Summary
The SARS-CoV-2 pandemic has unprecedented implications for public health, social life, and the world economy. Because approved drugs and vaccines are limited or not available, new options for COVID-19 treatment and prevention are in high demand. To identify SARS-CoV-2-neutralizing antibodies, we analyzed the antibody response of 12 COVID-19 patients from 8 to 69 days after diagnosis. By screening 4,313 SARS-CoV-2-reactive B cells, we isolated 255 antibodies from different time points as early as 8 days after diagnosis. Of these, 28 potently neutralized authentic SARS-CoV-2 with IC100 as low as 0.04 μg/mL, showing a broad spectrum of variable (V) genes and low levels of somatic mutations. Interestingly, potential precursor sequences were identified in naive B cell repertoires from 48 healthy individuals who were sampled before the COVID-19 pandemic. Our results demonstrate that SARS-CoV-2-neutralizing antibodies are readily generated from a diverse pool of precursors, fostering hope for rapid induction of a protective immune response upon vaccination.
Keywords: SARS-CoV-2, 2019-nCoV, COVID-19, neutralizing antibody, monoclonal antibody, single B cell analysis
Graphical Abstract
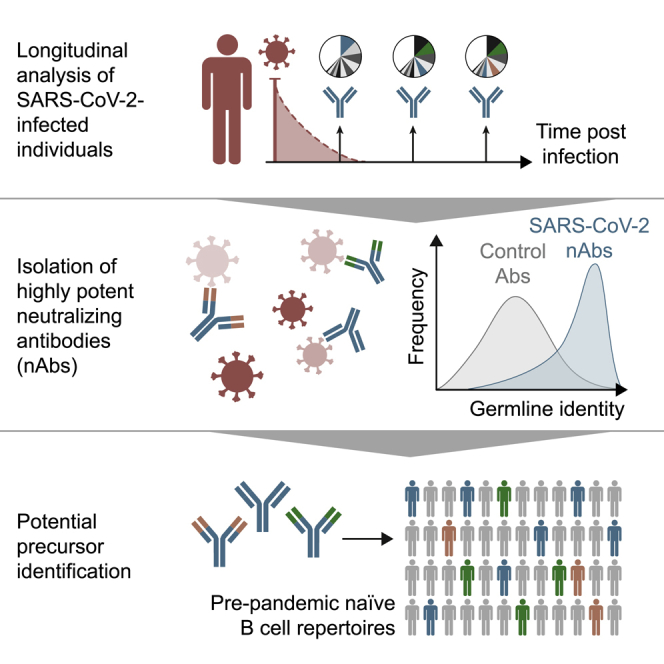
Highlights
-
•
Isolation of highly potent SARS-CoV-2-neutralizing antibodies
-
•
Longitudinal sampling reveals early class-switched neutralizing response
-
•
SARS-CoV-2 S-protein-reactive antibodies show little somatic mutation over time
-
•
Potential antibody precursor sequences identified in SARS-CoV-2-naive individuals
In a longitudinal analysis of SARS-CoV-2-infected people, Kreer et al. find highly potent neutralizing antibodies that use a broad spectrum of variable (V) genes and show low levels of somatic mutations. They also identify potential precursor sequences of these SARS-CoV-2-neutralizing antibodies from virus-naive individuals, sampled before the COVID-19 pandemic. This could indicate that neutralizing antibodies can be readily generated from existing germline antibody sequences found in the general population.
Introduction
By June 2020, over 8.4 million severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and over 450,000 casualties of the associated coronavirus disease 2019 (COVID-19) were reported (Dong et al., 2020; Huang et al., 2020; Zhou et al., 2020; Zhu et al., 2020). The exponential spread of the virus has caused countries to shut down public life with unprecedented social and economic consequences. Therefore, decoding SARS-CoV-2 immunity to promote development of vaccines as well as potent antiviral drugs is an urgent health need (Sanders et al., 2020).
Monoclonal antibodies (mAbs) targeting viral surface proteins have been demonstrated to effectively neutralize viruses such as Ebola virus (EBOV) (Ehrhardt et al., 2019; Flyak et al., 2016; Saphire et al., 2018), respiratory syncytial virus (RSV) (Kwakkenbos et al., 2010), influenza virus (Corti et al., 2011; Joyce et al., 2016; Kallewaard et al., 2016), or human immunodeficiency virus 1 (HIV-1) (Walker et al., 2009; Huang et al., 2016a, 2016b; Scheid et al., 2011; Schommers et al., 2020; Wu et al., 2010). The most prominent target for an antibody-mediated response on the surface of SARS-CoV-2 virions is the homotrimeric spike (S) protein. The S protein promotes cell entry through interaction of its receptor-binding domain (RBD) with angiotensin-converting enzyme 2 (ACE2) (Hoffmann et al., 2020; Walls et al., 2020). Antibodies that target the S protein are therefore of particular interest to combat the current pandemic (Burton and Walker, 2020; Sempowski et al., 2020).
SARS-CoV-2 infection induces a humoral immune response of varying magnitude (Duan et al., 2020; Ni et al., 2020), and antibody levels depend on several factors, including disease severity (Long et al., 2020a; Wang et al., 2020b). SARS-CoV-2-reactive as well as SARS-CoV-2-neutralizing antibodies have now been isolated from COVID-19 survivors (Brouwer et al., 2020; Cao et al., 2020; Hansen et al., 2020; Ju et al., 2020; Liu et al., 2020a; Robbiani et al., 2020; Seydoux et al., 2020; Shi et al., 2020; Wu et al., 2020; Zost et al., 2020), immunized animals (Hansen et al., 2020; Wang et al., 2020a; Wrapp et al., 2020a), and phage display libraries (Li et al., 2020; Liu et al., 2020b; Yuan, 2020; Zeng et al., 2020). Such antibodies are of great value to elucidate neutralization mechanisms, inform vaccination strategies, and potentially treat and prevent SARS-CoV-2 infection, as demonstrated in animal models (Cao et al., 2020; Rogers et al., 2020; Zost et al., 2020). The antibodies described target various sites on the S protein, including the RBD (Brouwer et al., 2020; Liu et al., 2020a; Seydoux et al., 2020). However, the affinities and neutralization activities of the reported antibodies vary strongly, and the potential for SARS-CoV-2 escape mutations highlights the need to carefully develop antibody-mediated strategies (Baum et al., 2020). Moreover, little is known about the likelihood of generating such neutralizing antibodies and how they evolve over time, which will be critical for the development of a broadly active SARS-CoV-2 vaccine.
Here, we isolated and sequenced 4,313 S-protein-reactive memory B cells from 12 SARS-CoV-2-infected individuals as early as 8 days after diagnosis (16 days after onset of symptoms). Five patients were followed over a period of 8–69 days after diagnosis to investigate the dynamics of antibody development against SARS-CoV-2. Besides presenting 28 neutralizing antibodies that are currently being evaluated for clinical application, we provide evidence that antibodies develop early after SARS-CoV-2 infection with limited ongoing somatic hypermutation. Finally, we identified potential precursor sequences of potent SARS-CoV-2-neutralizing antibodies in naive B cell repertoires from healthy individuals who were sampled before the SARS-CoV-2 pandemic.
Results
SARS-CoV-2-infected individuals develop a polyclonal memory B cell response against the S protein
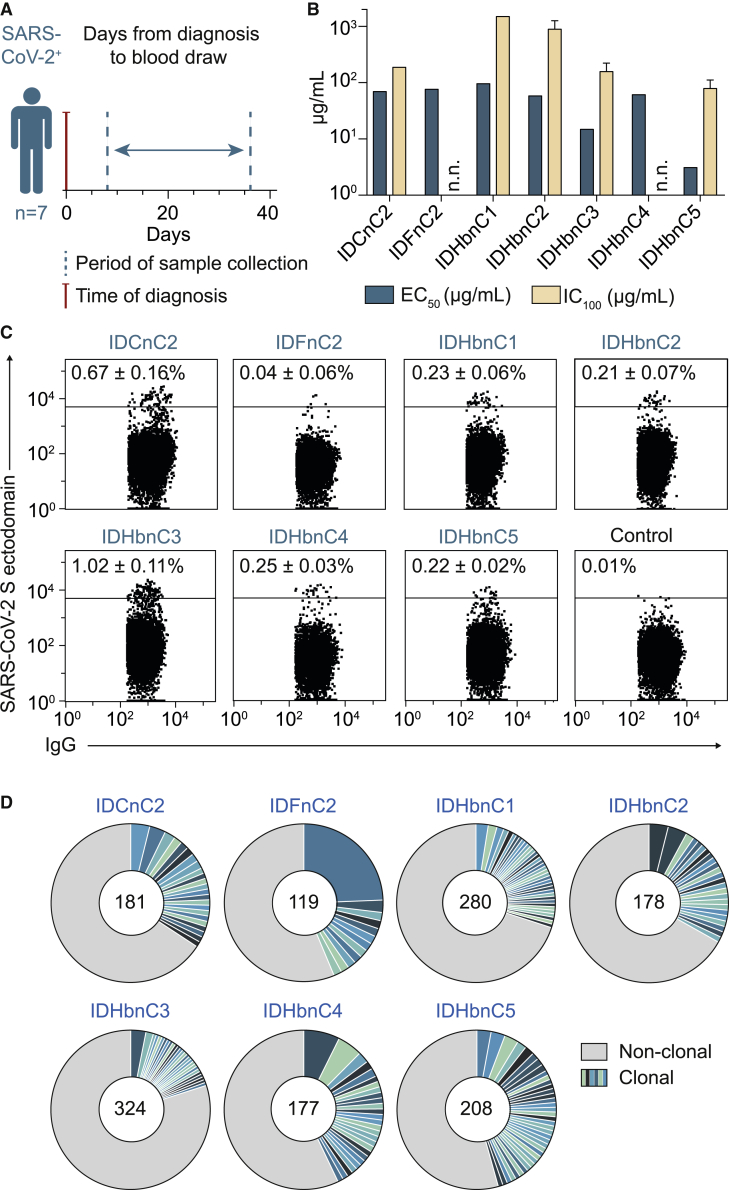
To investigate the antibody response against SARS-CoV-2, we collected blood samples from seven COVID-19 patients (38–59 years of age) between 8 and 36 days after diagnosis (Figure 1A; Table S1). Five patients presented with mild symptoms, including dry cough, fever, and dyspnea, whereas two patients were asymptomatic (Table S1). Purified plasma immunoglobulin G (IgG) of all seven individuals showed binding to the full trimeric S ectodomain (Wrapp et al., 2020b) by ELISA, and half-maximal effective concentrations (EC50) ranged from 3.1–96.1 μg/mL (Figure 1B; Table S2). Moreover, neutralizing IgG activity was determined against authentic SARS-CoV-2, showing 100% inhibitory concentrations (IC100) between 78.8 and 1,500 μg/mL in five of seven patients (Figure 1B; Table S2). To decipher SARS-CoV-2-specific B cells and antibody response on a molecular level, we performed single-B-cell sorting and sequence analysis of all individuals. Using flow cytometry, we detected that between 0.04% (±0.06) and 1.02% (±0.11) IgG+ B cells reacted with the S ectodomain (Figures 1C and S1). From these, we isolated a total of 1,751 single B cells and amplified IgG heavy and light chains using optimized PCR protocols (Figure 1C; Table S3) (Kreer et al., 2020a; Schommers et al., 2020). Sequence analysis revealed a polyclonal antibody response with 22%–45% clonally related sequences per individual and 2–29 members per identified B cell clone (Figure 1D; Table S3). We conclude that a polyclonal B cell response against the SARS-CoV-2 S protein was initiated in all studied COVID-19 patients.
Figure 1.
