Summary
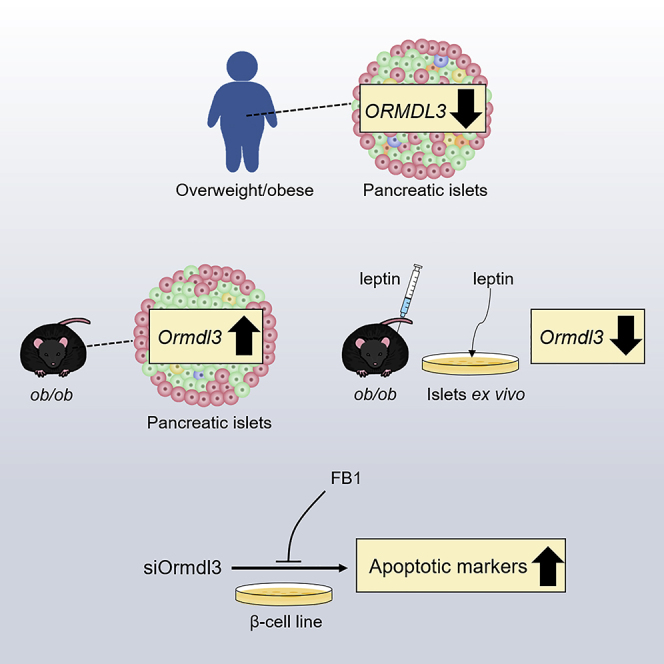
The orosomucoid-like (Ormdl) proteins play a critical role in sphingolipid homeostasis, inflammation, and ER stress, all of which are associated with obesity and βcell dysfunction. However, their roles in β cells and obesity remain unknown. Here, we show that islets from overweight/obese human donors displayed marginally reduced ORMDL1-2 expression, whereas ORMDL3 expression was significantly downregulated compared with islets from lean donors. In contrast, Ormdl3 was substantially upregulated in the islets of leptin-deficient obese (ob/ob) mice compared with lean mice. Treatment of ob/ob mice and their islets with leptin markedly reduced islet Ormld3 expression. Ormdl3 knockdown in a β cell line induced expression of pro-apoptotic markers, which was rescued by ceramide synthase inhibitor fumonisin B1. Our results reveal differential expression of Ormdl3 in the islets of a mouse model and humans with obesity, highlight the potential effect of leptin in this differential regulation, and suggest a role for Ormdl3 in β cell apoptosis.
Subject Areas: Biological Sciences, Endocrinology
Graphical Abstract
Highlights
-
•
Islets of overweight/obese human donors display markedly reduced ORMDL3 expression
-
•
Ormdl3 expression was significantly upregulated in the islets of ob/ob mice
-
•
Leptin treatment markedly reduced Ormld3 expression in the islets of ob/ob mice
-
•
Fumonisin B1 restores increased apoptotic marker levels induced by Ormdl3 silencing
Biological Sciences; Endocrinology
Introduction
Insulin resistance, often co-incident with obesity, dampens the brake on lipolysis, elevating plasma free fatty acid levels. Free fatty acids taken up from the plasma are the precursors for various species of intracellular lipids. Certain sphingolipids, most notably ceramide, accumulate within insulin-resistant tissues of animals (Holland and Summers, 2008; Summers, 2006) and humans (Adams et al., 2004; Straczkowski et al., 2007), including the pancreatic β cells (DeFronzo, 2004). There, they inhibit insulin action and activate processes including apoptosis, inflammation, and stress responses—a condition known as lipotoxicity (Ertunc and Hotamisligil, 2016; Kusminski et al., 2009; Schaffer, 2003; Summers, 2006; Unger et al., 2010; Ye et al., 2019). De novo sphingolipid synthesis, where fatty acids from exogenous sources are utilized as substrates, is primarily responsible for obesity-induced ceramide generation (Hu et al., 2009; Watt et al., 2012). Serine palmitoyltransferase (SPT) initiates de novo sphingolipid synthesis by catalyzing the decarboxylative condensation of L-serine and palmitoyl-CoA to 3-ketodihydrosphingosine. Surplus fatty acids not only lead to increased substrate availability but also alter the expression and activity of key enzymes in the sphingolipid synthetic pathway. SPT functions as a heterodimer of subunits SPTLC1 or SPTLC2 with SPTLC3, and high-fat diet feeding promotes both SPT subunit gene transcription and catalytic activity (Blachnio-Zabielska et al., 2010; Cinar et al., 2014; Longato et al., 2012). Yet, despite recent progress in the field, the molecular mechanisms of sphingolipid-mediated disease pathology and the pathways generating these pathogenic lipids remain poorly understood.
The members of the orosomucoids (Orm) gene family encode transmembrane proteins localized in the endoplasmic reticulum (ER). In the budding yeast S. cerevisiae, two Orm proteins, Orm1 and Orm2 (Han et al., 2010), have been identified as negative regulators of SPT (Breslow et al., 2010; Han et al., 2019). Orm proteins form a complex with SPT and inhibits its activity (Breslow et al., 2010). This association with SPT is regulated by Orm protein phosphorylation: an important factor for sphingolipid homeostasis (Breslow et al., 2010). Mammals, on the other hand, have three Orm-like proteins (Ormdl1–3) (Hjelmqvist et al., 2002). In vitro studies suggest that mammalian Ormdl3 alters ER-mediated calcium (Ca2+) homeostasis, facilitates the unfolded protein response (UPR), induces cellular stress responses, and plays a possible role in inflammation (Cantero-Recasens et al., 2010; Carreras-Sureda et al., 2013; Hsu and Turvey, 2013; Miller et al., 2012). In human genome-wide association studies (GWAS), ORMDL3 is strongly associated with inflammatory diseases, including asthma, Crohn's disease, and type 1 diabetes (T1D) (Barrett et al., 2009; Bouzigon et al., 2008; Galanter et al., 2008; Liu et al., 2010; McGovern et al., 2010; Moffatt et al., 2007, 2010). Additionally, GWAS has identified ORMDL3 as an obesity-related gene and its expression was negatively correlated with body mass index (BMI) (Pan et al., 2018). Although emerging data suggest Ormdl proteins are involved in sphingolipid homeostasis, chronic inflammation, and ER stress—all of which play critical roles in the development and progression of obesity, diabetes, and β cell dysfunction—the expression, regulation, function, and importance of Ormdl genes in β cell physiology and pathology remain unknown.
