ABSTRACT
There is emerging evidence that an unhealthy dietary pattern may increase the risk of developing depression or anxiety, whereas a healthy dietary pattern may decrease it. This nascent research suggests that dietary interventions could help prevent, or be an alternative or adjunct therapy for, depression and anxiety. The relation, however, is complex, affected by many confounding variables, and is also likely to be bidirectional, with dietary choices being affected by stress and depression. This complexity is reflected in the data, with sometimes conflicting results among studies. As the research evolves, all characteristics of the relation need to be considered to ensure that we obtain a full understanding, which can potentially be translated into clinical practice. A parallel and fast-growing body of research shows that the gut microbiota is linked with the brain in a bidirectional relation, commonly termed the microbiome–gut–brain axis. Preclinical evidence suggests that this axis plays a key role in the regulation of brain function and behavior. In this review we discuss possible reasons for the conflicting results in diet–mood research, and present examples of areas of the diet–mood relation in which the gut microbiota is likely to be involved, potentially explaining some of the conflicting results from diet and depression studies. We argue that because diet is one of the most significant factors that affects human gut microbiota structure and function, nutritional intervention studies need to consider the gut microbiota as an essential piece of the puzzle.
Keywords: microbiome–gut–brain axis, depression, anxiety, mood, mental health, nutritional psychiatry, microbiota, diet, nutrition
Introduction
Around 15–20% of people will experience mental health disorders such as a depressive episode or anxiety disorder in their lifetime (1, 2), and anxiety and depression are ranked in the top 10 causes of the global burden of disease (3, 4). Unfortunately, our understanding of these disorders and ability to effectively treat them are poor; for example ∼30%–40% of those with depression do not adequately respond to pharmacological or psychological treatment (5). The high impact on individual quality of life, as well as on the public health system, means that preventing and treating anxiety and depression is a global priority. Consequently, there has been a call for a broader research approach with more interdisciplinary efforts (6, 7). Recent approaches in depression and anxiety research are investigating 1) the links between diet and mood, and 2) the influence of gut microbiota on neurobiology and behavior, termed the microbiome–gut–brain axis (MGBA). There is emerging evidence showing a strong influence of both diet and gut microbiota on emotional behavior and neurological processes, and because the gut microbiota is strongly affected by diet (8), these 2 factors are also intertwined (Figure 1). With increasing understanding of the mechanisms involved in the complex interplay between diet and the gut microbiome and its impact on anxiety/depression, specific dietary patterns that can help prevent anxiety and mood disorders can be identified. In addition, the use of dietary intervention may prove to be an attractive and cost-effective alternative or adjuvant therapy to clinically treat these disorders. This review discusses the reasons for conflicting research on the link between diet and depression, and how any diet and depression relation is likely to be influenced by changes in the MGBA. The focus of the review is on whole diets and, and although interactions of the gut microbiota with individual components of the diet are discussed, separate dietary supplements (such as probiotics) are not included in detail.
FIGURE 1.
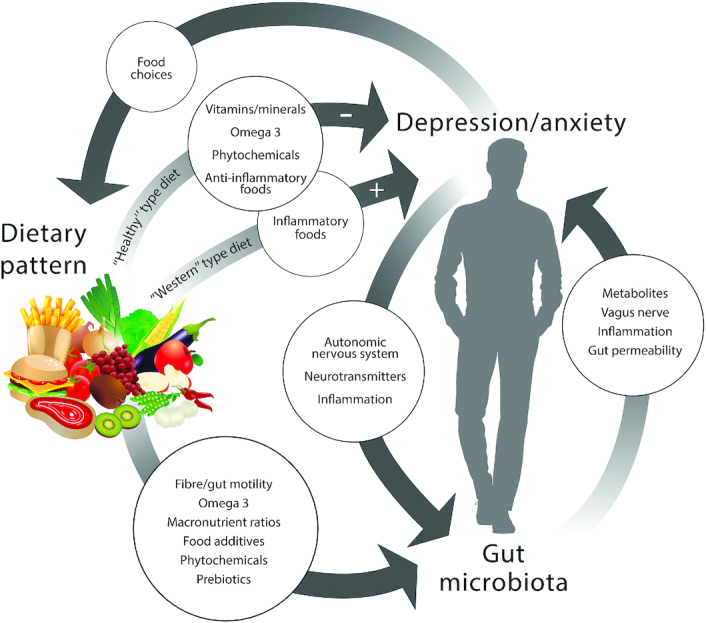
Diet has been linked with the risk of developing depression and anxiety; there are direct effects from dietary components which could mediate this relation. Emerging research suggests that the gut microbiota is also associated with depression and anxiety in a bidirectional relation. Because diet also has a large influence on gut microbiota, the gut microbiota should be considered a key variable in the diet–depression relation.
Current Status of Knowledge
The relation between diet and depression
Research shows that there is a relation between diet and depression; however, there are conflicting results from studies and the directionality and mechanism of the relation are currently unclear. Many correlative studies in healthy adults show that a lower incidence of depression occurs in those who eat according to “healthy” dietary patterns, characterized by an abundance of vegetables, fruits, cereals, nuts, seeds, and pulses, as well as moderate amounts of dairy, eggs, and fish and unsaturated fats (9), including the Mediterranean diet (10, 11), Japanese diet (12, 13), and Norwegian diet (14). In contrast, a “Western” dietary pattern, consisting of sweet and fatty foods, refined grains, fried and processed foods, red meat, high-fat dairy products, and low fruit and vegetable intake, is associated with higher depression incidence (10, 15, 16). However, not all studies show an association, with many finding no association (17–20) or showing an effect only from a specific food [e.g., tomatoes (18)].
Conflicting results from studies are potentially due to many factors. There is possible recall bias due to the use of FFQs, and difficulty in controlling for all confounding variables (21). The recall bias has not been addressed and may be a specific problem for assessing diet and depression, because depression can affect memory (22). Participant and researcher expectation bias is another issue in randomized controlled trials. Because the variables being measured rely on participant reporting, blinding is important to prevent expectation bias. However, blinding of the participants to the hypothesis is difficult. Dieticians/nutritionists and psychologists who deliver the separate arms of the trial should also be blinded as to the study hypothesis, but in practice this is rarely done.
Reverse causality is possible. Stress and depression can also alter taste thresholds (23), perception of sugary and fatty foods (24, 25), and food choices (26–28). A 10-y longitudinal study in France showed an association between depression incidence and poor diet, but found that there was probably reverse causality, with depression increasing the risk of poor eating behaviors (29). A reanalysis of a longitudinal study in Australia showed that those with an existing depressive episode had a poorer diet, but not those with only historical depression (30). The authors suggest that this could be due to reverse causality, but alternatively could be due to altered dietary habits after depression treatment in order to prevent depression recurrence, an interpretation that is supported by a study showing that 20% of people with depression intentionally improve their diet (31).
Attempts to determine the direction of causality from prospective studies and randomized controlled trials have shown similar mixed results. A meta-analysis of prospective studies identified that a high-quality diet, regardless of its type, as well as increased fish and vegetable intake, was associated with a lower incidence of depression, with a dose–type relation with compliance to the healthy diet. In contrast, however, this meta-analysis showed that a low-quality diet was not found to be associated with an increased risk of depression, and the results showed a high level of heterogeneity between studies (32).
A systematic review of randomized controlled trials by Opie et al. (33) found that study design varied in terms of delivery method, type of intervention, and the study population. Few studies have been done in medically healthy people, and over half of the studies showed no effect from the intervention. Interventions that were successful in improving depressive symptoms had a single delivery mode (e.g., face to face), had been delivered by a qualified nutritionist or dietitian, and were more likely to recommend an increase in vegetables as opposed to a cholesterol-lowering diet or a reduction in red meat.
The mixed results and difficulties in research design do not mean that there is not a clinically meaningful relation. There are plausible mechanisms for how diet can affect depression, and bidirectionality is probable. Depression is primarily a psychological illness and the strength and importance of a diet–depression relation will vary depending on individual psychological traits, including personality, thought patterns, and coping skills. Psychological variables are not usually adequately controlled or accounted for in nutrition studies. The finding that the diet–depression relation is stronger with a healthy diet than with an unhealthy diet could be due to people less susceptible to depression being more resilient to the effects of an unhealthy diet, and potentially diluting any measured effect. In addition, there are likely to be geographical differences in the strength of the relation, as variations in micronutrient content in soils, and consequently in foods, occur [e.g., low selenium content in New Zealand soils (34)]. Prospective studies often attempt to determine the direction of causality by excluding those who already have depression from the analysis, and then examining the rates of depression developed in the rest of the cohort against the different dietary patterns. The problem with this approach is that not everyone has an equal risk of developing depression, because there are strong genetic, epigenetic, and environmental components to being vulnerable to depression (35). By excluding those who already have depression, the sample is biased towards those who are less vulnerable to developing depression, and for whom any link between diet and depression is likely to be much weaker. In addition, Molendijk et al. (32) suggested that controlling for baseline depression severity could cancel out diet–depression effects because the influence of poor diet on depressive symptoms may have begun years earlier. It is also possible that poor diet increases vulnerability to developing depression under stress, rather than directly causing depression, and therefore a study undertaken in a cohort with low environmental stress may not reach the tipping point in numbers developing depression needed to reveal the relation if it exists.
Physiologically, there are multiple plausible mechanisms by which diet can directly influence symptoms of anxiety and depression. The etiology of depression itself is not fully established but many biological and neurological changes are linked to depressive symptoms. A reduction in monoamine neurotransmitters, especially serotonin, is the most well-known mechanism and the pharmacological target of most antidepressant drugs (36). But monoamine deficiency is most likely only a cause of depressive symptoms in a vulnerable population (37). Other mechanisms found to be linked to depression include a dysfunctional hypothalamic–pituitary–adrenal (HPA) axis (38); immune-inflammatory, oxidative, and nitrosative pathways (39–42, 30, 43–48); neuroinflammation (including activated microglial cells) (43); altered vagus nerve tone (49); neurotrophic changes, including structural changes such as decreased hippocampus volume (50, 51) and region-specific changes in brain-derived neurotrophic factor concentrations (52); an imbalance between neural excitation and inhibitory signaling (53–55); and alterations in tryptophan metabolism, including the kynurenine pathway (56).
A number of micronutrients have been found to be low in those with depression or an increased risk of depression, including zinc, magnesium, selenium, iron, and vitamins D, B-12, B-6, E, and folate (57–62). These micronutrients may affect depression risk via effects on the production and activity of monoamine neurotransmitters such as serotonin (63–68), alterations to the HPA system (62), glutamatergic signaling (62), or inflammatory and oxidative stress (62, 69). A diet high in fruits and vegetables has higher amounts of these micronutrients. Plants also contain effective antioxidant phytochemicals, such as vitamin C, polyphenols, and flavonoids, which have been shown to have antidepressant-like or anxiolytic effects (70–73). SFAs, and PUFAs such as DHA (22:6n–3), EPA (20:5n–3), and arachidonic acid (AA; 20:4n–6), are incorporated into neural tissue and are important for its function (74). The ratio of the different fatty acids (FAs) affects function; for example, increased SFA decreases cell membrane fluidity and permeability (75), and a low ratio of DHA to AA may increase systemic and brain inflammation (76, 77). Healthy-style diets, especially the Mediterranean diet, are anti-inflammatory (78) and may lower the risk of depression by reducing inflammation.
An understudied aspect in the diet–depression relation which could explain some of the inconsistencies is the diet–microbiota–mood relation. Emerging evidence shows that the gut microbiota is linked to emotional behaviors thought to represent symptoms of both depression and anxiety, and because the gut microbiota is highly influenced by diet, diet can influence this relation.
The link between the gut microbiota, depression, and anxiety
Research into the MGBA began with the observation that there is a high comorbidity of anxiety and depression in those with inflammatory bowel disease (79, 80) and irritable bowel syndrome (79–82). In addition, gut microbiota composition in individuals with anxiety or depression (including those in remission) differs from that in healthy controls (83–85), and animal models of depression show altered gut microbiota compared with controls (46).
