Abstract
Enterococcus cecorum is an emerging avian pathogen, particularly in chickens, but can be found in both diseased (clinical) and healthy (non-clinical) poultry. To better define differences between E. cecorum from the two groups, whole-genome sequencing (WGS) was used to identify and compare antimicrobial resistance genes as well as the pan-genome among the isolates. Eighteen strains selected from our previous study were subjected to WGS using Illumina MiSeq and comparatively analyzed. Assembled contigs were analyzed for resistance genes using ARG-ANNOT. Resistance to erythromycin was mediated by ermB, ermG, and mefA, in clinical isolates and ermB and mefA, in non-clinical isolates. Lincomycin resistance genes were identified as linB, lnuB, lnuC, and lnuD with lnuD found only in non-clinical E. cecorum; however, lnuB and linB were found in only one clinical isolate. For both groups of isolates, kanamycin resistance was mediated by aph3-III, while tetracycline resistance was conferred by tetM, tetO, and tetL. No mutations or known resistance genes were found for isolates resistant to either linezolid or chloramphenicol, suggesting possible new mechanisms of resistance to these drugs. A comparison of WGS results confirmed that non-clinical isolates contained more resistance genes than clinical isolates. The pan-genome of clinical and non-clinical isolates resulted in 3651 and 4950 gene families, respectively, whereas the core gene sets were comprised of 1559 and 1534 gene families in clinical and non-clinical isolates, respectively. Unique genes were found more frequently in non-clinical isolates than clinical. Phylogenetic analysis of the isolates and all the available complete and draft genomes showed no correlation between healthy and diseased poultry. Additional genomic comparison is required to elucidate genetic factors in E. cecorum that contribute to disease in poultry.
Keywords: Enterococcus, genomics, antimicrobial resistance, poultry
1. Introduction
Enterococcus cecorum has been implicated as a possible cause of disease in poultry, including spondylitis, vertebral osteoarthritis and femoral osteomyelitis. The bacterium normally resides as a member of the physiological microbiota of the intestinal tract of birds and mammals [1,2,3,4] and a dominant member of the enterococcal gastrointestinal microbiota of chickens [5]. It was first described as Streptococcus cecorum isolated from chickens in 1983 [6] and initially associated with clinical disease in poultry in Scotland in 2002 [7] followed by the Netherlands [8]. Since then, it has been reported as causing skeletal disease in broilers [9,10] and also in several outbreaks in Europe, [7,8,11,12,13,14,15,16] North America [17,18,19,20,21], Africa, [22], and eastern Asia [23].
As documented for many species within the Enterococcus genus, resistance to multiple classes of antimicrobials such as the aminoglycosides, β-lactams, macrolides, streptogramins, and tetracyclines have also been reported for E. cecorum [24]. These resistance phenotypes may partially explain why antimicrobial treatment has been ineffective in controlling mortality in poultry during outbreaks caused by E. cecorum [14]. In addition to antimicrobial resistance, genomic studies of Enterococcus in poultry are needed to compare the genetic composition of non-pathogenic and pathogenic E. cecorum strains to elucidate differences between the two groups. Presently there have been very few studies on comparison of resistance and genomes of E. cecorum from poultry [21,25] as well as comparison of non-pathogenic and pathogenic E. cecorum from different animal species [26] and chickens [25].
In our previous study, E. cecorum from diseased broiler chickens and poultry carcass rinsates were analyzed for antimicrobial resistance phenotype, virulence gene profile, and genetic relatedness [21].. Distinguishing the two groups of isolates was difficult based upon this phenotypic and genotypic characterization. The current study utilized comparative genomic analysis of selected E. cecorum isolates from diseased broiler chickens and poultry carcass rinsates to assess shared and unique genetic characteristics between the two groups. This data may aid in determining genetic relatedness and gene targets for the identification of pathogenic strains.
2. Materials and Methods
2.1. Bacterial Isolates and DNA Extraction
Isolates used in this analysis (n = 18) were selected from our previous study and included E. cecorum from non-clinical poultry collected by the animal arm of the National Antimicrobial Resistance Monitoring System (NARMS) from 2003–2011 and from diseased chickens from clinical cases submitted between 2008–2011 to the Animal Diagnostic Laboratory at the Pennsylvania State University [21]. E. cecorum genomic DNA was extracted using the blood and tissue genomic DNA extraction kit (Qiagen, Germantown, MD, USA). Extracted genomic DNA (gDNA) was quantified using Qubit double-stranded DNA (dsDNA) high-CHS) assay kit according to the manufacturer’s instructions (Life Technologies Inc., Carlsbad, CA, USA). The quality check of gDNA was performed using a NanoDrop™ spectrophotometer.
2.2. Sequencing, Assembly, and Annotation
E. cecorum sequencing libraries were prepared using a Nextera™ XT DNA Sample Preparation Kit and a Nextera™ XT Index Kit (Illumina Inc., San Diego, CA, USA). Illumina libraries were then quantified using a Qubit® DNA HS Assay Kit in a Qubit fluorometer (Thermo Fisher Scientific, Waltham, MA, USA), and the size of the fragment libraries was checked using an Agilent 2100 Bioanalyzer System with an Agilent HS DNA Kit (Agilent Technologies, Santa Clara, CA, USA). Illumina libraries were sequenced on an Illumina MiSeq platform (Illumina Inc., San Diego, CA, USA) using a MiSeq v2 reagent kit with 500 cycles and a paired-end read length of 2 × 250 bp. Resulting paired-end sequencing reads were de novo assembled into contigs using A5-miseq assembler [27] and contigs were annotated using Prokka [28]. Prophage analysis was done using PHASTER [29]. The list of the web-based and command line tools used in our study is provided in Table S1.
2.3. Antimicrobial Susceptibility Testing and Prediction of Antimicrobial Resistance Genes
Antimicrobial susceptibility phenotypes were determined as previously described using microbroth dilution according to Clinical and Laboratory Standards Institute (CLSI) [21]. Antimicrobial resistance genes were predicted using ARG-ANNOT [30].