In this study, we analyzed the expression of Ormdl genes in a genetic mouse model of obesity and type 2 diabetes prior to the onset of hyperglycemia and in human pancreatic islets isolated from lean and overweight/obese non-diabetic donors. Our results, for the first time, revealed that, although ORMDL3 expression in pancreatic islets was negatively correlated with BMI in humans, leptin-deficient obese mice displayed significant upregulation of Ormdl3 expression in their islets. Administration of leptin to leptin-deficient obese mice (ob/ob) and treatment of ob/ob islets ex vivo with leptin markedly reduced Ormdl3 expression, highlighting that leptin can potentially regulate Ormdl3 expression and providing an explanation for differential expression of this gene in ob/ob mouse model and human islets in the context of obesity. Finally, we demonstrated that knockdown of Ormdl3 causes substantial upregulation of pro-apoptotic markers in a β cell line, which could be rescued by pharmacological inhibition of ceramide synthase.
Result
ORMDL3 Expression Is Significantly Downregulated in the Islets of Overweight/Obese Female Donors
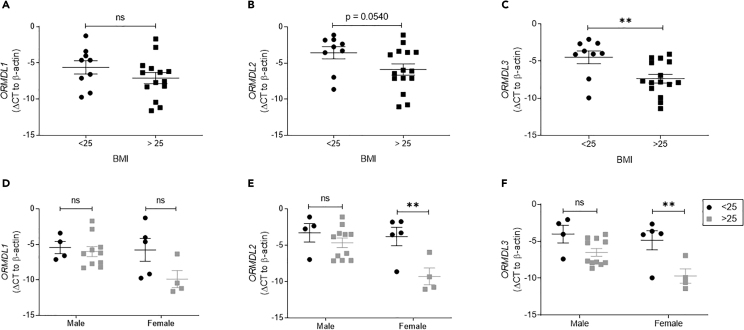
To identify pancreatic islet ORMDL expression in the context of obesity, we used pancreatic islets isolated from lean and overweight/obese human organ donors. We grouped donors as lean (BMI<25) and overweight/obese (BMI>25) (Table 1). All ORMDL genes showed a trend toward diminished mRNA expression in islets isolated from overweight/obese humans as compared with lean (as quantified by cycle threshold compared with β-actin), with the cycles necessary to amplify ORMDL3 PCR product being significantly reduced (approximately 3.5 cycles, or 11-fold) (Figures 1A–1C). We next examined the relationship between islet ORMDL expression and donor sex. Interestingly, the cycle threshold necessary to amplify ORMDL2 and ORMDL3 expression was significantly reduced (by approximately 5–5.5 cycles) in islets from overweight/obese female donors only, corresponding with a 32- to 45-fold decrease in mRNA expression with obesity (Figures 1D–1F). ORMDL1 expression level was non-significantly decreased in islets from female donors as a factor of overweight/obesity. Although no significant changes in the expression of any ORMDL family member were observed in islets isolated from male donors as a factor of overweight/obesity, the mean ORMDL3 cycle threshold in islets from overweight/obese male donors was reduced as compared with lean (Figures 1D–1F). Correlation analyses between the ORMDL genes with BMI further show the greater decrease in ORMDL expression in female donors with increasing BMI as compared with male donors (Figures S1A–S1C). As noted above, there was also a substantial trend toward a decrease in ORMDL3 expression in islets from male donors as a function of BMI (p = 0.05) (Figure S1C). To rule out a potential confounder in our human islet analyses, we examined the correlation between ORMDL expression and donor age but did not detect any significant correlation (Figures S1D–S1F).
Table 1.
Description of Human Islet Donors
| BMI Group | Donor | Age | Mean Age ± SEM | Sex | BMI | Mean BMI ± SEM | Ethnicity |
|---|---|---|---|---|---|---|---|
| <25 | 1 | 40 | 47.3 ± 4.01 | M | 19.6 | 22.62 ± 0.48 | Black |
| 2 | 21 | F | 21.6 | White | |||
| 3 | 53 | M | 21.8 | White | |||
| 4 | 42 | M | 22.8 | Black | |||
| 5 | 59 | F | 23.1 | White | |||
| 6 | 51 | F | 23.1 | White | |||
| 7 | 61 | F | 23.2 | White | |||
| 8 | 48 | F | 24.2 | White | |||
| 9 | 51 | M | 24.2 | Hispanic/Latino | |||
| >25 | 1 | 55 | 38.6 ± 3.74 | F | 25.8 | 30.63 ± 0.96 | White |
| 2 | 36 | M | 26.0 | White | |||
| 3 | 24 | F | 26.6 | Hispanic/Latino | |||
| 4 | 60 | M | 27.3 | Asian Indian | |||
| 5 | 36 | M | 28.7 | White | |||
| 6 | 25 | M | 29.3 | Hispanic/Latino | |||
| 7 | 43 | M | 29.6 | White | |||
| 8 | 38 | M | 29.8 | Black | |||
| 9 | 29 | M | 30.2 | Asian Indian | |||
| 10 | 58 | F | 31.1 | White | |||
| 11 | 36 | M | 33.8 | White | |||
| 12 | 21 | M | 33.8 | White | |||
| 13 | 19 | M | 34.1 | White | |||
| 14 | 36 | F | 34.8 | Hispanic/Latino | |||
| 15 | 63 | M | 38.6 | White |
Demographic and anthropometric data for each human islet donor is provided.
Figure 1.
The Expression of ORMDL Genes in the Islets of Overweight/Obese Human Donors
Quantitative PCR analyses of ORMDL1, ORMDL2, and ORMLD3 mRNA expression in islets from human organ donors. Expression levels of (A) ORMDL1, (B) ORMDL2, and (C) ORMLD3 for human donors divided into groups by BMI < 25 (lean; n = 9) (black circles) and BMI > 25 (overweight/obese; n = 15) (gray squares). Expression levels of (D) ORMDL1, (E) ORMDL2, and (F) ORMLD3 in male versus female donors. All data are expressed as ΔCT (versus β-actin) and are represented as mean ± SEM (∗∗p < 0.01). ns: non-significant.
