Abstract
Aim
Current literature lacks a definitive threshold of idiopathic premature ventricular complex (PVC) burden for predicting cardiomyopathy (CMP). The main objective of the present study was to evaluate relationship between the PVC burden and left ventricular ejection fraction (LVEF).
Method
This multicenter, cross‐sectional study included 341 consecutive patients with more than 1,000 idiopathic PVC in 24 hr of Holter monitoring admitted to the cardiology clinics between January 2019 and May 2019 in the nineteen different centers. The primary outcome was the LVEF measured during the echocardiographic examination.
Result
Overall, the median age was 50 (38–60) and 139 (49.4%) were female. Percentage of median PVC burden was 9% (IQR: 4%–17.4%). Median LVEF was found 60% (55–65). We used proportional odds logistic regression method to examine the relationship between continuous LVEF and candidate predictors. Increase in PVC burden (%) (regression coefficient (RE) −0.644 and 95% CI −1.063, –0.225, p < .001), PVC QRS duration (RE‐0.191 and 95% CI −0.529, 0.148, p = .049), and age (RE‐0.249 and 95% CI −0.442, −0.056, p = .018) were associated with decrease in LVEF. This inverse relationship between the PVC burden and LVEF become more prominent when PVC burden was above 5%. A nomogram developed to estimate the individual risk for decrease in LVEF.
Conclusion
Our study showed that increase in PVC burden %, age, and PVC QRS duration were independently associated with decrease in LVEF in patients with idiopathic PVC. Also, inverse relationship between PVC burden and LVEF was observed in lower PVC burden than previously known.
Keywords: cardiomyopathy, left ventricular ejection fraction, premature ventricular complexes
1. INTRODUCTION
Idiopathic premature ventricular complexes (PVCs) represent the most common ventricular arrhythmias in adult population. Premature ventricular complex can be detected in up to 70% of Holter recordings of ambulatory healthy and young adults (Von Rotz et al., 2017). In the literature, it was suggested that PVCs can lead to the deterioration of left ventricular (LV) function and subsequent LV dilatation which is defined as PVC induced cardiomyopathy (PVC‐CMP) (Agarwal et al., 2017; Nguyen et al., 2017; Niwano et al., 2009). Recent studies demonstrated that PVC‐CMP could be reversed upon the suppression of PVCs by antiarrhythmic drugs (AAD) or catheter ablation (Duffee, Shen, & Smith, 1998; Oomen, Dekker, & Meijer, 2018; Yarlagadda et al., 2005). Also, it was reported that PVC‐CMP was re‐observed in patients with recurrence of PVC after catheter ablation (Baser et al., 2015).
In order to identify the predictors for PVC‐CMP, several parameters such as sinus and PVC QRS duration, PVC coupling interval, mean heart rate, interpolation, polymorphic PVCs, circadian variability, SOO of PVC, symptom status and duration, and conflicting results were obtained (Bas et al., 2016; Del Carpio Munoz et al., 2011; deVries et al., 2018; Hasdemir et al., 2011; Olgun et al., 2011; Yokokawa, Kim, Good, Chugh, et al., 2012; Yokokawa, Kim, Good, Crawford, et al., 2012). In particular, high PVC burden is the only parameter that has consistently shown an association with PVC‐CMP (Latchamsetty et al., 2015; Lee, Denman, & Haqqani, 2019; Sekiguchi et al., 2005; Takemoto et al., 2005). According to current clinical practice, indication of treatment is obvious and robust for impaired LV functions and presence of symptoms with high PVC burden. But absolute threshold of PVC burden to initiate treatment for prevention of PVC‐CMP is not well defined in the current literature (Luebbert, Auberson, & Marchlinski, 2016; Panizo, Barra, Mellor, Heck, & Agarwal, 2018).
The main objective of the present study was to evaluate the relationship between PVC burden and LV ejection fraction (LVEF) with threshold of PVC burden in which this relationship becomes significantly apparent.
2. METHODS
2.1. Study population
This multicenter cross‐sectional study included older than eighteen years consecutive patients with more than 1,000 PVC in 24 hr of Holter monitoring admitted to the cardiology clinics between January 2019 and May 2019 in the nineteen different centers. The exclusion criteria were as follows: patients with less than 24 hr and/or inconclusive Holter recording, coronary artery disease (CAD), history of cardiac arrest, sustained ventricular tachycardia (VT), intra‐cardiac defibrillator (ICD), second‐ or third‐degree AV block, sick sinus syndrome, permanent cardiac pacemaker, known or suspected etiology of cardiomyopathies including, ischemic, restrictive, hypertrophic, diabetic, arrhythmogenic, and non‐compaction before or on admission, genetic cardiac channelopathies, pericardial disease, myocarditis, all forms of atrial fibrillation, thyroid disorders, anemia, electrolyte disorders, chronic pulmonary disease, pulmonary hypertension, moderate‐to‐severe valvular heart disease, or pulmonary embolism. After exclusion, a total of 341 patients without structural heart disease and with structural heart disease which was thought to be idiopathic PVC induced were included in the study. Antiarrhythmic drug use was defined as active using of β‐blocker, amiodarone, calcium channel blocker or propafenone for at least 1 month. The informed consent of each subject and the approval of the local ethics committee were obtained. The study protocol conforms to the Declaration of Helsinki (Rickham, 1964).
