Abstract
This study aimed to compare antimicrobial resistance (AMR) in extended-spectrum cephalosporin-resistant and generic Escherichia coli from a One Health continuum of the beef production system in Alberta, Canada. A total of 705 extended-spectrum cephalosporin-resistant E. coli (ESCr) were obtained from: cattle feces (CFeces, n = 382), catch basins (CBasins, n = 137), surrounding streams (SStreams, n = 59), beef processing plants (BProcessing, n = 4), municipal sewage (MSewage; n = 98) and human clinical specimens (CHumans, n = 25). Generic isolates (663) included: CFeces (n = 142), CBasins (n = 185), SStreams (n = 81), BProcessing (n = 159) and MSewage (n = 96). All isolates were screened for antimicrobial susceptibility to 9 antimicrobials and two clavulanic acid combinations. In ESCr, oxytetracycline (87.7%), ampicillin (84.4%) and streptomycin (73.8%) resistance phenotypes were the most common, with source influencing AMR prevalence (p < 0.001). In generic E. coli, oxytetracycline (51.1%), streptomycin (22.6%), ampicillin (22.5%) and sulfisoxazole (14.3%) resistance were most common. Overall, 88.8% of ESCr, and 26.7% of generic isolates exhibited multi-drug resistance (MDR). MDR in ESCr was high from all sources: CFeces (97.1%), MSewage (96.9%), CHumans (96%), BProcessing (100%), CBasins (70.5%) and SStreams (61.4%). MDR in generic E. coli was lower with CFeces (45.1%), CBasins (34.6%), SStreams (23.5%), MSewage (13.6%) and BProcessing (10.7%). ESBL phenotypes were confirmed in 24.7% (n = 174) ESCr and 0.6% of generic E. coli. Prevalence of bla genes in ESCr were blaCTXM (30.1%), blaCTXM-1 (21.6%), blaTEM (20%), blaCTXM-9 (7.9%), blaOXA (3.0%), blaCTXM-2 (6.4%), blaSHV (1.4%) and AmpC β-lactamase blaCMY (81.3%). The lower AMR in ESCr from SStreams and BProcessing and higher AMR in CHumans and CFeces likely reflects antimicrobial use in these environments. Although MDR levels were higher in ESCr as compared to generic E. coli, AMR to the same antimicrobials ranked high in both ESCr and generic E. coli sub-populations. This suggests that both sub-populations reflect similar AMR trends and are equally useful for AMR surveillance. Considering that MDR ESCr MSewage isolates were obtained without enrichment, while those from CFeces were obtained with enrichment, MSewage may serve as a hot spot for MDR emergence and dissemination.
Keywords: antimicrobial resistance, extended-spectrum beta-lactamase (ESBL), one health, beef, sewage
1. Introduction
Antimicrobial resistance (AMR) is a critical public health risk, estimated to account for 700,000 human mortalities per year [1,2]. Drivers of AMR include profuse use of antimicrobials in human and veterinary medicine, often misused in clinical settings or overused to improve the efficiency of livestock and crop production [3]. Genes associated with AMR can circulate among bacteria which may contaminate food or water consumed by humans [3,4,5].
Canada is ranked among the top 10 beef exporters worldwide. In 2016, Canada’s beef export industry was valued at $2.3 billion, contributing $33 billion worth of goods and services to the nation [6]. Antimicrobials (i.e., antibiotics) are administered to cattle to prevent and treat various diseases such as liver abscesses [7], foot rot [8] and bovine respiratory diseases (BRD) [9,10]. Owing to the routine use of antimicrobials common to the same class in both humans and beef, there is a possibility of antimicrobial use (AMU) in beef contributing to AMR in humans. Some studies have found similar AMR profiles and prevalence in bacterial species such as Escherichia coli and Salmonella isolates and AMR genes from cattle, humans, swine and waste streams [11,12,13]. Several studies have been narrower in scope, focusing on farms [14], processing plants [15,16] and retail meat [17]. Although a One Health approach to AMR monitoring has been advocated, most studies have not systematically compared AMR E. coli isolated across the beef production chain to those obtained from human sources. The One Health approach acknowledges the interconnectedness of health domains associated with humans, animals and the environment, and employs an integrated approach to risk management and decision making. This enables the identification of those segments along the continuum that can best disrupt the transmission of AMR from the environment to humans [2,18].
Extended-spectrum β-lactam (ESBL) resistant E. coli are of particular public health concern [19] as they are able to inactivate most β-lactam antimicrobials [20] used to treat associated infections. Presently, ESBL bacteria are a common source of therapeutic failure due to frequent co-resistance to multiple last resort antimicrobials [21,22] including colistin (i.e., polymyxin E), aminoglycoside, and 3rd and 4th generation cephalosporins that are used to treat a significant proportion of nosocomial infections [23,24,25]. In veterinary and human medicine, the extended-spectrum cephalosporins which are a sub-class of ESBL are regarded as critically important [26]. Resistance in extended-spectrum cephalosporin-resistant E. coli is often encoded by genes such as blaSHV, blaTEM and blaCTX-M [22,27].
Monitoring of cephalosporin-resistant bacteria in agricultural sectors could uncover crucial information for designing cost-effective actions to minimize the disease burden associated with these bacterial infections [28]. Considering the impact of such infections on human health, broadening the scope of monitoring to include AmpC-producing bacteria has also been recommended by the WHO [18]. A study by Horton et al. [29] found levels of CTX-M-positive E. coli in cattle, pigs and chickens at 1002, 800 and 5350 CFU/g of feces, respectively. Even though cephalosporin-resistant E. coli have been reported in cattle, cattle farms and retail beef [30,31], evidence supporting the direct contribution of livestock production to AMR emergence along the One Health continuum is lacking. In contrast to the low prevalence of extended-spectrum cephalosporin-resistant E. coli (ESCr) reported by the Canadian Integrated Program for Antimicrobial Resistance, Cormier et al. recently [32] found a high prevalence of ESCr in cattle using enrichment methods. The present study aimed to employ a One Health approach to examine similarities between patterns and prevalence of AMR typical of the entire food continuum using generic E. coli and cephalosporin-resistant E. coli in a defined geographic region of high beef production intensity in Canada.
2. Materials and Methods
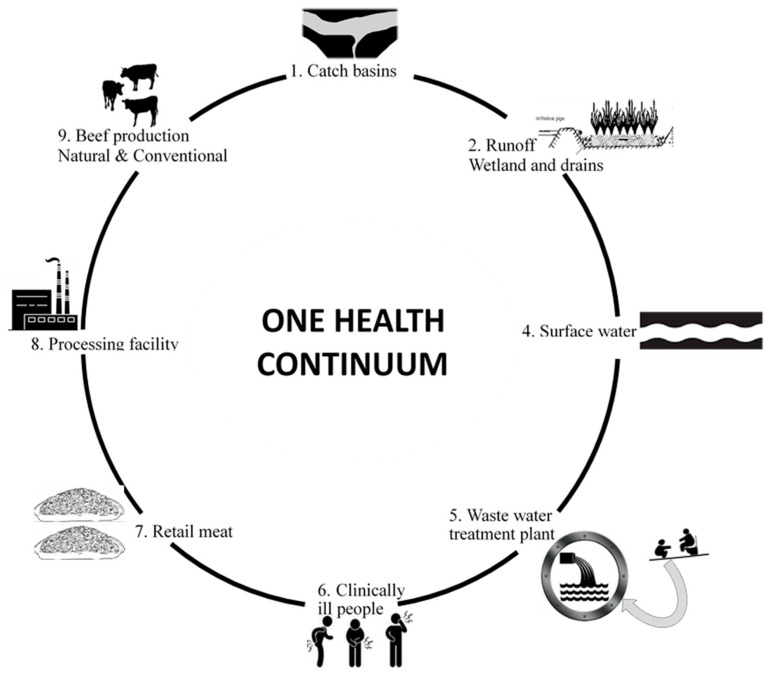
Sampling points and details of study area (Figure 1), associated sample collection and AMU records on farms are published elsewhere [33,34]. Samples were collected and transported to the lab on ice, with E. coli isolated within 24 h after arrival at the laboratory.
Figure 1.
Details of sample type and source of Escherichia coli isolates investigated for antimicrobial resistance in a One Health study of the beef production system. Sample location/sites comprised composite fecal samples collected from penned cattle in feedlots A, B, C and D as well as water samples from catch basins. Environmental samples comprised constructed wetlands and a creek (adjacent to feedlot C). Wastewater influent and effluent were collected from two water treatment plants while samples at a beef processing facility were obtained from carcasses after hide removal, after final wash, from ground beef and retail meat. Human samples were obtained from blood, urine and abdomen samples collected from hospital patients in southern Alberta.
2.1. Study Area and Description of Sampled Matrices
2.1.1. Fecal and Water Sampling from Cattle Feces, Catch Basins and Surrounding Streams
Four feedlots in Southern Alberta designated as A, B, and C used conventional production practices including the use of antimicrobials, while lot D was a commercial feedlot that employed both conventional and “raised without antimicrobials” practices. Feedlots had a one-time capacity of 15,000–40,000 cattle and were characterized by production conditions typical for western Canadian feedlots, with open air pens with dirt floors arranged side-by-side with central feed alleys. Animals used in this experiment were cared for in accordance with the Canadian Council of Animal Care [35]. All procedures and protocols used in this study were reviewed and approved by the University of Calgary’s Animal Care Committee (Protocol number AC14-0029). Fresh pen-floor fecal samples were collected every two months from April 2014 to April 2016. Twenty pens within each feedlot containing between 100 to 300 cattle were randomly selected, and the same pens were sampled throughout the two-year study. For each pen, a combined sample of 10 g of feces was obtained from 20 fecal pats to generate composite pen samples that were transported to the lab in Cary-Blair enteric transport medium (BD Canada, Inc., Mississauga, ON, Canada).
In each feedlot, runoff from the sampled pens drained into an adjacent catch basin. In feedlot C, water samples were also obtained from accumulated runoff periodically transferred into a constructed wetland and from an adjacent ephemeral creek into which the wetland drained. From mid-depth (≈0.75 m), 1 L of water was collected using a polyethylene bottle attached to a telescopic pole at four different locations per site, which were combined to generate a composite sample. For the wetland, one sample was generated by collecting and combining samples from four consistent locations throughout the study. Water samples were collected monthly from catch basins, the wetland and creeks from April to October of 2014 and 2015. Subsequently, samples from the creeks were collectively referred to as surface streams, while the wetland samples were categorized together with samples from the catch basin. Samples were not collected from these sources from November to March as they were often frozen.
2.1.2. Wastewater Treatment Plants (WWTP)
In tandem to farm sample collection, a composite of influent (post-grit tank) and effluent (just prior to release) sewage samples (1 L) was collected bimonthly from WWTP in Calgary and Medicine Hat from April 2014 to April 2016.
2.1.3. Processing Plants
Protocols for beef processing plant and retail meat sample collection followed procedures published elsewhere [36,37]. Samples were obtained from ~100 cm2 areas of hides, beef trim and conveyers; 1000 cm2 areas of washed carcasses; and from the whole of the distal surface of each chilled side (~12 × 500 cm2). Carcasses or conveyer samples were collected by randomly swabbing the surface with a 2 × 2 sterile gauze pad (Millerdale Pharmacy, Dukal Corporation, Red Deer, AB, Canada) moistened with 0·1% w/v peptone water (Becton Dickinson Co., Sparks, MD, USA). For ground beef, 200 g was obtained by aseptically removing ground beef from 4–5 kg packs of coarsely ground chub. Samples were placed in sterile stomacher bags on ice and transported in a cooler to the lab. Processing plant samples and feedlot fecal samples did not arise from the same animals, although the feedlots sampled did send cattle to the same processing plant.