Ormdl3 Expression Is Significantly Upregulated in the Islets of Leptin-Deficient Obese (ob/ob) Mice
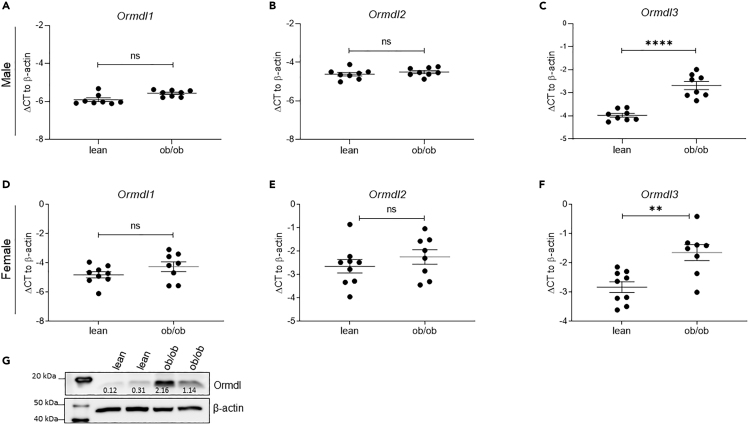
In rodent models of obesity and type 2 diabetes, increased islet ceramide and triglyceride production precede β cell dysfunction and demise (Lee et al., 1994; Unger, 2002). Since Ormdl genes were identified as negative regulators of sphingolipid biosynthesis (Davis et al., 2019; Siow et al., 2015), we asked whether the expression of these genes was altered in the pancreatic islets of leptin-deficient obese (ob/ob) mice, a model of severe insulin resistance and lipotoxicity. First, we analyzed the expression of Ormdl genes in the islets of lean and ob/ob male mice at 10 weeks of age, when ob/ob mice were still normoglycemic. Quantitative PCR analysis showed that expression of Ormdl1 and Ormdl2 was not significantly increased, whereas Ormdl3 expression was substantially upregulated in islets from male ob/ob mice (Figures 2A–2C). Next, we assessed the expression of the Ormdl genes in islets harvested from 10-week-old female lean and ob/ob mice. The expression levels of Ormdl1 and Ormdl2 were nearly identical between islets isolated from female lean and ob/ob mice, whereas the expression level of Ormdl3 was significantly increased in female ob/ob mice, similar to that of male ob/ob mice (Figures 2D–2F).
Figure 2.
The Expression of Ormdl Genes in the Islets of Leptin-Deficient Obese (ob/ob) Mice
Quantitative PCR analyses of Ormdl1, Ormdl2, and Ormdl3 mRNA expression in primary islets of 10-week-old male (n = 8 per group) (A–C) and female (D–F) lean (n = 9) and obese (ob/ob) (n = 8) mice. (G) Representative western blot image (of two independent experiments performed) reflecting Ormdl protein expression in the islets of lean and ob/ob male mice determined by western blot using the validated TPF-Ormdl antibody. All data are expressed as ΔCT (versus β-actin) and represented as mean ± SEM (∗∗∗p < 0.001). ns: non-significant.
Next, we investigated the expression of Ormdls at the protein level. Ormdl1, -2, and -3 share greater than 80% sequence homology. Currently, there are no commercially available antibodies that can detect specific expression of the individual Ormdl family members. In addition, three commercially available pan-Ormdl antibodies failed validation using knockdown lysates (data not shown). We obtained a TPF-Ormdl antibody from Dr. Petr Draber's group (Bugajev et al., 2016), and although the antibody had significant non-specific cross-reactivity, transfection with an Ormdl3 siRNA resulted in a substantial decrease in the abundance of a protein band at the expected molecular weight for Ormdl (17.5 kDa), whereas it did not affect any other “non-specific” bands, confirming the validity of this antibody for further analyses (Figures S2A–S2C). Using this antibody, we demonstrated that Ormdl protein levels were also significantly upregulated in islets from male ob/ob mice, consistent with the changes in mRNA expression (Figure 2G).
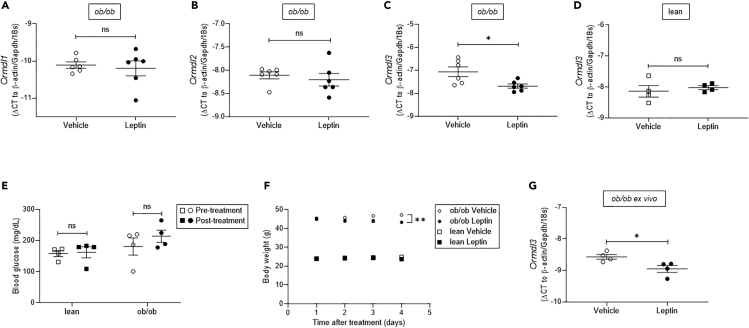
Leptin Administration Markedly Reduces Ormdl3 Expression in ob/ob Islets
Our data revealed that the expression of Ormdl3 had the opposite correlation to overweight/obesity in human islets compared with the mouse model. One possible explanation for the disparate results could be the difference in serum leptin levels in these models, such that obesity in humans is associated with increased circulating leptin (Al Maskari and Alnaqdy, 2006; Lonnqvist et al., 1997; Maffei et al., 1995), whereas ob/ob mice are leptin deficient (Moon and Friedman, 1997). To test whether leptin can regulate Ormdl3 expression, we treated 10-week-old male normoglycemic ob/ob mice with recombinant leptin for 4 days. Our qPCR results revealed that the expression level of housekeeping gene β-actin was altered with leptin treatment; thus, we supplemented our analysis with two other housekeeping genes, Gapdh and 18s, and employed the geometric mean of these three housekeeping genes for the following qPCR analyses (Vandesompele et al., 2002). Interestingly, the expression levels of Ormdl1 and Ormdl2 did not change upon leptin treatment, whereas the expression level of Ormld3 was significantly reduced in islets from ob/ob mice treated with leptin (Figures 3A–3C). The treatment of C57BL/6J lean mice with leptin did not alter Ormld3 expression in islets (Figure 3D). Mean blood glucose levels in lean and ob/ob mice did not significantly change upon leptin treatment, but an approximate 10% reduction in body weight was observed in ob/ob mice, as previously reported (Harris et al., 1998; Pelleymounter et al., 1995) (Figures 3E and 3F). To demonstrate that the decrease in Ormdl3 expression is leptin-dependent and not a result of the change in body weight, we isolated islets from 10-week-old ob/ob mice and treated them with leptin ex vivo. Consistent with our in vivo results, expression of Ormdl3 was significantly decreased in isolated islets upon leptin treatment (Figure 3G). Taken together, these results suggest that leptin can play a key role in Ormdl3 transcriptional regulation in pancreatic islets.
