Abstract
We aimed to systematically review the effectiveness of probiotic/synbiotic formulations to counteract cardiometabolic risk (CMR) in healthy people not receiving adjunctive medication. The systematic search (PubMed/MEDLINE/Embase) until 1 August 2019 was performed for randomized controlled trials in >20 adult patients. Random-effect meta-analysis subgroup and meta-regression analysis of co-primary (haemoglobin A1c (HbA1C), glucose, insulin, body weight, waist circumference (WC), body mass index (BMI), cholesterol, low-density lipoproteins (LDL), high-density lipoproteins (HDL), triglycerides, and blood pressure) and secondary outcomes (uric acid, plasminogen activator inhibitor-1–PAI-1, fibrinogen, and any variable related to inflammation/endothelial dysfunction). We included 61 trials (5422 persons). The mean time of probiotic administration was 67.01 ± 38.72 days. Most of probiotic strains were of Lactobacillus and Bifidobacterium genera. The other strains were Streptococci, Enterococci, and Pediococci. The daily probiotic dose varied between 106 and 1010 colony-forming units (CFU)/gram. Probiotics/synbiotics counteracted CMR factors (endpoint data on BMI: standardized mean difference (SMD) = −0.156, p = 0.006 and difference in means (DM) = −0.45, p = 0.00 and on WC: SMD = −0.147, p = 0.05 and DM = −1.21, p = 0.02; change scores on WC: SMD = −0.166, p = 0.04 and DM = −1.35, p = 0.03) in healthy persons. Overweight/obese healthy people might additionally benefit from reducing total cholesterol concentration (change scores on WC in overweight/obese: SMD: −0.178, p = 0.049). Poor quality of probiotic-related trials make systematic reviews and meta-analyses difficult to conduct and draw definite conclusions. “Gold standard” methodology in probiotic studies awaits further development.
Keywords: probiotic, synbiotic, microbiota, cardiometabolic
1. Introduction
Cardiovascular diseases (CVD) are the most prevalent noncommunicable disorders, with cardiometabolic risk factors (CMRF) including obesity [1], abnormal lipid profile and hypertension [2], insulin resistance, and aberrant glycaemia [3], playing a role in the pathogenesis. Increased consumption of unhealthy, high-calorie foods combined with a sedentary lifestyle further contribute to their poor outcomes [4,5]. In healthy persons, modestly skewed metabolic parameters may stand for the early onset CMRF [2].
Metabolic malfunctions of diverse nature, with epigenetic, hormonal, and infectious factors, are involved in the pathogenesis [6,7]. Intestinal microbiota actively participating in metabolism is an important factor regulating body metabolism [8]. Microorganisms, primarily bacteria, inhabiting our digestive tract actively participate in the digestion of nutrients and, through its metabolites, can regulate not only energy recovery from food but also lipogenesis or fat formation [9]. The mechanisms by which the gut microbiota can contribute to the pathogenesis of metabolic disorders include the short chain fatty acids (SCFAs) biosynthesis to triglycerides and glucose as well as the phenomenon of endotoxemia leading to increased blood levels of liposaccharide (LPS), which aggravates the process of systemic inflammation [10]. Both LPS and LPS-related inflammation have been linked to metabolic diseases, e.g., diabetes and insulin resistance (IR) [11].
The microbiota communicates with the host via toll-like receptors, nuclear factor-ĸB, and mitogen-activated protein kinase [12], which were shown to improve serum and glucose lipid concentration, to reduce insulin resistance [13,14], and to induce hypocholesterolemic effects [13]. Also, the products of the metabolic activity of the microbiota-predominant SCFAs were shown to regulate various metabolic processes [15]. These molecules after binding to G-protein-coupled receptors make the secretion of peptide YY, which lowers gut motility and augments nutrient absorption [16]. Also, butyrate serves as a source of energy for intestinal cells and improves tissue sensitivity to insulin, counteracting the development of type 2 diabetes. Together with propionic acid, it can stimulate the production of satiety hormones. Of note, butyrate can also stimulate the formation of fat cells and the storage of fat droplets in these cells, presumably through increased glucose uptake or participation in lipid formation. On the other hand, it may also inhibit lipolysis, which, together with stimulating glucose uptake and triglyceride synthesis, makes it a potential therapeutic agent in the fight against hyperglycemia and hyperlipidemia [17].
Considering these facts, metabolic impairment is at least a consequence of gut microbiota alteration. The use of probiotics and synbiotics to counteract metabolic disturbances has been reported. Probiotics are “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”, which has been confirmed in properly controlled studies [18]. Synbiotics are combinations of probiotics and prebiotics. Prebiotics are substrates that are selectively utilized by host microorganisms conferring a health benefit, which must be scientifically documented [19].
A few meta-analyses evaluating the efficacy of probiotics and synbiotics in persons diagnosed with diabetes or hypertension have been published [20,21,22]. However, early-onset CMRF have never been meta-analysed and reported in the literature. Therefore, we conducted the first systematic review and meta-analysis in healthy individuals. We hypothesized that probiotics/synbiotics would be superior to placebo yet would result in greater improvement of some metabolic indices—possibly via microbiota and/or inflammatory as well as gut barrier related pathways as assessed by biochemical parameter alterations—with very few adverse effects. We included studies in which clinically healthy people including those with excess body weight, those who are overweight, and those who are obese.
2. Methods
2.1. Search Strategy and Inclusion Criteria
Two independent authors (K.S.Z. and K.B.) searched PubMed/MEDLINE/Embase from database inception until 1 August 2019 for randomized controlled trials (RCTs) comparing probiotics and synbiotics with placebo/no-intervention/physical activity/diet to counteract cardiometabolic malfunctions in healthy people with normal weight or moderate/high-risk obesity (i.e., not exceeding 40 kg/m2).
The following search string was used in PubMed (probiotic* OR synbiotic* OR microbiota* OR lactobacillus OR bifidobacterium) AND (RCT OR random* OR placebo*) AND (“hemoglobin A1C” OR HbA1C OR glucose OR “fasting glucose” OR “glucose tolerance” OR hyperglycemia OR “oral glucose tolerance test” OR OGTT insulin OR hyperinsulinemia OR “insulin resistance” OR IR OR “insulin sensitivity” OR weight OR obesity OR obese OR overweight OR over-weight OR weight-gain OR “waist circumference” OR “body mass index” OR BMI OR cholesterol OR LDL OR HDL OR triglycerides OR dyslipidemia OR lipid OR “blood pressure” OR SBP OR DBP OR uric acid OR “Plasminogen activator inhibitor-1” OR PAI-1 OR PAI1 OR fibrinogen OR inflamma* OR C-reactive OR “C-reactive protein” OR CRP OR WBC OR leukocytes OR lymphoctes OR endothel* OR “endothelial dysfunction”). In the Embase database, the search string was (‘normal human’/exp OR ‘healthy adult’ OR ‘healthy human’ OR ‘healthy humans’ OR ‘healthy patient’ OR ‘healthy people’ OR ‘healthy person’ OR ‘healthy subject’ OR ‘healthy subjects’ OR ‘healthy volunteer’ OR ‘healthy volunteers’ OR ‘human, normal’ OR ‘normal human’ OR ‘normal humans’ OR ‘normal subject’ OR ‘normal subjects’ OR ‘normal volunteer’ OR ‘normal volunteers’) AND (‘probiotic agent’/exp OR ‘probiotic’ OR ‘probiotic agent’ OR ‘probiotics’ OR ‘synbiotic agent’/exp OR ‘synbiotic’ OR ‘synbiotic agent’ OR ‘synbiotics’ OR ‘microflora’/exp OR ‘microbial flora’ OR ‘microbiota’ OR ‘microflora’ OR ‘lactobacillus’/exp OR ‘bifidobacterium’/exp) AND (‘glycosylated hemoglobin’/exp OR ‘glycated haemoglobin’ OR ‘glycated hemoglobin’ OR ‘glycated hemoglobin a’ OR ‘glycohaemoglobin’ OR ‘glycohemoglobin’ OR ‘glycosyl haemoglobin’ OR ‘glycosyl hemoglobin’ OR ‘glycosylated haemoglobin’ OR ‘glycosylated hemoglobin’ OR ‘glycosylhaemoglobin’ OR ‘glycosylhemoglobin’ OR ‘glycosylised haemoglobin’ OR ‘glycosylized hemoglobin’ OR ‘haemoglobin a1’ OR ‘haemoglobin a 1’ OR ‘haemoglobin a, glycosylated’ OR ‘haemoglobin ai’ OR ‘haemoglobin alpha 1’ OR ‘haemoglobin glycoside’ OR ‘haemoglobin glycosylation’ OR ‘hemoglobin a, glycosylated’ OR ‘hemoglobin glycoside’ OR ‘glucose’/exp OR ‘glucose’ OR ‘fasting blood glucose’/exp OR ‘fasting plasma glucose’/exp OR ‘insulin’/exp OR ‘insulin’ OR ‘insuline’ OR ‘insulin resistance’/exp OR ‘insulin resistance’ OR ‘resistance, insuline’ OR ‘insulin sensitivity’/exp OR ‘insulin insensitivity’ OR ‘insulin sensitivity’ OR ‘insulin sensitivity test’ OR ‘insulin test’ OR ‘sensitivity, insulin’ OR ‘hyperglycemia’/exp OR ‘glucose blood level, elevated’ OR ‘glycemia, hyper’ OR ‘hyperglycaemia’ OR ‘hyperglycemia’ OR ‘hyperglycemic syndrome’ OR ‘glucose tolerance test’/exp OR ‘gtt’ OR ‘g.t.t.’ OR ‘glucogram’ OR ‘glucose load’ OR ‘glucose loading test’ OR ‘glucose test’ OR ‘glucose tolerance curve’ OR ‘glucose tolerance factor’ OR ‘glucose tolerance test’ OR ‘glucose toleration test’ OR ‘body weight’/exp OR ‘body weight’ OR ‘total body weight’ OR ‘weight, body’ OR ‘waist circumference’/exp OR ‘waist circumference’ OR ‘waist size’ OR ‘body mass’/exp OR ‘bmi (body mass index)’ OR ‘quetelet index’ OR ‘body ban mass’ OR ‘body mass’ OR ‘body mass index’ OR ‘cholesterol’/exp OR ‘cholesterol’ OR ‘low density lipoprotein cholesterol’/exp OR ‘ldl cholesterol’ OR ‘cholesterol, ldl’ OR ‘lipoproteins, ldl cholesterol’ OR ‘low density lipoprotein cholesterol’ OR ‘high density lipoprotein cholesterol’/exp OR ‘hdl cholesterol’ OR ‘cholesterol, hdl’ OR ‘high density lipoprotein cholesterol’ OR ‘lipoproteins, hdl cholesterol’ OR ‘triacylglycerol’/exp OR ‘triacylglycerol’ OR ‘triglyceride’ OR ‘triglycerides’ OR ‘tryglyceride’ OR ‘dyslipidemia’/exp OR ‘dyslipaemia’ OR ‘dyslipemia’ OR ‘dyslipidaemia’ OR ‘dyslipidaemias’ OR ‘dyslipidemia’ OR ‘dyslipidemias’ OR ‘blood pressure’/exp OR ‘blood pressure’ OR ‘blood tension’ OR ‘pressure, blood’ OR ‘vascular pressure’ OR ‘plasminogen activator’/exp OR ‘fibrinogen’/exp OR ‘factor 1’ OR ‘factor i’ OR ‘fibrinogen’ OR ‘human fibrinogen’ OR, OR ‘inflammation’/exp OR ‘acute inflammation’ OR ‘bacterial inflammation’ OR ‘inflammation’ OR ‘inflammation reaction’ OR ‘inflammation response’ OR ‘inflammatory condition’ OR ‘inflammatory lesion’ OR ‘inflammatory process’ OR ‘inflammatory reaction’ OR ‘inflammatory response’ OR ‘inflammatory syndrome’ OR ‘reaction, inflammation’ OR ‘response, inflammatory’ OR ‘serositis’ OR ‘sterile inflammation’) AND (‘randomized controlled trial’/exp OR ‘controlled trial, randomized’ OR ‘randomised controlled study’ OR ‘randomised controlled trial’ OR ‘randomized controlled study’ OR ‘randomized controlled trial’ OR ‘trial, randomized controlled’).
A manual review of reference lists from the most recent reviews followed the electronic search. Inclusion criteria were (i) full-text randomized controlled trial, (ii) populations containing >20 adult (>18 years old participants, excluding pregnant women), (iii) treatment with pro-/synbiotics for at least 4 weeks, (iv) randomization to probiotic/synbiotic vs. controls (placebo, no intervention, physical activity, and dietary elements, e.g., yoghurts and milk), and (v) available meta-analyzable change score/endpoint data on any of the following outcomes: HbA1C OR glucose OR OGTT OR insulin OR weight OR waist circumference OR BMI OR cholesterol OR LDL OR HDL OR triglycerides OR blood pressure OR SBP OR DBP OR uric acid OR Plasminogen activator inhibitor-1 OR fibrinogen OR any outcome related to inflammation/endothelial dysfunction. The exclusion criteria were as follows: (i) intervention with microbial agent and adjunctive medication aiming or known to prevent or counteract metabolic dysregulation, e.g., metformin, and (ii) disease, excluding morbid and super obese persons. Data from more than 2-arm studies were abstracted separately for particular comparators; however, placebos were preferentially selected, and regarding dietary comparators, products contained no lactic acid bacteria (e.g., milk vs. yoghurt).
2.2. Data Abstraction
We used the standard data extraction sheet according to our previous studies [23,24,25]. Due to a high number of studies included into metaanalysis, the abstraction stage was done by 4 independent authors. The study list was divided into two parts, and each was abstracted by 2 authors (the 1st part by K.S.Z. and K.B. and the 2nd part by D.M. and J.Ś.-D.). We abstracted data on the study design, the persons enrolled, and the probiotic intervention characteristics in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). For evaluation of the risk of bias (ROB) [26], we incorporated The Cochrane Collaboration’s tool and reported the number of low-risk assessments [26]. This was done by one investigator (D.M.). If some data were missing or difficult to abstract (e.g., from figures) for the review, authors were contacted via email twice, one week apart. All inconsistencies were resolved by senior author (W.M. and I.Ł.) consensus. Data from figures was extracted by means of WebPlotDigitizer software (https://automeris.io/WebPlotDigitizer/).
2.3. Outcomes
Co-primary outcomes were the changes within glycosylated haemoglobin A1c (HbA1C), glucose, insulin, Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), body weight, waist circumference (WC), body mass index (BMI), lipid profile (total cholesterol, low-density lipoproteins (LDL), high-density lipoproteins (HDL), and triglycerides), and blood pressure. Secondary outcomes included uric acid, plasminogen activator inhibitor-1, fibrinogen, and any outcome related to inflammation/endothelial dysfunction (e.g., C-reactive protein (CRP) and leukocyte count). Additionally, we abstracted all-cause and adverse-events discontinuation.
2.4. Data Synthesis and Statistical Analysis
We conducted a random-effects [27] meta-analysis of outcomes for which ≥3 studies contributed data, using Comprehensive Meta-Analysis V3 (http://www.meta-analysis.com). We explored study heterogeneity using the chi-square test of homogeneity, with p < 0.05 indicating significant heterogeneity. All analyses were two-tailed with alpha = 0.05.
