Abstract
Background
Community-based exercise rehabilitation programmes for chronic disease are an effective alternative to traditional hospital-based programmes. MedEx Wellness is a novel community-based exercise rehabilitation programme that integrates a range of chronic diseases. The aim of this trial was to investigate the effect of participating in MedEx Wellness on physical, clinical and psychological health.
Methods
A prospective cohort study was conducted. Participants were recruited at induction to the MedEx Wellness programme following referral from healthcare professionals. Participants underwent a baseline assessment before commencing the exercise programme and repeat assessments at 3, 6 and 12 months. The primary outcome was cardiorespiratory fitness (6 minute- time trial) at 12 months. Secondary outcomes included health-related quality of life (EuroQoL-5D, Satisfaction with Life Scale, Warwick Edinburgh Mental Wellbeing Scale, Patient Health Questionnaire8, Functional Assessment of Cancer Therapy Questionnaire), free living activity behavior (accelerometer) and healthcare utilization (recall questionnaire). Tertiary outcomes included blood pressure (24 h), biomarkers (lipids, glucose and C-reactive protein), other components of physical fitness, including strength (handgrip test, sit-to-stand test), flexibility (sit-and-reach test), body composition (body mass index and waist-to-hip ratio), and falls risk (timed up and go test), and claudication time (incremental treadmill walking test), cognitive function, including attention (Attention Network Task), memory (Luck & Vogel Visual Working Memory Task) and cognitive reserve. Exploratory outcomes included psychosocial determinants of physical activity (self-efficacy, social support, intentions).
Discussion
This trial will evaluate whether participation in the MedEx Wellness programme has positive effects on physical, clinical and psychological health in individuals with a range of chronic diseases.
Trial registration
ISRCTN Registry ISRCTN10351412.
Keywords: Chronic disease, Exercise rehabilitation, Community-based, Cardiorespiratory fitness
Abbreviations
- 6 MTT
6 Minute Time Trial
- BMI
Body Mass Index
- BP
Blood Pressure
- CBP
Community-Based Programme
- CVD
Cardiovascular Disease
- DCU
Dublin City University
- EQ-5D
EuroQoL-5D-3L questionnaire
- HSE
Health Service Executive
- NCD
Non-Communicable Disease
- PHQ-8
Patient Health Questionnaire 8
- SAE
Serious Adverse Event
- SWLS
Satisfaction with Life Scale
- TUG
Timed Get Up & Go Test
- WEMWS
Warwick Edinburgh Mental Wellbeing Scale
- HDL-C
High-Density Lipoprotein Cholesterol
- LDL-C
Low-Density Lipoprotein Cholesterol
- CRP
C-Reactive Protein
1. Introduction
Noncommunicable diseases, also known as chronic diseases, account for greater than half of the overall global disease burden [1] which is predicted to continue to rise [2] potentially overwhelm the current healthcare systems. Identifying effective approaches to implementing evidence-based treatment strategies is crucial. There is substantial evidence demonstrating for the beneficial effect of exercise in the secondary prevention of multiple chronic diseases [4,5]. Indeed, there are exceptionally few chronic diseases in which the burden of the disease, the comorbidities related to the disease, and the disease-related quality of life are not improved with exercise [4,5].
Exercise rehabilitation has traditionally been delivered in a hospital-based setting, primarily through cardiac or pulmonary rehabilitation programmes. Community-based programmes (CBP) are an effective alternative setting for the delivery of exercise-based rehabilitation [6,7]. CBP offer greater accessibility to patients and may help to address the poor participation rates in hospital-based exercise rehabilitation programmes [8].
Previous research involving CBP have focused primarily on single chronic disease populations [[9], [10], [11]] including cardiac [12], pulmonary [7,13], cancer [10,14], stroke [15], osteoarthritis [16], and peripheral arterial disease [17] cohorts. A systematic review of CBPs across chronic disease populations identified that the design and components of exercise programmes were similar, irrespective of disease [18]. Considering the overlap in these programmes and the high prevalence of multi-morbidity [19], defined as the co-existence of two or more chronic conditions within an individual [20], an integrated approach to CBP for chronic disease management represents a more resource efficient strategy.
MedEx Wellness (now rebranded as ExWell Medical) is a novel community-based exercise rehabilitation programme for individuals with chronic disease in Ireland. It offers group exercise classes with medical oversight for people with a range of chronic diseases, including cardiovascular disease (CVD), pulmonary disease, diabetes and cancer. The MedEx Wellness model provides a shared programme across chronic diseases and avoids duplication of programme infrastructure and content. The programme is a significant resource for healthcare professionals and individuals living with chronic disease and hosts approximately 700 participant visits per week. The MedEx model is also unique in that it does not have a fixed duration and p articipants can attend the programme on a continuous or intermittent basis. They are however, encouraged to establish a lifelong relationship with MedEx Wellness.
In this manuscript, we describe a cohort trial that investigated the effect of participating in the MedEx Wellness programme for 12 months on physical, clinical and psychological health in individuals living with chronic disease.
2. Aims
The aims of this trial were to evaluate the following hypotheses:
2.1. Primary hypothesis
Twelve months of participation in the MedEx Wellness programme will result in a significant improvement in physical health (cardiorespiratory fitness) assessed using the 6-min time trial in individuals with a range of established chronic diseases.
2.2. Secondary hypotheses
Twelve months of participation in the MedEx Wellness programme will result in a significant improvement in:
-
a)
Psychological health: assessed by EuroQoL-5D, Satisfaction with Life Scale, Warwick Edinburgh Mental Wellbeing Scale, the Patient Health Questionnaire 8 and the Functional Assessment of Cancer Therapy Questionnaire.
-
b)
Free Living Activity Behavior: physical activity and sedentary behaviors assessed by body-worn activity monitoring.
-
c)
Clinical health: healthcare utilization assessed by a recall questionnaire.
2.3. Tertiary hypotheses
Twelve months of participation in the MedEx Wellness programme will significantly improve:
-
a)
Clinical health: blood pressure assessed using a 24 h monitor and biomarkers including lipids, glucose and C-reactive protein.
