Abstract
Mitochondrial respiratory chain complexes I, III, and IV can associate into larger structures termed supercomplexes or respirasomes, thereby generating structural interdependences among the individual complexes yet to be understood. In patients, nonsense mutations in complex IV subunit genes cause severe encephalomyopathies randomly associated with pleiotropic complex I defects. Using complexome profiling and biochemical analyses, we have explored the structural rearrangements of the respiratory chain in human cell lines depleted of the catalytic complex IV subunit COX1 or COX2. In the absence of a functional complex IV holoenzyme, several supercomplex I+III2 species coexist, which differ in their content of COX subunits and COX7A2L/HIGD2A assembly factors. The incorporation of an atypical COX1‐HIGD2A submodule attenuates supercomplex I+III2 turnover rate, indicating an unexpected molecular adaptation for supercomplexes stabilization that relies on the presence of COX1 independently of holo‐complex IV formation. Our data set the basis for complex I structural dependence on complex IV, revealing the co‐existence of alternative pathways for the biogenesis of “supercomplex‐associated” versus individual complex IV, which could determine physiological adaptations under different stress and disease scenarios.
Keywords: mitochondrial biogenesis, mitochondrial complex IV assembly, mitochondrial respiratory chain, respirasomes, respiratory supercomplex stabilization
Subject Categories: Membrane & Intracellular Transport
Availability of complex IV subunit COX1 and assembly factor HIGD2A promotes the formation of submodules that directly bind and stabilize respiratory supercomplex I+III2 in human cells.

Introduction
Defects of the mitochondrial oxidative phosphorylation (OXPHOS) system are a frequent cause of severe encephalomyopathies and neurodegenerative conditions (Ghezzi & Zeviani, 2018). During OXPHOS, four enzymatic multiprotein complexes (CI–CIV) and two mobile electron carriers (coenzyme Q and cytochrome c) constituting the mitochondrial respiratory chain (MRC) act in concert to facilitate electron transfer from reducing equivalents to molecular oxygen. This cellular respiration is coupled to the generation of a proton gradient across the inner membrane that is used by the F 1 F o‐ATP synthase (complex V) to drive ATP synthesis. The MRC complexes may associate in higher‐order assemblies known as supercomplexes (SCs), which coexist with the individual complexes (Schägger & Pfeiffer, 2001; Lobo‐Jarne & Ugalde, 2018). In mammals, SCs are classified into two groups depending on whether they contain CI (SC I+III2, respirasomes, or SC I+III2+IV1–4 and megacomplex or SC I2+III2+IV2) or not (SC III2+IV1). In human cells, the practical totality of CI is contained within SCs (Lobo‐Jarne & Ugalde, 2018), whereas in mammalian tissues variable amounts of free CI exist (Schägger & Pfeiffer, 2001; Acín‐Pérez et al, 2008; Letts & Sazanov, 2017; Davies et al, 2018). The physiological significance or this organization in health and disease remains unsolved. The catalytic advantages claimed to be provided by SCs (Blanchi et al, 2004; Lapuente‐Brun et al, 2013) have been contested (Trouillard et al, 2011; Blaza et al, 2014; Milenkovic et al, 2017; Fedor & Hirst, 2018), and alternative hypotheses propose that SC organization could prevent deleterious random protein aggregations in the densely packed inner mitochondrial membrane (Hirst, 2018), assist CI assembly and stability (Schägger et al, 2004; Moreno‐Lastres et al, 2012), or prevent reactive oxygen species (ROS) generation (Maranzana et al, 2013; Lopez‐Fabuel et al, 2016).
In mammals, the predominant respirasome I+III2+IV1 contains a CIV unit positioned at the distal end of the CI membrane arm and adjacent to CIII2 (Gu et al, 2016; Letts et al, 2016; Sousa et al, 2016; Wu et al, 2016; Guo et al, 2017). Larger respirasomes I+III2+IV2–4 (Schägger & Pfeiffer, 2000) await structural characterization, but cryo‐EM analysis in HEK293T cells revealed a symmetrical respiratory megacomplex (MC) I2+III2+IV2 where CIII2 is centrally located and surrounded by two units of CI and CIV (Guo et al, 2017). CI also associates with CIII2 within SC I+III2 (Letts et al, 2019), where the contacts between CI and CIII2 are evolutionarily conserved (Davies et al, 2018), and are similar to the ones in SC I+III2+IV1 (Letts et al, 2016). In addition, CIII2 associates with CIV to form the scarce SC III2+IV1 that remains structurally uncharacterized in mammalian tissues. Despite most CIV (~ 80%) is unbound to other complexes (Lobo‐Jarne & Ugalde, 2018), CIV‐containing respirasomes are the prevalent SCs in mammals and their formation is assisted by two assembly factors, HIGD2A and COX7A2L (Chen et al, 2012; Ikeda et al, 2013; Lapuente‐Brun et al, 2013; Perez‐Perez et al, 2016; Lobo‐Jarne et al, 2018). HIGD2A primarily functions in CIV assembly (Chen et al, 2012), whereas COX7A2L promotes SC III2+IV formation and mediates CIV integration within a minor subset of respirasomes (Lobo‐Jarne et al, 2018), supporting different paths for SCs gathering via alternative COX7A isoforms (Cogliati et al, 2016; Letts & Sazanov, 2017). In this regard, two hypotheses have been proposed for respirasome biogenesis (Lobo‐Jarne & Ugalde, 2018). One hypothesis posits that SC assembly proceeds by the association of mature individual complexes (Acín‐Pérez et al, 2008; Guerrero‐Castillo et al, 2016). A second hypothesis proposes the sequential incorporation of CIII and CIV subassemblies into a partially assembled CI scaffold that will eventually result in a complete functional structure (Moreno‐Lastres et al, 2012). This is supported by experimental models of mitochondrial disease, where assembly intermediates of MRC complexes get stably associated with partially assembled SCs (Fernández‐Vizarra et al, 2009; Lazarou et al, 2009; Calvaruso et al, 2012; Alston et al, 2018) of uncertain significance.
Structural alterations of the MRC organization have major diagnostic implications in mitochondrial disorders, since mutations specifically targeting one given complex frequently alter the activities of the remainder MRC complexes. Mutations severely affecting CIII2 structure consistently cause a parallel CI enzyme defect in patients and disease models (Lamantea et al, 2002; Acín‐Pérez et al, 2004; Schägger et al, 2004; D'Aurelio et al, 2006; Diaz et al, 2012; Tucker et al, 2013; Wanschers et al, 2014; Fernández‐Vizarra & Zeviani, 2015; Gaignard et al, 2017; Protasoni et al, 2020), whereas CI or CIV structural defects rarely cause pleiotropic MRC deficiencies (Rak et al, 2016; Stroud et al, 2016; Hatakeyama & Goto, 2017). Few exceptions involve homoplasmic nonsense mutations leading to defective biosynthesis of the mitochondrial‐encoded CIV subunit COX1, usually associated with combined enzyme deficiencies of CI and CIV in cultured cells (D'Aurelio et al, 2006; Diaz et al, 2006; Li et al, 2007) and patients (Karadimas et al, 2000). These observations suggest that CI integrity and function mainly rely on its association with CIII2 within SC I+III2, but the functional relevance of the relatively small amount of CIV required for respirasome I+III2+IV1 biogenesis remains to be clarified. To this aim, we have analyzed the intricate MRC structural rearrangements of the OXPHOS system in human transmitochondrial cybrids carrying nonsense homoplasmic mutations that abolish the expression of CIV subunits COX1 (Bruno et al, 1999) and COX2 (Balsa et al, 2012), both causing the total loss of holo‐CIV. We show the existence of an alternative CIV biogenetic pathway that involves the formation of an atypical COX1‐HIGD2A submodule capable of binding directly to SC I+III2 to stabilize it and preserve its enzymatic activity. Our findings demonstrate that SC I+III2 stability relies on the presence of COX1 rather than on complete holo‐CIV, explaining the physiological relevance of COX subunits for CI maintenance and clarifying the pleiotropic CI defects observed in cellular models of CIV deficiency.
Results
Human cells lacking holo‐complex IV display several SC I+III2 species that differ in the presence of COX subunits and assembly factors
We first analyzed the steady‐state levels of MRC structural subunits in digitonin‐solubilized mitochondria (Fig 1A) and whole‐cell extracts (Fig EV1A) from control and mutant cybrids lacking CIV subunits COX1 (COX1Δ) or COX2 (COX2Δ). Both mutants showed similar levels of CI, CII, and CIII subunits, except for RISP that was significantly higher in the COX2Δ mutant. Regarding CIV subunits, COX4 and COX5A were detected at 50% of control values in both mutants, in agreement with their stabilization in early assembly intermediates (Nijtmans et al, 1998; Williams et al, 2004; Stiburek et al, 2005; Vidoni et al, 2017). In contrast, in COX2Δ cybrids, not only COX1 but also the COX2‐module subunit COX5B significantly accumulated relative to COX1Δ cybrids, whereas the remainder CIV subunits were barely detectable.
Figure 1. Mutant cybrids lacking CIV display different SC I+III2 species.
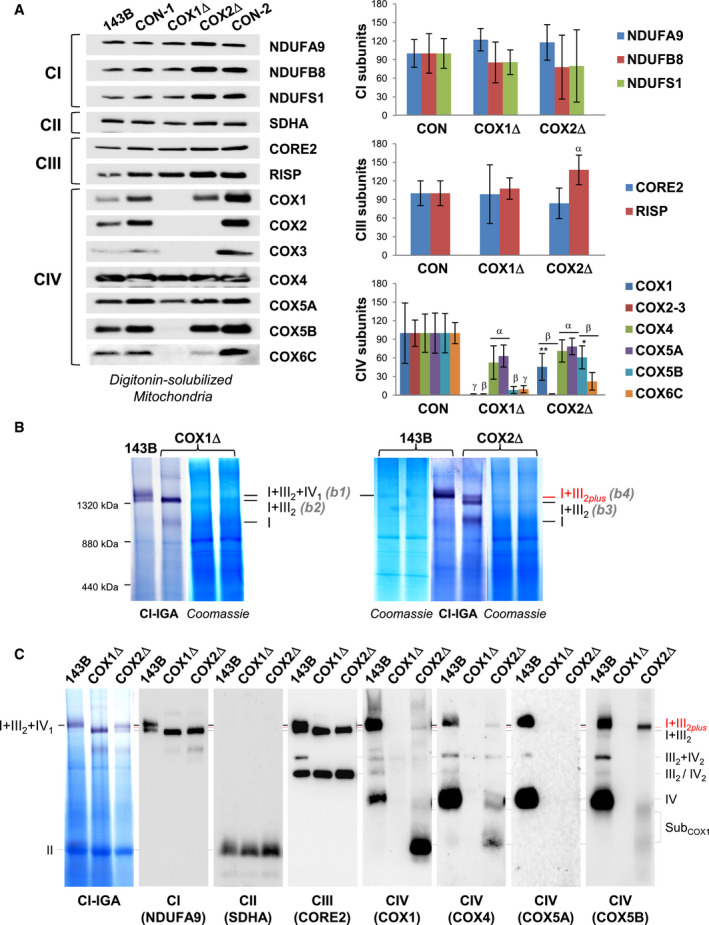
-
AMitochondrial extracts from 143B cells, and COX1Δ and COX2Δ mutant cybrids and their respective isogenic controls (CON‐1 and CON‐2) were analyzed by SDS–PAGE and immunoblotting with the indicated antibodies. The graphs (right) show densitometric quantifications of the signals normalized to CII subunit SDHA. The mean of three independent controls (CON) was set to 100, and all measurements were referenced to that value. The values represent mean ± SD (n = 3–8). Mann–Whitney U‐test was applied for statistical analyses. Variations between controls and mutants: α, P < 0.05; β, P < 0.01; and γ, P < 0.001. Specific variations between COX1Δ and COX2Δ mutants: *P < 0.05; **P < 0.01.
-
BDigitonized mitochondria (digitonin‐to‐protein ratio of 2:1) were analyzed by BN–PAGE, followed by CI in‐gel activity (IGA) assays and Coomassie staining. The SC I+III2+IV1 band (b1) was excised from the control (143B) lane, and the SC I+III2 bands (b2 to b4) were excised from the COX1Δ and COX2Δ lanes. Their protein compositions were subsequently analyzed by nano‐LC/ESI‐MS (see also Fig EV2). The relative position of the molecular weight marker is indicated on the left.
-
CBN–PAGE was followed by CI‐IGA assay and immunoblotting with the indicated antibodies (in brackets). The identity of the MRC complexes and SCs is I+III2+IV1, SC containing CI, CIII2, and CIV; I+III2, SC containing CI and CIII2; I+III2plus, SC containing CI, CIII2, and a COX1 submodule; III2+IV, SC containing CIII2 and CIV; I, complex I; III2, CIII dimer; IV2, CIV dimer; subCOX1, COX1‐containing subcomplexes.