2.2. Electrocardiographic assessment
Premature ventricular complexes were defined as premature beats with abnormally shaped and prolonged QRS complex (different QRS and T‐wave morphology compared with sinus beat and QRS duration >120 ms) arising from an ectopic focus within ventricles. QRS duration during sinus rhythm and PVC QRS duration in the lead with widest QRS complex were manually measured in the 12‐lead surface ECG. Premature ventricular complex coupling interval was calculated from the onset of the R wave of the preceding sinus beat to the onset of the PVC in the 12‐lead surface ECG. The anatomic site of origin (SOO) of PVC was determined by using the 12‐lead electrocardiographic criteria that were recently published by Enriquez, Baranchuk, Briceno, Saenz, and Garcia (2019). ECG interpretations were made by an electrophysiologist who was blinded to other clinical data.
2.3. Holter monitoring assessment
Twenty‐four hours of Holter monitoring was obtained by using a 12‐channel device. If the monitoring time was lower than 80% of the target time or the recording quality was considered low, repeat 24 hr of Holter monitoring was performed. Premature ventricular complex burden was assessed as percentage and number of PVCs per day. The percentage of PVCs was determined by dividing the total number of PVCs by the total number of beats recorded during Holter monitoring. Circadian variability was defined as the difference in the PVC burden between daytime and night. Polymorphic PVCs were defined as the presence of PVC with at least three different morphologies. Interpolation was defined as PVC without completely compensatory pause.
2.4. Echocardiographic assessment
The 16‐segment model was used for scoring the severity of segmental wall‐motion abnormalities according to the recent American Society of Echocardiography guideline (Lang et al., 2005). A modified Simpson's method was used to assess the LVEF. The echocardiographic evaluation of LV function was completed with the assessment of LV systolic and diastolic diameters, as well as systolic and diastolic volumes were evaluated according to the latest American Society of Echocardiography chamber quantification guideline.
2.5. Statistical analysis
All statistical analyses were performed using “rms,” “mgcv,” and “ggplot2” packages with R‐Software v. 3.5.1 (R statistical software, Institute for Statistics and Mathematics). Continuous variables were presented as median and interquartile range, whereas categorical variables were presented as counts and percentages.
Primary outcome: The primary outcome was the LVEF measured during the echocardiographic examination of patients.
Candidate predictors: It was important that the candidate predictors to be included in the model were supposed to be clinically and biologically plausible and that their association with LVEF had been demonstrated in previous studies. Variables with very low or very high frequency were not included in the model. The candidate predictors were chosen according to these principles. We evaluated two primary models. The first model included 12 predictor variables (full model). The candidate predictors were age, sex, PVC burden (%), antiarrhythmic drug use, interpolated PVC, polymorphic PVCs, circadian variability, sinus QRS duration, PVC QRS duration, PVC coupling interval, LVOT, and RVOT SOO. To decrease the complexity of full model and to yield reduced‐form models that would be more practical for bedside use, we performed step‐down backward variable selection with an alpha criterion 0.25. Variables with very low (<5%) or very high frequency (>95%), and variables with >50% missingness were not included in the model. The sample size must be at least 184 to estimate the entire distribution of LVEF with a global margin of error not exceeding 0.1
Statistical modeling: We used proportional odds logistic regression method to examine the relationship between primary outcome (continuous LVEF%) and candidate predictors (Liu, Shepherd, Li, & Harrell, 2017). Effects of individual predictors on LVEF were reported by using regression coefficient and 95% confidence interval (CI). Age, PVC burden (%), sinus QRS duration, PVC QRS duration, and PVC coupling interval were included in the model as flexible smooth parameters using with restricted cubic spline. The effects of continuous predictors were summarized using their interquartile range. Due to the expected interaction between PVC burden (%) and antiarrhythmic drug use, “PVC burden (%) * antiarrhythmic drug” interaction term was added to the regression model. The relative importance of each predictor in the models was estimated with partial chi‐square value for each predictor divided by the model's total chi‐square, which estimates the independent contribution of the predictor to the variance of the outcome. The generalized additive model was used to get plotting interaction effect of PVC burden (%) and age on the LVEF% (3‐dimensional perspective plot). The comparison between models was made with assessment of fit (likelihood ratio chi‐square), quality (Akaike and Bayesian information criteria—AIC and BIC) and predictive accuracy (Somers rank and Dxy) and R 2.
3. RESULTS
The study included 341 consecutive patients in accordance with the inclusion criteria. Overall, the median age was 50 (38–60) and 139 (49.4%) were female. Median PVC burden was 8,400 beat/d (IQR: 3,873–17,754), and median PVC burden was 9% (IQR: 4%–17.4%; Figure 1). Median LVEF was found 60% (55–65). Left ventricular ejection fraction was lower than 50% in 9.9% (34) of the patients. Among study patients, 298 (87.4%) had symptoms. Palpitation was identified as the most common symptom (58.1%). A total of 160 (46.9%) patients were on β‐blocker treatment, and 21 (6.1%) were using other antiarrhythmics during the enrollment phase. Also, 105 patients (30.8%) had a history of β‐blocker use and 18 (5.2%) had a history of at least one antiarrhythmic use. Only 17 (5.0%) patients had a history of PVC catheter ablation. Median PVC QRS duration and PVC coupling interval were 130 ms and 450 ms, respectively. Both polymorphic PVCs and circadian variability were identified in 52 (15.2%) patients. Interpolation was not observed in 89 (26.5%) patients. The most common anatomic SOO of PVCs that was determined from the 12‐lead surface ECG and Holter recordings was RVOT (47.5%) and LVOT (26.5%). Other demographic and clinical characteristics and medical therapy of the study patients were shown in Table 1. Electrocardiographic and Holter monitoring parameters were shown in Table 2.