SARS-CoV-2 infection induces a polyclonal B cell and antibody response
(A) Scheme of cross-sectional sample collection (see also Table S1).
(B) Binding to the trimeric SARS-CoV-2 S ectodomain (ELISA, EC50) and authentic SARS-CoV-2 neutralization activity (complete inhibition of VeroE6 cell infection, IC100) of cross-sectional plasma-purified IgG samples. Bar plots show arithmetic or geometric means ± SD of duplicates or quadruplicates for EC50 and IC100, respectively. Abbreviation is as follows: n.n., no neutralization as defined by IC100 > 1,500 g/mL IgG.
(C) Dot plots of IgG+ B cell analysis. Depicted numbers (percent ± SD) indicate average frequencies of S-reactive B cells (see also Tables S2, S3, and Figure S1).
(D) Clonal relationship of S ectodomain-reactive B cells. Individual clones are colored in shades of blue and green. Numbers of productive heavy-chain sequences are depicted in the center of the pie charts. Clone sizes are proportional to the total number of productive heavy chains per clone.
Figure S1.
Gating strategy for single-cell sorting, related to Figures 1 and 2
CD19+ B cells isolated by MACS were used and cell aggregates were excluded by FSC. Living CD20+ IgG+ cells were gated and cells with a positive SARS-CoV-2 S ectodomain staining were selected for single cell sort.
Longitudinal analysis of the SARS-CoV-2 antibody response
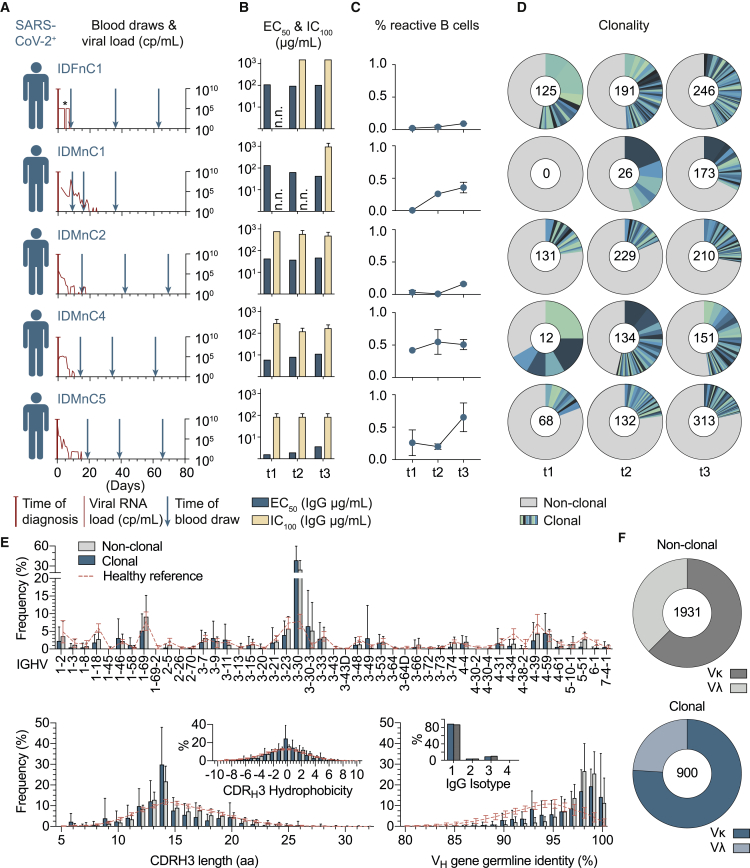
To delineate the dynamics of the SARS-CoV-2 antibody response, we obtained longitudinal blood samples from an additional five infected individuals at three time points spanning 8–69 days after diagnosis (Figure 2A; Table S1). Across the different individuals, EC50 (S ectodomain binding) and IC100 (SARS-CoV-2 neutralization) values of plasma IgG ranged from 1.54–129 μg/mL and 78.8–1,500 μg/mL, respectively (Figure 2B; Table S2). For each individual, however, this response remained almost unchanged over the studied period (Figures 2A and 2B).
Figure 2.
SARS-CoV-2-specific IgG+ B cells readily develop after infection with recurring B cell clones and a preference for the VH gene segment 3-30
(A) Scheme of longitudinal sample collection. The viral RNA load from nasopharyngeal swabs is indicated in red (copies [cp] per milliliter, right y axis). ∗The viral load for IDFnC1 is given as positive or negative result (see also Table S1).
(B) Binding to the trimeric SARS-CoV-2 S ectodomain (ELISA, EC50) and authentic SARS-CoV-2 neutralization activity (complete inhibition of VeroE6 cell infection, IC100) of longitudinal purified plasma IgG samples. n.n., no neutralization as defined by IC100 > 1,500 µg/mL IgG. Bar plots show arithmetic or geometric means ± SD of duplicates or quadruplicates for EC50 and IC100, respectively.
(C) Percentage of SARS-CoV-2 S ectodomain-reactive IgG+ B cells over time (mean ± SD; see also Tables S2, S3, and Figure S1).
(D) Clonal relationship over time. Individual clones are colored in shades of blue and green. Numbers of productive heavy-chain sequences per time point are given in the center of pie charts.
(E) Frequencies of VH gene segments (top), CDRH3 length and CDRH3 hydrophobicity (bottom left), as well as VH gene germline identity and IgG isotype of clonal and non-clonal sequences (bottom right) from all 12 subjects and time points. NGS reference data from 48 healthy individuals (collected before the outbreak of SARS-CoV-2) are depicted in red (see also Tables S1 and S2). Bar and line plots show mean ± SD.
(F) Ratio of κ and λ light chains in non-clonal (top, gray) and clonal (bottom, blue) sequences (see also Figure S2).
To investigate B cell clonality and antibody characteristics on a single-cell level, we proceeded to sort S ectodomain-reactive IgG+ B cells from all five subjects at the different time points (t1, t2, and t3). We found up to 0.65% SARS-CoV-2 S-reactive B cells, with higher frequencies at later time points and with the number of days after diagnosis being predictive of the fraction of SARS-CoV-2 S-reactive B cells (p = 0.0016) (Figure 2C). From a total of 2,562 B cells, we detected 254 B cell clones (Table S3). Fifty-one percent of these clones (129) were detected recurrently, suggesting persistence of SARS-CoV-2 S-reactive B cells over the investigation period of 2.5 months. When separated by individual time points, the fraction of clonally related sequences ranged from 18%–67% across patients and remained constant or showed only moderate decreases over time (Figure 2D).
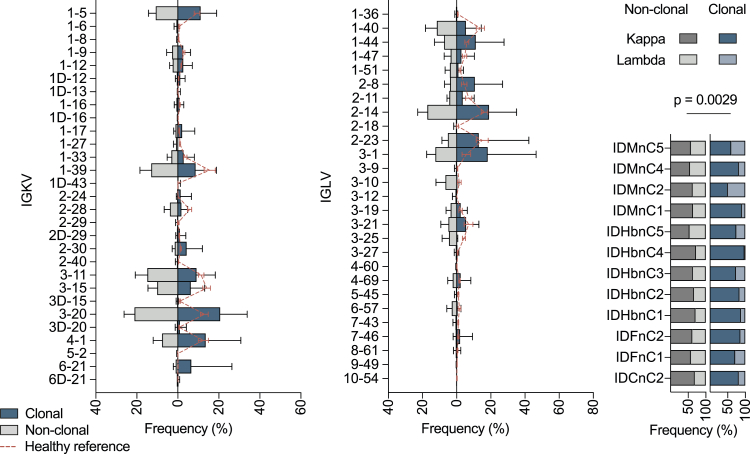
Next, we analyzed the single-cell Ig sequences (6,587 productive heavy and light chains) from all 12 patients (Figures 2E, 2F, and S2). Here, clonally related and non-clonal sequences similarly presented a broad spectrum of heavy-chain variable (VH) gene segments, normally distributed heavy-chain complementarity-determining region 3 (CDRH3) lengths, symmetrical CDRH3 hydrophobicity distributions, and a predominance of the IgG1 isotype (Figure 2E). However, in comparison with repertoire data from healthy individuals, IgVH 3-30 was overrepresented, and clonal sequences more often facilitated κ over λ light chains (76% in clonal versus 62.5% in non-clonal sequences, p = 0.0029) (Figures 2F and S2). Finally, VH genes of S-reactive B cells were, on average, less mutated than VH genes from healthy IgG+ repertoires (median identity of 98.3 versus 94.3, p < 0.0001) (Figures 2E and S2). We concluded that a SARS-CoV-2 S-reactive IgG+ B cell response readily develops after infection with the same B cell clones detectable over time and a preference for facilitating VH gene segment 3-30.
Figure S2.
Light-chain characteristics of sorted single cells, related to Figure 2
Left and middle graphics: frequencies of VL gene segments of clonal and non-clonal sequences are shown (κ left, λ middle). Shown on the right are ratios of κ and λ within the single sample sets in clonal and non-clonal sequences. A two-tailed Wilcoxon matched-pairs signed rank test was performed on κ / λ ratios to test for significance.
Isolation of highly potent near-germline SARS-CoV-2-neutralizing antibodies from COVID-19 patients
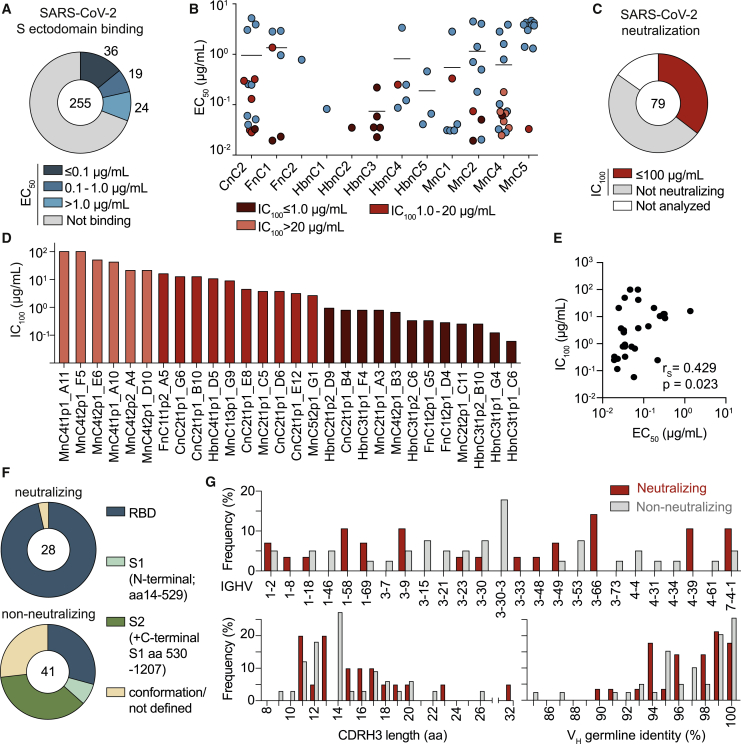
To determine antibody characteristics and isolate potent neutralizing antibodies, we cloned a total of 312 matched heavy- and light-chain pairs from all 12 patients. These antibodies were primarily selected on the basis of clonality with the aim to include at least one clonal member of several (but at least three) clones per individual (Table S3). However, the polyclonal response (median clone size of 2) (Table S3) and the lack of differences between clonal and non-clonal sequences suggested the presence of weakly expanded clones in the non-clonal fraction. We therefore also included about one-third randomly selected non-clonal sequences (83 antibodies) for production. From 255 successfully produced IgG1 antibodies, 79 (31%) bound to the full trimeric S ectodomain (Wrapp et al., 2020b) by ELISA with EC50 values ranging between 0.02 μg/mL and 5.20 μg/mL (Figure 3A). Of these, 30 antibodies showed SARS-CoV-2 reactivity via a commercial diagnostic system (Euroimmun IgG detection kit) (Figures 3A and 3B; Table S4). Surface plasmon resonance (SPR) analyses using the RBD as an analyte for 13 SARS-CoV-2-interacting antibodies gave dissociation constant (KD) values as low as 0.02 nM (Table S4). By determining the neutralization activity against authentic SARS-CoV-2, we found a total of 28 neutralizing antibodies among 9 of 12 individuals, and IC100 values ranged between 100 μg/mL (assay limit) and 0.04 μg/mL (Figures 3C and 3D). Of note, neutralizing activity was mainly detected among high-affinity antibodies (Figure 3B; Table S4), and a positive correlation between neutralization and binding could be detected (Spearman's correlation coefficient [rs] = 0.429, p = 0.023) (Figure 3E).
