Abstract
Purpose: Fertility preservation before therapy is underutilized for those diagnosed with cancer as an adolescent or young adult (AYA). The purpose of this study was to describe factors impacting utilization of fertility preservation consultations and procedures among AYAs at the University of Iowa Health Care (UIHC).
Methods: Patients were identified by the oncology registry at UIHC. Disease site, histology, date of diagnosis, sex, race, ethnicity, insurance, and zip code data were gathered by the registrars. UIHC's electronic medical record was queried for fertility preservation consultation. The Reproductive Endocrinology and Infertility clinical database captured information about patients who underwent fertility preservation. Rural-urban commuting area codes measured rurality. Descriptive statistics and multivariate linear probability models were used to predict the probability of fertility preservation consultation and procedure.
Results: From 2008 to 2017, 2932 AYAs were treated for an invasive malignancy at UIHC. Of the 440 (15%) who received a fertility preservation consultation, 156 (5%) underwent a fertility preservation procedure. Multivariate analyses showed that AYAs with public insurance coverage, those diagnosed with central nervous system (CNS) disease or melanoma, and those >30 years old at diagnosis had a significant decrease in the percentage point probability of having a consultation. The percentage point probability of undergoing a procedure was decreased for female patients, those with melanoma or carcinoma, those seen by a pediatric-based provider, and those diagnosed after 25 years of age.
Conclusion: This study has important implications for practice and policy, particularly regarding insurance coverage and patient and provider characteristics leading to fertility preservation consultations and procedures for AYAs with cancer.
Keywords: survivorship, oncofertility, fertility preservation, insurance coverage, referral and consultation
Introduction
There are 14 million cancer survivors in the United States; 2.8 million of those are under age 40.1 Cancer survivors face many side effects from treatment; a particularly impactful side effect is potential iatrogenic infertility.2 Both males and females face this possibility, specifically premature ovarian dysfunction and uterine damage for females and testosterone deficiency and impaired spermatogenesis for males.3,4 Even before treatment, pediatric patients display evidence of decreased gonadal function, suggesting that gonadal function is affected by cancer itself and not just the treatment received.5–7
The American Society of Reproductive Medicine (ASRM) and the American Society of Clinical Oncology (ASCO) issued joint clinical practice guidelines in 2006 surrounding patient care in the setting of anticancer treatment; these recommended that patients be counseled as early as possible about their risk of infertility from anticancer treatment and referred to a reproductive specialist.8,9 However, in practice the guideline is inconsistently followed, and if a specialist consultation leads to a desire to preserve fertility, procedures and storage fees are rarely covered by insurance.10
For many newly diagnosed adolescents and young adults (AYAs, ages 15–39) with cancer, fertility preservation may not be possible due to timing of therapy, preservation costs, lack of insurance coverage, and others.2 Treatment advances have minimized the impact of gonadotoxic therapy, such as limiting cumulative doses of alkylating agents and minimizing radiation exposure.2 However, the advances in reproductive technologies (e.g., in vitro fertilization, cryopreservation of oocytes, intrauterine insemination), sperm retrieval procedures (e.g., testicular sperm aspiration, percutaneous sperm aspiration, testicular sperm extraction), and cancer therapy have outpaced access to fertility preservation.2,11 Inconsistent referral to and coverage of fertility preservation before therapy present a unique health policy problem for AYAs with cancer.
This study aims to describe factors impacting utilization of fertility preservation consultations and procedures among AYA cancer patients at the University of Iowa Health Care (UIHC). UIHC holds both the Holden Comprehensive Cancer Center, the only National Cancer Institute (NCI)-designated comprehensive cancer center in the state of Iowa, and the Stead Family Children's Hospital, Iowa's only comprehensive children's hospital.
Methods
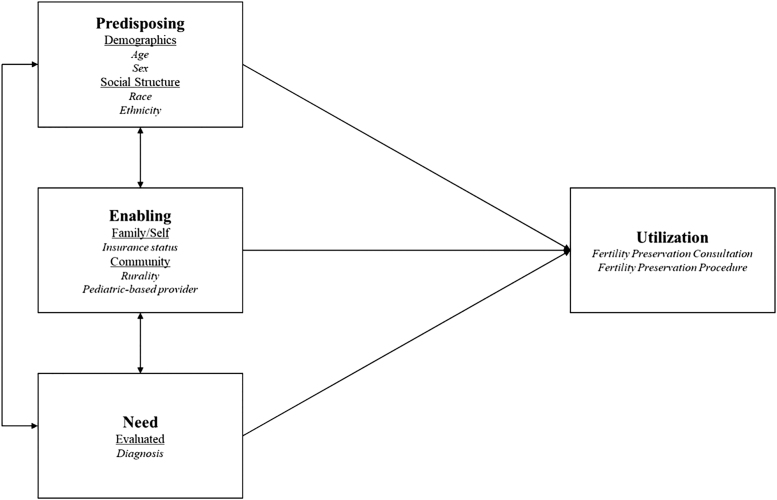
This descriptive study used Andersen's behavioral model as the overarching theory to examine factors affecting fertility preservation consultations and procedures (Fig. 1).12,13 This study was approved by the Institutional Review Board at the University of Iowa (201809719).
FIG. 1.
Conceptual model adapted from Andersen's behavioral model.12,13
Sample and data sources
The sample included all patients aged 15–39 diagnosed and treated at UIHC for any invasive malignancy between 2008 and 2017. Invasive malignancies are those that are required to be reported by cancer registrars, as agreed on by the North American Association of Central Cancer Registries.14 “Treated” was defined as those who received chemotherapy, immunotherapy, radiation therapy, surgery, hormone therapy, or stem cell transplant at UIHC. Data from UIHC's Reproductive Endocrinology and Infertility (REI) Division's clinical database captured detailed information about patients who underwent fertility preservation. Rural-urban commuting area (RUCA) codes were included using patient's zip code at diagnosis to capture rurality.15
Models and analyses
A multivariate linear probability model at the individual level predicted the percentage point change in the probability of fertility preservation consultation and procedure using the following equation:
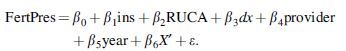
Andersen's predisposing characteristics were examined using demographic and social structure variables, including age at diagnosis, sex, race, and ethnicity; these were captured by X′ in the above equation.12 Patients were grouped by age at diagnosis into 5-year age categories.
According to Andersen, enabling factors encompass individual, family, and community characteristics.12 Enabling factors were insurance, rurality, and treatment by a pediatric-based provider. Insurance (ins) describes the type of patient insurance at diagnosis. Rurality was measured by RUCA code (RUCA) and grouped into the following categories: urban, large rural, small rural, and isolated.15 The patient's primary treating provider (provider) was examined as a binary variable indicating pediatric focus.
UIHC is an academic medical center serving pediatric and adult patients. Typically, patients <19 years old are treated in Pediatrics, and those >18 in the adult cancer center. However, institutional policy allows crossover, and the newly begun AYA Cancer Program works with clinicians in both groups to identify the most appropriate clinical treatment available to an AYA patient at diagnosis, with the ability to be treated by either Pediatric or Medical Oncology. In addition, the Urology and REI departments see both children and adults.
