Abstract
Septic shock can be defined as sepsis with persisting hypotension and is required for vasopressors after initial unsuccessful fluid resuscitation. Elevated lactate is a biomarker of tissue perfusion and oxygenation and a useful prognostic tool for resuscitation in septic shock, as it is a byproduct of anaerobic glycolysis due to inadequate oxygen delivery and tissue hypoxia. Early and serial systematic lactate measurement will prompt physician more rapid intervention and lactate normalization, which is associated with better outcome. However, lactate formation during septic shock is neither entirely related to tissue hypoxia, nor reversible by increasing oxygen delivery. Meanwhile, lactate can be oxidized via tricarboxylic acid cycle after being transferred into mitochondria via lactate shuttle, which indicates elevated lactate can be used rather than only accumulation. Glycolysis and elevated lactate can be initiated by hypoxia, but persistent hyperlactatemia may not only represent persistent hypoxia. Some other potential biomarkers have been reviewed in the article including intermediates of tricarboxylic acid cycle, malate-aspartate shuttle, the nicotinamide adenine dinucleotide/reduced nicotinamide adenine dinucleotide (NAD+/NADH) ratio, NAD+, NADH, malate, and malate dehydrogenase from the point of view of energy metabolism. Among them, malate dehydrogenase participates in both malate-aspartate shuttle and tricarboxylic acid cycle, and it can also indirectly reflex the NAD+/NADH ratio. It is reasonable to hypothesize that the combination of lactate and malate dehydrogenase will be more comprehensive to reflex hypoxia in septic shock.
Impact statement
Elevated lactate has been commonly considered as a biomarker and a useful prognostic tool for resuscitation in septic shock, facilitating physician more rapid intervention and treatment. However, it can be initiated by hypoxia, but persistent hyperlactatemia may not represent persistent hypoxia only. In the article, it is the first time to review potential biomarkers in septic shock from the point of view of energy metabolism including intermediates of TCA cycle, MAS, the NAD+/NADH ratio, NAD+, NADH, malate, and MDH. And the combination of lactate and MDH is also proposed in septic shock for the first time, as MDH in cytoplasm and mitochondria participates in both MAS and TCA cycle for ATP generation. Its feasibility in clinic has been analyzed at the end, although related research is still limited. It is reasonable the combination of lactate and MDH will be more comprehensive to reflex hypoxia in septic shock.
Keywords: Lactate, malate-aspartate shuttle, malate dehydrogenase, septic shock
Introduction
It is widely accepted that anaerobic glycolysis will take place upon hypoxia, and then more lactate will be produced from pyruvate catalyzed by lactic dehydrogenase (LDH). Overwhelming evidence demonstrates that hyperlactatemia and lactic acidosis are common in patients with severe sepsis or septic shock.1,2 And hyperlactatemia is also independently associated with significant morbidity and mortality of patients either with hyperlactatemia after volume resuscitation according to the Sepsis-3 definition or with initial hyperlactatemia later normalized after fluid resuscitation excluded from the Sepsis-3 definition.3 However, whether hyperlactatemia is only attributed to tissue hypoxia or anaerobic glycolysis is still not clear, as high serum lactate concentration can occur even when the whole body oxygen delivery is three times higher than the critical oxygen delivery point.4 Actually, there are many other causes leading to hyperlactatemia.5,6 (1) Lactate can participate in the Cori cycle for gluconeogenesis from muscle to liver or kidney via circulation. Thus, an array of biological events such as tissue perfusion, disordered glycolytic flux, and insulin resistance are involved in the concentration of lactate.7 (2) Lactate production can increase because of the reprogramming mitochondria‐dependent process to fulfill the elevated energy demands.8 For example, when the immune cells are exposed to an inflammatory environment, the activated neutrophils increase oxygen consumption and the activated macrophages and lymphocytes increase glycolysis to meet their increasing energy needs.9 For cancer energy metabolism, cancer cells gaining energy mainly via glycolysis have been wildly accepted, leading to elevated lactate (Warburg effect).10 And it is hypothesized that the elevated lactate can be further used by the neighbor cancer cells via the TCA cycle for ATP production.11 (3) Elevated lactate may facilitate the energy demand of cells. Lactate shuttle and oxidation via TCA after transforming lactate to pyruvate can take place in mitochondria. This shuttle can deliver protons from cytoplasm to mitochondria, as the similar role as MAS which is a dominant shuttle in liver and cardiac mitochondria.12 Furthermore, MAS has been demonstrated to promote lactate oxidation in mitochondria by controlling the homeostasis of NAD+ and NADH and maintaining the activity of mitochondrial LDH,13 which can also facilitate the TCA cycle and electron transport chain (ETC) by increasing the intermediates of TCA cycle. Therefore, glycolysis and the elevated lactate can be initiated by hypoxia, but persistent hyperlactatemia may not only represent persistent hypoxia. In addition, unchanged tissue oxygen tension and consumption were found in the skeletal muscles of septic patients and rodent models of sepsis, respectively.14 And hypometabolism in rats with endotoxic shock was also shown not consequential to hypoxia.15 Meanwhile, mitochondria may suffer from increasing production of reactive oxygen species (ROS), reprogramming metabolism of energy, impaired mitochondrial DNA (mtDNA), and mitophagy during sepsis.16,17 It all indicates that either delivery of oxygen or consumption sometimes cannot clearly reflect the real status of mitochondria and its energy metabolism. Therefore, some other potential biomarkers have been reviewed in the article including the intermediates of TCA cycle, MAS, the NAD+/NADH ratio, NAD+, NADH, malate, and MDH from the point of view of energy metabolism, which may play a role in evaluating the relationship between the delivery and consumption of oxygen of cells and mitochondria.
