Abstract
Purpose
To evaluate the clinical characteristics and prognostic factors in patients with adrenocortical carcinoma (ACC) in South Korea.
Methods
A nationwide, registry-based survey was conducted to identify pathologically proven ACC at 25 tertiary care centers in South Korea between 2000 and 2014. Cox proportional hazard model and log-rank test were adopted for survival analysis.
Results
Two hundred four patients with ACC were identified, with a median follow-up duration of 20 months (IQR 5–52 months). The median age at diagnosis was 51.5 years (IQR 40–65.8 years), and ACC was prevalent in women (n = 110, 53.9%). Abdominal pain was the most common clinical symptom (n = 70, 40.2%), and ENSAT stage 2 was most common (n = 62, 30.4%) at the time of diagnosis. One hundred sixty-nine patients underwent operation, while 17 were treated with other modalities. The remission rate was 48%, and median recurrence-free survival time was 46 months. Estimated 5-year recurrence-free rate was 44.7%. There were more women, large tumor, atypical mitosis, venous invasion, and higher mitotic count in cancer recurrence group. Estimated 5-year overall survival and disease-specific survival rates were 64.5 and 70.6%, respectively. Higher ENSAT stage and advanced pathologic characteristics were risk factors for all-cause mortality of ACC. Large tumor size and cortisol-secreting tumor were additional risk factors for ACC-specific death.
Conclusions
We report the first epidemiologic study regarding ACC in an Asian population. ENSAT stage 4; lymph node involvement; non-operative group; and invasion of vein, sinusoid, or capsule were associated with an increased risk for all-cause mortality.
Keywords: adrenocortical carcinoma, epidemiology, recurrence, survival, Korean
Introduction
Adrenocortical carcinoma (ACC) is a rare disease with an estimated annual incidence of 0.7–2.0 per million population (1, 2). The prevalence of ACC varies depending on the circumstances under which patient data are collected. The reported frequency of ACC is derived from highly selected patient populations and may not reflect the prevalence rates observed in population-based studies. Combining the studies reported from 1982 to 2008, the ACC etiology of adrenal incidentaloma ranged from 0 to 14% (3, 4, 5, 6, 7). Further, studies including patients with symptoms or signs caused by hormone excess or an abdominal mass showed a higher prevalence of ACC within the group of adrenal incidentalomas, 10–15% (8, 9, 10). ACC can occur at any age, with a peak incidence between 40 and 50 years, and has a female predominance (55–60%) (2, 11). Most ACCs occur sporadically but rarely are related to various hereditary syndromes, including Li Fraumeni syndrome (12), multiple endocrine neoplasia type 1 (13), Beckwith–Wiedemann syndrome (14), Lynch syndrome (15), and others (16). Moreover, at least 50–60% of those with ACC show clinical hormone excess; the most common form is hypercortisolism (Cushing syndrome) (17).
Although the most common genetic alterations in ACC were TP53 and CTNNB1 mutations and CDKN2A and ZNRF3 homozygous deletions (18), the molecular and cellular mechanisms underlying the development of ACC have not been fully clarified; multi-omic studies demonstrated that only a minority of patients with ACC have pathogenic driver mutations (19, 20). Complete surgical removal can lead to cure (17). However, the prognosis of ACC remains a challenge. The median overall survival (OS) of all patients with ACC is approximately 3–4 years; therapeutic outcomes are heterogeneous (17). There are several known prognostic factors in patients with ACC, including clinical, pathological, and molecular factors (18). Clinical prognostic factors include advanced tumor stage, cortisol excess, and older age (18). In addition, poor prognosis is associated with pathologic factors, including tumor grade, mitotic count, Ki-67 proliferation index (21), resection status (22), and results of p53 and CTNNB1 immunohistochemistry (18). Recently, progress in genomics has allowed research on the molecular prognostic markers of ACC (18). Nonetheless, there is a paucity of data on recurrence or survival in Asian patients with ACC. Understanding of the clinical characteristics and prognostic stratification of ACC in the Asian population is essential for proper management.
Here, we investigated epidemiologic data including clinical manifestations and imaging/pathologic findings of patients with ACC in South Korea. Based on these results, we aimed to analyze the differences in characteristics according to remission, recurrence, and overall survival to identify prognostic factors of Asian patients with ACC.
Materials and methods
Data collection
We aimed to identify all patients aged 18 years and older diagnosed with or treated for ACC in South Korea and designed a patient cohort study with a retrospectively collected dataset. Among all 43 tertiary care institutions in South Korea, 25 participated in the data search for patients with ACC managed at their institutions. Multiple endocrinologists at each institution reviewed the medical records of all patients registered in this study to validate the diagnosis of ACC based on the following criteria: (1) diagnosed or treated by an endocrinologist between January 2000 and December 2014 and (2) reported by a pathologist via surgical resection or biopsy specimen. The registration process proceeded between June 2015 and March 2018. Patients with ACC were identified based on the following International Classification of Diseases, 10th revision (ICD-10) codes: C740 (primary malignant neoplasm of adrenal cortex, nonfunctioning adrenal carcinoma) and C749 (primary malignant neoplasm of adrenal gland, other type of adrenal cancer, unspecified adrenal cancer). Patients with metastasis to the adrenal gland were excluded.
The following data were collected from registered ACC patients: age at diagnosis, sex, BMI, date of last visit, managing institution, clinical symptoms at diagnosis, comorbidities, results of biochemical and hormonal tests to confirm tumor functionality, abdominal CT findings including size and pre-contrast Hounsfield unit (HU), pathologic findings including Ki-67 index and Weiss score, presence and location of distant metastasis at diagnosis, the first-line treatment modality, postoperative adjuvant therapy, and current progress including recurrence and mortality. Endocrinologists at each institution confirmed the presence of remission after surgery and the disease recurrence. Patient date of birth, initials, and home address were also collected to exclude duplicated subjects. In this study, the European Network for the Study of Adrenal Tumors (ENSAT)-staging system was used to evaluate clinical stage (9) because, among all proposed classifications, this system may provide the best survival discrimination in patients with ACC (18).
The institutional review board of each participating institution approved the current study based on the study protocol of Seoul National University Hospital (No.1505-051-671). The study was performed in accordance with the Declaration of Helsinki. The need to obtain informed consent from participants was waived due to the retrospective nature of this study.
Statistical analysis
Data were analyzed using IBM SPSS, version 23.0 for Windows (SPSS Inc.). Continuous variables are presented as median (interquartile range, IQR). Categorical variables are presented as number (%). Comparisons between groups were conducted using chi-square test, Mann–Whitney U test, and Fisher’s exact test. In addition, Cox proportional hazard model and log-rank tests were used to evaluate the prognosis of patients with ACC. Two-sided P values less than 0.05 were considered statistically significant.
