Abstract
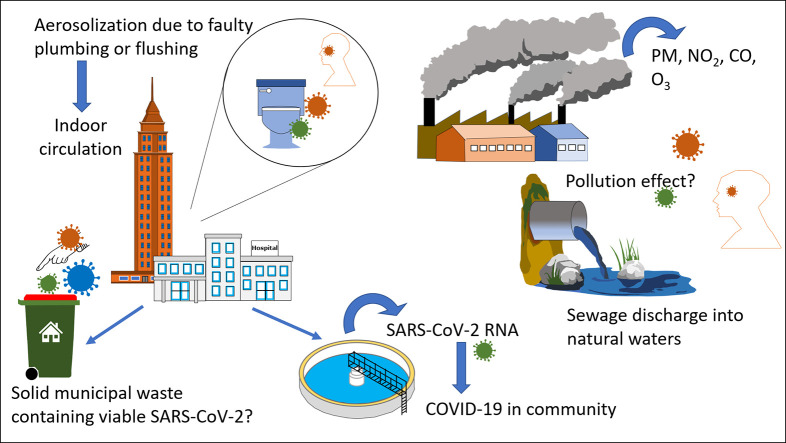
The coronavirus disease 2019 (COVID-19) is spreading globally having a profound effect on lives of millions of people, causing worldwide economic disruption. Curbing the spread of COVID-19 and future pandemics may be accomplished through understanding the environmental context of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and adoption of effective detection tools and mitigation policies. This article aims to examine the latest investigations on SARS-CoV-2 plausible environmental transmission modes, employment of wastewater surveillance for early detection of COVID-19, and elucidating the role of solid waste, water, and atmospheric quality on viral infectivity. Transmission of SARS-CoV-2 via faecal-oral or bio-aerosols lacks robust evidence and remains debatable. However, improper disinfection and defected plumbing systems in indoor environments such as hospitals and high-rise towers may facilitate the transport of virus-laden droplets of wastewater causing infection. Clinical and epidemiological studies are needed to present robust evidence that SARS-CoV-2 is transmissible via aerosols, though quantification of virus-laden aerosols at low concentrations presents a challenge. Wastewater surveillance of SARS-CoV-2 can be an effective tool in early detection of outbreak and determination of COVID-19 prevalence within a population, complementing clinical testing and providing decision makers guidance on restricting or relaxing movement. While poor air quality increases susceptibility to diseases, evidence for air pollution impact on COVID-19 infectivity is not available as infections are dynamically changing worldwide. Solid waste generated by households with infected individuals during the lockdown period may facilitate the spread of COVID-19 via fomite transmission route but has received little attention from the scientific community. Water bodies receiving raw sewage may pose risk of infection but this has not been investigated to date. Overall, our understanding of the environmental perspective of SARS-CoV-2 is imperative to detecting outbreak and predicting pandemic severity, allowing us to be equipped with the right tools to curb any future pandemic.
Keywords: COVID-19, Environmental context, Wastewater-based epidemiology, Air pollution, Modes of transmission, Solid waste
Graphical abstract
1. Introduction
The outbreak of the novel coronavirus SARS-CoV-2 was reported in Wuhan, China at the end of December 2019 where cases of pneumonia in people associated with the wet market had been confirmed as cases of the novel coronavirus (Holshue et al., 2020). Shortly after, it was declared as a pandemic by the World Health Organization (WHO) with over 8.7 million COVID-19 confirmed cases as of June 21, 2020 (WHO, 2020c). The spread of the pandemic resulted in worldwide lockdowns in effort to “flatten the curve” and not overwhelm health care institutions (Lau et al., 2020). The key to interrupting the chain of transmission is isolating infected people and tracing back those who they have interacted with (Khanna et al., 2020; Larsen et al., 2020). Human-to-human transmission occurs during the virus incubation period of 2–10 days, with spread being facilitated through droplets, contaminated hands and surfaces (Enyoh et al., 2020; Kampf et al., 2020). An inter-personal distance of at least 2 m was prescribed to minimize the risk of contagion through the droplets (CDC, 2020; Setti et al., 2020c).
