Abstract
Advances in cancer care have led to improved survival, which, coupled with demographic trends, have contributed to rapid growth in the number of patients needing cancer care services. However, with increasing caseload, care complexity, and administrative burden, the current workforce is ill equipped to meet these burgeoning new demands. These trends have contributed to clinician burnout, compounding a widening workforce shortage. Moreover, family caregivers, who have unique knowledge of patient preferences, symptoms, and goals of care, are infrequently appreciated and supported as integral members of the oncology “careforce.” A crisis is looming, which will hinder access to timely, high-quality cancer care if left unchecked. Stemming from the proceedings of a 2019 workshop convened by the National Cancer Policy Forum of the National Academies of Sciences, Engineering, and Medicine, this commentary characterizes the factors contributing to an increasingly strained oncology careforce and presents multilevel strategies to improve its efficiency, effectiveness, and resilience. Together, these will enable today’s oncology careforce to provide high-quality care to more patients while improving the patient, caregiver, and clinician experience.
R.A. is a 67-year-old male with metastatic non-small cell lung cancer. He returns to the oncology clinic for follow-up after his disease progressed on frontline chemoimmunotherapy. His daughter, who is his primary caregiver, accompanies him to the visit, necessitating another missed day of work. One hour after his scheduled appointment time, they finally meet with the oncologist, who relays the results of genomic testing that indicate a neurotrophic tyrosine receptor kinase (NTRK) gene fusion. Given the rarity of this genetic aberration, expert consultation is advised. His oncologist explains that her office will prepare and send records to the consulting oncologist while also initiating the prior authorization process for insurance approval of a new NTRK-targeted therapy. His daughter then reminds him to report his worsening cough and shortness of breath, reiterating concerns previously conveyed to the nurse practitioner weeks earlier. There is no discussion of his growing feelings of sadness or anxiety. The visit ends after 15 minutes with a tentative plan for treatment with an NTRK inhibitor pending expert consultation and insurance approval. The first available appointment with the expert consultant is in 3 weeks.
The experiences of this patient with cancer, his family caregiver, and their clinicians are not uncommon and reflect emerging trends in cancer care delivery: increasing caseload and complexity of cancer care combined with insufficient growth and adaptation of the cancer workforce to meet burgeoning new demands. These trends have contributed to clinician burnout, compounding a widening workforce shortage. Moreover, family caregivers, who have unique knowledge of patient preferences, symptoms, and goals of care, are infrequently appreciated and supported as integral members of the oncology “careforce.”
Inspired by the proceedings of a 2019 workshop convened by the National Cancer Policy Forum of the National Academies of Sciences, Engineering, and Medicine (1), this commentary characterizes the factors contributing to an increasingly strained oncology careforce and presents strategies and actions for improving its efficiency, effectiveness, and resilience.
Factors Contributing to a Stressed Oncology Careforce
Demographic and Workforce Trends
Current and projected trends in cancer incidence, survivorship, and mortality, coupled with population-based trends, portend a precipitous rise in the absolute number of patients with cancer in the future. Although age-specific cancer incidence rates have declined over the past 20 years, the growth and aging of the US population, increase in obesity-related cancers, changing screening and diagnostic practices, and improved survival and survivorship are contributing to an increased number of patients living with cancer (2,3). It is estimated that 2.1 million new cancer cases were diagnosed in the United States in 2018, and this figure is expected to grow to 2.7 million by 2030 (4). The absolute number of cancer survivors is expected to grow from 16.9 million in 2019 to 22.1 million by 2030 (5).
Meanwhile, the available supply of cancer clinicians has failed to keep pace with this rapid growth in demand for oncology care. A 2007 study commissioned by the American Society of Clinical Oncology (ASCO ) projected a substantial shortage of oncologists by 2020 due to an aging oncology workforce, declining pool of fellowship applicants, and increasing number of patients with cancer (6). This galvanized ASCO’s Workforce Implementation Group and a related National Cancer Policy Forum workshop in 2008, whose themes included increased use of nonphysician clinicians, better coordination of care with nononcologists, research in care delivery efficiency, and augmented efforts to train new oncologists (7,8). Although there have been modest improvements in the recruitment of oncologists since then (9), a workforce shortage of over 2000 oncologists is still projected by 2025 (10). This is partly due to the fact that aggregate workforce capacity is defined not only by the supply of clinicians but also by the efficiency of practice—that is, how many cancer patients can be cared for by a clinician or clinical team. Strategies promoting meaningful improvements in efficiency have been lacking.
An assessment of the nonphysician cancer workforce has proven methodologically challenging owing to limitations in identifying oncology-focused nonphysician clinicians. There are currently more than 3 million registered nurses in the United States, and this workforce is expected to grow to 3.9 million by 2030 (11). Variations in geographic and specialty distribution are projected to lead to both regional surpluses and shortages of oncology-focused registered nurses by 2030. Advanced practice providers (APPs), including nurse practitioners and physician assistants, are increasingly important members of the oncology care team. A recent survey identified at least 5350 APPs in oncology (and an additional 5400 who might practice oncology) and found that among 577 survey respondents, the majority (90%) reported satisfaction with career choice and spent the majority (80%) of time in direct patient care (12).
Informal caregivers—family members or friends who spend a substantial amount of time, energy, and costs caring for a loved one with cancer—represent vital members of the cancer careforce and historically have been underappreciated and unsupported. As of June 2016, there were an estimated 2.8 million informal cancer caregivers (mean age 53 years, 58% female), of whom nearly three-quarters were family members of the recipient of care (13). However, such caregivers are seldom integrated into the care team, formally trained, or compensated for their time and efforts (14–18). Moreover, high direct costs (ie, out of pocket) and indirect costs (ie, lost work productivity) are burdensome for informal caregivers, and their contributions to caregiving are far from optimized (19–23).