Figure 3.
Infected individuals can develop potent near-germline SARS-CoV-2-neutralizing antibodies that preferentially bind to the S-protein RBD
(A) Interaction of isolated antibodies with the SARS-CoV-2 S ectodomain by ELISA. Binding antibodies (blue) were defined by an EC50 of less than 30 μg/mL and an optical density 415–695 nm (OD415–695) of 0.25 or more (data not shown).
(B) EC50 values (mean of duplicates) of SARS-CoV-2 S ectodomain-interacting antibodies per individual. Neutralizing antibodies are labeled in shades of red (see also Figure S5 and Table S4).
(C) Authentic SARS-CoV-2 neutralization activity (complete inhibition of VeroE6 cell infection, IC100, in quadruplicates) of S-ectodomain-specific antibodies (red).
(D) Geometric mean potencies (IC100) of all neutralizing antibodies.
(E) Correlation between S ectodomain binding (EC50) and neutralization potency (IC100). The correlation coefficient rS and approximate p value were calculated by Spearman’s rank-order correlation (see also Figure S3).
(F) Epitope mapping of SARS-CoV-2 S ectodomain-specific antibodies against the RBD, truncated N-terminal the S1 subunit (aa 14–529), and a monomeric S ectodomain construct by ELISA. S2 binding was defined by interaction with monomeric S but not RBD or S1. Antibodies interacting with none of the subdomains were specified as conformational epitopes or not defined.
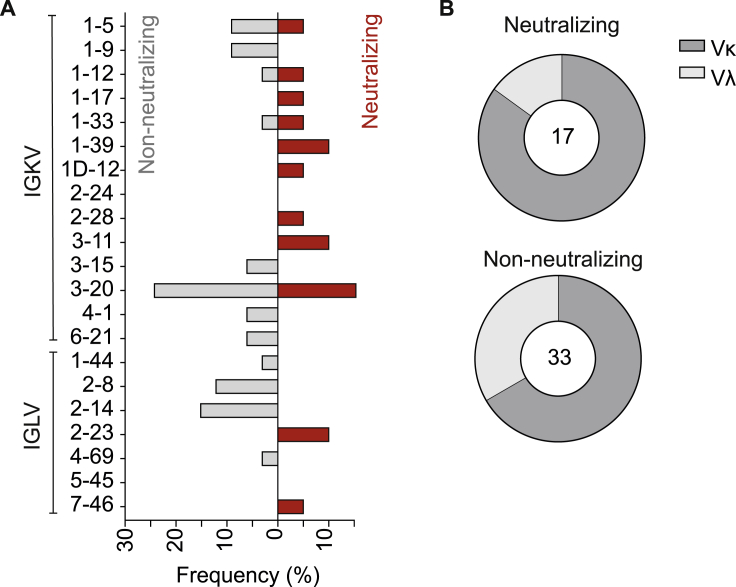
(G) Top: frequencies of VH gene segments for non-neutralizing and neutralizing antibodies. Clonal sequence groups were collapsed and treated as one sample for calculation of the frequencies. Shown on the bottom are the CDRH3 length (left) and VH gene germline identity (right) of non-neutralizing and neutralizing antibodies (see also Figure S4).
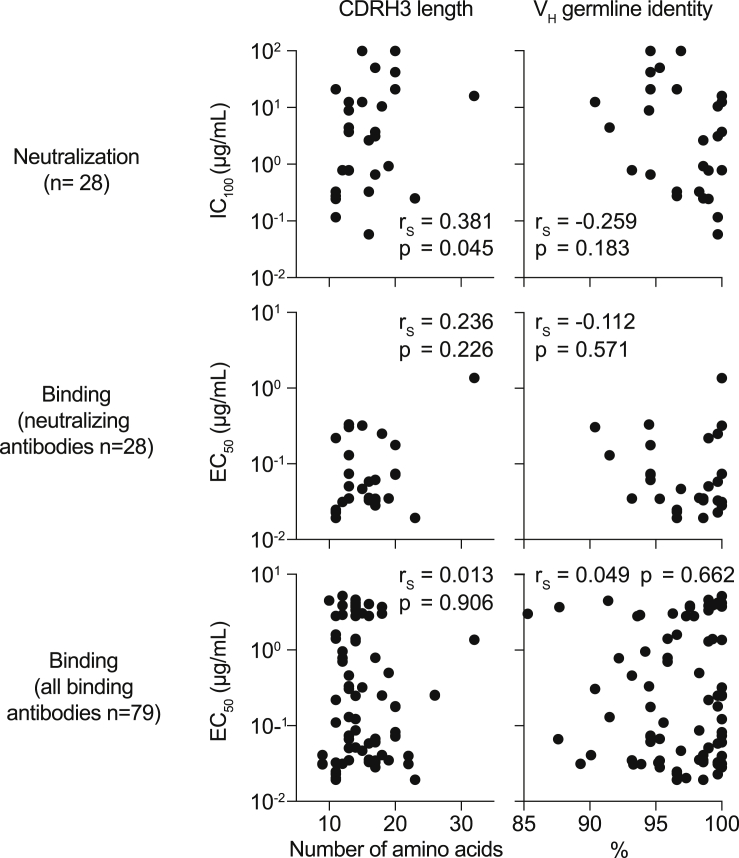
To better characterize the interaction between the SARS-CoV-2 S protein and reactive antibodies, we determined binding to a truncated N-terminal S1 subunit (including the RBD), the isolated RBD, and a monomeric S ectodomain. We found 27 of 28 neutralizing antibodies binding to the RBD but only 29% of the non-neutralizing antibodies, suggesting that the RBD is a major site of vulnerability on the S protein. Epitopes for non-neutralizing antibodies included the N-terminal S1 domain and conformational epitopes (Figure 3F; Table S4). Notably, neutralizing and non-neutralizing antibodies were characterized by a broad distribution of VH as well as light-chain variable (VL) gene segments and a preference for κ light chains (Figures 3G and S4). Moreover, 31 of 79 binding and 11 of 28 neutralizing antibodies demonstrated germline identities of 99%–100%, and no correlation was detected between neutralizing activity and the level of somatic mutation (Figure 3G; Table S4; Figure S3).
Figure S4.
VL gene distribution in non-neutralizing and neutralizing antibodies, related to Figure 3
(A) Frequencies of VL gene segments for non-neutralizing (left, gray) and neutralizing antibodies (right, red). Clonal sequence groups were collapsed and treated as one sample for calculation of the frequencies.
(B) Ratio of λ and κ light chains for neutralizing (left) and non-neutralizing S-ectodomain-specific antibodies (bottom, blue).
Figure S3.
Correlation of binding and neutralization with VH gene characteristics, related to Figure 3
Correlation plots of EC50 values of binding or neutralizing antibodies or IC100 values of neutralizing antibodies with CDRH3 lengths or VH germline identities. Spearman correlation coefficient rS and approximate p values are given.
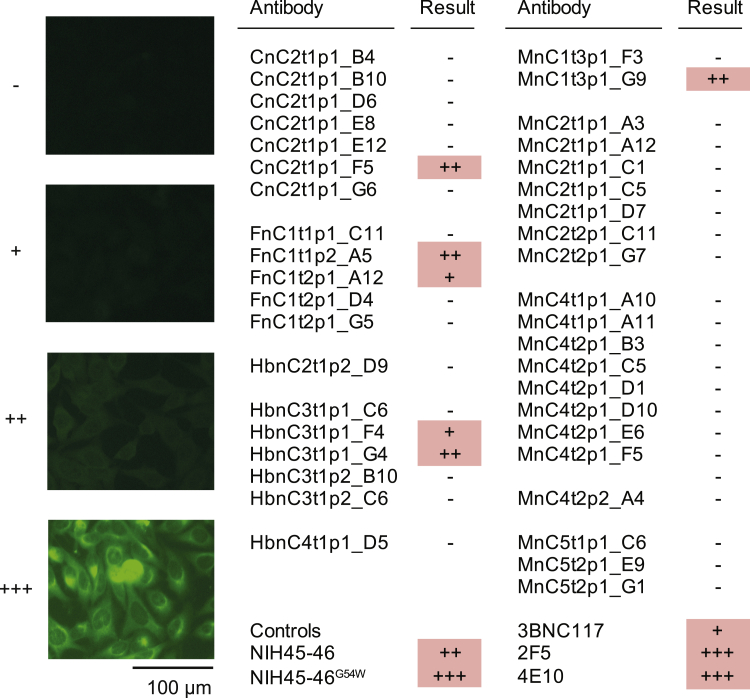
Finally, we performed a HEp-2 cell autoreactivity assay. Of 28 neutralizing antibodies, 4 showed low to moderate levels of autoreactivity (Figure S5; Table S4), and 2 of them also reacted with envelope proteins of other viruses (i.e., Ebola glycoprotein and HIV-1 gp140) (Table S4). In summary, these data show that SARS-CoV-2-neutralizing antibodies develop from a broad set of different V genes and are characterized by a low degree of somatic mutations. Moreover, we were able to isolate highly potent neutralizing antibodies that are promising candidates for antibody-mediated prevention and therapy of SARS-CoV-2 infection.
Figure S5.
Autoreactivity of selected SARS-CoV-2-binding and -neutralizing antibodies, related to Figure 3
HEp-2 cells were incubated with SARS-CoV-2 S-ectodomain antibodies at concentrations of 100 μg/mL and analyzed by indirect immunofluorescence. Representative pictures of the scoring system are shown.
Investigating ongoing somatic hypermutation in SARS-CoV-2 binding and neutralizing antibodies
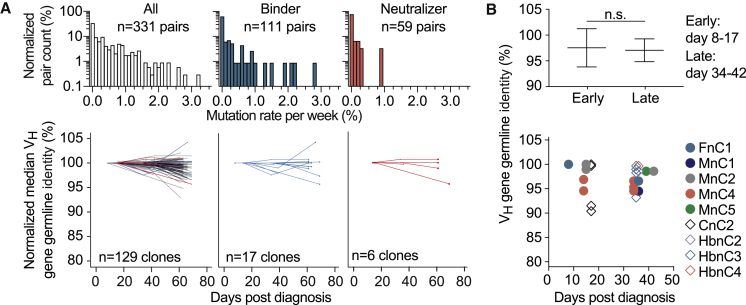
To investigate the development of somatic mutations over time, we longitudinally analyzed 129 recurring B cell clones that comprised 17 binding and 6 neutralizing antibodies. To this end, we phylogenetically matched all members of a B cell clone at a given time point with the most closely related member at the consecutive time point (331 pairings in total). Mean mutation frequencies in either direction (i.e., toward higher or lower V gene germline identities) were 0.51% ± 0.61%, 0.08% ± 0.51%, and 0.01% ± 0.19% per week for all, binding, and neutralizing clonal members, respectively (Figure 4A, top). When averaging the VH gene germline identity of concurrent clonal members, we found a moderate increase in somatic mutations over time (Figure 4A, bottom). Changes were similar for binding and neutralizing subsets, with one exception among the neutralizing antibodies that accumulated about 5% nucleotide mutations over the investigated period (Figure 4A, bottom). In line with this finding, neutralizing antibodies isolated on days 8–17 and days 34–42 after diagnosis showed VH gene germline identities of 97.5% and 97.0%, respectively (Figure 4B). We concluded that SARS-CoV-2-neutralizing antibodies carry similar levels of somatic hypermutation independent of the time of isolation.