Early studies in mice showed that gut infections or chemically induced colitis caused an increase in patterns of behavior thought to represent anxiety, including decreased exploration (86) and increased behavioral inhibition (87, 88). The direct effect of gut microbiota on emotional behaviors was shown in studies which identified that anxiety-like behaviors differ between germ-free (GF) rats and mice (born and raised in a microbiota-free environment) and animals with normal, specific pathogen–free (SPF) gut microbiota (89–92). Colonization of GF animals with SPF gut microbiota has been shown to ameliorate the behavioral differences (90, 92, 93). Fecal transplants from anxious-type mice into a more resilient strain increase anxiety-like behaviors in the resilient strain, and vice versa (94). Probiotic supplementation has also shown promise, with a reduction in anxiety and depression reported in many human and animal studies (95–99). Probiotics seem to also be protective against the development of anxiety due to gut infection (88) and immunodeficiency (100).
Although there is convincing evidence that microbiota can be linked to emotional behaviors, we do not fully understand the mechanisms, nor the clinical relevance. Studies in humans are still few, and often do not translate from animal studies, possibly because they often use healthy people without depressive symptoms (101). Inconsistencies in behavioral changes in animal studies often occur between stress-sensitive and stress-resilient rodent strains (89–92) and between males and females (90, 91), suggesting that the host–microbiota relation may depend on host genotype. There may be critical windows of development during which the gut microbiota have more effect: for example, early life (93), adolescence (90, 102, 103), or during gestation (104–106).
Microbial-mediated mechanisms of mood and neurological processes are still being elucidated (88). At the systemic level, immune modulation has been found in GF mice (90), antibiotic-treated mice (103), and with probiotic supplementation (88, 90, 98, 99, 103, 107–111). Some studies have shown no evidence of inflammation alongside behavioral changes (88, 108) or only a partial effect (98, 109, 110); however, even subclinical gut infection without overt inflammation caused behavioral changes in mice (87). Increased HPA axis activation (96, 102) and alterations to the tryptophan/kynurenine metabolism have also been found (88, 90, 107, 112–115). In the gut, changes have been found for SCFAs (113), gut motility (100), and gut permeability (110, 116–118). The vagus nerve may be required (97, 102, 119–121), but not always (88, 94). It is likely that multiple parallel mechanisms are at play, and the mechanisms of the effect of the gut microbiota are specific even to the species level.
Microbial metabolites may play a role, with some bacteria able to produce the same neuromodulating substances that are found in the nervous system of animals, including γ-aminobutyric acid (GABA), acetylcholine, dopamine, serotonin, and norepinephrine (122–126). GABA, acetylcholine, and noradrenaline are also all immunomodulatory (127, 128). SCFAs, particularly butyrate, contribute to decreased gut inflammation (129) and enhanced gut epithelial integrity (130). They stimulate the secretion of serotonin from enterochromaffin cells in the gut (115, 131, 132), which mainly affects gut motility but can also activate the vagus nerve, and enter the circulation (132). SCFAs activate free fatty acid receptors (FFARs), which seem to have a direct anti-inflammatory effect on microglial activation (133), as well as being generally anti-inflammatory, because FFARs are present on neutrophils and dendritic cells (134). No difference in SCFAs was found in the fecal samples of people with depression compared with healthy controls, but when the fecal samples were transplanted into mice, an increase in fecal acetate and total SCFAs was found (113). Gut bacteria are also a significant source of vitamins, including vitamin K-2 (menaquinone) and the B-vitamins niacin (B-3), biotin (B-7), folate (B-9), and pyroxidine (B-6) (135–139). Biotin and niacin are immunomodulatory, and deficiency could contribute to gut and systemic inflammation (129, 140). Serum folate is lower in those with depression (141) and may be associated with symptom severity (61) and responsiveness to antidepressant treatment (142). Pyroxidine is an essential cofactor in many enzymes in the kynurenine pathway, which is altered in those with depression (55). GF rats show an increased susceptibility to pyroxidine deficiency (137).
Changes in gut microbiota with depression and anxiety
The MGBA findings of behavioral effects with probiotic or inflammatory bacteria broadly fit with the microbial profiles associated with positive or negative mental health, although there is no specific gut microbiota composition profile linked to anxiety or depression. Comparisons of microbial changes in humans with depression show a variety of changes compared with healthy controls, but show a general pattern of increases in potentially harmful and inflammatory bacteria such as Proteobacteria, which are normally minor in relative abundance, alongside a decrease in commensal bacteria, which are normally more abundant (83, 84, 113, 143–145). In those with Generalized Anxiety Disorder, fewer changes were found but a similar reduction in commensal bacteria was seen (85). The lack of an identified depression or anxiety “gut microbiota profile” is likely to be due to variation in the methods used to evaluate gut microbiota composition and gene abundance, and individual variation in the human gut microbiota (146). Although there are still many unknowns relating to the MGBA and its mechanisms, the emerging evidence, combined with the effect of diet on microbiota, supports its important role in the diet–mood relation.
Interactions of Diet with the MGBA
Whole diet
There is a paucity of research measuring the effects of whole diet on microbiota as well as depressive symptoms, and studies on diet and anxiety in humans are still needed. However, dietary patterns associated with a risk of depression are in line with changes in microbial composition and functions, which MGBA research shows can affect emotional behavior in rodents. Adherence to the Mediterranean diet reduces the numbers of inflammatory/pathogenic bacteria such as Escherichia coli, and increases key commensal bacteria such as Bifidobacteria (147), Clostridium cluster XVIa, and Faecalibacterium prausnitzii (148). It also increases microbial metabolites including fecal SCFA concentrations (149), phenolic metabolites, benzoic acid, and 3-hydroxyphenylacetic acid (148). Vegetarian or entirely plant-based diets have been shown to alter microbial composition (150–152) and reduce gut inflammation (150). A dietary pattern defined by fast-food consumption reduced Lactobacilli (149). A high-fat/low-carbohydrate diet, regardless of the type of fat, decreases total bacteria (153).
Many of the individual dietary elements that are associated with an increased or decreased risk of developing depression also alter the gut microbiota (refer to Table 1). It is plausible that the effect of a dietary component on the gut microbiota may partially or wholly mediate the effect of that dietary component on mood.
TABLE 1.
Examples of published literature evidence (from in vitro, animal, and human studies) of components within dietary patterns related to depression in humans or emotional behaviors in animals, which directly affect the host but also interact with the gut microbiota1
| Dietary component | Effect | Summary | Subject | Ref. |
|---|---|---|---|---|
| Phytochemicals | ||||
| Cocoa polyphenols | Affected mood | In an RCT in adults, 500 mg supplement for 30 d increased self-rated calmness and contentedness compared with placebo. | Human | Pase et al. (183) |
| Altered microbial growth | A 6-wk diet with 10% cocoa in rats caused a decrease in Bacteroides, Clostridium, and Staphylococcus genera in feces. | Animal | Massot-Cladera et al. (184) | |
| In vitro digestion with 1 g cocoa powder/60 mL water. 38.6% of phenols were solubilized, and an increase in Bifidobacteria, Lactobacilli, and butyrate was found. | In vitro | Fogliano et al. (185) | ||
| Altered immune function | A 6-wk diet with 10% cocoa in rats caused an altered toll-like receptor pattern and increased gastrointestinal immunoglobin A secretion. | Animal | Massot-Cladera et al. (184) | |
| Blueberry extract (anthocyanins) | Affected mood and cognition | In a BCT, in children and young adults, a single drink containing 253 mg anthocyanins increased positive but did not change negative affect scores using the “Positive and Negative Affect Scale” compared with a placebo drink. | Human | Khalid et al. (186) |
| A 5% blueberry drink given to rats for 8 wk protected against cognitive impairment during chronic mild stress. | Animal | Guo et al. (187) | ||
| Altered host metabolites | Decreased plasma norepinephrine and dopamine concentrations, and brain concentrations of antioxidant compounds due to 8 wk of chronic mild stress were attenuated by a 5% blueberry drink. | Animal | Guo et al. (187) | |
| Fiber (prebiotic) | ||||
| GOS, PDX, and FOS | Attenuated stress-induced behaviors and mood, and gene expression in the brain | Male rats were fed diets containing GOS + PDX for 4 wk and then underwent inescapable stressors. The prebiotic reduced stress-induced exaggerated freezing and deficit in escape latency, and attenuated c-fos mRNA in parts of the brain. | Animal | Mika et al. (188) |
| Male and female rats underwent early-life stress (maternal separation model). Prebiotic supplementation of GOS + FOS for 5 wk after the stress attenuated stress-induced deficits in spatial memory and locomotion, but not anxiety-like behaviors. | Animal | McVey Neufeld et al. (189) | ||
| RCT, patients with depression: 8 wk supplementation with 5 g GOS resulted in decreases in scores on the Beck Depression Inventory compared with placebo. | Human | Kazemi et al. (190) | ||
| Healthy volunteers given either FOS or GOS daily for 3 wk. Salivary cortisol awakening response and emotional bias (attention to negative information) were decreased after GOS but not FOS. | Human | Schmidt et al. (191) | ||
| Altered the gut microbiota | Prebiotic diet of GOS + FOS increased Lactobacillus rhamnosus and also Lactobacillus spp. | Animal | Mika et al. (188) | |
| 44 elderly subjects, given 5.5 g/d GOS or placebo for 10 wk in a double-blind, placebo-controlled, crossover study. Increase in Bifidobacterium spp., Lactobacillus Enterococcus spp., Clostridium coccoides–Eubacterium rectale, and a decrease in Bacteroides spp., Clostridium histolyticum group, Escherichia coli, and Desulfovibrio spp. | Human | Vulevic et al. (192) | ||
| Altered immune function | Increases in immune function, including reduced proinflammatory cytokines and increased anti-inflammatory cytokines, phagocytosis, and NK cell activity. | Human | Vulevic et al. (192) | |
| Wheat arabinoxylan | May counteract effects of high-protein diet on the gut microbiota | In pigs fed a 4-wk Western-type diet, added soluble fiber (wheat arabinoxylan) increased carbohydrate fermentation and reduced protein fermentation and fermentation products such as ammonia. | Animal | Williams et al. (193) |
| Vitamins/minerals | ||||
| Vitamin D | Regulated gut physiological processes | Vitamin D receptors in the gut regulate processes including epithelial barrier function and immune processes. | Review | Barbáchano et al. (194) |
| Associated with changes in the gut microbiota | Plasma 25-hydroxyvitamin D and vitamin D supplementation in women in their 36th week of pregnancy were measured, and compared with fecal samples in their 1-mo-old infants. Increased concentrations of both were associated with decreased Bifidobacteriumspp. and Clostridium difficile and increased B. fragilis. | Human | Talsness et al. (182) | |
| Magnesium | Dietary deficiency altered behavior | 30 mice fed a magnesium-restricted diet for 6 wk had increased immobility in the forced swim test and increased hippocampal IL-6 compared with mice fed a normal diet. | Animal | Winther et al. (179) |
| Associated with changes in the gut microbiota | The cecal gut microbiota was also altered, with cluster analysis showing significant differences between the diets. | |||
| Vitamin A | Associated with changes in the gut microbiota and the gut mucosal barrier | A vitamin A–deficient diet in rats increased total bacteria, decreased Lactobacillus spp., and increased Escherichia coli. Mucin-producing goblet cells were altered and expression of toll-like receptors was increased. | Animal | Amit-Romach et al. (195) |
| Vitamin A deficiency in children aged 1–12 mo with persistent diarrhea showed significantly different gut microbiota than in those with normal serum vitamin A concentrations. | Human | Lv et al. (196) | ||
| Macronutrients | ||||
| ω-3 fatty acids | Immunomodulatory | The metabolic and inflammatory effects in wild-type mice fed a diet with a high ratio of ω-6 to ω-3 were able to be prevented with antibiotic treatment, or by cohousing mice with Fat-1 transgenic mice, which endogenously produce ω-3 fatty acids. | Animal | Kaliannan et al. (176) |
| Increased endogenous antimicrobial defenses | Fat-1 mice were found to produce increased intestinal alkaline phosphatase, an endogenous antimicrobial compound, which reduced gut permeability and LPS production. | Animal | Kaliannan et al. (176) | |
| Restored gut dysbiosis | Fat-1 transgenic mice were found to be protected against gut dysbiosis and obesity caused by a Western-style diet after early-life antibiotic exposure. | Animal | Kaliannan et al. (197) | |
| Supplementation of 100–250 mg/d ω-3 FA (80% EPA, 20% DHA) for 12 wk to female rats reversed stress-induced gut dysbiosis. | Animal | Pusceddu et al. (177) | ||
| Increased gut microbial metabolites (SCFAs) | An 8-wk open label trial using an EPA/DHA supplement drink or capsule in adult males and females reversibly increased SCFA-producing bacteria including Bifidobacterium,Roseburia, and Lactobacillus. | Human | Watson et al. (198) | |
