Abstract
There are now multiple targeted and immunotherapies available for the treatment of metastatic melanoma. Although these agents have dramatically improved the survival of patients, the appropriate sequencing and the safety during the transition between these drugs remains unknown. Recently two cases of cytokine release syndrome (CRS) following transition from immune‐checkpoint inhibitors to BRAF and MEK inhibitors (BRAFi/MEKi) in patients with metastatic melanoma have been reported. CRS is a systemic cytokine‐driven inflammatory reaction, previously well reported in chimeric antigen receptor T‐cell therapies for hematologic malignancies. Here, we report a third case in which severe CRS resistant to glucocorticoid therapy following transition to a MEKi/BRAFi was treated successfully with tocilizumab, an interleukin‐6 (IL‐6) inhibitor. CRS should be on the differential diagnosis of immune‐related adverse events of immunotherapies or targeted cancer therapies for metastatic melanoma, and clinicians in multiple disciplines should be aware of this rare complication and the potential benefits of IL‐6 blockade.
Short abstract
This brief report describes the unique case of a patient with metastatic melanoma who was transitioned from immune‐checkpoint to MEK/BRAF inhibitors and developed cytokine release syndrome, which did not completely respond to 5 days of high‐dose IV steroids, and for whom IV tocilizumab was used with excellent results. In this era of evolving knowledge of immune complications from targeted and immunotherapies, this report provides hopeful results.
Introduction
Targeted and immunotherapies have transformed the landscape in the adjuvant and advanced melanoma treatment settings 1. How these drugs should be optimally sequenced and the potential toxicities during the transition remain unknown 2. Recently, two cases of cytokine release syndrome (CRS) were reported in patients who were treated with immune‐checkpoint inhibitors followed by BRAF and MEK inhibitors, one of whom did not respond to high doses of glucocorticoids and required a single dose of IV tocilizumab 3. We present a third case of CRS following a transition from immune‐checkpoint inhibitor therapy to treatment with BRAF/MEK inhibitors (BRAFi/MEKi) successfully treated with tocilizumab.
Case Presentation
A 58‐year‐old man with a history of metastatic melanoma to his lungs presented to hospital in distributive shock. He had been diagnosed with metastatic melanoma 18 months prior to his presentation. Initial treatment included Interferon for induction followed by maintenance (15 months prior to current presentation, total duration 6 months). He developed a left axillary recurrence that was completely excised and then he was treated with nivolumab (8 months prior to current presentation, duration 6 months). Subsequently, he developed lung metastases, and as such, he was transitioned to trametinib (MEKi) and dabrafenib (BRAFi) (2 months prior to his presentation, duration 1.5 months). He had difficulty with dabrafenib and trametinib, developing fever, arthralgia, thrombocytopenia, hand and foot syndrome, transaminitis, and an increased creatine kinase. He recovered with discontinuation of therapy and supportive care. Eleven days prior to his presentation, he was started on cobimetinib (MEKi) and vemurafenib (BRAFi). Five days after initiation of this treatment he began to develop fatigue, malaise, and chills, and he discontinued therapy. Over the next 24 hours, he developed severe nausea, vomiting, diarrhea, fever of 39°C, and a purpuric eruption on his lower extremities, which progressed to involve his buttocks, trunk, arms, hands, nose, and left eyelid (Fig. 1A).
Figure 1.
