Abstract
The interaction between nutrition and human infectious diseases has always been recognized. With the emergence of molecular tools and post-genomics, high-resolution sequencing technologies, the gut microbiota has been emerging as a key moderator in the complex interplay between nutrients, human body, and infections. Much of the host–microbial and nutrition research is currently based on animals or simplistic in vitro models. Although traditional in vivo and in vitro models have helped to develop mechanistic hypotheses and assess the causality of the host–microbiota interactions, they often fail to faithfully recapitulate the complexity of the human nutrient–microbiome axis in gastrointestinal homeostasis and infections. Over the last decade, remarkable progress in tissue engineering, stem cell biology, microfluidics, sequencing technologies, and computing power has taken place, which has produced a new generation of human-focused, relevant, and predictive tools. These tools, which include patient-derived organoids, organs-on-a-chip, computational analyses, and models, together with multi-omics readouts, represent novel and exciting equipment to advance the research into microbiota, infectious diseases, and nutrition from a human-biology-based perspective. After considering some limitations of the conventional in vivo and in vitro approaches, in this review, we present the main novel available and emerging tools that are suitable for designing human-oriented research.
Keywords: microbiota, infectious diseases, nutrition, human-based methods, gut-on-a-chip, gut-organoids, third-generation sequencing
1. Introduction
The interaction between nutrition and infectious diseases has always been recognized. Before the era of antibiotics, diet was considered an essential part of the management of infections. Malnutrition, including undernutrition and overnutrition, can increase susceptibility to infectious diseases and amplify the severity of an infection, which in turn, can worsen malnutrition [1]. Thanks to the advancements in next-generation sequencing (NGS) technologies, the gut microbiota has been emerging as an integral mediator in the complex relations between food, the human body, and infectious diseases. The complex community of microorganisms inhabiting an animal’s or human’s digestive tract constitutes the gut microbiota and their collective genetic content constitutes the gut microbiome. High-throughput comparative metagenomics and meta-transcriptomics enabled by the development of NGS platforms have led to an unprecedented understanding of human, animal, and environmental microbiomes, and have shown that the gut microbiota is comparable to a virtual organ or emergent system, whose properties need to be integrated into host biology and physiology [2].
The microbiota that inhabits the human gut is crucial in regulating gut physiological functions and all body homeostasis. Such physiological functions include aiding digestion, producing metabolites from undigested fibers, regulating drug metabolism [3], impacting the development of the immune system during the early life stages [4], modulating immune responses, and protecting the host from pathogens and infections [5,6]. Changes in the gut-associated microbial community composition and diversity have been associated with several diseases [7,8,9,10,11], including infections in humans [12,13,14,15,16]. The composition of the microbiota can offer either resistance or assistance to invading pathogenic species. Differential susceptibility to Campylobacter jejuni infections was shown to depend on the species composition and diversity of the microbiotas in an epidemiological study of Swedish adults [17]. Several metabolites, such as short-chain fatty acids (SCFAs) that are produced by the microbiota, are important determinants of the interactions between the microbiota and pathogenic bacteria in the gut [18].
Nutrition profoundly affects the composition of the microbiota and the concentration of metabolites in the gut, which has repercussions for the physiology, immunity, and susceptibility to infectious diseases of the host [19]. The functions that the gut microbiota play in the human body can be quite easily disrupted or influenced by a wide variety of nutritional factors, including dietary habits [20], antibiotics, probiotics, and prebiotics [21]. Several nutritional studies described the impact on gut microbiota composition and the function of micro- and macro-nutrients, such as carbohydrates, novel food components, and food additives, as well as low-fat/high-fiber diets, high- or low-protein diets, diets containing high-fat/low-fiber fruits, and vegetables [20,22,23,24]. Nutrients can directly affect the gut microbiota by promoting or inhibiting microorganism growth or indirectly influencing the host’s metabolism and its immune system, or passively incorporating food-derived members into the microbiota, such as Candida and Penicillium fungi and lactic acid bacteria [25,26]. Likewise, if food is contaminated with pathogens, it can influence the development of gastrointestinal infections [27], food poisoning [28], and systemic infectious diseases [29,30].
On the other hand, infectious diseases could impact the microbiota diversity and composition by determining dysbiosis [31], which in turn, increases the susceptibility to infections.
Despite the microbiota emerging as an important modulator in the interaction between diet and infectious diseases, research findings into the role of the microbiota in the field of infectious diseases remain rudimentary.
The complexity of a holistic understanding of the cause-and-effect relationships in humans makes human studies difficult and limited, most notably because of the challenges in accessing the human gut, making disease modeling essential. Traditional 2D cell cultures or ex vivo models have been invaluable in the study of the gut microbiome and infectious diseases, but excessively simplistic cellular models, often utilizing nonhuman cells cultured under non-homeostatic and non-physiologic in vitro conditions, could have hampered, to some extent, the understanding of the complex relationships between the human host, gut microbiome, and pathogens in vivo. On the other hand, although animal models have provided extensive insights into the host–microbiota–pathogens interactions regarding diet, especially in developing mechanistic hypotheses, they fail to adequately recapitulate the complexity of human conditions and therapeutic responses.
In the last few years, there have been large steps forward in microfluidics, tissue engineering, computing power, artificial intelligence, etc., which have brought about an amazing array of tools and research approaches that are offering bold new ways to study the human gut microbiome regarding pathogens and the effect of nutrition in a human-biology-based setting. In addition, the field of metagenomics and other “omics” disciplines has greatly expanded due to improvement in sequencing technologies, namely “third-generation sequencing,” as well as analytical and single-cell techniques, allowing for a more comprehensive characterization of microbial communities and host–microbe interactions. These tools and approaches could potentially yield profuse and meaningful human-relevant data, allowing for a better understanding of human pathophysiology, while helping to reduce the number of animals employed in biomedical research, eventually replacing them. After discussing some of the major limitations associated with traditional in vivo and in vitro models, in this review, we present a list of the novel available and emerging tools and approaches used to study the interactions between the human host and the gut microbiome and pathogens through a more holistic representation of the human in vivo microenvironment. The potential opportunities regarding modifying and shaping the microbiota through nutritional interventions to treat or prevent infectious diseases will be also considered.
2. Limitations of Traditional In Vivo and In Vitro Models
Animal models, including mice, fish, and insects, have been extensively used to analyze host–microbiome interactions and their contributions to pathophysiology in infectious diseases regarding diet. Mice are the model of choice for most studies in this emerging field, allowing for manipulations in the gut microbiota and host to be studied in a controlled experimental setup [32]. Manipulations include host genetic background manipulation (gene knockouts); gut microbiota composition manipulation (controlled inoculation in germ-free or gnotobiotic mice, i.e., germ-free mice administered with human microbes); and ecosystem interventions, including dietary interventions, antibiotic treatment, and fecal transplantations.
Although animal models have provided critical insight into how host-microbiota homeostasis is constructed and maintained [33] or influenced by infections or dietary factors [34], they do not faithfully represent the human body and they are not suitable models to assess drug efficacy nor to evaluate complex research questions [35,36,37]. Translating results from murine models to humans remains elusive due to the existence of several key differences between the two systems [38,39], including the anatomy and function of the mouse and human intestinal tract, immune system and interaction with pathogens [40], gut microbiota composition, feeding pattern, genetic background. Besides, since crosstalk between gut microbiota and the host is host-specific, observations in mouse models might not be translatable to humans.
Although the gastrointestinal tracts in both humans and mice are composed of anatomically similar organs, the anatomy of the mouse and human intestinal tract also have prominent macroscopic and microscopic differences, which might be shaped by their diverging diets, feeding patterns, body sizes, and metabolic requirements; for example, the average ratio of the intestinal surface area/body surface area differs critically between the two species over different sections of the gut [41,42]. The intestinal tract of mice and humans also differs in histological features: the mouse colon is composed of thin muscularis mucosae with no distinct sub-mucosa, whereas the human colon is covered by a thicker mucosal wall. Another difference is the presence of transverse folds along the length of the colonic mucosa in humans, whereas these folds are limited to the cecum and proximal colon in mice. These differences in colonic micro-compartmentalization and structure might contribute to the creation of diverse ecological micro-niches presenting different microbial communities [43]. There are also several notable differences at the cellular level, e.g., the distribution of mucin-producing goblet cells and the Paneth cells. Paneth cells secrete antimicrobial compounds into the lumen of the small intestine. In mice, these cells are completely absent in the colonic mucosa and are exclusively found in the cecum, whereas they are present in the cecum and proximal colon of humans. Furthermore, mice and humans differ in the amount of defensins (peptides involved in the host defense) secreted by Paneth cells, as well as their storage and secretion [44,45,46]. These differences in the distribution and functioning of both goblet and Paneth cells between the two organisms suggest differences in local immune responses, which might shape the composition of the gut microbiota. In addition to the anatomical and histological differences, the physiology of the intestinal tract of mice and humans also differs, e.g., the overall intestinal transit time in mice is up to ten times as fast as humans. This is compatible with the total metabolic rate, which is approximately seven times higher in mice compared to humans [43]. Another factor to be considered is that mice are coprophagic. Coprophagy contributes to the nutritional value of their diet by ensuring some vitamins and fatty acids that are produced by microbiota in the cecum are not lost via defecation but re-enter the murine intestine to be absorbed [47].
Despite the known differences in various arrays of genetics, intestinal anatomy, gut physiology, enteric immune network, metabolism, dietary behavior, etc. (all of which may also correspond to microbiome differences), few independent studies have reported the features of gut microbiome configurations in different animal species [33]. An important limitation in mouse models of human microbiota studies is the difference in bacterial composition between the two species. In humans, three enterotypes can be identified, while only two can be found in mice [48,49]. Although the phylogenetic makeup of the bacterial communities in both humans and mice seems to be similar at the phylum level, at the species level, many differences are found [50] and 85% of the murine sequences represent species that have not been detected in humans [51].
Host tolerance to microbial infections varies greatly across different species [52,53] and the intestinal microbiome differentially modulates the susceptibility to infectious diseases in different species [54,55]; therefore, it remains a challenge to translate findings obtained from animal models to humans.
Humanization of the mouse microbiome is often used to address these problems. However, microbial species seem to be critically adapted to specific hosts. Human-microbiota-associated mice have low numbers of adaptive and innate intestinal immune cells and reduced antimicrobial peptide expression when compared with mice that harbor a murine microbiota. Importantly, the mouse microbiota is known to confer better protection against Salmonella infection than a human microbiota in mice. These data indicate a highly specific coexistence and mutual interaction between species-associated microbiota and the host immune system, and that humanized mice cannot adequately recapitulate microbiota–host interactions in humans [56].
Traditional cell-based in vitro systems have been extensively used to study intestinal barrier, host–microbiota, and pathogen interactions. Cell cultures, especially those of human origin, have been invaluable for gaining insights into bacterial adhesion/invasion, immune function, bacterial–host interaction mechanisms, and to study the protective effects of probiotics against pathogenic bacteria [57,58,59,60,61,62,63,64]. However, using cells on a 2D monolayer and/or under static non-physiologic condition (e.g., Transwell systems) could severely affect the relevance of the results. In particular, the reliability of traditional static monolayer models may be impaired by the lack of physiological stimuli, such as the biochemical signals from other cells and the extracellular matrix, the physical and structural stimuli from the three-dimensional (3D) microenvironment, and the mechanical stimuli derived from movement (e.g., peristalsis) and the physicochemical fluxes [65]. Other drawbacks of traditionally employed in vitro models are the cancerous and/or nonhuman origin of the cells [66]. Transformed colon carcinoma lines have been very popular and have led to rich insights into the host–microbe relationship and probiotic effects [60,61,62,63,67,68]. However, these transformed cell lines have many limitations, especially from a translational perspective. Many transformed cell lines have defects in innate immune signaling [69], which confound studies regarding the innate immune response to infectious diseases; for example, Caco-2 cells did not show observable innate immune responses in a conventional model of astrovirus infection [70,71].
Another limitation of the above cell models is the lack of mature cell types that can be differentiated from cell lines: the normal intestinal epithelium consists of diverse cell types, including enterocytes, goblet cells, stem cells, enteroendocrine cells, microfold (M) cells, Paneth and tuft cells, that are not accurately represented. On the other hand, cells obtained from nonhuman (animal) tissues are not species-specific, creating concerns about the translatability to humans [72,73,74,75]. Additionally, controlling the multi-species microbial communities and their growth has remained a notable technical challenge in static in vitro models [76].
The high number of therapeutic compounds that fail to translate in clinical trials [37,77,78], coupled with increasing awareness of the ethical and scientific issues surrounding the use of animal models [38,39,79,80], highlights the need and importance for models that are more physiologically relevant to the human body to personalize treatments and better predict patient outcomes.
3. Emerging Technologies and Opportunities for a Human-Based Research Approach
Advances in stem cell technology, microengineering, microfluidics, high-throughput third-generation sequencing techniques, computing power, machine learning (ML), and respective multidisciplinary cooperation has allowed for the development of new technologies and approaches, which were inaccessible until a few years ago. These technologies are beginning to provide more clinically relevant data and hold immense promise for studying complex regulatory networks and crosstalk between the host, gut microbiota, pathogens, and diet in a human-focused and physiologically-relevant setting. They comprise i) human-based multi-omics approaches, including (meta)genomics, (meta)transcriptomics, (meta)proteomics, metabolomics, and epigenomics, which result from global analyses of biological samples by high-throughput analytical approaches and databases; ii) computational models; iii) patient-derived cells, including induced pluripotent stem cells (iPSCs) and their differentiated derivatives, such as organoids; and iv) tissue engineering and advanced in vitro technologies (e.g., organs- or organoids-on-a-chip and microphysiological systems).
3.1. Multi-Omics Approaches and Computational Models
Omics disciplines include genomics, transcriptomics, proteomics, and metabolomics, which refer to the genome, transcriptome, proteome, and metabolome, respectively, of a species, population, or community. “Omics” aims at the collective characterization and quantification of pools of biological molecules that translate into the structure, function, and dynamics of an organism or population. The start of the 21st century was characterized by rapid advances in high-throughput sequencing, high-content- and single-cell technologies, mass spectrometry (MS), bioinformatics, and computational power. NGS techniques, also known as “second-generation sequencing,” which are capable of reading the code of millions of small fragments of DNA in parallel, enabled faster sequencing with increased throughput at falling costs, which allow for the assessment of genes and genomes contained within complex microbial communities. These techniques have entirely changed the perception of the human microbiome and have paved the way for the establishment of metagenomics and metatranscriptomics.