Figure 3.
Treatment of ob/ob Mice with Recombinant Leptin
(A–C) Quantitative PCR analyses of (A) Ormdl1, (B) Ormdl2, and (C) Ormdl3 mRNA expression in primary islets of 10-week-old male ob/ob mice treated with leptin or vehicle (n = 6 per group).
(D) Ormdl3 mRNA expression in primary islets of 10-week-old male C57BL/6J lean mice treated with leptin or vehicle (n = 4 per group).
(E) Fasting blood glucose levels of lean and ob/ob mice before and after leptin treatment (n = 4 per group).
(F) Daily body weight measurements following leptin- or vehicle-treatment of ob/ob and age, sex-matched control C57BL/6J mice (n = 8 per group).
(G) Ormdl3 expression in leptin- or vehicle-treated isolated islets of ob/ob mice (n = 4 per group). All data are expressed as ΔCT (versus geometric mean of β-actin, Gapdh, and 18s) and represented as mean ± SEM (∗p < 0.05, ∗∗p < 0.01). ns: non-significant.
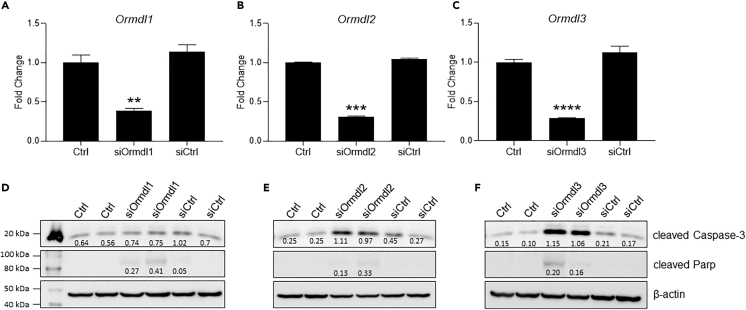
Knockdown of Ormdl3 Leads to Significant Upregulation of Apoptotic Markers in a β Cell Line
To investigate the physiological function of Ormdl genes in β cells, we knocked down all three members of the Ormdl family in the INS-1-derived 832/3 rat insulinoma cell line using siRNAs specific to each gene product. A knockdown efficiency of 80%–85% was confirmed by qPCR for each of the Ormdl genes (Figures 4A–4C). We assessed the expression levels of apoptotic markers in cells 48 h after knockdown of Ormdls by western blotting. The levels of pro-apoptotic markers cleaved Caspase-3 and cleaved Parp were markedly increased in Ormdl2- and Ormdl3-deficient cells, whereas these markers were only marginally increased in Ormdl1-deficient cells (Figures 4D–4F), suggesting that Ormdl2 and Ormdl3 can play a role in regulation of apoptosis in β cells under physiological conditions.
Figure 4.
Knockdown of Ormdl Genes in a β Cell Line
INS-1 832/3 β cells in triplicate wells were transfected with (A) Ormdl1, (B) Ormd2, and (C) Ormdl3 siRNAs, and the efficiency of knockdowns was determined by qPCR 24 h after the transfection. The expression levels of apoptotic markers, cleaved Caspase-3 and cleaved Parp in (D) Ormdl1-, (E) Ormdl2-, and (F) Ormdl3-deficient cells were assessed by western blotting. Signal intensity ratios are shown. Representative blots from two biological replicates are included from a total of three different experiments. Results are expressed as fold change relative to control siRNA-transfected, serum-free media alone conditions and represented as mean ± SEM, with statistical analysis performed by Student's t test (∗∗∗∗p < 0.0001, ∗∗p < 0.01).
Ormdl3 Silencing Does Not Alter the Expression of the UPR Markers in β Cells
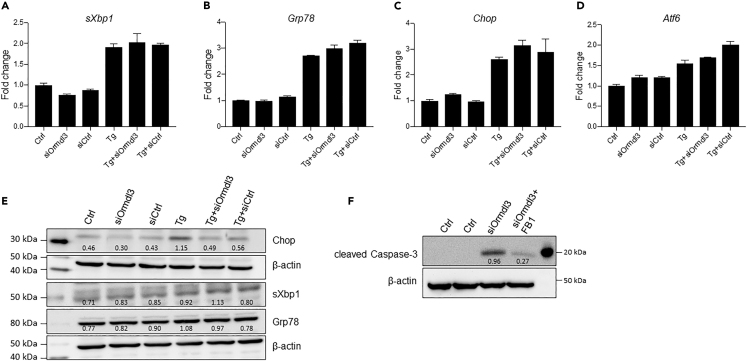
Ormdl3 can regulate ER calcium homeostasis via inhibition of ER calcium pump Serca2b (Cantero-Recasens et al., 2010) and modulate the UPR (Cantero-Recasens et al., 2010; McGovern et al., 2010). Moreover, knockdown of ORMDL3 in HEK293T cells was shown to induce a higher UPR following chemical ER stressors, indicating that ORMDL3 expression levels can regulate UPR and that ORMDL3 may play a role to ensure ER and cellular homeostasis (McGovern et al., 2010). Thus, we investigated whether altering Ormdl3 expression levels can affect ER stress and/or the UPR in a β cell line. Interestingly, we did not observe any significant changes in the mRNA (Figures 5A–5D) or protein levels (Figure 5E) of the UPR markers sXbp1, Grp78, Chop, or Atf6 in INS-1 832/3 cells transfected with siOrmdl3 alone or in the presence of ER stressor thapsigargin, suggesting that Ormdl3 deficiency does not trigger the UPR or ER stress-mediated apoptosis in INS-1 832/3 cells.
Figure 5.
