Abstract
Adipose tissue is a central regulator of metabolism and an important pharmacological target to treat the metabolic consequences of obesity, such as insulin resistance and dyslipidemia. Among the various cellular compartments, the adipocyte cell surface is especially appealing as a drug target as it contains various proteins that when activated or inhibited promote adipocyte health, change its endocrine function and eventually maintain or restore whole-body insulin sensitivity. In addition, cell surface proteins are readily accessible by various drug classes. However, targeting individual cell surface proteins in adipocytes has been difficult due to important functions of these proteins outside adipose tissue, raising various safety concerns. Thus, one of the biggest challenges is the lack of adipose selective surface proteins and/or targeting reagents. Here, we discuss several receptor families with an important function in adipogenesis and mature adipocytes to highlight the complexity at the cell surface and illustrate the problems with identifying adipose selective proteins. We then discuss that, while no unique adipocyte surface protein might exist, how splicing, posttranslational modifications as well as protein/protein interactions can create enormous diversity at the cell surface that vastly expands the space of potentially unique epitopes and how these selective epitopes can be identified and targeted.
Keywords: adipogenesis, brown adipose tissue, insulin resistance, obesity, surface markers, white adipose tissue
Introduction
Adipose tissue is the primary organ storing and releasing energy at times of energy surplus or demand and an important endocrine organ [1]. Adipose tissue can be divided into white (WAT) and brown adipose tissue (BAT). Both depots are morphologically and functionally different. WAT is organized in discrete depots largely categorized as subcutaneous or visceral adipose tissue depots [2]. On the other hand, BAT dissipates energy as heat through the action of the inner-mitochondrial uncoupling protein 1 (UCP-1). In addition, the third type of adipocytes termed beige or brite adipocytes can appear in white adipose depots under β-adrenergic stimulation or chronic cold exposure [3–5]. These cells express similar genes as brown adipocytes (e.g. UCP-1, CIDEA, PAT2 and PGC1α). However, beige adipocytes are thought to derive from different progenitor cells [2].
Importantly, too little and too much of adipose tissue, especially white adipose tissue, can cause severe metabolic complications, where lipodystrophy, referring to the lack of adipose tissue, causes similar metabolic impairments as obesity [6]. While hereditary and acquired lipodystrophy are relatively rare, the rates of obesity are steadily increasing worldwide [7]. In 2016, more than 1.9 billion adults were overweight and more than 650 million obese, with obesity defined by a body mass index (BMI) above 30 [8]. However, a greater health risk than the excessive body weight per se are the diseases associated with obesity, such as type 2 diabetes, cardiovascular disease, and certain types of cancer [9]. Interestingly, genes and pathways associated with the regulation of body weight associate with the central nervous system rather than adipose tissue [10–12]. In contrast, the development of insulin resistance, eventually resulting in the metabolic syndrome, is largely driven by changes in adipose tissue, as illustrated by the elegant work of Drs. C. Ronald Kahn and Philipp Scherer and many others, which showed that maintaining adipose endocrine and lipid storing function prevents the progression form obesity towards insulin resistance and the metabolic syndrome [13–18].
Thus, pharmacological approaches to maintain adipose function could dissociate body weight gain from the development of the metabolic syndrome and activation of brown and beige adipocytes could be utilized to reduce body weight gain and resolve metabolic abnormalities [19]. A steadily increasing amount of literature has identified potential therapeutic targets in adipose tissues, in various cellular compartments. However, for most, pharmacological utilization is limited by essential functions of these proteins in tissues outside adipose, raising safety concerns due to undesired side effects. To overcome this bottle neck, a crucial step is to identify adipose tissue-specific epitopes, allowing tissue-selective drug delivery. Cell surface proteins integrate all extracellular inputs to co-ordinate a cellular response and are ideally located at the outside of the cell, allowing easy access by drugs. Thus, targeting the cell surface does not only provide a unique opportunity to deliver cargo to adipocytes, but is an attractive target for pharmacotherapy itself. To date, more than 1200 cell surface proteins have been described. However, albeit we and others have tried extensively, no proteins were identified that are exclusively expressed in either brown or white adipocytes [20].
In the first part of this review, we will highlight some important and well-described cell surface proteins and their role in adipocyte differentiation and mature adipocytes, to underscore the significance and pharmacological potential of the cell surface. We do not discuss the advantages or disadvantages of targeting white versus brown or beige adipocytes in detail, as there are several recent reviews highlighting the functional differences and pharmacological benefits of either of those adipocyte types [3,21–23]. In the second part, we will discuss techniques that can be utilized to identify novel adipose selective cell surface epitopes distinguishing between distinct adipocyte subtypes and different progenitor populations.
Important cell surface regulators of (pre-)adipocyte function
Adipose tissue hypertrophy, in response to excessive caloric intake, can exceed the maximal lipid storing capacity of individual adipocytes, leading to adipocyte cell death and the development of local and systemic inflammation and insulin resistance [13]. However, hyperplasia, the de novo generation of adipocytes from precursors to store excessive calories, is not associated with these pathological changes. Thus, to maintain healthy adipose tissue in the context of obesity, one appealing approach is to promote the differentiation of preadipocytes into mature adipocytes, distributing lipid storage into more adipocytes thereby preventing lipid-induced cell death. Initially, Rodeheffer et al. identified and Berry et al. characterized a subpopulation of early adipocyte progenitors defined as Lineage (CD45, CD31 and/or not Ter119)−CD29+CD34+Sca-1 (Ly6A)+CD24+ in white adipose tissue of mice [24,25]. Since then, many studies found distinct adipocyte progenitor cells (APCs) with various cell surface proteins in white and BAT [26–32] (Table 1). Furthermore, preadipocytes with different functions were identified using cell surface proteins [28,34,35] and single-cell RNA sequencing (scRNAseq) [36–38,41–43]. For example, Ly6C−CD9−PDGFRβ+ cells were shown to be highly adipogenic [35], whereas CD142+ cells (Aregs) were shown to be anti-adipogenic APCs in human and mouse [37]. CD55 and CD34 were also identified as markers for APCs [36,37] and DPP4+ cells were demonstrated to give rise to both ICAM1+ and CD142+ preadipocytes [38]. Moving forward, methods such as cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) approaches that integrate cell surface markers with transcriptomics will allow even more detailed insights into the heterogeneity of adipocyte progenitor populations [44]. Together, these data suggest that selective enhancement of individual (pre-)adipocyte subpopulations could provide an interesting approach to maintain adipose function.
Table 1. Specific cell surface marker sets for different cell types in adipose tissue.
| Cell types | Markers | Fat depots | |
|---|---|---|---|
| White adipocytes | ASC-1 | S/VAT, S/VAT* | [20] |
| Prohibitin/annexin 2 complex | S/VAT | [33] | |
| Brown adipocytes | P2RX5 | BAT, CS/LC* | [20] |
| PAT2 | BAT, CS/LC* | [20] | |
| Adipocyte progenitor cells | Lin(−):Ter119(−)CD29(+):CD34(+):Sca-1(+):CD24(+) | S/VAT | [24] |
| iBAT | [26] | ||
| PDGFRα(+):Lin(−):CD29(+):CD34(+):Sca-1(+):CD24(+) | SAT | [25] | |
| PDGFRα(+):CD34(+):Sca-1(+):CD24(−):PDGFRβ(−) | VAT | [27] | |
| Lin(−):CD34(+):CD38(+) | S/VAT, iBAT | [29] | |
| Zfp423(+):CD45(−):CD29(+)Sca-1(+):CD24(−) | SAT | [30] | |
| Zfp423(+):CD45(−):CD29(+)Sca-1(−):CD24(+/−) | VAT | ||
| PDGFRα(+):PDGFRβ(−) | SAT | [31] | |
| PDGFRα(+):PDGFRβ(−), PDGFRα(−):PDGFRβ(−) | VAT | ||
| Lin(−):Gp38(+):PDGFRα(+):CD9(low) | VAT | [34] | |
| Lin(−):CD34(+):CD44(+):PDGFRα(+):CD9(low) | VAT* | ||
| Lin(−):Ly6c(−):CD9(−):PDGFRβ(+) | VAT | [35] | |
| Lin(−):Ter119(−):Sca-1(+):CD55(high) | VAT | [36] | |
| Lin(−):Ter119(−):Sca-1(+):CD29(+):CD34(+):CD142(−) | S/VAT, SAT* | [37] | |
| Lin(−):CD26/DPP4(+):CD142(−) | S/VAT, SAT* | [38] | |
| Lin(−):CD34(+):CD44(+):ΔDecorin(+) | WAT | [39] | |
| Preadipocytes | PDGFRα(+):Lin(−):CD29(+):CD34(+):Sca-1(+):CD24(−) | SAT | [25] |
| PDGFRα(+):PDGFRβ(−), PDGFRα(+):PDGFRβ(+) | SAT | [31] | |
| PDGFRα(+):PDGFRβ(−), PDGFRα(−):PDGFRβ(+) | VAT | ||
| Lin(−):PDGFRβ (+):Zfp423(+) | S/VAT | [32] | |
| Lin(−):Ter119(−):Sca-1(+):CD55(low) | VAT | [36] | |
| Lin(−):CD54/ICAM1(+):CD142(−) | S/VAT, iBAT | [38] | |
| Endothelial cells | Prohibitin | S/VAT, WAT* | [40] |
SAT, subcutaneous white adipose tissue; VAT, visceral white adipose tissue; iBAT, interscapular brown adipose tissue; CS, carotid sheath; LC, longus colli; Lin, CD45 and CD31; *, Human samples; WAT, white adipose tissue.
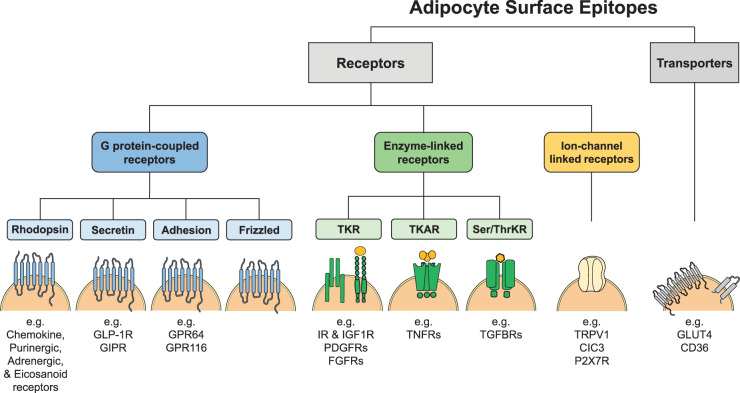
The surfome is composed of a very complex mixture of transmembrane, membrane-anchored and extracellular proteins that mediate cell–matrix interactions, signal transduction and shuttling of metabolites and ions, among other functions. Receptors can be divided into three main categories (Figure 1). The largest category of receptors are G protein-coupled receptors (GPCRs) [45], followed by enzyme-linked receptors [46] and ion-channel linked receptors [47]. However, it would not be possible to discuss all types of cell surface proteins and their function in adipose tissue. Therefore, we highlight here the role of some important transmembrane receptor families to underscore the complexity of signal integration and transduction as well as the heterogeneity of inputs received by the cells (Figure 2).
Figure 1. Receptor families expressed on adipocytes.
TKR, tyrosine kinase receptor; TKAR, tyrosine kinase-associated receptor; Ser/ThrKR, serine/threonine kinase receptor; GLP-1, Glucagen-like peptide 1; GIPR, Glucose-dependent insulinotropic polypeptide receptor; GPR, G protein-coupled receptor; IR, insulin receptor; IGF1R, insulin-like growth factor 1 receptor; PDGFRs, platelet-derived growth factor receptors; FGFRs, fibroblast growth factor receptors; TNFR, tumor necrosis factor receptor; TGFBR, transforming growth factor beta receptor; TRPV1, transient receptor potential vanilloid type 1 channel; CIC3, chloride channel 3; P2X7R, ionotropic purinergic receptor 7; GLUT4, glucose transporter 4.
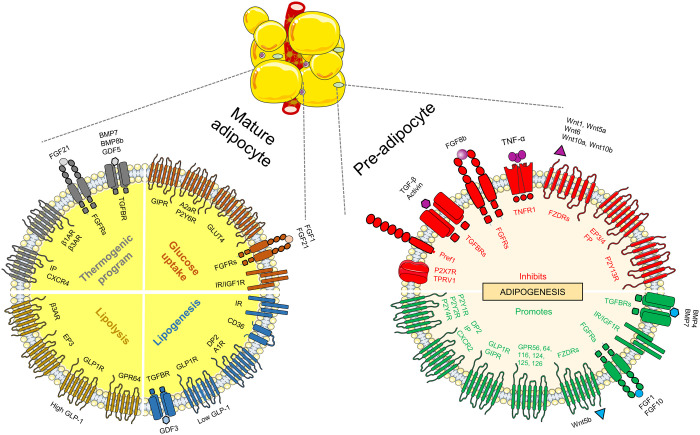
Figure 2. Receptors regulating pre- and mature adipocytes function.
Right side: receptors involved in preadipocyte differentiation. Left side: receptors promoting glucose uptake, thermogenesis, lipolysis and lipogenesis in mature adipocytes. IR, insulin receptor; IGF1R, insulin-like growth factor receptor; βAR, beta adrenergic receptor; AR, adenosine receptor; TGFBR, transforming growth factor beta receptor; P2YR, metabotropic purinergic receptor; P2XR, ionotropic purinergic receptor; FZDR, frizzled receptor; TNFR1, tumor necrosis factor alpha receptor 1; GLP1R, glucagon-like peptide-1 receptor; GIPR, glucose-dependent insulinotropic peptide receptor; CXCR2, CXC chemokine receptor 2; TPRV1, transient receptor potential vanilloid type-1; Pref1, preadipocyte factor 1; EP, prostaglandin E2 receptor; FP, prostaglandin F receptor; IP, prostaglandin I2 receptor; DP2, prostaglandin D2 receptor 2; GLUT4, glucose transporter type 4; BMP, bone morphogenetic protein; GDF, growth differentiation factor; TNF-α, tumor necrosis factor alpha; TGF-β, transforming growth factor beta; GLP-1, Glucagon-like peptide-1.
G protein-coupled receptors
GPCR are the most abundant receptor super family in the human genome with more than 800 genes coding for GPCRs. GPCRs are divided into five groups namely rhodopsin, secretin, adhesion, glutamate and frizzled/taste2 based on their structure and sequence homology [45]. More than one-third of all drugs target GPCRs [48,49], highlighting their pivotal role in cellular function. Thus, it is not surprising that they also play an important role in adipose tissue regulating various, and sometimes even opposing, effects in fat.
GPCRs consist of an extracellular N-terminus and three extracellular loops followed by seven transmembrane α helices. Intracellularly there are three loops, a short amphipathic α helix and the C-terminus [50]. A diverse set of ligands, from ions to nucleotides and proteins, can bind to GPCRs. Upon ligand binding, receptor conformational changes occur and the activated receptor interacts and activates heterotrimeric G proteins. Activated G protein subunits (Gα and Gβγ) transduce then the signal [50]. However, G protein independent pathways also exist, multiplying signaling complexity [51,52]. Here, we discuss examples of GPRCs playing important roles in adipose tissue.
Rhodopsin GPCRs
The biggest group of GPCRs are rhodopsin GPCRs [53]. We will discuss several receptor families to highlight the heterogeneity of these adipocyte cell surface receptors and their prominent role in adipose tissue.
Adenosine receptors
Adenosine and purinergic receptors fulfill various functions in the human body from the cardiovascular system to the central nervous system [54]. Within the adipose tissue, adenosine is released from adipocytes [55,56] and can bind to four different GPCRs (A1R, A2aR, A2bR and A3R). A1R and A3R are coupled to Gi/o proteins. Thus, their activation inhibits cyclic adenosine monophosphate (cAMP) production and decreases protein kinase A (PKA) activation while A2aR and A2bR are coupled to Gs proteins and their activation stimulates cAMP production and increases PKA activation. Additionally, some adenosine receptors can activate MAP kinases, PLC and Ca2+ signaling [57]. Earlier studies demonstrated that A1R is expressed in mature ob1771 and rat adipocytes while no expression was observed in undifferentiated ob1771 and rat preadipocytes. On the other hand, A2 receptors are expressed in preadipocytes and their expression decreases with differentiation [58,59]. A similar trend was seen with A2 receptors in 7F2 preosteoblasts, which can differentiate into adipocytes [60]. However, unlike murine white adipocytes, murine brown adipocytes show high A2aR expression, which was also reported for human adipocytes [61]. Interestingly, hamster brown adipocytes show similar levels of A1R and A2aR with no detectable expression of A2bR [61], indicating differences in adenosine receptor expression between different species.
With regards to adipogenesis, activation of the A2R with NECA (adenosine receptor agonist) in rat white preadipocytes increased differentiation in corticosterone treated ob1771 preadipocytes [58,59]. However, subsequent studies reported contradictory results, as activation of A2bR in human preadipocytes and murine stromal vascular fraction (SVF) inhibited adipogenesis. Moreover, knockdown of A2bR in mouse preadipocytes increased differentiation. This inhibition of differentiation by A2bR activation was associated with sustained krüppel like factor 4 (KLP4) expression as the ability of A2bR to inhibit differentiation is lost upon knockdown of KLP4 [62]. Furthermore, the transfection of 7F2 preosteoblasts with A1R promoted adipogenesis while transfection with A2bR decreased adipogenesis and increased osteogenesis [60]. The different effects of A2bR on differentiation in these studies could be explained by the different cell lines used and by the fact that NECA is a non-selective adenosine receptor agonist. Interestingly, no effect on brown preadipocyte differentiation was observed using brown preadipocytes from A2aR knockout mice [61]. A direct role of A3R in adipogenesis has not been reported so far. However, A3R knockout mice show less abdominal and total body fat [63].
Adenosine was shown to inhibit lipolysis in rat adipocytes [64]. A1R was later demonstrated to be required to inhibit lipolysis, as the administration of an adenosine analog to wild type mice reduced free fatty acid (FFA) and glycerol levels, which was blunted in A1R knockout mice. Furthermore, increased lipolysis was observed upon depletion of adenosine, using adenosine deaminase, in mouse adipocytes but not in adipocytes from A1R knockout mice [65]. Additionally, antagonizing the A1R receptor promoted lipolysis in rat adipocytes [66] further confirming the need for a functional A1R to inhibit lipolysis. On the other hand, mice overexpressing A1R exhibit reduced FFA. Moreover, these mice showed improved insulin sensitivity upon high-fat diet (HFD) feeding in comparison with controls [67]. Another well-characterized adenosine receptor is the A2b receptor. Chronic activation of A2bR for 4 weeks upon HFD feeding improved glucose tolerance and insulin sensitivity. This improvement in insulin sensitivity was accompanied by reduced adipose tissue inflammation, which is attributed to increased M2 macrophage activation [68,69]. In line with this, the deletion of the A2bR in HFD fed mice further impairs glucose tolerance and insulin sensitivity [69]. Interestingly, activation of A2aR improved glucose homeostasis and reduced adipose tissue inflammation in mice [70]. Moreover, agonizing A2aR protected mice from diet-induced obesity (DIO) and induced beiging of WAT [61].
Purinergic receptors
Purinergic receptors are divided into two types. The ionotropic ligand-gated cation channels (P2XR), which are mainly activated by adenosine tri-phosphate (ATP) and the metabotropic GPCRs (P2YR) which are activated by endogenous nucleotides. Seven P2XRs have been identified (P2X1–7) and eight P2YRs (P2YR1, 2, 3, 4, 6, 11, 12, 13 and 14). The P2YR1, 2, 4 and 6 are coupled to Gq proteins and activate PLC-β, increasing cytosolic Ca2+ levels. P2Y11R is coupled to Gs proteins and can, therefore, stimulate cAMP production while P2YR12, 13 and 14 are bound to Gi proteins and hence inhibit cAMP production [71,72]. All purinergic receptors are expressed in human adipose tissue-derived mesenchymal stem cells (MSCs) and mature adipocytes derived from these MSCs except P2X1R and P2X2R [73]. Another report examined human subcutaneous adipocytes and showed that they express all purinergic receptors except P2X1R, P2X2R, P2X3R and P2Y14R [74], while we could not detect the significant expression of P2X5R in white adipocytes [20].
ATP was shown to potentiate the differentiation of bone marrow-derived human MSCs into adipocytes. This effect is dependent on P2YRs as inhibition of P2YRs with pertussis toxin negated ATP effects on differentiation. Furthermore, P2Y1R and P2Y4R activation increased adipogenesis [75]. Another study showed that uridine triphosphate (UTP), which activates P2Y2R and P2Y4R, as well as uridine diphosphate (UDP), activating P2Y6R, promote adipogenesis and suppresses osteogenesis in rat bone marrow-derived MSCs. This effect was mediated by P2Y2R and not P2Y4R or P2Y6R as silencing P2Y4R (via siRNA) and antagonizing P2Y6R did not affect differentiation. Moreover, this pro-adipogenic effect of P2Y2R was mediated through ERK1/2 signaling [76]. Conversely, P2Y13R inhibits adipogenesis and MSCs from P2Y13R knockout mice showed increased adipogenesis [77].
Unlike adenosine receptors, purinergic receptors are not thoroughly characterized in mature adipocytes. Activation of P2Y6R increased glucose uptake via elevated glucose transporter (GLUT) 4 translocation in primary and 3T3-L1 adipocytes [78]. Moreover, purinergic receptors are implicated in the inflammatory response of adipocytes [79]. For a more detailed outlook on adenosine and purinergic receptors’ role in the adipose tissue, please see [80].
Eicosanoid receptors
Prostaglandin receptors are the best-described members of this receptor class in adipose tissue [81]. There are nine types of prostaglandin (PG) receptors: The PGD receptors (DP1–2), the PGE receptors (EP1–4), the PGF receptor (FP), the PGI receptor (IP) and the TXA receptor (TP) [82]. Several types of prostaglandin receptors are expressed in preadipocytes [83] and were shown to regulate adipogenesis. However, depending on the PG receptor activated, adipogenesis can be either promoted or inhibited. For example, activation of EP4 and EP3 receptors inhibit adipogenesis in both 3T3-L1 preadipocytes and mouse embryonic fibroblasts (MEFs) [84–86]. Similarly, activation of the FP receptor suppresses differentiation in 3T3-L1 preadipocytes [87], primary rat inguinal adipocyte precursor cells [88], human orbital preadipocytes [89] and mouse MSCs [90]. On the other hand, IP and DP receptor actions promote adipogenesis, which was demonstrated in various cell models, including human and mouse adipose precursor cells [91]. This pro-adipogenic effect is mediated via an increase in C/EBPβ and C/EBPδ [92,93]. Moreover, activation of DP2 enhances differentiation and lipid accumulation in 3T3-L1 and MEFs, where increased lipid accumulation is due to a suppression of lipolysis [94].