2.4. Pan-Genome Construction
The pan-genome of 18 E. cecorum isolates was constructed using the Prokka resultant general feature files 3 (.gff) as input file, and the families of homologous genes for E. cecorum were computed using Roary with identity cut-off of 95% [31]. The distribution of core (gene families commonly shared by all genomes), accessory (gene families not shared by all genomes), and unique (genes exclusive to one genome) genes were obtained from the Roary generated clusters of homologous gene groups. The E. cecorum genomes were functionally characterized using the Clusters of Orthologous Groups (COG) database [32]. For each E. cecorum genome, all the protein-coding sequences obtained from Prokka were subjected to reversed position specific blast (rps-blast) [33] against COG [32], and the rps-blast output was used to assign the COG function profile to the genes. Once the genes were assigned to the COGs, they were clustered into 20 of 25 functional categories, which were further grouped into four major classes, as described previously [34]. In brief, COG categories amino acid transport and metabolism (E), carbohydrate transport and metabolism (G), nucleotide transport and metabolism (F), energy production and conversion (C), coenzyme transport and metabolism (H), lipid transport and metabolism (I), inorganic ion transport and metabolism (P), and secondary metabolite biosynthesis, transport and catabolism (Q) were included in functional class Metabolism. COG categories cell wall/membrane/envelope biogenesis (M), cell motility (N), cell cycle control, cell division, chromosome partitioning (D), posttranslational modification, protein turnover, chaperones (O), signal transduction mechanisms (T), intracellular trafficking, secretion, and vesicular transport (U), and defense mechanisms (V) were included in functional class cellular processes and signaling. COG categories translation, ribosomal structure and biogenesis (J), transcription (K) and replication, recombination and repair (L) were included in functional class information storage, and processing and COG categories function unknown (S) and general function prediction only (R) were included in functional class poorly categorized. The core genes of clinical and non-clinical isolates were further categorized to different COG functional groups and compared with overall (clinical and non-clinical) core genes in different COG functional groups. The details of unique genes in all the clinical and non-clinical isolates is provided (Table S2).
2.5. Core-Genome Phylogenetic Tree
The phylogenetic tree was constructed using the core genes of 18 E. cecorum of our study, and available closed and draft genome sequences of E. cecorum retrieved from NCBI Gen-Bank. The retrieved genomes were annotated with Prokka for consistency. The Prokka generated .gff files were used as input in Roary to obtain core genome alignment. The core genome alignment was used for maximum likelihood (ML) phylogenetic tree generation using RAxML [35] with the general time-reversible (GTR) model of nucleotide evolution and gamma-distributed rate variation. FigTree (http://tree.bio.ed.ac.uk/software/figtree/) was used to graph the phylogenetic tree.
2.6. Nucleotide Accession Numbers
The Enterococcus cecorum genome sequences were deposited in GenBank under BioProject PRJNA580016 and accession numbers of WJEH00000000 to WJEY00000000 (Table S3).
3. Results
3.1. Genome Characteristics
Genome sizes of the 18 sequenced E. cecorum isolates ranged from 2.21 to 2.69 Mb, with 2120 to 2666 predicted coding genes and GC content from 36.0% to 36.6% (Table S3).
3.2. Antimicrobial Resistance Genes
Resistance genes reported were based on phenotypic results (Table 1). Differences in the antibiotic resistance patterns were noted between clinical and non-clinical isolates. Clinical isolates exhibited lower prevalence of resistance to antibiotics, with isolates displaying resistance to tetracycline (6/9), erythromycin (6/9), lincomycin (3/9), tylosin (2/9), kanamycin (1/9), and streptomycin (1/9). Alternatively, non-clinical isolates exhibited resistance to lincomycin (8/9) and tetracycline (8/9), followed by quinupristin–dalfopristin (Q/D; 7/9), tylosin (6/9), erythromycin (4/9), kanamycin (3/9), linezolid (3/9), and chloramphenicol (1/9). None of the clinical isolates were resistant to linezolid, Q/D, and chloramphenicol.
Table 1.
Antibiotic resistance profile of clinical and non-clinical Enterococcus cecorum isolates.
| Isolate No. | Resistance Profile | Antibiotics Resistance Genes |
|---|---|---|
| PS1 | Lincomycin-Tetracycline | lnuB, linB, tetL, tetM, tetO |
| PS2 | Erythromycin-Kanamycin-Lincomycin-Tylosin | ermG, ermB, mefA, msrD, aph3-III, lnuC |
| PS3 | Erythromycin-Streptomycin-Tetracycline | ermG, mefA, msrD, tetL, tetM |
| PS5 | Erythromycin-Tylosin-Tetracycline | ermG, mefA, msrD, tetL, tetM |
| PS6 | Erythromycin-Tetracycline | ermG, mefA, msrD, tetL, tetM |
| PS7 | Lincomycin | lnuC |
| PS8 | Erythromycin-Streptomycin-Tetracycline | ermG, mefA, msrD, tetL, tetM |
| PS11 | Erythromycin-Tetracycline | ermG, mefA, msrD, tetL, tetM |
| ARS9 | Linezolid-Quinupristin/Dalfopristin | ermB |
| ARS16 | Erythromycin-Kanamycin-Lincomycin-Quinupristin/Dalfopristin-Tetracycline | mefA, msrD, aph3-III, lnuB, linB, tetL, tetM, tetO |
| ARS48 | Erythromycin-Lincomycin-Quinupristin/Dalfopristin-Tylosin-Tetracycline | ermB, mefA, msrD, lnuB, linB, tetM |
| ARS57 | Lincomycin-Linezolid-Quinupristin/Dalfopristin-Tylosin-Tetracycline | ermB, mefA, msrD, lnuB, linB, lnuD |
| ARS60 | Kanamycin-Lincomycin-Quinupristin/Dalfopristin-Tetracycline | ermB, msrD, aph3-III, lnuB, linB, tetL, tetM |
| ARS62 | Kanamycin-Lincomycin-Quinupristin/Dalfopristin-Tylosin-Tetracycline | ermB, msrD, aph3-III, lnuB, linB, tetL, tetM, tetO |
| ARS64 | Erythromycin-Lincomycin-Tylosin-Tetracycline | ermB, tetL, tetM |
| ARS65 | Chloramphenicol-Lincomycin-Linezolid-Quinupristin/Dalfopristin-Tylosin-Tetracycline | ermB, mefA, msrD, lnuB, linB, tetL, tetM, tetO |
| ARS71 | Erythromycin-Lincomycin-Tylosin-Tetracycline | ermB, tetL, tetM |
Clinical isolates showed the highest resistance to erythromycin, and erythromycin resistance genes ermB and/or ermG were, in some instances, found in conjunction with efflux pumps such as mefA and/or msrD in both clinical and non-clinical isolates (Table 1). These efflux pumps could also play a role in the resistance to lincomycin in both sets of isolates. Both ermB or msrD also conferred resistance to the streptogramin B component of Q/D and were found in seven Q/D resistant non-clinical isolates. Tetracycline resistant isolates harbored tet(L), tet(M), or tet(O) while kanamycin resistance was conferred by aphIII in both sets of isolates. Approximately 33% (3/9) of clinical isolates and 89% (8/9) of non-clinical isolates were resistant to lincomycin, and corresponding lnu and lin genes were found in all of those isolates (Table 1). Only one clinical isolate was resistant to streptomycin and one non-clinical isolate resistant to chloramphenicol; no known resistance mechanism was detected to either drug.
