ABSTRACT
Tea flavonoids have been suggested to offer potential benefits to cardiovascular health. This review synthesized the evidence on the relation between tea consumption and risks of cardiovascular disease (CVD) and all-cause mortality among generally healthy adults. PubMed, EMBASE, Web of Science, Cochrane Central Register of Controlled Trials, Food Science and Technology Abstracts, and Ovid CAB Abstract databases were searched to identify English-language publications through 1 November 2019, including randomized trials, prospective cohort studies, and nested case-control (or case-cohort) studies with data on tea consumption and risk of incident cardiovascular events (cardiac or peripheral vascular events), stroke events (including mortality), CVD-specific mortality, or all-cause mortality. Data from 39 prospective cohort publications were synthesized. Linear meta-regression showed that each cup (236.6 mL) increase in daily tea consumption (estimated 280 mg and 338 mg total flavonoids/d for black and green tea, respectively) was associated with an average 4% lower risk of CVD mortality, a 2% lower risk of CVD events, a 4% lower risk of stroke, and a 1.5% lower risk of all-cause mortality. Subgroup meta-analysis results showed that the magnitude of association was larger in elderly individuals for both CVD mortality (n = 4; pooled adjusted RR: 0.89; 95% CI: 0.83, 0.96; P = 0.001), with large heterogeneity (I2 = 72.4%), and all-cause mortality (n = 3; pooled adjusted RR: 0.92; 95% CI: 0.90, 0.94; P < 0.0001; I2 = 0.3%). Generally, studies with higher risk of bias appeared to show larger magnitudes of associations than studies with lower risk of bias. Strength of evidence was rated as low and moderate (depending on study population age group) for CVD-specific mortality outcome and was rated as low for CVD events, stroke, and all-cause mortality outcomes. Daily tea intake as part of a healthy habitual dietary pattern may be associated with lower risks of CVD and all-cause mortality among adults.
Keywords: tea, Camellia sinensis, cardiovascular disease, all-cause mortality, systematic review
Introduction
Tea is one of the top dietary sources of flavonoids in the US diet and the second most widely consumed beverage worldwide (1, 2). Tea is produced from hot water infusion of dried Camellia sinensis to create a flavonoid-rich beverage. Differences in postharvest processing result in several categories of tea, including black (fermented), oolong (partially fermented), and green (unfermented), each with a different array of flavonoids including flavan-3-ol monomers (in green tea) to oxidized flavan-3-ols including theaflavins and thearubigens (in oolong and black tea). Black tea is the primary type of tea consumed in North America and Europe, whereas green tea is consumed principally in Asia and oolong tea in southeast China (2). Approximately 21% of Americans report consuming tea on a daily basis, and tea consumers were previously shown to have ∼20 times higher flavonoid intake compared with nonconsumers (3). Following consumption, flavan-3-ols and flavonols can be metabolized by both host and microbial systems to yield a mixture of complex metabolites that can be found in the circulation and throughout the body (1).
Tea has been investigated (via in vitro, animal, observational, preclinical, and clinical studies) for its potential to reduce the risk and/or progression of various chronic disease outcomes, particularly cardiovascular disease (CVD) and cerebrovascular disease (1, 2). Tea flavonoids can act as modulators of enzymes involved in oxidative and inflammatory stress, enhance NO status, and improve endothelial function, which may, in part, contribute to potential benefits on cardiovascular health (4). However, due to differences in tea profiles (i.e., concentrations of flavonoids) and variable doses, it has been difficult to compare results across studies. An early meta-analysis (literature searches through November 2009) found no significant association between black tea consumption and risk of coronary artery disease (CAD) and a small inverse association between green tea consumption and CAD (5). This meta-analysis, however, did not examine nonlinear relations and did not perform risk-of-bias (ROB) assessment of included studies. A more recent meta-analysis (literature searches through July 2014) found that increased tea consumption [i.e., 3 cups (709.8 mL)/d] was associated with a reduced risk of coronary heart disease, cardiac death, stroke, and total mortality (6). Both meta-analyses excluded studies that did not report sufficient data for the dose–response meta-analyses, which can thus introduce selection bias.
To provide comprehensive and updated evidence on the relation between tea consumption and risks of CVD and all-cause mortality, we conducted a systematic review and dose–response meta-analysis of prospective cohort studies and randomized controlled trials (RCTs).
Methods
We followed the methods for conducting a systematic review outlined in the National Academy of Medicine's Standards for Systematic Reviews (7) and reported the study results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (8). Two reviewers independently performed abstract and full-text screenings, ROB assessment, and data extraction. Disagreements between the reviewers were discussed until both parties were in agreement or by group consensus.
Data sources and searches
We searched PubMed, EMBASE, Web of Science, Cochrane Central Register of Controlled Trials, Food Science and Technology Abstracts, and Ovid CAB Abstract databases through 20 June 2019 for both 1) prospective cohort or nested case-control (or case-cohort) studies reporting ≥1 analysis of the association between tea consumption and risk of incident cardiovascular events (cardiac or peripheral vascular events), CVD-specific mortality, cerebrovascular (stroke) events or mortality, or all-cause mortality and 2) RCTs on the effect of increasing tea consumption on the same outcomes. Detailed search terms and search strategies used in each database are described in Supplemental Appendix 1.
Study selection
Study eligibility was restricted to peer-reviewed, English-language studies in adults aged ≥18 y. Only studies conducted in generally healthy adults (with the exception of hypertension, no more than 20% of study participants could have known CVD) were included. Exceptions were made for elderly populations (age >65 y) since the prevalence of CVD is high in this population. Prospective cohort studies and RCTs needed to have a minimal follow-up duration of 1 y and 4 wk, respectively, to be included. Reference lists of relevant systematic reviews were cross-checked with our list of included studies to ensure no relevant studies were missed. All CVD event or mortality outcomes (defined by the original authors) were included. Studies on alcohol or solvent extracts of tea leaves, other tea supplements, or herbal teas were excluded.
Data extraction and ROB assessment
A standardized data-extraction form was used to abstract data from each included study. The Newcastle-Ottawa Scale (NOS) (9) was used to assess the ROB for each included cohort study (no RCTs met the eligibility criteria of the present systematic review). Several NOS prompting questions must be tailored or defined for a specific systematic review topic, including “representative of exposed cohort,” “comparability of cohorts on the basis of the design and analysis,” and “adequacy of follow-up of cohorts.” In addition, the NOS “ascertainment of exposure” question was modified in order to assess the validity and uncertainty of intake assessment, which is one of the unique challenges that should be considered in nutrition-related systematic reviews (10). Specifically, the ROBs regarding the validity and uncertainty of daily tea intake assessment (i.e., the NOS “ascertainment of exposure” question) were rated on the basis of the reported dietary assessment methods using ratings of A–C as follows (Supplemental Appendix 2)—A (most valid or least uncertainty): multiple dietary exposure assessments during follow-ups and having internal or external calibration of the dietary assessment instrument, or single dietary exposure assessment at baseline and having internal or external calibration of dietary assessment for tea or flavonoid consumption specifically; B: single dietary exposure assessment at baseline and having internal or external calibration of the dietary assessment instrument; and C (least valid or most uncertainty): single dietary exposure assessment at baseline and unclear validity of the dietary assessment instrument. Details of the modified NOS and instructions are described in Supplemental Appendix 3. Moreover, we piloted ROB assessment among 5 reviewers to refine the instructions on how to interpret the NOS prompting questions, tailored to the present systematic review topic, to ensure interrater agreement.
Data synthesis
We planned to synthesize RCTs and observational studies separately. However, no RCTs met the eligibility criteria of the present systematic review. For each outcome, included studies were synthesized quantitatively using several meta-analytical methods (details are described in the “Meta-analysis” section), if there were sufficient quantitative data reported, or qualitatively using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach (11–13). Briefly, the GRADE approach entails an assessment of the quality of a body of evidence (also known as strength of evidence) for each individual outcome. Strength of a body of evidence involves consideration of within-study ROB (methodological quality), directness of evidence, heterogeneity, precision of effect or association estimates, and risk of publication bias to reach an overall strength of evidence rating of very low, low, moderate, or high for each outcome. This process was done by consensus among all investigators and the expert panel.
Meta-analysis
Many cohort studies had multiple analyses reporting different outcomes of interest. We planned our meta-analysis carefully to ensure that study populations did not overlap in each analysis. If >1 analysis model was reported in a study, we focused on the model that adjusted for the most potential confounders. Results for female and male participants were extracted, if reported separately, and considered as 2 independent “studies” in the analyses. We considered models adjusted only for age or sex as unadjusted analyses. Two studies reporting only unadjusted analyses (14, 15) were excluded due to the potential for confounding bias.
For cohort studies, we performed mixed-effects linear and nonlinear dose–response meta-regressions using a 2-stage hierarchical regression model implemented in the dosresmeta R software package (R Foundation for Statistical Computing) (16, 17). The same models are used to analyze the dose–response relations between tea flavonoid intake amounts and risks for cardiovascular events, CVD mortality, stroke events, and all-cause mortality. Nonlinearity was investigated by adopting quadratic models.
It is important to note that this dose–response meta-regression model requires categorical exposure data (≥3 exposure categories, including the reference category, within each study). The method was first formalized by Greenland and Longnecker (18); the authors described how to approximate the covariances of reported log RRs and how to use them to efficiently estimate an exposure–disease relation. The study-specific estimates (RRs per quantile intake category versus the referent intake category) are combined through multivariate random-effects meta-analytical models to obtain a pooled dose–response association. To estimate study-specific linear trends based on quantile-based intake data, several approximations were made; the mean or median value per exposure category of tea intake amounts is also needed for dose–response meta-regressions. When it was not reported, we selected the midpoint between exposure category thresholds; for the open categories, we imputed a mean intake that was 20% lower for the lowest quintile threshold or 20% higher for the highest quintile threshold, respectively. Total tea flavonoid intake amounts were estimated by multiplying the standard reference in the USDA database for the flavonoid content for green tea [Nutrient Databank (NDB) no. 14653; 137.9 mg/100 g)] and black tea (NDB no. 14355; 118.35 mg/100 g) (19) times the reported median or mean intake green or black tea consumption amounts per exposure category in each included study. In the USDA database, values for tea are given as milligrams per 100 g (100 mL) of tea infusions (as consumed) and are equivalent to 1 g of dry tea. Most included studies utilized food-frequency questionnaires (FFQs) that asked about the frequencies of daily or weekly cups of tea consumption. To calculate an estimated amount of total daily tea flavonoid intake, 1 cup (236.6 mL) of black tea was assumed to be 237 g and 1 cup (236.6 mL) of green tea was assumed to be 245 g (19). Sensitivity analysis was performed to test the robustness of our dose–response meta-regressions by changing the imputed mean of tea flavonoid intake for the open categories from 20% to 30% lower or higher for the lowest or highest category, respectively. None of the meta-analysis results presented in the present article were changed (data not shown).
