Abstract
Purpose:
Survival of CLL cells due to the presence of Bcl-2 and Mcl-1 has been established. Direct inhibition of Bcl-2 by venetoclax and indirect targeting of Mcl-1 with transcription inhibitors have been successful approaches for CLL. AMG-176 is a selective and direct antagonist of Mcl-1, which has shown efficacy in several hematological malignancies, however, its effect on CLL is elusive. We evaluated biological and molecular effects of AMG-176 in primary CLL cells.
Experimental Design:
Using samples from patients (n = 74) with CLL, we tested effects of AMG-176 on CLL and normal hematopoietic cell death and compared importance of CLL prognostic factors on this biological activity. We evaluated CLL cell apoptosis in the presence of stromal cells and identified cell death pathway including stabilization of Mcl-1 protein. Finally, we tested couplet of AMG-176 and venetoclax in CLL lymphocytes.
Results:
AMG-176 incubations resulted in time- and dose-dependent CLL cell death. At 100 and 300 nM, there was 30 and 45 % cell death at 24 hours. These concentrations did not result in significant cell death in normal hematopoietic cells. Presence of stroma did not affect AMG-176-induced CLL cell death. IGHV unmutated status, high β2M and Mcl-1 protein levels resulted in slightly lower cell death. Mcl-1 but not Bcl-2 protein levels in CLL cells increased with AMG-176. Low concentrations of venetoclax (1–30 nM) were additive or synergistic with AMG-176.
Conclusions:
AMG-176 is active in inducing CLL cell death while sparing normal blood cells. Combination with low dose venetoclax had at least additive effect.
Keywords: AMG-176, chronic lymphocytic leukemia, ibrutinib, Mcl-1 inhibitor, venetoclax
Introduction
Survival, maintenance, and cell death of many cancers are dependent on expression of Bcl-2 family proteins. Based on their functions and structures, three major subgroups of Bcl-2 family proteins have been identified which either promote or prevent apoptosis. The antiapoptotic Bcl-2 proteins have 6 members; Bcl-2, Bcl-XL, Bcl-w, Mcl-1, Bcl2A1 and Bcl-B (1). These members have the BH3 domain hydrophobic groove on the surface and sequester BH3-only proapoptotic proteins or bind to effector proteins such as BAK, BAX and BOK. Both pro-apoptotic and anti-apoptotic BCL-2 family members possess a transmembrane (TM) domain to promote connection with the outer mitochondrial membrane (2).
Bcl-2 family proteins are primary determinant of cellular fate as they maintain mitochondrial integrity and regulate the mitochondrial pathway of apoptosis which releases cytochrome-C from mitochondria into the cytoplasm, inducing cascade of events piloting proteolytic activation of caspase-9, caspase-3, and cleavage of PARP and other proteins leading to apoptosis (2).
A corollary to their expression and survival of cells, targeting of Bcl-2 anti-apoptotic proteins is a novel therapeutic intervention for cancer in general (3) and lymphoid malignancies in particular (4). Such innovations have resulted into synthesis, development, and clinical use of navitoclax (5,6). Recently, venetoclax, that selectively targets only Bcl-2 protein has shown success in preclinical (7) as well as clinical settings (8) (9). It is important to note that these approaches have been mostly used and successful in hematological malignancies especially chronic lymphocytic leukemia (CLL) (8) (9).
The success of Bcl-2 selective antagonist generated interest in targeting other anti-apoptotic molecules such as Mcl-1. Initial attempts resulted in Mcl-1 specific inhibitors; maritoclax (10,11) and A-1210477 (12), however, these were not ideal for clinical utility. Recently, three Mcl-1 specific clinical candidates have emerged; Astra Zeneca’s AZD-5991 (13,14), Amgen’s AMG-176 (15) and Novartis’ S63845 and S64315 (16,17). Clinical trials ( NCT03218683; NCT02675452; NCT02992483) in liquid tumors are ongoing with these agents. All three inhibitors have been tested in hematological malignancies with a focus on acute myeloid leukemia (14–16), acute lymphoid leukemia (13,17), and multiple myeloma (15,16). Data are not available for CLL cell lines or primary CLL lymphocytes.
We postulated that Mcl-1 inhibitors should be effective in CLL based on the following rationales. Mcl-1 is essential during early lymphoid development and then also later for the maintenance of mature T and B-lymphocytes (18). The level of expression of anti-apoptotic proteins in normal and malignant lymphocytes is consistent with their role in survival (19). Further, high levels of Mcl-1 and Bcl-2 mRNA (20) and protein (21) have been found in CLL. Additionally and specifically, the levels of Mcl-1 are inversely correlated with in vitro response to chemotherapeutic agents (21). High levels of Mcl-1 are observed in relapsed leukemias (22) and are associated with the failure of CLL patients to achieve complete responses to initial therapy with fludarabine (21). Consistent with these reports, down regulation of the expression of Mcl-1 protein by direct measures such as antisense oligonucleotides (23) or through indirect Mcl-1-transcription and translation inhibitor-approaches result in cell death during in vitro culture (24) (25) (26) or in vivo therapy (27). Conversely, over-expression of Mcl-1 prolongs the survival of cells exposed to a variety of stimuli, including cytokine withdrawal and treatment with chemo- and radio-therapeutic insults (28). Taken together, these lines of evidence establish Mcl-1 anti-apoptotic proteins as key survival factor for CLL. In fact, preliminary studies from our group suggested utility of both A-1210477 and S64315 during in vitro testing in primary CLL lymphocytes.
Based on these background information, we hypothesized that AMG-176 will induce apoptosis in CLL cells as a single agent and may be synergistic when combined with Bcl-2-inhibitor, venetoclax. To test this postulate we used AMG-176 during in vitro incubations in CLL lymphocytes obtained from >70 patients. This large number allowed us to compare impact of this agent in different cytogenetic background and prognostic factors. Further, we identified mechanism of action and interaction with venetoclax.
Materials and Methods
Patient sample collection and cell culture
Peripheral blood was obtained from patients with CLL who provided written informed consent as part of a protocol approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center in accordance with the Declaration of Helsinki. Altogether, blood samples were obtained from 74 patients and these were used for different experimental purposes. Baseline characteristics of these patients are summarized in Supplementary Table 1.
In all cases, peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density centrifugation (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) and suspended in Roswell Park Memorial Institute 1640 (RPMI 1640) media supplemented with 10% human serum (Sigma-Aldrich, St. Louis, MO). These samples were used fresh for each experiment. Previously, we have reported that the PBMCs isolated from blood primarily consisted of CLL lymphocytes (82 – 94%) (29).
Co-culturing CLL cells on stroma cells
CLL cells isolated from peripheral blood were either cultured in RPMI medium with human serum as described above or layered on stromal cells. For stromal cells, NK-Tert cell line (RIKEN cell bank, Tsukuba, Japan) was used at a ratio of 100 CLL cells to 1 stromal cell. For these experiments, we maintained continuous culture of stromal cells and plated them at a concentration of 1 x 105 cells / well 24 hr prior to adding CLL cells at a concentration of 1 x 107 per well. At the end of incubations, CLL cells were carefully removed and used for cell death assay leaving the adherent stromal layer undisturbed.