Results
Baseline characteristics and clinical manifestations of the study population
As shown in Table 1, 204 patients diagnosed with ACC from 25 hospitals were included in the study. The median follow-up duration was 20 months (IQR 5–52 months). The median age was 51.5 years (IQR 40–65.8 years) and female patients were dominant (n = 110, 53.9%). Among 188 patients with identifiable data of initial presentation at the time of diagnosis, 154 (81.9%) were symptomatic. The most common chief complaint was abdominal pain (n = 70, 40.2%), followed by palpable abdominal mass (n = 61, 33.9%). On abdominal CT images, the median tumor size was 85 mm (IQR 59–120 mm), with pre-contrast 34.4 HU (IQR 31.1–39.5 HU). Hypercortisolism (n = 62, 47.7%), elevated serum dehydroepiandrosterone-sulfate (DHEA-S; n = 21, 38.2%), elevated 24-h urine 17-ketosteroid (n = 16, 32.7%), and excessive aldosterone level (n = 14, 11.5%) within obtainable data.
Table 1.
Demographic characteristics of patients with ACC (total n = 204).
| Available | n (%) | Median (IQR) | |
|---|---|---|---|
| Sex | 204 | ||
| Male | 94 (46.1) | ||
| Female | 110 (53.9) | ||
| Age at diagnosis (years) | 204 | 51.5 (40–65.8) | |
| BMI (kg/m2) | 147 | 23.3 (21.2–25.5) | |
| Symptom and sign at diagnosis | 188 | 154 (81.9) | |
| Abdominal pain | 174 | 70 (40.2) | |
| Abdominal mass | 180 | 61 (33.9) | |
| Edema | 154 | 36 (23.4) | |
| Fatigue | 155 | 34 (21.9) | |
| Weight loss | 160 | 23 (14.4) | |
| Central obesity | 149 | 21 (14.1) | |
| Underlying disease | |||
| Hypertension | 198 | 81 (40.9) | |
| Diabetes mellitus | 197 | 40 (20.3) | |
| Other malignancy | 195 | 20 (10.3) | |
| Osteoporosis | 178 | 12 (6.7) | |
| Ischemic heart disease | 191 | 8 (4.2) | |
| Arrhythmia | 193 | 7 (3.6) | |
| Heart failure | 193 | 6 (3.1) | |
| Stroke | 191 | 4 (2.1) | |
| Functioning tumor | 130 | 74 (56.9) | |
| Hypercortisolism | 130 | 62 (47.7) | |
| Elevated serum DHEA-S | 55 | 21 (38.2) | |
| Elevated 24hr urine 17-KS | 49 | 16 (32.7) | |
| Aldosterone excess | 122 | 14 (11.5) | |
| Abdominal CT finding | |||
| Size (mm) | 187 | 85 (59–120) | |
| Pre-contrast HU | 73 | 34.4 (31.1–39.5) | |
| Right:Left:Bilateral | 199 | 91:103:5 | |
| Heterogeneity (yes) | 154 | 148 (96.1) | |
| Necrosis (yes) | 161 | 104 (64.6) | |
| Calcification (yes) | 162 | 40 (24.7) | |
| Hemorrhage (yes) | 161 | 31 (19.3) |
17-KS, 17-ketosteroid; ACC, adrenocortical carcinoma; BMI, body mass index; CT, computed tomography; DHEA-S, dehydroepiandosterone sulphate; HU, Hounsfield unit; IQR, interquartile range.
Table 2 shows the prevalence of ACC based on the ENSAT staging system and pathologic findings. By the ENSAT staging system, stage 2 (n = 62, 30.4%) was the most common at the time of diagnosis, followed in order by stage 4 (n = 58, 28.4%), stage 3 (n = 35, 17.2%), and stage 1 (n = 19, 9.3%). There were 56 available data of distant metastasis with multiple answers allowed: 34 in the lung, 32 in the liver, 13 in the bone, 3 in the brain, and 1 in the pericardial metastasis. The number of crude incidence cases is presented in Supplementary Fig. 1 (see section on supplementary materials given at the end of this article).
Table 2.
Stages and pathologic findings.
| Available | n (%) | Median (IQR) | |
|---|---|---|---|
| ENSAT stage | 174 | ||
| 1 | 19 (9.3) | ||
| 2 | 62 (30.4) | ||
| 3 | 35 (17.2) | ||
| 4 | 58 (28.4) | ||
| Unknown | 30 | 30 (14.7) | |
| Pathology | |||
| Weiss score | 61 | ||
| ≤3 | 21 (34.4) | ||
| >3 | 40 (65.6) | ||
| Size (mm) | 147 | 100 (64–130) | |
| Ki-67 index | 30 | 8 (4.5–16.3) | |
| Mitotic count (/50HPF) | 100 | 10 (5–28.8) | |
| High nuclear grade (yes) | 67 | 62 (92.5) | |
| Atypical mitosis (yes) | 72 | 26 (36.1) | |
| Diffuse architecture (yes) | 47 | 39 (83.0) | |
| Clear cell component (yes) | 63 | 47 (74.6) | |
| Necrosis (yes) | 137 | 122 (89.1) | |
| Venous invasion (yes) | 107 | 52 (48.61) | |
| Sinusoidal invasion (yes) | 60 | 23 (38.3) | |
| Capsular invasion (yes) | 123 | 80 (65) |
ENSAT, European Network for Study of Adrenal Tumors; IQR, interquartile range.
Treatment and overall prognosis
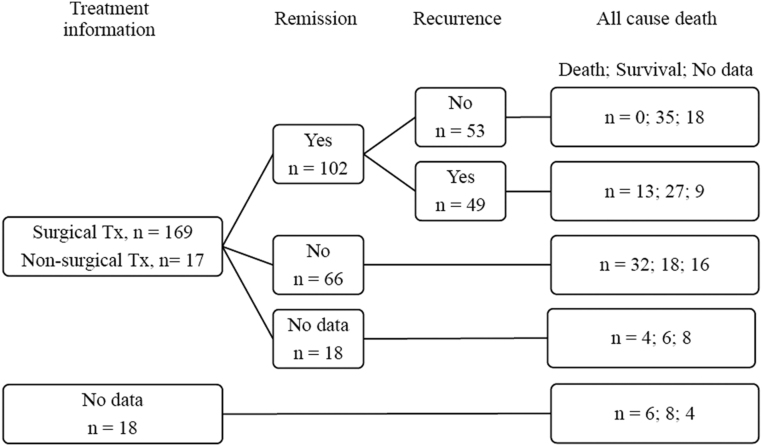
Figure 1 summarizes the prognosis of patients with ACC who had data for treatment modality using a flowchart. A total of 169 patients underwent surgery (84.1%), 149 of which were total adrenalectomy. Among 32 patients who did not undergo surgical treatment, 17 had identifiable data regarding one or more alternative palliative treatment methods: 5 for cytotoxic chemotherapy, 5 for mitotane therapy, 3 for both chemotherapy and mitotane, 3 for both mitotane and radiation therapy, and 1 for all three modalities.
Figure 1.
Treatment and prognosis of patients with ACC. The figures in each box indicates the number of patients. After the first-line treatment, 102 patients reached remission state. Among those with remission, 49 cases of disease recurrence were reported during the follow-up period. There were 55 mortality cases in total, 10 from the group of the missing data of treatment information. No mortality case of death was reported in patients without recurrence. ACC, adrenocortical carcinoma; Tx, treatment.
After the first-line surgical treatment, 102 patients have reached remission of ACC, while 66 had remnant disease. Forty-nine cases of disease recurrence were reported during the follow-up period. In total cohort, there were 55 cases of mortality, which was most common in the non-remission group. Death was not reported in patients without recurrence.