While metagenomics is the study of the genomes in a microbial community of a given sample and constitutes the first step toward studying the microbiome, metatranscriptomics involves sequencing the complete transcriptome of the microbial community and making it possible to infer the functional profile of this community under specific conditions [81]. The emergence of NGS technologies provides information about the composition and function of the entire community and the dynamics occurring between the taxa. NGS has been a source of basic biological and translational surprises, exposing a compelling range of crucial findings. Every human being appears to carry their own individual suite of microbial strains [82,83], which are acquired early in life [84,85]; differ between environments, age, and populations [86,87]; and can be altered by diet [26,88,89]. NGS has also led to the identification and characterization of metabolic and regulatory mechanisms through which hosts and microbes interact with each other to define a healthy or diseased state in the human host [90]. For example, Vázquez-Castellanos et al. [91] assessed functional modifications of HIV-associated microbiota by combining metagenomic and metatranscriptomic analyses. The transcriptionally active microbiota was shown to be well-adapted to the inflamed environment, overexpressing pathways related to resistance to oxidative stress. Furthermore, it has been demonstrated that gut inflammation is maintained by the Gram-negative nature of the HIV-associated microbiota and the under-expression of anti-inflammatory processes, such as short-chain fatty acid biosynthesis.
Meta-omics projects are largely based on short-read technologies for the taxonomic, phylogenetic, and functional evaluation of a gut bacterial community [92,93,94]. Short-read sequencing is cost-effective and accurate; however, challenges in the assembly of short reads has limited our ability to correctly assemble repeated genomic elements and place them into genomic context, failing to profile low abundance community members at the species/strains level.
Each microbial genus in the gut includes several species and strains that may harbor significant differences in their genomes and functional capacities and it has been recognized that strain-level diversity may contribute to discrepancies in genus and species associations with health and disease [95,96]. Our knowledge often relies on a genus- or species-level taxonomic assignments that, although useful, may not be sufficient for a comprehensive understanding of the complex interconnections between the gut microbiome and human health.
In recent years, new technologies that are capable of sequencing longer strands of DNA by reading single DNA molecules have advanced and become more prominent [97]. These technologies, which are also referred to as “third-generation sequencing” or “long-read sequencing,” coupled with the advancement in bioinformatics tools and single-cell sequencing methodologies, can produce genome assemblies of unprecedented quality, even for species with low abundances [94,98,99,100]. The characterization of microbial communities using short-read NGS approaches have revealed important shifts in microbiota associated with debilitating diseases, such as Clostridium difficile infection. However, due to limitations in sequence read length and sequence-biases, genus- and species-level classifications have been problematic. A comparison of short-read NGS and long-read, third-generation sequencing of samples from patients treated for C. difficile infection revealed similarities in community compositions at the phylum and family levels, but the long-read approach further allowed for species-level characterization, permitting a better understanding of the microbial ecology of this disease. Thus, as sequencing technologies continue to improve, new species-level insights can be gained in the study of complex and clinically-relevant microbial communities, as well as the relationships between gut microbiota and infectious diseases, to evaluate the effects of probiotics supplementation [101,102,103,104,105].
Metabolomics has emerged as a technique that focuses on defining the functional status of host– microbial relationships in biological specimens, providing a detailed picture of functionality and a better understanding of physiology through the identification of the metabolites produced within the gut by the host and/or the microbial community. Most microbial metabolic processes generate by-products that influence the microbiota as well as the host homeostasis. Many studies have reported that the intestinal metabolites (both from the host and microbiota) regulate pathogen infections through the use of genome-based analysis of bacteria and high-throughput metabolomics [106,107,108]. Traditionally, nuclear magnetic resonance (NMR), proton nuclear magnetic resonance (1H NMR) spectroscopy, and mass spectrometry (MS) have been the primary tools used in metabolomics analyses [109,110]. Advancements in these technologies, including matrix-assisted laser desorption ionization time of flight (MALDI-TOF), secondary ion mass spectrometry (SIMS), Fourier transform ion cyclotron resonance MS, in combination with the development of single-cell techniques, have improved the throughput and accuracy of metabolomics [111,112,113], which has opened up new avenues for the study of the dynamics of pathogen–microbiome–nutrients interactions and the metabolites involved in this process in a human-based setting. (Meta-)proteomics involves the high-throughput characterization of the entire constituent profile of microbial/host proteins within a biofluid or tissue sample. An important utility of metaproteomic studies is that the identification of the protein content of a sample, coupled with insight into their interactions, abundances, and modifications, gives direct information about the true functional activity of the gut microbiota. A range of different methodologies may be used for proteomic studies, including MS, NMR, microarray-based technologies, and the most recent single-cell and ultrasensitive protein analyses [114,115].
Practically, “omics” profiling has already been applied to study host–microbiota–pathogens relationships by employing a range of different human-derived sample types and experimental models (Table 1). It can be used to characterize the genome, transcriptome, proteome, or metabolome in samples from in vitro experiments, including complex gut models such as organs-on-a-chip and microphysiological systems [116].
Table 1.
Examples of the applications of human-based (meta)omics and multi-omics approaches to investigate host–microbiota–pathogen–nutrition relationships.
| Type of Omic Approach | Type of Model | Description/Major Findings | References |
|---|---|---|---|
| Multi-omics | hGoC | Identification of specific human microbiome metabolites modulating EHEC pathogenesis | [116] |
| Metagenomics/Metabolomics | HITChip/M-SHIME | In-depth microbial characterization of luminal and mucosal gut microbes | [122] |
| Metagenomics/Metabolomics | Human subjects/stool samples | Characterization of the gut microbiome of individuals living in the Amazon showed striking differences in the microbial communities from these two types of populations | [123] |
| Metabolomics | In vitro SIHUMIx | Analysis of the impact of functional food on the microbic metabolic pathways | [124] |
| Multi-omics | Human subjects/stool and plasma samples | Investigation of the interplay between the human gut microbiome and the host metabolism | [125] |
| Meta-proteomics | Human subjects/stool samples | Extensive microbiome comparison between infants and the identification of previously undetected microbial functional categories | [126] |
| Transcriptomics/Metatranscriptomics | hiOs | Exploration of the interaction of Salmonella enterica serovar Typhimurium with hiOs; clear changes in transcriptional signatures were detected | [127] |
Abbreviations: hGoC, human gut-on-a-chip; EHEC, enterohemorrhagic Escherichia coli; HITChip, human intestinal tract chip; M-SHIME, mucosal-simulator of a human intestinal microbial ecosystem; SIHUMIx, simplified intestinal human microbiota; HiOs, human intestinal organoids.
Validation and quantification of the data and insights from “omics” technologies depend on the computational sciences and scientists being able to bridge the biosciences and bioinformatics. To date, with advances in bioinformatics, researchers have access to a variety of computational methods to analyze “omics” data [117] and the combination of different “omic” layers, leading to a multi-omic approach [118]. Integrated multi-omics analysis has already been successfully carried out for the in-depth characterization of the human microbiome’s response to a specific nutritional intervention or environmental stimuli in both healthy and diseased conditions [119,120,121]. Some “omics” and multi-omics studies on host–microbiome interactions regarding pathogens/nutrition are mentioned in Table 1.
Nutrigenomics, which integrates different omics approaches to seek and explain the existing reciprocal interactions between genes and nutrients at the molecular level, has the potential to identify genetic predictors of disease-relevant responses to diet, which has wide appeal in the context of personalized nutrition [128,129]. Nutrigenomics has already been implemented to identify cellular and molecular targets to develop a novel hypothesis regarding the functional role of nutrition and microbiota in modulating intestinal inflammatory diseases [130]. A similar approach could be possible for other diseases, including infections.
These multi-omics approaches lead to unprecedented opportunities to comprehensively and accurately characterize microbial communities and their interactions with their environments and hosts. An important goal of multi-omics data integration is to generate and validate microbiome metabolic networks/models. Though this is still challenging, promising steps forward have been made, including the generation of over 700 genome-scale metabolic reconstructions [131], the development of tools for microbiome metabolic modeling/prediction [132,133], and the generation of interspecies metabolic network databases [134].
Information deriving from “omics” techniques may also provide a basis for employing machine learning (ML). ML consists of a series of algorithms that after being “trained,” can predict outcomes and future states in specific areas of the research arena, such as shifts in the microbiome structure and function as a result of certain factors (e.g., health vs. disease status, diet, drugs, etc.) [135,136]. ML applications in multi-omics datasets were scrutinized in a series of recent reviews [137,138,139,140].
As computational models are fairly reliant on the data they are trained on or are called upon to analyze, no model, regardless of its sophistication, can generate a useful analysis from low-quality data. Since the results returned by computational models are based exclusively on the input data and represent existing knowledge, these models are valid within the same context of that knowledge and their performance will be reduced if they are not regularly updated using novel, emerging, human-relevant data. Likewise, the development and adaptation of integrated software platforms are central to the efficient and effective use of data and for predictive computational modeling [141].
3.2. Human Intestinal Organoids
Organoids are stem-cell-derived and self-organized 3D clusters of organ-specific cells that incorporate many of the physiologically relevant features of the in vivo tissue, including the functionality, as well as molecular and cellular heterogeneity, of the originating organ [142]. Human organoids are suitable models for studying the mechanisms of morphogenesis and are promising platforms for disease modeling and drug screening [143]. Over the past decade, researchers have developed protocols to differentiate human stem cells into multiple lineages, obtaining several organoids, including (but not limited to) pancreas [144], liver [145], stomach [146], and intestine [147].
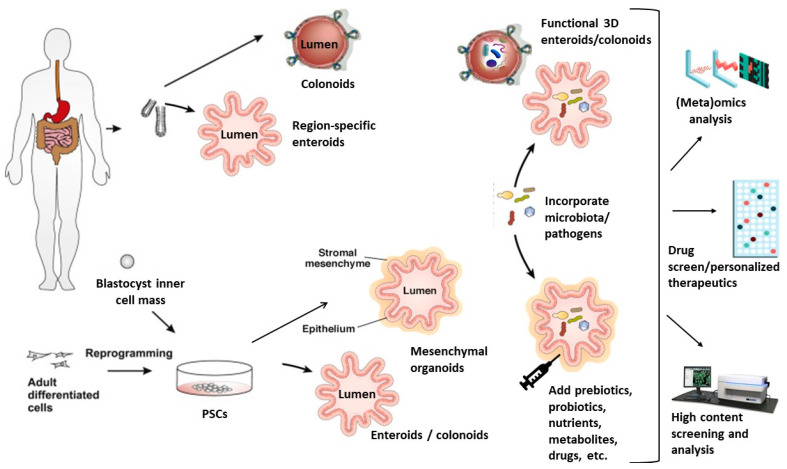
Tissue-derived stem cells isolated from human intestinal biopsies or surgical specimens can be differentiated into epithelial organoids, termed “enteroids” (when derived from the small intestine) or “colonoids” (when derived from the colon) [148]. Human embryonic stem cells (hESCs) or induced pluripotent stem cells (hiPSCs) can be directed to give rise to both intestinal organoids associated with mesenchymal cell types (the so-called “mini-guts”) and epithelial organoids [147,149,150,151,152]. In this review, we use “human intestinal organoids” (hiOs) as a generic term to indicate both epithelial organoids (enteroids and colonoids) and “mini-guts” (Figure 1).
Figure 1.
Human intestinal epithelial organoids (hiOs) generation and examples of their applications in the study of the relations between nutrition, infectious diseases, and microbiota. PSCs: Pluripotent stem cells.
hiOs are capable of undergoing self-renewal and self-organization for an extended period and replicate many of the physiologically relevant features of the in vivo human intestinal tissue [153]. These 3D intestinal-like structures imitate the villus and crypt microarchitecture, which is a polarized epithelial layer surrounding a functional lumen, as well as the presence of all of the cell types of the intestinal epithelium, including enterocytes, goblet, tuft, Paneth, M, and enteroendocrine cells, as well as intestinal stem cells. These cells are present in proportions and a relative spatial arrangement that imitates what is observed in vivo. Since hiOs functionally mimic normal human gastrointestinal tract physiology and pathophysiology [151], they represent an effective platform to study human gastrointestinal functions and diseases [154] and are already being successfully employed to model epithelial barrier function [155,156], nutrient transport physiology during digestion [157], celiac disease [158], inflammatory bowel disease [159], and cancer [160,161,162,163]. hiOs provide unprecedented opportunities for the generation of in vitro systems with a sufficient level of complexity to model physiological and pathological diet–microbiome–host conditions [164,165] and pathogen–host interactions [72,155,166,167,168,169,170,171,172,173,174,175]. Human microbiota suspensions, pathogenic organisms, and/or nutrients can indeed be microinjected into the pseudo-lumen of organoids, which can then be recovered and assayed for microbial composition, microbial transcriptomics, metabolites, and host gene expression profiles (Figure 1). Furthermore, the addition of automated injection and harvesting systems may provide a platform for high-throughput microbiome studies [176]. hiOs have already been successfully utilized to explore host–bacterial symbiotic interactions and human viral [167,175,177,178,179], bacterial [127,155,171,180,181], and protozoan [182,183] pathogens. Human enteroids have made it possible to study host–microbe interactions that were challenging because of species-specificity that led to tolerance to infection, poor infection, and/or replication rates in animal models, including human rotavirus [167] and enterohemorrhagic Escherichia coli (EHEC) [171,184].
Epithelial organoids (enteroids and colonoids) have been employed to model the effects of diet and nutrients on intestinal growth and development, ion and nutrient transport, secretory and absorption functions, the intestinal barrier, and location-specific functions of the intestine [165]. hiOs responses to gut-microbiota metabolites and microbes could provide novel insights into the mechanisms by which those agents may prevent or trigger diseases, including infections, significantly extending our knowledge of diet–microbiome–host interactions [164].