Drugs and cell line
AMG-176 and venetoclax were purchased from Chemietek (Indianapolis, IN) and Xcessbio Company (San Diego, CA), respectively. For both drugs, stock solutions were made in dimethyl sulfoxide (DMSO; Sigma-Aldrich). The pan caspase inhibitor, quinoline-Val-Asp (Ome)-CH2-O-Ph (Q-VD-Oph) was obtained from APExBIO (Houston, TX). Typically, incubations were performed for 24 hours and for all treatments, time-matched, DMSO-treated (i.e., vehicle only) cells were used as controls. MEC1 cell line was purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DMSZ, Braunschweig, Germany). It was authenticated using short-tandem repeat profiling and regularly tested for Mycoplasma contamination using Mycoalert Mycoplasma detection kit (Lonza Group Ltd, Basel, Switzerland). We use cells between 5th and 20th passage. The cells were used as a reference protein concentration for the Bcl-2 family proteins.
Cytotoxicity assays in CLL cells
CLL cells were treated with different concentrations of AMG-176 for indicated time points. For apoptosis assay, cells were stained with annexin V and propidium iodide (PI) and analyzed by flow cytometry. To calculate cell death, values from early apoptotic (Annexin+ PI−), late apoptotic (Annexin+ PI+) and necrotic (Annexin− PI+) cells were included and labelled as % Annexin V/PI +ve. Dual negative were considered live cells. Cell death of vehicle-treated samples was subtracted from drug-treated samples. For caspase activity assay, CLL cells were treated with AMG-176 or venetoclax and with or without 20 µM Q-VD-OPH. After 24 hours, cell-death was evaluated by annexin/PI assay.
Calculation of AMG-176 and venetoclax interaction and combination
To assess the combined effect of drugs and to calculate combination index, we used fractional analyses of the Bliss independence model (30). For this, each drug (AMG-176 or venetoclax) was tested alone and in combination. Survival fraction was used to calculate expected % cell death for the combination. To determine ratio, this expected value was divided by observed cell death percent obtained when both drugs were combined. Ratio of more than 1 was considered antagonistic, 1 was considered additive, and less than 1 was synergistic.
Cytotoxicity assays in normal hematopoietic cells
To test biological effect of AMG-176 on subsets of normal blood cells, peripheral blood samples were obtained from six healthy donors (Supplementary Table 2). All healthy individuals signed consent form of an IRB approved LAB protocol. PBMCs were isolated using ficoll gradient and were incubated either with DMSO or with 300 nM AMG-176 for 24 hours. Cells were surface stained with the following antibodies (all from Bio Legend, San Diego, CA): anti-human CD19, anti-human CD4, anti-human CD8, anti-human CD56, anti-human CD133 and anti-human CD34, followed by annexin V/PI staining and analyzed by BD Accuri C6 Plus flow cytometer.
Isolation of cytosolic and mitochondrial fractions
Untreated or drug treated CLL cells were harvested and washed with 1X PBS twice. Purified mitochondrial and cytosolic fractions were prepared by using Cell fractionation kit (Abcam; AB109719) following manufacturer’s guideline. Protein amount was quantified and 15–30 µg of total, cytosolic and mitochondrial fractions were used for immunoblot analysis. All kit components were supplemented with protease inhibitor cocktail (cOmplete, Protease and PhosSTOP inhibitors; Sigma-Aldrich) before use.
Immunoblot analyses
CLL cell pellets were suspended in modified RIPA buffer with protease inhibitors and were processed to extract total proteins. Immunoblots were performed with 20 – 30 µg of cellular protein extracts and were run on Criterion protein gels (4–12% gradient gels; Bio-Rad) and transferred onto nitrocellulose membrane ON. Blots were cut into slices and incubated with specific antibodies and visualized with the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). Antibodies for specific proteins are listed in Supplementary Table 3.
Quantitation of immunoblots
The band density of target protein and internal control (either Vinculin or GAPDH) were measured by using Licor Image Studio software following manufacturer’s guideline (www.Licor.com) and all normalized values were plotted into GraphPad Prism 7 for representation.
Statistical analysis
Student’s t-test and one- and two-way Analysis of Variance (ANOVA) were used for assessing the significance variable correlations. Specific statistical analyses are detailed in figure captions. All tests were conducted in R version 3.5.1.
Results
AMG-176 treatment induced dose- and time-dependent CLL cell death
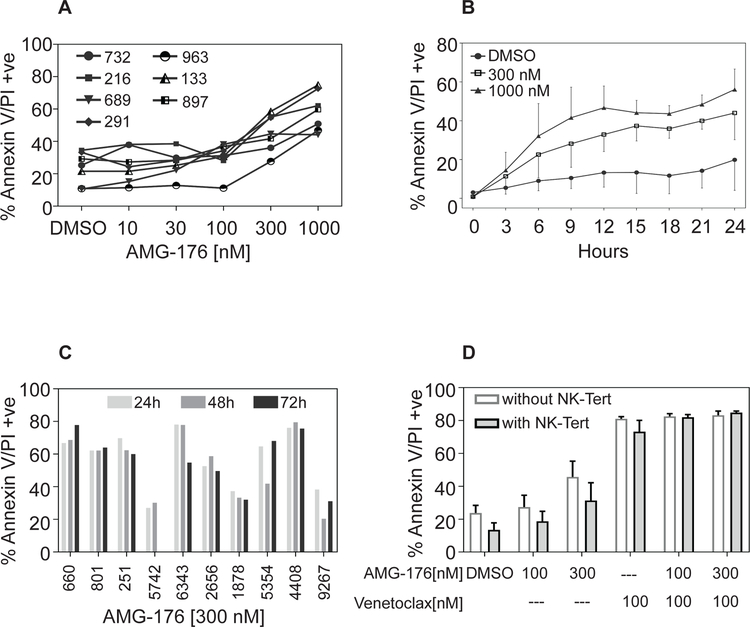
To determine if CLL lymphocytes are sensitive to AMG-176, peripheral blood mononuclear cells from seven patients were incubated with different concentration (10 – 1000 nM) of the drug for 24 hours. Time-matched vehicle treated (DMSO) cells were used as control samples. Annexin+/PI+ data suggest dose-dependent cell death was observed in all samples at 300 and 1000 nM AMG-176 (Figure 1A). To understand heterogeneity for cell death response, we evaluated the efficacy of either 100 or 300 nM AMG-176 to induce cell death in samples from 65 and 69 patients, respectively. At 100 nM AMG-176, the cell death was a median 30 % (range, 5–80 %; n=65) whereas at 300 nM the median value was 45 % (range 7–78 %; n=69) (Supplementary Table 1; Highlighted columns). To examine time-dependency, samples from five patients were incubated with 300 or 1000 nM drug for 24 hours and cells were collected every 3 hours. There was a time-dependent increase in extent of cell death but that appeared to plateau around 15 hours (Figure 1B). Cell death did not increase in 10 patient samples incubated for longer than 24 hours (2 or 3 days) (Figure 1C). Based on these results, in most experiments, we incubated CLL lymphocytes with 300 nM drug for 24 hours.
Fig 1. Chronic lymphocytic leukemia cells are sensitive to AMG-176-mediated cell death.
