Abstract
One of the major missions of the Taiwan Society of Cardiology is to publish practice guidelines that are suitable for local use in Taiwan. The ultimate purpose is to continuously improve cardiovascular health care from the implementation of the recommendations in the guidelines. Despite recent improvement of medical care, patients with ST-segment elevation myocardial infarction (STEMI) still carry a high morbidity and mortality. There have been many changes in the concepts of STEMI diagnosis and treatment in recent years. The 2020 focused update of the 2012 guidelines of the Taiwan Society of Cardiology for the management of STEMI is an amendment of the 2012 guidelines based on the newest published scientific data. The recommendations in this focused update provide the diagnosis and treatment strategy for STEMI that should be generally implemented in Taiwan. Nevertheless, guidelines never completely replace clinical judgment and medical decision still should be determined individually.
Keywords: Acute myocardial infarction, Guideline, Taiwan
INTRODUCTION
Through early revascularization with primary percutaneous coronary intervention (PCI) and use of guideline-recommended secondary preventive medications, the mortality of ST-segment elevation myocardial infarction (STEMI) continues to decrease in Taiwan.1 Since the publication of the 2012 Guidelines of the Taiwan Society of Cardiology (TSOC) for the Management of STEMI,2 some concepts in treating the disease have been modified due to emerging new evidences. The purpose of this "focused update" is to revise the 2012 guideline specifically in several areas that the new study results bring in changes of STEMI management. The focused update began with a short review of epidemiology and recent treatment trends of STEMI in Taiwan. STEMI equivalents, defined as patients without typical ST segment elevation on electrocardiography (ECG), but need immediate triage and management as STEMI, were added in the diagnosis section. Revision on the section of prehospital management added new recommendations of early identification of STEMI by emergency medical service (EMS) field triage with prehospital ECG and direct transportation to the nearest PCI-available hospitals. In addition to clopidogrel and ticagrelor, prasugrel was introduced into Taiwan in the end of 2018. There were revised recommendations about the choice of P2Y12 inhibitors for STEMI. There were also revisions to the section on primary PCI, including adequate time intervals from diagnosis to intervention, vascular access and complete revascularization strategy. Cardiogenic shock is still a major cause of mortality in STEMI. Revision on section of cardiogenic shock was added because of recent advances in therapeutic options. For secondary preventive strategies, new pharmacological therapies for diabetes and hypercholesterolemia have been developed and proved to further reduce recurrent cardiovascular (CV) events in high risk patients such as STEMI. The section of long-term pharmacological treatment after discharge was revised and included all these new medical therapies. Finally, a new section of quality care of STEMI was added to indicate the importance of guideline implementation and several quality indicators were proposed.
In 2019, the President and Executive Board of the TSOC decided to revise the 2012 Guidelines of the TSOC for the Management of STEMI and invited several members to form a writing group. The major topics in the guideline were assigned to the members of the writing group and the data from clinical trials as well as other publications in peer-reviewed journals related to the topics were reviewed. New or modified recommendations in this focused update reflect the latest progress in the diagnosis and treatment of STEMI. The readers may refer to the prior guideline about the clinical topics which were not addressed in this focused update.2 In order to demonstrate the intensities of recommendations, the evidence-based classification system, including class of recommendation (COR) and level of evidence (LOE), was adopted in the guideline. The definitions of COR I to III and LOE A to C were the same as those in the 2018 Guidelines of the TSOC for the management of non ST-segment elevation acute coronary syndrome (ACS).3
EPIDEMIOLOGY OF STEMI
Recent epidemiological studies in Taiwan using the Taiwan National Health Insurance Research Database showed that the overall adjusted incidence of acute myocardial infarction (MI) increased progressively from 1999 to 2008.1,4,5 The age- and sex-adjusted incidence rates (per 100,000 population) of acute MI increased from 30 in 1997 to 49.8 in 2009, which was mainly driven by the increase of non ST-segment elevation myocardial infarction (NSTEMI) and the increasing tendency was noted in both genders.1,5 However, the adjusted incidence of acute MI remained constant after 2008 and estimated to be 49.8 in 2009 to 50.7 in 2015.1 The adjusted incidence of STEMI decreased from 16.8 in 2009 to 14.4 in 2015, whereas NSTEMI continuously increased from 32.5 in 2009 to 35.7 in 2015 (Figure 1). The ratio of NSTEMI to STEMI incidence increased from 1.93 in 2009 to 2.47 in 2015.1 For STEMI, the incidence decreased across all age group except in the young population (< 55 years).1 There was a 7.7% increase of STEMI from 2009 to 2015 in young men and no sign of decreasing trend in young female.1 In Taiwan, individuals hospitalized for STEMI were more likely to be younger and were less likely to have diabetes mellitus and hypertension in comparison with NSTEMI patients.1 However, the incidence of dyslipidemia was higher in STEMI than NSTEMI patients.1 For STEMI, the mean age was 1.3 years younger in 2015 compared with that in 2009 and there was a 19.8% increase of dyslipidemia during these years.1 In Taiwan, most STEMI patients received primary PCI rather than fibrinolytic therapy.1 Two nationwide ACS registries in Taiwan among different time periods (2008-2010 and 2012-2015) showed that the median door-to-balloon (D2B) time in primary PCI decreased from 96 minutes in the first registry to 71 minutes in the second registry.6 One retrospective 10-year cohort study in a single medical center in Taiwan also showed that the median D2B time decreased from 142 minutes in 2005 to 69 minutes in 2014.7 In secondary preventive pharmacological therapies, the in-hospital use of dual antiplatelet therapy (DAPT) (95.1% to 99.6%), angiotensin converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) (63.8% to 77.5%), beta-blocker (48.8% to 71.4%), and statin (54.4% to 81.2%) all increased significantly between the two ACS registries.6 The overall crude in-hospital mortality rate of STEMI decreased from 2009 (9.3%) to 2015 (7.6%) in Taiwan.1 However, the mortality rate was persistently higher in female than male patients and there was no improving trend of mortality in STEMI for female patients.1 The prescription rate of secondary preventive medications for STEMI in Taiwan was still relatively low compared with the data from Western country.8
Figure 1.
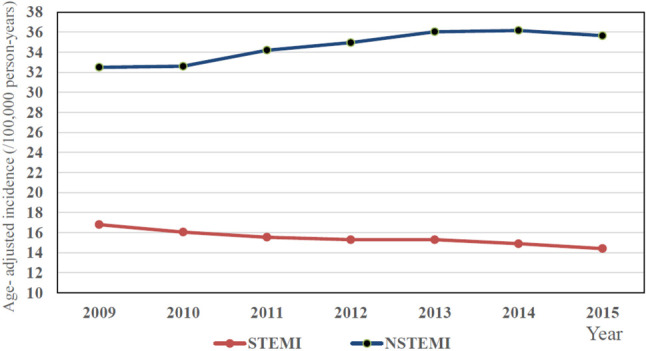
The recent trends of decreasing incidence of STEMI and increasing incidence of NSTEMI in Taiwan. NSTEMI, non ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction. Adapted from reference 1.
Recommendation
• More actions should be taken to control the increasing incidence of STEMI in young population in Taiwan. (COR I, LOE C)
• To increase prescription rate of secondary preventive medications for STEMI in Taiwan is necessary. (COR I, LOE C)
DIAGNOSIS OF STEMI
Definition
Diagnosis of acute MI in this guideline is based on definition of the Fourth Universal Definition of MI 2018.9 It defines acute MI as acute myocardial injury with clinical evidence of acute myocardial ischemia and with detection of a rise and/or fall of cardiac troponin values with at least one value above the 99th percentile upper reference limit with at least one of the following: symptoms of myocardial ischemia; new ischemic ECG changes; development of pathological Q waves; imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology; identification of a coronary thrombus by angiography or autopsy.9 Several types of acute MI were defined according to different pathophysiological mechanisms leading to ischemic myocardial injury. The typical STEMI is type 1 MI which is caused by coronary atherosclerosis with atherosclerotic plaque disruption, rupture or erosion and coronary thrombus formation. Most recommendations in this guideline can be applied to this type of MI. Some STEMIs fall into other types of MI which the managements are not included in this guideline.
ECG and biomarker
A 12-lead ECG is the most important diagnostic modality and determines the subsequent management pathways in patients presented with acute chest discomfort. No matter the patients are in an ambulance, local practitioner’s clinic, or emergency department, an ECG should be performed immediately if it is available. The ECG should be interpreted as soon as possible to identify any possibility of STEMI or its equivalents by experienced physician, nurse or trained EMS personnel. A rapid transmission of the ECG through smartphone or other ways to emergency physicians or cardiologists not only speeds up the diagnosis of STEMI but also shortens the time for activation of primary PCI team.10,11 Repeat ECG to follow up the ST-T changes is necessary if the first ECG is normal or equivocal when there are persistent symptoms of myocardial ischemia. Changes of biomarker confirm the presence of myocardial necrosis. Measurement of biomarker should not delay the process for primary PCI. High sensitivity cardiac troponin (hs-cTn) is the recommended biomarker because hs-cTn assays increase diagnostic accuracy for acute MI presenting early after chest pain and allow for a rapid rule-out of acute MI when there are doubts about the diagnosis.12,13 The detailed recommendations of hs-cTn assays were described previously in the 2018 Guidelines of the TSOC for the management of non ST-segment elevation ACS (Figure 2).3
Figure 2.
The detailed recommendations of using high sensitivity cardiac troponin assay to diagnose and rule out acute myocardial infarction. ACS, acute coronary syndrome; ECG, electrocardiography; hs-cTn, high sensitivity cardiac troponin. Adapted from reference 33.
Recommendation
• A 12-lead ECG should be performed immediately in patients presented with any symptom suggestive of STEMI. (COR I, LOE C)
• Rapid transmission of ECG by smartphone or other ways to emergency physicians or cardiologists for early diagnosis is recommended. (COR I, LOE B)
• Hs-cTn assay is recommended for rapid diagnosis and rule-out of acute MI. (COR I, LOE A)
STEMI equivalents
For rapid triage and primary PCI, this guideline adopts the conventional definition of STEMI as those with symptoms suggestive of myocardial ischemia and ST-segment elevation in at least two contiguous leads on ECG. However, there are several conditions that patients present without typical ST-segment elevation on a standard 12-lead ECG, but should be managed as STEMI equivalents. The first condition is posterior MI. The typical ECG changes of posterior MI include ST segment depression in precordial leads V1-V4 and a R/S ratio greater than 1 in leads V1 or V2.14 If posterior MI is suspected, posterior leads V7 to V9 of ECG should be done to look for ST segment elevation in these leads.14,15 Lead V7 is placed at the same level of V6 at the posterior axillary line; lead V8 is on the left side of the back at the tip of the scapula and V9 is placed at left paraspinal region at the same level as V6. The infarct related artery (IRA) in patients with typical ECG abnormalities of posterior MI is left circumflex artery. The second condition is left main MI. The typical ECG changes of left main MI include ST elevation in lead aVR with ST elevation in aVR ≥ V1 and extensive ST depression in leads I, II, and V4-6.16,17 Patients presented clinical symptoms with these ECG changes require early coronary angiography to define the left main coronary artery anatomy. The third condition is STEMI in preexisting left bundle branch block (LBBB). The ST segment changes in LBBB make it difficult to diagnose STEMI directly. The Sgarbossa ECG criteria, including (1) concordant ST-segment elevation > 1 mm in at least one lead or (2) concordant ST-segment depression > 1 mm in any of the V1 to V3 leads or (3) discordant ST-segment elevation > 5 mm, are used to identify patients with STEMI in patients with preexisting LBBB.18
Recommendation
• Even without typical ST segment elevation on standard 12-lead ECG, STEMI equivalents should be managed as STEMI. (COR I, LOE C)
PREHOSPITAL MANAGEMENT
Public awareness of acute MI
STEMI is a time-sensitive disease because prompt treatment after symptoms onset improves patients’ prognosis. Mortality of STEMI could be reduced 1.5% for every 30-minute decrease in reperfusion time.19 The key to successful treatment is early recognition of symptoms and rapid arrival at a hospital with PCI facility.20 Patient delay is a critical factor. It commonly due to poor awareness of heart attack symptoms and not calling an ambulance even when acute MI is really occuring.21 Previous study showed the awareness of acute MI symptoms in citizens ranged from 32.9% (arm or shoulder pain) to 70.2% (difficulty breathing) and 79.1% (chest pain and discomfort).20 Overall, 67% citizens would call an ambulance if someone had signs of acute MI.20 Currently, it is roughly estimated that only 10-20% STEMI patients were sent to hospitals by ambulance of EMS in Taiwan. Transportation of suspected STEMI patients by EMS ambulance is much safer because emergency medical technicians (EMT) received cardiopulmonary resuscitation (CPR) training and EMS ambulances are equipped with automated external defibrillator.22-25 Since 2011, EMS in more than 90% of cities and counties in Taiwan started to set up ECG telemetry system in ambulances which may assist in early diagnosis of STEMI and directly transferring patients to PCI available hospitals. Therefore, ambulance transfer is highly recommended for patients suspected of acute MI with typical chest pain with or without dyspnea, cold sweating or nausea.