Figure 4.
Dynamics of somatic mutations for SARS-CoV-2-specific antibodies
(A) Distribution of mutation rates per week for clonal members (top) and median change in VH germline identity normalized by the first measurement for each longitudinal clone (bottom).
(B) VH gene germline identity of neutralizing antibodies from different time points. Shown on top is the mean ± SD for groups of antibodies from early or late time points (two-tailed Mann-Whitney U test). Shown on the bottom are the VH germline identities of all isolated neutralizing antibodies depending on the time between diagnosis and blood sample collection (see also Table S4).
Potential precursor sequences of SARS-CoV-2-neutralizing antibodies can be identified among healthy individuals
The low rate of somatic mutations in the majority of binding and neutralizing antibodies emphasizes the requirement for the presence of distinct germline recombinations in the naive human B cell repertoire. To estimate the frequency of potential precursor B cells, we performed unbiased heavy- and light-chain next-generation sequencing (NGS) of the naive B cell receptor repertoires from 48 healthy donors (Table S5). All samples were collected before the SARS-CoV-2 outbreak and comprised a total of 1.7 million collapsed reads with 455,423 unique heavy, 170,781 κ, and 91,505 λ chain clonotypes (defined as identical V and joining (J) gene segment pairing and the same CDR3 amino acid sequence). Within this dataset, we searched for heavy and light chains that resemble the 79 SARS-CoV-2-binding antibodies (Figure 5A). For 14 of 79 tested antibodies, we found 61 heavy-chain clonotypes with identical V/J pairs and similar (±1 amino acid [aa] in length and up to 3 aa differences) CDRH3s in 28 healthy individuals (Figures 5B and 5C), including one exact CDRH3 match (MnC2t1p1_C12). For light chains, we identified 1,357 κ chain precursors with exact CDR3 matches that cover 41 of 62 antibodies and 109 λ chain precursors that represent 7 of 17 antibodies (Figures 5B and 5C). All 48 naive repertoires included at least one κ and one λ chain precursor. When combining heavy- and light-chain data, we found both precursor sequences of 9 antibodies in 14 healthy individuals (Figure 5C). Importantly, among these potential sequence precursors, we found close similarities to three potent neutralizing antibodies (CnC2t1p1_B4, HbnC3t1p1_G4, and HbnC3t1p2_B10). Although the NGS repertoire data did not include pairing information of heavy- and light-chain combinations, we found matched heavy- and light-chain sequences despite small sample sizes of, on average, 9,500 heavy and 2,000–3,500 light chain clonotypes per individual. We thus conclude that potential SARS-CoV-2-binding and -neutralizing antibody precursor sequences are likely to be abundant in naive B cell repertoires.
Figure 5.
Sequence precursor frequencies of SARS-CoV-2-specific antibodies in naive repertoires of healthy individuals
(A) Strategy for sequence precursor identification from healthy naive B cell receptor (BCR) repertoires. HC, heavy chain; KC, κ chain; LC, λ chain; VH and VL, heavy- and light-chain V gene; CDRH3 and CDRL3, heavy- and light-chain CDR3.
(B) Number of clonotypes in healthy naive B cell receptor repertoires (n = 48) with matched V/J genes from SARS-CoV-2-binding antibodies (n = 79), plotted against the CDR3 difference. Bars of included potential sequence precursors are highlighted in shades of blue. For heavy chains, CDR3s were allowed to differ 1 aa in length and contain up to 3 aa mutations. For light chains, only identical CDR3s were counted.
(C) Number of different antibody heavy and light chains for which precursors were identified and number of different individuals from which precursor sequences were isolated. Numbers in overlapping circles indicate that both heavy and light chains were detected.
See also Table S5.
Discussion
Neutralizing antibodies can effectively target pathogens, and their induction is a key objective of vaccination strategies (Fauci and Marston, 2015; Mascola and Montefiori, 2010; Walker and Burton, 2018; Zolla-Pazner et al., 2019). A detailed understanding of the human antibody response to SARS-CoV-2 is therefore critical for the development of effective immune-mediated approaches against the COVID-19 pandemic (Burton and Walker, 2020; Koff et al., 2013; Kreer et al., 2020b; Sempowski et al., 2020).
Using B cell microcultures, single-cell cloning, or high-throughput single-cell sequencing, SARS-CoV-2-neutralizing antibodies have been identified from a limited number of SARS-CoV-2-infected individuals and survivors of infection with the related betacoronavirus SARS-CoV-1. Most of these antibodies target the RBD of the S protein and interfere with viral binding to its host cell receptor angiotensin-converting enzyme 2 (ACE2) (Andreano et al., 2020; Brouwer et al., 2020; Cao et al., 2020; Hansen et al., 2020; Ju et al., 2020; Pinto et al., 2020; Robbiani et al., 2020; Seydoux et al., 2020; Shi et al., 2020; Wu et al., 2020; Zost et al., 2020). Demonstrating their potential for clinical application, SARS-CoV-2-neutralizing antibodies can dampen or prevent infection in animal models (Cao et al., 2020; Rogers et al., 2020; Zost et al., 2020), although antibody combinations might be needed to restrict the potential for viral escape and resistance (Baum et al., 2020). Through single-cell analysis of more than 4,000 SARS-CoV-2 S-protein-reactive B cells from 12 infected individuals, we identified highly potent human monoclonal SARS-CoV-2-neutralizing antibodies that preferentially target the RBD. These antibodies fully block authentic viral infection at concentrations as low as 0.04 μg/mL and therefore provide a potential option for prevention and treatment of SARS-CoV-2 infection.
Affinity maturation of antibodies through somatic hypermutation and clonal B cell selection is a hallmark of the adaptive immune response to pathogens. Chronic infections, such as HIV-1 infection, can result in extensive somatic mutation and amino acid substitutions in more than 30% of the V-gene-encoded region compared with their germline sequences (Klein et al., 2013; Scheid et al., 2011; Schommers et al., 2020; Wu et al., 2010). In contrast, SARS-CoV-2-neutralizing antibodies isolated from different individuals show a limited degree of somatic mutation (Brouwer et al., 2020; Ju et al., 2020; Robbiani et al., 2020; Rogers et al., 2020; Seydoux et al., 2020). However, previously described SARS-CoV-2 S-reactive antibodies were obtained from single time points, preventing conclusions regarding the molecular dynamics of the B cell response to SARS-CoV-2. By longitudinally analyzing the memory B cell response in five individuals for up to 2.5 months after SARS-CoV-2 transmission, we reveal that there is little additional somatic hypermutation or clonal B cell expansion over time. One explanation for this observation is the potentially limited antigenic B cell stimulation because of rapid viral clearance, to which the cellular response and additional immune factors might contribute (Grifoni et al., 2020; Ni et al., 2020). In contrast, somatic mutation over several months despite only a brief period of systemically circulating antigen can be observed after infection with EBOV (Davis et al., 2019) or the yellow fever vaccine strain 17D (Wec et al., 2020a). However, given the high binding affinity of near-germline IgG antibodies against SARS-CoV-2 in the range of nano- to picomolar KD values, these antibodies might limit antigen access to the germinal center, the site of affinity maturation (Zhang et al., 2013). Although recent data suggest that neutralizing titers correlate with severity of infection (Long et al., 2020b; Wang et al., 2020b), it remains elusive whether this effect is caused by ongoing somatic hypermutation that results in higher antibody potency or ongoing production of highly potent antibodies that were initially generated.
SARS-CoV-2 S-reactive antibodies could be isolated from three SARS-CoV-2-naive individuals, although these antibodies were only weakly binding (Wec et al., 2020b). Whether the observation of such antibodies is a consequence of prior exposure to other human coronaviruses, as suggested for SARS-CoV-2-reactive T cells from healthy donors (Braun et al., 2020; Grifoni et al., 2020), and whether such antibodies and cells can provide background immunity remains to be elucidated. Notably, our deep sequencing analysis of the naive B cell receptor repertoires of 48 individuals sampled before the pandemic identified potential heavy- and/or light-chain precursor sequences of potent SARS-CoV-2-neutralizing antibodies in every single individual. In addition to the limited mutation rate and broad use of antibody gene segments across potent SARS-CoV-2-neutralizing antibodies, these results suggest that protective antibodies can be widely and readily induced by vaccination.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Human IgG-APC (Clone G18-145) | BD Biosciences | Cat#550931; RRID: AB_398478 |
| Anti-Human CD20-Alexa Fluor 700 (Clone 2H7) | BD Biosciences | Cat#560631; RRID: AB_1727447 |
| Anti-Human IgD-Pe-Cy7 (Clone IA6-2) | BD Biosciences | Cat#561314; RRID: AB_10642457 |
| Anti-Human IgM-FITC (Clone G20-127) | BD Biosciences | Cat#555782; RRID: AB_396117 |
| Anti-Human CD27-PE (Clone M-T271) | BD Biosciences | Cat#560985; RRID: AB_10563213 |
| Peroxidase AffiniPure Goat Anti-Human IgG, Fcγ fragment specific | Jackson ImmunoResearch | Cat#109-035-098; RRID: AB_2337586 |
| AffiniPure Goat Anti-Human IgG, Fcγ fragment specific | Jackson ImmunoResearch | Cat#109-005-098; RRID: AB_2337541 |
| Anti-6X His tag antibody | Abcam | Cat#ab9108; RRID: AB_307016 |
| Bacterial and Virus Strains | ||
| E. coli DH5α | Thermo Fisher Scientific | Cat#18263012 |
| BavPat1/2020 | European Virus Archive global | Cat#026V-03883 |
| Biological Samples | ||
| PBMCs, Plasma, and IgGs of donor IDFnC1 | This paper | N/A |
| PBMCs, Plasma, and IgGs of donor IDFnC2 | This paper | N/A |
| PBMCs, Plasma, and IgGs of donor IDCnC2 | This paper | N/A |
| PBMCs, Plasma, and IgGs of donor IDMnC1 | This paper | N/A |
| PBMCs, Plasma, and IgGs of donor IDMnC2 | This paper | N/A |
| PBMCs, Plasma, and IgGs of donor IDMnC4 | This paper | N/A |
| PBMCs, Plasma, and IgGs of donor IDMnC5 | This paper | N/A |
| PBMCs, Plasma, and IgGs of donor IDHbnC1 | This paper | N/A |
| PBMCs, Plasma, and IgGs of donor IDHbnC2 | This paper | N/A |
| PBMCs, Plasma, and IgGs of donor IDHbnC3 | This paper | N/A |
| PBMCs, Plasma, and IgGs of donor IDHbnC4 | This paper | N/A |
| PBMCs, Plasma, and IgGs of donor IDHbnC5 | This paper | N/A |