| Deficiency affected mood as well as the gut microbiota | An ω-3 FA–deficient diet in pregnant mice and their male offspring resulted in an elevated ratio of Firmicutes to Bacteroidetes in the offspring, along with altered behavior and immune function. | Animal | Robertson et al. (178) | |
| Increased depressive behavior (immobility in forced swim test), decreased sociability (three chamber test), isolation-induced ultrasonic vocalizations in adulthood, and decreased memory (novel object recognition test) in both adolescence and adulthood. Increased contextual fear conditioning. | ||||
| High fat, particularly saturated fat | Altered microbiota composition | A high-fat diet in mice decreased Ruminococcaceae and increased Rikenellaceae compared with a carbohydrate diet. | Animal | Daniel et al. (199) |
| Increase in Firmicutes, particularly the family Erysipelotrichaceae, and decrease in Bacteroidetes in mice fed a high-fat diet. | Animal | Fleissner et al. (200) | ||
| Mice fed a low-fat diet who switched to a high-fat diet had a significant shift in microbiome composition within 1 d. Increased Firmicutes, particularly the Erysipelotrichi class, Bacilli, and decreased Bacteroidetes. | Animal | Turnbaugh et al. (201) | ||
| BALB/c mice fed a high-fat diet showed alterations in the gut microbiota including an increase in Firmicutes, particularly in the families Rumunococcaceae and Lachnospiraceae, a decrease in the Bacteroidetes phylum, and a resulting decrease in the ratio of Bacteroidetes to Firmicutes. | Animal | Pyndt Jørgensen et al. (202) | ||
| Altered anxiety-like behavior | Mice fed a high-fat diet displayed less burrowing (anxiety-like) behavior, and displayed reduced memory in the Morris water maze test compared with mice fed a control diet. The diets were not isocaloric, and the high-fat diet mice also gained more weight. | Animal | Pyndt Jørgensen et al. (202) | |
| High-fat, high-sugar diet | Altered microbiota composition | A Western-style diet in humanized mice resulted in increased Erysipelotrichi class (mainly Clostridium innocuum, Eubacterium dolichum, and Catenibacterium mitsuokai genera) and Bacilli class (mainly Enterococcus spp. genera). The microbial shift occurred after only a single day. | Animal | Turnbaugh et al. (201) |
| High-sugar diet | Positive change in behavior when the gut microbiota was not altered | A high-sucrose diet did not alter the gut microbiota in BALB/c mice compared with a control diet and did alter some behaviors, but in a positive direction (increased latency to immobility in the forced swim test, less goal-orientated burrowing, and less anxiety-like behavior in the triple test). | Animal | Pyndt Jørgensen et al. (202) |
| Red meat | Modified gut microbiota composition | A comparison between a diet rich in red meat or whole grains (10-wk crossover trial) showed that increased red meat consumption increased the genera Clostridium spp. from the phylum Firmicutes. | Human | Foerster et al. (203) |
| Microbial metabolism of heme-rich meat increases oxidative compounds | Comparison of meat types varying in heme content (beef, pork, chicken) in an in vitro digestion model showed that heme-rich meat caused higher concentrations of the nitrosoxide compound–derived DNA adduct O6-carboxymethylguanine. | In vitro | Vanden Bussche et al. (204) | |
| Food additives | ||||
| Emulsifiers CMC and P80 | Altered gut microbiota composition | C57Bl/6J mice were given either CMC or P80 emulsifiers at 1% in their drinking water from weaning until 3 mo old. The gut microbiota was altered by the treatment. Interestingly the outcomes differed between males and females. In males, Firmicutes phylum and Oscillospria, Coprococcus, and rc4_4 genera were reduced, as well as reduced Dorea with P80, and reduced Bacteroides, Burkholderia, Clostridium, and Veillonella with CMC. In females, Bacteroides, Sphingomonadales, Sphingomonas, and Ruminococcus were reduced, and there was an increase in Anaeroplasma with P80, and the Proteobacteria phylum and Clostridium and Burkholderia genera with CMC. | Animal | Holder et al. (205) |
| Altered anxiety-like behavior | Treatment with emulsifiers decreased sociability in the 3-chamber test in females only, and increased locomotion in the Elevated Plus Maze in males only. No difference found in forced swim test or light-dark box. | |||
1BCT, blinded crossover trial; CMC, carboxylmethylcellulose; FOS, fructooligosaccharide; GABA, γ-aminobutyric acid; GOS, galactooligosaccharide; PDX, polydextrose; P80, polysorbate 80; RCT, randomized controlled trial.
Fish and omega-3 FA intake
Although “healthy” dietary patterns containing fish are found to be associated with a lower risk of depression (10, 15, 29, 154–157), other studies show fish to be 1 component of dietary patterns that increase the odds of depression (13) or are inflammatory (158). When looked at in isolation, there is evidence for a decreased risk of depression (159), increased risk (160–162), or no relation (163–166). Randomized controlled trials comparing fish oil with olive oil found no difference in mood improvement (167, 168); however, neither group was initially deficient in ω-3 FAs. Meta-analyses have found that ω-3 FA dietary supplementation (from fish oil or added fish) has a positive effect on mood in those with symptoms of depression, but not in healthy controls (169, 170). There may be an optimal dose, because some studies have shown a nonlinear association between ω-3 FA and depressive symptoms, with the highest doses being less effective than moderate doses (162, 165). People with depression have lower concentrations of ω-3 FAs in their RBC membranes (171–173), possibly through oxidative damage (172) rather than lower ω-3 FA intake. This observation supports emerging evidence that those with depression have higher levels of inflammation and oxidative stress (174, 175).
Lower intake of fish or ω-3 FA may affect depression risk via microbiota-induced inflammation. Research in mice suggests that the anti-inflammatory effect of ω-3 FA may be due to its effect on microbiota (176). A diet comprising a high ratio of ω-6 FA to ω-3 FA (∼25:1), as is typical of a Western-style human diet, fed to wild-type mice caused elevated serum concentrations of the metabolic endotoxemia markers LPS and LPS-binding protein, as well as increased gut permeability compared with that in fat-1 transgenic mice fed the same diet. The fat-1 mice can endogenously produce ω-3 FA from ω-6 FA and therefore had a lower gut ratio of 4:1. The difference in serum LPS (but not the cytokine IL-1β and serum triglycerides) was eliminated when the mice were given antibiotics, or when ω-3 FA dietary intake was increased. The mechanism was found to be an ω-3 FA-dependent increase in production of an endogenous antimicrobial peptide, gut alkaline phosphatase, which suppresses proendotoxic bacteria.
Another study in rats showed that supplementation of the ω-3 FAs EPA and DHA was associated with restoration of disturbed gut microbiota caused by early-life stress (maternal separation) (177). In a follow-up study, pregnant mice and then their offspring were given diets that were either ω-3 FA deficient or ω-3 FA supplemented (178). The ω-3 FA–deficient diet caused increased fear-induced freezing behavior, decreased sociability, and increased depressive behavior in the offspring when they had become adults. The diets were, not surprisingly, associated with differences in FA composition in the brain, but also differences in fecal microbiota profiles. The changes in microbiota numbers showed the ratio of Firmicutes to Bacteroidetes was increased by ω-3 FA deficiency. In the ω-3 FA–supplemented group, Bifidobacterium and Lactobacillus were present in higher numbers in adult mice, the ratio of Bifidobacteriumto Enterobacteria was higher, and Anaeroplasma, Clostridium, and Peptostreptococcaceae numbers were lower, in both adolescents and adults.
Fish and ω-3 FAs may play additional roles to those previously assumed in the link with depression. Rather than there being only a straightforward relation between EPA or DPA concentrations and neural processes, dietary intake of ω-3 FA may also (or only) be important for those with gut dysbiosis–induced systemic inflammation, and the ratio of ω-3 FA to ω-6 FA in the diet may also be critical.
Micronutrient intake
Microorganisms require many of the same micronutrients that humans do, and obtain many of these micronutrients through the host diet. Subsequently, host micronutrient intake can affect the gut microbiota composition and function. Altered gut microbiota has been found in mice with a magnesium-deficient diet and was associated with increased depressive-like behavior (179). Whether the change in behavior was caused by or simply additional to a change in gut microbiota is unclear. Vitamin D intake also affects the gut microbiota; supplementation altered the gut microbiota in stool samples in patients with Crohn disease but not healthy controls (180), or caused a change in healthy adults but only in the stomach and duodenum (181). In infants, the vitamin D status of their mother during pregnancy influenced their gut microbiota at 1 mo old, but a supplement given directly to the infant did not, possibly due to different baseline concentrations of serum vitamin D in the infants (182). The different gut regions (stool sample compared with upper gastrointestinal tract) may explain the differences in these studies, and also suggest that a healthy gut may respond differently than an inflamed gut. Research into whether modulation of the gut microbiota by magnesium and vitamin D supplementation is via immune modulation, and whether vitamin D affects behavior, is lacking.
Iron is an essential nutrient for many bacteria, and dietary intake of iron affects the gut microbiota composition. Increased colonic iron due to dietary supplementation has been shown to increase gut inflammation and pathogenic bacteria, and a diet deficient in iron has been found to increase the relative abundance of Lactobacilli (nonsiderophilic bacteria, which do not require iron) in mice [reviewed in (206)]. Conversely, the relative abundance of many beneficial bacteria, including butyrate producers, was also reduced with an iron-deficient diet in rats, and subsequently SCFA production also decreased (207). Host iron status can influence the ability to fight pathogenic bacteria in the colon, and both high and low iron concentrations are associated with increased pathogen virulence (206). A change in gut microbial composition can also affect iron absorption owing to changes in pH (206). The Western diet contains much more iron than can be absorbed and so the concentration of iron found in feces is high (206). Studies with whole diets should examine how micronutrient intake relates to any changes in the gut microbiota.
Prebiotic foods
A “healthy” dietary pattern contains a larger amount of fruit, vegetables, and wholegrains, which contain prebiotics such as fermentable carbohydrates, polyols, and phytochemicals (208). Prebiotic compounds selectively promote the growth and microbial activity of beneficial bacteria and confer positive health outcomes (208). The higher prebiotic content characteristic of healthy diets may be why the association of depression with diet is stronger for healthy dietary patterns and more variable for poor dietary patterns (9, 32, 157). Prebiotic compounds typically have been shown to increase concentrations of Bifidobacteria and Lactobacillus but as microbial research techniques have become more sophisticated, we now understand that there are many other beneficial bacteria that are promoted with prebiotics, such as butyrate-producing bacteria. Some dietary fibers are considered prebiotic but not all. Dietary fiber that promotes the growth of all gut bacteria is not considered a prebiotic because numbers of pathogenic bacteria are also increased (208). The most well-researched prebiotics are the soluble fibers “fructans” [fructooligosaccharides (FOSs) and inulin] and galactans [galactooligosaccharides (GOSs)]. Mannanoligosaccharides and xylooligosaccharides are also considered prebiotic. Phenolics and phytochemicals show prebiotic effects, although some of the health benefits may be from microbially produced secondary metabolites. Conjugated linoleic acid (18:2n–6) and PUFAs are also considered candidate prebiotics (208).
Evidence to date for the impact of prebiotics on mood is mixed but mostly positive. Studies in rodents have found reduced baseline and stress-induced anxiety-like and depressive-like behaviors with FOSs and GOSs (individually or mixed) (209), a mixture of GOSs and polydextrose (PDX) (188), and the glycoprotein lactoferrin (188). In both these studies, the mixed supplements had a stronger effect on these behaviors. In rats, GOS and PDX supplementation improved scores in anxiety and memory tests more than a probiotic supplementation (Lactobacillus rhamnosus GG), but less than a synbiotic supplement (Lactobacillus rhamnosus GG, PDX, and GOSs) (189). In zebrafish, a tendency toward improved behaviors under stress was found with supplementation of mannanoligosaccharides and glucose (β-glucans) (210). A synbiotic (FOS, GOS, and inulin with a probiotic mixture containing Lactobacillus acidophilus strain T16, Bifidobacterium bifidum strain BIA-6, Bifidobacterium lactis strain BIA-6, and Bifidobacterium longum strain LAF-5) improved depressive symptoms more than the probiotic only in hemodialysis patients (211). Conversely, increased anxiety-like behaviors occurred in mice after supplementation with resistant starch (212). In a human study, prebiotic supplementation in healthy volunteers improved results in an emotional bias test with GOS, but not FOS (191). Prebiotic supplementation altered the microbiota in all these studies. In those studies in which positive behavioral results were found, increases in Lactobacillus species and decreases in the phylum Proteobacteria were found (209, 188, 210). Along with increased anxiety-like behavior with resistant starch supplementation, Lyte et al. (212) found an increase in the phylum Proteobacteria, but interestingly also an increase in Bifidobacterium.