Figure EV1. Related to Fig 1. Human cells lacking CIV display different SC I+III 2 species.

-
AWhole‐cell lysates from control 143B and HEK293T cells, COX1Δ and COX2Δ cybrids, COX18Δ HEK293T cells, and COX18Δ cells complemented with wild‐type COX18 (+COX18) were analyzed by SDS–PAGE and immunoblotting with antibodies targeting CIV subunits and SDHA as a loading control.
-
BDigitonin‐solubilized mitochondria from control and COX18Δ HEK293T cells were analyzed by BN–PAGE, followed by CI‐IGA assay and immunoblotting with the indicated antibodies. I+III2+IV1, SC containing CI, CIII2, and CIV; I+III2, SC containing CI and CIII2; I+III2plus, SC containing CI, CIII2, and a COX1 submodule; III2+IV1, SC containing CIII2 and CIV; III2, CIII dimer; IV2, CIV dimer; IV, CIV monomer.
Source data are available online for this figure.
Blue‐native gel electrophoresis (BN–PAGE) analyses revealed the co‐existence of different respirasome species displaying NADH dehydrogenase (NADH‐DH, CI) activity (Fig 1B). In control cells, the predominant structure corresponded to SC I+III2+IV1 (band 1), followed by the relatively less abundant SC I+III2. The COX1Δ mutant accumulated a single NADH‐DH‐reactive band (band 2) but, surprisingly, the COX2Δ mutant displayed two well‐differentiated NADH‐DH‐reactive bands (bands 3 and 4). Bands 1–4 were gel‐excised, and their protein composition was analyzed by high‐resolution nano‐LC/ESI‐MS (Fig EV2). Proteomics revealed the presence of the respirasome I+III2+IV1 in control cells (band 1), and of fully assembled SC I+III2 bound to COX7A2L in both mutants (bands 2 and 3), as previously reported (Perez‐Perez et al, 2016). Unexpectedly, band 4 from the COX2Δ cybrids (henceforth SC I+III2plus) also contained specific CIV subunits: the liver isoform of the COX7A subunits (COX7A2), COX4, and COX5B, but not COX5A despite its relatively high abundance. The SC I+III2plus co‐localization of CIV subunits (including COX1, which was not detected in our former MS analysis) was further confirmed by immunoblotting (Fig 1C). Since no CIV subunits were detected within SC I+III2 in the COX1Δ cybrids, we speculated that SC I+III2plus formation probably requires the presence of COX1 and that this structure accumulates in the absence of COX2. We validated this hypothesis in HEK293T cells deleted of COX18 (COX18Δ), an assembly factor essential for COX2 biogenesis (Bourens & Barrientos, 2017a). As expected, COX18Δ cells nearly lacked COX2, but expressed COX1 plus specific CIV subunits (Fig EV1A) that co‐localized with the unconventional SC I+III2plus species in the absence of holo‐CIV (Fig EV1B). These results confirm in different cellular models that, provided COX1 is present, additional CIV subunits may bind SCs even when the formation of holo‐CIV is fully impaired.
Figure EV2. Related to Fig 1. Proteomic identification of MRC subunits in supercomplexes from cells lacking CIV .
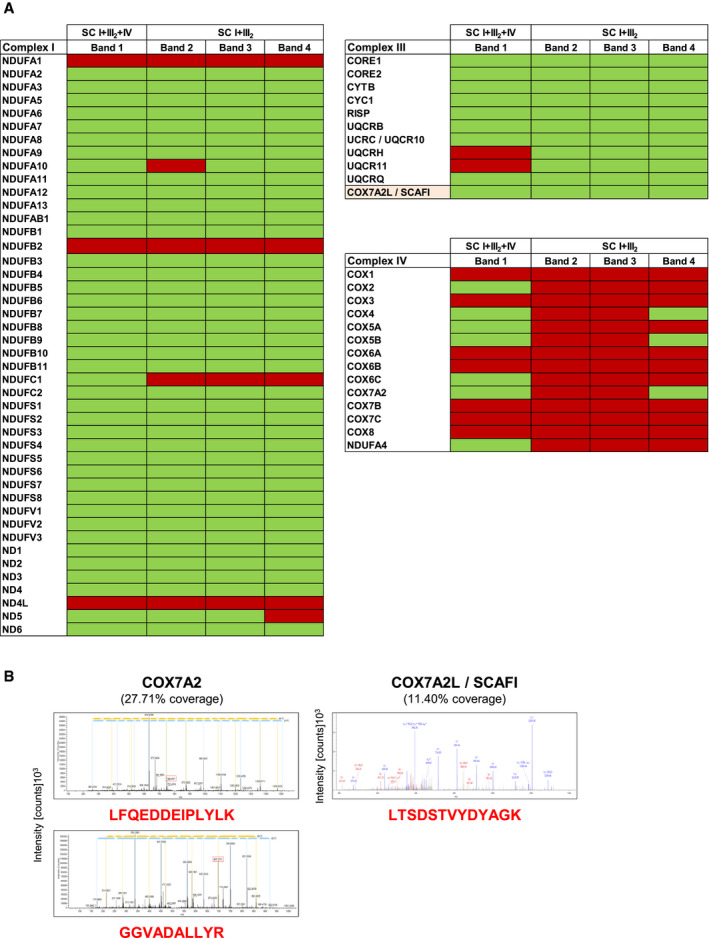
-
ADigitonin‐solubilized mitochondria from control, COX1Δ, and COX2Δ cybrids were separated by 1D‐BN–PAGE. CI‐containing bands 1–4 were identified by CI‐IGA assays, excised from Coomassie‐stained gels, and analyzed by nano‐LC/ESI‐MS in two independent experiments per sample. The presence (green) or absence (red) of MRC subunits detected on each band is indicated.
-
BMS/MS spectra from the doubly charged COX7A2 (left) and COX7A2L (right) peptides unambiguously detected by LC‐ESI/MS. The amino acid sequences of the identified unique peptides are highlighted in red. The most intense signals on the MS/MS spectra correspond to the main fragmentation series (b‐amino and y‐carboxy). I+III2+IV1, SC containing CI, CIII2, and CIV; I+III2, SCs containing CI and CIII2.
Complexome profiling reveals the accumulation of an atypical COX1 subcomplex co‐migrating with supercomplex I+III2 in COX2Δ cybrids
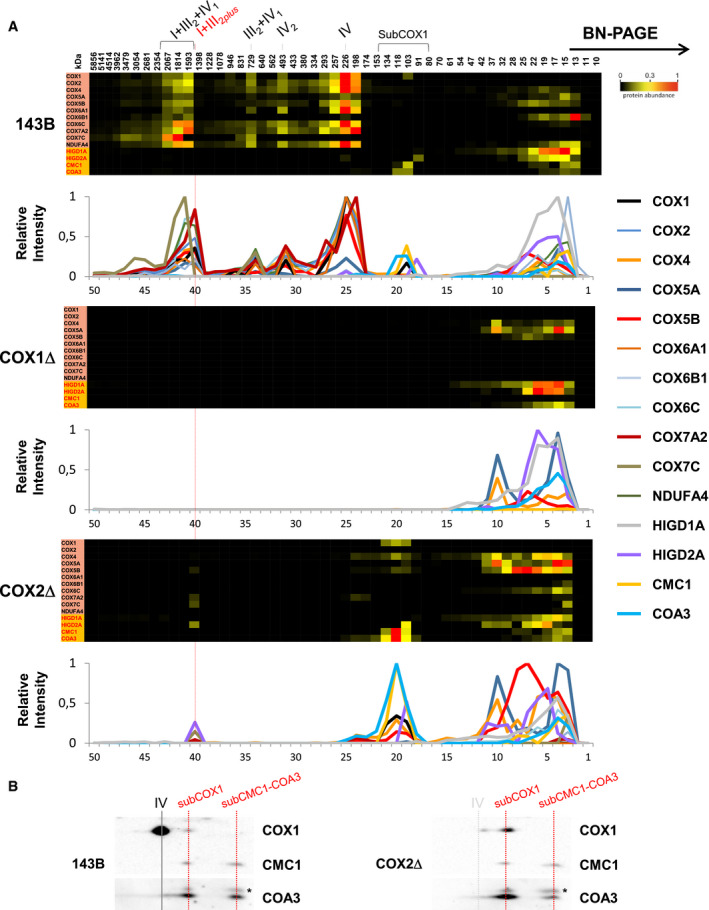
Comprehensive complexome profiling proteomics of BN–PAGE gels (Fig 2) recapitulated the MRC structural reorganization in the CIV‐KO cybrids. Control cells showed a major ~ 1,600 kDa respirasome I+III2+IV1 primarily containing CIV subunit COX7A2, and two less abundant respirasomes of ~ 1,810 and ~ 2,070 kDa mostly containing the SC assembly factor COX7A2L/SCAFI (Fig EV3A). Both COX1Δ and COX2Δ cybrids showed the accumulation of the ~ 1,400 kDa SC I+III2. The larger ~ 1,600 kDa SC I+III2plus present in COX2Δ cells accounted for ~ 25% of the total amount of I+III2 species and co‐migrated with subunits COX7A2, COX7C, COX5B, and COX4. Importantly, the assembly factor HIGD2A appeared upregulated within SC I+III2plus by complexome profiling (Figs 2A and EV3B) and Western blot (Fig EV3C), supporting its role in SC I+III2plus biosynthesis. In contrast, complexome profiling did not detect assembly factors COX7A2L/SCAFI and HIGD1A within SC I+III2plus.
Figure 2. Complexome profiling reveals a differential reorganization of the MRC in CIV‐KO cybrids.
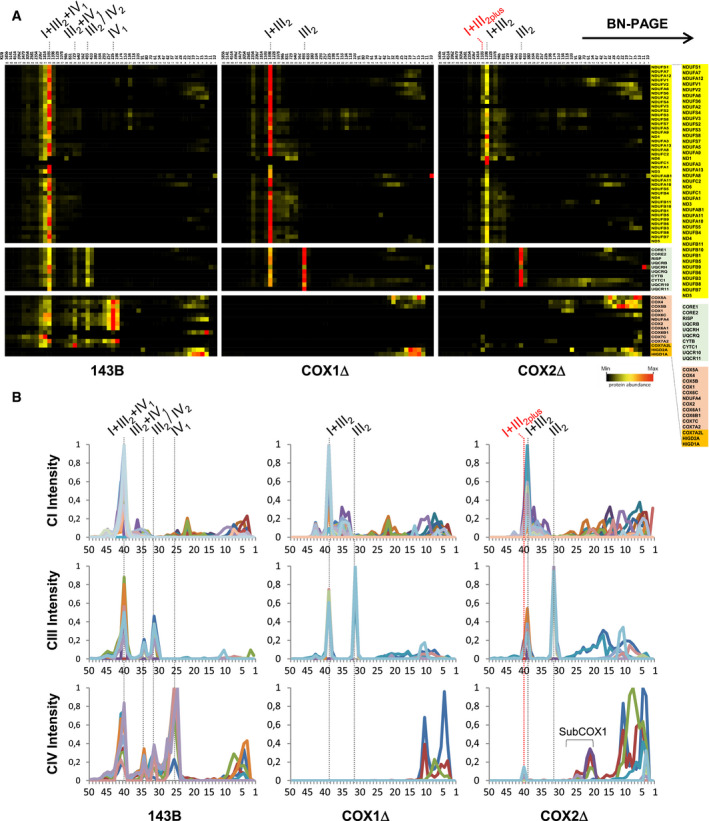
-
AControl 143B cells (left panels), COX1Δ cybrids (middle panels), and COX2Δ cybrids (right panels). The right lane highlights structural subunits of CI (yellow), CIII (light green), and CIV (orange brown), and assembly factors (orange). Intensity‐based absolute quantification (iBAQ) values were normalized to the sum of all control values. Top lanes indicate gel slice numbers and their relative native masses.
-
BIntensity profiles showing abundance and relative distribution of structural subunits from CI (upper graphs), CIII (middle graphs), and CIV (bottom graphs). The identities of MRC complexes and SCs are as in Fig 1.
Figure EV3. Related to Figs 2 and 3. Different respirasome species are defined by COX7A2, COX7A2L, and HIGD2A.