2.1.4. Humans
Human clinical samples were randomly selected from blood, urine and abdominal samples from anonymous individual patients and did not require patient approval or a clinical use permit. The samples were collected during the same time period as those described above by the Division of Medical Microbiology, Calgary Laboratory Services (CLS) biorepository. This laboratory services about 1.5 million people in Calgary and the surrounding rural area. Clinical strains were isolated and confirmed from samples as described by Pitout et al [38,39].
2.2. Isolation of E. coli
Processing of samples from cattle feces, catch basin, surrounding streams and sewage treatment followed procedures detailed by Adator et al. [40] and Tymensen et al. [41] to obtain generic E. coli and ESCr. To isolate ESCr from feedlot samples, 0.5 g of cattle feces was added to 4.5 mL E. coli broth-cefotaxime (2 μg/mL) and enriched overnight, followed by sub-culture onto MacConkey plates supplemented with 1 μg/mL of ceftriaxone (MilliporeSigma) [40]. Three distinct red/magenta lactose-fermenting colonies were subcultured onto tryptic soy agar (TSA)-ampicillin (32 μg/mL) (MilliporeSigma) and later archived at −80 °C. For catch basins and surrounding streams, ESCr were isolated by membrane filtration using the US EPA Method 1603 [34,40]. To obtain generic E. coli, the same procedure, excluding enrichment and selective plates, was used. For samples from the beef processing plant (BProcessing), swabs were mixed with 10 mL of buffered peptone water (BPW), stomached for 2 min and incubated overnight at 37 °C. For ground beef, ~25 g of ground beef was incubated overnight at 37 °C in 225 mL of BPW. One mL from each resulting culture was added to 9 mL of E. coli enrichment broth containing a Durham tube and 2 µg/mL of cefotaxime, followed by overnight incubation at 37 °C and plating onto MacConkey agar containing 1 µg/mL of ceftriaxone. The plates were then incubated overnight at 37 °C. Samples from catch basin (CBasins), surface streams (SStreams), sewage treatment (MSewage) and humans (CHumans) were not enriched overnight prior to plating onto ceftriaxone-supplemented MacConkey plates.
Subsequently, pure putative E. coli colonies obtained from feedlots A, B and D, MSewage and BProcessing were subjected to indole test (Indole Spot Reagent, Hardy Diagnostics; Santa Maria, CA, USA). Three colonies positive for the indole reaction were deposited in Brain Heart Infusion (BHI) broth containing 15% glycerol and in Tris-EDTA (TE) buffer (pH 7.6) for DNA template extraction and stored at −80 °C. E. coli ATCC 25,922 and M. haemolytica 33,396 were included as indole positive and negative controls, respectively. E. coli was confirmed using the arpA gene for PCR before archiving [34]. A single colony obtained from antibiotic-supplemented plates with or without enrichment were collectively referred to as extended-spectrum cephalosporin-resistant E. coli (ESCr). ESCr isolates originated from CFeces (n = 382), CBasins (n = 137), SStreams (n = 59), BProcessing (n = 4), MSewage (n = 98) and CHumans (n = 25), while generic E. coli isolates obtained on MacConkey agar originated from CFeces (n = 142), CBasins (n = 185), MSewage (n = 96), SStreams (n = 81) and BProcessing (n = 159).
2.3. Antibiograms
A comprehensive panel of antimicrobials was selected on the basis of: (i) use in beef production systems; (ii) category of medical importance to humans; (iii) adequate representation of antimicrobials in diverse classes; and (iv) use in phenotypic confirmation of extended-spectrum β-lactam -resistant E. coli. Resistance of ESCr and generic E. coli to an antibiotic panel (ampicillin, amoxicillin/clavulanic, ceftiofur, ceftazidime, ceftazidime/clavulanic acid, streptomycin, neomycin, oxytetracycline, florfenicol, trimethoprim/sulfamethoxazole and sulfisoxazole) was examined using the Kirby–Bauer disk diffusion susceptibility method according to documents M100-S26 and VET-01S of the Clinical and Laboratory Standards Institute (CLSI) [42,43]. Briefly, E. coli isolates were retrieved from −80 °C and streaked onto TSA supplemented with 5% sheep blood (PB75, Dalynn Biologicals, Calgary, AB) and incubated overnight at 37 °C. A single colony from each plate was subcultured onto PB75, incubated overnight at 37 °C and then used for ASTs. Isolates were resuspended in saline and density was calibrated to a 0.5 McFarland turbidity standard. The suspension was streaked onto Mueller Hinton Kirby agar plates (PM90K) (Dalynn Biologicals). BD BBL™ Sensi-Disc™ (BD) (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) or Thermo Scientific Oxoid (Dardilly, France) antimicrobial susceptibility disks were then placed onto the plates. The plates were incubated for 18 h and zone diameters of inhibition were recorded using BIOMIC V3 Microbiology System (Santa Barbara, CA) using standards set by the CLSI [42,43]. E. coli American Type Culture Collection (ATCC) 25,922, ATCC 35,218 and Staphylococcus aureus ATCC 29,213 were used as quality controls [42,44]. Putative extended-spectrum β-lactamase-resistant phenotypes were assigned if isolates showed an increased zone of inhibition of ≥5 mm for ceftazidime in combination with clavulanic acid versus the zone of inhibition obtained when tested against ceftazidime alone [42,45].
2.4. Genotypic Characterization of ESBL Resistance
A simplex and two multiplex PCR assays were used to screen 705 ESCr using the primer sets and conditions previously validated elsewhere, with minor modifications. Multiplex 1 comprised blaTEM [46], blaSHV [46], blaOXA [47], and blaCMY [48], multiplex 2 comprised blaCTX-M 1,2,9 [47], while the simplex identified blaCTX-M [46]. DNA template was prepared by heat lysing each E. coli colony in TE (pH 7.4) at 99 °C for 10 min and the lysate was centrifuged 21,130× g for 5 min (5424 R Eppendorf Centrifuge). Each 25 µL multiplex PCR reaction comprised 12.5 μL of 1× Qiagen HotStar Plus Multiplex PCR Master Mix (Qiagen GmbH, Hilden, Germany), 2.5 μL CoralLoad Concentrate, primer-specific concentration and 2 μL DNA template. Simplex reactions were comprised of 12.5 μL of Qiagen PCR Master Mix, 2.5 μL coral-load solution, primer-specific concentration and 2 μL DNA template. Amplification conditions for multiplex 1 were as follows: 95 °C for 15 min; 30 cycles of 94 °C for 1 min, annealing at 53 °C for 1 min, 72 °C for 1 min with a final extension for 10 min at 72 °C. For multiplex 2 and simplex reactions, annealing temperature was 60 °C. PCR products were subjected to electrophoresis in 1.5% agarose gel in 1X TAE buffer. E. coli strains previously sequenced with known β-lactamase genes of interest were used as positive controls and water was used as a no template control.
2.5. Data Management and Analysis
Descriptive analysis comparing the distributions of resistance (R), intermediate (I) and susceptible (S) E. coli, MDR and ESBL-resistant phenotypes as well as β-lactamase genotypes was performed with PROC FREQ (SAS software, version 9.4 SAS Institute, Cary, North Carolina, USA). Isolates were categorized as MDR if they showed resistance to antimicrobial agents in ≥2 different antimicrobial classes [49,50]. To compare the effect of isolate source on AMR in both ESCr and generic E. coli sub-populations, univariate multinomial logistic regression models were fitted to the data using the SAS GLIMMIX procedure [13,51]. E. coli population (ESCr or generic E. coli) was modeled as a random block factor, while antibiotic isolate source and their interaction were considered fixed effects. Differences in AMR for each antibiotic per source were expressed as odds ratios (OR); source-specific ORs were specified with a 95% confidence interval and declared significant at p < 0.05. Antimicrobials which were not modeled due to low prevalence of resistance or inability for the model to converge included neomycin in ESCr and ceftiofur, ceftazidime, trimethoprim-sulfamethoxazole and neomycin in generic E. coli. Since not all bla genes are indicative of ESBLs, isolates were designated as cephalosporin-resistant, β-lactamase genotype-positive if they possessed one or more of blaSHV, blaTEM, blaOXA and blaCTXM and designated as a pAmpC genotype-positive if they possessed blaCMY. To examine the associations between the number or type of genes present and MDR phenotype per source, separate binary logit analyses were performed using logistic regression [52] and effects were considered significant at p < 0.05 [53]. For source effect analyses of AMR phenotypes and β-lactamase/pAmpC genes in ESCr, CHumans and MSewage ESCr isolates were categorized together as human-associated E. coli designated HM (total n = 123). ESCr BProcessing isolates were not included in the analyses due to the low number of isolates obtained.
3. Results
3.1. Antimicrobial Resistance in Extended-Spectrum Cephalosporin-Resistant E. coli and Generic E. coli
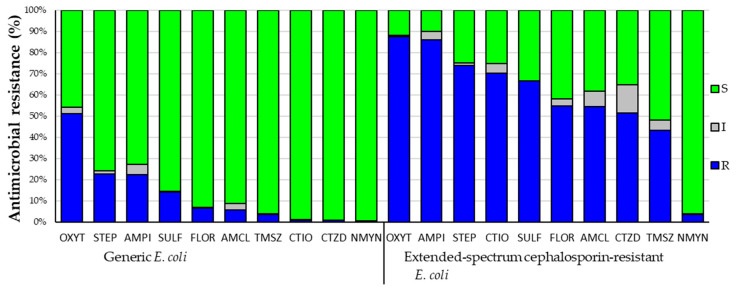
A total of 705 ESCr strains and 663 generic E. coli were examined for antimicrobial susceptibility to nine antimicrobial agents and two combinations. For ESCr, prevalence of resistance ranked as oxytetracycline (87.7%), followed by ampicillin (86%), streptomycin (73.9%), ceftiofur (70.2%), sulfisoxazole (66.8%), trimethoprim-sulfamethoxazole (43.4%) and neomycin (3.7%) (Figure 2). Generic E. coli exhibited the highest prevalence of resistance to oxytetracycline (51.1%), followed by streptomycin (22.6%), ampicillin (22.5%), sulfisoxazole (14.3%), trimethoprim-sulfamethoxazole (3.6%), ceftiofur (0.9%), ceftazidime (0.8%) and neomycin (0.5%) (Figure 2). In both populations, resistance to oxytetracycline and ampicillin was prevalent, while neomycin resistance was the least common. The overall prevalence of resistance to antimicrobials was higher (p < 0.001) for ESCr than for generic E. coli.
Figure 2.
Antimicrobial resistance distribution among generic (n = 663) and extended-spectrum cephalosporin-resistant (n = 705) Escherichia coli. Legends: S—Susceptible; I—Intermediate; R—Resistance. OXYT—Tetracycline; STEP—Streptomycin; AMPI—Ampicillin; SULF—Sulfisoxazole; FLOR—Florfenicol; AMCL—Amoxicillin/clavulanic acid; TMSZ—trimethoprim/sulfamethoxazole; CTIO—Ceftiofur; CTZD—Ceftazidime; NMYN—neomycin.
Overall AMR prevalence among ESCr from various sources (CFeces, CBasins, MSewage, BProcessing, MSewage, and CHumans) differed (p < 0.001; Table 1). ESCr from CFeces exhibited high levels of resistance to oxytetracycline (98.4%) followed by ampicillin (96.3%), streptomycin (89.0%), sulfisoxazole (85.1%), ceftiofur (78.2%), florfenicol (77.2%), amoxicillin clavulanate (71.7%), ceftazidime (65.4%), trimethoprim-sulfamethoxazole (51.0%) and neomycin (4.9%; Table 1). Although less common than in CFeces, resistance to oxytetracycline, ampicillin and streptomycin was also most prominent in CBasins and SStreams isolates. Resistance to neomycin was rare, regardless of isolate source. ESCr from MSewage and CHumans did not exhibit specific similarities in AMR trends. In MSewage, ESCr exhibited the highest resistance to ampicillin (99%) and ceftiofur (93.9%), while clinical isolates from humans showed the highest resistance to ampicillin (100%), ceftiofur (92%) and oxytetracycline (84%; Table 1).