Group differences in continuous outcomes were analysed as the pooled standardized mean difference (SMD) in either endpoint scores (preferred) or change scores from endpoint to baseline (if endpoint scores were not available) using observed cases (OC). For continuous metabolic outcomes, standardized mean difference (SMD) and, where applicable, differences in means (DM) were calculated. The additional analyses included studies with participants with proper BMI value (20–25 kg/m2) and trials including overweight and obese persons (BMI > 25 kg/m2, not exceeding 45 kg/m2)
To understand the relationship between effect sizes and various study-level predictors, we fit random-effect meta-regression (multiple) models without interaction term using DerSimonian–Laird estimator estimation of the amount of heterogeneity. The test statistics of the individual coefficient (and confidence intervals) for predictors were based on standard normal distribution (z), and the overall test was based on the chi-square distribution (Q statistics following the chi-square distribution with degrees of freedom representing the number of predictors). Meta-regression variables included (i) number of low ROB assessments, (ii) study duration, (iii) mono- vs. multi-strain probiotic intervention, (iv) sample size (analysed persons), and (v) age of participants (mean). Finally, we evaluated funnel plots and conducted Egger’s regression test [28] to detect whether publication bias could have influenced the results we obtained.
3. Results
3.1. Search Results
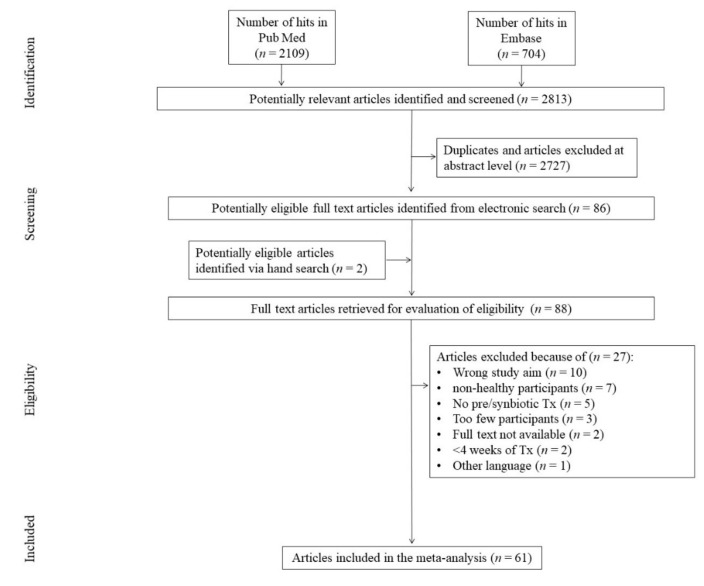
The initial search yielded 2813 hits. Almost 97% (n = 2727) of screened studies were excluded, being duplicates and/or after evaluation on the title/abstract level. Two (n = 2) additional articles were identified via hand search. After exclusion of duplicates between the initial search and hand search results, 88 (n = 88) full-text articles were reviewed. Of those, a total of 27 (n = 27) papers were excluded due to not fitting the inclusion criteria. The primary reasons for exclusion were wrong study aim (n = 10); non-healthy participants (n = 7); no probiotic treatment (n = 5); too few participants (n = 3); too short a study duration (n = 2); unavailability of full texts (n = 2); and another language other than English, German, and Polish (n = 1), yielding 61 (n = 61) studies that were included in the meta-analysis (Figure 1).
Figure 1.
Study flow chart. Tx—treatment.
3.2. Study, Treatment, and Patient Characteristics
As demonstrated in Table 1, altogether, 61 studies (n = 61) were included [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89], comprising 84 interventions. The mean probiotic administration was 67.01 ± 38.72 days (range = 28–186 days). Probiotic, not synbiotic, interventions were predominantly conducted (n = 54) [29,30,31,32,33,34,35,36,39,40,41,42,43,44,46,47,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,66,67,68,69,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,88,89]. Probiotic powders were administered in 15 studies [31,36,39,40,42,44,48,49,50,51,60,72,79,80,81], and in the cases of 14 [30,38,41,53,54,61,63,66,69,74,76,83,88,89] and 9 trials [29,33,43,52,56,57,58,84,86], yoghurt and milk products served as probiotic carriers, respectively. Almost all but eight of probiotic strains utilized in the trails belonged to Lactobacillus and Bifidobacterium genera. The other strains ingested by study participants were from Streptococcus, Enterococcus, and Pediococcus genera. The daily doses varied between 106 and 1010 CFU (colony-forming units). The trials were financed by only industry budgets in 20 (n = 20) [29,30,33,34,39,41,42,46,47,48,55,64,67,69,75,76,78,79,83,84]. Studies were financed only by academic resources in 10 studies (n = 10) [31,32,35,37,40,43,44,52,58,60]. The sponsorships in other studies were partially academic/industrial/government.
Table 1.
Study characteristics.
| No. | Study Description | Intervention | Study Characteristisc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference/Year/Country/Sponsorship | Blinding/Crossover (Y/N)/Multiarm > 2 | Focus on | ROB | Form/Probiotic Strain/Prebiotic | Probiotic Dose CFU | Duration of Probiotic Administration (Days)/Comparator | N Total Randomized and Allocated to Intervention/Analyzed | Age Years (Mean ± SD) | Males (n/%) | BMI Baseline (kg/m2): Probiotic Group (Mean ± SD) | BMI Baseline (kg/m2): Control group (Mean ± SD) | |
| 1 | Agerbaek et al./1995/Denmark/Industry [29] | DB/N/N | Lipoprotein levels | 2 | Naturally fermented milk/Enterococcus faecium (1 strain), Streptococcus termophilus (2 strains) | Daily: Enterococcus faecium 4 × 1010 ; Streptococcus termophilus 1.4 × 1011 | 42/chemically fermented milk | 58/57 | 44 (±ND) | 57/100 | 24.3 (±2) | 24.1 (±1.7) |
| 2a | Agerholm-Larsen et al./2000/Denmark/Industry [30] | DB/N/Y: 5 arms: 3 probiotic groups and 2 placebo groups (3 probiotic and PBO tablet arms were analyzed) |
Risk factors for cardiovascular disease | 2 | Yoghurt/2 strains of Streptococcus thermophilus and 2 strains of Lactobacillus acidophilus | 3 days/week: Streptococcus thermophilus 4.5 × 1010, Lactobacillus acidophilus 9 × 109 | 56/placebo tablets | 2626 | 38.49 (±2.58) | 7/26.92 | 30 (±2.8) | 29.9 (±3.48) |
| 2b | Yoghurt/2 strains of Streptococcus thermophilus and 1 strain of Lactobacillus rhamnosus | 3 days/week: Streptococcus thermophiles 3.6 × 1011, Lactobacillus rhamnosus 9 × 1010 | 56/placebo tablets | 2424 | 38.07 (±2.77) | 7/29.17 | 30.2 (±2.62) | 29.9 (±3.48) | ||||
| 2c | Yoghurt/1 strain of Enterococcus faecium and 2 strains of Streptococcus termophilus | 3 days/week: Enterococcus faecium 2.7 × 1010, Streptococcus thermophilus 4.5 × 1011 | 56/placebo tablets | 26/26 | 37.99 (±2.54) | 7/26.92 | 30.1 (±2.4) | 29.9 (±3.48) | ||||
| 3 | Ahn et al./2015/South Korea/Non-industry [31] | DB/N/N | Triglyceride level and fasting plasma metabolome | 2 | Powder/Lactobacillus curvatus HY7601, Lactobacillus plantarum KY1032 | Daily: Lactobacillus curvatus HY7601 5 × 109 and Lactobacillus plantarum KY1032 5 × 109 | 84/placebo powder | 92/92 | 53.4 (±8.38) | 30/32.61 | 24.7 (±2.91) | 24.9 (±2.26) |
| 4 | Ahn et al./2015a/South Korea/Non-industry [32] | DB/N/N | Triglyceride and apolipoprotein A-V levels | 2 | Powder/Lactobacillus curvatus HY7601, L. Plantarum KY1032 | Daily: Lactobacillus curvatus HY7601 0.5 × 1010 and Lactobacillus plantarum KY1032 0.5 × 1010 | 84/placebo powder | 128/121 | 52.87 (±9.02) | 33/27.27 | 24.9 (±3.2) | 24.8 (±2.62) |
| 5a | Andrade and Borges/2009/Portugal/industry [33] | DB/Y/N | Plasma lipids concentration | 0 | Fermented milk/L. Acidophilus 145 and Bifidobacterium longum BB536 | Daily: Lactobacillus acidophilus 145 5.25–7.88 × 1010 and Bifidobacterium longum BB536 1.01–3.75 × 1010 | 28-7 washout-28/regular yoghurt) | 41/34 | 35.44 (±11.17) | 0/0 | Baseline: Group probiotic-placebo 24.6 (±3.5) Group placebo-probiotic 24.9 (±3.40) |
|
| 5b | ||||||||||||
| 6 | Bjerg et al./2015/Denamark/industry [34] | DB/N/N | Blood lipids, fatty acids levels and stearoyl-coa desaturase−1 (SCD1) activity | 3 | Capsules/L. Casei W8 | Daily: 1 × 1010 | 28/placebo capsules contained rice flour | 70/64 | Range: 20–45 | 34/48.57 | 23.7 (±) | 23.7 (±) |
| 7 | Boesmans et al./2018/Belgium/Non-industry [35] | DB/Y/N | Blood parameters, fecal microbiota composition and metabolites | 7 | Capsules/Butyricicoccus pullicaecorum 25-3T | Daily: 1 × 108 | 28-21 washout-28-21 washout/placebo capsules | 30/28 | Group probiotic-placebo 32 (26–45) Group placebo- probiotic 28 (25–33) 30(±ND) Albo Range: 25–45 |
14/46.67 | Baseline: Group probiotic-placebo 23.6 (±2.1) Group placebo-probiotic 22.1 (±1.9) |
|
| 8 | Brahe et al./2015/Multicenter/Academic/industry [36] | SB/N/Y 3 arms: 1 probiotic 1 prebiotic and 1 placebo group (Probiotic and placebo groups were analyzed) |
The gut microbiota composition, fecal SCFA concentration and metabolic risk markers in obesity | 3 | Powder/Lactobacillus paracasei F19 | Daily: 9.4 × 1010 | 42/placebo powder | 39/35 | 59.92 (±6.09) | 0/0 | 34.2 (±3.1) | 34.3 (±3.8) |
| 9 | Bukowska et al./1998/poland/Non-industry [37] | DB/N/N | Metabolic parmeteres | 2 | Drink/Lactobacillus plantarum 299 v/oat fibers | Daily: Lactobacillus plantarum 299v 1 × 1010 and 160 mg oat fibers | 42/control drink (rose hip drink) | 30/30 | 42.65 (±2.57) | 30/100 | 26.6 (±3.7) | 25.9 (±2.6) |
| 10 | Chang et al./2011/South Korea/Non-industry/industry [38] | DB/N/N | Metabolic parameters | 2 | The functional yogurt: starters: S. thermophilus, L. acidophilus, B. infantis; probiotics: Enterococcus faecalis FK-23, Bifidobacterium breve; fibersol-2 (resistant maltodextrin); pine needle extract; whey protein hydroxylate; Rice germ extract powder; Yucca schidigera and Quillaja saponaria extract | ND | 87/(control yoghurt with starters) | 103/101 | 36.78 (±9.45) | 31/30.69 | 22.63 (±3.26) | 22.13 (±2.8) |
| 11a | Cox et al./2014/Multicenter/industry [39] | DB/N/Y: 3 arms: 2 probiotic groups and 1 placebo group | Routine haematology and clinical chemistry measures | 2 | Powder/Bifidobacterium animalis subsp. Lactis Bl-04, | Daily: 2 × 109 | 150/placebo powder | 87/84 | 40.43 (±13.72) | 44/52.38 | 24.6 (±3.2) | 24.1 (±3.1) |
| 11b | Powder/Lactobacillus acidophilus NCFM, Bifidobacterium animalis subsp. Lactis Bi-07 | Daily: total dose1 × 1010 (equal amount of each strain) | 91/90 | 38.14 (±11.17) | 42/50 | 24.4 (±3.8) | 24.1 (±3.1) | |||||
| 12 | De Roos et al./2017/netherlands/Non-industry [40] | DB/N/N | Migraine symptom reduction, an effect on intestinal permeability and inflammation markers | 4 | Powder/Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcuslactis W19, Lactococcus lactis W58 | Daily: 5 × 109 | 84/placebo powder | 63/60 | 40.07, Range: 18–70 | 4/6.67 | 24.2 (±NA) | 25.6 (±NA) |
| 13 | Fabian et al./2006/Austria/industry [41] | ND/N/N | Plasma lipid profile | 0 | Yoghurt/starter cultures: Streptococcus thermophilus and Lactobacillus bulgaricus; probiotic: L. casei DN-114 001 | Daily: Days 1–14: 3.6 × 1010/Days 15–28: 7.2 × 1010 | 28/placebo regular yoghurt | 34/33 | 24 ± 2.56 | 0/0 | 20.7 (±3) | 21 (±2.7) |
| 14 | Gleeson et al./2012/United Kingdom/Industry [42] | DB/N/N | Incidence of upper respiratory tract infections (URTI) and mucosal immune markers | 1 | Powder/Lactobacillus salivarius | Daily: 2 × 1010 | 112/placebo powder | 66/54 | 23.9 (±4.7) On PRO age 25 ± 5 years PBO age 24 ± 4 years |
28/42.42 (during randomization) | 24.2 (±3.4) | 23.2 (±2.7) |
| 15 | Gohel et al./2016/india/Non-industry [43] | DB/Y/N | Calcium level and haematological parameters | 3 | Fermented milk/Lactobacillus helveticus MTCC 5463 | Daily: min. 2 × 1010 | 28-28 washout-28-28 washout/placebo milk | 76/59 | 68.93 (±4.1) | 38/50 | ND | ND |
| 16 | Gomes et al./2017/Brazil/Non-industry [44] | DB/N/N | Body composition, lipid profile, endotoxemia, inflammation, and antioxidant profile | 5 | Powder/Lactobacillusacidophilus LA-14, Lactobacillus casei LC-11, Lactococcus lactis LL-23, Bifidobacterium bifidum BB-06, and Bifidobacteriumlactis BL-4 | Daily: 2 × 1010 (equal amount of each strain) | 56/(placebo powder) | 60/43 | Range: 20–59 | 0/0 | 31.7 (±3.9) | 33.34 (±4.69) |
| 17 | Greany et al./2008/USA/Non-industry/industry [45] | SB/N/N | Plasma lipids | 2 | Capsules/Lactobacillus acidophilus DDS-1, Bifidobacterium longum UABL-14/FOS | Daily: L. acidophilus DDS-1: 3.75 × 109; B. longum UABL-14: 3.75 109 plus 30–45 mg FOS | 52/placebo capsules | 64/55 | 26.77 (±5.07) | 22/40 | 24.1 (±3.1) | 22.8 (±3.5) |
