Background:
During the COVID-19 pandemic, ventilator sharing was suggested to increase availability of mechanical ventilation. The safety and feasibility of ventilator sharing is unknown.
Methods:
A single ventilator in pressure control mode was used with flow control valves to simultaneously ventilate two patients with different lung compliances. The system was first evaluated using high-fidelity human patient simulator mannequins and then tested for 1 h in two pairs of COVID-19 patients with acute respiratory failure. Patients were matched on positive end-expiratory pressure, fractional inspired oxygen tension, and respiratory rate. Tidal volume and peak airway pressure (PMAX) were recorded from each patient using separate independent spirometers and arterial blood gas samples drawn at 0, 30, and 60 min. The authors assessed acid-base status, oxygenation, tidal volume, and PMAX for each patient. Stability was assessed by calculating the coefficient of variation.
Results:
The valves performed as expected in simulation, providing a stable tidal volume of 400 ml each to two mannequins with compliance ratios varying from 20:20 to 20:90 ml/cm H2O. The system was then tested in two pairs of patients. Pair 1 was a 49-yr-old woman, ideal body weight 46 kg, and a 55-yr-old man, ideal body weight 64 kg, with lung compliance 27 ml/cm H2O versus 35 ml/cm H2O. The coefficient of variation for tidal volume was 0.2 to 1.7%, and for PMAX 0 to 1.1%. Pair 2 was a 32-yr-old man, ideal body weight 62 kg, and a 56-yr-old woman, ideal body weight 46 kg, with lung compliance 12 ml/cm H2O versus 21 ml/cm H2O. The coefficient of variation for tidal volume was 0.4 to 5.6%, and for PMAX 0 to 2.1%.
Conclusions:
Differential ventilation using a single ventilator is feasible. Flow control valves enable delivery of stable tidal volume and PMAX similar to those provided by individual ventilators.
Custom designed three-dimensional printed inspiratory flow control valves designed to allow individualized setting of tidal volume and airway pressure were evaluated using high-fidelity simulator mannequins with similar or different lung compliance and were found to perform as expected with stable tidal volumes delivered to each mannequin. Testing for 60 min in two pairs of volunteer COVID-19 patients with acute respiratory failure, matched on positive end-expiratory pressure level, fractional inspired oxygen tension level, and respiratory rate, demonstrated stable system performance. Continuous assessment of tidal volume and peak airway pressure in each patient during the study allowed for dynamic alteration of tidal volume in response to respiratory acidosis. This study suggests that custom designed flow control valves may facilitate the use of split ventilation techniques in a surge setting.
Supplemental Digital Content is available in the text.
Editor’s Perspective.
What We Already Know about This Topic
In previous mass casualty situations that have resulted in intensive care unit or emergency room surge conditions, the use of ventilator splitting to ventilate two or more patients has been proposed.
The concept has received renewed attention with the global COVID-19 pandemic.
The impaired respiratory mechanics similar to the acute respiratory distress syndrome seen in COVID-19 patients pose significant engineering challenges to optimally ventilate one patient while preventing damage to a paired patient.
What This Article Tells Us That Is New
Custom three-dimensional printed inspiratory flow control valves designed to allow individualized setting of tidal volume and airway pressure were evaluated using high-fidelity simulator mannequins with similar or different lung compliance and were found to perform as expected with stable tidal volumes delivered to each mannequin.
The system demonstrated stable performance when tested for 1 h in two pairs of volunteer COVID-19 patients with acute respiratory failure. Continuous assessment of tidal volume and peak airway pressure in each patient during the study allowed for dynamic alteration of tidal volume in response to respiratory acidosis.
This study suggests that custom designed flow control valves may facilitate the use of split ventilation techniques in a surge setting.
THE COVID-19 (SARS CoV-2) pandemic during early 2020 resulted in an unprecedented number of hospital and intensive care unit admissions, with patients requiring mechanical ventilation for prolonged periods of time.1,2 Our experience in the Mount Sinai Health System in New York City was similarly overwhelming. Between February 27 and April 9, 2020, hospitals in the Mount Sinai Health System admitted 4,241 COVID-19–positive patients, of whom ~10% required ventilation. The median duration of ongoing ventilation was 9.3 days. Of those ventilated, 26% died and only 25% had been successfully extubated, leaving nearly 50% in need of continuing ventilation. Although the Mount Sinai Health System had enough ventilators to meet demand at that time, the steady increase in the number of patients needing prolonged ventilation led to concern that there would be too few ventilators to meet the growing demand, and that potentially salvageable patients could be lost because ventilation would be unavailable for them.3
The United States Public Health Service Commissioned Corps’ statement on Optimizing Ventilator Use during the COVID-19 Pandemic asserted that a possible “crisis standard of care” strategy was the ventilation of two patients with a single mechanical ventilator, although such a strategy should only be considered as an absolute last resort.4 The Institute of Medicine defines crisis standards of care as “a substantial change in the usual health care operations and the level of care it is possible to deliver…justified by specific circumstances and…formally declared by a state government in recognition that crisis operations will be in effect for a sustained period.”5 The Institute of Medicine further stated that “CSC [crisis standards of care], planned and implemented in accordance with ethical values, are necessary for the allocation of scarce resources.”6
In light of the extraordinary circumstances and in accordance with the Institute of Medicine’s crisis standard of care guidelines, the Governor of New York, Andrew M. Cuomo, issued a statement at the end of March 2020 approving the use of ventilator sharing as a last resort.7 We therefore proceeded to design a novel system and method of differential ventilation that uses a custom-manufactured flow control valve to overcome the important challenges of safely ventilating two patients with one ventilator. This work is similar to that done by several other groups during the COVID-19 crisis, both domestically and internationally, and was inspired by earlier work done in the mid-2000s.8–12
The purpose of this study was to (1) test the feasibility, in a simulation laboratory, of ventilating two patients simultaneously using a single standard mechanical ventilator and a system that allows individualized setting of tidal volumes and airway pressures, and (2) test the system in consented COVID-19 patients as a proof of concept.