Numerous research articles have been recently published assessing the plausible modes of environmental transmission of SARS-CoV-2 and spread of COVID-19 through environmental analysis. There is no conclusive evidence for aerosol or faecal-oral transmission of SARS-CoV-2 despite several researchers considering them as plausible routes that may explain the high infectivity and global spread of COVID-19 (Chen et al., 2020; van Doremalen et al., 2020; Wang et al., 2020a). Additionally, environmental parameters have been utilized to assess the spread of COVID-19 within a population through wastewater surveillance as well as attempts to predict the severity of the pandemic through analysis of atmospheric pollution of cities. Solid waste management is another aspect of paramount importance that may contribute to the spread of the pandemic within the community but that has not received much attention within the scientific community. Only two studies were found analyzing SARS-CoV-2 RNA in natural water bodies receiving raw or treated sewage waters with differing results, and it is unclear if there is any risk of infection, particularly in recreational waters where people are in frequent contact (Guerrero-Latorre et al., 2020; Odih et al., 2020; Usman et al., 2020). From the literature studied, concerns of COVID-19 infection through environmental contact pertain mainly to areas that lack proper sanitation and wastewater treatment, lack adequate solid waste management infrastructure, in areas where raw sewage is discharged directly into natural water bodies, and in cities where air pollution is problematic. Although there has not been any evidence presented that validates environmental transmission routes, the lack of sanitary and waste infrastructure is likely to increase the probability of human contact with contaminated material, the impact of which is yet to be investigated.
The importance of the understanding of SARS-CoV-2 within the environmental context is to assist in establishing effective policies for mitigating the transmission of the disease and combating future pandemics. Only a small number of published articles have investigated the environmental perspective of COVID-19. Barcelo (2020) presented an opinion paper summarizing environmental and health aspects related to the monitoring, fate, and treatment solutions for COVID-19. The objectives of this review are to present the latest investigations on SARS-CoV-2 plausible environmental transmission modes (aerosol and faecal-oral pathways) and employment of environmental tools for early detection of COVID-19 (wastewater surveillance) and prediction of severity of viral infections through associations with atmospheric pollution. The overall aim is to assist in setting policy priorities for mitigating the current and future pandemics through environmental understanding of SARS-CoV-2.
2. Modes of environmental transmission
The major mode of transmission of SARS viruses is through exposure to droplets of respiratory secretions from an infected person (>5 μm), indirectly through fomite transmission from contact with contaminated objects, or possibly by faecal-oral routes and air borne transmission (Wang et al., 2005; Ye et al., 2016; Kitajima et al., 2020; Naddeo and Liu, 2020). Faecal-oral routes and aerosol transmission have not been validated as exposure routes as there is not enough evidence to prove that SARS-CoV-2 transmission is possible by aerosol or wastewater. However, alarming infection incidents among health care workers, cruise-ship and airplane passengers point to the likelihood of additional transmission mechanisms in confined spaces with dense populations (Nghiem et al., 2020). Wu et al. (2020b) agreed that potential faecal-oral transmission may pose risk in cruise ships, public transportation, hostels and dormitories but unlikely in hospitals or quarantine facilities. Although Grunig et al. (2020) concluded that faecal-oral transmission had likely played a role in amplifying the pandemic in Wuhan (Grunig et al., 2020), the data is based on literature review of SARS-CoV not on research investigation. On that basis, it is plausible that sewage could serve as vectors for coronavirus (Ye et al., 2016; Zhang et al., 2020). Researchers have also postulated that the spread of COVID-19 solely by respiratory droplets and close contact did not seem to explain the vast spread of the disease in various parts of the world such as Italy, and thus air borne transmission has been investigated (Setti et al., 2020c). Clearly, the alarming infection rate of SARS-CoV-2 may suggest several plausible transmission mechanisms. Table 1 summarizes the latest research articles proposing various modes of SARS-CoV-2 transmission and arguments presented.
Table 1.