The biological activity of many phytochemicals has been shown to have possible positive health effects, including antidepressant-like or anxiolytic effects (70) and a prebiotic effect (213). The actions of phytochemicals may also be due to secondary metabolites created by microbial utilization (213). Research examining the effect of phytochemicals on both mood and microbiota is lacking.
Macronutrients
A large driver of the effect of diet on the composition of the gut microbiota is variation in macronutrient ratios, amounts, and types. Carbohydrate fermentation tends to increase overall microbial fermentation and SCFA production. The amount of fermentation depends on how much reaches the colon, which is influenced by the amount and type of dietary fiber and prebiotic carbohydrates (214). A plant-rich diet promotes the phylum Bacteroidetes, specifically the genera Prevotella and Xylanibacter, which ferment plant fiber. One study found that a reduction in total carbohydrate in the diet reduced the butyrate-producing Roseburia/Eubacterium rectale group. Fermentation of protein generates SCFAs, branched-chain FAs, sulfides, and phenolic and indolic compounds. The sulfides are associated with gut diseases (214). The types of bacteria promoted by protein intake are not well established (214).
Increased dietary fat alters the gut microbiota composition (153, 215, 202, 216–219, 199–201, 220), possibly via the stimulation of bile and its modulation into secondary bile acid products (153). A high-fat diet (HFD) (72% fat kcal, corn oil and lard) may also affect metabolism, inflammation, and gut permeability via the gut microbiota, likely mediated by LPS and the CD14 receptor. These physiological effects of the HFD were able to be reduced with antibiotic treatment (221). Changes in the gut microbiota with an HFD (45% kcal fat) compared with a control diet (10%–12% kcal fat) in mice include decreases in the beneficial bacteria Bacteroidetes (222), Akkermansia (223), Bifidobacteria (223), and Lactobacillus (222, 223) and increases in Firmicutes, particularly the potentially inflammatory Clostridiales (222, 223), Enterobacteriaceae (223), and Proteobacteria (222). Counterintuitively, the relative abundances of the families Ruminococcaceaeand Lachnospiraceae (202) and the genus F. prausnitzii (153), which are considered beneficial gut bacteria (224), were increased by a 60% kcal HFD.
Some evidence for an effect of macronutrient intake on emotional behavior has been found in rodent studies, mostly with an HFD. Increased anxiety-like behavior has been found in mice fed an HFD comprising 60% kcal unspecified unsaturated FAs (225), 58% kcal hydrogenated coconut oil (226), or 45% kcal lard and soybean oil (227), compared with control diets of ∼10% kcal fat. Decreased anxiety-like behaviors have also been found with an HFD (Crisco and corn oil, 90% kcal), compared with a diet high in protein (90% kcal) or carbohydrate (90% kcal), which did not alter these behaviors (228). Another study found no change in anxiety-like behaviors with an HFD (60% kcal), but did find alterations in memory, and also found decreased anxiety-like behaviors with a high-sucrose diet (70% kcal) (202). Support for the role of the gut microbiota as a mediator of any behavioral changes with an HFD comes from a study where a fecal transplant from mice fed an HFD (60% kcal fat) into mice with antibiotic-depleted microbiota (fed a normal diet, 13% kcal fat) increased anxiety-like behaviors (229).
Dietary compounds also interact with each other and may offset their individual effects. Increasing dietary carbohydrate, particularly prebiotic compounds, may reduce some of the protein fermentation products because carbohydrate is a preferred substrate (214). Increased inflammation and endotoxemia in mice caused by an HFD were mitigated by polyphenol supplementation or polyphenol-rich plant extracts (215, 230–233). The polyphenol supplementation was also associated with differences in the gut bacteria, including an increase in Firmicutes and Verrucomicrobia (231), Akkermansia spp. (231, 233), Faecalibacterium (233), and Lachnospiraceae, specifically Coprococcus (215), and a decrease in Bacteroidetes (231) or microbial diversity (232). The fat content of these diets varied from 26% kcal (231) and 45% kcal (231) to 60% kcal (232). The fat content in the control diets was low at 0% (231) and 4.1% (232).
Food additives
Western diets include a high proportion of processed foods containing food additives to improve attributes such as shelf life, texture, and palatability. Studies in mice showed that emulsifiers can alter the gut microbiota composition (234, 205), increase the proinflammatory potential of the gut microbiota (234), increase microbiota infiltration of the gut mucosa layer (234), and alter anxiety-like behavior (205). Salt is another food additive that tends to be in high concentration in processed foods and Seck et al. (235) found that high fecal salinity alters gut microbe composition, including a decrease in the beneficial bacteria Akkermansia muciniphila and Bifidobacterium spp., specifically B. longum and B. adolescentis. Maltodextrin reduces mucus production and increases gut inflammation by increasing endoplasmic reticulum stress (236). The links between a Western diet and depression may include an effect of food additives on the gut microbiota. Evidence from human studies is needed.
Other considerations
Fermented foods typically contain strains of Lactobacillus as well as yeasts, and are likely to be important because they contain both probiotic microbiota and microbial metabolites. Most studies investigating the effect of fermented foods on the gut microbiota or mood have been undertaken using commercially produced yoghurts with very specific microbiota and fall more into the category of supplements than diet, so are not discussed here. However, there is huge scope for research into fermented drinks such as wine and kombucha, or foods such as breads, sauerkraut, kimchi, and yoghurt, and their effect on the gut microbiota and mood.
Individual variation in response to dietary changes needs to be considered. Differences in physiology and baseline microbiota composition may affect how the gut microbiota responds to dietary changes, or to disruption such as during stress or antibiotic treatment. For example, a high (palmitic) fat diet in mice increased Actinobacteria and Firmicutes, with a decrease in Bacteroides and Proteobacteria, as well as increasing gut permeability and systemic inflammation in mice that became obese. In contrast, the mice that were resistant to becoming obese with the same diet did not have the same changes in microbiota, or systemic effects (220). Age is a factor that also needs to be taken into account. Dietary changes may be more effective, at least in the short term, in younger subjects. Of a group of urban dwellers who spent 16 d living in a rainforest village and consuming the village diet, the change in gut microbiota composition was greater in the children (237). Because gut microbiota composition and stability change with age, different interventions may be more effective at different life stages or, conversely, disruption to microbiota may have more of an impact at different ages.
Childhood adversity increases susceptibility to developing mood disorders later in life, and episodes of major depressive disorder are commonly preceded by psychosocial stress (238, 239). Stress also alters the gut microbiota (240–244), and the effects of early-life stress on microbiota may extend to adulthood (245). It is therefore plausible that changes in microbiota due to stress at least partly mediate the onset of stress-related depressive or anxious episodes. Dietary intervention during or after stress is a promising area of research (241, 246).
Determining which factors help keep a healthy microbiota composition stable or help correct a dysbiosis may be beneficial. The low–Fermentable Oligosaccharides, Disaccharides, Monosaccharides, And Polyols (FODMAP) diet has a reduced amount of fermentable substrate compared with the amounts in normal diets, and can alleviate gut symptoms such as pain and bloating in those with irritable bowel syndrome, because of reduced gas production by gut microbiota (247). The diet is followed strictly for 2–6 wk until gut symptoms resolve, and foods are then reintroduced to determine individual tolerances. Because of the link between irritable bowel syndrome and depression/anxiety, it is plausible that the low-FODMAP diet could correct a dysbiosis related to altered mood. Initial research findings suggest that for those with irritable bowel syndrome symptoms, a low-FODMAP diet may reduce symptoms of anxiety (248). However, the low-FODMAP diet reduces total bacterial abundance, which may lower the production of bacterial metabolites such as SCFAs that are important in maintaining gut homeostasis. A decrease in Bifidobacteria and other beneficial bacteria may also occur (247, 249). The diet is designed as a temporary elimination diet for irritable bowel syndrome and adhering to it long-term is likely to compromise nutritional status. Long-term effects on the microbiota profile are unknown.
There is some evidence that altered gut motility may be associated with mood (250) and that the gut microbiota composition is altered by changes in motility and vice versa (251). Foods that directly affect factors such as gut motility, e.g., those containing soluble or insoluble fiber, may also be able to affect mood by correcting problems with motility, which could be a confounding variable or could be related to changes in the gut microbiota. More research in this area is needed, and it would be useful for food intervention studies to measure changes in gut function concurrently with assessing changes in mood. Other cofactors usually considered in depression research, such as exercise and sleep, also have independent impacts on microbiota (252, 253) and should be considered when assessing relations between foods, mood, and the microbiome.
Conclusions
Research shows that there is a link between diet and depression but conflicting results and limited research mean that we do not yet understand the nature of the relation. There is likely to be a bidirectional relation and it may be of more importance in vulnerable individuals. Because diet is a large influencer of the gut microbiota composition and function, it is likely that changes in the gut microbiota contribute to how diet (whole diet and individual components of diet) may affect depression and anxiety. Limited research in this area is sometimes contradictory and mostly in rodents but does show a pattern of results indicating that the gut microbiota may play a significant role and should be considered in dietary intervention studies. Dietary patterns for positive mental health will likely support the growth of commensal microbiota, decrease the growth of pathogenic and colitis-inducing bacteria, and affect gut barrier permeability and inflammation. In addition, because a change in whole dietary patterns changes the ratio of many dietary components, investigation into these individual components is also important. In dietary studies for depression and anxiety, types and amounts of dietary components (e.g., fat, prebiotics) within the dietary patterns should be identified.
Although examining the changes in microbial profile is interesting, it is important to remember that it is the collective function and characteristics of the gut microbiota that interact with the host, and that >1 microbe can occupy a particular ecological niche within their environment. Therefore, similar functions can be carried out by different microbiota structures and the same functional outcome could occur with different changes in microbiota. This particularly supports food as an effective intervention, because it can shift the microbial profile at all taxonomic levels and may also affect composition and function separately. The type and strength of the effect of diet on the gut microbiota will be determined by existing microbiota composition and function and the host phenotype, including interactions with immune function. Research needs to include examination of the gut microbiota function as well, using metabolomics and/or metagenomics techniques.
Research, in both humans and animals, into mechanisms of the MGBA will continue to help to elucidate the mechanisms by which the gut microbiota affect depression and anxiety symptoms, as well as other psychological and neurological effects. Food interventions have the dual benefit of a direct impact on gut and brain physiology and an indirect effect via the gut microbiota. With continued research investigating these aspects of the MGBA, we will further our understanding and make advances in obtaining a well-understood and well-guided holistic approach to treating and preventing anxiety and depression.
ACKNOWLEDGEMENTS
All authors read and approved the final manuscript.
Notes
Supported by the Riddet Institute, a New Zealand Centre of Research Excellence, funded by the Tertiary Education Commission (to TLKB).
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: AA, arachidonic acid; FA, fatty acid; FFAR, free fatty acid receptor; FODMAP, Fermentable Oligosaccharides, Disaccharides, Monosaccharides, And Polyols; FOS, fructooligosaccharide; GABA, γ-aminobutyric acid; GF, germ-free; GOS, galactooligosaccharide; HFD, high-fat diet; HPA, hypothalamic–pituitary–adrenal; MGBA, microbiome–gut–brain axis; PDX, polydextrose; SPF, specific pathogen–free.
Contributor Information
Tracey L K Bear, School of Food and Advanced Technology, Massey University, Palmerston North, New Zealand; Riddet Institute, Massey University, Palmerston North, New Zealand; The New Zealand Institute for Plant and Food Research Limited, Palmerston North, New Zealand.
Julie E Dalziel, Riddet Institute, Massey University, Palmerston North, New Zealand; AgResearch Ltd Grasslands Research Centre, Palmerston North, New Zealand.
Jane Coad, School of Food and Advanced Technology, Massey University, Palmerston North, New Zealand.