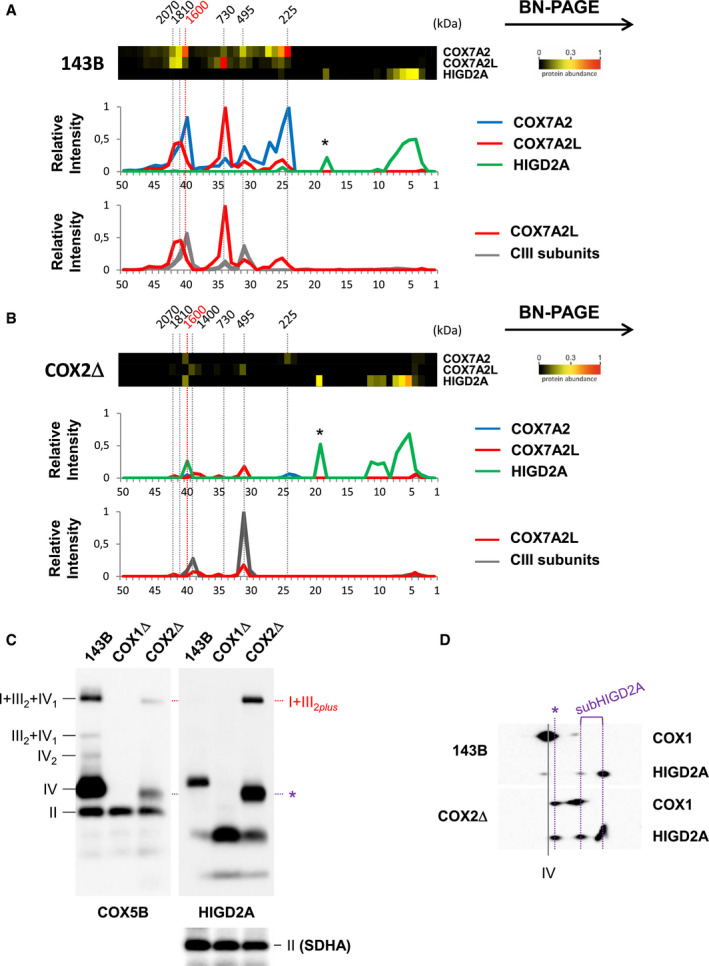
-
A, BExcerpts of complexome profiling data showing the relative distribution of COX7A2, COX7A2L, and HIGD2A in (A) control 143B cells and (B) COX2Δ cybrids. The molecular weight (˜ 1.6 MDa) of the COX7A2‐containing respirasomes (SC I+III2+IV1 in CON cells, and SC I+III2plus in COX2Δ cybrids) is highlighted in red. The relative abundance and distribution of COX7A2L relative to CIII subunits are shown in the lower graphs.
-
C, D(C) BN–PAGE or (D) 2D‐BN/SDS–PAGE from control (143B) and mutant cybrids followed by immunoblotting with the indicated antibodies. I+III2+IV1, SC containing CI, CIII, and CIV; I+III2plus, SC containing CI, CIII, and specific COX subunits; III2+IV, SC containing CIII and CIV; IV2, CIV dimer; IV, CIV monomer; II, complex II used as loading control. The presence of a COX1‐COX5B‐HIGD2A‐containing subcomplex is indicated with an asterisk.
Source data are available online for this figure.
The COX1Δ and COX2Δ cybrids additionally showed a differential accumulation of CIV assembly intermediates (Figs 2B and 3A). In COX1Δ cybrids, only subunits COX4 and COX5A appeared stabilized in common fast‐migrating subcomplexes of ~ 13–35 kDa, compatible with free subunits and heterodimers. However, in COX2Δ cybrids a major portion of COX1 was stabilized in a ~ 100–135 kDa subcomplex co‐migrating with subunits COX4 and COX5B, and with the CIV assembly factors CMC1 and COA3 (Bourens & Barrientos, 2017b). This subcomplex was detected also in control cells (Fig 3A and B), thereby representing a bona fide CIV assembly intermediate. Then, COX1‐COX4‐COX5B signals appeared in a less abundant subcomplex (of ~ 175–200 kDa) below CIV (~ 225 kDa) that also co‐migrated with COX7A2 and HIGD2A (Figs 2B, and EV3C and D). This atypical COX1‐COX4‐COX5B‐COX7A2‐HIGD2A subcomplex further co‐migrated with SC I+III2plus in COX2Δ cybrids. In addition, the COX7C subunit accumulated in a subcomplex of ~ 13 kDa and then in SC I+III2plus, suggesting its direct incorporation into SCs. Altogether, we conclude that the presence of COX1 is an essential requirement for the recruitment of specific COX subunits into an unconventional module that is directly targeted to SC I+III2.
Figure 3. CIV assembly factors co‐migrate with an atypical COX1 submodule that is present in control and COX2Δ cybrids.
-
AExcerpts of complexome profiling data showing the distribution of CIV subunits and assembly factors HIGD1A, HIGD2A, CMC1, and COA3 in control (143B), COX1Δ, and COX2Δ cybrids. The relative abundance and distribution of detected proteins are shown in the lower graphs. The localization of SC I+III2plus in COX2Δ cybrids is highlighted with a red line. The approximate molecular weights of CIV‐containing structures are indicated on the top (in kDa).
-
B2D‐BN/SDS–PAGE analysis from control (143B) and COX2Δ cybrids followed by immunoblotting with the indicated antibodies. I+III2+IV1, SC containing CI, CIII, and CIV; I+III2plus, SC containing CI, CIII, and a COX1 submodule; III2+IV, SC containing CIII and CIV; IV2, CIV dimer; IV, CIV monomer. The presence of a COX1‐CMC1‐COA3‐containing subcomplex is indicated.
Complex IV subunits physically interact with SC I+III2plus in COX2Δ cybrids
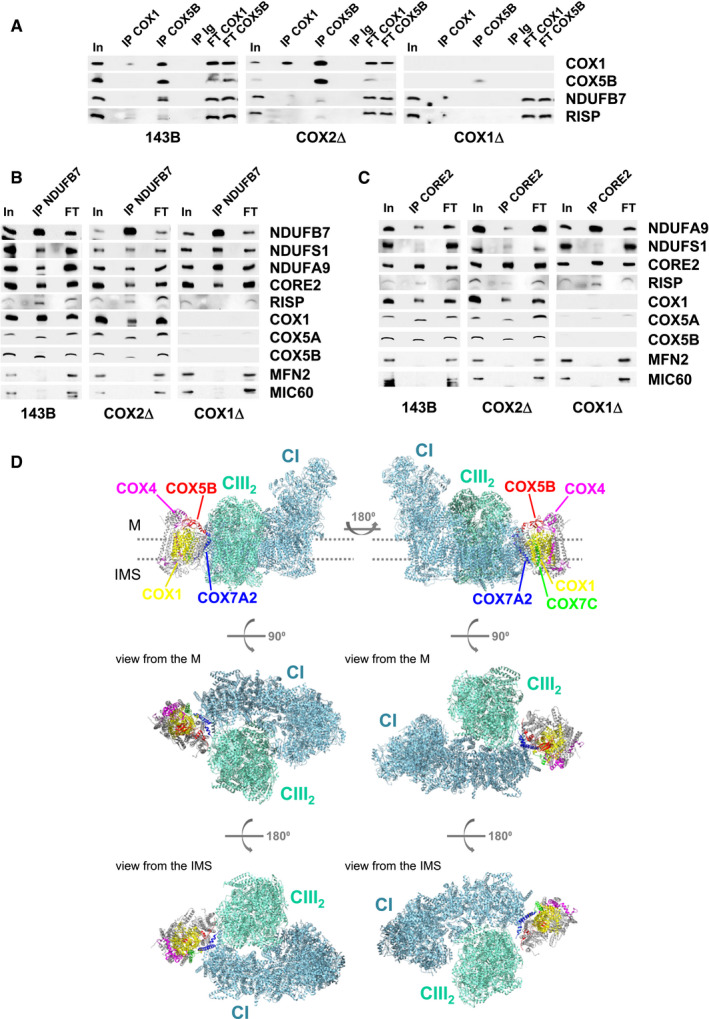
Co‐immunoprecipitation (coIP) assays of digitonin‐solubilized mitochondria from control and CIV‐KO cybrids (Fig 4) using an anti‐COX5B antibody pulled down CI (NDUFB7) and CIII (RISP) subunits in control and COX2Δ cybrids. The interaction with COX1 appeared to be weaker, but still detectable (Fig 4A). Reciprocal coIP assays using antibodies against CI (NDUFB7, Fig 4B) or CIII (CORE2, Fig 4C) showed their successful co‐purification with other CI and CIII subunits in control and mutant cybrids, but not with the OXPHOS‐unrelated mitochondrial proteins MIC60 and MFN2. Importantly, both coIPs specifically pulled down COX1 and COX5B in control and COX2Δ cybrids. These data showed that, even in the absence of holo‐CIV, specific COX subunits physically interact with SC I+III2 and that this interaction is driven by COX1.
Figure 4. COX subunits physically bind SC I+III2plus .
-
A–CMitochondrial extracts from control 143B cells (left), COX1Δ cybrids (right), and COX2Δ cybrids (middle) were immunoprecipitated using antibodies against (A) CIV subunits COX1 and COX5B. (B) CI subunit NDUFB7. (C) CIII subunit CORE2. Samples were subsequently analyzed by SDS–PAGE and immunoblotting with the indicated antibodies. In, input. IP, immunoprecipitate. FT, flow‐through. Ig, unrelated immunoglobulin used as a negative control.
-
DStructural arrangement of COX subunits detected in association with SC I+III2. Upper illustrations depict lateral views of human SC I+III2+IV1, where CI (aquamarine) and CIII2 (light green) are positioned along the inner membrane plane (dots). CIV appears as a smooth backbone with the associated COX subunits highlighted to show their positions relative to SC I+III2: COX7A2 (blue) and COX5B (red) attach COX1 (yellow) to the concave surface formed by CIII2 and the CI membrane arm, interaction further stabilized by COX7C (green) binding to CI. COX4 (magenta) interacts with COX1 on the distal side of CIV. Medium illustrations: matrix (M) view. Lower illustrations: intermembrane space (IMS) view. The figure was created with USCF Chimera v.1.12 using the 5XTH structure (Guo et al, 2017) from the PDB database.
Source data are available online for this figure.
This direct association correlates with the positions of the detected CIV subunits within the human respirasome (Guo et al, 2017; Fig 4D). Subunit COX7A2 attaches COX1 to the concave surface formed by CIII2 and the distal end of the CI membrane arm, an interaction that could be further stabilized by binding COX5B to CIII2 and COX7C to CI (Letts et al, 2016; Wu et al, 2016). In contrast, considering its position within the respirasome, the association of COX4 with SC I+III2plus is probably rather unstable, which is reflected in the relative low steady‐state levels of COX4 in this structure (Figs 1C and 2A).
Human cells lacking CIV display a differential accumulation of stabilized COX subunits
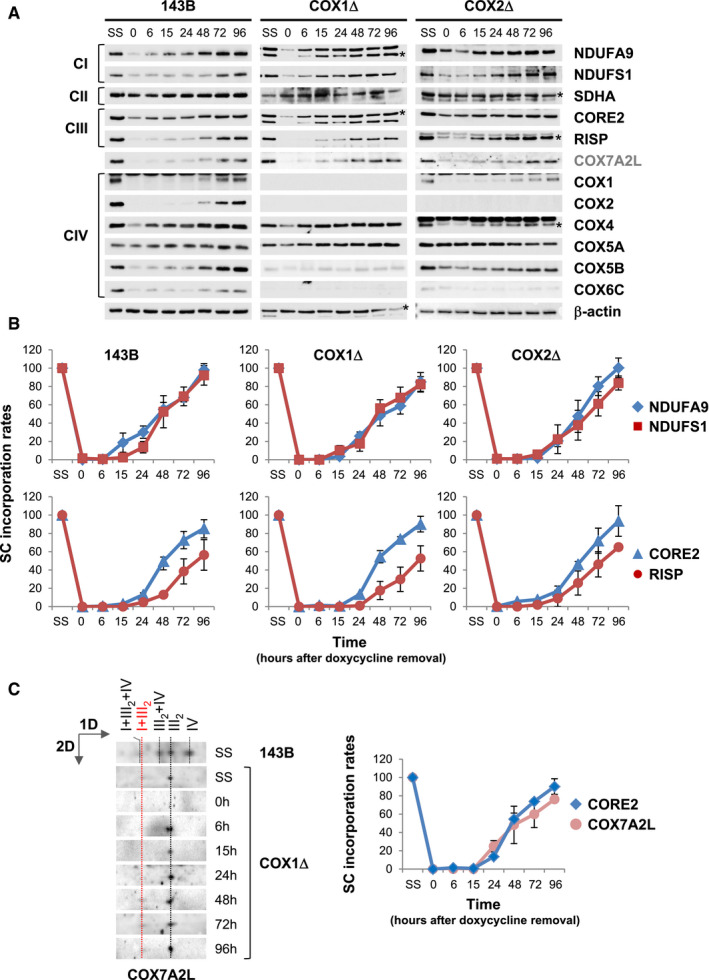
We next investigated the biogenetic dynamics of MRC subunits and COX7A2L in cultured cells by reversible inhibition of mitochondrial translation with doxycycline (Moreno‐Lastres et al, 2012; Perez‐Perez et al, 2016). The reappearance of MRC translation products was analyzed by SDS–PAGE and immunoblotting with a panel of twelve antibodies raised against MRC components (Fig EV4A). Subunits from CI, CIII2, and COX7A2L showed similar kinetics of reappearance in control and CIV‐KO cells. However, the accumulation rates of CIV subunits varied among cell lines, depending on whether these subunits are labile in the absence of either COX1 or COX2, or stabilized in preassembled modules (Figs 1C and 2). CII levels remained unaffected by doxycycline treatment, as expected since this complex lacks mtDNA‐encoded subunits.