Disease remission and recurrence
There were 36 patients who had missing data for remission state; the remaining 168 patients were included in the analysis of remission and recurrence. In 168 patients who had follow-up records, 102 (60%) achieved remission. Table 3 compares the characteristics of remission and remnant disease groups. Remission was more frequent in female patients (61.8 vs 42.4%, P = 0.017), patients without symptoms at diagnosis (26.5 vs 7.1%, P = 0.013), and patients without distant metastasis (95.6 vs 26.8%, P < 0.001). The ENSAT stage was significantly different between the remission and non-remission groups: the proportion of stages 1–4 of remission-reached group was 14.3, 53.8, 27.5, and 4.4% in order, while that of remnant disease group was 5.4, 14.3, 7.1, and 73.2% (P for intergroup difference <0.001). The remission group had smaller tumor size (91.5 mm vs 125 mm, P = 0.018) and lower mitotic count (9/50 HPF vs 19.5/50 HPF, P = 0.030) than the remnant disease group. Age, BMI, hormonal functionality, lymph node metastasis, and operation type did not show statistical differences.
Table 3.
Characteristics and findings according to remission after first-line treatment (n = 168).
| Remission (+), n = 102 | Remission (−), n = 66 | P value | |||
|---|---|---|---|---|---|
| Available data | Valuea | Available data | Valuea | ||
| Age at diagnosis (years) | 102 | 49.5 (39.0–63.0) | 66 | 50 (38.5–61.3) | 0.961 |
| BMI (kg/m2) | 75 | 23.1 (20.9–25.9) | 47 | 23.3 (21.4–25.5) | 0.839 |
| Male | 102 | 39 (38.2) | 66 | 38 (57.6) | 0.017 |
| Initial symptom (yes) | 68 | 50 (73.5) | 42 | 39 (92.9) | 0.013 |
| Functional tumor (yes) | 70 | 40 (57.1) | 42 | 26 (61.9) | 0.694 |
| Distant metastasis (yes) | 91 | 4 (4.4) | 56 | 41 (73.2) | <0.001 |
| Lymph node metastasis (yes) | 6 | 2 (33.3) | 44 | 17 (38.6) | 1.000 |
| CT | |||||
| Tumor size (mm) | 94 | 76.5 (54.8–114.8) | 61 | 100 (65–140) | 0.009 |
| Pre-contrast HU | 36 | 35.0 (28.8–39.7) | 18 | 34.4 (32.5–39.9) | 0.734 |
| Complete adrenalectomy (yes) | 102 | 90 (88.2) | 50 | 44 (88) | 1.000 |
| Pathology | |||||
| Weiss score | 42 | 4 (2–6) | 13 | 6 (4–7) | 0.103 |
| Tumor size (mm) | 92 | 91.5 (60.0–130.0) | 41 | 125 (79.5–155) | 0.018 |
| Ki67 index | 24 | 7.0 (3.5–10.8) | 3 | 20 (3–20) | 0.393 |
| Mitotic count (/50HPF) | 62 | 9.0 (3.8–24.3) | 28 | 19.5 (5.0–48.8) | 0.030 |
| ENSAT stage | 91 | 56 | <0.001 | ||
| 1 | 13 (14.3) | 3 (5.4) | |||
| 2 | 49 (53.8) | 8 (14.3) | |||
| 3 | 25 (27.5) | 4 (7.1) | |||
| 4 | 4 (4.4) | 41 (73.2) | |||
| Death (yes) | 75 | 13 (17.3) | 50 | 32 (64) | <0.001 |
Chi-square test, Fischer’s exact test, and Mann–Whitney U test were adopted for comparison.
aContinuous variables are presented as median (interquartile range), categorical variables as n (%).
HPF, high power field.
Among 102 cases of remission during the follow-up period, there were 49 recurrent cases. As shown in Table 4, ACC recurrence was reported more in the female population (73.5 vs 50.9%, P = 0.019), with larger tumors (110 mm vs 80 mm, P = 0.007), in tumors with venous invasion (60.6 vs 24.2%, P = 0.006), and with higher mitotic count (15/50 HPF vs 5/50 HPF, P = 0.015). There was no significant difference between groups regarding initial presentation with symptoms or signs (67.6 vs 79.4%, P = 0.272) or ENSAT stage (P = 0.948): stages 1 to 4 accounted for 16.3, 51.0, 26.5, and 6.1% in non-recurrent group and 11.9, 57.1, 28.6, and 2.4% in recurrent group, respectively.
Table 4.
Characteristics and findings according to presence of recurrence (n = 102).
| Recurrence (−), n = 53 | Recurrence (+), n = 49 | P value | |||
|---|---|---|---|---|---|
| Available data | Valuea | Available data | Valuea | ||
| Age at diagnosis (years) | 53 | 50 (39–63) | 49 | 48 (37.5–63.5) | 0.730 |
| BMI (kg/m2) | 43 | 23.3 (20.9–25.9) | 32 | 22.8 (20.9–25.9) | 0.748 |
| Male | 53 | 26 (49.1) | 49 | 13 (26.5) | 0.019 |
| Initial symptom (yes) | 34 | 23 (67.6) | 34 | 27 (79.4) | 0.272 |
| Functional tumor (yes) | 36 | 20 (55.6) | 34 | 20 (58.8) | 0.782 |
| CT | 51 | 48 | |||
| Right | 21 (41.2) | 17 (35.4) | 0.556 | ||
| Left | 30 (58.8) | 31 (64.6) | |||
| Tumor size (mm) | 49 | 67 (51–96.5) | 45 | 100 (58–120) | 0.008 |
| Pre-contrast HU | 21 | 35 (28–39.6) | 15 | 34.3 (31–39.8) | 0.810 |
| Necrosis (yes) | 41 | 26 (63.4) | 40 | 29 (72.5) | 0.381 |
| Hemorrhage (yes) | 40 | 10 (25.0) | 40 | 6 (15.0) | 0.264 |
| Calcification (yes) | 41 | 11 (26.8) | 41 | 8 (19.5) | 0.432 |
| ENSAT stage | 49 | 42 | |||
| 1 | 8 (16.3) | 5 (11.9) | 0.948 | ||
| 2 | 25 (51.0) | 24 (57.1) | |||
| 3 | 13 (26.5) | 12 (28.6) | |||
| 4 | 3 (6.1) | 1 (2.4) | |||
| Lymph node metastasis (yes) | 4 | 1 (25.0) | 2 | 1 (50.0) | 1.000 |
| Surgery | 53 | 49 | |||
| Complete adrenalectomy | 48 (90.6) | 42 (85.7) | 0.447 | ||
| Partial adrenalectomy | 5 (9.4) | 7 (14.3) | |||
| Adjuvant therapy after surgery (yes) | 50 | 13 (26.0) | 45 | 16 (35.6) | 0.313 |
| Pathology | |||||
| High nuclear grade (yes) | 24 | 21 (87.5) | 20 | 18 (90.0) | 1.000 |
| Atypical mitosis (yes) | 26 | 6 (23.1) | 20 | 10 (50.0) | 0.070 |
| Diffuse architecture (yes) | 14 | 12 (85.7) | 17 | 14 (82.4) | 1.000 |
| Clear cell component (yes) | 22 | 16 (72.7) | 18 | 14 (77.8) | 1.000 |
| Necrosis (yes) | 38 | 31 (81.6) | 41 | 37 (90.2) | 0.338 |
| Venous invasion (yes) | 33 | 8 (24.2) | 33 | 20 (60.6) | 0.006 |
| Sinusoid invasion (yes) | 21 | 5 (23.8) | 18 | 9 (50.0) | 0.108 |
| Capsular invasion (yes) | 43 | 23 (53.5) | 37 | 26 (70.3) | 0.168 |
| Weiss score | 20 | 4 (2–5) | 22 | 5 (3–7) | 0.174 |
| Tumor size (mm) | 47 | 80 (54–120) | 45 | 110 (80–140) | 0.007 |
| Ki 67 index | 13 | 6 (4–8) | 11 | 10 (3–20) | 0.123 |
| Mitotic count (/50HPF) | 29 | 5 (2–12) | 33 | 15 (5–35) | 0.012 |
| Death (yes) | 35 | 0 (0) | 40 | 13 (32.5) | <0.001 |
Chi-square test, Fisher’s exact test, and Mann–Whitney U tests were adopted for comparison.
