Vitamin D for prevention of respiratory tract infections: A systematic review and meta-analysis. J. Pharmacol. Pharm. 2012;3:300–303. doi: 10.4103/0976-500X.103685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bergman P., Lindh A.U., Bjorkhem-Bergman L., Lindh J.D. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE. 2013;8:e65835. doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hemila H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin. Interv. Aging. 2016;11:1379–1385. doi: 10.2147/CIA.S114515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ran L., Zhao W., Wang J., Wang H., Zhao Y., Tseng Y., Bu H. Extra Dose of Vitamin C Based on a Daily Supplementation Shortens the Common Cold: A Meta-Analysis of 9 Randomized Controlled Trials. Biomed. Res. Int. 2018;2018:1837634. doi: 10.1155/2018/1837634. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Poudel-Tandukar K., Chandyo R.K. Dietary B Vitamins and Serum C-Reactive Protein in Persons With Human Immunodeficiency Virus Infection: The Positive Living With HIV (POLH) Study. Food Nutr. Bull. 2016;37:517–528. doi: 10.1177/0379572116657268. [DOI] [PubMed] [Google Scholar]
- 96.Mossad S.B., Macknin M.L., Medendorp S.V., Mason P. Zinc gluconate lozenges for treating the common cold. A randomized, double-blind, placebo-controlled study. Ann. Intern. Med. 1996;125:81–88. doi: 10.7326/0003-4819-125-2-199607150-00001. [DOI] [PubMed] [Google Scholar]
- 97.Dhur A., Galan P., Hannoun C., Huot K., Hercberg S. Effects of iron deficiency upon the antibody response to influenza virus in rats. J. Nutr. Biochem. 1990;1:629–634. doi: 10.1016/0955-2863(90)90021-C. [DOI] [PubMed] [Google Scholar]
- 98.Goldson A.J., Fairweather-Tait S.J., Armah C.N., Bao Y., Broadley M.R., Dainty J.R., Furniss C., Hart D.J., Teucher B., Hurst R. Effects of selenium supplementation on selenoprotein gene expression and response to influenza vaccine challenge: A randomised controlled trial. PLoS ONE. 2011;6:e14771. doi: 10.1371/journal.pone.0014771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Droebner K., Ehrhardt C., Poetter A., Ludwig S., Planz O. CYSTUS052, a polyphenol-rich plant extract, exerts anti-influenza virus activity in mice. Antivir. Res. 2007;76:1–10. doi: 10.1016/j.antiviral.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 100.Melikian G., Mmiro F., Ndugwa C., Perry R., Jackson J.B., Garrett E., Tielsch J., Semba R.D. Relation of vitamin A and carotenoid status to growth failure and mortality among Ugandan infants with human immunodeficiency virus. Nutrition. 2001;17:567–572. doi: 10.1016/S0899-9007(01)00567-6. [DOI] [PubMed] [Google Scholar]
- 101.Institute of Medicine . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. The National Academies Press; Washington, DC, USA: 2005. [Google Scholar]
- 102.Amaral J.F., Foschetti D.A., Assis F.A., Menezes J.S., Vaz N.M., Faria A.M. Immunoglobulin production is impaired in protein-deprived mice and can be restored by dietary protein supplemntation. Braz J. Med. Biol. Res. 2006;39:1581–1586. doi: 10.1590/S0100-879X2006001200009. [DOI] [PubMed] [Google Scholar]
- 103.Jakulj F., Zernicke K., Bacon S.L., van Wielingen L.E., Key B.L., West S.G., Campbell T.S. A high-fat meal increases cardiovascular reactivity to psychological stress in healthy young adults. J. Nutr. 2007;137:935–939. doi: 10.1093/jn/137.4.935. [DOI] [PubMed] [Google Scholar]
- 104.O’Keefe J.H., Bell D.S. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am. J. Cardiol. 2007;100:899–904. doi: 10.1016/j.amjcard.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 105.Hruby A., Jacques P.F. Dietary Protein and Changes in Biomarkers of Inflammation and Oxidative Stress in the Framingham Heart Study Offspring Cohort. Curr. Dev. Nutr. 2019;3:nzz019. doi: 10.1093/cdn/nzz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kostovcikova K., Coufal S., Galanova N., Fajstova A., Hudcovic T., Kostovcik M., Prochazkova P., Jiraskova Zakostelska Z., Cermakova M., Sediva B., et al. Diet Rich in Animal Protein Promotes Pro-inflammatory Macrophage Response and Exacerbates Colitis in Mice. Front. Immunol. 2019;10:919. doi: 10.3389/fimmu.2019.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arora S.K., McFarlane S.I. The case for low carbohydrate diets in diabetes management. Nutr. Metab. (Lond.) 2005;2:16. doi: 10.1186/1743-7075-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chungchunlam S.M.S., Henare S.J., Ganesh S., Moughan P.J. Effects of whey protein and its two major protein components on satiety and food intake in normal-weight women. Physiol. Behav. 2017;175:113–118. doi: 10.1016/j.physbeh.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 109.Westerterp-Plantenga M.S., Lemmens S.G., Westerterp K.R. Dietary protein-its role in satiety, energetics, weight loss and health. Br. J. Nutr. 2012;108(Suppl. 2):S105–S112. doi: 10.1017/S0007114512002589. [DOI] [PubMed] [Google Scholar]
- 110.O’Keefe J.H., Gheewala N.M., O’Keefe J.O. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J. Am. Coll. Cardiol. 2008;51:249–255. doi: 10.1016/j.jacc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 111.Li P., Yin Y.L., Li D., Kim S.W., Wu G. Amino acids and immune function. Br. J. Nutr. 2007;98:237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- 112.Ren M., Zhang S.H., Zeng X.F., Liu H., Qiao S.Y. Branched-chain Amino Acids are Beneficial to Maintain Growth Performance and Intestinal Immune-related Function in Weaned Piglets Fed Protein Restricted Diet. Asian-Australas J. Anim. Sci. 2015;28:1742–1750. doi: 10.5713/ajas.14.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cruzat V., Macedo Rogero M., Noel Keane K., Curi R., Newsholme P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients. 2018;10:1564. doi: 10.3390/nu10111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim S.-H., Roszik J., Grimm E.A., Ekmekcioglu S. Impact of l-Arginine Metabolism on Immune Response and Anticancer Immunotherapy. Front. Oncol. 2018;8:67. doi: 10.3389/fonc.2018.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Curi R., Newsholme P., Marzuca-Nassr G.N., Takahashi H.K., Hirabara S.M., Cruzat V., Krause M., de Bittencourt P.I., Jr. Regulatory principles in metabolism-then and now. Biochem. J. 2016;473:1845–1857. doi: 10.1042/BCJ20160103. [DOI] [PubMed] [Google Scholar]
- 116.Curi R., Lagranha C.J., Doi S.Q., Sellitti D.F., Procopio J., Pithon-Curi T.C. Glutamine-dependent changes in gene expression and protein activity. Cell Biochem. Funct. 2005;23:77–84. doi: 10.1002/cbf.1165. [DOI] [PubMed] [Google Scholar]
- 117.Curi R., Lagranha C.J., Doi S.Q., Sellitti D.F., Procopio J., Pithon-Curi T.C., Corless M., Newsholme P. Molecular mechanisms of glutamine action. J. Cell Physiol. 2005;204:392–401. doi: 10.1002/jcp.20339. [DOI] [PubMed] [Google Scholar]
- 118.Mills E.L., Kelly B., O’Neill L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017;18:488–498. doi: 10.1038/ni.3704. [DOI] [PubMed] [Google Scholar]
- 119.Coeffier M., Claeyssens S., Hecketsweiler B., Lavoinne A., Ducrotte P., Dechelotte P. Enteral glutamine stimulates protein synthesis and decreases ubiquitin mRNA level in human gut mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G266–G273. doi: 10.1152/ajpgi.00385.2002. [DOI] [PubMed] [Google Scholar]
- 120.Jobin C., Hellerbrand C., Licato L.L., Brenner D.A., Sartor R.B. Mediation by NF-kappa B of cytokine induced expression of intercellular adhesion molecule 1 (ICAM-1) in an intestinal epithelial cell line, a process blocked by proteasome inhibitors. Gut. 1998;42:779–787. doi: 10.1136/gut.42.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roth E., Oehler R., Manhart N., Exner R., Wessner B., Strasser E., Spittler A. Regulative potential of glutamine--relation to glutathione metabolism. Nutrition. 2002;18:217–221. doi: 10.1016/S0899-9007(01)00797-3. [DOI] [PubMed] [Google Scholar]
- 122.Hiscock N., Petersen E.W., Krzywkowski K., Boza J., Halkjaer-Kristensen J., Pedersen B.K. Glutamine supplementation further enhances exercise-induced plasma IL-6. J. Appl. Physiol. (1985) 2003;95:145–148. doi: 10.1152/japplphysiol.00471.2002. [DOI] [PubMed] [Google Scholar]