-
b)
Physical health: strength assessed by handgrip test and sit-to-stand test; flexibility assessed by sit-and-reach test; body composition assessed by body mass index and waist-to-hip ratio; falls risk assessed by timed up and go test and claudication time assessed by an incremental treadmill walking test.
-
c)
Psychological health: cognitive function, including attention assessed by Attention Network Task and working memory assessed by Luck & Vogel Visual Working Memory Task.
2.4. Exploratory hypotheses
Twelve months of participation in the MedEx Wellness programme will significantly improve psychosocial determinants of physical activity assessed by self-efficacy, social support and intentions.
3. Methods
3.1. Study design
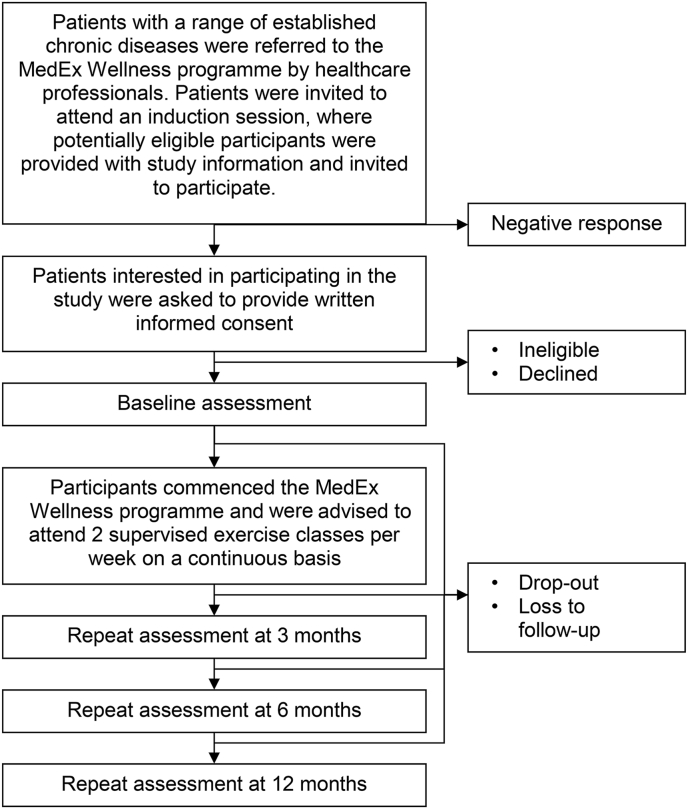
A prospective cohort study was conducted in participants recruited from patients referred to the MedEx Wellness programme by healthcare professionals, who were observed over the course of 12 months of participation in the programme. Outcomes were assessed at baseline and 3, 6 and 12 months (Fig. 1). The trial was funded by the Health Service Executive (HSE) and approved by Dublin City University Ethics Committee (DCUREC: 2014/227). ISRCTN Registry ISRCTN10351412. This manuscript is reported using the PRISMA reporting guidelines.
Fig. 1.
Trial algorithm.
3.2. Study setting
The trial was conducted at Dublin City University (DCU), Ireland. The MedEx Wellness programme operates as a partnership between members of academic staff from the School of Health and Human Performance DCU and the Sports Services department in the institution on DCU campus.
3.3. Participants
Eligibility criteria for inclusion were individuals aged >18 years with established non-communicable disease (NCD) referred to the MedEx Wellness programme by consultant physicians, phase III (outpatient) cardiac rehabilitation teams from three major tertiary hospitals and general practitioners in the local area. Exclusion criteria included uncontrolled cardiovascular disease (CVD), significant musculoskeletal or neurological conditions, cognitive decline and significant mental illness or intellectual disability that restricted participation in a physical training programme.
3.3.1. Recruitment
Participants were recruited at induction to the MedEx Wellness programme. Programme inductions took place weekly and involved 10–20 individuals per week. Potential participants were provided with a full oral explanation and a plain language statement detailing the trial. Participants provided written informed consent at this visit.
3.4. MedEx Wellness community-based exercise programme
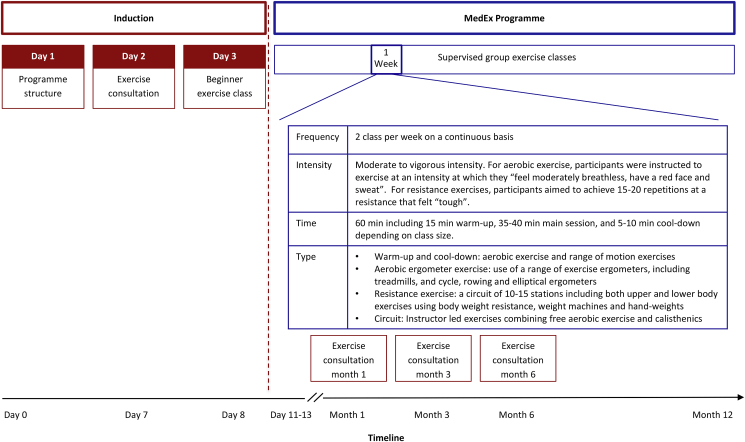
The exercise intervention was delivered as part of the MedEx Wellness programme, which is outlined in Fig. 2. Participants underwent an induction to the programme, which involved 3 visits that provided: i) information on the programme purpose, structure and logistics; ii) a group exercise consultation and iii) a beginner exercise class. The exercise consultation was delivered by researchers trained in exercise consultation delivery and adhered to a motivational interviewing style. The aim of the consultation was to use behaviour change techniques to improve exercise adherence. The consultations focused on the benefits and barriers to exercise, problem solving, goal setting and action planning. The exercise consultations were repeated at 1, 3 and 6 months and involved a review of behaviour goals and feedback on progress. The beginner class followed a similar format as regular MedEx Wellness classes but involved a smaller group size (approx. 10–15 participants). The beginner classes introduced the participants to the exercise equipment and demonstrated proper techniques with the aim to improve self-efficacy and foster social support to improve programme initiation and adherence.