Figure 1.
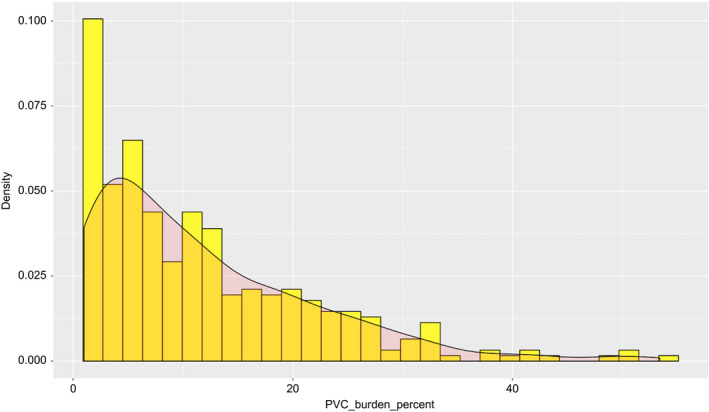
The frequency distribution of PVC burden (%). PVC, premature ventricular complex
Table 1.
Baseline demographic, clinical, and medical therapy of the study patients
| Variables | Study patients (n = 341) |
|---|---|
| Age (years) | 50 (38–60) |
| Male gender [n (%)] | 172 (50.4) |
| Hypertension [n (%)] | 114 (33.4) |
| Diabetes mellitus [n (%)] | 48 (14.1) |
| Smoking [n (%)] | 94 (27.4) |
| NYHA class | |
| NYHA class 1 | 246 (72.4) |
| NYHA class 2 | 95 (27.6 |
| BMI (kg/m2) | 25.9 (23.4–28.1) |
| Symptoms (=yes) [n (%)] | 298 (87.4) |
| Palpitations | 198 (58.1) |
| Skipped/dropped beats | 33 (9.7) |
| Chest discomfort | 26 (7.6) |
| Dizziness | 16 (4.7) |
| Fatigue | 20 (5.9) |
| Syncope | 5 (1.5) |
| Current medical therapy | |
| β‐blocker [n (%)] | 160 (46.9) |
| Calcium channel blocker [n(%)] | 38 (11.1) |
| Amiodarone [n (%)] | 10 (2.9) |
| Propafenone [n (%)] | 11 (3.2) |
| History of medical therapy | |
| β‐blocker [n (%)] | 105 (30.8) |
| Amiodarone [n (%)] | 8 (2.3) |
| Propafenone [n (%)] | 10 (2.9) |
| History of PVC Ablation [n(%)] | 17 (5.0) |
| EF (%) | 60 (55–65) |
Data are expressed as median interquartile range and count (percentage).
Abbreviations: BMI, body mass index; EF, ejection fraction; LV, left ventricle; PVC, premature ventricular complex.
Table 2.
Various electrocardiographic and Holter monitoring parameters of the study patients
| Variables | Study patients (n = 341) |
|---|---|
| Mean heart rate | 77 (69–83) |
| QT interval (ms) | 420 (390–430) |
| QTc interval (ms) | 390 (370–410) |
| Sinus QRS duration (ms) | 80 (80–100) |
| PVC QRS duration (ms) | 130 (120–140) |
| PVC coupling interval (ms) | 450 (420–500) |
| PVC burden (beat/24 hr) | 8,400 (3,786–17,857) |
| PVC burden (%) | 9.0 (4.0–17.4) |
| Multifocal PVC (=yes) [n (%)] | 52 (15.2) |
| Circadian variability (=yes) [n (%)] | 52 (15.2) |
| Interpolation (=yes) [n (%)] | 89 (26.5) |
| Anatomic SOO of PVC | |
| RVOT [n (%)] | 164 (47.5) |
| LVOT [n (%)] | 90 (26.5) |
| Parahisian [n (%)] | 15 (4.4) |
| TV [n (%)] | 8 (2.3) |
| Moderator band [n (%)] | 3 (0.9) |
| AMC [n (%)] | 10 (2.9) |
| MV [n (%)] | 24 (7.0) |
| Papillary muscles [n (%)] | 17 (5.0) |
| Fascicles [n (%)] | 8 (2.3) |
| Epicardial [n (%)] | 4 (1.2) |
| Features of electrocardiographic morphology of PVC | |
| Positive Lead 1 + Inferior ax [n (%)] | 155 (45.5) |
| Negative Lead 1 + Inferior ax [n (%)] | 146 (42.8) |
| LBBB + Superior ax [n (%)] | 12 (3.5) |
| RBBB + Superior ax [n (%)] | 28 (8.2) |
Data are expressed as median interquartile range and count (percentage).
Abbreviations: AMC, aortomitral continuity; LBBB, left bundle branch block; LV, left ventricle; LVOT, left ventricular outflow tract; MV, mitral valve; PVC, premature ventricular complex; RBBB, right bundle branch block; RVOT, right ventricular outflow tract; SOO, site of origin; TV, tricuspid valve.
