Abstract
BACKGROUND
Intractable neck and upper limb pain has historically been challenging to treat with conventional spinal cord stimulation (SCS) being limited by obtaining effective paresthesia coverage.
OBJECTIVE
To assess the safety and effectiveness of the 10-kHz SCS system, a paresthesia-independent therapy, in the treatment of neck and upper limb pain.
METHODS
Subjects with chronic, intractable neck and/or upper limb pain of ≥5 cm (on a 0-10 cm visual analog scale [VAS]) were enrolled in 6 US centers following an investigational device exemption from the Food and Drug Administration (FDA) and institutional review board approval. Each subject was implanted with 2 epidural leads spanning C2-C6 vertebral bodies. Subjects with successful trial stimulation were implanted with a Senza® system (Nevro Corp) and included in the evaluation of the primary safety and effectiveness endpoints.
RESULTS
In the per protocol population, the primary endpoint (≥50% pain relief at 3 mo) was achieved in 86.7% (n = 39/45) subjects. Compared to baseline, subjects reported a significant reduction (P < .001) in their mean (± standard error of the mean) VAS scores at 12-mo assessment for neck pain (7.6 ± 0.2 cm, n = 42 vs 1.5 ± 0.3 cm, n = 37) and upper limb pain (7.1 ± 0.3 cm, n = 24 vs 1.0 ± 0.2 cm, n = 20). At 12-mo assessment, 89.2% of subjects with neck pain and 95.0% with upper limb pain had ≥50% pain relief from baseline, 95.0% reported to be “satisfied/very satisfied” and 30.0% either eliminated or reduced their opioid intake.
CONCLUSION
In conclusion, 10-kHz SCS can treat intractable neck and upper limb pain with stable long-term outcomes.
Keywords: 10-kHz SCS, VAS, Upper limb pain, Neck pain and opioids
ABBREVIATIONS
- DNRS
dorsal nerve root stimulation
- FDA
Food and Drug Administration
- GAF
Global Assessment of Functioning
- GIC
global impression of change
- IDE
investigational device exemption
- MCID
minimum clinically important difference
- MCS
mental health component summary score
- MME
morphine milliequivalent
- PCS
physical component summary score
- PDI
Pain Disability Index
- PEA
primary endpoint assessment
- PPP
per protocol population
- PSQ-3
3 point pain and sleep questionnaire
- PSQI
pittsburgh sleep quality index
- RCT
randomized controlled trial
- SCS
spinal cord stimulation
- SD
standard deviation
- SEM
standard error of the mean
- SF-MPQ
Short Form McGill Pain Questionnaire
- SF-12
Short Form Questionnaire-12
- VAS
visual analog scale
Cervical spine disorders are common conditions that are frequently disabling and costly to treat.1,2 Some of the most common diagnoses in patients with chronic neck pain include cervical radiculopathy, discopathy, and spondylosis. Patients are typically managed with conservative care, including physiotherapy and exercise programs. Upon a lack of improvement with conservative care, interventional procedures such as epidural steroid injections, facet rhizotomies, or surgical procedures such as anterior cervical discectomy with or without fusion are employed.3,4 Other treatment options for axial neck pain include peripheral subcutaneous field stimulation or peripheral nerve field stimulation.5,6
Spinal cord stimulation (SCS) is currently indicated as an aid in the management of chronic, intractable pain of the trunk and/or limbs.7-10 Benefits of SCS for the treatment of neck and/or upper limb pain were demonstrated in a limited number of prospective studies and in multiple case-series reports.11-17 However, the efficacy of traditional low-frequency SCS (LF-SCS) is challenged by the difficulty in obtaining sensory paresthesias in the axial neck region and the variability of the paresthesias that come with the inherent, dynamic neck and upper limb movements in the human body. This variability may result in excessive stimulation and patient discomfort or less than optimal stimulation and loss of efficacy, leading to explant of the devices in some cases.15,16,18,19
High-frequency SCS at 10 kHz (10-kHz SCS) does not elicit paresthesias, thereby eliminating the need to establish paresthesia mapping and coverage. Ten-kilohertz SCS also eliminates the risk of uncomfortable stimulation due to positional variation, which can compromise neck and upper limb pain relief with LF-SCS.20 Ten-kilohertz SCS was previously shown to provide pain relief and improve quality of life in a retrospective chart review of patients with upper or lower limb pain, but the number of patients with upper limb pain included in the analysis was low and the study also did not include patients with neck pain.17 The goal of this study is to prospectively assess the safety and effectiveness of 10-kHz SCS in the treatment of upper limb and neck pain. In addition to the safety and pain relief assessments, the study also reports data from overall quality of life assessments, patient satisfaction, and changes in opioid medication usage.
METHODS
Study Design and Population
The report includes a prospective, single-arm multicenter study designed to assess the safety and effectiveness of the 10-kHz SCS therapy in subjects with chronic, intractable pain of the upper limbs and/or neck (Figure S1, and Methods 1 and 2 in Supplemental Digital Content). Investigational device exemption (IDE) approval was obtained from the Food and Drug Administration (FDA) prior to the enrollment of subjects. The investigational plan, amendments, and informed consent forms were reviewed and approved prior to implementation, and the study was conducted in compliance with US Code of Federal Regulations and recommendations guiding physicians in biomedical research adopted by the 18th World Medical Assembly, Helsinki, Finland. The protocols were listed on ClinicalTrials.gov (NCT02385201). Subjects were identified from the pool of candidates for SCS therapy affiliated with, or referred to, the clinical investigation sites, who were recruited from 6 geographically diverse centers in the United States. Study sites submitted redacted medical records and flexion-extension images of the cervical spine to 2 independent medical monitors, including a neurosurgeon and an anesthesiologist, for the study and determined enrollment eligibility. Subjects who signed an informed consent were evaluated for eligibility based on the inclusion and exclusion criteria (Tables S1 and S2, respectively, in Supplemental Digital Content). Enrollment in this study started on June 15, 2015, and the first permanent implant was on August 4, 2015. Enrollment in the study ended on January 9, 2017, and the final subject completed the 12-mo visit on March 7, 2018. Outcomes assessed at follow-up visits are listed in Table 1.21-29
TABLE 1.