Fig. 2.
The MedEx Wellness Programme
Following induction, participants were advised to attend 2 MedEx Wellness supervised group exercise classes per week. The number of participants at the exercise classes ranged from 30 to 75 (dependent on day of the week) and classes were supervised at a ratio of one instructor to 15 participants. MedEx Wellness staff are certified in Cardiac Prevention and Rehabilitation and the programme has medical oversight from the MedEx Wellness Chief Medical Officer (physician). The cost of participation was €7/8 (with/without a medical card) per session or €45/50 (with/without a medical card) per month (parking was included in this payment). The MedEx Wellness exercise intervention is described subsequently using the FITT (frequency, intensity, time, type) principle.
3.4.1. Frequency
Participants were advised to attend ≥2 supervised group exercise classes per week and encouraged to attend the same classes every week in order to foster social-support and habit formation.
3.4.2. Intensity
For the aerobic component of the exercise classes, participants were instructed to work at an intensity at which they “feel moderately breathless, have a red face and sweat”. For the resistance component of the exercise classes, participants aimed to achieve 15–20 repetitions at resistance that felt “tough” at each station.
3.4.3. Time
The class included a 15 min warm-up followed by a 40 min exercise training phase and a 5 min cool-down. For the exercise training phase, depending on the class size, participants were split into two or three groups: if ≤30 participants were present, the class was divided into two groups that alternated between 20 min of aerobic exercise and 20 min resistance exercise; and if >30 participants were present, the class was divided into three groups that rotated between 10 min of aerobic exercise, 10 min of resistance exercise and 10 min of a combined aerobic and resistance circuit. For the resistance component, participants performed exercises at each resistance station for 60 s aiming to achieve 15–20 repetitions.
3.4.4. Type
The aerobic component of the exercise classes involved the use of different equipment based on participant preference and ability, including treadmills, cycle ergometers, rowers and elliptical ergometers. Resistance training involved a circuit of 10–15 stations that included upper and lower body exercises using body weight resistance, weight machines and hand-weights. The combined aerobic and resistance circuit was led by an instructor and involved a combination of free aerobic exercises and calisthenics, for example, jumping jacks, toe taps, squats and wall press.
Following completion of the class, participants engaged in a social tea or coffee. Adherence to the programme was monitored as the number of sessions attended, which was electronically recorded by swipe access to the facility.
3.5. Outcome measures
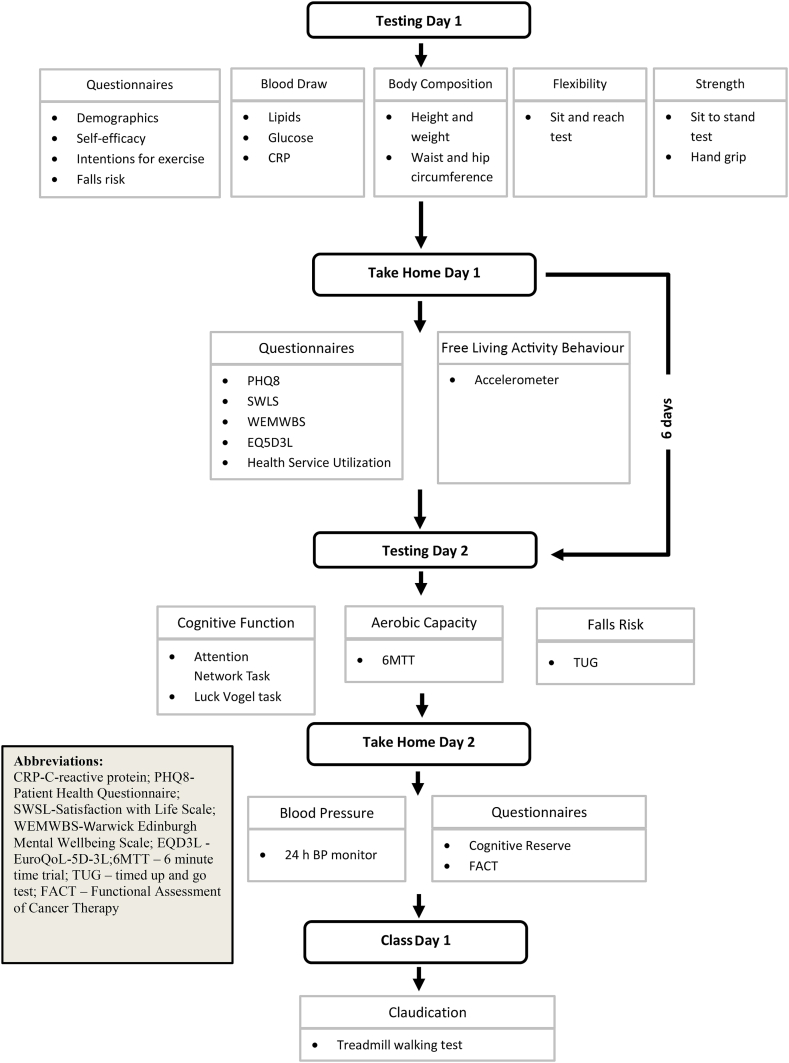
Participants underwent a series of outcome assessments at baseline and 3, 6 and 12 months. Outcome measurements are presented in Table 1. The assessments were conducted over 2 visits (and 3 visits for individuals with intermittent claudication). Fig. 3 provides an overview of the study flow algorithm. Visit 1 and 2 were separated by 6 days whilst visit 2 and 3 by 24 h. Visit 1 involved a fasting blood sample, questionnaire completion to obtain demographic information and the psychosocial determinants of physical activity, and assessment of body composition, strength and flexibility. Participants were provided with a take home questionnaire that assessed health-related quality of life and were provided with an activPAL3 micro activity monitor (PAL Technologies Ltd. Glasgow, Scotland) to wear for 6 days. Visit 2 involved assessments of cognitive function, cardiorespiratory fitness and falls risk. Participants were provided with an ambulatory blood pressure (BP) monitor to wear for 24 h and a take home questionnaire that assessed cognitive reserve. Participants were encouraged to complete questionnaires independently but where required, a member of the research team or a family member or friend provided assistance. Participants with intermittent claudication performed a treadmill test at visit 3, which was organized prior to a scheduled exercise class.