I is noteworthy that human enteroids are being used successfully to study the new severe acute respiratory syndrome coronaviruses (SARS-CoV and SARS-CoV-2). Since clinical evidence suggests that the intestine may present another viral target organ, in their very recent work, Lamers et al. have demonstrated that SARS-CoV and SARS-CoV-2 can readily infect human enterocytes and that the intestinal epithelium supports SARS-CoV-2 replication. In addition, gene expression studies on enteroids have shown that interferon-stimulated genes become activated [185]. This demonstrated that hiOs serve as an experimental model for coronavirus infection and biology. Another aspect is the potential evidence on the pathophysiology of coronavirus infections modulated through the gut microbiome [186]. hiOs approaches could soon take these aspects into account by integrating, e.g., the human microbiota and/or accounting for the effects of probiotics. The treatment of a human colonoid model with the probiotic Lactobacillus reuteri or its antimicrobial metabolite, reuterin, before or after challenge with S. typhimurium, reduced the adhesion, invasion, and intracellular survival of this pathogen compared to findings for untreated cells [187]. Since the isolation of fresh crypts that contain multipotent adult stem cells is invasive and there is limited availability of live primary human cells and tissues, organoids derived from mice have been often employed [165]. However, recent advancements in culturing protocols allow for generating both epithelial-mesenchymal organoids and epithelial region-specific intestinal organoids (enteroids or colonoids) from hiPSCs [150,152,188]. hiPSC-derived organoids offer tremendous advantages if hiPSCs are harvested non-invasively and obviate the ethical dilemmas surrounding the use of hESCs. hiPSCs represent a limitless source of nontransformed patient-specific somatic cells that can be used to study the nutrient–microbiome axis in gastrointestinal homeostasis and infectious diseases in vitro in a human-relevant setting. hiOs cultures can be provided by many donors. Since they retain the genetic and biological properties of the donors, they can lead to the discovery of host-specific factors that affect susceptibility to infectious diseases and result in personalized approaches to treat individuals [173].
Stem cell 3D derived organoids overcome and surpass the limitations of the traditional 2D colonic adenocarcinoma cell lines models while providing the potential to perform mechanistic studies within a “human model” system with the same scrutiny and depth of analysis that is customary for research with nonhuman model organisms [189].
Despite the significant advances in organoid technologies and their numerous advantages over other systems, there still exist several challenges to overcome. In 3D culture, microbiological research of organoids and enteroids is very difficult. The presence of an enclosed lumen is non-physiological since secreted material from host-cells and bacteria accumulates within this central space instead of being removed by peristalsis and luminal flow. In addition, the inaccessibility of the apical cell surface makes the use of organoids experimentally challenging for transport studies, as well as exposure to living commensal microbiota or pathogens for more than approximately one day in culture. Finally, organoid cultures lack a tissue–tissue interface, mechanical forces (fluid flow and peristalsis-like movement), immune cells, and a vascular compartment, which are all crucial factors in host–microbiota homeostasis, nutrient transport, and infectious disease development.
However, there have been large steps forward in hiOs technology, including the ability to polarize the organoid cells on a 2D monolayer [172,178,190], as well as the opportunity to co-culture them with immune cell lineages, vasculature, neurons, and other cell types to make a more physiologically relevant model system [191,192]. Recent successes in the co-culture of human enteroids and macrophages/neutrophils suggest the potential for even more robust modeling of the interaction between the host and commensal or pathogenic bacteria [193]. hiOs cultured with human neutrophils imitate innate cellular responses when exposed to Shiga toxin-producing E. coli, including intraepithelial macrophage projections, phagocytosis, cyotokine response, loss of epithelial integrity, and the activation of stress responses that involve oxygen species [171].
In addition, progress is being made in improving the capability of organoid culture to fully mimic in vivo responses by incorporating human organoids in a millifluidic system [194] or by combining the organoid technology with a microfluidic chip system [195,196].
Although there is still significant room to improve regarding modeling the true physiology of intestinal function, hiOs have already enabled new avenues of research into host–microbial interaction and are promising tools to study the nutrients–gut microbiota–infections triangle.
3.3. Organs-On-a-Chip/Microphysiological Systems
Organs-on-a-chip (OoCs) are microfluidic cell culture devices in which (human) cells are cultured in engineered microenvironments that imitate the essential aspects of multicellular architectures, dynamic, tissue-tissue interfaces, physicochemical microenvironments, flow, and gradients found in the human body [197]. A wide range of tissues and organs have been modeled, including heart [198], kidney [199], brain [200], liver [201], blood vessels [202], lymphoid follicle [203], and intestine [204]. For this review, we discuss the use of OoCs to study intestinal dynamics with a specific focus on the gut microbiome and infectious diseases. OoCs models of the human intestine have been developed and successfully employed to study intestinal physiology and pathophysiology. Over the past few years, human intestine-on-a-chip models have been engineered with increasing complexity that also include neighboring channels lined by human microvascular endothelium, commensal microbiota, pathogenic bacteria, immune cells, and some even allow for the application of cyclic mechanical forces that mimic peristalsis-like deformations experienced by the intestine in vivo [205]. Peristalsis-like mechanical forces induce epithelial cells to spontaneously form polarized 3D villus-like structures that contain all the specialized differentiated intestinal epithelial cells (including adsorptive enterocytes, mucus-producing goblet, Paneth, and enteroendocrine cells) [195,206,207,208]. The resulting epithelial layer shows the basic functional properties, such as mucus production, high barrier resistance, activity of the brush border, drug-metabolizing enzymes, and high efficiency in nutrient uptake. These features allow for studies focusing on nutrient uptake and digestion, barrier function, and drug metabolism [195,206,207,208,209], as well as for co-cultures with human commensal bacteria for extended periods (up to weeks) [206,210]. Human intestine-on-a-chip or human gut-on-a-chip (hGoC) have already been used to model invasion or infection by pathogenic entero-invasive E. coli strains into the commensal bacterial biofilm or the host intestinal endothelium [210,211], as well as to model Staphylococcus aureus, Pseudomonas aeruginosa, and Salmonella typhimurium infections [212,213]. Grassart et al. [214] have successfully modeled the impact of flow and peristalsis on the human pathogen Shigella within a 3D colonic epithelium using hGoC technology. The authors observed that Shigella invasion accurately imitates what has been previously reported from clinical data. Shigella was also shown to leverage the intestinal microenvironment by taking advantage of the microarchitecture and mechanical forces to efficiently invade the intestine. This study is the proof-of-concept that we can use hGoCs to gain insights into infection mechanisms of human-restricted pathogens and that such models could provide a viable alternative to animals, particularly where species differences can preclude accurate extrapolation to humans. In addition, some lactic acid bacteria have been used to model the presence or biochemical contribution of probiotics in human microbiota using hGoC models [206,212,215]. Notably, it has been shown that the probiotic Lactobacillus rhamnosus GG improved intestinal barrier function when co-cultured in the lumen of the intestinal epithelial channel of the hGoC [206], and that the VSL#3® probiotic formulation suppressed villus blunting and the loss of barrier function induced by infection with pathogenic E. coli in this model [210]. Thus, the hGoC approach may also be useful for the discovery of new microbiome-based therapeutics, such as genetically engineered commensal bacteria [216]. Furthermore, by integrating circulating and organ-specific human immune cells into hGoCs, they might be useful for the in vitro development of new mucosal vaccines [217]. Villenave et al. [218] demonstrated that human Enterovirus infection, replication, and infectious virus production can be analyzed in vitro in a hGoC microfluidic device. Since the analysis of Enterovirus infection is difficult in animals because they express different virus receptors than humans, hGoCs may provide suitable in vitro models for enteric virus infection and for investigating the mechanisms of enteroviruses’ pathogenesis.
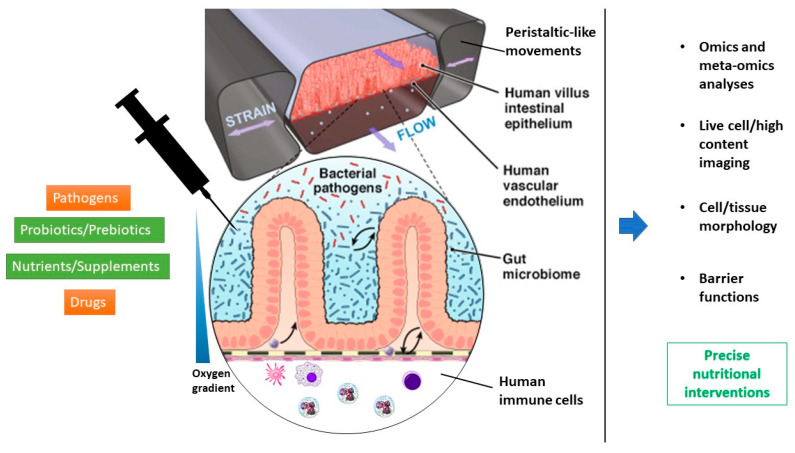
OoC technology integrated with multi-omics approaches has allowed for the identification of specific human microbiome metabolites modulating enterohemorrhagic Escherichia coli (EHEC) pathogenesis. It has been shown that epithelial injury was greater when exposed to metabolites derived from the human gut microbiome compared to those derived from mice. The active human microbiome metabolites preferentially induced the expression of flagellin, a bacterial protein associated with the motility of EHEC and increased epithelial damage. The authors concluded that the decreased tolerance to infection observed in humans versus other species might be due in part to the presence of compounds produced by the human intestinal microbiome that actively promote bacterial pathogenicity [116]. A noticeable advantage of microfluidic OoC is the ability to integrate analytical biosensors into the culture system, thus combining living cells and sensors for the non-invasive detection of cellular physiological parameters, including O2, pH, protein and metabolite secretion, and cell layer barrier and cell–cell interaction, via fluorescence and confocal microscopy [219]. Jalili-Firoozinezhad and colleagues set out to develop an experimental intestine-on-a-chip system that permits the control and real-time assessment of physiologically relevant oxygen gradients and can support dynamic interactions between living, mucus-producing human intestinal epithelial cells and a complex community of living human aerobic and anaerobic commensal gut microbes. When compared to traditional aerobic coculture conditions, the establishment of a transluminal hypoxia gradient in the chip increased intestinal barrier function and sustained a physiologically relevant level of microbial diversity, containing over 200 unique active taxonomic units from 11 diverse genera and an abundance of obligate anaerobic bacteria, with ratios of Firmicutes and Bacteroidetes similar to those observed in human intestines [220]. This model may serve as a human-relevant in vitro discovery tool for the development of microbiome-related therapeutics, probiotics, and nutraceuticals, as well as for examining the biological interactions of food products, the microbiome, and infectious diseases. Figure 2 shows a schematic representation of a hGoC.
Figure 2.
Scheme of a human gut-on-a-chip and its potential applications in the study of the interaction between the microbiome, infectious diseases, and nutrition.
Integrating patient-specific or hiPSC-derived intestinal 3D cell constructs, local immune cells, pathogens, and the commensal microbiota into OoCs might create a highly defined and controllable translation platform that should accelerate the discovery of new drugs and/or personalized precision probiotic therapeutics. Maurer et al. have established a 3D immunocompetent intestine-on-a-chip model as an in vitro platform for functional and microbial interaction studies [221]. This model allows for a detailed characterization of the immune response, microbial pathogenicity mechanisms, and quantification of cellular dysfunction attributed to alterations in the microbial composition. The authors examined the microbial interaction between probiotic Lactobacillus rhamnosus and the opportunistic pathogen Candida albicans, showing that pre-colonization of the intestinal lumen of the model by L. rhamnosus reduces C.-albicans-induced tissue damage, lowers its translocation, and limits the fungal burden. Utilizing a similar model, Graf et al. established an in vitro system that can be used to experimentally dissect the commensal-like interactions of C. albicans with a bacterial microbiota and the host epithelial barrier, discovering fungal shedding as a novel mechanism by which probiotics contribute to the protection of epithelial surfaces [5]. While an OoC construct is designed to imitate the structure and function of a single human organ or organ region, microphysiological systems (MPSs) consist of interacting OoCs or tissue-engineered, 3D organ constructs that use human cells. OoCs coupled together to create a MPS provide the ability to analyze multiorgan interactions and offer the opportunity of providing an unprecedented physiological accuracy for disease modeling and drug discovery in vitro, allowing for investigating the complex physiological and pathophysiological responses of cells and tissues at a multi-organ level [222]. MPSs have been developed for several organ systems, including liver–skin–intestine–kidney [223], gut–microbiome–brain [224], and gut–liver [225]. The interaction between the liver and the gut microbiota, with their reciprocal influence on biosynthesis pathways and the integrity of the intestinal epithelial barrier, has been documented. Dysbiosis or liver disorders lead to intestinal epithelial barrier dysfunction, altering membrane permeability [226]. The potential of MPSs to accurately model drug uptake and metabolism in the human body has recently been shown in the context of metabolites produced by the microbiota. Vernetti et al. [227] have demonstrated that an intestine–liver–kidney-on-a-chip system coupled with an intact blood–brain barrier/neurovascular unit adequately modeled in vivo trimethylamine (a by-product of the microbiome) metabolism. Specifically, trimethylamine that was microinjected into the intestinal compartment was found in the basolateral media and was subsequently metabolized to trimethylamine N-oxide by the liver module and then secreted into the lumen of the kidney module, which occurs in vivo. The study also revealed a novel finding that trimethylamine N-oxide crosses the blood–brain barrier, highlighting the potential of such systems to improve our understanding of human pathophysiology.
Integrating gut–liver organ-on-a-chip systems with pathobionts/pathogens and immune cells might allow for future study into the interaction between microbiota, pathogens, and the effect of nutrients in a more complex multi-organ context.
4. Discussion
While traditional animal and cell culture models of gut microbiota and infectious diseases have been useful for elucidating some of the mechanisms underlying microbiota and nutrition relationships, the use of non-human (animal) models to mimic the complex interplay between host–microbiota–pathogens and the effects of nutritional pro- and prebiotic interventions may potentially be misleading in light of the numerous interspecies differences. In addition, although some problems of external validity can be overcome by improving the animal models, the problem of species differences can never be overcome and will always undermine external validity and the reliable translation of preclinical findings to humans, emphasizing the need to focus on human-relevant research methods and technologies [37,228]
Here we described some new technologies, tools, and approaches that could be employed in an integrated human-focused framework that is suitable for investigating the interaction between gut microbiota, nutrients, and infectious diseases. To our knowledge, this is the first review discussing the applicability of human-based methods and models to microbiome research related to infectious diseases and nutrition.
In recent years, the shift toward a new human-biology-focused paradigm has been broadly encouraged in toxicology and regulatory testing [229], but also in other research fields, including immunology, human infectious diseases, and nutritional research [7,80,230,231,232,233,234,235,236,237,238,239,240,241].