In addition, we performed standard random-effects meta-analyses (20) to combine study-specific linear trend estimates per each cup (236.6 mL) of daily tea intake from the dose–response meta-regression analyses with those from studies that reported only linear trend estimates (and thus cannot be combined in the dose–response meta-analysis) assessing the association between tea intake amounts and the risks of CVD mortality, CVD events, stroke, or all-cause mortality outcomes.
Subgroup meta-analysis and meta-regression to explore heterogeneity
We performed post hoc subgroup meta-analyses to explore sources of heterogeneity in the random-effects meta-analyses, including study participants’ sex (both sexes, female, or male) and age groups [adults (mean/median age <65 y) or elderly (mean/median age ≥65 y)], countries or regions where studies were conducted (United States, Asia, Europe, or others), types of tea (black, green, or all teas), and ROB. We also performed random-effects meta-regression analyses (21) to examine whether studies’ follow-up durations or outcome incidence rates were associated with the magnitudes of linear trend estimates across studies.
Analytic data sets for the dose–response meta-regression analyses are available upon request. All analyses and charting were conducted using Stata SE 13 software (StataCorp) and R software version 3.2.5. We tested for heterogeneity using Cochran's Q statistic (considered significant when P < 0.10) and quantified the extent of heterogeneity with the I2 index. I2 values of 25%, 50%, and 75% were defined as low, moderate, and high heterogeneity, respectively. These cutoffs were arbitrary and were used for descriptive purposes only. All P values were 2-tailed and a P value <0.05 was considered statistically significant.
Role of the funding source
Funding for this project was provided through an unrestricted educational grant from Unilever to Think Healthy Group. The funder had no role in study selection, quality assessment, data analysis, or writing of the manuscript.
Results
Search results
No RCTs met the inclusion criteria. Of the 39 publications that were included in the present systematic review (22–60), 8 prospective cohort studies did not report sufficient quantitative data for meta-analyses (23–25, 32, 49, 51, 59, 60), and thus their results are summarized narratively and synthesized qualitatively with the respective meta-analysis results for each outcome. Supplemental Figure 1 shows the summary of literature searches and study selection flow. Characteristics of included studies are described in Table 1.
TABLE 1.
Characteristics of included prospective cohort studies reporting the relation between tea intake and risks of CVD mortality, CVD events, stroke events, or all-cause mortality1
| Author, year (ref) | Cohort name | Country | Analyzed/enrolled, n/N | Mean age, y | Age range, y | Men, % | Race/ethnicity | Mean BMI, kg/m2 | Mean follow-up, y | Funding source category | Confounders adjusted |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Andersen et al., 2006 (22) | Iowa's Women's Health Study Cohort | USA | 27,312/41,836 | 61.4 | 55–69 | 0 | NR | 26.7 | 15 | Government, nonprofit | Age, smoking, alcohol intake, BMI, waist-hip ratio, education, physical activity, use of estrogens, use of multivitamin supplements, energy intake, and intakes of whole and refined grains, red meat, fish and seafood, and total fruit and vegetables |
| Arts et al., 2001 (23) | Zutphen Elderly Study | Netherlands | 806/1266 | 71.3 | 65–84 | 100 | NR | 25.5 | NR | Government | Age, smoking status, total energy intake, BMI, alcohol intake and physical activity, coffee consumption, fish consumption, vitamin C, vitamin E, β-carotene, SFAs, PUFAs, dietary cholesterol, fiber, and prescribed diet |
| Baik et al., 2013 (24) | Korean Genome Epidemiology Study | Korea | 9026/9026 | 52.1 | 40–69 | 52 | NR | 24.6 | 8 | Government | Age, sex, SBP, hypertension, diabetes, smoking status, cholesterol, HDL, BMI, family history of CVD, alcohol drinking, vitamin and mineral intake, physical activity, calorie intake, food and beverage consumption |
| Bertoia et al., 2013 (25) | Women's Health Initiative (WHI) Observational Study | USA | 92,847/93,676 | 63.5 | 50–79 | 0 | White, 83%; black, 8%; Hispanic, 4%; other, 4% | 27.3 | 11 | Government | Age, total energy intake, race, income, smoking status, physical activity, waist-to-hip ratio, BMI, atrial fibrillation, coronary artery disease, heart failure, diabetes, high cholesterol, hypertension, pulse in 60 s, and hormone use |
| de Koning et al., 2010 (26) | EPIC-NL (European Prospective Investigation into Cancer and Nutrition–Netherlands) | Netherlands | 37,514/40,011 | 49 | 20–69 | 25 | NR | Mean waist circumference (cm) = 85.1 | 13 | Government, nonprofit | Sex, age, cohort (strata), educational level, physical activity, smoking status, waist circumference, hormone replacement therapy and menopausal status, alcohol intake, tea or coffee intake, total energy, and energy-adjusted intake of saturated fat, fiber, and vitamin C; total fluid intake, hypertension, hypercholesterolemia, and diabetes |
| Gaeini et al., 2019 (27) | Tehran Lipid and Glucose Study (TLGS) | Iran | 2369/3052 | 41.2 | ≥19 | 43.5 | NR | 26.6 | 6 | Not supported by any funding agency | CVD risk score, coffee, dietary fat, and total energy |
| Gardener et al., 2013 (28) | Northern Manhattan Study (NOMAS) | USA | 2461/3298 | 68.3 | >40 | 36 | Black, 23%; white, 19%; Hispanic, 56% | 28.0 | 11 | Government | Age, sex, race/ethnicity, education, pack-years of smoking, alcohol consumed/d, moderate–heavy physical activity, diet (total daily energy, protein, carbohydrates, total fat, saturated fat), BMI, vascular risk factors (history of cardiac disease, diabetes, hypertension, hypercholesterolemia), other nonwater beverage consumption, milk in coffee/tea, cream in coffee/tea, and nondairy creamer in coffee/tea, and mutually adjusted for coffee and tea |
| Geleijnse et al., 2002 (29) | Rotterdam Study | Netherlands | 4807/6521 | 67.4 | ≥55 | 38 | NR | 26.3 | 5.6 | For-profit | Age, sex, BMI, smoking status, pack-years of cigarette smoking, educational level, and daily intakes of alcohol, coffee, polyunsaturated fat, saturated fat, fiber, vitamin E, and total energy |
| Hertog et al., 1993 (30) | Zutphen Elderly Study | Netherlands | 805/939 | 71.3 | 65–84 | 100 | NR | 25.5 | 5 | Government, nonprofit | Age, history of myocardial infarction at baseline, intake of total energy and saturated fat, physical activity, BMI, smoking, total and HDL cholesterol, and SBP |
| Hertog et al., 1997 (31) | The Caerphilly Study | United Kingdom | 1900/2512 | 52.1 | 45–59 | 100 | NR | 26.2 | 14 | Government | Age, baseline IHD (for IHD mortality outcome only), smoking, social class, BMI, and intake of total energy, alcohol, fat, vitamin C, vitamin E, and β-carotene |
| Hirvonen et al., 2001 (32) | Alpha-Tocopherol, Beta-Carotene Cancer (ATBC) prevention study | Finland | 25,372/29,133 | NR | 50–69 | 100 | NR | Median = 26.0 | 6.1 | Government | Age, supplementation group, SBP and DBP, serum total cholesterol, serum HDL cholesterol, BMI, smoking years, number of cigarettes smoked daily, histories of diabetes mellitus and CHD, marital status, education, and leisure-time physical activity |
| Ivey et al., 2017 (33) | Nurses’ Health Study II | USA | 93,145/93,145 | 36.1 | 25–42 | 0 | ∼94% Caucasian | 24.6 | 18 | Government | Age, BMI, smoking status, menopausal status, family history (of diabetes, cancer, and myocardial infarction), multivitamin supplement use, aspirin use, race, type 2 diabetes, hypercholesterolemia, hypertension, physical activity, energy intake, alcohol consumption and the Alternative Health Eating Index (minus alcohol) score |
| Iwai et al., 2002 (34) | NR | Japan | 2855/4411 | 58.2 | 40–79 | 49.2 | Asian (assumed) | NR | 9.9 | Government | Age, history of selected diseases, physical activity level, education status, and additionally only in men, smoking status and habitual alcohol consumption |
| Keli et al., 1996 (35) | The Zutphen Study | Netherlands | 552/872 | 59.5 | 40–59 | 100 | NR | NR | 15 | Government | Age, average SBP, serum cholesterol, energy intake, lifetime cigarette smoking exposure, fish consumption, and alcohol consumption habits |
| Klatsky et al., 1990 (36) | NR | USA | 9986/101,774 | NR | <50, 53%; 50–59, 20%; >60 , 23% | 47 | Black, 27%; white and Hispanic, 73% | NR | 5 | Nonprofit | Age, sex, race, cigarette smoking, alcohol intake, education, coffee use, and baseline disease |
| Klatsky et al., 1993 (37) | NR, part of Kaiser Permanente Medical Care Program | USA | 125,356/128,934 | 40.36 | NR | NR | NR | NR | 8 | Government, nonprofit | Age, sex, race, BMI, smoking, alcohol, education, and marital status |
| Kokubo et al., 2013 (38) | The Japan Public Health Center–Based Study Cohort | Japan | 81,978/100,938 | 54 | 45–74 | 46 | Asian | 23.6 | 13 | Government | Age, sex, smoking, alcohol, BMI, history of diabetes mellitus, medication of antihypercholesterolemia and antihypertension, sports, dietary intake (of fruits, vegetables, fish, and energy), public health centers, and coffee consumption |
| Kuriyama et al., 2006 (39) | Ohsaki National Health Insurance Cohort Study | Japan | 40,530/52,029 | 60.2 | 40–79 | 47 | Asian (assumed) | 68.9% with normal BMI, 3.5% underweight, 27.6% overweight or obese | 11 | Government | Age, sex, job status, years of education, BMI, engaging in sports or exercise, walking duration, history of hypertension, diabetes mellitus, gastric ulcer, smoking status, alcohol drinking, total energy intake per day, daily consumption of rice, daily consumption of miso (soybean paste) soup, daily consumption of soybean products, total meat, total fish, dairy products, total fruits, total vegetables, and consumption of oolong tea, black tea, and coffee |