Can intermediates of TCA cycle be responsible for hypoxia in septic shock?
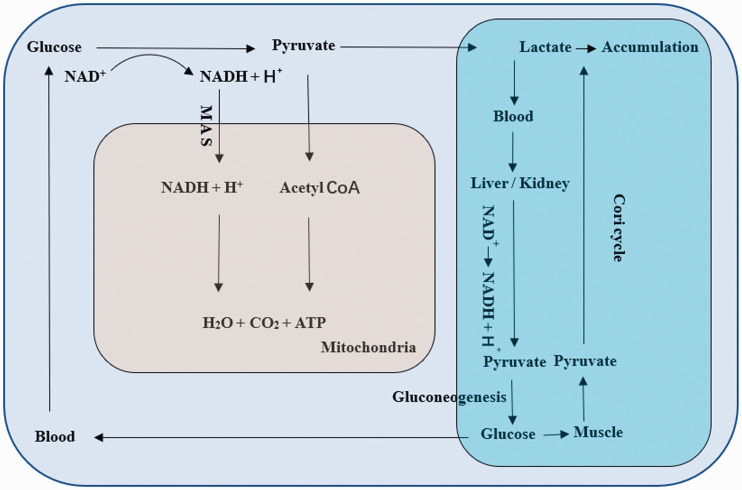
One TCA cycle in mitochondria can generate 38 ATP molecules as well as water and carbon dioxide for each molecule of metabolized glucose with oxygen, electrons, and protons transferred by ETC.18 During sepsis, lactate increases, often accompanied with lactic acidosis. Because the transformation of pyruvate to lactate will consume protons, and the NADH in cytoplasm will be dehydrogenized by LDH to NAD+.19 If the pyruvate transferred into mitochondria for TCA cycle decreases, the protons in acid and protons produced in cytoplasm transferred into mitochondria and participating in TCA cycle will decrease. And decreasing the TCA cycle and increasing the protons finally contribute to metabolic acidosis, just as the accumulation of lactate rather than entering the TCA cycle leading to lactic acidosis. If lactate is transferred into mitochondria via the lactate shuttle, the TCA cycle will continue with the transformation of lactate to pyruvate, and protons will be consumed.20 Therefore, whether compensated/decompensated lactic acidosis/metabolic acidosis exists is useful to estimate the persistent hypoxia. However, when it is during septic shock, the Cori cycle is inhibited due to the dysfunction of liver/kidney and muscle. Lactic acidosis is common, although the body compensatory mechanism of acid–base balance works including respiratory carbon dioxide and HCO3− regulated by kidney. Thus, the TCA cycle cannot be entirely reflected by lactic acidosis.21 In theory, intermediates of the TCA cycle cannot be consumed. Oxaloacetic acid (OAA) and α-ketoglutarate can be replenished by amino acid. Both of them are the main components of MAS which can transport NADH into mitochondria for ETC. It is reasonable to hypothesize the components of MAS may be the better index of hypoxia compared with other final metabolites and intermediates, because (1) there is overlap transformation between the MAS and TCA cycle which means the same enzymes catalyze the reaction; (2) OAA is the initiator of TCA cycle combined with acetyl CoA to citric acid; (3) and NADH provides protons to oxygen to generate water and release energy (Figure 1).
Figure 1.
During sepsis, the production of lactate increases, and then it crosses cell membrane and enters blood circulation, which will lead to accumulation when gluconeogenesis is impaired because of the liver/kidney injury, the increase of gluconeogenesis is not sufficient to deal with elevated lactate, or microcirculation disturbance leads to the retention of lactate, finally resulting in lactic acidosis. Meanwhile, pyruvate entering TCA cycle will decrease due to increasing glycolysis, without lactate shuttle. Further, the consumption of protons will decrease, because of the decrease of transfer of MAS and transfer of pyruvate into mitochondria. (A color version of this figure is available in the online journal.)