Outcomes Assessed
| Outcome | Variables |
|---|---|
| Pain relief | |
| Pain assessment (VAS) | 0-10 cm |
| Responder rates | % of subjects with ≥50% pain relief compared to baseline |
| Remitter rates | % of subjects with ≤2.5 cm VAS |
| Short Form McGill Pain Questionnaire (SF-MPQ-2) | 0-10 |
| Quality of life | |
| Pain Disability Index (PDI) | 0-70 |
| SF-12 (PCS & MCS subscales) | 0-50 |
| Global Assessment of Functioning (GAF) | 0-100 |
| Global impression of change (PGIC and CGIC) | No change, almost same, somewhat or a little bit better, better, moderately better, a great deal better |
| Sleep (PSQI and PSQ-3) | 0-21 for PSQI; 0-10 cm for PSQ-3 |
| Subject satisfaction | Dissatisfied, very dissatisfied, not sure, satisfied, very satisfied |
| Opioids | |
| Medication usage | Increased, no change, decreased, eliminated |
| Dosage | Morphine milliequivalents (MME) |
| Safety | |
| Neurological assessments | Deficit, maintained, improved |
| Adverse events | Grade I-IV |
Procedures
Enrolled subjects who met all of the inclusion criteria and none of the exclusion criteria underwent a temporary trial stimulation with 10-kHz SCS (Senza System, Nevro Corp, Redwood City, California). Leads were placed (Method 3 in Supplemental Digital Content) at varying vertebral levels ranging from C2 to C6 (Figure 1). The distal lead in the majority of the subjects was placed at the C2 vertebral level, and the contacts between mid-C2 and C3-C4 disc were identified as the most effective “sweet spots” in over 70% of the subjects. The sweet spots did not change through the study period. In few subjects, significant pain relief was seen with sweet spots between C4 and C7 discs. Subjects who experienced at least 40% reduction in their upper limb and/or neck pain during the trial compared to baseline (trial responders) were eligible for a permanent device implantation. Successful trial responders underwent a permanent implantation of the SCS device. Stimulation was delivered at a frequency of 10 kHz, pulse width of 30 μs, and amplitudes (mean ± standard deviation [SD], 0.9 ± 0.5 mA) adjusted to maximize the subject's pain relief. Follow-up visits were performed at 3, 6, and 12 mo after the permanent implant. If required, programming adjustments were offered throughout the follow-up period. Programming was done in a cephalad to caudad bipole search pattern starting at the tip of the most cephalad lead. This continued caudally, crossing to the second lead as needed until a positive response was obtained. Once a positive response in pain reduction was achieved, optimization was done by increasing or decreasing the amplitude, in an attempt to bring about further pain reduction. Typical wait time before moving to the next amplitude or new bipole was 8 h. During the trial phase, median time to reach at least 50% pain relief was 3 d, whereas during the permanent implant phase, it was 5 d.
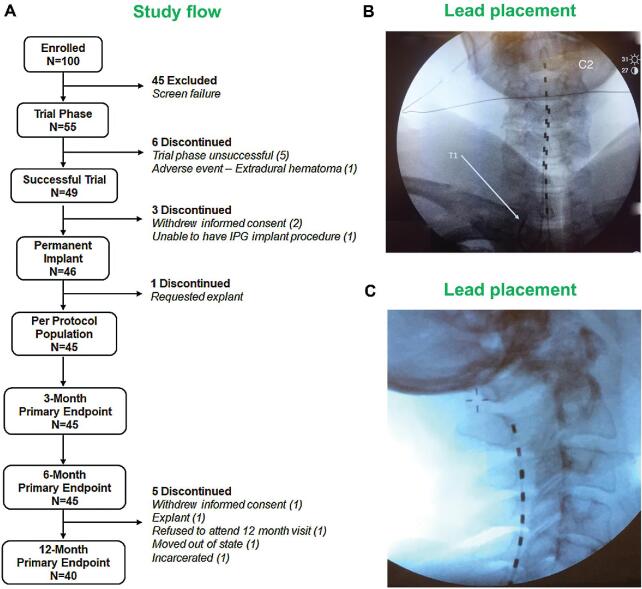
FIGURE 1.
A, Study flow chart. B and C, Representative X-ray images showing lead placement.
As is evident from Figure S2 in Supplemental Digital Content, in the initial phase of the study, some subjects needed a relatively higher number of programming sessions due to the investigational nature of neck pain treatment with 10-kHz SCS. However, as the study progressed, the number of programming sessions was markedly reduced. A total of 26 subjects out of 45 needed reprogramming during the study, and the median of the number of programming sessions in the study was 2 (max, 7; min, 0).
Statistics
Descriptive analysis of continuous variables included mean and standard error of the mean (SEM), or median, as appropriate. Categorical variables were reported as counts and percentages where possible. All the outcomes (Method 4 in Supplemental Digital Content) were analyzed by reporting descriptive statistics. All data were analyzed as observed. A 2-tailed paired t-test was used to compare the means, and a P-value less than .05 was considered as significant.
RESULTS
Trial Phase Results
Pain relief of ≥40% compared to baseline as measured by visual analog scale (VAS) scores was considered as success for the temporary trial phase of the study. The trial phase success rate of 89.1% was observed, with 46 subjects eligible to receive permanent implants (Figure 1).
Study Population
Of the subjects who received permanent implants and included in the intent-to-treat population (Method 5 in Supplemental Digital Content), 1 subject requested for a device explant 1 wk after it was implanted. The subject had a non-study-related adverse event (loss of consciousness) and requested to have the device explanted. As the device was never activated, there was no follow-up study data available for this subject and was not included in the per protocol population (PPP, Figure 1). Within the PPP, 42 of 45 subjects had a baseline neck pain score of ≥5.0 cm and were included in the neck pain subset and 24 of 45 subjects with a baseline upper limb pain score of ≥5.0 cm were included in the upper limb pain subset. Data from PPP outcome measures were used for statistical analysis and are reported in the following sections.
