Abstract
Background
We assessed cross-sectional differences in sleep quality and risk factors among Asian, Black, Latino, and White participants in the Kaiser Healthy Aging and Diverse Life Experiences (KHANDLE) Study.
Methods
KHANDLE enrolled community-dwelling adults aged ≥65 years living in northern California. Participants completed a modified Pittsburgh Sleep Quality Index to measure six sleep components and a global sleep score (scored 0-24). Covariates included age, sex, central adiposity, education, income, alcohol consumption, ever smoking, physical activity, and depression. Ordinal logistic regression was used to model sleep component scores across race/ethnic groups. Linear regression was used to assess racial/ethnic differences in global sleep score and the association between risk factors and global sleep score.
Results
1,664 participants with a mean age of 76 (SD=7) and mean global sleep score of 6 (SD=4) were analyzed. Using Latinos as reference (highest average sleep score), Blacks had an average .96 (.37, 1.54) unit higher global sleep score (worse sleep) while Asians [β: .04 (-.56, .63)] and Whites [β: .28 (-.29, .84)] did not significantly differ. Compared with Latinos, Blacks and Asians had greater odds of a worse score on the sleep duration component; Blacks and Whites had greater odds of a worse score on the sleep disturbances component; and, Whites had greater odds of a worse score on the medication component. Risk factors for poor sleep did not differ by race/ethnicity except alcohol consumption (interaction P=.04), which was associated with poor sleep in Blacks only.
Conclusions
In this cohort, racial/ethnic differences in sleep quality were common.
Keywords: Sleep, Disparities, Risk Factors, Older Adults
Introduction
Approximately 60% of US adults aged ≥65 years report chronic sleep disruptions and low quality sleep.1 Age-related sleep changes such as decreased total sleep time, decreased sleep efficiency, decreased slow-wave sleep, and increased wakefulness after sleep onset are particularly common in this population.1,2 However, these changes can have deleterious consequences on brain and body health.3 Poor sleep is associated with obesity,4 diabetes,5 stroke,6 cardiovascular disease,7 cognitive impairment,8,9 and all-cause mortality.10
Previous studies have found inadequate sleep to be especially prevalent among racial/ethnic minorities in the United States11; however, the majority of studies have only assessed racial/ethnic sleep differences in young or middle-age adults.12,13 Blacks and Latinos are more likely to report long or short sleep duration (both of which are associated with poor health outcomes14,15) as well as more likely to report diagnosis of a sleep disorder, such as obstructive sleep apnea, compared with Whites.11,12,14,16 Sleep patterns in Asians have been less extensively studied, but there is some evidence they report fewer sleep disturbances and less prevalent obstructive sleep apnea than Whites, Blacks, or Latinos.11,12,14 Risk factors for poor sleep quality include being female, depression, low physical activity, and low socioeconomic status.17 While a number of studies, including meta-analyses, have examined racial/ethnic (particularly Black/White) differences in sleep using both objective and subjective sleep measures, very few have focused on racial/ethnic disparities among older adults (aged ≥65 years) who are the most likely to report inadequate sleep and the most vulnerable to the chronic conditions associated with poor sleep.18-23
Using data from the Kaiser Healthy Aging and Diverse Life Experiences (KHANDLE) Study, we characterized differences in sleep quality among older adults in four racial/ethnic groups (Asian, Black, Latino, and White). We hypothesized that after adjustment for covariates, Asian participants would report the best quality sleep, followed by Whites, Latinos, and Blacks, and that differences in prevalence of socioeconomic, behavioral, and vascular risk factors associated with poor sleep would follow similar patterns.
Methods
The KHANDLE cohort comprises community-dwelling older adults residing in the San Francisco Bay Area and Sacramento, California. KHANDLE aims to evaluate how race/ethnicity, life course health, and sociocultural factors influence late-life brain health and cognitive decline. Individuals eligible for KHANDLE were long-term members of Kaiser Permanente Northern California, participated in multiphasic health checkups between 1964-1973 or 1977-1985 (prior to KHANDLE study wave 1), and were aged ≥65 years on January 1, 2017. Participants were excluded if they had a diagnosis of dementia or other neurodegenerative disease in their electronic health record. All procedures were in accordance with the ethical standards of the institutional review board (IRB) of the authors and with the Helsinki Declaration of 1975 (as revised in 2000). Informed consent was obtained from all participants included in the study. A total of 1,712 individuals were enrolled at wave 1 (April 2017 – December 2018) using stratified random sampling to recruit approximately equal proportions of Asian, Black, Latino, and White participants. Participants were excluded if they were missing self-reported race/ethnicity or were Native American due to small numbers (n = 4) or they were missing measures of self-reported sleep quality (n = 44) for a final analytic sample of 1,664 participants.