Recommendation
• Increased public awareness of the typical symptoms of acute MI is recommended. (COR I, LOE C)
• Ambulance transfer to PCI available hospitals is recommended for patients with typical symptoms of acute MI. (COR I, LOE C)
Transportation for primary PCI
Compared with fibrinolytic therapy, primary PCI is the preferred reperfusion strategy.26 In Taiwan, primary PCI is the routine reperfusion therapy for STEMI, whereas fibrinolysis is used only in some special occasions, such as during severe pandemic infectious diseases or in adjacent small islands without PCI available hospitals.6,27,28 Currently, with a population of 23 million in Taiwan, there are 103 PCI available hospitals. Approved by the national STEMI accreditation system from Taiwan Government, 58 hospitals provide 24-hour service of primary PCI, including 46 hospitals with high grade critical care ability and 12 hospitals with moderate grade critical care ability (Figure 3). Through convenient highway system, ambulances usually could reach a PCI available hospital within 2 hours. However, pre-hospital delay is still a challenging issue in Taiwan due to multifactorial reasons. A well-organized STEMI network among different hospitals and EMS system is critical for optimization of STEMI care.29-31 A city/county-based STEMI network should have a standard operating procedure to guide EMTs in the ambulance to transport STEMI patients directly to nearby PCI available hospitals and bypass non-PCI hospitals. EMTs could diagnose STEMI by ECG reading themselves or through on-line instruction from hospital staffs by ECG transmission via smartphone or telemetry ECG system in the field.23-25,32 The network can activate primary PCI team in nearby hospital as soon as possible. EMS ambulance system should be equipped with ECG recorders, automated external defibrillators and auto CPR machines, which could diagnose STEMI in the field and provide critical life-saving treatment.23,25,32
Figure 3.
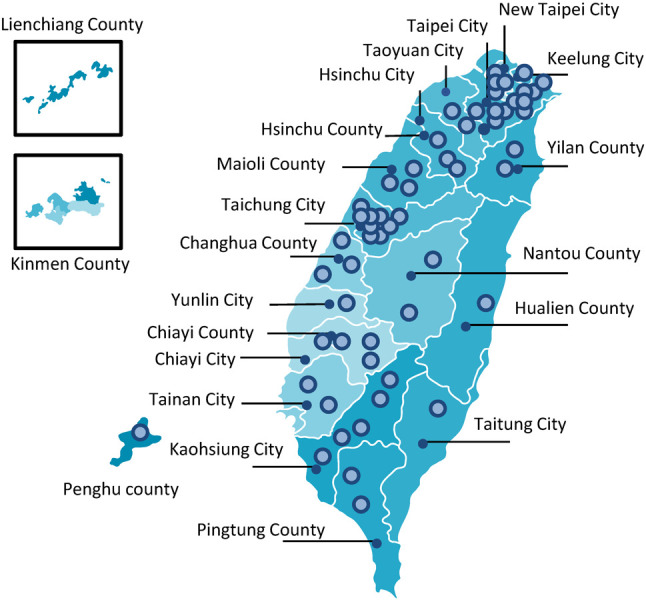
Distribution of hospitals that provide 24-hour primary PCI service in Taiwan. Fibrinolysis is only suggested in special occasions, including special pandemic infection or islands without PCI available hospitals. PCI, percutaneous coronary intervention.
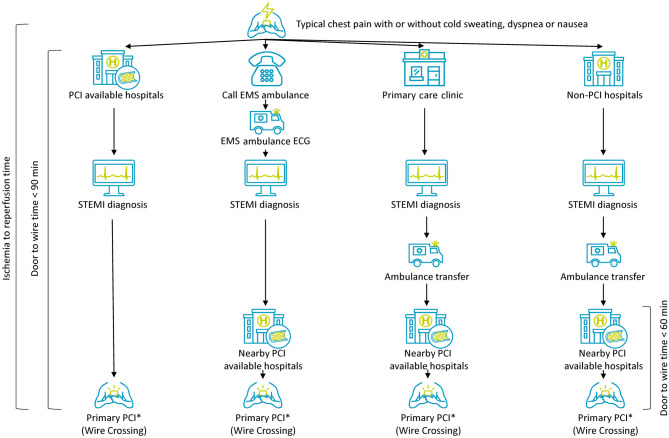
STEMI patients can be diagnosed in a hospital without PCI facility or 24-hour PCI capability. Hospitals with 24-hour PCI capability should establish inter-hospital STEMI network with other hospitals in order to transport STEMI patients safely and rapidly. Inter-hospital STEMI network was reported to shorten D2B time, and hospitals with 24-hour PCI capability should take a "no-refusal" principle for all patients transferred for consideration of primary PCI.33,34 The hospitals with 24-hour PCI capability should have a 24-hour hot line with other non-PCI hospitals. Inter-hospital transmission of ECG and/or laboratory data via smartphone or facsimile in order to early activation of PCI team is necessary. On arrival, the patients should be directly transferred to catheterization laboratory as soon as possible. There should be a feedback system inside the network between non-PCI and PCI hospitals. Figure 4 summarizes the transportation pathways for primary PCI from different places of patient presentation.
Figure 4.
Recommended transportation pathways and door-to-wire time for primary PCI from different places of patient presentation. PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction. ECG, electrocardiography; EMS, emergency medical service.
Recommendation
• Regional STEMI network should be established to guide direct transportation of STEMI patients to PCI available hospitals. (COR I, LOE B)
• The EMS ambulances should be equipped with ECG recorders, automated external defibrillators and auto CPR machines. (COR I, LOE C)
• The EMTs in ambulance should receive training for ECG recording, interpretation and transmission for early identification of STEMI. (COR I, LOE C)
• Inter-hospital network is recommended to facilitate transfer for primary PCI among non-PCI and PCI available hospitals. (COR I, LOE B)
Pre-hospital medication
Early observational studies showed that upstream clopidogrel treatment before arriving PCI hospitals was associated with a reduction of death or recurrent MI in patients with STEMI treated with primary PCI.35,36 A later small randomized trial found that clopidogrel 600 mg loading in prehospital phase could not increase the patency rate of IRA before primary PCI, but was associated with a trend toward less adverse clinical event.37 The ATALANTIC trial randomized STEMI patients to receive ticagrelor either during transfer to a primary PCI hospital or immediately before angiography. The study found early ticagrelor treatment could not increase ST-segment elevation resolution or Thrombolysis in Myocardial Infarction (TIMI) blood flow in the IRA at initial angiography. But the rates of definite stent thrombosis were lower in 24 hour and 30 days in the prehospital group.38 Currently, EMTs in 3 cities of Taiwan could administer DAPT in ambulance after on-line instruction by physicians in hospitals after reading ECG from telemetry transmission.
Recommendation
• Early use of DAPT (aspirin and P2Y12 inhibitor) in ambulance may be considered after on-line instruction by physicians in hospitals if no contraindication. (COR IIb, LOE C)
Out-of-hospital cardiac arrest
STEMI can be presented with out-of-hospital cardiac arrest (OHCA). After CPR and return of spontaneous circulation, emergency coronary angiography and PCI should be performed in awake or comatose patients with OHCA with suspected STEMI. Therapeutic hypothermia with targeted temperature management (TTM) should be considered because TTM after return of spontaneous circulation in cardiac arrest patients leads to improvements in mortality and neurological outcomes.39,40 Previous study showed, after cardiac catheterization and TTM, there was a survival rate of 56% of the OHCA patients and most of the survivors had good neurological outcome.41 However, previous studies using prehospital cold intravenous fluid resulted in worse outcomes including decreased rate of return of spontaneous circulation, increased chance of pulmonary edema, and increased incidence of diuretic use.42,43
Recommendation
• Primary PCI is recommended in STEMI patients with cardiac arrest post return of spontaneous circulation. (COR I, LOE B)
• TTM is recommended in comatose STEMI patients with cardiac arrest after return of spontaneous circulation. (COR I, LOE B)
• Prehospital cooling in ambulance with rapid infusion with cold saline is not recommended in STEMI patients with cardiac arrest post return of spontaneous circulation. (COR III, LOE B)
ANTIPLATELET THERAPY
DAPT with aspirin and P2Y12 inhibitor is the cornerstone therapy for STEMI. Clopidogrel was used in STEMI patients for many years and was the only P2Y12 inhibitor recommended in the 2012 Taiwan STEMI Guidelines.2 New generation P2Y12 inhibitors, prasugrel and ticagrelor, showed superior efficacy compared with clopidogrel in improving clinical outcomes but at the expense of bleeding. Choosing either clopidogrel or potent P2Y12 inhibitors needs more consideration of the balance between clinical benefit and bleeding risk.
Clopidogrel
In large randomized studies, combination of clopidogrel with aspirin was associated with reduced ischemic event and mortality rate for STEMI, regardless of reperfusion strategies.44,45 Four observational studies in Taiwan showed DAPT with aspirin and clopidogrel for more than 9 to 12 months significantly reduced ischemic risk and mortality rate in patients with ACS with or without PCI.46-49 In patients receiving elective PCI, clopidogrel 600 mg loading dose was associated with more rapid and higher platelet inhibition than 300 mg dose.50,51 But in the CURRENT–OASIS 7 trial, clopidogrel 600 mg loading dose did not reduce ischemic events but lead to higher major bleeding risk comparing with 300 mg loading dose in patients with ACS. However, in the subgroup analysis for patients receiving PCI, higher clopidogrel loading dose significantly reduced the rates of 30 days primary outcomes and stent thrombosis.52 A meta-analysis demonstrated that higher clopidogrel loading dose with 600 mg was associated with reduced ischemic events and similar bleeding risk comparing with standard clopidogrel 300 mg loading dose in patients undergoing PCI.53 In Taiwan, a loading dose of 300 mg to 600 mg clopidogrel is recommended for STEMI depending on patients’ clinical conditions. There are some drawbacks of clopidogrel including slower onset time and drug response variability. Clopidogrel is an inactive prodrug and requires a 2-step metabolism to become an active metabolite,54 which may be responsible to its slower drug onset and may lead to increased ischemic events in ACS patients with coronary thrombus formation. Furthermore, the prevalence of cytochrome P450 2C19 polymorphism with clopidogrel resistance is not uncommon and may increase CV risk in ACS patients.55,56 The percentage of CYP2C19 reduced function alleles carrier in Asian population is much higher than that in Caucasian population.57,58 Therefore, newer P2Y12 inhibitors such as prasugrel or ticagrelor have been developed to overcome these unmet clinical needs of clopidogrel.
Prasugrel
Comparing with clopidogrel, prasugrel (60 mg loading and 10 mg daily dose) provides faster and greater platelet inhibitory effects in patients undergoing PCI.59 The TRITON–TIMI 38 trial randomized 13608 ACS patients to receive either prasugrel or clopidogrel. The study found prasugrel reduced more ischemic events compared with clopidogrel [hazard ratio (HR) 0.82, 95% confidence interval (CI) 0.73-0.93], but also increased the risk of major bleeding (HR 1.40, 95% CI 1.05-1.88).60 In the subgroup analysis for STEMI patients, the efficacy of prasugrel was consistent (HR 0.79, 95% CI 0.65-0.97) irrespective of the PCI timing.61 It is worth of noticing that the post-hoc analysis of the TRITON-TIMI 38 study demonstrated that prasugrel was associated with net clinical harm due to a trend toward more major bleeding (p = 0.06) in patients with a history of ischemic stroke or transient ischemic attack (TIA). Patients aged 75 years or older and who were weighing less than 60 kg also had no clinical benefit from prasugrel.60 Therefore, prasugrel is contraindicated in patients with prior history of stroke/TIA and should be used cautiously in patients with old age or low body weight.