MAS and the overlap with TCA cycle
MAS consists of aspartate, glutamate, OAA, oxoglutarate, malate, oxoglutarate/malate carrier (OMC), aspartate/glutamate carrier (AGC), glutamic oxalacetic transaminase (GOT), and MDH.22,23 Aspartate and glutamate as amino acid can be taken in from food, and further transformed into OAA and oxoglutarate via GOT. Malate can be taken in from food and transformed from OAA via MDH.24 In addition, there is overlap between the MAS and TCA cycle (OAA, oxoglutarate, and malate).25 Oxoglutarate can also be supplied by other amino acid in TCA cycle. The crucial function of malate is to transfer cytosolic NADH into mitochondria. Alcohol dehydrogenase (ALDH) contributes to a large amount of ATP production following cytosolic NADH production. Inhibition of ALDH could result in up to 80% depletion of ATP production in cancer cells.26 It indicates MAS is required for transporting cytosolic NADH into mitochondria. And MAS will be influenced by any part of MAS mentioned above. For example, levels of OMC and AGC, as two transport proteins, can regulate the efficiency of MAS.23 Amino oxyacetate acid (AOAA) can inhibit MAS by inhibiting GOT, and lead to apoptosis, mitochondrial depolarization, increase in cytosolic Ca2+ concentrations, and decrease in intracellular ATP levels in microglia.27 Malate supplement can increase the efficiency of MAS further to reduce ROS generation by increasing the efficiency of electron transport.28 Although the level of lactate is elevated in septic shock and has been widely considered as a predominant indicator of glycolysis and hypoxia, simultaneous glycolysis and TCA cycle other than in tumor cells have been described in heart and muscles, which means anaerobic glycolysis and aerobic oxidation can be concurrent.29 MAS is a predominant indicator of TCA cycle, as well as contributing to the homeostasis of NAD+ and NADH and maintain the activity of mitochondrial LDH which enables aerobic oxidation of lactate in mitochondria. Therefore, in septic shock, MAS could be more specific for persistent hypoxia even after increasing the production of lactate induced by initial hypoxia. However, research on MAS is rather limited.
Can NAD+ and NADH be responsible for MAS?
MAS is used to transfer electrons from NADH into cytosolic side to generate NADH in mitochondrial side and transfer reducing equivalents from cytoplasm into mitochondrial matrix.30 NAD+ is a classical redox coenzyme working as a key cellular energy sensor,31 and a precursor for the phosphorylated dinucleotides NADP+ and NADPH, which play a key role in protecting cells from ROS. As a biological hydride acceptor that forms the reduced dinucleotide NADH, NAD+ is regulated by various NAD+ biosynthetic and degradative enzymes, such as nicotinamide phosphoribosyl transferase (NAMPT), sirtuins, and poly (ADP-ribose) polymerases (PARPs) because of its synthesized routes.32,33 NAD+ is also influenced by nutritional and environmental conditions.34 NAD+ can decline during aging and senescence of human cells,35 while NADH is produced as a byproduct from the conversion of aldehyde to carboxylic acid by ALDH, contributing significantly to ATP production.26 For NADH, increasing cytosolic NADH could lead to the inhibition of glycolysis. Decreasing NADH availability was showed in severe sepsis. Fluorescence lifetime imaging was extended to determine the concentration of NADH.36 The assessment of mitochondrial activity through NADH autofluorescence by live cell microscopy gives a range of outputs reflecting the activity of ETC as well as substrate supply which is conceptually and practically appealing.37