Treatment of Ormdl3 Knockdown β Cell Line with a Ceramide Synthase Inhibitor
INS-1 832/3 cells were transfected with siOrmdl3, and 24 h after transfection cells were treated with 10 nM Tg for 8 h. RNA was collected and qPCR was performed for (A) sXbp1, (B) Grp78 c. Chop, and (D) Atf6. (E) Cells were transfected with siOrmdl3, and protein expression of Chop, sXbp1, and Grp78 was determined by western blotting. (F) INS-1 832/3 cells transfected with siOrmdl3 and treated with 15 μM fumonisin B1 (FB1) for 24 h. Apoptosis was assessed by examining the protein levels of cleaved Caspase-3 by western blotting (representative image of two independent experiments). Signal intensity ratios are shown.
Induction of Pro-apoptotic Pathways by Ormdl3 Knockdown Can Be Rescued by a Ceramide Synthase Inhibitor
Ormdl proteins suppress sphingolipid biosynthesis and specific classes of sphingolipids, namely, ceramides, are known to be important mediators of β cell dysfunction and apoptosis (Boslem et al., 2012; Veret et al., 2014). Thus, we hypothesized that Ormdl3 deficiency might increase ceramide production and subsequently lead to β cell apoptosis. If this is the case, blocking ceramide synthesis downstream of sphingolipid synthesis by using a pharmacological inhibitor of ceramide synthase, fumonisin B1, should reduce the expression of apoptotic markers. To test this hypothesis, we treated Ormdl3-deficient INS-1 832/3 cells with fumonisin B1 and measured the protein levels of apoptotic markers by western blotting. Consistent with our hypothesis, expression levels of apoptotic marker cleaved Caspase-3 was markedly reduced upon inhibition of ceramide synthesis in INS-1 832/3 cells (Figure 5F). Taken together, these data suggest that Ormdl3 can play a role in the regulation of apoptosis in β cells likely by affecting cellular ceramide homeostasis.
Discussion
A growing body of evidence has suggested the genetic association of ORMDL3 gene polymorphisms with a diverse set of inflammatory disorders, including bronchial asthma, inflammatory bowel disease, ankylosing spondylitis, T1D, atherosclerosis, and obesity. We have recently shown that aberrant β cell ER stress is linked to T1D pathogenesis and that there is abnormal β cell UPR activity in type 1 and type 2 diabetes animal models and human patients, suggesting that conserved cellular mechanisms can play a critical role in the pathology of both types of diabetes (Engin, 2016; Engin et al., 2013, 2014). Hence, owing to the involvement of Ormdls with inflammatory diseases, regulation of sphingolipid biosynthesis, and ER stress, we hypothesized that Ormdls could play an important role in β cell homeostasis and investigated the regulation of these genes in pancreatic islets in the context of obesity. One of the most intriguing findings of our study was that islet ORMDL3 expression was significantly influenced by obesity in both mouse and human samples, albeit in opposing directions. ORMDL3 mRNA expression was significantly reduced in islets isolated from overweight/obese human female organ donors, whereas Ormdl3 expression was actually increased in islets from both female and male ob/ob mice. We reasoned that these contrasting results might be due to leptin as obese humans have significantly increased levels of circulating leptin (Al Maskari and Alnaqdy, 2006; Lonnqvist et al., 1997; Maffei et al., 1995), whereas ob/ob mice are deficient of this adipokine (Moon and Friedman, 1997). Indeed, administration of leptin to male ob/ob mice for only 4 days significantly reduced Ormdl3 expression in islets, indicating that leptin may have a regulatory role in Ormdl3 expression. We further supported this finding with an ex vivo experiment, in which leptin treatment of islets from ob/ob mice resulted in markedly diminished Ormdl3 expression. Interestingly, leptin action in the central nervous system represses SPT expression in white adipose tissue by 30% (Bonzon-Kulichenko et al., 2009) and decreases mRNA expression of enzymes involved in de novo ceramide synthesis (SPT-1, LASS2, LASS4) and ceramide production from sphingomyelin (SMPD-1/2) (Bonzon-Kulichenko et al., 2009). Leptin receptor overexpression in the islets of obese Zucker diabetic fatty (ZDF) rats with mutant leptin receptors leads to significantly reduced SPT mRNA levels and fat content (Shimabukuro et al., 1998; Unger and Roth, 2015). However, whether such regulation also exists in human islets is not yet known. Since ob/ob mice have elevated circulating free fatty acids and increased ceramide in their islets (Sloan et al., 2011), it is possible that Ormdl3 levels in ob/ob mice in the absence of leptin is upregulated to exert compensatory inhibitory effects on sphingolipid synthesis, whereas in obese, hyperleptinemic human subjects, downregulation of ORMDL3 may lead to increased ceramide synthesis and lipotoxicity in islets. Of note, although leptin resistance in the hypothalamus is well established in obesity and diabetes, such resistance has not been definitively demonstrated in pancreatic β cells. Leptin levels are also higher in women than in men using any given measure of obesity (Kennedy et al., 1997), a finding that may provide insight into more pronounced downregulation of ORMLD2 and ORMDL3 expression in the islets of female donors compared with those of male islets. Indeed, lack of leptin in male and female ob/ob mice might have abolished this variation, leading to no apparent differences in the expression of Ormdl3 in the islets of male and female mice.
A role for Ormdl3 in calcium homeostasis and the UPR has been reported in various cell types (Cantero-Recasens et al., 2010; Carreras-Sureda et al., 2013; Miller et al., 2012), although no such effect of Ormdl3 on the UPR was also reported (Hsu and Turvey, 2013). We demonstrated that silencing Ormdl3 in INS-1 832/3 cells neither caused a significant increase in the expression of UPR markers nor potentiated the effects of a chemical ER stressor, suggesting that changing Ormdl3 expression levels do not affect the UPR in a rat β cell line. We then showed that, even in the absence of additional stressors, Ormdl3 deficiency led to upregulation of apoptotic markers in INS-1 832/3 cells, which could be significantly reduced by administration of a pharmacological inhibitor of ceramide synthesis. Although we did not measure the ceramide levels in these cells, a recent report indicated significantly increased levels of total sphingolipids, including ceramides, in liver and serum of Ormdl3−/− mice and a marked decrease in these lipid species in Ormdl3 transgenic mice (Debeuf et al., 2019), supporting the idea that increased ceramide levels in β cells is a response to reduced Ormdl3 levels. Of note, no metabolic phenotype has yet been described in these mouse models. Whether reduced expression of ORMDL3 contributes to β cell apoptosis in overweight/obese individuals with impaired fasting glucose remains to be elucidated (Butler et al., 2003). Interestingly, silencing Ormdl3 in a mouse β cell line, Min6 cells, did not induce apoptosis (Yang et al., 2019). The discrepancy between our results and the published work can result from utilization of mouse versus rat cell line or different oligos to perform the gene silencing in these cells. Generating tissue-specific genetic loss and gain-of-function models for Ormdl3 will be essential to understand the exact function and regulation of this gene in physiological and pathological settings.