| Larsson et al., 2008 (40) | Alpha-Tocopherol, Beta-Carotene Cancer (ATBC) Prevention Study | Southwest Finland | 26,556/29,133 | 57.6 | 50–69 | 100 | NR | 26.3 | 13.6 | Government | Age, supplementation group, number of cigarettes smoked daily, BMI, SBP and DBP, serum total cholesterol, serum HDL cholesterol, histories of diabetes and CHD, leisure-time physical activity, alcohol intake, and coffee consumption |
| Larsson et al., 2013 (41) | The Swedish Mammography Cohort, The Cohort of Swedish Men | Sweden | 74,961/88,077 | 60.3 | 45–83 | 54 | NR | 25.4 | 10.2 | Government | Age, sex, smoking status and pack-years of smoking, education, BMI, total physical activity, aspirin use, history of hypertension, diabetes, family history of myocardial infarction, and intakes of total energy, alcohol, coffee, fruits, and vegetables |
| Leurs et al., 2010 (42) | Netherlands Cohort Study (NLCS) | Netherlands | Subcohort: 3970/5000 | 62.3 | 55–69 | 54 | NR | 53% with normal BMI; 1.0% underweight; 46% overweight or obese | 10 | Nonprofit | Age, sex, cigarette smoking, and energy intake |
| Li et al., 2017 (43) | China Kadoorie Biobank | China | 487,375/512,891 | 51 | 30–79 | 41 | Asian (assumed) | 23.6 | 7.2 | Government, nonprofit | Age, sex, education, marital status, alcohol consumption; smoking status, physical activity, intake frequencies of red meat, fruits, and vegetables; family history of heart attack; BMI; prevalent hypertension and diabetes at baseline |
| Lim et al., 2017 (44) | Calcium Intake Fracture Outcome Study (CAIFOS) | Australia | 1055/1055 | 80.0 | ≥70 | 0 | NR | 27.2 | 10 | Government, nonprofit | Smoking history, SES, diabetes status, hypertension, SBP, prevalent CVD, medications and treatment code (calcium supplementation vs. no calcium supplementation), fluid status, age, BMI, and eGFR |
| Liu et al., 2016 (45) | Chinese Prospective Smoking Study (CPSS) | China | 164,681/222,279 | 53.2 | >40 | 100 | Asian | 82.9% with normal weight; 8.0% underweight; 9.3% overweight or obese | 11 | Government, nonprofit | Age, BMI, marital status, urban locality, education, job status, smoking status, alcohol drinking, times of weekly fish consumption, times of weekly meat consumption, times of weekly poultry consumption, times of weekly egg consumption, times of weekly milk consumption, black tea drinker, jasmine tea drinker, and other tea drinker |
| Lopez-Garcia et al., 2009 (46) | Nurses’ Health Study | USA | 83,076/83,076 | Mean range, 55–56 | NR | 0 | NR | Mean ∼25 | 24 | Government | Age, smoking status, BMI, physical activity, alcohol intake, menopausal status and use of hormone replacement therapy, aspirin use, total caloric intake, intake quintiles (of calcium, potassium, sodium, and folate), glycemic load, whole grain intake, and tertiles of fruits, vegetables, and fish consumption |
| Miller et al., 2017 (47) | Multi-Ethnic Study of Atherosclerosis (MESA) | USA | 6508/6508 | 62.2 | 44–84 | 47.4 | White, 39%; Chinese, 12.3%; black, 26.8%; Hispanic, 21.8% | 28.2 | 11.1 | Government | Age, sex, race/ethnicity, and education, smoking, physical activity, total fat, alcohol consumption, fruits quartiles, vegetables quartiles, red meat quartiles, SBP and DBP, use of antihypertensive medications, lipid-lowering medication, antidiabetic medication, BMI, family history of CHD, diabetes, HDL cholesterol, total cholesterol, TGs, C-reactive protein, and fibrinogen |
| Mineharu et al., 2011 (48) | Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC Study) | Japan | 34,345 men and 48,310 women/82,655 | 57.1 | 40–79 | 42 | Asian (assumed) | NR | 13.1 | Government | Age, BMI, history of hypertension, history of diabetes, smoking status, alcohol intake, education, walking hours, hours of sports participation, perceived mental stress, multivitamin use, vitamin E supplement use, consumption of total fruits, total vegetable, total beans, total meat, total fish and seaweeds, total daily energy intake, and use of hormone therapy (in women) |
| Nakachi et al., 2000 (49) | NR | Japan | 8497/8552 | 54 | >40 | 48 | Asian (assumed) | NR | 11 | Government | Cigarette smoking, alcohol consumption, intake of meat, and relative body weight, using age as the fundamental time variable; analyses were stratified by sex |
| Odegaard et al., 2015 (50) | Singapore Chinese Health Study (SCHS) | Singapore | 52,584/63,257 | 55.8 | 45–74 | 44 | Asian | 23 | 16.3 | Government | Model includes all beverages simultaneously (coffee, black tea, alcohol, soft drinks, juice, and green tea) and was adjusted for age, sex, dialect, education, year of interview, moderate and vigorous activity, sleep, BMI, hypertension (except for cancer), nonbeverage vegetable-fruit-soy–rich dietary pattern score, and energy intake |
| Rimm et al., 1996 (51) | Health Professionals Follow-Up Study | USA | 34,789/51,529 | 54.1 | 40–75 | 100 | NR | 25.4 | 6 | NR | Age, obesity, smoking, intake of vitamin E, intake of alcohol, diabetes, hypertension, hypercholesterolemia, and family history of CHD |
| Saito et al., 2015 (52) | Japan Public Health Center-based Prospective Study (JPHC Study) | Japan | 90,914/140,420 | 50.9 | 40–69 | 47 | Asian | 23.4 | 18.7 | Government, nonprofit | Age, public health center area, smoking status, alcohol consumption, BMI, history of hypertension, history of diabetes, leisure-time physical activity, coffee intake, tea, black tea, soda or juice, energy intake, fruit intake, vegetable, fish, meat, dairy products, rice, miso soup, job status, excluding death ≤5 y |
| Sesso et al., 2003 (53) | College Alumni Health Study (CAHS) | USA | 17,228/35,924 | 59.5 | NR | 95.6 | NR | 24.4 | 15 | Government, nonprofit | Age, sex, BMI, physical activity, physician-diagnosed hypertension, physician-diagnosed diabetes mellitus, smoking status, alcohol consumption, and early parental death |
| Suzuki et al., 2009 (54) | Shizuoka Elderly Cohort Study | Japan | 12,251/14,001 | 74.3 | 65–84 | 50.7 | Asian | 21.8 | 5.2 | Government | Smoking status, alcohol consumption, BMI, and the frequency of physical activity |
| Tanabe et al., 2008 (55) | NR | Japan | 6358/7753 | NR | 40–89 | 49 | NR | ∼23 | 5 | Government | Age, sex, hypertension, diabetes, presence of medically treated disease, BMI, SBP, serum total cholesterol, smoking, intake of grain products, vegetables, salted vegetables, fruit, balance of meat and fish intake, soybean paste soup, milk, time spent walking each day, consumption of another type of tea, green or roasted, consumption of black or oolong tea, consumption of coffee |
| van den Brandt et al., 2018 (56) | Netherlands Cohort Study (NLCS) | Netherlands | 3166/4193 | 61 | 55–69 | 48 | NR | 24.9 | 10 | NR | Age at baseline, cigarette smoking status, number of cigarettes smoked per day, and years of smoking, history of physician-diagnosed hypertension and diabetes, body height, BMI, nonoccupational physical activity, highest level of education, intake (of alcohol, nuts, vegetables and fruit, coffee, energy), use of nutritional supplements, and, in women, postmenopausal HRT |
| Woodward and Tunstall-Pedoe, 1999 (57) | Scottish Heart Health Study (SHHS) | Scotland | 5724 men and 5842 women/11,566 | NR | 40–59 | 49.5 | NR | NR | 7.7 | Government | Age, housing tenure, activity at work, activity in leisure, cigarette smoking status, BMI, Bortner score, cotinine, SBP, fibrinogen, total cholesterol, HDL cholesterol, TGs, alcohol, vitamin C, and coffee |
| Yan et al., 2017 (58) | Aerobics Center Longitudinal Study | USA | 11,808/11,808 | 42.89 | 20–82 | 86.2 | Majority white | 25.3 | 16 | NR | Age, sex, baseline examination year, regular coffee use, decaffeinated coffee use, herbal tea use, physical inactivity, BMI, smoking and alcohol consumption, and fitness in metabolic equivalents |
| Yochum et al., 1999 (59) | Iowa Women's Health Study | USA | 34,492/34,492 | 61.5 | 55–69 | 0 | NR | 26.9 | 10 | Government, nonprofit | Age, total energy intake, BMI squared, waist-to-hip ratio, high blood pressure, diabetes, estrogen replacement therapy, alcohol intake, education, marital status, pack-years smoking, physical activity, intake of cholesterol, saturated fat, vitamin E, dietary fiber, whole grains |
| Zhao et al., 2017 (60) (males) | Shanghai Men's Health Study | Shanghai, China | 51,920/61,491 | 54.1 | 40–74 | 100 | Asian | 23.6 | 8.3 | Government, nonprofit | Age, education, income, smoking status, alcohol intake, energy intake, BMI, physical activity, history of hypertension, and gastritis |
| Zhao et al., 2017 (60) (females) | Shanghai Women's Health Study | Shanghai, China | 64,034/74,941 | 51.5 | 40–70 | 0 | Asian | 23.8 | 14.2 | Government, nonprofit | Age, education, income, smoking status, alcohol intake, energy intake, BMI, physical activity, history of hypertension, gastritis, and menopause status (women only) |
1CHD, coronary heart disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration ratio; HRT, hormone replacement therapy; IHD, ischemic heart disease; NR, not reported; ref, reference; SBP, systolic blood pressure; SES, socioeconomic status; TG, triglyceride.
Many cohort studies reported >1 outcome of interest, and overall ROBs were moderate and limitations were similar across outcomes (Supplemental Table 1). Results were synthesized outcome-by-outcome, including CVD mortality, cardiovascular events, stroke events, and all-cause mortality as discussed in the following sections.
CVD mortality
A total of 17 unique studies in 13 publications (22, 26, 30, 31, 39, 45, 48, 50, 52, 54, 56–58) were included in the mixed-effects dose–response meta-regression analyses of the relation between estimated total tea flavonoid intake amounts and risks of CVD mortality (Supplemental Figure 2A). Of these, 2 cohorts were based in the United States, 6 in Asia (Japan, China, and Singapore), and the rest in Europe (United Kingdom, Netherlands, and Scotland). The mean or median follow-up durations ranged from 5 to 18.7 y, and the incidence of CVD mortality ranged from 32 to 14,470 per 100,000 study participants per year.