Increasing Complexity of Cancer Care
Rapid advances in oncology research, new technologies, and an expanding clinical evidence base have contributed to an increasingly complex landscape of cancer care delivery. Precision oncology necessitates the use of advanced genomic and proteomic tumor profiling to identify and match actionable mutations and other biomarkers with immunotherapies or targeted therapies (24). The number of US Food and Drug Administration (FDA)-approved targeted and biomarker-driven therapies and indications has grown dramatically in recent years, and many more are in development and clinical trials (25–27). In 2017, pembrolizumab received the FDA’s first tumor site-agnostic approval, instead requiring evidence of high microsatellite instability and/or mismatch repair deficiency (28). The same year saw the first approvals for adoptive cellular therapies in aggressive childhood leukemias and adult lymphomas (29,30). Although these collective achievements represent remarkable progress, they present unique implementation challenges for clinicians. For example, sophisticated approaches to diagnostic testing and the growing use of precision therapies for cancer treatment will increasingly necessitate collaboration and expert interpretation to inform clinical decision-making (31,32). Strategies to foster multidisciplinary care with integrated teams of pathologists, radiologists, and oncologists will be critical to addressing the increasing complexity of cancer care, particularly within community oncology practices that are often staffed by general oncologists who care for patients with many different diseases and where most patients with cancer receive their care (33,34).
Increasing Administrative Burden
Clinicians and oncology practices also face unprecedented clerical burden—by some estimates consuming up to 25% of their time—in the form of inconsistent electronic health record (EHR) usability, documentation and billing requirements, and prior authorizations (35,36). Over the past two decades, rapid digitization of the health-care delivery system has led to almost all US hospitals and the majority (80%) of office-based practices using certified EHRs (37). Although meaningful EHR adoption has been associated with improvements in quality, safety, efficiency, and mortality (38,39), unintended consequences abound (40–43). In a recent commentary, one physician noted: “I am always multitasking… I am entering orders, checking labs, downloading information while I talk to the patient. It requires chronic hypervigilance, which is exhausting” (44).
Experts have argued that EHR documentation to fulfill billing and regulatory requirements is a root cause of suboptimal usability and contributes to clinician burnout (45–47). Excessive and redundant quality reporting requirements add considerable strain to oncology practices. A recent study indicated that physicians in four common specialties spend, on average, 785 hours per physician and more than $15.4 billion dealing with the reporting of quality measures each year (48). A 2015 Institute of Medicine consensus report similarly concluded that the surfeit of quality metrics in use today are contributing to burdensome data collection, unclear prioritization of measures, and suboptimal effectiveness in improving health (49).
Recent evidence also suggests that the burden of prior authorizations in oncology practices is growing (50). Although intended to curb inappropriate resource utilization, prior authorizations often result in increased clerical load and care delays. In a 2017 ASCO census survey completed by 395 practices, nearly two-thirds (58%) identified payer strains as the top pressure in their daily practice, driven first by prior authorizations and second by coverage denials (51). More recently, an American Society for Radiation Oncology survey of 3882 radiation oncologists indicated that nearly all (93%) of the 620 respondents believed prior authorizations were associated with delayed receipt of life-prolonging therapies in addition to contributing to patient stress and wasted physician time (52).
Clinician Burnout
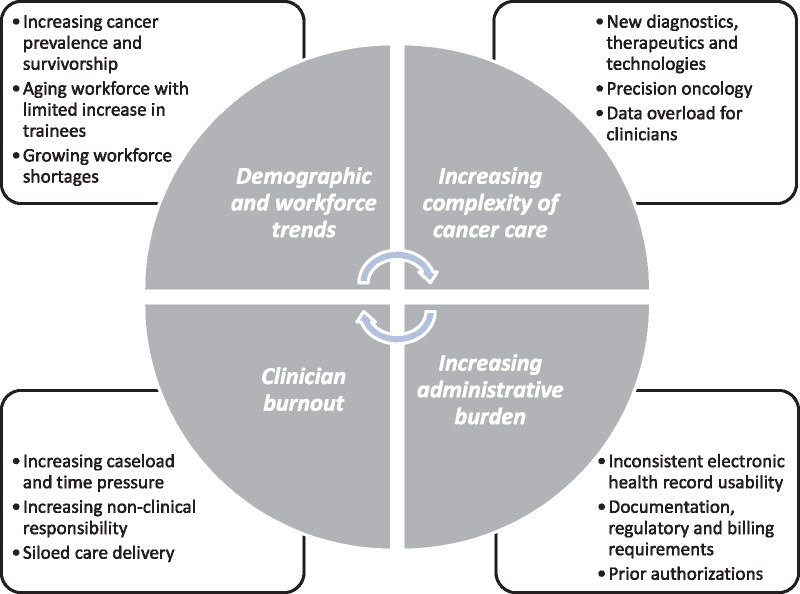
These conspiring workplace factors have contributed to clinician dissatisfaction and burnout (35). Burnout is a work-related stress syndrome, most commonly defined by emotional exhaustion, depersonalization, and decreased personal accomplishment (53). It is estimated that roughly one-third to one-half of oncologists experience burnout during their careers (54,55), which is comparable with the average rate of burnout among US physicians (56). Despite high job satisfaction relative to their physician colleagues, APPs experience comparable levels of burnout (57). Among oncology nurses, a recent meta-analysis found a burnout prevalence of approximately 30% (58). Such profound rates of burnout create a vicious cycle: limiting the clinical capacity of an already strained workforce, exacerbating existing shortages, and threatening the delivery of high-quality cancer care (59) (Figure 1).