The envisioned human-based framework will not only increase the human relevance and translatability but will also contribute to the reduction and/or replacement of animals conventionally used in microbiota and infectious diseases research, which is very important considering the rising concerns for the ethical justifications of the use of animals in research. The EU legislation governing animal experimentation (European Directive 2010/63/EU on the protection of animals used for scientific purposes) [242], as well as the U.S. regulatory and research agencies in both environmental and medical arenas [243], actively support animal replacement/reduction in accordance with the 3R principle (animal replacement, reduction, refinement).
Our ability to generate 3D engineered tissue models from human embryonic stem cells or induced pluripotent stem cells continues to make rapid progress. We may soon be able to assemble them into a miniature gastrointestinal system in a dish or even on a chip [244]. This would facilitate the manipulation and analysis of digestion in a reproducible, accurate, precise, and large-scale manner, and allow for exploration of the individual genetic and microbial diversity regarding pathogens and the effects of probiotics or nutrients.
The microbiota responds dynamically to nutritional cues, it changes with age, and has close interactions with its host through the immune system and metabolites. It is also extremely variable between individuals and societies, which represents the main source of metabolic unpredictability [245]. Although the in vitro modeling of the gut microbiome is still in the early stages, and some bacterial strains are not easily cultured in the lab, the ever-expanding fine-scale knowledge of microbiota functions and innovative culture systems is very promising [100,204,220]. Experimenting with this system will teach us about human–microbiota interactions and explore how metabolic variability relates to microbiome composition.
Since the stomach, duodenum, jejunum, ileum, and colon have different digestive functions and encounter food at different digestive stages, in vitro systems should be designed such that stem-cell-derived intestinal segments form separate chambers that pass food and chyme at diverse stages of digestion through the “lumen” of the system. Tailored culture conditions and chip mechanics will be essential to support the introduction of food and provide appropriate conditions for gut microbiota to flourish [244]. The various parameters could be well controlled to allow for collecting high quality, reproducible data. For example, it will be possible to sample fluids containing absorbed metabolites from different positions in the in vitro model and understand how they affect or are affected by gut microbiota, pathogens, probiotics, a sudden change in diet, antibiotics, or fasting and feeding. “Biopsies” or fluid samples from the in vitro system could teach us about the proteomic and transcriptomic profile in response to different cues, with a high temporal resolution. The ability to genetically modify stem cells that are employed to make the tissues and organs will be extremely valuable in defining which genes function in which cells to affect the phenotype. Advances in gene editing using TALEN (Transcription Activator-like Effector Nucleases) and CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technologies allow for testing both the loss and gain of function for specific genes and tissues [246]. Building reliable in vitro models capable of mimicking digestion and human gastrointestinal physiopathology is a monumental task demanding the collaborative efforts of labs from several fields in biology, chemistry, and engineering [247]. Some crucial parts of the system are missing and incorporation of the stem-cell-derived tissues in a bioengineered system will be challenging. However, considering the advancement in the last decade in stem cell and bioengineering arenas, there is good reason to believe that creating a human-biology-based and human-relevant model of gastrointestinal tract suitable for nutritional studies is possible. Moreover, it is important to notice that these devices do not attempt to recreate the highly complex composition and dynamics of the human gastrointestinal tract and the gut microbiota; rather, they aim to recapitulate key features of human physiology.
It has to be said that in the proposed strategic framework, the use of patient-derived cellular models, such as intestinal organoids or gut-on-a-chip, and the application of (meta)omics readouts while aiming for human relevance would still represent the lower level/scale of (“wet”) lab research. Consequently, wide-scale computational approaches, together with large-scale epidemiological data sets represent the crucial tools required to produce a higher level/scale and to establish systemic correlations between signaling pathways, (meta)-omics perturbations, humans heterogeneity, and the effect of diet and/or nutritional interventions on infectious diseases development and outcomes. The strategy should involve large, adequately powered international studies that recruit patients and controls to collect clinical data, detailed dietary assessments, host genetics, immune phenotyping, and multi-omic gut-microbiome markers. The international approach would enable the inclusion of populations from different regions with different backgrounds, various dietary patterns, and environmental exposures. This broad and collaborative approach is essential for unraveling the determinants of clinical outcomes of infectious diseases and for designing targeted therapeutic and preventative measures. The effects of high fiber, freshly fermented, and diverse foods or probiotics should also be examined as preventative and mitigating measures.
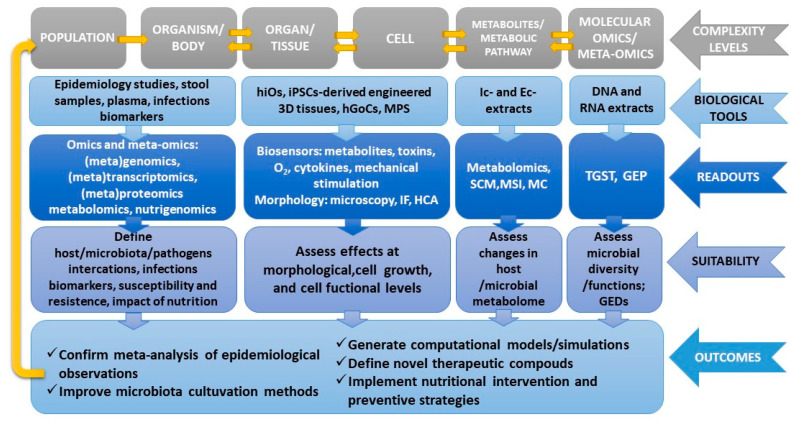
The feasibility of the envisioned human-based strategy necessarily requires the establishment of an integrated, collaborative strategy to investigate the relationship between nutrition, microbiota, and infectious diseases at multiple levels of complexity (from gene expression to protein, cells, and tissues/organs at the individual and population levels) (Figure 3).
Figure 3.
Overview of the novel available tools and readouts applicable to study the links between nutrition, infectious diseases, and microbiota in a human-relevant setting that accounts for multiple levels of complexity, from the molecular to the population level. MPS, microphysiological systems; iPSCs, induced pluripotent stem cells; IF, immunofluorescence; HCA, high-content analysis; MS, mass spectrometry; GEP, gene expression profiling; Ic and Ec, intracellular and extracellular; GEDs, gene expression dysregulations; MC, mass cytometry; MSI, high-resolution mass spectrometry imaging; SCM, Single-Cell Metabolomics; TGST, third-generation sequencing technologies; hiOs, human intestinal organoids; hGoCs, human gut-on-a-chip.
5. Conclusions
Advanced human-relevant 3D cellular models, high-throughput (“omics” and meta-omics) readouts, and computational models, together with data obtained from the meta-analysis of epidemiological and interventional studies, are among the ideal tools used to investigate the complex relations between nutrition, microbiota, and infectious diseases in a human-biology-based milieu, as well as to develop new microbiome-related therapeutics or to implement personalized nutritional interventions.
Although most of the methodologies and approaches described in this review are still in their infancy, they are already yielding meaningful human-relevant data. In our opinion, the development and application of these approaches should be encouraged, while funding for research focusing on human-centered models, rather than “improved” animal models, should be prioritized.
Acknowledgments
Tamara Y. Forbes-Hernández was supported by a “Juan de la Cierva-Formación” post-doctoral contract.
Author Contributions
Conceptualization: M.C. and T.Y.F.-H.; writing—original draft: M.C. and T.Y.F.-H.; visualization: R.C.I., R.R. and M.E.Z.; writing—review and editing: F.G. and M.B.; supervision: F.G. and M.B.; project administration: M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Krawinkel M.B. Interaction of nutrition and infections globally: An overview. Ann. Nutr. Metab. 2012;61:39–45. doi: 10.1159/000345162. [DOI] [PubMed] [Google Scholar]
- 2.Evans J.M., Morris L.S., Marchesi J.R. The gut microbiome: The role of a virtual organ in the endocrinology of the host. J. Endocrinol. 2013;218:R37–R47. doi: 10.1530/JOE-13-0131. [DOI] [PubMed] [Google Scholar]
- 3.Sharma A.K., Jaiswal S.K., Chaudhary N., Sharma V.K. A novel approach for the prediction of species-specific biotransformation of xenobiotic/drug molecules by the human gut microbiota. Sci. Rep. 2017;7:9751. doi: 10.1038/s41598-017-10203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sjögren Y.M., Tomicic S., Lundberg A., Böttcher M.F., Björkstén B., Sverremark-Ekström E., Jenmalm M.C. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2009;39:1842–1851. doi: 10.1111/j.1365-2222.2009.03326.x. [DOI] [PubMed] [Google Scholar]
- 5.Graf K., Last A., Gratz R., Allert S., Linde S., Westermann M., Gröger M., Mosig A.S., Gresnigt M.S., Hube B. Keeping Candida commensal: How lactobacilli antagonize pathogenicity of Candida albicans in an in vitro gut model. Dis. Model. Mech. 2019;12 doi: 10.1242/dmm.039719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pistollato F., Sumalla Cano S., Elio I., Masias Vergara M., Giampieri F., Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016;74:624–634. doi: 10.1093/nutrit/nuw023. [DOI] [PubMed] [Google Scholar]
- 8.Bekkering P., Jafri I., van Overveld F.J., Rijkers G.T. The intricate association between gut microbiota and development of type 1, type 2 and type 3 diabetes. Expert Rev. Clin. Immunol. 2013;9:1031–1041. doi: 10.1586/1744666X.2013.848793. [DOI] [PubMed] [Google Scholar]
- 9.Esteve E., Ricart W., Fernández-Real J.-M. Gut microbiota interactions with obesity, insulin resistance and type 2 diabetes: Did gut microbiote co-evolve with insulin resistance? Curr. Opin. Clin. Nutr. Metab. Care. 2011;14:483–490. doi: 10.1097/MCO.0b013e328348c06d. [DOI] [PubMed] [Google Scholar]
- 10.Horta-Baas G., Romero-Figueroa M.D.S., Montiel-Jarquín A.J., Pizano-Zárate M.L., García-Mena J., Ramírez-Durán N. Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. J. Immunol. Res. 2017;2017:4835189. doi: 10.1155/2017/4835189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuo T., Ng S.C. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front. Microbiol. 2018;9:2247. doi: 10.3389/fmicb.2018.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Nood E., Vrieze A., Nieuwdorp M., Fuentes S., Zoetendal E.G., de Vos W.M., Visser C.E., Kuijper E.J., Bartelsman J.F., Tijssen J.G., et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 13.Yooseph S., Kirkness E.F., Tran T.M., Harkins D.M., Jones M.B., Torralba M.G., O’Connell E., Nutman T.B., Doumbo S., Doumbo O.K., et al. Stool microbiota composition is associated with the prospective risk of Plasmodium falciparum infection. BMC Genom. 2015;16:631. doi: 10.1186/s12864-015-1819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libertucci J., Young V.B. The role of the microbiota in infectious diseases. Nat. Microbiol. 2019;4:35–45. doi: 10.1038/s41564-018-0278-4. [DOI] [PubMed] [Google Scholar]
- 15.Braun T., Di Segni A., BenShoshan M., Asaf R., Squires J.E., Farage Barhom S., Glick Saar E., Cesarkas K., Smollan G., Weiss B., et al. Fecal microbial characterization of hospitalized patients with suspected infectious diarrhea shows significant dysbiosis. Sci. Rep. 2017;7:1088. doi: 10.1038/s41598-017-01217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stecher B. The Roles of Inflammation, Nutrient Availability and the Commensal Microbiota in Enteric Pathogen Infection. Microbiol. Spectr. 2015;3 doi: 10.1128/microbiolspec.MBP-0008-2014. [DOI] [PubMed] [Google Scholar]
- 17.Kampmann C., Dicksved J., Engstrand L., Rautelin H. Composition of human faecal microbiota in resistance to Campylobacter infection. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016;22:61.e61–61.e68. doi: 10.1016/j.cmi.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y., O’Riordan M.X.D. Regulation of bacterial pathogenesis by intestinal short-chain Fatty acids. Adv. Appl. Microbiol. 2013;85:93–118. doi: 10.1016/B978-0-12-407672-3.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kau A.L., Ahern P.P., Griffin N.W., Goodman A.L., Gordon J.I. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolodziejczyk A.A., Zheng D., Elinav E. Diet–microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019;17:742–753. doi: 10.1038/s41579-019-0256-8. [DOI] [PubMed] [Google Scholar]
- 21.Preidis G.A., Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: Gastroenterology enters the metagenomics era. Gastroenterology. 2009;136:2015–2031. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leeming E.R., Johnson A.J., Spector T.D., Le Roy C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients. 2019;11:2862. doi: 10.3390/nu11122862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zmora N., Suez J., Elinav E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019;16:35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 24.Markowiak P., Śliżewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segre J.A. Microbial growth dynamics and human disease. Science. 2015;349:1058. doi: 10.1126/science.aad0781. [DOI] [PubMed] [Google Scholar]