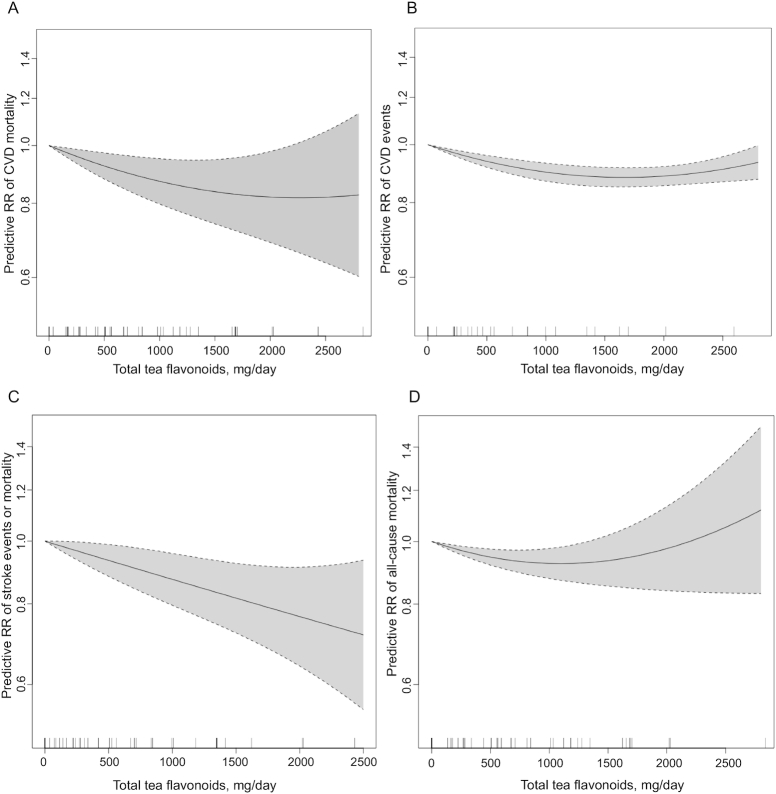
Results from both linear and nonlinear dose–response models were statistically significant overall (P < 0.01) with large residual heterogeneity (I2 = 73.9% and 63.3% for linear and nonlinear models, respectively). The nonlinear dose–response model did not significantly reduce the unexplained variance compared with the linear model. The nonlinear model showed wide CIs (large uncertainty) at very high intakes (total estimated tea flavonoids >2500 mg/d) due to sparse data, and thus the pooled RR became nonsignificant at high intake amounts (Figure 1A). Two additional cohort studies (1 in the United States and 1 in Australia) reported multivariable Cox regression analysis results for the relation between black tea intake and risks of CVD mortality outcomes (28, 44). Combining results from these studies with the individual study linear trend estimates from the linear dose–response meta-regression, the random-effects model meta-analysis of 19 studies showed that each cup (236.6 mL) increase in daily black or green tea consumption was associated with an average 4% lower risk of CVD mortality (pooled adjusted RR: 0.96; 95% CI: 0.94, 0.98; P = 0.0001), with large heterogeneity (I2 = 72.4%; P < 0 .0001) (Figure 2). Subgroup meta-analysis results showed similar pooled adjusted RRs between men and women, but the magnitude of association was larger in elderly individuals (n = 4; pooled adjusted RR: 0.89; 95% CI: 0.83, 0.96; P = 0.001; I2 = 59.5%; P = 0.06) compared with adults (n = 15; pooled adjusted RR: 0.98; 95% CI, 0.96, 0.99; P = 0.012; I2 = 58.5%; P = 0.002). Generally, studies with higher ROBs appeared to show larger magnitudes of associations than studies with lower ROBs (Table 2). Meta-regression analyses did not find associations between follow-up durations (I2 residue = 71.3%; P = 0.15) or incidence of CVD mortality (I2 residue = 73.9%; P = 0.76) and the magnitudes of associations.
FIGURE 1.
Mixed-effects dose–response meta-regression results (nonlinear models). (A) CVD mortality. (B) CVD events. (C) Stroke events or mortality. (D) All-cause mortality. Shaded areas correspond to the 95% CIs of the prediction. CVD, cardiovascular disease.
FIGURE 2.
Random-effects model meta-analysis of the associations between each cup (236.6 mL) increment of black or green tea consumption per day and risks of cardiovascular disease mortality outcome. Column headings indicate the following: adequate follow-up, adequacy of follow-up of cohorts; comparability, comparability of cohorts on the basis of the design or analysis; exposure, ascertainment of exposure; and no outcome at start, demonstration that outcome of interest was not present at start of study. See Supplemental Table 1 for details of risk-of-bias assessment questions depicted in the right columns of the forest plot. The size of the boxes represents the weight of the study in the random-effects meta-analysis.
TABLE 2.
Subgroup meta-analysis results for CVD-mortality and CVD-events outcomes1
| CVD mortality | CVD events | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Pooled adjusted RR (95% CI) | P for pooled RR | I 2, % | n | Pooled adjusted RR (95% CI) | P for pooled RR | I 2, % | |
| Overall | 19 | 0.962 (0.939, 0.984) | 0.001 | 72.4** | 7 | 0.982 (0.961, 1.003) | 0.085 | 76.5** |
| Sex | ||||||||
| Both sexes | 7 | 0.932 (0.893, 0.973) | 0.001 | 62.9** | 7 | 0.982 (0.961, 1.003) | 0.085 | 76.5** |
| Female | 5 | 0.973 (0.926, 1.021) | 0.267 | 72.0** | 0 | n/a | n/a | n/a |
| Male | 7 | 0.980 (0.945, 1.017) | 0.289 | 72.5** | 0 | n/a | n/a | n/a |
| Tea type | ||||||||
| Black tea | 11 | 0.974 (0.935, 1.015) | 0.212 | 59.5** | 4 | 0.985 (0.938, 1.034) | 0.542 | 80.8** |
| Green tea | 8 | 0.952 (0.923, 0.981) | 0.002 | 81.6** | 1 | 0.966 (0.951, 0.982) | <0.0001 | n/a |
| All tea | 0 | n/a | n/a | n/a | 2 | 0.927 (0.785, 1.095) | 0.0372 | 71.7* |
| Age group | ||||||||
| Adults | 15 | 0.975 (0.955, 0.994) | 0.012 | 58.5** | 6 | 0.983 (0.964, 1.003) | 0.097 | 76.9** |
| Elderly | 4 | 0.888 (0.825, 0.955) | 0.001 | 59.5* | 1 | 0.749 (0.570, 0.984) | 0.038 | n/a |
| Region | ||||||||
| United States | 3 | 0.991 (0.957, 1.027) | 0.637 | 0.0 | 2 | 0.925 (0.788, 1.085) | 0.338 | 69.1* |
| Asia | 8 | 0.952 (0.923, 0.981) | 0.002 | 81.6** | 2 | 0.978 (0.958, 0.999) | 0.045 | 83.5** |
| Europe | 7 | 0.978 (0.915, 1.046) | 0.520 | 70.2** | 2 | 0.881 (0.699, 1.109) | 0.281 | 67.8* |
| Australia | 1 | 0.920 (0.862, 0.982) | 0.012 | n/a | 0 | n/a | n/a | n/a |
| Iran | 0 | n/a | n/a | n/a | 1 | 1.040 (1.005, 1.076) | 0.023 | n/a |
| Risk of bias | ||||||||
| Exposure ascertainment2 | ||||||||
| A | 7 | 0.969 (0.918, 1.022) | 0.246 | 77.8** | 1 | 0.959 (0.926, 0.993) | 0.020 | n/a |
| B | 5 | 0.981 (0.949, 1.014) | 0.258 | 64.3** | 5 | 0.986 (0.957, 1.016) | 0.352 | 82.6** |
| C | 7 | 0.932 (0.884, 0.983) | 0.009 | 75.5** | 1 | 0.982 (0.955, 1.010) | 0.205 | n/1 |
| No outcomes at start3 | ||||||||
| A | 17 | 0.973 (0.953, 0.993) | 0.009 | 59.4** | 7 | 0.982 (0.961, 1.003) | 0.085 | 76.5** |
| B | 2 | 0.886 (0.830, 0.946) | <0.0001 | 62.7* | 0 | n/a | n/a | n/a |
| Comparability4 | ||||||||
| AB | 17 | 0.973 (0.953, 0.993) | 0.009 | 59.4** | 7 | 0.982 (0.961, 1.003) | 0.085 | 76.5** |
| A | 1 | 0.920 (0.862, 0.982) | 0.012 | n/a | 0 | n/a | n/a | n/a |
| B | 1 | 0.860 (0.820, 0.902) | <0.0001 | n/a | 0 | n/a | n/a | n/a |
| Adequate follow-up5 | ||||||||
| A | 8 | 0.979 (0.927, 1.035) | 0.463 | 68.3** | 1 | 0.982 (0.955, 1.010) | 0.205 | n/a |
| B | 10 | 0.964 (0.946, 0.983) | <0.0001 | 49.6** | 4 | 0.972 (0.951, 0.994) | 0.014 | 75.2** |
| C | 1 | 0.860 (0.820, 0.902) | <0.0001 | n/a | 1 | 1.040 (1.005, 1.076) | 0.023 | n/a |
| D | 0 | n/a | n/a | n/a | 1 | 0.828 (0.688, 0.995) | 0.044 | n/a |
n = number of studies. *,**Indicates significant heterogeneity based on Q-test: *P < 0.1, **P < 0.05. CVD, cardiovascular disease; n/a, not applicable.
Ascertainment of exposure: A, multiple dietary exposure assessments during follow-ups and having internal or external calibration of the dietary assessment instrument, or single dietary exposure assessment at baseline and having internal or external calibration of dietary assessment for tea or flavonoid consumption specifically; B, single dietary exposure assessment at baseline and having internal or external calibration of the dietary assessment instrument; and C, single dietary exposure assessment at baseline and unclear validity of the dietary assessment instrument.
Demonstration that the outcome of interest was not present at the start of the study: A, yes; B, no.
Comparability of cohorts on the basis of the design or analysis: A, study controls for age, sex, socioeconomic characteristics (e.g., education, income), any anthropometric measure (BMI, weight, etc.), medication for or history/existing diseases if applicable [choose this when 1) must include all of them or 2) if they describe having a variable selection process, and justify why they did not include some of these]; B, study controls for any additional factor such as other dietary factors or physical activity; C, both A and B; and D, neither A nor B. Note that AB indicates both A and B.
Adequacy of follow-up of cohorts: A, complete follow-up, all subjects accounted for (e.g., mortality as results and use death record linkage data); B, subjects lost to follow-up unlikely to introduce bias—small number lost (≤20%), or >20% lost to follow-up, with description provided of those lost; C, lost to follow-up rate ≥20% without description of those lost or likely to bring bias; and D, no statement.