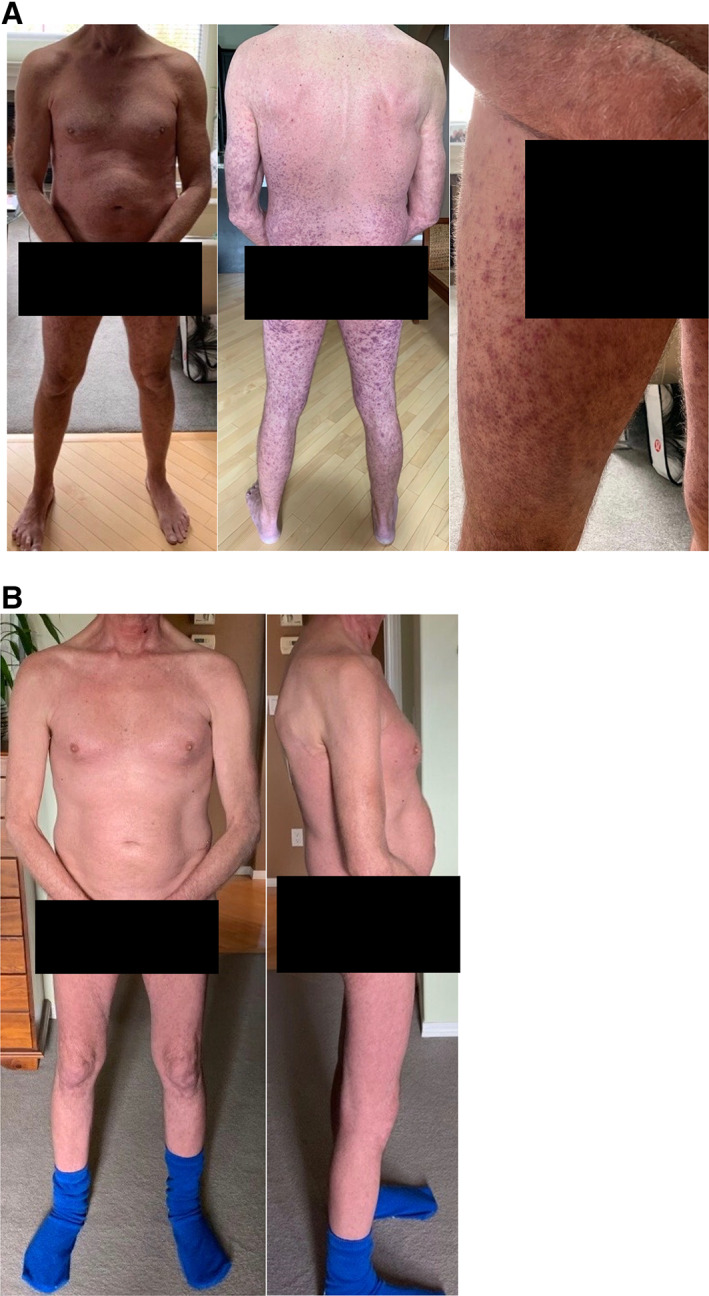
Pictures of patient's rash. (A): Rash prior to hospital admission. (B): Resolution of rash upon discharge from hospital.
He presented to hospital 3 days later in distributive shock unresponsive to 7 L of intravenous crystalloid. He was found to have transaminitis, atrial fibrillation, an oliguric acute kidney injury, worsened anemia and thrombocytopenia, and confusion. His presenting lab work is shown in Table 1. He was initially treated with broad spectrum antimicrobials for presumed septic shock and admitted to the intensive care unit. His skin biopsy showed an interface dermatitis with eosinophils suggestive of a drug reaction, and intraluminal thrombi in keeping with a coagulopathy on deeper sections, with no evidence of a vasculitis (online supplemetal Fig. 1). His bone marrow biopsy was not consistent with hemophagocytic lymphohistiocytosis. As no infection was found, he was started on treatment with intravenous methylprednisolone 1 g daily for 5 days on admission day 2, followed by 50 mg every 8 hours.
Table 1.
Key initial laboratory results on presentation to the emergency department
| Lab | Valuea | Normal range |
|---|---|---|
| Hemoglobin | 96 g/L | 137–180 g/L |
| WBC | 5.7 × 109 | 4–11 × 109 |
| Platelets | 80 × 10 9 | 150–400 × 109 |
| Creatinine | 375 μmol/L | 50–120 μmol/L |
| LD | 999 U/L | 100–235 U/L |
| Haptoglobin | 1.53 g/L | 0.3–2 g/L |
| Fibrinogen | 3.0 g/L | 1.6–4.1 g/L |
| Total bilirubin | 36 μmol/L | 0–24 μmol/L |
| ALT | 107 U/L | 1–60 U/L |
| AST | 228 U/L | 8–40 U/L |
| Calcium | 1.89 mmol/L | 2.1–2.6 mmol/L |
| Phosphate | 1.55 mmol/L | 0.8–1.5 mmol/L |
| Magnesium | 0.62 mmol/L | 0.65–1.05 mmol/L |
| Urate | 420 μmol/L | 210–490 μmol/L |
| Albumin | 29 g/L | 33–48 g/L |
| INR | 1.9 | 0.9–1.1 |
| CRP | 331.4 mg/L | 0–8 mg/L |
| Ferritin | 15,273 μg/L | 30–500 μg/L |
| CK | 670 U/L | 0–195 U/L |
Additional negative tests: antinuclear antibody, extractable nuclear antigen, anti–double‐stranded DNA, antiphospholipid antibodies, antineutrophil cytoplasmic antibody, rheumatoid factor, complement C3, complement C4, serum protein electrophoresis, cryoglobulins, blood, urine cultures, repiratory viral panel, hepatitis B panel, hepatitis C, and cytomegalovirus IgG.