Nicole C Roy, Riddet Institute, Massey University, Palmerston North, New Zealand; AgResearch Ltd Grasslands Research Centre, Palmerston North, New Zealand; High-Value Nutrition National Science Challenge, Auckland, New Zealand.
Christine A Butts, The New Zealand Institute for Plant and Food Research Limited, Palmerston North, New Zealand.
Pramod K Gopal, Riddet Institute, Massey University, Palmerston North, New Zealand; The New Zealand Institute for Plant and Food Research Limited, Palmerston North, New Zealand.
References
- 1. Somers JM, Goldner EM, Waraich P, Hsu L. Prevalence and incidence studies of anxiety disorders: a systematic review of the literature. Can J Psychiatry. 2006;51(2):100–13. [DOI] [PubMed] [Google Scholar]
- 2. Lépine J-P, Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat. 2011;7(Suppl 1):3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baxter AJ, Vos T, Scott KM, Ferrari AJ, Whiteford HA. The global burden of anxiety disorders in 2010. Psychol Med. 2014;44(11):2363–74. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Depression fact sheet. [Internet] Geneva: World Health Organization; 2016. [Accessed 2019 Jan 13]. Available from:www.who.int/mediacentre/factsheets/fs369/en/. [Google Scholar]
- 5. DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depressions: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9(10):788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marquez PV, Saxena S. Making mental health a global priority. Cerebrum. 2016:cer–10-16. [PMC free article] [PubMed] [Google Scholar]
- 7. McLaughlin KA. The public health impact of major depression: a call for interdisciplinary prevention efforts. Prev Sci. 2011;12(4):361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oriach CS, Robertson RC, Stanton C, Cryan JF, Dinan TG. Food for thought: the role of nutrition in the microbiota-gut–brain axis. Clin Nutr Exp. 2016;6:25–38. [Google Scholar]
- 9. Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. 2014;99(1):181–97. [DOI] [PubMed] [Google Scholar]
- 10. Ford PA, Jaceldo-Siegl K, Lee JW, Youngberg W, Tonstad S. Intake of Mediterranean foods associated with positive affect and low negative affect. J Psychosom Res. 2013;74(2):142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crichton GE, Bryan J, Hodgson JM, Murphy KJ. Mediterranean diet adherence and self-reported psychological functioning in an Australian sample. Appetite. 2013;70:53–9. [DOI] [PubMed] [Google Scholar]
- 12. Suzuki T, Miyaki K, Tsutsumi A, Hashimoto H, Kawakami N, Takahashi M, Shimazu A, Inoue A, Kurioka S, Kakehashi M et al.. Japanese dietary pattern consistently relates to low depressive symptoms and it is modified by job strain and worksite supports. J Affect Disord. 2013;150(2):490–8. [DOI] [PubMed] [Google Scholar]
- 13. Nanri A, Kimura Y, Matsushita Y, Ohta M, Sato M, Mishima N, Sasaki S, Mizoue T. Dietary patterns and depressive symptoms among Japanese men and women. Eur J Clin Nutr. 2010;64(8):832–9. [DOI] [PubMed] [Google Scholar]
- 14. Jacka FN, Mykletun A, Berk M, Bjelland I, Tell GS. The association between habitual diet quality and the common mental disorders in community-dwelling adults: the Hordaland Health study. Psychosom Med. 2011;73(6):483–90. [DOI] [PubMed] [Google Scholar]
- 15. Ruusunen A, Lehto SM, Mursu J, Tolmunen T, Tuomainen TP, Kauhanen J. Dietary patterns are associated with the prevalence of elevated depressive symptoms and the risk of getting a hospital discharge diagnosis of depression in middle-aged or older Finnish men. J Affect Disord. 2014;159:1–6. [DOI] [PubMed] [Google Scholar]
- 16. Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br J Psychiatry. 2009;195(5):408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sugawara N, Yasui-Furukori N, Tsuchimine S, Kaneda A, Tsuruga K, Iwane K, Okubo N, Takahashi I, Kaneko S. No association between dietary patterns and depressive symptoms among a community-dwelling population in Japan. Ann Gen Psychiatry. 2012;11(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Niu K, Guo H, Kakizaki M, Cui Y, Ohmori-Matsuda K, Guan L, Hozawa A, Kuriyama S, Tsuboya T, Ohrui T et al.. A tomato-rich diet is related to depressive symptoms among an elderly population aged 70 years and over: a population-based, cross-sectional analysis. J Affect Disord. 2013;144(1–2):165–70. [DOI] [PubMed] [Google Scholar]
- 19. O'Neil A, Quirk SE, Housden S, Brennan SL, Williams LJ, Pasco JA, Berk M, Jacka FN. Relationship between diet and mental health in children and adolescents: a systematic review. Am J Public Health. 2014;104(10):e31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chocano-Bedoya PO, O'Reilly EJ, Lucas M, Mirzaei F, Okereke OI, Fung TT, Hu FB, Ascherio A. Prospective study on long-term dietary patterns and incident depression in middle-aged and older women. Am J Clin Nutr. 2013;98(3):813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanchez-Villegas A, Martínez-González MA. Diet, a new target to prevent depression?. BMC Med. 2013;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psych Bull. 1995;117(2):285–305. [DOI] [PubMed] [Google Scholar]
- 23. Heath TP, Melichar JK, Nutt DJ, Donaldson LF. Human taste thresholds are modulated by serotonin and noradrenaline. J Neurosci. 2006;26(49):12664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Platte P, Herbert C, Pauli P, Breslin PA. Oral perceptions of fat and taste stimuli are modulated by affect and mood induction. PLoS One. 2013;8(6):e65006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noel C, Dando R. The effect of emotional state on taste perception. Appetite. 2015;95:89–95. [DOI] [PubMed] [Google Scholar]
- 26. Ouwens MA, van Strien T, van Leeuwe JFJ. Possible pathways between depression, emotional and external eating. A structural equation model. Appetite. 2009;53(2):245–8. [DOI] [PubMed] [Google Scholar]
- 27. Lang UE, Beglinger C, Schweinfurth N, Walter M, Borgwardt S. Nutritional aspects of depression. Cell Physiol Biochem. 2015;37(3):1029–43. [DOI] [PubMed] [Google Scholar]
- 28. Frost RO, Goolkasian GA, Ely RJ, Blanchard FA. Depression, restraint and eating behavior. Behav Res Ther. 1982;20(2):113–21. [DOI] [PubMed] [Google Scholar]
- 29. Le Port A, Gueguen A, Kesse-Guyot E, Melchior M, Lemogne C, Nabi H, Goldberg M, Zins M, Czernichow S. Association between dietary patterns and depressive symptoms over time: a 10-year follow-up study of the GAZEL cohort. PLoS One. 2012;7(12):e51593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatr. 2009;65(9):732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pirotta M, Densley K, Forsdike K, Carter M, Gunn J. St John's wort use in Australian general practice patients with depressive symptoms: their characteristics and use of other health services. BMC Complement Altern Med. 2014;14(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Molendijk M, Molero P, Ortuño Sánchez-Pedreño F, Van der Does W, Angel Martínez-González M. Diet quality and depression risk: a systematic review and dose-response meta-analysis of prospective studies. J Affect Disord. 2018;226:346–54. [DOI] [PubMed] [Google Scholar]
- 33. Opie RS, O'Neil A, Itsiopoulos C, Jacka FN. The impact of whole-of-diet interventions on depression and anxiety: a systematic review of randomised controlled trials. Public Health Nutr. 2015;18(11):2074–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomson CD, Robinson MF. Selenium in human health and disease with emphasis on those aspects peculiar to New Zealand. Am J Clin Nutr. 1980;33(2):303–23. [DOI] [PubMed] [Google Scholar]
- 35. American Psychiatric Association. Depressive disorders. In: Diagnostic and statistical manual of mental disorders. 5th ed Washington (DC): American Psychiatric Association; 2013. [Google Scholar]
- 36. Willner P, Scheel-Kruger J, Belzung C. The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev. 2013;37(10 Pt 1):2331–71. [DOI] [PubMed] [Google Scholar]
- 37. Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psych. 2007;12(4):331–59. [DOI] [PubMed] [Google Scholar]
- 38. Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–56. [DOI] [PubMed] [Google Scholar]
- 39. Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342–5. [DOI] [PubMed] [Google Scholar]
- 40. Arborelius L, Owens MJ, Nemeroff CB, Plotsky PM. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrin. 1999;160(1):1–12. [DOI] [PubMed] [Google Scholar]
- 41. Song C, Earley B, Leonard BE. Behavioral, neurochemical, and immunological responses to CRF administration. Is CRF a mediator of stress?. Ann N Y Acad Sci. 1995;771(1):55–72. [DOI] [PubMed] [Google Scholar]
- 42. Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N et al.. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135(5):373–87. [DOI] [PubMed] [Google Scholar]
- 43. Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein H-G, Sarnyai Z, Mawrin C, Brisch R, Bielau H, zu Schwabedissen LM et al.. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission?. J Neuroinflamm. 2011;8(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Müller N, Schwarz M. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psych. 2007;12:988–1000. [DOI] [PubMed] [Google Scholar]
- 45. Raison CL, Miller AH. Is depression an inflammatory disorder?. Curr Psychiatr Rep. 2011;13(6):467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Park AJ, Collins J, Blennerhassett PA, Ghia JE, Verdu EF, Bercik P, Collins SM. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil. 2013;25(9):733–e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang R, Asai M, Mahoney CE, Joachim M, Shen Y, Gunner G, Majzoub JA. Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Mol Psychiatry. 2017;22(5):733–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martin-Subero M, Anderson G, Kanchanatawan B, Berk M, Maes M. Comorbidity between depression and inflammatory bowel disease explained by immune-inflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gut–brain pathways. CNS Spectr. 2016;21(2):184–98. [DOI] [PubMed] [Google Scholar]
- 49. Chambers AS, Allen JJB. Vagal tone as an indicator of treatment response in major depression. Psychophysiology. 2002;39(6):861–4. [DOI] [PubMed] [Google Scholar]
- 50. Jayatissa MN, Bisgaard CF, West MJ, Wiborg O. The number of granule cells in rat hippocampus is reduced after chronic mild stress and re-established after chronic escitalopram treatment. Neuropharmacology. 2008;54(3):530–41. [DOI] [PubMed] [Google Scholar]
- 51. Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11):1957–66. [DOI] [PubMed] [Google Scholar]
- 52. Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ghosal S, Hare B, Duman RS. Prefrontal cortex GABAergic deficits and circuit dysfunction in the pathophysiology and treatment of chronic stress and depression. Curr Opin Behav Sci. 2017;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim J, Schmid-Burgk W, Claus D, Kornhuber H. Increased serum glutamate in depressed patients. Arch Psychiatr Nervenkr (1970). 1982;232(4):299–304. [DOI] [PubMed] [Google Scholar]
- 55. Myint A-M, Kim YK, Verkerk R, Scharpé S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord. 2007;98(1–2):143–51. [DOI] [PubMed] [Google Scholar]
- 56. O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2008;14(5):511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gougeon L, Payette H, Morais JA, Gaudreau P, Shatenstein B, Gray-Donald K. Intakes of folate, vitamin B6 and B12 and risk of depression in community-dwelling older adults: the Quebec Longitudinal Study on Nutrition and Aging. Eur J Clin Nutr. 2016;70(3):380–5. [DOI] [PubMed] [Google Scholar]
- 58. Jacka FN, Maes M, Pasco JA, Williams LJ, Berk M. Nutrient intakes and the common mental disorders in women. J Affect Disord. 2012;141(1):79–85. [DOI] [PubMed] [Google Scholar]
- 59. Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100–7. [DOI] [PubMed] [Google Scholar]
- 60. Vulser H, Wiernik E, Hoertel N, Thomas F, Pannier B, Czernichow S, Hanon O, Simon T, Simon JM, Danchin N et al.. Association between depression and anemia in otherwise healthy adults. Acta Psychiatr Scand. 2016;134(2):150–60. [DOI] [PubMed] [Google Scholar]
- 61. Alpert JE, Fava M. Nutrition and depression: the role of folate. Nutr Rev. 1997;55(5):145–9. [DOI] [PubMed] [Google Scholar]