Table 1.
Trends of antimicrobial resistance prevalence in extended-spectrum cephalosporin-resistant E. coli (ESCr) and generic E. coli populations with specificity to sample origin, expressed as percentages.
| Prevalence (%) of antimicrobial resistance in extended-spectrum cephalosporin E. coli per source | Environment | OXYT | AMPI | STEP | SULF | CTIO | FLOR | AMCL | CTZD | TMSZ | NMYN |
| CFeces | 98.4 | 96.3 | 89 | 85.1 | 78.3 | 77.2 | 71.7 | 65.4 | 51 | 5 | |
| CBasins | 88.3 | 62.8 | 64.2 | 43.1 | 39.4 | 45.3 | 33.6 | 33.6 | 27 | 0.7 | |
| SStreams | 71.2 | 45.8 | 52.5 | 44.1 | 39 | 28.8 | 23.7 | 27.1 | 27.1 | 0 | |
| BProcessing | 75 | 100 | 75 | 75 | 100 | 75 | 100 | 75 | 0 | 0 | |
| MSewage | 56.1 | 99 | 49 | 43.9 | 93.9 | 9.2 | 41.8 | 43.9 | 46.9 | 5.1 | |
| CHumans | 84 | 100 | 44 | 64 | 92 | 4 | 20 | 20 | 52 | 4 | |
| Pair wise comparison of resistance prevalence for sources of extended-spectrum cephalosporin E. coli | CBasins vs. CFeces | CB < CF | CB < CF | CB < CF | CB < CF | CB < CF | CB < CF | CB < CF | CB < CF | CB < CF | nd |
| CBasins vs. HM* | CB > HM | CB < HM | CB < HM | CB > HM | CB < HM | CB < HM | |||||
| CBasins vs. SStreams | |||||||||||
| CFeces vs. HM* | CF > HM | CF > HM | CF > HM | CF < HM | CF > HM | CF > HM | CF > HM | ||||
| CFeces vs. SStreams | CF > SS | CF > SS | CF > SS | CF > SS | CF > SS | CF > SS | CF > SS | CF > SS | CF > SS | ||
| HM* vs. SStreams | HM > SS | HM > SS | HM > SS | SS > HM | HM > SS | HM > SS | HM > SS | ||||
| Prevalence (%) of antimicrobial resistance in generic E. coli per source | CFeces | 88.7 | 13.4 | 41.5 | 25.4 | 0.7 | 12 | 1.4 | 0.7 | 6.3 | 0 |
| CBasins | 72.4 | 17.3 | 29.2 | 18.4 | 1.1 | 9.7 | 1.6 | 1.1 | 2.2 | 1.1 | |
| SStreams | 44.4 | 8.6 | 22.2 | 12.3 | 0 | 8.6 | 1.2 | 0 | 7.8 | 1.2 | |
| BProcessing | 19.5 | 44 | 5.7 | 3.8 | 0 | 1.3 | 18.9 | 0 | 1.9 | 0 | |
| MSewage | 12.5 | 21.9 | 10.4 | 9.4 | 3.1 | 0 | 1 | 2.1 | 4.2 | 0 | |
| Pair wise comparison of resistance prevalence for sources of generic E. coli | CBasin vs. CFeces | CB < CF | nd | nd | nd | nd | |||||
| CBasins vs. BProcessing | CB > BP | CB < BP | CB > BP | CB > BP | CB < BP | ||||||
| CBasins vs. MSewage | CB > MS | CB > MS | |||||||||
| CBasins vs. SStreams | CB > SS | ||||||||||
| CFeces vs. BProcessing | CF > BP | CF < BP | CF > BP | CF > BP | CF > BP | CF < BP | |||||
| CFeces vs. MSewage | CF > MS | CF > MS | CF > MS | ||||||||
| CFeces vs. SStreams | CF > SS | SS < CF | |||||||||
| BProcessing vs. MSewage | BP > MS | ||||||||||
| BProcessing vs. SStreams | BP > SS | BP > SS | BP < SS | BP > SS | |||||||
| MSewage vs. SStreams | MS < SS |
In the ESCr population, E. coli resistance to individual antibiotics differ across sources (p < 0.001), whereas in the generic population, E. coli did not differ across sources (p < 0.99), although differences were observed per source*antibiotic interaction in both populations (p < 0.001). Pairwise comparisons display significant differences in antibiotic resistance between locations (0.0 ≤ p ≤ 0.03 for ESCr E. coli, while 0.0 ≤ p ≤ 0.04 for generic E. coli; Table S3). CF—cattle feces, CB—catch basins, SS—Surface streams, BP—Beef processing, MS—Municipal sewage, CH—Human clinical isolates while HM* represents the total of human clinical and municipal sewage isolates. nd represents antibiotics which were not modeled due to low prevalence of resistance and as a consequence the model did not converge.
Comparison of source among ESCr (Table 1; Table S1a–i; Figure S1) revealed that for all antimicrobials, resistance was higher in CFeces than in CBasins and SStreams isolates (p ≤ 0.002), which did not differ (p > 0.2). CFeces isolates were more (p ≤ 0.001) resistant to oxytetracycline (98.4%), streptomycin (89.0%), sulfisoxazole (85.1%), florfenicol (77.2%), amoxicillin/clavulanate (71.7%) and ceftazidime (65.4%) than HM isolates (CHumans and MSewage isolates combined), which averaged 70.1%, 46.5%, 53.9%, 6.6%, 30.9% and 31.9%, respectively. In contrast, ceftiofur resistance was higher (p = 0.01) in HM than CFeces isolates. HM isolates also showed higher (p ≤ 0.01) resistance than CBasins and SStreams to ampicillin, ceftiofur, ceftazidime and trimethoprim-sulfamethoxazole. Florfenicol resistance levels were higher in CBasins (45.3%) and SStreams (28.8%) isolates than in HM isolates (6.6%). For almost all antimicrobials, HM isolates differed in AMR prevalence from those obtained directly from cattle or surrounding environments (Table 1; Table S1a–i). Additionally, an antibiotic*source interaction (p < 0.001) was observed for the prevalence of resistance to some antimicrobials, associated specifically with isolates from CBasins, CFeces and HM. Synergistic interactions associated with slightly higher resistance to specific antimicrobials included HM*amoxicillin/clavulanate, sulfisoxazole (p ≤ 0.001), both CBasins and HM*ampicillin, ceftazidime and ceftiofur (p ≤ 0.001) and CFeces*florfenicol (p < 0.04), while isolates from all three of these sources were associated with higher resistance to trimethoprim-sulfamethoxazole (p ≤ 0.001).
Generic E. coli isolates from all environments (CFeces, MSewage, SStreams, CBasins and BProcessing) were the least resistant to neomycin (0.0% to 1.2%), followed by ceftazidime (0.0% to 2.1%). Resistance to individual antimicrobials was higher for generic isolates from CFeces and CBasins (Table 1; Figure S1). Similar resistance trends in CFeces (oxytetracycline, 88.7% > streptomycin, 41.6% > sulfisoxazole, 25.4%), but at lower frequencies were observed in CBasins (oxytetracycline, 72.4% > streptomycin, 29.2% > sulfisoxazole, 18.4%), with low resistance to ceftazidime, ceftiofur and neomycin (Table 1; Figure S1). In BProcessing, MSewage and SStreams, resistance to ampicillin (44.0%, 21.9% and 8.6%) and oxytetracycline (19.5%, 12.5% and 44.4%) was most prevalent (Table 1). Independently, the source of generic E. coli did not affect the prevalence of AMR (p = 0.998), but an antibiotic*source interaction was observed (p < 0.001). Oxytetracycline resistance in CFeces (88.7%) was higher than BProcessing (19.5%; p = 0.03) and MSewage (12.5%; <0.001) (Table 1; Figure S1).
For generic isolates, ampicillin resistance was higher in BProcessing (44.0%; p < 0.001) and MSewage (21.9%; p = 0.01) than in CBasins isolates. Resistance to amoxicillin/clavulanate in CBasins isolates was also slightly higher than in CFeces and SStreams (p ≤ 0.02), but less than BProcessing isolates (p = 0.004). Streptomycin resistance was higher in CFeces (41.5%; p ≤ 0.0013) than MSewage and CBasins isolates, while sulfisoxazole resistance was higher (p = 0.04) for CFeces than CBasins isolates. Prevalence of florfenicol resistance did not differ across most sources (p ≥ 0.09), with no resistance to this antibiotic detected in isolates from MSewage. For most antimicrobials, prevalence of resistance in generic E. coli population did not differ between CBasins, SStreams and MSewage (Table 1; Table S1j–o; Figure S1).
3.2. Distribution of Multidrug Resistance in Extended-Spectrum Cephalosporin-Resistant E. coli and Generic E. coli
Altogether, 88.8% of ESCr were MDR, with the majority resistant to six different classes of antimicrobials (Table 2). Generic E. coli isolated on antibiotic-free media exhibited MDR in 26.7% of the isolates (Table 2). Altogether, ESCr collected from MSewage (96.9%), CHumans (96%), CFeces (97.1%) and BProcessing (100%) exhibited high MDR, with the least MDR observed in isolates from SStreams (54.2%) (Table 2; Table S3 and Figure S2). With generic E. coli, prevalence of MDR was highest in CFeces (45.1%), CBasins (34.6%), SStreams (23.5%), MSewage (13.6%) and BProcessing (10.7%) (Table 2; Figure S2). Confirmed ESBL phenotypes were more prevalent in ESCr isolates from CHumans (64%), followed by MSewage (48%), CFeces (22.5%), SStreams (15.3%) and CBasins (10.9%), with no ESBL phenotypes identified within BProcessing (Table 3). The most common MDR profiles in ESCr were oxytetracycline, streptomycin and sulfisoxazole with the majority of this group exhibiting the phenotypes AMPI–CTZD–AMCL–CTIO–STEP–SULF–FLOR–OXYT–TMSZ (41.3%) and AMPI–CMCL–CTZD–CTIO–STEP–SULF–FLOR–OXYT (35.2%) (Table S2). For generic E. coli the most common MDR profiles were to OXYT, AMPI and STEP. Combined, 24.7% of the ESCr were positive for ESBL phenotype, whereas only 0.6% (n = 3 MSewage; n = 1 from BP) of the generic E. coli isolates exhibited true ESBL phenotypes (data not shown). Hence, confirmed phenotypic ESBL E. coli were not found among generic E. coli isolated from feedlot environments.
Table 2.