Figure 1.
Factors contributing to a strained oncology careforce. Figure 1 depicts mutually reinforcing factors contributing to workforce and caregiver (“careforce”) stress. These include rising demand for oncology care services, inadequate growth in workforce supply, increasing complexity of cancer care, increasing administrative burden, and clinician burnout. Together, these create a vicious cycle of careforce strain.
Strategies to Build an Effective and Resilient Oncology Careforce
In response to these ominous trends in cancer care delivery, a 2019 National Cancer Policy Forum workshop convened a broad spectrum of stakeholders—including leadership from oncology physician and nursing societies, cancer care practitioners, researchers, payers, policymakers, and patient advocates—to discuss multilevel strategies to support and sustain an effective and resilient oncology careforce. There was agreement that solutions should be patient-centered, enhance both the patient and clinician experience, and improve the overall efficiency and quality of care delivery rather than simply augment workforce recruitment and supply. Moreover, there was agreement that optimizing the use of technology in routine care delivery should reinforce proposed solutions.
Strategies Focusing on Patients
Workshop attendees identified two strategies aimed primarily at improving the patient experience—enhancing patient navigation services and augmenting the patient voice in routine cancer care delivery—each with anticipated careforce benefits. The use of patient navigators seeks to improve the patient’s interaction and integration into the cancer care process, overcoming barriers to optimal care delivery. Navigators increase access to care across the cancer continuum and also serve as important liaisons and advocates, helping to streamline care. A recent review outlined numerous benefits of oncology patient navigator programs, including improved screening rates, adherence to recommended treatment, and timeliness of cancer care (60). Particularly when paired with enabling screening technologies and clinical pathways, patient navigator programs have the potential to improve both patient outcomes and practice efficiency (61), though additional research on the effects on workload and clinician well-being is warranted (62).
Once a patient initiates the cancer care process, growing evidence supports the routine collection and use of patient-reported outcomes (PROs), that is, reports of health status taken directly from patients without interpretation or amendment by clinicians or others (63). PROs capture the patient voice and experience in structured form and represent a vital data element within learning health-care systems (64). Systematic PRO collection has been associated with reduced acute care utilization, improved health-related quality of life, and lengthened overall survival among patients with advanced solid tumors (65–67). Despite their potential, PROs have not been widely implemented or adopted across oncology practices, in part due to concerns regarding detrimental impact on clinical workflows and clinician information overload (68–70). Workshop attendees emphasized the importance of making PRO data meaningful and actionable to clinical teams and in conducting further investigation of optimal implementation strategies.
Low-cost, technology-based approaches such as text messaging have shown promise as sustainable and scalable strategies for remote patient monitoring and engagement in oncology care (71,72). With advances in artificial intelligence, machine learning, and natural language processing, so-called “conversational agents”—systems that mimic human conversation using text or spoken language—have also entered the digital health landscape (73,74). These allow for real-time communication between patients and clinical teams and may assist teams in detecting symptoms and adverse effects earlier in their course, enabling proactive management. Preliminary data from the University of Pennsylvania on the use of a text-based conversational agent to support symptom management and oral anticancer agent adherence illustrate the potential of novel digital approaches to act as virtual adjuncts to the traditional care team, potentially improving both the quality and efficiency of care (75).
As new communication technologies are deployed, health-care organizations and oncology practices need to ensure they do not inadvertently perpetuate clinician burnout, practice inefficiencies, and patient or clinician dissatisfaction. Engaging clinicians and patients in the development of these tools, applying human-centered design principles, and optimizing implementation within clinical workflows will be critical to advancing progress (76).
Strategies Focusing on Informal Caregivers
Workshop attendees also identified opportunities to optimize the contributions of informal caregivers to the oncology careforce. These included formally recognizing them as central members of the care team, offering capacity assessment and skills training (eg, in symptom monitoring and management), and developing quality measures that reward team-based care centered on the patient and family caregivers. The Veterans Health Administration’s Program of Comprehensive Assistance for Family Caregivers—which has offered over 40 000 caregivers core curriculum training and direct financial assistance—could serve as a national model for caregiver support (77–80). For those with employer-sponsored insurance, insurance redesign (eg, lifetime patient cost-sharing caps) and improved workplace policy benefits (eg, paid family leave) could help address the direct and indirect financial hardships associated with informal caregiving.
The Symptom Care at Home (SCH) program (81) of the University of Utah’s Huntsman Cancer Institute illustrates the promise of dually engaging patients and family caregivers. The SCH program consists of electronic daily monitoring of 11 common patient symptoms and five indicators of caregiver well-being. Reports trigger automated, pathway-driven coaching to improve patient symptom management and caregiver well-being as well as automated alerts to clinicians. Preliminary results from randomized evaluation of the SCH program suggest a benefit to patients and caregivers alike, with reduced symptom burden and improved caregiver resilience compared with usual care (82,83). Moreover, the improvement in caregiver resilience and well-being was found to mediate the reduction in patient symptom burden, supporting the hypothesis that a caregiver’s health and well-being are integrally tied to patient outcomes.