Prostaglandin receptors also regulate mature adipocyte function. The deletion of the EP3 receptor in mice impaired insulin sensitivity and promoted adipose tissue inflammation. Moreover, EP3 knockout mice were obese with high levels of serum triglycerides and insulin. Interestingly, EP3 receptor is down-regulated in genetically and diet-induced obese mice, [86]. In line with this, antagonizing EP3 receptors in a human adipocyte cell line (SGBS) decreased pro-inflammatory gene expression, while activation of the EP3 receptor (via PGE2) promoted inflammatory gene expression [95]. Conversely, agonizing EP4 receptors in db/db mice improved insulin sensitivity and glucose tolerance concomitant with a decreased inflammatory profile in adipose tissue. This phenotype was mainly due to a switch from pro-inflammatory M1 to anti-inflammatory M2 macrophages in adipose tissue [96]. Activation of DP2 receptors in 3T3-L1 adipocytes inhibited lipolysis through inhibition of the cAMP/PKA pathway and promoted triglyceride accumulation [94]. This is in line with in vivo data, as mice overexpressing PGD2 (a ligand for the DP2 receptor) exhibited increased body weight gain under HFD in comparison with controls. Moreover, these mice showed decreased serum triglycerides and improved insulin sensitivity [97]. Lastly, activation of the IP receptor, in human multipotent adipose-derived stem cells, promoted the transition from white to beige adipocytes [98]. All in all, these results demonstrate that prostaglandin receptors can modulate adipose tissue function in various ways.
Adrenergic receptors
β-adrenergic receptors are among the best-described receptors in adipose tissue, regulating cold- and diet-induced thermogenesis in BAT [4]. There are three β-adrenergic receptors (β1AR, β2AR and β3AR) in mice and humans [99,100]. All three types are expressed in white and brown adipocytes with β3AR showing the highest expression of all β-adrenergic receptors in adipose of mice [101]. Activation of β-adrenergic receptors occurs via the release of catecholamines from the sympathetic nerve terminals. This leads to the induction of lipolysis and BAT thermogenesis [102]. The contribution of each receptor was dissected using knockout and overexpression studies.
β1AR plays a crucial role in both cold- and diet-induced thermogenesis. This was demonstrated using β1AR knockout mice. These mice were hypothermic when cold challenged and gained significantly more weight under HFD, in comparison with controls, indicating a deficit in cold- and diet-induced thermogenesis. Moreover, β1AR knockout mice developed insulin resistance [103]. Furthermore, overexpression of the β1AR, under the control of the aP2 promoter, partially protected mice from DIO [104]. Deletion of the β2AR did not impair cold- or diet-induced thermogenesis, but glucose homeostasis [105]. Activation of β3AR in brown adipocytes promoted lipolysis and increased oxygen consumption [106], and even when mice were housed at thermoneutrality, reduced fat mass and improved glucose tolerance upon HFD feeding [107]. Counterintuitively, β3AR knockout mice are cold tolerant with only a modest increase in adiposity [108], which is exacerbated under HFD [109]. This could be explained by increased β1AR and UCP-1 expression in BAT in comparison with control mice. Moreover, UCP1 expression can be induced by activation of β3AR or β1AR (but not β2AR) in human brown adipocytes derived from multipotent adipose-derived stem cells. Thus, β1AR can substitute for the action of β3AR in β3AR knockout mice [110].
Beige adipocytes are therapeutically interesting to reduce body fat and β3AR agonist treatment-induced beiging of certain WAT depots [111]. Moreover, β3AR knockout mice showed an inability to recruit beige adipocytes in WAT [112,113]. However, this was shown to be dependent on the genetic background, as β3AR knockout mice on a FVB/N background normally developed beige adipocytes upon cold exposure, while β3AR knockout mice on a C57BL/6 and 129Sv background did not [114]. Additional data showed that β1ARs are required for cold-induced beiging [115]. All in all, β-adrenergic receptors have a prominent role in adipose tissue and are interesting therapeutical targets for combating obesity. However, the great limitation for the use in humans is the important role of adrenergic receptors in the human heart raising strong safety concerns regarding side effects upon β-adrenergic receptor activation in humans [116]. Nevertheless, adipose restricted β3AR activation would be a promising therapeutic approach to reduce body weight and restore glucose and lipid homeostasis.
In addition to β-adrenergic receptors, two α-adrenergic receptors have been identified. α2-adrenergic receptor (α2AR) exhibits anti-lipolytic effects and inhibits cAMP production, thus, antagonizing the effects of β-adrenergic receptors [117–119]. An increase in α2AR and the ratio between α2AR/βAR was found in adipocytes from obese humans [120–126]. Moreover, in animal models, the α2AR/βAR ratio is correlated with obesity and an increase in α2AR is associated with adipose hypertrophy [120,121,123–128]. Overexpression of α2AR in the adipose tissue of mice lacking β3AR, which resembles the situation in humans where there is low β3AR and high α2AR expression, showed that these mice are more susceptible to HFD induced weight gain. Surprisingly, these mice exhibited normal glucose and insulin levels and the increase in fat mass was due to adipose tissue hyperplasia rather than hypertrophy [129]. Conversely, the α1-adrenergic receptor regulates lactate production and glycogenolysis and is not linked to lipolysis [118].
Chemokine receptors
Chemotactic cytokines or chemokines compose a family of secreted proteins that were classically thought to direct the migration of leucocytes. However, it is now clear that chemokines regulate the physiology of most cell types. Chemokine actions are mediated via binding to chemokine receptors, which are divided into typical chemokine receptors (GPCRs) and atypical chemokine receptors. To date, there are 18 typical chemokine GPCRs described [130] and here we will discuss selected examples that regulate adipocytes/adipose tissue function.
CXCR2 plays a role in neutrophil recruitment, as well as in adipocytes. The knockdown of CXCR2 inhibits adipogenesis in immortalized preadipocytes [131]. In line with this, CXCR2 knockout mice show smaller and fewer adipocytes in different fat depots, possibly due to a reduction in adipogenesis. Interestingly, this phenotype is only seen in female, and not male, mice [132]. Moreover, CXCR2 knockout mice are protected from HFD induced insulin resistance [133].
Another well-studied member of the chemokine GPCRs in adipose tissue is CXCR4. CXCR4 is expressed on adipocytes [134] and targeted deletion of CXCR4 in fat depots of mice using the aP2-Cre promoter (AdCXCR4KO mice) resulted in elevated body weight gain upon HFD feeding in comparison with controls, due to increased fat mass. Moreover, AdCXCR4KO mice are cold intolerant with reduced BAT activity upon HFD feeding, due to decreased mitochondrial biogenesis and expression of oxidative phosphorylation genes in BAT of mice fed a HFD and housed at room temperature (25°C) and upon acute cold exposure. However, AdCXCR4KO mice are not glucose intolerant or insulin-resistant despite increased adiposity and cold intolerance. This phenotype is only observed when CXCR4 is deleted in adipocytes and not in myeloid leucocytes (macrophages) [135]. Moreover, the administration of a CXCR4 antagonist in mice led to decreased M1 macrophage recruitment to WAT of obese mice, resulting in reduced inflammation and improved insulin sensitivity in WAT (and other tissues) and improved systemic glucose tolerance [136]. In line with this, antibody-mediated blocking of CXCL12 (a ligand for CXCR4) led to improved adipose tissue and whole-body insulin sensitivity [137].
Thus, these two examples demonstrate the intricate role of chemokine receptors in adipose tissue and that more studies are needed to further elucidate their potential as targets for combating obesity and its co-morbidities.
Secretin GPCRs
Secretin GPCRs include various receptors that are pharmacological targets for the treatment of cardiovascular disease, psychiatric disorders and diabetes [138]. Some of these receptors play an important role in maintaining adipose tissue function. Two good examples are glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors.
GLP-1 receptor
GLP-1 is produced from enteroendocrine L cells in the small intestine. It is produced after nutrient ingestion and has important effects on different organs [139] including adipose tissue. GLP-1 is well known for its anti-diabetic effect in healthy and diabetic individuals [140]. The actions of GLP-1 are mediated via the GLP-1 receptor which is expressed in several tissues like the pancreas, central nervous system and adipose tissue. Isolated plasma membranes from rat adipose tissue [141], 3T3-L1 adipocytes [142,143] as well as human adipose tissue [144] have been shown to express GLP-1 receptor. Interestingly, the adipose tissue GLP-1 receptor isoform seems to differ from the pancreatic isoform [145,146]. Early studies demonstrated that GLP-1 can have either lipolytic [145] or lipogenic effects [147] in isolated rat adipocytes and rat adipose tissue explants, respectively. Later it was demonstrated that low doses of GLP-1 have a lipogenic effect, while high doses have a lipolytic effect in isolated human adipocytes [146]. Another prominent role of GLP-1 is to enhance insulin-stimulated glucose uptake, which was demonstrated in 3T3-L1 [143] and isolated rat adipocytes [148]. In isolated rat adipocytes, GLP-1 stimulated glucose uptake on its own and had an additive effect together with insulin [149]. Moreover, GLP-1 treatment increased GLUT1 and GLUT4 protein levels in 3T3-L1 adipocytes [150] and increased the phosphorylation of the insulin receptor substrate 1 (IRS-1) and other signaling molecules downstream of the insulin receptor (IR) [151]. This increased insulin sensitivity is seen in ob/ob mice due to the alleviation of endoplasmic reticulum stress [152]. Furthermore, GLP-1 receptor activation promotes preadipocyte proliferation and differentiation by inhibiting apoptosis [153].
In vivo, administration of GLP-1 receptor agonist (exenatide) increased lipolysis in Wistar rats [154]. Additionally, the adenoviral administration of GLP-1 reduced adipose tissue inflammation in ob/ob mice [155]. In humans, GLP-1 receptor expression was increased in visceral fat of obese individuals with insulin resistance [144]. Moreover, exenatide administration ameliorated adipose tissue insulin resistance [156] and decreased inflammation in visceral fat [157]. However, the exact physiological role of the GLP-1 receptor in adipocytes remains to be elucidated.
GIP receptor
Another prominent member of the secretin receptor family is GIP. The GIP receptor is expressed on rat and 3T3-L1 adipocytes [158]. GIP, like GLP-1, is an incretin that stimulates insulin secretion from pancreatic β-cells upon meal ingestion. GIP increased glucose uptake and fatty acid synthesis in isolated rat adipocytes [159]. Moreover, GIP stimulated lipoprotein lipase activity in explants of rat adipose tissue [160]. Mechanistically, this is based on the activation of AKT and inhibition of AMP-dependent protein kinase (AMPK) and LKB1 phosphorylation in the presence of insulin, as seen in human adipocytes [161]. Like insulin, GIP promotes AKT phosphorylation leading to GLUT4 translocation in 3T3-L1 adipocytes. Additionally, knockdown of GIP receptor in 3T3-L1 preadipocytes inhibited adipogenesis [162]. Interestingly, global GIP receptor knockout mice are protected from DIO and insulin resistance [163]. Surprisingly, the deletion of the GIP receptor in adipose tissue via the aP2-Cre promoter (GIPRadipo−/−) did not result in differences in body fat upon HFD feeding. However, GIPRadipo−/− mice showed improved insulin sensitivity and a reduction in lean mass, as well as reduced interleukin (IL)-6 expression in adipose tissue and decreased hepatosteatosis upon HFD feeding [164].
Adhesion GPCRs
The human genome encodes more than 30 adhesion GPCRs. Adhesion GPCRs are characterized by long N-termini containing adhesion domains (e.g. epidermal growth factor-like repeats) capable of mediating cell–cell and cell–matrix interactions [165]. Adhesion GPCRs play different roles in adipocytes/adipose tissue physiology. Most adhesion GPCRs are expressed in human and mouse adipose tissues [166]. Knockdown of GPR56, GPR64, GPR116, GPR124, GPR125 and GPR126 decreased adipogenesis as seen by decreased lipid accumulation. Additionally, GPR64 activation decreased adiponectin secretion and glucose uptake and increased lipolysis in 3T3-L1 adipocytes [166]. Knockdown of GPR116 also inhibited the differentiation of 3T3-L1 preadipocytes and adipose tissue-specific deletion of GRP116 resulted in decreased epididymal adipocyte size. Furthermore, plasma adiponectin levels were decreased and resistin levels increased, suggesting impaired adipocyte function. Additionally, these mice were glucose intolerant upon chow diet and HFD feeding and insulin-resistant upon HFD feeding [167].
Frizzled GPCRs
Frizzled receptors
Frizzled receptors are crucial for cell proliferation and differentiation as well as regulation of cell polarity [168]. The 10 mammalian frizzled (FZD) receptors are seven transmembrane receptors, with best-known function in inhibiting adipogenesis. FZD receptors mainly act as receptors for the 19 Wnt proteins. The initiation of the signaling cascade begins when Wnts bind to two receptors. The first interaction is with the cysteine-rich domain of the FZD receptor and the second one is with the low-density lipoprotein receptor-related protein (LRP) 5 or 6 [169]. This results in the stabilization of β-catenin and its translocation to the nucleus where it regulates gene expression. In addition, FZD receptors also initiate non-canonical signaling independent of β-catenin [169]. Of note, not all Wnt actions are through FZD/LRP receptors [170].
In MSCs, Wnt signaling inhibits adipogenesis and stimulates osteoblastogenesis. Wnt1 also inhibited adipogenesis of 3T3-L1 preadipocytes. This was mediated by inhibition of PPARγ and C/EBPα. Similarly, 3T3-F442A preadipocytes overexpressing Wnt1, injected subcutaneously into athymic mice, failed to develop into adipose tissue [171]. In line with this, activation of the FZD1 receptor stabilized β-catenin, promoted osteoblastogenesis and inhibited adipogenesis. Activation of FZD2 receptors also inhibited adipogenesis but did not affect osteoblastogenesis, which appeared dependent on β-catenin in the case of FZD1 receptor and β-catenin independent in case of FZD2 receptor [172].
Enzyme-linked receptors
Enzyme-linked receptors are receptors with intrinsic intracellular kinase activity. These can be tyrosine kinase receptors (e.g. IR), serine/threonine kinase receptors (e.g. TGF-β receptors) or receptors which do not have intrinsic intracellular activity. However, they can associate with intracellular molecules possessing kinase activity (e.g. TNF-α receptor) (see below). In all of these categories, there are receptors that play a crucial role in adipose tissue and few selected examples of each are described below.
Tyrosine kinase receptors
IR and IGF-1R
IR and insulin-like growth factor (IGF-1) receptor 1 (IGF-1R) signaling are among the best-studied signaling cascades in preadipocytes and adipocytes. To this end, it is of important to highlight their role in adipose tissue here. However, a detailed review of their role in adipose tissue would exceed the scope of this review. IR and IGF-1R belong to the tyrosine kinase receptor superfamily. However, unlike other members of the family, they exist as a covalent disulfide-linked dimer prior to ligand binding. Upon ligand binding, the tyrosine kinase domain phosphorylates tyrosine residues on the intracellular part of the receptor [173]. These phosphorylated residues act as a binding region for a multitude of adaptor and signaling proteins that regulate the pleiotropic effects of insulin/IGF-1 action. Importantly, the IR exists as two splice variants (IR-A and IR-B) and both can form heterodimers with the IGF-1R, creating six different combinations, which have been shown to differentially regulate metabolic or mitogenic effects of insulin/IGF signaling [174–176]. Moreover, we previously showed that the surface proteoglycan Glypican-4 interacts with the IR in preadipocytes and thereby regulates IR binding affinity to insulin [177], providing further complexity in the regulation of insulin action in these cells.
With respect to adipogenesis, both the IR and IGF-1R are expressed in pre and mature adipocytes [178,179]. It was already shown in the 1980s that IGF-1 is required for the differentiation of 3T3-L1 preadipocytes into mature adipocytes. This could also be achieved by using supraphysiological amounts of insulin [180], which remains part of the standard differentiation cocktail for adipocytes. Antibody-mediated blockage of the IGF-1R in human MSCs decreased proliferation and lipid accumulation [181]. However, there is also a role of the IR in adipogenesis as pluripotent stem cells from IR knockout mice differentiated poorly in comparison with control cells, as assessed by lipid accumulation and gene expression [182]. Thus, insulin/IGF signaling plays an important role in adipogenesis and the complex regulation of this signaling network through multiple receptor heteromers and modulatory surface proteins suggests adipose selective combinations could be explored to selectively modulate adipose function.
The central role of insulin action in adipose tissue and the fact that most other signaling cascades in one way or another impact on insulin action, requires a brief overview over its impact on adipose tissue. More detailed information can be found elsewhere [183]. IR and IGF-1R both play a crucial role in adipose tissue. Their function has been studied in great detail using conditional ablation in adipose tissues using different Cre-expressing mouse lines. Using adiponectin-Cre mice, the IGF-1R knockout slightly reduces BAT mass, but does not impact on its function as assessed by its ability to maintain body temperature under cold exposure. Meanwhile, the size of WAT is reduced by 25% with concurrent reduction in leptin and adiponectin levels. The effect of IR deletion in adipose tissue is more pronounced. In adipose-specific IR knockout mice, WAT mass is greatly reduced (by 90%). These mice are insulin resistant and exhibit compensatory β-cell hyperplasia throughout life. Interestingly, BAT of IR knockout mice is increased (by 50%) with the appearance of large unilocular adipocytes. Moreover, BAT function is impaired. The deletion of both the IR and IGF-1R resulted in a more severe phenotype with an almost complete absence of WAT and an 85% reduction in BAT mass. These double knockout mice were also highly cold intolerant [184]. The deletion of the IGF-1R and IR using the aP2-Cre promoter resulted in different phenotypes than with the adiponectin-Cre promoter. aP2-Cre-mediated IGF-1R knockout mice showed an increase in WAT mass with an increase in overall growth related to a modest increase in IGF-1 levels [185]. Deletion of the IR or both the IR and IGF-1R using the aP2-Cre promoter resulted in a modest decrease in WAT with an improved glucose tolerance under HFD [186,187]. These differences are thought to results from incomplete deletion using the aP2 promoter, further highlighting the requirement of fine balanced insulin/IGF-1 action in adipose tissue.
The difference in the phenotype observed between the adiponectin-Cre IR knockout and IGF-1R knockout could be due to differences in expression of these receptors during adipogenesis. The IGF-1R is higher expressed in preadipocytes than the IR [188,189], while at this stage adiponectin expression is low and no gene deletion is expected [190,191]. However, IR expression increases with differentiation and is more expressed in mature adipocytes than the IGF-1R [192] and at this time adiponectin expression is high [193] ensuring high recombination efficacy. Interestingly, IR and IGF-1R regulate identical gene expression in murine brown adipocytes [188]. Thus, the differences seen in vivo could be a result of different ligand concentration and availability as well as different extent and timing of receptor expression.
PDGF receptors
Platelet-derived growth factor receptors (PDGFR) α and β are class III tyrosine kinase receptors. Upon ligand binding, dimerization of the receptor occurs followed by autophosphorylation of the receptor on tyrosine residues, initiating downstream signaling [194]. PDGFRα was suggested as a marker for adipocyte progenitors [195] and both PDGFRα and β are expressed in 3T3-L1 preadipocytes, while their expression diminishes upon differentiation [196]. The role of PDGFRs in adipogenesis is controversial. PDGF-AA promoted adipogenesis while PDGF-BB inhibited adipogenesis in 3T3-L1 cells [197]. Early studies suggested that PDGF enhances differentiation of 3T3-L1 preadipocytes [198] and acts anti-apoptotic [199]. Others showed that PDGF inhibits differentiation of human adipose stromal cells [200], human preadipocytes and murine 3T3-L1 preadipocytes [201]. Inhibition of adipogenesis was accompanied with an increase in the inhibitor κB kinase β (IKKβ) in human subcutaneous preadipocytes [202]. Moreover, blocking PDGFRα and β promoted adipogenesis via suppression of phosphatidylinositol-3-kinase (PI3K) in human MSCs [203]. Thus, increasing evidence suggests an inhibitory role of PDGFR signaling in adipogenesis. In addition, PDGFRα and β differentially impact on preadipocyte fate as PDGFRα+ cells give rise to both beige and white adipocytes in murine abdominal WAT under β3 adrenergic stimulation and HFD feeding [27]. This was further corroborated by another study showing that adipocyte precursors with dominant PDGFRα expression and signaling differentiated to beige adipocytes while adipocyte precursors with dominant PDGFRβ expression and signaling differentiated into white adipocytes in mice [31]. However, PDGFRβ+ mural preadipocytes can differentiate to beige adipocytes under prolonged cold exposure in mice, indicating that PDGFR expression does not mark distinct developmental lineages [32]. These data demonstrate the intricate role of PDGFRs in adipogenesis and cell fate determination and highlight the complex interplay between receptor/ligand combinations in the regulation of cellular outcome.
FGF receptors
Fibroblast growth factor (FGF) receptors (FGFRs) are single-pass transmembrane receptors with an intracellular tyrosine kinase domain. There are seven FGFRs made from four different genes (FGFR1–4) by alternative splicing. Activation of FGFRs results in activation of PI3K-AKT, RAS-RAF-MAPK, STAT and PLCγ pathways [204].
In murine and human WAT, FGFR1 is highest expressed, followed by FGFR2, while expression of FGFR3 and 4 were not detected [205–207]. FGFR1 and 2 expression levels gradually declined during differentiation in 3T3-L1 adipocytes [208]. FGFR1 was shown to regulate adipogenesis in murine and human preadipocytes as well as MSCs [209–211]. Pdgfrα-CreERT2;Fgfr1flox/flox mice lacking Fgfr1 in APCs showed adipocyte hypertrophy in response to HFD feeding to compensate deficits in adipogenesis [212].
FGFR1 also regulates glucose and lipid metabolism in mature adipocytes [213,214]. The FGF1-induced glucose-lowering effect was blunted in adipose FGFR1 knockout (aP2-Cre;Fgfr1flox/flox) mice [215]. Furthermore, antibody-mediated activation of FGFR1c increased glucose uptake in murine WAT and BAT [214]. FGFR1, but not FGFR2, deficiency in white adipocytes increased adipocyte lipase activity in response to fasting, resulting in hepatosteatosis [216]. Furthermore, lack of the FGFR1 in white adipocytes increased lipogenesis and lipid droplet size due to accelerated phospholipid biosynthesis [217]. These findings suggest that FGFR1 signaling regulates glucose and lipid metabolism in mature white adipocytes.
Paracrine FGFs, such as FGF1, 6 and 9, that have heparin-binding domains can directly activate FGFRs. In contrast, endocrine FGFs (FGF19 subfamily) including, FGF19, 21 and 23 lacking this domain require the co-receptor Klotho to activate FGFRs [205,206,218,219]. βKlotho, a type I membrane protein is up-regulated during adipocyte differentiation [208]. In contrast, obesity decreased the expression of βKlotho as well as FGFR1 in WAT [220,221]. The association of βKlotho with FGFR1 is necessary for FGF21-induced glucose uptake in WAT and BAT as well as cultured adipocytes [205,219,222–224]. FGFR1/βKlotho signaling also mediates the FGF21 effects on BAT thermogenesis [213,223,225], albeit its action includes non-adipocyte cells within BAT [226,227]. In contrast, FGFR3 regulates UCP1 dependent thermogenesis in brown adipocytes [228].