The NAD+/NADH ratio is crucial for driving a wide range of reduction and oxidation reactions in cellular bioenergetics. For example, LDH, MDH, pyruvate dehydrogenase (PDH), and enzymes in TCA cycle need NAD+ and NADH to catalyze the oxidation and reduction reactions. If the ratio is abnormal, the activity of enzymes will be influenced further to impair energy metabolism. The NAD+/NADH ratio is compartmentalized in cytoplasm and mitochondria.31 In cytoplasm and mitochondria of liver, it was found to be 725 and 8, respectively, which could be influenced by diabetes and change to 208 and 10 in diabetic rats, respectively.38 It indicates NAD+ is much more than NADH, and the ratio is more susceptible to NADH. A water-forming NADH oxidase from Lactobacillus brevis (LbNOX) as a genetically encoded tool was developed for raising NAD+/NADH ratios and showed it can complement impaired ETC in human cells.39 It also indicates the NAD+/NADH ratio is responsible for ETC, transferring protons to generate water with oxygen and release ATP.40 Therefore, the ratio can reflect TCA cycle. However, it can be influenced by many factors mentioned above. And also, its way of determination is indirect and inadequate. For example, the cytosolic-free NAD+/NADH ratio is determined by measuring lactate and pyruvate levels.41,42 The mitochondrial free NAD+/NADH ratio is determined by measuring the concentration of glutamate, oxoglutarate, and NH3.43,44
Malate and MDH, indispensable role in energy metabolism
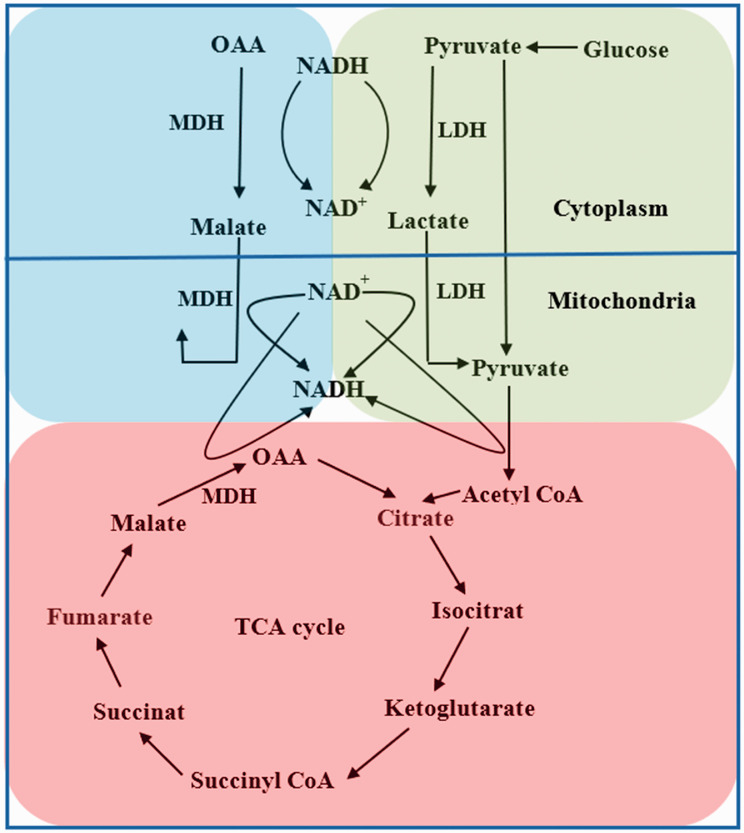
As an intermediate in TCA cycle, malate is a C4-dicarboxylic acid and an essential intermediate of cell metabolism.28 And its synthesis-associated enzymes MDH and malic enzyme (ME) are also essential in TCA cycle. (1) Malate is a trigger for the oxidation of acetyl CoA and can increase TCA cycle.45 Malate is a central kind of component in some kinds of fluid used for resuscitation that are recommended to serve as primary volume therapy in emergency medicine and fluid replacement in cases of moderate acidosis.46 Malate infusion was used to treat rats with moderate and severe acidosis.47 The intragastric administration of malate was showed to increase mitochondrial respiration and energy production in rats.28 Resuscitation with malate could also correct lactic acidosis in severe hemorrhagic shock rats.48 (2) Malate can be from two crucial pathways tightly associated with energy metabolism, MAS, and TCA cycle involving oxidation/reduction of malate/oxaloacetate catalyzed by MDH. It is known from kinetic studies that the reaction from malate to OAA is an ordered reaction with NAD+/NADH binding first, followed by combining OAA/malate.49 Physiologically, the direction of the reversal reaction in cytoplasm is from malate to oxaloacetate, binding NAD+ firstly and transforming it to NADH. And the direction is opposite in mitochondrial matrix which binds NADH,22 induces conformational changes, and results in protons release for TCA cycle.50 So, in order to ensure the physiological reaction, the condition needs to be reversal. It means that in different parts of one cell, NAD+, NADH, OAA, malate, and pH involved in the reaction should be appropriately reversal to ensure the activity of MDH. MDH can be regulated by many factors which exactly indicates its responsible role in reflecting the change of its reactional environment. This enzyme is inhibited by ATP, ADP, AMP, fumarate, aspartate, and high OAA concentrations.51 It can also be allosterically regulated by citrate. Citrate inhibits oxaloacetate reduction under all conditions, and malate oxidation at low malate or NAD+ concentrations, while promotes MDH activity at high malate and NAD+ concentrations.52 Studies showed a dramatic reduction of enzymatic activity on dissociation to monomers at low enzyme concentration at pH 5.0 and in the absence of substrates.53 This explains both intermediates in TCA cycle and pH influence the reaction velocity. Considering the indispensable role of MDH in energy metabolism, some of its inhibitors have been under research. A novel MDH2 inhibitor was showed to suppress HIF-1α accumulation via the reduction of oxygen consumption and ATP production.54 In activated T cells, a common MDH2 inhibitor, LW6 inhibited T cells proliferation and decreased the level of HIF‑1α, intracellular O2 consumption, and TCA cycle, and increased the level of pyruvate dehydrogenase leading to decreasing production of pyruvate.55 (3) Another malate-related enzyme is ME, a member of oxidative decarboxylase family catalyzing irreversible oxidative decarboxylation to yield CO2 and pyruvate, with