In a full model, increase in PVC burden (%) (regression coefficient −0.644 and 95% CI −1.063, −0.225 when PVC burden % change from 4.0% to 17.4%, p < .001), PVC QRS duration (regression coefficient −0.191 and 95% CI −0.529, 0.148 when PVC QRS duration change from 120 to 140 msn, p = .049), and age (regression coefficient −0.249 and 95% CI −0.442, −0.056 when PVC burden % change from 38 to 60 year, p = .018) were associated with decrease in LVEF (Table 3). The partial effect plot of PVC burden (%) and 3‐dimensional perspective plot for PVC burden (%) and age interaction were summarized in Figures 2 and 3. As easily noticed in Figure 2, decrease in LVEF become more prominent when PVC burden was above 5%, and PVC burden of more than 20% was associated with LVEF below the normal range.
Table 3.
Adjusted regression coefficient and SE for individual predictors included in full model
| Regression coefficient, 95% CI | p value | |
|---|---|---|
| PVC burden %, (change from 4.0% to 17.4%) | −0.644 (−1.063, −0.225) | <.001 |
| Antiarrhythmic drug use (yes:no) | −0.287 (−0.723, 0.149) | .091 |
| PVC burden %a antiarrhythmic use | – | .059 |
| Interpolated PVC (yes:no) | 0.070 (−0.223, 0.363) | .640 |
| Polymorphic PVCs (yes:no) | −0.329 (−0.689, 0.030) | .073 |
| Sinus QRS duration, msn (change from 80 to 100) | −0.031 (−0.227, 0.166) | .068 |
| PVC QRS duration, msn (change from 120 to 140) | −0.191 (−0.529, 0.148) | .049 |
| PVC coupling interval, msn (change from 420 to 500) | −0.201 (−0.546, 0.144) | .632 |
| Age, year (change from 38 to 60) | −0.249 (−0.442, −0.056) | .018 |
| Gender (female:male) | 0.116 (−0.144, 0.376) | .381 |
| LVOT origin (yes:no) | 0.007 (−0.349, 0.364) | .967 |
| RVOT origin (yes:no) | 0.072 (−0.253, 0.396) | .665 |
Abbreviations: LVOT, left ventricular outflow tract; PVC, premature ventricular complex; RVOT; right ventricular outflow tract.
PVC burden % antiarrhythmic use interaction.
Figure 2.

Partial effect plot of PVC burden (%). Red line represents the beginning of LVEF decline. Interacting with LVEF began when PVC burden was >5%. LVEF, left ventricular ejection fraction; PVC, premature ventricular complex
Figure 3.
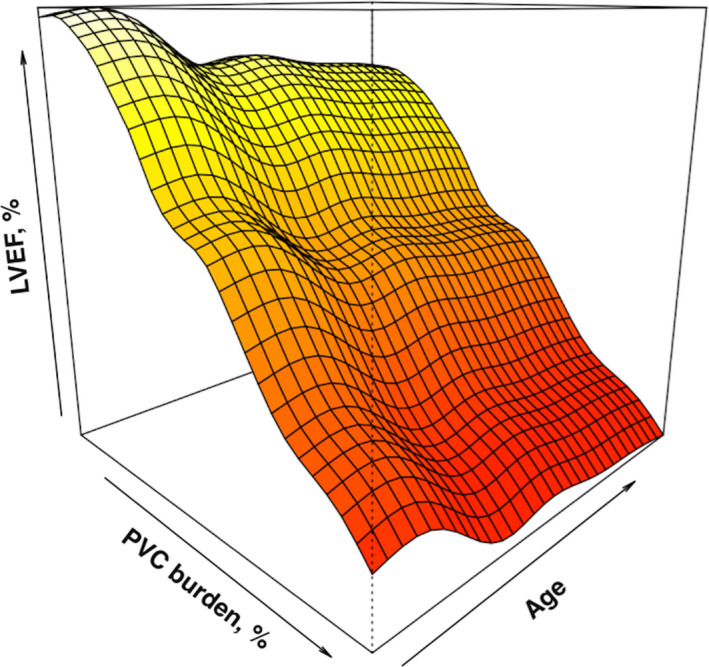
Three‐dimensional perspective plot for PVC burden (%) and age interaction. PVC, premature ventricular complex
In Figure 4, we summarized the relative importance of each predictor in the model. Premature ventricular complex burden (%) was ranked as the strongest predictor for decline in LVEF (PVC burden (%), contributing 74% of the explainable outcome in the model). Following step‐down backward variable selection, only PVC burden (%) remained in the model (reduced model). As it was shown in Table 4, differences in model fit, quality, and predictive accuracy were considered negligible and comparable.
Figure 4.

Importance of individual predictors. The importance of each predictor in the full model was calculated as the proportion of explainable outcome variation contributed by each predictor (partial chi‐square value for each predictor divided by the model's total chi‐square)
Table 4.
Model performances of both full and reduced models
| df | Likelihood ratio X2 | R 2 | Dxy | AIC | BIC | |
|---|---|---|---|---|---|---|
| Full model | 23 | 90.1 | 0.239 | 0.455 | 1,278 | 1,450 |
| Reduced modela | 7 | 88.2 | 0.233 | 0.423 | 1,249 | 1,360 |
PVC burden %* antiarrhythmic use interaction term was added.
We also developed a nomogram using reduced model and variable coefficient in estimating the probability of LVEF ≤ 40% and LVEF ≤ 50% (Figure 5). As an example, in a 24‐hr ambulatory Holter monitoring of a patient using an antiarrhythmic (metoprolol 50 mg) for 2 months, PVC burden was calculated as 25%. According to our nomogram, for this patient, the probability of LVEF to be equal or lower than 40% and 50% were found to be 0.04 and 0.25, respectively.