Tyrosine kinase-associated receptors
These receptors possess no intrinsic intracellular kinase activity, but signal through the recruitment of kinases upon ligand binding.
TNF-α receptors
Tumor necrosis factor alpha (TNF-α) is a pro-inflammatory cytokine [229] and high TNF-α levels in adipose tissue are associated with type 2 diabetes and insulin resistance [230,231]. In adipose tissue, the effects of TNF-α are mediated via two distinct receptors: TNF-α receptor (TNFR) 1 and 2. Both receptors belong to the TNFR superfamily and are expressed in many cell types including pre- and mature adipocytes [232]. Upon ligand binding to TNFR1 or TNFR2, homo-trimerization of these receptors occurs [233]. TNFR1 and 2 do not possess an intracellular catalytic domain and therefore depend on intracellular adaptor proteins to further transduce the signal. Activation of TNFRs can induce apoptosis or promote cell survival and a pro-inflammatory response. Both receptors are proteolytically cleaved to produce soluble forms [232]. These soluble receptor forms sequester ligands from binding to cell surface receptors inhibiting signal transduction [234].
TNF-α inhibits adipocyte differentiation [235–237], during the first 24–36 h after induction (commitment phase) as the addition of TNF-α after this time period did not impair differentiation [238]. The inhibitory action of TNF-α is mediated via TNFR1 as the deletion of TNFR1 in preadipocytes blocks TNF-α effects [237]. Mechanistically, TNF-α blocked C/EBPα and PPARγ expression and promoted expression of anti-adipogenic genes. Moreover, TNF-α induced the de-differentiation of mature adipocytes in vitro [239,240].
TNF-α also inhibited insulin action in murine adipocytes by inhibiting tyrosine phosphorylation of the IR and IRS-1 [241], which could mediate the observed de-differentiation/delipidation described above. Moreover, TNF-α down-regulates several proteins involved in insulin action (e.g. GLUT4) [242–244], which appears to be mediated by TNFR1, as deletion of TNFR1 blunted the effects of TNF-α treatment [245]. Moreover, ob/ob mice lacking TNFR1 showed improved insulin sensitivity [246] and global TNF-α knockout mice had improved insulin sensitivity in comparison with controls [247]. A more detailed review on the role of TNF-α in the adipose tissue can be found in [232].
Serine/threonine kinase receptors
Transforming growth factor beta (TGF-β) receptors (TGFBRs) are transmembrane serine/threonine kinase glycoproteins with well-established roles in adipocyte differentiation and function. There are two receptor types (I and II) with five type I and seven type II receptors. Binding of a TGFBR ligand results in an interaction of receptor type I and II. In the canonical signaling pathway, the signal is then propagated through the phosphorylation of Smad proteins. However, other non-canonical signaling pathways have been reported including β-catenin/tcf4, p38 MAPK, ERK and JNK [248]. Preadipocytes express both TGFBRs and expression of these receptors decreases during differentiation [249].
Activation of the TGF-β superfamily receptors has different effects on adipogenesis, depending on the ligand/receptors activated. Bone morphogenetic protein (BMP) 4 induced mouse embryonic stem cells to differentiate into adipocytes [250]. Moreover, the treatment of C3H10T1/2 pluripotent stem cells with BMP4 triggered commitment to the adipocyte lineage. Furthermore, treatment of C3H10T1/2 with BMP4 in culture followed by transplantation of those cells in the subcutaneous adipose tissue of athymic mice resulted in the formation of WAT indistinguishable from normal adipose tissue [251]. Interestingly, BMP4 treatment suppressed UCP-1 expression while increasing lipid accumulation in brown preadipocytes [223]. However, the role of BMP4 on the differentiation of brown and beige adipocytes is controversial [252]
BMP7, which is another member of the TGF-β superfamily, also promotes adipogenesis [253,254]. In brown preadipocytes, the addition of BMP7, in the absence of an induction cocktail, induced differentiation and induction of UCP-1. This pro-adipogenic role of BMP7 includes suppression of adipogenic inhibitors like Pref-1 and Wnt10a, while increasing expression of pro-adipogenic genes like PPARγ, C/EBPα and aP2. BMP7 also drove brown adipogenesis in mesenchymal progenitor cells [255].
Other members of the TGF-β superfamily inhibit adipogenesis. TGF-β1 inhibits adipogenesis in both 3T3-L1 [256] and 3T3-F442A cells [249]. TGF-β1 also reduced lipid accumulation in primary cultures of pig subcutaneous adipose tissue [257]. Interestingly, inhibition of TGFBR1 promoted beiging in undifferentiated cells of the epididymal murine SVF. Similarly, subcutaneous transplantation of SVF cells from adipose tissue-specific TGFBR1 knockout mice into nude mice showed that knockout of the TGFBR1increases beiging in HFD fed mice after β-adrenergic stimulation [258]. Moreover, there are additional receptors of this family that showed mixed effects on adipogenesis and are reviewed in detail elsewhere [248].
In adipose tissue, activin receptor-like kinase 7 (ALK7), is a TGFBR1that is activated by growth differentiation factor 3 (GDF3) [259,260]. Mice lacking ALK7 receptor have reduced fat mass upon HFD feeding reminiscent of Gdf3 knockout mice [259]. Conversely, activation of the ALK7 receptor increased adiposity by suppression of lipolysis [261]. These data demonstrate the crucial function of TGFBR superfamily in adipose tissue.
Ion-channel linked receptors
Ion-channel linked receptors are transmembrane proteins that undergo conformational changes upon activation, allowing selective ions to pass through the channel and across the membrane [262]. This group of receptors plays a role in various tissues including adipose. Activation of transient receptor potential vanilloid type 1 channel inhibits adipogenesis [263]. Similarly, blockage of the chloride channel 3 on human subcutaneous preadipocytes by tamoxifen inhibits the proliferation of these cells [264].
K+ channels regulate the proliferation of human preadipocytes [265]. Furthermore, activation of the ionotropic purinergic cation channel P2X7R decreased adipogenesis and increased osteogenesis in rat MSCs [266]. Our group also demonstrated that P2RX5 is highly expressed in BAT in comparison with WAT and other tissues and therefore could be used as a cell surface marker for brown adipocytes. Yet it's function remains unknown [20]. Many other ions channels exist in adipose tissue and could be considered as pharmacological targets, which are discussed in [267].
Transporters
Apart from the groups/categories mentioned above, there are transporters that are pivotal for adipose tissue and whole body normal physiology but do not fit in the above-mentioned classification. Two good examples of these receptors are carbohydrate and fatty acid transporters which have been shown to play a crucial role in the adipose tissue.
GLUT4
Insulin action is the most important regulator of glucose uptake in adipose tissue, by mediating the translocation of the GLUT4 to the plasma membrane [268]. GLUT4 is a part of 14 member protein family that mainly share structural similarities [269].
GLUT4 was first identified in rat adipocytes by screening for proteins that translocate from the intracellular fraction to the plasma membrane upon insulin stimulation [270]. The gene for GLUT4 (Slc2a4) was later cloned from rat adipose tissue [271,272]. The pivotal role of GLUT4 in adipose tissue was demonstrated by overexpression and knockout studies [273–275]. The knockout of GLUT4 in adipose tissue (using aP2-Cre mice) resulted in glucose intolerance and insulin resistance. This insulin resistance was also observed in skeletal muscle and liver where GLUT4 expression is intact [273]. Moreover, overexpression of GLUT4 in adipose tissue enhanced glucose tolerance while increasing adiposity. Interestingly, this increase in adiposity was due to adipocyte hyperplasia with no change in adipocyte size, further strengthening the conclusion that a shift from hypertrophy towards hyperplasia has beneficial metabolic effects even in the context of obesity [274]. Additionally, adipose tissue overexpression of GLUT4 in mice lacking muscle GLUT4 restored insulin sensitivity and improved glucose tolerance. However, these mice showed an increased fat mass, increased serum FFA and leptin levels, but decreased adiponectin levels [275]. These results underscore the important role of adipose GLUT4 and the IR (see above) in regulating systemic glucose homeostasis, but also highlight that the adipocyte surface is continuously remodeled depending on the metabolic state of the organism.
CD36
Like GLUTs, fatty acid transporters also play an important role in adipocyte function. In fact only a small proportion of triacylglycerides stored in adipocytes derive from glucose through de novo-lipogenesis, whereas the vast majority is esterified from circulating lipids. One prominent fatty acid transporter is CD36 [276–278]. CD36 is a scavenger receptor, which exhibits a hairpin-like topology with two transmembrane domains and both termini facing intracellularly. CD36 is widely expressed in various cell types including adipocytes [279] and is not the only fatty acid transporter expressed in adipocytes. Other important fatty acid transporters are reviewed elsewhere [280]. Of note, CD36 is expressed on other cell types and in these cells, it plays different roles [281]. CD36 is complexed with prohibitin and annexin 2 at the plasma membrane of endothelial cells as well as adipocytes, mediating transendothelial fatty acid transport to adipocytes [33].
CD36 is induced upon adipocyte differentiation [278], but can be also detected in human preadipocytes [282]. Uptake of labeled oleate was reduced in adipocytes isolated from CD36 knockout mice [283]. Moreover, injection of CD36 knockout mice with fatty acid analogs (BMIPP and IPPA) showed impaired uptake in adipose tissue [284]. CD36 knockout mice were protected from DIO exhibiting reduced fat mass [285]. Under HFD feeding, CD36 knockout mice showed improved glucose tolerance and insulin sensitivity compared with controls. Interestingly, primary adipocytes isolated from CD36 knockout mice showed a reduced pro-inflammatory response to lipopolysaccharides. In accordance with this, adipose tissue from these mice showed reduced inflammation with less macrophage infiltration [286]. Moreover, gonadal adipocytes from CD36 knockout mice showed increased lipolysis and elevated insulin sensitivity [287]. However, this is in contradiction to another report showing that knockdown of CD36 in 3T3-L1 adipocytes impairs isoproterenol (β-adrenergic receptor agonist) stimulated lipolysis, which was also observed using adipose tissue cultures from CD36 knockout mice ex vivo [288].
In humans, CD36 deficiency is accompanied with mild fasting hyperglycemia, hyperlipidemia and insulin resistance [289]. Furthermore, common variants in the CD36 gene are associated with the metabolic syndrome [290]. Expression of CD36 in adipocytes is positively correlated with systemic and adipose tissue insulin sensitivity in obese non-diabetic individuals [282]. However, other studies found an up-regulation of CD36 in insulin-resistant individuals [291–294]. Thus, additional studies are needed to understand the regulation and role of CD36 in human adipose tissue and its contribution to the metabolic syndrome.
All receptors and transporters described above have important roles in regulating adipose tissue function and could provide interesting pharmacological targets. However, we chose these receptors/transporters to illustrate that while they possess interesting functions in adipose tissues, they also play important functions in cell types outside adipose. Thus, targeting them will have potentially severe side effects. Therefore, novel strategies are urgently needed to achieve adipocyte selective drug targeting to generate effective and safe pharmacotherapy for the metabolic syndrome.
Chasing adipose selective drug delivery
A multitude of studies have identified marker genes distinguishing adipocyte subtypes, such as white versus beige versus brown adipocytes [295–297]. Identification of such markers is important for the characterization of dissected tissues or cells in culture to estimate the relative contribution of one cell type versus the others or changes in the abundance of certain cell types in disease states. However, most of the identified markers are intracellular proteins, which limit their use for cell sorting or other applications aiming to work with living cells. To this end, cell surface proteins have the clear advantage that they are readily accessible by various molecules (small molecules, antibodies, peptides, aptamers, etc.) to stain, interfere with their function or hijack their cell type-specific expression to facilitate tissue-selective drug delivery. Unfortunately, to date, no unique cell surface protein for adipose tissue has been identified. We previously identified three surface markers for white, beige and brown adipocytes, respectively, which remain the most adipocyte selective surface proteins [20]. However, ASC-1 is also expressed in the central nervous system and P2RX5 in skeletal muscle, albeit at lower levels than in adipose. Low mRNA expression of PAT2 can be found outside beige/brown fat, but how that translates to protein levels remains unknown. Furthermore, we recently showed that the surface location of PAT2 is highly dependent on extracellular amino acid concentrations, and could strongly fluctuate in vivo [298].
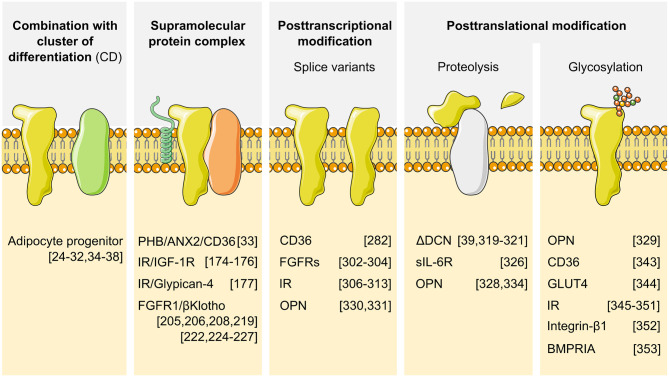
To this end, we came to the conclusion that, given the large number of distinct cell types in the mammalian organism, and the key cellular functions mediated by most surface proteins, the identification of a cell surface protein that is uniquely expressed in adipocytes will most likely be unsuccessful. However, at the same time, we are convinced that cell type-specific cell surface epitopes exist that can be utilized to facilitate target drug delivery. In contrast with cell surface proteins, surface epitopes could present protein/protein interactions, posttranslational modifications or even lipid modifications, thereby greatly extending the potential repertoire of possible targets (Figure 3). However, the great challenge is that we do not know the nature of these epitopes nor do we have knowledge of the potential targeting reagents. In the following part, we will discuss ways to extend the spectrum of surface epitopes and ways to identify and target these.
Figure 3. Extending cell surface epitope complexity beyond protein expression.
Diversity in cell surface epitopes is created through combination of protein expression and protein/protein interactions. Additional diversity in cell surface epitopes is achieved through posttranscriptional and posttranslational modifications. Splicing can be tissue and/or cell type specific. Proteolysis can generate tissue-specific fragments from ubiquitously expressed proteins. Glycosylation is one representative for posttranslational modifications further increasing surfome diversity.
Extending the complexity of surface epitopes
Alternative splicing
Alternative splicing is one of the most important mechanisms to expand the diversity of proteins. In the last decade, advances in next-generation sequencing technologies combined with computational analyses revealed that up to 95% of multi-exon transcripts undergo alternative splicing [299,300]. This, however, could be a strong underestimation as Vaquero-Garcia et al. suggest that the classical binary definition of splicing variants (e.g. include or skip an exon) is too simplified [301]. They developed novel algorithms to define previously annotated classical alternative splicing as well as unknown more complex transcript variants, which account for over 30% of tissue-dependent transcript variants [301]. In addition, splicing is highly tissue and cell type dependent [299,300]. Thus, this additional level of complexity amplifies the chance to detect cell type-specific protein isoforms.
FGFR2b is one of three FGFR2s and preferentially expressed on preadipocytes compared with mature adipocytes [302,303]. Its signaling is important for the commitment of preadipocytes towards beige/brown adipocytes [302,303]. On the other hand, FGFR2c is expressed on mature white adipocytes and represses thermogenic gene expression [304]. In line with this, adipocyte-specific FGFR2c knockout mice showed reduced hypertrophy in visceral WAT and reduced plasma FFA levels [304]. These findings indicate that splice variants of the same gene can facilitate very different or even opposing functions. Intriguingly, neutralization of FGFR2c with monoclonal antibodies did not reduce plasma FFA levels or induced thermogenic gene expression in WAT, although it suppressed body weight gain caused by social isolation in KKAy diabetic mice [305]. This suggests that systemic inhibition of FGFR2c has different effects than adipose selective inactivation, further strengthening our conclusion that tissue-selective targeting is essential for next-generation therapeutics.
Another example for a metabolically relevant alternatively spliced receptor is the IR [306,307]. As already mentioned above, the IR is found as two splice variants IR-A and IR-B. IR-B has 12 additional amino acids at the C-terminus of the alpha chain, which results from alternative splicing of exon 11 [306,308]. IR-A is predominantly expressed in fetal, tumor tissues and preadipocytes, whereas IR-B is preferentially expressed in postnatal tissues such as liver, muscle, fat and kidney. IR-A has been associated with the mitogenic function of insulin, whereas IR-B correlates with the metabolic aspect of insulin action [309,310]. Importantly, the abundance of IR-B in adipose is changed in obesity, type 2 diabetes and weight loss [311,312]. Moreover, the splicing of IR-B appears to be regulated by insulin but not glucose levels [311,313]. Nevertheless, currently, no animal models are available to verify potentially distinct functions of these splice variants in vivo.
Proteolytic cleavage
In addition to posttranscriptional modifications, posttranslational modifications, such as proteolysis and glycosylation that is described below, provide an additional layer of diversification. Proteolytic cleavage of cell surface proteins can result in shedding via sheddases to release the ectodomain of single-pass transmembrane proteins, such as Pref-1 [314]. Furthermore, processing by intracellular proteases can release intracellular domains as described for Notch [315]. However, it is important to keep in mind that albeit most attention is being paid to the liberated protein fragments, the residual transmembrane peptides provide potentially unique surface epitopes that could be targeted.
Decorin is a secreted proteoglycan mediating cell–matrix interaction [316,317]. Proteolytic cleavage generates different isoforms [318]. For example, a non-glycosylated isoform (termed ΔDCN) lacking the N-terminal methionine, suggests that it is generated by proteolysis rather than alternative splicing [39]. ΔDCN accumulates exclusively on the cell surface of human and murine perivascular PDGFRα−PDGFRβ+ APCs within WAT and is absent on MSCs in other tissues. Based on these characteristics a ΔDCN targeting peptide was generated and used to specifically deliver cargo into subsets of APCs [319–321]. Moreover, ΔDCN was shown to act as a resistin receptor to facilitate proliferation and migration of 3T3-L1 preadipocytes [39].
Development of obesity is associated with alterations in the expression of proteases, including matrix metalloproteases (MMPs) [322–324] and A disintegrin and metalloproteinases (ADAMs) [325–327] in WAT, locally changing the bioactivity of transmembrane proteins and cytokines [326,328]. IL-6 trans-signaling, where IL-6-bound soluble IL-6 receptor (IL-6R) binds to gp130 on the cell surface, but not classical transmembrane IL-6R signaling, contributes to diet-induced macrophage infiltration into WAT rather than liver [326]. Furthermore, osteopontin is heavily posttranslationally modified, including proteolysis [328–330]. Its levels increase in WAT upon HFD feeding and β3 adrenergic stimulation through secretion by macrophages [331–333]. This results in the recruitment of macrophages [332], T cells [334] and APCs expressing CD44 that acts as an osteopontin receptor [333]. Intriguingly, not only full-length osteopontin but also MMP-cleaved derivatives potentiate T cell viability [334]. Moreover, MMP-cleaved osteopontin blunts insulin sensitivity of adipocytes compared with full-length osteopontin [328].
Protein glycosylation
With increasingly advanced proteomics and metabolomics technologies, the number of identified posttranslational modifications is rapidly expanding [335–339]. Protein glycosylation is one of the best-studied posttranslational modification, generating large proteomic diversity. This diversity depends on glycan composition and complexity of branching, which is cell type-specific [340,341]. Glycosylation regulates the biological activity of proteins, such as cell–cell and cell–extracellular matrix interactions, ligand binding affinity, protein trafficking as well as subcellular localization and protein stability [177,342–344]. The IR, for example, is highly glycosylated in the ectodomains of the α and β subunit [345–351], which is essential for proper processing of the receptor [347,349,350]. Furthermore, site-specific mutation of N-glycosylation sites within the IR revealed that glycation of the α and β subunit regulate IR trafficking and tyrosine kinase activity [345,348].
Interestingly, N-glycosylation of the α subunit of the IR differs between adipocytes and the brain [346], highlighting that glycan structures are differently produced by numerous glycan-related enzymes in a tissue-dependent pattern [340]. Moreover, glycosyltransferases are differentially regulated in the adipose tissue upon obesity and directly impact on adipocyte differentiation and function through modulation of different cell surface proteins such as beta 1 integrin and BMP receptors [352,353]. Beta-galactoside α-2,6-sialytransferase-1 (ST6GAL1) was identified as the most down-regulated glycosyltransferase during adipocyte differentiation in visceral adipose tissue of DIO mice [352]. ST6GAL1 mediates sialylation of beta 1 integrin resulting in inhibition of adipogenesis. Expression of β1,4-galactosyltransferase 5 (B4GalT5) in subcutaneous adipose tissue is positively correlated with type 2 diabetes and obesity in human and mice [353], but is predominantly expressed in the SVF rather than mature adipocytes. B4GalIT5 inhibits adipocyte differentiation by modifying glycan structures of the type 1 BMP receptor [353].
Taken together, the combination of splice variants, tissue-specific posttranslational modifications and protein/protein interactions create a very large theoretical diversity at the cell surface that should provide adipocyte-specific cell surface epitopes. However, as the nature of these tissue-selective epitopes are very hard to predict, a diverse set of methods, outlined below need to be combined to not only identify these epitopes, but also to generate targeting vehicles for tissue-selective drug delivery.