aContinuous variables are presented as median (interquartile range), categorical variables as n (%).
Mortality
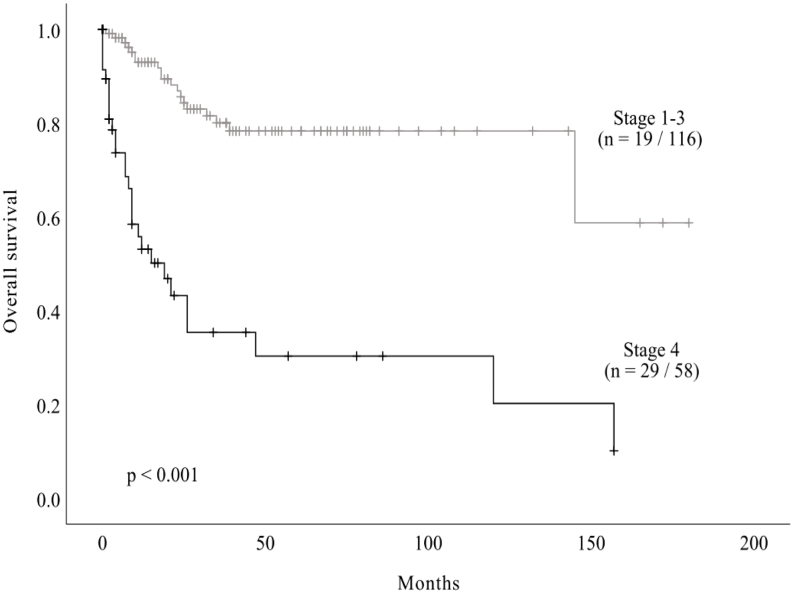
Fifty-five deaths (27%) were reported during the follow-up period. The median OS of total cohort was as 145 months (95% CI 101.8 – 188.2) and estimated 5-year OS rate was 64.5%. Among the total cohort, there were 174 cases with known ENSAT stage. There were 19 and 29 events in stages 1–3 and stage 4, respectively. Estimated 5-year OS rates were 91.7, 76.9, 78.3, and 30.3% from ENSAT stages 1 to 4, respectively. Kaplan–Meier curves and estimates of OS data according to ENSAT stage are plotted in Fig. 2. The median survival of stage 4 advanced disease was 19 months while that of less-advanced disease (stages 1–3) was 138.4 months, and the OS rates significantly differed (log-rank test, P < 0.001).
Figure 2.
Kaplan–Meier estimates of survival of patients with ACC according to ENSAT stage. Among a total cohort of 204 cases, 174 had known ENSAT stage. Median OS of these patients was 145 months (95% CI 101.8–188.2), and estimated 1-year, 2-year, and 5-year OS rates were 82.6, 75.0, and 64.5%, respectively. There were 19 and 29 events in stages 1–3 and 4, respectively. Mean survival of stages 1–3 was 138.4 months, and median survival of stage 4 was 19 months. ACC, adrenocortical carcinoma; CI, confidence interval; OS, overall survival.
Risk factors for mortality were assessed with univariable Cox proportional hazard models (Table 5). Since there were some missing values for variables regarding prognosis, it was not possible to create valid multivariable models due to small case numbers. For all-cause mortality, advanced ENSAT stage had the highest hazard ratio (HR) of 5.61 (95% CI 3.11–10.11), followed by sinus invasion in pathologic review (HR 5.50, 95% CI 1.93–15.71). The presence of lymph node metastasis, venous invasion, and capsular invasion were also statistically significant risk factors. Adrenalectomy was a protective factor (HR 0.11, 95% CI 0.06–0.19).
Table 5.
All-cause mortality and ACC-specific mortality Cox models (univariable analysis).
| Event/available (n) | HR | 95% CI | P value | |
|---|---|---|---|---|
| All-cause mortality | ||||
| ENSAT stage 4 (vs stage 1–3) | 48/174 | 5.61 | 3.11–10.11 | <0.001 |
| Lymph node metastasis (yes) | 32/65 | 2.45 | 1.19–5.05 | 0.016 |
| Adrenalectomy (yes) | 55/200 | 0.11 | 0.06–0.19 | <0.001 |
| Pathology | ||||
| Venous invasion (yes) | 25/107 | 2.67 | 1.17–6.06 | 0.019 |
| Sinusoid invasion (yes) | 17/60 | 5.50 | 1.93–15.71 | 0.001 |
| Capsular invasion (yes) | 27/123 | 3.28 | 1.13–9.54 | 0.029 |
| ACC-specific mortality | ||||
| CT size | 42/178 | 1.01 | 1.00–1.01 | 0.043 |
| CT pre-contrast HU | 16/73 | 1.04 | 1.01–1.08 | 0.009 |
| ENSAT stage 4 (vs. stage 1–3) | 38/174 | 5.62 | 2.89–10.90 | <0.001 |
| Hypercortisolism (yes) | 25/113 | 2.76 | 1.13–6.71 | 0.025 |
| Lymph node metastasis (yes) | 25/65 | 2.81 | 1.22–6.47 | 0.015 |
| Adrenalectomy (yes) | 44/200 | 0.08 | 0.044–0.16 | <0.001 |
| Adjuvant therapy after surgery (yes)a | 9/95 | 4.76 | 1.19–19.03 | 0.027 |
| Pathology | ||||
| Venous invasion (yes) | 19/107 | 3.29 | 1.24–8.71 | 0.016 |
| Sinusoid invasion (yes) | 13/60 | 12.77 | 2.81–58.03 | 0.001 |
Only statistically significant variables are listed.
aAdjuvant therapy was analyzed in patient with complete surgical removal.
HR was also calculated for ACC-specific mortality in univariable analysis (Table 5). Sinusoid invasion had the highest HR of 12.77 (95% CI 2.81–58.03), followed by ENSAT stage 4 disease (HR 5.62, 95% CI 2.89–10.90). Large mass size, higher pre-contrast HU on CT, and tumor functionality (especially hypercortisolism) were additional risk factors for ACC-specific mortality.