Our findings do not clarify whether the functions of Ormdl proteins themselves were altered under the conditions of obesity and/or inflammation. In yeast, Orm proteins are regulated through phosphorylation (Roelants et al., 2011; Sun et al., 2012). Although Orm proteins are highly conserved in higher organisms, Ormdl proteins have a truncated N-terminal domain and lack the phosphorylation motif found in yeast, indicating divergent post-translational regulatory mechanisms (Paulenda and Draber, 2016). Emerging data indicate that Ormdl genes might be transcriptionally regulated, such that, when sphingolipid degradation is compromised by deletion of the lyase enzyme, low SPT activity parallels a considerable elevation in Ormdl1 and Ormdl3 transcription (Hagen-Euteneuer et al., 2012). In addition, ORMDL expression strongly influences SPT activity and de novo ceramide synthesis in macrophages (Kiefer et al., 2015). The molecular mechanisms of regulation of Ormdl transcription and post-translational processing need further investigation.
To summarize, our data provide the first comprehensive analysis of ORMDL family gene expression in mouse and human islets in the context of obesity. We demonstrated that leptin can have a significant role in the regulation of islet expression of Ormdl3. Finally, our results indicate that loss of Ormdl3 leads to significantly increased expression of pro-apoptotic markers in a rat insulinoma cell line possibly owing to increased ceramide synthesis. The molecular mechanisms by which Ormdl proteins regulate β cell homeostasis under physiological and pathological conditions, including obesity and diabetes, remain to be determined and are worthy of future study.
Limitations of the Study
Our study reveals for the first time the expression of the Ormdl gene family members in both mouse and humans; however, it does not address the protein levels in human samples mainly because of the limitations stemming from human samples and the antibody. We also recognize the limited number of donors as a potential limitation of our study. Although this study paves the way for more detailed mechanistic studies, mechanistic work with primary islets and genetic models with tissue-specific loss and gain-of-function models of Ormdl genes will be necessary to definitively demonstrate function and regulation of these genes in β cell pathophysiology.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Feyza Engin (fengin@wisc.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate or analyze any new datasets or code.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Dr. Petr Draber for providing the Ormdl antibody and Dr. Mieke Baan from the Dawn Davis laboratory for her technical help in generating some of the ob/ob mouse islet samples. H.L. is supported by NIH National Research Service Award T32 GM007215. R.J.F. is supported by NIH F31 DK109698. F.E. is supported by grants from the JDRF-5-CDA-2014-184-A-N and NIH 5K01DK102488-03. M.E.K. is supported by NIH R01 DK102598 and VA BLR&D I01 BX003700. D.B.D. is supported by VA BLR&D I01 BX001880 and I01 BX004715 and NIH R01 DK110324. This work was conducted using facilities and resources from the William S. Middleton Memorial Veterans Hospital and does not represent the views of the Department of Veterans Affairs or the United States Government.
Author Contributions
H.L. designed and performed experiments, analyzed data, prepared the figures, and revised the manuscript. R.J.F. contributed to gene expression analyses, analyzed data, and prepared the figures. T.A. and E.D. contributed to experiments. M.E.K. and D.B.D. analyzed the data, supervised research, and edited the revised manuscript. F.E. conceived, supervised, and supported the project, designed experiments, interpreted results, and wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101324.
Supplemental Information
References
- Adams J.M., 2nd, Pratipanawatr T., Berria R., Wang E., DeFronzo R.A., Sullards M.C., Mandarino L.J. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- Al Maskari M.Y., Alnaqdy A.A. Correlation between serum leptin levels, body mass index and obesity in Omanis. Sultan Qaboos Univ. Med. J. 2006;6:27–31. [PMC free article] [PubMed] [Google Scholar]
- Barrett J.C., Clayton D.G., Concannon P., Akolkar B., Cooper J.D., Erlich H.A., Julier C., Morahan G., Nerup J., Nierras C. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachnio-Zabielska A., Baranowski M., Zabielski P., Gorski J. Effect of high fat diet enriched with unsaturated and diet rich in saturated fatty acids on sphingolipid metabolism in rat skeletal muscle. J. Cell Physiol. 2010;225:786–791. doi: 10.1002/jcp.22283. [DOI] [PubMed] [Google Scholar]
- Bonzon-Kulichenko E., Schwudke D., Gallardo N., Molto E., Fernandez-Agullo T., Shevchenko A., Andres A. Central leptin regulates total ceramide content and sterol regulatory element binding protein-1C proteolytic maturation in rat white adipose tissue. Endocrinology. 2009;150:169–178. doi: 10.1210/en.2008-0505. [DOI] [PubMed] [Google Scholar]
- Boslem E., Meikle P.J., Biden T.J. Roles of ceramide and sphingolipids in pancreatic beta-cell function and dysfunction. Islets. 2012;4:177–187. doi: 10.4161/isl.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzigon E., Corda E., Aschard H., Dizier M.H., Boland A., Bousquet J., Chateigner N., Gormand F., Just J., Le Moual N. Effect of 17q21 variants and smoking exposure in early-onset asthma. N. Engl. J. Med. 2008;359:1985–1994. doi: 10.1056/NEJMoa0806604. [DOI] [PubMed] [Google Scholar]
- Breslow D.K., Collins S.R., Bodenmiller B., Aebersold R., Simons K., Shevchenko A., Ejsing C.S., Weissman J.S. Orm family proteins mediate sphingolipid homeostasis. Nature. 2010;463:1048–1053. doi: 10.1038/nature08787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugajev V., Halova I., Draberova L., Bambouskova M., Potuckova L., Draberova H., Paulenda T., Junyent S., Draber P. Negative regulatory roles of ORMDL3 in the FcepsilonRI-triggered expression of proinflammatory mediators and chemotactic response in murine mast cells. Cell Mol. Life Sci. 2016;73:1265–1285. doi: 10.1007/s00018-015-2047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Cantero-Recasens G., Fandos C., Rubio-Moscardo F., Valverde M.A., Vicente R. The asthma-associated ORMDL3 gene product regulates endoplasmic reticulum-mediated calcium signaling and cellular stress. Hum. Mol. Genet. 2010;19:111–121. doi: 10.1093/hmg/ddp471. [DOI] [PubMed] [Google Scholar]