- 62. Wang J, Um P, Dickerman BA, Liu J. Zinc, magnesium, selenium and depression: a review of the evidence, potential mechanisms and implications. Nutrients. 2018;10(5):584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharm. 2005;19(1):59–65. [DOI] [PubMed] [Google Scholar]
- 64. Paul RTP, McDonnell AP, Kelly CB. Folic acid: neurochemistry, metabolism and relationship to depression. Hum Psychopharmacol. 2004;19(7):477–88. [DOI] [PubMed] [Google Scholar]
- 65. Spedding S. Vitamin D and depression: a systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients. 2014;6(4):1501–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Partonen T. Vitamin D and serotonin in winter. Med Hypotheses. 1998;51(3):267–8. [DOI] [PubMed] [Google Scholar]
- 67. Hartvig P, Lindner KJ, Bjurling P, Långström B, Tedroff J. Pyridoxine effect on synthesis rate of serotonin in the monkey brain measured with positron emission tomography. J Neural Transm. 1995;102(2):91–7. [DOI] [PubMed] [Google Scholar]
- 68. Dakshinamurti K, Sharma SK, Bonke D. Influence of B vitamins on binding properties of serotonin receptors in the CNS of rats. Klin Wochenschr. 1990;68(2):142–5. [DOI] [PubMed] [Google Scholar]
- 69. Rybka J, Kedziora-Kornatowska K, Banas-Lezanska P, Majsterek I, Carvalho LA, Cattaneo A, Anacker C, Kedziora J. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic Biol Med. 2013;63:187–94. [DOI] [PubMed] [Google Scholar]
- 70. Bouayed J. Polyphenols: a potential new strategy for the prevention and treatment of anxiety and depression. Curr Nutr Food Sci. 2010;6(1):13–8. [Google Scholar]
- 71. Hurley LL, Akinfiresoye L, Kalejaiye O, Tizabi Y. Antidepressant effects of resveratrol in an animal model of depression. Behav Brain Res. 2014;268:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chandrasekhar Y, Ramya EM, Navya K, Phani Kumar G, Anilakumar KR. Antidepressant like effects of hydrolysable tannins of Terminalia catappa leaf extract via modulation of hippocampal plasticity and regulation of monoamine neurotransmitters subjected to chronic mild stress (CMS). Biomed Pharmacother. 2017;86:414–25. [DOI] [PubMed] [Google Scholar]
- 73. Zhang M, Robitaille L, Eintracht S, Hoffer LJ. Vitamin C provision improves mood in acutely hospitalized patients. Nutrition. 2011;27(5):530–3. [DOI] [PubMed] [Google Scholar]
- 74. Harauma A, Hatanaka E, Yasuda H, Nakamura MT, Salem JN, Moriguchi T. Effects of arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid on brain development using artificial rearing of delta-6-desaturase knockout mice. Prostaglandins Leukot Essent Fatty Acids. 2017;127:32–9. [DOI] [PubMed] [Google Scholar]
- 75. Ibarguren M, López DJ, Escribá PV. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. BBA-Biomembranes. 2014;1838(6):1518–28. [DOI] [PubMed] [Google Scholar]
- 76. Kiecolt-Glaser JK, Belury MA, Porter K, Beversdorf DQ, Lemeshow S, Glaser R. Depressive symptoms, omega-6:omega-3 fatty acids, and inflammation in older adults. Psychosom Med. 2007;69(3):217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J Neurochem. 2007;101(3):577–99. [DOI] [PubMed] [Google Scholar]
- 78. Estruch R. Anti-inflammatory effects of the Mediterranean diet: the experience of the PREDIMED study. Proc Nutr Soc. 2010;69(3):333–40. [DOI] [PubMed] [Google Scholar]
- 79. Kurina L, Goldacre M, Yeates D, Gill L. Depression and anxiety in people with inflammatory bowel disease. J Epidemiol Community Health. 2001;55(10):716–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Addolorato G, Capristo E, Stefanini GF, Gasbarrini G. Inflammatory bowel disease: a study of the association between anxiety and depression, physical morbidity, and nutritional status. Scand J Gastroenterol. 1997;32(10):1013–21. [DOI] [PubMed] [Google Scholar]
- 81. Lydiard RB. Irritable bowel syndrome, anxiety, and depression: what are the links?. J Clin Psychiatry. 2001;62(Suppl 8):38–47. [PubMed] [Google Scholar]
- 82. Masand PS, Kaplan DS, Gupta S, Bhandary AN, Nasra GS, Kline MD, Margo KL. Major depression and irritable bowel syndrome: is there a relationship?. J Clin Psychiatry. 1995;56(8):363–7. [PubMed] [Google Scholar]
- 83. Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, Rudi K. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26(8):1155–62. [DOI] [PubMed] [Google Scholar]
- 84. Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J et al.. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–94. [DOI] [PubMed] [Google Scholar]
- 85. Jiang H-Y, Zhang X, Yu Z-H, Zhang Z, Deng M, Zhao J-H, Ruan B. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130–6. [DOI] [PubMed] [Google Scholar]
- 86. Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav. 2006;89(3):350–7. [DOI] [PubMed] [Google Scholar]
- 87. Lyte M, Varcoe JJ, Bailey MT. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiol Behav. 1998;65(1):63–8. [DOI] [PubMed] [Google Scholar]
- 88. Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA et al.. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139(6):2102–12..e1. [DOI] [PubMed] [Google Scholar]
- 89. Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Dauge V, Naudon L, Rabot S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–17. [DOI] [PubMed] [Google Scholar]
- 90. Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psych. 2013;18(6):666–73. [DOI] [PubMed] [Google Scholar]
- 91. Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23(3):255–e119. [DOI] [PubMed] [Google Scholar]
- 92. Nishino R, Mikami K, Takahashi H, Tomonaga S, Furuse M, Hiramoto T, Aiba Y, Koga Y, Sudo N. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol Motil. 2013;25(6):521–e371. [DOI] [PubMed] [Google Scholar]
- 93. Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609..e3. [DOI] [PubMed] [Google Scholar]
- 95. Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, Logan AC. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson J-F, Rougeot C, Pichelin M, Cazaubiel M et al.. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105(5):755–64. [DOI] [PubMed] [Google Scholar]
- 97. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–77. [DOI] [PubMed] [Google Scholar]
- 99. Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacteriuminfantis in the maternal separation model of depression. Neuroscience. 2010;170(4):1179–88. [DOI] [PubMed] [Google Scholar]
- 100. Smith CJ, Emge JR, Berzins K, Lung L, Khamishon R, Shah P, Rodrigues DM, Sousa AJ, Reardon C, Sherman PM. Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am J Physiol Gastrointest Liver Physiol. 2014;307(8):G793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Anglin R, Surette M, Moayyedi P, Bercik P. Lost in translation: the gut microbiota in psychiatric illness. Can J Psychiatry. 2015;60(10):460–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu X-N, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Leclercq S, Mian FM, Stanisz AM, Bindels LB, Cambier E, Ben-Amram H, Koren O, Forsythe P, Bienenstock J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. 2017;8:15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Degroote S, Hunting DJ, Baccarelli AA, Takser L. Maternal gut and fetal brain connection: increased anxiety and reduced social interactions in Wistar rat offspring following peri-conceptional antibiotic exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zijlmans MAC, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–45. [DOI] [PubMed] [Google Scholar]
- 106. Tormo-Badia N, Håkansson Å, Vasudevan K, Molin G, Ahrné S, Cilio CM. Antibiotic treatment of pregnant non-obese diabetic mice leads to altered gut microbiota and intestinal immunological changes in the offspring. Scand J Immunol. 2014;80(4):250–60. [DOI] [PubMed] [Google Scholar]
- 107. Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164–74. [DOI] [PubMed] [Google Scholar]
- 108. Kelly JR, Allen AP, Temko A, Hutch W, Kennedy PJ, Farid N, Murphy E, Boylan G, Bienenstock J, Cryan JF et al.. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun. 2017;61:50–9. [DOI] [PubMed] [Google Scholar]
- 109. D'Mello C, Ronaghan N, Zaheer R, Dicay M, Le T, MacNaughton WK, Surrette MG, Swain MG. Probiotics improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain. J Neurosci. 2015;35(30):10821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37(11):1885–95. [DOI] [PubMed] [Google Scholar]
- 111. Rajkumar H, Kumar M, Das N, Kumar SN, Challa HR, Nagpal R. Effect of probiotic Lactobacillussalivarius UBL S22 and prebiotic fructo-oligosaccharide on serum lipids, inflammatory markers, insulin sensitivity, and gut bacteria in healthy young volunteers: a randomized controlled single-blind pilot study. J Cardiovasc Pharmacol Ther. 2015;20(3):289–98. [DOI] [PubMed] [Google Scholar]
- 112. Desbonnet L, Clarke G, Traplin A, O'Sullivan O, Crispie F, Moloney RD, Cotter PD, Dinan TG, Cryan JF. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun. 2015;48:165–73. [DOI] [PubMed] [Google Scholar]
- 113. Kelly JR, Borre Y, O'Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–18. [DOI] [PubMed] [Google Scholar]
- 114. Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Bäckhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Mazmanian SK, Hsiao EY, Ma L, Ismagilov RF, Nagler CR. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P, Windey K, Tremaroli V, Backhed F, Verbeke K et al.. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111(42):E4485–E93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Griffiths EA, Duffy LC, Schanbacher FL, Qiao H, Dryja D, Leavens A, Rossman J, Rich G, Dirienzo D, Ogra PL. In vivo effects of bifidobacteria and lactoferrin on gut endotoxin concentration and mucosal immunity in Balb/c mice. Dig Dis Sci. 2004;49(4):579–89. [DOI] [PubMed] [Google Scholar]
- 118. Wang Z, Xiao G, Yao Y, Guo S, Lu K, Sheng Z. The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma Acute Care Surg. 2006;61(3):650–7. [DOI] [PubMed] [Google Scholar]
- 119. Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D et al.. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol Motil. 2011;23(12):1132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang X, Wang B-R, Zhang X-J, Xu Z, Ding Y-Q, Ju G. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J Gastroenterol. 2002;8(3):540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tanida M, Yamano T, Maeda K, Okumura N, Fukushima Y, Nagai K. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci Lett. 2005;389(2):109–14. [DOI] [PubMed] [Google Scholar]
- 122. Tsavkelova E, Klimova SY, Cherdyntseva T, Netrusov A. Hormones and hormone-like substances of microorganisms: a review. Appl Biochem Microbiol. 2006;42(3):229–35. [PubMed] [Google Scholar]
- 123. Roshchina VV. Evolutionary considerations of neurotransmitters in microbial, plant, and animal cells. In: Lyte M, Freestone PPE, editors. Microbial endocrinology: interkingdom signaling in infectious disease and health. Cham (Switzerland): Springer; 2010. pp. 17–52. [Google Scholar]
- 124. Ross RP, Mills S, Hill C, Fitzgerald GF, Stanton C. Specific metabolite production by gut microbiota as a basis for probiotic function. Int Dairy J. 2010;20(4):269–76. [Google Scholar]
- 125. Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574–81. [DOI] [PubMed] [Google Scholar]
- 126. Holzer P, Farzi A. Neuropeptides and the microbiota-gut-brain axis. In: Lyte M, Cryan JF, editors. Microbial endocrinology: the microbiota-gut-brain axis in health and disease. New York: Springer; 2014. pp. 195–219. [Google Scholar]
- 127. Bjurstöm H, Wang J, Ericsson I, Bengtsson M, Liu Y, Kumar-Mendu S, Issazadeh-Navikas S, Birnir B. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol. 2008;205(1–2):44–50. [DOI] [PubMed] [Google Scholar]
- 128. de Jonge WJ. The gut's little brain in control of intestinal immunity. ISRN Gastroenterol. 2013:630159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad Puttur D, Manicassamy S, Munn David H et al.. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis?. Neurochem Int. 2016;99:110–32. [DOI] [PubMed] [Google Scholar]
- 131. Reigstad CS, Salmonson CE, Rainey JF, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29(4):1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284(5):R1269–76. [DOI] [PubMed] [Google Scholar]