- 26.David L.A., Materna A.C., Friedman J., Campos-Baptista M.I., Blackburn M.C., Perrotta A., Erdman S.E., Alm E.J. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15:R89. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bintsis T. Foodborne pathogens. Aims Microbiol. 2017;3:529–563. doi: 10.3934/microbiol.2017.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang P., Dong J., Bai L., Qiu Z. [Clinical analysis of 53 patients with Clostridium botulinum food poisoning] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017;29:459–464. doi: 10.3760/cma.j.issn.2095-4352.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Antunes P., Mourão J., Campos J., Peixe L. Salmonellosis: The role of poultry meat. Clin. Microbiol. Infect. 2016;22:110–121. doi: 10.1016/j.cmi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Dadar M., Shahali Y., Whatmore A.M. Human brucellosis caused by raw dairy products: A review on the occurrence, major risk factors and prevention. Int. J. Food Microbiol. 2019;292:39–47. doi: 10.1016/j.ijfoodmicro.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Bandera A., De Benedetto I., Bozzi G., Gori A. Altered gut microbiome composition in HIV infection: Causes, effects and potential intervention. Curr. Opin. HIV Aids. 2018;13:73–80. doi: 10.1097/COH.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 32.Fois C.A.M., Le T.Y.L., Schindeler A., Naficy S., McClure D.D., Read M.N., Valtchev P., Khademhosseini A., Dehghani F. Models of the Gut for Analyzing the Impact of Food and Drugs. Adv. Healthc. Mater. 2019;8:e1900968. doi: 10.1002/adhm.201900968. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen T.L., Vieira-Silva S., Liston A., Raes J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015;8:1–16. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George N.S., Cheung L., Luthria D.L., Santin M., Dawson H.D., Bhagwat A.A., Smith A.D. Pomegranate peel extract alters the microbiome in mice and dysbiosis caused by Citrobacter rodentium infection. Food Sci. Nutr. 2019;7:2565–2576. doi: 10.1002/fsn3.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greek R., Hansen L.A. Questions regarding the predictive value of one evolved complex adaptive system for a second: Exemplified by the SOD1 mouse. Prog. Biophys. Mol. Biol. 2013;113:231–253. doi: 10.1016/j.pbiomolbio.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Greek R., Shanks N. Complex systems, evolution, and animal models. Stud. Hist. Philos. Biol. Biomed. Sci. 2011;42:542–544. doi: 10.1016/j.shpsc.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Van Norman G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019;4:845–854. doi: 10.1016/j.jacbts.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leist M., Hartung T. Inflammatory findings on species extrapolations: Humans are definitely no 70-kg mice. Arch. Toxicol. 2013;87:563–567. doi: 10.1007/s00204-013-1038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seok J., Warren H.S., Cuenca A.G., Mindrinos M.N., Baker H.V., Xu W., Richards D.R., McDonald-Smith G.P., Gao H., Hennessy L., et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mestas J., Hughes C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004;172:2731. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 41.Treuting P.M., Arends M.J., Dintzis S.M. 12—Lower Gastrointestinal Tract. In: Treuting P.M., Dintzis S.M., Montine K.S., editors. Comparative Anatomy and Histology. 2nd ed. Academic Press; San Diego, CA, USA: 2018. pp. 213–228. [DOI] [Google Scholar]
- 42.Casteleyn C., Rekecki A., Van der Aa A., Simoens P., Van den Broeck W. Surface area assessment of the murine intestinal tract as a prerequisite for oral dose translation from mouse to man. Lab. Anim. 2010;44:176–183. doi: 10.1258/la.2009.009112. [DOI] [PubMed] [Google Scholar]
- 43.Hugenholtz F., de Vos W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell. Mol. Life Sci. CMLS. 2018;75:149–160. doi: 10.1007/s00018-017-2693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunliffe R.N., Rose F.R.A.J., Keyte J., Abberley L., Chan W.C., Mahida Y.R. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut. 2001;48:176. doi: 10.1136/gut.48.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghosh D., Porter E., Shen B., Lee S.K., Wilk D., Drazba J., Yadav S.P., Crabb J.W., Ganz T., Bevins C.L. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat. Immunol. 2002;3:583–590. doi: 10.1038/ni797. [DOI] [PubMed] [Google Scholar]
- 46.Ouellette A.J., Selsted M.E. Paneth cell defensins: Endogenous peptide components of intestinal host defense. FASEB J. 1996;10:1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 47.Sakaguchi E. Digestive strategies of small hindgut fermenters. Anim. Sci. J. 2003;74:327–337. doi: 10.1046/j.1344-3941.2003.00124.x. [DOI] [Google Scholar]
- 48.Hildebrand F., Nguyen T.L., Brinkman B., Yunta R.G., Cauwe B., Vandenabeele P., Liston A., Raes J. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biol. 2013;14:R4. doi: 10.1186/gb-2013-14-1-r4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J., Linnenbrink M., Kunzel S., Fernandes R., Nadeau M.J., Rosenstiel P., Baines J.F. Dietary history contributes to enterotype-like clustering and functional metagenomic content in the intestinal microbiome of wild mice. Proc. Natl. Acad. Sci. USA. 2014;111:E2703–E2710. doi: 10.1073/pnas.1402342111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dethlefsen L., McFall-Ngai M., Relman D.A. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ley R.E., Backhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng Y.L., Song L.Q., Huang Y.M., Xiong Y.W., Zhang X.A., Sun H., Zhu X.P., Meng G.X., Xu J.G., Ren Z.H. Effect of enterohaemorrhagic Escherichia coli O157:H7-specific enterohaemolysin on interleukin-1beta production differs between human and mouse macrophages due to the different sensitivity of NLRP3 activation. Immunology. 2015;145:258–267. doi: 10.1111/imm.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perlman R.L. Mouse models of human disease: An evolutionary perspective. Evol. Med. Public Health. 2016;2016:170–176. doi: 10.1093/emph/eow014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung H., Pamp S.J., Hill J.A., Surana N.K., Edelman S.M., Troy E.B., Reading N.C., Villablanca E.J., Wang S., Mora J.R., et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eppinger M., Cebula T.A. Future perspectives, applications and challenges of genomic epidemiology studies for food-borne pathogens: A case study of Enterohemorrhagic Escherichia coli (EHEC) of the O157:H7 serotype. Gut Microbes. 2015;6:194–201. doi: 10.4161/19490976.2014.969979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arrieta M.-C., Walter J., Finlay B.B. Human Microbiota-Associated Mice: A Model with Challenges. Cell Host Microbe. 2016;19:575–578. doi: 10.1016/j.chom.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 57.van Nuenen M.H., de Ligt R.A., Doornbos R.P., van der Woude J.C., Kuipers E.J., Venema K. The influence of microbial metabolites on human intestinal epithelial cells and macrophages in vitro. FEMS Immunol. Med. Microbiol. 2005;45:183–189. doi: 10.1016/j.femsim.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Parlesak A., Haller D., Brinz S., Baeuerlein A., Bode C. Modulation of cytokine release by differentiated CACO-2 cells in a compartmentalized coculture model with mononuclear leucocytes and nonpathogenic bacteria. Scand. J. Immunol. 2004;60:477–485. doi: 10.1111/j.0300-9475.2004.01495.x. [DOI] [PubMed] [Google Scholar]
- 59.Toki S., Kagaya S., Shinohara M., Wakiguchi H., Matsumoto T., Takahata Y., Morimatsu F., Saito H., Matsumoto K. Lactobacillus rhamnosus GG and Lactobacillus casei suppress Escherichia coli-induced chemokine expression in intestinal epithelial cells. Int. Arch. Allergy Immunol. 2009;148:45–58. doi: 10.1159/000151505. [DOI] [PubMed] [Google Scholar]
- 60.Furrie E., Macfarlane S., Thomson G., Macfarlane G.T. Toll-like receptors-2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology. 2005;115:565–574. doi: 10.1111/j.1365-2567.2005.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zenhom M., Hyder A., de Vrese M., Heller K.J., Roeder T., Schrezenmeir J. Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco-2 cells via activation of PPARgamma and peptidoglycan recognition protein 3. J. Nutr. 2011;141:971–977. doi: 10.3945/jn.110.136176. [DOI] [PubMed] [Google Scholar]
- 62.Altamimi M., Abdelhay O., Rastall R.A. Effect of oligosaccharides on the adhesion of gut bacteria to human HT-29 cells. Anaerobe. 2016;39:136–142. doi: 10.1016/j.anaerobe.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 63.Resta-Lenert S., Barrett K.E. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC) Gut. 2003;52:988–997. doi: 10.1136/gut.52.7.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arboleya S., Bahrami B., Macfarlane S., Gueimonde M., Macfarlane G.T., de los Reyes-Gavilan C.G. Production of immune response mediators by HT-29 intestinal cell-lines in the presence of Bifidobacterium-treated infant microbiota. Benef. Microbes. 2015;6:543–552. doi: 10.3920/BM2014.0111. [DOI] [PubMed] [Google Scholar]
- 65.Di Nardo P., Minieri M., Ahluwalia A. Engineering the Stem Cell Niche and the Differentiative Micro- and Macroenvironment: Technologies and Tools for Applying Biochemical, Physical and Structural Stimuli and Their Effects on Stem Cells. In: Artmann G.M., Minger S., Hescheler J., editors. Stem Cell Engineering: Principles and Applications. Springer; Berlin/Heidelberg, Germany: 2011. pp. 41–59. [DOI] [Google Scholar]
- 66.Pamies D., Hartung T. 21st Century Cell Culture for 21st Century Toxicology. Chem. Res. Toxicol. 2017;30:43–52. doi: 10.1021/acs.chemrestox.6b00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nandakumar N.S., Pugazhendhi S., Ramakrishna B.S. Effects of enteropathogenic bacteria & lactobacilli on chemokine secretion & Toll like receptor gene expression in two human colonic epithelial cell lines. Indian J. Med. Res. 2009;130:170–178. [PubMed] [Google Scholar]
- 68.Raja S.B., Murali M.R., Devaraj H., Devaraj S.N. Differential expression of gastric MUC5AC in colonic epithelial cells: TFF3-wired IL1 β/Akt crosstalk-induced mucosal immune response against Shigella dysenteriae infection. J. Cell Sci. 2012;125:703–713. doi: 10.1242/jcs.092148. [DOI] [PubMed] [Google Scholar]
- 69.Rausell A., Muñoz M., Martinez R., Roger T., Telenti A., Ciuffi A. Innate immune defects in HIV permissive cell lines. Retrovirology. 2016;13:43. doi: 10.1186/s12977-016-0275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kolawole A.O., Mirabelli C., Hill D.R., Svoboda S.A., Janowski A.B., Passalacqua K.D., Rodriguez B.N., Dame M.K., Freiden P., Berger R.P., et al. Astrovirus replication in human intestinal enteroids reveals multi-cellular tropism and an intricate host innate immune landscape. PLoS Pathog. 2019;15:e1008057. doi: 10.1371/journal.ppat.1008057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kolawole A.O., Wobus C.E. Gastrointestinal organoid technology advances studies of enteric virus biology. PLoS Pathog. 2020;16:e1008212. doi: 10.1371/journal.ppat.1008212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saxena K., Blutt S.E., Ettayebi K., Zeng X.L., Broughman J.R., Crawford S.E., Karandikar U.C., Sastri N.P., Conner M.E., Opekun A.R., et al. Human Intestinal Enteroids: A New Model To Study Human Rotavirus Infection, Host Restriction, and Pathophysiology. J. Virol. 2016;90:43–56. doi: 10.1128/JVI.01930-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ginis I., Luo Y., Miura T., Thies S., Brandenberger R., Gerecht-Nir S., Amit M., Hoke A., Carpenter M.K., Itskovitz-Eldor J., et al. Differences between human and mouse embryonic stem cells. Dev. Biol. 2004;269:360–380. doi: 10.1016/j.ydbio.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 74.Andersson M.L., Karlsson-Sjöberg J.M.T., Pütsep K.L.A. CRS-peptides: Unique defense peptides of mouse Paneth cells. Mucosal Immunol. 2012;5:367–376. doi: 10.1038/mi.2012.22. [DOI] [PubMed] [Google Scholar]
- 75.Dimitrov V., White J.H. Species-specific regulation of innate immunity by vitamin D signaling. J. Steroid Biochem. Mol. Biol. 2016;164:246–253. doi: 10.1016/j.jsbmb.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 76.Park G.S., Park M.H., Shin W., Zhao C., Sheikh S., Oh S.J., Kim H.J. Emulating Host-Microbiome Ecosystem of Human Gastrointestinal Tract in Vitro. Stem Cell Rev. Rep. 2017;13:321–334. doi: 10.1007/s12015-017-9739-z. [DOI] [PubMed] [Google Scholar]
- 77.Seyhan A.A. Lost in translation: The valley of death across preclinical and clinical divide–identification of problems and overcoming obstacles. Transl. Med. Commun. 2019;4:18. doi: 10.1186/s41231-019-0050-7. [DOI] [Google Scholar]
- 78.Hartung T. Look back in anger—What clinical studies tell us about preclinical work. Altex. 2013;30:275–291. doi: 10.14573/altex.2013.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coleman R.A. Efficacy and safety of new medicines: A human focus. Cell Tissue Bank. 2011;12:3–5. doi: 10.1007/s10561-010-9200-x. [DOI] [PubMed] [Google Scholar]
- 80.Herrmann K., Pistollato F., Stephens M.L. Beyond the 3Rs: Expanding the use of human-relevant replacement methods in biomedical research. Altex. 2019;36:343–352. doi: 10.14573/altex.1907031. [DOI] [PubMed] [Google Scholar]