Abnormal values bolded.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CRP, C‐reactive protein; INR, international normalized ratio; LD, lactate dehyndrogenase; WBC, white blood cell count.
After 5 days of treatment, his purpura began to resolve. Additionally, his urine output improved and his creatinine clearance began to recover; however, his encephalopathy worsened, as did his thrombocytopenia and transaminitis, and he developed hypofibrinogenemia. At this point, the favored diagnosis was CRS, and thus IV tocilizumab 4 mg/kg was administered. Within hours of tocilizumab administration, the patient's encephalopathy resolved, and he was given a second dose after 12 hours. Both his transaminitis and thrombocytopenia began to improve within 4 days.
His stay was further complicated by atrial fibrillation and an acute aspiration event requiring intubation for 24 hours. His biochemistry continued to improve, and his glucocorticoids were tapered to oral prednisone 40 mg daily, and he was discharged home in stable condition on day 25 with a prednisone taper with resolution of his rash (Fig. 1B). A cytokine profile drawn before tocilizumab infusion (not available at the time of clinical decision making) did confirm that the expected cytokines in CRS (interleukin [IL]‐6, IL‐10, interferon [IFN]‐γ, and tumor necrosis factor [TNF]‐α) were elevated (online supplemental Table 1).
Discussion
To our knowledge, this is the third case of a patient with CRS during the transition from immunotherapy to targeted therapy with a BRAFi/MEKi and the second case successfully treated with tocilizumab.
CRS is a systemic cytokine‐driven inflammatory response described as a consequence of multiple antibody and non–protein‐based cancer therapies 4, 5. In the era of cancer immunotherapy, CRS has recently gained significant attention as a frequently observed and serious adverse effect among immunotherapies, including chimeric antigen receptor (CAR) T‐cell therapies used in the treatment of hematologic malignancy 4, 5. Patients with CRS can present with a wide range of symptoms, varying from mild flu‐like symptoms to severe multiorgan failure, multifactorial shock, and disseminated intravascular coagulation 4, 5. CRS is classically characterized by the release of a variety of cytokines, most notably IL‐6, IL‐10, IFN‐γ, and TNF‐α 4, 5.
The pathophysiology of CRS among patients treated with CAR T‐cell therapies is thought to be related to activation of bystander immune and nonimmune cells, such as endothelial cells, resulting in a massive release of cytokines 4. The pathophysiology of CRS among patients with melanoma who are sequentially treated with immunotherapies followed by targeted therapies remains unclear but has been recently hypothesized to be a result of simultaneous exposure to immune‐checkpoint and MAPK inhibition due to the long half‐life of immune‐checkpoint inhibitors, resulting in T‐cell activation and release of multiple cytokines including IL‐6 2, 4. IL‐6 causes a positive feedback loop and at high levels initiates a proinflammatory cascade through the trans‐signaling pathway 4, 5, 6. Notably, our patient as well as both published cases of this were exposed to T‐cell stimulating therapies prior to initiation of MEK and BRAF inhibition (Table 2).
Table 2.