Sleep was measured via a questionnaire using a modified version of the Pittsburgh Sleep Quality Index24 (PSQI). Questions assessed components of sleep quality, latency, duration, disturbances, use of sleep medication, and daytime dysfunction. Our modified version of the questionnaire omitted questions about typical bedtime, how long it took individuals to fall asleep, and typical wake time. Because of this, we could not calculate sleep efficiency (total time asleep/total time in bed) and had to omit this sleep component from our modified PSQI assessment. Participants’ responses were used to create a separate sum score for each of the six sleep components. Component scores ranged from 0-3 for five components (sleep quality, latency, duration, medication, and daytime dysfunction) and ranged from 0-9 for sleep disturbances. A higher score indicated poorer sleep quality. The six component scores were then summed to generate a global sleep score ranging from 0-24. Of note, a global sleep score of >5 on the original PSQI is indicative of poor sleep quality. Our modified PSQI questionnaire used the same score range (0-24), but was weighted differently, so the threshold for poor sleep quality may differ slightly.24
Our primary models adjusted for age (linear) and sex (male/female). Secondary models additionally adjusted for educational attainment, family income, alcohol consumption, tobacco smoking status, physical activity, and depression, which were all assessed via self-report using questionnaires. The physical activity questionnaire evaluated frequency of light and vigorous leisure and sport activity.25 Responses to these questions were assigned scores on a Likert scale ranging from 0 (no physical activity) to 4 (daily or almost daily activity). Question scores were then summed for a total range of 0-16 with a higher score indicating higher levels of physical activity. Depression was measured using the NIH Toolbox PROMIS® Depression assessment. Questions in the assessment corresponded to emotional, cognitive, and behavioral manifestations of depression experienced in the last 7 days.26,27 Question responses ranged from 1 (indicating “Never”) to 5 (indicating “Always”).26,27 The scores generated from participants’ responses were used to create a Ί-score metric with a Ί-score > 60 signifying clinically significant depression symptoms.27 Additionally, participant waist circumference was measured by trained study interviewers. Waist circumference was analyzed both continuously and as a dichotomous central adiposity variable. Central adiposity was defined as a waist circumference of ≥ 90 cm for Asian men, ≥ 80 cm for Asian women, ≥ 102 cm for Black, Latino, and White men, and ≥ 88 cm for Black, Latino, and White women.28
Statistical Analysis
We described means and prevalence of wave 1 characteristics including global sleep quality and sleep duration by race/ethnicity. We then examined racial/ethnic differences in individual sleep components (quality, latency, duration, disturbances, medication use, and daytime dysfunction) as well as the global sleep score. We estimated odds ratios (OR) and 95% confidence intervals (CI) using ordinal logistic regression to assess racial/ethnic differences in sleep component scores. The proportional odds assumption was met for our variable of interest, race/ethnic group. (ie, a single OR was appropriate to describe the associations of race/ethnicity with each increment in the sleep component score). We used generalized linear models with a normal distribution and identity link to estimate mean differences in global sleep score by race/ethnicity. Latinos were used as the reference because they reported the best overall sleep quality based on the global sleep score. Model 1 was adjusted for age and sex, and model 2 was additionally adjusted for waist circumference (cm), education, income, alcohol consumption, tobacco smoking status, physical activity, and depression. In a secondary analysis, we assessed racial/ethnic differences in mean sleep duration modeled as hours of sleep per night.
We also examined the association between demographic, behavioral, and vascular risk factors with mean global sleep score using generalized linear models with a normal distribution and identity link to estimate β coefficients (95% CI). We calculated race/ethnicity-specific estimates for risk factors of age (per 5 years), sex (male/female), central adiposity (yes/no), education (≤ high school/> high school), income (<$55,000/≥ $55,000), alcohol consumption (yes/no), ever tobacco smoker (yes/no), physical activity (per unit), and depression (yes/no). In addition to race/ethnicity stratified results, we analyzed pooled results adjusting for age and race/ethnicity as well as tested a race/ethnicity*risk factor interaction term.
Analyses were completed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Participants had a mean age of 76 (SD=7) and 41% were male (Table 1). Asian and White participants were more likely to have attained a college degree or higher level of education and report a family income of >$55,000 compared with Black or Latino participants. Asians were the least likely to have ever smoked tobacco while Whites were the most likely. Latino and Asian participants were more likely than Blacks or Whites to report clinically significant depression symptoms. Physical activity did not differ substantially by race/ethnicity. Whites reported the longest sleep duration (mean = 7.1; SD = 1.3 hours) while Blacks reported the shortest (mean = 6.1; SD = 1.5 hours). Based on the global sleep score, Latino participants had the highest quality of sleep (mean = 5.7; SD = 4.0) followed by Asians (mean = 5.8; SD = 3.9), Whites (mean = 6.0; SD = 3.8), and Blacks (mean = 6.8; SD = 4.3), (Table 1; Figure 1).