| PBMCs of 48 healthy blood donors | This paper | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| DMSO | Sigma-Aldrich | Cat#D2650; CAS: 67-68-5 |
| DAPI | Thermo Fisher | Cat#D1306; CAS: 581-88-4 |
| DTT | Promega | Cat#P1171 |
| RNasin | Promega | Cat#N2515 |
| RNaseOUT | Thermo Fisher | Cat#10777019 |
| SuperScript IV Reverse Transcriptase | Thermo Fisher | Cat#18090050 |
| Platinum Taq DNA Polymerase | Thermo Fisher | Cat#10966034 |
| Platinum Taq Green Hot Start | Thermo Fisher | Cat#11966034 |
| Q5 Hot Start High Fidelity DNA Polymerase | NEB | Cat#M0493L |
| T4 DNA Polymerase | New England Biolabs | Cat#M0203L |
| NP-40 | Thermo Fisher | Cat#85124 |
| dNTP Mix | Thermo Fisher | Cat#R1122 |
| DTT | Sigma Aldrich | Cat#GE17-1318-01 |
| SMARTScribe Reverse Transcriptase | Takara Bio | Cat#639537 |
| Branched Polyethylenimine, 25 kDa | Sigma-Aldrich | Cat#408727; CAS: 9002-98-6 |
| FreeStyle Expression Medium | Thermo Fisher | Cat#12338001 |
| Protein G Sepharose 4 Fast Flow | GE Life Sciences | Cat#17061805 |
| HiTrap MabSelect Protein A column | GE Life Sciences | Cat#8408255 |
| ABTS solution | Thermo Fisher | Cat#002024 |
| Dulbecco’s Modified Eagle Medium (DMEM) | GIBCO | Cat#11960-044 |
| Fetal bovine serum (FBS) | Sigma-Aldrich | Cat#F9665 |
| Sodium Pyruvate | Thermo Fisher | Cat#11360-070 |
| L-Glutamine | Thermo Fisher | Cat#25030024 |
| HEPES | Thermo Fisher | Cat#15630-080 |
| GlutaMAX | Thermo Fisher | Cat#35050-061 |
| MEM NEAA | Thermo Fisher | Cat#11140-050 |
| FBS, Qualified | GIBCO | Cat#10270-106 |
| 0.05% Trypsin-EDTA (1x) | GIBCO | Cat#25300-096 |
| Pen Strep | GIBCO | Cat#15070-063 |
| L-Glutamine 200 mM (100x) | GIBCO | Cat#250030-123 |
| Ni-NTA Agarose | Macherey-Nagel | Cat#745400.25 |
| Strep-Tactin®XT Superflow® 50% suspension | IBA lifesciences | Cat#2-4010-010 |
| 10x Buffer BXT; Strep-Tactin®XT Elution Buffer | IBA | Cat#2-1042-025 |
| 10x Buffer W; Strep-Tactin®/Strep-Tactin®XT Wash Buffer | IBA | Cat#2-1003-100 |
| Critical Commercial Assays | ||
| NOVA Lite Hep-2 ANA Kit | Inova Diagnostics / Werfen | Cat#066708100 |
| CD19-Microbeads | Miltenyi Biotec | Cat#130-050-301 |
| Microscale Antibody Kit (DyLight 488) | Thermo Fisher Scientific | Cat#53025 |
| Molecular Probes Alexa Fluor 488 NHS Ester (Succinimidyl Ester) | Thermo Fisher Scientific | Cat#10266262 |
| SARS-CoV-2 detection ELISA | Euroimmun | Cat#EI 2606-9601 G |
| Deposited Data | ||
| Cloned and tested SARS-CoV-2-neutralizing antibodies | This paper | GenBank: MT658806 - MT658861 |
| Experimental Models: Cell Lines | ||
| 293-6E cells | NRC | NRC file 11565 |
| HEK293T cells | ATCC | Cat#CRL-11268 |
| Vero C1008 | ATCC | Cat#CRL-1586, RRID: CVCL_0574 |
| Oligonucleotides | ||
| Single cell PCR Primer | Kreer et al., 2020a | N/A |
| Random Hexamer Primer | Thermo Fisher | Cat#SO142 |
| SLIC heavy chain reverse primer (GGGTGCCAGGGGGAAGACC GATGGGCCCTTGGTCGAGGC) |
This paper | N/A |
| SLIC kappa chain reverse primer (CTCATCAGATGGCGGGAAGA TGAAGACAGATGGTGCAGCCACCGTACG) |
This paper | N/A |
| SLIC lambda chain reverse primer (GAAGCTCCTCACTCGAGGG YGGGAACAGAGTG) |
This paper | N/A |
| Recombinant DNA | ||
| Human antibody expression vectors (IgG1, Igλ, Igκ) | Tiller et al., 2008 | N/A |
| Plasmid encoding SARS-CoV-2 S ectodomain (amino acids 1−1208 of SARS-CoV-2 S; GenBank: MN908947) | Wrapp et al., 2020b | N/A |
| pCAGGS Plasmid encoding RBD of the SARS-CoV-2 spike protein (GenBank: MN908947; aa:319-541) | Stadlbauer et al., 2020 | N/A |
| pCAGGS encoding SARS-CoV-2 S ectodomain “monomer” without trimerization domain (GenBank: MN908947; aa:1-1207) | This paper | N/A |
| pCAGGS encoding SARS-CoV-2 S1 subunit (GenBank: MN908947; aa:14-529) | This paper | N/A |
| pCAGGS-EBOV GPΔTM-GCN4-HIS-Avi, encoding the EBOV Makona glycoprotein (GP) ectodomain (GenBank: KJ660347; aa:1-651) | Ehrhardt et al., 2019 | N/A |
| pCAGGS-YU-2 gp140-GCN4-HIS-Avi, encoding the HIV-1YU2 gp140 ectodomain (GenBank: M93258; aa:1- 683) | Ehrhardt et al., 2019 | N/A |
| Software and Algorithms | ||
| Geneious R10 and Geneious Prime | Geneious | RRID: SCR_010519 |
| Prism | GraphPad | RRID: SCR_002798 |
| Python 3.6.8 | Python Software Foundation; https://www.python.org/ | RRID: SCR_008394 |
| SciPy | SciPy developers | RRID: SCR_008058 |
| IgBLAST 1.13.0 | Ye et al., 2013 | RRID: SCR_002873 |
| Clustal Omega 1.2.3 | Sievers et al., 2011 | RRID: SCR_001591 |
| pRESTO 0.5.11 | Vander Heiden et al., 2014 | RRID: SCR_001782 |
| FlowJo 10.5.3 | FlowJo, LLC | https://www.flowjo.com |
| Prism 7 | GraphPad | https://www.graphpad.com/ |
| MacVector 16.0.9 | MacVector | https://www.macvector.com |
| Adobe Illustrator CC 2018 | Adobe | https://www.adobe.com |
| Other | ||
| Amicon MWCO 30 kDa/10 kDa | Merck Millipore | Cat#Z677108 |
| Leica DMI-microscope | Leica Biosystem | N/A |
| BD FACSAria Fusion Cell Sorter | BD Bioscience | N/A |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Florian Klein (florian.klein@uk-koeln.de).
Materials Availability
Reasonable amounts of antibodies will be made available by the Lead Contact upon request under a Material Transfer Agreement (MTA) for non-commercial usage.
Data and Code Availability
Nucleotide sequences of all SARS-CoV-2-neutralizing antibodies were deposited at GenBank (accession numbers MT658806 - MT658861). Further antibody sequences and NGS data of healthy individuals will be shared by the Lead Contact upon request.
Experimental Models and Subject Details
SARS-CoV-2-infected individuals and sample collection
Samples were obtained under a study protocol approved by the Institutional Review Board of the University of Cologne and respective local IRBs (study protocol 16-054). All 12 participants (six females, six males; Table S1) provided written informed consent and were recruited at hospitals or as outpatients. Sites of recruitment were Munich Clinic Schwabing for IDMnC1, IDMnC2, IDMnC4, and IDMnC5; the University Hospital of Frankfurt for patients IDFnC1 and IDFnC2; and the Institute of Virology at the University Hospital Cologne for patient IDCnC2. Patients IDHbnC1-5 were recruited as outpatients in the county Heinsberg. Sample size was estimated based on previous studies (Corti et al., 2011; Ehrhardt et al., 2019; Schommers et al., 2020) to sufficiently inform on B cell receptor repertoires and yield neutralizing antibodies. Participants were enrolled and allocated to single blood draws or longitudinal follow-up based on the epidemiology of the infection and participants’ availability.
Method Details
Isolation of peripheral blood mononuclear cells (PBMCs), plasma and total IgG from whole blood
Blood draw collection was performed using EDTA tubes and/or syringes pre-filled with heparin. PBMC isolation was performed using Leucosep centrifuge tubes (Greiner Bio-one) prefilled with density gradient separation medium (Histopaque; Sigma-Aldrich) according to the manufacturer’s instructions. Plasma was collected and stored separately. For IgG isolation, 1 mL of the collected plasma was heat-inactivated (56°C for 40 min) and incubated with Protein G Sepharose (GE Life Sciences) overnight at 4°C. The suspension was transferred to chromatography columns and washed with PBS. IgGs were eluted from Protein G using 0.1 M glycine (pH = 3.0) and buffered in 0.1 M Tris (pH = 8.0). For buffer exchange to PBS, 30 kDa Amicon spin membranes (Millipore) were used. Purified IgG concentration was measured using a Nanodrop (A280) and samples were stored at 4°C.
SARS-CoV-2 S protein expression and purification
The construct encoding the prefusion stabilized SARS-CoV-2 S ectodomain (amino acids 1−1208 of SARS-CoV-2 S; GenBank: MN908947) was kindly provided by Jason McLellan (Texas, USA) and described previously (Wrapp et al., 2020b). In detail, two proline substitutions at residues 986 and 987 were introduced for prefusion state stabilization, a “GSAS” substitution at residues 682–685 to eliminate the furin cleavage site, and a C-terminal T4 fibritin trimerization motif. For purification, the protein is C-terminally fused to a TwinStrepTag and 8XHisTag. Protein production was done in HEK293-6E cells by transient transfection with polyethylenimine (PEI, Sigma-Aldrich) and 1 μg DNA per 1 mL cell culture medium at a cell density of 0.8 106 cells/mL in FreeStyle 293 medium (Thermo Fisher Scientific). After 7 days of culture at 37°C and 5% CO2, culture supernatant was harvested and filtered using a 0.45 μm polyethersulfone (PES) filter (Thermo Fisher Scientific). Recombinant protein was purified by Strep-Tactin affinity chromatography (IBA lifescience, Göttingen Germany) according to the Strep-Tactin XT manual. Briefly, filtered medium was adjusted to pH 8 by adding 100 mL 10x Buffer W (1 M Tris/HCl, pH 8.0, 1.5 M NaCl, 10 mM EDTA, IBA lifescience) and loaded with a low pressure pump at 1 mL/min on 5 mL bedvolume Strep-Tactin resin. The column was washed with 15 column volumes (CV) 1x Buffer W (IBA lifescience) and eluted with 6 × 2.5 mL 1x Buffer BXT (IBA lifescience). Elution fractions were pooled and buffer was exchanged to PBS pH 7.4 (Thermo Fisher Scientific) by filtrating four times over 100 kDa cut-off cellulose centrifugal filter (Merck).
Cloning and expression of different SARS-CoV-2 S protein subunits and Ebola surface glycoprotein
The RBD of the SARS-CoV-2 spike protein (GenBank: MN908947; aa: 319-541), fused to a hexahistidine tag, was expressed from a plasmid kindly provided by Florian Krammer (Stadlbauer et al., 2020) through transient calcium phosphate transfection of HEK293T cells. After 7 days of culture at 37°C and 5% CO2, culture supernatant was harvested and filtered using a 0.45 μm PES filter (Thermo Fisher Scientific). RBD protein was purified via the hexahistidine tag using Ni-NTA agarose (Macherey-Nagel). To this end, filtered culture supernatant was mixed with an equal volume of 2x NPI10 buffer (1x NPI10 buffer: 50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8). Ni-NTA beads were equilibrated with NPI10 and added to the cell culture supernatant/NPI10 mix (1 mL bed volume per 1000 mL original cell culture supernatant), followed by incubation at 4°C over night with constant rotation. Beads were harvested by centrifugation at 500 g for 5 min at 4°C and washed twice in NPI10 (100 mL per 1000 mL original cell culture supernatant), followed by centrifugation at 500 g for 5 min at 4°C. Beads were additionally washed three times in NPI20 (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, pH 8). Beads were then transferred in NPI20 to Polyprep chromatography columns (BioRad, 2 columns per 1000 mL original cell culture supernatant) and washed with 10 mL NPI20 per column. Protein was eluted with 5 mL NPI250 (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, pH 8). Buffer was exchanged to PBS using 10 kDa Amicon spin columns (Millipore).