Discussion
ACC is an extremely rare but aggressive disease for which it is essential to obtain epidemiologic data, such as clinical characteristics and prognostic factors. The incidence rate of ACC was 0.5–2 cases per 1,000,000 person-years in previous reports (1, 23). In a population-based study conducted in the United States, Sharma et al. found that ACC incidence was higher in Caucasians, with a ratio of 6.3:1 (23). While racial, ethnic, and regional differences may exist, underestimation of cases from hospitals that were not included in the survey could be another explanation for the low incidence rate (Supplementary Fig. 1). Additionally, the incidence rate has slightly increased in recent years. Recent high-resolution imaging studies and a nationwide health screening program could contribute to increased incidental identification of adrenal masses (24). ACC was diagnosed more frequently in females and in the fifth decade, consistent with previous studies (2, 22, 25, 26, 27, 28).
The more symptomatic cases were observed in our study. There were 111 cases of abdominal pain and/or palpable abdominal mass as symptoms at diagnosis (59.4% of known information), which is a higher rate than previous studies. Iñiguez-Ariza et al. reported the mode of ACC discovery as follows: 42% incidentally found, 32% hormone excess, and 20% mass effect (29). Several patients reported no symptoms even when the mass was larger than 8 cm. Since the data were collected by questionnaire retrospectively, the relationship between symptom and tumor size could not be concluded. In addition, our data contain the symptoms and signs at the time of diagnosis, which is not the same as the mode of ACC diagnosis. This point requires careful interpretation. In other words, it is hard to distinguish whether ACC was diagnosed due to the symptoms examined or whether ACC was found in testing for other symptoms or by accident in our dataset.
In diagnostic CT images, pre-contrast density was 34.4 HU, and median size was 85 mm. These findings were in accordance with known findings of ACC with higher pre-contrast HU and large size (30, 31, 32). Mass heterogeneity, necrosis, calcification, and hemorrhage were also observed in CT images. There are previous reports on tumor laterality (26, 33, 34, 35, 36), and left-side ACC was prevalent in the current study. ACC laterality is not fully explained in the current study. According to ENSAT stage (37), the majority of patients in this cohort presented with stage 2 (30.4%) ACC, consistent with previous findings (32). Common distant metastasis sites were lung, liver, and bone. Despite few (56 cases) identifiable records of the location of distant metastasis, the tendency of frequent metastatic sites was similar to previous studies (32, 38, 39, 40, 41, 42).
It is widely accepted that Weiss score of 3 or higher implies malignant potential of ACC, and Ki-67 index higher than 5% is only observed in malignancy. In the current study, there were 13 pathologic ACC cases with Weiss score of 1 or 2 and 11 cases with Ki-67 index of 1–5. We propose two possibilities for this result. First, there were some cases of Weiss score <3 with aggressive disease course. Initially low-Weiss score tumors can progress to metastatic lesions during follow up. Pohlink et al. reported a case of Weiss 2 tumor that recurred 6 years later with lung metastases (43), and Papotti et al. reported myxoid type ACC cases with low Weiss score (44). Similarly, one patient with Weiss 2 score in our cohort showed distant metastasis at the time of diagnosis (ENSAT stage 4). Also, there were three patients with Weiss <3 and ENSAT stage 3 and four patients with Weiss <3 in whom the disease recurred after initial remission. Second, even though a higher Ki-67 index indicates malignant behavior, a low Ki-67 index does not always define benign behavior (45). Stojadinovic et al. reported Ki-67 overexpression in 35.5% of ACC cases (46). Our data include one ENSAT stage 4 patient with Ki-67 3%. These findings suggest that we should carefully evaluate and follow the progress of patients with low Weiss score and/or low Ki-67.
In Korea, surgical resection remains the mainstay of therapy, occurring in 84.1% of treated cases, consistent with other reports (21, 23, 40). Surgical removal of primary disease is often curative and could yield survival benefits in advanced disease (21, 40). Of the 29 patients with information on adjuvant treatment modality, 4 received cytotoxic chemotherapy, 18 received mitotane, and 7 received radiation therapy. Data regarding mitotane dose, duration, and side effects were not included in the survey to determine therapeutic and adverse outcomes in a Korean ACC patient cohort. Although ACC was previously considered a radiotherapy-resistant disease, and there were contradictory results of adjuvant radiotherapy (47, 48), recent studies revealed 56–100% local disease control in the adjuvant setting (49, 50, 51, 52) without an advantage in OS. Radiation therapy should be considered in selected patients to prevent local recurrence.
In this study, all-cause mortality rate was 27%, and 5-year OS rate was 64.5%, which is higher than previous studies, stating 5-year OS rate of 35–48% (6, 38, 53, 54, 55, 56). Despite progress in understanding the molecular pathogenesis of ACC, tumor stage remains the main factor for predicting prognosis in patients with ACC. Five-year survival is reported to be 60–80% for tumors confined to the adrenal gland, 35–50% for locally advanced disease, and much lower in patients with metastatic disease (0–28%) (17, 18, 22, 31, 57, 58). As expected, higher ENSAT stage, lymph node metastasis, and the presence of venous, sinusoid, and capsular invasion were prognostic risk factors. Functioning tumors, especially ACC with cortisol excess, showed HR of 2.76 (95% CI 1.13–6.71, P = 0.025) for ACC-specific death but not for all-cause mortality. The poorer prognosis of cortisol-secreting tumors might be related to comorbidity with Cushing’s syndrome and its possible immunosuppressive effects that may promote tumor development and metastasis (6, 59). Nevertheless, the effect of cortisol secretion on survival of patients with ACC remains uncertain (18). Some studies reported that sex can affect survival rate (27, 36, 60), but we found no such relationship in this cohort. Therefore, prospective research with long-term follow-up is required to investigate the risk factors linked to survival and prognosis in Asian patients with ACC.
The major strength of our study is that we analyzed ACC data in an Asian population. To our knowledge, this is the first nationwide multicenter cohort study of ACC conducted in Asia. Although ACC is a rare disease, this study was initiated with awareness of the demand for a Korean population-specific database. However, there were some limitations as well. First, because it was a retrospective registry-based study, there were missing data of several clinical variables. Multivariable Cox-proportional hazard models for disease outcome were not constructed due to this shortage, although multivariable models with risk factors would help to predict the prognosis for an individual case. Secondly, there were no detailed data on surgical resection type and not enough pathologic data which are considered prognostic factors. Data regarding Ki-67 index, an independent prognostic factor for predicting the survival of patients with ACC (18), were insufficient because few hospitals performed the Ki-67 index test. Weiss score is also considered an informative factor to assess the prognosis of ACC; however, the majority of our data had partial data of Weiss score components or small number of Weiss score. Instead, we analyzed each pathologic characteristic as well as the Weiss score to make the best use of our dataset. Third, this study could not cover all incident ACC cases in Korea since 25 of 43 tertiary hospitals of the country participated in the survey. In addition, we consider the rather short duration of follow-up as another shortage of our study. Therefore, future studies should include more detailed data on surgical treatment and pathologic findings in longer duration.