Table 1. Baseline characteristics stratified by race/ethnicity, KHANDLE.
| Characteristic | Asian | Black | Latino | White |
| n = 400 | n = 429 | n = 342 | n = 493 | |
| Age, years | 75.6 ± 6.6 | 75.2 ± 6.5 | 76.0 ± 6.4 | 76.8 ± 7.2 |
| Male, % | 46.4 | 33.1 | 42.9 | 41.7 |
| Waist circumference, cm | 88.7 ± 12.2 | 99.3 ± 14.6 | 99.2 ± 15.3 | 96.8 ± 13.9 |
| Central adiposity,a % | 62.9 | 62.9 | 59.4 | 55.1 |
| Education, % | ||||
| High school grad less | 10.3 | 17.0 | 25.5 | 15.0 |
| Trade school or partial college | 23.0 | 48.7 | 42.5 | 28.2 |
| College grad | 37.8 | 16.3 | 17.0 | 28.6 |
| Grad school | 29.0 | 18.0 | 15.0 | 28.2 |
| Income < $55,000, % | 22.3 | 42.4 | 43.4 | 29.8 |
| Current Alcohol Drinker, % | 66.8 | 59.2 | 75.5 | 80.5 |
| Ever Tobacco Smoker, % | 32.0 | 43.4 | 48.5 | 52.1 |
| Physical Activity,b units | 8.8 ± 3.0 | 8.7 ± 3.3 | 8.7 ± 3.3 | 9.0 ± 3.1 |
| Depression,c % | 7.3 | 5.8 | 9.4 | 5.3 |
| Sleep Duration, hours | 6.4 ± 1.3 | 6.1 ± 1.5 | 6.6 ± 1.3 | 7.1 ± 1.3 |
| Global Sleep Score,d units | 5.8 ± 3.9 | 6.8 ± 4.3 | 5.7 ± 4.0 | 6.0 ± 3.8 |
Data are mean ± standard deviation unless shown otherwise.
a. Central adiposity was defined as a waist circumference of ≥ 90 cm for Asian men, ≥ 80 cm for Asian women, ≥ 102 cm for Black, Latino, and White men, and ≥ 88 cm for Black, Latino, and White women.
b. Physical activity included light and vigorous leisure and sport activity with a higher score indicating participants being more physically active.
c. Depression was ascertained using the NIH Toolbox PROMIS® Depression assessment with a Ί-score >60 indicating depression.
d. Global Sleep Score is the sum of 6 sleep components with a higher score indicating worse sleep (range 0-24).
Figure 1. Means of sleep quality components and global sleep by race/ethnicity, KHANDLE.
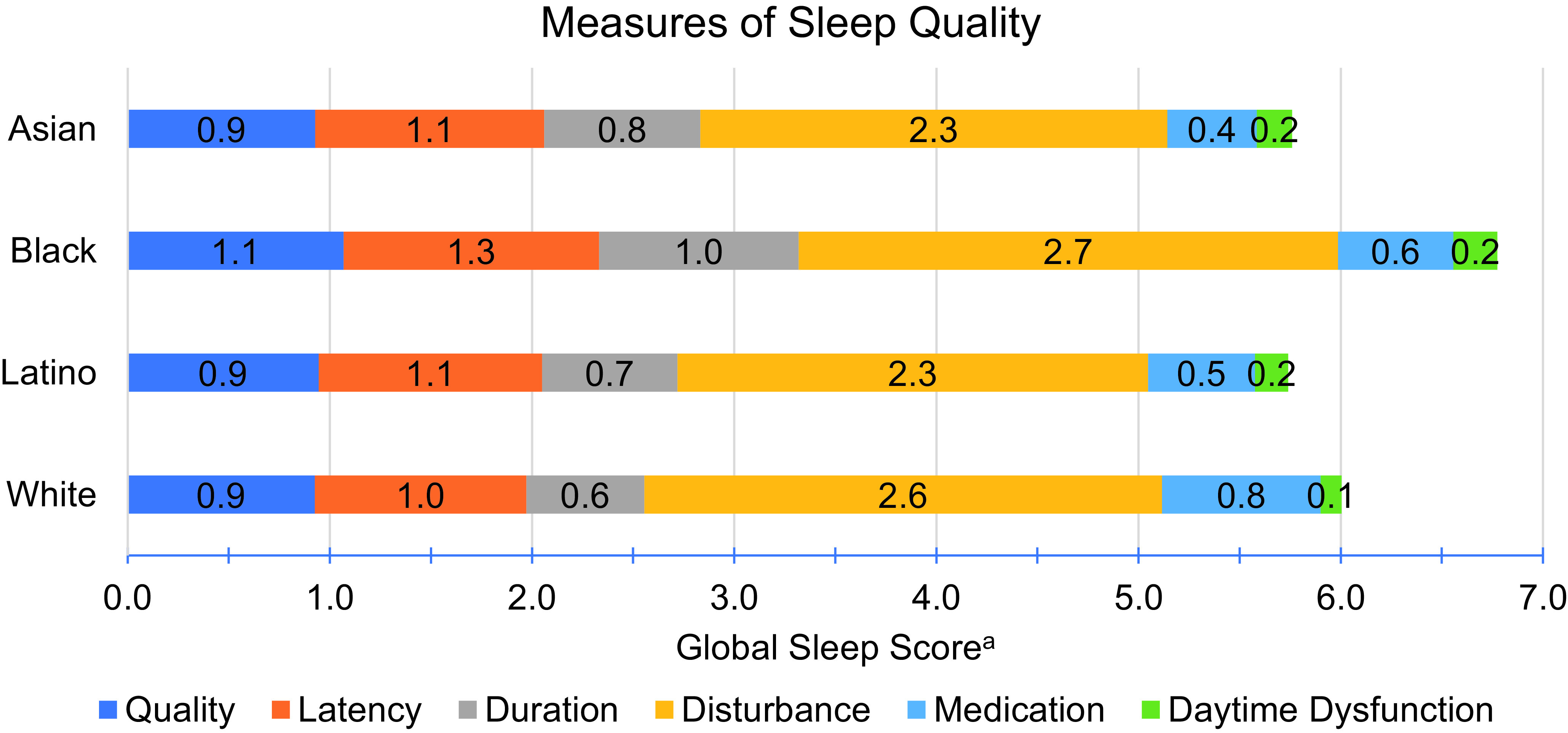
a. The Global Sleep Score is the sum of 6 sleep components with a higher score indicating worse sleep (range 0-24).
We assessed racial/ethnic differences in each sleep component with adjustment for covariates and using Latinos as the reference (Table 2). Associations were similar in our primary (Model 1) and fully adjusted (Model 2) analyses. Substantial racial/ethnic differences were seen for every sleep domain except sleep latency, although for sleep quality and daytime dysfunction confidence intervals were too wide to rule out chance differences. At any threshold of sleep duration component