In Taiwan and Japan, reduced-dose prasugrel (20 mg loading and 3.75 mg daily dose) is available due to the concern of bleeding risk in Asians. The reduced-dose regimen was studied in the PRASFIT-ACS trial which was conducted in Japan with the similar study design as the TRITON-TIMI 38 study. In the PRASFIT-ACS trial, about 50% of the study population was STEMI and those with prior ischemic stroke/TIA were excluded. After randomizing 1363 ACS patients undergoing PCI, the reduced-dose prasugrel was associated with a trend of 23% risk reduction (HR 0.77, 95% CI 0.56-1.07) of adverse CV events and similar risk of non-coronary artery bypass graft (CABG) major bleeding compared with clopidogrel.62 A post-marketing observational study (PRASFIT-Practice I) enrolled 732 Japanese ACS patients receiving both PCI and reduced-dose prasugrel from 2014 to 2015 and 60% of the patients were STEMI. The rates of TIMI major bleeding and major adverse CV events were 1.6% and 3.1%, respectively during the observational period indicating the safety and efficacy of this regimen.63 There was another larger nationwide registry study in Japan including 62737 ACS patients undergoing PCI in 2016. After propensity score matching, there were 12016 patients in the clopidogrel or prasugrel group, respectively. STEMI patients accounted for 30.7% in clopidogrel and 32.6% in prasugrel group. Compared with clopidogrel, reduced-dose prasugrel was associated with higher bleeding risk [odds ratio (OR) 1.65, 95% CI 1.10-2.51], similar rates of mortality (OR 1.11, 95% CI 0.89-1.38), and similar risk of stent thrombosis (OR 1.29, 95% CI 0.73-2.30). In subgroup analysis for STEMI patients, reduced-dose prasugrel was associated with numerically higher rate of bleeding than clopidogrel (0.67% vs. 0.47%; OR 1.44, 95% CI 0.76-2.78).64 Therefore, larger randomized controlled trials are needed in the future to confirm the efficacy and safety of reduced-dose prasugrel in ACS or STEMI patients.
Ticagrelor
Another newer P2Y12 inhibitor, ticagrelor (180 mg loading and 90 mg twice daily dose), is an active drug that does not require hepatic metabolism for activation. The platelet inhibitory ability of ticagrelor is greater and faster than clopidogrel. In addition, ticagrelor binds to P2Y12 inhibitors reversibly, which lead to faster onset after platelet binding and offset after discontinuing the drug.65 In the PLATO study, ticagrelor was associated with reduced combined risk of CV death, MI, or stroke compared with clopidogrel (HR 0.84, 95% CI 0.77-0.92) in 18,624 ACS patients. However, ticagrelor also increased the rate of non-CABG major bleeding (4.5% vs. 3.8%, p = 0.03).66 The benefits of ticagrelor over clopidogrel in STEMI subgroup were consistent with those from the overall PLATO results. In STEMI patients receiving primary PCI, ticagrelor also reduced risks of MI, total mortality and definite stent thrombosis without increasing major bleeding risk.67 In the TREAT study, STEMI patients receiving fibrinolysis were randomized to ticagrelor or clopidogrel with a median of 11.4 hours after fibrinolytic therapy. Ticagrelor was non-inferior to clopidogrel for TIMI major bleeding at 30 days in this study.68 After 12 months follow-up, ticagrelor was associated with similar ischemic and bleeding events compared with clopidogrel.69 The largest real world data comes from SWEDEHEART registry which included 45073 ACS patients in Sweden from 2010 to 2013. In this study, ticagrelor versus clopidogrel was associated with a lower composite ischemic risk (HR 0.85, 95% CI 0.78-0.93) and a higher risk for re-admission with bleeding (HR 1.20, 95% CI 1.04-1.40) which was similar to the results in the PLATO study.70 However, in Asia, data from small randomized control trials and observational studies comparing ticagrelor with clopidogrel in STEMI or ACS patients demonstrated conflicting results.71-76 In Taiwan, three observational studies comparing ticagrelor with clopidogrel in ACS patients showed reduced ischemic risk without increasing major bleeding risk in patients receiving ticagrelor.77-79
Comparisons between P2Y12 inhibitors
When comparing the efficacy and safety between newer P2Y12 inhibitors (prasugrel/ticagrelor) and clopidogrel in STEMI patients undergoing PCI, a meta-analysis demonstrated that newer P2Y12 inhibitors versus clopidogrel significantly reduced all-cause death, major adverse CV events and stent thrombosis with similar risk in bleeding.80 Another meta-analysis showed that both prasugrel and ticagrelor were associated with better clinical outcomes than clopidogrel, and prasugrel was even superior to ticagrelor in STEMI patients undergoing primary PCI.81 In addition, there was a retrospective analysis from Korea using claim data from the Health Insurance Review and Assessment Service with 40706 acute MI patients undergoing PCI between 2010 to 2015 and 35% of the study population were STEMI. In this study, newer P2Y12 inhibitors including prasugrel and ticagrelor both had favorable effect on reducing 30-day mortality.82 Furthermore, according to the data from KAMIR-NIH registry, treatment with both prasugrel and ticagrelor in ACS patients were shown to improve major adverse CV events-free survival rate compared to clopidogrel. The difference of major adverse CV events rate between prasugrel and ticagrelor in overall population and STEMI subgroups were not significant.83 For head to head comparison of the efficacy and safety between the two newer P2Y12 inhibitors in ACS patients, a retrospective cohort analysis from claims database showed that patients received ticagrelor had both reduced ischemic and bleeding events compared with patients treated with prasugrel.84 However, in a large randomized control trial, the ISAR-REACT 5 study, which recruited 4018 ACS subjects and 41% of whom were STEMI patients, prasugrel was associated with significantly reduced ischemic composite risks including death, MI, or stroke than ticagrelor, and the incidence of bleeding was statistically insignificant between the two groups.85
Based on current available evidences, standard-dose ticagrelor and prasugrel should be the preferred P2Y12 inhibitor in STEMI patients. However, if concerns about bleeding prevail over ischemia, it is reasonable to choose clopidogrel rather than newer P2Y12 inhibitors. High bleeding risk features include old age, chronic kidney disease, anemia, low body weight, combination therapy with oral anticoagulant, prior intracranial hemorrhage, or history of previous major bleeding. Furthermore, based on Asians’ data, reduced-dose prasugrel (20 mg loading and 3.75 mg daily maintenance dose) may also be considered in STEMI patients undergoing primary PCI in Taiwan. The selection of different P2Y12 inhibitors, different dose regimens, and the trade-off between ischemic and bleeding risk should be individualized to acquire the maximal net clinical benefit for STEMI patients.
Recommendation
• Ticagrelor (180 mg loading dose, 90 mg twice daily), prasugrel (60 mg loading dose, 10 mg daily dose), or clopidogrel (300-600 mg loading dose, 75 mg daily dose) is recommended in STEMI patients undergoing primary PCI unless contraindicated and ticagrelor or prasugrel is preferred to clopidogrel. (COR I, LOE B)
• Clopidogrel rather than ticagrelor or prasugrel may be considered in patients with increased bleeding risk features. (COR IIa, LOE C)
• Reduced dose of prasugrel (20 mg loading dose, 3.75 mg daily dose) may be considered in STEMI patients undergoing PCI based on Asian data. (COR IIa, LOE B)
PCI STRATEGY
Door-to-wire time
D2B time of primary PCI has been regarded as an important metric for STEMI patients because it could influence the clinical outcomes of the patients.86-88 Previous international guidelines as well as 2012 Taiwan STEMI guidelines have focused on the importance of D2B time.2 However, later studies found that the total ischemia time, including the time from symptom onset to STEMI diagnosis and the time from STEMI diagnosis to primary PCI, is a more important determinant of clinical outcomes.89-91 That is why much more effort now is devoted to shorten the total ischemia time by pre-hospital diagnosis of STEMI and early activation of PCI team. Due to the progress of PCI technique, balloon dilation is not always the first intervention step performed during primary PCI, therefore, the term has further evolved to medical contact-to-device time. Wire crossing the lesion in IRA is always necessary before any intracoronary procedure, therefore, the wire time instead of device time is preferred in our guideline. In Taiwan, if STEMI has already been diagnosed in primary care clinic, non-PCI hospital or in ambulance and transferred for primary PCI, a door-to-wire time ≤ 60 minutes is suggested in the transferred PCI available hospitals. In fresh cases that need time to diagnose STEMI, a door-to-wire time ≤ 90 minutes is suggested in PCI available hospitals.
Recommendation
• For patients with already diagnosed STEMI that are transferred for primary PCI, a door-to-wire time ≤ 60 minutes in PCI available hospitals is recommended. (COR I, LOE C)
• In fresh cases that need time to diagnose STEMI, a door-to-wire time ≤ 90 minutes in PCI available hospitals is recommended. (COR I, LOE B)
PCI procedures
The RIVAL trial was a large scale clinical trial that randomly allocated 7021 ACS patients including 1958 STEMI patients to radial or femoral artery access during PCI. The composite primary outcome of death, MI, stroke or non-CABG major bleeding at 30 days occurred significantly fewer in the radial group compared with the femoral group in patients with STEMI (HR 0.60, 95% CI 0.38-0.94) and in high volume radial centers (HR 0.49, 95% CI 0.28-0.87).92 The radial procedural volume was important and independently associated with the primary outcome.93 The MATRIX trial included a total of 8404 ACS patients (4008 STEMI) undergoing PCI and randomized to radial or femoral access. The 30-day co-primary outcomes were major adverse CV events (MACE), defined as all-cause mortality, MI and stroke. The net adverse clinical events were defined as MACE or non-CABG major bleeding. The radial access group was associated with reduced MACE [risk ratio (RR) 0.85, 95% CI 0.74-0.99] and net adverse clinical events (RR 0.83, 95% CI 0.73-0.96). The difference was mainly driven by fewer non-CABG major bleeding and reduction of all-cause death.94 Therefore, the radial access has become the favorable choice during primary PCI and is especially recommended to be performed by an experienced operator at high volume center.
The EXAMINATION study randomly assigned 1504 patients with STEMI to receive everolimus-eluting stent (EES) or bare-metal stent (BMS). The results showed both rates of target lesion revascularization and stent thrombosis were reduced in recipients of EES at 1 year.95 Similarly, the COMFORTABLE AMI trial randomly assigned 1161 STEMI patients to receive biolimus-eluting stents or BMS in primary PCI. The group of biolimus-eluting stents with a biodegradable polymer was associated with reduced composite of cardiac death, target vessel-related reinfarction, and ischemia-driven target-lesion revascularization in STEMI patients at 1 year.96 Based on these evidences, new generation drug-eluting stent (DES) is recommended during primary PCI for STEMI. The TASTE trial randomly assigned 7244 STEMI patients undergoing PCI to manual thrombus aspiration followed by PCI or to PCI only. The results revealed routine thrombus aspiration before PCI as compared with PCI alone did not reduce 30-day and 1-year mortality. In addition, this strategy did not reduce total mortality or the composite of death from any cause, rehospitalization for MI, or stent thrombosis up to 1 year.97,98 A later and larger scale TOTAL study with 10732 STEMI patients also reported routine manual thrombectomy did not reduce the risk of CV death, recurrent MI, cardiogenic shock, or class IV heart failure within 180 days. In addition, this strategy in TOTAL trial was associated with an increased rate of stroke within 30 days, which was not observed in TASTE trial.99-101 In summary, with compared to PCI alone, recent clinical trials have not shown any clinical benefit with routine thrombectomy during primary PCI.