Figure 5.
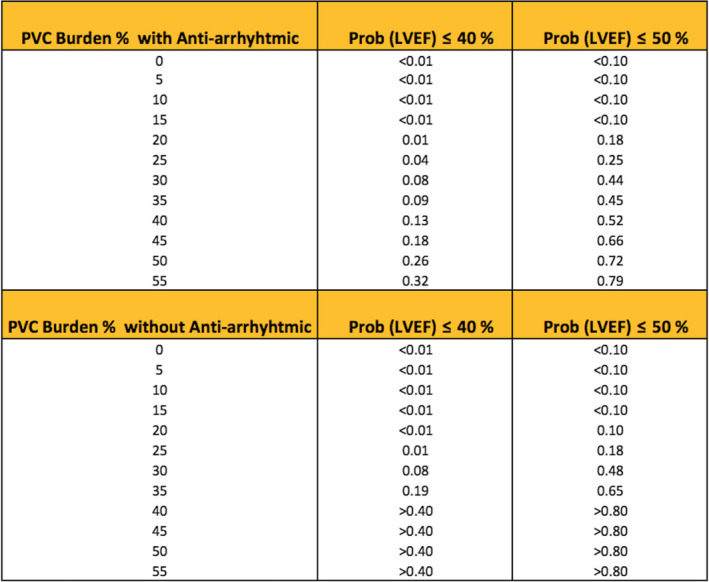
Nomogram for estimating the probability of LVEF ≤ 40%, LVEF ≤ 50%. AA, antiarrhythmic drug; LVEF, left ventricular ejection fraction
4. DISCUSSION
To the best of our knowledge, this is the first multicenter study evaluating the relation between idiopathic PVC burden and LVEF among patients with a prognostic model. In the present study, we found that increase in PVC burden %, age, and PVC QRS duration were independently associated with the decrease in LVEF in the study patients. This inverse relationship between the PVC burden and LVEF becomes more prominent when PVC burden was above 5%.
The exact pathophysiological mechanism for the development of PVC‐CMP is a still matter of debate. Tachycardia‐induced CMP was initially thought as the responsible mechanism (Bogun et al., 2007; Sekiguchi et al., 2005; Takemoto et al., 2005; Yarlagadda et al., 2005). In contradiction to this hypothesis, several studies demonstrated that the total number of heartbeats and mean heart rate during Holter recordings were similar in patients with and without CMP (Niwano et al., 2009; Olgun et al., 2011; Zhong et al., 2014). In an animal model, LVEF declined linearly as PVC burden increased. PVC‐CMP developed in some canines when PVC burden was 25% and 33%, but PVC‐CMP developed in all animals as PVC burden was increased to 50% (Tan et al., 2016). In other animal models, PVC‐CMP was shown to occur at lower heart rates than tachycardia‐induced CMP. Importantly, longer PVC QRS duration, greater LV dyssynchrony, hemodynamic deterioration, and de‐arranged cellular calcium handling were suggested as possible other mechanisms for the occurrence of CMP (Akoum, Daccarett, Wasmund, & Hamdan, 2011; Huizar et al., 2011; Walters et al., 2018; Wang et al., 2014).
Current literature lacks the definitive threshold of PVC burden for identifying high‐risk patients for the development of CMP. Studies that were aimed to investigate the relationship between threshold of PVC burden and CMP had two major designs; one of them is catheter ablation studies. In these studies, PVC‐CMP is often a diagnosis of successful PVC ablation that resulted complete recovery of LVEF with exclusion after ruling out other potential causes of CMP. Baman et al. (2010) reported PVC burden cutoff value of 24% predicted the development of CMP with sensitivity and specificity of 79% and 78%, respectively. Ban et al. (2013) found that this threshold was 26% with sensitivity and specificity of 70% and 78%, respectively. In another multicenter study reported by Penela et al. (2017), threshold of PVC burden was found 17% among patients with PVC. In one study, LV global longitudinal strain (GLS) measurements were evaluated instead of LVEF and PVC burden 8% was found to be associated with LV systolic dysfunction (Lie et al., 2017). Differences in the threshold of PVC burden among the studies might be attributed to several reasons including relatively small number of patients that were included and high mean or median PVC burden of the patients. Additionally, diagnostic confusion of PVC‐CMP and high PVC burden threshold may lead to inadvertently evaluated patients with low PVC burden as patients with CMP due to other causes. This may lead to less represented patients with low PVC burden in ablation studies. Another reason might be limitation of statistical models. In the analysis of these studies, patients were diagnosed with PVC‐CMP according to a specific LVEF value (<50%) or GLS value after catheter ablation, and then, a specific PVC burden was determined by ROC analysis with sensitivity and specificity determination. This statistical analysis model does not predict individual risk. Also, it is difficult to say which combination of sensitivity and specificity is optimal. It was reported by authors that patients who had PVC‐CMP with a lower PVC burden might not be able to be identified with threshold values determined by this method.