score, Asians [OR: 1.49 (1.12, 1.99)] and Blacks [OR: 2.16 (1.63, 2.87)] had elevated odds of a higher score (worse sleep) than Latinos (reference). At any threshold of sleep disturbance component score, Blacks [OR: 1.32 (1.02, 1.71)] and Whites [OR: 1.32 (1.01, 1.69)] had greater odds of a higher score compared with Latinos. For the medication use component, Whites [OR: 1.62 (1.18, 2.23)] had greater odds of a higher score compared to Latinos. Overall, Blacks [β: .96 (.37, 1.54)] had significantly higher mean global sleep scores (worse overall sleep) compared with Latinos while mean global sleep in Asians and Whites did not significantly differ.
Table 2. Odds ratios (95% CIs) for a one-unit higher sleep component score (indicating worse sleep) and linear regression coefficients for global sleep score associated with race/ethnicity, KHANDLE.
| Sleep Components | Asian | Black | Latino | White |
| n = 400 | n = 429 | n = 342 | n = 492 | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Sleep quality (range 0-3) | ||||
| Model 1 | 1.01 (.75, 1.35) | 1.34 (.99, 1.78) | 1 (Ref) | 1.04 (.79, 1.38) |
| Model 2 | 1.01 (.73, 1.39) | 1.35 (.99, 1.83) | 1 (Ref) | 1.04 (.77, 1.41) |
| Sleep (range 0-3) | ||||
| Model 1 | 1.01 (.77, 1.32) | 1.14 (.88, 1.50) | 1 (Ref) | .86 (.66, 1.12) |
| Model 2 | 1.06 (.79, 1.44) | 1.27 (.95, 1.69) | 1 (Ref) | .94 (.71, 1.24) |
| Sleep duration (range 0-3) | ||||
| Model 1 | 1.49 (1.12, 1.99) | 2.16 (1.63, 2.87) | 1 (Ref) | .78 (.59, 1.04) |
| Model 2 | 1.72 (1.25, 2.37) | 2.23 (1.64, 3.02) | 1 (Ref) | .87 (.64, 1.18) |
| Sleep disturbance (range 0-9) | ||||
| Model 1 | 1.01 (.77, 1.31) | 1.32 (1.02, 1.71) | 1 (Ref) | 1.32 (1.02, 1.69) |
| Model 2 | 1.08 (.80, 1.44) | 1.42 (1.07, 1.87) | 1 (Ref) | 1.32 (1.01, 1.73) |
| Medication use (range 0-3) | ||||
| Model 1 | .76 (.53, 1.08) | 1.03 (.73, 1.44) | 1 (Ref) | 1.62 (1.18, 2.23) |
| Model 2 | .82 (.55, 1.21) | 1.02 (.72, 1.47) | 1 (Ref) | 1.61 (1.15, 2.26) |
| Daytime dysfunction (range 0-3) | ||||
| Model 1 | 1.18 (.73, 1.90) | 1.44 (.90, 2.29) | 1 (Ref) | .71 (.43, 1.18) |
| Model 2 | 1.08 (.63, 1.84) | 1.42 (.87, 2.32) | 1 (Ref) | .66 (.38, 1.12) |
| Global sleep score (range 0-24) | ||||
| β(95% CI) | β (95% CI) | β (95% CI) | β(95% CI) | |
| Model 1 | .04 (-.56, 0.63) | .96 (.37, 1.54) | (Ref) | .28 (-.29, 0.84) |
| Model 2 | .27 (-.37, .90) | 1.13 (.52, 1.73) | (Ref) | .39 (-.20, .97) |
OR, odds ratio; CI, confidence interval.