Demographics
Baseline demographics and clinical characteristics of the PPP enrolled in the study are shown in Table 2. Briefly, the mean age of the subjects at the time of enrollment was 55.8 yr, 66.7% were female, and the mean time since diagnosis was 11.4 yr.
TABLE 2.
Baseline Demographics and Clinical Characteristics
| Characteristics | N = 45 |
|---|---|
| Gender - n (%) | |
| Female | 30 (66.7%) |
| Male | 15 (33.3%) |
| Age (years) at enrollment | |
| Mean ± SD | 55.8 ± 9.6 |
| Range | 30.9 to 70.5 |
| Years since diagnosis | |
| Mean ± SD | 11.4 ± 8.2 |
| Range | 1.0 to 39.0 |
| Diagnosisa - n (%) | |
| Chronic intractable neck pain | 45 (100.0%) |
| Chronic intractable upper limb pain | 24 (53.3%) |
| Upper limb pain - n (%) | |
| Bilateral | 15 (62.5%) |
| Unilateral | 9 (37.5%) |
| Pain etiologyb - n (%) | |
| Radiculopathy/neuropathic pain | 40 (88.9%) |
| Degenerative disc disease | 32 (71.1%) |
| Failed cervical spine surgery syndrome | 25 (55.6%) |
| Spondylosis | 19 (42.2%) |
| Mild or moderate spinal stenosis | 17 (37.8%) |
| Other chronic pain | 14 (31.1%) |
| Internal disc disruption/annular tear | 4 (8.9%) |
| Spondylolisthesis | 3 (6.7%) |
| Previous cervical spine surgery - n (%) | 30 (66.7%) |
| Baseline use of opioids - n (%) | 35 (77.8%) |
| Baseline VAS in subjects with pain ≥5 cm | |
| Neck pain (mean ± SD) | 7.6 ± 1.3 |
| Upper limb pain (mean ± SD) | 7.1 ± 1.4 |
| Baseline Pain Disability Index (mean ± SD) | 42.4 ± 11.8 |
aSubjects could have both diagnoses.
bSubject could have more than one etiology.
Paresthesia
Uncomfortable paresthesias or uncomfortable changes in stimulation related to changes in postures were not reported in the study at the primary endpoint assessment visit (PEA, 3-mo) or 12-mo visit.
Safety
The mean duration of 10-kHz SCS utilization in the study was 51.1 wk (range: 27.4-57.1 wk). Cumulatively, there was 44.1 yr of permanent implant experience among the subject population. During the study, subjects were assessed for possible neurological deficits and other safety events, and the data were compared with baseline. Overall, there were no stimulation-related neurological deficits reported. At PEA, neurological assessments showed “no change” in neurological function in 43 subjects (95.6%) and “improvement” in neurological function in 2 subjects (4.4%). Two study-related serious adverse events were reported in 2 different subjects during the trial phase (3.6%, Table 3). Details of adverse events and the number of subjects at each follow-up assessment are described in Result 1 in Supplemental Digital Content.
TABLE 3.
Study-Related Serious Adverse Events (SAEs)
| Cause | No. of SAEs | No. (%) of subjects with SAE (n = 55) |
|---|---|---|
| Total SAEs | 2 | 2 (3.6%) |
| Extradural hematoma | 1 | 1 (1.8%) |
| Medical device site infection | 1 | 1 (1.8%) |
Pain Relief
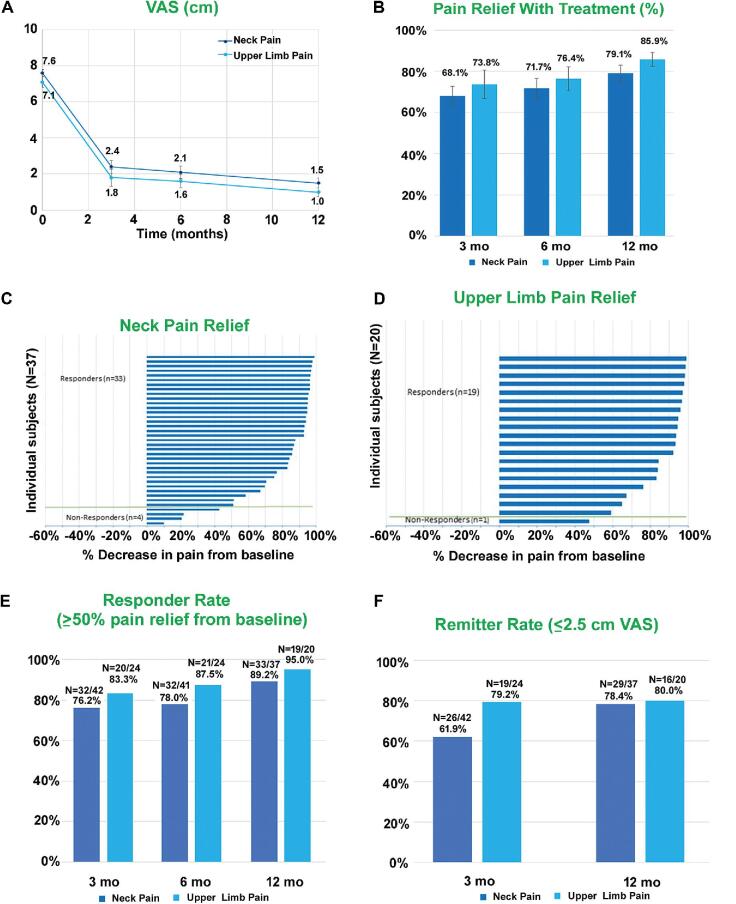
In subjects who received permanent implants, the baseline average VAS score for neck pain was 7.6 ± 0.2 cm, and for upper limb pain, it was 7.1 ± 0.3 cm, which were significantly (P < .001) reduced to 2.4 ± 0.4 cm and 1.8 ± 0.5 cm, respectively, at the 3-mo visit. Low pain scores were maintained at 6-mo and 12-mo endpoint assessments (Figure 2A-2D).
FIGURE 2.