3.3. Pan-Genome Analysis
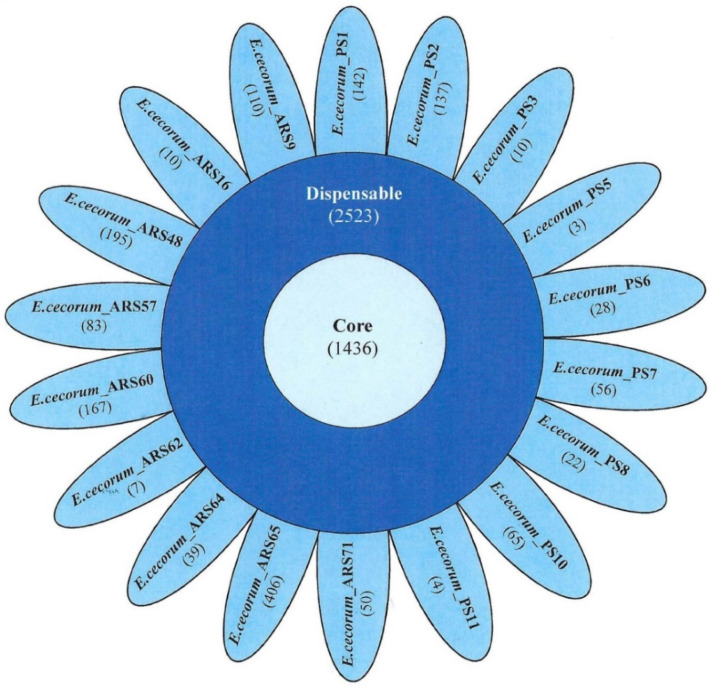
The pan-genome analysis for the E. cecorum isolates was initiated with 41,650 protein-coding sequences that resulted in 5493 gene families. The core gene set comprised 1436 genes, i.e., 62% of the average number of protein-coding sequences (2314 per genome), suggesting that more than half of the protein-coding sequences were part of the core and just over 1/3 of the protein-coding sequences in each genome were dispensable. The number of core, accessory, and unique genes in each genome is represented as a floral diagram in which the inner circle, outer circle, and petals represent core, accessory, and unique genes, respectively (Figure 1). The unique genes present in clinical isolates ranged from 3–142, whereas those in non-clinical isolates ranged from 7–406.
Figure 1.
Venn diagram showing the pan-genome of 18 Enterococcus cecorum clinical (PS) and non-clinical (ARS) isolates from chicken. The number of core genes is the number of common genes shown in the center, while genes common between isolates are shown in the periphery (accessory genes). Each petal represents the unique genes in the respective isolates.
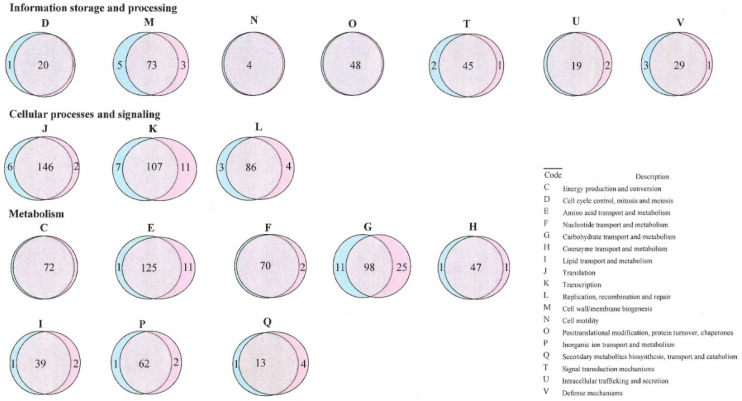
The comparison of core genes in different COG categories between clinical and non-clinical isolates is presented in the Venn diagram (Figure 2). Core genes of all of the E. cecorum isolates were assigned to 20 of 25 functional COG categories. Overall, the major class metabolism (CEFGHIPQ) was comprised of 36.63% of the core genes, while 23.61% and 16.57% of core genes were ascribed to class information storage and processing (JKL) and cellular process and signaling (DMNOTUV).
Figure 2.
Venn diagram showing the Clusters of Orthologous Groups (COG) function categories of 18 Enterococcus cecorum clinical (PS; shown in pink) and non-clinical (ARS; shown in teal) isolates from chicken.
The core gene comparison of the isolates between clinical and non-clinical isolates revealed that the majority of genes were common; however, a few genes were more frequent in either clinical or non-clinical isolates. A high number of the core genes from functional class metabolism (CEFGHIPQ) and information storage and processing (JKL) were detected in the clinical isolates, while the gene number was higher for functional class cellular processes and signaling (DMNOTUV) in non-clinical isolates (Table S4). This revealed variation in the functional profile among E. cecorum isolates.
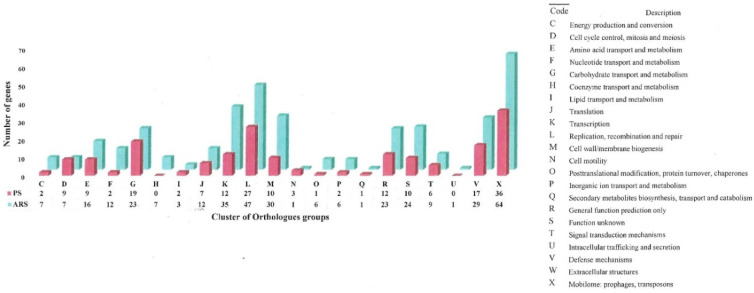
The unique genes were further assigned to different COG categories (Figure 3). Almost all of the COG categories were high in non-clinical isolates except for cell cycle control, mitosis and meiosis (D), and cell motility (N), which were slightly higher in clinical isolates. The most abundant COG category in both sets of isolates was mobilome: prophages, transposons (X). In the non-clinical isolates, the genes encoding for transcription (K), replication, recombination and repair (L), cell wall/membrane biogenesis (M), functions unknown (S), mobilome: prophages, transposons (X), and defense mechanisms (V) genes were significantly higher.