How to identify adipose selective surface epitopes
Database mining
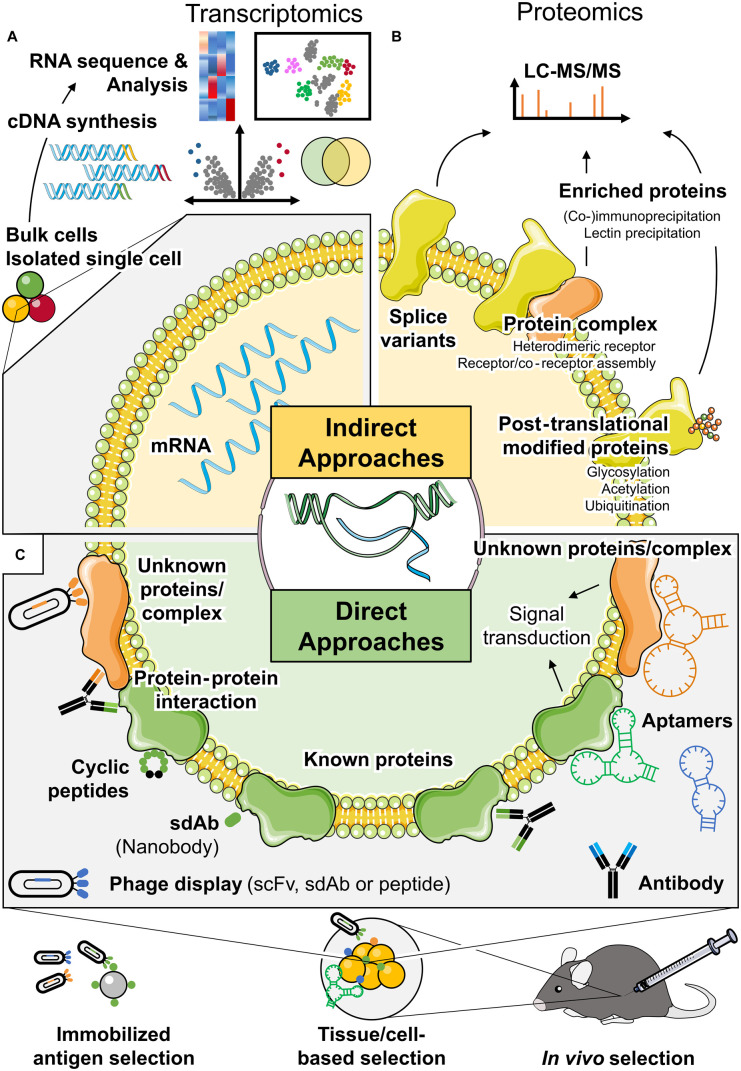
Comparative gene expression analysis has been a long-used tool to identify markers for tissues, cell types or disease states (Figure 4A). However, classically this approach is limited in its ability to predict tissue selectivity as a given data set is often only compared with one or a hand full of other cells, tissues or states. Nevertheless, comparisons of differentially expressed genes between adipose tissue depots, have revealed many interesting candidate genes to study adipose function [354]. To identify genes with adipose selective expression we also utilized multiple microarray datasets available from databases, such as the SymAtlas database (http://www.biogps.org) [20]. To identify genes with adipose selective expression encoding cell surface proteins we first selected all genes with high correlation to white and brown adipocyte-specific reference genes, such as Adiponectin and Ucp1 and then ranked those according to their adipose selectivity. Finally, transmembrane protein-encoding genes were selected and individually verified on mRNA and protein level from a large multi-tissue panel. However, this strategy did not consider splice variants or any other posttranscriptional modifications. Today, with rapidly increasing numbers of RNAseq data from numerous species, organs and even single cells, such as in the human cell atlas, and increasingly sophisticated mathematical models and computational tools, a more detailed analysis for potential selective surface proteins might be possible in the near future. Nevertheless, this simple approach was critically important to advance our understanding that tissue selectivity is most likely, at least in adipose tissue, not encoded by individual genes, but the result of a more complex interactions of protein modifications and interactions.
Figure 4. Approaches for identification and characterization of cell surface epitopes.
(A,B) Indirect approaches to identify the cell surface epitopes. (A) Transcriptomics can provide information to predict potential unique epitopes and splice variants. (B) Proteomics identifies protein complexes and posttranslational modifications as well as splice variants. (C) Approaches capable of not only identifying but also targeting adipocyte selective epitopes. Antibodies, peptides and aptamers can all recognize epitopes of unknown proteins and protein complexes in addition to known proteins. These high affinity molecules are obtained by enrichment from large libraries screened with in vitro and in vivo methods. scFv, single-chain variable fragments; sdAb, single-domain antibody.
Proteomics
While comparative gene expression analysis has a very high sensitivity, it is only an indirect method to identify cell surface proteins, not taking into account regulatory steps post transcription. Thus, liquid chromatography–tandem mass spectrometry (LC–MS/MS) has been used to successfully identify proteins in small amounts of crude protein lysate from culture cells and tissues (Figure 4B). Moreover, proteomics enables us to provide unbiased comprehensive information by matching the molecular mass of peptide fragments composing proteins in biological samples against databases [355]. Thus, mass spectrometry is an alternative approach to screen for adipose selective surface proteins, especially when combined with cell surface biotinylation or other approaches to enrich for cell membrane proteins [356,357], allowing the identification of modified proteins and profiling of posttranscriptional and posttranslational modifications, such as splicing, methylation, ubiquitination, acetylation, phosphorylation and glycosylation [335–339]. However, thus far these approaches have not been successfully used to identify tissue-selective surface epitopes, largely as they also require comparative analysis and reference data for the full surfome of all tissues and cell types is not available.
This should, however, by no means devalue the enormous progress that had been made in understanding cellular and organismal function using these methods. However, omics technologies always rely on a comparison between datasets and while comparisons to closely related cell types or organs will significantly increase the probability of identifying cell type-specific proteins or protein modifications, this remains the search for the needle in the haystack. Furthermore, identifying a tissue-specific epitope would be only the first step, as subsequently targeting vehicles need to be developed that then allow for tissue-selective drug delivery.
Selecting adipose tissue targeting reagents
The biggest challenge for identifying adipocyte selective targeting reagents is that neither the selective epitope nor the characteristics of the targeting reagent are known. To this end, selection methods are required where neither the reagent nor the target need to be known in advance. Thus, the only applicable methods are those, where pools of molecules are incubated with adipocytes or exposed to adipose tissue in vivo and selectively bound molecules can be extracted and subsequently characterized (Figure 4C).
Antibody-drug conjugates are the most frequently used active drug delivery moieties [358] and phage displays allow to screen large libraries of antibodies or peptides [359]. The technology is based on the finding that bacteriophages can display exogenous random peptides fused with their coating proteins on the surface [360]. Building upon these bacteriophages have been modified to display single-chain variable fragments (scFv) linking the light- and heavy-chain variable region (VL and VH, respectively) capable of binding to antigens [361]. However, antibodies and scFvs remain challenging to use due to low stability and low solubility. In this context, single-domain antibodies provide an attractive alternative (sdAb, also referred to as nanobody). They mimic heavy chain only antibodies naturally found in camelidae species. Due to their small size (∼15 kDa), sdAb could be used to target otherwise hidden epitopes [362–364].
Antibody phage libraries encoding various scFv are generated from nonimmune (naïve), immune and synthetic libraries. Naive libraries are constructed from antibody genes in lymphocytes of healthy subjects while immune libraries are built from that of immunized donors, infected or cancer patients. Naive libraries are unbiased despite low antigen specificity. Antibody repertoires in immune libraries have high antigen specificity even though the library size is small. The use of synthetic libraries allows displaying artificial unnatural scFvs by the replacement of genes encoding complementary determining regions in the VH with random oligonucleotides. These libraries are constructed from human as well as rodent genomes so that antibody phage displays can provide human and mouse antibodies. Similarly, random synthetic peptide libraries possess large structural diversity complementing these antibody approaches [39,40,319,320,365].
The real advantage of these libraries, however, is that they can be used for biopanning (reviewed in [366]), which is a method to screen phage libraries against immobilized antigen on plates or beads, cell-based screenings, tissue-based screenings and/or in vivo screenings. Using scFv phage libraries Edwards and colleagues identified a set of antibodies binding to the cell surface of human adipocytes [367]. Interestingly, however, none of the tested antibodies was selective to adipose tissue and cross-reacted with at least one additional tissue. In contrast with this in vitro biopanning, the use of in vivo biopanning has major advantages as it allows selecting for specific binding of a biomolecules to a target tissue, while in parallel negatively selecting against all other tissues and cell types in the body. Indeed, using this in vivo approach novel peptides targeting murine BAT [365], WAT [368], and the adipose tissue endothelium [40], APCs [39,319,320] as well as adipocytes [33] were developed. Furthermore, targeting the adipose vasculature allowed the delivery of functional peptides and liposome into the adipose intercellular space [40,369–373], suggesting that the surfome of the endothelium could also be a promising target in adipose tissue. Importantly, in vivo biopanning is not limited to phages but can be also performed with other ‘barcoded’/retrievable molecules such as aptamers [366].
Aptamers are randomly synthesized short (typically 40–100 nucleotides) single-stranded deoxy- or ribonucleic acids (ssDNA/ssRNA) that can fold into very heterogeneous three-dimensional structures and bind a wide variety of targets. Compared with antibodies, aptamers are much smaller, typically between 6 and 30 kDa, have greater stability and are cheaper to produce. In addition, aptamers can be easily conjugated to small molecules, siRNAs and used to decorate liposomes to function as drug delivery reagents. [374–376].
Similar to biopanning aptamers are selected through the systematic evolution of ligands by exponential enrichment (SELEX), which has been developed by Tuerk and Gold [377]. The SELEX protocol enriches potential aptamers through positive and negative selection, followed by PCR based amplification, single strand aptamer preparation and repeated selection. To date in vivo SELEX has not been utilized to identify aptamers binding to metabolically relevant tissues, but in vitro SELEX has been used to select white adipocyte selective DNA aptamers [378], albeit adipose selectivity was not shown for these sequences. Thus, both in vivo biopanning using phage libraries or in vivo SELEX provide interesting strategies to identify adipocyte cell surface epitopes that are truly selective for adipose tissue and target the full epitope space available.
Limitation and perspective
The human body contains many different adipose depots, some, such as brown and dermal adipose tissue [2,3,379], with specialized functions beyond energy storage that are not explicitly discussed here. These depots can have unique cell surface topology, gene expression, cytokine profiles and association with the metabolic syndrome [380,381]. Thus, much more research is needed to understand the heterogeneity between, but also within each adipose depot and its role in obesity and its associated co-morbidities to develop highly potent tissue-selective drugs combating the metabolic syndrome. The adipose surfome also contains many more interesting proteins and epitope classes than the few receptors/transporters discussed above. Examples for these are proteoglycans such as Glypican-4 [177] and Syndecan-1 [382], but also angiotensin-converting enzyme 2 (ACE2), which is currently extensively studied, as it serves as the docking receptor for SARS-CoV, NL63 and SARS-CoV-2 viruses [383–385]. ACE2 is a single-pass transmembrane glycoprotein [386,387] that is expressed on the surface of various cell types [388] and is higher expressed in adipose tissue than in the lungs [389]. Furthermore, increased adipose tissue mass (obesity) is linked to increased severity and worse outcome of COVID-19 [390–396]. However, the exact role of adipose ACE2 in physiology and disease remains to be largely determined.
Nevertheless, it is important to stress that while we highlight the high probability for adipose selective epitopes and discuss potential approaches to target these, proof that truly adipose selective epitopes exist remains to be experimentally verified.
Concluding remarks
Adipose tissue plays a central role in maintaining whole-body glucose and lipid homeostasis and the adipocyte cell surface is the central hub integrating various environmental and endocrine inputs to orchestrate its function. Moreover, the cell surface of adipocytes is the key target for future therapeutic attempts aiming to selectively deliver drugs to adipose tissue. To this end, a more detailed characterization of the composition of the adipocyte cell surface between different depots and healthy versus diseased states is pivotal to achieve this task. It is becoming increasingly clear, however, that posttranscriptional, posttranslational modification as well as protein/protein interactions need to be considered when aiming to identify unique cell surface epitopes.
Acknowledgements
We received support by the project Aging and Metabolic Programming (AMPro) and the BMBF within the framework of the European ERA-NET Marine Biotechnology project ‘CYANOBESITY—Cyanobacteria as a source of bioactive compounds with effects on obesity and obesity-related co-morbidities’. Y.O. received support through the Post-doctoral Fellowship program from The Uehara Memorial Foundation, Japan (201830047) and the Alexander von Humboldt-Stiftung.
Abbreviations
- APCs
adipocyte progenitor cells
- ATP
adenosine tri-phosphate
- BAT
brown adipose tissue
- cAMP
cyclic adenosine monophosphate
- DIO
diet-induced obesity
- FFA
free fatty acid
- GLUT
glucose transporter
- GPCRs
G protein-coupled receptors
- HFD
high-fat diet
- KLP4
krüppel like factor 4
- LRP
lipoprotein receptor-related protein
- MEFs
mouse embryonic fibroblasts
- MMPs
matrix metalloproteases
- MSCs
mesenchymal stem cells
- PKA
protein kinase A
- SVF
stromal vascular fraction
- UCP-1
uncoupling protein 1
- UTP
uridine triphosphate
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
All authors wrote the manuscript, edited the text and approved the final version of the manuscript.
References
- 1.Coelho M., Oliveira T. and Fernandes R. (2013) Biochemistry of adipose tissue: an endocrine organ. Arch. Med. Sci. 9, 191–200 10.5114/aoms.2013.33181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoettl T., Fischer I.P. and Ussar S. (2018) Heterogeneity of adipose tissue in development and metabolic function. J. Exp. Biol. 221, jeb162958 10.1242/jeb.162958 [DOI] [PubMed] [Google Scholar]
- 3.Ikeda K., Maretich P. and Kajimura S. (2018) The common and distinct features of brown and beige adipocytes. Trends Endocrinol. Metab. 29, 191–200 10.1016/j.tem.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachman E.S., Dhillon H., Zhang C.Y., Cinti S., Bianco A.C., Kobilka B.K. et al. (2002) betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845 10.1126/science.1073160 [DOI] [PubMed] [Google Scholar]
- 5.Wang Q.A., Tao C., Gupta R.K. and Scherer P.E. (2013) Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344 10.1038/nm.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang-Doran I., Sleigh A., Rochford J.J., O'Rahilly S. and Savage D.B. (2010) Lipodystrophy: metabolic insights from a rare disorder. J. Endocrinol. 207, 245–255 10.1677/JOE-10-0272 [DOI] [PubMed] [Google Scholar]
- 7.González-Muniesa P., Mártinez-González M.-A., Hu F.B., Després J.-P., Matsuzawa Y., Loos R.J.F. et al. (2017) Obesity. Nat. Rev. Dis. Primers 3, 17034 10.1038/nrdp.2017.34 [DOI] [PubMed] [Google Scholar]
- 8.WHO (2016) Obesity and overweight. World Health Organization
- 9.Visscher T.L. and Seidell J.C. (2001) The public health impact of obesity. Annu. Rev. Public Health 22, 355–375 10.1146/annurev.publhealth.22.1.355 [DOI] [PubMed] [Google Scholar]
- 10.Farooqi I.S. and O'Rahilly S. (2000) Recent advances in the genetics of severe childhood obesity. Arch. Dis. Child. 83, 31–34 10.1136/adc.83.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morton G.J., Cummings D.E., Baskin D.G., Barsh G.S. and Schwartz M.W. (2006) Central nervous system control of food intake and body weight. Nature 443, 289–295 10.1038/nature05026 [DOI] [PubMed] [Google Scholar]
- 12.Rohner-Jeanrenaud E. and Jeanrenaud B (1997) Central nervous system and body weight regulation. Ann. Endocrinol. (Paris) 58, 137–142 PMID: [PubMed] [Google Scholar]
- 13.Kahn C.R., Wang G. and Lee K.Y. (2019) Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Invest. 129, 3990–4000 10.1172/JCI129187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun K., Kusminski C.M. and Scherer P.E. (2011) Adipose tissue remodeling and obesity. J. Clin. Invest. 121, 2094–2101 10.1172/JCI45887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy S.M. (2015) Adipose tissue and metabolic syndrome: too much, too little or neither. Eur. J. Clin. Invest. 45, 1209–1217 10.1111/eci.12519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusminski C.M. and Scherer P.E. (2012) Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol. Metab. 23, 435–443 10.1016/j.tem.2012.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gesta S., Tseng Y.H. and Kahn C.R. (2007) Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256 10.1016/j.cell.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 18.Kim J.Y., van de Wall E., Laplante M., Azzara A., Trujillo M.E., Hofmann S.M. et al. (2007) Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 10.1172/JCI31021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kajimura S., Spiegelman B.M. and Seale P. (2015) Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22, 546–559 10.1016/j.cmet.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ussar S., Lee K.Y., Dankel S.N., Boucher J., Haering M.F., Kleinridders A. et al. (2014) ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci. Transl. Med. 6, 247ra103 10.1126/scitranslmed.3008490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S., Pan M.H., Hung W.L., Tung Y.C. and Ho C.T. (2019) From white to beige adipocytes: therapeutic potential of dietary molecules against obesity and their molecular mechanisms. Food Funct. 10, 1263–1279 10.1039/C8FO02154F [DOI] [PubMed] [Google Scholar]
- 22.Symonds M.E., Aldiss P., Pope M. and Budge H. (2018) Recent advances in our understanding of brown and beige adipose tissue: the good fat that keeps you healthy. F1000Res 7, F1000 10.12688/f1000research.14585.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park A., Kim W.K. and Bae K.H. (2014) Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem Cells 6, 33–42 10.4252/wjsc.v6.i1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodeheffer M.S., Birsoy K. and Friedman J.M. (2008) Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249 10.1016/j.cell.2008.09.036 [DOI] [PubMed] [Google Scholar]
- 25.Berry R. and Rodeheffer M.S. (2013) Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol. 15, 302–308 10.1038/ncb2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X., Ying Z., Cai M., Xu Z., Li Y., Jiang S.Y. et al. (2011) Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1115–R1125 10.1152/ajpregu.00806.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee Y.H., Petkova A.P., Mottillo E.P. and Granneman J.G. (2012) In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab. 15, 480–491 10.1016/j.cmet.2012.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan J.L., Marshall M.A., C C., Harmon M., Garmey D.B., Oldham J.C. et al. (2015) Adipocyte progenitor cells initiate monocyte chemoattractant protein-1-mediated macrophage accumulation in visceral adipose tissue. Mol. Metab. 4, 779–794 10.1016/j.molmet.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carriere A., Jeanson Y., Cote J.A., Dromard C., Galinier A., Menzel S. et al. (2017) Identification of the ectoenzyme CD38 as a marker of committed preadipocytes. Int. J. Obes. (Lond) 41, 1539–1546 10.1038/ijo.2017.140 [DOI] [PubMed] [Google Scholar]
- 30.Gupta R.K., Mepani R.J., Kleiner S., Lo J.C., Khandekar M.J., Cohen P. et al. (2012) Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 15, 230–239 10.1016/j.cmet.2012.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Z., Daquinag A.C., Su F., Snyder B. and Kolonin M.G. (2018) PDGFRalpha/PDGFRbeta signaling balance modulates progenitor cell differentiation into white and beige adipocytes. Development 145, dev155861 10.1242/dev.155861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vishvanath L., MacPherson K.A., Hepler C., Wang Q.A., Shao M., Spurgin S.B. et al. (2016) Pdgfrbeta+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab. 23, 350–359 10.1016/j.cmet.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salameh A., Daquinag A.C., Staquicini D.I., An Z., Hajjar K.A., Pasqualini R. et al. (2016) Prohibitin/annexin 2 interaction regulates fatty acid transport in adipose tissue. JCI Insight 1, e86351 10.1172/jci.insight.86351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcelin G., Ferreira A., Liu Y., Atlan M., Aron-Wisnewsky J., Pelloux V. et al. (2017) A PDGFRα-mediated switch toward CD9(high) adipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell Metab. 25, 673–685 10.1016/j.cmet.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 35.Hepler C., Shan B., Zhang Q., Henry G.H., Shao M., Vishvanath L. et al. (2018) Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife 7, e39636 10.7554/eLife.39636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho D.S., Lee B. and Doles J.D. (2019) Refining the adipose progenitor cell landscape in healthy and obese visceral adipose tissue using single-cell gene expression profiling. Life Sci. Alliance 2, e201900561 10.26508/lsa.201900561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwalie P.C., Dong H., Zachara M., Russeil J., Alpern D., Akchiche N. et al. (2018) A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559, 103–108 10.1038/s41586-018-0226-8 [DOI] [PubMed] [Google Scholar]
- 38.Merrick D., Sakers A., Irgebay Z., Okada C., Calvert C., Morley M.P. et al. (2019) Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364, eaav2501 10.1126/science.aav2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daquinag A.C., Zhang Y., Amaya-Manzanares F., Simmons P.J. and Kolonin M.G. (2011) An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell 9, 74–86 10.1016/j.stem.2011.05.017 [DOI] [PubMed] [Google Scholar]
- 40.Kolonin M.G., Saha P.K., Chan L., Pasqualini R. and Arap W. (2004) Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 10, 625–632 10.1038/nm1048 [DOI] [PubMed] [Google Scholar]