- 123.Chandra R.K. Nutrition, immunity and infection: From basic knowledge of dietary manipulation of immune responses to practical application of ameliorating suffering and improving survival. Proc. Natl. Acad. Sci. USA. 1996;93:14304–14307. doi: 10.1073/pnas.93.25.14304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ah O. Protein Energy Malnutrition and Susceptibility to Viral Infections as Zika and Influenza Viruses. J. Nutr. Food Sci. 2016;6:2. doi: 10.4172/2155-9600.1000489. [DOI] [Google Scholar]
- 125.Harbige L.S. Fatty acids, the immune response, and autoimmunity: A question of n-6 essentiality and the balance between n-6 and n-3. Lipids. 2003;38:323–341. doi: 10.1007/s11745-003-1067-z. [DOI] [PubMed] [Google Scholar]
- 126.Radzikowska U., Rinaldi A.O., Çelebi Sözener Z., Karaguzel D., Wojcik M., Cypryk K., Akdis M., Akdis C.A., Sokolowska M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients. 2019;11:2990. doi: 10.3390/nu11122990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Clarke R., Shipley M., Armitage J., Collins R., Harris W. Plasma phospholipid fatty acids and CHD in older men: Whitehall study of London civil servants. Br. J. Nutr. 2009;102:279–284. doi: 10.1017/S0007114508143562. [DOI] [PubMed] [Google Scholar]
- 128.Lennie T.A., Chung M.L., Habash D.L., Moser D.K. Dietary fat intake and proinflammatory cytokine levels in patients with heart failure. J. Card Fail. 2005;11:613–618. doi: 10.1016/j.cardfail.2005.06.434. [DOI] [PubMed] [Google Scholar]
- 129.Innes J.K., Calder P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids. 2018;132:41–48. doi: 10.1016/j.plefa.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 130.Serhan C.N., Levy B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018;128:2657–2669. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chowdhury R., Warnakula S., Kunutsor S., Crowe F., Ward H.A., Johnson L., Franco O.H., Butterworth A.S., Forouhi N.G., Thompson S.G., et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern Med. 2014;160:398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 132.Gabbs M., Leng S., Devassy J.G., Monirujjaman M., Aukema H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015;6:513–540. doi: 10.3945/an.114.007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dennis E.A., Norris P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bates E.J. Eicosanoids, fatty acids and neutrophils: Their relevance to the pathophysiology of disease. Prostaglandins Leukot Essent Fatty Acids. 1995;53:75–86. doi: 10.1016/0952-3278(95)90133-7. [DOI] [PubMed] [Google Scholar]
- 135.Magnusson J., Kull I., Westman M., Hakansson N., Wolk A., Melen E., Wickman M., Bergstrom A. Fish and polyunsaturated fat intake and development of allergic and nonallergic rhinitis. J. Allergy Clin. Immunol. 2015;136:1247–1253.e2. doi: 10.1016/j.jaci.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 136.Scaioli E., Liverani E., Belluzzi A. The Imbalance between n-6/n-3 Polyunsaturated Fatty Acids and Inflammatory Bowel Disease: A Comprehensive Review and Future Therapeutic Perspectives. Int. J. Mol. Sci. 2017;18:2619. doi: 10.3390/ijms18122619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Elten T.M., van Rossem L., Wijga A.H., Brunekreef B., de Jongste J.C., Koppelman G.H., Smit H.A. Breast milk fatty acid composition has a long-term effect on the risk of asthma, eczema, and sensitization. Allergy. 2015;70:1468–1476. doi: 10.1111/all.12703. [DOI] [PubMed] [Google Scholar]
- 138.Pischon T., Hankinson S.E., Hotamisligil G.S., Rifai N., Willett W.C., Rimm E.B. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 139.Simopoulos A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999;70:560S–569S. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- 140.Simopoulos A.P. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011;44:203–215. doi: 10.1007/s12035-010-8162-0. [DOI] [PubMed] [Google Scholar]
- 141.Rett B.S., Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: A systematic review. Nutr. Metab. 2011;8:36. doi: 10.1186/1743-7075-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Akbar U., Yang M., Kurian D., Mohan C. Omega-3 Fatty Acids in Rheumatic Diseases: A Critical Review. J. Clin. Rheumatol. 2017;23:330–339. doi: 10.1097/RHU.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 143.Kiecolt-Glaser J.K., Belury M.A., Andridge R., Malarkey W.B., Hwang B.S., Glaser R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: A randomized controlled trial. Brain Behav. Immun. 2012;26:988–995. doi: 10.1016/j.bbi.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kiecolt-Glaser J.K., Belury M.A., Andridge R., Malarkey W.B., Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: A randomized controlled trial. Brain Behav. Immun. 2011;25:1725–1734. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schwerbrock N.M., Karlsson E.A., Shi Q., Sheridan P.A., Beck M.A. Fish oil-fed mice have impaired resistance to influenza infection. J. Nutr. 2009;139:1588–1594. doi: 10.3945/jn.109.108027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jones G.J.B., Roper R.L. The effects of diets enriched in omega-3 polyunsaturated fatty acids on systemic vaccinia virus infection. Sci. Rep. 2017;7:15999. doi: 10.1038/s41598-017-16098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Milner J.J., Sheridan P.A., Karlsson E.A., Schultz-Cherry S., Shi Q., Beck M.A. Diet-induced obese mice exhibit altered heterologous immunity during a secondary 2009 pandemic H1N1 infection. J. Immunol. 2013;191:2474–2485. doi: 10.4049/jimmunol.1202429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu S., Manson J.E., Buring J.E., Stampfer M.J., Willett W.C., Ridker P.M. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am. J. Clin. Nutr. 2002;75:492–498. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- 149.Monnier L., Mas E., Ginet C., Michel F., Villon L., Cristol J.P., Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 150.Bullo M., Casas R., Portillo M.P., Basora J., Estruch R., Garcia-Arellano A., Lasa A., Juanola-Falgarona M., Aros F., Salas-Salvado J. Dietary glycemic index/load and peripheral adipokines and inflammatory markers in elderly subjects at high cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2013;23:443–450. doi: 10.1016/j.numecd.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 151.Neuhouser M.L., Schwarz Y., Wang C., Breymeyer K., Coronado G., Wang C.Y., Noar K., Song X., Lampe J.W. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J. Nutr. 2012;142:369–374. doi: 10.3945/jn.111.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Egger G., Dixon J. Should obesity be the main game? Or do we need an environmental makeover to combat the inflammatory and chronic disease epidemics? Obes. Rev. 2009;10:237–249. doi: 10.1111/j.1467-789X.2008.00542.x. [DOI] [PubMed] [Google Scholar]
- 153.Bo S., Ciccone G., Guidi S., Gambino R., Durazzo M., Gentile L., Cassader M., Cavallo-Perin P., Pagano G. Diet or exercise: What is more effective in preventing or reducing metabolic alterations? Eur. J. Endocrinol. 2008;159:685–691. doi: 10.1530/EJE-08-0334. [DOI] [PubMed] [Google Scholar]
- 154.Galland L. Diet and inflammation. Nutr. Clin. Pract. 2010;25:634–640. doi: 10.1177/0884533610385703. [DOI] [PubMed] [Google Scholar]
- 155.Stephen A.M., Champ M.M., Cloran S.J., Fleith M., van Lieshout L., Mejborn H., Burley V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017;30:149–190. doi: 10.1017/S095442241700004X. [DOI] [PubMed] [Google Scholar]
- 156.Costabile A., Klinder A., Fava F., Napolitano A., Fogliano V., Leonard C., Gibson G.R., Tuohy K.M. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: A double-blind, placebo-controlled, crossover study. Br. J. Nutr. 2008;99:110–120. doi: 10.1017/S0007114507793923. [DOI] [PubMed] [Google Scholar]