- Carreras-Sureda A., Cantero-Recasens G., Rubio-Moscardo F., Kiefer K., Peinelt C., Niemeyer B.A., Valverde M.A., Vicente R. ORMDL3 modulates store-operated calcium entry and lymphocyte activation. Hum. Mol. Genet. 2013;22:519–530. doi: 10.1093/hmg/dds450. [DOI] [PubMed] [Google Scholar]
- Cinar R., Godlewski G., Liu J., Tam J., Jourdan T., Mukhopadhyay B., Harvey-White J., Kunos G. Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology. 2014;59:143–153. doi: 10.1002/hep.26606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D.L., Gable K., Suemitsu J., Dunn T.M., Wattenberg B.W. The ORMDL/Orm-serine palmitoyltransferase (SPT) complex is directly regulated by ceramide: reconstitution of SPT regulation in isolated membranes. J. Biol. Chem. 2019;294:5146–5156. doi: 10.1074/jbc.RA118.007291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeuf N., Zhakupova A., Steiner R., Van Gassen S., Deswarte K., Fayazpour F., Van Moorleghem J., Vergote K., Pavie B., Lemeire K. The ORMDL3 asthma susceptibility gene regulates systemic ceramide levels without altering key asthma features in mice. J. Allergy Clin. Immunol. 2019;144:1648–1659.e9. doi: 10.1016/j.jaci.2019.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R.A. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int. J. Clin. Pract. 2004;Suppl:9–21. doi: 10.1111/j.1368-504x.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- Engin F. ER stress and development of type 1 diabetes. J. Investig. Med. 2016;64:2–6. doi: 10.1097/JIM.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin F., Nguyen T., Yermalovich A., Hotamisligil G.S. Aberrant islet unfolded protein response in type 2 diabetes. Sci. Rep. 2014;4:4054. doi: 10.1038/srep04054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin F., Yermalovich A., Nguyen T., Hummasti S., Fu W., Eizirik D.L., Mathis D., Hotamisligil G.S. Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Sci. Transl Med. 2013;5:211ra156. doi: 10.1126/scitranslmed.3006534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertunc M.E., Hotamisligil G.S. Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 2016;57:2099–2114. doi: 10.1194/jlr.R066514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanter J., Choudhry S., Eng C., Nazario S., Rodriguez-Santana J.R., Casal J., Torres-Palacios A., Salas J., Chapela R., Watson H.G. ORMDL3 gene is associated with asthma in three ethnically diverse populations. Am. J. Respir. Crit. Care Med. 2008;177:1194–1200. doi: 10.1164/rccm.200711-1644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen-Euteneuer N., Lutjohann D., Park H., Merrill A.H., Jr., van Echten-Deckert G. Sphingosine 1-phosphate (S1P) lyase deficiency increases sphingolipid formation via recycling at the expense of de novo biosynthesis in neurons. J. Biol. Chem. 2012;287:9128–9136. doi: 10.1074/jbc.M111.302380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G., Gupta S.D., Gable K., Bacikova D., Sengupta N., Somashekarappa N., Proia R.L., Harmon J.M., Dunn T.M. The ORMs interact with transmembrane domain 1 of Lcb1 and regulate serine palmitoyltransferase oligomerization, activity and localization. Biochim. Biophys. Acta. 2019;1864:245–259. doi: 10.1016/j.bbalip.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Lone M.A., Schneiter R., Chang A. Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control. Proc. Natl. Acad. Sci. U S A. 2010;107:5851–5856. doi: 10.1073/pnas.0911617107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.B., Zhou J., Redmann S.M., Jr., Smagin G.N., Smith S.R., Rodgers E., Zachwieja J.J. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology. 1998;139:8–19. doi: 10.1210/endo.139.1.5675. [DOI] [PubMed] [Google Scholar]
- Hjelmqvist L., Tuson M., Marfany G., Herrero E., Balcells S., Gonzalez-Duarte R. ORMDL proteins are a conserved new family of endoplasmic reticulum membrane proteins. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-6-research0027. RESEARCH0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland W.L., Summers S.A. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr. Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu K.J., Turvey S.E. Functional analysis of the impact of ORMDL3 expression on inflammation and activation of the unfolded protein response in human airway epithelial cells. Allergy Asthma Clin. Immunol. 2013;9:4. doi: 10.1186/1710-1492-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Bielawski J., Samad F., Merrill A.H., Jr., Cowart L.A. Palmitate increases sphingosine-1-phosphate in C2C12 myotubes via upregulation of sphingosine kinase message and activity. J. Lipid Res. 2009;50:1852–1862. doi: 10.1194/jlr.M800635-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A., Gettys T.W., Watson P., Wallace P., Ganaway E., Pan Q., Garvey W.T. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J. Clin. Endocrinol. Metab. 1997;82:1293–1300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
- Kiefer K., Carreras-Sureda A., Garcia-Lopez R., Rubio-Moscardo F., Casas J., Fabrias G., Vicente R. Coordinated regulation of the orosomucoid-like gene family expression controls de novo ceramide synthesis in mammalian cells. J. Biol. Chem. 2015;290:2822–2830. doi: 10.1074/jbc.M114.595116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusminski C.M., Shetty S., Orci L., Unger R.H., Scherer P.E. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14:1484–1495. doi: 10.1007/s10495-009-0352-8. [DOI] [PubMed] [Google Scholar]