Materials and Methods
Split Circuit
The split circuit for shared ventilation is intended to be used with the ventilator in pressure control mode with paralyzed patients only. The advantage of pressure control ventilation is that peak alveolar pressure cannot rise above the set peak inspiratory pressure.13 This is critical in our system so that changes in compliance in one patient do not affect the peak inspiratory pressures, and consequently the tidal volumes delivered to the paired patient. Gas flow to the less compliant patient will decrease or cease while continuing to the more compliant patient. The arrangement is as follows (fig. 1): the ventilator breathing circuit is split using standard T-pieces and connectors. Attached to either end of the T-piece is a custom-designed and manufactured flow control valve (fig. 2; Stryker Corporation, USA), followed by a one-way valve, and then the inspiratory limb of the breathing circuit. The flow control valve is a three-dimensional printed needle valve with an inner diameter of 9 mm and maximum flow rate at full open of 68 l/min, fitted with standard 22-mm connectors as per the International Standards Organization 5356-1:2015 standard.14 The valve has a zero mark, index markings for degrees open, click stops every 30 degrees, and a full stop position (fig. 2). It is an investigational device approved by the Food and Drug Administration under an Emergency Use Authorization. At the distal (patient) end, a standard spirometry sensor (GE D-lite++ Patient Spirometry Set, GE, USA) is placed in-line between the endotracheal tube elbow connector and the wye piece of the breathing circuit. The sensor is used to measure the peak inspiratory pressure and tidal volume delivered to each patient and is connected to the gas analyzer-spirometry module of a physiologic monitoring system (GE CARESCAPE Respiratory Module, GE, USA). A separate monitor is used for each patient. At the distal end of each expiratory limb, a one-way valve (fig. 1) is placed between the circuit wye piece and the expiratory limb of the breathing circuit. One-way valves are placed in the inspiratory and expiratory limbs of each patient’s breathing circuit to ensure unidirectional gas flow and prevent backflow and contamination between the two patient circuits. The ends of the two expiratory limbs are joined with a T-piece that is connected to the expiratory port of the ventilator. Bacterial/viral filters are placed between the elbow connector and the spirometry sensor, and at the inspiratory and expiratory connection ports on the ventilator.
Fig. 1.
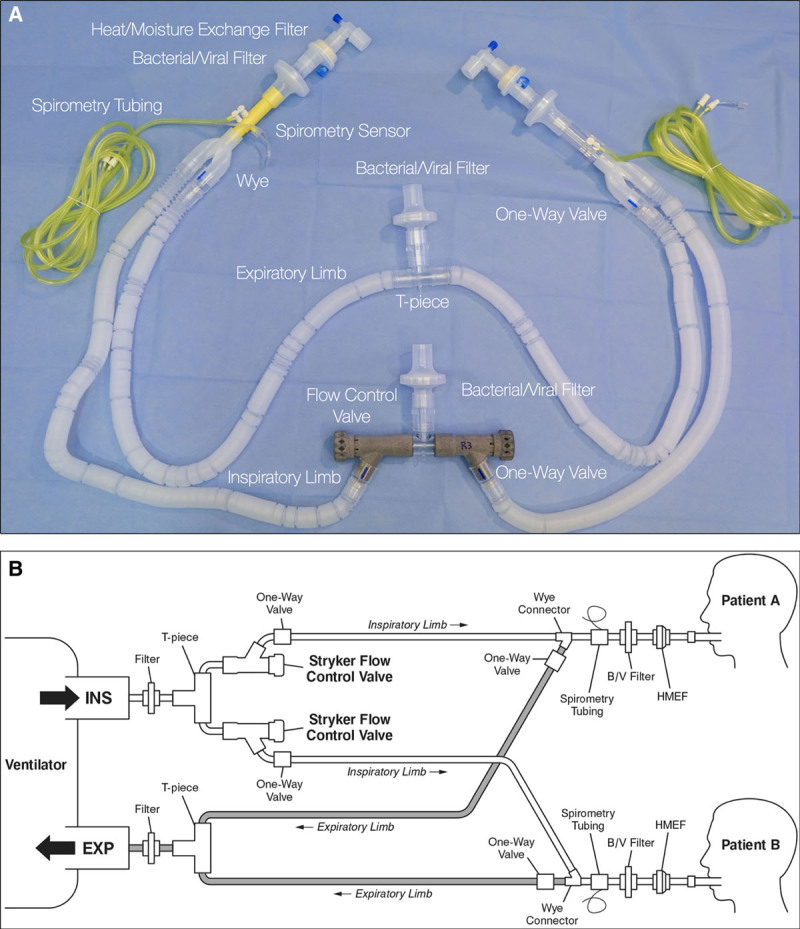
Split circuit with flow control valves. (A) Circuit as-built. (B) Line drawing of circuit. Note: all components shown are standard breathing circuit components, aside from the three-dimensional printed flow control valves. Image courtesy of Stryker Corporation, USA.
Fig. 2.
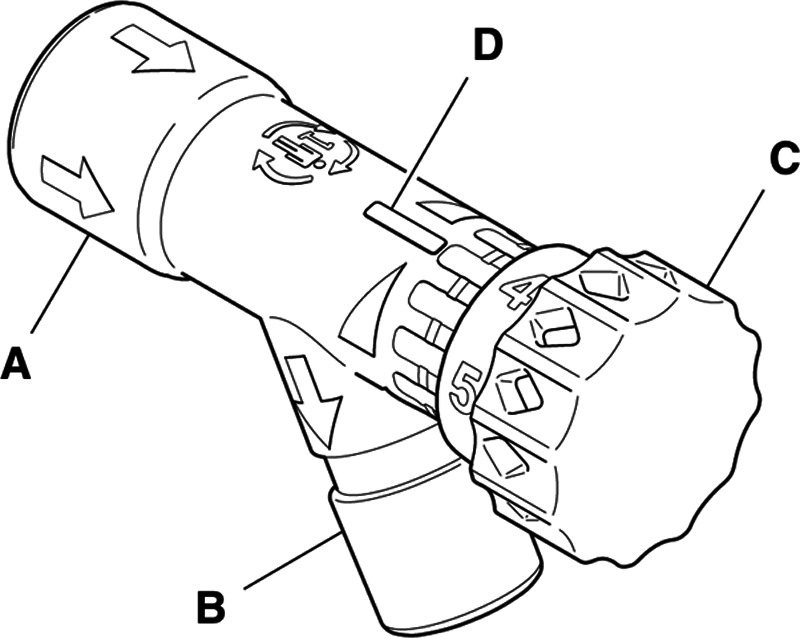
Flow control valve. (A) Inlet port; (B) outlet port; (C) flow control knob; (D) flow level indicator. Image courtesy of Stryker Corporation, USA.