Table 1.
Outline of outcome measurement assessment time points.
| Outcome and measurement tool | Baseline/ T1 | 3 mth/ T2 | 6 mth/ T3 | 12 mth/ T4 |
|---|---|---|---|---|
| Primary outcome: | ||||
| Physical health: 6MTT | x | x | x | x |
| Secondary outcomes: | ||||
| Psychological health | ||||
| EQ-5D-3L | x | x | x | x |
| PHQ-8 | x | x | x | x |
| SWLS | x | x | x | x |
| WEMWBS | x | x | x | x |
| FACT | x | x | x | x |
| Physical health: Free living activity behaviour | x | x | x | x |
| Clinical health: Healthcare utilization | x | x | ||
| Tertiary outcomes: | ||||
| Clinical health: Biomarkers | ||||
| Blood pressure | x | x | x | x |
| Lipids | x | x | x | x |
| Glucose | x | x | x | x |
| CRP | x | x | x | x |
| Physical health | ||||
| Body composition | x | x | x | x |
| Strength | x | x | x | x |
| Flexibility | x | x | x | x |
| Falls risk | x | x | x | x |
| Claudication time | x | x | x | x |
| Psychological health: Cognitive function | ||||
| Attentionand visual working memory | x | x | x | x |
| Cognitive reserve | x | |||
| Exploratory outcomes: | ||||
| Psychological health: Psychosocial determinants of physical activity | ||||
| Self-efficacy | x | x | x | x |
| Social support for exercise | x | x | x | x |
| Intentions for exercise | x | x | x | x |
Abbreviations: 6 MTT - 6 Minute Time Trial; EQ-5D - EuroQoL-5D-3L questionnaire; PHQ-8 - The Patient Health Questionnaire; SWLW - TheSatisfaction with Life Scale; WEMWS - Warwick Edinburgh Mental Wellbeing Scale; FACT - Functional Assessment of Cancer Therapy; CRP - C-Reactive Protein (CRP).
Fig. 3.
Outline of data collection.
3.6. Primary outcome
Physical health: cardiorespiratory fitness assessed using the 6-min time trial (6MTT) [21,22]. Participants were instructed to cover as much distance as possible in 6 min while walking, running or a combination, back and forth on a flat indoor 20 m course. Participants received a standard set of instructions adapted from the ATS guidelines for the 6-min walk test [23]. No warm-up was permitted. The position on the 20 m course at which the participant stopped at the end of the 6 min was marked with a cone and the distance covered in the final partial lap was measured to the nearest metre using a measuring tape. The total distance covered was recorded.
3.7. Secondary outcomes
Psychological health assessed by the following questionnaires:
-
•
The EuroQoL-5D-3L (EQ-5D), which consists of two components: health state description and health evaluation. The description component is comprised of five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension has 3 levels: no problems, some problems and extreme problems. The evaluation component records the patient's self-rated health on a vertical visual analogue scale where the endpoints are labelled ‘best imaginable health state’ and ‘worst imaginable health state’ [[24], [25]].
-
•
The Satisfaction with Life Scale (SWLS), a 5-item scale designed to measure global cognitive judgment of subjective wellbeing [26].
-
•
The Warwick Edinburgh Mental Wellbeing Scale (WEMWS), a 14-item positively worded scale covering both feeling and functional aspects of mental wellbeing [27].
-
•
The Patient Health Questionnaire (PHQ-8), an 8-item validated and widely used diagnostic and severity measure for depression [28].
-
•
The Functional Assessment of Cancer Therapy (FACT) questionnaire was completed by participants with cancer only. This is a 40-item questionnaire that includes five domains: physical, social/family, emotional, function and cancer-specific, to evaluate quality of life and fatigue in patients with cancer [29]. It is composed of a general component (FACT-G) and cancer specific subscales.
Free living activity behavior assessed using:
The activPAL3 micro accelerometer, a device that measures bodily accelerations using a triaxial accelerometer, sampling at 20 Hz. The device was worn on the midpoint of the anterior aspect of the thigh. It was covered with a water-resistant nitrile sleeve and attached to the skin using a Tegaderm film adhesive dressing. Participants were instructed to wear the device continuously for 6 consecutive days, except during water immersion activities (i.e. swimming and bathing).
Raw acceleration data were processed and stored in a range of file formats, including csv files or 15 s epoch summary files. Proprietary algorithms classify activities into sitting/lying time, standing time, stepping time, step count and activity counts. Only datasets that provide ≥4 valid days of activity data, including 1 weekend day, will be processed for analysis. A valid day will be defined as ≥600 min of recording during daytime hours (i.e. 7 a.m. to 11 p.m.) [18]. Non-wear time will be defined as ≥60 min of consecutive zero accelerometer counts [19]. Sedentary behavior characteristics will be examined using a customized MATLAB® (version 7.0.1, The Mathworks Inc, Natick, MA, USA) software programme [19]. The programme has been previously described [19]. Briefly, the number and duration of sedentary bouts per day will be calculated. Sedentary bouts will be categorized by specific durations, namely <5 min, 5–10 min, 11–20 min, 21–30 min, 31–40 min, 41–60 min, >60 min, >90 min. The number of sedentary bouts and the total duration spent in sedentary bouts in each category will be calculated.
Clinical health assessed by:
An 8-item healthcare utilization questionnaire developed based on The Irish Longitudinal Study on Ageing (TILDA) [30,31]. The questionnaire captured self-reported 12-month recall of health service use. Participants were asked about: i) medical card cover; ii) GP visits; iii) hospital emergency service visits; iv) outpatient hospital visits; v) whether an outpatient hospital visit was a public/private visit; vi) nights spent in hospital; vii) whether nights spent in hospital were a public/private visit; viii) days taken off work due to illness.