Strategies Focusing on Clinicians
Faced with growing demand for cancer services and increasing care complexity, workshop attendees looked to other sectors for inspiration on how to organize the oncology careforce optimally. Whereas cancer care is typically delivered by groups of siloed health-care professionals, each acting independently and contributing individual expertise (ie, within the confines of the so-called “multidisciplinary team”), there was acknowledgment that such groups frequently fall short of true team-based standards. Instead, attendees envisioned a future in which cancer care was delivered by “high-performance teams.” (84) Such teams would be internally and externally recognized as such, committed to a shared vision and team-level objectives, and would regularly reflect on ways to improve team-level processes and outcomes. Additionally, high-performance teams would operate in a fully integrated manner, with shared respect, trust and accountability, high emotional intelligence, and strong lines of communication.
In 2016, the National Cancer Institute and ASCO launched the Teams in Cancer Care Delivery project with the goal of applying team science (85) to oncology care delivery. More than 20 teams participated in the inaugural workshop, engaging in iterative feedback with team scientists and exploring the application of team principles to problems in oncology. The results of subsequent team-based care initiatives were published in a themed issue of the Journal of Oncology Practice, highlighting numerous benefits to high-performance teams in oncology, including enhanced productivity and high satisfaction among team members (86–88). Most importantly, this served as proof-of-concept that groups could intentionally develop and cultivate attributes of high-performance teams through the application of team science principles.
An important characteristic of high-performance teams is having well-defined (yet flexible) roles and responsibilities among members. In the context of oncology care, this implies utilizing team members to the maximum extent of their ability and training. This can take many forms: shared clinical encounters, independent APP- or pharmacist-led visits, and effective collaborations among oncology care and primary care practices. Paired with individualized patient risk assessments, this also creates an opportunity for dedicated clinics provided by the service most suited to the intensity of patient needs. This is an extension of precision health care in which delivery structures themselves are tailored to the precise needs of patients. Examples include APP-led, risk-adapted survivorship and palliative care clinics (89,90), demonstration projects of the oncology medical home concept (91), and remote specialist consultation (92–94). Such models hold great promise for improving team effectiveness and efficiency.
Workshop participants also discussed strategies for reducing administrative burdens and practice inefficiencies. First, attendees advocated for EHR usability and safety standards and for decoupling clinical documentation from billing, regulatory, and administrative requirements as has been advocated by the American Medical Informatics Association (95). Second, attendees stressed the importance of streamlined quality measurement and reporting, using a consolidated set of measures consistent with the vision of the Core Quality Measures Collaborative (96). Ideally, such measures would be collected directly from the EHR, eliminating the requirement for manual data entry and enabling real-time performance measurement and quality improvement within a learning health system (97). Finally, attendees proposed eliminating prior authorization requirements and processes for therapies that are evidence-based and pathway-driven, such as those endorsed by the National Comprehensive Cancer Network, and streamlining the approval process for off-pathway therapies.
Implementation of these strategies would dramatically improve the work lives of cancer clinicians while allowing teams to care for more patients with cancer. But efforts targeting clinician burnout and supporting careforce resilience are critical adjuncts. For example, mindfulness programs designed to promote professional well-being have shown benefit for primary care physicians (98), and their use in oncology and palliative care practices is under investigation (99). Moreover, strong organizational leadership committed to promoting positive workplace culture has been associated with increased professional satisfaction and decreased burnout and should be purposefully cultivated (100,101).
Conclusion: Opportunities for Action
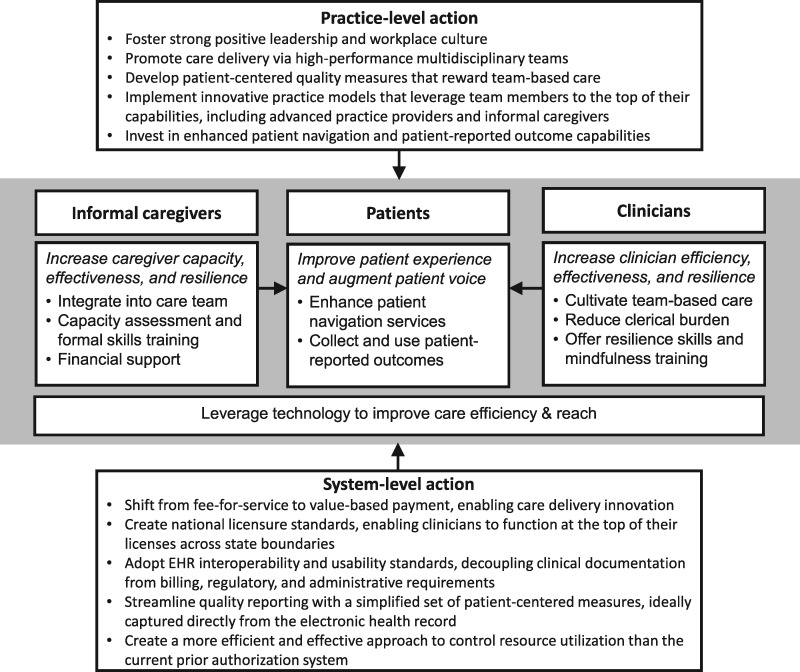
Considering the widening gap between the number of patients needing cancer care and the limited capacity of the current workforce to meet these demands, a crisis is looming, which will hinder access to timely, high-quality care if left unchecked. Because recruiting and training more cancer clinicians is unlikely to solve this problem alone, we posit that the most critical and feasible solution is to improve the efficiency with which cancer care is delivered, leveraging the strategies described above. Organizations should embark on practice-level changes to improve the effectiveness and resilience of their workforces, but system-level changes are also urgently needed and will require national will and coordinated efforts from regulatory agencies, payers, and practitioners. Stemming from the 2019 National Cancer Policy Forum workshop proceedings, we propose multilevel strategies focusing on patients, caregivers, and clinicians as well as practice- and system-level opportunities for action (Figure 2). Together, these will allow today’s oncology careforce to provide high-quality care to more patients while improving the patient, caregiver, and clinician experience.