Simulation Testing
Two high-fidelity Human Patient Simulator mannequins (HPS Anesthesia Simulator Mannequin Systems, CAE Healthcare, USA) were used for the simulation. We tested the system under a variety of simulated patient physiologies likely to be encountered in patients with acute respiratory failure due to COVID-19, using both a GE-Datex-Ohmeda S/5 anesthesia machine (GE, USA) and a Puritan Bennett 840 ventilator (Medtronic, USA). The ventilator system pre-use checkouts were performed, and the split breathing circuit was connected to the two mannequins, designated Mannequin A and Mannequin B. The ventilator was set to pressure control mode, and both mannequins were set to simulate identical compliance. With both flow control valves open, the driving pressure on the ventilator was adjusted to deliver a tidal volume of 4 to 6 ml/kg ideal body weight to Mannequin A. The flow control valves were characterized under these conditions first, to determine the degree of valve opening corresponding to no flow resistance and the range over which meaningful flow resistance could be introduced. Mannequin A was then adjusted to the minimum compliance and Mannequin B to the maximum compliance setting available on the simulator. Flow control valve B was opened, and then the ventilator was set to deliver approximately 420 ml tidal volume to Mannequin A (the less compliant mannequin). Thereafter, flow control valve B was turned clockwise to an increasingly closed position, until the same valve characteristics were determined as above. Tidal volume, peak inspiratory pressure, positive end-expiratory pressure (PEEP), and degree of valve opening were recorded throughout each simulation.
Clinical Study
Justification for Human Study.
This study was conducted under extraordinary circumstances at the height of the COVID-19 epidemic in New York City. Our hospital was almost at capacity with respect to ventilators and experiencing a surge of patients in acute respiratory failure. At the time we conducted this study, we believed that we might be required to implement shared ventilation in patients within a week. As such, we initiated a feasibility study to test our equipment and protocol before we might be compelled by events to implement it hospital-wide. We believed that it would be safer to do so when staff and equipment were still relatively available, rather than waiting to try an untested technique in the midst of a true crisis. Two anesthesiologists were present in the room throughout the studies to ensure patient safety.
Consent Process.
Before initiating the testing of shared ventilation in patients, a discussion was held with the chair of our Institutional Review Board (IRB), the Icahn School of Medicine at Mount Sinai, New York, New York. The very real potential need for shared ventilation was underscored by a letter from the New York State Health Commissioner to the Mount Sinai Health System authorizing the use of the flow control valve and any other devices/accessories that were subject to an Emergency Use Authorization to expand the use of ventilators for addressing the COVID-19 pandemic. The chair of the IRB reviewed the test design and intensive safety monitoring procedures (see Clinical Protocol and Preparation for Split Ventilation below and in Supplemental Digital Content 1, http://links.lww.com/ALN/C441) and concluded that, while this procedure (testing of shared ventilation) was not covered by the federal definition of regulated research under the common rule 45 C.F.R. § 46 (2018), written informed consent was needed.
Eligible patients were, by the nature of their severe critical illness, sedated and paralyzed. Furthermore, onsite visitation was suspended at the hospital during the pandemic. As such, informed consent was obtained from the legally authorized representative of all patients via telephone, using an emergency consent document created in conjunction with and approved by our IRB. The consent document stressed that participation in the testing was purely altruistic with no known benefit to the patient. It was read to the legally authorized representative over the telephone and, after appropriate discussion and answering of any and all questions from the legally authorized representative, the verbal consent was witnessed by a team member (author E.K.), and either the senior author (M.D.C.) or first author (M.A.L.). One copy of the witnessed consent document was then placed in each patient’s chart, a second copy was retained by the first author, and a third copy was made available to the patient’s legally authorized representative.
Inclusion and Exclusion Criteria.
Eligible patients were identified via chart review. Inclusion criteria were patients with acute respiratory failure due to COVID-19 who had been receiving mechanical ventilation with sedation and paralysis for 1 to 12 days, and were expected to require continued prolonged ventilatory care. The patients were required to have similar PEEP and fractional inspired oxygen tension (Fio2) requirements. Exclusion criteria were age less than 18 yr, use of inhaled pulmonary vasodilators, superimposed bacterial infection, and unstable patients on escalating PEEP or pressor requirements.
Clinical Protocol and Preparation for Split Ventilation.
The full clinical protocol used to manage patients during the study is shown in Supplemental Digital Content 1, Mount Sinai Health System Vent Sharing Protocol (http://links.lww.com/ALN/C441) and available online at https://bit.ly/3aYRxpb. The patients were moved into the same room in an intensive care unit and connected to individual physiologic monitoring. Each patient was placed on pressure control ventilation with the same respiratory rate, PEEP, and Fio2. The peak inspiratory pressure of each patient’s ventilator was adjusted to achieve a tidal volume of 4 to 6 ml/kg of ideal body weight. We used an Inspiratory:Expiratory ratio of 1:1 for all patients in this series for consistency and in order to deliver the targeted tidal volumes to both patients using relatively low peak inspiratory pressures. The patients were then observed for a period of 20 min to ensure they were hemodynamically stable, and a baseline arterial blood gas (ABG) sample was drawn. The split ventilation circuit including the flow control valves was fully assembled, and a third ventilator (to be shared) was set up between the two patient beds and self-tested with a single breathing circuit. The single circuit was then removed from the ventilator to be shared, and the split circuit was connected. The shared ventilator was then set with the parameters of the patient with the higher peak inspiratory pressure.
Initiation and Testing of Split Ventilation.
The patients were closely monitored by two anesthesiologists wearing appropriate personal protective equipment who were present at bedside throughout. The adequacy of sedation and paralysis was reassessed for both patients immediately before beginning the study. Shared ventilation was then initiated by clamping the endotracheal tube (ETT) of each patient (to prevent derecruitment as well as to decrease aerosolization of viral particles), disconnecting them from their individual ventilators, and quickly connecting them to the split circuit. The flow control valve of the patient with the lower peak inspiratory pressure was almost fully closed, and then slowly opened, titrating inspiratory pressure and volume delivered to that patient while monitoring individual spirometric values in order to achieve tidal volumes that were similar to their baseline value.
Thirty minutes after initiating shared ventilation, an ABG sample was drawn from each patient, and the flow control valves to each patient were adjusted as needed based on the results. Sixty minutes after initiating shared ventilation, another ABG sample was drawn. At this point, the patients were disconnected from the split circuit by clamping their ETTs and reconnected to their individual ventilators. After termination of the study, the patients were closely monitored for 1 h in order to ensure they remained stable and had returned to their baseline respiratory status.
Statistical Analysis.
Ventilator and physiologic data were manually recorded every 5 min throughout the study using Google Sheets (Google, USA). A data analysis and statistical plan were written after the data were collected. Data from the patient studies were analyzed for assessment of flow control valve stability over the 60-min study periods by calculating the coefficient of variation for three time periods throughout each study: the first 0 to 10 min after beginning split ventilation, the observation period from 10 to 30 min before drawing an ABG sample, and the period from 30 to 60 min after any adjustment to the ventilation, which was made based on the ABG result at 30 min. Coefficient of variation was calculated as the SD divided by the mean, multiplied by 100 to convert to a percentage.