Figure EV4. Related to Figs 5 and 6. Reappearance of MRC subunits and COX7A2L and assembly kinetics into respirasomes upon reversible inhibition of mitochondrial translation.
-
AWhole‐cell extracts were subsequently analyzed by SDS–PAGE and immunoblotting with the indicated antibodies. β‐actin was used as a loading control. Asterisks indicate the matching bands recognized by the antibodies.
-
BMitochondria were extracted with a digitonin‐to‐protein ratio of 2:1 g/g and analyzed by BN–PAGE. The assembly dynamics of CI subunits NDUFA9 and NDUFS1, involved in early and late steps of CI assembly, and of CIII subunits CORE2 and RISP, involved in early and late steps of CIII assembly, were compared in the three cell lines.
-
CAssembly kinetics of COX7A2L relative to CORE2 in the COX1Δ cybrids. The directions of electrophoresis on 2D‐BN/SDS–PAGE gels are indicated with arrows. I+III2+IV, SCs containing CI, CIII, and CIV; I+III2, SC containing CI and CIII; III2+IV, SC containing CIII and CIV; III2, CIII dimer; IV, CIV monomer.
COX7A2L‐containing supercomplex I+III2 is fully assembled prior to the insertion of complex IV subunits
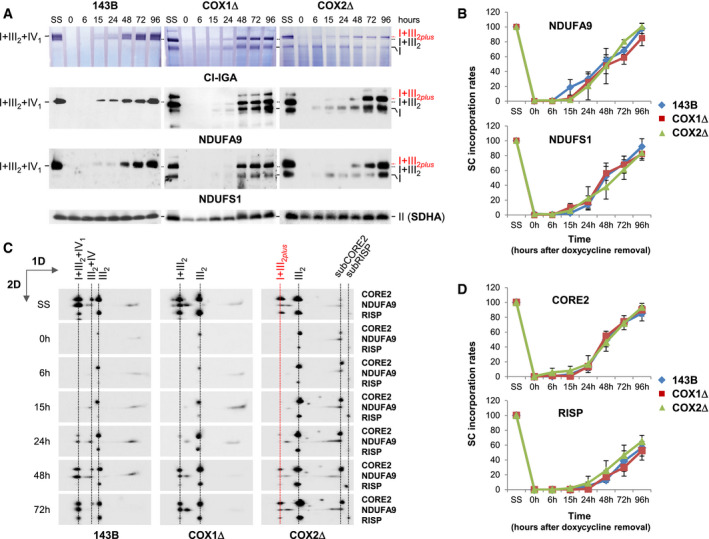
Then, we investigated the kinetics of SC formation in doxycycline‐treated cells (Figs 5 and EV4B). CI assembly and its integration into SCs were analyzed by BN–PAGE followed by CI‐IGA assays (Fig 5A, upper panels) or immunoblotting with antibodies targeting subunits NDUFA9 and NDUFS1 (Fig 5A, medium and lower panels), markers of intermediate and late CI assembly stages, respectively (Signes & Fernandez‐Vizarra, 2018). Signals were quantified, normalized to CII, and expressed relative to the steady levels in untreated cells (SS) (Fig 5B). The assembly rates of CI subunits within SCs were similar between control and mutant cybrids, and the incorporation of NDUFS1 (catalytic N‐module) into SCs was initially delayed relative to NDUFA9 (Fig EV4B, upper panels), as previously observed (Moreno‐Lastres et al, 2012). We next analyzed the assembly kinetics of newly synthesized CIII subunits by 2D‐BN/SDS–PAGE and immunoblotting using antibodies that recognize CORE2 and RISP (Fig 5C and D), markers of early and late CIII assembly stages, respectively (Fernández‐Vizarra & Zeviani, 2015). The assembly rates of CIII subunits within CIII2 and SCs were also similar between control and mutant cybrids, and the incorporation of RISP (catalytic) into SCs was initially delayed relative to CORE2 (Fig EV4B, lower panels). These results indicate that a RISP‐lacking pre‐SC I+III2 is formed at an early assembly stage, to which RISP is independently added to yield fully assembled SC I+III2, in agreement with previous studies (Moreno‐Lastres et al, 2012; Davoudi et al, 2016; Perez‐Perez et al, 2016). In addition, COX7A2L accumulated gradually within CIII2 and then moved toward SC I+III2 at similar assembly rates than CORE2, and before the incorporation of RISP (Fig EV4C), as expected (Moreno‐Lastres et al, 2012; Perez‐Perez et al, 2016). Altogether, we conclude that COX7A2L‐containing SC I+III2 is normally assembled prior to the insertion of CIV subunits.
Figure 5. Assembly kinetics of CI and CIII subunits into the COX7A2L‐containing SC I+III2 .
-
ADigitonin‐solubilized mitochondria from doxycycline‐treated 143B cells, and COX1Δ and COX2Δ cybrids were analyzed by BN–PAGE followed by CI‐IGA assays (upper panels) or immunoblotting (medium and lower panels) with antibodies against CI subunits NDUFA9 and NDUFS1.
-
BMean incorporation rates of NDUFA9 and NDUFS1 into SC I+III2+IV1 from 143B cells and into SC I+III2 from the CIV‐KO cybrids.
-
CSamples were subsequently analyzed by 2D‐BN/SDS–PAGE and immunoblotting with the indicated antibodies.
-
DMean incorporation rates of CIII subunits CORE2 and RISP into SC I+III2+IV1 from control cells and into SC I+III2 from CIV‐KO cybrids.
-
B–DSignals from at least three independent experiments were quantified and normalized by CII subunit SDHA levels. Time‐point values are expressed as percentages of untreated cells (SS) and indicated as mean ± SD.
-
A–CThe identities of MRC complexes and SCs are as in Fig 1.
An atypical COX1 module directly integrates into supercomplex I+III2plus
Remarkably, the co‐migration of a CIV subcomplex with SC I+III2plus suggests an unusual biogenetic regulation of CIV assembly in the respirasomes that largely depends on the presence of COX1 or specific COX1‐interacting proteins. To investigate the mechanism involved (Fig 6A), we analyzed the assembly kinetics into SC I+III2plus of newly synthesized CIV subunits considered to assemble at early stages (COX1, COX4, and COX5A, which conform the canonical COX1 module) or at intermediate stages (COX5B and COX6C, both belonging to the COX2 module) in human CIV assembly models (Fornuskova et al, 2010; Vidoni et al, 2017; Signes & Fernandez‐Vizarra, 2018; Timón‐Gómez et al, 2018). In control cells (Fig 6B), COX1 incorporation into SCs occurred in parallel with subunits COX4, COX5A, and unexpectedly with COX5B, clearly followed by COX6C. In COX2Δ cybrids, COX1 first accumulated in a subcomplex that also contained COX4 and COX5B, which was further incorporated into SCI+III2plus. In agreement, COX5B overexpression in COX2Δ cybrids led to the accumulation of the COX1‐dependent submodule and to its direct integration into SC I+III2plus (Fig 6C and D). Subunits COX5A and COX6C were not integrated in SCs despite being stabilized in smaller subcomplexes (Fig 6A and B). The faint COX4 signal into SCI+III2plus probably indicates that this subunit is rapidly detached from SCs due to the absence of COX5A. We were unable to evaluate the incorporation of subunits COX7A2 and COX7C due to the unavailability of efficient antibodies. However, complexome profiling (Fig 2) suggested that COX7A2 is incorporated into SCI+III2plus at an early stage together with COX1‐COX5B‐COX4. In contrast, COX7C would be directly integrated into SC I+III2plus at later stages, as it primarily interacts with COX1 within the respirasome (Letts et al, 2016), but was not detected in COX1 subcomplexes by proteomics. Altogether, these data indicate that CIV subunits are integrated into fully assembled SC I+III2 in a COX1‐dependent stepwise fashion that generates the SC I+III2plus intermediate in the absence of COX2.
Figure 6. Assembly kinetics of COX subunits into the COX7A2L‐containing SC I+III2 .
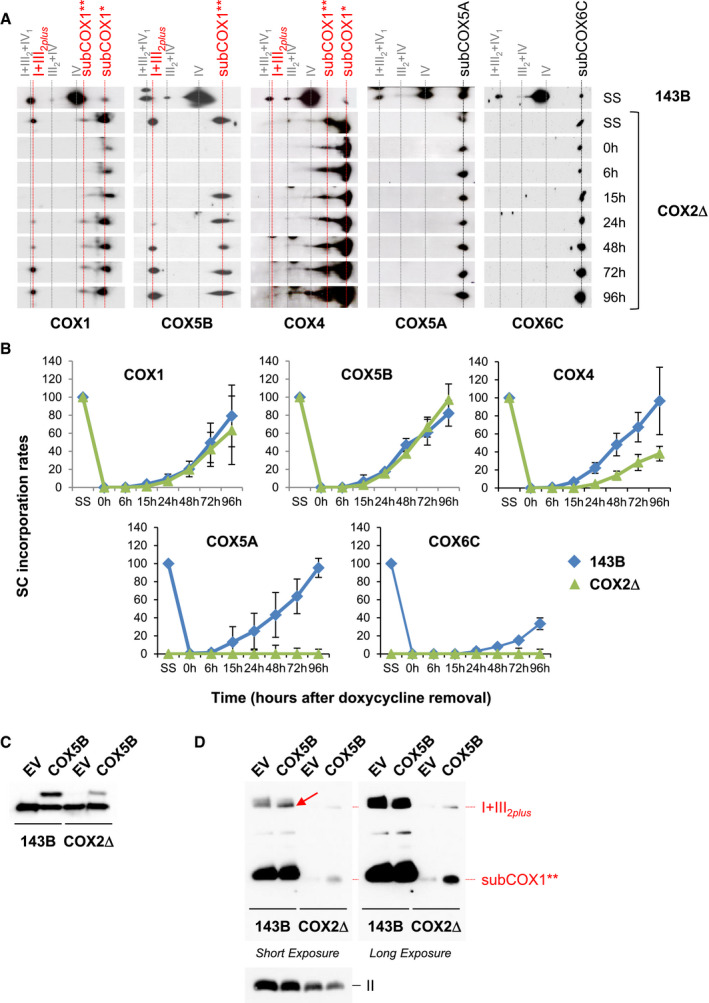
-
ADigitonized mitochondria from doxycycline‐treated 143B cells and COX2Δ cybrids were analyzed by 2D‐BN/SDS–PAGE and Western blot with the indicated antibodies.
-
BMean incorporation rates of COX subunits into SC I+III2plus from COX2Δ cybrids. Signals from three independent experiments were quantified and normalized by CII. Time‐point values are expressed as percentages of the untreated cells (SS) and indicated as mean ± SD. Control (143B) values for subunits COX4, COX5A, and COX6C were obtained from (Moreno‐Lastres et al, 2012).
-
C, DSDS–PAGE (C) and BN–PAGE (D) analyses from 143B cells and COX2Δ cybrids transiently transfected either with a COX5B‐Myc‐DDK construct or with the empty vector, followed by immunoblotting with antibodies against COX5B and CII subunit SDHA (loading control). The identities of MRC complexes and SCs are as in Fig 1. The red arrow points to the accumulation of overexpressed COX5B bound to SC I+III2 in control cells.
Binding of CIV subassemblies results in slower turnover of supercomplex I+III2
To test the structural stability of the different SC species, we performed digitonin titrations on mitochondrial membranes followed by BN–PAGE analysis (Fig EV5). Mild SC extraction conditions using digitonin‐to‐protein ratios (g/g) of 2:1 and 4:1 revealed no differences in SC levels between control and CIV‐KO cybrids. Stringent digitonin‐to‐protein ratios ranging between 8:1 and 20:1 led to the increasing dissociation of CIV subunits from SC I+III2, accumulating SC I+III2 in detriment of the respirasome in control cells, and of SC I+III2plus in COX2Δ cybrids. Consistently, SC I+III2 levels remained unaffected in COX1Δ cybrids even with a harsh 20:1 digitonin‐to‐protein ratio. Therefore, CI and CIII establish tight interactions within SC I+III2, to which CIV is loosely attached.