concomitant reduction of dinucleotide cofactor NAD+ or NADP+.25,56 And ME2, mitochondrial NAD+ dependent, is a mitochondrial enzyme that catalyzes the conversion of malate to pyruvate and CO2 in the mitochondria of tumor cells while absent in non-tumor tissues.57 ME2 can directly interact with MAS and generate NADH in mitochondria. Depleting ME2 induced an increase in the NAD+/NADH ratio and ROS, and a significant decrease in ATP levels in K562 cells.58 So, depletion of ME2 may prevent transferring malate from cytosol into mitochondria, render less effective function of MAS, and further prevent transferring reduced equivalents from extra-mitochondrial compartments into intra-mitochondrial compartments (Figure 2).
Figure 2.
The crucial proceed is shown in Figure 2 including MAS, lactate shuttle, and TCA cycle. MDH participates in both MAS and TCA cycle, and catalyzes reversible reaction of NAD+ and NADH in cytoplasm and mitochondria, which is also necessary for MDH, LDH, and PDH, key enzymes of the three proceeds. And also, it is like a bridge to facilitate the interaction between energy metabolism of one cell. (A color version of this figure is available in the online journal.)
Is it feasible to detect MDH in clinic?
As a crucial enzyme, MDH in cytoplasm and mitochondria participates in both MAS and TCA cycle, and plays an indispensable role in ATP generation. Cytosolic MDH (MDH1) remains in cytoplasm after synthesis, whereas mitochondrial MDH (MDH2) is translocated into mitochondrial matrix.59 MDH1 DNA, mRNA, and its protein can be detected. And mRNA was expressed at a high level in heart and skeletal muscle, correlated with changes in energy metabolism. Increasing expression of MDH1 could be adaptive to support the production of adequate ATP in relatively hypoxia.60 And MDH2 can be identified by specific antibodies with higher and higher specificity.61 As research showed, cytosolic and mitochondrial MDH activities and cytosolic ratio of MDH/LDH activity in leukocytes from the whole blood of race horses were significantly higher than those of riding horses. It was considered to reflect the elevation of energy metabolism in animal tissues.62 Therefore, if MDH of leukocytes from the whole blood can be detected and combined with the level of lactate to evaluate the hypoxia and TCA cycle in sepsis and shock, it will be better than one in clinic. For example, the combination can reflect the feature of energy metabolism during septic shock, as the level of lactate and MDH is responsible for anaerobic glycolysis and aerobic oxidation, respectively. Further, the level of MDH can also reflect MAS for transferring protons to combine with oxygen and generate ATP. Therefore, it can help evaluate whether hypoxia really exists and the degree of hypoxia. And also, dynamic monitoring of the combination of lactate and MDH can help estimate the evolution of septic shock. However, research related to the combination in septic shock is lacking, and further research is needed.
Conclusions
Elevated lactate is generally considered as a significant hint of septic shock induced by tissue hypoperfusion and associated with poor prognosis. However, lactate formation during septic shock is not entirely related to hypoxia. And also, hypoxia cannot be fully responsible for hyperlactatemia, as aforementioned reasons indicate. ATP is generated from efficient TCA cycle. Lactate can be transferred by lactate shuttle and oxidized in TCA cycle. Indeed, glycolysis and elevated lactate can be initiated by hypoxia, but persistent hyperlactatemia may not only represent persistent hypoxia. MDH serves as an indispensable role in TCA cycle. It participates in both MAS and TCA cycle by catalyzing the transformation between malate and OAA, and can also indirectly reflex the NAD+/DADH ratio. Therefore, it is reasonable to hypothesize that the combination of lactate and MDH will be more comprehensive to reflex hypoxia in septic shock. For example, if both lactate and MDH are elevated, it indicates TCA cycle and ATP are ensured. If lactate is elevated and MDH is inactive, it indicates TCA cycle and ATP are impaired.