Figure 2.
Multi-level strategies to sustain an effective and resilient oncology careforce. Figure 2 illustrates a conceptual model stemming from the National Cancer Policy Forum 2019 workshop proceedings, highlighting multi-level strategies to improve careforce capacity, effectiveness, efficiency, and resilience, as well as opportunities for action at the practice and system levels. EHR = electronic health record.
Funding
The activities of the National Cancer Policy Forum are supported by its sponsoring members, which currently include: the Centers for Disease Control and Prevention, the National Institutes of Health/National Cancer Institute, the American Association for Cancer Research, the American Cancer Society, the American College of Radiology, American Society of Clinical Oncology, the Association of Community Cancer Centers, the Association of American Cancer Institutes, Bristol-Myers Squibb, the Cancer Support Community, the CEO Roundtable on Cancer, Flatiron Health, Helsinn Therapeutics (US), Merck, the National Comprehensive Cancer Network, Novartis Oncology, the Oncology Nursing Society, and Pfizer.
Notes
Disclaimer: The funders had no role in the writing of this commentary or the decision to submit it for publication. The responsibility for the content of this article rests with the authors and does not necessarily represent the views of the National Academies of Sciences, Engineering, and Medicine or the institutional affiliations of the authors.
The authors thank the speakers and participants for their contributions to the workshop. The authors also extend their thanks to David A. Siegel for his contributions to an early draft of this manuscript.
EB and SN derive salary support from the pooled sponsor funds of the National Cancer Policy Forum, listed above. NJM is an employee of Flatiron Health, Inc., an independent subsidiary of the Roche Group. NJM holds equity interests in Flatiron Health and Roche. The authors otherwise report no conflicts of interest related to the content of this manuscript.
References
- 1.National Academies of Sciences, Engineering, and Medicine. Developing and sustaining an effective and resilient oncology careforce: A National Cancer Policy Forum Workshop. 2019. http://www.nationalacademies.org/hmd/Activities/Disease/NCPF/2019-FEB-11.aspx. Accessed August 23, 2019. [PubMed]
- 2. Siegel RL, Jemal A, Wender RC, Gansler T, Ma J, Brawley OW.. An assessment of progress in cancer control. CA Cancer J Clin. 2018;68(5):329–339. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 4.GLOBOCAN. 2018. http://gco.iarc.fr/tomorrow/home. Accessed July 16, 2019.
- 5. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. [DOI] [PubMed] [Google Scholar]
- 6. Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M.. Future supply and demand for oncologists: challenges to assuring access to oncology services . J Oncol Pract. 2007;3(2):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patlak M, Levit LA, National Cancer Policy Forum (U.S.) Ensuring Quality Cancer Care through the Oncology Workforce: Sustaining Care in the 21st Century: Workshop Summary. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 8. Levit L, Smith AP, Benz EJ, Ferrell B.. Ensuring quality cancer care through the oncology workforce. J Oncol Pract. 2010;6(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirkwood MK, Kosty MP, Bajorin DF, Bruinooge SS, Goldstein MA.. Tracking the workforce: the American Society of Clinical Oncology workforce information system. J Oncol Pract. 2013;9(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang W, Williams JH, Hogan PF, et al. Projected supply of and demand for oncologists and radiation oncologists through 2025: an aging, better-insured population will result in shortage. J Oncol Pract. 2014;10(1):39–45. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. Supply and Demand Projections of the Nursing Workforce: 2014-2030. 2017. https://bhw.hrsa.gov/sites/default/files/bhw/nchwa/projections/NCHWA_HRSA_Nursing_Report.pdf. Accessed July 17, 2019.
- 12. Bruinooge SS, Pickard TA, Vogel W, et al. Understanding the role of advanced practice providers in oncology in the United States. J Oncol Pract. 2018;14(9):e518–e532. [DOI] [PubMed] [Google Scholar]
- 13.National Alliance for Caregiving, in partnership with the National Cancer Institute and the Cancer Support Community. Cancer Caregiving in the U.S.: An Intense, Episodic, and Challenging Care Experience. 2016. https://www.caregiving.org/wp-content/uploads/2016/06/CancerCaregivingReport_FINAL_June-17-2016.pdf. Accessed July 17, 2019.