- 133. Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T et al.. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D et al.. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hill M. Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev. 1997;6(2):S43–5. [DOI] [PubMed] [Google Scholar]
- 136. Burgess CM, Smid EJ, van Sinderen D. Bacterial vitamin B2, B11 and B12 overproduction: an overview. Int J Food Microbiol. 2009;133(1–2):1–7. [DOI] [PubMed] [Google Scholar]
- 137. Sumi Y, Miyakawa M, Kanzaki M, Kotake Y. Vitamin B-6 deficiency in germfree rats. J Nutr. 1977;107(9):1707–14. [DOI] [PubMed] [Google Scholar]
- 138. LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotech. 2013;24:160–8. [DOI] [PubMed] [Google Scholar]
- 139. Rosenberg J, Ischebeck T, Commichau FM. Vitamin B6 metabolism in microbes and approaches for fermentative production. Biotechnol Adv. 2017;35(1):31–40. [DOI] [PubMed] [Google Scholar]
- 140. Agrawal S, Agrawal A, Said HM. Biotin deficiency enhances the inflammatory response of human dendritic cells. Am J Physiol Cell Physiol. 2016;311(3):C386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatr. 2000;69(2):228–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Owen RT. Folate augmentation of antidepressant response. Drugs Today (Barc). 2013;49(12):791–8. [DOI] [PubMed] [Google Scholar]
- 143. Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X et al.. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psych. 2016;21(6):786–96. [DOI] [PubMed] [Google Scholar]
- 144. Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C et al.. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019;4(4):623–32. [DOI] [PubMed] [Google Scholar]
- 145. Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, Ota M, Koga N, Hattori K, Kunugi H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord. 2016;202:254–7. [DOI] [PubMed] [Google Scholar]
- 146. The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Gow NAR, Yadav B. Microbe profile: Candida albicans: a shape-changing, opportunistic pathogenic fungus of humans. Microbiology. 2017;163(8):1145–7. [DOI] [PubMed] [Google Scholar]
- 148. Gutiérrez-Díaz I, Fernández-Navarro T, Salazar N, Bartolomé B, Moreno-Arribas MV, de Andres-Galiana EJ, Fernández-Martínez JL, de los Reyes-Gavilán CG, Gueimonde M, González S. Adherence to a Mediterranean diet influences the fecal metabolic profile of microbial-derived phenolics in a Spanish cohort of middle-age and older people. J Agric Food Chem. 2017;65(3):586–95. [DOI] [PubMed] [Google Scholar]
- 149. Mitsou EK, Kakali A, Antonopoulou S, Mountzouris KC, Yannakoulia M, Panagiotakos DB, Kyriacou A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br J Nutr. 2017;117(12):1645–55. [DOI] [PubMed] [Google Scholar]
- 150. Kim M-S, Hwang S-S, Park E-J, Bae J-W. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ Microbiol Rep. 2013;5(5):765–75. [DOI] [PubMed] [Google Scholar]
- 151. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA et al.. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2013;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Matijašić BB, Obermajer T, Lipoglavšek L, Grabnar I, Avguštin G, Rogelj I. Association of dietary type with fecal microbiota in vegetarians and omnivores in Slovenia. Eur J Nutr. 2014;53(4):1051–64. [DOI] [PubMed] [Google Scholar]
- 153. Fava F, Gitau R, Griffin BA, Gibson GR, Tuohy KM, Lovegrove JA. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int J Obes. 2013;37:216–23. [DOI] [PubMed] [Google Scholar]
- 154. Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O'Reilly SL, Nicholson GC, Kotowicz MA, Berk M. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167(3):305–11. [DOI] [PubMed] [Google Scholar]
- 155. Chatzi L, Melaki V, Sarri K, Apostolaki I, Roumeliotaki T, Georgiou V, Vassilaki M, Koutis A, Bitsios P, Kogevinas M. Dietary patterns during pregnancy and the risk of postpartum depression: the mother–child ‘Rhea’ cohort in Crete, Greece. Public Health Nutr. 2011;14(9):1663–70. [DOI] [PubMed] [Google Scholar]
- 156. Mamplekou E, Bountziouka V, Psaltopoulou T, Zeimbekis A, Tsakoundakis N, Papaerakleous N, Gotsis E, Metallinos G, Pounis G, Polychronopoulos E et al.. Urban environment, physical inactivity and unhealthy dietary habits correlate to depression among elderly living in eastern Mediterranean islands: the MEDIS (MEDiterranean ISlands Elderly) study. J Nutr Health Aging. 2010;14(6):449–55. [DOI] [PubMed] [Google Scholar]
- 157. Rienks J, Dobson AJ, Mishra GD. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: results from a large community-based prospective study. Eur J Clin Nutr. 2013;67(1):75–82. [DOI] [PubMed] [Google Scholar]
- 158. Lucas M, Chocano-Bedoya P, Schulze MB, Mirzaei F, O'Reilly ÉJ, Okereke OI, Hu FB, Willett WC, Ascherio A. Inflammatory dietary pattern and risk of depression among women. Brain Behav Immun. 2014;36:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351(9110):1213. [DOI] [PubMed] [Google Scholar]
- 160. Tanskanen A, Hibbeln JR, Tuomilehto J, Uutela A, Haukkala A, Viinamäki H, Lehtonen J, Vartiainen E. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv. 2001;52(4):529–31. [DOI] [PubMed] [Google Scholar]
- 161. Timonen M, Horrobin D, Jokelainen J, Laitinen J, Herva A, Räsänen P. Fish consumption and depression: the Northern Finland 1966 birth cohort study. J Affect Disord. 2004;82(3):447–52. [DOI] [PubMed] [Google Scholar]
- 162. Jacka FN, Pasco JA, Williams LJ, Meyer BJ, Digger R, Berk M. Dietary intake of fish and PUFA, and clinical depressive and anxiety disorders in women. Br J Nutr. 2013;109(11):2059–66. [DOI] [PubMed] [Google Scholar]
- 163. Lucas M, Mirzaei F, O'Reilly EJ, Pan A, Willett WC, Kawachi I, Koenen K, Ascherio A. Dietary intake of n−3 and n−6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. Am J Clin Nutr. 2011;93(6):1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Strøm M, Mortensen EL, Halldorsson TI, Thorsdottir I, Olsen SF. Fish and long-chain n-3 polyunsaturated fatty acid intakes during pregnancy and risk of postpartum depression: a prospective study based on a large national birth cohort. Am J Clin Nutr. 2009;90(1):149–55. [DOI] [PubMed] [Google Scholar]
- 165. Miyake Y, Sasaki S, Yokoyama T, Tanaka K, Ohya Y, Fukushima W, Saito K, Ohfuji S, Kiyohara C, Hirota Y. Risk of postpartum depression in relation to dietary fish and fat intake in Japan: the Osaka Maternal and Child Health Study. Psychol Med. 2006;36(12):1727–35. [DOI] [PubMed] [Google Scholar]
- 166. Browne JC, Scott KM, Silvers KM. Fish consumption in pregnancy and omega-3 status after birth are not associated with postnatal depression. J Affect Disord. 2006;90(2–3):131–9. [DOI] [PubMed] [Google Scholar]
- 167. Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids. 2005;72(3):211–8. [DOI] [PubMed] [Google Scholar]
- 168. Grenyer BFS, Crowe T, Meyer B, Owen AJ, Grigonis-Deane EM, Caputi P, Howe PRC. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1393–6. [DOI] [PubMed] [Google Scholar]
- 169. Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n−3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91(3):757–70. [DOI] [PubMed] [Google Scholar]
- 170. Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, Drago F, Caraci F. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One. 2014;9(5):e96905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48(2–3):149–55. [DOI] [PubMed] [Google Scholar]
- 172. Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatr. 1998;43(5):315–9. [DOI] [PubMed] [Google Scholar]
- 173. Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31(1Part2):S157–S61. [DOI] [PubMed] [Google Scholar]
- 174. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):676–92. [DOI] [PubMed] [Google Scholar]
- 176. Kaliannan K, Wang B, Li X-Y, Kim K-J, Kang JX. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci Rep. 2015;5:11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Pusceddu MM, El Aidy S, Crispie F, O'Sullivan O, Cotter P, Stanton C, Kelly P, Cryan JF, Dinan TG. N-3 polyunsaturated fatty acids (PUFAs) reverse the impact of early-life stress on the gut microbiota. PLoS One. 2015;10(10):e0139721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Robertson RC, Seira Oriach C, Murphy K, Moloney GM, Cryan JF, Dinan TG, Paul Ross R, Stanton C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav Immun. 2017;59:21–37. [DOI] [PubMed] [Google Scholar]
- 179. Winther G, Jorgensen BMP, Elfving B, Nielsen DS, Kihl P, Lund S, Sorensen DB, Wegener G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatrica. 2015;27(3):168–76. [DOI] [PubMed] [Google Scholar]
- 180. Schaffler H, Herlemann DP, Klinitzke P, Berlin P, Kreikemeyer B, Jaster R, Lamprecht G. Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn's disease patients, but not in healthy controls. J Dig Dis. 2018;19(4):225–34. [DOI] [PubMed] [Google Scholar]
- 181. Bashir M, Prietl B, Tauschmann M, Mautner S, Kump P, Treiber G, Wurm P, Gorkiewicz G, Högenauer C, Pieber T. Effects of high doses of vitamin D on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur J Nut. 2016;55(4):1479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Talsness CE, Penders J, Jansen EHJM, Damoiseaux J, Thijs C, Mommers M. Influence of vitamin D on key bacterial taxa in infant microbiota in the KOALA Birth Cohort Study. PLoS One. 2017;12(11):e0188011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Pase MP, Scholey AB, Pipingas A, Kras M, Nolidin K, Gibbs A, Wesnes K, Stough C. Cocoa polyphenols enhance positive mood states but not cognitive performance: a randomized, placebo-controlled trial. J Psychopharm. 2013;27(5):451–8. [DOI] [PubMed] [Google Scholar]
- 184. Massot-Cladera M, Pérez-Berezo T, Franch A, Castell M, Pérez-Cano FJ. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch Biochem Biophys. 2012;527(2):105–12. [DOI] [PubMed] [Google Scholar]
- 185. Fogliano V, Corollaro ML, Vitaglione P, Napolitano A, Ferracane R, Travaglia F, Arlorio M, Costabile A, Klinder A, Gibson G. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol Nutr Food Res. 2011;55(S1):S44–55. [DOI] [PubMed] [Google Scholar]
- 186. Khalid S, Barfoot KL, May G, Lamport DJ, Reynolds SA, Williams CM. Effects of acute blueberry flavonoids on mood in children and young adults. Nutrients. 2017;9(2):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Guo Q, Kim YN, Lee BH. Protective effects of blueberry drink on cognitive impairment induced by chronic mild stress in adult rats. Nutr Res Pract. 2017;11(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Mika A, Day HEW, Martinez A, Rumian NL, Greenwood BN, Chichlowski M, Berg BM, Fleshner M. Early life diets with prebiotics and bioactive milk fractions attenuate the impact of stress on learned helplessness behaviours and alter gene expression within neural circuits important for stress resistance. Eur J Neurosci. 2017;45(3):342–57. [DOI] [PubMed] [Google Scholar]
- 189. McVey Neufeld K-A, O'Mahony SM, Hoban AE, Waworuntu RV, Berg BM, Dinan TG, Cryan JF. Neurobehavioural effects of Lactobacillus rhamnosus GG alone and in combination with prebiotics polydextrose and galactooligosaccharide in male rats exposed to early-life stress. Nutr Neurosci. 2019;22(6):425–34. [DOI] [PubMed] [Google Scholar]
- 190. Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin Nutr. 2019;38(2):522–8. [DOI] [PubMed] [Google Scholar]
- 191. Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, Burnet PW. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl). 2015;232(10):1793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Vulevic J, Drakoularakou A, Yaqoob P, Tzortzis G, Gibson GR. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr. 2008;88(5):1438–46. [DOI] [PubMed] [Google Scholar]
- 193. Williams BA, Zhang D, Lisle AT, Mikkelsen D, McSweeney CS, Kang S, Bryden WL, Gidley MJ. Soluble arabinoxylan enhances large intestinal microbial health biomarkers in pigs fed a red meat–containing diet. Nutrition. 2016;32(4):491–7. [DOI] [PubMed] [Google Scholar]
- 194. Barbáchano A, Fernández-Barral A, Ferrer-Mayorga G, Costales-Carrera A, Larriba MJ, Muñoz A. The endocrine vitamin D system in the gut. Mol Cell Endocrinol. 2017;453:79–87. [DOI] [PubMed] [Google Scholar]
- 195. Amit-Romach E, Uni Z, Cheled S, Berkovich Z, Reifen R. Bacterial population and innate immunity-related genes in rat gastrointestinal tract are altered by vitamin A-deficient diet. J Nutr Biochem. 2009;20(1):70–7. [DOI] [PubMed] [Google Scholar]