Figure EV5. Related to Fig 7. Complex IV is loosely attached to SC I+III 2 .
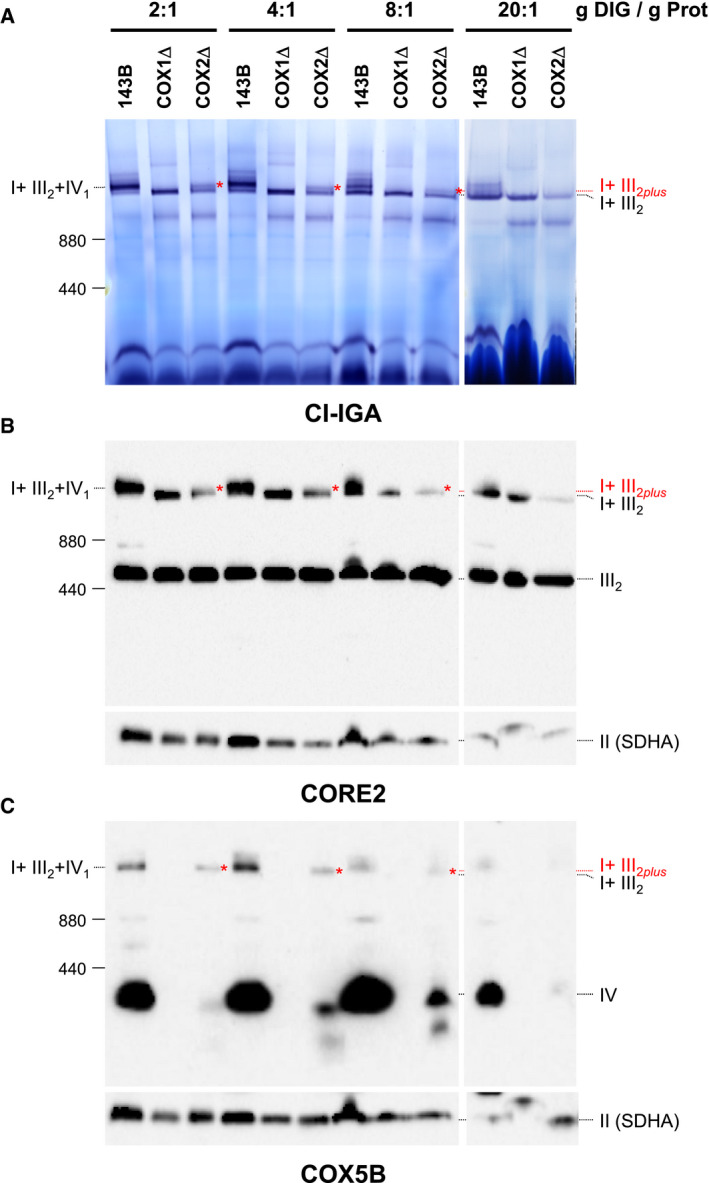
-
A–CMitochondria from control (143B), COX1Δ, and COX2Δ cybrids were extracted with increasing digitonin‐to‐mitochondrial protein ratios (ranging from 2 to 20 g/g) and analyzed by BN–PAGE followed by (A) CI‐IGA assays, or alternatively, by immunoblotting with antibodies against (B) the CIII subunit CORE2, or with (C) the CIV subunit COX5B. Red asterisks indicate the presence of SC I+III2plus in the COX2Δ cybrids. I+III2+IV1, SC containing CI, CIII2, and CIV; I+III2, SC containing CI and CIII2; I+III2plus, SC containing CI, CIII2, and a COX1 submodule.
Source data are available online for this figure.
We next performed radioactive pulse chase of mitochondrial translation products in cells grown in normal culture conditions and analyzed their incorporation into the individual MRC complexes and SCs by 2D‐BN/SDS–PAGE and autoradiography (Fig 7). After 30 min of pulse (T0) and a chase period of 4 h (T4), newly synthesized subunits of CI (ND1–ND6) and CIII (cytochrome b, CYTB) were mostly progressing from large CI subcomplexes (ND subunits) and from CIII2 (CYTB signal) to SCs. Between 4 and 8 h after chase initiation (times T4–T8), in control cells the COX1, COX2, and COX3 subunits were present in fully assembled CIV, but not yet integrated in SCs. As expected, COX2 and COX3 subunits were rapidly degraded in COX1Δ cybrids, losing their signal at T4. The COX2Δ cybrids, however, accumulated the COX1 and COX3 subunits in low molecular weight subcomplexes incapable to reach the CIV position. At 16 h after chase initiation (T16), control cells displayed newly synthesized and fully assembled respirasome I+III2+IV1. In both mutants, SC I+III2 was also fully assembled at T16, although there was a larger accumulation of CI subcomplexes and free CIII2 than in control cells, as previously observed (Figs 1C and 2B). In COX2Δ cybrids, at T16, the COX1 signal was still mostly accumulated in small subcomplexes, whereas the COX3 signal disappeared, suggesting its rapid degradation when COX2 is absent. After 24 h of chase (T24), the respirasome I+III2+IV1 remained clearly visible in control cells, as well as SC I+III2 in COX2Δ cybrids. However, in COX1Δ cybrids the SC I+III2 radioactive signals declined, while the relative proportion of subcomplexes increased. These results support the rapid turnover of SC I+III2 in the absence of COX1, as previously reported (Hornig‐Do et al, 2012). In contrast, in COX2Δ cybrids the SC I+III2 turnover rate was stabilized by binding of CIV subunits.
Figure 7. Stabilized turnover rate of SC I+III2 in COX2Δ cybrids.

Mitochondrial translation products were pulse‐labeled in control 143B cells (left panel), and COX1Δ (middle panel) and COX2Δ (right panel) cybrids with [35S]‐methionine for 30 min in the presence of anisomycin to inhibit cytosolic translation. For chase, cells were washed and incubated with fresh culture medium for the indicated time points (T0–T24 h). Radiolabeled mitochondrial proteins (indicated on the right) were separated by 2D‐BN/SDS–PAGE and visualized by autoradiography. An enlarged section of the SC region at T24 (delimited by a square) shows the co‐migration of COX1 with SC I+III2plus (black arrow) in the COX2Δ cybrids relative to the position of canonical SC I+III2 (gray arrow). The identities of MRC complexes and SCs (bottom) are as in Fig 1. Molecular weight markers are indicated on the left.
Data information: See also Fig EV5.Source data are available online for this figure.
Finally, we assessed whether the re‐distribution of the MRC complexes and SCs induced by the absence of COX1 and COX2 differentially affected the respiratory chain enzyme activities (Fig 8A). Besides a total loss of CIV and normal CII activities in both mutants, there was a significant decrease in CI and CIII activities only in COX1Δ cybrids. In agreement, the combined I+III activity was significantly decreased in COX1Δ cells, whereas it showed normal levels in the COX2Δ mutant, likely due to the stabilization and increased activation of CIII (Protasoni et al, 2020) as suggested by the increased levels of the catalytic RISP subunit (Fig 1A). These effects on CIII activation are interpreted as preferentially impacting the CIII fraction present in SC I+III2plus, given that CII+III activity, which relies on the free CIII2 fraction, is not affected in any of the cybrid lines (Fig 8A). These results indicate that the stabilization of SC I+III2 provided by the binding of a CIV submodule confers functional advantage to the remainder respiratory chain components in the COX2Δ mutants. Given the direct contact of specific CIV subunits with CI and CIII in the respirasome architecture as well as in the SC I+III2plus, this contact loss could presumably lead to a conformational change that would induce the active–deactive transition of CI within SC I+III2, in agreement with previous findings (Letts et al, 2019).
Figure 8. Multiple pathways for respirasome stabilization.
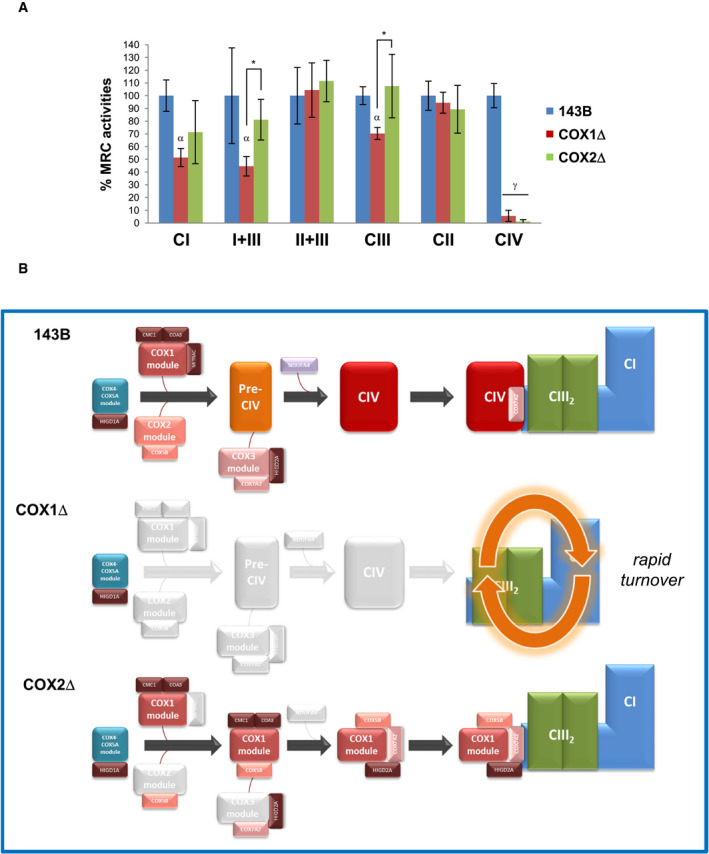
-
ARespiratory chain enzyme activities of control (blue), COX1Δ (red), and COX2Δ (green) cybrids. Enzyme activities were measured in three experimental replicates. Data are expressed as the percentages of control cells and indicated as means ± SD. Mann–Whitney U‐test was applied for statistical analyses. Variations between controls and mutants: α, P < 0.05; γ, P < 0.001. Specific variations between COX1Δ and COX2Δ mutants: *P < 0.05.
-
BStructural reorganization of SCs in the absence of CIV. In control cells (top), subunits COX4 and COX5A form an independent subcomplex that binds newly synthesized COX1, allowing the completion of CIV assembly and its further integration into respirasomes through canonical pathways. In COX1Δ cybrids (middle), SC I+III2 is rapidly degraded due to the absence of stabilizing CIV components. In COX2Δ cybrids (bottom), SC I+III2 is stabilized by the binding of a “non‐canonical” stable COX1 submodule (comprising subunits COX1, COX5B, and COX7A2 and the assembly factor HIGD2A), likely by keeping this SC in an assembly competent state for the further incorporation of CIV subunits/modules up to completion of respirasome biogenesis.
Discussion
Here, we investigated how cytochrome c oxidase (CIV) loss impacts the structural organization of the respiratory chain in human cultured cells depleted of subunits COX1 (Bruno et al, 1999) or COX2 (Balsa et al, 2012; Bourens & Barrientos, 2017a). We demonstrate that cells lacking holo‐CIV display alternative SC I+III2 species that differ in the presence of CIV subunits and SC assembly factors. The formation of alternative SCs requires the presence of COX1, which binds additional CIV subunits to form an unconventional module that is directly incorporated into fully assembled SC I+III2. This association yields a non‐canonical SC (SC I+III2plus) that is relatively unstable, but whose presence stabilizes SC I+III2 in an assembly competent state for the further incorporation of CIV subunits/modules. Therefore, our observations point toward a novel molecular adaptation through which the binding of a specific COX1 module to SC I+III2 is sufficient to stabilize it, necessarily implying the existence of an alternative pathway for CIV assembly in the SC context that likely occurs by the sequential incorporation of subunits and submodules to SC I+III2.