3.8. Tertiary outcomes
Clinical health assessed by:
-
•
An ambulatory BP monitor (Oscar 2, SunTech Medical, Inc, NC USA), issued to participants to wear for 24 h. Data was retrieved from the device using the AccWin Pro 3 PC (SunTech Medical, Inc, NC USA). Average systolic and diastolic daytime, night-time and 24-h blood pressures were derived.
-
•
Venous blood samples were taken following an overnight fast. Serum vacutainers were left to stand for 30 min before centrifugation at 3000 rpm (1600 g) for 15 min at 4 °C. Serum triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting glucose and C-reactive protein (CRP) were determined using spectrophotometric assays, performed on an automated bench-top clinical chemistry system (ACE®, Alfa Wassermann B.V., Netherlands) using the appropriate reagents, calibrators and controls (Randox Laboratories, UK).
Physical health assessed by:
-
•
Body composition: Height and body mass were measured using a stadiometer and electronic scale (model 707 balance scales: Seca GmbH, Hamburg, Germany), respectively. Body mass index (BMI) was calculated as body mass in kilograms divided by squared height in metres. Waist and hip circumferences were measured by a trained researcher using a tape measure and waist to hip ratio was derived.
-
•
Muscle strength: Lower body strength was assessed using the 10 repetition sit to stand test [32], which records the time taken to stand and sit 10 times as fast as possible. The best of two attempts was recorded. Handgrip strength was measured in the dominant arm using a hand-held dynamometer (Takei 5401 Handgrip Digital Dynamometer). The average of three attempts was recorded.
-
•
Flexibility: assessed using a modified sit and reach test [33], whereby a sit and reach box (Eveque Leisure Equipment Ltd, Cheshire, UK) was placed on a bench. While sitting on the bench,participants were instructed to extend their legs fully with feet flat against the box and were asked to flex forward to reach their fingertips as far as possible along the measurement scale. The best of three attempts was recorded.
-
•
Falls risk: A 3-item questionnaire was used to identify participants who reported falling one or more times in the previous year or reported a fear of falling or problems with balance or walking. A Timed Get Up and Go (TUG) Test [34] assessed the time taken to rise from a chair of standardized height, walk a fixed distance of 3m, turn, return to the chair, and sit down again. The bestof two attempts was recorded.
-
•
Claudication time: An incremental treadmill walking test using the Gardner protocol [35] of 3.2 km.h-1 and 0% grade, with a subsequent 2% increase in grade every 2 min was used to determine time to the onset of and absolute claudication pain in participants with symptoms of intermittent claudication resulting from peripheral arterial disease.
Psychological health assessed by:
-
•
Attention control: assessed using the Attention Network Task [36]. The computerised task measured three functionally and independent aspects of attention control crucial to everyday cognitive functioning by recording performance accuracy and average reaction time to a correctly detected target amongst flankers (i.e. a set of response inhibition tests used to assess the ability to suppress responses that are inappropriate in a particular context): (1) alertness, (2) attentional orienting, and (3) executive function.
-
•
Visual working memory: measured using the Luck & Vogel Visual Working Memory Task [37]. The computerised task measures performance accuracy and average reaction times to correct detection of a change in a visual array of objects. The set size of the object array varies from small to large.
Both computerised tasks are used with open permissions from the Psychology Experiment Building Language (PEBL) site: http://peblblog.blogspot.ie/2014/07/overview-of-some-of-new-tests-in-pebl.html. Benchmark tests have been reported within various populations to establish timing precision [38].
-
•
Cognitive reserve: assessed at baseline only using the Cognitive Reserve Questionnaire, http://www.cognitivereserveindex.org/ [39]. The questionnaire measures demographics, education, and working activity including physical, social and intellectually challenging activity.
3.9. Exploratory outcomes
Selected psychosocial determinants of physical activity were assessed. Barrier self-efficacy and self-regulatory self-efficacy for physical activity were assessed using an established 13-item scale [40] and a modified 11-item scale [41,42], respectively. Social support from family and friends for physical activity was assessed using a validated 10-item tool [43]. A modified 6-item measure was used to assess intentions for exercise [44].
3.10. Safety
Any adverse event was recorded and reported to the MedEx Wellness Chief Medical Officer and the principal investigator. Serious adverse events (SAE) were defined as all-cause mortality or hospitalization for cardiovascular complications. Other adverse events included training-related adverse events such as musculoskeletal problems that prevented exercise participation or other adverse events that interrupted the exercise intervention. Follow-up telephone calls to schedule repeat assessments provided an opportunity for participants to report adverse events. There was also an opportunity during the exercise classes for participants to report adverse events to the exercise instructors or the programme's Chief Medical Officer, which were then communicated to the research team.
3.11. Data analysis
3.11.1. Sample size
The critical determination of the sample size was the standard deviation of the change in 6MTT distance from baseline to 12 months. The standard deviation estimate used was 90 m [45]. The minimum difference to be detected was 25 m [46]. For a power of 80% and two-sided significance of 5%, a sample size of 104 participants was required. To account for drop out, 400 was the target sample size.
3.11.2. Procedures for data checking and entering
Primary and secondary outcome data for physical health and psychological health (except cognitive assessments) outcomes will be handled using double data entry and cleaning. Due to lack of resources, all other outcome measures will be handled using single data entry.
3.11.3. Statistical analyses
Analyses will be performed using IBM SPSS Statistics 24. Continuous variables will be reported as mean (range), mean (standard deviation) or median (inter-quartile range), depending on distribution, and categorical variables as frequency (%). Baseline demographics and number of chronic diseases (including type) will be reported descriptively. Prior to statistical analysis, the Shapiro-Wilks test will be applied to check for normality. To investigate longitudinal changes in repeated measures variables, linear mixed model analysis (MMA) will be used. A MMA is an appropriate approach to modelling time series data that contains repeated measures for numerous subjects [47]. It does not require complete data sets and it will not exclude participants missing a repeat assessment timepoint [48]. Bonferroni post-hoc stratified analysis comparing estimated marginal means at each timepoint will be performed for variables that indicate a significant main effect for time.