Other important aspect of the studies contributing to this controversy was their design which was prospective observational cohort. A study published by Niwano et al. (2009) revealed that PVC prevalence was the only major determinant for the development of LV systolic dysfunction during the long‐term follow‐up in patients with initial normal LVEF. The group with initial PVC burden less than 5.000 beats/d did not exhibit any significant changes in the LVEF. In contrary, the group with initial PVC burden of >20.000 beats/d exhibited a significant decline in LVEF after 4th year and latest of follow‐up. Patients with initial PVC burden between 5.000 and 20.000 beats/d which was third group did not exhibit any significant decline in the LVEF at the 4‐year time point, but there was a significant decline in the LVEF at the latest follow‐up point (5.6 years). Despite these findings, they suggested that the most powerful cutoff point was 31.268 beats/d with a sensitivity of 0.692 and a specificity of 0.929. Another prospective cohort which included 1,139 healthy subjects with PVC and without structural heart disease reported that the 15‐year risk for chronic heart failure rose abruptly as the percentage of PVCs increased between 0% and 0.5%, with tapering of the risk curve for PVC percentage >10% (Dukes et al., 2015). They concluded that this effect was observed at a lower PVC percentage than expected and previously recognized. The most important limitation of these studies was the possibility of many unpredictable confounders' parameters affecting results in long‐term follow‐up.
Our study had a multicenter cross‐sectional design that included more patients than most of other studies. Therefore, our study included patients with lower PVC burden. Our study showed that threshold of PVC burden which is associated decrease in LVEF was observed lower than the amount requiring the treatment according to the current recommendations (Pedersen et al., 2014). In observational cohort studies, the threshold of PVC burden which related the beginning of decrease in LVEF was close to our result. In a study evaluating PVC‐CMP recurrence following PVC catheter ablation, the mean PVC burden was found below 5% in the non‐recurrent group (Lee, Hoffmayer, et al., 2019). This finding supports our study result.
These findings of our study do not allow for individual risk estimation, as in other studies. In the above‐mentioned studies, the relationship between PVC number and LVEF was investigated by using descriptive models. For this reason, in our study the relationship between PVC burden and LVEF was investigated by using the prognostic model in addition to descriptive model. We developed a nomogram by using reduce model and variable coefficient for estimating the individual risk. This model may be useful in determining the risk for PVC‐CMP and making treatment decisions for patients with idiopathic PVC.
5. CONCLUSION
Our study showed that inverse relationship between PVC burden and LVEF become more prominent in lower PVC burden than previously known. Also, our study was the first study which included a prognostic model showing the relationship between LVEF and idiopathic PVC burden. This prognostic model may contribute to reduce uncertainty in the treatment and follow‐up of patients with especially in lower PVC burden.
6. STUDY LIMITATIONS
Our study had several limitations. It is a cross‐sectional design which might not eliminate effects of unknown confounders. 24‐hr of Holter recordings might not be sufficient to evaluate the day‐to‐day variability. A possible referral bias toward patients with compromised LVEF cannot be excluded. Cardiac MRIs were not performed in the majority of patients during the enrollment phase. Using ECG to identify the anatomic site of origin has significant limitations.
CONFLICT OF INTEREST
None.
Altıntaş B, Özkalaycı F, Çinier G, et al. The effect of idiopathic premature ventricular complexes on left ventricular ejection fraction. Ann Noninvasive Electrocardiol. 2020;25:e12702 10.1111/anec.12702
REFERENCES
- Agarwal, V. , Vittinghoff, E. , Whitman, I. R. , Dewland, T. A. , Dukes, J. W. , & Marcus, G. M. (2017). Relation between ventricular premature complexes and incident heart failure. American Journal of Cardiology, 119(8), 1238–1242. [DOI] [PubMed] [Google Scholar]
- Akoum, N. W. , Daccarett, M. , Wasmund, S. L. , & Hamdan, M. H. (2011). An animal model for ectopy‐induced cardiomyopathy. Pacing and Clinical Electrophysiology, 34, 291–295. 10.1111/j.1540-8159.2010.02947.x [DOI] [PubMed] [Google Scholar]
- Baman, T. S. , Lange, D. C. , Ilg, K. J. , Gupta, S. K. , Liu, T.‐Y. , Alguire, C. , … Bogun, F. (2010). Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 7, 865–869. 10.1016/j.hrthm.2010.03.036 [DOI] [PubMed] [Google Scholar]
- Ban, J. E. , Park, H. C. , Park, J. S. , Nagamoto, Y. , Choi, J. I. , Lim, H. E. , … Kim, Y. H. (2013). Electrocardiographic and electrophysiological characteristics of premature ventricular complexes associated with left ventricular dysfunction in patients without structural heart disease. Europace, 15(5), 735–741. 10.1093/europace/eus371 [DOI] [PubMed] [Google Scholar]