Primary diagnosis (dx) was captured by the International Classification of Diseases for Oncology (ICD-O-3) code.16 Osseous and chondromatous neoplasms were combined with soft tissue sarcomas, consistent with common malignancies seen in AYAs (referred to as “sarcoma”). The variable indicating year (year) captured temporal changes in service availability.
The consultation outcome was derived using the ICD 10 codes for fertility-related encounters (Appendix A1).17 Procedure outcomes came from the UIHC REI database and were validated with corresponding data from the EMR using Current Procedural Terminology codes.18 To be more inclusive of fertility preservation consultations and procedures, those that occurred after the date of diagnosis were set equal to 1.
Subgroup analyses were performed by pediatric-based provider and sex. Reference categories for age and year at diagnosis were set at 15–19 years and 2008, respectively. Sarcoma was used as the diagnosis reference group because these AYA patients typically have more time to begin treatment and a higher need for fertility preservation compared with those with other malignancies. Carcinoma was the highest frequency diagnosis due to the inclusion of many different carcinoma subtypes in the AYA Site Recode.16,19 For all other variables, the group with the highest frequency was made the reference. Robust standard errors were used in all models to address heteroskedasticity. Robustness checks assessed utilization of linear probability modeling instead of logistic regression, and these showed consistent signs and significance levels.
Results
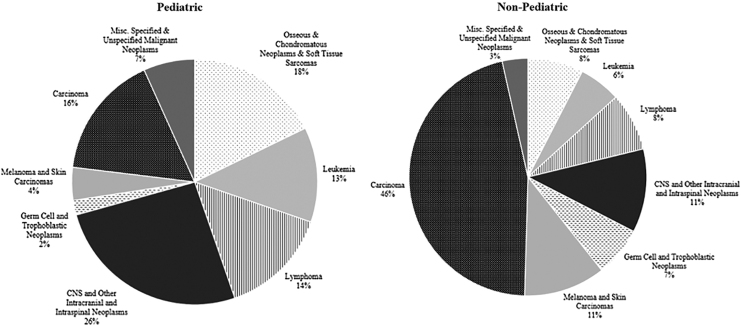
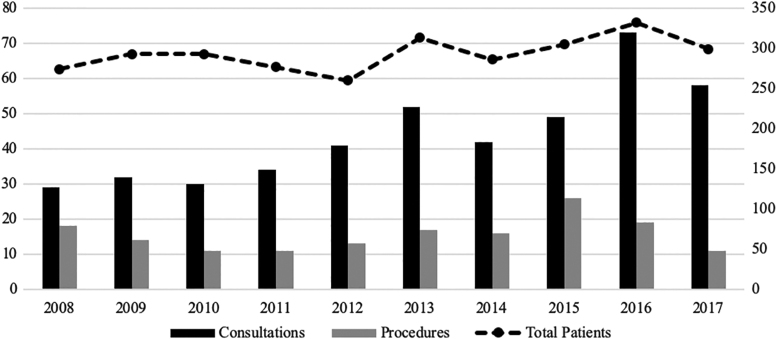
From 2008 to 2017, 2932 AYA patients were treated for an invasive malignancy at UIHC. Of the 440 (15%) who received a fertility preservation consultation, 156 (5%) underwent a preservation procedure (Table 1). The majority of patients were privately insured (51%), diagnosed with carcinoma (44%), treated by a nonpediatric-based provider (93%; Figs. 2 and 3), resided in an urban area (61%), white race (90%), non-Hispanic ethnicity (94%), female (58%), in the oldest age group of 35–39 (34%), and diagnosed in the year 2016 (11%). The distributions were similar across those who received a fertility preservation consultation and underwent a procedure, with the exception of age and year (Table 1).
Table 1.
Characteristics of Sample Overall and by Fertility Preservation Consultation and Procedure Status
| Overall |
Consultation (n = 2932) |
Procedure (n = 440) |
|||||
|---|---|---|---|---|---|---|---|
| No |
Yes |
Significanceb | No |
Yes |
Significanceb | ||
| n = 2932 | n = 2492 (84.9%) | n = 440 (15.0%) | n = 284 (64.5%) | n = 156 (35.5%) | |||
| Insurance, n (%) | |||||||
| Private | 1480 (50.5) | 1219 (48.9) | 261 (59.3) | *** | 176 (62.0) | 85 (54.5) | ** |
| Public | 643 (21.9) | 581 (23.3) | 62 (14.1) | 46 (16.2) | 16 (10.3) | ||
| Insured, NOS | 521 (17.8) | 439 (17.6) | 82 (18.6) | 46 (16.2) | 36 (23.1) | ||
| Uninsured and unknown | 288 (9.8) | 253 (10.2) | 35 (8.0) | 16 (5.6) | 19 (12.2) | ||
| Diagnosis, n (%) | |||||||
| Soft tissue sarcoma and osseous and chondromatous neoplasms | 243 (8.3) | 202 (8.1) | 41 (9.3) | *** | 20 (7.0) | 21 (13.5) | *** |
| Leukemia | 181 (6.2) | 127 (5.1) | 54 (12.3) | 30 (10.6) | 24 (15.4) | ||
| Lymphoma | 246 (8.4) | 177 (7.1) | 69 (15.7) | 33 (11.6) | 36 (23.1) | ||
| CNS and other intracranial and intraspinal neoplasms | 364 (12.4) | 333 (13.4) | 31 (7.0) | 19 (6.7) | 12 (7.7) | ||
| Germ cell and trophoblastic neoplasms | 189 (6.4) | 132 (5.3) | 57 (13.0) | 10 (3.5) | 47 (30.1) | ||
| Melanoma and skin carcinomas | 311 (10.6) | 285 (11.4) | 26 (5.9) | 25 (8.8) | 1 (0.6) | ||
| Carcinomas | 1289 (44.0) | 1137 (45.6) | 152 (34.5) | 140 (49.3) | 12 (7.7) | ||
| Miscellaneous neoplasmsa | 109 (3.7) | 99 (4.0) | 10 (2.3) | 7 (2.5) | 3 (1.9) | ||
| Oncologist, n (%) | |||||||
| Nonpediatric | 2724 (92.9) | 2340 (93.9) | 384 (87.3) | *** | 252 (88.7) | 132 (84.6) | |
| Pediatric | 208 (7.1) | 152 (6.1) | 56 (12.7) | 32 (11.3) | 24 (15.4) | ||
| RUCA, n (%) | |||||||
| Urban | 1785 (60.9) | 1503 (60.3) | 282 (64.1) | 184 (64.8) | 98 (62.8) | ||
| Large rural | 459 (15.7) | 404 (16.2) | 55 (12.5) | 37 (13.0) | 18 (11.5) | ||
| Small rural | 351 (12.0) | 305 (12.2) | 46 (10.5) | 30 (10.6) | 16 (10.3) | ||
| Isolated | 322 (11.0) | 268 (10.8) | 54 (12.3) | 30 (10.6) | 24 (15.4) | ||
| Unknown or international | 15 (0.5) | 12 (0.5) | 3 (0.7) | 3 (1.1) | 0 (0.0) | ||
| Race, n (%) | |||||||
| Non-White | 240 (8.2) | 202 (8.1) | 38 (8.6) | 28 (9.9) | 10 (6.4) | ||
| White | 2642 (90.1) | 2248 (90.2) | 394 (89.5) | 250 (88.0) | 144 (92.3) | ||
| Unknown | 50 (1.7) | 42 (1.7) | 8 (1.8) | 6 (2.1) | 2 (1.3) | ||
| Ethnicity, n (%) | |||||||
| Hispanic | 104 (3.5) | 90 (3.6) | 14 (3.2) | 7 (2.5) | 7 (4.5) | ||
| Non-Hispanic | 2769 (94.4) | 2352 (94.4) | 417 (94.8) | 272 (95.8) | 145 (92.9) | ||
| Unknown | 59 (2.0) | 50 (2.0) | 9 (2.0) | 5 (1.8) | 4 (2.6) | ||
| Sex, n (%) | |||||||