Our results provide an explanation for the unusual co‐migration of COX subassemblies with SC I+III2 previously observed by several research groups in cellular models of CIV deficiency in which COX2 biogenesis was severely impaired (Moreno‐Lastres et al, 2012; Stroud et al, 2015; Bourens & Barrientos, 2017a; Aich et al, 2018), supporting the idea that alternative CIV subcomplexes may bind directly to respirasome intermediates (Moreno‐Lastres et al, 2012). In fact, in mitochondria from patients with severe CIV deficiency, COX subunits are preferentially incorporated into SC I+III2 rather than into an unstable holo‐CIV (Lazarou et al, 2009; Kovářová et al, 2012), and here, we show that COX1 is essential for this process. Although the specific early steps of mammalian CIV biogenesis remain unconcluded, subunit COX1 is traditionally considered as the seed around which monomeric CIV is assembled (Nijtmans et al, 1998; Mick et al, 2012; Dennerlein et al, 2015; Signes & Fernandez‐Vizarra, 2018; Timón‐Gómez et al, 2018). In control cells, subunits COX4 and COX5A form an independent subcomplex that binds newly synthesized COX1, in agreement with the initial steps of the modular CIV biosynthetic pathway (Vidoni et al, 2017; Signes & Fernandez‐Vizarra, 2018; Fig 8B). Although this canonical pathway is commonly observed in CIV‐deficient cell lines (Williams et al, 2004; Stiburek et al, 2005; Fornuskova et al, 2010; Bourens et al, 2014), in all cases COX2 was expressed albeit at reduced levels, allowing completion of holo‐CIV assembly. We propose that the direct incorporation of nuclear‐encoded COX subunits into SC I+III2 occurs when COX2 levels are heavily compromised, limiting the progression of CIV assembly, but COX1 expression remains above the respiratory defect threshold (i.e., 40–50% of control values) (D'Aurelio et al, 2006). In this case, COX1 is partially stabilized together with subunits COX4, COX5B, and COX7A2 in a “non‐canonical” submodule that gets directly integrated within SC I+III2, to which COX7C is subsequently incorporated. Strikingly, subunits COX5B, COX7A2, and COX7C are generally believed to enter the holo‐COX biogenetic pathway at intermediate/late assembly stages (Fornuskova et al, 2010; Vidoni et al, 2017; Signes & Fernandez‐Vizarra, 2018). Therefore, our data indicate that the assembly of CIV within SCs may be a sequential process, that alternative assembly pathways may coexist for the formation of “free” versus “respirasome‐bound” CIV, and that there is no need for full assembly of holo‐CIV in order to interact with complexes I and III in SCs. Importantly, these possibilities are consistent with the heterogeneity of CIV species found in mouse mitochondria (Cogliati et al, 2016), and with the highly resolved structures of the mammalian respirasomes (Gu et al, 2016; Letts et al, 2016; Sousa et al, 2016; Wu et al, 2016; Guo et al, 2017). Our data also support the co‐existence of different respirasome species, where the integration of CIV in SCs could be alternatively mediated by COX7A protein isoforms (Letts & Sazanov, 2017) in collaboration with HIGD2A (Chen et al, 2012). This assembly factor accumulates within the unconventional COX1 submodule as well as within SC I+III2plus, suggesting its stabilizing role to allow the further association of CIV submodules.
The use of cybrid cells maintained in conditions that could differ in vivo, and which mostly express liver‐type CIV subunit isoforms, may not fully reflect what occurs in mammalian tissues (i.e., heart/skeletal muscle, lung, or testis) expressing their own specific isoforms. However, our study provides a rationale for the mechanisms governing the structural interdependency of MRC complexes I and IV that can be re‐examined in experimental models and patients with mitochondrial disorders. CI stability was hypothesized to be affected by the loss of CIV (Diaz et al, 2006; Li et al, 2007), but most experimental and clinical models of CIV deficiency show normal assembly or enzyme activities of CI and CIII (Rak et al, 2016). Instead, we show that CI instability is specifically driven by a severe reduction in COX1 levels, as formerly evidenced in mammalian cultured cells (D'Aurelio et al, 2006; Diaz et al, 2006; Li et al, 2007; Hornig‐Do et al, 2012) and patients (Karadimas et al, 2000). Although SC I+III2 becomes rapidly degraded in COX1Δ mutants (Hornig‐Do et al, 2012), small levels of holo‐CIV are sufficient to stabilize CI and SCs (D'Aurelio et al, 2006; Fornuskova et al, 2010), suggesting that a severe COX1 defect may only lead to combined CI and CIV deficiencies over a certain threshold. Overall evidence thus indicates that SC I+III2 formation is not sufficient to fully stabilize CI within SCs, as this process requires the binding of CIV structural components. In this regard, the minimal SC I+III2 stabilizing unit would require the association of a specific COX1 submodule to prevent its turnover, an unexpected mechanism for CI stabilization in the respirasomes with potential physiological implications. For instance, a CIV regulatory checkpoint could occur at the level of SCs through a mechanism for COX1 subunit stabilization when CIV biogenesis is compromised, i.e., to prevent the accumulation of redox reactive assembly intermediates, or to provide CIV repair mechanisms using the SC platform to yield optimal respiratory structures in an energetically inexpensive timely manner. In this way, alternative SC species could not only adjust the respiratory capacity to metabolic variations, but also allow turnover adaptations in diverse intra‐mitochondrial redox conditions to the availability of specific MRC structural components.
Materials and Methods
Cell lines and culture conditions
The COX1 deletion mutant (COX1Δ) lacks complex IV due to the homoplasmic m.6930G>A transition in the MT‐COI gene, which creates a stop codon that results in a predicted loss of the last 170 amino acids of the COX1 polypeptide (Bruno et al, 1999). The COX2 deletion mutant (COX2Δ) lacks holo‐COX due to the homoplasmic m.7896 G>A nonsense mutation in the MT‐COII gene, which presumably causes a loss of 123 amino acids at the C‐terminus of COX2 (Campos et al, 2001; Balsa et al, 2012). The COX18Δ HEK293T cells were previously described (Bourens & Barrientos, 2017a).
Cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco™) supplemented with 10% fetal bovine serum (FBS), 2 mM l‐glutamine, 1 mM sodium pyruvate, and antibiotics. To block mitochondrial translation, 15 μg/ml doxycycline was added for 6 days to the culture medium. Cells were grown in exponential conditions and harvested at different time points (0, 6, 15, 24, 48, 72, and 96 h) after doxycycline removal. Untreated cells (SS) were included as a positive control.
Cells were transiently transfected with the pCMV6‐Entry and pCMV6‐COX5B‐Myc‐DDK vectors using Lipofectamine 2000 (Thermo Fisher) according to the manufacturer's instructions, and collected after 48 h for subsequent analyses.
Respiratory chain enzyme activities
Mitochondrial respiratory chain enzyme activities were performed according to established methods (Medja et al, 2009), except for inhibition of the combined complex I+III activity with 12.5 μM rotenone and 18 μM antimycin A. Activities are expressed as nmol/min/mg protein.
Protein separation by SDS–PAGE and immunoblotting
Cells were homogenized in an extraction buffer containing 20 mM HEPES NaOH pH 7,4, 150 mM NaCl, 10% glycerol, and 1% Triton X‐100, supplemented with a Protease Inhibitor Cocktail (Roche). Protein concentration was measured with the BCA protein assay kit (Thermo Fisher). Protein extracts (30–50 μg) were separated on 10% SDS–PAGE gels and transferred to PROTAN (Schleicher & Schuell) nitrocellulose membranes by conventional procedures.
Blue‐native electrophoresis and in‐gel activity assays
Mitochondrial pellets were isolated from P150 cell culture flasks or 15‐cm dishes (Thermo Fisher) as described before (Perez‐Perez et al, 2016). In brief, pellets were resuspended in 200 μl buffer containing 1.5 M aminocaproic acid, 50 mM Bis–Tris (pH 7.0). Unless otherwise indicated, samples were solubilized using digitonin at a detergent‐to‐protein ratio of 2:1 and incubated on ice for 15 min. After centrifugation for 30 min at 20,000 g at 4°C, the supernatant was combined with 20 μl of sample buffer (750 mM aminocaproic acid, 50 mM Bis–Tris, 0.5 mM EDTA, 5% SERVA Blue G‐250) prior to loading. Pre‐cast NativePAGE™ 3–12% Bis–Tris gels (Invitrogen™) were loaded with 60–80 μg of mitochondrial protein and processed for blue‐native electrophoresis (BN–PAGE) as previously described (Moreno‐Lastres et al, 2012). Duplicate gels were further used for IGA assays and for second‐dimension electrophoresis (2D‐BN/SDS–PAGE) on 10% SDS–PAGE gels. After electrophoresis, proteins were transferred to nitrocellulose membranes at 40 V overnight and probed with antibodies.
Immunoprecipitation
One milligram of mitochondrial protein was solubilized in 600 μl of 4 g/g digitonin‐to‐protein buffer as for BNE analyses. After centrifugation for 30 min at 20,000 g at 4°C, 50 μg of the supernatant was separated as the input fraction. The remainder supernatant was co‐immunoprecipitated in resin spin columns (Pierce™ Co‐Immunoprecipitation Kit, Thermo Fisher) where 15 μg of primary antibodies had been previously immobilized. The mixture was gently incubated overnight at 4°C in a rotating shaker and centrifuged at 1,000 g for 1 min to separate the flow‐through fraction. The column was washed three times with lysis buffer containing 1% NP‐40, and proteins were eluted. The immunoprecipitate was divided into three aliquots, treated with 5× loading sample buffer, and heated at 95°C for 5 min prior to loading.
Antibody detection
Western blot was performed using primary antibodies raised against the following human OXPHOS subunits: NDUFS1 (GeneTex); NDUFA9, NDUFB8, CORE2, RISP, COX1, COX2, COX3, COX4, COX5A, and SDHA (Abcam‐MitoSciences); COX5B, COX6C, and NDUFB7 (Santa Cruz); β‐actin, CMC1, COA3, and HIGD2A (Sigma‐Aldrich); and COX7A2L (ProteinTech). Peroxidase‐conjugated anti‐mouse and anti‐rabbit IgGs were used as a secondary antibody (Invitrogen). Immunoreactive bands were detected with an ECL prime Western Blotting Detection Reagent (Amersham) in a ChemiDoc™ MP Imager (Bio‐Rad). Optical densities of the immunoreactive bands were measured using the Image Lab™ analysis software (Bio‐Rad) and the ImageJ software.
Protein identification by liquid chromatography coupled to tandem mass spectrometry
Gel bands of interest were excised from blue‐native gels. All samples were reduced by adding 10 mM DTT for 30 min at 37°C and alkylated with 55 mM iodoacetamide during 20 min in the dark. Next, digestion was performed by adding recombinant sequencing grade Trypsin (Roche) 1:20 (w/w) overnight at 37°C. The produced peptides were cleaned up with OMIX tips (Agilent technologies), eluted with 80% ACN in 0.1% TFA, dried in a SpeedVac, and resuspended in 0.1% formic acid. To identify proteins, the resulting tryptic peptide mixtures were analyzed by nanoliquid chromatography coupled to mass spectrometry. Peptides were loaded onto a C18‐A1 ASY‐Column 2 cm precolumn (Thermo Fisher) and then eluted onto a Biosphere C18 analytic column (C18, inner diameter 75 μm, 15 cm long, 3 μm particle size) (Nanoseparations) and separated using a 150 min gradient from 0 to 45% Buffer B (Buffer A: 0.1% formic acid/2% ACN; Buffer B: 0.1% formic acid in ACN) at a flow rate of 250 nl/min on a nanoEasy HPLC coupled to a nanoelectrospray ion source (Proxeon). Mass spectra were acquired on the LTQ‐Orbitrap Velos (Thermo Fisher) in the positive ion mode. Full‐scan MS spectra (m/z 400–1,800) were acquired in the Orbitrap at a resolution of 60,000 at m/z 400, and the 15 most intense ions were selected for collision‐induced dissociation (CID) fragmentation in the LTQ with a normalized collision energy of 35%. Precursor ion charge state screening and monoisotopic precursor selection were enabled. Singly charged ions and unassigned charge states were rejected. Dynamic exclusion was enabled with a repeat count of 1 and exclusion duration of 45 s. Peptide identification from raw data (MS/MS spectra) was carried out using a licensed version of search engine MASCOT 2.3.0 through Proteome Discoverer Software 1.2.0.208 (Thermo Fisher). Database search was performed against a UniProt–SwissProt with taxonomy restriction to human (date 2012/12/11; 20,233 sequences). The following parameters were used for the searches: tryptic cleavage after Arg and Lys, up to two missed cleavage sites allowed, tolerances of 10 ppm for precursor ions, and 0.8 Da for MS/MS fragment ions. Oxidation of methionine was selected as dynamic modification and carbamidomethylation of cysteine as fixed modification. Search against decoy database (integrated decoy approach in MASCOT) was used for FDR calculation, and this filter was applied to MASCOT results. The acceptance criteria for protein identification were FDR < 1% and at least one peptide identified with high confidence (CI > 95%).
Complexome profiling
Sample preparation and blue‐native electrophoresis (BNE) of cultured cell pellets were previously described (Wittig et al, 2006). Blue‐native gels were fixed in 50% (v/v) methanol, 10% (v/v) acetic acid, and 10 mM ammonium acetate for 30 min and stained with Coomassie (0.025% SERVA Blue G, 10% (v/v) acetic acid). Each lane was cut into 60 equal fractions (50 shown in the figures for simplification) and collected in 96 filter well plates (30–40 μm PP/PE, Pall Corporation). The gel pieces were distained in 60% methanol, 50 mM ammonium bicarbonate (ABC). Solutions were removed by centrifugation for 2 min at 1,500 g. Proteins were reduced in 10 mM DTT and 50 mM ABC for 1 h at 56°C and alkylated for 45 min in 30 mM iodoacetamide. Samples were digested for 16 h with trypsin (sequencing grade, Promega) at 37°C in 50 mmol/l ABC, 0.01% Protease Max (Promega), and 1 mM CaCl2. Peptides were eluted in 30% acetonitrile and 3% formic acid, centrifuged into a fresh 96‐well plate, dried in SpeedVac, and resolved in 1% acetonitrile and 0.5% formic acid.