In conclusion, we report the first patient-based cohort study of 204 cases of ACC in Korea. Higher ENSAT stage and advanced pathologic characteristics were risk factors for all-cause mortality, and large tumor size and cortisol-secreting tumor were additional risk factors for ACC-specific death. The results of the current study may help with disease prognostication in an Asian population. Prognostic factors of ENSAT stage 4, advanced pathologic characteristics, and cortisol excess were generally in accordance with previously reported Western-population-based research. Identifying such prognostic factors and risk stratification are essential for ACC treatment. In this context, patients with ACC should be managed by a multidisciplinary team including endocrinologists, surgeons, pathologists, oncologists, and radiologists.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
The Korean Society of Endocrinology and Metabolism (EnM) and the Korean Adrenal Gland and Endocrine Hypertension Study Group supported the current study.
Acknowledgements
The authors thank all 25 hospitals and contributors for their cooperation in the study. The full list of all participating university hospitals and contributors is as follows: The Korean Adrenal Gland and Endocrine Hypertension Study Group, Korean Endocrine Society. Dong Seop Choi (Korea University Anam Hospital, Seoul), Choon Hee Chung (Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju), Dong Jin Chung (Chonnam National University Hospital, Gwangju), Ho Yeon Chung (Kyung Hee University Hospital at Gangdong, Seoul), Ho-Cheol Kang (Chonnam National University Hwasun Hospital, Hwasun), Chul Hee Kim (Soonchunhyang University Bucheon Hospital, Bucheon), Doo Man Kim (Hallym University Kangdong Sacred Heart Hospital, Seoul), Hye Sun Kim (Keimyung University Dongsan Medical Center, Daegu), In Joo Kim (Pusan National University Hospital, Busan), Jae Hyeon Kim (Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul), Jin Taek Kim (Nowon Eulji Medical Center, Eulji University School of Medicine, Seoul), Jung Guk Kim (Kyungpook National University Hospital, Daegu), Jung Hee Kim (Seoul National University Hospital, Seoul), Kyoung Min Kim (Seoul National University Bundang Hospital, Seongnam), Kyung Ah Kim (Dongguk University Ilsan Hospital, Goyang), Sang Wan Kim (Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul), Seung-Hyun Ko (St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon), Sang Ah Lee (Jeju National University Hospital, Jeju), Sung Dae Moon (Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Incheon), Jong Suk Park (Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul), Sang Youl Rhee (Kyung Hee University Medical Center, Seoul), Yumie Rhee (Severance Hospital, Yonsei University College of Medicine, Seoul), Kee Ho Song (Konkuk University Medical Center, Seoul), Ju Hee Lee (Chungnam National University Hospital, Daejeon), and So Young Oak (Kosin University Gospel Hospital, Busan).
References
- 1.Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World Journal of Surgery 2006. 30 872–878. ( 10.1007/s00268-005-0329-x) [DOI] [PubMed] [Google Scholar]
- 2.Kerkhofs TM, Verhoeven RH, Van der Zwan JM, Dieleman J, Kerstens MN, Links TP, Van de Poll-Franse LV, Haak HR. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. European Journal of Cancer 2013. 49 2579–2586. ( 10.1016/j.ejca.2013.02.034) [DOI] [PubMed] [Google Scholar]
- 3.Bernardino ME, Walther MM, Phillips VM, Graham SD, Jr, Sewell CW, Gedgaudas-McClees K, Baumgartner BR, Torres WE, Erwin BC. CT-guided adrenal biopsy: accuracy, safety, and indications. American Journal of Roentgenology 1985. 144 67–69. ( 10.2214/ajr.144.1.67) [DOI] [PubMed] [Google Scholar]
- 4.Hong AR, Kim JH, Park KS, Kim KY, Lee JH, Kong SH, Lee SY, Shin CS, Kim SW, Kim SY. Optimal follow-up strategies for adrenal incidentalomas: reappraisal of the 2016 ESE-ENSAT guidelines in real clinical practice. European Journal of Endocrinology 2017. 177 475–483. ( 10.1530/EJE-17-0372) [DOI] [PubMed] [Google Scholar]
- 5.Ahn SH, Kim JH, Baek SH, Kim H, Cho YY, Suh S, Kim BJ, Hong S, Koh JM, Lee SH, et al Characteristics of adrenal incidentalomas in a large, prospective computed tomography-based multicenter study: the COAR study in Korea. Yonsei Medical Journal 2018. 59 501–510. ( 10.3349/ymj.2018.59.4.501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abiven G, Coste J, Groussin L, Anract P, Tissier F, Legmann P, Dousset B, Bertagna X, Bertherat J. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. Journal of Clinical Endocrinology and Metabolism 2006. 91 2650–2655. ( 10.1210/jc.2005-2730) [DOI] [PubMed] [Google Scholar]
- 7.Kasperlik-Zeluska AA, Roslonowska E, Slowinska-Srzednicka J, Migdalska B, Jeske W, Makowska A, Snochowska H. Incidentally discovered adrenal mass (incidentaloma): investigation and management of 208 patients. Clinical Endocrinology 1997. 46 29–37. ( 10.1046/j.1365-2265.1997.d01-1751.x) [DOI] [PubMed] [Google Scholar]
- 8.Cawood TJ, Hunt PJ, O'Shea D, Cole D, Soule S. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? European Journal of Endocrinology 2009. 161 513–527. ( 10.1530/EJE-09-0234) [DOI] [PubMed] [Google Scholar]
- 9.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Tabarin A, Terzolo M, Tsagarakis S, Dekkers OM. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. European Journal of Endocrinology 2016. 175 G1–G34. ( 10.1530/EJE-16-0467) [DOI] [PubMed] [Google Scholar]
- 10.Terzolo M, Ali A, Osella G, Mazza E. Prevalence of adrenal carcinoma among incidentally discovered adrenal masses. A retrospective study from 1989 to 1994. Gruppo Piemontese Incidentalomi Surrenalici. Archives of Surgery 1997. 132 914–919. ( 10.1001/archsurg.1997.01430320116020) [DOI] [PubMed] [Google Scholar]
- 11.Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. Journal of Clinical Endocrinology and Metabolism 2013. 98 4551–4564. ( 10.1210/jc.2013-3020) [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez KD, Noltner KA, Buzin CH, Gu D, Wen-Fong CY, Nguyen VQ, Han JH, Lowstuter K, Longmate J, Sommer SS, et al Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. Journal of Clinical Oncology 2009. 27 1250–1256. ( 10.1200/JCO.2008.16.6959) [DOI] [PubMed] [Google Scholar]