Distribution of multidrug resistance in extended-spectrum cephalosporin-resistant E. coli and generic E. coli population per source.
| E. coli Population | Multidrug Resistance (%) | R6 | R5 | R4 | R3 | R2 | R1 | S |
|---|---|---|---|---|---|---|---|---|
| Extended-spectrum cephalosporin-resistant E. coli (n = 705) | Overall MDR in ESCr E. coli | 45.2 | 17.9 | 2.3 | 15.6 | 7.8 | 7.4 | 3.8 |
| Cattle feces (n = 382) | 64.4 | 19.6 | 0.3 | 12.0 | 0.8 | 2.6 | 0.3 | |
| Catch basin (n = 137) | 34.3 | 6.6 | 0.0 | 19.0 | 13.1 | 19.7 | 7.3 | |
| Surface streams (n = 59) | 28.8 | 6.8 | 0.0 | 11.9 | 6.8 | 18.6 | 27.1 | |
| Municipal sewage (n = 98) | 5.1 | 29.6 | 12.2 | 22.4 | 27.6 | 3.1 | 0.0 | |
| Beef processing (n = 4) | 75.0 | 0.0 | 0.0 | 0.0 | 25.0 | 0.0 | 0.0 | |
| Human (n = 25) | 4.0 | 36.0 | 12.0 | 36.0 | 8.0 | 4.0 | 0.0 | |
| Generic E. coli (n = 663) | Overall MDR in generic E. coli | 0.6 | 2.9 | 4.4 | 9.2 | 9.7 | 37.9 | 35.4 |
| Cattle feces (n = 142) | 0.7 | 4.9 | 7.7 | 14.1 | 17.6 | 45.1 | 9.9 | |
| Catch basin (n = 185) | 1.6 | 3.8 | 4.9 | 11.9 | 12.4 | 40.5 | 24.9 | |
| Surface streams (n = 81) | 0.0 | 3.7 | 4.9 | 7.4 | 7.4 | 21.0 | 55.6 | |
| Municipal sewage (n = 96) | 0.0 | 0.0 | 5.2 | 5.2 | 3.1 | 15.6 | 70.8 | |
| Beef processing (n = 159) | 0.0 | 1.3 | 0.0 | 5.0 | 4.4 | 50.3 | 39.0 |
S—% susceptible; R—% resistance to specific number of antimicrobial classes, represented 1–6. Total of 88.8% of extended-spectrum cephalosporin-resistant isolates showed MDR phenotypes and were resistant to antimicrobials belonging to at least two different antimicrobial classes, while 26.7% of generic E. coli isolates were MDR.
Table 3.
Proportion of true ESBL phenotypes and β-lactamase among extended-spectrum cephalosporin-resistant E. coli isolates from multiple sources.
| Source | Phenotypic Confirmatory Test | β-Lactamase Genes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sources | ESBL | non-ESBL | SHV | TEM | OXA | CTXM | CTXM 1 | CTX-M 2 | CTX-M 9 | CMY |
| Human | 64.0 | 36.0 | 0.0 | 48.0 | 28.0 | 96.0 | 72.0 | 24.0 | 24.0 | 32.0 |
| Municipal sewage | 48.0 | 52.0 | 3.1 | 34.7 | 14.3 | 67.3 | 33.7 | 19.4 | 28.6 | 50.0 |
| Cattle feces | 22.5 | 77.5 | 1.8 | 15.4 | 0.0 | 25.4 | 18.6 | 5.2 | 5.8 | 87.2 |
| Catch basin | 11.7 | 88.3 | 0.0 | 19.7 | 0.0 | 11.7 | 11.7 | 0.0 | 0.0 | 91.2 |
| Surface water | 15.3 | 84.7 | 0.0 | 15.3 | 0.0 | 15.3 | 15.3 | 0.0 | 0.0 | 93.2 |
| Processing plant | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 50.0 |
Total ESBL prevalence was 24.7% in extended-spectrum cephalosporin-resistant E. coli sub-population (n = 705). ESBL phenotypic confirmation was achieved using the combination disk method such that a ≥5-mm increase in zone diameter for ceftazidime tested in combination with clavulanate vs the zone diameter of ceftazidime when tested alone equaled confirmed phenotypes.
3.3. AMR Determinants of Extended-Spectrum Cephalosporin-Resistant E. coli
Overall, 40.9% of the ESCr population possessed at least one cephalosporin-resistant β-lactamase gene, with 81.3% of isolates possessing pAmpC blaCMY. All (100%) E. coli isolates of human origin possessed ≥1 cephalosporin-resistant β-lactamase gene, followed by isolates from MSewage (80.6%), CFeces (35.3%), CBasins (27.0), SStreams (22.0%) and BProcessing (0%) (Table S3). Trends of ESBL phenotypes followed a similar pattern as we found for β-lactamase genotypes CHumans > MSewage > CFeces > CBasins > SStreams > BProcessing, but prevalence of ESBL phenotypes were generally lower than the identification of β-lactamase determinants (Table 3; Table S3), with a higher diversity of genes occurring in MSewage isolates (Table 3; Table S3). However, no apparent trends were observed between β-lactamase and pAmpC genotypes and MDR phenotypes relative to source, although the top three sources (CHumans, MSewage and CBasins) remained consistent for both MDR and β-lactamase genotypes (Table 3, Figure S2 and Table S3). Assessment of individual bla genes per source revealed that most were blaCTX-M genes. Extended-spectrum cephalosporin-resistant E. coli from CFeces harbored blaCTX-M (25.4%), followed by blaCTX-M-1 (18.6%), blaTEM (15.4%), blaCTX-M-9 (5.8%), blaCTX-M-2 (5.2%) and blaSHV (1.8%), while blaOXA was not detected. Clinical isolates tended to be higher in blaCTX-M (96%) and blaCTX-M-1 (72%), followed by blaTEM (48%) and blaOXA (28%) (Table 3). For the remaining sources, MSewage isolates also harbored blaCTX-M (67.3%), SStreams isolates blaCTX-M and blaTEM (15.3% each) and CBasins isolates blaTEM (19.7%). SStreams and CBasins isolates also exhibited the highest prevalence of blaCMY at 93.2% and 91.2% respectively, with a lower level of this determinant (32%) in CHumans isolates.
Generally, the source of isolate was found to influence MDR phenotypes in the ESCr population (p < 0.004), although the exact or relative number of genes did not influence MDR. Additionally, when the effect of the presence or absence of a single gene was examined, statistical analyses revealed that MDR prevalence was not affected by the presence of just a single gene. Examination of the effect of specific bla/pAmpC gene types also revealed that the presence of the genes (SHV, TEM, OXA, CTX-M, CTXM 1, CTX-M 2, CTX-M 9 and CMY) did not influence the prevalence of MDR.
4. Discussion
4.1. Overall and Longitudinal Antimicrobial Resistance
In this investigation, enrichment procedures were used to increase the likelihood of isolating extended-spectrum cephalosporin-resistant E. coli (ESCr) within the beef production system. This study is the first to comprehensively assess AMR ESCr and generic E. coli in beef production systems from the perspective of a One Health continuum within the same geographical region.
In the present study, oxytetracycline, ampicillin and streptomycin resistance were the highest in both ESCr and generic E. coli. The generally high levels of AMR in ESCr as opposed to generic E. coli is a potential reflection of the enrichment procedure selecting for ESCr. Genes such as blaSHV, blaTEM and blaCTX-M are often borne on mobile genetic elements that code for resistance to multiple antimicrobials [54,55,56]. Although enrichment for ESCr was expected to only isolate β-lactamase resistant E. coli, 4.1% of isolates were susceptible to ampicillin. Factors such as silencing of resistance genes in E. coli [57] and the loss of plasmids in the absence of selective pressure may explain this finding [58,59,60].
Overall, high prevalence of AMR in ESCr agrees with reports of resistance to 3rd generation cephalosporin E. coli (3GCr) isolated from the beef processing continuum [15], as well as wild birds and associated nearby water sources [61]. Schmidt et al. [15] reported AMR for ampicillin, ceftiofur, tetracycline and streptomycin (100%, 100%, 97.3% and 65.3%) in 3GCr E. coli, while our study found that 85.6%, 69.7%, 87.1%, 73.4% and 66.4% of ESCr were resistant to ampicillin, ceftiofur, oxytetracycline, streptomycin and sulfisoxazole, respectively. Similarly, in generic E. coli isolated from conventionally raised beef cattle [62] and from dairy cattle, swine, horses, sheep, goats, chickens, cats, dogs, deer, ducks, geese, human sewage and surface water, resistance to tetracycline, streptomycin and sulfisoxazole was most common [13].
Significant differences in AMR profiles associated with various isolate sources coincide with findings by Ojer-Usoz et al. [63] where they investigated ESBL E. coli from food, wastewater, rivers, farms and humans and found higher cefepime (a 4th GC) resistance in food and human isolates (94.2% and 94.6%, respectively), compared to farm isolates (2.0%). In our study, prevalence of AMR was higher in CFeces compared to CBasins, SStreams, BProcessing and HM (MSewage and CHumans combined), with the exception of ampicillin, ceftiofur and trimethoprim/sulfonamide. In North America and the United Kingdom, tetracyclines, penicillins and aminoglycosides tend to be among the major classes of antimicrobials used in beef production [7,64]. Widespread use of tetracyclines in livestock increases tetracycline-resistant E. coli in feces [62,65]. In this study, the conventional feedlots used tetracycline so it is not surprising that tetracycline resistance was the highest in E. coli isolated at the point source of utilization and declined with movement from CFeces to CBasins and surrounding water ways.
Runoff water from pens collects in CBasins, and from there it may enter the surrounding environments, resulting in the prevalence of resistance to oxytetracycline, ampicillin, and streptomycin and neomycin being similar in E. coli isolated from these environments. Considering that AMR prevalence of CFeces > CBasins > SStreams isolates, CFeces may serve as a point source from which antibiotic residues as well as AMR bacteria and genes may be disseminated. The decreasing AMR proportions in CBasins and SStreams could reflect decreasing selective pressure as a result of lower antibiotic residue concentrations in these environments as compared to CFeces [66,67]. According to Sayah et al. [13], similarities of AMR patterns in different farm sources suggest a common source of resistant bacteria, an assertion that agrees with our findings. Likewise, Ibekwe et al. [68] found that elevated levels of AMR E. coli in surface water coincided with nonpoint sources of fecal contamination from both agricultural and human sources.
ESCr from MSewage and CHumans differed in the top-three resistant phenotypes (ampicillin > ceftiofur > oxytetracycline) as compared to ESCr (oxytetracycline > ampicillin > streptomycin) isolated from cattle. Symptomatic correlations between AMR and selective antibiotic pressures are expected when both antimicrobials and bacterial isolates are derived from a common source, as is the case when human feces enter surface waters in the form of sewage effluent [69]. The similarity in AMR phenotypes in MSewage and CHumans E. coli isolates is consistent with this observation.
Generic E. coli isolated from CFeces, CBasins, SStreams and MSewage were most often resistant to oxytetracycline, streptomycin, sulfisoxazole and ampicillin. Antibiograms of CFeces isolates, which aligned with those from CBasins suggests that catch basins restricted the flow of AMR E. coli into surrounding surface waters. Generic BProcessing isolates were resistant to ampicillin, oxytetracycline and amoxicillin-clavulanic acid, likely reflecting the frequent use of penicillins and tetracyclines in beef production [13].
4.2. Resistance to Antimicrobial Agents of Clinical Relevance Relative to Source
4.2.1. Oxytetracycline
High prevalence of resistance to oxytetracycline in E. coli has been reported in several other studies related to beef cattle and the environment [13,62,66,68]. This finding is not surprising as tetracycline is a first-line antibiotic used in beef cattle to control liver abscesses and bovine respiratory disease [7,13]. Interestingly, oxytetracycline resistance in generic MSewage isolates in this study was less than in a study by Ibekwe et al. [68], where the highest tetracycline resistance was found in isolates from WWTPs (~27%), followed by urban and agricultural runoff.
4.2.2. Penicillins
Schmidt et al. (2013) and Volkova et al. (2012) reported 100% ampicillin and 64.7% amoxicillin resistance in 3GCr E. coli from CFeces, hides, pre-evisceration and final carcasses. In our study, 96.3% and 71.7% of ESCr isolates from CFeces were resistant to ampicillin and amoxicillin/clavulanic acid, respectively, while all human isolates in our study were resistant to ampicillin. A USDA report examining E. coli isolates from 1950–2002 consistently found more resistance to antimicrobials such as penicillin, which have been used for decades in clinical and veterinary medicine [70]. In another report, ≤2% of 600 isolates from agricultural sources and urban runoff were resistant to amoxicillin-clavulanic [68]. Consequently, it can be speculated that there are other prevailing conditions such as antibiotic residues at the point of isolation which may impact ampicillin and amoxicillin/clavulanic acid resistance in specific populations.