Liquid chromatography/mass spectrometry (LC/MS) was performed on Thermo Fisher™ Q Exactive Plus equipped with an ultra‐high performance liquid chromatography unit (Dionex Ultimate 3000) and a Nanospray Flex Ion Source (Thermo Fisher). Peptides were loaded on a C18 reversed‐phase precolumn (Thermo Fisher) followed by separation on a with 2.4 μm Reprosil C18 resin (Dr. Maisch GmbH) in‐house packed PicoTip emitter tip (diameter 100 μm, 15 cm from New Objectives) using a gradient from 4% acetonitrile, 0.1% formic acid to 50% eluent B (99% acetonitrile, 0.1% formic acid) for 30 min with a flow rate 400 nl/min and washout with 99% B for 5 min. MS data were recorded by data‐dependent acquisition. The full MS scan range was 300 to 2,000 m/z with resolution of 70,000 and an automatic gain control (AGC) value of 3*10E6 total ion counts with a maximal ion injection time of 160 ms. Only higher charged ions (2+) were selected for MS/MS scans with a resolution of 17,500, an isolation window of 2 m/z, and an automatic gain control value set to 10E5 ions with a maximal ion injection time of 150 ms. MS1 data were acquired in profile mode.
Xcalibur Raw files were analyzed by proteomics software Max Quant (1.6.1.0) (Cox & Mann, 2008). The enzyme specificity was set to trypsin, and missed cleavages were limited to 2. Following variable modifications were selected: at N‐terminus acetylation (+42.01), oxidation of methionine (+15.99), as fixed modification carbamidomethylation (+57.02) on cysteines. Human reference proteome set from UniProt (download 2/2018, 71,785 entries) was used to identify peptides and proteins. False discovery rate (FDR) was set to 1%. Identifications from reverse decoy database, by site, and known contaminants were excluded. Abundance profiles were generated by NOVA software (Giese et al, 2015) using intensity‐based absolute quantification (IBAQ) values from MaxQuant (Schwanhäusser et al, 2011). The sum of all IBAQ values of data sets was normalized to the corresponding control set. Protein abundance within native lanes was normalized to maximum appearance to enable comparison of mitochondrial complexes between controls and mutants. Slice number of the maximum appearance of mitochondrial CIII dimer (483,695 Da), CIV (220,156 Da), CV (618,824 Da), and respiratory SC I+III2+IV1 (1,663,827 Da) was used for native mass calibration. The equation [f(x) = 8,716.6*e^(0.1302x), R 2 = 0.9985] obtained by exponential regression was used to calculate the native masses of each slice. Gel slices 51–60 (molecular masses ranging between 6,770 and 21,530 kDa) were excluded from the final figures for space reasons, as they did not contain proteins of interest.
Mitochondrial protein synthesis
Mitochondrial protein synthesis was analyzed by pulse labeling in 80% confluent cells, as described before (Moreno‐Lastres et al, 2012) with some adaptations. Approximately 2.5 million cybrids were incubated for 20 min in DMEM (supplemented with 10% FBS, 50 μg/ml uracil, 1 mM sodium pyruvate) devoid of methionine. The media were then supplemented with 100 μg/ml anisomycin for 10 min to inhibit cytoplasmic protein synthesis, followed by 30‐min pulse labeling in the presence of 100 μCi of [35S]‐methionine. For pulse samples, after incubation cells were washed once with PBS and collected. For chase samples, cells were washed three times with PBS and incubated in regular culture medium for the indicated chase times. Cells were harvested by trypsinization, and mitochondria‐enriched fractions were prepared for 2D‐BN/SDS–PAGE as described above. After electrophoresis, gels were transferred to nitrocellulose membranes and exposed to X‐ray films.
Statistical data analysis
Experiments were performed at least in triplicate, and results were presented as mean ± standard deviation (SD) values. Statistical P values were obtained by the application of the Mann–Whitney U‐test using the GraphPad Prism v.7.0e and SPSS v.21.0 softwares.
Author contributions
Conceptualization: AB and CU; Methodology, Investigation, and Validation: TL‐J, RP‐P, FF, AT‐G, IW, AP, PS‐L, IG‐C, JA, MAM, AB, and CU; Software: IW; Formal Analysis: TL‐J, RP‐P, IW, and CU; Resources: IW, AB, and CU; Writing—Original Draft: CU; Writing—Reviewing and Editing: TL‐J, FF, AT‐G, IW, AP, JA, MAM, AB, and CU; Supervision: AB and CU; Funding Acquisition: FF, IW, MAM, AB, and CU.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 6
Source Data for Figure 7
Acknowledgements
We thank Dr. Erika Fernández‐Vizarra for critically reading the manuscript, and Prof. G. Manfredi and Prof. J.A. Enríquez for providing the mutant cybrids. Nano‐LC/ESI‐MS analyses were carried out at the Proteomics Facility UCM‐FPCM/ProteoRed (Spain). Research was supported by Instituto de Salud Carlos III‐MINECO/European FEDER Funds grants PI14‐00209 and PI17‐00048 (to CU) and PI18‐00374 (to MAM), by Comunidad Autónoma de Madrid/ERDF‐ESF grant P2018/BAA‐4403 (to CU), by NIH‐RO1 grants GM105781 (to AB and CU) and GM112179 (to AB), NIH‐R35 grant GM118141 (to AB), MDA grant MDA‐381828 (to AB), an AHA development grant 14SDG20040003 (to FF), by Bundesministerium für Bildung und Forschung (BMBF 01GM1906D; mitoNET–Deutsches Netzwerk für mitochondriale Erkrankungen), and by Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 815, Project Z1 (to IW).
The EMBO Journal (2020) 39: e103912
Data availability
The complexome profiling data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD017840 (http://www.ebi.ac.uk/pride/archive/projects/PXD017840).
References
- Acín‐Pérez R, Bayona‐Bafaluy MP, Fernández‐Silva P, Moreno‐Loshuertos R, Pérez‐Martos A, Bruno C, Moraes CT, Enríquez JA (2004) Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol Cell 13: 805–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acín‐Pérez R, Fernández‐Silva P, Peleato ML, Pérez‐Martos A, Enriquez JA (2008) Respiratory active mitochondrial supercomplexes. Mol Cell 32: 529–539 [DOI] [PubMed] [Google Scholar]
- Aich A, Wang C, Chowdhury A, Ronsör C, Pacheu‐Grau D, Richter‐Dennerlein R, Dennerlein S, Rehling P (2018) COX16 promotes COX2 metallation and assembly during respiratory complex IV biogenesis. Elife 7: e32572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alston CL, Heidler J, Dibley MG, Kremer LS, Taylor LS, Fratter C, French CE, Glasgow RIC, Feichtinger RG, Delon I et al (2018) Bi‐allelic mutations in NDUFA6 establish its role in early‐onset isolated mitochondrial complex I deficiency. Am J Hum Genet 103: 592–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsa E, Marco R, Perales‐Clemente E, Szklarczyk R, Calvo E, Landázuri MO, Enríquez JA (2012) NDUFA4 is a subunit of complex IV of the mammalian electron transport chain. Cell Metab 16: 378–386 [DOI] [PubMed] [Google Scholar]
- Blanchi C, Genova ML, Castelli GP, Lenaz G, Bianchi C, Genova ML, Parenti Castelli G, Lenaz G (2004) The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. J Biol Chem 279: 36562–36569 [DOI] [PubMed] [Google Scholar]
- Blaza JN, Serreli R, Jones AJY, Mohammed K, Hirst J (2014) Kinetic evidence against partitioning of the ubiquinone pool and the catalytic relevance of respiratory‐chain supercomplexes. Proc Natl Acad Sci USA 111: 15735–15740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourens M, Boulet A, Leary SC, Barrientos A (2014) Human COX20 cooperates with SCO1 and SCO2 to mature COX2 and promote the assembly of cytochrome c oxidase. Hum Mol Genet 23: 2901–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourens M, Barrientos A (2017a) Human mitochondrial cytochrome c oxidase assembly factor COX18 acts transiently as a membrane insertase within the subunit 2 maturation module. J Biol Chem 292: 7774–7783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourens M, Barrientos A (2017b) A CMC1 ‐knockout reveals translation‐independent control of human mitochondrial complex IV biogenesis. EMBO Rep 18: 477–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno C, Martinuzzi A, Tang Y, Andreu AL, Pallotti F, Bonilla E, Shanske S, Fu J, Sue CM, Angelini C et al (1999) A stop‐codon mutation in the human mtDNA cytochrome c oxidase I gene disrupts the functional structure of complex IV. Am J Hum Genet 65: 611–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvaruso MA, Willems P, den Brand M, Valsecchi F, Kruse S, Palmiter R, Smeitink J, Nijtmans L (2012) Mitochondrial complex III stabilizes complex I in the absence of NDUFS4 to provide partial activity. Hum Mol Genet 21: 115–120 [DOI] [PubMed] [Google Scholar]
- Campos Y, García‐Redondo A, Fernández‐Moreno MA, Martínez‐Pardo M, Goda G, Rubio JC, Martín MA, del Hoyo P, Cabello A, Bornstein B et al (2001) Early‐onset multisystem mitochondrial disorder caused by a nonsense mutation in the mitochondrial DNA cytochrome c oxidase II gene. Ann Neurol 50: 409–413 [DOI] [PubMed] [Google Scholar]
- Chen YC, Taylor EB, Dephoure N, Heo JM, Tonhato A, Papandreou I, Nath N, Denko NC, Gygi SP, Rutter J (2012) Identification of a protein mediating respiratory supercomplex stability. Cell Metab 15: 348–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogliati S, Calvo E, Loureiro M, Guaras AM, Nieto‐Arellano R, Garcia‐Poyatos C, Ezkurdia I, Mercader N, Vázquez J, Enriquez JA (2016) Mechanism of super‐assembly of respiratory complexes III and IV. Nature 539: 579–582 [DOI] [PubMed] [Google Scholar]
- Cox J, Mann M (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.‐range mass accuracies and proteome‐wide protein quantification. Nat Biotechnol 26: 1367–1372 [DOI] [PubMed] [Google Scholar]
- D'Aurelio M, Gajewski CD, Lenaz G, Manfredi G (2006) Respiratory chain supercomplexes set the threshold for respiration defects in human mtDNA mutant cybrids. Hum Mol Genet 15: 2157–2169 [DOI] [PubMed] [Google Scholar]
- Davies KM, Blum TB, Kühlbrandt W (2018) Conserved in situ arrangement of complex I and III2 in mitochondrial respiratory chain supercomplexes of mammals, yeast, and plants. Proc Natl Acad Sci USA 115: 3024–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoudi M, Kotarsky H, Hansson E, Kallijärvi J, Fellman V (2016) COX7A2L/SCAFI and pre‐complex III modify respiratory chain supercomplex formation in different mouse strains with a Bcs1 l mutation. PLoS ONE 11: e0168774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennerlein S, Oeljeklaus S, Jans D, Hellwig C, Bareth B, Jakobs S, Deckers M, Warscheid B, Rehling P (2015) MITRAC7 acts as a COX1‐specific chaperone and reveals a checkpoint during cytochrome c oxidase assembly. Cell Rep 12: 1644–1655 [DOI] [PubMed] [Google Scholar]
- Diaz F, Fukui H, Garcia S, Moraes CT (2006) Cytochrome c oxidase is required for the assembly/stability of respiratory complex I in mouse fibroblasts. Mol Cell Biol 26: 4872–4881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz F, Enríquez JA, Moraes CT (2012) Cells lacking Rieske iron‐sulfur protein have a reactive oxygen species‐associated decrease in respiratory complexes I and IV. Mol Cell Biol 32: 415–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor JG, Hirst J (2018) Mitochondrial supercomplexes do not enhance catalysis by quinone channeling. Cell Metab 28: 525–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Vizarra E, Tiranti V, Zeviani M (2009) Assembly of the oxidative phosphorylation system in humans: what we have learned by studying its defects. Biochim Biophys Acta 1793: 200–211 [DOI] [PubMed] [Google Scholar]