Last, findings regarding the associations between tea consumption and CVD mortality outcomes from 8 cohort publications (4 in the United States, and 1 each in Finland, Netherlands, Japan, and China) that could not be meta-analyzed were inconsistent (23, 25, 32, 33, 49, 51, 59, 60); however, the characteristics of these studies were heterogeneous, limiting their comparability. Detailed results are shown in Supplemental Table 2. Briefly, 5 studies (4 cohorts in the United States and 1 cohort in Finland) found no significant associations in men and women (25, 32, 33, 51, 59). In contrast, 3 other cohort studies (1 cohort study in elderly men in the Netherlands, 1 cohort in Japan, and 1 cohort in China) found inverse associations between tea consumption and risk of CVD mortality (23, 49, 60).
Cardiovascular events
Seven unique studies in 7 publications (26, 27, 29, 38, 43, 47, 53) were included in the mixed-effects dose–response meta-regression analyses (Supplemental Figure 2B). Of these, 2 cohorts were based in the United States, 2 in Asia (Japan and China), 2 in the Netherlands, and 1 in Iran. The mean and median follow-up durations ranged from 5.6 to 15 y, and the incidence of CVD events (including CVD-specific mortality) ranged from 332 to 1165 per 100,000 study participants per year.
Results from both linear and nonlinear dose–response models were statistically significant overall (P < 0.001), but the nonlinear model had smaller residual heterogeneity (I2 = 20.3%) than the linear model (I2 = 66.9%) (Figure 1B). The random-effects model meta-analysis of the linear trend estimates from these studies showed that each cup (236.6 mL) increase in daily black or green tea consumption was associated with a 2% lower risk of CVD (n = 7; pooled adjusted RR: 0.98; 95% CI: 0.96, 1.00; P = 0.085), with large heterogeneity (I2 = 76.5%; P < 0.0001) (Figure 3). Consistent with the dose–response meta-regression findings, 1 cohort in Korea (Korean Genome Epidemiology Study) that cannot be included in the meta-analysis reported that green tea consumption (>1 serving/wk) was associated with a lower risk of CVD (adjusted HR: 0.72; 95% CI: 0.57, 0.92) (24).
FIGURE 3.
Random-effects model meta-analysis of the associations between each cup (236.6 mL) increment of black or green tea consumption per day and risks of cardiovascular disease event outcome. Column headings indicate the following: adequate follow-up, adequacy of follow-up of cohorts; comparability, comparability of cohorts on the basis of the design or analysis; exposure, ascertainment of exposure; and no outcome at start, demonstration that outcome of interest was not present at start of study. See Supplemental Table 1 for details of risk-of-bias assessment questions depicted in the right columns of the forest plot. The size of the boxes represents the weight of the study in the random-effects meta-analysis.
Because of the small number of included studies, subgroup analysis results should be interpreted with caution (Table 2). Meta-regression analyses did not find associations between follow-up durations (I2 residue = 70.5%; P = 0.32) or incidence of CVD events (I2 residue = 78.1%; P = 0.54) and the magnitudes of associations for CVD event outcome.
Stroke events
Thirteen unique studies in 11 publications (26, 35, 38–42, 46, 48, 53, 55) were included in the mixed-effects dose–response meta-regression analyses (Supplemental Figure 2C). Of these, 3 cohorts were based in the United States, 4 in Japan, and the rest in Europe (countries including the Netherlands, Finland, and Sweden). The mean and median follow-up durations ranged from 5 to 24 y, and the incidence of stroke events (including stroke-specific mortality) ranged from 120 to 2261 per 100,000 study participants per year.
Results from both linear and nonlinear dose–response models were statistically significant overall (P < 0.001), but the nonlinear model had smaller residual heterogeneity (I2 = 38.5%) than the linear model (I2 = 61.9%) (Figure 1C). The random-effects model meta-analysis of the linear trend estimates from these studies showed that each cup (236.6 mL) increase in daily black or green tea consumption was associated with a 4% lower risk of stroke (n = 13; pooled adjusted RR: 0.96; 95% CI: 0.93, 0.99; P = 0.002), with large heterogeneity (I2 = 63.9%; P = 0.001) (Figure 4).
FIGURE 4.
Random-effects model meta-analysis of the associations between each cup (236.6 mL) increment of black or green tea consumption per day and risks of stroke event outcome. Column headings indicate the following: adequate follow-up, adequacy of follow-up of cohorts; comparability, comparability of cohorts on the basis of the design or analysis; exposure, ascertainment of exposure; and no outcome at start, demonstration that outcome of interest was not present at start of study. See Supplemental Table 1 for details of risk-of-bias assessment questions depicted in the right columns of the forest plot. The size of the boxes represents the weight of the study in the random-effects meta-analysis.
Subgroup meta-analysis results showed similar pooled adjusted RRs between men and women, and no studies were conducted in elderly individuals. Generally, studies with higher ROBs appeared to show larger magnitudes of associations than studies with lower ROBs (Table 3). Meta-regression analyses did not find associations between follow-up durations (I2 residue = 66.7%; P = 0.63) or incidence of stroke events (I2 residue = 66.9%; P = 0.96) and the magnitudes of associations.
TABLE 3.
Subgroup meta-analysis results for stroke and all-cause mortality outcomes1
| Stroke | All-cause mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Pooled adjusted RR (95% CI) | P for pooled RR | I 2, % | n | Pooled adjusted RR (95% CI) | P for pooled RR | I 2, % | |
| Overall | 13 | 0.959 (0.934, 0.985) | 0.002 | 63.9** | 18 | 0.979 (0.967, 0.991) | 0.001 | 73.7** |
| Sex | ||||||||
| Both sexes | 7 | 0.956 (0.922, 0.992) | 0.016 | 74.3** | 9 | 0.967 (0.946, 0.989) | 0.003 | 75.6** |
| Female | 3 | 0.956 (0.913, 1.001) | 0.056 | 0.0 | 4 | 0.992 (0.968, 1.017) | 0.549 | 58.3* |
| Male | 3 | 0.972 (0.890, 1.062) | 0.526 | 77.1** | 5 | 0.986 (0.963, 1.009) | 0.0223 | 77.8** |
| Tea type | ||||||||
| Black tea | 5 | 0.971 (0.936, 1.008) | 0.119 | 57.8** | 10 | 0.991 (0.966, 1.016) | 0.484 | 74.7** |
| Green tea | 8 | 0.943 (0.902, 0.986) | 0.009 | 70.1** | 8 | 0.969 (0.957, 0.981) | <0.0001 | 65.3** |
| All tea | 0 | n/a | n/a | n/a | 0 | n/a | n/a | n/a |
| Age group | ||||||||
| Adults | 13 | 0.959 (0.934, 0.985) | 0.002 | 63.9** | 15 | 0.985 (0.975, 0.996) | 0.006 | 60.4** |
| Elderly | 0 | n/a | n/a | n/a | 3 | 0.920 (0.898, 0.942) | <0.0001 | 0.3 |
| Region | ||||||||
| United States | 2 | 0.969 (0.926, 1.013) | 0.0165 | 0.0 | 5 | 0.979 (0.951, 1.007) | 0.140 | 69.4** |
| Asia | 5 | 0.943 (0.902, 0.986) | 0.009 | 70.1** | 8 | 0.969 (0.957, 0.981) | <0.0001 | 65.3** |
| Europe | 6 | 0.971 (0.922, 1.023) | 0.266 | 68.8** | 4 | 1.024 (0.975, 1.075) | 0.349 | 80.0** |
| Australia | 0 | n/a | n/a | n/a | 1 | 0.900 (0.814, 0.995) | 0.040 | n/a |
| Iran | 0 | n/a | n/a | n/a | 0 | n/a | n/a | n/a |
| Risk of bias | ||||||||
| Exposure ascertainment2 | ||||||||
| A | 5 | 0.978 (0.918, 1.041) | 0.480 | 66.6** | 4 | 1.005 (0.972, 1.040) | 0.751 | 85.8** |
| B | 6 | 0.954 (0.929, 0.980) | 0.001 | 52.0* | 5 | 0.988 (0.968, 1.009) | 0.023 | 64.8** |
| C | 2 | 0.917 (0.794, 1.058) | 0.236 | 84.7** | 9 | 0.957 (0.937, 0.979) | <0.0001 | 71.3** |
| No outcomes at start3 | ||||||||
| A | 13 | 0.959(0.934, 0.985) | 0.002 | 63.9** | 14 | 0.985 (0.974, 0.997) | 0.012 | 68.7** |
| B | 0 | n/a | n/a | n/a | 4 | 0.927 (0.907, 0.948) | <0.0001 | 0.0 |
| Comparability4 | ||||||||
| AB | 11 | 0.972 (0.948, 0.997) | 0.026 | 63.9** | 14 | 0.985 (0.974, 0.997) | 0.012 | 68.7** |
| A | 0 | n/a | n/a | n/a | 1 | 0.900 (0.814, 0.995) | 0.040 | n/a |
| B | 2 | 0.887 (0.834, 0.943) | <0.0001 | 30.2 | 3 | 0.929 (0.908, 0.950) | <0.0001 | 0.0 |
| Adequate follow-up5 | ||||||||
| A | 6 | 0.959 (0.903, 1.008) | 0.094 | 66.4** | 6 | 0.981 (0.936, 1.028) | 0.423 | 80.7** |
| B | 7 | 0.962 (0.931, 0.994) | 0.019 | 66.9** | 11 | 0.982 (0.972, 0.991) | <0.0001 | 48.3** |
| C | 0 | n/a | n/a | n/a | 1 | 0.925 (0.902, 0.949) | <0.0001 | n/a |
| D | 0 | n/a | n/a | n/a | 0 | n/a | n/a | n/a |
n = number of studies. *,**Indicates significant heterogeneity based on Q-test: *P < 0.1, **P < 0.05. CVD, cardiovascular disease; n/a, not applicable.
Ascertainment of exposure: A, multiple dietary exposure assessments during follow-ups and having internal or external calibration of the dietary assessment instrument, or single dietary exposure assessment at baseline and having internal or external calibration of dietary assessment for tea or flavonoid consumption specifically; B, single dietary exposure assessment at baseline and having internal or external calibration of the dietary assessment instrument; and C, single dietary exposure assessment at baseline and unclear validity of the dietary assessment instrument.
Demonstration that the outcome of interest was not present at the start of the study: A, yes; B, no.
Comparability of cohorts on the basis of the design or analysis: A, study controls for age, sex, socioeconomic characteristics (e.g., education, income), any anthropometric measure (BMI, weight, etc.), medication for or history/existing diseases if applicable [choose this when 1) must include all of them or 2) if they describe having a variable selection process, and justify why they did not include some of these]; B, study controls for any additional factor such as other dietary factors or physical activity; C, both A and B; and D, neither A nor B. Note that AB indicates both A and B.