Recommendation
• Radial access is recommended over femoral access in experienced operators and high volume centers for primary PCI. (COR I, LOE A)
• New generation DESs are recommended for primary PCI. (COR I, LOE A)
• Thrombus aspiration during primary PCI is an optional but not a routine procedure during primary PCI. (COR I, LOE A)
Complete revascularization
About 40% of patients with acute MI carried multiple complex coronary lesions in multiple coronary arteries on angiography and these patients had poorer left ventricular function and increased risk for recurrent ischemia.102 Whether to completely revascularize these non-culprit lesions or only treat culprit lesion during the index procedure of primary PCI is a common dilemma.103,104 In addition, the optimal timing of complete revascularization also remains uncertain. Previous randomized trials have compared routine non-culprit lesion PCI with optimal medical therapy alone in patients with multivessel disease (MVD) who underwent primary PCI for STEMI, regardless of whether revascularization was performed during index or staged procedure. Although the results almost consistently revealed significant reduction in repeat revascularizations in the group of complete revascularization, none of which were powered for the hard end points of death or MI.105-109 In a meta-analysis, compared to culprit lesion-only PCI, complete revascularization significantly reduced the combined end points of death or MI (OR 0.71, 95% CI 0.52-0.96). In addition, the benefit of routine non-culprit lesion PCI was only observed among patients who performed during the index primary PCI but not staged procedure.110 Recently, the COMPLETE trial randomized 4041 STEMI patients with MVD but without cardiogenic shock who had undergone successful culprit-lesion primary PCI to a strategy of either complete revascularization with PCI of angiographically significant non-culprit lesions or no further revascularization. At a median follow-up of 3 years, the first co-primary outcome of CV death or MI was significantly lower in the complete-revascularization group than culprit-lesion-only PCI group (HR 0.74, 95% CI 0.60-0.91). In this study, all non-culprit lesion PCIs were staged procedures either during the index hospitalization or within 45 days after discharge. This study was statistically powered to concluded that routine staged complete revascularization was superior to culprit lesion only PCI in reducing the risk of CV death, new MI, or ischemia driven revascularization in STEMI patients with MVD and without cardiogenic shock.111
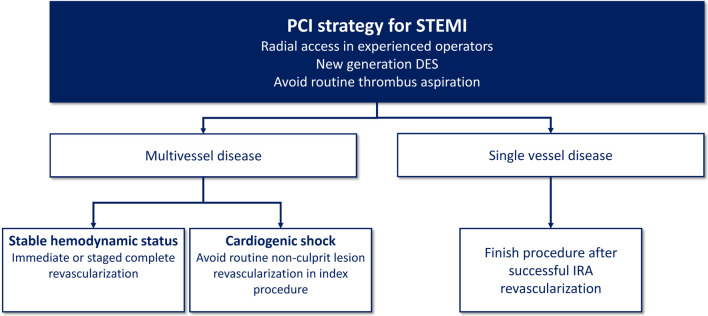
In STEMI patients with MVD and cardiogenic shock (CS), the CULPRIT-SHOCK trial randomized 706 patients to either PCI of the culprit lesion only or multivessel PCI. At 30 days, the end points of death or renal replacement therapy were significantly lower in the culprit lesion only group and these benefits were maintained at 1 year.112,113 In addition, a large-scale observational study also showed that culprit lesion only PCI in STEMI patients with CS had lower mortality rate than multivessel PCI at 30 days and at 1 year.114 Figure 5 summarizes the current recommendations for PCI strategy for STEMI in Taiwan.
Figure 5.
Recommended PCI strategy for STEMI in Taiwan. DES, drug-eluting stent; IRA, infarct-related artery; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Recommendation
• In hemodynamically stable STEMI patients with MVD, immediate or staged PCI (< 45 days) for complete revascularization is recommended. (COR I, LOE A)
• In STEMI patients with CS and MVD, routine non-culprit lesion revascularization during primary PCI is not recommended. (COR III, LOE B)
CARDIOGENIC SHOCK
Definition and classification
Although there were some variations of the definition, CS complicating acute MI is generally defined as systolic blood pressure (BP) < 90 mmHg for ≥ 30 minutes with end-organ hypoperfusion ± hemodynamic criteria (cardiac index ≤ 2.2 L/min/m2 and wedge pressure ≥ 15 mmHg) based on largest randomized controlled trials.112,115,116 Recently, the Society of Cardiovascular Angiography and Interventions unified CS definition and recommended a classification system with 5 stages: A: at risk for CS development (e.g., anterior wall MI), B: beginning or pre-shock (clinical evidence of relative hypotension or tachycardia without hypoperfusion), C: classical CS (hypoperfusion requiring pharmacological or mechanical intervention), D: doom (worse with failing to respond to initial interventions), and E: extremis [cardiac arrest with ongoing CPR and/or extracorporeal membrane oxygenation (ECMO)].1117 The classification system not only makes different CS trials more comparable but also emphasizes the effectiveness of early intervention on the phase of pre-shock or early shock.118,119
Pathophysiology
The incidence of CS with acute MI ranges from 3% to 13%.120,121 Ventricular myocardial dysfunction following acute MI accounts for the majority of CS, comprising around 80% and 7% for left and right ventricular failure, respectively. The remaining 13% of CS victims result from mechanical complications, including acute mitral regurgitation (~7%), ventricular septal defect (~4%) and free wall rupture (~2%).122 Impaired myocardial systolic and diastolic function subsequent to acute MI induce hypoperfusion and lung congestion leading to tissue ischemia and hypoxia.123 It also chemically or biologically elicits a serial release of inflammatory cytokines or inducible nitric oxide synthase resulting in vasodilation which further decreases systemic and coronary perfusions and aggravates pre-existing pumping impairment.124 The catastrophic vicious cycle results in 40-50% mortality rate of CS even in the era of early revascularization and mechanical circulatory support (MCS).124,125
Management
Management should be initiated at pre-shock phase when hypotension appears as the first sign of CS. Echocardiography should be performed immediately to exclude mechanical complications of acute MI as the potential causes of CS. Intra-aortic balloon pumping (IABP) can be considered as a strategy to unload left ventricle and increase coronary perfusion if there is unstable hemodynamics due to mechanical complications. However, IABP should not be performed routinely in patients with CS complicating acute MI.116 Emergent coronary angiography is strongly recommended and revascularization strategy should be determined after heart team evaluation. PCI to open the occluded culprit vessel can be performed but routine non-culprit lesions revascularization during the initial PCI is not recommended.112,113 In patients with CS after initial treatment, complete revascularization during the index hospitalization maybe beneficial.126
Patients with CS should be treated with fluids, vasopressors, and inotropes to prevent or rescue multiple organ dysfunction syndrome (MODS).127 If hemodynamic instability remains after fluid resuscitation with saline or Ringer’s lactate > 200 mL in 15-30 minutes, pharmacological circulatory support with vasopressor (e.g., norepinephrine) and/or ventricular support with inodilator (e.g., dobutamine or levosimendan) should be considered.128 The patients are closely monitored in intensive care units. Pulmonary artery catheter for diagnostic evaluation and therapeutic guidance may be considered if necessary.129 Ultrafiltration may be considered for organ decongestion in acute cardiorenal syndrome with fluid overload and poor response to forceful diuretic therapy.130 Although those resuscitated from cardiac arrest or lethal arrhythmia are generally treated with hypothermia for neuroprotection, the effects of therapeutic hypothermia on hemodynamic or clinical outcomes in CS are still controversial.131,132 For those with refractory shock or deteriorating organ dysfunctions, growing evidences have shown appropriate use of MCS could reduce catecholamine dosage, improve hemodynamics, and serve as bridge to recovery, destination, transplantation, or family’s decision.133-135 Nevertheless, there are still limited data from randomized clinical trials on when and how to use MCS devices properly in CS during acute MI. The survival outcome of CS with acute MI might be affected by different study designs, indications, and limitations of the MSC devices.136
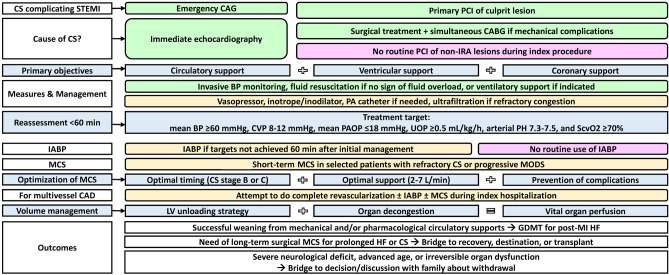
Several reviews have concluded that a successful management of CS with acute MI depends on the following important components including early reperfusion therapy, early hemodynamic support, adequate left ventricular unload, and good quality of post-MI care.137-139 A meta-analysis of 1,866 CS patients supported with ECMO has shown the MCS-associated complication rates were up to 50%, 40%, 30% and 15% for acute kidney injury, major bleeding, infection, and limb ischemia/neurologic deficit, respectively, implicating ECMO can result in survival to discharge but may be associated with considerable morbidity.140 Prevention or prompt management of MCS-associated complications probably increases the chance of survival of CS. Although recent systematic review and meta-analysis have found MODS following CS could be prevented by short-term MCS if it is used at optimal timing at stages B or C of CS, with sufficient blood flow support with 2-7 L/min and without MCS-related complications, the overall survival rate was similar compared with control group.137,141 Therefore, routine use of MCS in unselected patients with CS complicating acute MI is still not suggested. The MCS should be applied after heart team evaluation and according to operator’s experience and patient’s conditions. Figure 6 summarizes the recommendations of evaluation and management of CS complicating acute MI.
Figure 6.
Evidence-based algorithm for evaluation and management of cardiogenic shock complicating AMI. The recommendations were mainly based on the latest reviews and international guidelines. Class I, II, III recommendations were depicted with light green, light yellow, and pink colors, respectively. AMI, acute myocardial infarction; BP, blood pressure; CABG, coronary artery bypass grafting surgery; CAD, coronary artery disease; CAG, coronary angiography; CS, cardiogenic shock; CVP, central venous pressure; GDMT, guideline-directed medical therapy; HF, heart failure; IABP, intra-aortic balloon pumping; IRA, infarct-related artery; LV, left ventricular; MCS, mechanical circulatory support; MODS, multiple organ dysfunction syndrome; PAOP, pulmonary artery obstructive pressure; PCI, percutaneous coronary intervention; ScvO2, central venous oxygen saturation; STEMI, ST-segment elevation myocardial infarction; UOP, urine output.