- 13.Gatta-Cherifi B, Chabre O, Murat A, Niccoli P, Cardot-Bauters C, Rohmer V, Young J, Delemer B, Du Boullay H, Verger MF, et al Adrenal involvement in MEN1. Analysis of 715 cases from the Groupe d’Etude des Tumeurs Endocrines database. European Journal of Endocrinology 2012. 166 269–279. ( 10.1530/EJE-11-0679) [DOI] [PubMed] [Google Scholar]
- 14.Steenman M, Westerveld A, Mannens M. Genetics of Beckwith-Wiedemann syndrome-associated tumors: common genetic pathways. Genes, Chromosomes and Cancer 2000. 28 1–13. () [DOI] [PubMed] [Google Scholar]
- 15.Raymond VM, Everett JN, Furtado LV, Gustafson SL, Jungbluth CR, Gruber SB, Hammer GD, Stoffel EM, Greenson JK, Giordano TJ, et al Adrenocortical carcinoma is a lynch syndrome-associated cancer. Journal of Clinical Oncology 2013. 31 3012–3018. ( 10.1200/JCO.2012.48.0988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creemers SG, Hofland LJ, Korpershoek E, Franssen GJ, van Kemenade FJ, de Herder WW, Feelders RA. Future directions in the diagnosis and medical treatment of adrenocortical carcinoma. Endocrine-Related Cancer 2016. 23 R43–R69. ( 10.1530/ERC-15-0452) [DOI] [PubMed] [Google Scholar]
- 17.Fassnacht M, Dekkers OM, Else T, Baudin E, Berruti A, de Krijger R, Haak HR, Mihai R, Assie G, Terzolo M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. European Journal of Endocrinology 2018. 179 G1–G46. ( 10.1530/EJE-18-0608) [DOI] [PubMed] [Google Scholar]
- 18.Jouinot A, Bertherat J. MANAGEMENT OF ENDOCRINE DISEASE: Adrenocortical carcinoma: differentiating the good from the poor prognosis tumors. European Journal of Endocrinology 2018. 178 R215–R230. ( 10.1530/EJE-18-0027) [DOI] [PubMed] [Google Scholar]
- 19.Assie G, Letouze E, Fassnacht M, Jouinot A, Luscap W, Barreau O, Omeiri H, Rodriguez S, Perlemoine K, Rene-Corail F, et al Integrated genomic characterization of adrenocortical carcinoma. Nature Genetics 2014. 46 607–612. ( 10.1038/ng.2953) [DOI] [PubMed] [Google Scholar]
- 20.Juhlin CC, Goh G, Healy JM, Fonseca AL, Scholl UI, Stenman A, Kunstman JW, Brown TC, Overton JD, Mane SM, et al Whole-exome sequencing characterizes the landscape of somatic mutations and copy number alterations in adrenocortical carcinoma. Journal of Clinical Endocrinology and Metabolism 2015. 100 E493–E502. ( 10.1210/jc.2014-3282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berruti A, Baudin E, Gelderblom H, Haak HR, Porpiglia F, Fassnacht M, Pentheroudakis G. & ESMO Guidelines Working Group. Adrenal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2012. 23 (Supplement 7) vii131–vii138. ( 10.1093/annonc/mds231) [DOI] [PubMed] [Google Scholar]
- 22.Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, Sturgeon C. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer 2008. 113 3130–3136. ( 10.1002/cncr.23886) [DOI] [PubMed] [Google Scholar]
- 23.Sharma E, Dahal S, Sharma P, Bhandari A, Gupta V, Amgai B, Dahal S. The characteristics and trends in adrenocortical carcinoma: a United States population based study. Journal of Clinical Medicine Research 2018. 10 636–640. ( 10.14740/jocmr3503w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JM, Kim MK, Ko SH, Koh JM, Kim BY, Kim SW, Kim SK, Kim HJ, Ryu OH, Park J, et al Clinical guidelines for the management of adrenal incidentaloma. Endocrinology and Metabolism 2017. 32 200–218. ( 10.3803/EnM.2017.32.2.200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tauchmanov L, Colao A, Marzano LA, Sparano L, Camera L, Rossi A, Palmieri G, Marzano E, Salvatore M, Pettinato G, et al Andrenocortical carcinomas: twelve-year prospective experience. World Journal of Surgery 2004. 28 896–903. ( 10.1007/s00268-004-7296-5) [DOI] [PubMed] [Google Scholar]
- 26.Crucitti F, Bellantone R, Ferrante A, Boscherini M, Crucitti P. The Italian Registry for Adrenal Cortical Carcinoma: analysis of a multiinstitutional series of 129 patients. The ACC Italian Registry study group. Surgery 1996. 119 161–170. ( 10.1016/s0039-6060(96)80164-4) [DOI] [PubMed] [Google Scholar]
- 27.Luton JP, Cerdas S, Billaud L, Thomas G, Guilhaume B, Bertagna X, Laudat MH, Louvel A, Chapuis Y, Blondeau P. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. New England Journal of Medicine 1990. 322 1195–1201. ( 10.1056/NEJM199004263221705) [DOI] [PubMed] [Google Scholar]
- 28.Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Practice and Research: Clinical Endocrinology and Metabolism 2009. 23 273–289. ( 10.1016/j.beem.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 29.Iniguez-Ariza NM, Kohlenberg JD, Delivanis DA, Hartman RP, Dean DS, Thomas MA, Shah MZ, Herndon J, McKenzie TJ, Arlt W, et al Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clinic Proceedings: Innovations, Quality, and Outcomes 2018. 2 30–39. ( 10.1016/j.mayocpiqo.2017.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson PT, Horton KM, Fishman EK. Adrenal mass imaging with multidetector CT: pathologic conditions, pearls, and pitfalls. RadioGraphics 2009. 29 1333–1351. ( 10.1148/rg.295095027) [DOI] [PubMed] [Google Scholar]
- 31.Sturgeon C, Shen WT, Clark OH, Duh QY, Kebebew E. Risk assessment in 457 adrenal cortical carcinomas: how much does tumor size predict the likelihood of malignancy? Journal of the American College of Surgeons 2006. 202 423–430. ( 10.1016/j.jamcollsurg.2005.11.005) [DOI] [PubMed] [Google Scholar]
- 32.Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocrine Reviews 2014. 35 282–326. ( 10.1210/er.2013-1029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hajjar RA, Hickey RC, Samaan NA. Adrenal cortical carcinoma. A study of 32 patients. Cancer 1975. 35 549–554. () [DOI] [PubMed] [Google Scholar]
- 34.Lipsett MB, Hertz R, Ross GT. Clinical and pathophysiologic aspects of adrenocortical carcinoma. American Journal of Medicine 1963. 35 374–383. ( 10.1016/0002-9343(63)90179-7) [DOI] [PubMed] [Google Scholar]
- 35.Soreide JA, Brabrand K, Thoresen SO. Adrenal cortical carcinoma in Norway, 1970–1984. World Journal of Surgery 1992. 16 663–667; discussion 668 ( 10.1007/BF02067349) [DOI] [PubMed] [Google Scholar]
- 36.Venkatesh S, Hickey RC, Sellin RV, Fernandez JF, Samaan NA. Adrenal cortical carcinoma. Cancer 1989. 64 765–769. () [DOI] [PubMed] [Google Scholar]
- 37.Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, Terzolo M, Mueller HH, Hahner S, Allolio B, et al Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a revised TNM classification. Cancer 2009. 115 243–250. ( 10.1002/cncr.24030) [DOI] [PubMed] [Google Scholar]