Figure 3.
Distribution of unique genes in each Cluster of Orthologous Groups (COG) categories in clinical (PS; shown in pink) and non-clinical (ARS; shown in teal) Enterococcus cecorum isolates from chicken. The number of unique genes for each cluster is shown below the cluster code.
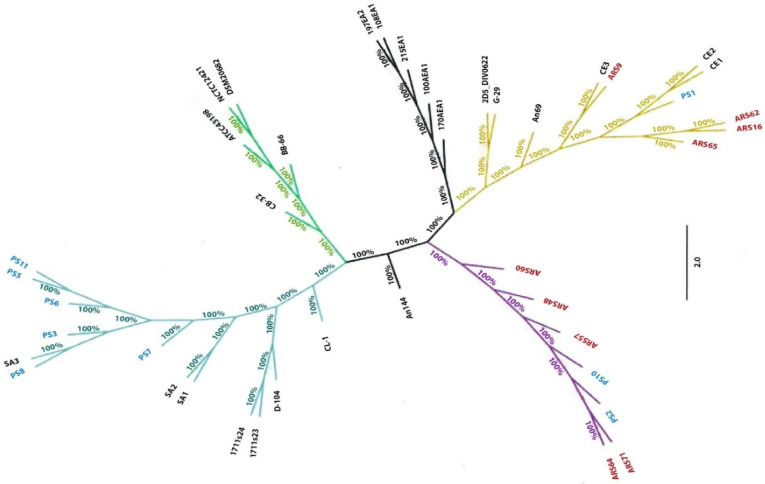
3.4. Core Genome Phylogenetic Analysis
The core genome phylogenetic tree was generated using the core genome of the E. cecorum isolates from this study and 24 E. cecorum genomes from NCBI GenBank (Table S5). Some of the clinical isolates (PS3, PS5, PS6, PS7, PS8, and PS11) clustered with pathogenic reference isolates (SA1, SA2, and SA3) from chickens, while non-clinical (ARS9, ARS16, ARS62, and ARS65) isolates, including a single clinical isolate PS1, clustered with commensal reference isolates (CE1, CE2, and CE3) and formed a separate clade (Figure 4). Remaining clinical (PS2, PS10) and non-clinical isolates (ARS48, ARS57, ARS60, ARS64, ARS71) clustered together and branched out in the phylogenetic tree.
Figure 4.
Core genome phylogenetic tree of 42 Enterococcus cecorum strains. The core genome phylogenetic tree was generated using the core-genome of the E. cecorum isolates from this study and 24 E. cecorum genomes from NCBI GenBank.
3.5. Prophage Analysis
The total number of intact prophages found in clinical and non-clinical isolates were nine and eight, respectively (Table 2). Intact prophages were not detected in all the isolates. Non-clinical isolate ARS65 had the highest number of intact prophages (four), whereas three clinical isolates (PS5, PS6, and PS11) had two each. There was no correlation between clinical and non-clinical isolates and the occurrence of intact prophages. The most frequent intact prophage detected was associated with Streptococcus genera. Three Enterococcus prophages were detected in non-clinical isolates only. The other prophages detected were associated with Listeria and Bacillus genera.
Table 2.
List of intact phage in Enterococcus cecorum isolates from healthy and diseased chickens.
| Isolate | Length | GC | CDS | Best Match | |
|---|---|---|---|---|---|
| Phage | Accession Number | ||||
| ARS16 | 36.3 | 37.17 | 47 | PHAGE_Strept_5093 | NC_012753 |
| ARS48 | 31.1 | 37.87 | 43 | PHAGE_Entero_EFC_1 | NC_025453 |
| ARS57 | 47.3 | 37.59 | 52 | PHAGE_Entero_EFC_1 | NC_025453 |
| ARS62 | 36.3 | 37.17 | 47 | PHAGE_Strept_5093 | NC_012753 |
| ARS64 | 36.1 | 35.41 | 43 | PHAGE_Lister_vB_LmoS_188 | NC_028871 |
| ARS65 | 33.6 | 37.60 | 43 | PHAGE_Entero_EFC_1 | NC_025453 |
| 34.4 | 36.85 | 23 | PHAGE_Bacter_Diva | NC_028788 | |
| 14.7 | 39.26 | 17 | PHAGE_Strept_5093 | NC_012753 | |
| 14.5 | 38.74 | 16 | PHAGE_Strept_5093 | NC_012753 | |
| PS2 | 23.9 | 37.30 | 35 | PHAGE_Strept_5093 | NC_012753 |
| PS5 | 30.7 | 35.37 | 36 | PHAGE_Bacill_phBC6A52 | NC_004821 |
| 35.8 | 36.8 | 47 | PHAGE_Strept_5093 | NC_012753 | |
| PS6 | 30.7 | 35.37 | 36 | PHAGE_Bacill_phBC6A52 | NC_004821 |
| 35.8 | 36.8 | 47 | PHAGE_Strept_5093 | NC_012753 | |
| PS8 | 32.2 | 36.34 | 39 | PHAGE_Strept_5093 | NC_012753 |
| PS11 | 30.7 | 35.37 | 36 | PHAGE_Bacill_phBC6A52 | NC_004821 |
| 35.8 | 36.8 | 47 | PHAGE_Strept_5093 | NC_012753 | |
4. Discussion
In this study, eighteen E. cecorum were sequenced and compared based upon antibiotic resistance mechanisms and genome characteristics. The genome size and GC content of E. cecorum used in this study were similar to the generally observed genome sizes of other Enterococcus species. The average size of E. cecorum genomes was 2.38 Mb, and the average GC content was 36.35%. However, it was interesting to note that the average genome size of clinical isolates was less (2.33 Mb) than non-clinical isolates (2.44 Mb), which contradicts a previous study in which the size of commensal E. cecorum genomes were less than pathogenic genomes [25]. As a result, the average number of protein-coding sequences was also less in the clinical isolates (2264) as compared to non-clinical isolates (2363). The present study attempted to decipher the concordance between phenotypic resistance and genetic determinants. Results from this study correlated resistance determinants to their phenotypic expression in agreement with other studies on the use of WGS as a surveillance tool to detect antibiotic resistance [36,37]. Our results showed that non-clinical isolates exhibited resistance to more antibiotics and, thus, the presence of more antibiotic resistance genes in comparison to clinical isolates.