Recommendation
• Early heart team approach is recommended for treatment of mechanical complications as well as revascularization strategy in acute MI with CS. (COR I, LOE C)
• Emergency coronary angiography is recommended for acute MI patients with CS, followed by PCI of culprit vessel if coronary lesion is suitable, otherwise, emergency CABG is an alternative. (COR I, LOE B)
• For MI patients with CS and MVD, attempt of complete revascularization during index hospitalization is reasonable. (COR IIa, LOE B)
• Short-term mechanical support device (e.g., percutaneous cardiopulmonary support, ECMO, or ventricular assist device) with/without IABP may be considered as a rescue therapy in patients with refractory CS. (COR IIb, LOE C)
POST-MI MEDICAL THERAPIES
Aggressive control of risk factors with evidence-based medical therapies substantially improved prognosis of STEMI. Recent report from the TSOC ACS-Diabetes Mellitus Registry revealed a higher adherence rate of guideline-directed medical therapies could reduce about 40% CV events.142 Repetitive and careful assessment of treatment goal achievement and medical adherence is crucial from acute to chronic stage of MI.143 Additional efforts to enhance patients’ drug adherence, such as using single-pill combination or participation of educational programs, are necessary.144,145
Hypertension
The Taiwan STEMI and hypertension guidelines both recommend a BP goal < 130/80 mmHg in STEMI patients.2,146 Although more intensive treatment with systolic BP < 120 mmHg appeared to benefit high-risk individuals in the SPRINT study,147 implementing this result to all STEMI patients in Taiwan might not be practical because of the method of BP measurement used in the SPRINT study and the concern of J-curve phenomenon of BP.147-149 Effective medical regimens, including long-acting beta-blocker, ACEI/ARB and calcium channel blocker (CCB) are recommended to optimize BP control.146 Beta-blocker, ACEI/ARB and/or mineralocorticoid receptor antagonist (MRA) are especially beneficial in STEMI patients with heart failure or depressed left ventricular ejection fraction.146 MRA should not be used in those with creatinine clearance < 30 mL/min/1.73 m2 or potassium level > 5 mEq/L. Routine administration of beta-blocker, ACEI or ARB in all STEMI patients was supported by international guidelines.150,151 But baseline heart rate, comorbidity and beta receptor selectivity are likely to influence the efficacy of beta-blocker.152-155
Recommendation
• It is recommended to control BP < 130/80 mmHg in STEMI patients by long-acting beta-blocker, ACEI/ARB and/or CCB. (COR I, LOE B)
• Beta-blocker, ACEI/ARB and/or mineralocorticoid receptor antagonist are recommended for STEMI patients with heart failure and reduced ejection fraction if there is no contraindication. (COR I, LOE B)
• Routine use of ACEI/ARB for all STEMI patients is reasonable if no contraindication. (COR IIa, LOE A)
• Routine use of beta-blocker for all STEMI patients may be beneficial if no contraindication. (COR IIa, LOE B)
Diabetes
A treatment goal of HbA1c < 7% is recommended in patients with STEMI. However, clinicians can modify the HbA1c target based on patient’s general condition and risk of hypoglycemia. A less stringent HbA1c target < 8% is reasonable if patients had hypoglycemic history, limited life expectancy, advanced micro- or macrovascular complications and extensive comorbid conditions. In contrast, individuals with least hypoglycemic risk are also eligible to have more stringent target of < 6.5%.156 Metformin remains the usual first-line anti-diabetic agent.157 Thiazolidinedione (TZD), sodium/glucose co-transporter 2 inhibitor (SGLT-2i) or glucagon-like peptide-1 receptor agonist (GLP-1 RA) are recommended as second-line therapies in coronary artery disease since favorable CV outcomes had been observed in relevant clinical studies.157 Concerning the increased risk of development of post-MI heart failure, TZD is not generally recommended to all STEMI patients as a second-line agent and should be administered with caution. In patients with STEMI and heart failure, SGLT-2i can be considered as the first line therapy because the drug has been proved to improve the prognosis of heart failure with reduced ejection fraction in recent clinical trial.158
Recommendation
• It is recommended to control HbA1c < 7% in STEMI patients. The target of HbA1c could be individualized according to patient’s condition (< 6.5% to < 8%). (COR I, LOE B)
• Metformin is recommended as the first-line therapy. (COR I, LOE B)
• The priority for add-on therapy includes SGLT-2i and GLP-1 RA. (COR IIa, LOE A)
• SGLT-2i is recommended for STEMI patients with heart failure and reduced ejection fraction. (COR I, LOE B)
Hypercholesterolemia
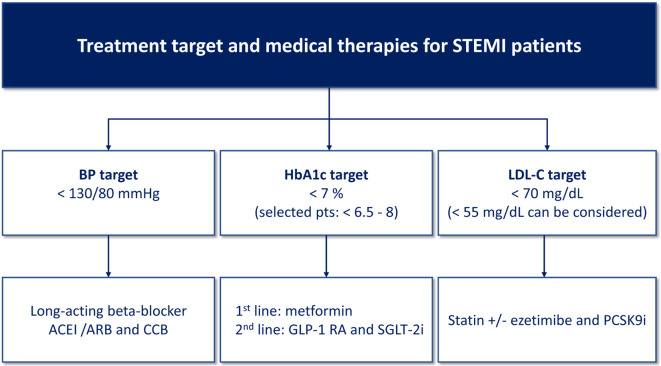
Low-density lipoprotein cholesterol (LDL-C) remains the primary target for screening, diagnosis and management of dyslipidemia.159,160 Current Taiwan lipid guidelines for high risk patients recommend a LDL-C goal < 70 mg/dl for patients with acute MI and < 55 mg/dl for acute MI in diabetic patients.160 The major evidence-based lipid-lowering medications for ACS were limited to statin and ezetimibe in the past. Previous meta-analysis demonstrated that reduction of LDL-C with statin or nonstatin therapy was associated with similar risk reduction of major vascular events.161 Due to its potency and scientific evidences, statin is the first-line therapy. In Taiwan, titration to high intensity statin (atorvastatin 40 mg/day or rosuvastatin 20 mg/day) or use statin plus ezetimibe combination therapy is suggested to treat to LDL-C goal in patients with acute MI.160 A number of patients may develop adverse effects after taking high or any dose of statins.162 This population has suboptimal LDL-C control and increased risk of CV events.163 Recently published randomized controlled trials, FOURIER and ODYSSEY OUTCOMES studies, showed that add-on proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) to statin therapy could further reduce 50-60% of LDL-C and obtain a 15% relative risk reduction of CV events in patients with stable ASCVD and recent ACS.164,165 In the ODYSSEY OUTCOMES trial with recent ACS, the preset LDL-C target of PCSK9i group was 25 to 50 mg/dL and the mean achieved LDL-C level at 48-month follow-up was 53 mg/dL.165 Intensification of LDL-C control by these potent agents could help STEMI patients to attain the current treatment LDL-C goal much more easier. However, due to the drugs are still very expensive, PCSK9i is usually suggested as the last resort after using maximally tolerated statin and ezetimibe.166,167 Figure 7 summarizes the recommendations of the treatment target and drug of choice for long term post-MI medical therapies.
Figure 7.
Recommended treatment targets and medical therapies for STEMI patients. ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CCB, calcium channel blocker; GLP-1 RA, glucagon-like peptide-1 receptor agonist; LDL-C, low-density lipoprotein cholesterol; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor; SGLT-2i, sodium/glucose co-transporter 2 inhibitor.
Recommendation
• It is recommended to control LDL-C < 70 mg/dL in STEMI patients. (COR I, LOE A)
• A lower target of LDL-C < 55 mg/dL can be considered in STEMI patients. (COR IIa, LOE B)
• LDL-C control should be intensified by statin plus nonstatin therapies, including ezetimibe and/or PCSK9i with full consideration of clinical efficacy, patient’s preference as well as cost-effectiveness. (COR I, LOE A)
QUALITY CARE OF STEMI
To reduce the gap between clinical practice and guideline recommendations in STEMI care, a quality evaluation system should be established.168,169 The quality care of STEMI depends on regularly tracking time intervals of reperfusion therapy, monitoring measurable quality indicators and timely feedback to network stakeholders. The major purpose is for continuous quality improvement of STEMI. The quality indicators, which can measure and compare the quality of health service provider, are divided into three dimensions, including structure, process and outcome indicators (Table 1).170,171 The pre-hospital and hospital-based treatment delays or in-hospital medications can be monitored by routine audit meeting and updated STEMI management protocols. If the targets of quality indicators are not met, quality improvement program should be initiated to improve performance of the system and further maintain quality targets.
Table 1. STEMI quality indicators.
| Dimension | Process | Setting | Indicators |
| Structure indicator | Diagnosis | Pre-hospital | • Ambulance equipped with telemetry pre-hospital ECG recorders |
| • Ambulance equipped with automated external defibrillators | |||
| • Ambulance equipped with auto CPR machines | |||
| • City-based network system to record key factors and time to reperfusion | |||
| Treatment | Hospital | • Single call to activate STEMI team | |
| • 24-hour service of catheterization laboratory | |||
| Process indicator | Diagnosis | Pre-hospital | • EMTs perform telemetry pre-hospital ECG for patients with typical symptoms of acute MI |
| Diagnosis | Hospital | • Door to ECG time | |
| • Evaluation of LVEF before discharge | |||
| Treatment | Emergent department | • Aspirin at arrival | |
| • P2Y12 receptor inhibitor at arrival | |||
| Treatment | Catheterization laboratory | • Symptom onset to door time | |
| • Door to wire time | |||
| Treatment | In-hospital | • Aspirin prescribed at discharge | |
| • P2Y12 receptor inhibitor prescribed at discharge | |||
| • Beta blocker prescribed at discharge | |||
| • Statin prescribed at discharge | |||
| • ACEI or ARB or ARNI prescribed at discharge for LV systolic dysfunction (EF ≤ 40%) | |||
| • Aldosterone antagonist prescribed at discharge for LV systolic dysfunction (EF ≤ 40%) | |||
| • Cardiac rehabilitation referral from an inpatient setting | |||
| • Immediate angiography for resuscitated OHCA in STEMI patients | |||
| • Therapeutic hypothermia for comatose STEMI patients with OHCA | |||
| • Smoking cessation advice or counseling before discharge | |||
| Quality | Follow up | • Participation in ≥ 1 national STEMI accreditation system and/or other STEMI certification system | |
| Outcome indicator | Quality | In-hospital | • In-hospital mortality |
| Quality | Follow up | • 30-day mortality | |
| • 30-day readmission rate | |||
| • 30-day re-infarction rate | |||
| • 1-year mortality | |||
| • 1-year readmission rate | |||
| • 1-year re-infarction rate |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; CPR, cardiopulmonary resuscitation; ECG, electrocardiography; EF, ejection fraction; EMT, emergency medical technicians; LV, left ventricle; MI, myocardial infarction; OHCA, out-of-hospital cardiac arrest; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
In Taiwan, a national STEMI accreditation system was developed by government to monitor the care quality of STEMI in hospitals that performs primary PCI every 3 years. The monitored items include door to ECG time, DAPT use, door to wire time in primary PCI, and other standard operation procedures of STEMI care. The hospitals with 24-hour PCI capability have to reach the following targets: (1) more than 80% of STEMI patients have door to ECG time < 10 minutes, (2) more than 80% of STEMI patients received DAPT, (3) more than 75% of STEMI patients have door to wire time in primary PCI < 90 minutes for those initially presented with ischemic symptoms and more than 70% of STEMI patients have door to wire time in primary PCI < 60 minutes for those diagnosed STEMI referred for primary PCI. The national STEMI accreditation system established the basic requirements for STEMI quality care in Taiwan.27 The Joint Commission of Taiwan is a non-government organization with the missions of hospital accreditation, certification and healthcare quality inspection. The Joint Commission of Taiwan built up a Taiwan AMI Certification Hospitals system to help the hospitals to pursue a better quality of care for AMI. Taiwan AMI Certification Hospitals system has 3 categories and 27 detailed items. The three categories include STEMI teamwork and management, STEMI patients and family care and STEMI quality improvement. The ultimate purpose is to further improve the comprehensive care of AMI.
Recommendation
• Participation of national STEMI accreditation system and other certification system are recommended to audit the time delay and critical quality targets achievement for STEMI. (COR I, LOE C)
• STEMI quality control system, including monitoring quality indicators and regular quality care meetings, should be established in hospitals. (COR I, LOE C)
CONFLICTS OF INTEREST
This study was supported by the Taiwan Society of Cardiology.