CLL lymphocytes from seven CLL patients were collected and cultured in media supplemented with 10% human serum. Each sample was treated with either dimethyl sulfoxide (DMSO) (0.1%), or indicated concentration of AMG-176 for indicated times. Cell death was determined using flow cytometry after Annexin V/propidium iodide (PI) staining. A. Concentration-dependent cell death. CLL cells were incubated with 3, 10, 30, 100, 300, and 1000 nM AMG-176 for 24 hours. B. Time-dependent cell death. CLL cells were incubated with 300 or 1000 nM AMG-176 for 0, 3, 6, 9, 12, 15, 18, 21, 24 hours. We fit a two way ANOVA model using treatment and patient as fixed effects at 12 hours and obtained a p-value of 0.00007 using an F-test of no treatment effect versus treatment effect. We performed the same analysis for 24 hours and obtained a p-value of 0.00013. C. Impact of prolonged incubation on cell death. CLL cells from 10 patients were incubated with 300 nM AMG-176 for 24, 48, and 72 hours. D. Influence of microenvironment on AMG-176 mediated cell death. CLL lymphocytes from 4 CLL patients were collected and cultured either in suspension or on NK-Tert cells as indicated in the Methods section. Each sample was treated with either dimethyl sulfoxide (DMSO) (0.1%), or indicated concentration of AMG-176 for indicated times. Cell death was determined using flow cytometry after Annexin V/propidium iodide (PI) staining. We conducted two-sample paired t-tests between the two groups (without NK-Tert and with NK-Tert). The p values were 0.067, 0.12; 0.02; 0.33, 0.57, 0.59 for DMSO, 100 nM AMG-176, 300 nM AMG-176, 100 nM venetoclax, and two combinations, respectively.
Previous investigations from our group (29) (31) (32) and others (33) have established that NK-Tert stromal cells provide survival advantage to CLL cells and these malignant lymphocytes show relative-drug resistance. To test NK-Tert mediated survival advantage, we compared CLL cell death when cultured in suspension or co-cultured with stroma for 24-hour with 100 or 300 nM AMG-176 alone or in combination with venetoclax. Cells incubated with 100 nM venetoclax served as positive control. As shown in Figure 1D apoptosis levels were similar in CLL cells with or without NK-Tert cells. The p value suggested that there may be some difference at 300 nM AMG-176, although the difference is minor.
To determine if AMG-176 has any direct effect on stromal cells, we incubated NK-Tert cells with either 100 or 300 nM drug alone or with venetoclax for 24 hours. Data presented in the Supplementary Figure 1 suggest that AMG-176 or venetoclax did not induce any significant NK-Tert cell death. This may be due to the fact that these cells do not express detectable level of Mcl-1, Bcl-2, and Bcl-xL (34).
Impact of CLL prognostic factors on AMG-176-induced cell death
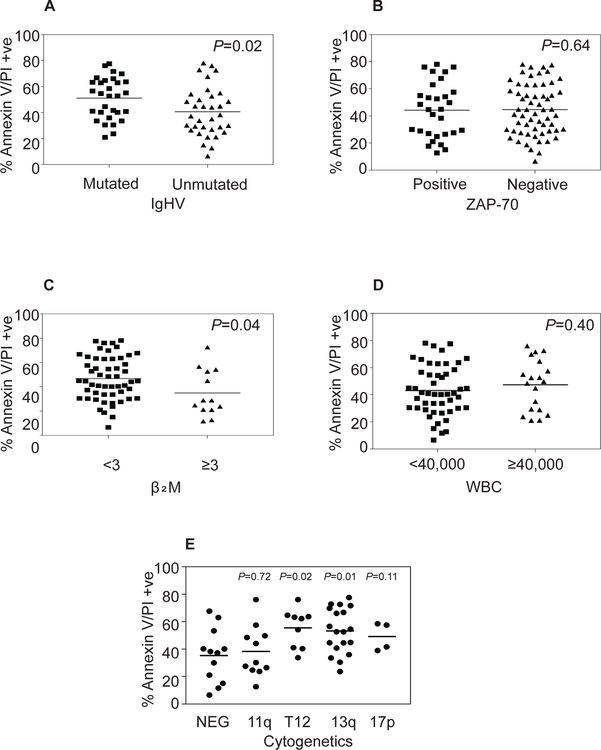
Annexin/PI data in figures 1A–C in several patients suggested heterogeneity among samples. This differential apoptotic response among the primary CLL patient samples may be related to their respective prognostic features. To explore this possibility, we analyzed data presented in Supplementary Table 1 and compared these values with different prognostic features of CLL patients. Mutated IgHV status is considered good prognostic features compared to the counterpart. Data in >30 patients in each subset suggested that heterogeneity in the levels of apoptosis is similar with each category, however unmutated group had slightly lesser extent of cell death which was significantly different (Figure 2A). Such difference was not reflected in ZAP-70 positivity group (Figure 2B). Although the numbers are small, higher level of β2M, Rai and Binet stage of the disease showed slightly inferior extent of cell death (Figure 2C, Supplementary Figure 2) but this was not reflected in the peripheral blood cell count suggesting that the extent of cell death was not altered by tumor load (Figure 2D and Supplementary Figure 2C–E). Finally, we compared the outcome of AMG-176-induced cell death in these samples with genetic variants such as normal cytogenetics (NEG), trisomy 12, 11q deletion, 17p deletion, and 13q deletion. Within 55 patients, AMG-176 induced cell death in all subgroups which was slightly higher than the diploid cohort and significantly different in trisomy 12 and 13q del subsets. We also compared these prognostic parameters and correlated their response to 100 nM AMG-176-induced cell death. These data were consistent with the results obtained at 300 nM AMG-176 (Supplementary Table 1, Supplementary Figure 3–4). These data suggest that AMG-176 incubations generally resulted in similar cell death in all prognostic and genetic subtypes of CLL; even when there was statistical significance, the differences were minor.
Fig 2. AMG-176 induced cell death and CLL prognostic markers.
CLL lymphocytes from 68 patients were treated in vitro with 300 nM AMG-176 for 24 h, and Annexin V/PI positivity was determined after the incubation period. The relationship between cytotoxicity and prognostic markers was plotted for immunoglobulin heavy-chain variable-region (IGHV) mutation, zeta-chain-associated protein kinase 70 (ZAP 70) status, β2M levels, WBC count in the blood, and cytogenetics status. For each prognostic factor in Figures 2 A-D, we conducted two-sample Student’s t-test assuming equal group variances. The p values are listed in each graph. For Figure 2 E, we computed p-values based on two-sample equal variance t-tests for negative (NEG) against four cytogenetic groups. The p-values are 0.72, 0.02, 0.01, and 0.11 for 11q del, trisomy 12, 13q del, and 17 p del, respectively.