Reports on possible modes of transmission of COVID-19.
| Studies | Proposed mode of transmission | Presented argument |
|---|---|---|
| Santarpia et al. (2020); van Doremalen et al. (2020) | Fomite transmission | Detected SARS-CoV-2 on surfaces including personal items, toilet, room surfaces and floor surfaces plastic, stainless steel, copper, and cardboard up to days. |
| Enyoh et al. (2020) | Fomite transmission | Persistence on inanimate surfaces such as wood, ceramics, aluminium, glass, waste containers, bags for days. |
| Mol and Caldas (2020); Nghiem et al. (2020) | Fomite (transmission via solid waste handling) | |
| Wang et al. (2020b) | Possibility of faecal – oral routes | Detected live SARS-CoV-2 in faeces, suggesting possible faecal oral transmission. Information on how frequently viable virus is present in patient stool and the range of viral loads were not present. |
| Xu et al. (2020) | Plausible faecal-oral transmission | Rectal swab was tested positive with SARS-CoV- 2 RNA persistently when negative nasopharyngeal tests were found. Evidence of replication-competent virus in faecal swabs was not present. |
| Randazzo et al. (2020b) | Unlikely faecal – oral (wastewater) | Disinfection effect is likely to inactivate SARS-CoV-2. |
| Chen et al. (2020) | Plausible faecal-oral transmission | COVID-19 with a positive result of virus nucleic acid in a faecal specimen and negative results on multiple pharyngeal and sputum samples. The study did not report presence of live SARS-CoV-2 virus and other evidences to prove the transmission path. |
| Xiao et al. (2020) | Plausible faecal-oral transmission | Showed angiotensin converting enzyme (ACE)2 is abundantly expressed in the glandular cells of gastric, duodenal, and rectal epithelia through immunofluorescent staining of gastrointestinal tissues. Data on SARS-CoV-2 live virus was not published. Precise mechanisms by which SARS-CoV-2 interacts with the gastrointestinal tract was not presented. |
| Heller et al. (2020); Nghiem et al. (2020); Qu et al. (2020); Wong et al. (2020); Enyoh et al. (2020) | Plausible faecal-oral routes (transmission via sewage) | Review studies postulating that presence of SARS-CoV-2 in sewage and stool could lead to possible faecal- oral transmission via aerosolization from toilet flushing, leaky plumbing system etc., similar to SARS- CoV. |
| van Doremalen et al. (2020) | Aerosol transmission | Detected viable SARS-CoV-2 in aerosols for hours with results echoing SARS CoV. |
| Barcelo (2020) | Aerosols (wastewater treatment) | Postulated aerosol transmission by providing SARS-CoV- 2 virus in stool and probable exposure to aerosols. |
| Coccia (2020) | Air pollution to human transmission | Italian cities with more air pollution had higher number of infected individuals. |
| Frontera et al. (2020) | Air pollution to human transmission | PM2.5 mean concentrations in areas of China and Italy most affected by COVID-19, had vastly exceed the hourly standard of 75 μg/m3. |
| Gormley et al., 2020 | Aerosol transmission (transmission via engineered water systems) | Aerosolization from defective plumbing system with wastewater containing viral load due to interconnectedness of all parts of the building by the wastewater plumbing system. |
| Setti et al. (2020b) | Aerosol/air borne transmission | Detection of SARS-CoV-2 RNA on particulate matter. |
| Morawska and Cao (2020) | Review study postulating the aerosol transmission based on researches on SARS-CoV-2 similarity with SARS-COV, droplet dynamics and airflow in buildings. | |
| Santarpia et al. (2020) | Detection of SARS CoV-2 in air samples both in the rooms and in the hallway spaces of COVID -19 patient rooms. |
2.1. SARS-CoV-2 in stool & sewage
Coronavirus RNA has been detected in the stool of symptomatic and asymptomatic SARS and COVID-19 patients, indicating that transmission may be possible via the raw sewage network (faecal-oral) (Wang et al., 2005; Gundy et al., 2009; Ahmed et al., 2020; Holshue et al., 2020., Wang et al., 2020a; Wölfel et al., 2020; Wu et al., 2020b; Zhang et al., 2020). The incidence of diarrhea in SARS cases raised concern about its potential environmental transmission where viruses are more stable in diarrheal stool due to the higher pH, reaching upto 4 days as opposed to 1–2 days for normal stool (Gundy et al., 2009). Nevertheless, live SARS-CoV-2 was observed in stool of 2 patients who did not have diarrhea (Wang et al., 2020a). SARS-CoV can persist in sewage for 14 days at 4 °C and for 2 days at 20 °C, and its RNA can be detected for 8 days. (Gundy et al., 2009; Wang et al., 2005). SARS-CoV RNA was detected in 97% of stool samples from patients infected with SARS (Peiris et al., 2003), while detected in 57% of samples in another study by He et al. (2004).