- 81.Aguiar-Pulido V., Huang W., Suarez-Ulloa V., Cickovski T., Mathee K., Narasimhan G. Metagenomics, Metatranscriptomics, and Metabolomics Approaches for Microbiome Analysis. Evol. Bioinform. Online. 2016;12:5–16. doi: 10.4137/EBO.S36436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schloissnig S., Arumugam M., Sunagawa S., Mitreva M., Tap J., Zhu A., Waller A., Mende D.R., Kultima J.R., Martin J., et al. Genomic variation landscape of the human gut microbiome. Nature. 2013;493:45–50. doi: 10.1038/nature11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Franzosa E.A., Huang K., Meadow J.F., Gevers D., Lemon K.P., Bohannan B.J.M., Huttenhower C. Identifying personal microbiomes using metagenomic codes. Proc. Natl. Acad. Sci. USA. 2015;112:2930. doi: 10.1073/pnas.1423854112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N., Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA. 2010;107:11971. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koenig J.E., Spor A., Scalfone N., Fricker A.D., Stombaugh J., Knight R., Angenent L.T., Ley R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA. 2011;108:4578. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., Magris M., Hidalgo G., Baldassano R.N., Anokhin A.P., et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pasolli E., Asnicar F., Manara S., Zolfo M., Karcher N., Armanini F., Beghini F., Manghi P., Tett A., Ghensi P., et al. Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle. Cell. 2019;176:649–662.e620. doi: 10.1016/j.cell.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keilbaugh S.A., Bewtra M., Knights D., Walters W.A., Knight R., et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shreiner A.B., Kao J.Y., Young V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015;31:69–75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vázquez-Castellanos J.F., Serrano-Villar S., Jiménez-Hernández N., Soto Del Rio M.D., Gayo S., Rojo D., Ferrer M., Barbas C., Moreno S., Estrada V., et al. Interplay between gut microbiota metabolism and inflammation in HIV infection. ISME J. 2018;12:1964–1976. doi: 10.1038/s41396-018-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huttenhower C., Gevers D., Knight R., Abubucker S., Badger J.H., Chinwalla A.T., Creasy H.H., Earl A.M., FitzGerald M.G., Fulton R.S., et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salipante S.J., Kawashima T., Rosenthal C., Hoogestraat D.R., Cummings L.A., Sengupta D.J., Harkins T.T., Cookson B.T., Hoffman N.G. Performance comparison of Illumina and ion torrent next-generation sequencing platforms for 16S rRNA-based bacterial community profiling. Appl. Environ. Microbiol. 2014;80:7583–7591. doi: 10.1128/AEM.02206-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malla M.A., Dubey A., Kumar A., Yadav S., Hashem A., Abd Allah E.F. Exploring the Human Microbiome: The Potential Future Role of Next-Generation Sequencing in Disease Diagnosis and Treatment. Front. Immunol. 2019;9:2868. doi: 10.3389/fimmu.2018.02868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Filippis F., Pasolli E., Tett A., Tarallo S., Naccarati A., De Angelis M., Neviani E., Cocolin L., Gobbetti M., Segata N., et al. Distinct Genetic and Functional Traits of Human Intestinal Prevotella copri Strains Are Associated with Different Habitual Diets. Cell Host Microbe. 2019;25:444–453.e443. doi: 10.1016/j.chom.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 96.Wu G., Zhang C., Wu H., Wang R., Shen J., Wang L., Zhao Y., Pang X., Zhang X., Zhao L., et al. Genomic Microdiversity of Bifidobacterium pseudocatenulatum Underlying Differential Strain-Level Responses to Dietary Carbohydrate Intervention. mBio. 2017;8 doi: 10.1128/mBio.02348-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Dijk E.L., Jaszczyszyn Y., Naquin D., Thermes C. The Third Revolution in Sequencing Technology. Trends Genet. TIG. 2018;34:666–681. doi: 10.1016/j.tig.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 98.Kuleshov V., Jiang C., Zhou W., Jahanbani F., Batzoglou S., Snyder M. Synthetic long-read sequencing reveals intraspecies diversity in the human microbiome. Nat. Biotechnol. 2016;34:64–69. doi: 10.1038/nbt.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kang D.D., Froula J., Egan R., Wang Z. MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ. 2015;3:e1165. doi: 10.7717/peerj.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tolonen A.C., Xavier R.J. Dissecting the human microbiome with single-cell genomics. Genome Med. 2017;9:56. doi: 10.1186/s13073-017-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sadowsky M.J., Staley C., Heiner C., Hall R., Kelly C.R., Brandt L., Khoruts A. Analysis of gut microbiota—An ever changing landscape. Gut Microbes. 2017;8:268–275. doi: 10.1080/19490976.2016.1277313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao J., Nian L., Kwok L.Y., Sun T., Zhao J. Reduction in fecal microbiota diversity and short-chain fatty acid producers in Methicillin-resistant Staphylococcus aureus infected individuals as revealed by PacBio single molecule, real-time sequencing technology. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2017;36:1463–1472. doi: 10.1007/s10096-017-2955-2. [DOI] [PubMed] [Google Scholar]
- 103.Baumgartner M., Bayer F., Pfrunder-Cardozo K.R., Buckling A., Hall A.R. Resident microbial communities inhibit growth and antibiotic-resistance evolution of Escherichia coli in human gut microbiome samples. PLoS Biol. 2020;18:e3000465. doi: 10.1371/journal.pbio.3000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leggett R.M., Alcon-Giner C., Heavens D., Caim S., Brook T.C., Kujawska M., Martin S., Peel N., Acford-Palmer H., Hoyles L., et al. Rapid MinION profiling of preterm microbiota and antimicrobial-resistant pathogens. Nat. Microbiol. 2020;5:430–442. doi: 10.1038/s41564-019-0626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hou Q., Zhao F., Liu W., Lv R., Khine W.W.T., Han J., Sun Z., Lee Y.-K., Zhang H. Probiotic-directed modulation of gut microbiota is basal microbiome dependent. Gut Microbes. 2020:1–20. doi: 10.1080/19490976.2020.1736974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Browne H.P., Neville B.A., Forster S.C., Lawley T.D. Transmission of the gut microbiota: Spreading of health. Nat. Rev. Microbiol. 2017;15:531–543. doi: 10.1038/nrmicro.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hirata S.I., Kunisawa J. Gut microbiome, metabolome, and allergic diseases. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2017;66:523–528. doi: 10.1016/j.alit.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 108.Li Z., Quan G., Jiang X., Yang Y., Ding X., Zhang D., Wang X., Hardwidge P.R., Ren W., Zhu G. Effects of Metabolites Derived From Gut Microbiota and Hosts on Pathogens. Front. Cell. Infect. Microbiol. 2018;8:314. doi: 10.3389/fcimb.2018.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garcia A., Barbas C. Gas chromatography-mass spectrometry (GC-MS)-based metabolomics. Methods Mol. Biol. 2011;708:191–204. doi: 10.1007/978-1-61737-985-7_11. [DOI] [PubMed] [Google Scholar]
- 110.Larive C.K., Barding G.A., Jr., Dinges M.M. NMR spectroscopy for metabolomics and metabolic profiling. Anal. Chem. 2015;87:133–146. doi: 10.1021/ac504075g. [DOI] [PubMed] [Google Scholar]
- 111.Duncan K.D., Fyrestam J., Lanekoff I. Advances in mass spectrometry based single-cell metabolomics. Analyst. 2019;144:782–793. doi: 10.1039/C8AN01581C. [DOI] [PubMed] [Google Scholar]
- 112.Junot C., Fenaille F., Colsch B., Bécher F. High resolution mass spectrometry based techniques at the crossroads of metabolic pathways. Mass Spectrom. Rev. 2014;33:471–500. doi: 10.1002/mas.21401. [DOI] [PubMed] [Google Scholar]
- 113.Dunham S.J.B., Ellis J.F., Li B., Sweedler J.V. Mass Spectrometry Imaging of Complex Microbial Communities. Acc. Chem. Res. 2017;50:96–104. doi: 10.1021/acs.accounts.6b00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jin P., Wang K., Huang C., Nice E.C. Mining the fecal proteome: From biomarkers to personalised medicine. Expert Rev. Proteom. 2017;14:445–459. doi: 10.1080/14789450.2017.1314786. [DOI] [PubMed] [Google Scholar]
- 115.Zhang X., Chen W., Ning Z., Mayne J., Mack D., Stintzi A., Tian R., Figeys D. Deep Metaproteomics Approach for the Study of Human Microbiomes. Anal. Chem. 2017;89:9407–9415. doi: 10.1021/acs.analchem.7b02224. [DOI] [PubMed] [Google Scholar]
- 116.Tovaglieri A., Sontheimer-Phelps A., Geirnaert A., Prantil-Baun R., Camacho D.M., Chou D.B., Jalili-Firoozinezhad S., de Wouters T., Kasendra M., Super M., et al. Species-specific enhancement of enterohemorrhagic E. coli pathogenesis mediated by microbiome metabolites. Microbiome. 2019;7:43. doi: 10.1186/s40168-019-0650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Efremova M., Teichmann S.A. Computational methods for single-cell omics across modalities. Nat. Methods. 2020;17:14–17. doi: 10.1038/s41592-019-0692-4. [DOI] [PubMed] [Google Scholar]
- 118.Jiang D., Armour C.R., Hu C., Mei M., Tian C., Sharpton T.J., Jiang Y. Microbiome Multi-Omics Network Analysis: Statistical Considerations, Limitations, and Opportunities. Front. Genet. 2019;10:995. doi: 10.3389/fgene.2019.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Benítez-Páez A., Kjølbæk L., Gómez del Pulgar E.M., Brahe L.K., Astrup A., Matysik S., Schött H.-F., Krautbauer S., Liebisch G., Boberska J., et al. A Multi-omics Approach to Unraveling the Microbiome-Mediated Effects of Arabinoxylan Oligosaccharides in Overweight Humans. mSystems. 2019;4:e00209-19. doi: 10.1128/mSystems.00209-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lloyd-Price J., Arze C., Ananthakrishnan A.N., Schirmer M., Avila-Pacheco J., Poon T.W., Andrews E., Ajami N.J., Bonham K.S., Brislawn C.J., et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang Z.-Z., Chen G., Hong Q., Huang S., Smith H.M., Shah R.D., Scholz M., Ferguson J.F. Multi-Omic Analysis of the Microbiome and Metabolome in Healthy Subjects Reveals Microbiome-Dependent Relationships Between Diet and Metabolites. Front. Genet. 2019;10:454. doi: 10.3389/fgene.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Van den Abbeele P., Belzer C., Goossens M., Kleerebezem M., De Vos W.M., Thas O., De Weirdt R., Kerckhof F.M., Van de Wiele T. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013;7:949–961. doi: 10.1038/ismej.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pires E.S., Hardoim C.C.P., Miranda K.R., Secco D.A., Lobo L.A., de Carvalho D.P., Han J., Borchers C.H., Ferreira R.B.R., Salles J.F., et al. The Gut Microbiome and Metabolome of Two Riparian Communities in the Amazon. Front. Microbiol. 2019;10:2003. doi: 10.3389/fmicb.2019.02003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Farag M.A., Abdelwareth A., Sallam I.E., El Shorbagi M., Jehmlich N., Fritz-Wallace K., Serena Schäpe S., Rolle-Kampczyk U., Ehrlich A., Wessjohann L.A., et al. Metabolomics reveals impact of seven functional foods on metabolic pathways in a gut microbiota model. J. Adv. Res. 2020;23:47–59. doi: 10.1016/j.jare.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Visconti A., Le Roy C.I., Rosa F., Rossi N., Martin T.C., Mohney R.P., Li W., de Rinaldis E., Bell J.T., Venter J.C., et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019;10:4505. doi: 10.1038/s41467-019-12476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xiong W., Giannone R.J., Morowitz M.J., Banfield J.F., Hettich R.L. Development of an enhanced metaproteomic approach for deepening the microbiome characterization of the human infant gut. J. Proteome Res. 2015;14:133–141. doi: 10.1021/pr500936p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Forbester J.L., Goulding D., Vallier L., Hannan N., Hale C., Pickard D., Mukhopadhyay S., Dougan G. Interaction of Salmonella Enterica Serovar Typhimurium with Intestinal Organoids Derived from Human Induced Pluripotent Stem Cells. Infect. Immun. 2015;83:2926. doi: 10.1128/IAI.00161-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dimitrov D.V. The Human Gutome: Nutrigenomics of the Host–Microbiome Interactions. Omics A J. Integr. Biol. 2010;15:419–430. doi: 10.1089/omi.2010.0109. [DOI] [PubMed] [Google Scholar]
- 129.Aruoma O.I., Hausman-Cohen S., Pizano J., Schmidt M.A., Minich D.M., Joffe Y., Brandhorst S., Evans S.J., Brady D.M. Personalized Nutrition: Translating the Science of NutriGenomics Into Practice: Proceedings From the 2018 American College of Nutrition Meeting. J. Am. Coll. Nutr. 2019;38:287–301. doi: 10.1080/07315724.2019.1582980. [DOI] [PubMed] [Google Scholar]
- 130.Haller D. Nutrigenomics and IBD: The Intestinal Microbiota at the Cross-road Between Inflammation and Metabolism. J. Clin. Gastroenterol. 2010;44:S6–S9. doi: 10.1097/MCG.0b013e3181dd8b76. [DOI] [PubMed] [Google Scholar]
- 131.Magnúsdóttir S., Heinken A., Kutt L., Ravcheev D.A., Bauer E., Noronha A., Greenhalgh K., Jäger C., Baginska J., Wilmes P., et al. Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nat. Biotechnol. 2017;35:81–89. doi: 10.1038/nbt.3703. [DOI] [PubMed] [Google Scholar]
- 132.Shoaie S., Ghaffari P., Kovatcheva-Datchary P., Mardinoglu A., Sen P., Pujos-Guillot E., de Wouters T., Juste C., Rizkalla S., Chilloux J., et al. Quantifying Diet-Induced Metabolic Changes of the Human Gut Microbiome. Cell Metab. 2015;22:320–331. doi: 10.1016/j.cmet.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 133.Noecker C., Eng A., Srinivasan S., Theriot C.M., Young V.B., Jansson J.K., Fredricks D.N., Borenstein E. Metabolic Model-Based Integration of Microbiome Taxonomic and Metabolomic Profiles Elucidates Mechanistic Links between Ecological and Metabolic Variation. mSystems. 2016;1 doi: 10.1128/mSystems.00013-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sung J., Kim S., Cabatbat J.J.T., Jang S., Jin Y.S., Jung G.Y., Chia N., Kim P.J. Global metabolic interaction network of the human gut microbiota for context-specific community-scale analysis. Nat. Commun. 2017;8:15393. doi: 10.1038/ncomms15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yazdani M., Taylor B.C., Debelius J.W., Li W., Knight R., Smarr L. Using machine learning to identify major shifts in human gut microbiome protein family abundance in disease; Proceedings of the 2016 IEEE International Conference on Big Data (Big Data); Washington, DC, USA. 5–8 December 2016; pp. 1272–1280. [Google Scholar]
- 136.Eetemadi A., Rai N., Pereira B.M.P., Kim M., Schmitz H., Tagkopoulos I. The Computational Diet: A Review of Computational Methods Across Diet, Microbiome, and Health. Front. Microbiol. 2020;11:393. doi: 10.3389/fmicb.2020.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ching T., Himmelstein D.S., Beaulieu-Jones B.K., Kalinin A.A., Do B.T., Way G.P., Ferrero E., Agapow P.M., Zietz M., Hoffman M.M., et al. Opportunities and obstacles for deep learning in biology and medicine. J. R. Soc. Interface. 2018;15 doi: 10.1098/rsif.2017.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Libbrecht M.W., Noble W.S. Machine learning applications in genetics and genomics. Nat. Rev. Genet. 2015;16:321–332. doi: 10.1038/nrg3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim M., Tagkopoulos I. Data integration and predictive modeling methods for multi-omics datasets. Mol. Omics. 2018;14:8–25. doi: 10.1039/C7MO00051K. [DOI] [PubMed] [Google Scholar]