Results
Simulation Study
The split circuit performed as expected in both simulator scenarios (i.e., with identical compliance and disparate compliances). Results of the simulation are shown in figure 3. As the flow control valve was progressively closed from a fully open position, the tidal volume delivered to the attached mannequin began to decrease in a nonlinear but controllable fashion. Figure 4 shows the valve position (number of turns opened) required to achieve a tidal volume of 400 ml each in two mannequins with different compliances.
Fig. 3.
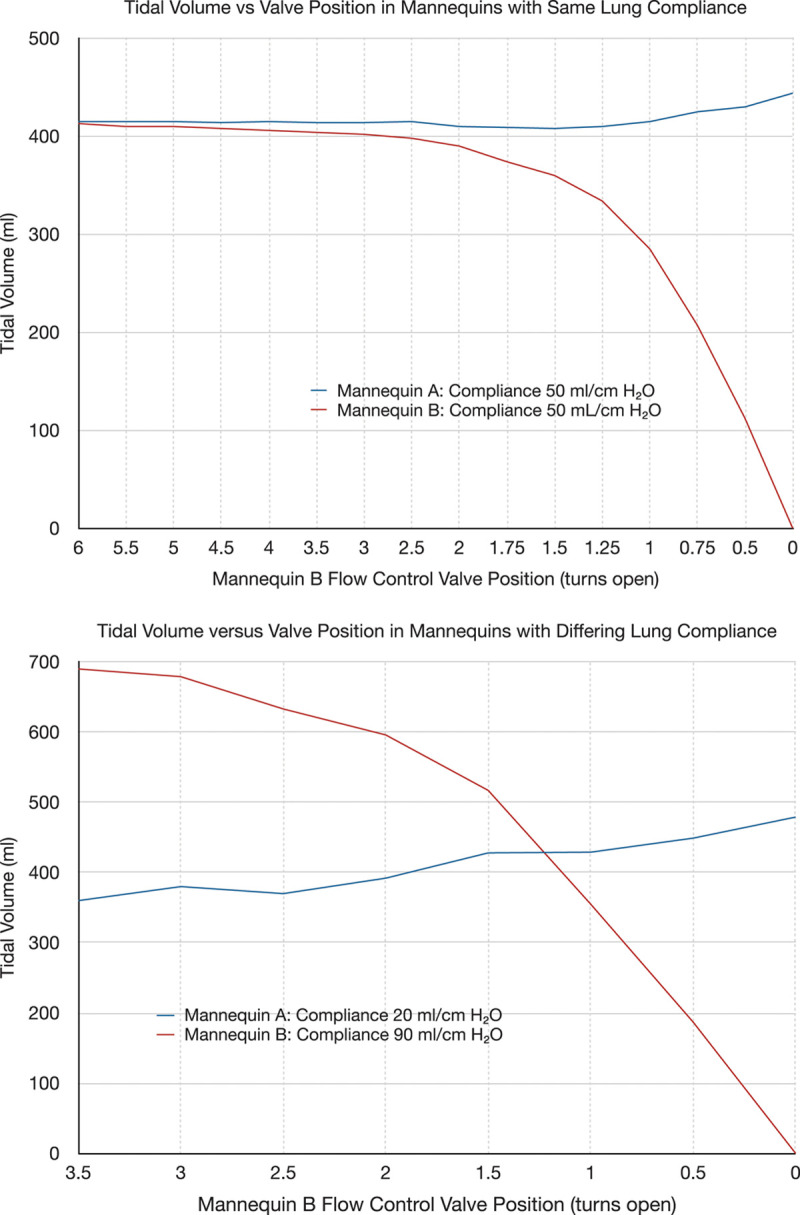
Tidal volumes during simulation. Top panel: Mannequins set to equal compliance. Bottom panel: Mannequins with differing compliance. As the flow control valve for Mannequin B is slowly closed, the tidal volume delivered to mannequin B decreases, while the tidal volume delivered to Mannequin A stays nearly the same.
Fig. 4.
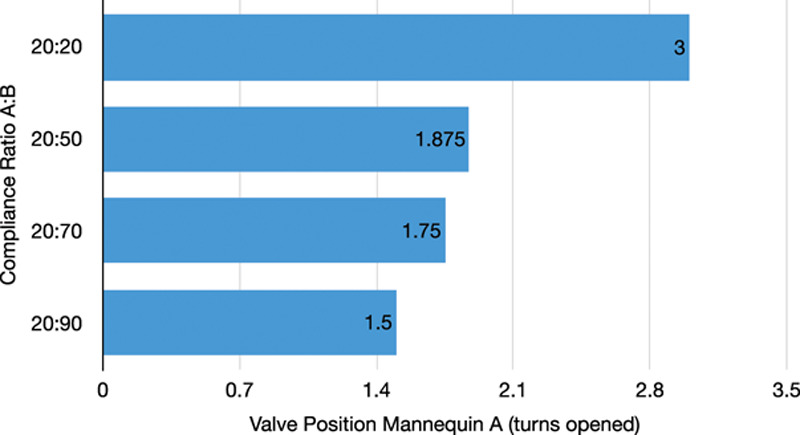
Valve position to maintain tidal volume of 400 ml in mannequins with differing lung compliances. Compliance of Mannequin A is fixed at 20 ml/cm H2O while the compliance of Mannequin B is incrementally increased. The length of the bar indicates the number of turns open for the flow control valve for Mannequin A. Top bar: Equal compliance, flow control valve for Mannequin A is completely open at 3 turns. Bottom bar: Compliance ratio of 20:90, flow control valve for Mannequin A is open 1.5 turns. Middle bars: Intermediate ratios.
Patient Study
We screened 120 patients, identified 30 eligible patients, and were able to contact 24 legally authorized representatives. Eight legally authorized representatives gave their consent; one subsequently withdrew consent before enrollment. Of the seven potential patients, four were selected based on their hemodynamic stability, as well as physical proximity to one another.
Baseline patient demographics are shown in table 1. In the first study, the patients differed in both ideal body weight, 46 versus 64 kg, and lung compliance, 27 versus 35 ml/cm H2O. Both patients were stable on a moderate degree of hemodynamic support (Patient A: norepinephrine 130 ng · kg-1 · min-1, Patient B: norepinephrine 100 ng · kg-1 · min-1). The results of an ABG drawn at time 0 just before initiation of shared ventilation are shown in table 2.
Table 1.