Numerous researchers have attempted to assess whether sewage systems were plausible transmission pathways of coronavirus (Barcelo, 2020; Gormley et al., 2020; Grunig et al., 2020). It is unlikely for SARS-CoV-2 to be transmitted via wastewater or present any significant risk of infection due to viral sensitivity to disinfectants and poor stability in environmental conditions (Randazzo et al., 2020b; Rimoldi et al., 2020). Coronaviruses are enveloped viruses that have a lipid bilayer membrane outside the viral protein capsid, which contains proteins or glycoproteins. The structural difference with nonenveloped viruses may have an impact on their survival in aqueous environments, where their lipid layers are sensitive to the detergents and organic solvents (Ye et al., 2016). Gundy et al. (2009) found that coronavirus dies off very rapidly in wastewater, with a 99.9% reduction in 2–3 days due to action of solvents and detergents. Thus, while genetic fragments remain detectable in wastewater, the virus likely becomes non-viable once the envelope is damaged (Ye et al., 2016; Nghiem et al., 2020). At two hospitals receiving SARS patients in Beijing, China, SARS-CoV RNA was detectable in concentrates of sewage prior to, and occasionally after disinfection by chlorine but there was no live SARS-CoV (Wang et al., 2005). SARS-CoV-2 was detected in faeces (Wang et al., 2020a) and its RNA was detected in both faeces and urine (Sun et al., 2020; Xiao et al., 2020), while other attempts failed to cultivate the virus from faeces (Wölfel et al., 2020) or treated wastewater (Rimoldi et al., 2020). Raw sewage with improper disinfection in hospitals containing patients' excrements may be a possible transmission path (Wang et al., 2005). However, accidental contact with treated wastewater through aerosols or droplets should not call for public concern as potential risk of infection seems to be negligible (Rimoldi et al., 2020). Contact with raw wastewater particularly in areas with poor sanitation may be potential routes of transmission posing risk of infection (Grunig et al., 2020; Lodder and Husman, 2020; Rimoldi et al., 2020). In fact, contact with, and contamination by sewage water has been reported to be responsible for an outbreak of SARS CoV-1. The outbreak occurred in Hong Kong in 2003 involving 341 patients and 42 deaths due to a faulty wastewater plumbing system in a high-rise building (Peiris et al., 2003). The defected plumbing system facilitated the transport of virus laden droplets of wastewater originating in the bathroom pipelines to air ducts via the bathroom extraction ventilation (Gormley et al., 2020). Gormley et al. (2017) presented evidence that pathogens can be aerosolized and transported on airstreams within sanitary plumbing systems from one part of a building to another on different floors. One important factor contributing to the travel of contaminated air throughout a building is the interconnectedness of building's plumbing system (Gormley et al., 2020). One study found that while toilets may generate a large number of aerosols, the total volume of aerosolized liquid was extremely small, indicating that the potential for inhalation was low. (Lin and Marr, 2017). Nevertheless, the possibility of infection by contact with large splashing droplets has not been negated and requires further research (Lin and Marr, 2017). Heller et al. (2020) presented a framework to test the faecal-oral hypothesis, and proposed different environmental pathways of exposure from faeces to mouth: transmission by ingestion of a pathogen present in water, transmission by hand contact with surface washed with water containing pathogen, and transmission by excreta-related insect vectors.