4. Discussion
Exercise is an evidence-based secondary prevention strategy for chronic disease. It is associated with reductions in recurrent events, future complications, hospital readmissions and mortality in several chronic diseases [4,49]. Exercise can also restore health, including improving physical function, functional independence and health-related quality of life [3,5]. Despite the established benefits, referral, uptake and adherence rates to exercise rehabilitation programmes in the hospital setting are suboptimal, with less than 35% of eligible patients participating in cardiac rehabilitation [12,50]. Commonly cited barriers to participation in exercise rehabilitation programmes include environmental factors, such as distance to the facility, lack of transport and health system resources [51,52]. A quantitative review of 32 cardiac rehabilitation studies found that patients were more likely to participate when the programmes were easily accessible [34]. Embedding exercise rehabilitation services within the community may improve uptake and compliance, as well as shift the resource burden from the healthcare system.
CBPs are effective models of exercise rehabilitation, with some research reporting similar benefits compared to hospital-based programmes [6,7]. They have been associated with improvements in physical function and health-related quality of life in a variety of clinical populations, including cardiac [12], pulmonary [7,13], cancer [10,14], stroke [15], osteoarthritis [16], and peripheral arterial disease [17]. These previous studies have focused on the effect of CBPs on a single chronic disease. However, multi-morbidity, commonly defined as the co-existence of two or more chronic conditions within an individual [20], is a growing challenge for healthcare systems and is associated with the ageing population [19]. The increasing levels of mutli-morbidity threaten the sustainability of current healthcare approaches and necessitate fundamental change to the current model [53]. In relation to exercise rehabilitation, a common chronic disease model is warranted.
A systematic review of the structure and delivery of CBPs across chronic disease populations identified that the design and components of programmes were similar, irrespective of chronic disease [18]. Specifically, the primary components of >85% of the programmes included a combination of aerobic and resistance exercise. The frequency of exercise sessions was typically 2–3 days per week and sessions were generally 40–60 min in duration. These findings highlight that it is unnecessary to have separate programmes for single chronic conditions and that such programmes can be easily applied across a range of conditions with minimal disease-specific adjustments. An integrated model is likely to represent a more resource efficient approach.
The novelty of the MedEx Wellness model is that it integrates a range of chronic diseases with a shared common infrastructure and content, including facilities, staffing, operating procedures and standards, patient information handling, exercise prescription and programme content, and the programme is financially self-sufficient; its business model has been described in the National Exercise Referral Framework [54]. It is also unique in terms of programme duration; it is not a fixed duration, participants can attend the programme on a continuous or intermittent basis. Other CBP range from 8 weeks to 18 months in duration [15]. The MedEx Wellness approach supports the long-term maintenance of physical activity and exercise. All of these features combine to make the MedEx Wellness model scalable and sustainable and it represents a potential public health model for chronic disease rehabilitation.
4.1. Study limitations
The primary limitation of this study is the lack of a usual care comparison group. As the study setting was within an established programme in the community, withholding service to allow for a controlled trial was not considered ethically appropriate. Maturation may threaten the study validity given the population included, as changes in health status may have occurred over the 12-month period. Selection bias is present as participants were recruited from those who attended induction to the community-based exercise rehabilitation programme and no data was collected on those that were not referred or declined to attend. Additionally, study uptake rates (screening logs) were not recorded. Study limitations also include that the assessor was not blinded to outcome measures.
5. Conclusion
This trial investigated the clinical effectiveness of the MedEx Wellness programme. The extent of the trial outcome measures will provide a holistic view of the physical, clinical and psychological effect of the programme.
Ethical approval and consent to participate
The trial protocol has been approved by the Dublin City University Research Ethics Committee (DCUREC: 2014/227).
Consent for publication
Availability of data and material
Funding
This work was supported by the Health Service Executive.
Author’s contributions
NMcC, NM, and CW conceived the study. BK, LB, LD, NM, CW and NMc contributed to the study design. BK, FS, MC, NMcC and LL conducted data collection. BK, FS and LL prepared the manuscript, which underwent revision by all authors.
Trial status
Data collection is complete.
Declaration of competing interest
None to declare.
Contributor Information
Bróna Kehoe, Email: bkehoe@wit.ie.
Fiona Skelly, Email: fiona.skelly2@mail.dcu.ie.
Niall Moyna, Email: niall.moyna@dcu.ie.
Mairéad Cantwell, Email: mairead.cooney4@mail.dcu.ie.
Lorraine Boran, Email: lorraine.boran@dcu.ie.
Andrew McCarren, Email: andrew.mccarren@dcu.ie.
Kieran Dowd, Email: kdowd@ait.ie.
Catherine Woods, Email: catherine.woods@ul.ie.
Noel McCaffrey, Email: noel.mccaff@gmail.com.
Lisa Loughney, Email: lloughney@exwell.ie.