Resistance to ampicillin and amoxicillin/clavulanic acid was consistently higher in both ESCr and generic BProcessing isolates. As such, it makes sense to surmise that irrespective of sub-population, β-lactamase-resistant E. coli could potentially contaminate beef products.
4.2.3. Aminoglycoside
Streptomycin resistance was found to range between 44% (CHumans) and 89.1% (CFeces) in ESCr. Coincidentally, high streptomycin (84.2%) and low kanamycin resistance (5.3%) were reported in generic E. coli from surface water. [66]. It is interesting to note that our isolates showed neomycin resistance ranging between 0% and 5.1% for ESCr, while very few generic E. coli were neomycin resistant. In generic isolates, streptomycin resistance ranged from 5.7% in BProcessing isolates to 41.5% in CFeces. These results are consistent with the observation of very low neomycin and higher streptomycin resistance in E. coli from feedlot cattle [62]. In the current investigation, CHumans ESCr exhibited low prevalence of streptomycin and neomycin resistance, a finding consistent with those of others [71]. E. coli from CFeces and CBasins in both sub-populations showed high resistance to streptomycin even though it was not administered to cattle, suggesting possible co-selection for this resistance when other antimicrobials such as tetracyclines are administered [70,72,73].
4.2.4. Trimethoprim/Sulfonamide
Trimethoprim-sulfamethoxazole is a first-line therapy for acute urinary tract infection (UTI), one of the most common infections in outpatients [74,75]. We found sulfamethoxazole-trimethoprim resistance to be rare in both generic and ESCr, as have others [13]. Sulfamethoxazole-trimethoprim resistances between 0.0% and 2.2% and sulfisoxazole between 0.0% and 13.98% have been reported in generic E. coli from livestock, companion animals, farm environments, surface water and human septage [13], compared to 1.9% (BProcessing) to 6.3% (CFeces) in generic E. coli in our study. Since this antibiotic is mainly used in human medicine, it is unsurprising that clinical human isolates demonstrated a higher prevalence of resistance than those from the beef production system.
4.2.5. Phenicol
It has been reported that florfenicol use in livestock co-selects for chloramphenicol resistance [76,77,78]. Florfenicol is approved for use in treating BRD. Donaldson et al. [76] reported 93% florfenicol and 94% chloramphenicol resistance in 3GCr E. coli isolated from dairy cattle, while 92% of E. coli isolated at necropsy or from fecal samples of calves with diarrhea were florfenicol resistant [79]. In the present study, florfenicol resistance in ESCr was 77.4% in CFeces, while it was low ≤ 45% in isolates from other environments. Similarly, Escherichia/Shigella, Pseudomonas, Sphingobactaria, and Brevundimonas obtained from a university hospital outlet and municipal sewage inlet on sulfamethoxazole/trimethoprim and streptomycin-selective plates exhibited florfenicol resistance in ≤45% of the isolates [80]. Results from our study suggest that florfenicol resistance in E. coli was generally infrequent in human-associated sources, likely due to limited use of phenicols in humans.
4.2.6. Third-Generation Cephalosporins and ESBL Phenotype Distribution
In cattle feedlots, commensal 3GCr E. coli represent a sub-population of generic E. coli associated with hide and fecal populations [81,82]. We observed that ceftiofur resistance (93.9%) in MSewage ESCr isolates was substantially higher than in all other sources except for CHumans isolates (92%). Ceftiofur (38.9%) and ceftazidime (27.1%) resistance in SStreams ESCr isolates was comparably lower than cefotaxime-resistant (77%) ESBL E. coli isolated from rivers and lakes in Switzerland [83]. Schmidt et al. [81] and Volkova et al. [82] also reported ceftiofur and ceftriaxone resistance of 100% in 3GCr E. coli from cattle feces, hide and carcasses as compared to 66.7% of isolates from BProcessing and 65.5% from CFeces being resistant to ceftazidime in our study. It is possible that differences in the prevalence of 3GC resistance within ESCr populations are attributable to differences in isolation methods, including the specific type and concentration of antimicrobial used for enrichment. The use of enrichment broths containing cephalosporins may also enhance bacterial conjugation and exchange of resistance plasmids between bacteria [28].
The β-lactam antimicrobials including cephalosporins are routinely used for treating bacterial infections in humans so that hospitals are a significant source of cephalosporin antimicrobials in wastewater [84]. Korzeniewska et al. [84] found that the majority of generic E. coli in three different hospital sewage plants were resistant to cefotaxime and ceftazidime, a result similar to ours with MSewage ESCr isolates, although this contrasted the low resistance in our generic E. coli isolates. ESBL E. coli were readily obtained from MSewage even without enrichment with ceftriaxone. Discharge of untreated sewage containing antimicrobial residues and resistant bacteria from humans and animals may contribute to the emergence and spread of AMR bacteria in aquatic environments [80]. It is possible that antimicrobial residue levels in MSewage exert sufficient selective pressure for the development of MDR phenotypes. It was not surprising that clinical isolates obtained without enrichment showed the highest ESBL phenotype, as β-lactams are widely used in Canadian clinics.
Phenotypic ESBL isolates were not detected in BProcessing samples, an observation that agrees with others that did not detect ESBL E. coli in raw milk or minced beef, despite enrichment [85]. Furthermore, these observations suggest that there is a decrease in ESBL bacteria after defecation as one moves down the production chain [86]. The overall low levels of phenotypic ESBL E. coli reported suggests that without enrichment, negligible levels would have been detected within the beef production environment.
4.3. Prevalence of Multidrug Resistance
Of the 88.8% MDR ESCr in the current study, most (45.1%) were resistant to antimicrobials in six different drug classes. The most common MDR patterns in ESCr involved combinations of oxytetracycline, ampicillin, streptomycin, ceftiofur, florfenicol and sulfisoxazole, whereas oxytetracycline, streptomycin and sulfisoxazole were common in generic E. coli. Others have also found high levels of oxytetracycline, ampicillin and streptomycin resistance in generic E. coli isolates from feedlot cattle, farm environments, human septage and surface water [13,62]. In concordance with results from Ojer-Usoz et al. [63], MDR ESCr exceeded 60% in all sources. Ojer-Usoz et al. [63] found levels of MDR in ESBL E. coli ranging between 58% and 86.2% in food, WWTP, rivers, farms and humans. Numerous investigators note that the administration and presence of even a single antimicrobial can select for MDR strains in both humans and animals [87,88,89]. Consequently, it is likely that the high MDRs reflect long-term exposure of bacteria to specific antimicrobials [87]. Long-term tetracycline use confers resistance to other antimicrobial agents via co-selection, as tet genes and other resistance genes often share common integrons, plasmids or transposons [65,90].
4.4. AMR Determinants Associated with Extended-Spectrum Cephalosporin-Resistant E. coli
4.4.1. Occurrence of Bla Genes
Per source, bla genes from CFeces, MSewage and CHumans isolates were mostly blaCTX-M in agreement with studies conducted in Canada [27,32] and other countries [91,92]. Enterobacteriaceae possessing blaCTX-M variants have been isolated from food [93], poultry [94], companion animals [95,96], clinical settings [97,98,99] and wastewater [100,101]. Most clinical isolates in the current study possessed blaCTX-M (96%) a finding that agrees with Ojer-Usoz et al [63]. In MSewage isolates, we also detected blaCTX-M (67.3%), a result that aligns with clinical and human sewage isolates obtained by others [84,102].
The SHV- and TEM-type genes have also been detected in livestock and meat products [63,103], while OXA has been rarely reported in livestock. Brinas et al. [104] detected SHV- or OXA-type β-lactamases in only 3% of 124 ampicillin-resistant E. coli recovered from food and feces of healthy animals and humans. This is only slightly higher than the level of SHV (1.4%) we observed in ESCr. Interestingly, the OXA-type genes in our study were unique to MSewage and clinical isolates, while SHV-type were specific to CFeces and MSewage. Agga et al. [11] also reported a higher diversity of resistance genes in municipal wastewater than in livestock environments. In wastewater, the commonality of homologous regions within complex DNA elements enhances recombination [105,106], a response that may account for the high diversity of bla genes in MSewage. The complete absence of blaOXA in our isolates from the beef production environment agrees with Ojer-Usoz et al. [63], who found blaOXA-1 (20%) only in clinical E. coli isolates and not in isolates from food, farm or aquatic environments. OXA-type β-lactamases have often been reported in clinical Enterobactreacea around the world as well as from WWTP, with some variants implicated in hospital outbreaks [107,108,109].
4.4.2. Occurrence of AmpC Gene
Few investigations have assessed the frequency of blaCMY genes in E. coli isolated from food and environmental matrices as compared to isolates obtained from clinical settings. Of ESCr from cattle sources, we found blaCMY ranging from 87.2% to 93.2%, in contrast to the lower prevalence of 32% to 50% in human sources. In Canada, blaCMY has mostly been detected in clinical or hospital-associated isolates and infrequently in food-associated Enterobacteriaceae, while others have suggested that food sources may be an emerging concern [110,111,112].
5. Conclusions
Overall, AMR profiles of E. coli from the various sources reflected corresponding antimicrobial use in those segments of the continuum. A point source effect on AMR occurrence, where sources with antibiotic use reflected high AMR E. coli was further underscored by the similarity of AMR patterns between CFeces and CBasins. As such, continuing to challenge both the human medical and veterinary communities to monitor use protocols and improve antimicrobial stewardship practices in line with community, national and global AMR reduction goals. Continuous monitoring of critically important antimicrobials such as neomycin should be considered as a means of detecting early changes in resistance trends and onset of AMR emergence. E. coli isolation with enrichment enhanced the sensitivity of detecting ESBL-producing bacteria. ESBL phenotypes within ESCr were more frequently associated with human than cattle sources. In generic E. coli, MDR was lowest in BProcessing isolates, suggesting that strategies employed during beef processing reduce the risk of MDR isolates in final meat products.
Acknowledgments
We thank Patrick Boerlin for providing E. coli control isolates for various β-lactamase genes, and Ruth Barbieri and Wendi Smart for technical assistance. We also gratefully acknowledge Francis Zvomuya, Dept. of Soil Science, University of Manitoba for his assistance with data analysis.
Abbreviations
| CFeces | Cattle feces |
| CBasins | Catch basins |
| SStreams | Surrounding streams |
| BProcessing | Beef processing plants |
| MSewage | Municipal sewage treatment |
| CHumans | Humans clinical isolates |
| CLSI | Clinical and Laboratory Standards Institute |
| AMR | Antimicrobial resistance |
| MDR | Multidrug resistance |
| ESBL | Extended-spectrum beta-lactamase |
| ESCr | Extended-spectrum cephalosporin-resistant E. coli |
| 3GCr | Third generation cephalosporin resistance |
| HM | Human and Municipal Sewage isolates combined |
| WWTP | Wastewater treatment plant |
Supplementary Materials
The supplementary materials are available online at https://www.mdpi.com/2076-2607/8/6/885/s1.
Author Contributions
E.H.A.: study conceptualization, methodology, investigation, formal analysis, visualization, writing—original draft; C.N.-B.: study conceptualization, resources, supervision, writing—review; R.Z.: study conceptualization, methodology, writing—review; S.R.C.: methodology, writing—review; L.T.: methodology; writing—review; S.J.H.: investigation, methodology, writing—review, C.W.B.: investigation, methodology, resources, writing-review, D.C.: methodology, writing—review, R.R.R.: methodology, resources, writing—review; T.A.M.: conceptualization, project administration, resources, supervision, writing—review. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by funding from the Beef Cattle Research Council (BCRC)-Agriculture and Agri-Food Canada beef cluster (FOS 10 13) and the Genomics Research and Development Initiative of the Government of Canada. E.H.A. is supported by the University of Manitoba PhD Graduate Fellowship. The funding agencies had no role in study design, sample collection, analysis, interpretation, and in writing of the manuscript.