AMG-176-induced cell death in normal hematopoietic cells
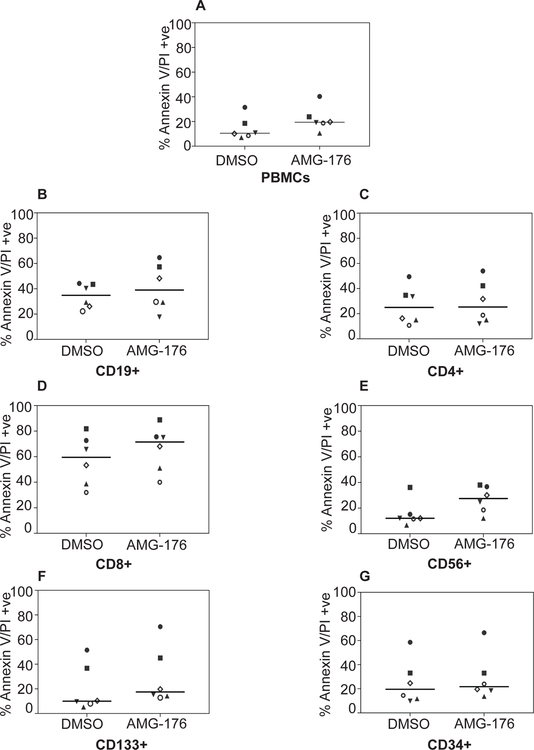
Biological impact of AMG-176 treatment in normal hematopoietic cells was determined in the peripheral blood mononuclear cells (PBMC) isolated from 6 healthy donors. PBMCs were incubated with 300 nM AMG-176 for 24 hours and total population as well as different cell types were analyzed for annexin/PI positivity. Total PBMCs showed a median of 10% cell death (DMSO) which increased to 20% after AMG-176 treatment (Figure 3A). Among hematopoietic subset, CD19+ cells which represent healthy B-lymphocytes (normal counterpart of CLL lymphocytes) showed no further increase in cell death after AMG-176 (Figure 3B). CD4+ T-cells had similar cell death in control and drug-treated condition (Figure 3C). In contrast, CD8+ cells experienced high cell death in DMSO–treated PBMCs which increased about 20% in drug-treated population (Figure 3D). Both CD56+ NK cells, CD133+ and CD34+ cells showed only 10% increase in cell death after treatment with AMG-176 (Figure 3E–G). Collectively, AMG-176 had negligible impact on normal hematopoietic cellular subsets.
Fig 3. Biological impact of AMG-176 in normal PBMCs.
Normal PBMCs were isolated from peripheral blood sample of healthy donors. PBMCs were cultured in media supplemented with 10% human serum. Each sample was treated with either dimethyl sulfoxide (DMSO) (0.1%) or 300 nM AMG-176 for 24 hours. Cell death was determined using flow cytometry after Annexin V/propidium iodide (PI) staining and cell surface marker staining. A. PBMCs, B. CD19+ B-cells. C. CD4+ T-cells. D. CD8+ T-cells. E. CD56+ NK-cells. F. CD133+ plasma cells and G. CD34+ myeloid cells.
Mcl-1 inhibitor AMG-176 induces CLL cell-death via mitochondrial pathway
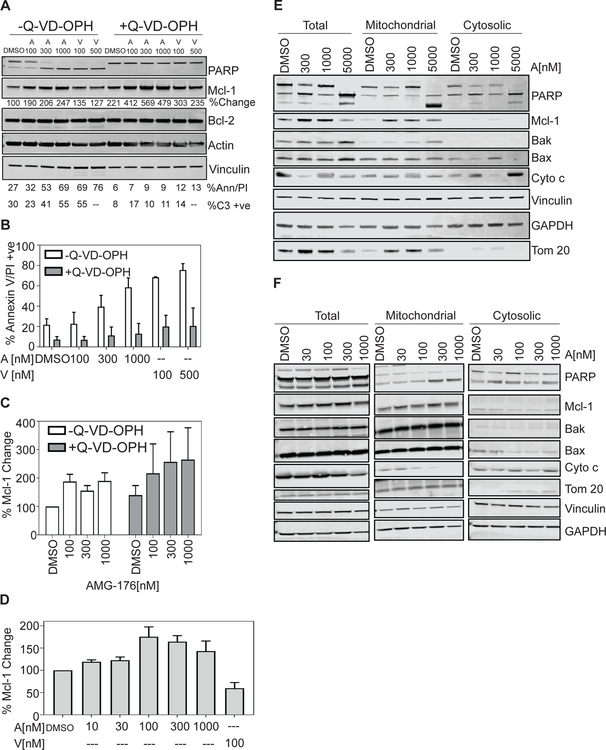
To determine the role of caspases in AMG-176 toxicity to CLL cells, we evaluated the effect of the pan-caspase inhibitor, quinoline-Val-Asp (Ome)-CH2-O-Ph (Q-VD-OPH) on AMG-176-mediated cell death. Primary CLL cells were treated with either AMG-176 or venetoclax alone or in combination with Q-VD-OPH. Both AMG-176 and venetoclax-induced cell death was completely abrogated by Q-VD-OPH treatment (Figure 4A). To further validate role of caspases, we simultaneously detected caspase-3 activity. Again, we show that caspase-3 activity was abolished when cells were co-treated with Q-VD-OPH (Figure 4A). Down-stream effector of caspase 3 activity is cleaved PARP; PARP proteolysis and cleavage of procaspase-3 were examined from above experiments. Using antibodies that detect both the full-length (119 KDa) and cleaved (89 KDa) PARP as well as cleaved caspase-3 (17-kDa and 19-KDa subunit), we demonstrate that both AMG-176 and venetoclax caused a dose-dependent PARP proteolysis and caspase-3 cleavage, however, co-incubation of cells with Q-VD-OPH abolished both PARP and caspase-3 proteolysis (Figure 4A).
Fig 4. Change in Mcl-1 protein level and caspase-dependent cell death by AMG-176.
CLL lymphocytes from 4 patients were treated in vitro with DMSO, indicated concentrations of AMG-176 or venetoclax for 24 h in presence or absence of 20 µM Q-VD-OPH. (A) Sample from one patient (#493) were stained and Annexin V/PI positivity was quantitated (% Ann/PI). Cells from same patient were used for caspase 3 activation assay (% C3 +ve) and were also analyzed for protein expression of PARP, cleaved caspase 3, Mcl-1 and Bcl-2 along with loading control. Numbers under Mcl-1 immunoblot indicate levels of Mcl-1 protein compared to DMSO (control) (B) Samples from 4 patients were treated and analyzed for cell death. For statistical analyses, we used three factor ANOVA with patient, absence or presence of caspase inhibitor and drug dosage as fixed effects. (C) Samples from 4 patients were treated and analyzed for change in Mcl-1 protein levels. For statistical analyses, we computed log2 ratios of fold changes relative to DMSO using a two factor ANOVA with patient and AMG-176 dosage as fixed effects. (D) CLL lymphocytes from 4 −8 patients were treated in vitro with DMSO, 10, 30, 100, 300 or 1000 nM AMG-176 or 100 nM venetoclax for 24 h. These were analyzed for Mcl-1 protein expression using immunoblot assay which is shown in Supplemental Figure 5. Quantitated protein levels are normalized to untreated control and plotted. Sample numbers are n=4 at 10 nM, n=8 at 30, 100, and 300 nM, and n=5 at 1000 nM of AMG-176 and n=6 at 100 nM of venetoclax. For statistical analyses, we used log2 ratios of fold change relative to DMSO using two factor ANOVA with patient and AMG-176 or venetoclax dosage as fixed effects. (E and F) Mitochondrial and cytosolic components were fractionated as described under Methods section for 10 patient samples. Immunoblots from two patients are shown to provide variability among patients. Additional two patients’ immunoblots are included in Supplementary Figure 8. Total cell, cytosolic, and mitochondrial fractions were used for western blot analyses for Mcl-1, Bax, Bak, and cytochrome C (Cyto c) along with normalizing loading control proteins. Abbreviations used are A = AMG-176; V = venetoclax.