SARS-CoV-2 S ectodomain “monomer” without trimerization domain (GenBank: MN908947; aa:1-1207) and S1 subunit (GenBank: MN908947; aa:14-529) regions of the spike DNA were amplified from a synthetic gene plasmid (furin site mutated) (Wrapp et al., 2020b) by PCR. PCR products were cloned into a modified sleeping beauty transposon expression vector containing a C-terminal thrombin cleavage and a double Strep II purification tag. For the S1 subunit, the tag was added at the 5′ end and a BM40 signal peptide was included. For recombinant protein production, stable HEK293 EBNA cell lines were generated employing the sleeping beauty transposon system (Kowarz et al., 2015). Briefly, expression constructs were transfected into the HEK293 EBNA cells using FuGENE HD transfection reagent (Promega). After selection with puromycin, cells were induced with doxycycline. Supernatants were filtered and the recombinant proteins purified via Strep-Tactin®XT (IBA Lifescience) resin. Proteins were then eluted by biotin-containing TBS-buffer (IBA Lifescience), and dialyzed against TBS-buffer.
Ebola surface glycoprotein (EBOV Makona, GenBank: KJ660347; GP aa:1-651) and HIV-1 gp140 (strain YU2, GenBank: M93258; Env aa:1-683), both lacking the transmembrane domain and containing a GCN4 trimerization domain (Ehrhardt et al., 2019), were expressed by transient transfection of HEK293-6E cells using PEI, following the same protocol and culture conditions as for the prefusion stabilized SARS-CoV-2 S ectodomain described above. After 7 days, supernatants were filtered using a 0.45 μm polyethersulfone (PES) filter (Thermo Fisher Scientific) and proteins were purified by affinity chromatography through their hexahistidine tag with Ni-NTA agarose (Macherey-Nagel) following the same protocol as for the RBD purification described above.
Isolation of SARS-CoV S ectodomain-specific IgG+ B cells
B cells were isolated from PBMCs using CD19-microbeads (Miltenyi Biotec) according to the manufacturer’s instruction. Isolated B cells were stained for 20 min on ice with a fluorescence staining-mix containing 4’,6-Diamidin-2-phenylindol (DAPI; Thermo Fisher Scientific), anti-human CD20-Alexa Fluor 700 (BD), anti-human IgG-APC (BD), anti-human CD27-PE (BD) and DyLight488-labeled SARS-CoV-2 spike protein (10μg/mL). Dapi−, CD20+, IgG+, SARC-CoV-2 spike protein positive cells were sorted using a FACSAria Fusion (Becton Dickinson) in a single cell manner into 96-well plates. All wells contained 4 μL buffer, consisting of 0.5x PBS, 0.5 U/μL RNAsin (Promega), 0.5 U/μL RNaseOUT (Thermo Fisher Scientific), and 10 mM DTT (Thermo Fisher Scientific). After sorting, plates were immediately stored at −80°C until further processing.
Antibody heavy/light chain amplification and sequence analysis
Single cell amplification of antibody heavy and light chains was mainly performed as previously described (Kreer et al., 2020a; Schommers et al., 2020). Briefly, reverse transcription was performed with Random Hexamers (Invitrogen), and Superscript IV (Thermo Fisher Scientific) in the presence of RNaseOUT (Thermo Fisher Sicentific) and RNasin (Promega). cDNA was used to amplify heavy and light chains using PlatinumTaq HotStart polymerase (Thermo Fisher Scientific) with 6% KB extender and optimized V gene-specific primer mixes (Kreer et al., 2020a) in a sequential semi-nested approach with minor modifications to increase throughput (unpublished data). PCR products were analyzed by gel electrophoresis for correct sizes and subjected to Sanger sequencing. For sequence analysis, chromatograms were filtered for a mean Phred score of 28 and a minimal length of 240 nucleotides (nt). Sequences were annotated with IgBLAST (Ye et al., 2013) and trimmed to extract only the variable region from FWR1 to the end of the J gene. Base calls within the variable region with a Phred score below 16 were masked and sequences with more than 15 masked nucleotides, stop codons, or frameshifts were excluded from further analyses. Clonal analysis was performed separately for each patient. All productive heavy chain sequences were grouped by identical VH/JH gene pairs and the pairwise Levenshtein distance for their CDRH3s was determined. Starting from a random sequence, clone groups were assigned to sequences with a minimal CDRH3 amino acid identity of at least 75% (with respect to the shortest CDRH3). 100 rounds of input sequence randomization and clonal assignment were performed and the result with the lowest number of remaining unassigned (non-clonal) sequences was selected for downstream analyses. All clones were cross-validated by the investigators taking shared mutations and light chain information into account.
Next generation sequencing and evaluation of healthy control IgG+ and naive B cell repertoires
B cell receptor repertoire sequence data were generated by an unbiased template-switch-based approach as previously described (Ehrhardt et al., 2019; Schommers et al., 2020). In brief, PBMCs from 48 healthy individuals (samples collected before the SARS-CoV-2 outbreak from the Institute of Transfusion Medicine of the University Hospital of Cologne under protocol 16-054 approved by the Institutional Review Board of the University of Cologne) were enriched for CD19+ cells with CD19-microbeads (Miltenyi Biotec). For each individual, 100,000 CD20+IgG+ and 100,000 CD20+IgD+IgM+CD27−IgG− B cells were sorted into FBS (Sigma-Aldrich) using a BD FACSAria Fusion. RNA was isolated with the RNeasy Micro Kit (QIAGEN) on a QiaCube (QIAGEN) instrument. cDNA was generated by template-switch reverse transcription according to the SMARTer RACE 5′/3′ manual using the SMARTScribe Reverse Transcriptase (Takara) with a template-switch oligo including an 18-nucleotide unique molecular identifier (UMI). Heavy and light chain variable regions were amplified in a constant region-specific nested PCR and amplicons were used for library preparation and Illumina MiSeq 2 × 300 bp sequencing. Raw NGS reads were pre-processed and assembled with an in-house pipeline based on custom Python scripts, IgBLAST (Ye et al., 2013), Clustal Omega (Sievers et al., 2011), and the pRESTO toolkit (Vander Heiden et al., 2014) as previously described (Ehrhardt et al., 2019). In brief, raw reads were initially filtered by Phred quality score (mean of 25 or higher) and read length (250 bp or longer). Unique molecular identifiers (UMIs) were extracted and paired reads were pre-annotated with IgBLAST. Based on IgBLAST pre-annotation, an additional molecular identifier (MID) was extracted by taking consecutive 18 nucleotides (nt) starting 12 nt downstream of the end of framework region (FWR) 3. For error correction, reads with the same UMIs were grouped. Reads that did not match the most abundant V gene call or had more than 1 nt difference to any other read were removed from their assigned UMI group. Assuming that ungrouped and removed reads may result from RT, PCR, or sequencing errors within the UMI, the remaining single as well as the removed reads were re-grouped by their MID. MID groups with a unique V gene call and no more than 1 nt difference between included UMIs were re-defined as a novel UMI group. Clustal omega was used to align all reads within each corrected UMI group and aligned sequences were collapsed to build consensus reads. For consensus building, base calls were weighted by their quality (1 - error probability) and bases with the highest quality-weighted frequencies were taken as the consensus. Paired consensus reads were assembled with the pRESTO AssemblePairs module and a minimal overlap set to 6 nt. Assembled sequences were annotated with IgBLAST and productive sequences were kept for analyses. To minimize the influence of sequencing and PCR errors, NGS-derived sequences were only evaluated for UMI groups with at least three reads. For the identification of overlapping clonotypes in healthy individuals a maximum of one amino acid length difference and three or less differences in absolute amino acid composition of CDR3s were considered as similar.
Cloning and production of monoclonal antibodies
Antibody cloning from 1st PCR products was performed as previously described (Schommers et al., 2020; Tiller et al., 2008) by sequence and ligation-independent cloning (SLIC; von Boehmer et al., 2016) with a minor modification. In brief, 1 μL of the 1st PCR product was used for amplification with Q5 Hot Start High Fidelity DNA Polymerase (New England Biolabs) as well as specific forward- and reverse primers including overhangs for subsequent SLIC into expression vectors (IgG1, Igλ, Igκ; Tiller et al., 2008). In contrast to the published protocol, PCR amplification for SLIC assembly was performed with extended forward primers based on 2nd PCR primers (Kreer et al., 2020a) covering the complete endogenous leader sequence of all heavy and light chain V genes (unpublished data) and reverse primers specific for the 5′ end of heavy and light chain constant regions. PCR conditions were 98°C for 30 s; 35 cycles of 98°C for 10 s, 72°C for 45 s; and 72°C for 2 min. PCR products were purified (NucleoSpin 96 PCR Clean-up, Macherey Nagel), cloned into expression vectors by SLIC using T4 DNA polymerase (NEB) and chemical competent Escherichia coli DH5α. After verification of positive colonies by colony PCR and Sanger sequencing, plasmids were amplified and purified from midi cultures (Macherey Nagel).
Monoclonal antibodies were produced by transient co-transfection of 293-6E cells with human heavy chain (IgG1 isotype) and light chain antibody expression plasmids using polyethylenimine (PEI) (Sigma-Aldrich). Following transfection, cells were maintained in FreeStyle 293 Expression Medium (Thermo Fisher) and 0.2% penicillin/streptomycin (Thermo Fisher) at 37°C and 6% CO2 and kept under constant shaking at 90-120 rpm. Seven days after transfection, supernatants were harvested and clarified by centrifugation and subsequent filtration using PES filters. For antibody purification, Protein G-coupled Sepharose beads (GE Life Sciences) were incubated with culture supernatants. After washing, antibodies were eluted from the Protein G-coupled beads in chromatography columns using 0.1 M glycine (pH = 3) and buffered using 1 M Tris (pH = 8). Finally, buffer exchange to PBS was performed using Amicon spin membranes (Millipore). Antibody concentrations were determined using UV spectrophotometry (Nanodrop, Thermo Fisher) and antibodies were stored at 4°C until further use.
ELISA analysis to determine antibody binding activity to SARS-CoV-2 S and subunit binding
ELISA plates (Corning 3369) were coated with 2 μg/mL of protein in PBS (SARS-CoV-2 spike ectodomain, RBD, or N-terminal truncated S1) or in 2 M Urea (SARS-CoV-2 spike ectodomain “monomer” lacking the trimerization domain) at 4°C overnight. For SARS-CoV-2 spike ectodomain ELISA, plates were blocked with 5% BSA in PBS for 60 min at RT, incubated with primary antibody in 1% BSA in PBS for 90 min, followed by anti-human IgG-HRP (Southern Biotech 2040-05) diluted 1:2500 in 1% BSA in PBS for 60 min at RT. SARS-CoV-2 spike subunit ELISAs were done following a published protocol (Stadlbauer et al., 2020). ELISAs were developed with ABTS solution (Thermo Fisher 002024) and absorbance was measured at 415 nm and 695 nm. Positive binding was defined by an OD ≥ 0.25 and an EC50 < 30 μg/mL. The commercial anti-SARS-CoV-2 ELISA kit for immunoglobulin class G was provided by Euroimmun (Euroimmun Diagnostik, Lübeck, Germany). Antibody detection was done according to manufacturer’s instructions and a concentration of 50 μg/mL of antibodies and 2 mg/mL of plasma IgG was used. The samples were tested using the automated platform Euroimmun Analyzer 1.