- Bas, H. D. , Baser, K. , Hoyt, J. , Yokokawa, M. , LaBounty, T. , Morady, F. , & Bogun, F. (2016). Effect of circadian variability in frequency of premature ventricular complexes on left ventricular function. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 13, 98–102. 10.1016/j.hrthm.2015.07.038 [DOI] [PubMed] [Google Scholar]
- Baser, K. , Bas, H. D. , LaBounty, T. , Yokokawa, M. , Good, E. , Latchamsetty, R. , … Bogun, F. (2015). Recurrence of PVCs in patients with PVC‐induced cardiomyopathy. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 12(7), 1519–1523. 10.1016/j.hrthm.2015.03.027 [DOI] [PubMed] [Google Scholar]
- Bogun, F. , Crawford, T. , Reich, S. , Koelling, T. M. , Armstrong, W. , Good, E. , … Morady, F. (2007). Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: Comparison with a control group without intervention. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 4, 863–867. 10.1016/j.hrthm.2007.03.003 [DOI] [PubMed] [Google Scholar]
- Del Carpio Munoz, F. , Syed, F. F. , Noheria, A. , Cha, Y.‐M. , Friedman, P. A. , Hammill, S. C. , … Asirvatham, S. J. (2011). Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: Study of the burden, duration, coupling interval, morphology and site of origin of PVCs. Journal of Cardiovascular Electrophysiology, 22(7), 791–798. 10.1111/j.1540-8167.2011.02021.x [DOI] [PubMed] [Google Scholar]
- deVries, L. J. , Martirosyan, M. , van Domburg, R. T. , Wijchers, S. A. , Géczy, T. , & Szili‐Torok, T. (2018). Coupling interval variability of premature ventricular contractions in patients with different underlying pathology: An insight into the arrhythmia mechanism. Journal of Interventional Cardiac Electrophysiology, 51(1), 25–33. 10.1007/s10840-017-0309-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffee, D. F. , Shen, W. K. , & Smith, H. C. (1998). Suppression of frequent premature ventricular contractions and improvement of left ventricular function in patients with presumed idiopathic dilated cardiomyopathy. Mayo Clinic Proceedings, 73, 430–433. 10.1016/S0025-6196(11)63724-5 [DOI] [PubMed] [Google Scholar]
- Dukes, J. W. , Dewland, T. A. , Vittinghoff, E. , Mandyam, M. C. , Heckbert, S. R. , Siscovick, D. S. ,. … Marcus, G. M. (2015). Ventricular ectopy as a predictor of heart failure and death. Journal of the American College of Cardiology, 66(2), 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez, A. , Baranchuk, A. , Briceno, D. , Saenz, L. , & Garcia, F. (2019). How to use the 12‐lead ECG to predict the site of origin of idiopathic ventricular arrhythmias. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 10.1016/j.hrthm.2019.04.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hasdemir, C. , Ulucan, C. , Yavuzgil, O. , Yuksel, A. , Kartal, Y. , Simsek, E. , … Can, L. H. (2011). Tachycardia‐induced cardiomyopathy in patients with idiopathic ventricular arrhythmias: The incidence, clinical and electrophysiologic characteristics and the predictors. Journal of Cardiovascular Electrophysiology, 22(6), 663–668. 10.1111/j.1540-8167.2010.01986.x [DOI] [PubMed] [Google Scholar]
- Huizar, J. F. , Kaszala, K. , Potfay, J. , Minisi, A. J. , Lesnefsky, E. J. , Abbate, A. , … Wood, M. A. (2011). Left ventricular systolic dysfunction induced by ventricular ectopy: A novel model for Ventricular premature contraction‐induced cardiomyopathy. Circulation: Arrhythmia and Electrophysiology, 4, 543–549. 10.1161/CIRCEP.111.962381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, R. M. , Bierig, M. , Devereux, R. B. , Flachskampf, F. A. , Foster, E. , Pellikka, P. A. , … Stewart, W. J. (2005). Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography, 18, 1440–1463. 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Latchamsetty, R. , Yokokawa, M. , Morady, F. , Kim, H. M. , Mathew, S. , Tilz, R. , … Bogun, F. (2015). Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACC: Clinical Electrophysiology, 1(3), 116–123. 10.1016/j.jacep.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Lee, A. , Denman, R. , & Haqqani, H. M. (2019). Ventricular ectopy in the context of left ventricular systolic dysfunction: Risk factors and outcomes following catheter ablation. Heart, Lung and Circulation, 28(3), 379–388. 10.1016/j.hlc.2018.01.012 [DOI] [PubMed] [Google Scholar]
- Lee, D. , Hoffmayer, K. S. , Hsu, J. C. , Schricker, A. , Birgersdotter‐Green, U. , Raissi, F. , … Krummen, D. E. (2019). Long‐term mode and timing of premature ventricular complex recurrence following successful catheter ablation. Journal of Interventional Cardiac Electrophysiology, 55(2), 153–160. 10.1007/s10840-019-00520-3. [DOI] [PubMed] [Google Scholar]
- Lie, Ø. H. , Saberniak, J. , Dejgaard, L. A. , Stokke, M. K. , Hegbom, F. , Anfinsen, O. G. , … Haugaa, K. H. (2017). Lower than expected burden of premature ventricular contractions impairs myocardial function. ESC Heart Failure, 4(4), 585–594. 10.1002/ehf2.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. , Shepherd, B. E. , Li, C. , & Harrell, F. E. Jr (2017). Modeling continuous response variables using ordinal regression. Statistics in Medicine, 36(27), 4316–4335. 10.1002/sim.7433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebbert, J. , Auberson, D. , & Marchlinski, F. (2016). Premature ventricular complexes in apparently normal hearts. Cardiac Electrophysiology Clinics, 8(3), 503–514. 10.1016/j.ccep.2016.04.001 [DOI] [PubMed] [Google Scholar]