Patient Characteristics
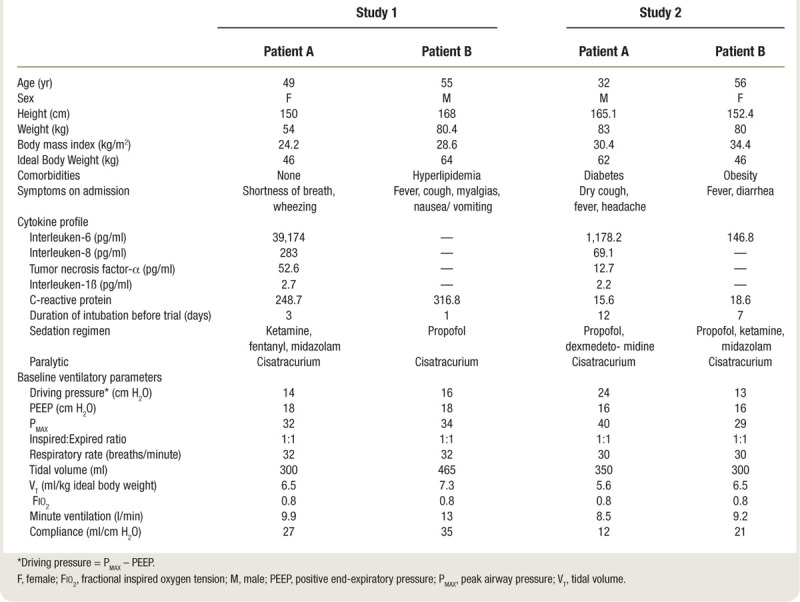
Table 2.
Arterial Blood Gas, Set, and Measured Ventilator Parameters during Human Studies
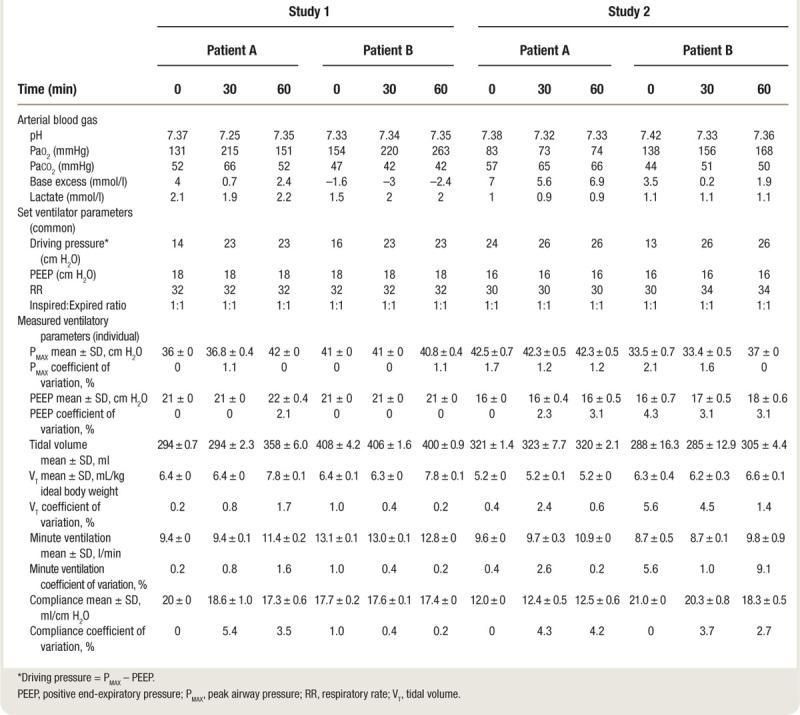
When shared ventilation was initiated, tidal volumes for both patients initially decreased and airway pressures increased (fig. 5, top panel). Spirometry, oxygenation, and acid-base data are presented in table 2. At 30 min, Patient A had developed a respiratory acidosis, so the flow control valve for Patient A was slowly opened until a tidal volume of ~8 ml/kg ideal body weight was achieved (fig. 5). The patients were observed for a further 30 min at the new setting, and by 60 min, the respiratory acidosis of Patient A had been corrected (table 2). The patients were then reconnected to their original ventilators, and the study was terminated.
Fig. 5.
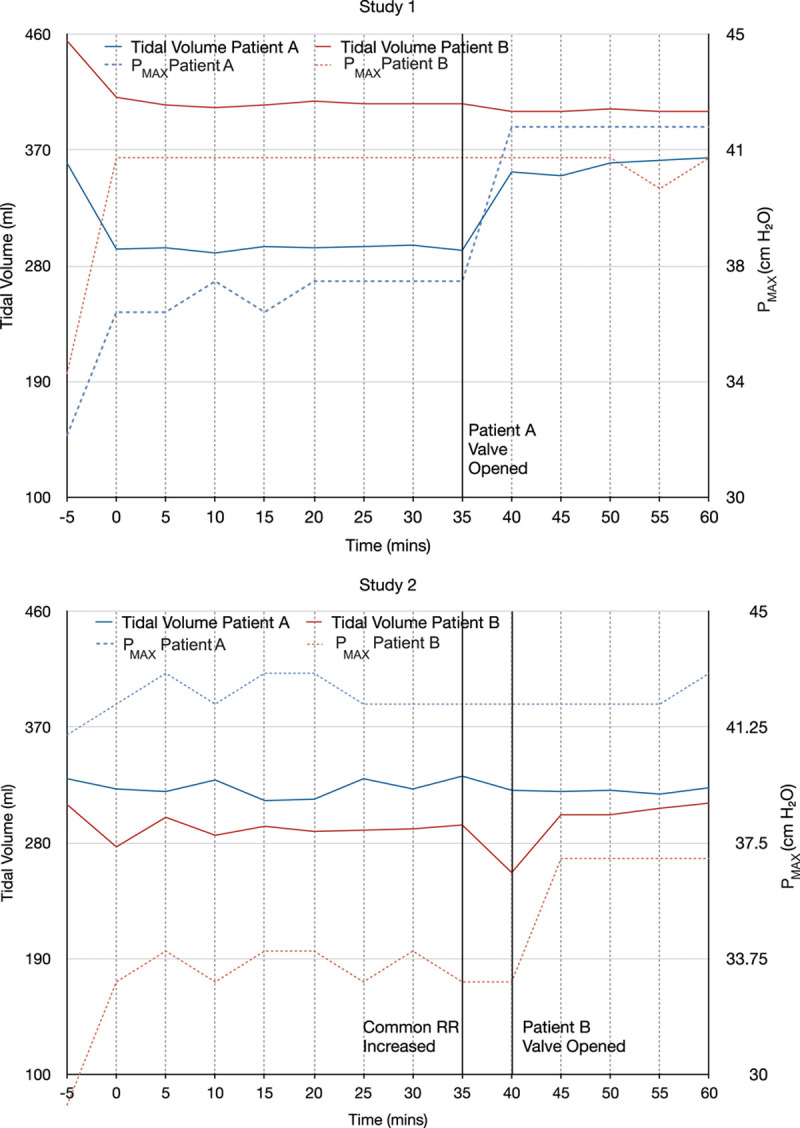
Tidal volumes and peak airway pressures (Pmax) during human studies. Tidal volumes are shown in solid lines, and peak inspiratory pressures are shown in dashed lines. Top panel: Study 1. At the start of the study, tidal volumes fell for both patients, and Pmax rose. Both parameters then stabilized. The black line indicates when the flow control valve for Patient A was further opened by three fourths of a turn. The tidal volume and Pmax for Patient A subsequently rose, while the tidal volume and Pmax for Patient B remained unchanged. Bottom panel: Study 2. Tidal volume fell and Pmax initially rose, as in study 1. At 35 min, the common respiratory rate (RR) was increased, and tidal volumes fell for both patients, but more so for Patient B. The black line at 40 min indicates when the flow control valve for Patient B was opened to allow more flow. Tidal volume for Patient B recovered and the Pmax rose.