- Lee Y., Hirose H., Ohneda M., Johnson J.H., McGarry J.D., Unger R.H. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc. Natl. Acad. Sci. U S A. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Invernizzi P., Lu Y., Kosoy R., Lu Y., Bianchi I., Podda M., Xu C., Xie G., Macciardi F. Genome-wide meta-analyses identify three loci associated with primary biliary cirrhosis. Nat. Genet. 2010;42:658–660. doi: 10.1038/ng.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longato L., Tong M., Wands J.R., de la Monte S.M. High fat diet induced hepatic steatosis and insulin resistance: role of dysregulated ceramide metabolism. Hepatol. Res. 2012;42:412–427. doi: 10.1111/j.1872-034X.2011.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnqvist F., Nordfors L., Jansson M., Thorne A., Schalling M., Arner P. Leptin secretion from adipose tissue in women. Relationship to plasma levels and gene expression. J. Clin. Invest. 1997;99:2398–2404. doi: 10.1172/JCI119422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M., Halaas J., Ravussin E., Pratley R.E., Lee G.H., Zhang Y., Fei H., Kim S., Lallone R., Ranganathan S. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- McGovern D.P., Gardet A., Torkvist L., Goyette P., Essers J., Taylor K.D., Neale B.M., Ong R.T., Lagace C., Li C. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat. Genet. 2010;42:332–337. doi: 10.1038/ng.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Tam A.B., Cho J.Y., Doherty T.A., Pham A., Khorram N., Rosenthal P., Mueller J.L., Hoffman H.M., Suzukawa M. ORMDL3 is an inducible lung epithelial gene regulating metalloproteases, chemokines, OAS, and ATF6. Proc. Natl. Acad. Sci. U S A. 2012;109:16648–16653. doi: 10.1073/pnas.1204151109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt M.F., Gut I.G., Demenais F., Strachan D.P., Bouzigon E., Heath S., von Mutius E., Farrall M., Lathrop M., Cookson W.O. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt M.F., Kabesch M., Liang L., Dixon A.L., Strachan D., Heath S., Depner M., von Berg A., Bufe A., Rietschel E. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- Moon B.C., Friedman J.M. The molecular basis of the obese mutation in ob2J mice. Genomics. 1997;42:152–156. doi: 10.1006/geno.1997.4701. [DOI] [PubMed] [Google Scholar]
- Pan D.Z., Garske K.M., Alvarez M., Bhagat Y.V., Boocock J., Nikkola E., Miao Z., Raulerson C.K., Cantor R.M., Civelek M. Integration of human adipocyte chromosomal interactions with adipose gene expression prioritizes obesity-related genes from GWAS. Nat. Commun. 2018;9:1512. doi: 10.1038/s41467-018-03554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulenda T., Draber P. The role of ORMDL proteins, guardians of cellular sphingolipids, in asthma. Allergy. 2016;71:918–930. doi: 10.1111/all.12877. [DOI] [PubMed] [Google Scholar]
- Pelleymounter M.A., Cullen M.J., Baker M.B., Hecht R., Winters D., Boone T., Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Roelants F.M., Breslow D.K., Muir A., Weissman J.S., Thorner J. Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U S A. 2011;108:19222–19227. doi: 10.1073/pnas.1116948108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer J.E. Lipotoxicity: when tissues overeat. Curr. Opin. Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- Shimabukuro M., Higa M., Zhou Y.T., Wang M.Y., Newgard C.B., Unger R.H. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J. Biol. Chem. 1998;273:32487–32490. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- Siow D., Sunkara M., Dunn T.M., Morris A.J., Wattenberg B. ORMDL/serine palmitoyltransferase stoichiometry determines effects of ORMDL3 expression on sphingolipid biosynthesis. J. Lipid Res. 2015;56:898–908. doi: 10.1194/jlr.M057539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan C., Tuinei J., Nemetz K., Frandsen J., Soto J., Wride N., Sempokuya T., Alegria L., Bugger H., Abel E.D. Central leptin signaling is required to normalize myocardial fatty acid oxidation rates in caloric-restricted ob/ob mice. Diabetes. 2011;60:1424–1434. doi: 10.2337/db10-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straczkowski M., Kowalska I., Baranowski M., Nikolajuk A., Otziomek E., Zabielski P., Adamska A., Blachnio A., Gorski J., Gorska M. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50:2366–2373. doi: 10.1007/s00125-007-0781-2. [DOI] [PubMed] [Google Scholar]
- Summers S.A. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sun Y., Miao Y., Yamane Y., Zhang C., Shokat K.M., Takematsu H., Kozutsumi Y., Drubin D.G. Orm protein phosphoregulation mediates transient sphingolipid biosynthesis response to heat stress via the Pkh-Ypk and Cdc55-PP2A pathways. Mol. Biol. Cell. 2012;23:2388–2398. doi: 10.1091/mbc.E12-03-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R.H. Lipotoxic diseases. Annu. Rev. Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- Unger R.H., Clark G.O., Scherer P.E., Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta. 2010;1801:209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Unger R.H., Roth M.G. A new biology of diabetes revealed by leptin. Cell Metab. 2015;21:15–20. doi: 10.1016/j.cmet.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veret J., Bellini L., Giussani P., Ng C., Magnan C., Le Stunff H. Roles of sphingolipid metabolism in pancreatic beta cell dysfunction induced by lipotoxicity. J. Clin. Med. 2014;3:646–662. doi: 10.3390/jcm3020646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M.J., Barnett A.C., Bruce C.R., Schenk S., Horowitz J.F., Hoy A.J. Regulation of plasma ceramide levels with fatty acid oversupply: evidence that the liver detects and secretes de novo synthesised ceramide. Diabetologia. 2012;55:2741–2746. doi: 10.1007/s00125-012-2649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Sheng F., Sun B., Fischbach S., Xiao X. The role of ORMDL3/ATF6 in compensated beta cell proliferation during early diabetes. Aging (Albany NY) 2019;11:2787–2796. doi: 10.18632/aging.101949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R., Onodera T., Scherer P.E. Lipotoxicity and beta cell maintenance in obesity and type 2 diabetes. J. Endocr. Soc. 2019;3:617–631. doi: 10.1210/js.2018-00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate or analyze any new datasets or code.