Model 1 adjusted for age and sex.
Model 2 adjusted for Model 1 + waist circumference (cm), education, income, current alcohol drinker, ever tobacco smoker, physical activity, and depression.
As a secondary analysis, we examined racial/ethnic differences in mean sleep duration (hours per night) and found that Asians [β: -.35 (-.57, -.13)] and Blacks [β: -.60 (-.82, -.39)] slept significantly fewer hours compared with Latinos. Whites [β: .29 (.09, .50)] slept significantly more hours per night than Latinos.
We then analyzed racial/ethnic differences in the association between risk factors for poor sleep and average global sleep score (Table 3). There was no association between age (per 5 years) or ever smoking tobacco and global sleep score overall or for any race/ethnic group. There was a statistically significant race*alcohol consumption interaction (P = .04) indicating the association between alcohol consumption and global sleep score differed by race/ethnicity. Alcohol consumption was not associated with global sleep score among Asians, Latinos, or Whites, but among Blacks, alcohol consumption was associated with a significantly higher global sleep score (worse sleep) [β: .93 (.09, 1.78) ]. All other risk factors did not differ by race/ethnicity (ie, no significant race*risk factors interactions). Being male was associated with a lower mean global sleep score (better quality sleep) [Pooled β: -.73 (-1.13, -.34)]. Central adiposity was associated with a higher global sleep score (worse quality sleep) [Pooled β: .51 (.12, .91)]. Education above high school [Pooled β: -.90 (-1.42, -.37)], higher income [Pooled β: -1.21 (-1.64, -.78)], and physical activity [Pooled β: -.22 (-.28, -.15)] were associated with better quality sleep, and depression [Pooled β: 2.73 (1.98, 3.49)] was associated with significantly worse quality sleep.
Table 3. Age-adjusted linear regression coefficients (95% CI) for Global Sleep Scorea (higher scores indicate worse sleep) associated with risk factors for poor sleep and stratified by race/ethnicity, KHANDLE.
| Asian | Black | Latino | White | Pooledc | |
| n = 400 | n = 429 | n = 342 | n = 492 | n = 1,664 | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β(95% CI) | |
| Age (per 5 years) | |||||
| -.15 (-.44, .14) | .11 (-0.21, 0.42) | .18 (-.14, .51) | .04 (-.19, .27) | .04 (-.10, .18) | |
| Sex | |||||
| Female | (Ref) | (Ref) | (Ref) | (Ref) | (Ref) |
| Male | -.53 (-1.29, .23) | -1.20 (-2.07, -0.34) | -.34 (-1.22, .54) | -.77 (-1.45, -.10) | -.73 (-1.13, -.34) |
| Central adiposityb | |||||
| No | (Ref) | (Ref) | (Ref) | (Ref) | (Ref) |
| Yes | .43 (-.37, 1.22) | 1.17 (.32, 2.03) | -.28 (-1.18, .61) | .44 (-.24, 1.11) | .51 (.12, .91) |
| Education | |||||
| ≤ High school | (Ref) | (Ref) | (Ref) | (Ref) | (Ref) |
| > High school | -.90 (-2.17, .37) | -.57 (-1.68, .54) | -.82 (-1.77, .14) | -1.34 (-2.30, -.39) | -.90 (-1.42, -.37) |
| Income | |||||
| < $55,000 | (Ref) | (Ref) | (Ref) | (Ref) | (Ref) |
| ≥ $55,000 | -1.18 (-2.12, -.25) | -1.88 (-2.73, -1.02) | -1.05 (-1.96, -.13) | -.85 (-1.61, -.09) | -1.21 (-1.64, -.78) |
| Alcohol drinker | |||||
| No | (Ref) | (Ref) | (Ref) | (Ref) | -- |
| Yes | -.17 (-.98, .64) | .93 (.09, 1.78) | -.43 (-1.42, .57) | -.60 (-1.45, .25) | -- |
| Ever tobacco smoker | |||||
| No | (Ref) | (Ref) | (Ref) | (Ref) | (Ref) |
| Yes | .50 (-.32, 1.32) | .49 (-.34, 1.32) | .08 (-.78, .93) | -.02 (-.70, .65) | .26 (-.14, .65) |
| Physical activity (per unit) | |||||
| -.24 (-.37, -.12) | -.18 (-.31, -0.05) | -.21 (-.35, -.08) | -.24 (-.35, -.13) | -.22 (-.28, -.15) | |
| Depression | |||||
| No | (Ref) | (Ref) | (Ref) | (Ref) | (Ref) |
| Yes | 2.87 (1.42, 4.32) | 2.77 (1.05, 4.50) | 3.75 (2.37, 5.13) | 1.36 (-.13, 2.85) | 2.73 (1.98, 3.49) |
CI, confidence interval.