E. cecorum from poultry appear to be highly resistant to macrolides [24] and, in this study, erythromycin-resistance was mediated by either the presence of one or more resistance genes (ermB, ermG), and/or efflux genes (mefA, msrD) in both clinical and non-clinical isolates. The erythromycin resistance gene, ermB, is present in both humans and animal isolates and can be harbored on transposons and plasmids [38,39,40]. In the present study, ermB was associated on a plasmid in a clinical isolate or was likely to be harbored on a mobile genetic element in non-clinical isolates. The finding was similar to previous studies in which a higher prevalence of resistance to erythromycin was noticed in pathogenic isolates [19,41,42]. Genes conferring resistance to erythromycin and streptogramin B antibiotics such as mefA and msrD, respectively, and lincomycin resistance genes such as linB, lnuB, lnuC, and lnuD were identified. The linB, lnuB genes were common in both sets of isolates exhibiting resistance; however, two clinical E. cecorum isolates harbored lnuC, and only one non-clinical isolate contained lnuD along with other resistance genes. Upon further analysis of the contigs harboring lincomycin resistance genes, some of them were present on a plasmid in non-clinical isolates, but not in any of the clinical isolates where the genes were simply present in the genome.
Notably, three non-clinical E. cecorum isolates were simultaneously resistant to erythromycin, lincomycin, and Q/D, suggesting a possible acquired macrolide-lincosamide-streptogramin type B (MLSB) and streptogramin type A co-resistance. Although we have previously detected streptogramin type A resistance genes vatB, vatD, vatE, and vgaB from enterococci from poultry carcass rinsates [43,44], none of these genes were present in the E. cecorum in this study.
In both groups of isolates, kanamycin resistance was mediated by the aminoglycoside-modifying enzyme, aph3-III, and was associated with a plasmid in a single non-clinical isolate. Tetracycline resistance genes for ribosomal protection (tet(M), tet(O)) or tet(L) for efflux were detected in both sets of isolates. The tet(M) gene is most frequently found in Enterococcus [45] and, not surprising, overall resistance to tetracycline is linked to the use of this antibiotic as a therapeutic agent in poultry [42].
Phenotypic linezolid resistance in non-clinical isolates was not linked to any known mechanisms for resistance to linezolid, such as 23S rRNA gene mutation [46] or horizontally acquired cfr and optrA resistance genes [47,48]. However, linezolid resistance could also be due to cell wall thickness and biofilm formation [49] or overestimation of minimum inhibitory concentration [50]. Similarly, no mechanisms of resistance to chloramphenicol were detected in the one chloramphenicol resistant non-clinical isolate. Interestingly, this isolate was also resistant to linezolid. This could suggest possible new and/or alternative mechanisms of resistance to these antibiotics, which should be further examined.
The pan-genome of the 18 E. cecorum isolates comprised 41,650 protein-coding sequences, of which 1436 genes were conserved across all studied isolates. This finding is in concordance with the previous study on E. faecalis strains [51,52]. The pan-genome of clinical and non-clinical isolates resulted in 3651 and 4950 gene families, respectively, whereas the core gene sets were comprised of 1559 and 1534 gene families in clinical and non-clinical isolates, respectively. Comparison of the core genes of clinical and non-clinical isolates with the core genes of all the isolates revealed that there were 123 additional genes included in the core genes of clinical isolates, while 98 additional genes were included in the core genes of non-clinical isolates. The high number of accessory genes in non-clinical isolates compared to clinical isolates was attributed to a large number of accessory genes for maintaining replication and cell division that these commensal isolates possessed.
The pan-genome size increased when genomes of clinical and non-clinical isolates were assessed, which indicated an open pan-genome for the E. cecorum isolates and reduced core-genome size. The minute influence on the size of core genes with the addition of genomes indicated that the analyzed E. cecorum genomes were sufficient to construct the representative core-genome. The results were in agreement with previous studies of E. faecalis [53,54]. The small pan-genome size and inclusion of 43% gene families in core genes in the clinical isolates showed their conserved nature. Non-clinical isolates had large pan-genomes as compared to clinical isolates, and only 31% of gene families were part of the core genes and a higher number of unique genes. This data suggested that these isolates acquired a diverse set of genes to perform novel functions for better sustainability [55]. The distribution of the genes in different functional categories revealed variation in the functional profile among E. cecorum isolates. The functional analysis of core genes revealed that 6.82% (98/1436) of genes were ascribed to carbohydrate metabolism, which contrasted to an earlier report where 11.3% of core genes were ascribed to carbohydrate metabolism in E. faecium [54]. Moreover, about 7.88% (123/1559) and 7.11% (109/1534) of core genes were ascribed to carbohydrate metabolism in clinical and non-clinical isolates, respectively, which clearly indicates low intra-species and high inter-species metabolic gene variations in Enterococcus sp. The relative higher number of core genes were ascribed to carbohydrate metabolism in pathogenic isolates, and this indicates that these genes are essential to utilize various carbohydrates [56]. The relatively high number of genes in the core genome of the clinical isolates in class metabolism and class information storage and processing indicated that these genes are essential and conserved in clinical isolates. Alternatively, the relatively high number of genes in the core genome of the non-clinical isolates in class cellular processes and signaling showed that these genes are essential for better adaptability. A large number of genes were hypothetical genes which were assigned with unknown function. These genes may have additional functions associated with adaptability and survival, and this requires further research.
Among unique genes, high fractions (100/550) of genes were assigned to the mobilome group, and about 2/3 of this group of genes were in non-clinical isolates, suggesting that non-clinical isolates are contributing more to the population [57]. A unique sortase (surface protein transpeptidase) gene was present in clinical isolate PS8, and this surface protein may play a key role in the infection process [58]. Further studies will be required to examine the unexplored attributes.
Phylogenetic analysis of the core-genome of our 18 isolates along with core-genomes of 24 E. cecorum available in NCBI was performed. Some of the clinical and non-clinical isolates clustered, respectively, with previously reported pathogenic and commensal E. cecorum strains. However, no profound correlation between the strain origins to its phylogeny was established, which was in accordance with the inference drawn by earlier studies [59].