Sustained relief from neck and upper limb pain with 10-kHz SCS. A, VAS scores (mean ± SEM). B, Pain relief (mean ± SEM). C, Tornado chart for neck pain relief in individual subjects at 12 mo. D, Tornado chart for upper limb pain relief in individual subjects at 12 mo. E, Responder rates at 3, 6, and 12 mo. F, Remitter rates at 3 and 12 mo.
Responder Rates
Subjects who had ≥50% pain relief as assessed by VAS were considered as responders in the study. At 3-mo assessment, >75% of subjects and at 12-mo assessment, >85% subjects responded to the 10-kHz SCS therapy (Figure 2E). For neck pain, the responder rates at 3-mo and 12-mo visits were 76.2% and 89.2%, respectively. For upper limb pain, the responder rates at 3 and 12 mo were 83.3% and 95.0%, respectively.
Remitter Rates
Subjects with VAS scores ≤2.5 cm were defined as remitters, and the remitter rate of subjects was calculated.30,31 The remitter rates for neck pain at 3-mo and 12-mo assessments were 61.9% and 78.4%, respectively, and for upper limb pain were 79.2% and 80.0%, respectively (Figure 2F).
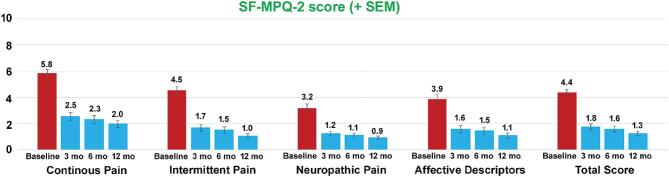
Short Form McGill Pain Questionnaire
As seen in Figure 3, the 10-kHz SCS therapy resulted in improved Short Form McGill Pain Questionnaire (SF-MPQ) scores at 3-mo assessment, which further improved at 6-mo and 12-mo assessments. Compared to baseline, average score for “continuous pain” decreased by 3.8 points (66.3%), “intermittent pain” decreased by 3.0 points (76.9%), “neuropathic pain” decreased by 2.3 points (71.3%), “affective descriptors” decreased by 2.8 points (72.0%), and “total score” decreased by 3.1 points (71.2%) at 12-mo assessment (Figure 3).
FIGURE 3.
Reduction in SF-MPQ scores after the 10-kHz SCS treatment. Data shown include mean ± SEM at indicated assessment times.
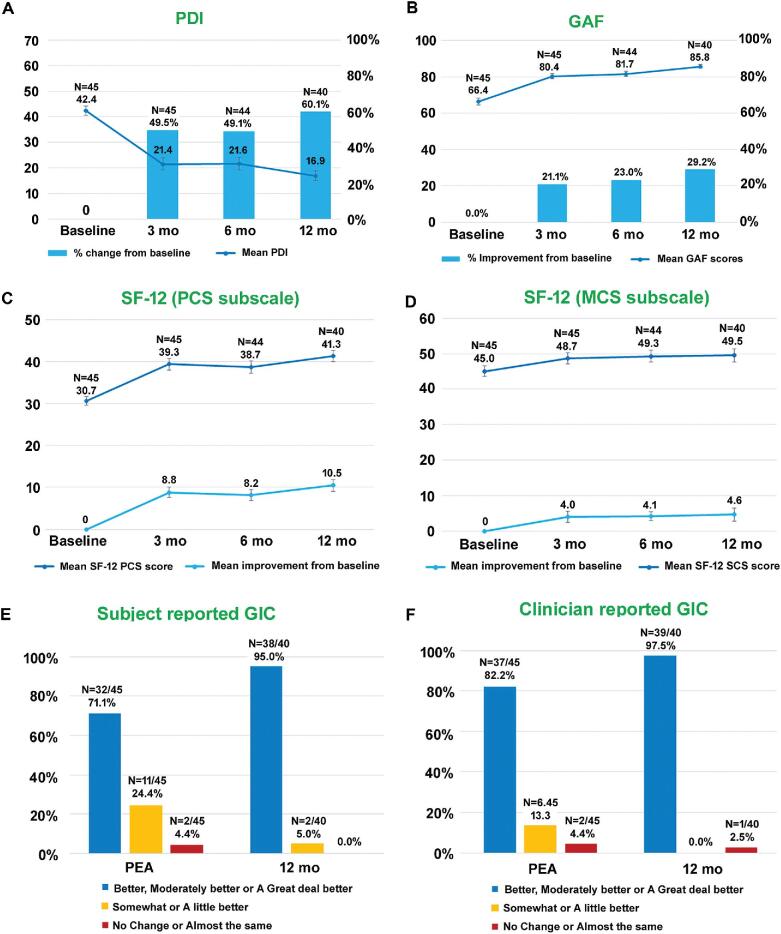
Improvement in Disability and Quality of Life
Improvement in disability and quality of life following the 10-kHz SCS treatment for neck and upper limb pain was assessed using multiple questionnaires including Pain Disability Index (PDI), Short Form Questionnaire-12 (SF-12), Global Assessment of Functioning (GAF), and global impression of change (GIC). Significant improvements in all the disability and quality of life related assessments were seen with the 10-kHz SCS therapy (Figure 4A-4F).
FIGURE 4.
Improvement in quality of life and functioning after the 10-kHz SCS treatment. A, PDI scores and percentage change from baseline. B, GAF scores and percentage improvement from baseline. C, SF-12 (PCS subscale) scores and mean improvement from baseline. D, SF-12 (MCS subscale) scores and mean improvement from baseline. E, Subject reported GIC. F, Clinician reported GIC. Data shown include mean ± SEM at indicated assessment times.
Sleep
Quality of sleep was significantly improved with the 10-kHz SCS treatment as seen by the lower pittsburgh sleep quality index (PSQI) (Figure 5A) and 3 point pain and sleep questionnaire (PSQ-3) (Figure 5B) scores. At 12-mo assessment, 20% of subjects (8/41) had PSQI <5 compared to 2% (1/45) at baseline.
FIGURE 5.