a. Global Sleep Score is the sum of 6 sleep components with a higher score indicating worse sleep (range 0-24).
b. Central adiposity was defined as a waist circumference of ≥ 90 cm for Asian men, ≥ 80 cm for Asian women, ≥ 102 cm for Black, Latino, and White men, and ≥ 88 cm for Black, Latino, and White women.
c. Pooled results adjusted for age and race/ethnicity.
Discussion
In this sample of community-dwelling adults aged ≥65 years, there were significant racial/ethnic differences in sleep quality. Overall, Blacks had the poorest global sleep quality, followed by Whites, with very similar global scores for Asians and Latinos. With respect to sleep components, Blacks had shorter sleep duration and more sleep disturbance than Latinos; Whites had more sleep disturbances and medication use than Latinos; and Asians had shorter sleep duration than Latinos. While there were significant racial/ethnic disparities in sleep components, global sleep scores were only significantly different in Blacks (compared with Latinos) including after adjustment for covariates.
Risk factors for poor sleep included being female, central adiposity, having a high school degree or less, income <$55,000, physical inactivity, and depression. There were no significant race interactions for these risk factors suggesting that the associations between risk factors and global sleep quality did not differ across race/ethnicity more than might be expected by chance. However, there was a significant race interaction for alcohol consumption. Black participants who reported consuming alcohol had significantly worse sleep compared with those who did not; whereas among Asians, Latinos, and Whites, there was no statistically significant association and point estimates indicating that those who drank alcohol averaged better sleep.
Previous studies of racial/ethnic disparities in sleep have primarily found that Blacks and Latinos report worse quality sleep compared with Whites, while Asians report better sleep quality.12,14 In KHANDLE, we found Latinos reported the highest quality sleep overall followed by Asians, Whites, and Blacks. Asian and Black participants reported the shortest average sleep duration, which is a pattern other studies have noted.11,29,30 Additionally, findings from the National Health and Nutrition Examination Survey (NHANES) and the Chicago Area Sleep Study (CASS) showed Latinos were more likely to report sleep disturbances and daytime dysfunction than Whites.30,31 By contrast, we found that Whites were more likely to report sleep disturbances than Latinos, though we did not find a statistically significant difference in daytime dysfunction between Latinos and Whites. In KHANDLE, Black participants were the most likely to report sleep disturbances (in addition to short sleep duration), a finding also noted by CASS.30 There were no significant differences in sleep between Asians and Whites in NHANES; however, we found Asians reported significantly shorter sleep duration than Whites, as was observed in CASS.30,31 Whites in KHANDLE were more likely than any other race/ethnic group to report taking medication to sleep, similar to what has been reported elsewhere.32,33
Age differences may explain why the racial/ethnic patterns in KHANDLE differ from previous studies such as NHANES and CASS. KHANDLE participants were aged ≥65 years (mean age of 76) whereas CASS participants were aged 35-64 years (mean age of 48) and NHANES participants were aged ≥18 years (mean age of 47).30,31 To our knowledge, the only prior study that has examined racial/ethnic differences in sleep quality among older adults is the Multi-Ethnic Study of Atherosclerosis (MESA) study (age range 54-93; mean age 69).11 MESA examined racial/ethnic differences using objectively measured sleep components, and, similar to KHANDLE, found that racial/ethnic minorities (Chinese, Hispanic, and Black participants) reported more characteristics of poor sleep, particularly Black participants.11 While there is evidence that sleep quality differs by race/ethnicity from childhood through middle age, whether sleep quality differs by race/ethnicity among older adults needs further study using both subjective and objective sleep measures.13,31
Being female, having central adiposity, lower education, lower income, physical inactivity, and depression were associated with poor sleep, consistent with findings in prior literature.11,34,35 The prevalence of these risk factors was highest in Black and Latino KHANDLE participants, which is also consistent with previous work.30,34,36 The relationship between these risk factors and global sleep score did not differ by race/ethnicity with the exception of alcohol consumption. Alcohol consumption was associated with significantly worse sleep in Blacks only. We could not account for quantity of alcohol consumed or patterns of alcohol consumption, which may explain these findings.