5. Conclusions
In conclusion, comparison of antimicrobial resistance and the genome of clinical and non-clinical E. cecorum isolates provided valuable insight about fundamental genetic differences observed between the two groups, which were different from previous findings on pathogenic and commensal E. cecorum isolates. The comparison of WGS results confirmed that non-clinical isolates contained more resistance genes than clinical isolates. Resistance genes were both shared and exclusive to each group indicating varying genetic characteristics among E. cecorum isolates. The pan-genome analysis revealed that the non-clinical genomes were comparatively diverse due to the acquisition of additional genes, while genome reduction in the conserved clinical genomes suggested better host adaptability. Further comparative investigation of additional genomes of non-clinical and clinical E. cecorum isolates may reveal attributes in clinical E. cecorum responsible for their pathogenic nature in poultry.
Acknowledgments
The authors would like to thank Calvin L. Williams for technical support.
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-8158/9/6/686/s1. Table S1: List of online tools used in this study; Table S2: Clusters of Orthologous Groups (COG) in the clinical and non-clinical Enterococcus cecorum isolates; Table S3: Genome assembly statistics and metadata of clinical and non-clinical Enterococcus cecorum; Table S4: List of core gene predominantly present in Enterococcus cecorum isolates from healthy and diseased chickens; Table S5: List of Enterococcus cecorum genomes used for phylogenetic analysis.
Author Contributions
Conceptualization, P.S., S.K.G., S.K., J.G.F., and C.R.J.; methodology, P.S., S.K.G., J.B.B., L.M.H., T.A.W., S.K., J.G.F., and C.R.J.; validation, P.S., S.K.G., and C.R.J.; formal analysis, P.S., S.K.G., J.B.B., L.M.H., T.A.W., S.K., and C.R.J.; investigation, P.S., S.K.G., and C.R.J.; resources, J.G.F. and C.R.J.; data curation, P.S. and S.K.G.; writing—original draft preparation, P.S., S.K.G., and C.R.J.; writing—review and editing, P.S., S.K.G., J.B.B., L.M.H., T.A.W., S.K., J.G.F., and C.R.J.; visualization, C.R.J.; supervision, C.R.J.; project administration, C.R.J.; funding acquisition, J.G.F. and C.R.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the U.S. Department of Agriculture–Agricultural Research Service (USDA–ARS) project No. 6040-32000-009-00D.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Devriese L.A., Hommez J., Wijfels R., Haesebrouck F. Composition of the enterococcal and streptococcal intestinal flora of poultry. J. Appl. Bacteriol. 1991;71:46–50. doi: 10.1111/j.1365-2672.1991.tb04480.x. [DOI] [PubMed] [Google Scholar]
- 2.Devriese L.A., Laurier L., De Herdt P., Haesebrouck F. Enterococcal and streptococcal species isolated from faeces of calves, young cattle and dairy cows. J. Appl. Bacteriol. 1992;72:29–31. doi: 10.1111/j.1365-2672.1992.tb04877.x. [DOI] [PubMed] [Google Scholar]
- 3.Devriese L.A., Hommez J., Pot B., Haesebrouck F. Identification and composition of the streptococcal and enterococcal flora of tonsils, intestines and faeces of pigs. J. Appl. Bacteriol. 1994;77:31–36. doi: 10.1111/j.1365-2672.1994.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 4.Baele M., Devriese L.A., Butaye P., Haesebrouck F. Composition of enterococcal and streptococcal flora from pigeon intestines. J. Appl. Microbiol. 2002;92:348–351. doi: 10.1046/j.1365-2672.2002.01537.x. [DOI] [PubMed] [Google Scholar]
- 5.Gong J., Forster R.J., Yu H., Chambers J.R., Wheatcroft R., Sabour P.M., Chen S. Molecular analysis of bacterial populations in the ileum of broiler chickens and comparison with bacteria in the cecum. FEMS Microbiol. Ecol. 2002;41:171–179. doi: 10.1111/j.1574-6941.2002.tb00978.x. [DOI] [PubMed] [Google Scholar]
- 6.Devriese L.A., Dutta G.N., Farrow J.A.E., van de Kerckhove A., Phillips B.A. Streptococcus cecorum, a new species isolated from chickens. Int. J. Syst. Bacteriol. 1983;33:772–776. doi: 10.1099/00207713-33-4-772. [DOI] [Google Scholar]
- 7.Wood A.M., MacKenzie G., McGiliveray N.C., Brown L., Devriese L.A., Baele M. Isolation of Enterococcus cecorum from bone lesions in broiler chickens. Vet. Rec. 2002;150:27. [PubMed] [Google Scholar]
- 8.Devriese L.A., Cauwerts K., Hermans K., Wood A. Enterococcus cecorum septicemia as a cause of bone and joint lesions resulting in lameness in broiler chickens. Vlaam. Diergeneeskd. Tijdschr. 2002;71:219–221. [Google Scholar]
- 9.Borst L.B., Suyemoto M.M., Sarsour A.H., Harris M.C., Martin M.P., Strickland J.D., Oviedo E.O., Barnes H.J. Pathogenesis of Enterococcal Spondylitis Caused by Enterococcus cecorum in Broiler Chickens. Vet. Pathol. 2017;54:61–73. doi: 10.1177/0300985816658098. [DOI] [PubMed] [Google Scholar]
- 10.Jung A., Petersen H., Teske L., Rautenschlein S. Colonization patterns of Enterococcus cecorum in two different broiler production cycles detected with a newly developed quantitative real-time PCR. BMC Microbiol. 2017;17:106. doi: 10.1186/s12866-017-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Herdt P.D.P., Van Steelant J., Swam H., Tanghe L., Van Goethem S., Vanrobaeys M. Enterococcus cecorum osteomyelitis and arthritis in broiler chickens. Vlaams Diergeneeskd. Tijdschr. 2008;78:44–48. [Google Scholar]
- 12.Jung A., Rautenschlein S. Comprehensive report of an Enterococcus cecorum infection in a broiler flock in Northern Germany. BMC Vet. Res. 2014;10:311. doi: 10.1186/s12917-014-0311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makrai L., Nemes C., Simon A., Ivanics E., Dudas Z., Fodor L., Glavits R. Association of Enterococcus cecorum with vertebral osteomyelitis and spondylolisthesis in broiler parent chicks. Acta Vet. Hung. 2011;59:11–21. doi: 10.1556/avet.59.2011.1.2. [DOI] [PubMed] [Google Scholar]