- 157.Napolitano A., Costabile A., Martin-Pelaez S., Vitaglione P., Klinder A., Gibson G.R., Fogliano V. Potential prebiotic activity of oligosaccharides obtained by enzymatic conversion of durum wheat insoluble dietary fibre into soluble dietary fibre. Nutr. Metab. Cardiovasc. Dis. 2009;19:283–290. doi: 10.1016/j.numecd.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 158.Gaskins A.J., Mumford S.L., Rovner A.J., Zhang C., Chen L., Wactawski-Wende J., Perkins N.J., Schisterman E.F., BioCycle Study G. Whole grains are associated with serum concentrations of high sensitivity C-reactive protein among premenopausal women. J. Nutr. 2010;140:1669–1676. doi: 10.3945/jn.110.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gogebakan O., Kohl A., Osterhoff M.A., van Baak M.A., Jebb S.A., Papadaki A., Martinez J.A., Handjieva-Darlenska T., Hlavaty P., Weickert M.O., et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: The diet, obesity, and genes (DiOGenes) study: A randomized, controlled trial. Circulation. 2011;124:2829–2838. doi: 10.1161/CIRCULATIONAHA.111.033274. [DOI] [PubMed] [Google Scholar]
- 160.Goletzke J., Buyken A.E., Joslowski G., Bolzenius K., Remer T., Carstensen M., Egert S., Nothlings U., Rathmann W., Roden M., et al. Increased intake of carbohydrates from sources with a higher glycemic index and lower consumption of whole grains during puberty are prospectively associated with higher IL-6 concentrations in younger adulthood among healthy individuals. J. Nutr. 2014;144:1586–1593. doi: 10.3945/jn.114.193391. [DOI] [PubMed] [Google Scholar]
- 161.Herder C., Peltonen M., Koenig W., Sutfels K., Lindstrom J., Martin S., Ilanne-Parikka P., Eriksson J.G., Aunola S., Keinanen-Kiukaanniemi S., et al. Anti-inflammatory effect of lifestyle changes in the Finnish Diabetes Prevention Study. Diabetologia. 2009;52:433–442. doi: 10.1007/s00125-008-1243-1. [DOI] [PubMed] [Google Scholar]
- 162.Pol K., Christensen R., Bartels E.M., Raben A., Tetens I., Kristensen M. Whole grain and body weight changes in apparently healthy adults: A systematic review and meta-analysis of randomized controlled studies. Am. J. Clin. Nutr. 2013;98:872–884. doi: 10.3945/ajcn.113.064659. [DOI] [PubMed] [Google Scholar]
- 163.Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014;121:91–119. doi: 10.1016/b978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 165.Tungland B. Chapter 2-Short-Chain Fatty Acid Production and Functional Aspects on Host Metabolism. In: Tungland B., editor. Human Microbiota in Health and Disease. Academic Press; Cambridge, UK: 2018. pp. 37–106. [DOI] [Google Scholar]
- 166.Davie J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003;133:2485s–2493s. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 167.Candido E.P., Reeves R., Davie J.R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978;14:105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- 168.Vidali G., Boffa L.C., Bradbury E.M., Allfrey V.G. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc. Natl. Acad. Sci. USA. 1978;75:2239–2243. doi: 10.1073/pnas.75.5.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Boffa L.C., Vidali G., Mann R.S., Allfrey V.G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J. Biol. Chem. 1978;253:3364–3366. [PubMed] [Google Scholar]
- 170.Riggs M.G., Whittaker R.G., Neumann J.R., Ingram V.M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977;268:462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- 171.Le Poul E., Loison C., Struyf S., Springael J.Y., Lannoy V., Decobecq M.E., Brezillon S., Dupriez V., Vassart G., Van Damme J., et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 172.Brown A.J., Goldsworthy S.M., Barnes A.A., Eilert M.M., Tcheang L., Daniels D., Muir A.I., Wigglesworth M.J., Kinghorn I., Fraser N.J., et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 173.Miyauchi S., Gopal E., Fei Y.J., Ganapathy V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na(+)-coupled transporter for short-chain fatty acids. J. Biol. Chem. 2004;279:13293–13296. doi: 10.1074/jbc.C400059200. [DOI] [PubMed] [Google Scholar]
- 174.Ritzhaupt A., Wood I.S., Ellis A., Hosie K.B., Shirazi-Beechey S.P. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: Its potential to transport L-lactate as well as butyrate. J. Physiol. 1998;513 Pt 3:719–732. doi: 10.1111/j.1469-7793.1998.719ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rechkemmer G., von Engelhardt W. Concentration- and pH-dependence of short-chain fatty acid absorption in the proximal and distal colon of guinea pig (Cavia porcellus) Comp. Biochem. Physiol. A Comp. Physiol. 1988;91:659–663. doi: 10.1016/0300-9629(88)90944-9. [DOI] [PubMed] [Google Scholar]
- 176.Thorburn A.N., Macia L., Mackay C.R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. 2014;40:833–842. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 177.Saemann M.D., Bohmig G.A., Osterreicher C.H., Burtscher H., Parolini O., Diakos C., Stockl J., Horl W.H., Zlabinger G.J. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000;14:2380–2382. doi: 10.1096/fj.00-0359fje. [DOI] [PubMed] [Google Scholar]
- 178.Ni Y.F., Wang J., Yan X.L., Tian F., Zhao J.B., Wang Y.J., Jiang T. Histone deacetylase inhibitor, butyrate, attenuates lipopolysaccharide-induced acute lung injury in mice. Respir. Res. 2010;11:33. doi: 10.1186/1465-9921-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Segain J.P., Raingeard de la Bletiere D., Bourreille A., Leray V., Gervois N., Rosales C., Ferrier L., Bonnet C., Blottiere H.M., Galmiche J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Carlson J.L., Erickson J.M., Lloyd B.B., Slavin J.L. Health Effects and Sources of Prebiotic Dietary Fiber. Curr. Dev. Nutr. 2018;2:nzy005. doi: 10.1093/cdn/nzy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Myhrstad M.C.W., Tunsjo H., Charnock C., Telle-Hansen V.H. Dietary Fiber, Gut Microbiota, and Metabolic Regulation-Current Status in Human Randomized Trials. Nutrients. 2020;12:859. doi: 10.3390/nu12030859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.van den Munckhof I.C.L., Kurilshikov A., Ter Horst R., Riksen N.P., Joosten L.A.B., Zhernakova A., Fu J., Keating S.T., Netea M.G., de Graaf J., et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: A systematic review of human studies. Obes. Rev. 2018;19:1719–1734. doi: 10.1111/obr.12750. [DOI] [PubMed] [Google Scholar]
- 183.Qu H., Zhang Y., Chai H., Gao Z.Y., Shi D.Z. Effects of microbiota-driven therapy on inflammatory responses in elderly individuals: A systematic review and meta-analysis. PLoS ONE. 2019;14:e0211233. doi: 10.1371/journal.pone.0211233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zmora N., Suez J., Elinav E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019;16:35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 185.Wilson A.S., Koller K.R., Ramaboli M.C., Nesengani L.T., Ocvirk S., Chen C., Flanagan C.A., Sapp F.R., Merritt Z.T., Bhatti F., et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020;65:723–740. doi: 10.1007/s10620-020-06112-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dubourg G., Edouard S., Raoult D. Relationship between nasopharyngeal microbiota and patient’s susceptibility to viral infection. Expert Rev. Anti. Infect. Ther. 2019;17:437–447. doi: 10.1080/14787210.2019.1621168. [DOI] [PubMed] [Google Scholar]
- 187.Schenck L.P., Surette M.G., Bowdish D.M.E. Composition and immunological significance of the upper respiratory tract microbiota. FEBS Lett. 2016;590:3705–3720. doi: 10.1002/1873-3468.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Budden K.F., Gellatly S.L., Wood D.L., Cooper M.A., Morrison M., Hugenholtz P., Hansbro P.M. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 189.Marsland B.J., Trompette A., Gollwitzer E.S. The Gut-Lung Axis in Respiratory Disease. Ann. Am. Thorac. Soc. 2015;12(Suppl. 2):S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 190.Hanada S., Pirzadeh M., Carver K.Y., Deng J.C. Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia. Front. Immunol. 2018;9:2640. doi: 10.3389/fimmu.2018.02640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen C.J., Wu G.H., Kuo R.L., Shih S.R. Role of the intestinal microbiota in the immunomodulation of influenza virus infection. Microbes Infect. 2017;19:570–579. doi: 10.1016/j.micinf.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 192.Li N., Ma W.-T., Pang M., Fan Q.-L., Hua J.-L. The Commensal Microbiota and Viral Infection: A Comprehensive Review. Front. Immunol. 2019;10:1551. doi: 10.3389/fimmu.2019.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Botic T., Klingberg T.D., Weingartl H., Cencic A. A novel eukaryotic cell culture model to study antiviral activity of potential probiotic bacteria. Int. J. Food Microbiol. 2007;115:227–234. doi: 10.1016/j.ijfoodmicro.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 194.Bandoro C., Runstadler J.A. Bacterial Lipopolysaccharide Destabilizes Influenza Viruses. mSphere. 2017;2:e00267-17. doi: 10.1128/mSphere.00267-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen H.W., Liu P.F., Liu Y.T., Kuo S., Zhang X.Q., Schooley R.T., Rohde H., Gallo R.L., Huang C.M. Nasal commensal Staphylococcus epidermidis counteracts influenza virus. Sci. Rep. 2016;6:27870. doi: 10.1038/srep27870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tuyama A.C., Cheshenko N., Carlucci M.J., Li J.H., Goldberg C.L., Waller D.P., Anderson R.A., Profy A.T., Klotman M.E., Keller M.J., et al. ACIDFORM inactivates herpes simplex virus and prevents genital herpes in a mouse model: Optimal candidate for microbicide combinations. J. Infect Dis. 2006;194:795–803. doi: 10.1086/506948. [DOI] [PubMed] [Google Scholar]