How long the novel SARS-CoV-2 can survive in wastewater and remain infectious is yet to be assessed (Naddeo and Liu, 2020). Aboubakr et al. (2020) reported that information on SARS-CoV-2 stability in excrements is important in elucidating their role in transmitting the disease and assessing the plausibility of a faecal-oral route. In addition to the impact of disinfection, survival of coronavirus in water depends on a number of factors, including temperature, light exposure (solar or UV inactivation), organic matter, total dissolved solids (TDS), hardness, turbidity, pH, nitrate concentrations, and the presence of antagonist microorganisms (John and Rose, 2005; Naddeo and Liu, 2020). Temperature is the most critical factor influencing survival of coronavirus where higher water temperatures decrease the virus survival rate due to denaturation of proteins and activity of extracellular enzymes (John and Rose, 2005; Gundy et al., 2009). A 99.9% reduction of coronavirus was exhibited within 10 days in filtered tap water at room temperature, while over 100 days were required to reach that same level of virus inactivation for tap water at 4 °C (Gundy et al., 2009). Coronavirus were found to be more susceptible to higher temperatures than nonenveloped viruses and more strongly associated with wastewater solids (Gundy et al., 2009; Ye et al., 2016). The latter indicates that the primary treatment of wastewater and adsorption of coronavirus onto organic matter and suspended solids may provide protection against the virus (Gundy et al., 2009; Ye et al., 2016; Rimoldi et al., 2020; Wurtzer et al., 2020). Further investigations are required to assess environmental conditions of wastewater that may impact SARS-CoV-2 viability.
2.2. SARS-CoV-2 in air environment
The question currently remains as to the possibility of SARS-CoV-2 transmission and spread by air, indoor or outdoor. One method by which that could take place is through transport of virus-laden particles in the air where very small droplets (<5 μm) are formed after the liquid content of respiratory secretions evaporates allowing their transport by air currents carrying the viral content (Morawska and Cao, 2020). Current knowledge indicates an unlikely probability in outdoor environments and an increase in probability under specific indoor environments, like hospitals and areas where patients are quarantined (Contini and Costabile, 2020; Liu et al., 2020a, Liu et al., 2020b). Tabula (2020) stressed that evidence is limited on SARS-CoV-2 transmission via airborne route contending that studies are conflicting, and that robust evidence is yet to be presented. The difficulty in validating SARS-CoV-2 aerosol transmission stems from difficulty in sampling virus-laden aerosols and quantification at low concentrations (Liu et al., 2020b; Morawska and Cao, 2020).
2.2.1. Indoor environment
Aerosol transmission is currently a matter of intense debate and more studies are proposing it as plausible despite lack of robust evidence. Aerosol generation was reported under specific medical procedures (such as endotracheal intubation, non-invasive positive-pressure ventilation, etc) and aerosol transmission could be more probable route than faecal-oral transmission (Tran et al., 2012; Hussain et al., 2020). Some researchers based their support for aerosol transmission on experimental research while others based it on reasoning to explain the vast global spread of COVID-19 or spread by asymptomatic individuals. Morawska and Cao (2020) present previous studies confirming SARS-CoV spread by air as the main transmission route in specific indoor environments and plausibility for similar transmission for the novel coronavirus. van Doremalen et al. (2020) showed that SARS-CoV-2 can remain viable and infectious in aerosols for hours and on surfaces for up to days, highlighting the plausibility of both aerosol and fomite transmission. Similarly, Fears et al. (2020) reported that SARS-CoV-2 generally maintains infectivity when airborne over short distances and is persistent over longer periods of time than when generated as respiratory particles. Peters et al. (2020) in their Letter to the Editor contend that although aerosols may be generated through specific clinical procedures as reported by van Doremalen et al. (2020), the mechanism in transmission of SARS-CoV-2 remains through droplets and contact with contaminated surfaces. Nevertheless, Anderson et al. (2020) presented lines of reasoning supporting the aerosol transmission pathway of SARS-CoV-2 despite limited empirical data on aerosolized SARS-CoV-2 being transported long distances. Reasoning used by researchers to support aerosol transmission of SARS-CoV-2 include case reports of asymptomatic individuals infecting others through small droplets subject to aerosol transport (Anderson et al., 2020) and outbreak of COVID-19 in poorly ventilated restaurant possibly involving aerosol transmission (Li et al., 2020). In terms of faecal bio-aerosol transmission in indoor environment, Liu et al. (2020b) reported elevated concentrations of SARS-CoV-2 RNA in aerosols of hospital toilets used by COVID-19 patients, indicating that toilets may act as sources of airborne SARS-CoV-2, though infectivity remains unknown. Further discussion on faecal-oral transmission in indoor environments is presented in Section 2.1 of this review. Asadi et al. (2020) reported that aerosol transmission through regular speech may explain the spread of COVID-19 by asymptomatic and pre-symptomatic individuals who do not cough or sneeze to any appreciable extent to allow spread by respiratory excretions. Other researchers have supported the possibility of an airborne aerosol form of SARS-CoV-2 generated during speech and that COVID-19 patients may spread infection by talking or breathing through the resulting aerosol droplets lingering in the air (Anfinrud et al., 2020; Anderson et al., 2020). In terms of precautionary measures, it was reported that particles containing the virus can spread up to 10 m from emission source in indoor environments and thus natural ventilation and avoiding air recirculation should be implemented (Morawska and Cao, 2020; Setti et al., 2020c). Effective measures in minimizing airborne SARS-CoV-2 included negative pressure ventilation and high air exchange rates as shown for intensive care and critical care units of Renmin hospital in Wuhan, China (Liu et al., 2020a).