| Female | 1704 (58.1) | 1502 (60.3) | 202 (45.9) | *** | 202 (71.1) | 0 (0.0) | *** |
| Male | 1227 (41.8) | 989 (39.7) | 238 (54.1) | 82 (28.9) | 156 (100.0) | ||
| Age at diagnosis (years), n (%) | |||||||
| 15–19 | 305 (10.4) | 225 (9.0) | 80 (18.2) | *** | 37 (13.0) | 43 (27.6) | *** |
| 20–24 | 365 (12.4) | 291 (11.7) | 74 (16.8) | 36 (12.7) | 38 (24.4) | ||
| 25–29 | 555 (18.9) | 442 (17.7) | 113 (25.7) | 68 (23.9) | 45 (28.8) | ||
| 30–34 | 714 (24.4) | 624 (25.0) | 90 (20.5) | 71 (25.0) | 19 (12.2) | ||
| 35–39 | 993 (33.9) | 910 (36.5) | 83 (18.9) | 72 (25.4) | 11 (7.1) | ||
| Year of diagnosis, n (%) | |||||||
| 2008 | 274 (9.3) | 245 (9.8) | 29 (6.6) | *** | 11 (3.9) | 18 (11.5) | ** |
| 2009 | 293 (10.0) | 261 (10.5) | 32 (7.3) | 18 (6.3) | 14 (9.0) | ||
| 2010 | 293 (10.0) | 263 (10.6) | 30 (6.8) | 19 (6.7) | 11 (7.1) | ||
| 2011 | 277 (9.4) | 243 (9.8) | 34 (7.7) | 23 (8.1) | 11 (7.1) | ||
| 2012 | 260 (8.9) | 219 (8.8) | 41 (9.3) | 28 (9.9) | 13 (8.3) | ||
| 2013 | 313 (10.7) | 261 (10.5) | 52 (11.8) | 35 (12.3) | 17 (10.9) | ||
| 2014 | 286 (9.8) | 244 (9.8) | 42 (9.5) | 26 (9.2) | 16 (10.3) | ||
| 2015 | 305 (10.4) | 256 (10.3) | 49 (11.1) | 23 (8.1) | 26 (16.7) | ||
| 2016 | 332 (11.3) | 259 (10.4) | 73 (16.6) | 54 (19.0) | 19 (12.2) | ||
| 2017 | 299 (10.2) | 241 (9.7) | 58 (13.2) | 47 (16.5) | 11 (7.1) | ||
Miscellaneous neoplasm includes: “Miscellaneous Specified Neoplasms, Not Otherwise Specified” and “Unspecified Malignant Neoplasms”
Significance level corresponds to **p < 0.01, ***p < 0.001.
CNS, central nervous system; NOS, not otherwise specified; RUCA, rural-urban commuting area.
FIG. 2.
Distribution of age at diagnosis and diagnosis category by pediatric-based oncologist.
FIG. 3.
Trend of fertility preservation consultations, procedures, and total patients. Fertility preservation consultations and procedures graphed on the primary vertical axis by year; total patients by year graphed on the secondary vertical axis.
Fertility preservation consultation
Almost 18% of privately insured patients had a fertility preservation consultation, compared with 12% of those with either public or unspecified insurance, and 12% of uninsured patients. The linear probability model showed that after adjustment for all covariates (Table 2), having public insurance coverage or being uninsured decreased the probability of receiving a fertility preservation consultation by 7.1% points and 4% points, respectively, in comparison with those with private insurance. As compared with AYAs diagnosed with sarcomas (17% consultation rate), those diagnosed with leukemia (30% rate), lymphoma (28% rate), or germ cell or trophoblastic neoplasms (“germ cell”; 18% rate) had a combined 11% point increase in the probability of having a fertility preservation consultation after adjusting for all covariates.
Table 2.
Probability of Fertility Preservation Consultation and Procedure for Adolescents and Young Adults Ages 15–39 at Diagnosis from 2008 to 2017
| Consultation (n = 2932) |
Procedure (n = 440)a |
|||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | Significanceb | Coefficient | 95% CI | Significanceb | |
| Insurance | ||||||
| Private | Ref. | Ref. | ||||
| Public | −0.0706 | −0.10 to −0.04 | *** | −0.0354 | −0.15 to 0.08 | |
| Insured, NOS | 0.0319 | −0.01 to 0.07 | 0.0296 | −0.10 to 0.16 | ||
| Uninsured and unknown | −0.0434 | −0.09 to −0.00 | * | 0.0782 | −0.07 to 0.23 | |
| Diagnosis | ||||||
| Osseous and chondromatous neoplasms and soft tissue sarcomas | Ref. | Ref. | ||||
| Leukemia | 0.1392 | 0.06 to 0.22 | ** | −0.0250 | −0.23 to 0.18 | |
| Lymphoma | 0.1128 | 0.04 to 0.18 | ** | 0.0039 | −0.18 to 0.19 | |
| CNS and other intracranial and intraspinal neoplasms | −0.0678 | −0.12 to −0.01 | * | −0.1095 | −0.33 to 0.11 | |
| Germ cell and trophoblastic neoplasms | 0.1232 | 0.04 to 0.20 | ** | 0.3097 | 0.13 to 0.49 | ** |
| Melanoma and skin carcinomas | −0.0654 | −0.12 to −0.01 | * | −0.4076 | −0.58 to −0.24 | *** |
| Carcinomas | −0.0034 | −0.05 to 0.05 | −0.3570 | −0.52 to −0.20 | *** | |
| Misc. specified and unspecified neoplasms | −0.0544 | −0.13 to 0.02 | −0.0936 | −0.41 to 0.23 | ||
| Oncologist | ||||||
| Nonpediatric | Ref. | Ref. | ||||
| Pediatric | 0.0504 | −0.02 to 0.12 | −0.2293 | −0.42 to −0.03 | * | |
| RUCA | ||||||
| Urban | Ref. | Ref. | ||||
| Large rural | −0.0208 | −0.05 to 0.01 | 0.0081 | −0.11 to 0.12 | ||
| Small rural | −0.0182 | −0.06 to 0.02 | 0.0016 | −0.13 to 0.13 | ||
| Isolated | 0.0151 | −0.03 to 0.06 | 0.0463 | −0.08 to 0.17 | ||
| Unknown or international | 0.0263 | −0.18 to 0.23 | −0.4764 | −0.73 to −0.22 | *** | |
| Race | ||||||
| White | Ref. | Ref. | ||||
| Non-White | 0.0139 | −0.04 to 0.06 | 0.0314 | −0.10 to 0.16 | ||
| Unknown | 0.0322 | −0.17 to 0.23 | −0.2216 | −0.49 to 0.04 | ||
| Ethnicity | ||||||
| Non-Hispanic | Ref. | Ref. | ||||
| Hispanic | −0.0438 | −0.11 to 0.02 | 0.0196 | −0.26 to 0.30 | ||
| Unknown | 0.0052 | −0.17 to 0.18 | 0.2178 | −0.04 to 0.48 | ||
| Sex | ||||||
| Female | Ref. | c | ||||
| Male | 0.0425 | 0.02 to 0.07 | ** | |||