- Fernández‐Vizarra E, Zeviani M (2015) Nuclear gene mutations as the cause of mitochondrial complex III deficiency. Front Genet 6: 134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornuskova D, Stiburek L, Wenchich L, Vinsova K, Hansikova H, Zeman J (2010) Novel insights into the assembly and function of human nuclear‐encoded cytochrome c oxidase subunits 4, 5a, 6a, 7a and 7b. Biochem J 428: 363–374 [DOI] [PubMed] [Google Scholar]
- Gaignard P, Eyer D, Lebigot E, Oliveira C, Therond P, Boutron A, Slama A (2017) UQCRC2 mutation in a patient with mitochondrial complex III deficiency causing recurrent liver failure, lactic acidosis and hypoglycemia. J Hum Genet 62: 729–731 [DOI] [PubMed] [Google Scholar]
- Ghezzi D, Zeviani M (2018) Human diseases associated with defects in assembly of OXPHOS complexes. Essays Biochem 62: 271–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese H, Ackermann J, Heide H, Bleier L, Drose S, Wittig I, Brandt U, Koch I (2015) NOVA: a software to analyze complexome profiling data. Bioinformatics 31: 440–441 [DOI] [PubMed] [Google Scholar]
- Gu J, Wu M, Guo R, Yan K, Lei J, Gao N, Yang M (2016) The architecture of the mammalian respirasome. Nature 537: 639–643 [DOI] [PubMed] [Google Scholar]
- Guerrero‐Castillo S, Baertling F, Kownatzki D, Wessels HJ, Arnold S, Brandt U, Nijtmans L (2016) The assembly pathway of mitochondrial respiratory chain complex I. Cell Metab 25: 1–12 [DOI] [PubMed] [Google Scholar]
- Guo R, Zong S, Wu M, Gu J, Yang M (2017) Architecture of human mitochondrial respiratory megacomplex I 2 III 2 IV 2. Cell 170: 1247–1257 [DOI] [PubMed] [Google Scholar]
- Hatakeyama H, Goto Y (2017) Respiratory chain complex disorganization impairs mitochondrial and cellular integrity. Am J Pathol 187: 110–121 [DOI] [PubMed] [Google Scholar]
- Hirst J (2018) Open questions: respiratory chain supercomplexes—why are they there and what do they do? BMC Biol 16: 111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig‐Do H‐T, Tatsuta T, Buckermann A, Bust M, Kollberg G, Rötig A, Hellmich M, Nijtmans L, Wiesner RJ (2012) Nonsense mutations in the COX1 subunit impair the stability of respiratory chain complexes rather than their assembly. EMBO J 31: 1293–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Shiba S, Horie‐Inoue K, Shimokata K, Inoue S (2013) A stabilizing factor for mitochondrial respiratory supercomplex assembly regulates energy metabolism in muscle. Nat Commun 4: 2147 [DOI] [PubMed] [Google Scholar]
- Karadimas CL, Greenstein P, Sue CM, Joseph JT, Tanji K, Haller RG, Taivassalo T, Davidson MM, Shanske S, Bonilla E et al (2000) Recurrent myoglobinuria due to a nonsense mutation in the COX I gene of mitochondrial DNA. Neurology 55: 644–649 [DOI] [PubMed] [Google Scholar]
- Kovářová N, Čížková Vrbacká A, Pecina P, Stránecký V, Pronicka E, Kmoch S, Houštěk J (2012) Adaptation of respiratory chain biogenesis to cytochrome c oxidase deficiency caused by SURF1 gene mutations. Biochim Biophys Acta 1822: 1114–1124 [DOI] [PubMed] [Google Scholar]
- Lamantea E, Carrara F, Mariotti C, Morandi L, Tiranti V, Zeviani M (2002) A novel nonsense mutation (Q352X) in the mitochondrial cytochrome b gene associated with a combined deficiency of complexes I and III. Neuromuscul Dis 12: 49–52 [DOI] [PubMed] [Google Scholar]
- Lapuente‐Brun E, Moreno‐Loshuertos R, Acin‐Perez R, Latorre‐Pellicer A, Colas C, Balsa E, Perales‐Clemente E, Quiros PMM, Calvo E, Rodriguez‐Hernandez MAA et al (2013) Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340: 1567–1570 [DOI] [PubMed] [Google Scholar]
- Lazarou M, Smith SM, Thorburn DR, Ryan MT, McKenzie M (2009) Assembly of nuclear DNA‐encoded subunits into mitochondrial complex IV, and their preferential integration into supercomplex forms in patient mitochondria. FEBS J 276: 6701–6713 [DOI] [PubMed] [Google Scholar]
- Letts JA, Fiedorczuk K, Sazanov LA (2016) The architecture of respiratory supercomplexes. Nature 537: 644–648 [DOI] [PubMed] [Google Scholar]
- Letts JA, Sazanov LA (2017) Clarifying the supercomplex: the higher‐order organization of the mitochondrial electron transport chain. Nat Struct Mol Biol 24: 800–808 [DOI] [PubMed] [Google Scholar]
- Letts JA, Fiedorczuk K, Degliesposti G, Skehel M, Sazanov LA (2019) Structures of respiratory supercomplex I+III2 reveal functional and conformational crosstalk. Mol Cell 75: 1131–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, D'Aurelio M, Deng JH, Park JS, Manfredi G, Hu P, Lu J, Bai Y (2007) An assembled complex IV maintains the stability and activity of complex I in mammalian mitochondria. J Biol Chem 282: 17557–17562 [DOI] [PubMed] [Google Scholar]
- Lobo‐Jarne T, Ugalde C (2018) Respiratory chain supercomplexes: structures, function and biogenesis. Semin Cell Dev Biol 76: 179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo‐Jarne T, Nývltová E, Pérez‐Pérez R, Timón‐Gómez A, Molinié T, Choi A, Mourier A, Fontanesi F, Ugalde C, Barrientos A (2018) Human COX7A2L regulates complex III biogenesis and promotes supercomplex organization remodeling without affecting mitochondrial bioenergetics. Cell Rep 25: 1786–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Fabuel I, Le Douce J, Logan A, James AM, Bonvento G, Murphy MP, Almeida A, Bolaños JP (2016) Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc Natl Acad Sci USA 113: 13063–13068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranzana E, Barbero G, Falasca AI, Lenaz G, Genova ML (2013) Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid Redox Signal 19: 1469–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medja F, Allouche S, Frachon P, Jardel C, Malgat M, de Camaret BM, Slama A, Lunardi J, Mazat JP, Lombès A (2009) Development and implementation of standardized respiratory chain spectrophotometric assays for clinical diagnosis. Mitochondrion 9: 331–339 [DOI] [PubMed] [Google Scholar]
- Mick DU, Dennerlein S, Wiese H, Reinhold R, Pacheu‐Grau D, Lorenzi I, Sasarman F, Weraarpachai W, Shoubridge EA, Warscheid B et al (2012) MITRAC links mitochondrial protein translocation to respiratory‐chain assembly and translational regulation. Cell 151: 1528–1541 [DOI] [PubMed] [Google Scholar]
- Milenkovic D, Blaza JN, Larrson N‐G, Hirst J (2017) The enigma of the respiratory chain supercomplex. Cell Metab 25: 765–776 [DOI] [PubMed] [Google Scholar]
- Moreno‐Lastres D, Fontanesi F, García‐Consuegra I, Martín MA, Arenas J, Barrientos A, Ugalde C (2012) Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab 15: 324–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijtmans LGJ, Taanman JW, Muijsers AO, Speijer D, Van Den Bogert C (1998) Assembly of cytochrome‐c oxidase in cultured human cells. Eur J Biochem 254: 389–394 [DOI] [PubMed] [Google Scholar]
- Perez‐Perez R, Lobo‐Jarne T, Milenkovic D, Mourier A, Bratic A, Garcia‐Bartolome A, Fernandez‐Vizarra E, Cadenas S, Delmiro A, Garcia‐Consuegra I et al (2016) COX7A2L is a mitochondrial complex III binding protein that stabilizes the III2 + IV supercomplex without affecting respirasome formation. Cell Rep 16: 2387–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protasoni M, Pérez‐Pérez R, Lobo‐Jarne T, Harbour ME, Ding S, Peñas A, Diaz F, Moraes CT, Fearnley IM, Zeviani M et al (2020) Respiratory supercomplexes act as a platform for complex III‐mediated maturation of human mitochondrial complexes I and IV. The EMBO Journal 39: e102817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak M, Benit P, Chretien D, Bouchereau J, Schiff M, El‐Khoury R, Tzagoloff A, Rustin P (2016) Mitochondrial cytochrome c oxidase deficiency. Clin Sci 130: 393–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H, Pfeiffer K (2000) Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J 19: 1777–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H, Pfeiffer K (2001) The ratio of oxidative phosphorylation complexes I–V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. J Biol Chem 276: 37861–37867 [DOI] [PubMed] [Google Scholar]
- Schägger H, de Coo R, Bauer MF, Hofmann S, Godinot C, Brandt U (2004) Significance of respirasomes for the assembly/stability of human respiratory chain complex I. J Biol Chem 279: 36349–36353 [DOI] [PubMed] [Google Scholar]
- Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M (2011) Global quantification of mammalian gene expression control. Nature 473: 337–342 [DOI] [PubMed] [Google Scholar]
- Signes A, Fernandez‐Vizarra E (2018) Assembly of mammalian oxidative phosphorylation complexes I–V and supercomplexes. Essays Biochem 62: 255–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa JS, Mills DJ, Vonck J, Kühlbrandt W (2016) Functional asymmetry and electron flow in the bovine respirasome. Elife 5: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiburek L, Vesela K, Hansikova H, Pecina P, Tesarova M, Cerna L, Houstek J, Zeman J (2005) Tissue‐specific cytochrome c oxidase assembly defects due to mutations in SCO2 and SURF1. Biochem J 392: 625–632 16083427 [Google Scholar]
- Stroud DA, Maher MJ, Lindau C, Vögtle F‐N, Frazier AE, Surgenor E, Mountford H, Singh AP, Bonas M, Oeljeklaus S et al (2015) COA6 is a mitochondrial complex IV assembly factor critical for biogenesis of mtDNA‐encoded COX2. Hum Mol Genet 24: 5404–5415 [DOI] [PubMed] [Google Scholar]
- Stroud DA, Surgenor EE, Formosa LE, Reljic B, Frazier AE, Dibley MG, Osellame LD, Stait T, Beilharz TH, Thorburn DR et al (2016) Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature 538: 1–17 [DOI] [PubMed] [Google Scholar]
- Timón‐Gómez A, Nývltová E, Abriata LALA, Vila AJAJ, Hosler J, Barrientos A (2018) Mitochondrial cytochrome c oxidase biogenesis: recent developments. Semin Cell Dev Biol 76: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillard M, Meunier B, Rappaport F (2011) Questioning the functional relevance of mitochondrial supercomplexes by time‐resolved analysis of the respiratory chain. Proc Natl Acad Sci USA 108: E1027–E1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker EJ, Wanschers BFJ, Szklarczyk R, Mountford HS, Wijeyeratne XW, van den Brand MAM, Leenders AM, Rodenburg RJ, Reljić B, Compton AG et al (2013) Mutations in the UQCC1‐interacting protein, UQCC2, cause human complex III deficiency associated with perturbed cytochrome b protein expression. PLoS Genet 9: e1004034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni S, Harbour ME, Guerrero‐Castillo S, Signes A, Ding S, Fearnley IM, Taylor RW, Tiranti V, Arnold S, Fernandez‐Vizarra E et al (2017) MR‐1S interacts with PET100 and PET117 in module‐based assembly of human cytochrome c oxidase. Cell Rep 18: 1727–1738 [DOI] [PubMed] [Google Scholar]
- Wanschers BFJ, Szklarczyk R, van den Brand MAM, Jonckheere A, Suijskens J, Smeets R, Rodenburg RJ, Stephan K, Helland IB, Elkamil A et al (2014) A mutation in the human CBP4 ortholog UQCC3 impairs complex III assembly, activity and cytochrome b stability. Hum Mol Genet 23: 6356–6365 [DOI] [PubMed] [Google Scholar]
- Williams SL, Valnot I, Rustin P, Taanman JW (2004) Cytochrome c oxidase subassemblies in fibroblast cultures from patients carrying mutations in COX10, SCO1, or SURF1. J Biol Chem 279: 7462–7469 [DOI] [PubMed] [Google Scholar]
- Wittig I, Carrozzo R, Santorelli FMM, Schägger H (2006) Supercomplexes and subcomplexes of mitochondrial oxidative phosphorylation. Biochim Biophys Acta 1757: 1066–1072 [DOI] [PubMed] [Google Scholar]
- Wu M, Gu J, Guo R, Huang Y, Yang M (2016) Structure of mammalian respiratory supercomplex I1III2IV1. Cell 167: 1598–1609 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 6
Source Data for Figure 7
Data Availability Statement
The complexome profiling data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD017840 (http://www.ebi.ac.uk/pride/archive/projects/PXD017840).