| 18 | Guillemard et al.2010/Multicenter/Industry [46] | DB/N/N | Incidence of respiratory and gastrointestinal common infectious diseases (cids) and immune functions | 3 | Fermented dairy drink/Lactobacillus casei DN-114 001 | Daily: 2 × 1010 | 84/placebo diary drink) | 1000/962 | 32.15 (±8.91) | 435/43.5 (during randomization) | 24 (±2.8) | 24.2 (±2.9) |
| 19 | Hatakka et al./2008/Finland/Industry [47] | DB/Y/N | Serum cholesterol and triglyceride levels | 2 | Capsules/L. rhamnosus LC705 and P. freudenreichii JS | Daily: L. rhamnosus LC70 5 2 × 1010; P. freudenreichii JS 2 × 1010 | 28-28/(placebo capsules) | 38/38 | 42 (±7.28) | 38/100 | Baseline: Group probiotic-placebo 25.2 (±3.4) Group placebo-probiotic 24.5 (±2.6) |
|
| 20a | Hibberd et al./2019/Multicenter/industry [48] | DB/N/Y: 4 arms: 1 probiotic group, 1 synbiotic group and 2 control groups (Prebiotic and synbiotic and placebo arms were analyzed) |
Body fat mass and obesity-related markers | 1 | Powder/Bifidobacterium animalis subsp. Lactis 420 | Daily: Bifidobacterium animalis subsp. Lactis 420, 1 × 1010 | 168/placebo powder | 61/61 | 48.63 (±10.09) | 17/27.87 | 30.9 (±1.9) | 31 (±2.2) |
| 20b | Powder/Bifidobacterium animalis subsp. Lactis 420 and Litesse Ultra (refined polydextrose) | Daily: Bifidobacterium animalis subsp. Lactis 420, 1 × 1010 + 12 g Litesse Ultra (refined polydextrose) | 73/73 | 47.69 (±9.85) | 16/21.92 | 31.2 (±2) | 31 (±2.2) | |||||
| 21a | Higashikawa et al./2016/Japan/Non-industry [49] | DB/N/Y: 3 arms: probiotic group, killed bacteria group and control group | Body fat and body weight | 6 | Powder/Pediococcus pentosaceus LP28 (live) | Daily: LP28 1 × 1011 | 84/placebo powder | 41/41 | 52.65 (±11.7) | 15/36.58 | 26.84 (±1.15) | 27.37 (±1.43) |
| 21b | Powder/Pediococus pentosaceus LP28 (heat-killed) | 41/41 | 54.18 (±10.89) | 27.1 (±1.24) | ||||||||
| 22a | Ibrahim et al./2018/Malaysia/Non-industry/industry [50] | ND/N/Y: 4 arms: 2 sedentary groups: probiotic group, placebo group; 2 circuit training groups: probiotic, placebo | Muscular strength and power and cytokine responses | 1 | Powder/Lacidophilusacidophilus BCMC 12130, L. casei BCMC 12313, L. lactis BCMC 12451, Bifidobacterium bifidum BCMC 02290, B. infantis BCMC 02129, and B. longum BCMC 02120) | Sedentary groups Daily: 6 × 1010 |
84/placebo powder | 24/20 | 22.5 (±1.66) | 24/100 | 21.8 (±3.4) | 21.1 (±2.8) |
| 22b | Circuit training groups Daily: 6 × 1010 | 84/placebo powder plus circuit training | 24/21 | 21.43 (±2.53) | 22.1 ±3.4 | 21.1 ±2.7 | ||||||
| 23 | Inoue et al./2018/Japan/Non-industry [51] | DB/N/N | Cognitive function, mental state, body composition, and bowel movement were measured | 4 | Powder/B. longum BB536, B. infantis M-63, B. breve M-16V and B. breve B-3 | Daily: 1.25 × 1010 of each strain | 84/placebo powder+ | 39/38 | 70.3 (±3.1) | 14/36.82 | 24 (±2.8) | 23 (±2.7) |
| 24 | Ito et al./2017/Japan/Non-industry [52] | DB/N/N | Serum lipids level | 5 | Fermented milk/Streptococcus thermophilus YIT 2001 | Daily: ≥1 × 1011 | 84/placebo non-fermented milk | 60/59 | 47.35 (±8.25) | 30/50.84 | 22.4 (±2.8) | 23.3 (±2.8) |
| 25 | Ivey et al./2014/Australia/Non-industry/Industry [53] | DB/N/Y: 4 arms: 2 probiotic yoghurt groups: probiotic capsules group, placebo group; 2 control milk: probiotic capsules, placebo (probiotic yoghurt, control milk | Biomarkers of glycaemic control | 4 | Yoghurt and capsules/Lactobacillus acidophilus La5, Bifidobacterium animalis subsp lactis Bb12 | Daily: 3 × 109 (both yoghurt and capsules) | 42/probiotic yoghurt placebo capsules, | 77/77 | 68.4 (±8.25) | 50/64.93 | 30.6 (±3.8) | 30.2 (±4.3) |
| Capsules/Lactobacillus acidophilus La5, Bifidobacterium animalis subsp lactis Bb12 | 42/control milk, placebo capsules | 79/79 | 65.05 (±7.79) | 46/58.23 | 30.8 (±3.5) | 30.8 (±3.5) | ||||||
| 26 | Ivey et al./2015/Australia/Non-industry/industry [54] | DB/N/Y: 4 arms: 2 probiotic yoghurt groups: probiotic capsules group, placebo group; 2 control milk groups: probiotic capsules, placebo | Blood pressure and serum lipid profile | 4 | Yoghurt and capsules/Lactobacillus acidophilus La5, Bifidobacterium animalis subsp lactis Bb12 | Daily: 3 × 109 (both yoghurt and capsules) | 42/placebo capsules, control milk | Probiotic yoghurt 77/77 |
68 (±8.34) | 50/64.93 | 31 (±4) | 30 (±4) |
| Control milk 79/79 |
65 (±7.52) | 46/58.23 | 31 (±4) | 31 (±4) | ||||||||
| 27 | Jones et al./2016/Canada/industry [55] | DB/N/N | Blood cholesterol concentration | 5 | Capsules/Lactobacillus. reuteri NCIMB 30242 | Daily: min. 4.0 × 109 | 63/(placebo capsules) | 131/127 | 49.09 (±13.57) | 55/43.31 | 26.83 (±3.05) | 27.62 (±2.81) |
| 28 | Kadooka et al./2010/Japan/ND [56] | DB/N | Abdominal adiposity, body weight and other body measures in adults with obese tendencies | 2 | Fermented milk/Lactobacillus gasseri SBT2055 (LG2055) | Daily: 1 × 1011 | 84/placebo fermented milk | 87/87 | 48.76 (±9.21) | 59/67.82 | 27.5 (±1.67) | 27.2 (±1.69) |
| 29a | Kadooka et al./2013/Japan/Non-industry [57] | DB/N/Y: 3 arms: lower dose probiotic group, higher dose probiotic group, control group | Abdominal adiposity, anthropometric measures and body composition | 2 | Fermented milk/Lactobacillus gasseri SBT2055 | Daily: 2 × 109 | 84/fermented placebo milk | 139/139 | 47.15 (±7.21) | 69/49.64 | 27.5 (±1.9) | 27.2 (±1.9) |
| 29b | Daily: 2 × 108 | 141/141 | 47.29 (±7.21) | 72/51.06 | 27.2 (±1.8) | 27.2 (±1.9) | ||||||
| 30 | Kawase et al./2000/Japan/Non-industry [58] | SB/N/N | Serum lipid level | 2 | Fermented milk/Lactobacillus casei subsp. casei TMC0409, Streptococcus thermophilus TMC1543 | Daily: L. casei TMC0409 2.44 × 1011, S. thermophilus TMC1543 1.04 × 1010 | 59/fermented placebo milk | 20/20 | 40.1 (±ND) | 20/100 | ND | ND |
| 31a | Kim et al./2018/South Korea/Non-industry/Non-industry [59] | DB/N/Y: 3 arms: lower dose probiotic group, higher dose probiotic group, control group | Adiposity | 5 | Capsules/Lactobacillus gasseri BNR17 | Daily: 1 × 109 | 84/placebo capsules | 60/60 | 38.7 (±11.76) | 20/33.3 | 27.9 (±1.07) | 28.6 (±1.96) |
| 31b | Daily: 1 × 1010 | 60/60 | 38 (±10.4) | 21/35 | 28.8 (±2.24) | 28.6 (±1.96) | ||||||
| 32 | Kim et al./2017/South Korea/Non-industry [60] | DB/N/N | Adiposity parameters and metabolomic profile | 5 | Powder/L. curvatus HY7601 and L. Plantarum KY1032 | Daily: Lactobacillus curvatus HY7601 2.5 × 109, Lactobacillus plantarum KY1032 2.5 × 109 | 84/placebo powder | 120/66 | 38.99 (±1.93) | ND | 26.6 (±1.3) | 27.1 (±1.57) |
| 33 | Klein et al./2008/Germany/Non-industry [61] | DB/Y/N | Blood lipids, faecal microbiota, and immunological parameters | 3 | Yoghurt/B. lactis DGCC420, L. acidophilus 74-2 | Daily:B. lactis 9 × 108; L. acidophilus 74-2 2.79 × 1011 | 35-35/placebo yoghurt | 26/26 | 25 (±3) | 13/50 | Baseline: Group probiotic-placebo 21.3(±2.1) Group placebo-probiotic21.5(±2.0) |
|
| 34 | Lambert et al./2017/Denmark/industry/Non-industry [62] | DB/N/N | Anthropometric data, lipids level, and menopausal symptoms | 4 | Drink/Heterogeneous culture of probiotic lactic acid bacteria in red clover drink | ND | 84/placebo water based drink | 62/59 | 52.34 (±3.66) | 0/0 | 26.02 (±5.38) | 25.45 (±3.34) |
| 35a | Lee et al./2017/USA/Non-industry [63] | Partially SB/Y/Y: 4 arms: (1) placebo yogurt, (2) yogurt with probiotic added pre-fermentation, (3) yogurt with probiotic added post-fermentation, and (4) probiotic capsules | Blood lipids level and fecal excretion of scfas | 3 | Yoghurt, capsules/Bifidobacterium animalis subsp. Lactis BB-12® | Daily: 3.16 × 109 | Each treatment period 28-washout 14/placebo capsules | 36/30 | 28.2 (±6.4) | 11/36.67 | All crossover groups 24.2 (±2.6) | |
| 35b | ||||||||||||
| 35c | ||||||||||||
| 36 | Lin et al./1989/USA/industry [64] | DB/Y/N | Serum lipids level | 4 | Tablets/L. acidophilus (ATCC 4962) and L. Bulgaricus (ATCC 33409) | Daily: 8 × 106 | 42-21 washout-42/placebo tablets | 334/334 | ND | ND | ND | |
| 37 | Macfarlane et al./2013/United Kingdom/Non-industry [65] | DB/Y/N | Colonic microbiota composition, immune function and health status | 5 | Capsules/Bifidobacterium longum/Powder/Synergy I mixture of inulin and oligofructose (DP2-60) | Daily: 4 × 1011 B. longum + 12 gof prebiotic) | 28-28 washout-28/placebo capsules and powder | 47/43 | 71.9 (±5.4) | 21/48.83 | All crossover groups 26.9 (±4.2) | |
| 38 | Madjd et al./2016/Multicenter/Non-industry [66] | SB/N/N | Body weight and cardiometabolic risk factors | 3 | Yoghurt/Lactobacillus acidophilus LA5, Bifidobacterium lactis BB12 | ND | 84/low fat yoghurt | 89/89 | 31.98 (±6.88) | 0/0 | 32.14 (±3.2) | 32.05 (±3.94) |
| 39a | Mohammad Moradi et al./2015.Iran/non-industry [67] | TB/N/Y: 3 arms: (1) probiotic cheese and extract of chicory root, (2) probiotic cheese, and (3) control | Lipid profile | 5 | Cheese/Lactobacillus acidophilus LA5, Bifidobacterium lactis BB12and raw chicory root | ND | 49/no intervention | 120/120 | 37.55 (±15.97) | 60/50 | 22.38 (±2.01) | 22.14 (±0.97) |
| 39b | Cheese/Lactobacillus acidophilus LA5, Bifidobacterium lactis BB12 | 120/120 | 39.4 (±15.92) | 60/50 | 21.94 (±2.19) | 22.14 (±0.97) | ||||||
| 40 | Naruszewicz et al./2002/poland/Non-industry [68] | DB/N/N | Lipid profiles, inflammatory markers, and monocyte function | 3 | Drink/Lactobacillus plantarum 299v | Daily: 2 × 1010 | 42/placebo drink | 36/36 | 42.3 (±3.9) | 18/50 | 24.8 (±4.8) | 25.8 (±3.7) |
| 41 | Nishiyama et al./2018/Japan/industry [69] | DB/N/N | Immunity and metabolic syndrome parameters | 2 | Yoghurt/L. lactis 11/19-B1, B. Lactis BB-12 | No data available | 56/placebo yoghurt | 79/76 | 42.35 (±11.15) | 29/38.15 | ND | ND |
| 42 | Nova et al./2011/Spain/Non-industry [70] | DB/N/N | Self-perceived gastrointestinal well-being and immunoinflammatory status | 3 | Tablets/L. Acidophilus La5, B. animalis Ssp. Lactis Bb-12, Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, and Lactobacillus paracasei ssp. paracasei and FOS | Daily: 2.4 × 109 | 42/placebo tablets with no probiotics) | 37/36 | Range 25–45 | 16/44.4 | 23.74 (±2.19) | 23.06(±2.32) |
| 43 | Ostan et al./2015/Multicenter/Non-industry [71] | ND/N/Y: from 4 arms only probiotic and control arm were analysed | Inflammageing, oxidative stress, and gut microbiota composition | 3 | Capsules/Lactobacillus paracasei, L. plantarum, L. acidophilus, L. delbrueckii subsp bulgaricus, bifidobacterium longum, B. breve, B. infantis, Streptococcus thermophilus | Daily: 2.24 × 1011 | 56/Ristomed diet alone | 69/59 | 70.4 (±3.9) | 58/46.4 | 26.7(±3.8) | 26.9(±3.4) |
| 44 | Osterberg et al./2015/USA/Non-industry [72] | DB/N/N | Body and fat mass, insulin sensitivity, and skeletal muscle substrate oxidation | 3 | Powder/Streptococcus thermophilus DSM24731, Lactobacillus acidophilus DSM24735, Lactobacillus delbrueckii ssp. Bulgaricus DSM24734, Lactobacillus paracasei DSM24733, Lactobacillus plantarum DSM24730, Bifidobacterium longum DSM24736, Bifidobacterium infantis DSM24737, and Bifidobacterium breve DSM24732 | Daily: 9 × 1011 | 28/placebo powder | 20/20 | 22.6 (±3.59) | 20/100 | 23.9 (±2.7) | 23.2 (±1.99) |