During this process, treatment of AMG-176 resulted in upregulation of Mcl-1 protein levels with little effect on Bcl-2 expression levels. Increase in Mcl-1 protein levels were more pronounced when cell death was blocked by Q-VD-OPH (Figure 4A). We analyzed samples from four patients to establish Q-VD-OPH effect on cell death (Figure 4B) which suggested that pan-caspase inhibitor had significant inhibition of cell death (p= 0.10e-5). In same four patient samples we compared induction of Mcl-1 protein levels (Figure 4C); data demonstrated a significant increase in Mcl-1 protein without (p = 0.002) and with (p = 0.009) Q-VD-OPH.
AMG-176-mediated changes and translocation of Bcl-2 family proteins in CLL cells
Prior studies have elucidated that treatment with AMG-176 and other Mcl-1 antagonists result in increased Mcl-1 protein levels (15). We evaluated impact of escalating doses of AMG-176 on the Mcl-1 protein expression from 8 CLL patients (Supplementary Figure 5). In these CLL lymphocytes, there was dose-dependent induction of Mcl-1 protein reaching a plateau level at 100 nM in 8 patient samples (Figure 4D); in contrast treatment with venetoclax (n = 6) resulted in a 50% reduction in Mcl-1 levels compared to DMSO control.
To determine if there were changes in other anti-apoptotic family, we analyzed six patient samples after incubating with 10 to 1000 nM of AMG-176 (Supplementary Figure 6). Data from these samples suggest that Bcl-2 and Bcl-XL did not change at these concentrations. Longer incubations (48 hours) also did not impact these two proteins. Immunoblots from six patient samples were quantitated and these data suggested that the changes were only in the Mcl-1 protein; however, this was not statistically significant (Supplementary Figure 7).
To determine relationship with cell death and localization of Mcl-1 protein, we isolated mitochondrial and cytosolic fractions (Figure 4E–F and Supplementary Figure 8A–B) from CLL cells before and after treatment with AMG-176. AMG-176 treatment resulted in increased Mcl-1 protein in mitochondrial fraction. After treatment, Bax and Bak protein levels increased in mitochondrial fraction which may represent oligomerization of these proteins (Figure 4E and F). Cytochrome C, on the other hand, decreased in the mitochondria and increased in cytosol (Figure 4E–F and Supplementary Figure 8) and was associated with cell death.
Influence of Bcl-2 family proteins on AMG-176-mediated CLL cell death
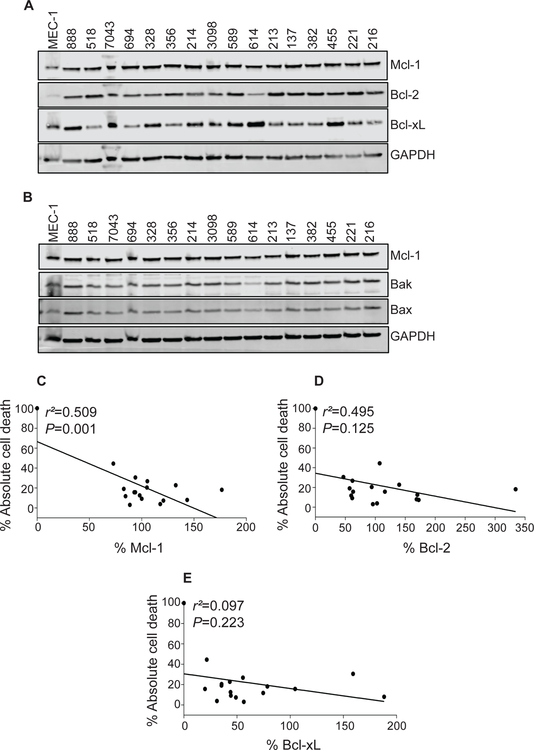
For CLL, Mcl-1, Bcl-2, and Bcl-xL are the primary survival proteins in the Bcl-2 family. Endogenous level of these proteins alone and together may influence cell death by AMG-176. In other heme-malignancies, level of Bak was associated with AMG-176 mediated cell death. To determine such relationship, we quantitated levels of these three proteins as well as Bak and Bax in 16 patient samples. To facilitate comparison, levels of these proteins in MEC-1 cell line was used as a reference point (Figure 5A–B). Expression level of these proteins was correlated with absolute cell death (i.e. endogenous cell death was not subtracted) in the same sample using linear regression. Data suggest that Mcl-1 and Bcl-2 members and not Bcl-xL, Bax or Bak show an indirect relationship to cell demise (Figure 5C–E and Supplementary Figure 9A–B); although only Mcl-1 correlation was statistically significant (p=0.001). The dependency on Bcl-2 and Mcl-1 prompted us to test dual targeting of these two molecules as described below.
Fig 5. Impact of baseline endogenous levels of Bcl-2 family proteins on AMG-176-induced cell death.
(A - B) CLL lymphocytes from peripheral blood of 16 patients were isolated to measure basal levels of Bcl-2 family proteins. Total protein was purified from these lymphocytes and MEC-1 cells and immunoblots were performed for the indicated Bcl-2 family proteins. (C - E) Protein levels were quantitated and normalized with GAPDH and compared with the levels in MEC-1 cells. The % Mcl-1, Bcl-2, and Bcl-xL levels were correlated with % apoptosis after a 24 hour incubation with 300 nM AMG-176. Linear regression and statistical analyses were performed using prism software, which required to put data point 0 on X-axis at 100% Y-axis value. Quantitation graphs of Bak and Bax proteins are shown in Supplemental Figure 9.
Dual targeting of Bcl-2 and Mcl-1 was superior to single agents for CLL cell death
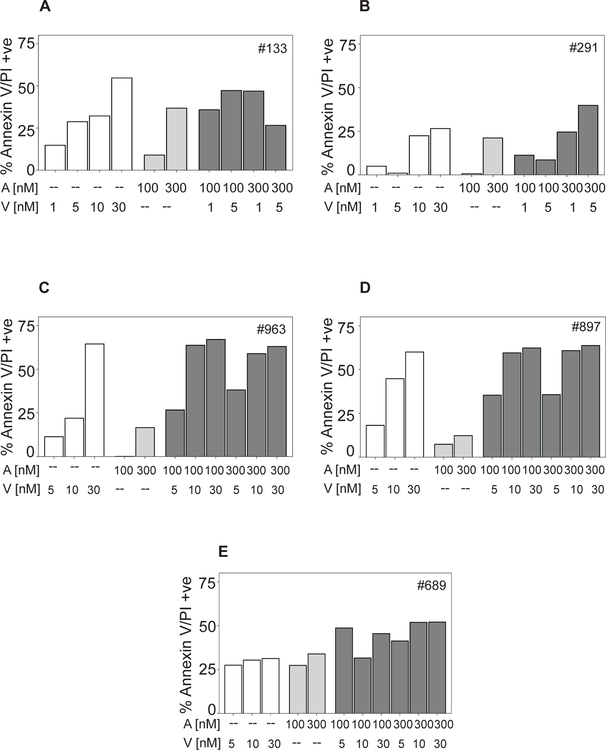
To examine dual inhibition of Bcl-2 and Mcl-1, CLL lymphocytes from five patients were incubated with low concentrations (1–30 nM) of venetoclax, 100 or 300 nM AMG-176 or combination of these two agents (Figure 6A–E). At these concentrations of venetoclax, generally the combination was synergistic (ratio <1; n = 14; Supplementary Table 4) or additive (ratio 1; n = 7; Supplementary Table 4). Additional samples from 29 CLL patients were incubated with higher concentration of venetoclax (100 nM), with AMG-176 (100 and 300 nM), or combinations (Supplementary Table 5). At higher concentrations of these agents; a varying degree of apoptosis was observed. While 18 CLL samples showed synergy between these two agents (ratio <1.0), 11 combinations were additive and 29 were antagonistic (Supplementary Table 5). There were more synergistic combination values with 100 nM AMG-176 and more antagonistic values with 300 nM AMG-176.