| Age at diagnosis (years) | ||||||
| 15–19 | Ref. | Ref. | ||||
| 20–24 | −0.0249 | −0.09 to 0.04 | −0.1008 | −0.27 to 0.07 | ||
| 25–29 | −0.0174 | −0.08 to 0.05 | −0.2312 | −0.39 to −0.07 | ** | |
| 30–34 | −0.0807 | −0.14 to −0.02 | * | −0.2784 | −0.45 to −0.11 | ** |
| 35–39 | −0.1194 | −0.18 to −0.06 | *** | −0.2933 | −0.47 to −0.12 | ** |
| Year of diagnosis | ||||||
| 2008 | Ref. | Ref. | ||||
| 2009 | 0.0138 | −0.04 to 0.06 | −0.2125 | −0.43 to 0.01 | ||
| 2010 | 0.0230 | −0.03 to 0.07 | −0.1137 | −0.33 to 0.11 | ||
| 2011 | 0.0424 | −0.01 to 0.10 | −0.2575 | −0.46 to −0.06 | * | |
| 2012 | 0.0765 | 0.02 to 0.14 | * | −0.2017 | −0.42 to 0.02 | |
| 2013 | 0.0819 | 0.03 to 0.14 | ** | −0.2719 | −0.48 to −0.06 | * |
| 2014 | 0.0751 | 0.02 to 0.13 | * | −0.1353 | −0.36 to 0.09 | |
| 2015 | 0.0789 | 0.02 to 0.14 | ** | −0.0717 | −0.28 to 0.14 | |
| 2016 | 0.1361 | 0.08 to 0.20 | *** | −0.2080 | −0.40 to −0.02 | * |
| 2017 | 0.1241 | 0.06 to 0.18 | *** | −0.2457 | −0.44 to −0.06 | * |
Model predicting fertility preservation procedure only includes those who had a consultation.
Significance level corresponds to *p < 0.05, **p < 0.01, and ***p < 0.001.
No female patients underwent any fertility preservation procedure during the study time period; the variable sex was removed from the analyses.
CI, confidence interval.
Those diagnosed with central nervous system and other intracranial and intraspinal neoplasms (CNS; 30% rate), and those diagnosed with melanoma and skin cancers (“melanoma”; 8% rate), both had a 7% point decrease in the probability of consultation when all other factors were considered. In the model, being male (19% consultation rate) increased the probability of consultation by 4% points in comparison with females (12% rate). Finally, those diagnosed between 30 and 34 (13% rate) or 35 and 39 (8% rate) had an 8% point and 12% point lower probability of consultation compared with younger patients (22% rate).
Fertility preservation procedure
Because all preserving AYAs were male, the variable indicating sex was removed from models predicting preservation procedure. In the fully adjusted linear probability model, AYAs diagnosed with carcinoma or melanoma had a decreased probability of undergoing a preservation procedure when compared with those diagnosed with sarcoma by 41% points and 36% points, respectively; however, those diagnosed with a germ cell tumor had 31% point increase in the probability of procedure when compared with those with sarcoma (Table 2). AYAs treated by a pediatric-based provider had a decreased probability of undergoing a procedure by >22% points in comparison with those treated by nonpediatric-based providers.
Subgroup analyses: Treatment by a pediatric versus nonpediatric oncologist
Subgroup analyses by pediatric-based provider showed that those diagnosed with lymphoma had a 44% point increase in the probability of receiving a fertility preservation consultation compared with those with sarcoma (Table 3). When looking at those treated by a nonpediatric-based oncologist, AYAs with leukemia (by 12% points) and germ cell tumors (by 13% points) had an increase in the probability of undergoing a procedure; AYAs with a CNS neoplasm (by 6% points) or melanoma (by 7% points) had a decreased probability of consultation. Similar to the overall model, AYAs treated by a nonpediatric-based provider and those publicly insured had a 7% point decrease in the probability of consultation compared with those with private coverage. AYAs treated by a nonpediatric-based provider had an ∼10% point decrease in the probability of consultation if they were 35–39 years of age at diagnosis. Across both models, males had a higher probability of having a fertility preservation consultation.
Table 3.
Probability of Fertility Preservation Consultation and Procedure by Treatment by Pediatric and Nonpediatric Oncologist
| Consultation (n = 2932) |
Procedure (n = 440)a |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pediatric oncologist (n = 208) |
Nonpediatric oncologist (n = 2724) |
Pediatric oncologist (n = 47) |
Nonpediatric oncologist (n = 393) |
|||||||||
| Coefficient | 95% CI | Significanceb | Coefficient | 95% CI | Significanceb | Coefficient | 95% CI | Significanceb | Coefficient | 95% CI | Significanceb | |
| Insurance | ||||||||||||
| Private | Ref. | Ref. | Ref. | Ref. | ||||||||
| Public | −0.0765 | −0.22 to 0.07 | −0.0684 | −0.10 to −0.04 | *** | −0.0658 | −0.48 to 0.34 | −0.0126 | −0.13 to 0.10 | |||
| Insured, NOS | 0.0042 | −0.20 to 0.21 | 0.0329 | −0.01 to 0.08 | −0.2145 | −0.97 to 0.54 | 0.0587 | −0.08 to 0.19 | ||||
| Uninsured and unknown | 0.0169 | −0.27 to 0.31 | −0.0407 | −0.08 to 0.00 | 0.3943 | −0.33 to 1.11 | 0.0841 | −0.08 to 0.25 | ||||
| Diagnosis | ||||||||||||
| Osseous and chondromatous neoplasms and soft tissue sarcomas | Ref. | Ref. | Ref. | Ref. | ||||||||
| Leukemia | 0.2199 | −0.03 to 0.47 | 0.1237 | 0.04 to 0.21 | ** | −0.3432 | −0.91 to 0.22 | 0.1517 | −0.18 to 0.48 | |||
| Lymphoma | 0.4377 | 0.22 to 0.66 | *** | 0.0625 | −0.01 to 0.13 | −0.0873 | −0.60 to 0.43 | 0.0593 | −0.27 to 0.38 | |||