In the second study, the patients again had dissimilar ideal body weight, 62 versus 46 kg, and differed greatly in both baseline lung compliance (12 vs. 21 ml/cm H2O) and peak inspiratory pressure (40 vs. 29 ml/cm H2O). Neither patient was receiving hemodynamic support. After 30 min of shared ventilation, both patients were hypercarbic; therefore, at 35 min the (common) respiratory rate was increased from 30 to 34 breaths/min. At 40 min, the flow control valve for Patient B was closed slightly to decrease the delivered tidal volume (table 2 and fig. 5).
As of May 1, 2020, only one of the four patients had been discharged home. Three patients required tracheostomy and continued ventilation; two had been discharged to long-term acute care facilities; and one remained in the intensive care unit.
Stability of Flow Control Valve
The flow control valves exhibited good stability during both studies. In the first study, the coefficient of variation for tidal volume ranged between 0.2 and 5.6%, and for peak airway pressure between 0 and 1.1% (table 2), indicating that the SD was very small relative to the mean. The greatest variation was seen in Patient A during the 30- to 60-min period after her flow control valve was opened to increase the delivered tidal volume. In the second study, the coefficient of variation for tidal volume ranged between 1.4 and 7.7%, and for peak airway pressure between 0 and 2.1% (table 2). In this study, the greatest variation was seen for Patient A during the initial 10-min period after initiating split ventilation. The coefficients of variation for other respiratory parameters are shown in table 2.
Discussion
Using a rapid bench-to-bedside approach, we developed a split breathing circuit for shared ventilation that utilizes custom-manufactured three-dimensional printed flow control valves to deliver differential ventilation to two patients connected to a single ventilator. The system performed as predicted by the simulation study, and no patient suffered adverse sequelae. We were able to successfully ventilate the two pairs of patients for 1 h from a single ventilator, with independent adjustment of tidal volume and peak inspiratory pressure for each patient. Our study demonstrates the feasibility of this approach to shared ventilation in a small series of COVID-19 patients who differed significantly in size and lung compliance. The small coefficients of variation for both tidal volume and peak inspiratory pressure indicate that the flow control valve had very stable performance characteristics.
As the number of patients with COVID-19 who required prolonged ventilation was increasing, the potential unavailability of mechanical ventilators became of great national concern.3 States and institutions have existing protocols for the allocation of mechanical ventilators such that some salvageable patients by default may receive only palliative care. The New York State Ventilator Allocation Guidelines are but one example.15 Availability of ventilation can be increased if ventilator sharing can be safely achieved. The concept of ventilator sharing is not new, and simulated ventilator sharing has been previously described.8,9,16–18 However, the only report describing the use of a control valve in a breathing circuit to achieve differential lung ventilation in clinical practice was in a single patient with a broncho-pleural fistula, in whom each lung was separately ventilated.19
The safety and efficacy of shared ventilation is unknown. A Consensus Statement of six professional organizations, the Society of Critical Care Medicine (Mount Prospect, Illinois), American Association for Respiratory Care (Irving, Texas), American Society of Anesthesiologists (Schaumburg, Illinois), Anesthesia Patient Safety Foundation (Rochester, Minnesota), American Association of Critical-Care Nurses (Aliso Viejo, California), and American College of Chest Physicians (Glenview, Illinois), advised clinicians that sharing mechanical ventilators “should not be attempted because it cannot be done safely with current equipment” (appendix 1).20 We believe that our split circuit shared ventilation system design addresses many of the concerns expressed in the consensus statement (appendix 1). The flow control valves allow the peak inspiratory pressure and tidal volume delivered to each patient to be continuously individualized and titrated to changes in lung compliance. Spirometry sensors placed between the circuit wye and the patient’s ETT enable continuous monitoring of the delivered tidal volume, airway pressure, compliance, and respired gases for each patient individually. These data can be displayed on a wall-mounted or portable monitor. The one-way valves in both the inspiratory and expiratory limbs prevent reverse gas flow in the circuits and mixing of respired gases between patients.
Caveats in the consensus statement that are not addressed by the split circuit shared ventilator design outlined in this report include the requirement for a spare ventilator in case of emergencies requiring removal from split ventilation, the potential need for increased staffing ratios to ensure safe conduct of split ventilation, a requirement for individual spirometry, which may be challenging in a crisis, and the potential for leaks in the new circuit.