- 197.Ferrey A.J., Choi G., Hanna R.M., Chang Y., Tantisattamo E., Ivaturi K., Park E., Nguyen L., Wang B., Tonthat S., et al. A Case of Novel Coronavirus Disease 19 in a Chronic Hemodialysis Patient Presenting with Gastroenteritis and Developing Severe Pulmonary Disease. Am. J. Nephrol. 2020;51:337–342. doi: 10.1159/000507417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kan H., Stevens J., Heiss G., Rose K.M., London S.J. Dietary fiber, lung function, and chronic obstructive pulmonary disease in the atherosclerosis risk in communities study. Am. J. Epidemiol. 2008;167:570–578. doi: 10.1093/aje/kwm343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Weaver C.T., Elson C.O., Fouser L.A., Kolls J.K. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu. Rev. Pathol. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yang H., Sun Y., Cai R., Chen Y., Gu B. The impact of dietary fiber and probiotics in infectious diseases. Microb. Pathog. 2020;140:103931. doi: 10.1016/j.micpath.2019.103931. [DOI] [PubMed] [Google Scholar]
- 201.Araujo G.V., Oliveira Junior M.H., Peixoto D.M., Sarinho E.S. Probiotics for the treatment of upper and lower respiratory-tract infections in children: Systematic review based on randomized clinical trials. J. Pediatr. (Rio J.) 2015;91:413–427. doi: 10.1016/j.jped.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 202.Harrington D. Laboratory Assessment of Vitamin Status. Academic Press; Cambridge, MA, USA: 2018. [Google Scholar]
- 203.Semba R.D. Vitamin A, immunity, and infection. Clin. Infect. Dis. 1994;19:489–499. doi: 10.1093/clinids/19.3.489. [DOI] [PubMed] [Google Scholar]
- 204.Huang Z., Liu Y., Qi G., Brand D., Zheng S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018;7:258. doi: 10.3390/jcm7090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Müller O., Krawinkel M. Malnutrition and health in developing countries. CMAJ. 2005;173:279–286. doi: 10.1503/cmaj.050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ross A.C. Diet in vitamin A research. Methods Mol. Biol. 2010;652:295–313. doi: 10.1007/978-1-60327-325-1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Grune T., Lietz G., Palou A., Ross A.C., Stahl W., Tang G., Thurnham D., Yin S.-A., Biesalski H.K. Beta-carotene is an important vitamin A source for humans. J. Nutr. 2010;140:2268S–2285S. doi: 10.3945/jn.109.119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sommer A., Vyas K.S. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr. 2012;96:1204s–1206s. doi: 10.3945/ajcn.112.034868. [DOI] [PubMed] [Google Scholar]
- 209.McCullough F.S., Northrop-Clewes C.A., Thurnham D.I. The effect of vitamin A on epithelial integrity. Proc. Nutr. Soc. 1999;58:289–293. doi: 10.1017/S0029665199000403. [DOI] [PubMed] [Google Scholar]
- 210.Wang J.L., Swartz-Basile D.A., Rubin D.C., Levin M.S. Retinoic acid stimulates early cellular proliferation in the adapting remnant rat small intestine after partial resection. J. Nutr. 1997;127:1297–1303. doi: 10.1093/jn/127.7.1297. [DOI] [PubMed] [Google Scholar]
- 211.Rochette-Egly C., Germain P. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs) Nucl. Recept Signal. 2009;7:e005. doi: 10.1621/nrs.07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hiemstra I.H., Beijer M.R., Veninga H., Vrijland K., Borg E.G., Olivier B.J., Mebius R.E., Kraal G., den Haan J.M. The identification and developmental requirements of colonic CD169(+) macrophages. Immunology. 2014;142:269–278. doi: 10.1111/imm.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Shrestha S., Kim S.Y., Yun Y.J., Kim J.K., Lee J.M., Shin M., Song D.K., Hong C.W. Retinoic acid induces hypersegmentation and enhances cytotoxicity of neutrophils against cancer cells. Immunol. Lett. 2017;182:24–29. doi: 10.1016/j.imlet.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 214.Chang H.K., Hou W.S. Retinoic acid modulates interferon-gamma production by hepatic natural killer T cells via phosphatase 2A and the extracellular signal-regulated kinase pathway. J. Interferon Cytokine Res. 2015;35:200–212. doi: 10.1089/jir.2014.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wynn T.A., Vannella K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Beijer M.R., Molenaar R., Goverse G., Mebius R.E., Kraal G., den Haan J.M. A crucial role for retinoic acid in the development of Notch-dependent murine splenic CD8- CD4- and CD4+ dendritic cells. Eur. J. Immunol. 2013;43:1608–1616. doi: 10.1002/eji.201343325. [DOI] [PubMed] [Google Scholar]
- 217.Duriancik D.M., Hoag K.A. Vitamin A deficiency alters splenic dendritic cell subsets and increases CD8(+)Gr-1(+) memory T lymphocytes in C57BL/6J mice. Cell Immunol. 2010;265:156–163. doi: 10.1016/j.cellimm.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Klebanoff C.A., Spencer S.P., Torabi-Parizi P., Grainger J.R., Roychoudhuri R., Ji Y., Sukumar M., Muranski P., Scott C.D., Hall J.A., et al. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J. Exp. Med. 2013;210:1961–1976. doi: 10.1084/jem.20122508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Worbs T., Hammerschmidt S.I., Forster R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017;17:30–48. doi: 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- 220.Stephensen C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 221.Timoneda J., Rodríguez-Fernández L., Zaragozá R., Marín M.P., Cabezuelo M.T., Torres L., Viña J.R., Barber T. Vitamin A Deficiency and the Lung. Nutrients. 2018;10:1132. doi: 10.3390/nu10091132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Siddiqi H.K., Mehra M.R. COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal. J. Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Villamor E., Fawzi W.W. Effects of Vitamin A Supplementation on Immune Responses and Correlation with Clinical Outcomes. Clin. Microbiol. Rev. 2005;18:446–464. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gardner E.M., Bernstein E.D., Popoff K.A., Abrutyn E., Gross P., Murasko D.M. Immune response to influenza vaccine in healthy elderly: Lack of association with plasma β-carotene, retinol, α-tocopherol, or zinc. Mech. Ageing Dev. 2000;117:29–45. doi: 10.1016/S0047-6374(00)00134-2. [DOI] [PubMed] [Google Scholar]
- 225.Penkert R.R., Rowe H.M., Surman S.L., Sealy R.E., Rosch J., Hurwitz J.L. Influences of Vitamin A on Vaccine Immunogenicity and Efficacy. Front. Immunol. 2019;10:1576. doi: 10.3389/fimmu.2019.01576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mosekilde L. Vitamin D and the elderly. Clin. Endocrinol. (Oxf.) 2005;62:265–281. doi: 10.1111/j.1365-2265.2005.02226.x. [DOI] [PubMed] [Google Scholar]
- 227.von Essen M.R., Kongsbak M., Schjerling P., Olgaard K., Odum N., Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat. Immunol. 2010;11:344–349. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 228.Sigmundsdottir H., Pan J., Debes G.F., Alt C., Habtezion A., Soler D., Butcher E.C. DCs metabolize sunlight-induced vitamin D3 to ’program’ T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 229.Aranow C. Vitamin D and the immune system. J. Investig. Med. 2011;59:881–886. doi: 10.2310/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gruber-Bzura B.M. Vitamin D and Influenza-Prevention or Therapy? Int. J. Mol. Sci. 2018;19:2419. doi: 10.3390/ijms19082419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhou J., Du J., Huang L., Wang Y., Shi Y., Lin H. Preventive Effects of Vitamin D on Seasonal Influenza A in Infants: A Multicenter, Randomized, Open, Controlled Clinical Trial. Pediatr. Infect. Dis. J. 2018;37:749–754. doi: 10.1097/INF.0000000000001890. [DOI] [PubMed] [Google Scholar]