- 14.Kense M.J., Landman W.J. Enterococcus cecorum infections in broiler breeders and their offspring: Molecular epidemiology. Avian Pathol. 2011;40:603–612. doi: 10.1080/03079457.2011.619165. [DOI] [PubMed] [Google Scholar]
- 15.Szeleszczuk P.D.B., Zbikowski A., Dolka I., Peryga M. First case of enterococcal spondylitis in broiler chickens in Poland. Med. Weter. 2013;69:298–303. [Google Scholar]
- 16.Albini F., Lobsiger S.T., Renggli H. Infection with the capnophilic bacteria Enterococcus cecorum in broiler chickens. Schweiz Arch. Tierheilkd. 2014;156:295–298. doi: 10.1024/0036-7281/a000594. [DOI] [PubMed] [Google Scholar]
- 17.Stalker M.J., Brash M.L., Weisz A., Ouckama R.M., Slavic D. Arthritis and osteomyelitis associated with Enterococcus cecorum infection in broiler and broiler breeder chickens in Ontario, Canada. J. Vet. Diagn. Investig. 2010;22:643–645. doi: 10.1177/104063871002200426. [DOI] [PubMed] [Google Scholar]
- 18.Robbins K.M., Suyemoto M.M., Lyman R.L., Martin M.P., Barnes H.J., Borst L.B. An outbreak and source investigation of enterococcal spondylitis in broilers caused by Enterococcus cecorum. Avian Dis. 2012;56:768–773. doi: 10.1637/10253-052412-Case.1. [DOI] [PubMed] [Google Scholar]
- 19.Borst L.B., Suyemoto M.M., Robbins K.M., Lyman R.L., Martin M.P., Barnes H.J. Molecular epidemiology of Enterococcus cecorum isolates recovered from enterococcal spondylitis outbreaks in the southeastern United States. Avian Pathol. 2012;41:479–485. doi: 10.1080/03079457.2012.718070. [DOI] [PubMed] [Google Scholar]
- 20.Wijetunge D.S., Dunn P., Wallner-Pendleton E., Lintner V., Lu H., Kariyawasam S. Fingerprinting of poultry isolates of Enterococcus cecorum using three molecular typing methods. J. Vet. Diagn. Investig. 2012;24:1166–1171. doi: 10.1177/1040638712463563. [DOI] [PubMed] [Google Scholar]
- 21.Jackson C.R., Kariyawasam S., Borst L.B., Frye J.G., Barrett J.B., Hiott L.M., Woodley T.A. Antimicrobial resistance, virulence determinants and genetic profiles of clinical and nonclinical Enterococcus cecorum from poultry. Lett. Appl. Microbiol. 2015;60:111–119. doi: 10.1111/lam.12374. [DOI] [PubMed] [Google Scholar]
- 22.Aitchison H., Poolman P., Coetzer M., Griffiths C., Jacobs J., Meyer M., Bisschop S. Enterococcal-related vertebral osteoarthritis in South African broiler breeders: A case report. J. S. Afr. Vet. Assoc. 2014;85:1077. doi: 10.4102/jsava.v85i1.1077. [DOI] [PubMed] [Google Scholar]
- 23.Zeshan B., Khaing A.T., Daud N.H. Enterococcal-Associated Vertebral Osteoarthritis (Evoa) in Broiler Chicken in Malaysia: A Case Report; Proceedings of the 2nd World Veterinary Poultry Association (WVPA) and World Poultry Science Association (WPSA) (Malaysia Branch) Scientifix Conference; Kuala Lumpur, Malaysia. 21–23 September 2015; pp. 77–80. [Google Scholar]
- 24.Jung A., Chen L.R., Suyemoto M.M., Barnes H.J., Borst L.B. A Review of Enterococcus cecorum Infection in Poultry. Avian Dis. 2018;62:261–271. doi: 10.1637/11825-030618-Review.1. [DOI] [PubMed] [Google Scholar]
- 25.Borst L.B., Suyemoto M.M., Scholl E.H., Fuller F.J., Barnes H.J. Comparative genomic analysis identifies divergent genomic features of pathogenic Enterococcus cecorum including a type IC CRISPR-Cas system, a capsule locus, an epa-like locus, and putative host tissue binding proteins. PLoS ONE. 2015;10:e0121294. doi: 10.1371/journal.pone.0121294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung A., Metzner M., Ryll M. Comparison of pathogenic and non-pathogenic Enterococcus cecorum strains from different animal species. BMC Microbiol. 2017;17:33. doi: 10.1186/s12866-017-0949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coil D., Jospin G., Darling A.E. A5-miseq: An updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics. 2015;31:587–589. doi: 10.1093/bioinformatics/btu661. [DOI] [PubMed] [Google Scholar]
- 28.Seemann T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 29.Arndt D., Grant J.R., Marcu A., Sajed T., Pon A., Liang Y., Wishart D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44:W16–W21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S.K., Padmanabhan B.R., Diene S.M., Lopez-Rojas R., Kempf M., Landraud L., Rolain J.M. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob. Agents Chemother. 2014;58:212–220. doi: 10.1128/AAC.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page A.J., Cummins C.A., Hunt M., Wong V.K., Reuter S., Holden M.T., Fookes M., Falush D., Keane J.A., Parkhill J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galperin M.Y., Makarova K.S., Wolf Y.I., Koonin E.V. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res. 2015;43:D261–D269. doi: 10.1093/nar/gku1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchler-Bauer A., Panchenko A.R., Shoemaker B.A., Thiessen P.A., Geer L.Y., Bryant S.H. CDD: A database of conserved domain alignments with links to domain three-dimensional structure. Nucleic Acids Res. 2002;30:281–283. doi: 10.1093/nar/30.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prabha R., Singh D.P., Somvanshi P., Rai A. Functional profiling of cyanobacterial genomes and its role in ecological adaptations. Genom. Data. 2016;9:89–94. doi: 10.1016/j.gdata.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDermott P.F., Tyson G.H., Kabera C., Chen Y., Li C., Folster J.P., Ayers S.L., Lam C., Tate H.P., Zhao S. Whole-Genome Sequencing for detecting antimicrobial resistance in nontyphoidal Salmonella. Antimicrob. Agents Chemother. 2016;60:5515–5520. doi: 10.1128/AAC.01030-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao S., Tyson G.H., Chen Y., Li C., Mukherjee S., Young S., Lam C., Folster J.P., Whichard J.M., McDermott P.F. Whole-Genome Sequencing analysis accurately predicts antimicrobial resistance phenotypes in Campylobacter spp. Appl. Environ. Microbiol. 2016;82:459–466. doi: 10.1128/AEM.02873-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen L.B., Frimodt-Moller N., Aarestrup F.M. Presence of erm gene classes in gram-positive bacteria of animal and human origin in Denmark. FEMS Microbiol. Lett. 1999;170:151–158. doi: 10.1111/j.1574-6968.1999.tb13368.x. [DOI] [PubMed] [Google Scholar]