Limitations
Our split circuit shared ventilation design has several limitations. First, it does not allow for individualized control of respiratory rate, PEEP, or Fio2. In our limited clinical experience, this did not prove to be a significant issue in critically ill COVID-19 patients in the acute phase of their disease. We set an Inspiratory:Expiratory ratio of 1:1 to deliver the targeted tidal volumes to both patients using relatively low peak inspiratory pressures and decrease the likelihood of auto-PEEP. Second, it requires prolonged sedation and paralysis, although many COVID-19 patients already require paralysis for optimal ventilation. Third, if one patient in the pair decompensates, there may be a significant increase in their ventilatory requirement, requiring emergent separation from the split circuit and connection to an independent means of ventilation. Fourth, even with bacterial/viral filters, it is possible that bacterial super-infection could be spread between patients. The U.S. Public Health Service Commissioned Corps guideline on Optimizing Ventilator Use during the COVID-19 Pandemic, however, includes a statement from the Centers for Disease Control and Prevention stating the following with regard to infection control of co-vented patients: “...with the criteria specified and if done with currently established infection control interventions to reduce healthcare-associated infections, including ventilator associated infections, any additional risk is likely to be small and would likely be appropriate in a crisis standard of care.”21 Fifth, it will not be possible to wean patients from controlled ventilation while they are on the split circuit. This is a known limitation, and we do not expect weaning to be attempted while patients are sharing a ventilator. Sixth, the short duration (60 min) of our studies in human patients did not allow us to evaluate responses to common complications of prolonged mechanical ventilation such as bronchospasm, secretions, occlusion of an ETT, or kinking or obstruction of the breathing circuit tubing. Any of these conditions could potentially prove life-threatening in practice and would require careful monitoring and increased vigilance. We believed that a 1-h period was a suitable balance between demonstrating the stability and adjustability of the system, and limiting the resource usage and exposure of staff. Two attending anesthesiologists spent the full 1 h in the room with both patients, an extraordinary exposure risk despite the use of appropriate personal protection equipment. Seventh, in the simulation study we did not test the possible impact of changes in airway resistance because of the compressed development cycle dictated by the COVID-19 pandemic. We do not believe that changes in airway resistance in one patient should affect peak inspiratory pressure or tidal volume delivered to the other patient because of our use of one-way valves and pressure control ventilation. A decrease in tidal volume caused by an increase in airway resistance in one patient would be detected by the individual spirometric monitoring, and then the patient’s flow control valve would be adjusted to compensate. Finally, our design adds an additional layer of complexity that may be unfamiliar to many clinicians, which increases the potential for use error.
Conclusions
We have demonstrated that a single ventilator and a split circuit system that incorporates custom-designed and -manufactured flow control valves can be used to ventilate two patients of differing size and lung compliance, with individualized tidal volumes and pressures. The clinical performance of this system was predicted using high-fidelity simulation. In human studies, we were able to compensate for respiratory acidosis in one patient by increasing that patient’s minute ventilation without affecting the other patient. All patients remained hemodynamically stable without any negative effect on oxygenation. This proof of concept study demonstrates this technique is feasible as a potential response to a crisis situation where lack of individual ventilators would lead to the death of salvageable patients in respiratory failure. Further evaluation is needed to confirm the efficacy and safety of this technique for longer periods of time.
Acknowledgments
Shams Ranginwala, M.B.A., R.R.T.-N.P.S., C.P.F.T., The Mount Sinai Hospital, New York, New York—respiratory supplies, ventilator setup. Michael McCarry, R.N., M.S., The Mount Sinai Hospital, New York, New York—introduction to manufacturing partner, idea conception. Shweta Golhar, M.D., Institute for Critical Care Medicine, Icahn School of Medicine at Mount Sinai, New York, New York—patient screening. Benjamin Salter, D.O., Department of Anesthesiology, Perioperative and Pain Medicine, Icahn School of Medicine at Mount Sinai, New York, New York—manuscript advice. Hung-Mo Lin, Sc.D., Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, New York—statistical advice. David L. Reich, M.D., Department of Anesthesiology, Perioperative and Pain Medicine, Icahn School of Medicine at Mount Sinai, New York, New York—administrative support and oversight.
Research Support
Support was provided solely from institutional and/or departmental sources.
Competing Interests
Dr. Levin reports having received publication fees from the McMahon Group (New York, New York) and consultant fees from American Society of Anesthesiologists Practice Management 2020 (Schaumburg, Illinois), and has filed a provisional patent for the split ventilation circuit design. Dr. Levin received no fee or equity interest from Stryker Corporation. Dr. Eisenkraft reports having received royalties from Elsevier as co-editor of the textbook Anesthesia Equipment: Principles and Applications, and consultant fees from Shaub Ahmuty Citrin & Spratt L.L.P. for serving as a malpractice defense expert at trial. The other authors declare no competing interests.
Supplementary Material
Appendix 1: Response to Consensus Statement on Multiple Patients per Ventilator
Differential ventilation using one ventilator and a split circuit with flow control valves is feasible and addresses many of the concerns expressed in the consensus statement that recommends against shared ventilation.7
-
Volumes would go to the most compliant lung segments.
Placement of flow control valves in both limbs of the split circuit and measurement of individual patient tidal volumes enables separate control of each tidal volume.
-
PEEP, which is of critical importance in these patients, would be impossible to manage.
This can be addressed by choosing patients requiring similar levels of PEEP. If it is determined that PEEP requirements for the patients have begun to diverge significantly, the patients should be removed from split ventilation and placed back on individual ventilators.
Monitoring patients and measuring pulmonary mechanics would be challenging, if not impossible. Individual patient monitoring is accomplished using separate gas analyzer/spirometry tubing for each patient, connected to separate monitors.
-
Alarm monitoring and management would not be feasible.
Individual spirometry monitoring alarms are available and can be used.
-
Individualized management for clinical improvement or deterioration would be impossible.
With a flow control valve in each inspiratory limb, driving pressure and tidal volumes and to some extent minute ventilation (via changes in tidal volume) can be individualized for each patient. It remains true that major changes in respiratory status requiring significant changes in PEEP or Fio2 for one patient and not the other would require discontinuation of shared ventilation.
In the case of a cardiac arrest, ventilation to all patients would need to be stopped to allow the change to bag ventilation without aerosolizing the virus and exposing healthcare workers. This circumstance also would alter breath delivery dynamics to the other patients. In the case of cardiac arrest, the arresting patient would need to be emergently separated from the ventilator. In this case, the patient’s flow control valve could be closed and the expiratory limb of the circuit clamped just distal to the T-piece at the expiratory limb of the ventilator and the patient removed from the ventilator to allow for bag ventilation. This would carry the same risk of viral aerosolization as removing a patient in cardiac arrest from a single ventilator for bag ventilation or removing any patient from the ventilator for routine care or suctioning. Because we use pressure control ventilation exclusively, clamping and removing one patient should not affect ventilation to the other patient during this procedure.
-
The added circuit volume defeats the operational self-test (the test fails). The clinician would be required to operate the ventilator without a successful test, adding to errors in the measurement.
The ventilator would be tested with a single circuit before sharing. Measurements can be individualized.
Additional external monitoring would be required. The ventilator monitors the average pressures and volumes. In this protocol, we used individual spirometry modules to measure airway pressures, delivered tidal volumes, and inspired and end-tidal gas concentrations. While this is feasible in most first world institutions, we acknowledge that a split ventilation strategy such as ours would most likely be deployed amid significant equipment shortages and stress that it is extremely dangerous to implement without some form of individualized monitoring of delivered pressures and tidal volumes. Several groups have developed low-cost or open source flow and pressure monitoring solutions.