- 232.Li X., He J., Yu M., Sun J. The efficacy of vitamin D therapy for patients with COPD: A meta-analysis of randomized controlled trials. Ann. Palliat. Med. 2020;9:286–297. doi: 10.21037/apm.2020.02.26. [DOI] [PubMed] [Google Scholar]
- 233.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rhodes J.M., Subramanian S., Laird E., Kenny R.A. Editorial: Low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment. Pharmacol. Ther. 2020 doi: 10.1111/apt.15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Laird E., Rhodes J., Kenny R.A. Vitamin D and Inflammation: Potential Implications for Severity of Covid-19. Ir. Med. J. 2020;113:81. [PubMed] [Google Scholar]
- 236.Strain J.J., Mulholland C.W. Vitamin C and vitamin E--synergistic interactions in vivo? Exs. 1992;62:419–422. doi: 10.1007/978-3-0348-7460-1_40. [DOI] [PubMed] [Google Scholar]
- 237.Lee G.Y., Han S.N. The Role of Vitamin E in Immunity. Nutrients. 2018;10:1614. doi: 10.3390/nu10111614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Buendia P., Ramirez R., Aljama P., Carracedo J. Klotho Prevents Translocation of NFkappaB. Vitam Horm. 2016;101:119–150. doi: 10.1016/bs.vh.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 239.Tan P.H., Sagoo P., Chan C., Yates J.B., Campbell J., Beutelspacher S.C., Foxwell B.M., Lombardi G., George A.J. Inhibition of NF-kappa B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J. Immunol. 2005;174:7633–7644. doi: 10.4049/jimmunol.174.12.7633. [DOI] [PubMed] [Google Scholar]
- 240.Xuan N.T., Trang P.T., Van Phong N., Toan N.L., Trung D.M., Bac N.D., Nguyen V.L., Hoang N.H., Van Hai N. Klotho sensitive regulation of dendritic cell functions by vitamin E. Biol. Res. 2016;49:45. doi: 10.1186/s40659-016-0105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Stiff A., Trikha P., Mundy-Bosse B., McMichael E., Mace T.A., Benner B., Kendra K., Campbell A., Gautam S., Abood D., et al. Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor-Mediated Natural Killer Cell Function. Clin. Cancer Res. 2018;24:1891–1904. doi: 10.1158/1078-0432.CCR-17-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Beharka A.A., Han S.N., Adolfsson O., Wu D., Smith D., Lipman R., Cao G., Meydani M., Meydani S.N. Long-term dietary antioxidant supplementation reduces production of selected inflammatory mediators by murine macrophages. Nutr. Res. 2000;20:281–296. doi: 10.1016/S0271-5317(99)00160-8. [DOI] [Google Scholar]
- 243.Meydani S.N., Meydani M., Blumberg J.B., Leka L.S., Siber G., Loszewski R., Thompson C., Pedrosa M.C., Diamond R.D., Stollar B.D. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277:1380–1386. doi: 10.1001/jama.1997.03540410058031. [DOI] [PubMed] [Google Scholar]
- 244.Adolfsson O., Huber B.T., Meydani S.N. Vitamin E-enhanced IL-2 production in old mice: Naive but not memory T cells show increased cell division cycling and IL-2-producing capacity. J. Immunol. 2001;167:3809–3817. doi: 10.4049/jimmunol.167.7.3809. [DOI] [PubMed] [Google Scholar]
- 245.Marko M.G., Ahmed T., Bunnell S.C., Wu D., Chung H., Huber B.T., Meydani S.N. Age-associated decline in effective immune synapse formation of CD4(+) T cells is reversed by vitamin E supplementation. J. Immunol. 2007;178:1443–1449. doi: 10.4049/jimmunol.178.3.1443. [DOI] [PubMed] [Google Scholar]
- 246.Marko M.G., Pang H.J., Ren Z., Azzi A., Huber B.T., Bunnell S.C., Meydani S.N. Vitamin E reverses impaired linker for activation of T cells activation in T cells from aged C57BL/6 mice. J. Nutr. 2009;139:1192–1197. doi: 10.3945/jn.108.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Meydani S.N., Han S.N., Wu D. Vitamin E and immune response in the aged: Molecular mechanisms and clinical implications. Immunol. Rev. 2005;205:269–284. doi: 10.1111/j.0105-2896.2005.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tantcheva L.P., Stoeva E.S., Galabov A.S., Braykova A.A., Savov V.M., Mileva M.M. Effect of vitamin E and vitamin C combination on experimental influenza virus infection. Methods Find Exp. Clin. Pharmacol. 2003;25:259–264. doi: 10.1358/mf.2003.25.4.769673. [DOI] [PubMed] [Google Scholar]
- 249.Han S.N., Meydani S.N. Vitamin E and infectious diseases in the aged. Proc. Nutr. Soc. 1999;58:697–705. doi: 10.1017/S0029665199000919. [DOI] [PubMed] [Google Scholar]
- 250.Han S.N., Wu D., Ha W.K., Beharka A., Smith D.E., Bender B.S., Meydani S.N. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology. 2000;100:487–493. doi: 10.1046/j.1365-2567.2000.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kowdley K.V., Mason J.B., Meydani S.N., Cornwall S., Grand R.J. Vitamin E deficiency and impaired cellular immunity related to intestinal fat malabsorption. Gastroenterology. 1992;102:2139–2142. doi: 10.1016/0016-5085(92)90344-X. [DOI] [PubMed] [Google Scholar]
- 252.Lewis E.D., Meydani S.N., Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019;71:487–494. doi: 10.1002/iub.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ghani S.M.A., Goon J.A., Azman N., Zakaria S.N.A., Hamid Z., Ngah W.Z.W. Comparing the effects of vitamin E tocotrienol-rich fraction supplementation and alpha-tocopherol supplementation on gene expression in healthy older adults. Clinics (Sao Paulo) 2019;74:e688. doi: 10.6061/clinics/2019/e688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Neupane B., Walter S.D., Krueger P., Marrie T., Loeb M. Predictors of inhospital mortality and re-hospitalization in older adults with community-acquired pneumonia: A prospective cohort study. BMC Geriatr. 2010;10:22. doi: 10.1186/1471-2318-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Carr A.C., Shaw G.M., Fowler A.A., Natarajan R. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin C administration in severe sepsis and septic shock? Crit. Care. 2015;19:418. doi: 10.1186/s13054-015-1131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Englard S., Seifter S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 1986;6:365–406. doi: 10.1146/annurev.nu.06.070186.002053. [DOI] [PubMed] [Google Scholar]
- 257.Kuiper C., Vissers M.C. Ascorbate as a co-factor for fe- and 2-oxoglutarate dependent dioxygenases: Physiological activity in tumor growth and progression. Front. Oncol. 2014;4:359. doi: 10.3389/fonc.2014.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mandl J., Szarka A., Banhegyi G. Vitamin C: Update on physiology and pharmacology. Br. J. Pharmacol. 2009;157:1097–1110. doi: 10.1111/j.1476-5381.2009.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Young J.I., Zuchner S., Wang G. Regulation of the Epigenome by Vitamin C. Annu. Rev. Nutr. 2015;35:545–564. doi: 10.1146/annurev-nutr-071714-034228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kim K.P., Shin K.O., Park K., Yun H.J., Mann S., Lee Y.M., Cho Y. Vitamin C Stimulates Epidermal Ceramide Production by Regulating Its Metabolic Enzymes. Biomol. Ther. (Seoul) 2015;23:525–530. doi: 10.4062/biomolther.2015.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pasonen-Seppanen S., Suhonen T.M., Kirjavainen M., Suihko E., Urtti A., Miettinen M., Hyttinen M., Tammi M., Tammi R. Vitamin C enhances differentiation of a continuous keratinocyte cell line (REK) into epidermis with normal stratum corneum ultrastructure and functional permeability barrier. Histochem. Cell Biol. 2001;116:287–297. doi: 10.1007/s004180100312. [DOI] [PubMed] [Google Scholar]
- 262.Ponec M., Weerheim A., Kempenaar J., Mulder A., Gooris G.S., Bouwstra J., Mommaas A.M. The formation of competent barrier lipids in reconstructed human epidermis requires the presence of vitamin C. J. Investig. Dermatol. 1997;109:348–355. doi: 10.1111/1523-1747.ep12336024. [DOI] [PubMed] [Google Scholar]
- 263.Savini I., Catani M.V., Rossi A., Duranti G., Melino G., Avigliano L. Characterization of keratinocyte differentiation induced by ascorbic acid: Protein kinase C involvement and vitamin C homeostasis. J. Investig. Dermatol. 2002;118:372–379. doi: 10.1046/j.0022-202x.2001.01624.x. [DOI] [PubMed] [Google Scholar]