REFERENCES
- 1.Lee CH, Fang CC, Tsai LM, et al. Patterns of acute myocardial infarction in Taiwan from 2009 to 2015. Am J Cardiol. 2018;122:1996–2004. doi: 10.1016/j.amjcard.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 2.Li YH, Yeh HI, Tsai CT, et al. 2012 Guidelines of the Taiwan Society of Cardiology (TSOC) for the management of ST-segment elevation myocardial infarction. Acta Cardiol Sin. 2012;28:63–89. [Google Scholar]
- 3.Li YH, Wang YC, Wang YC, et al. 2018 Guidelines of the Taiwan Society of Cardiology, Taiwan Society of Emergency Medicine and Taiwan Society of Cardiovascular Interventions for the management of non ST-segment elevation acute coronary syndrome. J Formos Med Assoc. 2018;117:766–790. doi: 10.1016/j.jfma.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Lee CH, Cheng CL, Kao Yang YH, et al. Trends in the incidence and management of acute myocardial infarction from 1999 to 2008: get with the guidelines performance measures in Taiwan. J Am Heart Assoc. 2014;3:pii:e001066. doi: 10.1161/JAHA.114.001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin WH, Lu TH, Chen KC, et al. The temporal trends of incidence, treatment, and in-hospital mortality of acute myocardial infarction over 15 years in a Taiwanese population. Int J Cardiol. 2016;209:103–113. doi: 10.1016/j.ijcard.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Li YH, Chiu YW, Cheng JJ, et al. Changing practice pattern of acute coronary syndromes in Taiwan from 2008 to 2015. Acta Cardiol Sin. 2019;35:1–10. doi: 10.6515/ACS.201901_35(1).20180716B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee PT, Chao TH, Huang YL, et al. Analysis of the clinical characteristics, management, and causes of death in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention from 2005 to 2014. Int Heart J. 2016;57:541–546. doi: 10.1536/ihj.15-454. [DOI] [PubMed] [Google Scholar]
- 8.Qian F, Ling FS, Deedwania P, et al. Care and outcomes of Asian-American acute myocardial infarction patients: findings from the American Heart Association Get With The Guidelines-Coronary Artery Disease program. Circ Cardiovasc Qual Outcomes. 2012;5:126–133. doi: 10.1161/CIRCOUTCOMES.111.961987. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 10.Chao CC, Chen YC, Shih CM, et al. Smartphone transmission of electrocardiography images to reduce time of cardiac catheterization laboratory activation. J Chin Med Assoc. 2018;81:505–510. doi: 10.1016/j.jcma.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Chen KC, Yen DH, Chen CD, et al. Effect of emergency department in-hospital tele-electrocardiographic triage and interventional cardiologist activation of the infarct team on door-to-balloon times in ST-segment-elevation acute myocardial infarction. Am J Cardiol. 2011;107:1430–1435. doi: 10.1016/j.amjcard.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Lee CC, Huang SS, Yeo YH, et al. High-sensitivity-cardiac troponin for accelerated diagnosis of acute myocardial infarction: a systematic review and meta-analysis. Am J Emerg Med. 2019:pii: S0735-6757(19)30774-0. doi: 10.1016/j.ajem.2019.11.035. [DOI] [PubMed] [Google Scholar]
- 13.Tan WCJ, Inoue K, AbdelWareth L, et al. The Asia-Pacific Society of Cardiology (APSC) expert committee consensus recommendations for assessment of suspected acute coronary syndrome using high-sensitivity cardiac troponin T in the emergency department. Circ J. 2020;84:136–143. doi: 10.1253/circj.CJ-19-0874. [DOI] [PubMed] [Google Scholar]
- 14.Levis JT. ECG diagnosis: isolated posterior wall myocardial infarction. Perm J. 2015;19:e143–e144. doi: 10.7812/TPP/14-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briosa E, Gala A, Rawlins J. Posterior-wall myocardial infarction. N Engl J Med. 2019;381:e32. doi: 10.1056/NEJMicm1901367. [DOI] [PubMed] [Google Scholar]
- 16.Yamaji H, Iwasaki K, Kusachi S, et al. Prediction of acute left main coronary artery obstruction by 12-lead electrocardiography. ST segment elevation in lead aVR with less ST segment elevation in lead V(1). J Am Coll Cardiol. 2001;38:1348–1354. doi: 10.1016/s0735-1097(01)01563-7. [DOI] [PubMed] [Google Scholar]
- 17.Sen F, Ozeke O, Kirbas O, et al. Classical electrocardiographic clues for left main coronary artery disease. Indian Heart J. 2016;68 Suppl 2:S226–S227. doi: 10.1016/j.ihj.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sgarbossa EB, Pinski SL, Barbagelata A, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. N Engl J Med. 1996;334:481–487. doi: 10.1056/NEJM199602223340801. [DOI] [PubMed] [Google Scholar]
- 19.McNamara RL, Wang Y, Herrin J, et al. Effect of door-to-balloon time on mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2006;47:2180–2186. doi: 10.1016/j.jacc.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 20.Kim HS, Lee H, Kim K, et al. The general public’s awareness of early symptoms of and emergency responses to acute myocardial infarction and related factors in South Korea: a national public telephone survey. J Epidemiol. 2016;26:233–241. doi: 10.2188/jea.JE20150074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finn JC, Bett JH, Shilton TR, et al. Patient delay in responding to symptoms of possible heart attack: can we reduce time to care? Med J Aust. 2007;187:293–298. doi: 10.5694/j.1326-5377.2007.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 22.Ryan D, Craig AM, Turner L, Verbeek PR. Clinical events and treatment in prehospital patients with ST-segment elevation myocardial infarction. Prehosp Emerg Care. 2013;17:181–186. doi: 10.3109/10903127.2012.744783. [DOI] [PubMed] [Google Scholar]
- 23.Tanguay A, Brassard E, Lebon J, et al. Effectiveness of a prehospital wireless 12-lead electrocardiogram and cardiac catheterization laboratory activation for ST-elevation myocardial infarction. Am J Cardiol. 2017;119:553–559. doi: 10.1016/j.amjcard.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 24.Mercuri M, Welsford M, Schwalm JD, et al. Providing optimal regional care for ST-segment elevation myocardial infarction: a prospective cohort study of patients in the Hamilton Niagara Haldimand Brant Local Health Integration Network. CMAJ Open. 2015;3:E1–E7. doi: 10.9778/cmajo.20140035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross G, Alsayed T, Turner L, et al. Assessment of the safety and effectiveness of emergency department STEMI bypass by defibrillation-only emergency medical technicians/primary care paramedics. Prehosp Emerg Care. 2015;19:191–201. doi: 10.3109/10903127.2014.959226. [DOI] [PubMed] [Google Scholar]
- 26.Boden WE, Eagle K, Granger CB. Reperfusion strategies in acute ST-segment elevation myocardial infarction: a comprehensive review of contemporary management options. J Am Coll Cardiol. 2007;50:917–929. doi: 10.1016/j.jacc.2007.04.084. [DOI] [PubMed] [Google Scholar]
- 27.Wu CK, Juang JMJ, Chiang JY, et al. The Taiwan Heart Registries: its influence on cardiovascular patient care. J Am Coll Cardiol. 2018;71:1273–1283. doi: 10.1016/j.jacc.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Chou LP, Zhao P, Kao C, et al. Women were noninferior to men in cardiovascular outcomes among patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention from Taiwan acute coronary syndrome full-spectrum registry. Medicine (Baltimore) 2018;97:e12998. doi: 10.1097/MD.0000000000012998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmes DR, Jr., Bell MR, Gersh BJ, et al. Systems of care to improve timeliness of reperfusion therapy for ST-segment elevation myocardial infarction during off hours: the Mayo Clinic STEMI protocol. JACC Cardiovasc Interv. 2008;1:88–96. doi: 10.1016/j.jcin.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Fosbol EL, Granger CB, Jollis JG, et al. The impact of a statewide pre-hospital STEMI strategy to bypass hospitals without percutaneous coronary intervention capability on treatment times. Circulation. 2013;127:604–612. doi: 10.1161/CIRCULATIONAHA.112.118463. [DOI] [PubMed] [Google Scholar]
- 31.Le May MR, So DY, Dionne R, et al. A citywide protocol for primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2008;358:231–240. doi: 10.1056/NEJMoa073102. [DOI] [PubMed] [Google Scholar]
- 32.Cantor WJ, Hoogeveen P, Robert A, et al. Prehospital diagnosis and triage of ST-elevation myocardial infarction by paramedics without advanced care training. Am Heart J. 2012;164:201–206. doi: 10.1016/j.ahj.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Kuo FY, Huang WC, Chiou KR, et al. The effect of failure mode and effect analysis on reducing percutaneous coronary intervention hospital door-to-balloon time and mortality in ST segment elevation myocardial infarction. BMJ Qual Saf. 2013;22:626–638. doi: 10.1136/bmjqs-2012-001288. [DOI] [PubMed] [Google Scholar]
- 34.Wong GC, Welsford M, Ainsworth C, et al. 2019 Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology Guidelines on the acute management of ST-elevation myocardial infarction: focused update on regionalization and reperfusion. Can J Cardiol. 2019;35:107–132. doi: 10.1016/j.cjca.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 35.Koul S, Smith JG, Scherstén F, et al. Effect of upstream clopidogrel treatment in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J. 2011;32:2989–2997. doi: 10.1093/eurheartj/ehr202. [DOI] [PubMed] [Google Scholar]
- 36.Dörler J, Edlinger M, Alber HF, et al. Clopidogrel pre-treatment is associated with reduced in-hospital mortality in primary percutaneous coronary intervention for acute ST-elevation myocardial infarction. Eur Heart J. 2011;32:2954–2961. doi: 10.1093/eurheartj/ehr360. [DOI] [PubMed] [Google Scholar]
- 37.Zeymer U, Arntz HR, Mark B, et al. Efficacy and safety of a high loading dose of clopidogrel administered prehospitally to improve primary percutaneous coronary intervention in acute myocardial infarction: the randomized CIPAMI trial. Clin Res Cardiol. 2012;101:305–312. doi: 10.1007/s00392-011-0393-1. [DOI] [PubMed] [Google Scholar]
- 38.Montalescot G, van't Hof AW, Lapostolle F, et al. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med. 2014;371:1016–1027. doi: 10.1056/NEJMoa1407024. [DOI] [PubMed] [Google Scholar]
- 39.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 40.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 41.Mooney MR, Unger BT, Boland LL, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest: evaluation of a regional system to increase access to cooling. Circulation. 2011;124:206–214. doi: 10.1161/CIRCULATIONAHA.110.986257. [DOI] [PubMed] [Google Scholar]
- 42.Kim F, Nichol G, Maynard C, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA. 2014;311:45–52. doi: 10.1001/jama.2013.282173. [DOI] [PubMed] [Google Scholar]
- 43.Yajnik V, Gomez H. Prehospital induction of mild hypothermia with cold normal saline for cardiac arrest: more harm than good? Crit Care. 2014;18:559. doi: 10.1186/s13054-014-0559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo controlled trial. Lancet. 2005;366:1607–1621. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- 45.Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352:1179–1189. doi: 10.1056/NEJMoa050522. [DOI] [PubMed] [Google Scholar]
- 46.Cheng CI, Chen CP, Kuan PL, et al. The causes and outcomes of inadequate implementation of existing guidelines for antiplatelet treatment in patients with acute coronary syndrome: the experience from Taiwan Acute Coronary Syndrome Descriptive Registry (T-ACCORD Registry). Clin Cardiol. 2010;33:E40–E48. doi: 10.1002/clc.20730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiang FT, Shyu KG, Wu CJ, et al. Predictors of 1-year outcomes in the Taiwan Acute Coronary Syndrome Full Spectrum Registry. J Formos Med Assoc. 2014;113:794–802. doi: 10.1016/j.jfma.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Chen SC, Hsiao FY, Lee CM, et al. Duration of dual antiplatelet therapy following percutaneous coronary intervention on re-hospitalization for acute coronary syndrome. BMC Cardiovasc Disord. 2014;14:21. doi: 10.1186/1471-2261-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li YH, Chiu YW, Cheng JJ, et al. Duration of clopidogrel-based dual antiplatelet therapy and clinical outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention - a real-world observation in Taiwan from 2012 to 2015. Circ J. 2019;83:1317–1323. doi: 10.1253/circj.CJ-18-1283. [DOI] [PubMed] [Google Scholar]
- 50.Gurbel PA, Bliden KP, Zaman KA, et al. Clopidogrel loading with eptifibatide to arrest the reactivity of platelets: results of the Clopidogrel Loading With Eptifibatide to Arrest the Reactivity of Platelets (CLEAR PLATELETS) study. Circulation. 2005;111:1153–1159. doi: 10.1161/01.CIR.0000157138.02645.11. [DOI] [PubMed] [Google Scholar]
- 51.von Beckerath N, Taubert D, Pogatsa-Murray G, et al. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) trial. Circulation. 2005;112:2946–2950. doi: 10.1161/CIRCULATIONAHA.105.559088. [DOI] [PubMed] [Google Scholar]