Comparison of subject patient with other published cases of cytokine release syndrome with MEKi/BRAFi
| Characteristic | Subject patient | Case 1 | Case 2A | Case 2B |
|---|---|---|---|---|
| Age (years) | 58 | 47 | 48 | 48 |
| Sex | Male | Male | Female | Female |
| Melanoma stage | IV | IV | IV | IV |
| Previous Treatment | Interferon, anti–PD‐1, MEKi/BRAFi | anti–PD‐1 | anti–PD‐1, T‐VEC, anti‐LAG3 | anti–PD‐1, T‐VEC, anti–PD‐1 again, anti‐LAG3, MEKi/BRAFi |
| MEKi/BRAFi |
Cobimetinib/ Vemurafenib |
Cobimetinib/ Vemurafenib |
Trametinib/ Dabrafenib |
Cobimetinib/ Vemurafenib |
| Time to CRS onseta | 11 days | 21 days | 10 days | 10 days |
| Fever | Yes | Yes | Yes | Yes |
| Hypotension | Yes | Yes | No | No |
| Cardiac | SVT, atrial fibrillation | Tachycardia, ventricular extrasystoles | Tachycardia | Tachycardia |
| Dermatologic | Purpura, blisters/bullae, Mucositis | Diffuse maculopapular | Generalized macular rash, erosive mucositis | Blisters/bullae, erosive stomatitis |
| Renal Insufficiency | Yes | Yes | No | No |
| Neurologic | Confusion | No | No | No |
| Gastrointestinal | Nausea, vomiting, diarrhea | No | No | No |
| Transaminitis | Yes | Yes | No | No |
| Cytopenias | Anemia, thrombocytopenia | Unknown | Leukopenia | Unknown |
| ↑ inflammatory markers | Ferritin, CRP | CRP | Unknown | Unknown |
| ↑Cytokines | IL‐1, IL‐2, IL‐4, IL‐5, IL‐6, IL‐8, IL‐10, IL‐13, IFN‐γ, TNF‐α | IL‐6, IFN‐γ, TNF‐α | IL‐6, IFN‐γ, TNF‐α | IL‐6, IFN‐γ |
| Treatment | Systemic CS, TCZ 400 mg × 2 doses | Local and systemic CS | Systemic CS | Systemic CS, TCZ 400 mg |
| Outcome | Discharged home | Developed tolerance to MEKi/BRAFi | Switched to cobimetinib/vemurafeni, had relapse of CRS (Case 2B) | Dramatic improvement following TCZ |
Time to CRS following MEKi/BRAFi treatment.
Abbreviations: BRAFi, BRAF inhibitor; CRP, C‐reactive protein; CRS, cytokine release syndrome; CS, corticosteroids; IFN, interferon; IL, interleukin; LAG3, lymphocyte activation gene 3; MEKi, MEK inhibitor; PD‐1, programmed cell death protein 1; SVT, supraventricular tachycardia; TNF, tumor necrosis factor; T‐VEC, talimogene laherparepvec, TCZ, tocilizumab.
There is a paucity of guidance with regard to management of patients such as ours in the long term. From our experience, ongoing management with tocilizumab was not necessary, and we also recommend tapering the glucocorticoids rapidly, given the potential for complications in some nonhematologic malignancies 7. With respect to further cancer therapies, rechallenging patients with the same agents has been described 2, 4. We suggest that the decision to rechallenge should be shared between the patient and health team based on risks and benefits of other therapeutic options.
As the use of targeted and immunotherapies becomes more commonplace, clinicians need to be aware of CRS, and tocilizumab should be considered in cases unresponsive to corticosteroids.
Disclosures
Jose Monzon: Amgen, Bristol‐Myers Squibb, Merck, Novartis, Sanofi (C/A), Merck (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure
Supplemental Table
Acknowledgments
The authors would like to thank Dr. Mitchell Garry Rohatensky for his contributions to this manuscript. The authors would also like to thank pathologist Dr. Michelle Schneider for providing the slides for this case.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Luke JJ, Flaherty KT, Ribas A et al. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017;14:463–482. [DOI] [PubMed] [Google Scholar]
- 2. Gide TN, Wilmott JS, Scolyer RA et al. Primary and acquired resistance to immune checkpoint inhibitors in metastatic melanoma. Clin Cancer Res 2018;24:1260–1270. [DOI] [PubMed] [Google Scholar]
- 3. Dimitriou F, Matter AV, Mangana J et al. Cytokine release syndrome during sequential treatment with immune checkpoint inhibitors and kinase inhibitors for metastatic melanoma. J Immunother 2019;42:29–32. [DOI] [PubMed] [Google Scholar]
- 4. Shimabukuro‐Vornhagen A, Gödel P, Subklewe M et al. Cytokine release syndrome. J Immunother Cancer 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee DW, Gardner R, Porter DL et al. Current concepts in the diagnosis and management of cytokine release syndrome [correction in Blood 2016;128:1533]. Blood 2014;124:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dimitriou F, Mangana J, Micaletto S et al. Cytokine release syndrome as an important differential diagnosis of severe skin toxicity with organ damage during switch from immunotherapy to targeted therapy in metastatic melanoma. Melanoma Res 2019;29:107–108. [DOI] [PubMed] [Google Scholar]
- 7. Keith BD. Systematic review of the clinical effect of glucocorticoids on nonhematologic malignancy. BMC Cancer 2008;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure
Supplemental Table