- 264.Uchida Y., Behne M., Quiec D., Elias P.M., Holleran W.M. Vitamin C stimulates sphingolipid production and markers of barrier formation in submerged human keratinocyte cultures. J. Investig. Dermatol. 2001;117:1307–1313. doi: 10.1046/j.0022-202x.2001.01555.x. [DOI] [PubMed] [Google Scholar]
- 265.Duarte T.L., Cooke M.S., Jones G.D. Gene expression profiling reveals new protective roles for vitamin C in human skin cells. Free Radic. Biol. Med. 2009;46:78–87. doi: 10.1016/j.freeradbiomed.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 266.Mohammed B.M., Fisher B.J., Kraskauskas D., Ward S., Wayne J.S., Brophy D.F., Fowler A.A., 3rd, Yager D.R., Natarajan R. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int. Wound J. 2016;13:572–584. doi: 10.1111/iwj.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Bozonet S.M., Carr A.C., Pullar J.M., Vissers M.C. Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold kiwifruit. Nutrients. 2015;7:2574–2588. doi: 10.3390/nu7042574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Carr A.C., Maggini S. Vitamin C and Immune Function. Nutrients. 2017;9:1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Parker W.H., Rhea E.M., Qu Z.C., Hecker M.R., May J.M. Intracellular ascorbate tightens the endothelial permeability barrier through Epac1 and the tubulin cytoskeleton. Am. J. Physiol. Cell Physiol. 2016;311:C652–c662. doi: 10.1152/ajpcell.00076.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sharma P., Raghavan S.A., Saini R., Dikshit M. Ascorbate-mediated enhancement of reactive oxygen species generation from polymorphonuclear leukocytes: Modulatory effect of nitric oxide. J. Leukoc Biol. 2004;75:1070–1078. doi: 10.1189/jlb.0903415. [DOI] [PubMed] [Google Scholar]
- 271.Huijskens M.J., Walczak M., Koller N., Briede J.J., Senden-Gijsbers B.L., Schnijderberg M.C., Bos G.M., Germeraad W.T. Technical advance: Ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J. Leukoc. Biol. 2014;96:1165–1175. doi: 10.1189/jlb.1TA0214-121RR. [DOI] [PubMed] [Google Scholar]
- 272.Manning J., Mitchell B., Appadurai D.A., Shakya A., Pierce L.J., Wang H., Nganga V., Swanson P.C., May J.M., Tantin D., et al. Vitamin C promotes maturation of T-cells. Antioxid. Redox Signal. 2013;19:2054–2067. doi: 10.1089/ars.2012.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Huijskens M.J., Walczak M., Sarkar S., Atrafi F., Senden-Gijsbers B.L., Tilanus M.G., Bos G.M., Wieten L., Germeraad W.T. Ascorbic acid promotes proliferation of natural killer cell populations in culture systems applicable for natural killer cell therapy. Cytotherapy. 2015;17:613–620. doi: 10.1016/j.jcyt.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 274.Hemila H. Vitamin C and Infections. Nutrients. 2017;9:339. doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Cai Y., Li Y.F., Tang L.P., Tsoi B., Chen M., Chen H., Chen X.M., Tan R.R., Kurihara H., He R.R. A new mechanism of vitamin C effects on A/FM/1/47(H1N1) virus-induced pneumonia in restraint-stressed mice. Biomed. Res. Int. 2015;2015:675149. doi: 10.1155/2015/675149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hemila H., Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD005532.pub3. [DOI] [PubMed] [Google Scholar]
- 277.Hunt C., Chakravorty N.K., Annan G., Habibzadeh N., Schorah C.J. The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int. J. Vitam Nutr. Res. 1994;64:212–219. [PubMed] [Google Scholar]
- 278.Vorilhon P., Arpajou B., Vaillant Roussel H., Merlin E., Pereira B., Cabaillot A. Efficacy of vitamin C for the prevention and treatment of upper respiratory tract infection. A meta-analysis in children. Eur. J. Clin. Pharmacol. 2019;75:303–311. doi: 10.1007/s00228-018-2601-7. [DOI] [PubMed] [Google Scholar]
- 279.Keil S.D., Bowen R., Marschner S. Inactivation of Middle East respiratory syndrome coronavirus (MERS-CoV) in plasma products using a riboflavin-based and ultraviolet light-based photochemical treatment. Transfusion. 2016;56:2948–2952. doi: 10.1111/trf.13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Jones H.D., Yoo J., Crother T.R., Kyme P., Ben-Shlomo A., Khalafi R., Tseng C.W., Parks W.C., Arditi M., Liu G.Y., et al. Nicotinamide exacerbates hypoxemia in ventilator-induced lung injury independent of neutrophil infiltration. PLoS ONE. 2015;10:e0123460. doi: 10.1371/journal.pone.0123460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Willis-Carr J.I., St Pierre R.L. Effects of vitamin B6 deficiency on thymic epithelial cells and T lymphocyte differentiation. J. Immunol. 1978;120:1153–1159. [PubMed] [Google Scholar]
- 282.Ha C., Miller L.T., Kerkvliet N.I. The effect of vitamin B6 deficiency on cytotoxic immune responses of T cells, antibodies, and natural killer cells, and phagocytosis by macrophages. Cell. Immunol. 1984;85:318–329. doi: 10.1016/0008-8749(84)90246-6. [DOI] [PubMed] [Google Scholar]
- 283.Axelrod A.E. Immune processes in vitamin deficiency states. Am. J. Clin. Nutr. 1971;24:265–271. doi: 10.1093/ajcn/24.2.265. [DOI] [PubMed] [Google Scholar]
- 284.Sergeev A.V., Bykovskaja S.N., Luchanskaja L.M., Rauschenbach M.O. Pyridoxine deficiency and cytotoxicity of T lymphocytes in vitro. Cell. Immunol. 1978;38:187–192. doi: 10.1016/0008-8749(78)90045-X. [DOI] [PubMed] [Google Scholar]
- 285.Cheng C.H., Chang S.J., Lee B.J., Lin K.L., Huang Y.C. Vitamin B6 supplementation increases immune responses in critically ill patients. Eur. J. Clin. Nutr. 2006;60:1207–1213. doi: 10.1038/sj.ejcn.1602439. [DOI] [PubMed] [Google Scholar]
- 286.Morris M.S., Sakakeeny L., Jacques P.F., Picciano M.F., Selhub J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J. Nutr. 2010;140:103–110. doi: 10.3945/jn.109.114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Paniz C., Bertinato J.F., Lucena M.R., De Carli E., Amorim P., Gomes G.W., Palchetti C.Z., Figueiredo M.S., Pfeiffer C.M., Fazili Z., et al. A Daily Dose of 5 mg Folic Acid for 90 Days Is Associated with Increased Serum Unmetabolized Folic Acid and Reduced Natural Killer Cell Cytotoxicity in Healthy Brazilian Adults. J. Nutr. 2017;147:1677–1685. doi: 10.3945/jn.117.247445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Tamura J., Kubota K., Murakami H., Sawamura M., Matsushima T., Tamura T., Saitoh T., Kurabayshi H., Naruse T. Immunomodulation by vitamin B12: Augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin. Exp. Immunol. 1999;116:28–32. doi: 10.1046/j.1365-2249.1999.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ford T.C., Downey L.A., Simpson T., McPhee G., Oliver C., Stough C. The Effect of a High-Dose Vitamin B Multivitamin Supplement on the Relationship between Brain Metabolism and Blood Biomarkers of Oxidative Stress: A Randomized Control Trial. Nutrients. 2018;10:1860. doi: 10.3390/nu10121860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Christen W.G., Cook N.R., Van Denburgh M., Zaharris E., Albert C.M., Manson J.E. Effect of Combined Treatment With Folic Acid, Vitamin B6, and Vitamin B12 on Plasma Biomarkers of Inflammation and Endothelial Dysfunction in Women. J. Am. Heart Assoc. 2018;7:e008517. doi: 10.1161/JAHA.117.008517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Alpert P.T. The Role of Vitamins and Minerals on the Immune System. Home Health Care Manag. Pract. 2017;29:199–202. doi: 10.1177/1084822317713300. [DOI] [Google Scholar]
- 292.Maggini S., Pierre A., Calder P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients. 2018;10:1531. doi: 10.3390/nu10101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Abdelhaleim A.F., Abdo Soliman J.S., Amer A.Y., Abdo Soliman J.S. Association of Zinc Deficiency with Iron Deficiency Anemia and its Symptoms: Results from a Case-control Study. Cureus. 2019;11:e3811. doi: 10.7759/cureus.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wessells K.R., Brown K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE. 2012;7:e50568. doi: 10.1371/journal.pone.0050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Black R.E. Zinc deficiency, infectious disease and mortality in the developing world. J. Nutr. 2003;133:1485s–1489s. doi: 10.1093/jn/133.5.1485S. [DOI] [PubMed] [Google Scholar]