References
- 1.Global Burden Collaborative Network . Inst. Heal. Metrics Eval.; 2018. Global Burden of Disease Study 2018 (GBD 2017) Results. [Google Scholar]
- 2.Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006 doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburton D.E.R., Nicol C.W., Bredin S.S.D. Health benefits of physical activity: the evidence. CMAJ (Can. Med. Assoc. J.) 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen B.K., Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports. 2015;25:1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 5.Pasanen T., Tolvanen S., Heinonen A., Kujala U.M. Exercise therapy for functional capacity in chronic diseases: an overview of meta-analyses of randomised controlled trials. Br. J. Sports Med. 2017;51 doi: 10.1136/bjsports-2016-097132. [DOI] [PubMed] [Google Scholar]
- 6.Clark R.A., Conway A., Poulsen V., Keech W., Tirimacco R., Tideman P. Alternative models of cardiac rehabilitation: a systematic review. Eur. J. Prev. Cardiol. 2015;22:35–74. doi: 10.1177/2047487313501093. [DOI] [PubMed] [Google Scholar]
- 7.Neves L.F., dos Reis M.H., Goncalves T.R. Home or community-based pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Cad. Saúde Pública. 2016 doi: 10.1590/0102-311X00085915. [DOI] [PubMed] [Google Scholar]
- 8.Dalal H.M., Doherty P., Taylor R.S. Cardiac rehabilitation. BMJ. 2015:351. doi: 10.1136/bmj.h5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mccarthy B., Casey D., Devane D., Murphy K., Murphy E., Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015 doi: 10.1002/14651858.CD003793.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swartz M.C., Lewis Z.H., Lyons E.J., Jennings K., Middleton A., Deer R.R., Arnold D., Dresser K., Ottenbacher K.J., Goodwin J.S. Effect of home- and community-based physical activity interventions on physical function among cancer survivors: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2017;98:1652–1665. doi: 10.1016/j.apmr.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson L., Thompson D.R., Oldridge N., Zwisler A.-D., Rees K., Martin N., Taylor R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2016 doi: 10.1002/14651858.CD001800.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosleh S.M., Bond C.M., Lee A.J., Kiger A., Campbell N.C. Effects of community based cardiac rehabilitation: comparison with a hospital-based programme. Eur. J. Cardiovasc. Nurs. 2015 doi: 10.1177/1474515113519362. [DOI] [PubMed] [Google Scholar]
- 13.Beauchamp M.K., Evans R., Janaudis-Ferreira T., Goldstein R.S., Brooks D. Systematic review of supervised exercise programs after pulmonary rehabilitation in individuals with COPD. Chest. 2013 doi: 10.1378/chest.12-2421. [DOI] [PubMed] [Google Scholar]
- 14.Rajotte E.J., Yi J.C., Baker K.S., Gregerson L., Leiserowitz A., Syrjala K.L. Community-based exercise program effectiveness and safety for cancer survivors. J. Canc. Surv. 2012 doi: 10.1007/s11764-011-0213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desveaux L., Beauchamp M., Goldstein R., Brooks D. Community-based exercise programs as a strategy to optimize function in chronic disease. Med. Care. 2014;52:216–226. doi: 10.1097/MLR.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 16.a Kelley G., Kelley K.S., Hootman J.M., Jones D.L. Effects of community-deliverable exercise on pain and physical function in adults with arthritis and other rheumatic diseases: a meta-analysis. Arthritis Care Res. 2011 doi: 10.1002/acr.20347. Hoboken. [DOI] [PubMed] [Google Scholar]
- 17.Bendermacher B.L., Willigendael E.M., Nicolaï S.P., Kruidenier L.M., Welten R.J., Hendriks E., Prins M.H., Teijink J. a W., a de Bie R. Supervised exercise therapy for intermittent claudication in a community-based setting is as effective as clinic-based. J. Vasc. Surg. Off. Publ. Soc. Vasc. Surg. [and] Int. Soc. Cardiovasc. Surgery, North Am. 2007:1192–1196. doi: 10.1016/j.jvs.2007.01.059. Chapter. 45. [DOI] [PubMed] [Google Scholar]
- 18.Desveaux L., Beauchamp M., Goldstein R., Brooks D. Community-based exercise programs as a strategy to optimize function in chronic disease. Med. Care. 2014;52:216–226. doi: 10.1097/MLR.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 19.Violan C., Foguet-Boreu Q., Flores-Mateo G., Salisbury C., Blom J., Freitag M., Glynn L., Muth C., Valderas J.M. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PloS One. 2014 doi: 10.1371/journal.pone.0102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Den Akker M., Buntinx F., Knottnerus J.A. Comorbidity or multimorbidity: what's in a name? A review of literature. Eur. J. Gen. Pract. 1996 doi: 10.3109/13814789609162146. [DOI] [Google Scholar]
- 21.Ayán-Pérez C., Martínez-Lemos R.I., Cancela-Carral J.M. Reliability and convergent validity of the 6-min run test in young adults with Down syndrome. Disabil. Health J. 2017 doi: 10.1016/j.dhjo.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Bergmann G., Bergmann M., De Castro A., Lorenzi T., Pinheiro E., Moreira R., Marques A., Gaya A. Use of the 6-minute walk/run test to predict peak oxygen uptake in adolescents. Rev. Bras. Atividade Física Saúde. 2014;19 doi: 10.12820/rbafs.v.19n1p64. [DOI] [Google Scholar]
- 23.American Thoracic Society ATS Statement : guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/rccm.166/1/111. [DOI] [PubMed] [Google Scholar]
- 24.Brooks R. EuroQol: the current state of play. Health Pol. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 25.EuroQol A new facility for the measurement of health-related quality of life. Health Pol. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 26.Diener E., Emmons R.A., Larsen R.J., Griffin S. The satisfaction with life scale. J. Pers. Assess. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 27.Tennant R., Hiller L., Fishwick R., Platt S., Joseph S., Weich S., Parkinson J., Secker J., Stewart-Brown S. The Warwick-Edinburgh mental well-being scale (WEMWBS): development and UK validation. Health Qual. Life Outcome. 2007;5:63. doi: 10.1186/1477-7525-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroenke K., Strine T.W., Spitzer R.L., Williams J.B.W., Berry J.T., Mokdad A.H. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 2009;114:163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Cella D.F., Tulsky D.S., Gray G., Sarafian B., Linn E., Bonomi A., Silberman M., Yellen S.B., Winicour P., Brannon J., Eckberg K., Lloyd S., Purl S., Blendowski C., Goodman M., Barnicle M., Stewart I., McHale M., Bonomi P., Kaplan E., Taylor IV S., Thomas C.R., Harris J. The functional assessment of cancer therapy scale: development and validation of the general measure. J. Clin. Oncol. 1993 doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 30.Whelan B.J., Savva G.M. 2013. Design and Methodology of the Irish Longitudinal Study on Ageing. [DOI] [PubMed] [Google Scholar]