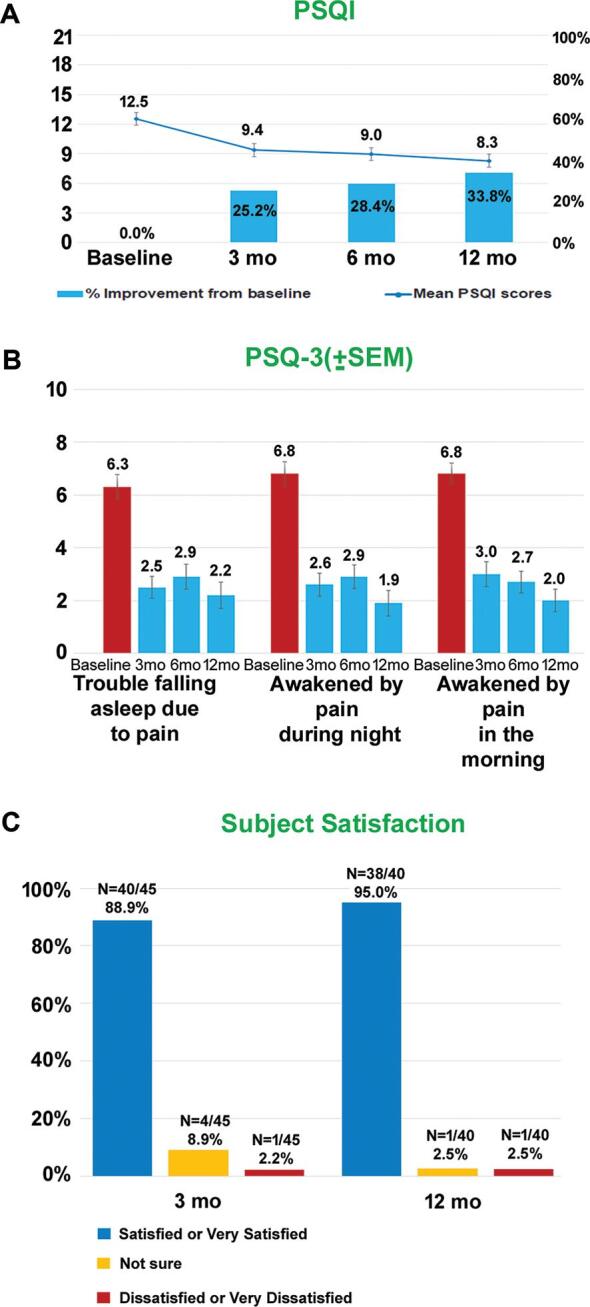
Improved sleep and subject satisfaction. A, PSQI scores (mean ± SEM) and percentage improvement from baseline. B, PSQ-3 scores (mean ± SEM) at indicated assessment times. C, Subject satisfaction at 3-mo and 12-mo assessments.
Medication and Opioid Usage
At baseline, 77.8% subjects were taking at least 1 opioid medication (Table 2). At 12-mo visit, 30.0% of subjects decreased or eliminated their opioid medication (Table 4) and average morphine equivalent dose was reduced from 63.1 morphine milliequivalents (MME) at baseline to 42.1 MME at 12-mo assessment (P = .14).
TABLE 4.
Medication Change Following the 10-kHz SCS Therapy
| 3 mo | 6 mo | 12 mo | |
|---|---|---|---|
| Increased | 8.6% (3) | 11.4% (4) | 6.7% (2) |
| No change | 80.0% (28) | 65.7% (23) | 63.3% (19) |
| Reduced or eliminated | 11.4% (4) | 22.8% (8) | 30.0% (9) |
Satisfaction
Subject satisfaction to the 10-kHz SCS therapy as assessed by the percentage of subjects responding as “satisfied” or “very satisfied” was 95.0% at 12-mo endpoint assessment (Figure 5C).
DISCUSSION
Cervical SCS with traditional paresthesia-based setting has been used to treat upper limb and/or neck pain, but the success was not satisfactory due to lack of adequate and consistent paresthesia coverage on a long-term basis.12-17,32-39 An extensive literature review revealed that level I evidence for the use of SCS for upper limb and/or neck pain is not currently available within peer reviewed publications, but level II evidence from 3 prospective studies evaluating the benefits of traditional cervical SCS and 1 study testing the benefits of dorsal nerve root stimulation (DNRS) for upper limb and/or neck pain were available for reference and comparison.12-14 Pain relief reported in subjects treated with 10-kHz SCS in the current study is higher than previously reported data at 12-mo endpoint assessment (79.2% for neck pain and 85.9% for upper limb pain, vs 36.8%-66.8% with cervical SCS and 52.6% with DNRS).12-14 More importantly, the percent pain relief gradually increased from 3-mo to 12-mo endpoint assessment with 10-kHz SCS, which to the best of our knowledge has never been reported with traditional SCS. Responder rate as determined by percentage of subjects with ≥50% pain relief was similarly higher (89% for neck pain and 95% for upper limb pain with 10-kHz SCS vs 67% with traditional cervical SCS as well as DNRS) in subjects treated with 10-kHz SCS compared to previously reported studies.14 Strikingly, at 12-mo assessment ∼80% of subjects with upper limb and/or neck pain had achieved VAS scores of ≤2.5 cm, a cut-off defined as the point of remission in chronic pain patients.30,31 Pain relief, responder rates, and remitter rates observed in the current study are comparable to the results reported in 10-kHz SCS-treated subjects with chronic low back and leg pain in randomized controlled trial (RCT) and to retrospective, real-world data.30,31,40,41 The results from 12-mo SF-MPQ-2 assessments in 10-kHz SCS-treated subjects further supported the VAS, pain relief, and responder rates analysis.
Loss of efficacy and other complications leading to explant of the devices is a major concern in the field of neuromodulation as it causes excess burden to the patients and increases the treatment costs. Lead migration leading to loss of efficacy, discomfort due to positioning of the leads (positional effect), and device related complications are commonly encountered in patients with upper limb and neck pain.15-17,34,39 In the current study, only 1 in 45 subjects required an explant (2.2%) and there were no cases of lead migration or other complications despite positioning the leads at C2 vertebral level in all enrolled subjects. More importantly, all the device-related adverse events were manageable and resolved shortly after reprogramming.