- 41.Rajbhandari P., Arneson D., Hart S.K., Ahn I.S., Diamante G., Santos L.C. et al. (2019) Single cell analysis reveals immune cell-adipocyte crosstalk regulating the transcription of thermogenic adipocytes. eLife 8, e49501 10.7554/eLife.49501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song A., Dai W., Jang M.J., Medrano L., Li Z., Zhao H. et al. (2020) Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J. Clin. Invest. 130, 247–257 10.1172/JCI129167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burl R.B., Ramseyer V.D., Rondini E.A., Pique-Regi R., Lee Y.H. and Granneman J.G. (2018) Deconstructing adipogenesis induced by beta3-adrenergic receptor activation with single-cell expression profiling. Cell Metab. 28, 300–309 10.1016/j.cmet.2018.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoeckius M., Hafemeister C., Stephenson W., Houck-Loomis B., Chattopadhyay P.K., Swerdlow H. et al. (2017) Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 14, 865–868 10.1038/nmeth.4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fredriksson R., Lagerstrom M.C., Lundin L.G. and Schioth H.B. (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63, 1256–1272 10.1124/mol.63.6.1256 [DOI] [PubMed] [Google Scholar]
- 46.Gahan P. B. (2005) Molecular Biology of the Cell, 4th edn (Alberts B., Johnson A., Lewis J., Roberts K. and Walter P., eds), 1463 pp., Garland Science, New York: ISBN 0-8153-4072-9 (paperback) (2002). Cell Biochemistry and Function. 23, Chapter 15, 150-150 [Google Scholar]
- 47.(2011) Ligand-gated ion channels. Br. J. Pharmacol. 164, S115–S135 10.1111/j.1476-5381.2011.01649_4.x [DOI] [Google Scholar]
- 48.Hauser A.S., Attwood M.M., Rask-Andersen M., Schioth H.B. and Gloriam D.E. (2017) Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 16, 829–842 10.1038/nrd.2017.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flower D.R. (1999) Modelling G-protein-coupled receptors for drug design. Biochim. Biophys. Acta 1422, 207–234 10.1016/S0304-4157(99)00006-4 [DOI] [PubMed] [Google Scholar]
- 50.Zhao J., Deng Y., Jiang Z. and Qing H. (2016) G protein-coupled receptors (GPCRs) in Alzheimer's disease: a focus on BACE1 related GPCRs. Front. Aging Neurosci. 8, 58 10.3389/fnagi.2016.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritter S.L. and Hall R.A. (2009) Fine-tuning of GPCR activity by receptor-interacting proteins. Nat. Rev. Mol. Cell Biol. 10, 819–830 10.1038/nrm2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenbaum D.M., Rasmussen S.G. and Kobilka B.K. (2009) The structure and function of G-protein-coupled receptors. Nature 459, 356–363 10.1038/nature08144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu G.M., Mai T.L. and Chen C.M. (2017) Visualizing the GPCR network: classification and evolution. Sci. Rep. 7, 15495 10.1038/s41598-017-15707-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheth S., Brito R., Mukherjea D., Rybak L.P. and Ramkumar V. (2014) Adenosine receptors: expression, function and regulation. Int. J. Mol. Sci. 15, 2024–2052 10.3390/ijms15022024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fredholm B.B. (1976) Release of adenosine-like material from isolated perfused dog adipose tissue following sympathetic nerve stimulation and its inhibition by adrenergic alpha-receptor blockade. Acta Physiol. Scand. 96, 122–130 10.1111/j.1748-1716.1976.tb10177.x [DOI] [PubMed] [Google Scholar]
- 56.Fredholm B.B. and Sollevi A. (1981) The release of adenosine and inosine from canine subcutaneous adipose tissue by nerve stimulation and noradrenaline. J. Physiol. 313, 351–367 10.1113/jphysiol.1981.sp013670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fredholm B.B., I A.P., Jacobson J., Linden K.A., and Muller J. and E C. (2011) International union of basic and clinical pharmacology. LXXXI. Nomenclature and classification of adenosine receptors–an update. Pharmacol. Rev. 63, 1–34 10.1124/pr.110.003285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vassaux G., Gaillard D., Mari B., Ailhaud G. and Negrel R. (1993) Differential expression of adenosine A1 and adenosine A2 receptors in preadipocytes and adipocytes. Biochem. Biophys. Res. Commun. 193, 1123–1130 10.1006/bbrc.1993.1742 [DOI] [PubMed] [Google Scholar]
- 59.Borglum J.D., Vassaux G., Richelsen B., Gaillard D., Darimont C., Ailhaud G. et al. (1996) Changes in adenosine A1- and A2-receptor expression during adipose cell differentiation. Mol. Cell. Endocrinol. 117, 17–25 10.1016/0303-7207(95)03728-4 [DOI] [PubMed] [Google Scholar]
- 60.Gharibi B., Abraham A.A., Ham J. and Evans B.A. (2012) Contrasting effects of A1 and A2b adenosine receptors on adipogenesis. Int. J. Obes. (Lond) 36, 397–406 10.1038/ijo.2011.129 [DOI] [PubMed] [Google Scholar]
- 61.Gnad T., Scheibler S., von Kugelgen I., Scheele C., Kilic A., Glode A. et al. (2014) Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516, 395–399 10.1038/nature13816 [DOI] [PubMed] [Google Scholar]
- 62.Eisenstein A., Carroll S.H., Johnston-Cox H., Farb M., Gokce N. and Ravid K. (2014) An adenosine receptor-Kruppel-like factor 4 protein axis inhibits adipogenesis. J. Biol. Chem. 289, 21071–21081 10.1074/jbc.M114.566406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang T., Zollbrecht C., Winerdal M.E., Zhuge Z., Zhang X.M., Terrando N. et al. (2016) Genetic abrogation of adenosine a3 receptor prevents uninephrectomy and high salt-induced hypertension. J. Am. Heart Assoc. 5, e003868 10.1161/JAHA.116.003868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fredholm B.B. (1978) Effect of adenosine, adenosine analogues and drugs inhibiting adenosine inactivation on lipolysis in rat fat cells. Acta Physiol. Scand. 102, 191–198 10.1111/j.1748-1716.1978.tb06062.x [DOI] [PubMed] [Google Scholar]
- 65.Johansson S.M., Lindgren E., Yang J.N., Herling A.W. and Fredholm B.B. (2008) Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue-interactions with insulin. Eur. J. Pharmacol. 597, 92–101 10.1016/j.ejphar.2008.08.022 [DOI] [PubMed] [Google Scholar]
- 66.Szkudelski T., Szkudelska K. and Nogowski L. (2009) Effects of adenosine A1 receptor antagonism on lipogenesis and lipolysis in isolated rat adipocytes. Physiol. Res. 58, 863–871 PMID: [DOI] [PubMed] [Google Scholar]
- 67.Dong Q., Ginsberg H.N. and Erlanger B.F. (2001) Overexpression of the A1 adenosine receptor in adipose tissue protects mice from obesity-related insulin resistance. Diabetes Obes. Metab. 3, 360–366 10.1046/j.1463-1326.2001.00158.x [DOI] [PubMed] [Google Scholar]
- 68.Csoka B., Koscso B., Toro G., Kokai E., Virag L., Nemeth Z.H. et al. (2014) A2b adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes 63, 850–866 10.2337/db13-0573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnston-Cox H., Koupenova M., Yang D., Corkey B., Gokce N., Farb M.G. et al. (2012) The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS One 7, e40584 10.1371/journal.pone.0040584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DeOliveira C.C., Paiva Caria C.R., Ferreira Gotardo E.M., Ribeiro M.L. and Gambero A. (2017) Role of A1 and A2A adenosine receptor agonists in adipose tissue inflammation induced by obesity in mice. Eur. J. Pharmacol. 799, 154–159 10.1016/j.ejphar.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 71.Abbracchio M.P., Burnstock G., Boeynaems J.M., Barnard E.A., Boyer J.L., Kennedy C. et al. (2006) International union of pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 58, 281–341 10.1124/pr.58.3.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burnstock G. (2007) Purine and pyrimidine receptors. Cell. Mol. Life Sci. 64, 1471–1483 10.1007/s00018-007-6497-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zippel N., Limbach C.A., Ratajski N., Urban C., Luparello C., Pansky A. et al. (2012) Purinergic receptors influence the differentiation of human mesenchymal stem cells. Stem Cells Dev. 21, 884–900 10.1089/scd.2010.0576 [DOI] [PubMed] [Google Scholar]
- 74.Ali S.B., Turner J.J.O. and Fountain S.J. (2018) Constitutive P2Y2 receptor activity regulates basal lipolysis in human adipocytes. J. Cell Sci. 131, jcs221994 10.1242/jcs.221994 [DOI] [PubMed] [Google Scholar]
- 75.Ciciarello M., Zini R., Rossi L., Salvestrini V., Ferrari D., Manfredini R. et al. (2013) Extracellular purines promote the differentiation of human bone marrow-derived mesenchymal stem cells to the osteogenic and adipogenic lineages. Stem Cells Dev. 22, 1097–1111 10.1089/scd.2012.0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li W., Wei S., Liu C., Song M., Wu H. and Yang Y. (2016) Regulation of the osteogenic and adipogenic differentiation of bone marrow-derived stromal cells by extracellular uridine triphosphate: the role of P2Y2 receptor and ERK1/2 signaling. Int. J. Mol. Med. 37, 63–73 10.3892/ijmm.2015.2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Biver G., Wang N., Gartland A., Orriss I., Arnett T.R., Boeynaems J.M. et al. (2013) Role of the P2Y13 receptor in the differentiation of bone marrow stromal cells into osteoblasts and adipocytes. Stem Cells 31, 2747–2758 10.1002/stem.1411 [DOI] [PubMed] [Google Scholar]
- 78.Balasubramanian R., Robaye B., Boeynaems J.M. and Jacobson K.A. (2014) Enhancement of glucose uptake in mouse skeletal muscle cells and adipocytes by P2Y6 receptor agonists. PLoS One 9, e116203 10.1371/journal.pone.0116203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu Z. and Jin T. (2010) Extracellular high dosages of adenosine triphosphate induce inflammatory response and insulin resistance in rat adipocytes. Biochem. Biophys. Res. Commun. 402, 455–460 10.1016/j.bbrc.2010.10.028 [DOI] [PubMed] [Google Scholar]
- 80.Tozzi M. and Novak I. (2017) Purinergic receptors in adipose tissue as potential targets in metabolic disorders. Front. Pharmacol. 8, 878 10.3389/fphar.2017.00878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Richelsen B. (1992) Release and effects of prostaglandins in adipose tissue. Prostaglandins Leukot Essent Fatty Acids 47, 171–182 10.1016/0952-3278(92)90235-B [DOI] [PubMed] [Google Scholar]
- 82.Matsuoka T. and Narumiya S. (2007) Prostaglandin receptor signaling in disease. ScientificWorldJournal 7, 1329–1347 10.1100/tsw.2007.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borglum J.D., Pedersen S.B., Ailhaud G., Negrel R. and Richelsen B. (1999) Differential expression of prostaglandin receptor mRNAs during adipose cell differentiation. Prostaglandins Other Lipid Mediat. 57, 305–317 10.1016/S0090-6980(98)00082-3 [DOI] [PubMed] [Google Scholar]
- 84.Tsuboi H., Sugimoto Y., Kainoh T. and Ichikawa A. (2004) Prostanoid EP4 receptor is involved in suppression of 3T3-L1 adipocyte differentiation. Biochem. Biophys. Res. Commun. 322, 1066–1072 10.1016/j.bbrc.2004.08.018 [DOI] [PubMed] [Google Scholar]
- 85.Inazumi T., Shirata N., Morimoto K., Takano H., Segi-Nishida E. and Sugimoto Y. (2011) Prostaglandin E(2)-EP4 signaling suppresses adipocyte differentiation in mouse embryonic fibroblasts via an autocrine mechanism. J. Lipid Res. 52, 1500–1508 10.1194/jlr.M013615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu H., Fu J.L., Miao Y.F., Wang C.J., Han Q.F., Li S. et al. (2016) Prostaglandin E2 receptor EP3 regulates both adipogenesis and lipolysis in mouse white adipose tissue. J. Mol. Cell Biol. 8, 518–529 10.1093/jmcb/mjw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Casimir D.A., Miller C.W. and Ntambi J.M. (1996) Preadipocyte differentiation blocked by prostaglandin stimulation of prostanoid FP2 receptor in murine 3T3-L1 cells. Differentiation 60, 203–210 10.1046/j.1432-0436.1996.6040203.x [DOI] [PubMed] [Google Scholar]
- 88.Serrero G. and Lepak N.M. (1997) Prostaglandin F2alpha receptor (FP receptor) agonists are potent adipose differentiation inhibitors for primary culture of adipocyte precursors in defined medium. Biochem. Biophys. Res. Commun. 233, 200–202 10.1006/bbrc.1997.6433 [DOI] [PubMed] [Google Scholar]
- 89.Choi H.Y., Lee J.E., Lee J.W., Park H.J., Lee J.E. and Jung J.H. (2012) In vitro study of antiadipogenic profile of latanoprost, travoprost, bimatoprost, and tafluprost in human orbital preadiopocytes. J. Ocul. Pharmacol. Ther. 28, 146–152 10.1089/jop.2011.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fujimori K., Ueno T. and Amano F. (2010) Prostaglandin F(2alpha) suppresses early phase of adipogenesis, but is not associated with osteoblastogenesis in mouse mesenchymal stem cells. Prostaglandins Other Lipid Mediat. 93, 52–59 10.1016/j.prostaglandins.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 91.Negrel R., Gaillard D. and Ailhaud G. (1989) Prostacyclin as a potent effector of adipose-cell differentiation. Biochem. J. 257, 399–405 10.1042/bj2570399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Negrel R. (1999) Prostacyclin as a critical prostanoid in adipogenesis. Prostaglandins Leukot Essent Fatty Acids 60, 383–386 10.1016/S0952-3278(99)80017-9 [DOI] [PubMed] [Google Scholar]
- 93.Aubert J., Saint-Marc P., Belmonte N., Dani C., Negrel R. and Ailhaud G. (2000) Prostacyclin IP receptor up-regulates the early expression of C/EBPbeta and C/EBPdelta in preadipose cells. Mol. Cell. Endocrinol. 160, 149–156 10.1016/S0303-7207(99)00210-5 [DOI] [PubMed] [Google Scholar]
- 94.Wakai E., Aritake K., Urade Y. and Fujimori K. (2017) Prostaglandin D2 enhances lipid accumulation through suppression of lipolysis via DP2 (CRTH2) receptors in adipocytes. Biochem. Biophys. Res. Commun. 490, 393–399 10.1016/j.bbrc.2017.06.053 [DOI] [PubMed] [Google Scholar]
- 95.Chan P.C., Hsiao F.C., Chang H.M., Wabitsch M. and Hsieh P.S. (2016) Importance of adipocyte cyclooxygenase-2 and prostaglandin E2-prostaglandin E receptor 3 signaling in the development of obesity-induced adipose tissue inflammation and insulin resistance. FASEB J. 30, 2282–2297 10.1096/fj.201500127 [DOI] [PubMed] [Google Scholar]
- 96.Yasui M., Tamura Y., Minami M., Higuchi S., Fujikawa R., Ikedo T. et al. (2015) The prostaglandin E2 receptor EP4 regulates obesity-related inflammation and insulin sensitivity. PLoS One 10, e0136304 10.1371/journal.pone.0136304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fujitani Y., Aritake K., Kanaoka Y., Goto T., Takahashi N., Fujimori K. et al. (2010) Pronounced adipogenesis and increased insulin sensitivity caused by overproduction of prostaglandin D2 in vivo. FEBS J. 277, 1410–1419 10.1111/j.1742-4658.2010.07565.x [DOI] [PubMed] [Google Scholar]
- 98.Ghandour R.A., Giroud M., Vegiopoulos A., Herzig S., Ailhaud G., Amri E.Z. et al. (2016) IP-receptor and PPARs trigger the conversion of human white to brite adipocyte induced by carbaprostacyclin. Biochim. Biophys. Acta 1861, 285–293 10.1016/j.bbalip.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 99.Collins S., Daniel K.W. and Rohlfs E.M. (1999) Depressed expression of adipocyte β-adrenergic receptors is a common feature of congenital and diet-induced obesity in rodents. Int. J. Obes. 23, 669–677 10.1038/sj.ijo.0800894 [DOI] [PubMed] [Google Scholar]
- 100.Katsarou M.-S., Karathanasopoulou A., Andrianopoulou A., Desiniotis V., Tzinis E., Dimitrakis E. et al. (2018) Beta 1, beta 2 and beta 3 adrenergic receptor gene polymorphisms in a southeastern European population. Front. Genet. 9, 560 10.3389/fgene.2018.00560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Collins S., Daniel K.W., Rohlfs E.M., Ramkumar V., Taylor I.L. and Gettys T.W. (1994) Impaired expression and functional activity of the beta 3- and beta 1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Mol. Endocrinol. 8, 518–527 10.1210/mend.8.4.7914350 [DOI] [PubMed] [Google Scholar]
- 102.Collins S. (2011) β-Adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front. Endocrinol. (Lausanne) 2, 102 10.3389/fendo.2011.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ueta C.B., Fernandes G.W., Capelo L.P., Fonseca T.L., Maculan F.D.A., Gouveia C.H.A. et al. (2012) Β(1) Adrenergic receptor is key to cold- and diet-induced thermogenesis in mice. J. Endocrinol. 214, 359–365 10.1530/JOE-12-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Soloveva V., Graves R.A., Rasenick M.M., Spiegelman B.M. and Ross S.R. (1997) Transgenic mice overexpressing the β1-adrenergic receptor in adipose tissue are resistant to obesity. Mol. Endocrinol. 11, 27–38 [DOI] [PubMed] [Google Scholar]
- 105.Fernandes G.W., Ueta C.B., Fonseca T.L., Gouveia C.H., Lancellotti C.L., Brum P.C. et al. (2014) Inactivation of the adrenergic receptor beta2 disrupts glucose homeostasis in mice. J. Endocrinol. 221, 381–390 10.1530/JOE-13-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Atgié C., D'Allaire F. and Bukowiecki L.J. (1997) Role of β1- and β3-adrenoceptors in the regulation of lipolysis and thermogenesis in rat brown adipocytes. Am. J. Physiol.-Cell Physiol. 273, C1136–C1142 10.1152/ajpcell.1997.273.4.C1136 [DOI] [PubMed] [Google Scholar]
- 107.Xiao C., Goldgof M., Gavrilova O. and Reitman M.L. (2015) Anti-obesity and metabolic efficacy of the beta3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22 degrees C. Obesity (Silver Spring) 23, 1450–1459 10.1002/oby.21124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Susulic V.S., Frederich R.C., Lawitts J., Tozzo E., Kahn B.B., Harper M.E. et al. (1995) Targeted disruption of the beta 3-adrenergic receptor gene. J. Biol. Chem. 270, 29483–29492 10.1074/jbc.270.49.29483 [DOI] [PubMed] [Google Scholar]
- 109.Revelli J.P., Preitner F., Samec S., Muniesa P., Kuehne F., Boss O. et al. (1997) Targeted gene disruption reveals a leptin-independent role for the mouse beta3-adrenoceptor in the regulation of body composition. J. Clin. Invest. 100, 1098–1106 10.1172/JCI119620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mattsson C.L., Csikasz R.I., Chernogubova E., Yamamoto D.L., Hogberg H.T., Amri E.Z. et al. (2011) Β(1)-Adrenergic receptors increase UCP1 in human MADS brown adipocytes and rescue cold-acclimated beta(3)-adrenergic receptor-knockout mice via nonshivering thermogenesis. Am. J. Physiol. Endocrinol. Metab. 301, E1108–E1118 10.1152/ajpendo.00085.2011 [DOI] [PubMed] [Google Scholar]
- 111.Himms-Hagen J., Cui J., Danforth E. Jr, Taatjes D.J., Lang S.S., Waters B.L. et al. (1994) Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am. J. Physiol. 266, R1371–R1382 10.1152/ajpregu.1994.266.4.R1371 [DOI] [PubMed] [Google Scholar]
- 112.Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K. et al. (2010) The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298, E1244–E1253 10.1152/ajpendo.00600.2009 [DOI] [PubMed] [Google Scholar]
- 113.Jimenez M., Barbatelli G., Allevi R., Cinti S., Seydoux J., Giacobino J.P. et al. (2003) Beta 3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. Eur. J. Biochem. 270, 699–705 10.1046/j.1432-1033.2003.03422.x [DOI] [PubMed] [Google Scholar]
- 114.de Jong J.M.A., Wouters R.T.F., Boulet N., Cannon B., Nedergaard J. and Petrovic N. (2017) The beta3-adrenergic receptor is dispensable for browning of adipose tissues. Am. J. Physiol. Endocrinol. Metab. 312, E508–E518 10.1152/ajpendo.00437.2016 [DOI] [PubMed] [Google Scholar]
- 115.Jiang Y., Berry D.C. and Graff J.M. (2017) Distinct cellular and molecular mechanisms for beta3 adrenergic receptor-induced beige adipocyte formation. eLife 6, e30329 10.7554/eLife.30329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arch J.R. (2011) Challenges in beta(3)-adrenoceptor agonist drug development. Ther. Adv. Endocrinol. Metab. 2, 59–64 10.1177/2042018811398517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lafontan M. and Berlan M. (1981) Alpha-adrenergic receptors and the regulation of lipolysis in adipose tissue. Trends Pharmacol. Sci. 2, 126–129 10.1016/0165-6147(81)90286-8 [DOI] [Google Scholar]
- 118.Lafontan M., Barbe P., Galitzky J., Tavernier G., Langin D., Carpene C. et al. (1997) Adrenergic regulation of adipocyte metabolism. Hum. Reprod. 12 Suppl 1, 6–20 10.1093/humrep/12.suppl_1.6 [DOI] [PubMed] [Google Scholar]
- 119.Robertson J.S. (1977) Future of child health services. Br. Med. J. 1, 375 10.1136/bmj.1.6057.375-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mauriege P., Prud'homme D., Lemieux S., Tremblay A. and Despres J.P. (1995) Regional differences in adipose tissue lipolysis from lean and obese women: existence of postreceptor alterations. Am. J. Physiol. 269, E341–E350 10.1152/ajpcell.1995.269.2.C341 [DOI] [PubMed] [Google Scholar]
- 121.Berman D.M., Nicklas B.J., Rogus E.M., Dennis K.E. and Goldberg A.P. (1998) Regional differences in adrenoceptor binding and fat cell lipolysis in obese, postmenopausal women. Metabolism 47, 467–473 10.1016/S0026-0495(98)90061-0 [DOI] [PubMed] [Google Scholar]
- 122.Lafontan M. and Berlan M. (1995) Fat cell alpha 2-adrenoceptors: the regulation of fat cell function and lipolysis. Endocr. Rev. 16, 716–738 10.1210/edrv-16-6-716 [DOI] [PubMed] [Google Scholar]
- 123.Berlan M. and Lafontan M. (1985) Evidence that epinephrine acts preferentially as an antilipolytic agent in abdominal human subcutaneous fat cells: assessment by analysis of beta and alpha 2 adrenoceptor properties. Eur. J. Clin. Invest. 15, 341–348 10.1111/j.1365-2362.1985.tb00282.x [DOI] [PubMed] [Google Scholar]
- 124.Galitzky J., Larrouy D., Berlan M. and Lafontan M. (1990) New tools for human fat cell alpha-2A adrenoceptor characterization. identification on membranes and on intact cells using the new antagonist [3H]RX821002. J. Pharmacol. Exp. Ther. 252, 312–319 PMID: [PubMed] [Google Scholar]
- 125.Mauriege P., Despres J.P., Prud'homme D., Pouliot M.C., Marcotte M., Tremblay A. et al. (1991) Regional variation in adipose tissue lipolysis in lean and obese men. J. Lipid Res. 32, 1625–1633 PMID: [PubMed] [Google Scholar]
- 126.Mauriege P., Marette A., Atgie C., Bouchard C., Theriault G., Bukowiecki L.K. et al. (1995) Regional variation in adipose tissue metabolism of severely obese premenopausal women. J. Lipid Res. 36, 672–684 PMID: [PubMed] [Google Scholar]
- 127.Carpene C., Berlan M. and Lafontan M. (1983) Influence of development and reduction of fat stores on the antilipolytic alpha 2-adrenoceptor in hamster adipocytes: comparison with adenosine and beta-adrenergic lipolytic responses. J. Lipid Res. 24, 766–774 PMID: [PubMed] [Google Scholar]