Virus neutralization test
SARS-CoV-2 neutralizing activity of poly-IgG samples or human monoclonal antibodies was investigated based on a previously published protocol for MERS-CoV Koch and Dahlke (2020). Briefly, samples were serially diluted in 96-well plates starting from a concentration of 1,500 μg/mL for plasma-isolated IgG and 100 μg/mL for monoclonal antibodies. Samples were incubated for 1 h at 37°C together with 100 50% tissue culture infectious doses (TCID50) SARS-CoV-2 (BavPat1/2020 isolate, European Virus Archive Global # 026V-03883). Cytopathic effect (CPE) on VeroE6 cells (ATCC CRL-1586) was analyzed 4 days after infection. Neutralization was defined as absence of CPE compared to virus controls. For each test, a positive control (neutralizing COVID-19 patient plasma) was used in duplicates as an inter-assay neutralization standard.
Surface Plasmon Resonance (SPR) measurements
For SPR measurement, the RBD was additionally purified by size exclusion chromatography (SEC) purification with a Superdex200 10/300 column (GE Healthcare). Binding of the RBD to the various mAbs was measured using single-cycle kinetics experiments with a Biacore T200 instrument (GE Healthcare). Purified mAbs were first immobilized at coupling densities of 800-1200 response units (RU) on a series S sensor chip protein A (GE Healthcare) in PBS and 0.02% sodium azide buffer. One of the four flow cells on the sensor chip was empty to serve as a blank. Soluble RBD was then injected at a series of concentrations (i.e., 0.8, 4, 20, 100, and 500 nM) in PBS at a flow rate of 60 μL/min. The sensor chip was regenerated using 10 mM Glycine-HCl pH 1.5 buffer. A 1:1 binding model was used to describe the experimental data and to derive kinetic parameters. For some mAbs, a 1:1 binding model did not provide an adequate description for binding. In these cases, we fitted a two-state binding model that assumes two binding constants due to conformational change. In these cases, we report the first binding constants (KD1).
HEp-2 Cell Assay
Monoclonal antibodies were tested at a concentration of 100 μg/mL in PBS using the NOVA Lite HEp-2 ANA Kit (Inova Diagnostics) according to the manufacturer’s instructions, including positive and negative kit controls on each substrate slide. HIV-1-reactive antibodies with known reactivity profiles were included as additional controls. Images were acquired using a DMI3000 B microscope (Leica) and an exposure time of 3.5 s, intensity of 100%, and a gain of 10.
Quantification and Statistical Analysis
Flow cytometry analysis and quantifications were done with FlowJo10. Statistical analyses were performed using GraphPad Prism (v7), Microsoft Excel for Mac (v14.7.3), Python (v3.6.8), and R (v4.0.0). To test for a significant increase in S-reactive B cells over time (Figure 2C and results section ‘Longitudinal analysis of the SARS-CoV-2 antibody response’) Linear Mixed Effects Models (R-function nlme::lme(), Pinheiro et al., 2020) were applied to the composite of all longitudinal data points, with individuals having their own intercept. V gene usage, CDRH3 length and V gene germline identity distributions for clonal sequences (Figure 2E) were determined for all input sequences without further collapsing. CDRH3 hydrophobicity (Figure 2E) was calculated based on the Eisenberg-scale (Eisenberg et al., 1984). To test for a significant difference of κ / λ ratios between clonal versus non-clonal sequences, D’Agostino-Pearson normality test (Prism, GraphPad) was used to test for normality and a Wilcoxon matched-pairs signed rank test was performed (Prism, GraphPad) on κ / λ ratios of each subset (Figure S2). V gene statistics for neutralizer and non-neutralizer (Figure 3G) were calculated from collapsed clonal sequences. For correlation analyses (Figures 3E and S3), spearman’s rank correlation coefficients were calculated in Prism (GraphPad). For longitudinal analyses on mutation frequencies of recurring clones (Figure 4A), a multiple sequence alignment for the B cell sequences was calculated with Clustal Omega (version 1.2.3) (Sievers et al., 2011) using standard parameters. From this, a phylogenetic tree of the sequences was estimated with RAxML through the raxmlGUI (version 2.0.0-beta.11) (Edler et al., 2019) using the GTRGAMMA substitution model (RAxML version 8.2.12) (Stamatakis, 2014). Based on the phylogenetic tree distances, all variants of a clone at a given time point were matched to variants at the consecutive time point and the slope between the pairs was computed. Hamming distances between the pairs were determined and normalized for sequence length and time difference to calculate the mean mutation frequency per day. Given the median slope per clone, a one-sided Wilcoxon Signed Rank Test was applied to test whether the slopes are equal to zero, with the alternative hypothesis that the slopes are smaller than zero. For visualizing the change of VH gene germline identity over time (Figure 4A, bottom), the germline identity for each clone was normalized by its median value at the first-time measurement and the median slope was plotted. To test for a difference in VH gene germline identity between neutralizing antibodies that were isolated at early or late time points, antibodies were separated into two groups (8–17 and 34–42 days, respectively). D’Agostino-Pearson normality test (Prism, GraphPad) was used to test for normality and a two-tailed unpaired Mann-Whitney U test was performed (Prism, GraphPad).
Acknowledgments
We thank all study participants who devoted time to our research; members of the Klein and Becker Laboratories for continuous support and helpful discussions; Jason McLellan, Nianshuang Wang, and Daniel Wrapp for sharing the SARS-CoV-2 S ectodomain plasmid; Florian Krammer for sharing the RBD plasmid; Simon Pöpsel and Robert Hänsel-Hertsch for helpful discussions and technical support; Daniela Weiland and Nadine Henn for lab management and assistance; Birgit Gathof and Sabine Adam of the Institute of Transfusion Medicine of the University Hospital of Cologne for providing blood samples for NGS analysis; as well as Heidrun Schößler and Ralf Ortmanns of the health department of Heinsberg for support with patient recruitment. This work was funded by grants from the German Center for Infection Research (DZIF) to F.K. and S.B., the German Research Foundation (DFG) (CRC 1279 and CRC 1310 to F.K., FOR2722 to M.K.; EXC 2064/1, project no. 390727645 to N.P.), the European Research Council (ERC-StG639961 to F.K.), the German Federal Ministry of Education and Research (BMBF) within the Medical Informatics Initiative (DIFUTURE) (reference 01ZZ1804D to S.L. and N.P.), the Ben B. and Joyce E. Eisenberg Foundation to R.D., the Ernst I. Ascher Foundation and Natan Sharansky to R.D.
Author Contributions
Conceptualization, F.K.; Methodology, F.K., S.B., C.K., M.Z., M.S.E., L.G., C.R., S.H., S.L., and N.P.; Investigation, C.K., M.Z., T. Weber, L.G., M.S.E., C.R., S.H., M. Korenkov, H.G., P.S., K.V., V.D.C., H.J., R.B., A.A., V.K., A.K., H.C.-D., M. Koch, J.M.E., T. Wolf, M.J.G.T.V., and C.W.; Software, C.K., S.L., and N.P.; Formal Analysis, C.K., M.Z., S.L., N.P., and F.K.; Resources, F.K., S.B., and R.D.; Writing – Original Draft, F.K., C.K., M.Z., T. Weber, and H.G.; Writing – Review and Editing, all authors; Supervision, F.K., S.B., and R.D.
Declaration of Interests
A patent application encompassing aspects of this work has been filed by the University of Cologne, listing F.K., S.B., C.K., M.Z., and H.G. as inventors.
Published: July 7, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.cell.2020.06.044.
Supplemental Information
References
- Andreano E., Nicastri E., Paciello I., Pileri P., Manganaro N., Piccini G., Manenti A., Pantano E., Kabanova A., Troisi M. Identification of neutralizing human monoclonal antibodies from Italian Covid-19 convalescent patients. bioRxiv. 2020 doi: 10.1101/2020.05.05.078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A., Fulton B.O., Wloga E., Copin R., Pascal K.E., Russo V., Giordano S., Lanza K., Negron N., Ni M. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020 doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., Hippenstiel S., Dingeldey M., Kruse B., Fauchere F. Presence of SARS-CoV-2 reactive T cells in COVID-19 patients and healthy donors. MedRxiv. 2020 doi: 10.1101/2020.04.17.20061440. [DOI] [PubMed] [Google Scholar]
- Brouwer P.J.M., Caniels T.G., van der Straten K., Snitselaar J.L., Aldon Y., Bangaru S., Torres J.L., Okba N.M.A., Claireaux M., Kerster G. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020 doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D.R., Walker L.M. Rational Vaccine Design in the Time of COVID-19. Cell Host Microbe. 2020;27:695–698. doi: 10.1016/j.chom.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Su B., Guo X., Sun W., Deng Y., Bao L., Zhu Q., Zhang X., Zheng Y., Geng C. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 2020 doi: 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D., Voss J., Gamblin S.J., Codoni G., Macagno A., Jarrossay D., Vachieri S.G., Pinna D., Minola A., Vanzetta F. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- Davis C.W., Jackson K.J.L., McElroy A.K., Halfmann P., Huang J., Chennareddy C., Piper A.E., Leung Y., Albariño C.G., Crozier I. Longitudinal Analysis of the Human B Cell Response to Ebola Virus Infection. Cell. 2019;177:1566–1582.e17. doi: 10.1016/j.cell.2019.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edler D., Klein J., Antonelli A., Silvestro D. raxmlGUI 2.0 beta: a graphical interface and toolkit for phylogenetic analyses using RAxML. bioRxiv. 2019 doi: 10.1101/2020.05.05.078154. [DOI] [Google Scholar]
- Ehrhardt S.A., Zehner M., Krähling V., Cohen-Dvashi H., Kreer C., Elad N., Gruell H., Ercanoglu M.S., Schommers P., Gieselmann L. Polyclonal and convergent antibody response to Ebola virus vaccine rVSV-ZEBOV. Nat. Med. 2019;25:1589–1600. doi: 10.1038/s41591-019-0602-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J. Mol. Biol. 1984;179:125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Fauci A.S., Marston H.D. PUBLIC HEALTH. Toward an HIV vaccine: A scientific journey. Science. 2015;349:386–387. doi: 10.1126/science.aac6300. [DOI] [PubMed] [Google Scholar]