- 14. Rosland AM, Piette JD.. Emerging models for mobilizing family support for chronic disease management: a structured review. Chronic Illn. 2010;6(1):7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Ryn M, Sanders S, Kahn K, et al. Objective burden, resources, and other stressors among informal cancer caregivers: a hidden quality issue? Psychooncology. 2011;20(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schulz R, Eden J, National Academies of Sciences Engineering and Medicine (U.S.) Committee on Family Caregiving for Older Adults. Families Caring for an Aging America. Washington, DC: The National Academies Press; 2016. [PubMed] [Google Scholar]
- 17. Wolff JL, Spillman BC, Freedman VA, Kasper JD.. A national profile of family and unpaid caregivers who assist older adults with health care activities. JAMA Intern Med. 2016;176(3):372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mollica MA, Litzelman K, Rowland JH, Kent EE.. The role of medical/nursing skills training in caregiver confidence and burden: a CanCORS study. Cancer. 2017;123(22):4481–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yabroff KR, Kim Y.. Time costs associated with informal caregiving for cancer survivors. Cancer. 2009;115(S18):4362–4373. [DOI] [PubMed] [Google Scholar]
- 20. Van Houtven CH, Ramsey SD, Hornbrook MC, Atienza AA, van Ryn M.. Economic burden for informal caregivers of lung and colorectal cancer patients. Oncologist. 2010;15(8):883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Moor JS, Dowling EC, Ekwueme DU, et al. Employment implications of informal cancer caregiving. J Cancer Surviv. 2017;11(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamal KM, Covvey JR, Dashputre A, et al. A systematic review of the effect of cancer treatment on work productivity of patients and caregivers. J Manag Care Spec Pharm. 2017;23(2):136–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Applebaum A. Cancer Caregivers. New York: Oxford University Press; 2019. [Google Scholar]
- 24. Schilsky RL. Implementing personalized cancer care. Nat Rev Clin Oncol. 2014;11(7):432–438. [DOI] [PubMed] [Google Scholar]
- 25. Ciardiello F, Arnold D, Casali PG, et al. Delivering precision medicine in oncology today and in future-the promise and challenges of personalised cancer medicine: a position paper by the European Society for Medical Oncology (ESMO). Ann Oncol. 2014;25(9):1673–1678. [DOI] [PubMed] [Google Scholar]
- 26. Madhavan S, Subramaniam S, Brown TD, Chen JL.. Art and challenges of precision medicine: interpreting and integrating genomic data into clinical practice. Am Soc Clin Oncol Educ Book. 2018;38:546–553. [DOI] [PubMed] [Google Scholar]
- 27.Global Oncology Trends. 2019. Therapeutics, clinical development and health system implications https://www.iqvia.com/institute/reports/global-oncology-trends-2019. Accessed August 6, 2019.
- 28.U.S. Food & Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication. Accessed July 17, 2019.
- 29.U.S. Food & Drug Administration. FDA approves axicabtagene ciloleucel for large B-cell lymphoma. 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-axicabtagene-ciloleucel-large-b-cell-lymphoma. Accessed July 17, 2019.
- 30.U.S. Food & Drug Administration. FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-b-cell-all-and-tocilizumab-cytokine-release-syndrome. Accessed July 17, 2019.
- 31. Bruinooge SS, Sherwood S, Grubbs S, Schilsky RL.. Determining if a somatic tumor mutation is targetable and options for accessing targeted therapies. J Oncol Pract. 2019;15(11):575–583. [DOI] [PubMed] [Google Scholar]
- 32. DeLeonardis K, Hogan L, Cannistra SA, Rangachari D, Tung N.. When should tumor genomic profiling prompt consideration of germline testing? J Oncol Pract. 2019;15(9):465–473. [DOI] [PubMed] [Google Scholar]
- 33. Levit LA, Kim ES, McAneny BL, et al. Implementing precision medicine in community-based oncology programs: three models. J Oncol Pract. 2019;15(6):325–329. [DOI] [PubMed] [Google Scholar]
- 34. Nass SJ, Cohen MB, Nayar R, et al. Improving cancer diagnosis and care: patient access to high-quality oncologic pathology. Oncologist. 2019;24(10):1287–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Woolhandler S, Himmelstein DU.. Administrative work consumes one-sixth of U.S. physicians' working hours and lowers their career satisfaction. Int J Health Serv. 2014;44(4):635–642. [DOI] [PubMed] [Google Scholar]
- 36. Rao SK, Kimball AB, Lehrhoff SR, et al. The impact of administrative burden on academic physicians: results of a hospital-wide physician survey. Acad Med. 2017;92(2):237–243. [DOI] [PubMed] [Google Scholar]
- 37. Washington V, DeSalvo K, Mostashari F, Blumenthal D.. The HITECH era and the path forward. N Engl J Med. 2017;377(10):904–906. [DOI] [PubMed] [Google Scholar]
- 38. Jones SS, Rudin RS, Perry T, Shekelle PG.. Health information technology: an updated systematic review with a focus on meaningful use. Ann Intern Med. 2014;160(1):48–54. [DOI] [PubMed] [Google Scholar]
- 39. Lin SC, Jha AK, Adler-Milstein J.. Electronic health records associated with lower hospital mortality after systems have time to mature. Health Aff (Millwood). 2018;37(7):1128–1135. [DOI] [PubMed] [Google Scholar]
- 40. Gawande A. Why doctors hate their computers. In: New Yorker. November 12, 2018.
- 41. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91(7):836–848. [DOI] [PubMed] [Google Scholar]
- 42. Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med. 2017;15(5):419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tai-Seale M, Olson CW, Li J, et al. Electronic health record logs indicate that physicians split time evenly between seeing patients and desktop medicine. Health Aff (Millwood). 2017;36(4):655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sinsky CA, Beasley JW.. Texting while doctoring: a patient safety hazard. Ann Intern Med. 2013;159(11):782–783. [DOI] [PubMed] [Google Scholar]
- 45. Balogh E, Miller BT, Ball J; Institute of Medicine (U.S.) Committee on Diagnostic Error in Health Care. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 46. Wachter RM. The Digital Doctor: Hope, Hype, and Harm at the Dawn of Medicine's Computer Age. New York: McGraw-Hill Education; 2017. [Google Scholar]
- 47. Downing NL, Bates DW, Longhurst CA.. Physician burnout in the electronic health record era: are we ignoring the real cause? Ann Intern Med. 2018;169(1):50–51. [DOI] [PubMed] [Google Scholar]
- 48. Casalino LP, Gans D, Weber R, et al. US physician practices spend more than $15.4 billion annually to report quality measures. Health Aff (Millwood). 2016;35(3):401–406. [DOI] [PubMed] [Google Scholar]
- 49.Institute of Medicine (U.S.). Committee on core metrics for better health at lower cost In: Blumenthal D, Malphrus E, McGinnis JM., Vital Signs: Core Metrics for Health and Health Care Progress. Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- 50. Lin NU, Bichkoff H, Hassett MJ.. Increasing burden of prior authorizations in the delivery of oncology care in the United States. J Oncol Pract. 2018;14(9):525–528. [DOI] [PubMed] [Google Scholar]
- 51. Kirkwood MK, Hanley A, Bruinooge SS, et al. The state of oncology practice in America, 2018: results of the ASCO Practice Census Survey. J Oncol Pract. 2018;14(7):e412–e420. [DOI] [PubMed] [Google Scholar]
- 52.Prior authorization and cancer patient care: a nationwide physician survey. 2019. https://www.astro.org/ASTRO/media/ASTRO/News and Publications/PDFs/ASTROPriorAuthorizationPhysician-SurveyBrief.pdf. Accessed July 19, 2019.