| CNS and other intracranial and intraspinal neoplasms | −0.1492 | −0.33 to 0.04 | −0.0626 | −0.12 to −0.01 | * | −0.5360 | −1.23 to 0.15 | 0.0529 | −0.30 to 0.40 | |||
| Germ cell and trophoblastic neoplasms | −0.1649 | −0.67 to 0.34 | 0.1285 | 0.05 to 0.21 | ** | −0.7772 | −1.30 to −0.26 | ** | 0.4335 | 0.12 to 0.75 | ** | |
| Melanoma and skin carcinomas | −0.1323 | −0.41 to 0.15 | −0.0674 | −0.13 to −0.01 | * | −0.4950 | −1.15 to 0.16 | −0.2956 | −0.60 to 0.01 | |||
| Carcinomas | −0.1399 | −0.34 to 0.06 | −0.0075 | −0.06 to 0.04 | 0.0000 | 0.00 to 0.00 | −0.2654 | −0.56 to 0.03 | ||||
| Misc specified and unspecified neoplasms | −0.2555 | −0.43 to −0.08 | ** | −0.0304 | −0.11 to 0.05 | 0.0000 | 0.00 to 0.00 | 0.0000 | 0.00 to 0.00 | |||
| RUCA | ||||||||||||
| Urban | Ref. | Ref. | Ref. | Ref. | ||||||||
| Large rural | −0.1065 | −0.25 to 0.04 | −0.0194 | −0.05 to 0.01 | 0.3161 | −0.35 to 0.99 | −0.0394 | −0.16 to 0.08 | ||||
| Small rural | 0.1269 | −0.06 to 0.31 | −0.0261 | −0.06 to 0.01 | −0.3401 | −0.89 to 0.21 | 0.0390 | −0.10 to 0.18 | ||||
| Isolated | 0.0855 | −0.09 to 0.26 | 0.0055 | −0.04 to 0.05 | −0.0932 | −0.61 to 0.42 | 0.0622 | −0.07 to 0.19 | ||||
| Unknown or international | 0.0373 | −0.16 to 0.23 | −0.4884 | −0.73 to −0.25 | *** | |||||||
| Race | ||||||||||||
| White | Ref. | Ref. | Ref. | Ref. | ||||||||
| Non-White | −0.1469 | −0.33 to 0.04 | 0.0286 | −0.02 to 0.08 | −0.4461 | −1.16 to 0.26 | 0.0580 | −0.07 to 0.19 | ||||
| Unknown | 0.0277 | −0.35 to 0.41 | 0.0226 | −0.19 to 0.24 | −0.1854 | −0.44 to 0.07 | ||||||
| Ethnicity | ||||||||||||
| Non-Hispanic | Ref. | Ref. | Ref. | Ref. | ||||||||
| Hispanic | −0.1432 | −0.37 to 0.08 | −0.0290 | −0.09 to 0.04 | 0.3899 | −0.08 to 0.86 | −0.0794 | −0.40 to 0.24 | ||||
| Unknown | −0.1374 | −0.36 to 0.08 | 0.0298 | −0.16 to 0.22 | 0.1957 | −0.06 to 0.45 | ||||||
| Sex | ||||||||||||
| Female | Ref. | Ref. | c | c | ||||||||
| Male | 0.1320 | 0.01 to 0.26 | * | 0.0289 | 0.00 to 0.06 | * | ||||||
| Age at diagnosis (years) | ||||||||||||
| 15–19 | Ref. | Ref. | Ref. | Ref. | ||||||||
| 20–24 | −0.0679 | −0.20 to 0.07 | 0.0108 | −0.07 to 0.09 | 0.3493 | −0.38 to 1.08 | −0.1499 | −0.33 to 0.03 | ||||
| 25–29 | 0.0416 | −0.13 to 0.21 | 0.0094 | −0.06 to 0.08 | −0.2719 | −0.43 to −0.11 | ** | |||||
| 30–34 | −0.0582 | −0.13 to 0.01 | −0.3118 | −0.48 to −0.14 | *** | |||||||
| 35–39 | −0.1572 | −0.51 to 0.20 | −0.0968 | −0.16 to −0.03 | ** | −0.3187 | −0.49 to −0.15 | *** | ||||
| Year of diagnosis | ||||||||||||
| 2008 | Ref. | Ref. | Ref. | Ref. | ||||||||
| 2009 | 0.0975 | −0.15 to 0.34 | 0.0117 | −0.04 to 0.06 | −0.2126 | −1.16 to 0.73 | −0.2479 | −0.47 to −0.03 | * | |||
| 2010 | 0.0907 | −0.17 to 0.35 | 0.0172 | −0.03 to 0.07 | −0.1622 | −1.10 to 0.78 | −0.1442 | −0.37 to 0.08 | ||||
| 2011 | −0.0043 | −0.28 to 0.27 | 0.0462 | −0.01 to 0.10 | −0.4920 | −1.39 to 0.41 | −0.2299 | −0.43 to −0.03 | * | |||
| 2012 | −0.0194 | −0.26 to 0.22 | 0.0795 | 0.02 to 0.14 | * | −0.2144 | −1.27 to 0.84 | −0.2191 | −0.44 to 0.01 | |||
| 2013 | 0.1491 | −0.16 to 0.46 | 0.0794 | 0.02 to 0.14 | ** | −0.4834 | −1.74 to 0.78 | −0.2727 | −0.49 to −0.06 | * | ||
| 2014 | −0.0451 | −0.28 to 0.19 | 0.0870 | 0.03 to 0.15 | ** | −0.0680 | −1.27 to 1.13 | −0.1810 | −0.41 to 0.04 | |||
| 2015 | 0.1899 | −0.08 to 0.46 | 0.0688 | 0.01 to 0.13 | * | −0.1586 | −1.32 to 1.00 | −0.0944 | −0.30 to 0.11 | |||
| 2016 | 0.1496 | −0.09 to 0.39 | 0.1358 | 0.07 to 0.20 | *** | −0.4408 | −1.46 to 0.58 | −0.2108 | −0.40 to −0.02 | * | ||
| 2017 | 0.0384 | −0.20 to 0.28 | 0.1302 | 0.07 to 0.19 | *** | −0.2218 | −1.43 to 0.98 | −0.2610 | −0.45 to −0.07 | ** | ||
Model predicting fertility preservation procedure only includes those who had a consultation.
Significance level corresponds to *p < 0.05, **p < 0.01, and ***p < 0.001.
No female patients underwent any fertility preservation procedure during the study time period; the variable sex was removed from the analyses.
AYAs treated by a pediatric-based provider and diagnosed with a germ cell tumor had a significantly decreased probability of preservation (by 78% points compared with those with sarcoma); those with germ cell tumors treated by a nonpediatric-based provider had an increase in the probability of preservation (by 43% points compared with those with sarcoma). Those treated by a nonpediatric-based provider and diagnosed over the age of 25 had a decreased probability of going through a preservation procedure compared with their youngest counterparts.
Subgroup analyses: sex
When modeling the probability of consultation among females and males separately (Table 4), AYAs who were publicly insured had a decreased probability of receiving a fertility preservation consultation (females by 6% points, males by 10% points); among males, those with “other” insurance coverage (“insured, NOS”) had about an 8% point increase in the probability of consultation than those with private coverage. Females with lymphoma had about an 11% point increase in the probability of consultation.
Table 4.