Authors’ contributions
All authors participated in the work. Hang Yang contributed to the design and draft of the manuscript. Linlin Du contributed to review the manuscript. And Zhaocai Zhang contributed to design and review the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (No.81772110) and Zhejiang Provincial Natural Science Foundation of China (No. LGD20H150003).
ORCID iD
Zhaocai Zhang https://orcid.org/0000-0002-4709-066X
References
- 1.Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care 2014; 18:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott HF, Brou L, Deakyne SJ, Kempe A, Fairclough DL, Bajaj L. Association between early lactate levels and 30-Day mortality in clinically suspected sepsis in children. JAMA Pediatr 2017; 171:249–55 [DOI] [PubMed] [Google Scholar]
- 3.Han X, Edelson DP, Snyder A, Pettit N, Sokol S, Barc C, Howell MD, Churpek MM. Implications of centers for medicare & medicaid services severe sepsis and septic shock early management bundle and initial lactate measurement on the management of sepsis. Chest 2018; 154:302–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronco JJ, Fenwick JC, Tweeddale MG, Wiggs BR, Phang PT, Cooper DJ, Cunningham KF, Russell JA, Walley KR. Identification of the critical oxygen delivery for anaerobic metabolism in critically ill septic and nonseptic humans. JAMA 1993; 270:1724–30 [PubMed] [Google Scholar]
- 5.Suetrong B, Walley KR. Lactic acidosis in sepsis: it’s not all anaerobic: implications for diagnosis and management. Chest 2016; 149:252–61 [DOI] [PubMed] [Google Scholar]
- 6.Gomez H, Kellum JA. Lactate in sepsis. JAMA 2015; 313:194–5 [DOI] [PubMed] [Google Scholar]
- 7.Levy B. Lactate and shock state: the metabolic view. Curr Opin Crit Care 2006; 12:315–21 [DOI] [PubMed] [Google Scholar]
- 8.Van Wyngene L, Vandewalle J, Libert C. Reprogramming of basic metabolic pathways in microbial sepsis: therapeutic targets at last? EMBO Mol Med 2018; 10:e8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol 2017; 17:774–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SY. Cancer energy metabolism: shutting power off cancer factory. Biomol Ther 2018; 26:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, Li H, Huet G, Yuan Q, Wigal T, Butt Y, Ni M, Torrealba J, Oliver D, Lenkinski RE, Malloy CR, Wachsmann JW, Young JD, Kernstine K, DeBerardinis RJ. Lactate metabolism in human lung tumors. Cell 2017; 171:358–71.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu JL, Wu QP, Huang JM, Chen R, Cai M, Tan JB. Effects of L-malate on physical stamina and activities of enzymes related to the malate-aspartate shuttle in liver of mice. Physiol Res 2007; 56:213–20 [DOI] [PubMed] [Google Scholar]
- 13.Altinok O, Poggio JL, Stein DE, Bowne WB, Shieh AC, Snyder NW, Orynbayeva Z. Malate-aspartate shuttle promotes l-lactate oxidation in mitochondria. J Cell Physiol 2020; 235:2569–81 [DOI] [PubMed] [Google Scholar]
- 14.Kohoutova M, Dejmek J, Tuma Z, Kuncova J. Variability of mitochondrial respiration in relation to sepsis-induced multiple organ dysfunction. Physiol Res 2018; 67:S577–s92 [DOI] [PubMed] [Google Scholar]
- 15.Corrigan JJ, Fonseca MT, Flatow EA, Lewis K, Steiner AA. Hypometabolism and hypothermia in the rat model of endotoxic shock: independence of circulatory hypoxia. J Physiol 2014; 592:3901–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu TF, Vachharajani VT, Yoza BK, McCall CE. NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem 2012; 287:25758–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bar-Or D, Bar-Or R, Rael LT, Brody EN. Oxidative stress in severe acute illness. Redox Biol 2015; 4:340–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akram M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem Biophys 2014; 68:475–8 [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto T, Brooks GA. Mitochondrial lactate oxidation complex and an adaptive role for lactate production. Med Sci Sports Exerc 2008; 40:486–94 [DOI] [PubMed] [Google Scholar]
- 20.Kane DA. Lactate oxidation at the mitochondria: a lactate-malate-aspartate shuttle at work. Front Neurosci 2014; 8:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraut JA, Madias NE. Lactic acidosis. N Engl J Med 2014; 371:2309–19 [DOI] [PubMed] [Google Scholar]