Conflicts of Interest
C.W.B. would like to declare that he is part owner and managing partner of Feedlot Health Management Services and Southern Alberta Veterinary Services. S.J.H. is an employee at Feedlot Health Management Services, Okotoks, Alberta, Canada. Feedlot Health is a private company that provides expert consultation regarding production and management of feedlot cattle and calf grower calves, including developing veterinary protocols to support animal health. They also conduct in-house and contract research related to dairy calf grower and feedlot production. All authors declare that the research was conducted without conflict of interest.
References
- 1.Jasovský D., Littmann J., Zorzet A., Cars O. Antimicrobial resistance-a threat to the world’s sustainable development. Upsala J. Med. Sci. 2016;121:159–164. doi: 10.1080/03009734.2016.1195900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Commission . A European One Health Action Plan Against Antimicrobial Resistance (AMR) European Commission; Brussels, Belgium: 2017. [Google Scholar]
- 3.Holmes A.H., Moore L.S., Sundsfjord A., Steinbakk M., Regmi S., Karkey A., Guerin P.J., Piddock L.J. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387:176–187. doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- 4.Rousham E.K., Unicomb L., Islam M.A. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: Integrating behavioural, epidemiological and One Health approaches. Proc. R. Soc. B Biol. Sci. 2018;285:20180332. doi: 10.1098/rspb.2018.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain A., Shaik S., Ranjan A., Nandanwar N., Tiwari S.K., Majid M., Baddam R., Qureshi I.A., Semmler T., Wieler L.H., et al. Risk of transmission of antimicrobial resistant Escherichia coli from commercial broiler and free-range retail chicken in India. Front. Microbiol. 2017;8:2120. doi: 10.3389/fmicb.2017.02120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Canadian Cattleman’s Association . Industry Stats. The Canadian Cattleman’s Association; Calgary, AB, Canada: 2017. [Google Scholar]
- 7.Cameron A., McAllister T.A. Antimicrobial usage and resistance in beef production. J. Anim. Sci. Biotechnol. 2016;7:68. doi: 10.1186/s40104-016-0127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanford K., Gibb D.J., Schwartzkopf-Genswein K.S., Van Herk F., McAllister T.A. Feeding subtherapeutic antimicrobials to low-risk cattle does not confer consistent performance benefits. Can. J. Anim. Sci. 2015;95:589–597. doi: 10.4141/cjas-2015-008. [DOI] [Google Scholar]
- 9.Abutarbush S.M., Schunicht O.C., Wildman B.K., Hannon S.J., Jim G.K., Ward T.I., Booker C.W. Comparison of enrofloxacin and ceftiofur sodium for the treatment of relapse of undifferentiated fever/bovine respiratory disease in feedlot cattle. Can. Vet. J. 2012;53:57–62. [PMC free article] [PubMed] [Google Scholar]
- 10.Stegner J.E., Lucas M.J., McLaughlin C.L., Davis M.S., Alaniz G.R., Weigel D.J., Szasz J.I. Comparative effects of therapeutic programs on bovine respiratory disease, performance, carcass, and profitability of high-risk feedlot heifers. Prof. Anim. Sci. 2013;29:208–218. doi: 10.15232/S1080-7446(15)30226-6. [DOI] [Google Scholar]
- 11.Agga G.E., Arthur T.M., Durso L.M., Harhay D.M., Schmidt J.W. Antimicrobial-resistant bacterial populations and antimicrobial resistance genes obtained from environments impacted by livestock and municipal waste. PLoS ONE. 2015;10:e0132586. doi: 10.1371/journal.pone.0132586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro-Gonzalez N., Porrero M.C., Mentaberre G., Serrano E., Mateos A., Domínguez L., Lavín S. Antimicrobial resistance in indicator Escherichia coli isolates from free-ranging livestock and sympatric wild ungulates in a natural environment (Northeastern Spain) Appl. Environ. Microbiol. 2013;79:6184–6186. doi: 10.1128/AEM.01745-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sayah R.S., Kaneene J.B., Johnson Y., Miller R. Patterns of antimicrobial resistance observed in Escherichia coli isolates obtained from domestic- and wild-animal fecal samples, human septage, and surface water. Appl. Environ. Microbiol. 2005;71:1394–1404. doi: 10.1128/AEM.71.3.1394-1404.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima A., Ishii Y., Ishihara K., Esaki H., Asai T., Oda C., Tamura Y., Takahashi T., Yamaguchi K. Extended-spectrum-β-lactamase-producing Escherichia coli strains isolated from farm animals from 1999 to 2002: Report from the Japanese Veterinary Antimicrobial Resistance Monitoring Program. Antimicrob. Agents Chemother. 2005;49:3533–3537. doi: 10.1128/AAC.49.8.3533-3537.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt J.W., Agga G.E., Bosilevac J.M., Brichta-Harhay D.M., Shackelford S.D., Wang R., Wheeler T.L., Arthur T.M. Occurrence of antimicrobial-resistant Escherichia coli and Salmonella enterica in the beef cattle production and processing continuum. Appl. Environ. Microbiol. 2015;81:713–725. doi: 10.1128/AEM.03079-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslam M., Diarra M.S., Service C., Rempel H. Antimicrobial resistance genes in Escherichia coli isolates recovered from a commercial beef processing plant. J. Food Prot. 2009;72:1089–1093. doi: 10.4315/0362-028X-72.5.1089. [DOI] [PubMed] [Google Scholar]
- 17.Sheikh A.A., Checkley S., Avery B., Chalmers G., Bohaychuk V., Boerlin P., Reid-Smith R., Aslam M. Antimicrobial resistance and resistance genes in Escherichia coli isolated from retail meat purchased in Alberta, Canada. Foodborne Pathog. Dis. 2012;9:625–631. doi: 10.1089/fpd.2011.1078. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organisation . Report of the 6th Meeting of the WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance with AGISAR 5-year Strategic Framework to Support Implementation of the Global Action Plan on Antimicrobial Resistance (2015–2019), Seoul, Korea, 10–12 June 2015. World Health Organisation; Geneva, Switzerland: 2015. pp. 25–30. [Google Scholar]
- 19.Korzeniewska E., Harnisz M. Extended-spectrum beta-lactamase (ESBL)-positive Enterobacteriaceae in municipal sewage and their emission to the environment. J. Environ. Manag. 2013;128:904–911. doi: 10.1016/j.jenvman.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 20.Bhoomika, Shakya S., Patyal A., Gade N.E. Occurrence and characteristics of extended-spectrum β-lactamases producing Escherichia coli in foods of animal origin and human clinical samples in Chhattisgarh, India. Vet. World. 2016;9:996–1000. doi: 10.14202/vetworld.2016.996-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebrahimi F., Mózes J., Monostori J., Gorácz O., Fésűs A., Majoros L., Szarka K., Kardos G. Comparison of rates of fecal colonization with extended-spectrum beta-lactamase-producing enterobacteria among patients in different wards, outpatients and medical students. Microbiol. Immunol. 2016;60:285–294. doi: 10.1111/1348-0421.12373. [DOI] [PubMed] [Google Scholar]
- 22.Shaikh S., Fatima J., Shakil S., Rizvi S.M., Kamal M.A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci. 2015;22:90–101. doi: 10.1016/j.sjbs.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkey P.M., Warren R.E., Livermore D.M., McNulty C.A.M., Enoch D.A., Otter J.A., Wilson A.P.R. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: Report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J. Antimicrob. Chemother. 2018;73:iii2–iii78. doi: 10.1093/jac/dky027. [DOI] [PubMed] [Google Scholar]
- 24.Heavner M.S., Claeys K.C., Masich A.M., Gonzales J.P. Pharmacokinetic and pharmacodynamic considerations of antibiotics of last resort in treating gram-negative infections in adult critically ill patients. Curr. Infect. Dis. Rep. 2018;20:10. doi: 10.1007/s11908-018-0614-0. [DOI] [PubMed] [Google Scholar]
- 25.Sherry N., Howden B. Emerging Gram negative resistance to last-line antimicrobial agents fosfomycin, colistin and ceftazidime-avibactam—epidemiology, laboratory detection and treatment implications. Expert Rev. Anti-Infect. Ther. 2018;16:289–306. doi: 10.1080/14787210.2018.1453807. [DOI] [PubMed] [Google Scholar]
- 26.WHO . Critically Important Antimicrobials for Human Medicine. 5th ed. World Health Organisation; Geneva, Switzerland: 2017. [Google Scholar]
- 27.Cormier A.C., Chalmers G., Cook S.R., Zaheer R., Hannon S.J., Booker C.W., Read R.R., Gow S.P., McAllister T.A., Boerlin P. Presence and diversity of extended-spectrum cephalosporin resistance among Escherichia coli from urban wastewater and feedlot cattle in Alberta, Canada. Microb. Drug Resist. 2020;26:300–309. doi: 10.1089/mdr.2019.0112. [DOI] [PubMed] [Google Scholar]
- 28.EFSA. European Food Safety Authority. Technical specifications on harmonised monitoring and reporting of antimicrobial resistance in Salmonella, Campylobacter, and indicator Escherichia coli and Enterococcus spp. bactria transmitted through food. EFSA J. 2012;10:2742. [Google Scholar]
- 29.Horton R.A., Randall L.P., Snary E.L., Cockrem H., Lotz S., Wearing H., Duncan D., Rabie A., McLaren I., Watson E., et al. Fecal carriage and shedding density of CTX-M extended-spectrum β-lactamase-producing Escherichia coli in cattle, chickens, and pigs: Implications for environmental contamination and food production. Appl. Environ. Microbiol. 2011;77:3715–3719. doi: 10.1128/AEM.02831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmid A., Hörmansdorfer S., Messelhäusser U., Käsbohrer A., Sauter-Louis C., Mansfeld R. Prevalence of extended-spectrum β-lactamase-producing Escherichia coli on Bavarian dairy and beef cattle farms. Appl. Environ. Microbiol. 2013;79:3027–3032. doi: 10.1128/AEM.00204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis M.A., Sischo W.M., Jones L.P., Moore D.A., Ahmed S., Short D.M., Besser T.E. Recent emergence of Escherichia coli with cephalosporin resistance conferred by blaCTX-M on washington state dairy farms. Appl. Environ. Microbiol. 2015;81:4403–4410. doi: 10.1128/AEM.00463-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cormier A.C., Chalmers G., McAllister T.A., Cook S., Zaheer R., Scott H.M., Booker C., Read R., Boerlin P. Extended-spectrum-cephalosporin resistance genes in Escherichia coli from beef cattle. Antimicrob. Agents Chemother. 2016;60:1162–1163. doi: 10.1128/AAC.02516-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beukers A.G., Zaheer R., Cook S.R., Chaves A.V., Ward M.P., Tymensen L., Morley P.S., Hannon S., Booker C.W., Read R.R., et al. Comparison of antimicrobial resistance genes in feedlots and urban wastewater. Can. J. Vet. Res. 2018;82:24–38. [PMC free article] [PubMed] [Google Scholar]
- 34.Tymensen L., Booker C.W., Hannon S.J., Cook S.R., Zaheer R., Read R., McAllister T.A. Environmental growth of Enterococci and Escherichia coli in feedlot catch basins and a constructed wetland in the absence of fecal input. Environ. Sci. Technol. 2017;51:5386–5395. doi: 10.1021/acs.est.6b06274. [DOI] [PubMed] [Google Scholar]
- 35.Canadian Council on Animal Care . CCAC Guidelines on: The Care and Use of Farm Animals in Research, Teaching and Testing. CCAC; Ottawa, ON, Canada: 2009. [Google Scholar]
- 36.Gill C.O., Jones T. Microbiological sampling of carcasses by excision or swabbing. J. Food Prot. 2000;63:167–173. doi: 10.4315/0362-028X-63.2.167. [DOI] [PubMed] [Google Scholar]