Adequacy of follow-up of cohorts: A, complete follow-up, all subjects accounted for (e.g., mortality as results and use death record linkage data); B, subjects lost to follow-up unlikely to introduce bias—small number lost (≤20%), or >20% lost to follow-up, with description provided of those lost; C, lost to follow-up rate ≥20% without description of those lost or likely to bring bias; and D, no statement.
All-cause mortality
Fifteen unique studies in 12 publications (22, 26, 31, 34, 36, 39, 45, 50, 52, 54, 56, 58) were included in the mixed-effects dose–response meta-regression analyses of the relation between estimated total tea flavonoid intake amounts and risks of all-cause mortality (Supplemental Figure 2D). Of these, 3 cohorts were in the United States, 6 in Asia (Japan, China, and Singapore), 1 in the United Kingdom, and the rest in the Netherlands. The mean or median follow-up durations ranged from 5 to 18.7 y, and the incidence of all-cause mortality ranged from 364 to 40,879 per 100,000 study participants per year.
Results from both linear and nonlinear dose–response models were statistically significant overall (P < 0.05), with large residual heterogeneity (I2 = 72.6% and 63.9% for linear and nonlinear models, respectively). The nonlinear dose–response model did not significantly reduce the unexplained variance compared with the linear model. The nonlinear model showed wide CIs (large uncertainty) at high intakes (total tea flavonoids >2000 mg/d) due to sparse data, and thus the pooled RR became nonsignificant (Figure 1D). Three cohort studies (2 in the United States and 1 in Australia) reported multivariable Cox regression analysis results for the relation between black tea intake and risks of all-cause mortality outcomes (28, 37, 44). Combining results from these studies with the individual study linear trend estimates from the linear dose–response meta-regression, the random-effects model meta-analysis of 18 studies showed that each cup (236.6 mL) increase in daily black or green tea consumption was associated with an average 2% lower risk of all-cause mortality (pooled adjusted RR: 0.98; 95% CI: 0.97, 0.99; P = 0.001), with large heterogeneity (I2 = 73.71%; P < 0.0001) (Figure 5). Subgroup meta-analysis results showed the inverse association in men but not in women, and the magnitude of association was larger in elderly individuals (n = 3; pooled adjusted RR: 0.92; 95% CI: 0.90, 0.94; P < 0.0001; I2 = 0.3%; P = 0.37) compared with adults (n = 15; pooled adjusted RR: 0.985; 95% CI: 0.975, 0.996; P < 0.0001; I2 = 60.4%; P = 0.001). Generally, studies with higher ROBs appeared to show larger magnitudes of associations than studies with lower ROBs (Table 3). Meta-regression analyses did not find associations between follow-up durations (I2 residue = 72.9%; P = 0.180) or incidence of all-cause mortality (I2 residue = 74.8%; P = 0.71) and the magnitudes of associations.
FIGURE 5.
Random-effects model meta-analysis of the associations between each cup (236.6 mL) increment of black or green tea consumption per day and risks of all-cause mortality outcome. Column headings indicate the following: adequate follow-up, adequacy of follow-up of cohorts; comparability, comparability of cohorts on the basis of the design or analysis; exposure, ascertainment of exposure; and no outcome at start, demonstration that outcome of interest was not present at start of study. See Supplemental Table 1 for details of risk-of-bias assessment questions depicted in the right columns of the forest plot. The size of the boxes represents the weight of the study in the random-effects meta-analysis.
Last, consistent with the meta-analysis results, 1 publication that could not be meta-analyzed reported that drinking green tea regularly was inversely associated with the risk of all-cause mortality in 2 prospective cohorts of Chinese adults (pooled HR: 0.95; 95% CI: 0.90, 1.01) (60).
Strength of evidence
The strength of evidence was rated as low and moderate (depending on the age groups of the study participants) for the inverse dose–response relation between tea consumption amounts and risks of CVD and all-cause mortality outcomes, and was rated as low for the inverse dose–response relation between tea consumption amounts and risks of CVD and stroke outcomes (Table 4). Although prospective cohort studies are a strong study design and significant dose–response relations were demonstrated in our meta-analyses, limitations in dietary assessment methods, ROB due to confounding and incomplete follow-ups, and heterogeneity in population characteristics and outcome definitions limited our confidence in the meta-analysis results. Furthermore, there is no protocol registration for observational studies, so reporting bias is difficult to assess. Because only published literature was included in the present systematic review, publication bias should be suspected.
TABLE 4.
GRADE evidence profile1
| Outcome | Number of studies and study design | Limitations2 | Inconsistency | Imprecision | Dose–response gradient | Summary of findings | Strength of evidence3 |
|---|---|---|---|---|---|---|---|
| CVD mortality | 27 prospective cohort studies | About half of included studies had high uncertainty regarding dietary assessment, 20% did not adequately control for confounding, and 5% had high ROB due to incomplete follow-up | Large heterogeneity; heterogeneity can be partially explained by age groups and ROB | Meta-analysis results were precise but sparse data at higher intake amounts | Present | Adults: Pooled adjusted RR of 15 studies = 0.98 (95% CI: 0.96, 0.99; P = 0.012) for each cup (236.6 mL) increase in daily tea intake; findings from 7 additional cohort studies were inconsistent | Adults: ⊕⊕◯◯; low |
| Elderly: Pooled adjusted RR of 4 studies = 0.89 (95% CI: 0.83, 0.96; P = 0.001) for each cup (236.6 mL) increase in daily tea intake; 1 additional cohort study in elderly men reported that tea intake was associated with a lower risk of IHD mortality (adjusted RR = 0.78; P = 0.056) | Elderly: ⊕⊕⊕◯; moderate | ||||||
| CVD events | 8 prospective cohort studies | 25% of included studies had high uncertainty regarding dietary assessment, 13% did not adequately control for confounding, and 48% had high ROB due to incomplete follow-up | Moderate heterogeneity | Meta-analysis results were precise but sparse data at higher intake amounts | Present | Pooled adjusted RR of 7 studies = 0.98 (95% CI: 0.97, 1.00; P = 0.085); finding from 1 additional cohort study was consistent with the meta-analysis result | Adults: ⊕⊕◯◯; low |
| Stroke events | 6 prospective cohort studies | 18% of included studies had high uncertainty regarding dietary assessment and 18% did not adequately control for confounding | Large heterogeneity; heterogeneity can be partially explained by ROB | Meta-analysis results were precise but sparse data at higher intake amounts | Present | Pooled adjusted RR of 6 studies = 0.96 (95% CI: 0.93, 0.99; P = 0.002) | Adults: ⊕⊕◯◯; low |
| All-cause mortality | 19 prospective cohort studies | 56% of included studies had high uncertainty regarding dietary assessment, 19% did not adequately control for confounding, and 6% had high ROB due to incomplete follow-up | Large heterogeneity; heterogeneity can be partially explained by sex, age groups, and ROB | Meta-analysis results were precise but sparse data at higher intake amounts | Present | Adults: Pooled adjusted RR of 15 studies = 0.985 (95% CI: 0.975, 0.996; P = 0.006) for each cup (236.6 mL) increase in daily tea intake; 1 additional cohort study reported that drinking green tea regularly was inversely associated with the risk of all-cause mortality in 2 prospective cohorts of Chinese adults (pooled HR = 0.95; 95% CI: 0.90, 1.01) | Adults: ⊕⊕◯; low |
| Elderly: Pooled adjusted RR of 3 studies = 0.92 (95% CI: 0.90, 0.94; P ≤ 0.0001) for each cup (236.6 mL) increase in daily tea intake | Elderly: ⊕⊕◯◯; low |
CVD, cardiovascular disease; GRADE, Grades of Recommendation, Assessment, Development, and Evaluation; IHD, ischemic heart disease; ROB, risk of bias.
See Supplemental Table 1 for details.
Symbols indicate the following strength of evidence: ⊕⊕⊕⊕, high (further research is very unlikely to change our confidence in the estimate of association); ⊕⊕⊕◯, moderate (further research is likely to have an important impact on our confidence in the estimate of association and may change the estimate); ⊕⊕◯◯, low (further research is very likely to have an important impact on our confidence in the estimate of association and is likely to change the estimate); and ⊕◯◯◯, very low (any estimate of association is very uncertain).
Discussion
Tea is the second most consumed beverage worldwide after water (61), and due to such high frequency of intake, even a modest impact of tea on human health could have large implications for public health. This systematic review and meta-analysis followed the same approach that was used by the most recent DRI committee to review current evidence and update intake recommendations for sodium and potassium (62). Although no RCTs met the inclusion criteria, we found that a moderate level of evidence from prospective cohort studies suggests that daily tea intake is associated with a lower risk of all-cause mortality among adults and lower risk of CVD-specific mortality in elderly populations. The stronger findings among elderly populations is not surprising given the higher rates of CVD mortality. Evidence for the CVD events and stroke outcomes is currently more limited; this is also somewhat expected since CVD events often go unreported (e.g., nonfatal myocardial infarction while sleeping) and are more difficult to adjudicate compared with mortality. The inverse dose–response (intake-response) relations suggest that the small risk reduction in CVD, stroke, and all-cause mortality may become larger with an increase in daily tea intake amounts. Our nonlinear dose–response meta-analyses suggest that the relation between daily tea intake amounts and risks of CVD and stroke events may be nonlinear, although the data at very high intake amounts are limited. Subgroup meta-analysis showed that population characteristics (e.g., age group and sex) and ROB can partially explain the heterogeneity (differences in study findings) in the meta-analyses. This suggests that baseline CVD risk, dietary assessment methods, and adequacy of confounding adjustment and follow-up can bias the study results. However, the impact of these biases (away or toward the null) cannot be reliably evaluated using meta-analytical techniques. Variables such as baseline and changes in BMI affect CVD development and incidence; it is likely that habitual tea consumption may have a larger effect over time in these subpopulations.
Several possible biological mechanisms support an inverse association between tea flavonoid intake amounts and risks of CVD. The most important potential biological mechanism is the ability of tea flavonoids to reduce both systolic and diastolic blood pressure (63, 64). Tea flavonoids improve endothelium-dependent vasorelaxation (i.e., NO-dependent vasorelaxation) in healthy individuals as well as in various human pathogenic conditions (65–69). Their ability to activate endothelial NO synthase seems to be the likely mechanism underlying improvement in flow-mediated dilation and blood pressure reduction. Colonic microbes convert flavonoids into smaller, more bioavailable compounds with ultimate conversion to hippuric acid, a major compound detected in human urine after consumption of both green and black tea (70). Thus, one might expect the bioefficacy of green and black tea to be similar, as seen throughout these analyses.