- 31.Mc Hugh S., O'Neill C., Browne J., Kearney P.M. Body mass index and health service utilisation in the older population: results from the Irish Longitudinal Study on Ageing. Age Ageing. 2015;44:428–434. doi: 10.1093/ageing/afu177. [DOI] [PubMed] [Google Scholar]
- 32.Csuka M., McCarty D.J. Simple method for measurement of lower extremity muscle strength. Am. J. Med. 1985;78:77–81. doi: 10.1016/0002-9343(85)90465-6. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner T., Jackson S. Dubuque: Wm. C. Brown Communications; 1995. Measurement for Evaluation in Physical Education and Exercise Science. [Google Scholar]
- 34.Podsiadlo D., Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 35.Gardner A.W., Skinner J.S., Cantwell B.W., Smith L.K. Progressive vs single-stage treadmill tests for evaluation of claudication. Med. Sci. Sports Exerc. 1991;23:402–408. [PubMed] [Google Scholar]
- 36.Fan J., McCandliss B.D., Sommer T., Raz A., Posner M.I. Testing the efficiency and independence of attentional networks. J. Cognit. Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 37.Luck S.J., Vogel E.K. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- 38.Mueller S.T., Piper B.J. The Psychology experiment Building Language (PEBL) and PEBL test battery. J. Neurosci. Methods. 2014;222:250–259. doi: 10.1016/j.jneumeth.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nucci M., Mapelli D., Mondini S. Cognitive Reserve Index questionnaire (CRIq): a new instrument for measuring cognitive reserve. Aging Clin. Exp. Res. 2012;24:218–226. doi: 10.3275/7800. [DOI] [PubMed] [Google Scholar]
- 40.McAuley E. The role of efficacy cognitions in the prediction of exercise behavior in middle-aged adults. J. Behav. Med. 1992 doi: 10.1007/BF00848378. [DOI] [PubMed] [Google Scholar]
- 41.Luszczynska A., Sutton S. Physical activity after cardiac rehabilitation: evidence that different types of self-efficacy are important in maintainers and relapsers. Rehabil. Psychol. 2006 doi: 10.1037/0090-5550.51.4.314. [DOI] [Google Scholar]
- 42.Shields C.A., Brawley L.R. Preferring proxy-agency: impact on self-efficacy for exercise. J. Health Psychol. 2006 doi: 10.1177/1359105306069092. [DOI] [PubMed] [Google Scholar]
- 43.Sallis J.F., Grossman R.M., Pinski R.B., Patterson T.L., Nader P.R. The development of scales to measure social support for diet and exercise behaviors. Prev. Med. 1987 doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 44.Sniehotta F.F., Schwarzer R., Scholz U., Schüz B. Action planning and coping planning for long-term lifestyle change: theory and assessment. Eur. J. Soc. Psychol. 2005 doi: 10.1002/ejsp.258. [DOI] [Google Scholar]
- 45.Polkey M.I., Spruit M.A., Edwards L.D., Watkins M.L., Pinto-Plata V., Vestbo J., Calverley P.M.A., Tal-Singer R., Agustí A., Bakke P.S., Coxson H.O., Lomas D.A., MacNee W., Rennard S., Silverman E.K., Miller B.E., Crim C., Yates J., Wouters E.F.M., Celli B. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am. J. Respir. Crit. Care Med. 2013;187:382–386. doi: 10.1164/rccm.201209-1596OC. [DOI] [PubMed] [Google Scholar]
- 46.Holland A.E., Nici L. The return of the minimum clinically important difference for 6-minute-walk distance in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013;187:335–336. doi: 10.1164/rccm.201212-2191ED. [DOI] [PubMed] [Google Scholar]
- 47.Haapalainen E., Laurinen P., Siirtola P., Röning J., Kinnunen H., Jurvelin H. 2008 IEEE Int. Conf. Inf. Reuse Integr. IEEE IRI-2008. 2008. Exercise energy expenditure estimation based on acceleration data using the linear mixed model. [DOI] [Google Scholar]
- 48.Armstrong R.A. Recommendations for analysis of repeated-measures designs: testing and correcting for sphericity and use of manova and mixed model analysis. Ophthalmic Physiol. Optic. 2017;37 doi: 10.1111/opo.12399. [DOI] [PubMed] [Google Scholar]
- 49.Heran B.S., Chen J.M., Ebrahim S., Moxham T., Oldridge N., Rees K., Thompson D.R., Taylor R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2011:CD001800. doi: 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bethell H., Lewin R., Evans J., Turner S., Allender S., Petersen S. Outpatient cardiac rehabilitation attendance in England: variability by region and clinical characteristics. J. Cardiopulm. Rehabil. Prev. 2008 doi: 10.1097/HCR.0b013e31818c3b44. [DOI] [PubMed] [Google Scholar]
- 51.Ruano-Ravina A., Pena-Gil C., Abu-Assi E., Raposeiras S., van ’t Hof A., Meindersma E., Bossano Prescott E.I., González-Juanatey J.R. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int. J. Cardiol. 2016 doi: 10.1016/j.ijcard.2016.08.120. [DOI] [PubMed] [Google Scholar]
- 52.Cox N.S., Oliveira C.C., Lahham A., Holland A.E. Pulmonary rehabilitation referral and participation are commonly influenced by environment, knowledge, and beliefs about consequences: a systematic review using the Theoretical Domains Framework. J. Physiother. 2017 doi: 10.1016/j.jphys.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Moffat K., Mercer S.W. Challenges of managing people with multimorbidity in today's healthcare systems. BMC Fam. Pract. 2015 doi: 10.1186/s12875-015-0344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woods C., McCaffrey N., Furlong B., Fitzsimons-D’Arcy L., Murphy M., Harrison M., Glynn L., O'Riordan J., O'Neill B., Jennings S., Peppard C. 2016. The National Exercise Referral Framework. Dublin, Ireland. [Google Scholar]