- 52.Mehta SR, Bassand JP, Chrolavicius S, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med. 2010;363:930–942. doi: 10.1056/NEJMoa0909475. [DOI] [PubMed] [Google Scholar]
- 53.Siller-Matula JM, Huber K, Christ G, et al. Impact of clopidogrel loading dose on clinical outcome in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Heart. 2011;97:98–105. doi: 10.1136/hrt.2010.195438. [DOI] [PubMed] [Google Scholar]
- 54.Schömig A. Ticagrelor--is there need for a new player in the antiplatelet-therapy field? N Engl J Med. 2009;361:1108–1111. doi: 10.1056/NEJMe0906549. [DOI] [PubMed] [Google Scholar]
- 55.Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–3175. doi: 10.1161/01.CIR.0000130846.46168.03. [DOI] [PubMed] [Google Scholar]
- 56.Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 57.Hu XP, Xu JM, Hu YM, et al. Effects of CYP2C19 genetic polymorphism on the pharmacokinetics and pharmacodynamics of omeprazole in Chinese people. J Clin Pharm Ther. 2007;32:517–524. doi: 10.1111/j.1365-2710.2007.00851.x. [DOI] [PubMed] [Google Scholar]
- 58.Veiga MI, Asimus S, Ferreira PE, et al. Pharmacogenomics of CYP2A6, CYP2B6, CYP2C19, CYP2D6, CYP3A4, CYP3A5 and MDR1 in Vietnam. Eur J Clin Pharmacol. 2009;65:355–363. doi: 10.1007/s00228-008-0573-8. [DOI] [PubMed] [Google Scholar]
- 59.Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation. 2007;116:2923–2932. doi: 10.1161/CIRCULATIONAHA.107.740324. [DOI] [PubMed] [Google Scholar]
- 60.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 61.Udell JA, Braunwald E, Antman EM, et al. Prasugrel versus clopidogrel in patients with ST-segment elevation myocardial infarction according to timing of percutaneous coronary intervention: a TRITON-TIMI 38 subgroup analysis (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis In Myocardial Infarction 38). JACC Cardiovasc Interv. 2014;7:604–612. doi: 10.1016/j.jcin.2014.01.160. [DOI] [PubMed] [Google Scholar]
- 62.Saito S, Isshiki T, Kimura T, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J. 2014;78:1684–1692. doi: 10.1253/circj.cj-13-1482. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura M, Iizuka T, Sagawa K, et al. Prasugrel for Japanese patients with acute coronary syndrome in short-term clinical practice (PRASFIT-Practice I): a postmarketing observational study. Cardiovasc Interv Ther. 2018;33:135–145. doi: 10.1007/s12928-017-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akita K, Inohara T, Yamaji K, et al. Impact of reduced-dose prasugrel vs. standard-dose clopidogrel on in-hospital outcomes of percutaneous coronary intervention in 62,737 patients with acute coronary syndromes:a nationwide registry study in Japan. Eur Heart J Cardiovasc Pharmacother. 2019:pii: pvz056. doi: 10.1093/ehjcvp/pvz056. [DOI] [PubMed] [Google Scholar]
- 65.Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 66.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 67.Steg PG, James S, Harrington RA, et al. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: A Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122:2131–2141. doi: 10.1161/CIRCULATIONAHA.109.927582. [DOI] [PubMed] [Google Scholar]
- 68.Berwanger O, Nicolau JC, Carvalho AC, et al. Ticagrelor vs clopidogrel after fibrinolytic therapy in patients with ST-elevation myocardial infarction: a randomized clinical trial. JAMA Cardiol. 2018;3:391–399. doi: 10.1001/jamacardio.2018.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berwanger O, Lopes RD, Moia DDF, et al. Ticagrelor versus clopidogrel in patients with STEMI treated with fibrinolysis: TREAT trial. J Am Coll Cardiol. 2019;73:2819–2828. doi: 10.1016/j.jacc.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 70.Sahlén A, Varenhorst C, Lagerqvist B, et al. Outcomes in patients treated with ticagrelor or clopidogrel after acute myocardial infarction: experiences from SWEDEHEART registry. Eur Heart J. 2016;37:3335–3342. doi: 10.1093/eurheartj/ehw284. [DOI] [PubMed] [Google Scholar]
- 71.Goto S, Huang CH, Park SJ, et al. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome -- randomized, double-blind, phase III PHILO study. Circ J. 2015;79:2452–2460. doi: 10.1253/circj.CJ-15-0112. [DOI] [PubMed] [Google Scholar]
- 72.Park DW, Kwon O, Jang JS, et al. Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: a randomized clinical trial. Circulation. 2019;140:1865–1877. doi: 10.1161/CIRCULATIONAHA.119.041766. [DOI] [PubMed] [Google Scholar]
- 73.Tang X, Li R, Jing Q, et al. Assessment of ticagrelor versus clopidogrel treatment in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Cardiovasc Pharmacol. 2016;68:115–120. doi: 10.1097/FJC.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 74.Xin YG, Zhang HS, Li YZ, et al. Efficacy and safety of ticagrelor versus clopidogrel with different dosage in high-risk patients with acute coronary syndrome. Int J Cardiol. 2017;228:275–279. doi: 10.1016/j.ijcard.2016.11.160. [DOI] [PubMed] [Google Scholar]
- 75.Park KH, Jeong MH, Ahn Y, et al. Comparison of short-term clinical outcomes between ticagrelor versus clopidogrel in patients with acute myocardial infarction undergoing successful revascularization; from Korea Acute Myocardial Infarction Registry-National Institute of Health. Int J Cardiol. 2016;215:193–200. doi: 10.1016/j.ijcard.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 76.Wang H, Wang X. Efficacy and safety outcomes of ticagrelor compared with clopidogrel in elderly Chinese patients with acute coronary syndrome. Ther Clin Risk Manag. 2016;12:1101–1105. doi: 10.2147/TCRM.S108965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen IC, Lee CH, Fang CC, et al. Efficacy and safety of ticagrelor versus clopidogrel in acute coronary syndrome in Taiwan: a multicenter retrospective pilot study. J Chin Med Assoc. 2016;79:521–530. doi: 10.1016/j.jcma.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 78.Lee CH, Cheng CL, Yang KYH, et al. Cardiovascular and bleeding risks in acute myocardial infarction newly treated with ticagrelor vs. clopidogrel in Taiwan. Circ J. 2018;82:747–756. doi: 10.1253/circj.CJ-17-0632. [DOI] [PubMed] [Google Scholar]
- 79.Lee CH, Tsai TH, Lin CJ, et al. Efficacy and safety of ticagrelor compared with clopidogrel in patients with end-stage renal disease with acute myocardial infarction. Am J Cardiovasc Drugs. 2019;19:325–334. doi: 10.1007/s40256-018-00318-0. [DOI] [PubMed] [Google Scholar]
- 80.Sun J, Xiang Q, Li C, et al. Efficacy and safety of novel oral P2Y12 receptor inhibitors in patients with ST-segment elevation myocardial infarction undergoing PCI: a systematic review and meta-analysis. J Cardiovasc Pharmacol. 2017;69:215–227. doi: 10.1097/FJC.0000000000000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rafique AM, Nayyar P, Wang TY, et al. Optimal P2Y12 inhibitor in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: a network meta-analysis. JACC Cardiovasc Interv. 2016;9:1036–1046. doi: 10.1016/j.jcin.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 82.Kim C, Shin DH, Ahn CM, et al. The use pattern and clinical impact of new antiplatelet agents including prasugrel and ticagrelor on 30-day outcomes after acute myocardial infarction in Korea: Korean Health Insurance Review and Assessment Data. Korean Circ J. 2017;47:888–897. doi: 10.4070/kcj.2017.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choe JC, Cha KS, Ahn J, et al. Comparison of prescription rates and clinical outcomes in acute coronary syndrome patients who underwent percutaneous coronary intervention using different P2Y12 inhibitors in a large observational study. Int J Cardiol. 2019;274:21–26. doi: 10.1016/j.ijcard.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 84.Dawwas GK, Dietrich E, Winchester DE, et al. Comparative effectiveness and safety of ticagrelor versus prasugrel in patients with acute coronary syndrome: a retrospective cohort analysis. Pharmacotherapy. 2019;39:912–920. doi: 10.1002/phar.2311. [DOI] [PubMed] [Google Scholar]
- 85.Schüpke S, Neumann FJ, Menichelli M, et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381:1524–1534. doi: 10.1056/NEJMoa1908973. [DOI] [PubMed] [Google Scholar]
- 86.Rathore SS, Curtis JP, Chen J, et al. Association of door-to-balloon time and mortality in patients admitted to hospital with ST elevation myocardial infarction: national cohort study. BMJ. 2009;338:b1807. doi: 10.1136/bmj.b1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McNamara RL, Wang Y, Herrin J, et al. Effect of door-to-balloon time on mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2006;47:2180–2186. doi: 10.1016/j.jacc.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 88.Nallamothu BK, Normand SL, Wang Y, et al. Relation between door-to-balloon times and mortality after primary percutaneous coronary intervention over time: a retrospective study. Lancet. 2015;385:1114–1122. doi: 10.1016/S0140-6736(14)61932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brodie BR, Gersh BJ, Stuckey T, et al. When is door-to-balloon time critical? Analysis from the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) and CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) trials. J Am Coll Cardiol. 2010;56:407–413. doi: 10.1016/j.jacc.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 90.Shiomi H, Nakagawa Y, Morimoto T, et al. Association of onset to balloon and door to balloon time with long term clinical outcome in patients with ST elevation acute myocardial infarction having primary percutaneous coronary intervention: observational study. BMJ. 2012;344:e3257. doi: 10.1136/bmj.e3257. [DOI] [PubMed] [Google Scholar]
- 91.Lai CC, Chang KC, Liao PC, et al. Effects of door-to-balloon times on outcomes in Taiwanese patients receiving primary percutaneous coronary intervention: a report of Taiwan Acute Coronary Syndrome Full Spectrum Registry. Acta Cardiol Sin. 2015;31:215–225. doi: 10.6515/ACS20140721E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–1420. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 93.Jolly SS, Cairns J, Yusuf S, et al. Procedural volume and outcomes with radial or femoral access for coronary angiography and intervention. J Am Coll Cardiol. 2014;63:954–963. doi: 10.1016/j.jacc.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 94.Valgimigli M, Gagnor A, Calabró P, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465–2476. doi: 10.1016/S0140-6736(15)60292-6. [DOI] [PubMed] [Google Scholar]
- 95.Sabate M, Cequier A, Iñiguez A, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomized controlled trial. Lancet. 2012;380:1482–1490. doi: 10.1016/S0140-6736(12)61223-9. [DOI] [PubMed] [Google Scholar]
- 96.Räber L, Kelbæk H, Ostojic M, et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308:777–787. doi: 10.1001/jama.2012.10065. [DOI] [PubMed] [Google Scholar]
- 97.Frobert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587–1597. doi: 10.1056/NEJMoa1308789. [DOI] [PubMed] [Google Scholar]
- 98.Lagerqvist B, Frobert O, Olivecrona GK, et al. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. 2014;371:1111–1120. doi: 10.1056/NEJMoa1405707. [DOI] [PubMed] [Google Scholar]
- 99.Jolly SS, Cairns JA, Yusuf S, et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372:1389–1398. doi: 10.1056/NEJMoa1415098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jolly SS, Cairns JA, Yusuf S, et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction:1-year follow-up of the prospective randomized TOTAL trial. . Lancet. 2016;387:127–135. doi: 10.1016/S0140-6736(15)00448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jolly SS, Cairns JA, Yusuf S, et al. Stroke in the TOTAL trial: a randomized trial of routine thrombectomy vs. percutaneous coronary intervention alone in ST elevation myocardial infarction. Eur Heart J. 2015;36:2364–2372. doi: 10.1093/eurheartj/ehv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goldstein JA, Demetriou D, Grines CL, et al. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343:915–922. doi: 10.1056/NEJM200009283431303. [DOI] [PubMed] [Google Scholar]
- 103.Bates ER, Tamis-Holland JE, Bittl JA, et al. PCI strategies in patients with ST-segment elevation myocardial infarction and multivessel coronary artery disease. J Am Coll Cardiol. 2016;68:1066–1081. doi: 10.1016/j.jacc.2016.05.086. [DOI] [PubMed] [Google Scholar]
- 104.Wood DA, Cairns JA, Mehta SR. Multivessel revascularization and ST segment-elevation myocardial infarction: do we have the complete answer? Circ Cardiovasc Interv. 2017;10:pii: e005215. doi: 10.1161/CIRCINTERVENTIONS.117.005215. [DOI] [PubMed] [Google Scholar]
- 105.Engstrom T, Kelbaek H, Helqvist S, et al. Complete revascularization versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomized controlled trial. Lancet. 2015;386:665–671. doi: 10.1016/s0140-6736(15)60648-1. [DOI] [PubMed] [Google Scholar]
- 106.Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963–972. doi: 10.1016/j.jacc.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional flow reserve guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234–1244. doi: 10.1056/NEJMoa1701067. [DOI] [PubMed] [Google Scholar]