- 196. Lv Z, Yang T, Li T, Chen J, Wang Y, Zhan X, Li Z, Hu H. Vitamin A deficiency impacts the structural segregation of gut microbiota in children with persistent diarrhea. J Clin Biochem Nutr. 2016;59(2):113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Kaliannan K, Wang B, Li X-Y, Bhan AK, Kang JX. Omega-3 fatty acids prevent early-life antibiotic exposure-induced gut microbiota dysbiosis and later-life obesity. Int J Obes. 2016;40(6):1039–42. [DOI] [PubMed] [Google Scholar]
- 198. Watson H, Mitra S, Croden FC, Taylor M, Wood HM, Perry SL, Spencer JA, Quirke P, Toogood GJ, Lawton CL et al.. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. 2018;67(11):1974–83. [DOI] [PubMed] [Google Scholar]
- 199. Daniel H, Gholami AM, Berry D, Desmarchelier C, Hahne H, Loh G, Mondot S, Lepage P, Rothballer M, Walker A et al.. High-fat diet alters gut microbiota physiology in mice. ISME J. 2013;8:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Fleissner CK, Huebel N, Abd El-Bary MM, Loh G, Klaus S, Blaut M. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br J Nutr. 2010;104(6):919–29. [DOI] [PubMed] [Google Scholar]
- 201. Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Pyndt Jørgensen B, Hansen JT, Krych L, Larsen C, Klein AB, Nielsen DS, Josefsen K, Hansen AK, Sørensen DB. A possible link between food and mood: dietary impact on gut microbiota and behavior in BALB/c mice. PLoS One. 2014;9(8):e103398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Foerster J, Maskarinec G, Reichardt N, Tett A, Narbad A, Blaut M, Boeing H. The influence of whole grain products and red meat on intestinal microbiota composition in normal weight adults: a randomized crossover intervention trial. PLoS One. 2014;9(10):e109606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Vanden Bussche J, Hemeryck LY, Van Hecke T, Kuhnle GGC, Pasmans F, Moore SA, Van de Wiele T, De Smet S, Vanhaecke L. O6-carboxymethylguanine DNA adduct formation and lipid peroxidation upon in vitro gastrointestinal digestion of haem-rich meat. Mol Nutr Food Res. 2014;58(9):1883–96. [DOI] [PubMed] [Google Scholar]
- 205. Holder MK, Peters NV, Whylings J, Fields CT, Gewirtz AT, Chassaing B, de Vries GJ. Dietary emulsifiers consumption alters anxiety-like and social-related behaviors in mice in a sex-dependent manner. Sci Rep. 2019;9(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Kortman GAM, Raffatellu M, Swinkels DW, Tjalsma H. Nutritional iron turned inside out: intestinal stress from a gut microbial perspective. FEMS Microbiol Rev. 2014;38(6):1202–34. [DOI] [PubMed] [Google Scholar]
- 207. Dostal A, Chassard C, Hilty FM, Zimmermann MB, Jaeggi T, Rossi S, Lacroix C. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J Nutr. 2011;142(2):271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD et al.. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. [DOI] [PubMed] [Google Scholar]
- 209. Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatr. 2017;82(7):472–87. [DOI] [PubMed] [Google Scholar]
- 210. Forsatkar MN, Nematollahi MA, Rafiee G, Farahmand H, Lawrence C. Effects of the prebiotic mannan-oligosaccharide on the stress response of feed deprived zebrafish (Danio rerio). Physiol Behav. 2017;180:70–7. [DOI] [PubMed] [Google Scholar]
- 211. Haghighat N, Rajabi S, Mohammadshahi M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: a randomized, double-blinded, clinical trial. Nutri Neurosci. 2019:1–10. [DOI] [PubMed] [Google Scholar]
- 212. Lyte M, Chapel A, Lyte JM, Ai Y, Proctor A, Jane JL, Phillips GJ. Resistant starch alters the microbiota-gut brain axis: implications for dietary modulation of behavior. PLoS One. 2016;11(1):e0146406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Dueñas M, Muñoz-González I, Cueva C, Jiménez-Girón A, Sánchez-Patán F, Santos-Buelga C, Moreno-Arribas MV, Bartolomé B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res Int. 2015:850902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacolog Res. 2013;69(1):52–60. [DOI] [PubMed] [Google Scholar]
- 215. Collins B, Hoffman J, Martinez K, Grace M, Lila MA, Cockrell C, Nadimpalli A, Chang E, Chuang CC, Zhong W et al.. A polyphenol-rich fraction obtained from table grapes decreases adiposity, insulin resistance and markers of inflammation and impacts gut microbiota in high-fat-fed mice. J Nutr Biochem. 2016;31:150–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Chu D, Antony K, Ma J, Prince A, Moller M, Boggan B, Aagaard K. A maternal high fat diet (HFD) during gestation alters the neonatal gut microbiome in a human population based longitudinal cohort. Am J Obstet Gynecol. 2016;214(1):S79. [Google Scholar]
- 217. Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K, Blundell P, Harris RA, Frias AE, Grove KL et al.. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun. 2014;5:3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Yang BG, Hur KY, Lee MS. Alterations in gut microbiota and immunity by dietary fat. Yonsei Med J. 2017;58(6):1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Volynets V, Louis S, Pretz D, Lang L, Ostaff MJ, Wehkamp J, Bischoff SC. Intestinal barrier function and the gut microbiome are differentially affected in mice fed a Western-style diet or drinking water supplemented with fructose. J Nutr. 2017;147(5):770–80. [DOI] [PubMed] [Google Scholar]
- 220. Zhang P, Yu Y, Qin Y, Zhou Y, Tang R, Wang Q, Li X, Wang H, Weston-Green K, Huang X-F et al.. Alterations to the microbiota–colon–brain axis in high-fat-diet-induced obese mice compared to diet-resistant mice. J Nutr Biochem. 2019;65:54–65. [DOI] [PubMed] [Google Scholar]
- 221. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. [DOI] [PubMed] [Google Scholar]
- 222. Hildebrandt MA, Hoffmann C, Sherrill–Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137(5):1716–24..e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Baboota RK, Murtaza N, Jagtap S, Singh DP, Karmase A, Kaur J, Bhutani KK, Boparai RK, Premkumar LS, Kondepudi KK et al.. Capsaicin-induced transcriptional changes in hypothalamus and alterations in gut microbial count in high fat diet fed mice. J Nutr Biochem. 2014;25(9):893–902. [DOI] [PubMed] [Google Scholar]
- 224. Martín R, Miquel S, Benevides L, Bridonneau C, Robert V, Hudault S, Chain F, Berteau O, Azevedo V, Chatel JM et al.. Functional characterization of novel Faecalibacterium prausnitzii strains isolated from healthy volunteers: a step forward in the use of F. prausnitzii as a next-generation probiotic. Front Microbiol. 2017;8:1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Buchenauer T, Behrendt P, Bode FJ, Horn R, Brabant G, Stephan M, Nave H. Diet-induced obesity alters behavior as well as serum levels of corticosterone in F344 rats. Physiol Behav. 2009;98(5):563–9. [DOI] [PubMed] [Google Scholar]
- 226. Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes. 2013;37(3):382–9. [DOI] [PubMed] [Google Scholar]
- 227. Del Rosario A, McDermott MM, Panee J. Effects of a high-fat diet and bamboo extract supplement on anxiety- and depression-like neurobehaviours in mice. Br J Nutr. 2012;108(7):1143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Prasad A, Prasad C. Short-term consumption of a diet rich in fat decreases anxiety response in adult male rats. Physiol Behav. 1996;60(3):1039–42. [DOI] [PubMed] [Google Scholar]
- 229. Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E, Taylor CM, Welsh DA, Berthoud H-R. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatr. 2015;77(7):607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Gil-Cardoso K, Gines I, Pinent M, Ardevol A, Blay M, Terra X. The co-administration of proanthocyanidins and an obesogenic diet prevents the increase in intestinal permeability and metabolic endotoxemia derived to the diet. J Nutr Biochem. 2018;62:35–42. [DOI] [PubMed] [Google Scholar]
- 231. Zhang N-N, Guo W-H, Hu H, Zhou AR, Liu Q-P, Zheng B-D, Zeng S-X. Effect of a polyphenol-rich Canarium album extract on the composition of the gut microbiota of mice fed a high-fat diet. Molecules. 2018;23(9):2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Huang JC, Lin XH, Xue B, Luo JM, Gao LJ, Wang Y, Ou SY, Peng XC. Impact of polyphenols combined with high-fat diet on rats’ gut microbiota. J Funct Foods. 2016;26:763–71. [Google Scholar]
- 233. Heyman-Linden L, Kotowska D, Sand E, Bjursell M, Plaza M, Turner C, Holm C, Fak F, Berger K. Lingonberries alter the gut microbiota and prevent low-grade inflammation in high-fat diet fed mice. Food Nutr Res. 2016;60:29993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Seck EH, Senghor B, Merhej V, Bachar D, Cadoret F, Robert C, Azhar EI, Yasir M, Bibi F, Jiman-Fatani AA et al.. Salt in stools is associated with obesity, gut halophilic microbiota and Akkermansia muciniphila depletion in humans. Int J Obes (Lond). 2019;43(4):862–71. [DOI] [PubMed] [Google Scholar]
- 236. Laudisi F, Di Fusco D, Dinallo V, Stolfi C, Di Grazia A, Marafini I, Colantoni A, Ortenzi A, Alteri C, Guerrieri F et al.. The food additive maltodextrin promotes endoplasmic reticulum stress–driven mucus depletion and exacerbates intestinal inflammation. Cell Mol Gastroenterol Hepatol. 2018;7(2):457–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Ruggles KV, Wang J, Volkova A, Contreras M, Noya-Alarcon O, Lander O, Caballero H, Dominguez-Bello MG. Changes in the gut microbiota of urban subjects during an immersion in the traditional diet and lifestyle of a rainforest village. mSphere. 2018;3(4):e00193–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156(6):837–41. [DOI] [PubMed] [Google Scholar]
- 239. Tambs K, Czajkowsky N, Røysamb E, Neale MC, Reichborn-Kjennerud T, Aggen SH, Harris JR, Ørstavik RE, Kendler KS. Structure of genetic and environmental risk factors for dimensional representations of DSM–IV anxiety disorders. Br J Psychiatry. 2009;195(4):301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25(3):397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Bangsgaard Bendtsen KM, Krych L, Sørensen DB, Pang W, Nielsen DS, Josefsen K, Hansen LH, Sørensen SJ, Hansen AK. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS One. 2012;7(10):e46231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Galley JD, Yu Z, Kumar P, Dowd SE, Lyte M, Bailey MT. The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor. Gut Microbes. 2014;5(6):748–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Tannock GW, Savage DC. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun. 1974;9(3):591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, Wu M, Overall CC, Kipnis J, Gaultier A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep. 2017;7:43859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatr. 2009;65(3):263–7. [DOI] [PubMed] [Google Scholar]
- 246. Tsilimigras MCB, Gharaibeh RZ, Sioda M, Gray L, Fodor AA, Lyte M. Interactions between stress and sex in microbial responses within the microbiota-gut-brain axis to stress in a mouse model. Psychosom Med. 2018;80(4):361–9. [DOI] [PubMed] [Google Scholar]
- 247. Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64(1):93. [DOI] [PubMed] [Google Scholar]
- 248. Piacentino D, Rossi S, Piretta L, Badiali D, Pallotta N, Corazziari ES. Low-FODMAP-diet in irritable bowel syndrome offers benefits not only in terms of gastrointestinal symptoms, but also in terms of psychopathology in the medium- and long-term. Eur Psychiatry. 2016;33:S395. [Google Scholar]
- 249. Staudacher HM, Lomer MCE, Anderson JL, Barrett JS, Muir JG, Irving PM, Whelan K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142(8):1510–8. [DOI] [PubMed] [Google Scholar]
- 250. Haug TT, Mykletun A, Dahl AA. Are anxiety and depression related to gastrointestinal symptoms in the general population?. Scand J Gastroenterol. 2002;37(3):294–8. [DOI] [PubMed] [Google Scholar]
- 251. Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61(3):355–61. [DOI] [PubMed] [Google Scholar]
- 252. Kang SS, Jeraldo PR, Kurti A, Miller MEB, Cook MD, Whitlock K, Goldenfeld N, Woods JA, White BA, Chia N et al.. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegener. 2014;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N et al.. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015;17(5):681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