- 37.Aslam M., Service C. Antimicrobial resistance and genetic profiling of Escherichia coli from a commercial beef packing plant. J. Food Prot. 2006;69:1508–1513. doi: 10.4315/0362-028X-69.7.1508. [DOI] [PubMed] [Google Scholar]
- 38.Pitout J.D., Hanson N.D., Church D.L., Laupland K.B. Population-based laboratory surveillance for Escherichia coli-producing extended-spectrum beta-lactamases: Importance of community isolates with blaCTX-M genes. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2004;38:1736–1741. doi: 10.1086/421094. [DOI] [PubMed] [Google Scholar]
- 39.Pitout J.D., Gregson D.B., Church D.L., Laupland K.B. Population-based laboratory surveillance for AmpC beta-lactamase-producing Escherichia coli, Calgary. Emerg. Infect. Dis. 2007;13:443–448. doi: 10.3201/eid1303.060447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adator E.H., Walker M., Narvaez-Bravo C., Zaheer R., Goji N., Cook S.R., Tymensen L., Hannon S.J., Church D., Booker C.W., et al. Whole genome sequencing differentiates presumptive extended spectrum beta-Lactamase producing Escherichia coli along segments of the One Health continuum. Microorganisms. 2020;8:448. doi: 10.3390/microorganisms8030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tymensen L., Booker C.W., Hannon S.J., Cook S.R., Jokinen C.C., Zaheer R., Read R., Boerlin P., McAllister T.A. Plasmid distribution among Escherichia coli from livestock and associated wastewater: Unraveling factors that shape the presence of genes conferring Third-Generation Cephalosporin resistance. Environ. Sci. Technol. 2019;53:11666–11674. doi: 10.1021/acs.est.9b03486. [DOI] [PubMed] [Google Scholar]
- 42.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. Volume 36 Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2016. Twenty-Fifth Informational Supplement; CLSI document M100S-26. [Google Scholar]
- 43.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. 3rd ed. Volume 35 Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2016. CLSI document VET01S. [Google Scholar]
- 44.Feßler A.T., Turnidge J., Schwarz S. Quality control ranges for cefoperazone 30 μg disks for Staphylococcus aureus ATCC® 25923 and Escherichia coli ATCC® 25922. Vet. Microbiol. 2014;171:284–289. doi: 10.1016/j.vetmic.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 45.Shrestha A., Bajracharya A.M., Subedi H., Turha R.S., Kafle S., Sharma S., Neupane S., Chaudhary D.K. Multi-drug resistance and extended spectrum beta lactamase producing Gram negative bacteria from chicken meat in Bharatpur Metropolitan, Nepal. BMC Res. Notes. 2017;10:574. doi: 10.1186/s13104-017-2917-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang H., Ataker F., Hedin G., Dornbusch K. Molecular epidemiology of extended-spectrum beta-lactamases among Escherichia coli isolates collected in a Swedish hospital and its associated health care facilities from 2001 to 2006. J. Clin. Microbiol. 2008;46:707–712. doi: 10.1128/JCM.01943-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dallenne C., Da Costa A., Decré D., Favier C., Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010;65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 48.Kozak G.K., Boerlin P., Janecko N., Reid-Smith R.J., Jardine C. Antimicrobial resistance in Escherichia coli isolates from swine and wild small mammals in the proximity of swine farms and in natural environments in Ontario, Canada. Appl. Environ. Microbiol. 2009;75:559–566. doi: 10.1128/AEM.01821-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaheer R., Cook S.R., Barbieri R., Goji N., Cameron A., Petkau A., Polo R.O., Tymensen L., Stamm C., Song J., et al. Surveillance of Enterococcus spp. reveals distinct species and antimicrobial resistance diversity across a One-Health continuum. Sci. Rep. 2020;10:3937. doi: 10.1038/s41598-020-61002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carvalho I., Campo R.D., Sousa M., Silva N., Carrola J., Marinho C., Santos T., Carvalho S., Nóvoa M., Quaresma M., et al. Antimicrobial-resistant Escherichia coli and Enterococcus spp. isolated from Miranda donkey (Equus asinus): An old problem from a new source with a different approach. J. Med. Microbiol. 2017;66:191–202. doi: 10.1099/jmm.0.000423. [DOI] [PubMed] [Google Scholar]
- 51.Österberg J., Wingstrand A., Nygaard Jensen A., Kerouanton A., Cibin V., Barco L., Denis M., Aabo S., Bengtsson B. Antibiotic resistance in Escherichia coli from pigs in organic and conventional farming in four European countries. PLoS ONE. 2016;11:e0157049. doi: 10.1371/journal.pone.0157049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanwar N., Scott H.M., Norby B., Loneragan G.H., Vinasco J., McGowan M., Cottell J.L., Chengappa M.M., Bai J., Boerlin P. Effects of ceftiofur and chlortetracycline treatment strategies on antimicrobial susceptibility and on tet(A), tet(B), and bla CMY-2 resistance genes among E. coli isolated from the feces of feedlot cattle. PLoS ONE. 2013;8:e80575. doi: 10.1371/journal.pone.0080575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ashayeri-Panah M., Feizabadi M.M., Eftekhar F. Correlation of multi-drug resistance, integron and blaESBL gene carriage with genetic fingerprints of extended-spectrum β-lactamase producing Klebsiella pneumoniae. Jundishapur J. Microbiol. 2014;7:e8747. doi: 10.5812/jjm.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poirel L., Kieffer N., Liassine N., Thanh D., Nordmann P. Plasmid-mediated carbapenem and colistin resistance in a clinical isolate of Escherichia coli. Lancet Infect. Dis. 2016;16:281. doi: 10.1016/S1473-3099(16)00006-2. [DOI] [PubMed] [Google Scholar]
- 55.Shrivastav A., Sharma R.K., Sahni Y.P., Shrivastav N., Gautam V., Jain S. Study of antimicrobial resistance due to extended spectrum beta-lactamase-producing. Vet. World. 2016;9:1259–1263. doi: 10.14202/vetworld.2016.1259-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falgenhauer L., Waezsada S.E., Yao Y., Imirzalioglu C., Käsbohrer A., Roesler U., Michael G.B., Schwarz S., Werner G., Kreienbrock L., et al. Colistin resistance gene mcr-1 in extended-spectrum β-lactamase-producing and carbapenemase-producing Gram-negative bacteria in Germany. Lancet Infect. Dis. 2016;16:282–283. doi: 10.1016/S1473-3099(16)00009-8. [DOI] [PubMed] [Google Scholar]
- 57.Enne V.I., Delsol A.A., Roe J.M., Bennett P.M. Evidence of antibiotic resistance gene silencing in Escherichia coli. Antimicrob. Agents Chemother. 2006;50:3003–3010. doi: 10.1128/AAC.00137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raum E., Lietzau S., Von Baum H., Marre R., Brenner H. Changes in Escherichia coli resistance patterns during and after antibiotic therapy: A longitudinal study among outpatients in Germany. Clin. Microbiol. Infect. 2008;14:41–48. doi: 10.1111/j.1469-0691.2007.01841.x. [DOI] [PubMed] [Google Scholar]
- 59.Sundqvist M. Reversibility of antibiotic resistance. Upsala J. Med. Sci. 2014;119:142–148. doi: 10.3109/03009734.2014.903323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subbiah M., Top E.M., Shah D.H., Call D.R. Selection pressure required for long-term persistence of blaCMY-2-positive IncA/C plasmids. Appl. Environ. Microbiol. 2011;77:4486–4493. doi: 10.1128/AEM.02788-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rashid M., Rakib M.M., Hasan B. Antimicrobial-resistant and ESBL-producing Escherichia coli in different ecological niches in Bangladesh. Infect. Ecol. Epidemiol. 2015;5:26712. doi: 10.3402/iee.v5.26712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benedict K.M., Gow S.P., McAllister T.A., Booker C.W., Hannon S.J., Checkley S.L., Noyes N.R., Morley P.S. Antimicrobial resistance in Escherichia coli recovered from feedlot cattle and associations with antimicrobial use. PLoS ONE. 2015;10:e0143995. doi: 10.1371/journal.pone.0143995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ojer-Usoz E., González D., Vitas A.I. Clonal diversity of ESBL-Producing Escherichia coli isolated from environmental, human and food samples. Int. J. Environ. Res. Public Health. 2017;14:676. doi: 10.3390/ijerph14070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.UK-VARSS . UK Veterinary Antibiotic Resistance and Sales Surveillance Report. 2018. Veterinary Medicines Directorate; New Haw, UK: 2019. [Google Scholar]
- 65.Shin S.W., Shin M.K., Jung M., Belaynehe K.M., Yoo H.S. Prevalence of antimicrobial resistance and transfer of tetracycline resistance genes in Escherichia coli isolates from beef cattle. Appl. Environ. Microbiol. 2015;81:5560–5566. doi: 10.1128/AEM.01511-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vital P.G., Zara E.S., Paraoan C.E.M., Dimasupil M.A.Z., Abello J.J.M., Santos I.T.G., Rivera W.L. Antibiotic resistance and extended-spectrum beta-lactamase production of Escherichia coli isolated from irrigation waters in selected urban farms in Metro Manila, Philippines. Water. 2018;10:548. doi: 10.3390/w10050548. [DOI] [Google Scholar]
- 67.Díaz-Cruz M.S., De Alda M.J.L., Barcelo D. Environmental behavior and analysis of veterinary and human drugs in soils, sediments and sludge. TrAC Trends Anal. Chem. 2003;22:340–351. doi: 10.1016/S0165-9936(03)00603-4. [DOI] [Google Scholar]
- 68.Ibekwe A.M., Murinda S.E., Graves A.K. Genetic diversity and antimicrobial resistance of Escherichia coli from human and animal sources uncovers multiple resistances from human sources. PLoS ONE. 2011;6:e20819. doi: 10.1371/journal.pone.0020819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jury K.L., Vancov T., Stuetz R.M., Khan S.J. Antibiotic resistance dissemination and sewage treatment plants. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010;2:509–510. [Google Scholar]
- 70.Tadesse D.A., Zhao S., Tong E., Ayers S., Singh A., Bartholomew M.J., McDermott P.F. Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950–2002. Emerg. Infect. Dis. 2012;18:741–749. doi: 10.3201/eid1805.111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Literak I., Petro R., Dolejska M., Gruberova E., Dobiasova H., Petr J., Cizek A. Antimicrobial resistance in fecal Escherichia coli isolates from healthy urban children of two age groups in relation to their antibiotic therapy. Antimicrob. Agents Chemother. 2011;55:3005–3007. doi: 10.1128/AAC.01724-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chudobova D., Dostalova S., Blazkova I., Michalek P., Ruttkay-Nedecky B., Sklenar M., Nejdl L., Kudr J., Gumulec J., Tmejova K., et al. Effect of ampicillin, streptomycin, penicillin and tetracycline on metal resistant and non-resistant Staphylococcus aureus. Int. J. Environ. Res. Public Health. 2014;11:3233–3255. doi: 10.3390/ijerph110303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chopra I., Roberts M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001;65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taur Y., Smith M.A. Adherence to the Infectious Diseases Society of America guidelines in the treatment of uncomplicated urinary tract infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007;44:769–774. doi: 10.1086/511866. [DOI] [PubMed] [Google Scholar]