| 45a | Rajkumar et al./2014/india/Non-industry [73] | SB/N/Y: 4 arms: placebo, probiotic, omega-3 fatty acid, omega-3 fatty acid + probiotic (probiotics and placebo arms were analyzed) | Insulin sensitivity, blood lipids, and inflammation | 6 | Capsules/Bifidobacterium longum, B. infantis, B. breve, Lactobacillus acidophilus, L. paracasei, L. delbrueckii subsp. bulgaricus, L. Plantarum, Streptococcus salivarius subsp. thermophilus; | Daily: 1.13 × 1011 | 42/placebo capsules | 30/30 | 49 (40-60) | 30/50 | 28.79 (Range: 27–30) | |
| 45b | Capsules/Bifidobacterium longum, B. infantis, B. breve, Lactobacillus acidophilus, L. paracasei, L. delbrueckii subsp. bulgaricus, L. plantarum, Streptococcus salivarius subsp. thermophilus, Omega 3 fatty acids | Daily: 1.13 × 1011 + Omega 3 (360 mg EPA and 240 mg DHA) | ||||||||||
| 46 | Sadrzadeh-Yeganeh et al./2010/Multicenter/industry [74] | TB/N/Y: 3 arms: placebo yoghurt, probiotic yoghurt, and no intervention (probiotic and placebo arms were analysed) | Lipid profile | 2 | Yoghurt/Placebo: Lactobacillus bulgaricus and Streptococcus thermophilus. Probiotic: Placebo + Lactobacillus acidophilus La5, Bifidobacterium lactis Bb12 | ND | 42/placebo yoghurt, | 60/59 | 34.06 (±5.74) | ND | Placebo yoghurt 23 (±2.4) Probiotic yoghurt 24 (±2.4) |
|
| 47 | Sanchez et al./2014/Multicenter/industry [75] | DB/N/N | Weight loss and weight maintenance | 4 | Capsules/Lactobacillus rhamnosus CGMCC1.3724 and mix of oligofructose and inulin | 2 Daily: 3.24 × 108 and 600 mg of a mix of oligofructose and inulin (70:30, v/v) | 168/placebo capsules | 125/93 | 36 (±79.06) | 48/38.4 | 33.8 (±25.98) | 33.3 (±25.39) |
| 48a | Savard et al./2011/Canada/industry [76] | DB/N/Y: 3 arms: lower dose of probiotics and green tea extract, higher dose of probiotic and green tea extract, and placebo | Fecal bacterial counts of Lactobacillus acidophilus LA-5 and Bifidobacterium animalis subsp. Lactis BB-12 and lipid profile | 3 | Yoghurt/Starters: Lactobacillus delbrueckii subsp. Bulgaricus and Streptococcus thermophilus; Probiotics: Bifidobacterium animalis subsp. Lactis BB-12, Lactobacillus acidophilus LA-5 and green tea extract | 1 arm, daily: Bifidobacterium animalis subsp. Lactis BB-12 1 × 109, Lactobacillus acidophilus LA-5 1 × 109 and 40 mg of green tea extract | 28/placebo yoghurt containing no starter culture, no probiotic, and no green tea extract | 40/38 | 32 (±11.9) | 12/30 | 22.8 (±3.8) | 23.8 (±4.1) |
| 48b | 2 arm, daily: Bifidobacterium animalis subsp. Lactis BB-12 1 × 1010, Lactobacillus acidophilus LA-5 1 × 109 and 40 mg of green tea extract | 38/36 | 33.27 (±12.37) | 12/31.6 | 23.7 (±2.7) | 23.8 (±4.1) | ||||||
| 49 | Simon et al./2015/Multicenter/Non-industry [77] | DB/N/Y: 4 arms: lean group: 1) probiotics, 2) placebo; obese group: 1) probiotics, 2) placebo | Insulin sensitivity | 4 | Capsules/Lactobacillus reuteri SD5865 | Daily: 2 × 1010 | 28/placebo capsules | Lean: 11/11 Obese:10/10 |
50 (±7) | Lean: 5/45 Obese:5/50 |
Lean: 23.6 (±6 1.7) Obese: 35.5±4.9 |
|
| 50 | Simons et al./2006/Australia/Industry [78] | DB/N/N | LDL cholesterol and other lipid fractions level | 3 | Capsules/Lactobacillus fermentum | Daily: 8 × 109 | 70/placebo capsules | 46/44 | 51.5 (±11.5) | 16/36.36 | 27 (±5.7) | 24.4 (±3.7) |
| 51a | Stenman et al./2016/Multicenter/industry [79] | DB/N/Y: 4 arms: (1) placebo, (2) prebiotic, (3) probiotic (4) synbiotic (probiotic, synbiotic vs. Placebo arms were analyzed) | Body fat mass and other obesity-related parameters | 4 | Powder/Probiotic: Bifidobacterium animalis ssp. Lactis 420 (B420); Prebiotic: polydextrose; Synbiotic: combination of above | Daily: B420 1 × 1010; | 186/placebo powder, | 112/61 | 48.67 (±10.23) | 17/27.86 | 30.9 (±1.9) 31.2 (±2) |
31 (±2.2) 31 (±2.2) |
| 51b | Synbiotic: B420 1 × 1010 +; polydextrose 12g | 113/73 | 47.75 (±9.75) | 16/21.92 | ||||||||
| 52a | Szulińska et al./2018/Poland/Non-industry [80] | DB DB/N/Y: 3 arms: (1) lower dose probiotic, (2) higher dose probiotic, and (3) placebo |
Functional (primary endpoint) and biochemical parameters (secondary endpoint) of endothelial dysfunction | 5 | Powder/Bifidobacterium bifidum W23, Bifidobacterium lactis W51, B. Lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19, Lactococcus lactis W58 | Daily: 1 × 109 | 84/placebo powder | 54/48 | 57.55 (±7) | 0/0 | 36 (±5.2) | 36.1 (±4.37) |
| 52b | Daily: 2.5 × 1010 | 54/47 | 56.94 (±7.28) | 36.57 (±5.95 | 36.1 (±4.37) | |||||||
| 53a | Szulińska et al./2018a/Poland/Non-industry [81] | DB/N/Y: 3 arms: (1) lower dose probiotic, (2) higher dose probiotic, (3) placebo | Cardiometabolic biochemical parameters, and lipopolysaccharide levels | 5 | Powder/Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19, and Lactococcus lactis W58. | Daily: 1 × 109 | 84/placebo powder | 54/48 | 57.55 (±7) | 0/0 | 36 (±5.2) | 36.1 (±4.37) |
| 53b | Daily: 2.5 × 1010 | 54/47 | 56.94 (±7.28) | 36.57 (±5.95) | 36.1 (±4.37) | |||||||
| 54a | Tenore et al./2019/Italy/Non-industry [82] | DB/N/Y: 3 arms: lactofermented Annurca apple puree, probiotic, unfermented apple puree | Lipid profile and oxidative metabolites level | 6 | Capsules/Lactobacillus rhamnosus LRH11, Lactobacillus plantarum SGL07 | 1 arm, daily: 3 × 108 | 112/Annurca apple puree with no probiotics | 54/42 | 46.65 (±10.36) | 30/71.43 | ≤30 | |
| 54b | Annurca apple puree fermeted with Lactobacillus rhamnosus LRH11, Lactobacillus plantarum SGL07 | Daily: probiotics 3.0 × 108 + Annurca apple puree 125 2 arm, Daily: probiotics 3.0 × 108 + Annurca apple puree 125 g | 53/41 | 45.64 (±10.51) | 31/75.61 | |||||||
| 55 | Trautvetter et al./2012/Germany/industry [83] | DB/Y/N | Intestinal colonisation of L. Paracasei and blood cholesterol level | 3 | Yoghurt/Lactobacillus paracasei LPC37 and bread containing pentacalcium hydroxy-triphosphate | Daily: probiotic 1 × 1012 and calcium 1 g | 28-28 washout-28/placebo yoghurt and bread) | 32/32 | 25 (±5) | ND | 22 (±3) | |
| 56a | Usinger et al./2010/Denmark/Industry [84] | DB/N/Y:3 arms: 150mL probiotic milk, 300 mL of probiotic milk, chemically acidifies milk | Blood pressure | 5 | Milk/Lactobacillus helveticus Cardi-04 | Daily 150 mL of milk fermented with probiotic strain (dose not available) and contains 1.25 mg Val–Pro–Pro (VPP) and 0.55 mg Ile–Pro–Pro (IPP) | 56/chemically acidified milk | 47/45 | 53.3 (±7.41) | 36/60 | 26 (±4) | 26 (±4) |
| 56b | Daily 300 mL of milk fermentem with probiotic strain (dose not available) and contains 2.5 mg Val–Pro–Pro (VPP) and 1.1 mg Ile–Pro–Pro (IPP) | 47/44 | 28/46.67 | 27 (±4) | 26 (±4) | |||||||
| 57 | Valentini et al./2015/Multicenter/Non-industry [85] | SB/N/N | Biomarkers of inflammation, nutrition, oxidative stress and intestinal microbiota | 4 | Capsules/Bifidobacterium infantis DSM24737, Bifidobacterium longum DSM24736, Bifidobacterium breve DSM24732, Lactobacillus acidophilus DSM24735, Lactobacillus delbruckii ssp.bulgaricus DSM 27734, Lacctobacillus paracasei DSM 24733, Lactobacillus plantarum DSM24730, Streptococcus thermophilus DSM 24731 | Daily: 2.24 × 1011 | 56/Ristomed diet | 69/62 | 70.1(±3.9) | 29/46.77 | 26.8 (±3.59) | |
| 58 | Välimäki et al./2012/finland/Non-industry [86] | DB/N/N | Oxidized LDL lipids, serum antioxidant potential (s-TRAP) and serum antioxidants (s-α-tocopherol, s-γ-tocopherol, s-retinol, s-β-carotene, and s-ubiquinone-10) | 4 | Milk drink or capsules/Lactobacillus rhamnosus GG | Daily: drink 4x × 1010 or capsules 1 × 1010 | 84/placebo drink or capsules | 141/119 | 40 (23–69) | 105/88.2 | 22 (Range: 18–26) | |
| 59 | Venkataraman et al./2018/India/ND [87] | SB/N/N | Blood glycemic markers concentration | 2 | Capsules/Lactobacillus salivarius UBLS22, Lactobacillus casei UBLC 42, Lactobacillus plantarum UBLP 40, Lactobacillus acidophilus UBLA 34, Bifidobacteriu breve UBBR 01, Bacillus coagulans Unique-IS2/FOS | 3.0 × 108 cfu/30 × 109 CFU/capsule | 84/placebo capsule | 80/80 | ND | ND | ND | ND |
| 60 | Xiao et al./2003/Japan/industry/Non-industry [88] | SB/N/N | Blood lipids level | 2 | Yoghurt/Bifidobacterium longum BL1 | Daily: 3 × 1010 | 28/placebo yoghurt | 32/32 | 43.85 (±8.05) | 32/100 | ND | ND |
| 61a | Zarrati et al./2014/Iran/Non-industry [89] | DB/N/Y: 3 arms: probiotic yoghurt with low calorie diet (LCD), probiotic yoghurt without LCD, regular yoghurt with LCD | Body fat percentage, blood proinflammatory markers and cytokines content | 3 | Yoghurt/Lactobacillus acidophilus LA5, Lactobacillus casei DN001, Bifidobacterium lactis BB12 without LCD | Daily: 2 × 1010 | 56/regular yoghurt with LCD | 50/50 | 35.5 (±9.27) | 24/32 | 32 (±3.62) | 33.9 (±6.73) |
| 61b | Yoghurt/Lactobacillus acidophilus LA5, Lactobacillus casei DN001, Bifidobacterium lactis BB12 with lcd | 50/50 | 36 (±9.07) | 33.8 (±6.35) | 33.9 (±6.73) | |||||||
DB—double blind, SB—single blind, TB—triple blind, N—no, Y—yes, NA—not applicable, CFU—colony-forming units, ROB—risk of bias, SD—standard deviation, URTI—upper respiratory tract infection, CIDs—common infectious diseases, LDL—low-density lipoprotein, TRAP—total reactive antioxidant potential, EPA—eicosapentaenoic acid, DHA—decosahexaenoic acid, ND—not determined, PBO—placebo, PRO—probiotic, FOS—fructooligosaccharides.
All studies included healthy subjects (including overweight and obese but excluding morbidly obese persons), with a total of 6820 subjected to randomization and 5422 subjected to analysis. The overall mean age was 44.26 ± 12.87 (range: 21.43–71.9) years. The majority of studied persons were females (n = 2934, 57.22%). Baseline metabolic parameters of included persons are presented in Table S1, and the smoking status and diet along with physical activity are in Table S2. When analysing discontinuation events being consequences of adverse events, we found that the probiotic intervention was linked to very few adverse effects, the majority of which were of gastrointestinal origin. Apart from the most common bowel discomforts, i.e., nausea, diarrhea, constipation, and flatulence, there were also cardiac-related events, dental infections, chest tightness, sleep dysregulation, as well as hives. The details on are presented in Supplementary Table S3).
3.3. Risk of Bias Assessment
As evaluated by means of a ROB assessment tool, the mean number of low risks of bias assessment was 3 (median 2.5). The highest score, i.e., 7 low ROB assessments was detected in only one study [35] and 6 low ROB assessments were detected in two studies only [73,82]. Additionally, while analysing the papers, we detected a number of unclear risks of bias. The exact ROB evaluation in particular domains is in Table S4.
3.4. Effects on Metabolic Indices
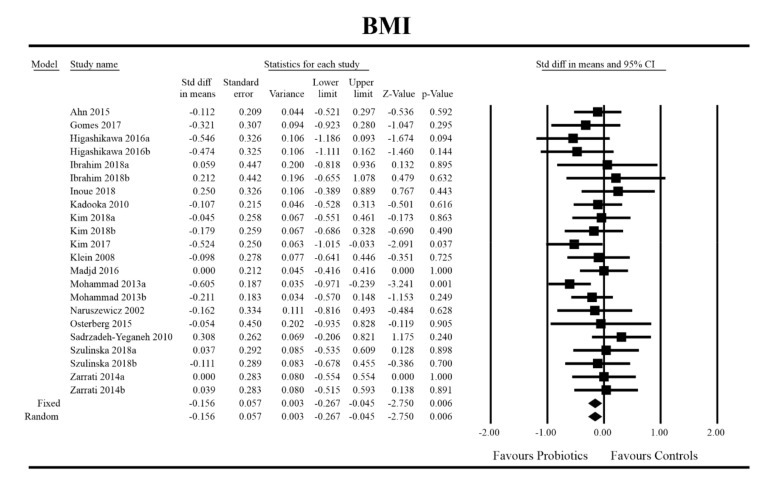
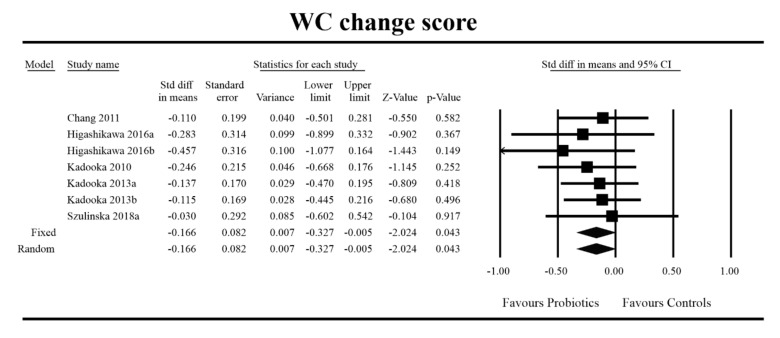
Out of all the metabolic indices that we evaluated, BMI and waist circumference decrease were significantly lower with the probiotic compared to controls. For endpoint data, the results were as follows: BMI studies = 16, n = 1256, SMD = −0.156, 95%CI = −0.27 to −0.04, p = 0.006 and DM = −0.45, 95%CI = −0.69 to −0.21, p = 0.00 and WC studies = 8, n = 690, SMD = −0.147, 95%CI = −0.30 to 0.03, p = 0.05 and DM = −1.21, 95%CI = −2.27 to −0.16, p = 0.02. In the case of the meta-analysis using change scores, the following results were obtained: WC studies = 5, n = 711, SMD = −0.166, 95%CI = −0.32 to −0.005, p = 0.04 and DM = −1.35, 95%CI = −2.59 to −2.15, p = 0.03 (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7). In one case, Egger’s test did indicate publication bias (DM for BMI: t value = 2.37, p = 0.02). For complete results, see Figures S1–S6.