Fig 6. Combination of venetoclax and AMG-176 in CLL cells.
CLL lymphocytes from 5 patients were either vehicle (DMSO) treated or treated with 1, 5, 10, or 30 nM venetoclax (white bars), 100 and 300 nM AMG-176 (light grey bars) or combination of both (dark grey bars) for 24 h. (A - E) Samples from patient 133, 291, 963, 897, and 689 were treated and annexin/PI positivity was measured as described in the Methods section. DMSO value was subtracted from % cell death obtained after each treatment. Abbreviations used are A = AMG-176; V = venetoclax.
Discussion
Mcl-1 is a member of Bcl-2 anti-apoptotic family with 5 other associates. On one hand, it shares structural and functional homologies with other constituents of this family; such as having a transmembrane domain and conservation of BH domains including hydrophobic BH3 domain that sequesters pro-apoptotic proteins of this family (35). On the other hand, Mcl-1 has several unique properties. The transcript has several A-U rich elements (AREs); which make the transcript inherently short-lived. Further, the protein also gets an accelerated turnover due to the presence of two proline, glutamic acid, serine, and threonine (PEST) motifs which signal for ubiquitylation and degradation by the proteasome (36), (37) making it an intrinsically short-lived protein. In addition, Mcl-1 protein has two caspase proteolytic cut sites, which result in cleavage and dissipation of Mcl-1 when apoptosis is initiated. Further, the C-terminal domain of Mcl-1 acts as a pro-apoptotic molecule after caspase-3 cleavage which propels mitochondrial apoptosis (38). Mcl-1 is highly expressed in many human cancers (39) and was one of the top genes identified as having the most frequent somatic copy number alterations in many tumor types (40). This short-lived protein controls the activation of downstream caspases which are the major effectors of apoptosis. Collectively, these data underscore the importance of Mcl-1 in human cancers and its centrality in tumor development and maintenance and its role in controlling and opposing cell death.
Mcl-1 has been identified as an early response gene and its transcription is regulated by super-enhancer-driven transcription (41). This feature provides an impetus and rationale for development of transcription inhibitors as a strategy to target Mcl-1 transcription and dissipation of mRNA leading to decreased protein. The fact that Mcl-1 has short transcript half-life, further facilitated utilization of such strategy. In concert with these reports and observations, recent high throughput investigations to target Mcl-1 suggested transcription inhibition a top approach to study (36). Several inhibitors were identified through this approach and many transcription inhibitors showed preferential dissipation of Mcl-1 transcript.
CDK9 and CDK7 are key kinases that phosphorylate serine residues on the heptamer repeats on the C-terminal of RNA polymerase II which is critical for mRNA syntheses (42). Because of their key role in transcription, inhibitors of CDK7 and 9 have been tested in preclinical and clinical settings. Mechanistic investigations established that such agents induced cell death (26) due to a decline in the level of Mcl-1 transcript and protein during in vitro incubations (25) as well as during therapy (43) (44).
Because Mcl-1 protein half-life is short, inhibition of translation could also play a role in dissipation of Mcl-1 protein and induction of apoptosis. This was first tested with cycloheximide which induced rapid degradation of Mcl-1, activation of Bak and Bax, and induction of mitochondrial pathway of apoptosis in a variety of cancer cell lines (37). Such temporal events were blocked by usage of proteasomal inhibitors. Cycloheximide treatment promoted apoptosis in primary CLL cells without a biological effect on healthy lymphocytes, although protein synthesis was suppressed in both malignant and normal cell types (45). Mechanism of this differential cytotoxicity was not known then, it could be postulated now that the CLL lymphocytes are Mcl-1 addicted (46). Other clinically-oriented drugs such as omacetaxine, a natural product, which blocks elongation phase of protein synthesis was utilized as protein translation inhibitor in primary leukemia cells and acted synergistically when combined with transcription inhibitor (47). Silvestrol, another natural product and inhibitor of protein synthesis caused rapid decline in Mcl-1 expression followed by mitochondrial catastrophe and intrinsic cell death pathway initiation (48). Collectively, these data demonstrate that global protein translation inhibition is another potential strategy for Mcl-1 targeting. Because transcription and translation repressors are not selective for specific gene, mRNA, or protein, off target effects of these agents have been identified and recognized. Further, CDK9 inhibitors also have an impact on structurally similar other CDKs that have functionality in cell cycle. An alternative approach is to directly target Mcl-1.
In recent years, significant progress has been made in design and development of Mcl-1 inhibitors that directly bind to the protein and act as antagonists. Further, some of these agents are clinical candidates and are currently in Phase I trials ( NCT02675452; NCT02979366; NCT02992483; NCT03218683). Three of these clinically-relevant compounds are S64315, AMG-176, and AZD5991. AMG-176 was developed by Amgen and was selected from several similar congeners for its favorable pharmacokinetics, potency, and selectivity. It inhibits Mcl-1 in cell-free system with a Ki of <1 nM. When tested against a variety of cell lines, impressive biological activity was observed in hematological cell lines, especially acute leukemias, myeloma, and lymphomas. In whole cell systems, the IC50 values were much higher. We observed CLL cell death induction when 100 nM or higher (300 and 1000 nM) concentration of AMG-176 was used for in vitro incubations. These concentrations are similar to the levels used in AMG-sensitive AML and myeloma cell lines (15) and resulted in similar level of cell death (Figure 1, 2). The extent of cell death, time of induction, and concentration of drug comparison in CLL and ‘hematopoietic-sensitive-cell lines’ suggest that CLL cells are equally sensitive to AMG-176 treatment. Although, it is important to note that we used clinically relevant compound AMG-176 (a congener of AM-8621), while in the published paper (15) tool compound AM-8621 was used. Also, we investigated primary CLL lymphocytes while prior investigations mostly focused on established cell lines.
Although CLL appears to be sensitive to AMG-176 treatment, it is worthy to note that the extent of cell death varied among 74 patient samples; at 100 and 300 nM it ranged between 5 and 80% (Supplementary Table 1). Among prognostic factors, IGHV unmutated group and higher level of β2M showed slightly inferior extent of cell death (Figure 2A–D). Similarly, although the numbers are small, higher stage of disease responded poorly (Supplementary Figure 2 and 4) but the number of circulating cells did not make a difference (Figure 2; Supplementary Figure 3).