In addition to pain relief, the current study evaluated additional quality of life related outcomes such as PDI, SF-12, GIC, GAF, and patient satisfaction.21,22,42,43 In the current study, the 10-kHz SCS treatment resulted in nearly 25-point reduction (∼2.5 × minimum clinically important difference (MCID)) in PDI scores at 12-mo assessment compared to baseline, which is slightly higher than previously reported improvement with LF-SCS.12 Similarly, 10-kHz SCS-treated subjects had 10-point reduction (∼2.8 × MCID) in SF-12 physical component summary score (PCS) subscale and 5-point reduction (∼1.2 × MCID) in SF-12 mental health component summary score (MCS) subscale. Although previous studies attempted to measure quality of life in upper limb and neck patients using SF-36 and euroqol 5 dimensions questionnaire questionnaires, the findings were inconclusive as the number of subjects was relatively small.14,17 Current study included information from 40 subjects with upper limb and/or neck pain for the longitudinal analysis, and clearly showed the benefits of 10-kHz SCS in the improvement of quality of life in the enrolled subjects. Furthermore, previous studies also did not attempt to directly assess the impression of change and global functioning in upper limb and neck pain patients using GIC and GAF questionnaires, respectively. Deer et al12 used patient rated “greatly improved,” “improved,” “neither improved nor deteriorated,” and “deteriorated” categories to estimate the quality of life in subjects treated with cervical SCS and reported ∼63% subjects in improved or greatly improved category at 12-mo endpoint. In the current study, 95% of subjects treated with 10-kHz SCS had a positive GIC seen as “better,” “moderately better,” or “a significantly better” and had a 19-point improvement on GAF scores at 12-mo endpoint assessment underscoring the benefits of 10-kHz SCS for the treatment of upper limb and neck pain. The improvement in quality of life at 12-mo assessment was further reflected in patient satisfaction rate (95%), which was higher than previously reported patient satisfaction with traditional cervical SCS.12 The findings on quality of life in chronic pain subjects in the current study are comparable to the previously reported results from randomized controlled study in chronic low back and leg pain subjects.40
Lack of uninterrupted and restful sleep is a common concern for patients with chronic pain. Continued reliance on opioids by chronic pain patients may partly be due to their ability to induce sleep.44 Results from the current study indicate that subjects treated with 10-kHz SCS reported continued improvement in their sleep patterns with a 4-point improvement in both PSQI scores and PSQ-3 scores at 12-mo endpoint. Moreover, at that same time of assessment 30% of the subjects enrolled in the study reduced or eliminated their opioid medication, pointing to the overall improvement in quality of life in 10-kHz SCS-treated subjects. Current findings on PSQI scores and changes in opioid medication are similar to previously reported results from studies in chronic back and leg pain subjects and to retrospective analysis of real-world data.30,31,40,41,45,46 The apparent disconnect between modest changes in opioid medication despite profound pain relief could be mainly because the study was not designed to test the effect of changes in opioid medication following 10-kHz SCS treatment. In order to translate the pain relief and quality of life outcomes into changes in opioid medication, reduction or elimination of opioids needs to be part of the treatment plan and the patient must understand and agree to the objectives of the treatment possibly before offering the trial. Though the current study did not include any active encouragement of changing opioid dose, 30% of the subjects either reduced their dose or eliminated opioids completely, indicating the potential of 10-kHz SCS as an alternative to opioids.
Finally, the exclusion criterion listed subjects who “are currently requiring or are likely to require” an MRI. All subjects had an MRI prior to enrollment. The subsequent medical review was intended to prevent implantation of a patient with near-term expectations of a required MRI. The safety of the Senza system for cervical MRI was not established at the time of study initiation. However, the Senza system now has full-body conditional MRI compatibility.
Additional discussion on rationale for lower (≥40%) pain relief cut-off in the trial phase and exclusion criteria can be found in Discussions 1 and 2 in Supplemental Digital Content. Additional references can be found in the Supplemental Digital Content.
Limitations
This study was not an RCT. An RCT is an important tool for clinicians to evaluate the efficacy of a new treatment or to compare treatments. However, efficacy of SCS, including10-kHz SCS, was documented in an RCT and further supported through a large real-world retrospective review.30,31,41 In the discussion, multiple published prospective trials of cervical SCS were reviewed and the findings were compared with 10-kHz SCS to determine if similar safety and efficacy in neck pain with or without arm pain was seen in the current study.
Secondly, the study excluded subjects with “any previous history of surgery on the posterior elements (laminectomy, posterior fusion).” This criterion may be considered too restrictive since in the absence of laminectomy, laminotomy, or laminoplasty posterior surgery, such as stand-alone fusion or foraminotomy, would not have been a contraindication for safe placement of SCS leads. A new MRI and careful neurosurgical review could have been considered to include these patients. Nonetheless, this would not affect the conclusions of the study.
CONCLUSION
This study demonstrates the effectiveness of 10-kHz SCS for the treatment of neck and upper limb pain by providing durable long-lasting relief and significant improvement in quality of life. Follow-up prospective RCTs and real-world studies in clinical setting are desirable to further replicate results from this study.
Disclosures
The study was sponsored by Nevro Corp. Drs Amirdelfan, Benyamin, Yu, and Bundschu are consultants to Nevro Corp. Mr Gliner, Drs Subbaroyan, Rotte, and Caraway are employees of Nevro Corp. Dr Vallejo is an employee of Stimgenics, and a consultant for Medtronic and Avanos.
Supplementary Material
Acknowledgements
Authors thank the medical monitors of the study, Dr Gary A Heit, Consultant Neurosurgeon, Redwood City, California and Dr Jeff Tiede, Consultant Anesthesiologist, Fort Gordon, Georgia for review of medical reports; Alex Zinner and Alice Yan of Nevro Corp. for help with the analyses and Dr Madhuri Bhandaru for help with illustrations.
Contributor Information
Kasra Amirdelfan, IPM Medical Group Inc., Walnut Creek, California.