- 108.Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–1123. doi: 10.1056/NEJMoa1305520. [DOI] [PubMed] [Google Scholar]
- 109.Lonborg J, Engstrom T, Kelbaek H, et al. Fractional flow reserve-guided complete revascularization improves the prognosis in patients with ST segment-elevation myocardial infarction and severe nonculprit disease: a DANAMI 3-PRIMULTI substudy (Primary PCI in Patients With ST Elevation Myocardial Infarction and Multivessel Disease: Treatment of Culprit Lesion Only or Complete evascularization). Circ Cardiovasc Interv. 2017;10:e004460. doi: 10.1161/CIRCINTERVENTIONS.116.004460. [DOI] [PubMed] [Google Scholar]
- 110.Bainey KR, Welsh RC, Toklu B, et al. Complete vs culprit-only percutaneous coronary intervention in STEMI with multivessel disease: a meta-analysis and trial sequential analysis of randomized trials. Can J Cardiol. 2016;32:1542–1551. doi: 10.1016/j.cjca.2016.02.077. [DOI] [PubMed] [Google Scholar]
- 111.Mehta SR, Wood DA, Storey RF, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381:1411–1421. doi: 10.1056/NEJMoa1907775. [DOI] [PubMed] [Google Scholar]
- 112.Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419–2432. doi: 10.1056/NEJMoa1710261. [DOI] [PubMed] [Google Scholar]
- 113.Thiele H, Akin I, Sandri M, et al. One-year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. 2018;379:1699–1710. doi: 10.1056/NEJMoa1808788. [DOI] [PubMed] [Google Scholar]
- 114.McNeice A, Nadra IJ, Robinson SD, et al. The prognostic impact of revascularization strategy in acute myocardial infarction and cardiogenic shock: insights from the British Columbia Cardiac Registry. Catheter Cardiovasc Interv. 2018;92:E356–E367. doi: 10.1002/ccd.27648. [DOI] [PubMed] [Google Scholar]
- 115.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 116.Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 117.Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94:29–37. doi: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 118.Asleh R, Resar JR. Utilization of percutaneous mechanical circulatory support devices in cardiogenic shock complicating acute myocardial infarction and high-risk percutaneous coronary interventions. J Clin Med. 2019;8:1209. doi: 10.3390/jcm8081209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vahdatpour C, Collins D, Goldberg S. Cardiogenic shock. J Am Heart Assoc. 2019;8:e011991. doi: 10.1161/JAHA.119.011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aissaoui N, Puymirat E, Tabone X, et al. Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST-MI French nationwide registries. Eur Heart J. 2012;33:2535–2543. doi: 10.1093/eurheartj/ehs264. [DOI] [PubMed] [Google Scholar]
- 121.Rathod KS, Koganti S, Iqbal MB, et al. Contemporary trends in cardiogenic shock: incidence, intra-aortic balloon pump utilisation and outcomes from the London Heart Attack Group. Eur Heart J Acute Cardiovasc Care. 2018;7:16–27. doi: 10.1177/2048872617741735. [DOI] [PubMed] [Google Scholar]
- 122.Hochman JS, Buller CE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction--etiologies, management and outcome: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol. 2000;36:1063–1070. doi: 10.1016/s0735-1097(00)00879-2. [DOI] [PubMed] [Google Scholar]
- 123.van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 124.Werdan K, Gielen S, Ebelt H, Hochman JS. Mechanical circulatory support in cardiogenic shock. Eur Heart J. 2014;35:156–167. doi: 10.1093/eurheartj/eht248. [DOI] [PubMed] [Google Scholar]
- 125.Thiele H, Ohman EM, de Waha-Thiele S, et al. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40:2671–2683. doi: 10.1093/eurheartj/ehz363. [DOI] [PubMed] [Google Scholar]
- 126.Lee JM, Rhee TM, Hahn JY, et al. Multivessel percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2018;71:844–856. doi: 10.1016/j.jacc.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 127.Vallabhajosyula S, Dunlay SM, Prasad A, et al. Acute noncardiac organ failure in acute myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2019;73:1781–1791. doi: 10.1016/j.jacc.2019.01.053. [DOI] [PubMed] [Google Scholar]
- 128.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 129.Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA. 2005;294:1664–1670. doi: 10.1001/jama.294.13.1664. [DOI] [PubMed] [Google Scholar]
- 130.Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stegman BM, Newby LK, Hochman JS, Ohman EM. Post-myocardial infarction cardiogenic shock is a systemic illness in need of systemic treatment: is therapeutic hypothermia one possibility. J Am Coll Cardiol. 2012;59:644–647. doi: 10.1016/j.jacc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 132.Fuernau G, Beck J, Desch S, et al. Mild hypothermia in cardiogenic shock complicating myocardial infarction. Circulation. 2019;139:448–457. doi: 10.1161/CIRCULATIONAHA.117.032722. [DOI] [PubMed] [Google Scholar]
- 133.Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol. 2011;57:1890–1898. doi: 10.1016/j.jacc.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 134.Sheu JJ, Tsai TH, Lee FY, et al. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med. 2010;38:1810–1817. doi: 10.1097/CCM.0b013e3181e8acf7. [DOI] [PubMed] [Google Scholar]
- 135.Esposito ML, Zhang Y, Qiao X, et al. Left ventricular unloading before reperfusion promotes functional recovery after acute myocardial infarction. J Am Coll Cardiol. 2018;72:501–514. doi: 10.1016/j.jacc.2018.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hajjar LA, Teboul JL. Mechanical circulatory support devices for cardiogenic shock: state of the art. Crit Care. 2019;23:76. doi: 10.1186/s13054-019-2368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thiele H, Jobs A, Ouweneel DM, et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J. 2017;38:3523–3531. doi: 10.1093/eurheartj/ehx363. [DOI] [PubMed] [Google Scholar]
- 138.Esposito M, Bader Y, Pedicini R, et al. The role of acute circulatory support in ST-segment elevation myocardial infarction complicated by cardiogenic shock. Indian Heart J. 2017;69:668–674. doi: 10.1016/j.ihj.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tehrani BN, Truesdell AG, Sherwood MW, et al. Standardized team-based care for cardiogenic shock. J Am Coll Cardiol. 2019;73:1659–1669. doi: 10.1016/j.jacc.2018.12.084. [DOI] [PubMed] [Google Scholar]
- 140.Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97:610–616. doi: 10.1016/j.athoracsur.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 141.Potapov EV, Antonides C, Crespo-Leiro MG, et al. 2019 EACTS expert consensus on long-term mechanical circulatory support. Eur J Cardiothorac Surg. 2019;56:230–270. doi: 10.1093/ejcts/ezz098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen KC, Yin WH, Wu CC, et al. In-hospital implementation of evidence-based medications is associated with improved survival in diabetic patients with acute coronary syndrome - data from TSOC ACS-DM Registry. Acta Cardiol Sin. 2018;34:211–223. doi: 10.6515/ACS.201805_34(3).20180207B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34:2940–2948. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 144.Castellano JM, Sanz G, Peñalvo JL, et al. A polypill strategy to improve adherence: results from the FOCUS project. J Am Coll Cardiol. 2014;64:2071–2082. doi: 10.1016/j.jacc.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 145.Lee CK, Lai CL, Lee MH, et al. Reinforcement of patient education improved physicians’ adherence to guideline-recommended medical therapy after acute coronary syndrome. PLoS One. 2019;14:e0217444-e44. doi: 10.1371/journal.pone.0217444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chiang CE, Wang TD, Ueng KC, et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J Chin Med Assoc. 2015;78:1–47. doi: 10.1016/j.jcma.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 147.Group SR, Wright JT, Jr., Williamson JD, et al. A randomized trial of intensive versus standard blood pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lip S, Tan LE, Jeemon P, et al. Diastolic blood pressure J-curve phenomenon in a tertiary-care hypertension clinic. Hypertension. 2019;74:767–775. doi: 10.1161/HYPERTENSIONAHA.119.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Flint AC, Conell C, Ren X, et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243–251. doi: 10.1056/NEJMoa1803180. [DOI] [PubMed] [Google Scholar]
- 150.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 151.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 152.Park JJ, Kim SH, Kang SH, et al. Differential effect of β-blockers according to heart rate in acute myocardial infarction without heart failure or left ventricular systolic dysfunction: a cohort study. Mayo Clin Proc. 2019;94:2476–2487. doi: 10.1016/j.mayocp.2019.05.033. [DOI] [PubMed] [Google Scholar]
- 153.Wu VC, Chen SW, Ting PC, et al. Selection of β-blocker in patients with cirrhosis and acute myocardial infarction: a 13-year nationwide population-based study in Asia. J Am Heart Assoc. 2018;7:e008982. doi: 10.1161/JAHA.118.008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Su TH, Chang SH, Kuo CF, et al. β-blockers after acute myocardial infarction in patients with chronic obstructive pulmonary disease: a nationwide population-based observational study. PLoS One. 2019;14:e0213187. doi: 10.1371/journal.pone.0213187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang CC, Wu CK, Tsai ML, et al. 2019 focused update of the guidelines of the Taiwan Society of Cardiology for the diagnosis and treatment of heart failure. Acta Cardiol Sin. 2019;35:244–283. doi: 10.6515/ACS.201905_35(3).20190422A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.American Diabetes Association. Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S66–S76. doi: 10.2337/dc20-S006. [DOI] [PubMed] [Google Scholar]
- 157.Chiang CE, Lin SY, Lin TH, et al. 2018 consensus of the Taiwan Society of Cardiology and the Diabetes Association of Republic of China (Taiwan) on the pharmacological management of patients with type 2 diabetes and cardiovascular diseases. J Chin Med Assoc. 2018;81:189–222. doi: 10.1016/j.jcma.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 158.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 159.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 160.Li YH, Ueng KC, Jeng JS, et al. 2017 Taiwan lipid guidelines for high risk patients. J Formos Med Assoc. 2017;116:217–248. doi: 10.1016/j.jfma.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 161.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 162.Chien SC, Chen PS, Huang YH, et al. 2019 Taiwan Society of Lipids and Atherosclerosis expert consensus statement on statin intolerance. J Formos Med Assoc. 2019;118:1385–1392. doi: 10.1016/j.jfma.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 163.Serban MC, Colantonio LD, Manthripragada AD, et al. Statin intolerance and risk of coronary heart events and all-cause mortality following myocardial infarction. J Am Coll Cardiol. 2017;69:1386–1395. doi: 10.1016/j.jacc.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 164.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. New Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 165.Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. New Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 166.Virani SS, Akeroyd JM, Nambi V, et al. Estimation of eligibility for proprotein convertase subtilisin/kexin type 9 inhibitors and associated costs based on the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk): insights from the department of veterans affairs. Circulation. 2017;135:2572–2574. doi: 10.1161/CIRCULATIONAHA.117.028503. [DOI] [PubMed] [Google Scholar]
- 167.Cannon CP, Khan I, Klimchak AC, et al. Simulation of lipid-lowering therapy intensification in a population with atherosclerotic cardiovascular disease. JAMA Cardiology. 2017;2:959–966. doi: 10.1001/jamacardio.2017.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fox KA, Goodman SG, Klein W, et al. Management of acute coronary syndromes. Variations in practice and outcome; findings from the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2002;23:1177–1189. doi: 10.1053/euhj.2001.3081. [DOI] [PubMed] [Google Scholar]
- 169.Lenfant C. Shattuck lecture--clinical research to clinical practice--lost in translation? N Engl J Med. 2003;349:868–874. doi: 10.1056/NEJMsa035507. [DOI] [PubMed] [Google Scholar]
- 170.Schiele F, Gale CP, Bonnefoy E, et al. Quality indicators for acute myocardial infarction: a position paper of the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care. 2017;6:34–59. doi: 10.1177/2048872616643053. [DOI] [PubMed] [Google Scholar]
- 171.Jneid H, Addison D, Bhatt DL, et al. 2017 AHA/ACC clinical performance and quality measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association task force on performance measures. J Am Coll Cardiol. 2017;70:2048–2090. doi: 10.1016/j.jacc.2017.06.032. [DOI] [PubMed] [Google Scholar]