Figure 2.
Effect size and standardized mean difference for BMI in persons taking probiotics vs. controls (endpoint data). Q = 18.487, df (Q) = 21, p = 0.618, I-squared = 0.0. BMI—body mass index. [31,44,49,50,51,56,59,60,61,66,67,68,72,74,81,89].
Figure 3.
Effect size and difference in means for BMI in persons taking probiotics vs. controls (endpoint data). Q = 12.996, df (Q) = 21, p = 0.909, I-squared = 0.0. BMI—body mass index. [31,44,49,50,51,56,59,60,61,66,67,68,72,74,81,89].
Figure 4.
Effect size and standardized mean difference for waist circumference (WC) in persons taking probiotics vs. controls (endpoint data). Q = 9.773, df (Q) = 12, p = 0.636, I-squared = 0.0. WC—waist circumference. [44,49,56,59,66,76,80,81].
Figure 5.
Effect size and difference in means for WC in persons taking probiotics vs. controls (endpoint data). Q = 8.698, df (Q) = 12, p = 0.729, I-squared = 0.0. WC—waist circumference. [44,49,56,59,66,76, 80,81].
Figure 6.
Effect size and standardized mean difference for WC in persons taking probiotics vs. controls (change scores). Q = 1.539, df (Q) = 6, p = 0.959, I-squared = 0.0. WC—waist circumference. [38,49,56,57,81].
Figure 7.
Effect size and difference in means for WC in persons taking probiotics vs. controls (change scores). Q = 1.102, df (Q) = 6 p = 0.981, I-squared = 0.0. WC—waist circumference. [38,49,56,57,81].
As for the other metabolic indices, we found that probiotic ingestion in clinically healthy subjects did not affect those (Table S5).
3.5. Effects on Metabolic Indices Regarding Obesity Status
When conducting analysis by BMI status, i.e., in persons with BMI within normal (BMI: 20–25 kg/m2) and abnormal (BMI: >25 kg/m2) range, we were able to demonstrate that probiotic intake significantly affected total cholesterol (endpoint analyses) in persons with normal BMI value (SMD: −0.974; 95% CI: −1.661 to −0.286, p = 0.006). However, Egger’s test did indicate publication bias (SMD for total cholesterol (endpoint data): t value = 5.38, p = 0.000006). On the other hand, the analyses on the same parameter but regarding change scores depicted that probiotics significantly lowered the parameter in persons with abnormal BMI only (SMD: −0.206, 95% CI: −0.395 to −0.018, p = 0.032). In this case, no publication bias was detected (SMD for total cholesterol (change sores): t value = 1.64, p = 0.137). At last, we evaluated that WC (change score) was significant also in persons with abnormal BMI (SMD: −0.178, 95% CI: −0.354 to −0.001, p = 0.049). In this case, no publication bias was detected (SMD for total cholesterol (change sores): t value = 1.29, p = 0.265).
3.6. Metaregression Analyses
For endpoint data (SMD) regarding diastolic blood pressure (DBP), p values for all predictors were significant (Q = 19.22, df = 7, p = 0.0075): ROB (−0.31, z = −2.18, p = 0.029) and age (0.03, z = 2.07, p = 0.038), indicating that the predicted effect size decreases with increasing risk of bias and increases with age. The model explained 96% of the heterogeneity; however, the permutation test did not confirm the significance of predictors (p = 0.103 and p = 0.093, for ROB and age, respectively). In the case of insulin, p values for all predictors were found to be significant (Q = 16.37, df = 6, p = 0.012): number of low ROB assessments (−0.59, z = −2.57, p = 0.010), number of persons analysed (−0.04, z = −2.43, p = 0.015), duration of probiotic intervention (0.02, z = 2.27, p = 0.023), and BMI of analysed subjects (0.37, z = 3.15, p = 0.0016), indicating that the predicted effect size tended to decrease with ROB and study sample size, whereas with increasing duration and BMI, the effect size tended to be greater. The model did not explain heterogeneity, and the permutation test was nonsignificant. When analysing the triglycerides level, p values for all predictors were also significant (Q = 19.76, df = 7, p = 0.0061): monostrain vs. multistrain probiotics (−0.28, z = −2.47, p = 0.014) and BMI (0.056, z = 3.00, p = 0.003), indicating that the predicted effect size tended to be smaller for multistrain formulas whereas the effect size increased with increasing BMI. The model explained 90% of heterogeneity, and permutation tests were significant (p = 0.022 and p = 0.005 for type of formula and BMI, respectively). Finally, for total cholesterol, p values for all predictor were significant (Q = 90.55, df = 7, p < 0.0001): number of low ROB assessments (−0.67, z = −4.67, p < 0.0001), number of persons analysed (−0.06, z = −7.01, p < 0.0001), duration of probiotic intervention (0.02, z = 3.69, p = 0.0002), age (0.04, z = 2.60, p = 0.009), and BMI of participants (0.19, z = 2.98, p = 0.003). For BMI, HOMA-IR, LDL, and systolic blood pressure (SBP), p values for all predictors and for the effect sizes calculated for change score data were found to be nonsignificant (Q).
3.7. Microbiota Parameters
In 18 studies [35,36,40,48,55,61,63,65,72,75,76,77,79,81,82,83,85,90], microbiota and gut-barrier-related outcomes were evaluated following probiotic intervention. Composition and/or metabolites and/or immunological and/or gut-barrier-related outcomes were evaluated following probiotic intervention. These were data on faecal microbiota composition (n = 13), bacterial metabolites analyses (n = 9), as well as gut barrier integrity markers (mostly LPS, CRP, and zonulin) (n = 13) and various blood immune markers (mostly cytokines) (n = 12). Table 2 presents major results on these parameters. In the analysed studies, particular genera abundance was reported. Only in four studies, microbiota by means of next generation sequencing (NGS) technique was evaluated. Other trials utilized the culture-dependent technique and quantitative polymerase chain reaction (qPCR). We analysed also the association between clinical outcome, microbiota changes, anti-inflammatory effects, and gut barrier markers caused by probiotics administration (Supplementary Table S6). Clinical outcome was associated, in six studies, with microbial changes; in two studies, with microbial metabolites changes; and in two studies, with anti-inflammatory or gut barrier markers alterations. In four studies, changes of microbiota were observed despite lack of clinical efficacy of probiotic treatment.
Table 2.
Microbiota and gut-barrier-related outcomes.
| No. | Reference/Year/Country/Sponsorship | Microbiota | Microbiota Related (Metabolites) | Gut Barrier and Inflammatory Markers | Methods |
|---|---|---|---|---|---|
| 1 | Boesmans et al./2018/Belgium/Non-industry [35] | No impact on the microbiota richness and single genera abundances, only transcient gut colonization by probiotic strain used in the study | No influence on microbiota metabolic activity as well as on saccharolytic and proteolytic fermentation processes markers (SCFAs and dimethyl sulfide, p-cresol, indole, and the branched-chain fatty acids) | No influence on faecal calprotectin concentrations | NGS, GC-MS |
| 2 | Brahe et al./2015/Multicenter/Academic/Industry [36] | Probiotc group: alterations in faecal abundance of 2493 bacterial genes ≥ ↑ Eubacterium rectale and ↑Ruminococcus torques. Placebo group: altered faecal abundance of 7436 genes ≥ ↑Roseburia hominis, ↑two Clostridiales, ↑ one unknown species, ↓ Eubacterium ventriosum, and ↓ one unknown species. |
No impact on faecal total SCFAs and butyric acid | No impact on lipopolysaccharide-(LPS)-binding protein and inflammatory markers (plasma high-sensitivity C-reactive protein (CRP), serum tumor necrosis factor-α (TNFα), and plasma interleukin (IL)-6 | metagenomics, ethyl chloroformate NEFA method and GC |
| 3 | de Roos et al./2017/Netherlands/Non-industry [40] | No impact on zonulin concentration and intestinal permeability measured by means of lactulose-mannitol test; no changes of IL -6, IL-10, TNFα, and CRP | ELISA, GC | ||
| 4 | Hibberd et al./2019/Multicenter/Industry [48] | Probiotic group ↑Akkermansia muciniphila, ↑Lactobacillus, ↑ Bifidobacterium OTU, ↑Akkermansia, ↑ Streptococcus, ↑ S24-7, ↑Methanobrevibacter, ↑Clostridiaceae spp., ↑Clostridium, ↑Phascolarctobacterium, ↑Dialister; ↓Bacteroides, ↓ Erysipelotrichaceae spp., ↓ Enterobacteriaceae spp., ↓ RF39 spp. Bifidobacterium was positively correlated to lean body mass (total, arms, legs, trunk, and android). Paraprevotella negatively correlated with fat mass. Synbiotic group The most pronounced changes of microbiota alterations in clustering analysis (long-term effect). Phylum Bacteroidetes: ↑ taxa: S24-7, Barnesiellaceae spp., Parabacteroides, and Rickenellaceae spp.; ↓ taxa: Paraprevotella. Phylum Firmicutes: ↑ taxa: Christensenellaceae, Ruminococcaceae spp., Oscillospira, Phascolarctobacterium, Erysipelotrichaceae spp. ↓ taxa: Lactobacillus, Lactococcus, Turicibacter Streptococcus, Clostridiales spp., Lachnospira Phylum Actinobacteria: ↓ taxa: Adlercreutzia, Collinsella, Eggerthella Others: ↑Methanobrevibacter, ↑ Akkermansia; ↓ Enterobacteriaceae spp., and ↓ RF39 spp. Christensenellaceae spp. abundance was correlated negatively to WHR and energy intake at baseline, and waist-area body fat and cholesterol markers were correlated to android fat, trunk fat, and lipid parameters Christensenellaceae spp. was positively correlated to the faecal branched-chain fatty acids (BCFAs), isobutyric acid, isovaleric acid, 2-methyl-butyric acid, and 3-methyl-2-oxovalerate, and to the plasma bile acids. |
Probiotic group ↓ propionate Synbiotic group Metabolites: ↓ primary conjugated plasma bile acid glycocholic acid (GCA) ↓ secondary conjugated bile acids glycoursodeoxycholic acid (GUDCA) and taurohyodeoxycholic acid and tauroursodeoxycholic acid (THDCA + TUDCA). ↑ carbohydrates/polysaccharides PICRUSt: “Cellular Processes” and “Metabolism”-KEGG pathways differentially abundant from placebo group No significant changes in short-chained fatty acids (SCFA) or amino acids for any treatment group |
NGS, NMR | |
| 5 | Jones et al./2016/Canada/Industry [55] | Highly sensitive (hs) CRP was unchanged. | LC-MS, GC-MS | ||
| 6 | Klein et al./2008/Germany/Non-industry [61] | L. acidophilus and B. lactis elevation | No impact on SCFAs | ↑ phagocytic activity as a marker for the unspecific cellular immune response | EUB-positive, DAPI-staining, FISH-based quantification, GC |
| 7 | Lee et al./2017/USA/Non-industry [63] | ↑fecal acetate in control yoghurt group and in probiotic added before fermentation group; other SCFAs were unchanged. | CRP level was unchanged. | GC-MS | |
| 8 | Macfarlane et al./2013/United Kingdom/Non-industry [65] | ↑Actinobacteria, ↑ some species of Firmicutes,↑ total bifidobacterial population, ↑ B. angulatum, ↑ B. longum, ↑ B. adolescentis, ↑ B. bifidum. ↓Proteobacteria.↑ Firmicutes/Bacteroidetes ratio. | ↑ butyrate, succinate, total acetate, propionate. | ↓TNF-α | FISH, GC |
| 9 | Osterberg et al./2015/USA/Non-industry [72] | ↑Streptococcus thermophiles, ↑ Lactobacillus acidophilus | No changes of LPS Binding Protein (LBP), LBP/sCD14, IL6, TNFα, hsCRP | qPCR | |
| 10 | Rajkumar et al./2014/India/Non-industry [73] | Probiotic group and probiotic + omega-3 group ↑ total aerobes, ↑total anaerobes, ↑lactobacillus, ↑ bifidobacteria, ↑ streptococcus in the. Probiotic + omega-3 group significant effect on↑ Bacteroides, ↓coliforms, and ↓E. coli. |
↓hsCRP | culture-dependent | |
| 11 | Sanchez et al./2014/Multicenter/Industry [75] | Males: No changes in gut microbiota Females: ↓, Lachnospiraceae family↓ Subdoligranulum genus. |
No change of β-hydroxybutyrate level. | No change of CRP and LPS level | NGS, ELISA |
| 12 | Savard et al./2011/Canada/Industry [76] | ↑B. animalis subsp. Lactis, ↑L. acidophilus LA-5, ↑Bifidobacteria, ↑ Lactobacilli, ↓Enterococci | qPCR | ||
| 13 | Simon et al./2015/Multicenter/Non-industry [77] | No impact on microbiota, only ↑L. reuterii-probiotic bacteria used in the study | No changes of LPS and cytokines | NGS | |
| 14 | Stenman et al./2016/Multicenter/Industry [79] | Probiotic: ↑propionic acid, butyric acid, and valeric acid, | Synbiotic: changes in zonulin and hsCRP were statistically significantly correlated with changes in trunk fat mass; ↑ LPS level, but no effect on inflammatory markers. | Limulus Amebocyte Lysate assay | |
| 15 | Szulińska et al./2018a/Poland/Non-industry [81] | ↓LPS level | kinetic assay | ||
| 16 | Tenore et al./2019/Italy/Non-industry [82] | ↑ Bifidobacterium and ↑Lactobacillus population, and ↓ Bacteroides and ↓Enterococcus genera but predominantly in the control group followed by probiotic one and lactofermented control meal | ↓TMAO blood level | culture-dependent | |
| 17 | Trautvetter et al./2012/Germany/Industry [83] | ↑L. paracasei and ↑ Lactobacilli | qPCR | ||
| 18 | Valentini et al./2015/Multicenter/Non-industry [85] | No significan influence on gut microbiota. | qPCR |
qPCR—quantitative polymerase chain reaction, NGS—next generation sequencing, ELISA—enzyme-linked immunosorbent assays, FISH—fluorescent in situ hybridization, GC-MS—gas chromatography-mass spectrometry, LC-MS—liquid chromatography-mass spectrometry, DAPI—4′,6-diamidino-2-phenylindole, NMR—nuclear magnetic resonance, Tx—treatment, NEFA—non-estrified fatty acid, GC—gas chromatography, SCFAs—short chain fatty acids, EUB-positive—Eubacteria positive, HD—high dose, LPS—lipopolysaccharide, TMAO—trimethylamine-N-oxide, OTU—operational taxonomic unit, PICRUSt—Phylogenetic Investigation of Communities by Reconstruction of Unobserved States, KEGG—Kyoto Encyclopedia of Genes and Genomes, WHR—waist to hip ratio, hsCRP—high sensitivity C-reactive protein, ↑—elevated, ↓—lowered.