- 128.Carpene C., Rebourcet M.C., Guichard C., Lafontan M. and Lavau M. (1990) Increased alpha 2-adrenergic binding sites and antilipolytic effect in adipocytes from genetically obese rats. J. Lipid Res. 31, 811–819 PMID: [PubMed] [Google Scholar]
- 129.Valet P., Grujic D., Wade J., Ito M., Zingaretti M.C., Soloveva V. et al. (2000) Expression of human alpha 2-adrenergic receptors in adipose tissue of beta 3-adrenergic receptor-deficient mice promotes diet-induced obesity. J. Biol. Chem. 275, 34797–34802 10.1074/jbc.M005210200 [DOI] [PubMed] [Google Scholar]
- 130.Hughes C.E. and Nibbs R.J.B. (2018) A guide to chemokines and their receptors. FEBS J. 285, 2944–2971 10.1111/febs.14466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kusuyama J., Komorizono A., Bandow K., Ohnishi T. and Matsuguchi T. (2016) CXCL3 positively regulates adipogenic differentiation. J. Lipid Res. 57, 1806–1820 10.1194/jlr.M067207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dyer D.P., Nebot J.B., Kelly C.J., Medina-Ruiz L., Schuette F. and Graham G.J. (2019) The chemokine receptor CXCR2 contributes to murine adipocyte development. J. Leukoc. Biol. 105, 497–506 10.1002/JLB.1A0618-216RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chavey C., Lazennec G., Lagarrigue S., Clape C., Iankova I., Teyssier J. et al. (2009) CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab. 9, 339–349 10.1016/j.cmet.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hazan U., Romero I.A., Cancello R., Valente S., Perrin V., Mariot V. et al. (2002) Human adipose cells express CD4, CXCR4, and CCR5 [corrected] receptors: a new target cell type for the immunodeficiency virus-1? FASEB J. 16, 1254–1256 10.1096/fj.01-0947fje [DOI] [PubMed] [Google Scholar]
- 135.Yao L., Heuser-Baker J., Herlea-Pana O., Zhang N., Szweda L.I., Griffin T.M. et al. (2014) Deficiency in adipocyte chemokine receptor CXCR4 exacerbates obesity and compromises thermoregulatory responses of brown adipose tissue in a mouse model of diet-induced obesity. FASEB J. 28, 4534–4550 10.1096/fj.14-249797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim D., Kim J., Yoon J.H., Ghim J., Yea K., Song P. et al. (2014) CXCL12 secreted from adipose tissue recruits macrophages and induces insulin resistance in mice. Diabetologia 57, 1456–1465 10.1007/s00125-014-3237-5 [DOI] [PubMed] [Google Scholar]
- 137.Shin J., Fukuhara A., Onodera T., Kita S., Yokoyama C., Otsuki M. et al. (2018) SDF-1 is an autocrine insulin-desensitizing factor in adipocytes. Diabetes 67, 1068–1078 10.2337/db17-0706 [DOI] [PubMed] [Google Scholar]
- 138.Bortolato A., Dore A.S., Hollenstein K., Tehan B.G., Mason J.S. and Marshall F.H. (2014) Structure of class B GPCRs: new horizons for drug discovery. Br. J. Pharmacol. 171, 3132–3145 10.1111/bph.12689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ranganath L.R. (2008) Incretins: pathophysiological and therapeutic implications of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1. J. Clin. Pathol. 61, 401–409 10.1136/jcp.2006.043232 [DOI] [PubMed] [Google Scholar]
- 140.Gutniak M., Orskov C., Holst J.J., Ahren B. and Efendic S. (1992) Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N. Engl. J. Med. 326, 1316–1322 10.1056/NEJM199205143262003 [DOI] [PubMed] [Google Scholar]
- 141.Valverde I., Merida E., Delgado E., Trapote M.A. and Villanueva-Penacarrillo M.L. (1993) Presence and characterization of glucagon-like peptide-1(7-36) amide receptors in solubilized membranes of rat adipose tissue. Endocrinology 132, 75–79 10.1210/endo.132.1.8380388 [DOI] [PubMed] [Google Scholar]
- 142.Montrose-Rafizadeh C., Yang H., Wang Y., Roth J., Montrose M.H. and Adams L.G. (1997) Novel signal transduction and peptide specificity of glucagon-like peptide receptor in 3T3-L1 adipocytes. J. Cell Physiol. 172, 275–283 [DOI] [PubMed] [Google Scholar]
- 143.Egan J.M., Montrose-Rafizadeh C., Wang Y., Bernier M. and Roth J. (1994) Glucagon-like peptide-1(7-36) amide (GLP-1) enhances insulin-stimulated glucose metabolism in 3T3-L1 adipocytes: one of several potential extrapancreatic sites of GLP-1 action. Endocrinology 135, 2070–2075 10.1210/endo.135.5.7956929 [DOI] [PubMed] [Google Scholar]
- 144.Vendrell J., El Bekay R., Peral B., Garcia-Fuentes E., Megia A., Macias-Gonzalez M. et al. (2011) Study of the potential association of adipose tissue GLP-1 receptor with obesity and insulin resistance. Endocrinology 152, 4072–4079 10.1210/en.2011-1070 [DOI] [PubMed] [Google Scholar]
- 145.Ruiz-Grande C., Alarcon C., Merida E. and Valverde I. (1992) Lipolytic action of glucagon-like peptides in isolated rat adipocytes. Peptides 13, 13–16 10.1016/0196-9781(92)90134-O [DOI] [PubMed] [Google Scholar]
- 146.Villanueva-Penacarrillo M.L., Marquez L., Gonzalez N., Diaz-Miguel M. and Valverde I. (2001) Effect of GLP-1 on lipid metabolism in human adipocytes. Horm. Metab. Res. 33, 73–77 10.1055/s-2001-12428 [DOI] [PubMed] [Google Scholar]
- 147.Oben J., Morgan L., Fletcher J. and Marks V. (1991) Effect of the entero-pancreatic hormones, gastric inhibitory polypeptide and glucagon-like polypeptide-1(7-36) amide, on fatty acid synthesis in explants of rat adipose tissue. J. Endocrinol. 130, 267–272 10.1677/joe.0.1300267 [DOI] [PubMed] [Google Scholar]
- 148.Miki H., Namba M., Nishimura T., Mineo I., Matsumura T., Miyagawa J. et al. (1996) Glucagon-like peptide-1(7-36)amide enhances insulin-stimulated glucose uptake and decreases intracellular cAMP content in isolated rat adipocytes. Biochim. Biophys. Acta 1312, 132–136 10.1016/0167-4889(96)00032-8 [DOI] [PubMed] [Google Scholar]
- 149.Perea A., Vinambres C., Clemente F., Villanueva-Penacarrillo M.L. and Valverde I. (1997) GLP-1 (7-36) amide: effects on glucose transport and metabolism in rat adipose tissue. Horm. Metab. Res. 29, 417–421 10.1055/s-2007-979068 [DOI] [PubMed] [Google Scholar]
- 150.Wang Y., Kole H.K., Montrose-Rafizadeh C., Perfetti R., Bernier M. and Egan J.M. (1997) Regulation of glucose transporters and hexose uptake in 3T3-L1 adipocytes: glucagon-like peptide-1 and insulin interactions. J. Mol. Endocrinol. 19, 241–248 10.1677/jme.0.0190241 [DOI] [PubMed] [Google Scholar]
- 151.Gao H., Wang X., Zhang Z., Yang Y., Yang J., Li X. et al. (2007) GLP-1 amplifies insulin signaling by up-regulation of IRbeta, IRS-1 and Glut4 in 3T3-L1 adipocytes. Endocrine 32, 90–95 10.1007/s12020-007-9011-4 [DOI] [PubMed] [Google Scholar]
- 152.Jiang Y., Wang Z., Ma B., Fan L., Yi N., Lu B. et al. (2018) GLP-1 improves adipocyte insulin sensitivity following induction of endoplasmic reticulum stress. Front. Pharmacol. 9, 1168 10.3389/fphar.2018.01168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Challa T.D., Beaton N., Arnold M., Rudofsky G., Langhans W. and Wolfrum C. (2012) Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J. Biol. Chem. 287, 6421–6430 10.1074/jbc.M111.310342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tanaka K., Masaki Y., Tanaka M., Miyazaki M., Enjoji M., Nakamuta M. et al. (2014) Exenatide improves hepatic steatosis by enhancing lipid use in adipose tissue in nondiabetic rats. World J. Gastroenterol. 20, 2653–2663 10.3748/wjg.v20.i10.2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee Y.S., Park M.S., Choung J.S., Kim S.S., Oh H.H., Choi C.S. et al. (2012) Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 55, 2456–2468 10.1007/s00125-012-2592-3 [DOI] [PubMed] [Google Scholar]
- 156.Gastaldelli A., Gaggini M., Daniele G., Ciociaro D., Cersosimo E., Tripathy D. et al. (2016) Exenatide improves both hepatic and adipose tissue insulin resistance: a dynamic positron emission tomography study. Hepatology 64, 2028–2037 10.1002/hep.28827 [DOI] [PubMed] [Google Scholar]
- 157.Izaguirre M., Gomez-Ambrosi J., Rodriguez A., Ramirez B., Becerril S., Valenti V. et al. (2019) GLP-1 limits adipocyte inflammation and its low circulating pre-operative concentrations predict worse type 2 diabetes remission after bariatric surgery in obese patients. J. Clin. Med. 8, 479 10.3390/jcm8040479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yip R.G., Boylan M.O., Kieffer T.J. and Wolfe M.M. (1998) Functional GIP receptors are present on adipocytes. Endocrinology 139, 4004–4007 10.1210/endo.139.9.6288 [DOI] [PubMed] [Google Scholar]
- 159.Hauner H., Glatting G., Kaminska D. and Pfeiffer E.F. (1988) Effects of gastric inhibitory polypeptide on glucose and lipid metabolism of isolated rat adipocytes. Ann. Nutr. Metab. 32, 282–288 10.1159/000177467 [DOI] [PubMed] [Google Scholar]
- 160.Knapper J.M., Puddicombe S.M., Morgan L.M. and Fletcher J.M. (1995) Investigations into the actions of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1(7-36)amide on lipoprotein lipase activity in explants of rat adipose tissue. J. Nutr. 125, 183–188 10.1093/jn/125.2.183 [DOI] [PubMed] [Google Scholar]
- 161.Kim S.J., Nian C. and McIntosh C.H. (2007) Activation of lipoprotein lipase by glucose-dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP-activated protein kinase cascade. J. Biol. Chem. 282, 8557–8567 10.1074/jbc.M609088200 [DOI] [PubMed] [Google Scholar]
- 162.Song D.H., Getty-Kaushik L., Tseng E., Simon J., Corkey B.E. and Wolfe M.M. (2007) Glucose-dependent insulinotropic polypeptide enhances adipocyte development and glucose uptake in part through Akt activation. Gastroenterology 133, 1796–1805 10.1053/j.gastro.2007.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miyawaki K., Yamada Y., Ban N., Ihara Y., Tsukiyama K., Zhou H. et al. (2002) Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat. Med. 8, 738–742 10.1038/nm727 [DOI] [PubMed] [Google Scholar]
- 164.Joo E., Harada N., Yamane S., Fukushima T., Taura D., Iwasaki K. et al. (2017) Inhibition of gastric inhibitory polypeptide receptor signaling in adipose tissue reduces insulin resistance and hepatic steatosis in high-fat diet-fed mice. Diabetes 66, 868–879 10.2337/db16-0758 [DOI] [PubMed] [Google Scholar]
- 165.Paavola K.J. and Hall R.A. (2012) Adhesion G protein-coupled receptors: signaling, pharmacology, and mechanisms of activation. Mol. Pharmacol. 82, 777–783 10.1124/mol.112.080309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Suchy T., Zieschang C., Popkova Y., Kaczmarek I., Weiner J., Liebing A. D. et al. (2020) The repertoire of adhesion G protein-coupled receptors in adipocytes and their functional relevance. Int. J. Obes. (Lond) 10.1038/s41366-020-0570-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nie T., Hui X., Gao X., Li K., Lin W., Xiang X. et al. (2012) Adipose tissue deletion of Gpr116 impairs insulin sensitivity through modulation of adipose function. FEBS Lett. 586, 3618–3625 10.1016/j.febslet.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 168.Huang H.C. and Klein P.S. (2004) The Frizzled family: receptors for multiple signal transduction pathways. Genome Biol. 5, 234 10.1186/gb-2004-5-7-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ackers I. and Malgor R. (2018) Interrelationship of canonical and non-canonical Wnt signalling pathways in chronic metabolic diseases. Diab. Vasc. Dis. Res. 15, 3–13 10.1177/1479164117738442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van Amerongen R., Mikels A. and Nusse R. (2008) Alternative wnt signaling is initiated by distinct receptors. Sci. Signal. 1, re9 10.1126/scisignal.135re9 [DOI] [PubMed] [Google Scholar]
- 171.Ross S.E., Hemati N., Longo K.A., Bennett C.N., Lucas P.C., Erickson R.L. et al. (2000) Inhibition of adipogenesis by Wnt signaling. Science 289, 950–953 10.1126/science.289.5481.950 [DOI] [PubMed] [Google Scholar]
- 172.Kennell J.A. and MacDougald O.A. (2005) Wnt signaling inhibits adipogenesis through beta-catenin-dependent and -independent mechanisms. J. Biol. Chem. 280, 24004–24010 10.1074/jbc.M501080200 [DOI] [PubMed] [Google Scholar]
- 173.Gutmann T., Kim K.H., Grzybek M., Walz T. and Coskun U. (2018) Visualization of ligand-induced transmembrane signaling in the full-length human insulin receptor. J. Cell Biol. 217, 1643–1649 10.1083/jcb.201711047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Federici M., Porzio O., Zucaro L., Giovannone B., Borboni P., Marini M.A. et al. (1997) Increased abundance of insulin/IGF-I hybrid receptors in adipose tissue from NIDDM patients. Mol. Cell. Endocrinol. 135, 41–47 10.1016/S0303-7207(97)00185-8 [DOI] [PubMed] [Google Scholar]
- 175.Belfiore A., Malaguarnera R., Vella V., Lawrence M.C., Sciacca L., Frasca F. et al. (2017) Insulin receptor isoforms in physiology and disease: an updated view. Endocr. Rev. 38, 379–431 10.1210/er.2017-00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cignarelli A., Genchi V.A., Perrini S., Natalicchio A., Laviola L. and Giorgino F. (2019) Insulin and insulin receptors in adipose tissue development. Int. J. Mol. Sci. 20, 759 10.3390/ijms20030759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ussar S., Bezy O., Bluher M. and Kahn C.R. (2012) Glypican-4 enhances insulin signaling via interaction with the insulin receptor and serves as a novel adipokine. Diabetes 61, 2289–2298 10.2337/db11-1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee J. and Pilch P.F. (1994) The insulin receptor: structure, function, and signaling. Am. J. Physiol. 266, C319–C334 10.1152/ajpcell.1994.266.2.C319 [DOI] [PubMed] [Google Scholar]
- 179.Bluher S., Kratzsch J. and Kiess W. (2005) Insulin-like growth factor I, growth hormone and insulin in white adipose tissue. Best Pract. Res. Clin. Endocrinol. Metab. 19, 577–587 10.1016/j.beem.2005.07.011 [DOI] [PubMed] [Google Scholar]
- 180.Smith P.J., Wise L.S., Berkowitz R., Wan C. and Rubin C.S. (1988) Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 263, 9402–9408 PMID: [PubMed] [Google Scholar]
- 181.Scavo L.M., Karas M., Murray M. and Leroith D. (2004) Insulin-like growth factor-I stimulates both cell growth and lipogenesis during differentiation of human mesenchymal stem cells into adipocytes. J. Clin. Endocrinol. Metab. 89, 3543–3553 10.1210/jc.2003-031682 [DOI] [PubMed] [Google Scholar]
- 182.Gupta M.K., De Jesus D.F., Kahraman S., Valdez I.A., Shamsi F., Yi L. et al. (2018) Insulin receptor-mediated signaling regulates pluripotency markers and lineage differentiation. Mol. Metab. 18, 153–163 10.1016/j.molmet.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Boucher J., Kleinridders A. and Kahn C.R. (2014) Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 6, a009191 10.1101/cshperspect.a009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Boucher J., Softic S., El Ouaamari A., Krumpoch M.T., Kleinridders A., Kulkarni R.N. et al. (2016) Differential roles of insulin and IGF-1 receptors in adipose tissue development and function. Diabetes 65, 2201–2213 10.2337/db16-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kloting N., Koch L., Wunderlich T., Kern M., Ruschke K., Krone W. et al. (2008) Autocrine IGF-1 action in adipocytes controls systemic IGF-1 concentrations and growth. Diabetes 57, 2074–2082 10.2337/db07-1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bluher M., Michael M.D., Peroni O.D., Ueki K., Carter N., Kahn B.B. et al. (2002) Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell 3, 25–38 10.1016/S1534-5807(02)00199-5 [DOI] [PubMed] [Google Scholar]
- 187.Boucher J., Mori M.A., Lee K.Y., Smyth G., Liew C.W., Macotela Y. et al. (2012) Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signalling. Nat. Commun. 3, 902 10.1038/ncomms1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Boucher J., Tseng Y.H. and Kahn C.R. (2010) Insulin and insulin-like growth factor-1 receptors act as ligand-specific amplitude modulators of a common pathway regulating gene transcription. J. Biol. Chem. 285, 17235–17245 10.1074/jbc.M110.118620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Entingh-Pearsall A. and Kahn C.R. (2004) Differential roles of the insulin and insulin-like growth factor-I (IGF-I) receptors in response to insulin and IGF-I. J. Biol. Chem. 279, 38016–38024 10.1074/jbc.M313201200 [DOI] [PubMed] [Google Scholar]
- 190.Korner A., Wabitsch M., Seidel B., Fischer-Posovszky P., Berthold A., Stumvoll M. et al. (2005) Adiponectin expression in humans is dependent on differentiation of adipocytes and down-regulated by humoral serum components of high molecular weight. Biochem. Biophys. Res. Commun. 337, 540–550 10.1016/j.bbrc.2005.09.064 [DOI] [PubMed] [Google Scholar]
- 191.Hu E., Liang P. and Spiegelman B.M. (1996) Adipoq is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 271, 10697–10703 10.1074/jbc.271.18.10697 [DOI] [PubMed] [Google Scholar]
- 192.Back K. and Arnqvist H.J. (2009) Changes in insulin and IGF-I receptor expression during differentiation of human preadipocytes. Growth Horm. IGF Res. 19, 101–111 10.1016/j.ghir.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 193.Scherer P.E., Williams S., Fogliano M., Baldini G. and Lodish H.F. (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 10.1074/jbc.270.45.26746 [DOI] [PubMed] [Google Scholar]
- 194.Demoulin J.B. and Montano-Almendras C.P. (2012) Platelet-derived growth factors and their receptors in normal and malignant hematopoiesis. Am. J. Blood. Res. 2, 44–56 PMID: [PMC free article] [PubMed] [Google Scholar]
- 195.Farahani R.M. and Xaymardan M. (2015) Platelet-derived growth factor receptor alpha as a marker of mesenchymal stem cells in development and stem cell biology. Stem Cells Int. 2015, 362753 10.1155/2015/362753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vaziri C. and Faller D.V. (1996) Down-regulation of platelet-derived growth factor receptor expression during terminal differentiation of 3T3-L1 pre-adipocyte fibroblasts. J. Biol. Chem. 271, 13642–13648 10.1074/jbc.271.23.13642 [DOI] [PubMed] [Google Scholar]
- 197.Krieger-Brauer H.I. and Kather H. (1995) Antagonistic effects of different members of the fibroblast and platelet-derived growth factor families on adipose conversion and NADPH-dependent H2O2 generation in 3T3 L1-cells. Biochem. J. 307, 549–556 10.1042/bj3070549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bachmeier M. and Loffler G. (1995) Influence of growth factors on growth and differentiation of 3T3-L1 preadipocytes in serum-free conditions. Eur. J. Cell Biol. 68, 323–329 PMID: [PubMed] [Google Scholar]
- 199.Staiger H. and Loffler G. (1998) The role of PDGF-dependent suppression of apoptosis in differentiating 3T3-L1 preadipocytes. Eur. J. Cell Biol. 77, 220–227 10.1016/S0171-9335(98)80110-6 [DOI] [PubMed] [Google Scholar]
- 200.Hauner H., Rohrig K. and Petruschke T. (1995) Effects of epidermal growth factor (EGF), platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) on human adipocyte development and function. Eur. J. Clin. Invest. 25, 90–96 10.1111/j.1365-2362.1995.tb01532.x [DOI] [PubMed] [Google Scholar]
- 201.Artemenko Y., Gagnon A., Aubin D. and Sorisky A. (2005) Anti-adipogenic effect of PDGF is reversed by PKC inhibition. J. Cell Physiol. 204, 646–653 10.1002/jcp.20314 [DOI] [PubMed] [Google Scholar]
- 202.Gagnon A., Landry A. and Sorisky A. (2009) IKKbeta and the anti-adipogenic effect of platelet-derived growth factor in human abdominal subcutaneous preadipocytes. J. Endocrinol. 201, 75–80 10.1677/JOE-08-0411 [DOI] [PubMed] [Google Scholar]
- 203.Fitter S., Vandyke K., Gronthos S. and Zannettino A.C. (2012) Suppression of PDGF-induced PI3 kinase activity by imatinib promotes adipogenesis and adiponectin secretion. J. Mol. Endocrinol. 48, 229–240 10.1530/JME-12-0003 [DOI] [PubMed] [Google Scholar]
- 204.Ohta H. and Itoh N. (2014) Roles of FGFs as adipokines in adipose tissue development, remodeling, and metabolism. Front. Endocrinol. (Lausanne) 5, 18 10.3389/fendo.2014.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kurosu H., Choi M., Ogawa Y., Dickson A.S., Goetz R., Eliseenkova A.V. et al. (2007) Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 282, 26687–26695 10.1074/jbc.M704165200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang C., Jin C., Li X., Wang F., McKeehan W.L. and Luo Y. (2012) Differential specificity of endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in complex with KLB. PLoS One 7, e33870 10.1371/journal.pone.0033870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gabrielsson B.G., Johansson J.M., Jennische E., Jernas M., Itoh Y., Peltonen M. et al. (2002) Depot-specific expression of fibroblast growth factors in human adipose tissue. Obes. Res. 10, 608–616 10.1038/oby.2002.83 [DOI] [PubMed] [Google Scholar]
- 208.Suzuki M., Uehara Y., Motomura-Matsuzaka K., Oki J., Koyama Y., Kimura M. et al. (2008) Betaklotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol. Endocrinol. 22, 1006–1014 10.1210/me.2007-0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Widberg C.H., Newell F.S., Bachmann A.W., Ramnoruth S.N., Spelta M.C., Whitehead J.P. et al. (2009) Fibroblast growth factor receptor 1 is a key regulator of early adipogenic events in human preadipocytes. Am. J. Physiol. Endocrinol. Metab. 296, E121–E131 10.1152/ajpendo.90602.2008 [DOI] [PubMed] [Google Scholar]
- 210.Kahkonen T.E., Ivaska K.K., Jiang M., Buki K.G., Vaananen H.K. and Harkonen P.L. (2018) Role of fibroblast growth factor receptors (FGFR) and FGFR like-1 (FGFRL1) in mesenchymal stromal cell differentiation to osteoblasts and adipocytes. Mol. Cell Endocrinol. 461, 194–204 10.1016/j.mce.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 211.Patel N.G., Kumar S. and Eggo M.C. (2005) Essential role of fibroblast growth factor signaling in preadipoctye differentiation. J. Clin. Endocrinol. Metab. 90, 1226–1232 10.1210/jc.2004-1309 [DOI] [PubMed] [Google Scholar]
- 212.Wang S., Cao S., Arhatte M., Li D., Shi Y., Kurz S. et al. (2020) Adipocyte Piezo1 mediates obesogenic adipogenesis through the FGF1/FGFR1 signaling pathway in mice. Nat. Commun. 11, 2303 10.1038/s41467-020-16026-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wu A.L., Kolumam G., Stawicki S., Chen Y., Li J., Zavala-Solorio J. et al. (2011) Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci. Transl. Med. 3, 113ra126 10.1126/scitranslmed.3002669 [DOI] [PubMed] [Google Scholar]