Probability of Fertility Preservation Consultation by Sex
| Consultation (n = 2932) |
||||||
|---|---|---|---|---|---|---|
| Female (n = 1704) |
Male (n = 1228) |
|||||
| Coefficient | 95% CI | Significancea | Coefficient | 95% CI | Significancea | |
| Insurance | ||||||
| Private | Ref. | Ref. | ||||
| Public | −0.0581 | −0.10 to −0.02 | ** | −0.1016 | −0.15 to −0.05 | *** |
| Insured, NOS | 0.0046 | −0.05 to 0.06 | 0.0773 | 0.01 to 0.15 | * | |
| Uninsured and unknown | −0.0422 | −0.10 to 0.01 | −0.0521 | −0.12 to 0.01 | ||
| Diagnosis | ||||||
| Osseous and chondromatous neoplasms and soft tissue sarcomas | Ref. | Ref. | ||||
| Leukemia | 0.0789 | −0.03 to 0.19 | 0.2184 | 0.11 to 0.33 | *** | |
| Lymphoma | 0.1089 | 0.01 to 0.21 | * | 0.1430 | 0.05 to 0.24 | ** |
| CNS and other intracranial and intraspinal neoplasms | −0.0514 | −0.12 to 0.02 | −0.0562 | −0.14 to 0.02 | ||
| Germ cell and trophoblastic neoplasms | −0.0785 | −0.20 to 0.04 | 0.1452 | 0.05 to 0.24 | ** | |
| Melanoma and skin carcinomas | −0.0178 | −0.09 to 0.05 | −0.0912 | −0.17 to −0.01 | * | |
| Carcinomas | 0.0238 | −0.04 to 0.09 | −0.0310 | −0.10 to 0.04 | ||
| Misc. specified and unspecified malignant neoplasms | 0.0086 | −0.10 to 0.12 | −0.1013 | −0.19 to −0.01 | * | |
| Oncologist | ||||||
| Nonpediatric | Ref. | Ref. | ||||
| Pediatric | 0.0324 | −0.04 to 0.11 | 0.0462 | −0.09 to 0.18 | ||
| RUCA | ||||||
| Urban | Ref. | Ref. | ||||
| Large rural | −0.0075 | −0.05 to 0.03 | −0.0354 | −0.09 to 0.02 | ||
| Small rural | −0.0152 | −0.06 to 0.03 | −0.0223 | −0.09 to 0.04 | ||
| Isolated | −0.0129 | −0.06 to 0.04 | 0.0673 | −0.01 to 0.14 | ||
| Unknown or international | 0.1389 | −0.16 to 0.43 | −0.0634 | −0.36 to 0.23 | ||
| Race | ||||||
| White | Ref. | Ref. | ||||
| Non-White | 0.0502 | −0.02 to 0.12 | −0.0133 | −0.09 to 0.06 | ||
| Unknown | 0.1422 | −0.11 to 0.39 | −0.1295 | −0.38 to 0.12 | ||
| Ethnicity | ||||||
| Non-Hispanic | Ref. | Ref. | ||||
| Hispanic | −0.0525 | −0.12 to 0.02 | −0.0365 | −0.14 to 0.06 | ||
| Unknown | −0.0970 | −0.32 to 0.12 | 0.1890 | −0.04 to 0.42 | ||
| Age at diagnosis (years) | ||||||
| 15–19 | Ref. | Ref. | ||||
| 20–24 | 0.0008 | −0.07 to 0.07 | −0.0775 | −0.20 to 0.05 | ||
| 25–29 | 0.0160 | −0.05 to 0.08 | −0.0752 | −0.20 to 0.05 | ||
| 30–34 | −0.0045 | −0.07 to 0.06 | −0.2083 | −0.33 to −0.09 | ** | |
| 35–39 | −0.0430 | −0.10 to 0.02 | −0.2417 | −0.36 to −0.13 | *** | |
| Year of diagnosis | ||||||
| 2008 | Ref. | Ref. | ||||
| 2009 | 0.0128 | −0.04 to 0.06 | 0.0267 | −0.07 to 0.12 | ||
| 2010 | 0.0519 | −0.01 to 0.11 | −0.0134 | −0.10 to 0.07 | ||
| 2011 | 0.0606 | −0.01 to 0.13 | 0.0276 | −0.06 to 0.12 | ||
| 2012 | 0.0839 | 0.01 to 0.16 | * | 0.0792 | −0.02 to 0.17 | |
| 2013 | 0.0834 | 0.01 to 0.15 | * | 0.0858 | −0.01 to 0.18 | |
| 2014 | 0.0475 | −0.02 to 0.11 | 0.1190 | 0.02 to 0.22 | * | |
| 2015 | 0.0356 | −0.03 to 0.10 | 0.1570 | 0.06 to 0.26 | ** | |
| 2016 | 0.1695 | 0.09 to 0.24 | *** | 0.0783 | −0.02 to 0.17 | |
| 2017 | 0.1598 | 0.08 to 0.23 | *** | 0.0685 | −0.03 to 0.16 | |
Significance level corresponds to *p < 0.05, **p < 0.01, and ***p < 0.001.
Males diagnosed with leukemia, lymphoma, or germ cell tumor had an increased probability of consultation by 22% points, 14% points, and 15% points, respectively, when compared with males diagnosed with sarcoma. Males with melanoma had a 9% point decrease in the probability of consultation, and those with miscellaneous neoplasm had a 10% point decrease in the probability of having a consultation. In addition, males diagnosed between 30 and 39 had a significant decrease in the probability of consultation compared with their youngest counterparts (by ∼20% points). No significant differences were seen by RUCA code across the multivariate models.
Discussion
Multivariate analyses showed that AYAs with public insurance coverage, those diagnosed with CNS disease or melanoma, and those >30 years of age at diagnosis had a significant decrease in the probability receiving a fertility preservation consultation. For those who received a consultation, AYAs with melanoma or carcinoma, those seen by a pediatric-based provider, those diagnosed after 25 years of age, and females experienced a decrease in the probability of preserving.
Overall differences in fertility preservation services are seen across teenage and young adult patients. This is not surprising, given the developmental changes that occur between these two life phases, as well as the evolution from dependence on family for information, support, and decision making, to more independent care decisions. However, if we are to provide high-quality, comprehensive cancer care to all AYAs, we must acknowledge and mitigate the role these factors play in affecting fertility preservation consultations and procedures throughout the ages that this population encompasses.
Insurance coverage
While AYAs with public insurance were shown to have a lower probability of consultation in comparison with their counterparts with private insurance, there was no effect of insurance coverage on the probability of undergoing a procedure. Although insurance was not a significant factor across all models, the findings are aligned with other studies, which show that inadequate insurance coverage influences fertility preservation.20–22
At UIHC before 2017, female patients' insurance coverage for fertility preservation procedures and storage was assessed before consultation, which likely resulted in many patients declining consultation. Currently, while fertility preservation coverage is not required in the state of Iowa, the initial consultation is often covered by insurance. After the creation of the AYA program in 2016, an initial fertility preservation consultation was provided for all AYAs enrolled in the AYA program. The overall percentage of AYA patients with documented consultations and preservation did not increase with the implementation of the program. However, only five cancer types were included in the initial program, and not every AYA patient was enrolled by their provider.