4. Discussion
In past years, many studies revealed that probiotics and synbiotics, through interactions with hosts, could affect nutrient metabolism and energy balance. Our current meta-analysis of 61 clinical trials and 5422 persons exclusively investigated the impact of probiotic and synbiotic interventions to reduce cardiovascular risk factors in otherwise healthy adults. The only factor we decided not to exclude was overweight and obesity, as their prevalence is worldwide and as they impact human’s health [91]. Morbidly obese persons (BMI ≥ 45 kg/m2) were excluded from the present analysis. We also decided to exclude studies with adjunct medications with reported efficacy against metabolic dysregulation (e.g., metformin [92]). Similarly, we excluded patients with diagnosed diseases, as meta-analyses in such patients have already been published [93,94,95] The results of the present meta-analysis indicated that probiotics may reduce the BMI by 0.5 unit (provide stats) and decrease waist circumference by more than 1.5 cm (stats). The effect sizes were/were not affected by meta-regression statistics. The up-to-date published data indicate that probiotics may reduce body weight, BMI, and other anthropometric indices, e.g., fat mass and waist circumference, via several mechanisms. While restoring the microecological ecosystem, probiotics diminish the inflammation responsible for insulin sensibility in the hypothalamus [96]. This in turn, together with increased concentration of glucagon-like peptide-1 (GLP-1) as well as peptide YY (PYY), improve satiety and suppress appetite by delaying gastric emptying. It should be emphasized that gut-derived GLP-1 is able to attenuate gut motility and to facilitate the aggregation of the constitutive flora to ferment more polysaccharides [97]. Furthermore, healthy microbiomes within the gut upregulate the expression of fasting-induced adipocyte factor (FIAF) and thus limits the degradation of lipoproteins and the deposition of free fatty acids in adipose tissue. Together with reduced food intake, the abovementioned healthy microbiome can promote reduction of body weight [96,98]. The systematic review by Crovesy et al. [96] indicated that strains of Lactobacillus gasseri and Lactobacillus amylovorus may promote decrease of body weight in the overweight population. The meta-analysis by John et al. [97] confirmed that probiotic therapy was associated with a significant reduction of BMI and, thus, body weight and fat mass. The study group consisted of overweight and obese persons. Notwithstanding, another systematic review and meta-analysis in a similar group of subjects showed that administration of probiotics was related to reduction of body weight in comparison to the placebo; however, the effect sizes were small (weighted mean difference (95% confidence interval); −0.60 (−1.19, −0.01) kg, I2 = 49%), BMI (−0.27 (−0.45, −0.08) kg m−2, I2 = 57%) and fat percentage (−0.60 (1.20, −0.01) %, I2 = 19%). Similarly to our findings, the effect of probiotics on fat mass was not significant (−0.42 (−1.08, 0.23) kg, I2 = 84%) [99]. Also, a study by Depommier et al. [100] demonstrated that supplementation with Akkermansia Muciniphila in overweight and obese human volunteers improved insulin sensitivity and total plasma cholesterol with a small reduction of body mass compared to controls. In contrast, in healthy, but overweight subjects, the administration of Lactobacillus amylovorus and Lactobacillus fermentum strains reduced this body fat mass [101].
The current meta-analysis did not confirm the efficacy of probiotics administration in reduction of other cardiovascular risk in healthy people. Of note, carbohydrate and lipid metabolism was not significantly affected by this type of intervention. In contrary to diabetic patients, we did not find any effect of probiotic therapy on carbohydrate metabolism. A study by Raygan et al. [102] which was conducted in patients with type 2 diabetes mellitus (T2DM) and coronary heart disease found that the intervention, during which the strains of Bifidobacterium bifidum, Lactobacillus casei, and Lactobacillus acidophilus were ingested for 12 weeks, significantly decreased the plasma glucose and insulin resistance. In a meta-analysis by Samah et al., [103] moderately hypoglicaemic properties (lower levels of fasting blood glucose) of microbial agents were confirmed. As in previously quoted studies, the meta-analysis cohort coincided with T2DM patients. Probiotics were demonstrated to affect glucose metabolism via several mechanisms, including antioxidant activity, and thus diminished gut-barrier integrity disruption, enhanced NK cells activity in the liver cells, and diminished insulin resistance by modulating the expression of proinflammatory cytokines and NF-kB-binding activity. Indeed, eubiosis within the gut may serve as a protective point for the preDM and DM onsets, diminishing low-grade inflammation which characterizes all metabolic diseases [104,105]. As concerns inflammation status, we did not find the relationship between common inflammatory markers (CRP and leukocytes count) as well as other indices associated with insulin resistance, including endothelial markers and uric acid. In T2DM patients, probiotics were found to lower the concentrations of hs-CRP, IL-6, and TNF-α [106]. Similar results, regarding hs-CRP, were demonstrated lately in a meta-analysis by Zheng et al. [107] and by Tabrizi et al. [108]. At last, the increase of the bioavailability of gliclazide regulating the intestinal absorption of glucose may also play a role [93].
In our study, we found that probiotics can decrease the total cholesterol level in persons with increased BMI, but other lipid parameters were not affected by probiotics and synbiotics administration. In Wang et al.’s meta-analysis including 32 randomized controlled trials (1971 participants with various metabolic entities), it was proved that probiotics significantly reduced serum total cholesterol (MD = −13.27, 95% CI (−16.74–9.80), p < 0.05) in comparison to controls [109]. Similar results were obtained in the meta-analyses by Chao et al. [110] and Shimizu et al. [111] (30 RCTs and 33 RCTs, respectively; hypocholesterolemic effects of probiotics–mean net change of total cholesterol: 7.8 mg/dL and 6.57 mg/dL, respectively, both in persons with mild lipid malfunctions). There are many hypotheses regarding mechanisms in which probiotics may lower the cholesterol level, such as binding of cholesterol to the probiotic cellular surface and incorporation of cholesterol molecules into the probiotic cellular membrane. However, the deconjugation of bile via bile salt hydrolase (BSH) activity seems to be the most profound mechanism in which probiotics reduce cholesterol level [112]. Bile salt hydrolase is the enzyme that catalyses the hydrolysis of glycine- and/or taurine-conjugated bile salts into amino acids residues and free bile acids. The most BSH-active probiotics belong to the genera of Lactobacillus, Lactococcus, and Bifidobacterium. These probiotics increase the production of bile salts from cholesterol in their colonized area and, as a consequence, contribute to reduced risk of coronary heart diseases [112].
The administration of probiotics improved blood pressure in humans, which was confirmed in Khalesi et al.’s meta-analysis including 9 randomized, controlled trials [113]. The consumption of probiotics significantly decreased systolic blood pressure by 3.56 mmHg and diastolic blood pressure by 2.38 mmHg in comparison to control groups (the duration of intervention is ≥8 weeks or daily dose > 1011 CFU). In contrast to our study, the authors included studies evaluating people with metabolic syndrome, hypertension, and hypercholesterolemia. As the menopause period is a strong contributor of CVD [114], we looked for metabolic effects on probiotic intake in this particular subgroup of participants. We were able to demonstrate that probiotic intake decreased the vascular stiffness in obese postmenopausal women [80]. Also, as reported by Lambert et al. [62], probiotics significantly diminished vasomotor symptoms of menopause. In a study by Szulińska et al. [81] was found that probiotics administration favorably affected the risk factors in a dose-dependent manner, showing beneficial effects on the cardiometabolic parameters and gut permeability of obese postmenopausal women. However, Brahe et al. [36] did not record that metabolic index was affected by microbial agent administration. Only these three studies reported on metabolic effects in the perimenopausal period; thus, we did not conduct a subgroup analysis. More studies are needed to clarify if and how probiotics can affect CVD risk in women at the menopause period.
Last but not least, we abstracted data related to the influence of probiotic administration on gut microbiota and immunological markers. The most frequently studied variables were (i) the effects of probiotic administration on the composition of the microbiota and (ii) colonization with probiotics. Among microbial metabolites, mostly faecal SCFAs were analyzed. The authors analyzed also markers of gut-barrier integrity—mostly LPS and different cytokines as well as inflammatory markers. CRP measured in few studies can be considered as an inflammatory marker as well as a gut integrity marker. Based on the results obtained, no definite association can be found between the use of probiotics, microbiota changes, modulation of the immune system, and either presence or lack of clinical effects (Table 2 and Table S6). Of note, the results cannot be subjected to meta-analysis due to very diverse methods used to analyze the microbiota. Therefore, the results are difficult to compare. For this reason, in order to fully assess the causal relationship between the microbiota and the function of the immune system and gut-integrity markers with relation to cardiovascular risk prevention, a multifactorial analysis should be performed, which was not performed in the works described in this systematic review. In only one study, the correlation between microbiota changes and cardiovascular risk factors was demonstrated [48]; however, in this study, no preventive outcome of probiotics administration was observed. In addition, the results of metabolomic studies did not contribute to elucidation of the mechanism of action of probiotics studied. Therefore, it cannot be determined whether the effect of probiotics in cardiovascular risk prevention is related to their effect on microbiota or the immune system or gut-barrier function. The relationship observed in some studies is rather based on association and not causation. We conclude that mechanistic studies should be an important point in analysis of probiotics/synbiotics efficacy.
Limitations
Several limitations of this meta-analysis need to be underlined. These include (i) a relatively small number of high-quality double-blinded studies comparing probiotic intervention to controls with a wide range within the number of participants preceded by no sample size calculations; (ii) heterogeneous study inclusion criteria (various age, profession of participants, and dietary and physical activity add-on interventions), and (iii) various type of strains and duration of probiotic intervention. In studies incorporated into the present meta-analysis, the association between the probiotic effect in relation to supplement dose and treatment duration was not analyzed. At last, most of the studies were financed by the industry and include products combined with different ingredients. These all are confounding factors for probiotic efficacy, which may have resulted in some publication bias as evaluated by Eagerr’s test and funnel plots [115]. Consequently, in order to draw some evidence-based conclusions and to give some guidelines regarding probiotic intake in healthy adults, strict inclusion criteria and homogenous intervention protocols are needed. Lastly, during meta-analysis, we did not use intent-to-treat data but adopted per-protocol evaluation as the majority of studies reported on that. We could have introduced potential bias during the review process and could have missed studies not clearly aimed at reducing cardiovascular risk but possibly reporting such outcomes.
5. Conclusions
Probiotics may counteract some CMRF (e.g., BMI and waist circumference) in clinically healthy participants. Overweight/obese persons might benefit from the reduction of total cholesterol serum concentration. Poor quality of probiotic-related trials make systematic reviews and meta-analyses difficult to conduct and draw exact conclusions. “Gold standard” methodology in probiotic studies awaits further development.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/6/1788/s1, Table S1. Baseline metabolic parameters in studies persons, Table S2. Smoking status and physical activity, Table S3. Trial discontinuations and adverse effects, Table S4. Risk of bias, Table S5. Effect sizes regarding metabolic outcomes, Table S6. Summary of the preventive outcome and changes in microbial composition and metabolites as well as anti-inflammatory effects and gut-barrier markers associated with probiotics administration; Figure S1. Funnel plot for endpoint BMI (SMD) in the present meta-analysis, Figure S2. Funnel plot for endpoint BMI (DM) in the present meta-analysis, Figure S3. Funnel plot for endpoint WC (SMD) in the present meta-analysis, Figure S4. Funnel plot for endpoint WC (DM) in the present meta-analysis, Figure S5. Funnel plot for WC change scores (SMD) in the present meta-analysis, Figure S6. Funnel plot for WC change scores (DM) in the present meta-analysis.
Author Contributions
Conceptualization, I.Ł. and K.S.-Ż.; methodology, K.S.-Ż. and M.K.; software, K.S.-Ż. and M.K.; validation, K.S.-Ż., I.Ł., and W.M. ; formal analysis, K.S.-Ż., I.Ł., and W.M.; investigation, K.S.-Ż., K.J., J.Ś.-D., D.M. (Damian Malinowski), K.B., and D.M. (Dominika Maciejewska); resources, K.S.-Ż.; data curation, K.S.-Ż., K.K.-S., and M.K.; writing—preparation, K.S.-Ż., K.K.-S., E.S., and D.M. (Dominika Maciejewska); writing—review and editing, B.Ł., W.M., and I.Ł.; visualization, K.S.-Ż. and M.K.; supervision, W.M. and I.Ł.; project administration, K.S.Ż.; funding acquisition, W.M. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
Igor Łoniewski and Wojciech Marlicz are probiotic company shareholders. Joanna Śliwa-Dominiak is a probiotic company employee. Karolina Skonieczna-Żydecka and Mariusz Kaczmarczyk receive renumeration from a probiotic company. The other authors declare no conflict of interest.