Our dose–response meta-analysis is limited by the validity of the dietary assessment methods in the original studies as well as the uncertainties surrounding the flavonoid contents in tea. Specifically, we estimated the total flavonoid content for green and black tea using the USDA standard reference and the reported frequencies of tea consumption. This approach may overestimate amounts of flavonoids present in some ready-to-drink products and those served in food service operations and underestimate amounts contained when tea is steeped for longer periods of time (each scenario is not captured by FFQs). The development of novel biomarkers of exposure for future research would assist researchers in overcoming measurement error from assessing tea consumption via FFQs. The amount of flavonoids consumed in tea beverages depends on several factors, including the following: 1) their concentration in the tea leaves; 2) the mass of tea leaves used to prepare the infusion; 3) the volume of water used to prepare the infusion; 4) the water temperature, brew time, and agitation used to prepare the infusion; 5) the pH of the water used to prepare the infusion, thermal processing (for commercial tea infusions), and duration between preparation and consumption; and 6) the volume of the infusion consumed, as reviewed and described by Ho et al. (71). Last, due to multiple testing in subgroup analyses that increased the risk of type I error (false positive), the findings from subgroup meta-analyses and meta-regression analyses should be interpreted with cautions as they are mainly for hypothesis-generating purposes.
The challenges in conducting RCTs to examine the effects of dietary interventions on long-term chronic disease outcomes have been discussed (72). Often, RCTs of dietary interventions examine only intermediate outcomes of chronic disease. Data synthesis from population-based, prospective cohort studies of the dose–response relation between dietary exposure and long-term chronic disease outcomes may provide the best available clinical outcome evidence for clinical or policy decision making. However, for observational research to be of the most utility, standardization and optimization of study design, accurate and reliable measurement of key variables, and appropriate data analysis and data reporting are paramount (73). Observational studies also help determine potential effective doses to use in RCTs, which can often only test 1 dose; RCTs are often funded before sufficient evidence is apparent to determine the effective dose (74). Synthesis of observational studies can complement evidence synthesis of shorter-duration RCTs that assess dietary exposure on validated biomarkers of disease. Our group is currently conducting a separate systematic review of RCTs to examine effects of tea consumption on surrogate markers of CVD.
In conclusion, daily tea intake as part of a healthy habitual dietary pattern may be associated with lower risks of CVD and all-cause mortality among adults. No adverse effects were shown at normal consumption amounts. Our systematic review provides evidence to begin developing dietary guidance and public health messaging around the consumption of tea, although future, rigorously designed RCTs would greatly strengthen the evidence base and certainty of our findings. Incorporating tea as part of a healthy diet is a simple dietary modification that may have positive public health implications on chronic disease risk reduction worldwide.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Kelly Copeland Cara, Ebuwa Igho-Osagie, Mengyuan Ruan, and Qisi Yao for assistance with the ROB assessment and data checking. The authors’ responsibilities were as follows—MC, DW, and TCW: were responsible for the study design; MC, NZ, DW, MS-W, MK, and TCW: were responsible for study execution and writing; AC, MF, PFJ, and EJJ: were members of the expert panel and helped guide the study execution; MC: conducted the meta-analyses; MC, NZ, DW, MS-W, MK, AC, MF, PFJ, EJJ, and TCW: were responsible for the final content; and all authors: read and approved the final manuscript.
Notes
Funding for this project was provided through an unrestricted educational grant from Unilever to the Think Healthy Group.
Author disclosures: MC contributed efforts without receiving salary support or compensation. AC, MF, PFJ, and EJJ served as expert panel members for this systematic review. MF is on the scientific board of Sensient Technologies and International Life Sciences Institute (ILSI) North America and has received scientific consulting fees from The Coca-Cola Company and travel support from Unilever. TCW has received scientific consulting fees from Unilever. The other authors reported no conflicts of interest.
The funder had no role in study selection, quality assessment, data analysis, or writing of the manuscript.
Supplemental Appendices 1–3, Supplemental Tables 1 and 2, and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CAD, coronary artery disease; CVD, cardiovascular disease; FFQ, food-frequency questionnaire; GRADE, Grades of Recommendation, Assessment, Development, and Evaluation; NOS, Newcastle-Ottawa Scale; RCT, randomized controlled trial; ROB, risk-of-bias.
Contributor Information
Mei Chung, Department of Public Health and Community Medicine, School of Medicine, Tufts University, Boston, MA, USA.
Naisi Zhao, Department of Public Health and Community Medicine, School of Medicine, Tufts University, Boston, MA, USA.
Deena Wang, D&V Systematic Evidence Review Consulting, LLC, Bronx, NY, USA.
Marissa Shams-White, University of New England, Portland, ME, USA.
Micaela Karlsen, University of New England, Portland, ME, USA; American College of Lifestyle Medicine, Chesterfield, MO, USA.
Aedín Cassidy, Department of Nutrition and Preventive Medicine, Norwich Medical School, University of East Anglia, Norwich, United Kingdom.
Mario Ferruzzi, Plants for Human Health Institute, North Carolina State University, Kannapolis, NC, USA.
Paul F Jacques, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Elizabeth J Johnson, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Taylor C Wallace, Department of Nutrition and Food Studies, George Mason University, Fairfax, VA, USA; Think Healthy Group, Inc., Washington, DC, USA.
References
- 1. Haufe TC, Ho KKHY, Ferruzzi MG, Neilson AP. Potential health effects of tea. Nutrition Today. 2018;53(5):213–28. [Google Scholar]
- 2. McKay DL, Blumberg JB. The role of tea in human health: an update. J Am Coll Nutr. 2002;21(1):1–13. [DOI] [PubMed] [Google Scholar]
- 3. Song WO, Chun OK. Tea is the major source of flavan-3-ol and flavonol in the U.S. diet. J Nutr. 2008;138(8):1543S–7S. [DOI] [PubMed] [Google Scholar]
- 4. Hodgson JM, Croft KD. Tea flavonoids and cardiovascular health. Mol Aspects Med. 2010;31(6):495–502. [DOI] [PubMed] [Google Scholar]
- 5. Wang ZM, Zhou B, Wang YS, Gong QY, Wang QM, Yan JJ, Gao W, Wang LS. Black and green tea consumption and the risk of coronary artery disease: a meta-analysis. Am J Clin Nutr. 2011;93(3):506–15. [DOI] [PubMed] [Google Scholar]
- 6. Zhang C, Qin YY, Wei X, Yu FF, Zhou YH, He J. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30(2):103–13. [DOI] [PubMed] [Google Scholar]
- 7. Institute of Medicine Finding what works in health care: standards for systematic reviews. Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- 8. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for ass. essing the quality of nonrandomised studies in meta-analyses. [Internet]. 2013. [Cited 2020 Jan 30]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 10. Lichtenstein AH, Yetley EA, Lau J. Application of systematic review methodology to the field of nutrition. J Nutr. 2008;138(12):2297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. [DOI] [PubMed] [Google Scholar]
- 12. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ; GRADE Working Group. What is “quality of evidence” and why is it important to clinicians?. BMJ. 2008;336(7651):995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sato Y, Nakatsuka H, Watanabe T, Hisamichi S, Shimizu H, Fujisaku S, Ichinowatari Y, Ida Y, Suda S, Kato K et al. Possible contribution of green tea drinking habits to the prevention of stroke. Tohoku J Exp Med. 1989;157(4):337–43. [DOI] [PubMed] [Google Scholar]
- 15. Stensvold I, Tverdal A, Solvoll K, Foss OP. Tea consumption. Relationship to cholesterol, blood pressure, and coronary and total mortality. Prev Med. 1992;21(4):546–53. [DOI] [PubMed] [Google Scholar]
- 16. Crippa A. Multivariate dose-response meta-analysis. [Internet]. [Cited 2019 May 1].Available from: https://cran.r-project.org/web/packages/dosresmeta/index.html. [Google Scholar]
- 17. Liu Q, Cook NR, Bergström A, Hsieh C-C. A two-stage hierarchical regression model for meta-analysis of epidemiologic nonlinear dose–response data. Comput Stat Data Anal. 2009;53(12):4157–67. [Google Scholar]
- 18. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. [DOI] [PubMed] [Google Scholar]
- 19. Bhagwat S, Haytowitz DB, Holden JM. Methods and application of food composition laboratory: Beltsville (MD). [Internet]. [Cited 2019 May 2]. Available from: http://www.ars.usda.gov/nutrientdata/flav. [Google Scholar]
- 20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 21. Harbord R, Higgins J. Meta-regression in Stata. The Stata Journal. 2008;8(4):493–519. [Google Scholar]
- 22. Andersen LF, Jacobs DR Jr, Carlsen MH, Blomhoff R. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women's Health Study. Am J Clin Nutr. 2006;83(5):1039–46. [DOI] [PubMed] [Google Scholar]
- 23. Arts IC, Hollman PC, Feskens EJ, Bueno de Mesquita HB, Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am J Clin Nutr. 2001;74(2):227–32. [DOI] [PubMed] [Google Scholar]
- 24. Baik I, Cho NH, Kim SH, Shin C. Dietary information improves cardiovascular disease risk prediction models. Eur J Clin Nutr. 2013;67(1):25–30. [DOI] [PubMed] [Google Scholar]
- 25. Bertoia ML, Triche EW, Michaud DS, Baylin A, Hogan JW, Neuhouser ML, Freiberg MS, Allison MA, Safford MM, Li W et al. Long-term alcohol and caffeine intake and risk of sudden cardiac death in women. Am J Clin Nutr. 2013;97(6):1356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Koning Gans JM, Uiterwaal CS, van der Schouw YT, Boer JM, Grobbee DE, Verschuren WM, Beulens JW. Tea and coffee consumption and cardiovascular morbidity and mortality. Arterioscler Thromb Vasc Biol. 2010;30(8):1665–71. [DOI] [PubMed] [Google Scholar]