- 140.Wainberg M., Merico D., Delong A., Frey B.J. Deep learning in biomedicine. Nat. Biotechnol. 2018;36:829. doi: 10.1038/nbt.4233. [DOI] [PubMed] [Google Scholar]
- 141.Ghosh S., Matsuoka Y., Asai Y., Hsin K.-Y., Kitano H. Software for systems biology: From tools to integrated platforms. Nat. Rev. Genet. 2011;12:821–832. doi: 10.1038/nrg3096. [DOI] [PubMed] [Google Scholar]
- 142.Fatehullah A., Tan S.H., Barker N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 143.Lehmann R., Lee C.M., Shugart E.C., Benedetti M., Charo R.A., Gartner Z., Hogan B., Knoblich J., Nelson C.M., Wilson K.M. Human organoids: A new dimension in cell biology. Mol. Biol. Cell. 2019;30:1129–1137. doi: 10.1091/mbc.E19-03-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Korytnikov R., Nostro M.C. Generation of polyhormonal and multipotent pancreatic progenitor lineages from human pluripotent stem cells. Methods. 2016;101:56–64. doi: 10.1016/j.ymeth.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 145.Fiorotto R., Amenduni M., Mariotti V., Fabris L., Spirli C., Strazzabosco M. Liver diseases in the dish: IPSC and organoids as a new approach to modeling liver diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865:920–928. doi: 10.1016/j.bbadis.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McCracken K.W., Cata E.M., Crawford C.M., Sinagoga K.L., Schumacher M., Rockich B.E., Tsai Y.H., Mayhew C.N., Spence J.R., Zavros Y., et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400–404. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Spence J.R., Mayhew C.N., Rankin S.A., Kuhar M.F., Vallance J.E., Tolle K., Hoskins E.E., Kalinichenko V.V., Wells S.I., Zorn A.M., et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Barker N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 149.Yoo J.-H., Donowitz M. Intestinal enteroids/organoids: A novel platform for drug discovery in inflammatory bowel diseases. World J. Gastroenterol. 2019;25:4125–4147. doi: 10.3748/wjg.v25.i30.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jung K.B., Lee H., Son Y.S., Lee M.-O., Kim Y.-D., Oh S.J., Kwon O., Cho S., Cho H.-S., Kim D.-S., et al. Interleukin-2 induces the in vitro maturation of human pluripotent stem cell-derived intestinal organoids. Nat. Commun. 2018;9:3039. doi: 10.1038/s41467-018-05450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zachos N.C., Kovbasnjuk O., Foulke-Abel J., In J., Blutt S.E., de Jonge H.R., Estes M.K., Donowitz M. Human Enteroids/Colonoids and Intestinal Organoids Functionally Recapitulate Normal Intestinal Physiology and Pathophysiology. J. Biol. Chem. 2016;291:3759–3766. doi: 10.1074/jbc.R114.635995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mithal A., Capilla A., Heinze D., Berical A., Villacorta-Martin C., Vedaie M., Jacob A., Abo K., Szymaniak A., Peasley M., et al. Generation of mesenchyme free intestinal organoids from human induced pluripotent stem cells. Nat. Commun. 2020;11:215. doi: 10.1038/s41467-019-13916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fujii M., Matano M., Toshimitsu K., Takano A., Mikami Y., Nishikori S., Sugimoto S., Sato T. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell. 2018;23:787–793.e786. doi: 10.1016/j.stem.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 154.Dedhia P.H., Bertaux-Skeirik N., Zavros Y., Spence J.R. Organoid Models of Human Gastrointestinal Development and Disease. Gastroenterology. 2016;150:1098–1112. doi: 10.1053/j.gastro.2015.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Leslie J.L., Huang S., Opp J.S., Nagy M.S., Kobayashi M., Young V.B., Spence J.R. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect. Immun. 2015;83:138–145. doi: 10.1128/IAI.02561-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pearce S.C., Coia H.G., Karl J.P., Pantoja-Feliciano I.G., Zachos N.C., Racicot K. Intestinal in vitro and ex vivo Models to Study Host-Microbiome Interactions and Acute Stressors. Front. Physiol. 2018;9:1584. doi: 10.3389/fphys.2018.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Foulke-Abel J., In J., Yin J., Zachos N.C., Kovbasnjuk O., Estes M.K., de Jonge H., Donowitz M. Human Enteroids as a Model of Upper Small Intestinal Ion Transport Physiology and Pathophysiology. Gastroenterology. 2016;150:638–649.e638. doi: 10.1053/j.gastro.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Freire R., Ingano L., Serena G., Cetinbas M., Anselmo A., Sapone A., Sadreyev R.I., Fasano A., Senger S. Human gut derived-organoids provide model to study gluten response and effects of microbiota-derived molecules in celiac disease. Sci. Rep. 2019;9:7029. doi: 10.1038/s41598-019-43426-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Angus H.C.K., Butt A.G., Schultz M., Kemp R.A. Intestinal Organoids as a Tool for Inflammatory Bowel Disease Research. Front. Med. 2020;6:334. doi: 10.3389/fmed.2019.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Michels B.E., Mosa M.H., Grebbin B.M., Yepes D., Darvishi T., Hausmann J., Urlaub H., Zeuzem S., Kvasnicka H.M., Oellerich T., et al. Human colon organoids reveal distinct physiologic and oncogenic Wnt responses. J. Exp. Med. 2019;216:704–720. doi: 10.1084/jem.20180823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fernández-Barral A., Costales-Carrera A., Buira S.P., Jung P., Ferrer-Mayorga G., Larriba M.J., Bustamante-Madrid P., Domínguez O., Real F.X., Guerra-Pastrián L., et al. Vitamin D differentially regulates colon stem cells in patient-derived normal and tumor organoids. FEBS J. 2020;287:53–72. doi: 10.1111/febs.14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Engel R.M., Chan W.H., Nickless D., Hlavca S., Richards E., Kerr G., Oliva K., McMurrick P.J., Jardé T., Abud H.E. Patient-Derived Colorectal Cancer Organoids Upregulate Revival Stem Cell Marker Genes following Chemotherapeutic Treatment. J. Clin. Med. 2020;9:128. doi: 10.3390/jcm9010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cristobal A., van den Toorn H.W.P., van de Wetering M., Clevers H., Heck A.J.R., Mohammed S. Personalized Proteome Profiles of Healthy and Tumor Human Colon Organoids Reveal Both Individual Diversity and Basic Features of Colorectal Cancer. Cell Rep. 2017;18:263–274. doi: 10.1016/j.celrep.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 164.Rubert J., Schweiger P.J., Mattivi F., Tuohy K., Jensen K.B., Lunardi A. Intestinal Organoids: A Tool for Modelling Diet–Microbiome–Host Interactions. Trends Endocrinol. Metab. 2020 doi: 10.1016/j.tem.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 165.Yin Y.-B., de Jonge H.R., Wu X., Yin Y.-L. Enteroids for Nutritional Studies. Mol. Nutr. Food Res. 2019;63:1801143. doi: 10.1002/mnfr.201801143. [DOI] [PubMed] [Google Scholar]
- 166.Schlaermann P., Toelle B., Berger H., Schmidt S.C., Glanemann M., Ordemann J., Bartfeld S., Mollenkopf H.J., Meyer T.F. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut. 2016;65:202–213. doi: 10.1136/gutjnl-2014-307949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yin Y., Bijvelds M., Dang W., Xu L., van der Eijk A.A., Knipping K., Tuysuz N., Dekkers J.F., Wang Y., de Jonge J., et al. Modeling rotavirus infection and antiviral therapy using primary intestinal organoids. Antivir. Res. 2015;123:120–131. doi: 10.1016/j.antiviral.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 168.Bartfeld S. Modeling infectious diseases and host-microbe interactions in gastrointestinal organoids. Dev. Biol. 2016;420:262–270. doi: 10.1016/j.ydbio.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 169.Yin Y., Zhou D. Organoid and Enteroid Modeling of Salmonella Infection. Front. Cell Infect. Microbiol. 2018;8:102. doi: 10.3389/fcimb.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kovbasnjuk O., Zachos N.C., In J., Foulke-Abel J., Ettayebi K., Hyser J.M., Broughman J.R., Zeng X.L., Middendorp S., de Jonge H.R., et al. Human enteroids: Preclinical models of non-inflammatory diarrhea. Stem Cell Res. Ther. 2013;4:S3. doi: 10.1186/scrt364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Karve S.S., Pradhan S., Ward D.V., Weiss A.A. Intestinal organoids model human responses to infection by commensal and Shiga toxin producing Escherichia coli. PLoS ONE. 2017;12:e0178966. doi: 10.1371/journal.pone.0178966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.In J., Foulke-Abel J., Zachos N.C., Hansen A.M., Kaper J.B., Bernstein H.D., Halushka M., Blutt S., Estes M.K., Donowitz M., et al. Enterohemorrhagic Escherichia coli reduce mucus and intermicrovillar bridges in human stem cell-derived colonoids. Cell. Mol. Gastroenterol. Hepatol. 2016;2:48–62.e43. doi: 10.1016/j.jcmgh.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Blutt S.E., Crawford S.E., Ramani S., Zou W.Y., Estes M.K. Engineered Human Gastrointestinal Cultures to Study the Microbiome and Infectious Diseases. Cell. Mol. Gastroenterol. Hepatol. 2017;5:241–251. doi: 10.1016/j.jcmgh.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Foulke-Abel J., In J., Kovbasnjuk O., Zachos N.C., Ettayebi K., Blutt S.E., Hyser J.M., Zeng X.L., Crawford S.E., Broughman J.R., et al. Human enteroids as an ex-vivo model of host-pathogen interactions in the gastrointestinal tract. Exp. Biol. Med. 2014;239:1124–1134. doi: 10.1177/1535370214529398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Finkbeiner S.R., Zeng X.L., Utama B., Atmar R.L., Shroyer N.F., Estes M.K. Stem cell-derived human intestinal organoids as an infection model for rotaviruses. mBio. 2012;3:e00159-12. doi: 10.1128/mBio.00159-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Williamson I.A., Arnold J.W., Samsa L.A., Gaynor L., DiSalvo M., Cocchiaro J.L., Carroll I., Azcarate-Peril M.A., Rawls J.F., Allbritton N.L., et al. A High-Throughput Organoid Microinjection Platform to Study Gastrointestinal Microbiota and Luminal Physiology. Cell. Mol. Gastroenterol. Hepatol. 2018;6:301–319. doi: 10.1016/j.jcmgh.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Saxena K., Simon L.M., Zeng X.L., Blutt S.E., Crawford S.E., Sastri N.P., Karandikar U.C., Ajami N.J., Zachos N.C., Kovbasnjuk O., et al. A paradox of transcriptional and functional innate interferon responses of human intestinal enteroids to enteric virus infection. Proc. Natl. Acad. Sci. USA. 2017;114:E570–E579. doi: 10.1073/pnas.1615422114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ettayebi K., Crawford S.E., Murakami K., Broughman J.R., Karandikar U., Tenge V.R., Neill F.H., Blutt S.E., Zeng X.-L., Qu L., et al. Replication of human noroviruses in stem cell–derived human enteroids. Science. 2016;353:1387. doi: 10.1126/science.aaf5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Drummond C.G., Bolock A.M., Ma C., Luke C.J., Good M., Coyne C.B. Enteroviruses infect human enteroids and induce antiviral signaling in a cell lineage-specific manner. Proc. Natl. Acad. Sci. USA. 2017;114:1672. doi: 10.1073/pnas.1617363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kuhlmann F.M., Santhanam S., Kumar P., Luo Q., Ciorba M.A., Fleckenstein J.M. Blood Group O–Dependent Cellular Responses to Cholera Toxin: Parallel Clinical and Epidemiological Links to Severe Cholera. Am. J. Trop. Med. Hyg. 2016;95:440–443. doi: 10.4269/ajtmh.16-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Koestler B.J., Ward C.M., Fisher C.R., Rajan A., Maresso A.W., Payne S.M. Human Intestinal Enteroids as a Model System of Shigella Pathogenesis. Infect. Immun. 2019;87:e00733-18. doi: 10.1128/IAI.00733-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Heo I., Dutta D., Schaefer D.A., Iakobachvili N., Artegiani B., Sachs N., Boonekamp K.E., Bowden G., Hendrickx A.P.A., Willems R.J.L., et al. Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat. Microbiol. 2018;3:814–823. doi: 10.1038/s41564-018-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Au-Dutta D., Au-Heo I., Au-O’Connor R. Studying Cryptosporidium Infection in 3D Tissue-derived Human Organoid Culture Systems by Microinjection. JoVE. 2019:e59610. doi: 10.3791/59610. [DOI] [PubMed] [Google Scholar]
- 184.Conlan J.W., Bardy S.L., KuoLee R., Webb A., Perry M.B. Ability of Escherichia coli O157:H7 isolates to colonize the intestinal tract of conventional adult CD1 mice is transient. Can. J. Microbiol. 2001;47:91–95. doi: 10.1139/w00-124. [DOI] [PubMed] [Google Scholar]
- 185.Lamers M.M., Beumer J., van der Vaart J., Knoops K., Puschhof J., Breugem T.I., Ravelli R.B.G., Paul van Schayck J., Mykytyn A.Z., Duimel H.Q., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020 doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gao Q.Y., Chen Y.X., Fang J.Y. 2019 Novel coronavirus infection and gastrointestinal tract. J. Dig. Dis. 2020;21:125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.De Weirdt R., Crabbé A., Roos S., Vollenweider S., Lacroix C., van Pijkeren J.P., Britton R.A., Sarker S., Van de Wiele T., Nickerson C.A. Glycerol supplementation enhances L. reuteri’s protective effect against S. Typhimurium colonization in a 3-D model of colonic epithelium. PLoS ONE. 2012;7:e37116. doi: 10.1371/journal.pone.0037116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Forbester J.L., Hannan N., Vallier L., Dougan G. Derivation of Intestinal Organoids from Human Induced Pluripotent Stem Cells for Use as an Infection System. Methods Mol. Biol. 2019;1576:157–169. doi: 10.1007/7651_2016_7. [DOI] [PubMed] [Google Scholar]