As the AYA program has grown, all cancer types are now eligible for enrollment in the AYA program, and education to cancer providers about the importance of fertility preservation continues. In addition, the institutional policy has been changed, so that only after this consultation is insurance coverage assessed to determine whether subsequent fertility preservation visits, testing, and procedures will be covered. This is a vital consideration when reviewing the results of the insurance subgroup analyses. The UIHC AYA and REI programs assist patients in seeking philanthropic funds to pay for preservation fees not covered by insurance, as recommended by Besharati et al.23 Importantly, a recent study showed that when fertility preservation consultations are a standard part of the AYA program, the type of insurance coverage may no longer be a factor driving decisions to undergo fertility preservation procedures, as uninsured patients made decisions to undergo fertility preservation procedures in a manner similar to their insured counterparts.24
Pediatric-based oncology physician
Before and during cancer treatment, fertility issues are inadequately addressed by physicians.25 This study uniquely included patients with diverse cancer types treated by both pediatric and adult providers at one institution, allowing for closer examination of differences across provider types. The multivariate results show that treatment by a pediatric-based physician did not change the probability of having a consultation; however, it did decrease the probability of undergoing a fertility preservation procedure by 22%.
There are several possible explanations for this. Pediatricians and younger AYA patients and their families may be less comfortable discussing fertility issues than young adults. Many younger AYA patients and their families may not be considering and prioritizing future fertility at the time of cancer diagnosis. However, for those who did seek consultation, AYAs treated by a pediatric-based physician, diagnosed with lymphoma, and of male sex were more likely to receive consultation. These findings are consistent with other studies, indicating that male AYAs are more likely to be referred to fertility specialists and undergo a procedure than their female counterparts.26–28
Diagnosis
There were a wide range of diagnoses across the sample. Our data did not include staging and treatment data, so information regarding gonadotoxicity cannot be analyzed. In light of this limitation, we used diagnosis category to capture some of that variation; however, it is difficult to interpret the results given the heterogeneity in stage and prognosis that was likely present across the sample. For example, those diagnosed with a CNS neoplasm may have a worse prognosis, which may influence referral to a fertility specialist. However, in an article published by Stone et al. examining fertility preservation for those diagnosed with primary brain tumors, patients indicated that they would like the option to see a fertility specialist.29
AYAs diagnosed with advanced melanoma have historically had poor outcomes before the advent of immunotherapy. This may have discouraged oncologists from consulting with fertility specialists in the past. With the advent of modern immunotherapy and precision medicine, referral patterns may change dramatically and should be further explored. The need for additional study, particularly for those diagnosed with melanoma, is being supported by the National Comprehensive Cancer Network.30
Males and females
Surprisingly, no female AYA patients underwent fertility preservation procedures during this time period. Preservation for females takes longer, and is more invasive and expensive than male preservation. Providers or female patients may feel that treatment delays to allow for preservation are not medically advisable. Both patients and providers may more closely consider the probability of infertility based on planned cancer treatment, given the complexity of preservation. Financial factors are also important, as insurance coverage for fertility preservation is not mandated in Iowa and is highly variable across insurers. Therefore, many female AYA patients are unable to afford the out-of-pocket costs. After implementation of the UIHC AYA Cancer Program, a dedicated AYA nurse coordinator now works with AYA patients and fertility staff to investigate external resources to help cover costs. Also, initiatives have begun in many states, including Iowa, to enact legislation that would mandate coverage for these services.
Limitations
This study is limited by its descriptive nature, the lack of depth in understanding patient-specific information, and the absence of the patient perspective. Although the type of insurance coverage at diagnosis was known, we do not know whether the specific plan covered fertility preservation consultations or procedures. This likely has significant influence on patient choices. Second, we do not know if the urgency to begin treatment, either patient- or provider driven, influenced referral. Third, this study was not structured to examine various types of therapies that patients received and does not take into account the range of gonadotoxic treatments that might influence provider or patient views on the importance of fertility preservation. Fourth, because this is a single-institution study, generalizability to a wider population is a concern.
Because of the nature of oncology practice in Iowa (e.g., high referral rates of young people with cancer to UIHC), and the homogenous sampling from a single state, arguably, the data are representative of the AYA population with cancer in Iowa. Fifth, patient-specific factors were not directly examined, such as sexual maturity level, patient wishes, or family dynamics and opinions, which could play an important role in fertility preservation decisions, particularly when comparing adolescents with young adults. Finally, we do not know the outcomes of preservation treatment or lack of treatment on assisted or unassisted fertility, or the reproductive history of the patient (e.g., whether the patient had children before their cancer diagnosis).
Conclusion
For AYAs treated at UIHC from 2008 to 2017, insurance coverage appears to influence the probability of receiving a fertility preservation consultation. Insurers typically cover the costs of managing iatrogenic side effects from treatment (e.g., nausea, pain, fatigue, neutropenia); however, despite clinical practice guidelines and recommendations by ASCO and ASRM, fertility preservation is typically not a covered service. From the perspective of the insurer, infertility cannot be clearly defined unless reproductive organs are removed and, therefore, fertility preservation coverage can be a difficult issue for payers.31 As of May 2019, six states had passed legislation to ensure coverage of fertility preservation for those with cancer and 11 states had active legislation.32
The patient's primary diagnosis, sex, age, year of diagnosis, and treatment by a pediatric-based physician were influential in predicting the probability of having a fertility preservation consultation and undergoing a procedure. In an effort to effectively and efficiently influence providers regarding the importance of fertility preservation in maintaining quality of life for patients, continuing medical education or maintenance of certification requirements could be used, and sharing of best practices and discussion of new and innovative ways to reach patients could be disseminated.
From the patient perspective, increased education and outreach from advocacy groups and other nonprofit organizations can increase visibility in the cancer community to facilitate patients asking about fertility preservation before, during, and after cancer treatment. From the practice perspective, this study illuminates the importance of having a care team member dedicated to helping patients navigate the very burdensome cancer process (e.g., nurse coordinator affiliated with an AYA cancer program).
This study has important practice and policy implications particularly regarding insurance coverage, rural differences, and patient and provider characteristics leading to fertility preservation consultations and procedures for AYAs with cancer. Ultimately, policies surrounding payment and reimbursement are driving these issues. Variation in costs for males versus females, lack of consistent and effective insurance coverage, the absence of a truly integrated health system, and other issues inherent in U.S. health care result in an inaccurate understanding of the future implications for family building when patients face a cancer diagnosis and treatment.
Acknowledgments
We express our sincere gratitude to the doctoral dissertation committee for Erin Mobley who included Keith J. Mueller, PhD (chair and advisor), Kanika Arora, PhD, Dan Shane, PhD, William W. Terry, MD, MPH, and Marcia M. Ward, PhD at the University of Iowa College of Public Health, Department of Health Management and Policy, for their time, expertise, and ongoing support, particularly concerning methodological ideas, study design questions, and guidance about practice and policy implications. We thank the UIHC Oncology Registry, particularly Tania Viet, and the UIHC Institute for Clinical and Translational Science, especially Ashlee Wilson and Michael Wright.