Ricardo Vallejo, Millennium Pain Center, Bloomington, Illinois.
Ramsin Benyamin, Millennium Pain Center, Bloomington, Illinois.
Cong Yu, Swedish Pain Center, Seattle, Washington.
Thomas Yang, Swedish Pain Center, Seattle, Washington.
Richard Bundschu, Coastal Orthopedics and Pain Medicine, Bradenton, Florida.
Thomas L Yearwood, Comprehensive Pain and Rehabilitation, Pascagoula, Mississippi.
B Todd Sitzman, Advanced Pain Therapy, PLLC, Hattiesburg, Mississippi.
Bradford Gliner, Nevro Corp, Redwood City, California.
Jeyakumar Subbaroyan, Nevro Corp, Redwood City, California.
Anand Rotte, Nevro Corp, Redwood City, California.
David Caraway, Nevro Corp, Redwood City, California.
Supplemental Digital Content. Method 1. Key inclusion and exclusion criteria; Method 2. Sample size; Method 3. Procedures; Method 4. Outcomes; Method 5. Study definitions; Result 1. Study flow; Discussion 1. Rationale for lower (≥40%) pain relief cut-off in trial phase; Discussion 2. Rationale for exclusion criteria; Table S1. Inclusion criteria; Table S2. Exclusion criteria; Figure S1. Study design flow chart; Figure S2. Number of reprogramming during the study; References.
REFERENCES
- 1. Cote P, Cassidy JD, Carroll L. The Saskatchewan Health and Back Pain Survey. The prevalence of neck pain and related disability in Saskatchewan adults. Spine (Phila Pa 1976). 1998;23(15):1689-1698. [DOI] [PubMed] [Google Scholar]
- 2. Makela M, Heliovaara M, Sievers K, Impivaara O, Knekt P, Aromaa A. Prevalence, determinants, and consequences of chronic neck pain in Finland. Am J Epidemiol. 1991;134(11):1356-1367. [DOI] [PubMed] [Google Scholar]
- 3. Yeung SST, Visintini S. Percutaneous Rhizotomy for Chronic Back or Neck Pain: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2018. [PubMed] [Google Scholar]
- 4. Cohen SP, Hooten WM. Advances in the diagnosis and management of neck pain. BMJ. 2017;358:j3221. [DOI] [PubMed] [Google Scholar]
- 5. Lipov EG, Joshi JR, Sanders S, Slavin KV. Use of peripheral subcutaneous field stimulation for the treatment of axial neck pain: a case report. Neuromodulation. 2009;12(4):292-295. [DOI] [PubMed] [Google Scholar]
- 6. Verrills P, Vivian D, Mitchell B, Barnard A. Peripheral nerve field stimulation for chronic pain: 100 cases and review of the literature. Pain Med. 2011;12(9):1395-1405. [DOI] [PubMed] [Google Scholar]
- 7. Medtronic Indications, Safety & Warnings for Spinal Cord Stimulation. Med-tronic. 2018. http://www.medtronic.com/us-en/healthcare-professionals/therapies-procedures/neurological/spinal-cord-stimulation/indications-safety-warnings.html#.VHymwjHF98E. Accessed June 11, 2019 [Google Scholar]
- 8. BostonScientific Spectra WaveWriter™ Spinal Cord Stimulator System. BostonScientific 2018. http://www.bostonscientific.com/content/gwc/en-US/products/spinal-cord-stimulator-systems/spectra-wavewriter-indications-of-use.html. Accessed June 11, 2019 [Google Scholar]
- 9. Abbott St. Jude Medical™ Invisible Trial System for SCS. Abbott; 2016. https://www.neuromodulation.abbott/us/en/hcp/products/sjm-invisible-trial-system-scs.html. Accessed June 11, 2019 [Google Scholar]
- 10. Nevro HF10™ Therapy: A Combination of Four Key Attributes Yield Superior Clinical Outcomes. Nevro. https://www.nevro.com/English/Physicians/Senza-System/default.aspx. [Google Scholar]
- 11. Oakley JC. Spinal cord stimulation in axial low back pain: solving the dilemma. Pain Med. 2006;7(suppl 1):S58-S63. [Google Scholar]
- 12. Deer TR, Skaribas IM, Haider N et al.. Effectiveness of cervical spinal cord stimulation for the management of chronic pain. Neuromodulation. 2014;17(3):265-271; discussion 271. [DOI] [PubMed] [Google Scholar]
- 13. Haider S, Owusu-Sarpong S, Peris Celda M et al.. A single center prospective observational study of outcomes with tonic cervical spinal cord stimulation. Neuromodulation. 2017;20(3):263-268. [DOI] [PubMed] [Google Scholar]
- 14. Levine AB, Parrent AG, MacDougall KW. Cervical spinal cord and dorsal nerve root stimulation for neuropathic upper limb pain. Can J Neurol Sci. 2017;44(1):83-89. [DOI] [PubMed] [Google Scholar]
- 15. Wolter T, Kieselbach K. Cervical spinal cord stimulation: an analysis of 23 patients with long-term follow-up. Pain Physician. 2012;15(3):203-212. [PubMed] [Google Scholar]
- 16. Chivukula S, Tempel ZJ, Weiner GM et al.. Cervical and cervicomedullary spinal cord stimulation for chronic pain: efficacy and outcomes. Clin Neurol Neurosurg. 2014;127:33-41. [DOI] [PubMed] [Google Scholar]
- 17. Al-Kaisy A, Palmisani S, Smith T, Harris S, Pang D. The use of 10-kilohertz spinal cord stimulation in a cohort of patients with chronic neuropathic limb pain refractory to medical management. Neuromodulation. 2015;18(1):18-23. [DOI] [PubMed] [Google Scholar]
- 18. Penn DL, Zussman BM, Wu C, Sharan AD. Anterograde revision of cervical spinal cord stimulator paddle electrode: a case report. Neuromodulation. 2012;15(6):581-585; discussion 584-585. [DOI] [PubMed] [Google Scholar]
- 19. Vallejo R, Kramer J, Benyamin R. Neuromodulation of the cervical spinal cord in the treatment of chronic intractable neck and upper extremity pain: a case series and review of the literature. Pain Physician. 2007;10(2):305-311. [PubMed] [Google Scholar]