References
- 1.Cercato C., Fonseca F.A. Cardiovascular risk and obesity. Diabetol. Metab. Syndr. 2019;11:74. doi: 10.1186/s13098-019-0468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y., Vittinghoff E., Pletcher M.J., Allen N.B., Hazzouri A.Z.A., Yaffe K., Balte P.P., Alonso A., Newman A.B., Ives D.G., et al. Associations of Blood Pressure and Cholesterol Levels During Young Adulthood With Later Cardiovascular Events. J. Am. Coll. Cardiol. 2019;74:330–341. doi: 10.1016/j.jacc.2019.03.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ormazabal V., Nair S., Elfeky O., Aguayo C., Salomon C., Zuñiga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018;17:122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa Pereira L.M., Aidar F.J., de Matos D.G., de Farias Neto J.P., de Souza R.F., Sobral Sousa A.C., de Almeida R.R., Prado Nunes M.A., Nunes-Silva A., da Silva Júnior W.M. Assessment of Cardiometabolic Risk Factors, Physical Activity Levels, and Quality of Life in Stratified Groups up to 10 Years after Bariatric Surgery. Int. J. Environ. Res. Public Health. 2019;16:1975. doi: 10.3390/ijerph16111975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguilar-Salinas C.A., Viveros-Ruiz T. Recent advances in managing/understanding the metabolic syndrome. F1000Res. 2019;8 doi: 10.12688/f1000research.17122.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzika E., Dreker T., Imhof A. Epigenetics and Metabolism in Health and Disease. Front. Genet. 2018;9:361. doi: 10.3389/fgene.2018.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhurandhar E.J., Keith S.W. The aetiology of obesity beyond eating more and exercising less. Best Pract. Res. Clin. Gastroenterol. 2014;28:533–544. doi: 10.1016/j.bpg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Parekh P.J., Balart L.A., Johnson D.A. The Influence of the Gut Microbiome on Obesity, Metabolic Syndrome and Gastrointestinal Disease. Clin. Transl. Gastroenterol. 2015;6:e91. doi: 10.1038/ctg.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muscogiuri G., Cantone E., Cassarano S., Tuccinardi D., Barrea L., Savastano S., Colao A., Education Research and Assessment (OPERA) Group on behalf of the Obesity Programs of Nutrition Gut microbiota: A new path to treat obesity. Int. J. Obes. Suppl. 2019;9:10–19. doi: 10.1038/s41367-019-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 12.Bermudez-Brito M., Plaza-Díaz J., Muñoz-Quezada S., Gómez-Llorente C., Gil A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012;61:160–174. doi: 10.1159/000342079. [DOI] [PubMed] [Google Scholar]
- 13.He M., Shi B. Gut microbiota as a potential target of metabolic syndrome: The role of probiotics and prebiotics. Cell Biosci. 2017;7:54. doi: 10.1186/s13578-017-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagarolli R.A., Tobar N., Oliveira A.G., Araújo T.G., Carvalho B.M., Rocha G.Z., Vecina J.F., Calisto K., Guadagnini D., Prada P.O., et al. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J. Nutr. Biochem. 2017;50:16–25. doi: 10.1016/j.jnutbio.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 15.den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.-J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown A.J., Goldsworthy S.M., Barnes A.A., Eilert M.M., Tcheang L., Daniels D., Muir A.I., Wigglesworth M.J., Kinghorn I., Fraser N.J., et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 17.Chambers E.S., Preston T., Frost G., Morrison D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018;7:198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 19.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 20.Dong Y., Xu M., Chen L., Bhochhibhoya A. Probiotic Foods and Supplements Interventions for Metabolic Syndromes: A Systematic Review and Meta-Analysis of Recent Clinical Trials. ANM. 2019;74:224–241. doi: 10.1159/000499028. [DOI] [PubMed] [Google Scholar]
- 21.Sivamaruthi B.S., Kesika P., Suganthy N., Chaiyasut C. A Review on Role of Microbiome in Obesity and Antiobesity Properties of Probiotic Supplements. [(accessed on 3 December 2019)]; doi: 10.1155/2019/3291367. Available online: https://www.hindawi.com/journals/bmri/2019/3291367/ [DOI] [PMC free article] [PubMed]
- 22.Koutnikova H., Genser B., Monteiro-Sepulveda M., Faurie J.-M., Rizkalla S., Schrezenmeir J., Clément K. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: A systematic review and meta-analysis of randomised controlled trials. BMJ. Open. 2019;9:e017995. doi: 10.1136/bmjopen-2017-017995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skonieczna-Żydecka K., Kaczmarczyk M., Łoniewski I., Lara L.F., Koulaouzidis A., Misera A., Maciejewska D., Marlicz W. A Systematic Review, Meta-Analysis, and Meta-Regression Evaluating the Efficacy and Mechanisms of Action of Probiotics and Synbiotics in the Prevention of Surgical Site Infections and Surgery-Related Complications. J. Clin. Med. 2018;7:556. doi: 10.3390/jcm7120556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marlicz W., Skonieczna-Zydecka K., Yung D.E., Loniewski I., Koulaouzidis A. Endoscopic findings and colonic perforation in microscopic colitis: A systematic review. Dig. Liver Dis. 2017;49:1073–1085. doi: 10.1016/j.dld.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Skonieczna-Żydecka K., Łoniewski I., Misera A., Stachowska E., Maciejewska D., Marlicz W., Galling B. Second-generation antipsychotics and metabolism alterations: A systematic review of the role of the gut microbiome. Psychopharmacology. 2018:1–22. doi: 10.1007/s00213-018-5102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agerbaek M., Gerdes L.U., Richelsen B. Hypocholesterolaemic effect of a new fermented milk product in healthy middle-aged men. Eur. J. Clin. Nutr. 1995;49:346–352. [PubMed] [Google Scholar]
- 30.Agerholm-Larsen L., Raben A., Haulrik N., Hansen A.S., Manders M., Astrup A. Effect of 8 week intake of probiotic milk products on risk factors for cardiovascular diseases. Eur. J. Clin. Nutr. 2000;54:288–297. doi: 10.1038/sj.ejcn.1600937. [DOI] [PubMed] [Google Scholar]
- 31.Ahn H.Y., Kim M., Ahn Y.-T., Sim J.-H., Choi I.-D., Lee S.-H., Lee J.H. The triglyceride-lowering effect of supplementation with dual probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032: Reduction of fasting plasma lysophosphatidylcholines in nondiabetic and hypertriglyceridemic subjects. Nutr. Metab. Cardiovasc. Dis. 2015;25:724–733. doi: 10.1016/j.numecd.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Ahn H.Y., Kim M., Chae J.S., Ahn Y.-T., Sim J.-H., Choi I.-D., Lee S.-H., Lee J.H. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduces fasting triglycerides and enhances apolipoprotein A-V levels in non-diabetic subjects with hypertriglyceridemia. Atherosclerosis. 2015;241:649–656. doi: 10.1016/j.atherosclerosis.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 33.Andrade S., Borges N. Effect of fermented milk containing Lactobacillus acidophilus and Bifidobacterium longum on plasma lipids of women with normal or moderately elevated cholesterol. J. Dairy Res. 2009;76:469–474. doi: 10.1017/S0022029909990173. [DOI] [PubMed] [Google Scholar]
- 34.Bjerg A.T., Kristensen M., Ritz C., Stark K.D., Holst J.J., Leser T.D., Wellejus A., Astrup A. Four weeks supplementation with Lactobacillus paracasei subsp. paracasei L. casei W8® shows modest effect on triacylglycerol in young healthy adults. Benef. Microbes. 2015;6:29–39. doi: 10.3920/BM2014.0058. [DOI] [PubMed] [Google Scholar]
- 35.Boesmans L., Valles-Colomer M., Wang J., Eeckhaut V., Falony G., Ducatelle R., Van Immerseel F., Raes J., Verbeke K. Butyrate Producers as Potential Next-Generation Probiotics: Safety Assessment of the Administration of Butyricicoccus pullicaecorum to Healthy Volunteers. mSystems. 2018;3:e00094-18. doi: 10.1128/mSystems.00094-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brahe L.K., Le Chatelier E., Prifti E., Pons N., Kennedy S., Blædel T., Håkansson J., Dalsgaard T.K., Hansen T., Pedersen O., et al. Dietary modulation of the gut microbiota--a randomised controlled trial in obese postmenopausal women. Br. J. Nutr. 2015;114:406–417. doi: 10.1017/S0007114515001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bukowska H., Pieczul-Mróz J., Jastrzebska M., Chełstowski K., Naruszewicz M. Decrease in fibrinogen and LDL-cholesterol levels upon supplementation of diet with Lactobacillus plantarum in subjects with moderately elevated cholesterol. Atherosclerosis. 1998;137:437–438. doi: 10.1016/s0021-9150(97)00283-9. [DOI] [PubMed] [Google Scholar]
- 38.Chang B.J., Park S.U., Jang Y.S., Ko S.H., Joo N.M., Kim S.I., Kim C.-H., Chang D.K. Effect of functional yogurt NY-YP901 in improving the trait of metabolic syndrome. Eur. J. Clin. Nutr. 2011;65:1250–1255. doi: 10.1038/ejcn.2011.115. [DOI] [PubMed] [Google Scholar]
- 39.Cox A.J., West N.P., Horn P.L., Lehtinen M.J., Koerbin G., Pyne D.B., Lahtinen S.J., Fricker P.A., Cripps A.W. Effects of probiotic supplementation over 5 months on routine haematology and clinical chemistry measures in healthy active adults. Eur. J. Clin. Nutr. 2014;68:1255–1257. doi: 10.1038/ejcn.2014.137. [DOI] [PubMed] [Google Scholar]
- 40.de Roos N.M., van Hemert S., Rovers J.M.P., Smits M.G., Witteman B.J.M. The effects of a multispecies probiotic on migraine and markers of intestinal permeability-results of a randomized placebo-controlled study. Eur. J. Clin. Nutr. 2017;71:1455–1462. doi: 10.1038/ejcn.2017.57. [DOI] [PubMed] [Google Scholar]
- 41.Fabian E., Elmadfa I. Influence of daily consumption of probiotic and conventional yoghurt on the plasma lipid profile in young healthy women. Ann. Nutr. Metab. 2006;50:387–393. doi: 10.1159/000094304. [DOI] [PubMed] [Google Scholar]
- 42.Gleeson M., Bishop N.C., Oliveira M., McCauley T., Tauler P., Lawrence C. Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2012;22:235–242. doi: 10.1123/ijsnem.22.4.235. [DOI] [PubMed] [Google Scholar]
- 43.Gohel M.K., Prajapati J.B., Mudgal S.V., Pandya H.V., Singh U.S., Trivedi S.S., Phatak A.G., Patel R.M. Effect of Probiotic Dietary Intervention on Calcium and Haematological Parameters in Geriatrics. J. Clin. Diagn. Res. 2016;10:LC05–LC09. doi: 10.7860/JCDR/2016/18877.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomes A.C., de Sousa R.G.M., Botelho P.B., Gomes T.L.N., Prada P.O., Mota J.F. The additional effects of a probiotic mix on abdominal adiposity and antioxidant Status: A double-blind, randomized trial. Obesity (Silver Spring) 2017;25:30–38. doi: 10.1002/oby.21671. [DOI] [PubMed] [Google Scholar]
- 45.Greany K.A., Bonorden M.J.L., Hamilton-Reeves J.M., McMullen M.H., Wangen K.E., Phipps W.R., Feirtag J., Thomas W., Kurzer M.S. Probiotic capsules do not lower plasma lipids in young women and men. Eur. J. Clin. Nutr. 2008;62:232–237. doi: 10.1038/sj.ejcn.1602719. [DOI] [PubMed] [Google Scholar]
- 46.Guillemard E., Tanguy J., Flavigny A., de la Motte S., Schrezenmeir J. Effects of consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114 001 on common respiratory and gastrointestinal infections in shift workers in a randomized controlled trial. J. Am. Coll. Nutr. 2010;29:455–468. doi: 10.1080/07315724.2010.10719882. [DOI] [PubMed] [Google Scholar]
- 47.Hatakka K., Mutanen M., Holma R., Saxelin M., Korpela R. Lactobacillus rhamnosus LC705 together with Propionibacterium freudenreichii ssp shermanii JS administered in capsules is ineffective in lowering serum lipids. J. Am. Coll. Nutr. 2008;27:441–447. doi: 10.1080/07315724.2008.10719723. [DOI] [PubMed] [Google Scholar]
- 48.Hibberd A.A., Yde C.C., Ziegler M.L., Honoré A.H., Saarinen M.T., Lahtinen S., Stahl B., Jensen H.M., Stenman L.K. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes. 2019;10:121–135. doi: 10.3920/BM2018.0028. [DOI] [PubMed] [Google Scholar]
- 49.Higashikawa F., Noda M., Awaya T., Danshiitsoodol N., Matoba Y., Kumagai T., Sugiyama M. Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2016;70:582–587. doi: 10.1038/ejcn.2016.17. [DOI] [PubMed] [Google Scholar]
- 50.Ibrahim N.S., Muhamad A.S., Ooi F.K., Meor-Osman J., Chen C.K. The effects of combined probiotic ingestion and circuit training on muscular strength and power and cytokine responses in young males. Appl. Physiol. Nutr. Metab. 2018;43:180–186. doi: 10.1139/apnm-2017-0464. [DOI] [PubMed] [Google Scholar]
- 51.Inoue T., Kobayashi Y., Mori N., Sakagawa M., Xiao J.-Z., Moritani T., Sakane N., Nagai N. Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef. Microbes. 2018;9:843–853. doi: 10.3920/BM2017.0193. [DOI] [PubMed] [Google Scholar]
- 52.Ito M., Kusuhara S., Yokoi W., Sato T., Ishiki H., Miida S., Matsui A., Nakamori K., Nonaka C., Miyazaki K. Streptococcus thermophilus fermented milk reduces serum MDA-LDL and blood pressure in healthy and mildly hypercholesterolaemic adults. Benef. Microbes. 2017;8:171–178. doi: 10.3920/BM2016.0102. [DOI] [PubMed] [Google Scholar]
- 53.Ivey K.L., Hodgson J.M., Kerr D.A., Lewis J.R., Thompson P.L., Prince R.L. The effects of probiotic bacteria on glycaemic control in overweight men and women: A randomised controlled trial. Eur. J. Clin. Nutr. 2014;68:447–452. doi: 10.1038/ejcn.2013.294. [DOI] [PubMed] [Google Scholar]
- 54.Ivey K.L., Hodgson J.M., Kerr D.A., Thompson P.L., Stojceski B., Prince R.L. The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015;25:46–51. doi: 10.1016/j.numecd.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 55.Jones M.L., Martoni C.J., Prakash S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: A randomized controlled trial. Eur. J. Clin. Nutr. 2012;66:1234–1241. doi: 10.1038/ejcn.2012.126. [DOI] [PubMed] [Google Scholar]
- 56.Kadooka Y., Sato M., Imaizumi K., Ogawa A., Ikuyama K., Akai Y., Okano M., Kagoshima M., Tsuchida T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010;64:636–643. doi: 10.1038/ejcn.2010.19. [DOI] [PubMed] [Google Scholar]
- 57.Kadooka Y., Sato M., Ogawa A., Miyoshi M., Uenishi H., Ogawa H., Ikuyama K., Kagoshima M., Tsuchida T. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br. J. Nutr. 2013;110:1696–1703. doi: 10.1017/S0007114513001037. [DOI] [PubMed] [Google Scholar]
- 58.Kawase M., Hashimoto H., Hosoda M., Morita H., Hosono A. Effect of administration of fermented milk containing whey protein concentrate to rats and healthy men on serum lipids and blood pressure. J. Dairy Sci. 2000;83:255–263. doi: 10.3168/jds.S0022-0302(00)74872-7. [DOI] [PubMed] [Google Scholar]
- 59.Kim J., Yun J.M., Kim M.K., Kwon O., Cho B. Lactobacillus gasseri BNR17 Supplementation Reduces the Visceral Fat Accumulation and Waist Circumference in Obese Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Med. Food. 2018;21:454–461. doi: 10.1089/jmf.2017.3937. [DOI] [PubMed] [Google Scholar]
- 60.Kim M., Kim M., Kang M., Yoo H.J., Kim M.S., Ahn Y.-T., Sim J.-H., Jee S.H., Lee J.H. Effects of weight loss using supplementation with Lactobacillus strains on body fat and medium-chain acylcarnitines in overweight individuals. Food Funct. 2017;8:250–261. doi: 10.1039/C6FO00993J. [DOI] [PubMed] [Google Scholar]
- 61.Klein A., Friedrich U., Vogelsang H., Jahreis G. Lactobacillus acidophilus 74-2 and Bifidobacterium animalis subsp lactis DGCC 420 modulate unspecific cellular immune response in healthy adults. Eur. J. Clin. Nutr. 2008;62:584–593. doi: 10.1038/sj.ejcn.1602761. [DOI] [PubMed] [Google Scholar]