- 189.Wallach T.E., Bayrer J.R. Intestinal Organoids: New Frontiers in the Study of Intestinal Disease and Physiology. J. Pediatr. Gastroenterol. Nutr. 2017;64:180–185. doi: 10.1097/MPG.0000000000001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rajan A., Vela L., Zeng X.L., Yu X., Shroyer N., Blutt S.E., Poole N.M., Carlin L.G., Nataro J.P., Estes M.K., et al. Novel Segment- and Host-Specific Patterns of Enteroaggregative Escherichia coli Adherence to Human Intestinal Enteroids. mBio. 2018;9 doi: 10.1128/mBio.02419-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Holloway E.M., Capeling M.M., Spence J.R. Biologically inspired approaches to enhance human organoid complexity. Development. 2019;146:dev166173. doi: 10.1242/dev.166173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Grebenyuk S., Ranga A. Engineering Organoid Vascularization. Front. Bioeng. Biotechnol. 2019;7:39. doi: 10.3389/fbioe.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Noel G., Baetz N.W., Staab J.F., Donowitz M., Kovbasnjuk O., Pasetti M.F., Zachos N.C. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci. Rep. 2017;7:45270. doi: 10.1038/srep45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sidar B., Jenkins B.R., Huang S., Spence J.R., Walk S.T., Wilking J.N. Long-term flow through human intestinal organoids with the gut organoid flow chip (GOFlowChip) Lab Chip. 2019;19:3552–3562. doi: 10.1039/C9LC00653B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kasendra M., Tovaglieri A., Sontheimer-Phelps A., Jalili-Firoozinezhad S., Bein A., Chalkiadaki A., Scholl W., Zhang C., Rickner H., Richmond C.A., et al. Development of a primary human Small Intestine-on-a-Chip using biopsy-derived organoids. Sci. Rep. 2018;8:2871. doi: 10.1038/s41598-018-21201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sontheimer-Phelps A., Chou D.B., Tovaglieri A., Ferrante T.C., Duckworth T., Fadel C., Frismantas V., Sutherland A.D., Jalili-Firoozinezhad S., Kasendra M., et al. Human Colon-on-a-Chip Enables Continuous In Vitro Analysis of Colon Mucus Layer Accumulation and Physiology. Cell. Mol. Gastroenterol. Hepatol. 2020;9:507–526. doi: 10.1016/j.jcmgh.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bhatia S.N., Ingber D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 198.Zhang Y.S., Aleman J., Arneri A., Bersini S., Piraino F., Shin S.R., Dokmeci M.R., Khademhosseini A. From cardiac tissue engineering to heart-on-a-chip: Beating challenges. Biomed. Mater. 2015;10:034006. doi: 10.1088/1748-6041/10/3/034006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lee J., Kim S. Kidney-on-a-Chip: A New Technology for Predicting Drug Efficacy, Interactions, and Drug-induced Nephrotoxicity. Curr. Drug Metab. 2018;19:577–583. doi: 10.2174/1389200219666180309101844. [DOI] [PubMed] [Google Scholar]
- 200.Jahromi M.A.M., Abdoli A., Rahmanian M., Bardania H., Bayandori M., Moosavi Basri S.M.M., Kalbasi A., Aref A.R., Karimi M., Hamblin M.R. Microfluidic Brain-on-a-Chip: Perspectives for Mimicking Neural System Disorders. Mol. Neurobiol. 2019;56:8489–8512. doi: 10.1007/s12035-019-01653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Knowlton S., Tasoglu S. A Bioprinted Liver-on-a-Chip for Drug Screening Applications. Trends Biotechnol. 2016;34:681–682. doi: 10.1016/j.tibtech.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 202.de Graaf M.N.S., Cochrane A., van den Hil F.E., Buijsman W., van der Meer A.D., van den Berg A., Mummery C.L., Orlova V.V. Scalable microphysiological system to model three-dimensional blood vessels. APL Bioeng. 2019;3:026105. doi: 10.1063/1.5090986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Goyal G., Long J., Levy O., Ingber D.E. Biologically Inspired, iterative engineering of a Human Lymphoid Follicle Chip. J. Immunol. 2018;200:120–134. [Google Scholar]
- 204.Poceviciute R., Ismagilov R.F. Human-gut-microbiome on a chip. Nat. Biomed. Eng. 2019;3:500–501. doi: 10.1038/s41551-019-0425-0. [DOI] [PubMed] [Google Scholar]
- 205.Bein A., Shin W., Jalili-Firoozinezhad S., Park M.H., Sontheimer-Phelps A., Tovaglieri A., Chalkiadaki A., Kim H.J., Ingber D.E. Microfluidic Organ-on-a-Chip Models of Human Intestine. Cell. Mol. Gastroenterol. Hepatol. 2018;5:659–668. doi: 10.1016/j.jcmgh.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kim H.J., Huh D., Hamilton G., Ingber D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12:2165–2174. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 207.Workman M.J., Gleeson J.P., Troisi E.J., Estrada H.Q., Kerns S.J., Hinojosa C.D., Hamilton G.A., Targan S.R., Svendsen C.N., Barrett R.J. Enhanced Utilization of Induced Pluripotent Stem Cell-Derived Human Intestinal Organoids Using Microengineered Chips. Cell. Mol. Gastroenterol. Hepatol. 2018;5:669–677.e662. doi: 10.1016/j.jcmgh.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kim H.J., Ingber D.E. Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Biol. Quant. Biosci. Nano Macro. 2013;5:1130–1140. doi: 10.1039/c3ib40126j. [DOI] [PubMed] [Google Scholar]
- 209.de Haan P., Ianovska M.A., Mathwig K., van Lieshout G.A.A., Triantis V., Bouwmeester H., Verpoorte E. Digestion-on-a-chip: A continuous-flow modular microsystem recreating enzymatic digestion in the gastrointestinal tract. Lab Chip. 2019;19:1599–1609. doi: 10.1039/C8LC01080C. [DOI] [PubMed] [Google Scholar]
- 210.Kim H.J., Li H., Collins J.J., Ingber D.E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl. Acad. Sci. USA. 2016;113:E7–E15. doi: 10.1073/pnas.1522193112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kim J., Hegde M., Jayaraman A. Co-culture of epithelial cells and bacteria for investigating host-pathogen interactions. Lab Chip. 2010;10:43–50. doi: 10.1039/B911367C. [DOI] [PubMed] [Google Scholar]
- 212.Costello C.M., Sorna R.M., Goh Y.L., Cengic I., Jain N.K., March J.C. 3-D intestinal scaffolds for evaluating the therapeutic potential of probiotics. Mol. Pharm. 2014;11:2030–2039. doi: 10.1021/mp5001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Costello C.M., Hongpeng J., Shaffiey S., Yu J., Jain N.K., Hackam D., March J.C. Synthetic small intestinal scaffolds for improved studies of intestinal differentiation. Biotechnol. Bioeng. 2014;111:1222–1232. doi: 10.1002/bit.25180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Grassart A., Malardé V., Gobaa S., Sartori-Rupp A., Kerns J., Karalis K., Marteyn B., Sansonetti P., Sauvonnet N. Bioengineered Human Organ-on-Chip Reveals Intestinal Microenvironment and Mechanical Forces Impacting Shigella Infection. Cell Host Microbe. 2019;26:565. doi: 10.1016/j.chom.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 215.Shah P., Fritz J.V., Glaab E., Desai M.S., Greenhalgh K., Frachet A., Niegowska M., Estes M., Jäger C., Seguin-Devaux C., et al. A microfluidics-based in vitro model of the gastrointestinal human-microbe interface. Nat. Commun. 2016;7:11535. doi: 10.1038/ncomms11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Charbonneau M.R., Isabella V.M., Li N., Kurtz C.B. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun. 2020;11:1738. doi: 10.1038/s41467-020-15508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lycke N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 218.Villenave R., Wales S.Q., Hamkins-Indik T., Papafragkou E., Weaver J.C., Ferrante T.C., Bahinski A., Elkins C.A., Kulka M., Ingber D.E. Human Gut-On-A-Chip Supports Polarized Infection of Coxsackie B1 Virus In Vitro. PLoS ONE. 2017;12:e0169412. doi: 10.1371/journal.pone.0169412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kratz S.R.A., Höll G., Schuller P., Ertl P., Rothbauer M. Latest Trends in Biosensing for Microphysiological Organs-on-a-Chip and Body-on-a-Chip Systems. Biosensors. 2019;9:110. doi: 10.3390/bios9030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Jalili-Firoozinezhad S., Gazzaniga F.S., Calamari E.L., Camacho D.M., Fadel C.W., Bein A., Swenor B., Nestor B., Cronce M.J., Tovaglieri A., et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. Biomed. Eng. 2019;3:520–531. doi: 10.1038/s41551-019-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Maurer M., Gresnigt M.S., Last A., Wollny T., Berlinghof F., Pospich R., Cseresnyes Z., Medyukhina A., Graf K., Gröger M., et al. A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. Biomaterials. 2019;220:119396. doi: 10.1016/j.biomaterials.2019.119396. [DOI] [PubMed] [Google Scholar]
- 222.Wikswo J.P. The relevance and potential roles of microphysiological systems in biology and medicine. Exp. Biol. Med. 2014;239:1061–1072. doi: 10.1177/1535370214542068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Maschmeyer I., Lorenz A.K., Schimek K., Hasenberg T., Ramme A.P., Hübner J., Lindner M., Drewell C., Bauer S., Thomas A., et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip. 2015;15:2688–2699. doi: 10.1039/C5LC00392J. [DOI] [PubMed] [Google Scholar]
- 224.Raimondi M.T., Albani D., Giordano C. An Organ-On-A-Chip Engineered Platform to Study the Microbiota–Gut–Brain Axis in Neurodegeneration. Trends Mol. Med. 2019;25:737–740. doi: 10.1016/j.molmed.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 225.Choe A., Ha S.K., Choi I., Choi N., Sung J.H. Microfluidic Gut-liver chip for reproducing the first pass metabolism. Biomed. Microdevices. 2017;19:4. doi: 10.1007/s10544-016-0143-2. [DOI] [PubMed] [Google Scholar]
- 226.Philips C.A., Pande A., Shasthry S.M., Jamwal K.D., Khillan V., Chandel S.S., Kumar G., Sharma M.K., Maiwall R., Jindal A., et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2017;15:600–602. doi: 10.1016/j.cgh.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 227.Vernetti L., Gough A., Baetz N., Blutt S., Broughman J.R., Brown J.A., Foulke-Abel J., Hasan N., In J., Kelly E., et al. Functional Coupling of Human Microphysiology Systems: Intestine, Liver, Kidney Proximal Tubule, Blood-Brain Barrier and Skeletal Muscle. Sci. Rep. 2017;7:42296. doi: 10.1038/srep42296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Pound P., Ritskes-Hoitinga M. Is it possible to overcome issues of external validity in preclinical animal research? Why most animal models are bound to fail. J. Transl. Med. 2018;16:304. doi: 10.1186/s12967-018-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.National Research Council . Toxicity Testing in the 21st Century: A Vision and a Strategy. The National Academies Press; Washington, DC, USA: 2007. p. 216. [DOI] [Google Scholar]
- 230.Langley G.R. Considering a new paradigm for Alzheimer’s disease research. Drug Discov. Today. 2014;19:1114–1124. doi: 10.1016/j.drudis.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 231.van der Worp H.B., Howells D.W., Sena E.S., Porritt M.J., Rewell S., O’Collins V., Macleod M.R. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mak I.W., Evaniew N., Ghert M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014;6:114–118. [PMC free article] [PubMed] [Google Scholar]
- 233.Begley C.G., Ellis L.M. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483:531–533. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 234.Geerts H. Of mice and men: Bridging the translational disconnect in CNS drug discovery. CNS Drugs. 2009;23:915–926. doi: 10.2165/11310890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 235.van de Stolpe A., Kauffmann R.H. Innovative human-specific investigational approaches to autoimmune disease. RSC Adv. 2015;5:18451–18463. doi: 10.1039/C4RA15794J. [DOI] [Google Scholar]
- 236.Pistollato F., Cavanaugh S.E., Chandrasekera P.C. A Human-Based Integrated Framework for Alzheimer’s Disease Research. J. Alzheimers Dis. 2015;47:857–868. doi: 10.3233/JAD-150281. [DOI] [PubMed] [Google Scholar]
- 237.Archibald K., Tsaioun K., Kenna J.G., Pound P. Better science for safer medicines: The human imperative. J. R. Soc. Med. 2018 doi: 10.1177/0141076818812783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Khanna R., Burrows S.R. Human immunology: A case for the ascent of non-furry immunology. Immunol. Cell Biol. 2011;89:330–331. doi: 10.1038/icb.2010.173. [DOI] [PubMed] [Google Scholar]
- 239.Langley G.R., Adcock I.M., Busquet F., Crofton K.M., Csernok E., Giese C., Heinonen T., Herrmann K., Hofmann-Apitius M., Landesmann B., et al. Towards a 21st-century roadmap for biomedical research and drug discovery: Consensus report and recommendations. Drug Discov. Today. 2017;22:327–339. doi: 10.1016/j.drudis.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 240.Chandrasekera P.C., Pippin J.J. The human subject: An integrative animal model for 21(st) century heart failure research. Am. J. Transl. Res. 2015;7:1636–1647. [PMC free article] [PubMed] [Google Scholar]
- 241.Pistollato F., Iglesias R.C., Ruiz R., Aparicio S., Crespo J., Lopez L.D., Manna P.P., Giampieri F., Battino M. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer’s disease: A focus on human studies. Pharmacol. Res. 2018;131:32–43. doi: 10.1016/j.phrs.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 242.Scientific Committee on Health and Environmental Risks (SCHER) Scientific Committee on Consumer Safety (SCCS) Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) Addressing the New Challenges for Risk Assessment. European Commission; Brussels, Belgium: 2014. [Google Scholar]
- 243.Collins F.S. Reengineering translational science: The time is right. Sci. Transl. Med. 2011;3:90cm17. doi: 10.1126/scitranslmed.3002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ben-Zvi D., Melton D.A. Modeling Human Nutrition Using Human Embryonic Stem Cells. Cell. 2015;161:12–17. doi: 10.1016/j.cell.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 245.Parfrey L.W., Knight R. Spatial and temporal variability of the human microbiota. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012;18:8–11. doi: 10.1111/j.1469-0691.2012.03861.x. [DOI] [PubMed] [Google Scholar]
- 246.Nie J., Hashino E. Organoid technologies meet genome engineering. EMBO Rep. 2017;18:367–376. doi: 10.15252/embr.201643732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Costa J., Ahluwalia A. Advances and Current Challenges in Intestinal in vitro Model Engineering: A Digest. Front. Bioeng. Biotechnol. 2019;7:144. doi: 10.3389/fbioe.2019.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]