- 22.Korla K, Vadlakonda L, Mitra CK. Kinetic simulation of malate-aspartate and citrate-pyruvate shuttles in association with Krebs cycle. J Biomol Struct Dyn 2015; 33:2390–403 [DOI] [PubMed] [Google Scholar]
- 23.Zeng X, Wu J, Wu Q, Zhang J. L-malate enhances the gene expression of carried proteins and antioxidant enzymes in liver of aged rats. Physiol Res 2015; 64:71–8 [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Li J, Shin HD, Liu L, Du G, Chen J. Protein and metabolic engineering for the production of organic acids. Biores Technol 2017; 239:412–21 [DOI] [PubMed] [Google Scholar]
- 25.Pu Z, Zhao M, Zhang Y, Sun W, Bao Y. Dynamic description of the catalytic cycle of malate enzyme: stereoselective recognition of substrate, chemical reaction, and ligand release. J Phys Chem B 2018; 122:12241–50 [DOI] [PubMed] [Google Scholar]
- 26.Kang JH, Lee SH, Hong D, Lee JS, Ahn HS, Ahn JH, Seong TW, Lee CH, Jang H, Hong KM, Lee C, Lee JH, Kim SY. Aldehyde dehydrogenase is used by cancer cells for energy metabolism. Exp Mol Med 2016; 48:e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H, Wang C, Wei X, Ding X, Ying W. Malate-Aspartate shuttle inhibitor aminooxyacetate acid induces apoptosis and impairs energy metabolism of both resting microglia and LPS-Activated microglia. Neurochem Res 2015; 40:1311–8 [DOI] [PubMed] [Google Scholar]
- 28.Wu JL, Wu QP, Peng YP, Zhang JM. Effects of L-malate on mitochondrial oxidoreductases in liver of aged rats. Physiol Res 2011; 60:329–36 [DOI] [PubMed] [Google Scholar]
- 29.Safer B, Smith CM, Williamson JR. Control of the transport of reducing equivalents across the mitochondrial membrane in perfused rat heart. J Mol Cell Cardiol 1971; 2:111–24 [DOI] [PubMed] [Google Scholar]
- 30.Wang T, Yao W, Li J, He Q, Shao Y, Huang F. Acetyl-CoA from inflammation-induced fatty acids oxidation promotes hepatic malate-aspartate shuttle activity and glycolysis. Am J Physiol Endocrinol Metab 2018; 315:E496–e510 [DOI] [PubMed] [Google Scholar]
- 31.Nikiforov A, Kulikova V, Ziegler M. The human NAD metabolome: functions, metabolism and compartmentalization. Crit Rev Biochem Mol Biol 2015; 50:284–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hershberger KA, Martin AS, Hirschey MD. Role of NAD(+) and mitochondrial sirtuins in cardiac and renal diseases. Nat Rev Nephrol 2017; 13:213–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Islam BU, Habib S, Ali SA, Moinuddin AA. Role of Peroxynitrite-Induced activation of poly(ADP-Ribose) polymerase (PARP) in circulatory shock and related pathological conditions. Cardiovasc Toxicol 2017; 17:373–83 [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 2007; 130:1095–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol 2014; 24:464–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blacker TS, Sewell MDE, Szabadkai G, Duchen MR. Metabolic profiling of live cancer tissues using NAD(P)H fluorescence lifetime imaging. Meth Mol Biol 2019; 1928:365–87 [DOI] [PubMed] [Google Scholar]
- 37.Mayevsky A, Chance B. Oxidation-reduction states of NADH in vivo: from animals to clinical use. Mitochondrion 2007; 7:330–9 [DOI] [PubMed] [Google Scholar]
- 38.Williamson DH, Lund P, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 1967; 103:514–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Titov DV, Cracan V, Goodman RP, Peng J, Grabarek Z, Mootha VK. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 2016; 352:231–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao W, Wang RS, Handy DE, Loscalzo J. NAD(H) and NADP(H) Redox couples and cellular energy metabolism. Antioxidants Redox Signal 2018; 28:251–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edington DW, McCafferty WB. Mitochondrial size distribution analysis in the soleus muscle of trained and aged rats. Experientia 1973; 29:692–3 [DOI] [PubMed] [Google Scholar]
- 42.Sun F, Dai C, Xie J, Hu X. Biochemical issues in estimation of cytosolic free NAD/NADH ratio. PLoS One 2012; 7:e34525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White AT, Schenk S. NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise. Am J Physiol Endocrinol Metab 2012; 303:E308–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodman RP, Calvo SE, Mootha VK. Spatiotemporal compartmentalization of hepatic NADH and NADPH metabolism. J Biol Chem 2018; 293:7508–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu JL, Wu QP, Yang XF, Wei MK, Zhang JM, Huang Q, Zhou XY. L-malate reverses oxidative stress and antioxidative defenses in liver and heart of aged rats. Physiol Res 2008; 57:261–8 [DOI] [PubMed] [Google Scholar]