- 296.Bhutta Z.A. Iron and zinc deficiency in children in developing countries. BMJ (Clin. Res. ed.) 2007;334:104–105. doi: 10.1136/bmj.39094.513924.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wintergerst E.S., Maggini S., Hornig D.H. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metab. 2007;51:301–323. doi: 10.1159/000107673. [DOI] [PubMed] [Google Scholar]
- 299.Wintergerst E.S., Maggini S., Hornig D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006;50:85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 300.Bulyk M.L., Huang X., Choo Y., Church G.M. Exploring the DNA-binding specificities of zinc fingers with DNA microarrays. Proc. Natl. Acad. Sci. USA. 2001;98:7158–7163. doi: 10.1073/pnas.111163698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Andreini C., Bertini I., Cavallaro G. Minimal functional sites allow a classification of zinc sites in proteins. PLoS ONE. 2011;6:e26325. doi: 10.1371/journal.pone.0026325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gammoh N.Z., Rink L. Zinc in Infection and Inflammation. Nutrients. 2017;9:624. doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Barnett J.B., Hamer D.H., Meydani S.N. Low zinc status: A new risk factor for pneumonia in the elderly? Nutr. Rev. 2010;68:30–37. doi: 10.1111/j.1753-4887.2009.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Shaw J.G., Friedman J.F. Iron deficiency anemia: Focus on infectious diseases in lesser developed countries. Anemia. 2011;2011:260380. doi: 10.1155/2011/260380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Oppenheimer S.J. Iron and its relation to immunity and infectious disease. J. Nutr. 2001;131:616S–633S. doi: 10.1093/jn/131.2.616S. [DOI] [PubMed] [Google Scholar]
- 306.Semba R.D., Bloem M.W. The anemia of vitamin A deficiency: Epidemiology and pathogenesis. Eur. J. Clin. Nutr. 2002;56:271–281. doi: 10.1038/sj.ejcn.1601320. [DOI] [PubMed] [Google Scholar]
- 307.Bloem M.W. Interdependence of vitamin A and iron: An important association for programmes of anaemia control. Proc. Nutr. Soc. 1995;54:501–508. doi: 10.1079/PNS19950018. [DOI] [PubMed] [Google Scholar]
- 308.Thurnham D.I. Vitamin A, iron, and haemopoiesis. Lancet. 1993;342:1312–1313. doi: 10.1016/0140-6736(93)92239-P. [DOI] [PubMed] [Google Scholar]
- 309.Cherayil B.J. The role of iron in the immune response to bacterial infection. Immunologic. Res. 2011;50:1–9. doi: 10.1007/s12026-010-8199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Drakesmith H., Prentice A. Viral infection and iron metabolism. Nat. Rev. Microbiol. 2008;6:541–552. doi: 10.1038/nrmicro1930. [DOI] [PubMed] [Google Scholar]
- 311.Ali M.K., Kim R.Y., Karim R., Mayall J.R., Martin K.L., Shahandeh A., Abbasian F., Starkey M.R., Loustaud-Ratti V., Johnstone D., et al. Role of iron in the pathogenesis of respiratory disease. Int. J. Biochem. Cell Biol. 2017;88:181–195. doi: 10.1016/j.biocel.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 312.Ahluwalia N., Sun J., Krause D., Mastro A., Handte G. Immune function is impaired in iron-deficient, homebound, older women. Am. J. Clin. Nutr. 2004;79:516–521. doi: 10.1093/ajcn/79.3.516. [DOI] [PubMed] [Google Scholar]
- 313.Jayaweera J., Reyes M., Joseph A. Childhood iron deficiency anemia leads to recurrent respiratory tract infections and gastroenteritis. Sci. Rep. 2019;9:12637. doi: 10.1038/s41598-019-49122-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 314.Besold A.N., Culbertson E.M., Culotta V.C. The Yin and Yang of copper during infection. JBIC J. Biol. Inorganic Chem. 2016;21:137–144. doi: 10.1007/s00775-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Saeed F., Nadeem M., Ahmed R.S., Tahir Nadeem M., Arshad M.S., Ullah A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds—A review. Food Agric. Immunol. 2016;27:205–229. doi: 10.1080/09540105.2015.1079600. [DOI] [Google Scholar]
- 316.Weiss G., Carver P.L. Role of divalent metals in infectious disease susceptibility and outcome. Clin. Microbiol. Infect. 2018;24:16–23. doi: 10.1016/j.cmi.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 317.Steinbrenner H., Al-Quraishy S., Dkhil M.A., Wunderlich F., Sies H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv. Nutr. 2015;6:73–82. doi: 10.3945/an.114.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Harthill M. Review: Micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011;143:1325–1336. doi: 10.1007/s12011-011-8977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Stoffaneller R., Morse N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients. 2015;7:1494–1537. doi: 10.3390/nu7031494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Steinbrenner H., Speckmann B., Klotz L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016;595:113–119. doi: 10.1016/j.abb.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 321.Zoidis E., Seremelis I., Kontopoulos N., Danezis G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants (Basel) 2018;7:66. doi: 10.3390/antiox7050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hoffmann P.R., Berry M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008;52:1273–1280. doi: 10.1002/mnfr.200700330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Stranges S., Marshall J.R., Natarajan R., Donahue R.P., Trevisan M., Combs G.F., Cappuccio F.P., Ceriello A., Reid M.E. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: A randomized trial. Ann. Intern. Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 324.Lee Y.-H., Lee S.J., Lee M.K., Lee W.-Y., Yong S.J., Kim S.-H. Serum selenium levels in patients with respiratory diseases: A prospective observational study. J. Thorac. Dis. 2016;8:2068–2078. doi: 10.21037/jtd.2016.07.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Mahmoodpoor A., Hamishehkar H., Shadvar K., Ostadi Z., Sanaie S., Saghaleini S.H., Nader N.D. The Effect of Intravenous Selenium on Oxidative Stress in Critically Ill Patients with Acute Respiratory Distress Syndrome. Immunol. Investig. 2019;48:147–159. doi: 10.1080/08820139.2018.1496098. [DOI] [PubMed] [Google Scholar]
- 326.Esmaillzadeh A., Kimiagar M., Mehrabi Y., Azadbakht L., Hu F.B., Willett W.C. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am. J. Clin. Nutr. 2006;84:1489–1497. doi: 10.1093/ajcn/84.6.1489. [DOI] [PubMed] [Google Scholar]
- 327.Gao X., Bermudez O.I., Tucker K.L. Plasma C-reactive protein and homocysteine concentrations are related to frequent fruit and vegetable intake in Hispanic and non-Hispanic white elders. J. Nutr. 2004;134:913–918. doi: 10.1093/jn/134.4.913. [DOI] [PubMed] [Google Scholar]
- 328.Nanri A., Yoshida D., Yamaji T., Mizoue T., Takayanagi R., Kono S. Dietary patterns and C-reactive protein in Japanese men and women. Am. J. Clin. Nutr. 2008;87:1488–1496. doi: 10.1093/ajcn/87.5.1488. [DOI] [PubMed] [Google Scholar]
- 329.di Giuseppe R., Di Castelnuovo A., Centritto F., Zito F., De Curtis A., Costanzo S., Vohnout B., Sieri S., Krogh V., Donati M.B., et al. Regular consumption of dark chocolate is associated with low serum concentrations of C-reactive protein in a healthy Italian population. J. Nutr. 2008;138:1939–1945. doi: 10.1093/jn/138.10.1939. [DOI] [PubMed] [Google Scholar]
- 330.Stote K.S., Clevidence B.A., Novotny J.A., Henderson T., Radecki S.V., Baer D.J. Effect of cocoa and green tea on biomarkers of glucose regulation, oxidative stress, inflammation and hemostasis in obese adults at risk for insulin resistance. Eur. J. Clin. Nutr. 2012;66:1153–1159. doi: 10.1038/ejcn.2012.101. [DOI] [PubMed] [Google Scholar]
- 331.Chun O.K., Chung S.J., Claycombe K.J., Song W.O. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. J. Nutr. 2008;138:753–760. doi: 10.1093/jn/138.4.753. [DOI] [PubMed] [Google Scholar]
- 332.Macready A.L., George T.W., Chong M.F., Alimbetov D.S., Jin Y., Vidal A., Spencer J.P., Kennedy O.B., Tuohy K.M., Minihane A.M., et al. Flavonoid-rich fruit and vegetables improve microvascular reactivity and inflammatory status in men at risk of cardiovascular disease--FLAVURS: A randomized controlled trial. Am. J. Clin. Nutr. 2014;99:479–489. doi: 10.3945/ajcn.113.074237. [DOI] [PubMed] [Google Scholar]
- 333.Martinez-Lopez S., Sarria B., Sierra-Cinos J.L., Goya L., Mateos R., Bravo L. Realistic intake of a flavanol-rich soluble cocoa product increases HDL-cholesterol without inducing anthropometric changes in healthy and moderately hypercholesterolemic subjects. Food Funct. 2014;5:364–374. doi: 10.1039/c3fo60352k. [DOI] [PubMed] [Google Scholar]