- 20. De Carolis G, Paroli M, Tollapi L et al.. Paresthesia-independence: an assessment of technical factors related to 10 kHz paresthesia-free spinal cord stimulation. Pain Physician. 2017;20(4):331-341. [PubMed] [Google Scholar]
- 21. Tait RC, Chibnall JT, Krause S. The Pain Disability Index: psychometric properties. Pain. 1990;40(2):171-182. [DOI] [PubMed] [Google Scholar]
- 22. Chibnall JT, Tait RC. The Pain Disability Index: factor structure and normative data. Arch Phys Med Rehabil. 1994;75(10):1082-1086. [DOI] [PubMed] [Google Scholar]
- 23. Hall RC. Global assessment of functioning. Psychosomatics. 1995;36(3):267-275. [DOI] [PubMed] [Google Scholar]
- 24. Dworkin RH, Turk DC, Revicki DA et al.. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144(1):35-42. [DOI] [PubMed] [Google Scholar]
- 25. Jenkinson C, Layte R, Jenkinson D et al.. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19(2):179-186. [DOI] [PubMed] [Google Scholar]
- 26. Fischer D, Stewart AL, Bloch DA, Lorig K, Laurent D, Holman H. Capturing the patient's view of change as a clinical outcome measure. JAMA. 1999;282(12):1157-1162. [DOI] [PubMed] [Google Scholar]
- 27. Guy W. ECDEU Assessment Manual for Psychopharmacology . U.S. Department of Health Education, and Welfare Publication (ADM). Rockville, MD: National Institute of Mental Health; 1976. [Google Scholar]
- 28. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. [DOI] [PubMed] [Google Scholar]
- 29. Ayearst L, Harsanyi Z, Michalko KJ. The Pain and Sleep Questionnaire three-item index (PSQ-3): a reliable and valid measure of the impact of pain on sleep in chronic nonmalignant pain of various etiologies. Pain Res Manag. 2012;17(4):281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kapural L, Yu C, Doust MW et al.. Novel 10-kHz high-frequency therapy (HF10 Therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851-860. [DOI] [PubMed] [Google Scholar]
- 31. Kapural L, Yu C, Doust MW et al.. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery. 2016;79(5):667-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dam-Hieu P, Magro E, Seizeur R, Simon A, Quinio B. Cervical cord compression due to delayed scarring around epidural electrodes used in spinal cord stimulation. J Neurosurg Spine. 2010;12(4):409-412. [DOI] [PubMed] [Google Scholar]
- 33. Wada E, Kawai H. Late onset cervical myelopathy secondary to fibrous scar tissue formation around the spinal cord stimulation electrode. Spinal Cord. 2010;48(8):646-648. [DOI] [PubMed] [Google Scholar]
- 34. Falowski S, Ooi YC, Sabesan A, Sharan A. Spinal cord injury induced by a cervical spinal cord stimulator. Neuromodulation. 2011;14(1):34-37; discussion 36-37. [DOI] [PubMed] [Google Scholar]
- 35. Issa MA, Kim CH. Cervical spinal cord stimulation with 5-column paddle lead in Raynaud's disease. Pain Physician. 2012;15(4):303-309. [PubMed] [Google Scholar]
- 36. Wloch A, Capelle HH, Saryyeva A, Krauss JK. Cervical myelopathy due to an epidural cervical mass after chronic cervical spinal cord stimulation. Stereotact Funct Neurosurg. 2013;91(4):265-269. [DOI] [PubMed] [Google Scholar]
- 37. Al Tamimi M, Aoun SG, Gluf W. Spinal cord compression secondary to epidural fibrosis associated with percutaneously placed spinal cord stimulation electrodes: case report and review of the literature. World Neurosurg. 2017;104:1051.e1-1051.e5. [DOI] [PubMed] [Google Scholar]
- 38. Chivukula S, Tomycz ND, Moossy JJ. Paddle lead cervical spinal cord stimulation for failed neck surgery syndrome. Clin Neurol Neurosurg. 2013;115(10):2254-2256. [DOI] [PubMed] [Google Scholar]
- 39. Chan AK, Winkler EA, Jacques L. Rate of perioperative neurological complications after surgery for cervical spinal cord stimulation. J Neurosurg Spine. 2016;25(1):31-38. [DOI] [PubMed] [Google Scholar]
- 40. Amirdelfan K, Yu C, Doust MW et al.. Long-term quality of life improvement for chronic intractable back and leg pain patients using spinal cord stimulation: 12-month results from the SENZA-RCT. Qual Life Res. 2018;27(8):2035-2044. [DOI] [PubMed] [Google Scholar]
- 41. Stauss T, El Majdoub F, Sayed D et al.. A multicenter real-world review of 10 kHz SCS outcomes for treatment of chronic trunk and/or limb pain. Ann Clin Transl Neurol. 2019:6(3):496-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soer R, Koke AJ, Speijer BL et al.. Reference values of the pain disability index in patients with painful musculoskeletal and spinal disorders: a cross-national study. Spine (Phila Pa 1976). 2015;40(9):E545-E551. [DOI] [PubMed] [Google Scholar]
- 43. Soer R, Reneman MF, Vroomen PC, Stegeman P, Coppes MH. Responsiveness and minimal clinically important change of the Pain Disability Index in patients with chronic back pain. Spine (Phila Pa 1976). 2012;37(8):711-715. [DOI] [PubMed] [Google Scholar]
- 44. Severino AL, Shadfar A, Hakimian JK et al.. Pain therapy guided by purpose and perspective in light of the opioid epidemic. Front Psychiatry. 2018;9:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Buyten J-P, Al-Kaisy A, Smet I, Palmisani S, Smith T. high-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation. 2013;16(1):59-66. [DOI] [PubMed] [Google Scholar]
- 46. Al-Kaisy A, Van Buyten J-P, Smet I, Palmisani S, Pang D, Smith T. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014;15(3):347-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.