- 46.Schoeneberg C, Schilling M, Keitel J, Kauther MD, Burggraf M, Hussmann B, Lendemans S, [TraumaNetwork, Trauma Registry of the DGU(R), Whitebook, S3 guideline on treatment of polytrauma/severe Injuries - An approach for validation by a retrospective analysis of 2304 patients (2002-2011) of a level 1 trauma Centre]. Zentralbl Chir 2017; 142:199–208 [DOI] [PubMed] [Google Scholar]
- 47.Waack IN, Himmen S, Mueller F, Rohrig R, Roehrborn F, Teloh JK, de Groot H. L-Malate’s plasma and excretion profile in the treatment of moderate and severe hemorrhagic shock in rats. Biomed Res Int 2016; 2016:5237148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai ZL, Wu J, Meng C, Zeng F, Yang Y, Yao SL. Ringer’s malate solution protects against the multiple organ injury and dysfunction caused by hemorrhagic shock in rats. Shock 2012; 38:268–74 [DOI] [PubMed] [Google Scholar]
- 49.Wiseman MS, McKay D, Crow KE, Hardman MJ. Rat liver mitochondrial malate dehydrogenase: purification, kinetic properties, and role in ethanol metabolism. Arch Biochem Biophys 1991; 290:191–6 [DOI] [PubMed] [Google Scholar]
- 50.Wood DC, Hodges CT, Howell SM, Clary LG, Harrison JH. The N-ethylmaleimide-sensitive cysteine residue in the pH-dependent subunit interactions of malate dehydrogenase. J Biol Chem 1981; 256:9895–900 [PubMed] [Google Scholar]
- 51.Dasika SK, Vinnakota KC, Beard DA. Determination of the catalytic mechanism for mitochondrial malate dehydrogenase. Biophys J 2015; 108:408–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minarik P, Tomaskova N, Kollarova M, Antalik M. Malate dehydrogenases–structure and function. General Physiol Biophys 2002; 21:257–65 [PubMed] [Google Scholar]
- 53.Wood DC, Jurgensen SR, Geesin JC, Harrison JH. Subunit interactions in mitochondrial malate dehydrogenase. Kinetics and mechanism of reassociation. J Biol Chem 1981; 256:2377–82 [PubMed] [Google Scholar]
- 54.Ban HS, Xu X, Jang K, Kim I, Kim BK, Lee K, Won M. A novel malate dehydrogenase 2 inhibitor suppresses Hypoxia-Inducible factor-1 by regulating mitochondrial respiration. PLoS One 2016; 11:e0162568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eleftheriadis T, Pissas G, Antoniadi G, Liakopoulos V, Stefanidis I. Malate dehydrogenase-2 inhibitor LW6 promotes metabolic adaptations and reduces proliferation and apoptosis in activated human T-cells. Exp Ther Med 2015; 10:1959–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hung HC, Chien YC, Hsieh JY, Chang GG, Liu GY. Functional roles of ATP-binding residues in the catalytic site of human mitochondrial NAD(P)+-dependent malic enzyme. Biochemistry 2005; 44:12737–45 [DOI] [PubMed] [Google Scholar]
- 57.Moreadith RW, Lehninger AL. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J Biol Chem 1984; 259:6215–21 [PubMed] [Google Scholar]
- 58.Ren JG, Seth P, Everett P, Clish CB, Sukhatme VP. Induction of erythroid differentiation in human erythroleukemia cells by depletion of malic enzyme 2. PLoS One 2010; 5:e12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartmann CM, Gehring H, Christen P. The mature form of imported mitochondrial proteins undergoes conformational changes upon binding to isolated mitochondria. Eur J Biochem 1993; 218:905–10 [DOI] [PubMed] [Google Scholar]
- 60.Lo AS, Liew CT, Ngai SM, Tsui SK, Fung KP, Lee CY, Waye MM. Developmental regulation and cellular distribution of human cytosolic malate dehydrogenase (MDH1). J Cell Biochem 2005; 94:763–73 [DOI] [PubMed] [Google Scholar]
- 61.Gabay-Maskit S, Schuldiner M, Zalckvar E. Validation of a yeast malate dehydrogenase 2 (Mdh2) antibody tested for use in Western blots. F1000Res 2018; 7:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arai T, Hosoya M, Nakamura M, Magoori E, Uematsu Y, Sako T. Cytosolic ratio of malate dehyrogenase/lactate dehydrogenase activity in peripheral leukocytes of race horses with training. Res Veter Sci 2002; 72:241–4 [DOI] [PubMed] [Google Scholar]