- 334.McFarlin B.K., Venable A.S., Henning A.L., Prado E.A., Best Sampson J.N., Vingren J.L., Hill D.W. Natural cocoa consumption: Potential to reduce atherogenic factors? J. Nutr. Biochem. 2015;26:626–632. doi: 10.1016/j.jnutbio.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 335.Tokede O.A., Gaziano J.M., Djousse L. Effects of cocoa products/dark chocolate on serum lipids: A meta-analysis. Eur. J. Clin. Nutr. 2011;65:879–886. doi: 10.1038/ejcn.2011.64. [DOI] [PubMed] [Google Scholar]
- 336.Flammer A.J., Sudano I., Wolfrum M., Thomas R., Enseleit F., Periat D., Kaiser P., Hirt A., Hermann M., Serafini M., et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur. Heart J. 2012;33:2172–2180. doi: 10.1093/eurheartj/ehr448. [DOI] [PubMed] [Google Scholar]
- 337.Grassi D., Desideri G., Necozione S., di Giosia P., Barnabei R., Allegaert L., Bernaert H., Ferri C. Cocoa consumption dose-dependently improves flow-mediated dilation and arterial stiffness decreasing blood pressure in healthy individuals. J. Hypertens. 2015;33:294–303. doi: 10.1097/HJH.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 338.West S.G., McIntyre M.D., Piotrowski M.J., Poupin N., Miller D.L., Preston A.G., Wagner P., Groves L.F., Skulas-Ray A.C. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. Br. J. Nutr. 2014;111:653–661. doi: 10.1017/S0007114513002912. [DOI] [PubMed] [Google Scholar]
- 339.Kaul T.N., Middleton E., Jr., Ogra P.L. Antiviral effect of flavonoids on human viruses. J. Med. Virol. 1985;15:71–79. doi: 10.1002/jmv.1890150110. [DOI] [PubMed] [Google Scholar]
- 340.Zamora-Ros R., Knaze V., Rothwell J.A., Hémon B., Moskal A., Overvad K., Tjønneland A., Kyrø C., Fagherazzi G., Boutron-Ruault M.-C., et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016;55:1359–1375. doi: 10.1007/s00394-015-0950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Franco M.N., Galeano-Diaz T., Lopez O., Fernandez-Bolanos J.G., Sanchez J., De Miguel C., Gil M.V., Martin-Vertedor D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014;163:289–298. doi: 10.1016/j.foodchem.2014.04.091. [DOI] [PubMed] [Google Scholar]
- 342.Gimeno E., Fito M., Lamuela-Raventos R.M., Castellote A.I., Covas M., Farre M., de La Torre-Boronat M.C., Lopez-Sabater M.C. Effect of ingestion of virgin olive oil on human low-density lipoprotein composition. Eur. J. Clin. Nutr. 2002;56:114–120. doi: 10.1038/sj.ejcn.1601293. [DOI] [PubMed] [Google Scholar]
- 343.Arranz S., Chiva-Blanch G., Valderas-Martinez P., Medina-Remon A., Lamuela-Raventos R.M., Estruch R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients. 2012;4:759–781. doi: 10.3390/nu4070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Rodrigo R., Miranda A., Vergara L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin. Chim. Acta. 2011;412:410–424. doi: 10.1016/j.cca.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 345.Gonzalez-Gallego J., Sanchez-Campos S., Tunon M.J. Anti-inflammatory properties of dietary flavonoids. Nutr. Hosp. 2007;22:287–293. [PubMed] [Google Scholar]
- 346.Covas M.I., Nyyssonen K., Poulsen H.E., Kaikkonen J., Zunft H.J., Kiesewetter H., Gaddi A., de la Torre R., Mursu J., Baumler H., et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006;145:333–341. doi: 10.7326/0003-4819-145-5-200609050-00006. [DOI] [PubMed] [Google Scholar]
- 347.Estruch R., Ros E., Salas-Salvado J., Covas M.I., Corella D., Aros F., Gomez-Gracia E., Ruiz-Gutierrez V., Fiol M., Lapetra J., et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 348.Hernaez A., Fernandez-Castillejo S., Farras M., Catalan U., Subirana I., Montes R., Sola R., Munoz-Aguayo D., Gelabert-Gorgues A., Diaz-Gil O., et al. Olive oil polyphenols enhance high-density lipoprotein function in humans: A randomized controlled trial. Arterioscler. Thromb. Vasc. Biol. 2014;34:2115–2119. doi: 10.1161/ATVBAHA.114.303374. [DOI] [PubMed] [Google Scholar]
- 349.Cardona F., Andrés-Lacueva C., Tulipani S., Tinahones F.J., Queipo-Ortuño M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013;24:1415–1422. doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 350.Kaulmann A., Bohn T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases-Promises, Perspectives, and Pitfalls. Oxid. Med. Cell Longev. 2016;2016:9346470. doi: 10.1155/2016/9346470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Bahramsoltani R., Sodagari H.R., Farzaei M.H., Abdolghaffari A.H., Gooshe M., Rezaei N. The preventive and therapeutic potential of natural polyphenols on influenza. Expert Rev. Anti Infect. Ther. 2016;14:57–80. doi: 10.1586/14787210.2016.1120670. [DOI] [PubMed] [Google Scholar]
- 352.Lin L.-T., Hsu W.-C., Lin C.-C. Antiviral Natural Products and Herbal Medicines. J. Tradit. Complementary Med. 2014;4:24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Mishra S., Pandey A., Manvati S. Coumarin: An emerging antiviral agent. Heliyon. 2020;6:e03217. doi: 10.1016/j.heliyon.2020.e03217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Zu M., Yang F., Zhou W., Liu A., Du G., Zheng L. In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antiviral Res. 2012;94:217–224. doi: 10.1016/j.antiviral.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 355.Fioravanti R., Celestino I., Costi R., Cuzzucoli Crucitti G., Pescatori L., Mattiello L., Novellino E., Checconi P., Palamara A.T., Nencioni L., et al. Effects of polyphenol compounds on influenza A virus replication and definition of their mechanism of action. Bioorg. Med. Chem. 2012;20:5046–5052. doi: 10.1016/j.bmc.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 356.Panahi Y., Hosseini M.S., Khalili N., Naimi E., Majeed M., Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015;34:1101–1108. doi: 10.1016/j.clnu.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 357.Krinsky N.I., Johnson E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 358.Bohn T. Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases. Antioxidants (Basel) 2019;8:179. doi: 10.3390/antiox8060179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Walston J., Xue Q., Semba R.D., Ferrucci L., Cappola A.R., Ricks M., Guralnik J., Fried L.P. Serum antioxidants, inflammation, and total mortality in older women. Am. J. Epidemiol. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- 360.Dalgard C., Nielsen F., Morrow J.D., Enghusen-Poulsen H., Jonung T., Horder M., de Maat M.P. Supplementation with orange and blackcurrant juice, but not vitamin E, improves inflammatory markers in patients with peripheral arterial disease. Br. J. Nutr. 2009;101:263–269. doi: 10.1017/S0007114508995660. [DOI] [PubMed] [Google Scholar]
- 361.Naithani R., Huma L.C., Holland L.E., Shukla D., McCormick D.L., Mehta R.G., Moriarty R.M. Antiviral activity of phytochemicals: A comprehensive review. Mini Rev. Med. Chem. 2008;8:1106–1133. doi: 10.2174/138955708785909943. [DOI] [PubMed] [Google Scholar]
- 362.Chew B.P., Park J.S. Carotenoid action on the immune response. J. Nutr. 2004;134:257s–261s. doi: 10.1093/jn/134.1.257S. [DOI] [PubMed] [Google Scholar]
- 363.Huang J., Weinstein S.J., Yu K., Männistö S., Albanes D. Serum Beta Carotene and Overall and Cause-Specific Mortality. Circ. Res. 2018;123:1339–1349. doi: 10.1161/CIRCRESAHA.118.313409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Bates C.J., Hamer M., Mishra G.D. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: The National Diet and Nutrition Survey of people aged 65 years and over. Br. J. Nutr. 2011;105:123–132. doi: 10.1017/S0007114510003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Omenn G.S., Goodman G., Thornquist M., Grizzle J., Rosenstock L., Barnhart S., Balmes J., Cherniack M.G., Cullen M.R., Glass A., et al. The beta-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high risk populations: Smokers and asbestos-exposed workers. Cancer Res. 1994;54:2038s–2043s. [PubMed] [Google Scholar]
- 366.Ulaszewska M.M., Weinert C.H., Trimigno A., Portmann R., Andres Lacueva C., Badertscher R., Brennan L., Brunius C., Bub A., Capozzi F., et al. Nutrimetabolomics: An Integrative Action for Metabolomic Analyses in Human Nutritional Studies. Mol. Nutr. Food Res. 2019;63:e1800384. doi: 10.1002/mnfr.201800384. [DOI] [PubMed] [Google Scholar]