- 27. Gaeini Z, Bahadoran Z, Mirmiran P, Azizi F. Tea, coffee, caffeine intake and the risk of cardio-metabolic outcomes: findings from a population with low coffee and high tea consumption. Nutr Metab (Lond). 2019;16:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gardener H, Rundek T, Wright CB, Elkind MS, Sacco RL. Coffee and tea consumption are inversely associated with mortality in a multiethnic urban population. J Nutr. 2013;143(8):1299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Geleijnse JM, Launer LJ, Van der Kuip DA, Hofman A, Witteman JC. Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. Am J Clin Nutr. 2002;75(5):880–6. [DOI] [PubMed] [Google Scholar]
- 30. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet North Am Ed. 1993;342(8878):1007–11. [DOI] [PubMed] [Google Scholar]
- 31. Hertog MG, Sweetnam PM, Fehily AM, Elwood PC, Kromhout D. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly Study. Am J Clin Nutr. 1997;65(5):1489–94. [DOI] [PubMed] [Google Scholar]
- 32. Hirvonen T, Pietinen P, Virtanen M, Ovaskainen ML, Hakkinen S, Albanes D, Virtamo J. Intake of flavonols and flavones and risk of coronary heart disease in male smokers. Epidemiology. 2001;12(1):62–7. [DOI] [PubMed] [Google Scholar]
- 33. Ivey KL, Jensen MK, Hodgson JM, Eliassen AH, Cassidy A, Rimm EB. Association of flavonoid-rich foods and flavonoids with risk of all-cause mortality. Br J Nutr. 2017;117(10):1470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iwai N, Ohshiro H, Kurozawa Y, Hosoda T, Morita H, Funakawa K, Okamoto M, Nose T. Relationship between coffee and green tea consumption and all-cause mortality in a cohort of a rural Japanese population. J Epidemiol. 2002;12(3):191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996;156(6):637–42. [PubMed] [Google Scholar]
- 36. Klatsky AL, Friedman GD, Armstrong MA. Coffee use prior to myocardial infarction restudied: heavier intake may increase the risk. Am J Epidemiol. 1990;132(3):479–88. [DOI] [PubMed] [Google Scholar]
- 37. Klatsky AL, Armstrong MA, Friedman GD. Coffee, tea, and mortality. Ann Epidemiol. 1993;3(4):375–81. [DOI] [PubMed] [Google Scholar]
- 38. Kokubo Y, Iso H, Saito I, Yamagishi K, Yatsuya H, Ishihara J, Inoue M, Tsugane S. The impact of green tea and coffee consumption on the reduced risk of stroke incidence in Japanese population: the Japan Public Health Center-Based Study cohort. Stroke. 2013;44(5):1369–74. [DOI] [PubMed] [Google Scholar]
- 39. Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 2006;296(10):1255–65. [DOI] [PubMed] [Google Scholar]
- 40. Larsson SC, Mannisto S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Coffee and tea consumption and risk of stroke subtypes in male smokers. Stroke. 2008;39(6):1681–7. [DOI] [PubMed] [Google Scholar]
- 41. Larsson SC, Virtamo J, Wolk A. Black tea consumption and risk of stroke in women and men. Ann Epidemiol. 2013;23(3):157–60. [DOI] [PubMed] [Google Scholar]
- 42. Leurs LJ, Schouten LJ, Goldbohm RA, van den Brandt PA. Total fluid and specific beverage intake and mortality due to IHD and stroke in the Netherlands Cohort Study. Br J Nutr. 2010;104(8):1212–21. [DOI] [PubMed] [Google Scholar]
- 43. Li X, Yu C, Guo Y, Bian Z, Si J, Yang L, Chen Y, Ren X, Jiang G, Chen J et al. Tea consumption and risk of ischaemic heart disease. Heart. 2017;103(10):783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lim WH, Wong G, Lewis JR, Lok CE, Polkinghorne KR, Hodgson J, Lim EM, Prince RL. Total volume and composition of fluid intake and mortality in older women: a cohort study. BMJ Open. 2017;7(3):e011720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu J, Liu S, Zhou H, Hanson T, Yang L, Chen Z, Zhou M. Association of green tea consumption with mortality from all-cause, cardiovascular disease and cancer in a Chinese cohort of 165,000 adult men. Eur J Epidemiol. 2016;31(9):853–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM, Logroscino G, Hu FB, van Dam RM. Coffee consumption and risk of stroke in women. Circulation. 2009;119(8):1116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miller PE, Zhao D, Frazier-Wood AC, Michos ED, Averill M, Sandfort V, Burke GL, Polak JF, Lima JAC, Post WS et al. Associations of coffee, tea, and caffeine intake with coronary artery calcification and cardiovascular events. Am J Med. 2017;130(2):188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mineharu Y, Koizumi A, Wada Y, Iso H, Watanabe Y, Date C, Yamamoto A, Kikuchi S, Inaba Y, Toyoshima H et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J Epidemiol Comm Health. 2011;65(3):230–40. [DOI] [PubMed] [Google Scholar]
- 49. Nakachi K, Matsuyama S, Miyake S, Suganuma M, Imai K. Preventive effects of drinking green tea on cancer and cardiovascular disease: epidemiological evidence for multiple targeting prevention. Biofactors. 2000;13(1–4):49–54. [DOI] [PubMed] [Google Scholar]
- 50. Odegaard AO, Koh WP, Yuan JM, Pereira MA. Beverage habits and mortality in Chinese adults. J Nutr. 2015;145(3):595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rimm EB, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Ann Intern Med. 1996;125(5):384–9. [DOI] [PubMed] [Google Scholar]
- 52. Saito E, Inoue M, Sawada N, Shimazu T, Yamaji T, Iwasaki M, Sasazuki S, Noda M, Iso H, Tsugane S et al. Association of green tea consumption with mortality due to all causes and major causes of death in a Japanese population: the Japan Public Health Center-based Prospective Study (JPHC Study). Ann Epidemiol. 2015;25(7):512–8. e3. [DOI] [PubMed] [Google Scholar]
- 53. Sesso HD, Paffenbarger RS Jr, Oguma Y, Lee IM. Lack of association between tea and cardiovascular disease in college alumni. Int J Epidemiol. 2003;32(4):527–33. [DOI] [PubMed] [Google Scholar]
- 54. Suzuki E, Yorifuji T, Takao S, Komatsu H, Sugiyama M, Ohta T, Ishikawa-Takata K, Doi H. Green tea consumption and mortality among Japanese elderly people: the prospective Shizuoka elderly cohort. Ann Epidemiol. 2009;19(10):732–9. [DOI] [PubMed] [Google Scholar]
- 55. Tanabe N, Suzuki H, Aizawa Y, Seki N. Consumption of green and roasted teas and the risk of stroke incidence: results from the Tokamachi-Nakasato cohort study in Japan. Int J Epidemiol. 2008;37(5):1030–40. [DOI] [PubMed] [Google Scholar]
- 56. van den Brandt PA. Coffee or tea? A prospective cohort study on the associations of coffee and tea intake with overall and cause-specific mortality in men versus women. Eur J Epidemiol. 2018;33(2):183–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Woodward M, Tunstall-Pedoe H. Coffee and tea consumption in the Scottish Heart Health Study follow up: conflicting relations with coronary risk factors, coronary disease, and all cause mortality. J Epidemiol Comm Health. 1999;53(8):481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yan Y, Sui X, Yao B, Lavie CJ, Blair SN. Is there a dose-response relationship between tea consumption and all-cause, CVD, and cancer mortality. J Am Coll Nutr. 2017;36(4):281–6. [DOI] [PubMed] [Google Scholar]
- 59. Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol. 1999;149(10):943–9. [DOI] [PubMed] [Google Scholar]
- 60. Zhao LG, Li HL, Sun JW, Yang Y, Ma X, Shu XO, Zheng W, Xiang YB. Green tea consumption and cause-specific mortality: results from two prospective cohort studies in China. J Epidemiol. 2017;27(1):36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113(Suppl 9B):71. [DOI] [PubMed] [Google Scholar]
- 62. National Academies of Sciences, Medicine, and Engineering Dietary Reference Intakes for sodium and potassium. Washington (DC): National Academies Press; 2019. [PubMed] [Google Scholar]
- 63. Greyling A, Ras RT, Zock PL, Lorenz M, Hopman MT, Thijssen DH, Draijer R. The effect of black tea on blood pressure: a systematic review with meta-analysis of randomized controlled trials. PLoS One. 2014;9(7):e103247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Khalesi S, Sun J, Buys N, Jamshidi A, Nikbakht-Nasrabadi E, Khosravi-Boroujeni H. Green tea catechins and blood pressure: a systematic review and meta-analysis of randomised controlled trials. Eur J Nutr. 2014;53(6):1299–311. [DOI] [PubMed] [Google Scholar]
- 65. Duffy SJ, Keaney JF Jr., Holbrook M, Gokce N, Swerdloff PL, Frei B, Vita JA. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001;104(2):151–6. [DOI] [PubMed] [Google Scholar]
- 66. Jochmann N, Lorenz M, Krosigk A, Martus P, Bohm V, Baumann G, Stangl K, Stangl V. The efficacy of black tea in ameliorating endothelial function is equivalent to that of green tea. Br J Nutr. 2008;99(4):863–8. [DOI] [PubMed] [Google Scholar]
- 67. Hodgson JM, Puddey IB, Burke V, Watts GF, Beilin LJ. Regular ingestion of black tea improves brachial artery vasodilator function. Clin Sci (Lond). 2002;102(2):195–201. [PubMed] [Google Scholar]
- 68. Widlansky ME, Hamburg NM, Anter E, Holbrook M, Kahn DF, Elliott JG, Keaney JF Jr, Vita JA. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J Am Coll Nutr. 2007;26(2):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ardalan MR, Tarzamni MK, Shoja MM, Tubbs RS, Rahimi-Ardabili B, Ghabili K, Khosroshahi HT. Black tea improves endothelial function in renal transplant recipients. Transplant Proc. 2007;39(4):1139–42. [DOI] [PubMed] [Google Scholar]
- 70. Mulder TP, Rietveld AG, van Amelsvoort JM. Consumption of both black tea and green tea results in an increase in the excretion of hippuric acid into urine. Am J Clin Nutr. 2005;81(Suppl):256S–60S. [DOI] [PubMed] [Google Scholar]
- 71. Ho KKHY, Haufe TC, Ferruzzi MG, Neilson AP. Production and polyphenolic composition of tea. Nutrition Today. 2018;53(6):268–78. [Google Scholar]
- 72. Blumberg J, Heaney RP, Huncharek M, Scholl T, Stampfer M, Vieth R, Weaver CM, Zeisel SH. Evidence-based criteria in the nutritional context. Nutr Rev. 2010;68(8):478–84. [DOI] [PubMed] [Google Scholar]
- 73. Bailey RL, Sahni S, Chocano-Bedoya P, Daly RM, Welch AA, Bischoff-Ferrari H, Weaver CM. Best practices for conducting observational research to assess the relation between nutrition and bone: an international working group summary. Adv Nutr. 2019;10(3):391–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Trikalinos TA, Moorthy D, Chung M, Yu WW, Lee J, Lichtenstein AH, Lau J. Concordance of randomized and nonrandomized studies was unrelated to translational patterns of two nutrient-disease associations. J Clin Epidemiol. 2012;65(1):16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.