- 214.Lewis J.E., Samms R.J., Cooper S., Luckett J.C., Perkins A.C., Dunbar J.D. et al. (2017) Antibody-mediated targeting of the FGFR1c isoform increases glucose uptake in white and brown adipose tissue in male mice. Endocrinology 158, 3090–3096 10.1210/en.2017-00591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Suh J.M., Jonker J.W., Ahmadian M., Goetz R., Lackey D., Osborn O. et al. (2014) Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature 513, 436–439 10.1038/nature13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yang C., Wang C., Ye M., Jin C., He W., Wang F. et al. (2012) Control of lipid metabolism by adipocyte FGFR1-mediated adipohepatic communication during hepatic stress. Nutr. Metab. (Lond) 9, 94 10.1186/1743-7075-9-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ye M., Lu W., Wang X., Wang C., Abbruzzese J.L., Liang G. et al. (2016) FGF21-FGFR1 coordinates phospholipid homeostasis, lipid droplet function, and ER stress in obesity. Endocrinology 157, 4754–4769 10.1210/en.2016-1710 [DOI] [PubMed] [Google Scholar]
- 218.Dolegowska K., Marchelek-Mysliwiec M., Nowosiad-Magda M., Slawinski M. and Dolegowska B. (2019) FGF19 subfamily members: FGF19 and FGF21. J. Physiol. Biochem. 75, 229–240 10.1007/s13105-019-00675-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ogawa Y., Kurosu H., Yamamoto M., Nandi A., Rosenblatt K.P., Goetz R. et al. (2007) Betaklotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl. Acad. Sci. U.S.A. 104, 7432–7437 10.1073/pnas.0701600104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fisher F.M., Chui P.C., Antonellis P.J., Bina H.A., Kharitonenkov A., Flier J.S. et al. (2010) Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 59, 2781–2789 10.2337/db10-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nygaard E. B., Moller C. L., Kievit P., Grove K. L. and Andersen B (2014) Increased fibroblast growth factor 21 expression in high-fat diet-sensitive non-human primates (Macaca mulatta). Int. J. Obes. (Lond) 38, 183–191 10.1038/ijo.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Foltz I.N., Hu S., King C., Wu X., Yang C., Wang W. et al. (2012) Treating diabetes and obesity with an FGF21-mimetic antibody activating the betaKlotho/FGFR1c receptor complex. Sci. Transl. Med. 4, 162ra153 10.1126/scitranslmed.3004690 [DOI] [PubMed] [Google Scholar]
- 223.Adams A.C., Yang C., Coskun T., Cheng C.C., Gimeno R.E., Luo Y. et al. (2012) The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Mol. Metab. 2, 31–37 10.1016/j.molmet.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ding X., Boney-Montoya J., Owen B.M., Bookout A.L., Coate K.C., Mangelsdorf D.J. et al. (2012) Betaklotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 16, 387–393 10.1016/j.cmet.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kolumam G., Chen M.Z., Tong R., Zavala-Solorio J., Kates L., van Bruggen N. et al. (2015) Sustained brown fat stimulation and insulin sensitization by a humanized bispecific antibody agonist for fibroblast growth factor receptor 1/betaKlotho complex. EBioMedicine 2, 730–743 10.1016/j.ebiom.2015.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.BonDurant L.D., Ameka M., Naber M.C., Markan K.R., Idiga S.O., Acevedo M.R. et al. (2017) FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab. 25, 935–944 10.1016/j.cmet.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chen M.Z., Chang J.C., Zavala-Solorio J., Kates L., Thai M., Ogasawara A. et al. (2017) FGF21 mimetic antibody stimulates UCP1-independent brown fat thermogenesis via FGFR1/betaKlotho complex in non-adipocytes. Mol. Metab. 6, 1454–1467 10.1016/j.molmet.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shamsi F., Xue R., Huang T.L., Lundh M., Liu Y., Leiria L.O. et al. (2020) FGF6 and FGF9 regulate UCP1 expression independent of brown adipogenesis. Nat. Commun. 11, 1421 10.1038/s41467-020-15055-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Idriss H.T. and Naismith J.H. (2000) TNFα and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 50, 184–195 [DOI] [PubMed] [Google Scholar]
- 230.Hotamisligil G.S., Shargill N.S. and Spiegelman B.M. (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 10.1126/science.7678183 [DOI] [PubMed] [Google Scholar]
- 231.Xu H., Uysal K.T., Becherer J.D., Arner P. and Hotamisligil G.S. (2002) Altered tumor necrosis factor-alpha (TNF-α) processing in adipocytes and increased expression of transmembrane TNF-α in obesity. Diabetes 51, 1876–1883 10.2337/diabetes.51.6.1876 [DOI] [PubMed] [Google Scholar]
- 232.Cawthorn W.P. and Sethi J.K. (2008) TNF-α and adipocyte biology. FEBS Lett. 582, 117–131 10.1016/j.febslet.2007.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bremer E. (2013) Targeting of the tumor necrosis factor receptor superfamily for cancer immunotherapy. ISRN Oncol. 2013, 371854 10.1155/2013/371854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gatanaga T., Hwang C.D., Kohr W., Cappuccini F., Lucci J.A. III, Jeffes E.W. et al. (1990) Purification and characterization of an inhibitor (soluble tumor necrosis factor receptor) for tumor necrosis factor and lymphotoxin obtained from the serum ultrafiltrates of human cancer patients. Proc. Natl. Acad. Sci. U.S.A. 87, 8781–8784 10.1073/pnas.87.22.8781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ninomiya-Tsuji J., Torti F.M. and Ringold G.M. (1993) Tumor necrosis factor-induced c-myc expression in the absence of mitogenesis is associated with inhibition of adipocyte differentiation. Proc. Natl. Acad. Sci. U.S.A. 90, 9611–9615 10.1073/pnas.90.20.9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cawthorn W.P., Heyd F., Hegyi K. and Sethi J.K. (2007) Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ. 14, 1361–1373 10.1038/sj.cdd.4402127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Xu H., Sethi J.K. and Hotamisligil G.S. (1999) Transmembrane tumor necrosis factor (TNF)-alpha inhibits adipocyte differentiation by selectively activating TNF receptor 1. J. Biol. Chem. 274, 26287–26295 10.1074/jbc.274.37.26287 [DOI] [PubMed] [Google Scholar]
- 238.Castro-Munozledo F., Beltran-Langarica A. and Kuri-Harcuch W. (2003) Commitment of 3T3-F442A cells to adipocyte differentiation takes place during the first 24-36 h after adipogenic stimulation: TNF-α inhibits commitment. Exp. Cell Res. 284, 163–172 10.1016/S0014-4827(02)00036-8 [DOI] [PubMed] [Google Scholar]
- 239.Torti F.M., Dieckmann B., Beutler B., Cerami A. and Ringold G.M. (1985) A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science 229, 867–869 10.1126/science.3839597 [DOI] [PubMed] [Google Scholar]
- 240.Torti F.M., Torti S.V., Larrick J.W. and Ringold G.M. (1989) Modulation of adipocyte differentiation by tumor necrosis factor and transforming growth factor beta. J. Cell Biol. 108, 1105–1113 10.1083/jcb.108.3.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Peraldi P., Hotamisligil G.S., Buurman W.A., White M.F. and Spiegelman B.M. (1996) Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J. Biol. Chem. 271, 13018–13022 10.1074/jbc.271.22.13018 [DOI] [PubMed] [Google Scholar]
- 242.Ruan H., Hacohen N., Golub T.R., Van Parijs L. and Lodish H.F. (2002) Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-κB activation by TNF-α is obligatory. Diabetes 51, 1319–1336 10.2337/diabetes.51.5.1319 [DOI] [PubMed] [Google Scholar]
- 243.Ruan H., Miles P.D., Ladd C.M., Ross K., Golub T.R., Olefsky J.M. et al. (2002) Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha: implications for insulin resistance. Diabetes 51, 3176–3188 10.2337/diabetes.51.11.3176 [DOI] [PubMed] [Google Scholar]
- 244.Stephens J.M., Lee J. and Pilch P.F. (1997) Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J. Biol. Chem. 272, 971–976 10.1074/jbc.272.2.971 [DOI] [PubMed] [Google Scholar]
- 245.Sethi J.K., Xu H., Uysal K.T., Wiesbrock S.M., Scheja L. and Hotamisligil G.S. (2000) Characterisation of receptor-specific TNFα functions in adipocyte cell lines lacking type 1 and 2 TNF receptors. FEBS Lett. 469, 77–82 10.1016/S0014-5793(00)01250-3 [DOI] [PubMed] [Google Scholar]
- 246.Uysal K.T., Wiesbrock S.M. and Hotamisligil G.S. (1998) Functional analysis of tumor necrosis factor (TNF) receptors in TNF-α-mediated insulin resistance in genetic obesity. Endocrinology 139, 4832–4838 10.1210/endo.139.12.6337 [DOI] [PubMed] [Google Scholar]
- 247.Uysal K.T., Wiesbrock S.M., Marino M.W. and Hotamisligil G.S. (1997) Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389, 610–614 10.1038/39335 [DOI] [PubMed] [Google Scholar]
- 248.Cawthorn W.P. and Sethi J.K. (2008) TNF-α and adipocyte biology. FEBS Lett. 582, 117–131 10.1016/j.febslet.2007.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Choy L., Skillington J. and Derynck R. (2000) Roles of autocrine TGF-β receptor and smad signaling in adipocyte differentiation. J. Cell Biol. 149, 667–682 10.1083/jcb.149.3.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Taha M.F., Valojerdi M.R. and Mowla S.J. (2006) Effect of bone morphogenetic protein-4 (BMP-4) on adipocyte differentiation from mouse embryonic stem cells. Anat. Histol. Embryol. 35, 271–278 10.1111/j.1439-0264.2006.00680.x [DOI] [PubMed] [Google Scholar]
- 251.Tang Q.Q., Otto T.C. and Lane M.D. (2004) Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. U.S.A. 101, 9607–9611 10.1073/pnas.0403100101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Modica S. and Wolfrum C. (2017) The dual role of BMP4 in adipogenesis and metabolism. Adipocyte 6, 141–146 10.1080/21623945.2017.1287637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chen T.L., Shen W.J. and Kraemer F.B. (2001) Human BMP-7/OP-1 induces the growth and differentiation of adipocytes and osteoblasts in bone marrow stromal cell cultures. J. Cell Biochem. 82, 187–199 10.1002/jcb.1145 [DOI] [PubMed] [Google Scholar]
- 254.Neumann K., Endres M., Ringe J., Flath B., Manz R., Haupl T. et al. (2007) BMP7 promotes adipogenic but not osteo-/chondrogenic differentiation of adult human bone marrow-derived stem cells in high-density micro-mass culture. J. Cell Biochem. 102, 626–637 10.1002/jcb.21319 [DOI] [PubMed] [Google Scholar]
- 255.Tseng Y.H., Kokkotou E., Schulz T.J., Huang T.L., Winnay J.N., Taniguchi C.M. et al. (2008) New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454, 1000–1004 10.1038/nature07221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ignotz R.A. and Massague J. (1985) Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 82, 8530–8534 10.1073/pnas.82.24.8530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Richardson R.L., Campion D.R., Hausman G.J. and Wright J.T. (1989) Transforming growth factor type beta (TGF-β) and adipogenesis in pigs. J. Anim. Sci. 67, 2171–2180 10.2527/jas1989.6782171x [DOI] [PubMed] [Google Scholar]
- 258.Wankhade U.D., Lee J.H., Dagur P.K., Yadav H., Shen M., Chen W. et al. (2018) TGF-β receptor 1 regulates progenitors that promote browning of white fat. Mol. Metab. 16, 160–171 10.1016/j.molmet.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Andersson O., Korach-Andre M., Reissmann E., Ibanez C.F. and Bertolino P. (2008) Growth/differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc. Natl. Acad. Sci. U.S.A. 105, 7252–7256 10.1073/pnas.0800272105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bu Y., Okunishi K., Yogosawa S., Mizuno K., Irudayam M.J., Brown C.W. et al. (2018) Insulin regulates lipolysis and fat mass by upregulating growth/differentiation factor 3 in adipose tissue macrophages. Diabetes 67, 1761–1772 10.2337/db17-1201 [DOI] [PubMed] [Google Scholar]
- 261.Yogosawa S., Mizutani S., Ogawa Y. and Izumi T. (2013) Activin receptor-like kinase 7 suppresses lipolysis to accumulate fat in obesity through downregulation of peroxisome proliferator-activated receptor gamma and C/EBPalpha. Diabetes 62, 115–123 10.2337/db12-0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Barker B. S., Young G. T., Soubrane C. H., Stephens G. J., Stevens E. B. and Patel M. K. (2017) Chapter 2 - Ion Channels In Conn's Translational Neuroscience (P. M. Conn, ed.). pp. 11–43, Academic Press, San Diego [Google Scholar]
- 263.Zhang L.L., Yan Liu D., Ma L.Q., Luo Z.D., Cao T.B., Zhong J. et al. (2007) Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ. Res. 100, 1063–1070 10.1161/01.RES.0000262653.84850.8b [DOI] [PubMed] [Google Scholar]
- 264.Hu H., Li D.L., Fan L., Ren J., Wang S.P., Jia B. et al. (2010) Involvement of volume-sensitive Cl- channels in the proliferation of human subcutaneous pre-adipocytes. Clin. Exp. Pharmacol. Physiol. 37, 29–34 10.1111/j.1440-1681.2009.05223.x [DOI] [PubMed] [Google Scholar]
- 265.Hu H., He M.L., Tao R., Sun H.Y., Hu R., Zang W.J. et al. (2009) Characterization of ion channels in human preadipocytes. J. Cell Physiol. 218, 427–435 10.1002/jcp.21617 [DOI] [PubMed] [Google Scholar]
- 266.Li W., Li G., Zhang Y., Wei S., Song M., Wang W. et al. (2015) Role of P2 × 7 receptor in the differentiation of bone marrow stromal cells into osteoblasts and adipocytes. Exp. Cell Res. 339, 367–379 10.1016/j.yexcr.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 267.Vasconcelos L.H., Souza I.L., Pinheiro L.S. and Silva B.A. (2016) Ion channels in obesity: pathophysiology and potential therapeutic targets. Front. Pharmacol. 7, 58 10.3389/fphar.2016.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Dimitriadis G., Mitrou P., Lambadiari V., Maratou E. and Raptis S.A. (2011) Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pract. 93 Suppl 1, S52–S59 10.1016/S0168-8227(11)70014-6 [DOI] [PubMed] [Google Scholar]
- 269.Mueckler M. and Thorens B. (2013) The SLC2 (GLUT) family of membrane transporters. Mol. Aspects Med. 34, 121–138 10.1016/j.mam.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.James D.E., Brown R., Navarro J. and Pilch P.F. (1988) Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature 333, 183–185 10.1038/333183a0 [DOI] [PubMed] [Google Scholar]
- 271.Birnbaum M.J. (1989) Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell 57, 305–315 10.1016/0092-8674(89)90968-9 [DOI] [PubMed] [Google Scholar]
- 272.James D.E., Strube M. and Mueckler M. (1989) Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature 338, 83–87 10.1038/338083a0 [DOI] [PubMed] [Google Scholar]
- 273.Abel E.D., Peroni O., Kim J.K., Kim Y.B., Boss O., Hadro E. et al. (2001) Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409, 729–733 10.1038/35055575 [DOI] [PubMed] [Google Scholar]
- 274.Shepherd P.R., Gnudi L., Tozzo E., Yang H., Leach F. and Kahn B.B. (1993) Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J. Biol. Chem. 268, 22243–22246 PMID: [PubMed] [Google Scholar]
- 275.Carvalho E., Kotani K., Peroni O.D. and Kahn B.B. (2005) Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am. J. Physiol. Endocrinol. Metab. 289, E551–E561 10.1152/ajpendo.00116.2005 [DOI] [PubMed] [Google Scholar]
- 276.Abumrad N.A., Perkins R.C., Park J.H. and Park C.R. (1981) Mechanism of long chain fatty acid permeation in the isolated adipocyte. J. Biol. Chem. 256, 9183–9191 PMID: [PubMed] [Google Scholar]
- 277.Abumrad N.A., Park J.H. and Park C.R. (1984) Permeation of long-chain fatty acid into adipocytes. kinetics, specificity, and evidence for involvement of a membrane protein. J. Biol. Chem. 259, 8945–8953 PMID: [PubMed] [Google Scholar]
- 278.Abumrad N.A., el-Maghrabi M.R., Amri E.Z., Lopez E. and Grimaldi P.A. (1993) Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. homology with human CD36. J. Biol. Chem. 268, 17665–17668 PMID: [PubMed] [Google Scholar]
- 279.Pepino M.Y., Kuda O., Samovski D. and Abumrad N.A. (2014) Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 34, 281–303 10.1146/annurev-nutr-071812-161220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Thompson B.R., Lobo S. and Bernlohr D.A. (2010) Fatty acid flux in adipocytes: the in's and out's of fat cell lipid trafficking. Mol. Cell Endocrinol. 318, 24–33 10.1016/j.mce.2009.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Silverstein R.L. and Febbraio M. (2009) CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2, re3 10.1126/scisignal.272re3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Pietka T.A., Schappe T., Conte C., Fabbrini E., Patterson B.W., Klein S. et al. (2014) Adipose and muscle tissue profile of CD36 transcripts in obese subjects highlights the role of CD36 in fatty acid homeostasis and insulin resistance. Diabetes Care 37, 1990–1997 10.2337/dc13-2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Febbraio M., Abumrad N.A., Hajjar D.P., Sharma K., Cheng W., Pearce S.F. et al. (1999) A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J. Biol. Chem. 274, 19055–19062 10.1074/jbc.274.27.19055 [DOI] [PubMed] [Google Scholar]
- 284.Coburn C.T., Knapp F.F. Jr, Febbraio M., Beets A.L., Silverstein R.L. and Abumrad N.A. (2000) Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 275, 32523–32529 10.1074/jbc.M003826200 [DOI] [PubMed] [Google Scholar]
- 285.Hajri T., Hall A.M., Jensen D.R., Pietka T.A., Drover V.A., Tao H. et al. (2007) CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes 56, 1872–1880 10.2337/db06-1699 [DOI] [PubMed] [Google Scholar]
- 286.Cai L., Wang Z., Ji A., Meyer J.M. and van der Westhuyzen D.R. (2012) Scavenger receptor CD36 expression contributes to adipose tissue inflammation and cell death in diet-induced obesity. PLoS One 7, e36785 10.1371/journal.pone.0036785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Vroegrijk I.O., van Klinken J.B., van Diepen J.A., van den Berg S.A., Febbraio M., Steinbusch L.K. et al. (2013) CD36 is important for adipocyte recruitment and affects lipolysis. Obesity (Silver Spring) 21, 2037–2045 10.1002/oby.20354 [DOI] [PubMed] [Google Scholar]
- 288.Zhou D., Samovski D., Okunade A.L., Stahl P.D., Abumrad N.A. and Su X. (2012) CD36 level and trafficking are determinants of lipolysis in adipocytes. FASEB J. 26, 4733–4742 10.1096/fj.12-206862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Yamashita S., Hirano K., Kuwasako T., Janabi M., Toyama Y., Ishigami M. et al. (2007) Physiological and pathological roles of a multi-ligand receptor CD36 in atherogenesis; insights from CD36-deficient patients. Mo.l Cell Biochem. 299, 19–22 10.1007/s11010-005-9031-4 [DOI] [PubMed] [Google Scholar]
- 290.Love-Gregory L., Sherva R., Sun L., Wasson J., Schappe T., Doria A. et al. (2008) Variants in the CD36 gene associate with the metabolic syndrome and high-density lipoprotein cholesterol. Hum. Mol. Genet. 17, 1695–1704 10.1093/hmg/ddn060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bonen A., Tandon N.N., Glatz J.F., Luiken J.J. and Heigenhauser G.J. (2006) The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int. J. Obes. (Lond) 30, 877–883 10.1038/sj.ijo.0803212 [DOI] [PubMed] [Google Scholar]
- 292.Fisher R.M., Hoffstedt J., Hotamisligil G.S., Thorne A. and Ryden M. (2002) Effects of obesity and weight loss on the expression of proteins involved in fatty acid metabolism in human adipose tissue. Int. J. Obes. Relat. Metab. Disord. 26, 1379–1385 10.1038/sj.ijo.0802110 [DOI] [PubMed] [Google Scholar]
- 293.Gertow K., Pietilainen K.H., Yki-Jarvinen H., Kaprio J., Rissanen A., Eriksson P. et al. (2004) Expression of fatty-acid-handling proteins in human adipose tissue in relation to obesity and insulin resistance. Diabetologia 47, 1118–1125 10.1007/s00125-004-1417-4 [DOI] [PubMed] [Google Scholar]
- 294.Gertow K., Rosell M., Sjogren P., Eriksson P., Vessby B., de Faire U. et al. (2006) Fatty acid handling protein expression in adipose tissue, fatty acid composition of adipose tissue and serum, and markers of insulin resistance. Eur. J. Clin. Nutr. 60, 1406–1413 10.1038/sj.ejcn.1602471 [DOI] [PubMed] [Google Scholar]
- 295.Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H. et al. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Walden T.B., Hansen I.R., Timmons J.A., Cannon B. and Nedergaard J. (2012) Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am. J. Physiol. Endocrinol. Metab. 302, E19–E31 10.1152/ajpendo.00249.2011 [DOI] [PubMed] [Google Scholar]
- 297.Gesta S., Bluher M., Yamamoto Y., Norris A.W., Berndt J., Kralisch S. et al. (2006) Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl. Acad. Sci. U.S.A. 103, 6676–6681 10.1073/pnas.0601752103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wang J., Krueger M., Hauck S.M. and Ussar S. (2020) PAT2 regulates autophagy through vATPase assembly and lysosomal acidification in brown adipocytes. bioRxiv 10.1101/2020.01.24.918078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Pan Q., Shai O., Lee L.J., Frey B.J. and Blencowe B.J. (2008) Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 40, 1413–1415 10.1038/ng.259 [DOI] [PubMed] [Google Scholar]
- 300.Wang E.T., Sandberg R., Luo S., Khrebtukova I., Zhang L., Mayr C. et al. (2008) Alternative isoform regulation in human tissue transcriptomes. Nature 456, 470–476 10.1038/nature07509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Vaquero-Garcia J., Barrera A., Gazzara M.R., Gonzalez-Vallinas J., Lahens N.F., Hogenesch J.B. et al. (2016) A new view of transcriptome complexity and regulation through the lens of local splicing variations. eLife 5, e11752 10.7554/eLife.11752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Fischer C., Seki T., Lim S., Nakamura M., Andersson P., Yang Y. et al. (2017) A miR-327-FGF10-FGFR2-mediated autocrine signaling mechanism controls white fat browning. Nat. Commun. 8, 2079 10.1038/s41467-017-02158-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Yamasaki M., Emoto H., Konishi M., Mikami T., Ohuchi H., Nakao K. et al. (1999) FGF-10 is a growth factor for preadipocytes in white adipose tissue. Biochem. Biophys. Res. Commun. 258, 109–112 10.1006/bbrc.1999.0594 [DOI] [PubMed] [Google Scholar]
- 304.Konishi M., Nakamura H., Miwa H., Chambon P., Ornitz D.M. and Itoh N. (2008) Role of Fgf receptor 2c in adipocyte hypertrophy in mesenteric white adipose tissue. Mol. Cell Endocrinol. 287, 13–19 10.1016/j.mce.2008.02.010 [DOI] [PubMed] [Google Scholar]