It is feasible that the levels of all pro-and anti-apoptotic Bcl-2 family may be determinant of AMG-176 induced cell death in CLL lymphocytes due to heterogeneous intracellular levels of these proteins. Such differential addiction to anti-apoptotic Bcl-2 family has been identified in small-cell lung cancer (49). Evasion of apoptosis has been identified as a primary mechanism of neoplastic cell survival in CLL patients. Bcl-2 family pro- and anti-apoptotic proteins are the arbitrators of the programmed cell death. Among the Bcl-2 survival members, in addition to Mcl-1, Bcl-2, and Bcl-xL have been shown to be primary mediators of cell fate. There was inverse correlation of AMG-176-induced cell death and Mcl-1 (p = 0.001) and perhaps also Bcl-2 (p = 0.125) protein levels (Figure 5). This may also explain why samples from CLL patients with unmutated IGHV, which are known to have higher Mcl-1 levels (44) were less susceptible to AMG-176 (Figure 2A and Supplementary Figure 3A).
Mcl-1 protein has been shown to have functions in controlling and opposing apoptosis as well as regulating cellular bioenergetics. However, in both cases the localization of the protein has been in the mitochondria (50). To determine if AMG-176-induced Mcl-1 protein also resides in mitochondria, we did subcellular fractionation. Our data in several patient samples demonstrate that AMG-176 treatment resulted in an increase in Mcl-1 protein levels (Figure 4C–D, Supplementary Figure 5) without any impact on Bcl-2 or Bcl-XL protein expression (Supplementary Figure 6–7). Further, the augmented Mcl-1 protein was also residing in mitochondrial fraction (Figure 4E–F and Supplementary Figure 8). Protein levels started to decline when cells initiated cell death. This was due to triggering of caspase mediated proteolysis of Mcl-1 protein. In fact, when caspase activity was inhibited by pan-caspase inhibitor, the levels of Mcl-1 protein increased further by AMG-176 (Figure 4A–C). It is not clear if the increase in Mcl-1 protein specifically in mitochondria may inhibit apoptosis.
Combination of venetoclax and AZD5991 during in vitro incubations showed beneficiary effects (14). Similarly, venetoclax and AMG-176 combination was effective for primary AML samples (15). Ibrutinib therapy resulted in a decrease in intracellular Mcl-1 protein levels in peripheral blood CLL lymphocytes and when combined with venetoclax was effective for CLL cells in vitro and during therapy (51). For AMG-176 in CLL lymphocytes, mostly combination was synergistic or additive when it was added to low concentration of venetoclax (Figure 6; Supplementary Table 4). Because Mcl-1 levels have been shown to predict for venetoclax resistance, combination of ibrutinib, venetoclax, and AMG-176 (or other Mcl-1 inhibitors) may provide a multi-faceted approach to target CLL.
When tested among same patient samples, it is clear that venetoclax treatment resulted with greater level of apoptosis compared to AMG-176. It is known that CLL cells have much higher levels of Bcl-2 protein than Mcl-1 levels. Further, compared to AMG-176’s action on Mcl-1, venetoclax is much more potent inhibitor of Bcl-2. It has been demonstrated that leukemia cells are resistant to venetoclax if they possess high levels of Mcl-1. Under these conditions, Mcl-1 antagonists such as AMG-176 may prove to be beneficial as a therapeutic agent. Such scenarios need to be tested.
In summary, current investigations in primary CLL lymphocyte establish utility of AMG-176 in CLL. The biological response in CLL cells was comparable to that in hematological cell lines treated with AMG-176 or cell lines and primary cells treated with AZD-5991. Except for preliminary data with A-1210477 and S63845 (52), this is the first detailed investigation of Mcl-1 inhibitor in CLL setting. Molecular responses were similar to that observed in other cancers with these agents and the pathway of cell death involved mitochondrial intrinsic route. Encouragingly, AMG-176 induced cell death was not influenced much by good or poor prognostic features or in genetic subsets of CLL disease. Finally, at low concentrations of venetoclax, AMG-176 added synergistic or additive cytotoxicity to CLL lymphocytes. Collectively, these data suggest that Mcl-1 inhibitor such as AMG-176 should be evaluated for patients with CLL especially in combination with venetoclax and/or ibrutinib.
Supplementary Material
Translational Relevance.
AMG-176, a Mcl-1 antagonist, is in clinical trials for hematological malignancies. Recent preclinical investigations with AMG-176 showed promise on hematological malignancies including acute leukemias and multiple myeloma, however, investigations in chronic lymphocytic leukemia (CLL) are lacking. It has been well established that CLL cell survival is predicted by the presence of Bcl-2 family anti-apoptotic family members including Bcl-2 and Mcl-1. Venetoclax, an FDA approved agent, that targets Bcl-2 has shown success in preclinical and clinical settings and was approved for CLL. Indirect manipulation of Mcl-1 using transcription and translation inhibitors have shown utility of targeting Mcl-1 in CLL cells. Whether direct Mcl-1 antagonists, such as AMG-176, can also eradicate CLL cells is of prime importance for translation of these agents to clinic either alone or in combination.
Acknowledgments
This work was supported in part by a CLL Global Research Foundation Alliance grant and MD Anderson’s CLL Moon Shot program. Authors thank Yuling Chen for collection of blood from healthy donors and Mark Hess, Data Analysis Supervisor, for providing patient characteristics.
Financial Support: This work was supported in part by Alliance grant from the CLL-Global Research Foundation and by The University of Texas MD Anderson Cancer Center Moon Shot Program. Core facilities are supported in part by the NIH through MD Anderson’s Cancer Center Support Grant CA016672
Footnotes
Conflict of Interest Disclosure: The authors do not have conflicts of interest.