- 38.Ayala-Ramirez M, Jasim S, Feng L, Ejaz S, Deniz F, Busaidy N, Waguespack SG, Naing A, Sircar K, Wood CG, et al Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. European Journal of Endocrinology 2013. 169 891–899. ( 10.1530/EJE-13-0519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Datrice NM, Langan RC, Ripley RT, Kemp CD, Steinberg SM, Wood BJ, Libutti SK, Fojo T, Schrump DS, Avital I. Operative management for recurrent and metastatic adrenocortical carcinoma. Journal of Surgical Oncology 2012. 105 709–713. ( 10.1002/jso.23015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erdogan I, Deutschbein T, Jurowich C, Kroiss M, Ronchi C, Quinkler M, Waldmann J, Willenberg HS, Beuschlein F, Fottner C, et al The role of surgery in the management of recurrent adrenocortical carcinoma. Journal of Clinical Endocrinology and Metabolism 2013. 98 181–191. ( 10.1210/jc.2012-2559) [DOI] [PubMed] [Google Scholar]
- 41.Bellantone R, Ferrante A, Boscherini M, Lombardi CP, Crucitti P, Crucitti F, Favia G, Borrelli D, Boffi L, Capussotti L, et al Role of reoperation in recurrence of adrenal cortical carcinoma: results from 188 cases collected in the Italian National Registry for Adrenal Cortical Carcinoma. Surgery 1997. 122 1212–1218. ( 10.1016/s0039-6060(97)90229-4) [DOI] [PubMed] [Google Scholar]
- 42.Allolio B, Hahner S, Weismann D, Fassnacht M. Management of adrenocortical carcinoma. Clinical Endocrinology 2004. 60 273–287. ( 10.1046/j.1365-2265.2003.01881.x) [DOI] [PubMed] [Google Scholar]
- 43.Young WF., Jr Management approaches to adrenal incidentalomas. A view from Rochester, Minnesota. Endocrinology and Metabolism Clinics of North America 2000. 29 159–185, x ( 10.1016/s0889-8529(05)70122-5) [DOI] [PubMed] [Google Scholar]
- 44.Papotti M, Volante M, Duregon E, Delsedime L, Terzolo M, Berruti A, Rosai J. Adrenocortical tumors with myxoid features: a distinct morphologic and phenotypical variant exhibiting malignant behavior. American Journal of Surgical Pathology 2010. 34 973–983. ( 10.1097/PAS.0b013e3181e2b726) [DOI] [PubMed] [Google Scholar]
- 45.McNicol AM. Update on tumours of the adrenal cortex, phaeochromocytoma and extra-adrenal paraganglioma. Histopathology 2011. 58 155–168. ( 10.1111/j.1365-2559.2010.03613.x) [DOI] [PubMed] [Google Scholar]
- 46.Stojadinovic A, Brennan MF, Hoos A, Omeroglu A, Leung DH, Dudas ME, Nissan A, Cordon-Cardo C, Ghossein RA. Adrenocortical adenoma and carcinoma: histopathological and molecular comparative analysis. Modern Pathology 2003. 16 742–751. ( 10.1097/01.MP.0000081730.72305.81) [DOI] [PubMed] [Google Scholar]
- 47.Polat B, Fassnacht M, Pfreundner L, Guckenberger M, Bratengeier K, Johanssen S, Kenn W, Hahner S, Allolio B, Flentje MJC. Radiotherapy in adrenocortical carcinoma. Cancer 2009. 115 2816–2823. ( 10.1002/cncr.24331) [DOI] [PubMed] [Google Scholar]
- 48.Else T, Williams AR, Sabolch A, Jolly S, Miller BS, Hammer GDJT. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. Journal of Clinical Endocrinology and Metabolism 2014. 99 455–461. ( 10.1210/jc.2013-2856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fassnacht M, Hahner S, Polat B, Koschker AC, Kenn W, Flentje M, Allolio BJT. Efficacy of adjuvant radiotherapy of the tumor bed on local recurrence of adrenocortical carcinoma. Journal of Clinical Endocrinology and Metabolism 2006. 91 4501–4504. ( 10.1210/jc.2006-1007) [DOI] [PubMed] [Google Scholar]
- 50.Hermsen IG, Groenen YE, Dercksen MW, Theuws J, Haak HR. Response to radiation therapy in adrenocortical carcinoma. Journal of Endocrinological Investigation 2010. 33 712–714. ( 10.1007/BF03346675) [DOI] [PubMed] [Google Scholar]
- 51.Sabolch A, Feng M, Griffith K, Hammer G, Doherty G, Ben-Josef E. Adjuvant and definitive radiotherapy for adrenocortical carcinoma. International Journal of Radiation Oncology, Biology, Physics 2011. 80 1477–1484. ( 10.1016/j.ijrobp.2010.04.030) [DOI] [PubMed] [Google Scholar]
- 52.Habra MA, Ejaz S, Feng L, Das P, Deniz F, Grubbs EG, Phan A, Waguespack SG, Ayala-Ramirez M, Jimenez C, et al A retrospective cohort analysis of the efficacy of adjuvant radiotherapy after primary surgical resection in patients with adrenocortical carcinoma. Journal of Clinical Endocrinology and Metabolism 2013. 98 192–197. ( 10.1210/jc.2012-2367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Icard P, Goudet P, Charpenay C, Andreassian B, Carnaille B, Chapuis Y, Cougard P, Henry JF, Proye C. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World Journal of Surgery 2001. 25 891–897. ( 10.1007/s00268-001-0047-y) [DOI] [PubMed] [Google Scholar]
- 54.Vassilopoulou‐Sellin R, Schultz PN. Adrenocortical carcinoma: clinical outcome at the end of the 20th century. Cancer 2001. 92 1113–1121. ( 10.1002/1097-0142(20010901)92:53.0.co;2-i) [DOI] [PubMed] [Google Scholar]
- 55.Paton BL, Novitsky YW, Zerey M, Harrell AG, Norton HJ, Asbun H, Kercher KW, Heniford BTJS. Outcomes of adrenal cortical carcinoma in the United States. Surgery 2006. 140 914–920; discussion 919 ( 10.1016/j.surg.2006.07.035) [DOI] [PubMed] [Google Scholar]
- 56.Ip JC, Pang TC, Glover AR, Soon P, Clarke S, Richardson A, Campbell P, Robinson BG, Sidhu SB. Improving outcomes in adrenocortical cancer: an Australian perspective. Annals of Surgical Oncology 2015. 22 2309–2316. ( 10.1245/s10434-014-4133-4) [DOI] [PubMed] [Google Scholar]
- 57.Kerkhofs TM, Ettaieb MH, Hermsen IG, Haak HR. Developing treatment for adrenocortical carcinoma. Endocrine-Related Cancer 2015. 22 R325–R338. ( 10.1530/ERC-15-0318) [DOI] [PubMed] [Google Scholar]
- 58.Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, et al Combination chemotherapy in advanced adrenocortical carcinoma. New England Journal of Medicine 2012. 366 2189–2197. ( 10.1056/NEJMoa1200966) [DOI] [PubMed] [Google Scholar]
- 59.Vanbrabant T, Fassnacht M, Assie G, Dekkers OM. Influence of hormonal functional status on survival in adrenocortical carcinoma: systematic review and meta-analysis. European Journal of Endocrinology 2018. 179 429–436. ( 10.1530/EJE-18-0450) [DOI] [PubMed] [Google Scholar]
- 60.King DR, Lack EE. Adrenal cortical carcinoma. A clinical and pathologic study of 49 cases. Cancer 1979. 44 239–244. () [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a