- Nguyen, K. T. , Vittinghoff, E. , Dewland, T. A. , Dukes, J. W. , Soliman, E. Z. , Stein, P. K. , … Marcus, G. M. (2017). Ectopy on a single 12‐Lead ECG, incident cardiac myopathy, and death in the community. Journal of the American Heart Association, 6(8), e006028 10.1161/JAHA.117.006028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwano, S. , Wakisaka, Y. , Niwano, H. , Fukaya, H. , Kurokawa, S. , Kiryu, M. , … Izumi, T. (2009). Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart, 95(15), 1230–1237. 10.1136/hrt.2008.159558 [DOI] [PubMed] [Google Scholar]
- Olgun, H. , Yokokawa, M. , Baman, T. , Kim, H. M. , Armstrong, W. , Good, E. , … Bogun, F. (2011). The role of interpolation in pvc‐induced cardiomyopathy. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 8, 1046–1049. 10.1016/j.hrthm.2011.02.034 [DOI] [PubMed] [Google Scholar]
- Oomen, A. W. G. J. , Dekker, L. R. C. , & Meijer, A. (2018). Catheter ablation of symptomatic idiopathic ventricular arrhythmias: A five year single centre experience. Netherlands Heart Journal, 26(4), 210–216. 10.1007/s12471-018-1085-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizo, J. G. , Barra, S. , Mellor, G. , Heck, P. , & Agarwal, S. (2018). Premature ventricular complex‐induced cardiomyopathy. Arrhythmia and Electrophysiology Review, 7(2), 128–134. 10.15420/aer.2018.23.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, C. T. , Kay, G. N. , Kalman, J. , Borggrefe, M. , Della‐Bella, P. , Dickfeld, T. , … Savelieva, I. (2014). EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 11(10), e166–196. 10.1016/j.hrthm.2014.07.024 [DOI] [PubMed] [Google Scholar]
- Penela, D. , Fernández‐Armenta, J. , Aguinaga, L. , Tercedor, L. , Ordoñez, A. , Bisbal, F. , … Berruezo, A. (2017). Clinical recognition of pure premature ventricular complex‐induced cardiomyopathy at presentation. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 14(12), 1864–1870. 10.1016/j.hrthm.2017.07.025 [DOI] [PubMed] [Google Scholar]
- Rickham, P. P. (1964). Human experimentation. Code of ethics of the World Medical Association. Declaration of Helsinki. British Medical Journal, 2, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi, Y. , Aonuma, K. , Yamauchi, Y. , Obayashi, T. , Niwa, A. , Hachiya, H. , … Isobe, M. (2005). Chronic hemodynamic effects after radiofrequency catheter ablation of frequent monomorphic ventricular premature beats. Journal of Cardiovascular Electrophysiology, 16, 1057–1063. 10.1111/j.1540-8167.2005.40786.x [DOI] [PubMed] [Google Scholar]
- Takemoto, M. , Yoshimura, H. , Ohba, Y. , Matsumoto, Y. , Yamamoto, U. , Mohri, M. , … Origuchi, H. (2005). Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. Journal of the American College of Cardiology, 45, 1259–1265. 10.1016/j.jacc.2004.12.073 [DOI] [PubMed] [Google Scholar]
- Tan, A. Y. , Hu, Y. L. , Potfay, J. , Kaszala, K. , Howren, M. , Sima, A. P. , … Huizar, J. F. (2016). Impact of ventricular ectopic burden in a premature ventricular contraction‐induced cardiomyopathy animal model. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 13(3), 755–761. 10.1016/j.hrthm.2015.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Rotz, M. , Aeschbacher, S. , Bossard, M. , Schoen, T. , Blum, S. , Schneider, S. , … Conen, D. (2017). Risk factors for premature ventricular contractions in young and healthy adults. Heart, 103(9), 702–707. 10.1136/heartjnl-2016-309632 [DOI] [PubMed] [Google Scholar]
- Walters, T. E. , Rahmutula, D. , Szilagyi, J. , Alhede, C. , Sievers, R. , Fang, Q. , … Gerstenfeld, E. P. (2018). Left ventricular dyssynchrony predicts the cardiomyopathy associated with premature ventricular contractions. Journal of the American College of Cardiology, 72, 2870–2882. 10.1016/j.jacc.2018.09.059 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Eltit, J. M. , Kaszala, K. , Tan, A. , Jiang, M. , Zhang, M. , … Huizar, J. F. (2014). Cellular mechanism of premature ventricular contraction induced cardiomyopathy. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 11, 2064–2072. 10.1016/j.hrthm.2014.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlagadda, R. K. , Iwai, S. , Stein, K. M. , Markowitz, S. M. , Shah, B. K. , Cheung, J. W. , … Mittal, S. (2005). Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation, 112, 1092–1097. 10.1161/CIRCULATIONAHA.105.546432 [DOI] [PubMed] [Google Scholar]
- Yokokawa, M. , Kim, H. M. , Good, E. , Chugh, A. , Pelosi, F. , Alguire, C. , … Bogun, F. (2012). Relation of symptoms and symptom duration to premature ventricular complex‐induced cardiomyopathy. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 9, 92–95. 10.1016/j.hrthm.2011.08.015 [DOI] [PubMed] [Google Scholar]
- Yokokawa, M. , Kim, H. M. , Good, E. , Crawford, T. , Chugh, A. , Pelosi, F. , … Bogun, F. (2012). Impact of QRS duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 9(9), 1460–1464. 10.1016/j.hrthm.2012.04.036 [DOI] [PubMed] [Google Scholar]
- Zhong, L. I. , Lee, Y.‐H. , Huang, X.‐M. , Asirvatham, S. J. , Shen, W.‐K. , Friedman, P. A. , … Cha, Y.‐M. (2014). Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: A single center retrospective study. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 11, 187–193. 10.1016/j.hrthm.2013.10.033 [DOI] [PubMed] [Google Scholar]