References
- 1.Birkinshaw RW, Czabotar PE. The BCL-2 family of proteins and mitochondrial outer membrane permeabilisation. 2017. Elsevier; p 152–62. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. New England Journal of Medicine 2009;361(16):1570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nature reviews Drug discovery 2008;7(12):989. [DOI] [PubMed] [Google Scholar]
- 4.Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. Journal of clinical oncology 2012;30(25):3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005;435(7042):677. [DOI] [PubMed] [Google Scholar]
- 6.Moore VDG, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. The Journal of clinical investigation 2007;117(1):112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nature medicine 2013;19(2):202. [DOI] [PubMed] [Google Scholar]
- 8.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. New England Journal of Medicine 2016;374(4):311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. The Lancet Oncology 2016;17(6):768–78. [DOI] [PubMed] [Google Scholar]
- 10.Doi K, Li R, Sung S-S, Wu H, Liu Y, Manieri W, et al. Discovery of marinopyrrole A (maritoclax) as a selective Mcl-1 antagonist that overcomes ABT-737 resistance by binding to and targeting Mcl-1 for proteasomal degradation. Journal of Biological Chemistry 2012;287(13):10224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey MK, Gowda K, Doi K, Sharma AK, Wang H-G, Amin S. Proteasomal degradation of Mcl-1 by maritoclax induces apoptosis and enhances the efficacy of ABT-737 in melanoma cells. PloS one 2013;8(11):e78570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leverson J, Zhang H, Chen J, Tahir S, Phillips D, Xue J, et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell death & disease 2015;6(1):e1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch R, Christie AL, Crombie JL, Palmer AC, Plana D, Shigemori K, et al. Biomarker-driven strategy for MCL1 inhibition in T-cell lymphomas. Blood 2019;133(6):566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tron AE, Belmonte MA, Adam A, Aquila BM, Boise LH, Chiarparin E, et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nature communications 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caenepeel S, Brown SP, Belmontes B, Moody G, Keegan KS, Chui D, et al. AMG 176, a selective MCL1 inhibitor, is effective in hematologic cancer models alone and in combination with established therapies. Cancer discovery 2018;8(12):1582–97. [DOI] [PubMed] [Google Scholar]
- 16.Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016;538(7626):477. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, He S, Look AT. The MCL1-specific inhibitor S63845 acts synergistically with venetoclax/ABT-199 to induce apoptosis in T-cell acute lymphoblastic leukemia cells. Leukemia 2019;33(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 2003;426(6967):671. [DOI] [PubMed] [Google Scholar]
- 19.Krajewski S, Blomqvist C, Franssila K, Krajewska M, Wasenius V-M, Niskanen E, et al. Reduced expression of proapoptotic gene BAX is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer research 1995;55(19):4471–8. [PubMed] [Google Scholar]
- 20.Gottardi D, Alfarano A, De Leo A, Stacchini A, Aragno M, Rigo A, et al. In leukaemic CD5+ B cells the expression of BCL-2 gene family is shifted toward protection from apoptosis. British journal of haematology 1996;94(4):612–8. [DOI] [PubMed] [Google Scholar]
- 21.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. nature 1998;392(6676):605. [DOI] [PubMed] [Google Scholar]
- 22.Kaufmann SH, Karp JE, Svingen PA, Krajewski S, Burke PJ, Gore SD, et al. Elevated expression of the apoptotic regulator Mcl-1 at the time of leukemic relapse. Blood 1998;91(3):991–1000. [PubMed] [Google Scholar]
- 23.Derenne S, Monia B, Dean NM, Taylor JK, Rapp M-J, Harousseau J-L, et al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-xL is an essential survival protein of human myeloma cells. Blood 2002;100(1):194–9. [DOI] [PubMed] [Google Scholar]
- 24.Balakrishnan K, Stellrecht CM, Genini D, Ayres M, Wierda WG, Keating MJ, et al. Cell death of bioenergetically compromised and transcriptionally challenged CLL lymphocytes by chlorinated ATP. Blood 2005;105(11):4455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen R, Keating MJ, Gandhi V, Plunkett W. Transcription inhibition by flavopiridol: mechanism of chronic lymphocytic leukemia cell death. Blood 2005;106(7):2513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitada S, Zapata JM, Andreeff M, Reed JC. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood 2000;96(2):393–7. [PubMed] [Google Scholar]
- 27.Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461): presented in part at the 43rd annual meeting of the American Society of Hematology, Orlando, FL, December 10, 2001, and published in abstract form. 59. Blood 2002;100(13):4325–36. [DOI] [PubMed] [Google Scholar]
- 28.Zhou P, Qian L, Kozopas KM, Craig RW. Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood 1997;89(2):630–43. [PubMed] [Google Scholar]
- 29.Patel V, Chen LS, Wierda WG, Balakrishnan K, Gandhi V. Impact of bone marrow stromal cells on Bcl-2 family members in chronic lymphocytic leukemia. Leukemia & lymphoma 2014;55(4):899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacology research & perspectives 2015;3(3):e00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balakrishnan K, Burger JA, Fu M, Doifode T, Wierda WG, Gandhi V. Regulation of Mcl-1 expression in context to bone marrow stromal microenvironment in chronic lymphocytic leukemia. Neoplasia 2014;16(12):1036–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurtova AV, Balakrishnan K, Chen R, Ding W, Schnabl S, Quiroga MP, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood 2009;114(20):4441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell–derived factor-1. Blood 2000;96(8):2655–63. [PubMed] [Google Scholar]
- 34.Balakrishnan K, Burger JA, Wierda WG, Gandhi V. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell–mediated Mcl-1 induction and drug resistance. Blood 2009;113(1):149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007;26(9):1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei G, Margolin AA, Haery L, Brown E, Cucolo L, Julian B, et al. Chemical genomics identifies small-molecule MCL1 repressors and BCL-xL as a predictor of MCL1 dependency. Cancer cell 2012;21(4):547–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams KW, Cooper GM. Rapid turnover of mcl-1 couples translation to cell survival and apoptosis. Journal of Biological Chemistry 2007;282(9):6192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weng C, Li Y, Xu D, Shi Y, Tang H. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. Journal of Biological Chemistry 2005;280(11):10491–500. [DOI] [PubMed] [Google Scholar]
- 39.Krajewska M, Moss SF, Krajewski S, Song K, Holt PR, Reed JC. Elevated expression of Bcl-X and reduced Bak in primary colorectal adenocarcinomas. Cancer Research 1996;56(10):2422–7. [PubMed] [Google Scholar]
- 40.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010;463(7283):899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 2013;153(2):307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Q, Li T, Price DH. RNA polymerase II elongation control. Annual review of biochemistry 2012;81:119–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood 2007;109(2):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tong W-G, Chen R, Plunkett W, Siegel D, Sinha R, Harvey RD, et al. Phase I and pharmacologic study of SNS-032, a potent and selective Cdk2, 7, and 9 inhibitor, in patients with advanced chronic lymphocytic leukemia and multiple myeloma. Journal of clinical oncology 2010;28(18):3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collins R, Harmon B, Souvlis T, Pope J, Kerr J. Effects of cycloheximide on B-chronic lymphocytic leukaemic and normal lymphocytes in vitro: induction of apoptosis. British journal of cancer 1991;64(3):518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pepper C, Lin TT, Pratt G, Hewamana S, Brennan P, Hiller L, et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood 2008;112(9):3807–17. [DOI] [PubMed] [Google Scholar]
- 47.Chen R, Guo L, Chen Y, Jiang Y, Wierda WG, Plunkett W. Homoharringtonine reduced Mcl-1 expression and induced apoptosis in chronic lymphocytic leukemia. Blood 2011;117(1):156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucas DM, Edwards RB, Lozanski G, West DA, Shin JD, Vargo MA, et al. The novel plant-derived agent silvestrol has B-cell selective activity in chronic lymphocytic leukemia and acute lymphoblastic leukemia in vitro and in vivo. Blood 2009;113(19):4656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inoue-Yamauchi A, Jeng PS, Kim K, Chen H-C, Han S, Ganesan YT, et al. Targeting the differential addiction to anti-apoptotic BCL-2 family for cancer therapy. Nature communications 2017;8:16078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perciavalle RM, Opferman JT. Delving deeper: MCL-1’s contributions to normal and cancer biology. Trends in cell biology 2013;23(1):22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cervantes-Gomez F, Lamothe B, Woyach JA, Wierda WG, Keating MJ, Balakrishnan K, et al. Pharmacological and protein profiling suggests venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Clinical cancer research 2015;21(16):3705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen LS, Keating MJ, Wierda WG, Gandhi V. Induction of apoptosis by MCL-1 inhibitors in chronic lymphocytic leukemia cells. Leukemia & lymphoma 2019:1–4. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.