Study Limitations
There are some limitations to our analyses. We relied on self-reported measures of sleep which may not be as accurate in describing sleep habits and behaviors. Subjective measures tend to overestimate sleep duration and are modestly correlated with actigraphy-based sleep.37 If the measurement error in self-reports is non-differential, it would likely bias our results toward the null as was seen in a similarly diverse cohort study of middle-age adults.37 We were also unable to account for formally diagnosed sleep disorders (eg, obstructive sleep apnea, insomnia, etc.). Symptoms of these disorders would likely be captured, albeit indirectly, as part of the PSQI assessment. Moreover, in participants whose sleep disorders were being effectively treated, evidence suggests risk of comorbidities would not be elevated when compared with individuals without a sleep disorder.38,39 Another limitation was that we assessed risk factors cross-sectionally and cannot determine temporality in the relationship between risk factors and sleep quality; most of the risk factors we evaluated may themselves be influenced by past history of poor sleep quality. Risk factors were also largely based on self-report, so there may be issues of recall bias and measurement error.
Despite these limitations, our study is one of few that have examined racial/ethnic disparities in sleep quality among older adults aged ≥65 years. Strengths of this analysis include a diverse cohort of approximately equal proportions of Asian, Black, Latino, and White participants of varying sociodemographic backgrounds. We add to a growing body of evidence of racial/ethnic disparities in sleep, a modifiable risk factor related to several chronic conditions for which older adults are at increased risk.
Conclusions
We found that there were significant racial/ethnic differences in overall sleep quality and in individual sleep components among Asian, Black, Latino, and White older adults in KHANDLE. Exploring disparities in sleep components and the consequences of poor sleep quality among diverse, older adults using objective measures of sleep and risk factors is an important next step to better understand and address racial/ethnic differences in sleep quality.
Acknowledgments
This work was funded by the National Institutes of Health, National Institute on Aging under grant number RF1AG052132.
References
- 1. Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425-432. 10.1093/sleep/18.6.425 [DOI] [PubMed] [Google Scholar]
- 2. Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255-1273. 10.1093/sleep/27.7.1255 10.1093/sleep/27.7.1255 [DOI] [PubMed] [Google Scholar]
- 3. Mander BA, Winer JR, Walker MP. Sleep and Human Aging. Neuron. 2017;94(1):19-36. 10.1016/j.neuron.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3(5):383-388. 10.1016/j.sleh.2017.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Larcher S, Benhamou PY, Pépin JL, Borel AL. Sleep habits and diabetes. Diabetes Metab. 2015;41(4):263-271. 10.1016/j.diabet.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 6. Mims KN, Kirsch D. Sleep and Stroke. Sleep Med Clin. 2016;11(1):39-51. 10.1016/j.jsmc.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 7. Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S; INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists) . Lorenzi- Filho G, Redline S; INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists). Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840-1850. 10.1161/CIRCULATIONAHA.117.029400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wennberg AMV, Wu MN, Rosenberg PB, Spira AP. Sleep disturbance, cognitive decline, and dementia: a review. Semin Neurol. 2017;37(4):395-406. 10.1055/s-0037-1604351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leng Y, McEvoy CT, Allen IE, Yaffe K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol. 2017;74(10):1237-1245. 10.1001/jamaneurol.2017.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65(1):63-73. https://doi.org/ 10.1097/01. PSY.0000039756.23250.7C PMID:12554816 [DOI] [PubMed]
- 11. Chen X, Wang R, Zee P, et al. Racial/ ethnic differences in sleep disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep (Basel). 2015;38(6):877-888. 10.5665/sleep.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adenekan B, Pandey A, McKenzie S, Zizi F, Casimir GJ, Jean-Louis G. Sleep in America: role of racial/ethnic differences. Sleep Med Rev. 2013;17(4):255-262. 10.1016/j.smrv.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grandner MA, Williams NJ, Knutson KL, Roberts D, Jean-Louis G. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Med. 2016;18:7-18. 10.1016/j.sleep.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. 2015;36(1):417-440. 10.1146/annurev-publhealth-031914-122838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rangaraj VR, Knutson KL. Association between sleep deficiency and cardiometabolic disease: implications for health disparities. Sleep Med. 2016;18:19-35. 10.1016/j.sleep.2015.02.