- 305.Nonogaki K., Kaji T., Yamazaki T. and Murakami M. (2016) Treatment with FGFR2-IIIc monoclonal antibody suppresses weight gain and adiposity in KKA(y) mice. Nutr. Diabetes 6, e233 10.1038/nutd.2016.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Seino S. and Bell G.I. (1989) Alternative splicing of human insulin receptor messenger RNA. Biochem. Biophys. Res. Commun. 159, 312–316 10.1016/0006-291X(89)92439-X [DOI] [PubMed] [Google Scholar]
- 307.Vidal H., Auboeuf D., Beylot M. and Riou J.P. (1995) Regulation of insulin receptor mRNA splicing in rat tissues. effect of fasting, aging, and diabetes. Diabetes 44, 1196–1201 10.2337/diab.44.10.1196 [DOI] [PubMed] [Google Scholar]
- 308.Escribano O., Beneit N., Rubio-Longas C., Lopez-Pastor A.R. and Gomez-Hernandez A. (2017) The role of insulin receptor isoforms in diabetes and its metabolic and vascular complications. J. Diabetes Res. 2017, 1403206 10.1155/2017/1403206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Sciacca L., Prisco M., Wu A., Belfiore A., Vigneri R. and Baserga R. (2003) Signaling differences from the A and B isoforms of the insulin receptor (IR) in 32D cells in the presence or absence of IR substrate-1. Endocrinology 144, 2650–2658 10.1210/en.2002-0136 [DOI] [PubMed] [Google Scholar]
- 310.Vienberg S.G., Bouman S.D., Sorensen H., Stidsen C.E., Kjeldsen T., Glendorf T. et al. (2011) Receptor-isoform-selective insulin analogues give tissue-preferential effects. Biochem. J. 440, 301–308 10.1042/BJ20110880 [DOI] [PubMed] [Google Scholar]
- 311.Kaminska D., Hamalainen M., Cederberg H., Kakela P., Venesmaa S., Miettinen P. et al. (2014) Adipose tissue INSR splicing in humans associates with fasting insulin level and is regulated by weight loss. Diabetologia 57, 347–351 10.1007/s00125-013-3097-4 [DOI] [PubMed] [Google Scholar]
- 312.Sesti G., Marini M.A., Tullio A.N., Montemurro A., Borboni P., Fusco A. et al. (1991) Altered expression of the two naturally occurring human insulin receptor variants in isolated adipocytes of non-insulin-dependent diabetes mellitus patients. Biochem. Biophys. Res. Commun. 181, 1419–1424 10.1016/0006-291X(91)92097-4 [DOI] [PubMed] [Google Scholar]
- 313.Kaminska D. and Pihlajamaki J. (2013) Regulation of alternative splicing in obesity and weight loss. Adipocyte 2, 143–147 10.4161/adip.24751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Wang Y. and Sul H.S. (2006) Ectodomain shedding of preadipocyte factor 1 (Pref-1) by tumor necrosis factor alpha converting enzyme (TACE) and inhibition of adipocyte differentiation. Mol. Cell Biol. 26, 5421–5435 10.1128/MCB.02437-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Shan T., Liu J., Wu W., Xu Z. and Wang Y. (2017) Roles of notch signaling in adipocyte progenitor cells and mature adipocytes. J. Cell Physiol. 232, 1258–1261 10.1002/jcp.25697 [DOI] [PubMed] [Google Scholar]
- 316.Jarvelainen H., Puolakkainen P., Pakkanen S., Brown E.L., Hook M., Iozzo R.V. et al. (2006) A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 14, 443–452 10.1111/j.1743-6109.2006.00150.x [DOI] [PubMed] [Google Scholar]
- 317.Fiedler L.R., Schonherr E., Waddington R., Niland S., Seidler D.G., Aeschlimann D. et al. (2008) Decorin regulates endothelial cell motility on collagen I through activation of insulin-like growth factor I receptor and modulation of alpha2beta1 integrin activity. J. Biol. Chem. 283, 17406–17415 10.1074/jbc.M710025200 [DOI] [PubMed] [Google Scholar]
- 318.Imai K., Hiramatsu A., Fukushima D., Pierschbacher M.D. and Okada Y. (1997) Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem. J. 322, 809–814 10.1042/bj3220809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Daquinag A.C., Tseng C., Salameh A., Zhang Y., Amaya-Manzanares F., Dadbin A. et al. (2015) Depletion of white adipocyte progenitors induces beige adipocyte differentiation and suppresses obesity development. Cell Death Differ. 22, 351–363 10.1038/cdd.2014.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Daquinag A.C., Tseng C., Zhang Y., Amaya-Manzanares F., Florez F., Dadbin A. et al. (2016) Targeted proapoptotic peptides depleting adipose stromal cells inhibit tumor growth. Mol. Ther. 24, 34–40 10.1038/mt.2015.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Daquinag A.C., Dadbin A., Snyder B., Wang X., Sahin A.A., Ueno N.T. et al. (2017) Non-glycanated decorin is a drug target on human adipose stromal cells. Mol. Ther. Oncolytics 6, 1–9 10.1016/j.omto.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Maquoi E., Munaut C., Colige A., Collen D. and Lijnen H.R. (2002) Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes 51, 1093–1101 10.2337/diabetes.51.4.1093 [DOI] [PubMed] [Google Scholar]
- 323.Chavey C., Mari B., Monthouel M.N., Bonnafous S., Anglard P., Van Obberghen E. et al. (2003) Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J. Biol. Chem. 278, 11888–11896 10.1074/jbc.M209196200 [DOI] [PubMed] [Google Scholar]
- 324.Berg G., Barchuk M. and Miksztowicz V. (2019) Behavior of metalloproteinases in adipose tissue, liver and arterial wall: an update of extracellular matrix remodeling. Cells 8, 158 10.3390/cells8020158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Liu M.J., Bao S., Bolin E.R., Burris D.L., Xu X., Sun Q. et al. (2013) Zinc deficiency augments leptin production and exacerbates macrophage infiltration into adipose tissue in mice fed a high-fat diet. J. Nutr. 143, 1036–1045 10.3945/jn.113.175158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kraakman M.J., Kammoun H.L., Allen T.L., Deswaerte V., Henstridge D.C., Estevez E. et al. (2015) Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metab. 21, 403–416 10.1016/j.cmet.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 327.Weerasekera L., Rudnicka C., Sang Q.X., Curran J.E., Johnson M.P., Moses E.K. et al. (2017) ADAM19: a novel target for metabolic syndrome in humans and mice. Mediators Inflamm. 2017, 7281986 10.1155/2017/7281986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Leitner L., Schuch K., Jurets A., Itariu B.K., Keck M., Grablowitz V. et al. (2015) Immunological blockade of adipocyte inflammation caused by increased matrix metalloproteinase-cleaved osteopontin in obesity. Obesity (Silver Spring) 23, 779–785 10.1002/oby.21024 [DOI] [PubMed] [Google Scholar]
- 329.Christensen B., Nielsen M.S., Haselmann K.F., Petersen T.E. and Sorensen E.S. (2005) Post-translationally modified residues of native human osteopontin are located in clusters: identification of 36 phosphorylation and five O-glycosylation sites and their biological implications. Biochem. J. 390, 285–292 10.1042/BJ20050341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Chapman J., Miles P.D., Ofrecio J.M., Neels J.G., Yu J.G., Resnik J.L. et al. (2010) Osteopontin is required for the early onset of high fat diet-induced insulin resistance in mice. PLoS One 5, e13959 10.1371/journal.pone.0013959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Kiefer F.W., Zeyda M., Todoric J., Huber J., Geyeregger R., Weichhart T. et al. (2008) Osteopontin expression in human and murine obesity: extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology 149, 1350–1357 10.1210/en.2007-1312 [DOI] [PubMed] [Google Scholar]
- 332.Nomiyama T., Perez-Tilve D., Ogawa D., Gizard F., Zhao Y., Heywood E.B. et al. (2007) Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J. Clin. Invest. 117, 2877–2888 10.1172/JCI31986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Lee Y.H., Petkova A.P. and Granneman J.G. (2013) Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 18, 355–367 10.1016/j.cmet.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wanko B., Tardelli M., Jurets A., Neuhofer A., Prager G., Morser J. et al. (2019) Antibody-mediated targeting of cleavage-specific OPN-T cell interactions. PLoS One 14, e0214938 10.1371/journal.pone.0214938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Guo A., Gu H., Zhou J., Mulhern D., Wang Y., Lee K.A. et al. (2014) Immunoaffinity enrichment and mass spectrometry analysis of protein methylation. Mol. Cell Proteomics 13, 372–387 10.1074/mcp.O113.027870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Azkargorta M., Escobes I., Elortza F., Matthiesen R. and Rodriguez M.S. (2016) TUBEs-mass spectrometry for identification and analysis of the ubiquitin-proteome. Methods Mol. Biol. 1449, 177–192 10.1007/978-1-4939-3756-1_9 [DOI] [PubMed] [Google Scholar]
- 337.Dittenhafer-Reed K.E., Richards A.L., Fan J., Smallegan M.J., Fotuhi Siahpirani A., Kemmerer Z.A. et al. (2015) SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab. 21, 637–646 10.1016/j.cmet.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Sharma K., D'Souza R.C., Tyanova S., Schaab C., Wisniewski J.R., Cox J. et al. (2014) Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell Rep. 8, 1583–1594 10.1016/j.celrep.2014.07.036 [DOI] [PubMed] [Google Scholar]
- 339.Zou X., Yoshida M., Nagai-Okatani C., Iwaki J., Matsuda A., Tan B. et al. (2017) A standardized method for lectin microarray-based tissue glycome mapping. Sci. Rep. 7, 43560 10.1038/srep43560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Nairn A.V., York W.S., Harris K., Hall E.M., Pierce J.M. and Moremen K.W. (2008) Regulation of glycan structures in animal tissues: transcript profiling of glycan-related genes. J. Biol. Chem. 283, 17298–17313 10.1074/jbc.M801964200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Goh J.B. and Ng S.K. (2018) Impact of host cell line choice on glycan profile. Crit. Rev. Biotechnol. 38, 851–867 10.1080/07388551.2017.1416577 [DOI] [PubMed] [Google Scholar]
- 342.Xia J., Zhang H., Gao X., Guo J., Hou J., Wang X. et al. (2016) E-cadherin-mediated contact of endothelial progenitor cells with mesenchymal stem cells through beta-catenin signaling. Cell Biol. Int. 40, 407–418 10.1002/cbin.10579 [DOI] [PubMed] [Google Scholar]
- 343.Hoosdally S.J., Andress E.J., Wooding C., Martin C.A. and Linton K.J. (2009) The human scavenger receptor CD36: glycosylation status and its role in trafficking and function. J. Biol. Chem. 284, 16277–16288 10.1074/jbc.M109.007849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Haga Y., Ishii K. and Suzuki T. (2011) N-glycosylation is critical for the stability and intracellular trafficking of glucose transporter GLUT4. J. Biol. Chem. 286, 31320–31327 10.1074/jbc.M111.253955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Collier E., Carpentier J.L., Beitz L., Carol H., Taylor S.I. and Gorden P. (1993) Specific glycosylation site mutations of the insulin receptor alpha subunit impair intracellular transport. Biochemistry 32, 7818–7823 10.1021/bi00081a029 [DOI] [PubMed] [Google Scholar]
- 346.Heidenreich K.A. and Brandenburg D. (1986) Oligosaccharide heterogeneity of insulin receptors. Comparison of N-linked glycosylation of insulin receptors in adipocytes and brain. Endocrinology 118, 1835–1842 10.1210/endo-118-5-1835 [DOI] [PubMed] [Google Scholar]
- 347.Hwang J.B., Hernandez J., Leduc R. and Frost S.C. (2000) Alternative glycosylation of the insulin receptor prevents oligomerization and acquisition of insulin-dependent tyrosine kinase activity. Biochim. Biophys. Acta 1499, 74–84 10.1016/S0167-4889(00)00109-9 [DOI] [PubMed] [Google Scholar]
- 348.Leconte I., Auzan C., Debant A., Rossi B. and Clauser E. (1992) N-linked oligosaccharide chains of the insulin receptor beta subunit are essential for transmembrane signaling. J. Biol. Chem. 267, 17415–17423 PMID: [PubMed] [Google Scholar]
- 349.Reed B.C., Ronnett G.V. and Lane M.D. (1981) Role of glycosylation and protein synthesis in insulin receptor metabolism by 3T3-L1 mouse adipocytes. Proc. Natl. Acad. Sci. U.S.A. 78, 2908–2912 10.1073/pnas.78.5.2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Ronnett G.V., Knutson V.P., Kohanski R.A., Simpson T.L. and Lane M.D. (1984) Role of glycosylation in the processing of newly translated insulin proreceptor in 3T3-L1 adipocytes. J. Biol. Chem. 259, 4566–4575 PMID: [PubMed] [Google Scholar]
- 351.Sparrow L.G., Lawrence M.C., Gorman J.J., Strike P.M., Robinson C.P., McKern N.M. et al. (2008) N-linked glycans of the human insulin receptor and their distribution over the crystal structure. Proteins 71, 426–439 10.1002/prot.21768 [DOI] [PubMed] [Google Scholar]
- 352.Kaburagi T., Kizuka Y., Kitazume S. and Taniguchi N. (2017) The inhibitory role of α2,6-sialylation in adipogenesis. J. Biol. Chem. 292, 2278–2286 10.1074/jbc.M116.747667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Li S.F., Zhu C.S., Wang Y.M., Xie X.X., Xiao L.L., Zhang Z.C. et al. (2018) Downregulation of beta1,4-galactosyltransferase 5 improves insulin resistance by promoting adipocyte commitment and reducing inflammation. Cell Death Dis. 9, 196 10.1038/s41419-017-0239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yamamoto Y., Gesta S., Lee K.Y., Tran T.T., Saadatirad P. and Kahn C.R. (2010) Adipose depots possess unique developmental gene signatures. Obesity 18, 872–878 10.1038/oby.2009.512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Aebersold R. and Mann M. (2016) Mass-spectrometric exploration of proteome structure and function. Nature 537, 347–355 10.1038/nature19949 [DOI] [PubMed] [Google Scholar]
- 356.Loh K.H., Stawski P.S., Draycott A.S., Udeshi N.D., Lehrman E.K., Wilton D.K. et al. (2016) Proteomic analysis of unbounded cellular compartments: synaptic clefts. Cell 166, 1295–1307 10.1016/j.cell.2016.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Li J., Han S., Li H., Udeshi N.D., Svinkina T., Mani D.R. et al. (2020) Cell-surface proteomic profiling in the fly brain uncovers wiring regulators. Cell 180, 373–386 10.1016/j.cell.2019.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Beck A., Goetsch L., Dumontet C. and Corvaia N. (2017) Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 16, 315–337 10.1038/nrd.2016.268 [DOI] [PubMed] [Google Scholar]
- 359.Hoogenboom H.R. (2005) Selecting and screening recombinant antibody libraries. Nat. Biotechnol. 23, 1105–1116 10.1038/nbt1126 [DOI] [PubMed] [Google Scholar]
- 360.Smith G.P. (1985) Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 228, 1315–1317 10.1126/science.4001944 [DOI] [PubMed] [Google Scholar]
- 361.McCafferty J., Griffiths A.D., Winter G. and Chiswell D.J. (1990) Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348, 552–554 10.1038/348552a0 [DOI] [PubMed] [Google Scholar]
- 362.Muyldermans S. (2013) Nanobodies: natural single-domain antibodies. Annu. Rev. Biochem. 82, 775–797 10.1146/annurev-biochem-063011-092449 [DOI] [PubMed] [Google Scholar]
- 363.Zabeau L., Verhee A., Catteeuw D., Faes L., Seeuws S., Decruy T. et al. (2012) Selection of non-competitive leptin antagonists using a random nanobody-based approach. Biochem. J. 441, 425–434 10.1042/BJ20110438 [DOI] [PubMed] [Google Scholar]
- 364.Doshi R., Chen B.R., Vibat C.R., Huang N., Lee C.W. and Chang G. (2014) In vitro nanobody discovery for integral membrane protein targets. Sci. Rep. 4, 6760 10.1038/srep06760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Azhdarinia A., Daquinag A.C., Tseng C., Ghosh S.C., Ghosh P., Amaya-Manzanares F. et al. (2013) A peptide probe for targeted brown adipose tissue imaging. Nat. Commun. 4, 2472 10.1038/ncomms3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Gustafson H.H., Olshefsky A., Sylvestre M., Sellers D.L. and Pun S.H. (2018) Current state of in vivo panning technologies: designing specificity and affinity into the future of drug targeting. Adv. Drug Deliv. Rev. 130, 39–49 10.1016/j.addr.2018.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Edwards B.M., Main S.H., Cantone K.L., Smith S.D., Warford A. and Vaughan T.J. (2000) Isolation and tissue profiles of a large panel of phage antibodies binding to the human adipocyte cell surface. J. Immunol. Methods 245, 67–78 10.1016/S0022-1759(00)00275-1 [DOI] [PubMed] [Google Scholar]
- 368.Lee N.K., Kim H.S., Kim K.H., Kim E.B., Cho C.S., Kang S.K. et al. (2011) Identification of a novel peptide ligand targeting visceral adipose tissue via transdermal route by in vivo phage display. J. Drug Target. 19, 805–813 10.3109/1061186X.2011.572974 [DOI] [PubMed] [Google Scholar]
- 369.Kim D.H., Sartor M.A., Bain J.R., Sandoval D., Stevens R.D., Medvedovic M. et al. (2012) Rapid and weight-independent improvement of glucose tolerance induced by a peptide designed to elicit apoptosis in adipose tissue endothelium. Diabetes 61, 2299–2310 10.2337/db11-1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Hossen M.N., Kajimoto K., Akita H., Hyodo M., Ishitsuka T. and Harashima H. (2010) Ligand-based targeted delivery of a peptide modified nanocarrier to endothelial cells in adipose tissue. J. Control. Release 147, 261–268 10.1016/j.jconrel.2010.07.100 [DOI] [PubMed] [Google Scholar]
- 371.Hossen N., Kajimoto K., Akita H., Hyodo M. and Harashima H. (2013) A comparative study between nanoparticle-targeted therapeutics and bioconjugates as obesity medication. J. Control. Release 171, 104–112 10.1016/j.jconrel.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 372.Sakurai Y., Kajimoto K. and Harashima H. (2015) Anti-angiogenic nanotherapy via active targeting systems to tumors and adipose tissue vasculature. Biomater. Sci. 3, 1253–1265 10.1039/C5BM00113G [DOI] [PubMed] [Google Scholar]
- 373.Chung J.Y., Ain Q.U., Song Y., Yong S.B. and Kim Y.H. (2019) Targeted delivery of CRISPR interference system against Fabp4 to white adipocytes ameliorates obesity, inflammation, hepatic steatosis, and insulin resistance. Genome Res. 29, 1442–1452 10.1101/gr.246900.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Gray P., Kelly B., Ahrens L., Barry D.P., Kratschmer A.P., Levy C. et al. (2018) Tunable cytotoxic aptamer-drug conjugates for the treatment of prostate cancer. Proc. Natl. Acad. Sci. U.S.A. 115, 4761–4766 10.1073/pnas.1717705115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.McNamara J.O. II, Andrechek E.R., Wang Y., Viles K.D., Rempel R.E., Gilboa E. et al. (2006) ) cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 24, 1005–1015 10.1038/nbt1223 [DOI] [PubMed] [Google Scholar]
- 376.Zhen S., Takahashi Y., Narita S., Yang Y.C. and Li X. (2017) Targeted delivery of CRISPR/Cas9 to prostate cancer by modified gRNA using a flexible aptamer-cationic liposome. Oncotarget 8, 9375–9387 10.18632/oncotarget.14072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Tuerk C. and Gold L. (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249, 505–510 10.1126/science.2200121 [DOI] [PubMed] [Google Scholar]
- 378.Kim E.Y., Kim J.W., Kim W.K., Han B.S., Park S.G., Chung B.H. et al. (2014) Selection of aptamers for mature white adipocytes by cell SELEX using flow cytometry. PLoS One 9, e97747 10.1371/journal.pone.0097747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Kruglikov I.L. and Scherer P.E. (2016) Dermal adipocytes: from irrelevance to metabolic targets? Trends Endocrinol. Metab. 27, 1–10 10.1016/j.tem.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Zhang M., Hu T., Zhang S. and Zhou L. (2015) Associations of different adipose tissue depots with insulin resistance: a systematic review and meta-analysis of observational studies. Sci. Rep. 5, 18495 10.1038/srep18495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Dodson M.V., Du M., Wang S., Bergen W.G., Fernyhough-Culver M., Basu U. et al. (2014) Adipose depots differ in cellularity, adipokines produced, gene expression, and cell systems. Adipocyte 3, 236–241 10.4161/adip.28321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Kasza I., Suh Y., Wollny D., Clark R.J., Roopra A., Colman R.J. et al. (2014) Syndecan-1 is required to maintain intradermal fat and prevent cold stress. PLoS Genet. 10, e1004514 10.1371/journal.pgen.1004514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. et al. (2003) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450–454 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T. and Veesler D. (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181, 281–292 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N. et al. (2000) A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 87, E1–E9 10.1161/01.RES.87.5.e1 [DOI] [PubMed] [Google Scholar]
- 387.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G. and Turner A.J. (2000) A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 275, 33238–33243 10.1074/jbc.M002615200 [DOI] [PubMed] [Google Scholar]
- 388.Gembardt F., Sterner-Kock A., Imboden H., Spalteholz M., Reibitz F., Schultheiss H.P. et al. (2005) Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides 26, 1270–1277 10.1016/j.peptides.2005.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Li M.Y., Li L., Zhang Y. and Wang X.S. (2020) Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis. Poverty 9, 45 10.1186/s40249-020-00662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Liu M., He P., Liu H.G., Wang X.J., Li F.J., Chen S. et al. (2020) Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 43, E016 10.3760/cma.j.issn.1001-0939.2020.0016 [DOI] [PubMed] [Google Scholar]
- 391.Peng Y.D., Meng K., Guan H.Q., Leng L., Zhu R.R., Wang B.Y. et al. (2020) Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi 48, E004 10.3760/cma.j.cn112148-20200220-00105 [DOI] [PubMed] [Google Scholar]
- 392.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O'Donnell L., Chernyak Y. et al. (2020) Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York city: prospective cohort study. Br. Med. J. 369, m1966 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A. et al. (2020) High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 28, 1195–1199 10.1002/oby.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Caussy C., Pattou F., Wallet F., Simon C., Chalopin S., Telliam C. et al. (2020) Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 8, P562–P564 10.1016/S2213-8587(20)30160-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Kassir R. (2020) Risk of COVID-19 for patients with obesity. Obes. Rev. 21, e13034 10.1111/obr.13034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Stefan N., Birkenfeld A.L., Schulze M.B. and Ludwig D.S. (2020) Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 16, 341–342 10.1038/s41574-020-0364-6 [DOI] [PMC free article] [PubMed] [Google Scholar]