- 62.Lambert M.N.T., Thorup A.C., Hansen E.S.S., Jeppesen P.B. Combined Red Clover isoflavones and probiotics potently reduce menopausal vasomotor symptoms. PLoS ONE. 2017;12:e0176590. doi: 10.1371/journal.pone.0176590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee Y., Ba Z., Roberts R.F., Rogers C.J., Fleming J.A., Meng H., Furumoto E.J., Kris-Etherton P.M. Effects of Bifidobacterium animalis subsp. lactis BB-12® on the lipid/lipoprotein profile and short chain fatty acids in healthy young adults: A randomized controlled trial. Nutr. J. 2017;16:39. doi: 10.1186/s12937-017-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin S.Y., Ayres J.W., Winkler W., Sandine W.E. Lactobacillus effects on cholesterol: In vitro and in vivo results. J. Dairy Sci. 1989;72:2885–2899. doi: 10.3168/jds.S0022-0302(89)79439-X. [DOI] [PubMed] [Google Scholar]
- 65.Macfarlane S., Cleary S., Bahrami B., Reynolds N., Macfarlane G.T. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: A randomised, double-blind, placebo-controlled crossover study. Aliment. Pharmacol. Ther. 2013;38:804–816. doi: 10.1111/apt.12453. [DOI] [PubMed] [Google Scholar]
- 66.Madjd A., Taylor M.A., Mousavi N., Delavari A., Malekzadeh R., Macdonald I.A., Farshchi H.R. Comparison of the effect of daily consumption of probiotic compared with low-fat conventional yogurt on weight loss in healthy obese women following an energy-restricted diet: A randomized controlled trial. Am. J. Clin. Nutr. 2016;103:323–329. doi: 10.3945/ajcn.115.120170. [DOI] [PubMed] [Google Scholar]
- 67.Mohammad Moradi S., Javidan A., Naji Isfahani H. Effects of probiotic ultra-filtered feta cheese and raw chicory root extract on lipid profile in healthy adult volunteers: A triple-blinded randomized controlled trial. Mediterr. J. Nutr. Metab. 2013;6:199–206. doi: 10.1007/s12349-013-0130-6. [DOI] [Google Scholar]
- 68.Naruszewicz M., Johansson M.-L., Zapolska-Downar D., Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am. J. Clin. Nutr. 2002;76:1249–1255. doi: 10.1093/ajcn/76.6.1249. [DOI] [PubMed] [Google Scholar]
- 69.Nishiyama K., Kobayashi T., Sato Y., Watanabe Y., Kikuchi R., Kanno R., Koshizuka T., Miyazaki N., Ishioka K., Suzutani T. A Double-Blind Controlled Study to Evaluate the Effects of Yogurt Enriched with Lactococcus lactis 11/19-B1 and Bifidobacterium lactis on Serum Low-Density Lipoprotein Level and Antigen-Specific Interferon-γ Releasing Ability. Nutrients. 2018;10:1778. doi: 10.3390/nu10111778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nova E., Viadel B., Wärnberg J., Carreres J.E., Marcos A. Beneficial effects of a synbiotic supplement on self-perceived gastrointestinal well-being and immunoinflammatory status of healthy adults. J. Med. Food. 2011;14:79–85. doi: 10.1089/jmf.2008.0328. [DOI] [PubMed] [Google Scholar]
- 71.Ostan R., Béné M.C., Spazzafumo L., Pinto A., Donini L.M., Pryen F., Charrouf Z., Valentini L., Lochs H., Bourdel-Marchasson I., et al. Impact of diet and nutraceutical supplementation on inflammation in elderly people. Results from the RISTOMED. study, an open-label randomized control trial. Clin. Nutr. 2016;35:812–818. doi: 10.1016/j.clnu.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 72.Osterberg K.L., Boutagy N.E., McMillan R.P., Stevens J.R., Frisard M.I., Kavanaugh J.W., Davy B.M., Davy K.P., Hulver M.W. Probiotic supplementation attenuates increases in body mass and fat mass during high-fat diet in healthy young adults. Obesity (Silver Spring) 2015;23:2364–2370. doi: 10.1002/oby.21230. [DOI] [PubMed] [Google Scholar]
- 73.Rajkumar H., Mahmood N., Kumar M., Varikuti S.R., Challa H.R., Myakala S.P. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: A randomized, controlled trial. Mediators Inflamm. 2014;2014:348959. doi: 10.1155/2014/348959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sadrzadeh-Yeganeh H., Elmadfa I., Djazayery A., Jalali M., Heshmat R., Chamary M. The effects of probiotic and conventional yoghurt on lipid profile in women. Br. J. Nutr. 2010;103:1778–1783. doi: 10.1017/S0007114509993801. [DOI] [PubMed] [Google Scholar]
- 75.Sanchez M., Darimont C., Drapeau V., Emady-Azar S., Lepage M., Rezzonico E., Ngom-Bru C., Berger B., Philippe L., Ammon-Zuffrey C., et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 2014;111:1507–1519. doi: 10.1017/S0007114513003875. [DOI] [PubMed] [Google Scholar]
- 76.Savard P., Lamarche B., Paradis M.-E., Thiboutot H., Laurin É., Roy D. Impact of Bifidobacterium animalis subsp. lactis BB-12 and, Lactobacillus acidophilus LA-5-containing yoghurt, on fecal bacterial counts of healthy adults. Int. J. Food Microbiol. 2011;149:50–57. doi: 10.1016/j.ijfoodmicro.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 77.Simon M.-C., Strassburger K., Nowotny B., Kolb H., Nowotny P., Burkart V., Zivehe F., Hwang J.-H., Stehle P., Pacini G., et al. Intake of Lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: A proof of concept. Diabetes Care. 2015;38:1827–1834. doi: 10.2337/dc14-2690. [DOI] [PubMed] [Google Scholar]
- 78.Simons L.A., Amansec S.G., Conway P. Effect of Lactobacillus fermentum on serum lipids in subjects with elevated serum cholesterol. Nutr. Metab. Cardiovasc. Dis. 2006;16:531–535. doi: 10.1016/j.numecd.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 79.Stenman L.K., Lehtinen M.J., Meland N., Christensen J.E., Yeung N., Saarinen M.T., Courtney M., Burcelin R., Lähdeaho M.-L., Linros J., et al. Probiotic With or Without Fiber Controls Body Fat Mass, Associated With Serum Zonulin, in Overweight and Obese Adults-Randomized Controlled Trial. EBioMedicine. 2016;13:190–200. doi: 10.1016/j.ebiom.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Szulińska M., Łoniewski I., Skrypnik K., Sobieska M., Korybalska K., Suliburska J., Bogdański P. Multispecies Probiotic Supplementation Favorably Affects Vascular Function and Reduces Arterial Stiffness in Obese Postmenopausal Women-A 12-Week Placebo-Controlled and Randomized Clinical Study. Nutrients. 2018;10:1672. doi: 10.3390/nu10111672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szulińska M., Łoniewski I., van Hemert S., Sobieska M., Bogdański P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients. 2018;10:773. doi: 10.3390/nu10060773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tenore G.C., Caruso D., Buonomo G., D’Avino M., Ciampaglia R., Maisto M., Schisano C., Bocchino B., Novellino E. Lactofermented Annurca Apple Puree as a Functional Food Indicated for the Control of Plasma Lipid and Oxidative Amine Levels: Results from a Randomised Clinical Trial. Nutrients. 2019;11:122. doi: 10.3390/nu11010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trautvetter U., Ditscheid B., Kiehntopf M., Jahreis G. A combination of calcium phosphate and probiotics beneficially influences intestinal lactobacilli and cholesterol metabolism in humans. Clin. Nutr. 2012;31:230–237. doi: 10.1016/j.clnu.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 84.Usinger L., Jensen L.T., Flambard B., Linneberg A., Ibsen H. The antihypertensive effect of fermented milk in individuals with prehypertension or borderline hypertension. J. Hum. Hypertens. 2010;24:678–683. doi: 10.1038/jhh.2010.4. [DOI] [PubMed] [Google Scholar]
- 85.Valentini L., Pinto A., Bourdel-Marchasson I., Ostan R., Brigidi P., Turroni S., Hrelia S., Hrelia P., Bereswill S., Fischer A., et al. Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota - The “RISTOMED. project”: Randomized controlled trial in healthy older people. Clin. Nutr. 2015;34:593–602. doi: 10.1016/j.clnu.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 86.Välimäki I.A., Vuorimaa T., Ahotupa M., Kekkonen R., Korpela R., Vasankari T. Decreased training volume and increased carbohydrate intake increases oxidized LDL levels. Int. J. Sports Med. 2012;33:291–296. doi: 10.1055/s-0031-1291223. [DOI] [PubMed] [Google Scholar]
- 87.Venkataraman R., Juwal J., Princy J. The effect of probiotics on glycemic index. Panminerva Med. 2018;60:234–235. doi: 10.23736/S0031-0808.18.03454-7. [DOI] [PubMed] [Google Scholar]
- 88.Xiao J.Z., Kondo S., Takahashi N., Miyaji K., Oshida K., Hiramatsu A., Iwatsuki K., Kokubo S., Hosono A. Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers. J. Dairy Sci. 2003;86:2452–2461. doi: 10.3168/jds.S0022-0302(03)73839-9. [DOI] [PubMed] [Google Scholar]
- 89.Zarrati M., Salehi E., Nourijelyani K., Mofid V., Zadeh M.J.H., Najafi F., Ghaflati Z., Bidad K., Chamari M., Karimi M., et al. Effects of probiotic yogurt on fat distribution and gene expression of proinflammatory factors in peripheral blood mononuclear cells in overweight and obese people with or without weight-loss diet. J. Am. Coll. Nutr. 2014;33:417–425. doi: 10.1080/07315724.2013.874937. [DOI] [PubMed] [Google Scholar]
- 90.Rajkumar H., Kumar M., Das N., Kumar S.N., Challa H.R., Nagpal R. Effect of Probiotic Lactobacillus salivarius UBL S22 and Prebiotic Fructo-oligosaccharide on Serum Lipids, Inflammatory Markers, Insulin Sensitivity, and Gut Bacteria in Healthy Young Volunteers: A Randomized Controlled Single-Blind Pilot Study. J. Cardiovasc. Pharmacol. Ther. 2015;20:289–298. doi: 10.1177/1074248414555004. [DOI] [PubMed] [Google Scholar]
- 91.Blüher M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 92.Piera-Mardemootoo C., Lambert P., Faillie J.-L. Efficacy of metformin on glycemic control and weight in drug-naive type 2 diabetes mellitus patients: A systematic review and meta-analysis of placebo-controlled randomized trials. Therapie. 2018 doi: 10.1016/j.therap.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Q., Wu Y., Fei X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina. 2016;52:28–34. doi: 10.1016/j.medici.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 94.Pan J., Pan Q., Chen Y., Zhang H., Zheng X. Efficacy of probiotic supplement for gestational diabetes mellitus: A systematic review and meta-analysis. J. Matern. Fetal. Neonatal. Med. 2019;32:317–323. doi: 10.1080/14767058.2017.1376318. [DOI] [PubMed] [Google Scholar]
- 95.Ardeshirlarijani E., Tabatabaei-Malazy O., Mohseni S., Qorbani M., Larijani B., Baradar Jalili R. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: A meta-analysis of randomized trials. Daru. 2019;27:827–837. doi: 10.1007/s40199-019-00302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crovesy L., Ostrowski M., Ferreira D.M.T.P., Rosado E.L., Soares-Mota M. Effect of Lactobacillus on body weight and body fat in overweight subjects: A systematic review of randomized controlled clinical trials. Int. J. Obes. (Lond) 2017;41:1607–1614. doi: 10.1038/ijo.2017.161. [DOI] [PubMed] [Google Scholar]
- 97.John G.K., Wang L., Nanavati J., Twose C., Singh R., Mullin G. Dietary Alteration of the Gut Microbiome and Its Impact on Weight and Fat Mass: A Systematic Review and Meta-Analysis. Genes (Basel) 2018;9:167. doi: 10.3390/genes9030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.(12) (PDF) Lactobacillus rhamnosus CNCMI-4317 Modulates Fiaf/Angpt14 in Intestinal Epithelial Cells and Circulating Level in Mice. [(accessed on 16 February 2020)]; Available online: https://www.researchgate.net/publication/282646122_Lactobacillus_rhamnosus_CNCMI-4317_Modulates_FiafAngpt14_in_Intestinal_Epithelial_Cells_and_Circulating_Level_in_Mice.
- 99.Borgeraas H., Johnson L.K., Skattebu J., Hertel J.K., Hjelmesaeth J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2018;19:219–232. doi: 10.1111/obr.12626. [DOI] [PubMed] [Google Scholar]
- 100.Depommier C., Everard A., Druart C., Plovier H., Van Hul M., Vieira-Silva S., Falony G., Raes J., Maiter D., Delzenne N.M., et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019;25:1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Omar J.M., Chan Y.-M., Jones M.L., Prakash S., Jones P.J.H. Lactobacillus fermentum and Lactobacillus amylovorus as probiotics alter body adiposity and gut microflora in healthy persons. J. Funct. Foods. 2013;5:116–123. doi: 10.1016/j.jff.2012.09.001. [DOI] [Google Scholar]
- 102.Raygan F., Rezavandi Z., Bahmani F., Ostadmohammadi V., Mansournia M.A., Tajabadi-Ebrahimi M., Borzabadi S., Asemi Z. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol. Metab. Syndr. 2018;10:51. doi: 10.1186/s13098-018-0353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Samah S., Ramasamy K., Lim S.M., Neoh C.F. Probiotics for the management of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2016;118:172–182. doi: 10.1016/j.diabres.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 104.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M., et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang X., Shen D., Fang Z., Jie Z., Qiu X., Zhang C., Chen Y., Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE. 2013;8:e71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Akram Kooshki A., Tofighiyan T., Rakhshani M.H. Effects of Synbiotics on Inflammatory Markers in Patients With Type 2 Diabetes Mellitus. Glob. J. Health Sci. 2015;7:1–5. doi: 10.5539/gjhs.v7n7p1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng H.J., Guo J., Jia Q., Huang Y.S., Huang W.-J., Zhang W., Zhang F., Liu W.J., Wang Y. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019;142:303–313. doi: 10.1016/j.phrs.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 108.Tabrizi R., Ostadmohammadi V., Lankarani K.B., Akbari M., Akbari H., Vakili S., Shokrpour M., Kolahdooz F., Rouhi V., Asemi Z. The effects of probiotic and synbiotic supplementation on inflammatory markers among patients with diabetes: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2019;852:254–264. doi: 10.1016/j.ejphar.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 109.Wang L., Guo M.-J., Gao Q., Yang J.-F., Yang L., Pang X.-L., Jiang X.-J. The effects of probiotics on total cholesterol. Medicine (Baltimore) 2018;97:e9679. doi: 10.1097/MD.0000000000009679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cho Y.A., Kim J. Effect of Probiotics on Blood Lipid Concentrations: A Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore) 2015;94:e1714. doi: 10.1097/MD.0000000000001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shimizu M., Hashiguchi M., Shiga T., Tamura H., Mochizuki M. Meta-Analysis: Effects of Probiotic Supplementation on Lipid Profiles in Normal to Mildly Hypercholesterolemic Individuals. PLoS ONE. 2015;10:e0139795. doi: 10.1371/journal.pone.0139795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ishimwe N., Daliri E.B., Lee B.H., Fang F., Du G. The perspective on cholesterol-lowering mechanisms of probiotics. Mol. Nutr. Food Res. 2015;59:94–105. doi: 10.1002/mnfr.201400548. [DOI] [PubMed] [Google Scholar]
- 113.Khalesi S., Sun J., Buys N., Jayasinghe R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 114.Newson L. Menopause and cardiovascular disease. Post. Reprod. Health. 2018;24:44–49. doi: 10.1177/2053369117749675. [DOI] [PubMed] [Google Scholar]
- 115.Sterne J.A.C., Sutton A.J., Ioannidis J.P.A., Terrin N., Jones D.R., Lau J., Carpenter J., Rücker G., Harbord R.M., Schmid C.H., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.