- 53. Maslach C, Jackson SE, Leiter MP.. Maslach Burnout Inventory Manual. 3rd ed Palo Alto, CA: Consulting Psychologists Press; 1996. [Google Scholar]
- 54. Shanafelt TD, Gradishar WJ, Kosty M, et al. Burnout and career satisfaction among US oncologists. J Clin Oncol. 2014;32(7):678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Medisauskaite A, Kamau C.. Prevalence of oncologists in distress: systematic review and meta-analysis. Psychooncology. 2017;26(11):1732–1740. [DOI] [PubMed] [Google Scholar]
- 56. Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377–1385. [DOI] [PubMed] [Google Scholar]
- 57. Essary AC, Bernard KS, Coplan B, et al. Burnout and Job and Career Satisfaction in the Physician Assistant Profession. A Review of the Literature NAM Perspectives. Washington, DC: National Academy of Medicine; 2018.
- 58. Canadas-De la Fuente GA, Gomez-Urquiza JL, Ortega-Campos EM, Canadas GR, Albendin-Garcia L, De la Fuente-Solana EI.. Prevalence of burnout syndrome in oncology nursing: a meta-analytic study. Psychooncology. 2018;27(5):1426–1433. [DOI] [PubMed] [Google Scholar]
- 59. Panagioti M, Geraghty K, Johnson J, et al. Association between physician burnout and patient safety, professionalism, and patient satisfaction: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(10):1317–1330. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60. Lopez D, Pratt-Chapman ML, Rohan EA, et al. Establishing effective patient navigation programs in oncology. Support Care Cancer. 2019;27(6):1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Burris HA. Leveraging organizational culture and leadership to promote change. Paper presented at: National Cancer Policy Forum: Workshop on Developing and Sustaining an Effective and Resilient Oncology Careforce; Washington, DC; February 11–12, 2019.
- 62. Kline RM, Rocque GB, Rohan EA, et al. Patient navigation in cancer: the business case to support clinical needs. J Oncol Pract. 2019;15(11):585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. LeBlanc TW, Abernethy AP.. Patient-reported outcomes in cancer care - hearing the patient voice at greater volume. Nat Rev Clin Oncol. 2017;14(12):763–772. [DOI] [PubMed] [Google Scholar]
- 64. Wysham NG, Wolf SP, Samsa G, Abernethy AP, LeBlanc TW.. Integration of electronic patient-reported outcomes into routine cancer care: an analysis of factors affecting data completeness. J Clin Oncol Clin Cancer Inform. 2017;1:1–10. [DOI] [PubMed] [Google Scholar]
- 65. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Denis F, Basch E, Septans AL, et al. Two-year survival comparing web-based symptom monitoring vs routine surveillance following treatment for lung cancer. JAMA. 2019;321(3):306–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Basch E. Patient-reported outcomes - harnessing patients' voices to improve clinical care. N Engl J Med. 2017;376(2):105–108. [DOI] [PubMed] [Google Scholar]
- 69. Rotenstein LS, Huckman RS, Wagle NW.. Making patients and doctors happier - the potential of patient-reported outcomes. N Engl J Med. 2017;377(14):1309–1312. [DOI] [PubMed] [Google Scholar]
- 70. Ivatury SJ, Hazard-Jenkins HW, Brooks GA, McCleary NJ, Wong SL, Schrag D.. Translation of patient-reported outcomes in oncology clinical trials to everyday practice. Ann Surg Oncol. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Darlow S, Wen KY.. Development testing of mobile health interventions for cancer patient self-management: a review. Health Informatics J. 2016;22(3):633–650. [DOI] [PubMed] [Google Scholar]
- 72. Fishbein JN, Nisotel LE, MacDonald JJ, et al. Mobile application to promote adherence to oral chemotherapy and symptom management: a protocol for design and development. JMIR Res Protoc. 2017;6(4):e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Laranjo L, Dunn AG, Tong HL, et al. Conversational agents in healthcare: a systematic review. J Am Med Inform Assoc. 2018;25(9):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stead WW. Clinical implications and challenges of artificial intelligence and deep learning. JAMA. 2018;320(11):1107. [DOI] [PubMed] [Google Scholar]
- 75. Berges BM, Cambareri C, Takvorian SU, et al. Leveraging a conversational agent to support adherence to oral anticancer agents: a usability study. J Clin Oncol. 2019;37(15_suppl):6534. [Google Scholar]
- 76.The National Academies of Sciences Engi (Washington District of Columbia). Taking Action Against Clinician Burnout: A Systems Approach to Professional Well-Being. Washington: The National Academies Press; 2019. [PubMed] [Google Scholar]
- 77. Miller KEM, Lindquist JH, Olsen MK, et al. Invisible partners in care: snapshot of well-being among caregivers receiving comprehensive support from Veterans Affairs. Health Sci Rep. 2019;2(3):e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Smith VA, Lindquist J, Miller KEM, et al. Comprehensive family caregiver support and caregiver well-being: preliminary evidence from a pre-post-survey study with a non-equivalent control group. Front Public Health. 2019;7:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sperber NR, Boucher NA, Delgado R, et al. Including family caregivers in seriously ill veterans' care: a mixed-methods study. Health Aff (Millwood). 2019;38(6):957–963. [DOI] [PubMed] [Google Scholar]
- 80. Van Houtven CH, Smith VA, Stechuchak KM, et al. Comprehensive support for family caregivers: impact on veteran health care utilization and costs. Med Care Res Rev. 2019;76(1):89–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mooney K, Whisenant MS, Beck SL.. Symptom care at home: a comprehensive and pragmatic PRO system approach to improve cancer symptom care. Med Care. 2019;57(suppl 5) (suppl 1):S66–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mooney KH, Beck SL, Wong B, et al. Automated home monitoring and management of patient-reported symptoms during chemotherapy: results of the symptom care at home RCT. Cancer Med. 2017;6(3):537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mooney K. Family caregivers: key members of the oncology careforce. Paper presented at: National Cancer Policy Forum: Workshop on Developing and Sustaining an Effective and Resilient Oncology Careforce; Washington, DC; February 11–12, 2019.