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096-1103. 10.1093/sleep/30.9.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kang JM, Lee JA, Jang JW, Kim YS, Sunwoo S. Factors associated with poor sleep quality in primary care. Korean J Fam Med. 2013;34(2):107-114. 10.4082/kjfm.2013.34.2.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruiter ME, Decoster J, Jacobs L, Lichstein KL. Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep Med. 2011;12(3):209-214. 10.1016/j.sleep.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 19. Rao U, Poland RE, Lutchmansingh P, Ott GE, McCracken JT, Lin KM. Relationship between ethnicity and sleep patterns in normal controls: implications for psychopathology and treatment. J Psychiatr Res. 1999;33(5):419-426. 10.1016/S0022-3956(99)00019-9 [DOI] [PubMed] [Google Scholar]
- 20. Nunes J, Jean-Louis G, Zizi F, et al. Sleep duration among black and white Americans: results of the National Health Interview Survey. J Natl Med Assoc. 2008;100(3):317-322. 10.1016/S0027-9684(15)31244-X [DOI] [PubMed] [Google Scholar]
- 21. Mokhlesi B, Pannain S, Ghods F, Knutson KL. Predictors of slow-wave sleep in a clinic-based sample. J Sleep Res. 2012;21(2):170-175. 10.1111/j.1365-2869.2011.00959.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep (Basel). 2014;37(3):601-611. 10.5665/sleep.3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164(4):406-418. 10.1001/archinte.164.4.406 [DOI] [PubMed] [Google Scholar]
- 24. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 25. Brewster PW, Melrose RJ, Marquine MJ, et al. Life experience and demographic influences on cognitive function in older adults. Neuropsychology. 2014;28(6):846-858. 10.1037/neu0000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D; PROMIS Cooperative Group . Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18(3):263-283. 10.1177/1073191111411667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McAlister C, Schmitter-Edgecombe M. Everyday functioning and cognitive correlates in healthy older adults with subjective cognitive concerns. Clin Neuropsychol. 2016;30(7):1087-1103. 10.1080/13854046.2016.1190404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alberti KG, Eckel RH, Grundy SM, et al. ; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640-1645. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 29. Cunningham TJ, Wheaton AG, Ford ES, Croft JB. Racial/ethnic disparities in self-reported short sleep duration among US-born and foreign-born adults. Ethn Health. 2016;21(6):628-638. 10.1080/13557858.2016.1179724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carnethon MR, De Chavez PJ, Zee PC, et al. Disparities in sleep characteristics by race/ethnicity in a population-based sample: Chicago Area Sleep Study. Sleep Med. 2016;18:50-55. https://doi.org/ 10.1016/j. sleep.2015.07.005 PMID:26459680 [DOI] [PMC free article] [PubMed]
- 31. Grandner MA, Petrov ME, Rattanaumpawan P, Jackson N, Platt A, Patel NP. Sleep symptoms, race/ethnicity, and socioeconomic position. J Clin Sleep Med. 2013;9(9):897-905; 905A-905D. https://doi.org/ 10.5664/jcsm.2990. [DOI] [PMC free article] [PubMed]
- 32. Allen KD, Renner JB, DeVellis B, Helmick CG, Jordan JM. Racial differences in sleep medication use: a cross-sectional study of the Johnston County Osteoarthritis Project. Ann Pharmacother. 2008;42(9):1239-1246. 10.1345/aph.1L111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alegria M, Takeuchi D, Canino G, et al. Considering context, place and culture: the National Latino and Asian American Study. Int J Methods Psychiatr Res. 2004;13(4):208-220. 10.1002/mpr.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mezick EJ, Matthews KA, Hall M, et al. Influence of race and socioeconomic status on sleep: pittsburgh SleepSCORE project. Psychosom Med. 2008;70(4):410-416. 10.1097/PSY.0b013e31816fdf21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luo J, Zhu G, Zhao Q, et al. Prevalence and risk factors of poor sleep quality among Chinese elderly in an urban community: results from the Shanghai aging study. PLoS One. 2013;8(11):e81261. 10.1371/journal.pone.0081261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Egan KJ, Knutson KL, Pereira AC, von Schantz M. The role of race and ethnicity in sleep, circadian rhythms and cardiovascular health. Sleep Med Rev. 2017;33:70-78. 10.1016/j.smrv.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jackson CL, Patel SR, Jackson WB II, Lutsey PL, Redline S. Agreement between self-reported and objectively measured sleep duration among white, black, Hispanic, and Chinese adults in the United States: Multi-Ethnic Study of Atherosclerosis. Sleep (Basel). 2018;41(6). 10.1093/sleep/zsy057 10.1093/sleep/zsy057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 2008;134(4):686-692. 10.1378/chest.08-0556 [DOI] [PubMed] [Google Scholar]
- 39. Bombois S, Derambure P, Pasquier F, Monaca C. Sleep disorders in aging and dementia. J Nutr Health Aging. 2010;14(3):212-217. 10.1007/s12603-010-0052-7 10.1007/s12603-010-0052-7 [DOI] [PubMed] [Google Scholar]