- Flyak A.I., Shen X., Murin C.D., Turner H.L., David J.A., Fusco M.L., Lampley R., Kose N., Ilinykh P.A., Kuzmina N. Cross-Reactive and Potent Neutralizing Antibody Responses in Human Survivors of Natural Ebolavirus Infection. Cell. 2016;164:392–405. doi: 10.1016/j.cell.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Baum A., Pascal K.E., Russo V., Giordano S., Wloga E., Fulton B.O., Yan Y., Koon K., Patel K. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020 doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Kang B.H., Ishida E., Zhou T., Griesman T., Sheng Z., Wu F., Doria-Rose N.A., Zhang B., McKee K. Identification of a CD4-Binding-Site Antibody to HIV that Evolved Near-Pan Neutralization Breadth. Immunity. 2016;45:1108–1121. doi: 10.1016/j.immuni.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Yu J., Lanzi A., Yao X., Andrews C.D., Tsai L., Gajjar M.R., Sun M., Seaman M.S., Padte N.N. Engineered Bispecific Antibodies with Exquisite HIV-1-Neutralizing Activity. Cell. 2016;165:1621–1631. doi: 10.1016/j.cell.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce M.G., Wheatley A.K., Thomas P.V., Chuang G.Y., Soto C., Bailer R.T., Druz A., Georgiev I.S., Gillespie R.A., Kanekiyo M. Vaccine-Induced Antibodies that Neutralize Group 1 and Group 2 Influenza A Viruses. Cell. 2016;166:609–623. doi: 10.1016/j.cell.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020 doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- Kallewaard N.L., Corti D., Collins P.J., Neu U., McAuliffe J.M., Benjamin E., Wachter-Rosati L., Palmer-Hill F.J., Yuan A.Q., Walker P.A. Structure and Function Analysis of an Antibody Recognizing All Influenza A Subtypes. Cell. 2016;166:596–608. doi: 10.1016/j.cell.2016.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F., Mouquet H., Dosenovic P., Scheid J.F., Scharf L., Nussenzweig M.C. Antibodies in HIV-1 vaccine development and therapy. Science. 2013;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T., Dahlke C. Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: an open-label, phase 1 trial. The Lancet. 2020;20:827–838. doi: 10.1016/S1473-3099(20)30248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff W.C., Burton D.R., Johnson P.R., Walker B.D., King C.R., Nabel G.J., Ahmed R., Bhan M.K., Plotkin S.A. Accelerating next-generation vaccine development for global disease prevention. Science. 2013;340:1232910. doi: 10.1126/science.1232910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowarz E., Löscher D., Marschalek R. Optimized Sleeping Beauty transposons rapidly generate stable transgenic cell lines. Biotechnol. J. 2015;10:647–653. doi: 10.1002/biot.201400821. [DOI] [PubMed] [Google Scholar]
- Kreer C., Döring M., Lehnen N., Ercanoglu M.S., Gieselmann L., Luca D., Jain K., Schommers P., Pfeifer N., Klein F. openPrimeR for multiplex amplification of highly diverse templates. J. Immunol. Methods. 2020;480:112752. doi: 10.1016/j.jim.2020.112752. [DOI] [PubMed] [Google Scholar]
- Kreer C., Gruell H., Mora T., Walczak A.M., Klein F. Exploiting B Cell Receptor Analyses to Inform on HIV-1 Vaccination Strategies. Vaccines (Basel) 2020;8:13. doi: 10.3390/vaccines8010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakkenbos M.J., Diehl S.A., Yasuda E., Bakker A.Q., van Geelen C.M.M., Lukens M.V., van Bleek G.M., Widjojoatmodjo M.N., Bogers W.M.J.M., Mei H. Generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells by genetic programming. Nat. Med. 2010;16:123–128. doi: 10.1038/nm.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Drelich A., Martinez D.R., Gralinski L., Chen C., Sun Z., Liu X., Zhelev D., Zhang L., Peterson E.C. Potent neutralization of SARS-CoV-2 in vitro and in an animal model by a human monoclonal antibody. bioRxiv. 2020 doi: 10.1101/2020.05.13.093088. [DOI] [Google Scholar]
- Liu L., Wang P., Nair M.S., Yu J., Huang Y., Rapp M.A., Wang Q., Luo Y., Sahi V., Figueroa A. Potent Neutralizing Monoclonal Antibodies Directed to Multiple Epitopes on the SARS-CoV-2 Spike. bioRxiv. 2020 doi: 10.1101/2020.06.17.153486. [DOI] [PubMed] [Google Scholar]
- Liu X., Gao F., Gou L., Chen Y., Gu Y., Ao L., Shen H., Hu Z., Guo X., Gao W. Neutralizing Antibodies Isolated by a site-directed Screening have Potent Protection on SARS-CoV-2 Infection. bioRxiv. 2020 doi: 10.1101/2020.05.03.074914. [DOI] [Google Scholar]
- Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J., Hu J.-L., Xu W., Zhang Y., Lv F.-J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020 doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Long Q.-X., Liu B.-Z., Deng H.-J., Wu G.-C., Deng K., Chen Y.-K., Liao P., Qiu J.-F., Lin Y., Cai X.-F. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Mascola J.R., Montefiori D.C. The role of antibodies in HIV vaccines. Annu. Rev. Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- Ni L., Ye F., Cheng M.-L., Feng Y., Deng Y.-Q., Zhao H., Wei P., Ge J., Gou M., Li X. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity. 2020;52:971–977.e3. doi: 10.1016/j.immuni.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., DebRoy S., Sarkar D., R Core Team . 2020. {nlme}: Linear and Nonlinear Mixed Effects Models. R package version 3.1-148.https://CRAN.R-project.org/package=nlme [Google Scholar]
- Pinto D., Park Y.J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020 doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Finkin S., Hagglof T. Convergent Antibody Responses to SARS-CoV-2 Infection in Convalescent Individuals. Nature. 2020 doi: 10.1101/2020.05.13.092619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T.F., Zhao F., Huang D., Beutler N., Burns A., He W., Limbo O., Smith C., Song G., Woehl J. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020 doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Saphire E.O., Schendel S.L., Fusco M.L., Gangavarapu K., Gunn B.M., Wec A.Z., Halfmann P.J., Brannan J.M., Herbert A.S., Qiu X., Viral Hemorrhagic Fever Immunotherapeutic Consortium Systematic Analysis of Monoclonal Antibodies against Ebola Virus GP Defines Features that Contribute to Protection. Cell. 2018;174:938–952.e13. doi: 10.1016/j.cell.2018.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid J.F., Mouquet H., Ueberheide B., Diskin R., Klein F., Oliveira T.Y.K., Pietzsch J., Fenyo D., Abadir A., Velinzon K. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schommers P., Gruell H., Abernathy M.E., Tran M.K., Dingens A.S., Gristick H.B., Barnes C.O., Schoofs T., Schlotz M., Vanshylla K. Restriction of HIV-1 Escape by a Highly Broad and Potent Neutralizing Antibody. Cell. 2020;180:471–489.e22. doi: 10.1016/j.cell.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempowski G.D., Saunders K.O., Acharya P., Wiehe K.J., Haynes B.F. Pandemic Preparedness: Developing Vaccines and Therapeutic Antibodies For COVID-19. Cell. 2020;181:1458–1463. doi: 10.1016/j.cell.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux E., Homad L.J., MacCamy A.J., Parks K.R., Hurlburt N.K., Jennewein M.F., Akins N.R., Stuart A.B., Wan Y.-H., Feng J. Analysis of a SARS-CoV-2 infected individual reveals development of potent neutralizing antibodies to distinct epitopes with limited somatic mutation. Immunity. 2020 doi: 10.1016/j.immuni.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R., Shan C., Duan X., Chen Z., Liu P., Song J., Song T., Bi X., Han C., Wu L. A human neutralizing antibody targets the receptor binding site of SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- Sievers F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer D., Amanat F., Chromikova V., Jiang K., Strohmeier S., Arunkumar G.A., Tan J., Bhavsar D., Capuano C., Kirkpatrick E. SARS-CoV-2 Seroconversion in Humans: A Detailed Protocol for a Serological Assay, Antigen Production, and Test Setup. Curr. Protoc. Microbiol. 2020;57:e100. doi: 10.1002/cpmc.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T., Meffre E., Yurasov S., Tsuiji M., Nussenzweig M.C., Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden J.A., Yaari G., Uduman M., Stern J.N.H., O’Connor K.C., Hafler D.A., Vigneault F., Kleinstein S.H. pRESTO: a toolkit for processing high-throughput sequencing raw reads of lymphocyte receptor repertoires. Bioinformatics. 2014;30:1930–1932. doi: 10.1093/bioinformatics/btu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer L., Liu C., Ackerman S., Gitlin A.D., Wang Q., Gazumyan A., Nussenzweig M.C. Sequencing and cloning of antigen-specific antibodies from mouse memory B cells. Nat. Protoc. 2016;11:1908–1923. doi: 10.1038/nprot.2016.102. [DOI] [PubMed] [Google Scholar]
- Walker L.M., Burton D.R. Passive immunotherapy of viral infections: ‘super-antibodies’ enter the fray. Nat. Rev. Immunol. 2018;18:297–308. doi: 10.1038/nri.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.M., Phogat S.K., Chan-Hui P.Y., Wagner D., Phung P., Goss J.L., Wrin T., Simek M.D., Fling S., Mitcham J.L., Protocol G Principal Investigators Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Li W., Drabek D., Okba N.M.A., van Haperen R., Osterhaus A.D.M.E., van Kuppeveld F.J.M., Haagmans B.L., Grosveld F., Bosch B.J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020;11:2251. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Liu L., Nair M.S., Yin M.T., Luo Y., Wang Q., Yuan T., Mori K., Solis A.G., Yamashita M. SARS-CoV-2 Neutralizing Antibody Responses Are More Robust in Patients with Severe Disease. bioRxiv. 2020 doi: 10.1101/2020.06.13.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wec A.Z., Haslwanter D., Abdiche Y.N., Shehata L., Pedreño-Lopez N., Moyer C.L., Bornholdt Z.A., Lilov A., Nett J.H., Jangra R.K. Longitudinal dynamics of the human B cell response to the yellow fever 17D vaccine. Proc. Natl. Acad. Sci. USA. 2020;117:6675–6685. doi: 10.1073/pnas.1921388117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wec A.Z., Wrapp D., Herbert A.S., Maurer D.P., Haslwanter D., Sakharkar M., Jangra R.K., Dieterle M.E., Lilov A., Huang D. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science, 2020:eabc7424. doi: 10.1126/science.abc7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., De Vlieger D., Corbett K.S., Torres G.M., Wang N., Van Breedam W., Roose K., van Schie L., Hoffmann M., Pöhlmann S. Structural Basis for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid Antibodies. Cell. 2020;181:1004–1015.e15. doi: 10.1016/j.cell.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Yang Z.Y., Li Y., Hogerkorp C.M., Schief W.R., Seaman M.S., Zhou T., Schmidt S.D., Wu L., Xu L. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Wang F., Shen C., Peng W., Li D., Zhao C., Li Z., Li S., Bi Y., Yang Y. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020 doi: 10.1126/eabc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Ma N., Madden T.L., Ostell J.M. IgBLAST: an immunoglobulin variable domain sequence analysis tool. Nucleic Acids Res. 2013;41 doi: 10.1093/nar/gkt382. W34-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A.Q. Isolation of and Characterization of Neutralizing Antibodies to Covid-19 from a Large Human Naïve scFv Phage Display Library. bioRxiv. 2020 doi: 10.1101/2020.05.19.104281. [DOI] [Google Scholar]
- Zeng X., Li L., Lin J., Li X., Liu B., Kong Y., Zeng S., Du J., Xiao H., Zhang T. Blocking antibodies against SARS-CoV-2 RBD isolated from a phage display antibody library using a competitive biopanning strategy. bioRxiv. 2020 doi: 10.1101/2020.04.19.049643. [DOI] [Google Scholar]
- Zhang Y., Meyer-Hermann M., George L.A., Figge M.T., Khan M., Goodall M., Young S.P., Reynolds A., Falciani F., Waisman A. Germinal center B cells govern their own fate via antibody feedback. J. Exp. Med. 2013;210:457–464. doi: 10.1084/jem.20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S., Alvarez R., Kong X.P., Weiss S. Vaccine-induced V1V2-specific antibodies control and or protect against infection with HIV, SIV and SHIV. Curr. Opin. HIV AIDS. 2019;14:309–317. doi: 10.1097/COH.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zost S.J., Gilchuk P., Case J.B., Binshtein E., Chen R.E., Reidy J.X., Trivette A., Nargi R.S., Sutton R.E., Suryadevara N. Potently neutralizing human antibodies that block SARS-CoV-2 receptor binding and protect animals. bioRxiv. 2020 doi: 10.1101/2020.04.19.049643. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Nucleotide sequences of all SARS-CoV-2-neutralizing antibodies were deposited at GenBank (accession numbers MT658806 - MT658861). Further antibody sequences and NGS data of healthy individuals will be shared by the Lead Contact upon request.