Appendix A1. Codes from the International Classification of Diseases Selected to Indicate Fertility Preservation Consultation
XXI. Factors influencing health status and contact with health services:
-
(1)
Encounter for procreative investigation and testing (Z31.4)
-
(2)
Encounter for procreative genetic counseling (Z31.5)
-
(3)
Encounter for general counseling and advice on procreation (Z31.6)
-
(4)
Encounter for procreative management and counseling for gestational carrier (Z31.7)
-
(5)
Encounter for other procreative management (Z31.8)
-
(6)
Encounter for other procreative management before cancer treatment (Z31.98)
-
(7)
Documentation of fertility issues—at risk of infertility (Z91.89)
XIV. Diseases of the genitourinary system:
-
(1)
Fertility problems—male (N46.9)
-
(2)
Fertility problems—female (N97.9)
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work received funding from the University of Iowa Dance Marathon.
References
- 1. National Cancer Institute. Report of the adolescent and young adult oncology progress review group. Bethesda, MD: National Cancer Institute, National Institutes of Health; 2006 [Google Scholar]
- 2. Hudson MM. Reproductive outcomes for survivors of childhood cancer. Obstet Gynecol. 2010;116(5):1171–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skinner R, Mulder RL, Kremer LC, et al. Recommendations for gonadotoxicity surveillance in male childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Lancet Oncol. 2017;18(2):e75–90 [DOI] [PubMed] [Google Scholar]
- 4. van Dorp W, Mulder R, Kremer L, et al. Recommendations for premature ovarian insufficiency surveillance for female survivors of childhood, adolescent, and young adult cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. J Clin Oncol. 2016;34(28):3440–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Kooi A, van den Heuvel-Eibrink F, van den Berg S, et al. Changes in anti-Müllerian hormone and inhibin B in children treated for cancer. J Adolesc Young Adult Oncol. 2019;8(3); DOI: 10.1089/jayao.2018.0130 [DOI] [PubMed] [Google Scholar]
- 6. de Vries ACH, Wigny KMGJ, Pluijm SMF, et al. Gonadal function in boys with newly diagnosed cancer before the start of treatment. Hum Reprod. 2016;31(11):2613–8 [DOI] [PubMed] [Google Scholar]
- 7. Laven JSE, van Dorp W, de Vries ACH, et al. Decreased serum anti-Müllerian hormone levels in girls with newly diagnosed cancer. Hum Reprod. 2013;29(2):337–42 [DOI] [PubMed] [Google Scholar]
- 8. American Society of Clinical Oncology. ASCO recommendations on fertility preservation in cancer patients: guideline summary. J Oncol Pract. 2006;2(3):143–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(19):1994–2001 [DOI] [PubMed] [Google Scholar]
- 10. Campo-Engelstein L. Consistency in insurance coverage for iatrogenic conditions resulting from cancer treatment including fertility preservation. J Clin Oncol. 2010;28(8):1284–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Letourneau JM, Smith JF, Ebbel EE, et al. Racial, socioeconomic, and demographic disparities in access to fertility preservation in young women diagnosed with cancer. Cancer. 2012;118(18):4579–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andersen R, Newman JF. Societal and individual determinants of medical care utilization in the United States. Milbank Q. 1973;51(1):95–124 [PubMed] [Google Scholar]
- 13. Andersen R, Newman JF. Societal and individual determinants of medical care utilization in the United States. Milbank Q. 2005;83(4):1–28 [PubMed] [Google Scholar]
- 14. North American Association of Central Cancer Registries. Standards for cancer registries: volume II—data standards and data dictionary. http://datadictionary.naaccr.org/?c=10 (accessed May25, 2019)
- 15. Larson E, Skillman S.. Rural urban commuting area code. Seattle, WA: WWAMI Rural Health Research Center, University of Washington; 2005 [Google Scholar]
- 16. World Health Organization. International classification of diseases for oncology (ICD-O-3), 3rd edition, 1st revision. Geneva: World Health Organization; 2013 [Google Scholar]
- 17. World Health Organization. International classification of diseases and related health problems—clinical modifications, tenth revision (ICD-10-CM). Geneva: World Health Organization; 2012 [Google Scholar]
- 18. American Medical Association. Current procedural terminology (CPT). Chicago, IL: American Medical Association; 2019 [Google Scholar]
- 19. National Cancer Institute. AYA site recode/World Health Organization 2008 definition. Accessed January3, 2019 from: https://seer.cancer.gov/ayarecode/aya-who2008.html
- 20. Kim J, Deal AM, Balthazar U, Kondapalli LA, Gracia C, Mersereau JE. Fertility preservation consultation for women with cancer: are we helping patients make high-quality decisions? Reprod BioMed Online. 2013;27(1):96–103 [DOI] [PubMed] [Google Scholar]
- 21. Schon SB, Shapiro M, Gracia C, Senapati S. Medical and elective fertility preservation: impact of removal of the experimental label from oocyte cryopreservation. J Assist Reprod Genet. 2017;34:1207–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quinn G, Vadaparampil S, McGowan Lowery K, et al. State laws and regulations addressing third-party reimbursement for infertility treatment: implications for cancer survivors. J Fertil Steril. 2011;95(1):72–8 [DOI] [PubMed] [Google Scholar]
- 23. Besharati M, Woodruff T, Victorson D. Young adults' access to fertility preservation services at National Cancer Institute Community Oncology Research Program Minority/Underserved Community Sites: a qualitative study. J Adolesc Young Adult Oncol. 2016;5(2):187–200 [DOI] [PubMed] [Google Scholar]
- 24. Flink DM, Sheeder J, Kondapalli LA. Do patient characteristics decide if young adult cancer patients undergo fertility preservation? J Adolesc Young Adult Oncol. 2017;6(2):223–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yee S, Abrol K, McDonald M, et al. Addressing oncofertility needs: views of female cancer patients in fertility preservation. J Psychosoc Oncol. 2012;30(3):331–46 [DOI] [PubMed] [Google Scholar]
- 26. Köhler TS, Kondapalli LA, Shah A, et al. Results from the survey for preservation of adolescent reproduction (SPARE) study: gender disparity in delivery of fertility preservation message to adolescents with cancer. J Assist Reprod Genet. 2011;28(3):269–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Armuand GM, Rodriguez-Wallberg KA, Wettergren L, et al. Sex differences in fertility-related information received by young adult cancer survivors. J Clin Oncol. 2012;30(17):2147–53 [DOI] [PubMed] [Google Scholar]
- 28. Bann CM, Treiman K, Squiers L, et al. Cancer survivors' use of fertility preservation. J Adolesc Young Adult Oncol. 2015;24(12):1030–7 [DOI] [PubMed] [Google Scholar]
- 29. Stone JB, Kelvin JF, DeAngelis LM. Fertility preservation in primary brain tumor patients. Neuro Oncol Pract. 2016;4(1):40–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Comprehensive Cancer Network. NCCN announces new guidelines for adolescent and young adult (AYA) oncology. 2012. https://www.nccn.org/about/news/newsinfo.aspx?NewsID=310 (accessed December30, 2016)
- 31. Raposa B. Maria's law: extending insurance coverage for fertility preservation to cancer patients in Massachusetts. University of Massachusetts Law Review 2014;9(2):334–359 [Google Scholar]
- 32. Alliance for Fertility Preservation. State legislation. Alliance for fertility preservation. Accessed May25, 2019 from: www.allianceforfertilitypreservation.org/advocacy/state-legislation