- 84. Katzenbach JR, Smith DK.. The Wisdom of Teams: Creating the High-Performance Organization. Cambridge, MA: Harvard Business Review Press; 2015. [Google Scholar]
- 85. Stokols D, Hall KL, Taylor BK, Moser RP.. The science of team science: overview of the field and introduction to the supplement. Am J Prev Med. 2008;35(2):S77–S89. [DOI] [PubMed] [Google Scholar]
- 86. Taplin SH, Weaver S, Chollette V, et al. Teams and teamwork during a cancer diagnosis: interdependency within and between teams. J Oncol Pract. 2015;11(3):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Taplin SH, Weaver S, Salas E, et al. Reviewing cancer care team effectiveness. J Oncol Pract. 2015;11(3):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kosty MP, Hanley A, Chollette V, Bruinooge SS, Taplin SH.. National Cancer Institute-American Society of Clinical Oncology teams in cancer care project. J Oncol Pract. 2016;12(11):955–958. [DOI] [PubMed] [Google Scholar]
- 89. Shulman LN, Jacobs LA, Greenfield S, et al. Cancer care and cancer survivorship care in the United States: will we be able to care for these patients in the future? J Oncol Pract. 2009;5(3):119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Waller A, Girgis A, Johnson C, et al. Improving outcomes for people with progressive cancer: interrupted time series trial of a needs assessment intervention. J Pain Symptom Manage. 2012;43(3):569–581. [DOI] [PubMed] [Google Scholar]
- 91.Oncology care model. https://innovation.cms.gov/initiatives/oncology-care/. Accessed November 26, 2019.
- 92. Vimalananda VG, Gupte G, Seraj SM, et al. Electronic consultations (e-consults) to improve access to specialty care: a systematic review and narrative synthesis. J Telemed Telecare. 2015;21(6):323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wachter RM, Judson TJ, Mourad M.. Reimagining specialty consultation in the digital age: the potential role of targeted automatic electronic consultations. JAMA. 2019;322(5):399. [DOI] [PubMed] [Google Scholar]
- 94. Shipman S. eConsults: a tool to extend careforce capacity in cancer care? Paper presented at National Cancer Policy Forum: Workshop on Developing and Sustaining an Effective and Resilient Oncology Careforce; February 11–12 2019; Washington, DC.
- 95. Fridsma DB. American Medical Informatics Association’s comment on the Department of Health and Human Services’ “Strategy on Reducing Regulatory and Administrative Burden Relating to the Use of Health IT and EHRs.” 2019. https://www.amia.org/sites/default/files/AMIA-Response-to-ONC-HIT-Burden-Reduction-Strategy.pdf. Accessed August 30, 2019.
- 96. Conway P. The core quality measures collaborative: a rationale and framework for public-private quality measure alignment. In: Health Affairs Blog, July 23, 2015. Available at: https://www.healthaffairs.org/do/10.1377/hblog20150623.048730/full/. Accessed February 20, 2020.
- 97. Kraut J, Mooney K, Sweetenham JW, et al. Implementing an electronic end-of-life chemotherapy utilization measure. J Oncol Pract. 2019;15(4):220–223. [DOI] [PubMed] [Google Scholar]
- 98. Krasner MS, Epstein RM, Beckman H, et al. Association of an educational program in mindful communication with burnout, empathy, and attitudes among primary care physicians. JAMA. 2009;302(12):1284–1293. [DOI] [PubMed] [Google Scholar]
- 99. Back AL, Steinhauser KE, Kamal AH, Jackson VA.. Building resilience for palliative care clinicians: an approach to burnout prevention based on individual skills and workplace factors. J Pain Symptom Manage. 2016;52(2):284–291. [DOI] [PubMed] [Google Scholar]
- 100. Shanafelt TD, Gorringe G, Menaker R, et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90(4):432–440. [DOI] [PubMed] [Google Scholar]
- 101. Shanafelt TD, Noseworthy JH.. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129–146. [DOI] [PubMed] [Google Scholar]