-
Even if all patients connected to a single ventilator have the same clinical features at initiation, they could deteriorate and recover at different rates, and distribution of gas to each patient would be unequal and unmonitored. The sickest patient would get the smallest tidal volume, and the improving patient would get the largest tidal volume. The greatest risks occur with sudden deterioration of a single patient (e.g., pneumothorax, kinked endotracheal tube), with the balance of ventilation distributed to the other patients.
The use of flow control valves, one-way valves, and individual patient monitoring and alarms overcomes these risks.
-
Finally, there are ethical issues. If the ventilator can be lifesaving for a single individual, using it on more than one patient at a time risks life-threatening treatment failure for all of them.
The Institute of Medicine defines crisis standard of care as “a substantial change in the usual health care operations and the level of care it is possible to deliver…justified by specific circumstances and…formally declared by a state government in recognition that crisis operations will be in effect for a sustained period.”5 The Institute of Medicine further states that “CSC [crisis standards of care], planned and implemented in accordance with ethical values, are necessary for the allocation of scarce resources.”6 Use of shared ventilation would be covered by crisis standard of care and as such would be a difficult but ethically defensible decision.
Appendix 2: Mount Sinai HELPS Innovate Group Collaborators
Daniel Katz, M.D., Chang Park, M.D., Cheuk Yin Lai, M.D., Garrett Burnett, M.D., Christopher Gidicsin, M.D., Jonathan Madek, M.D., Suzan Uysal, Ph.D., Roopa Kohli-Seth, M.D., Andrew B. Leibowitz, M.D.
From the Department of Anesthesiology, Perioperative and Pain Medicine (D.K., C.P., C.Y.L., G.B., C.G., J.M., S.U., A.B.L), Institute for Critical Care Medicine (R.K.-S., A.B.L.), and Department of Surgery (R.K.-S., A.B.L.), Icahn School of Medicine at Mount Sinai, New York, New York.
Footnotes
Members of the Mount Sinai Human Emulation Education and Evaluation Lab for Patient Safety and Professional Study (HELPS) Innovate Group are listed in appendix 2.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are available in both the HTML and PDF versions of this article. Links to the digital files are provided in the HTML text of this article on the Journal’s Web site (www.anesthesiology.org).
References
- 1.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A; COVID-19 Lombardy ICU Network: Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020; 323:1574–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Yu Y, Xu J, Shu H, Xia J ’an, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8:475–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranney ML, Griffeth V, Jha AK. Critical supply shortages - The need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020; 382:e41. [DOI] [PubMed] [Google Scholar]
- 4.U.S. PUBLIC HEALTH SERVICE COMMISSIONED CORPS, March 31, 2020. Optimizing Ventilator Use during the COVID-19 Pandemic. Available at: https://www.hhs.gov/sites/default/files/optimizing-ventilator-use-during-covid19-pandemic.pdf. Accessed June 16, 2020.
- 5.Institute of Medicine (US) Committee on Guidance for Establishing Standards of Care for Use in Disaster Situations: Guidance for Establishing Crisis Standards of Care for Use in Disaster Situations: A Letter Report. Edited by Altevogt BM, Stroud C, Hanson SL, Hanfling D, Gostin LO. 2009Washington (DC)National Academies Press (US)doi:10.17226/12749 [PubMed] [Google Scholar]
- 6.Committee on Guidance for Establishing Crisis Standards of Care for Use in Disaster Situations, Institute of Medicine: Crisis Standards of Care: A Systems Framework for Catastrophic Disaster Response. 2014Washington (DC)National Academies Press (US)doi:10.17226/13351 [PubMed] [Google Scholar]
- 7.Amid Ongoing COVID-19 Pandemic, Governor Cuomo Announces State Department of Health Has Approved New Protocol to Allow BiPAP Machines to be Converted Into Ventilators 2020. Available at: https://www.governor.ny.gov/news/amid-ongoing-covid-19-pandemic-governor-cuomo-announces-state-department-health-has-approved. Accessed June 16, 2020.
- 8.Neyman G, Irvin CB. A single ventilator for multiple simulated patients to meet disaster surge. Acad Emerg Med. 2006; 13:1246–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith R, Brown JM. Simultaneous ventilation of two healthy subjects with a single ventilator. Resuscitation. 2009; 80:1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overview - Differential Multiventilation Guide. Available at: https://www.differentialmultivent.org/. Accessed June 16, 2020.
- 11.Vent Multiplexor: Co-ventilation device, home. Available at: https://www.ventmultiplexor.com/. Accessed June 16, 2020.
- 12.Galway VentShare. Available at: https://www.galwayventshare.com/. Accessed June 16, 2020.
- 13.Amato MBP, Marini JJ. Tobin MJ. Pressure-controlled and inverse-ratio ventilation, Principles and Practice of Mechanical Ventilation. 2013, 3rd editionNew York: McGraw-Hill; pp 227–52 [Google Scholar]
- 14.Technical Committee: ISO/TC 121/SC 1 Breathing attachments and anaesthetic machines: ISO 5356-1:2015 Anaesthetic and respiratory equipment — Conical connectors — Part 1: Cones and sockets. Chemin de Blandonnet 8 CP 401 - 1214 Vernier, Geneva, Switzerland, International Organization for Standardization, 2015. Available at: https://www.iso.org/standard/54851.html. Accessed June 16, 2020
- 15.New York State Task Force on Life and the Law New York State Department of Health: VENTILATOR ALLOCATION GUIDELINES 11/2015. Available at: https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf. Accessed June 16, 2020.
- 16.Charan NB, Carvalho CG, Hawk P, Crowley JJ, Carvalho P. Independent lung ventilation with a single ventilator using a variable resistance valve. Chest. 1995; 107:256–60 [DOI] [PubMed] [Google Scholar]
- 17.Branson RD, Blakeman TC, Robinson BR, Johannigman JA. Use of a single ventilator to support 4 patients: Laboratory evaluation of a limited concept. Respir Care. 2012; 57:399–403 [DOI] [PubMed] [Google Scholar]
- 18.Lai BK, Erian JL, Pew SH, Eckmann MS. Emergency open-source three-dimensional printable ventilator circuit splitter and flow regulator during the COVID-19 pandemic. Anesthesiology. 2020; 133:246–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carvalho P, Thompson WH, Riggs R, Carvalho C, Charan NB. Management of bronchopleural fistula with a variable-resistance valve and a single ventilator. Chest. 1997; 111:1452–4 [DOI] [PubMed] [Google Scholar]
- 20.Joint Statement on Multiple Patients Per Ventilator. Available at: https://www.apsf.org/news-updates/joint-statement-on-multiple-patients-per-ventilator/. Accessed June 16, 2020.
- 21.Rutala WA, Weber Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Update: May 2019. Available at: https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf. Accessed June 16, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