- 75.Nordstrom L., Liu C.M., Price L.B. Foodborne urinary tract infections: A new paradigm for antimicrobial-resistant foodborne illness. Front. Microbiol. 2013;4:29. doi: 10.3389/fmicb.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Donaldson S.C., Straley B.A., Hegde N.V., Sawant A.A., DebRoy C., Jayarao B.M. Molecular epidemiology of ceftiofur-resistant Escherichia coli isolates from dairy calves. Appl. Environ. Microbiol. 2006;72:3940–3948. doi: 10.1128/AEM.02770-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nhung N.T., Cuong N.V., Campbell J., Hoa N.T., Bryant J.E., Truc V.N., Kiet B.T., Jombart T., Trung N.V., Hien V.B., et al. High levels of antimicrobial resistance among Escherichia coli isolates from livestock farms and synanthropic rats and shrews in the Mekong Delta of Vietnam. Appl. Environ. Microbiol. 2015;81:812–820. doi: 10.1128/AEM.03366-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao S., Blickenstaff K., Bodeis-Jones S., Gaines S.A., Tong E., McDermott P.F. Comparison of the prevalences and antimicrobial resistances of Escherichia coli isolates from different retail meats in the United States, 2002 to 2008. Appl. Environ. Microbiol. 2012;78:1701–1707. doi: 10.1128/AEM.07522-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.White D.G., Hudson C., Maurer J.J., Ayers S., Zhao S., Lee M.D., Bolton L., Foley T., Sherwood J. Characterization of chloramphenicol and florfenicol resistance in Escherichia coli associated with bovine diarrhea. J. Clin. Microbiol. 2000;38:4593–4598. doi: 10.1128/JCM.38.12.4593-4598.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Czekalski N., Berthold T., Caucci S., Egli A., Bürgmann H. Increased levels of multiresistant bacteria and resistance genes after wastewater treatment and their dissemination into lake geneva, Switzerland. Front. Microbiol. 2012;3:106. doi: 10.3389/fmicb.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmidt J.W., Griffin D., Kuehn L.A., Brichta-Harhay D.M. Influence of therapeutic ceftiofur treatments of feedlot cattle on fecal and hide prevalences of commensal Escherichia coli resistant to expanded-spectrum cephalosporins, and molecular characterization of resistant isolates. Appl. Environ. Microbiol. 2013;79:2273–2283. doi: 10.1128/AEM.03592-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Volkova V.V., Lanzas C., Lu Z., Gröhn Y.T. Mathematical model of plasmid-mediated resistance to ceftiofur in commensal enteric Escherichia coli of cattle. PLoS ONE. 2012;7:e36738. doi: 10.1371/journal.pone.0036738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zurfluh K., Hächler H., Nüesch-Inderbinen M., Stephan R. Characteristics of extended-spectrum β-lactamase- and carbapenemase-producing Enterobacteriaceae Isolates from rivers and lakes in Switzerland. Appl. Environ. Microbiol. 2013;79:3021–3026. doi: 10.1128/AEM.00054-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Korzeniewska E., Korzeniewska A., Harnisz M. Antibiotic resistant Escherichia coli in hospital and municipal sewage and their emission to the environment. Ecotoxicol. Environ. Saf. 2013;91:96–102. doi: 10.1016/j.ecoenv.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 85.Geser N., Stephan R., Hächler H. Occurrence and characteristics of extended-spectrum β-lactamase (ESBL) producing Enterobacteriaceae in food producing animals, minced meat and raw milk. BMC Vet. Res. 2012;8:21. doi: 10.1186/1746-6148-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blaak H., Van Hoek A.H., Hamidjaja R.A., Van der Plaats R.Q., Kerkhof-de Heer L., De Roda Husman A.M., Schets F.M. Distribution, numbers, and diversity of ESBL-producing E. coli in the poultry farm environment. PLoS ONE. 2015;10:e0135402. doi: 10.1371/journal.pone.0135402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li D., Yu T., Zhang Y., Yang M., Li Z., Liu M., Qi R. Antibiotic resistance characteristics of environmental bacteria from an oxytetracycline production wastewater treatment plant and the receiving river. Appl. Environ. Microbiol. 2010;76:3444–3451. doi: 10.1128/AEM.02964-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levy S.B., FitzGerald G.B., Macone A.B. Changes in intestinal flora of farm personnel after introduction of a tetracycline-supplemented feed on a farm. N. Engl. J. Med. 1976;295:583–588. doi: 10.1056/NEJM197609092951103. [DOI] [PubMed] [Google Scholar]
- 89.Shea K.M. Antibiotic resistance: What is the impact of agricultural uses of antibiotics on children’s health? Pediatrics. 2003;112:253–258. [PubMed] [Google Scholar]
- 90.Belaynehe K.M., Shin S.W., Yoo H.S. Interrelationship between tetracycline resistance determinants, phylogenetic group affiliation and carriage of class 1 integrons in commensal Escherichia coli isolates from cattle farms. BMC Vet. Res. 2018;14:340. doi: 10.1186/s12917-018-1661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramadan A.A., Abdelaziz N.A., Amin M.A., Aziz R.K. Novel blaCTX-M variants and genotype-phenotype correlations among clinical isolates of extended spectrum beta lactamase-producing Escherichia coli. Sci. Rep. 2019;9:4224. doi: 10.1038/s41598-019-39730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gay E., Bour M., Cazeau G., Jarrige N., Martineau C., Madec J.Y., Haenni M. Antimicrobial usages and antimicrobial resistance in commensal Escherichia coli from veal calves in France: Evolution during the fattening process. Front. Microbiol. 2019;10:792. doi: 10.3389/fmicb.2019.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cantón R., González-Alba J.M., Galán J.C. CTX-M Enzymes: Origin and Diffusion. Front. Microbiol. 2012;3:110. doi: 10.3389/fmicb.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dierikx C.M., Van der Goot J.A., Smith H.E., Kant A., Mevius D.J. Presence of ESBL/AmpC-producing Escherichia coli in the broiler production pyramid: A descriptive study. PLoS ONE. 2013;8:e79005. doi: 10.1371/journal.pone.0079005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O’Keefe A., Hutton T.A., Schifferli D.M., Rankin S.C. First detection of CTX-M and SHV extended-spectrum beta-lactamases in Escherichia coli urinary tract isolates from dogs and cats in the United States. Antimicrob. Agents Chemother. 2010;54:3489–3492. doi: 10.1128/AAC.01701-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dierikx C.M., Van Duijkeren E., Schoormans A.H., Van Essen-Zandbergen A., Veldman K., Kant A., Huijsdens X.W., Van der Zwaluw K., Wagenaar J.A., Mevius D.J. Occurrence and characteristics of extended-spectrum-β-lactamase- and AmpC-producing clinical isolates derived from companion animals and horses. J. Antimicrob. Chemother. 2012;67:1368–1374. doi: 10.1093/jac/dks049. [DOI] [PubMed] [Google Scholar]
- 97.Alves M., Lemire A., Decré D., Margetis D., Bigé N., Pichereau C., Ait-Oufella H., Baudel J.L., Offenstadt G., Guidet B., et al. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in the intensive care unit: Acquisition does not mean cross-transmission. BMC Infect. Dis. 2016;16:147. doi: 10.1186/s12879-016-1489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arhoune B., Oumokhtar B., Hmami F., Barguigua A., Timinouni M., El Fakir S., Chami F., Bouharrou A. Rectal carriage of extended-spectrum β-lactamase- and carbapenemase-producing Enterobacteriaceae among hospitalised neonates in a neonatal intensive care unit in Fez, Morocco. J. Glob. Antimicrob. Resist. 2017;8:90–96. doi: 10.1016/j.jgar.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 99.Ewers C., Bethe A., Stamm I., Grobbel M., Kopp P.A., Guerra B., Stubbe M., Doi Y., Zong Z., Kola A., et al. CTX-M-15-D-ST648 Escherichia coli from companion animals and horses: Another pandemic clone combining multiresistance and extraintestinal virulence? J. Antimicrob. Chemother. 2014;69:1224–1230. doi: 10.1093/jac/dkt516. [DOI] [PubMed] [Google Scholar]
- 100.Reinthaler F.F., Posch J., Feierl G., Wüst G., Haas D., Ruckenbauer G., Mascher F., Marth E. Antibiotic resistance of E. coli in sewage and sludge. Water Res. 2003;37:1685–1690. doi: 10.1016/S0043-1354(02)00569-9. [DOI] [PubMed] [Google Scholar]
- 101.Franz E., Veenman C., Van Hoek A.H., De Roda Husman A., Blaak H. Pathogenic Escherichia coli producing extended-spectrum β-Lactamases isolated from surface water and wastewater. Sci. Rep. 2015;5:14372. doi: 10.1038/srep14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reinthaler F.F., Feierl G., Galler H., Haas D., Leitner E., Mascher F., Melkes A., Posch J., Winter I., Zarfel G., et al. ESBL-producing E. coli in Austrian sewage sludge. Water Res. 2010;44:1981–1985. doi: 10.1016/j.watres.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 103.Mir R.A., Weppelmann T.A., Johnson J.A., Archer D., Morris J.G., Jeong K.C. Identification and characterization of cefotaxime resistant bacteria in beef cattle. PLoS ONE. 2016;11:e0163279. doi: 10.1371/journal.pone.0163279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Briñas L., Zarazaga M., Sáenz Y., Ruiz-Larrea F., Torres C. Beta-lactamases in ampicillin-resistant Escherichia coli isolates from foods, humans, and healthy animals. Antimicrob. Agents Chemother. 2002;46:3156–3163. doi: 10.1128/AAC.46.10.3156-3163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stokes H.W., Gillings M.R. Gene flow, mobile genetic elements and the recruitment of antibiotic resistance genes into Gram-negative pathogens. FEMS Microbiol. Rev. 2011;35:790–819. doi: 10.1111/j.1574-6976.2011.00273.x. [DOI] [PubMed] [Google Scholar]
- 106.Lood R., Ertürk G., Mattiasson B. Revisiting antibiotic resistance spreading in wastewater treatment plants—Bacteriophages as a much neglected potential transmission vehicle. Front. Microbiol. 2017;8:2298. doi: 10.3389/fmicb.2017.02298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mataseje L.F., Boyd D.A., Hoang L., Imperial M., Lefebvre B., Miller M., Poutanen S.M., Roscoe D., Willey B.M., Mulvey M.R. Carbapenem-hydrolyzing oxacillinase-48 and oxacillinase-181 in Canada, 2011. Emerg. Infect. Dis. 2013;19:157–160. doi: 10.3201/eid1901.120706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mathers A.J., Hazen K.C., Carroll J., Yeh A.J., Cox H.L., Bonomo R.A., Sifri C.D. First clinical cases of OXA-48-producing carbapenem-resistant Klebsiella pneumoniae in the United States: The "menace" arrives in the new world. J. Clin. Microbiol. 2013;51:680–683. doi: 10.1128/JCM.02580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thomas C.P., Moore L.S., Elamin N., Doumith M., Zhang J., Maharjan S., Warner M., Perry C., Turton J.F., Johnstone C., et al. Early (2008–2010) hospital outbreak of Klebsiella pneumoniae producing OXA-48 carbapenemase in the UK. Int. J. Antimicrob. Agents. 2013;42:531–536. doi: 10.1016/j.ijantimicag.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 110.Allen K.J., Poppe C. Occurrence and characterization of resistance to extended-spectrum cephalosporins mediated by beta-lactamase CMY-2 in Salmonella isolated from food-producing animals in Canada. Can. J. Vet. Res. 2002;66:137–144. [PMC free article] [PubMed] [Google Scholar]
- 111.Baudry P.J., Mataseje L., Zhanel G.G., Hoban D.J., Mulvey M.R. Characterization of plasmids encoding CMY-2 AmpC beta-lactamases from Escherichia coli in Canadian intensive care units. Diagn. Microbiol. Infect. Dis. 2009;65:379–383. doi: 10.1016/j.diagmicrobio.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 112.Mataseje L.F., Baudry P.J., Zhanel G.G., Morck D.W., Read R.R., Louie M., Mulvey M.R. Comparison of CMY-2 plasmids isolated from human, animal, and environmental Escherichia coli and Salmonella spp. from Canada. Diagn. Microbiol. Infect. Dis. 2010;67:387–391. doi: 10.1016/j.diagmicrobio.2010.02.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.