- 39.Roberts M.C., Sutcliffe J., Courvalin P., Jensen L.B., Rood J., Seppala H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 1999;43:2823–2830. doi: 10.1128/AAC.43.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rollins L.D., Lee L.N., LeBlanc D.J. Evidence for a disseminated erythromycin resistance determinant mediated by Tn917-like sequences among group D streptococci isolated from pigs, chickens, and humans. Antimicrob. Agents Chemother. 1985;27:439–444. doi: 10.1128/AAC.27.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boerlin P., Nicholson V., Brash M., Slavic D., Boyen F., Sanei B., Butaye P. Diversity of Enterococcus cecorum from chickens. Vet. Microbiol. 2012;157:405–411. doi: 10.1016/j.vetmic.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Dolka B., Chrobak-Chmiel D., Makrai L., Szeleszczuk P. Phenotypic and genotypic characterization of Enterococcus cecorum strains associated with infections in poultry. BMC Vet. Res. 2016;12:129. doi: 10.1186/s12917-016-0761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackson C.R., Fedorka-Cray P.J., Barrett J.B., Hiott L.M., Woodley T.A. Prevalence of streptogramin resistance in enterococci from animals: Identification of vatD from animal sources in the USA. Int. J. Antimicrob. Agents. 2007;30:60–66. doi: 10.1016/j.ijantimicag.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Jackson C.R., Fedorka-Cray P.J., Barrett J.B., Hiott L.M., Woodley T.A. First report of vatB and vgaB from Enterococcus gallinarum in the USA. Int. J. Antimicrob. Agents. 2008;31:175–176. doi: 10.1016/j.ijantimicag.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 45.Torres C., Alonso C.A., Ruiz-Ripa L., Leon-Sampedro R., Del Campo R., Coque T.M. Antimicrobial Resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. 2018;6 doi: 10.1128/microbiolspec.ARBA-0032-2018. [DOI] [PubMed] [Google Scholar]
- 46.Miller W.R., Munita J.M., Arias C.A. Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti-Infect. Ther. 2014;12:1221–1236. doi: 10.1586/14787210.2014.956092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen J., Wang Y., Schwarz S. Presence and dissemination of the multiresistance gene cfr in Gram-positive and Gram-negative bacteria. J. Antimicrob. Chemother. 2013;68:1697–1706. doi: 10.1093/jac/dkt092. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Lv Y., Cai J., Schwarz S., Cui L., Hu Z., Zhang R., Li J., Zhao Q., He T., et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J. Antimicrob. Chemother. 2015;70:2182–2190. doi: 10.1093/jac/dkv116. [DOI] [PubMed] [Google Scholar]
- 49.Tian Y., Li T., Zhu Y., Wang B., Zou X., Li M. Mechanisms of linezolid resistance in staphylococci and enterococci isolated from two teaching hospitals in Shanghai, China. BMC Microbiol. 2014;14:292. doi: 10.1186/s12866-014-0292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mendes R.E., Deshpande L.M., Jones R.N. Linezolid update: Stable in vitro activity following more than a decade of clinical use and summary of associated resistance mechanisms. Drug Resist. Updat. 2014;17:1–12. doi: 10.1016/j.drup.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 51.He Q., Hou Q., Wang Y., Li J., Li W., Kwok L.Y., Sun Z., Zhang H., Zhong Z. Comparative genomic analysis of Enterococcus faecalis: Insights into their environmental adaptations. BMC Genom. 2018;19:527. doi: 10.1186/s12864-018-4887-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raven K.E., Reuter S., Gouliouris T., Reynolds R., Russell J.E., Brown N.M., Torok M.E., Parkhill J., Peacock S.J. Genome-based characterization of hospital-adapted Enterococcus faecalis lineages. Nat. Microbiol. 2016;1:15033. doi: 10.1038/nmicrobiol.2015.33. [DOI] [PubMed] [Google Scholar]
- 53.Bakshi U., Sarkar M., Paul S., Dutta C. Assessment of virulence potential of uncharacterized Enterococcus faecalis strains using pan genomic approach—Identification of pathogen-specific and habitat-specific genes. Sci. Rep. 2016;6:38648. doi: 10.1038/srep38648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghattargi V.C., Gaikwad M.A., Meti B.S., Nimonkar Y.S., Dixit K., Prakash O., Shouche Y.S., Pawar S.P., Dhotre D.P. Comparative genome analysis reveals key genetic factors associated with probiotic property in Enterococcus faecium strains. BMC Genom. 2018;19:652. doi: 10.1186/s12864-018-5043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Medini D., Donati C., Tettelin H., Masignani V., Rappuoli R. The microbial pan-genome. Curr. Opin. Genet. Dev. 2005;15:589–594. doi: 10.1016/j.gde.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Ghattargi V.C., Nimonkar Y.S., Burse S.A., Davray D., Kumbhare S.V., Shetty S.A., Gaikwad M.A., Suryavanshi M.V., Doijad S.P., Utage B., et al. Genomic and physiological analyses of an indigenous strain, Enterococcus faecium 17OM39. Funct. Integr. Genom. 2018;18:385–399. doi: 10.1007/s10142-018-0596-x. [DOI] [PubMed] [Google Scholar]
- 57.Naito M., Pawlowska T.E. The role of mobile genetic elements in evolutionary longevity of heritable endobacteria. Mob. Genet. Elem. 2016;6:e1136375. doi: 10.1080/2159256X.2015.1136375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacobitz A.W., Kattke M.D., Wereszczynski J., Clubb R.T. Sortase Transpeptidases: Structural Biology and Catalytic Mechanism. Adv. Protein Chem. Struct. Biol. 2017;109:223–264. doi: 10.1016/bs.apcsb.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonacina J., Suarez N., Hormigo R., Fadda S., Lechner M., Saavedra L. A genomic view of food-related and probiotic Enterococcus strains. DNA Res. 2017;24:11–24. doi: 10.1093/dnares/dsw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.