2.2.2. Outdoor environment
It is more difficult to validate aerosol transmission of SARS-CoV-2 in outdoor environments than in indoors. The probability of inhaling airborne viable virus generated from a distance and being infected is very low (Contini and Costabile, 2020). Hospital settings for example host infected individuals occupying limited space with likely poor air exchange creating a favorable environment for virus survival and assessment of aerosol transmission (Contini and Costabile, 2020). Liu et al. (2020a) detected SARS-CoV-2 aerosol at two outdoor crowd gatherings where asymptomatic carriers could pose potential source of airborne SARS-CoV-2 but stated that public venues pose low risk when avoiding crowded gatherings.
2.2.3. Air pollution and COVID-19
The relationship between COVID-19 and air quality can be viewed from various aspects. First, the lockdown procedures around the world have resulted in less anthropogenic activities and subsequent decreases in air pollution. From other aspects, the level of air pollution in a city may seem to influence the severity of COVID-19, either due to health susceptibility of individuals living in polluted cities to COVID-19 infection, or air pollution as a mode of transmission which is not supported by evidence despite being proposed by some researchers. There are numerous studies from around the globe showing significant decrease in ambient air pollution following lock down and quarantine measures to combat COVID-19. The overall air quality in Northern China was improved during the control of COVID-19 due to reduced emissions from transportation and industry (Wang et al., 2020b). A total of 322 of the 366 cities studied in Northern China exhibited a decline in the air quality index (AQI) and reduction of PM2.5, PM10, SO2, NO2, and CO (Wang et al., 2020b). Similarly, Sharma et al. (2020) reported a decline in AQI and reduction in concentrations of pollutants expect for SO2 when comparing March and April of 2020 with previous years. In these studies, it was reported that O3 concentrations had increased during the COVID-19 control period possibly due to reduction of PM concentrations causing more sunlight to pass through the atmosphere encouraging more higher O3 production (Sharma et al., 2020). Other researchers have reported that concentrations of PM2.5 exhibited significant reduction in several cities in China, but not enough to avoid severe air pollution events during lockdown likely attributed to unfavorable meteorological conditions (Wang et al., 2020c). The decrease in air pollution may have reduced the number of non-communicable diseases and fatalities, where 6% reduction in mortality was postulated due to air pollution reduction, equivalent to saving 100,000 lives in China alone (Dutheil et al., 2020).
Air pollution has been linked to SARS-CoV-2 viral infections through the impact of poor air quality on human health and susceptibility to viral infection (Wu et al., 2020c), and evidence is not available for pollutant particles such as PM and NO2 acting as vectors despite suggested by some researchers (Pansini and Fornacca, 2020). Air pollution is known to cause inflammation, cellular damage, respiratory diseases and may suppress early immune response to infection (Wu et al., 2020c). For example, PM10 and PM2.5 have been linked to pneumonia and chronic pulmonary diseases (Pansini and Fornacca, 2020; Wu et al., 2020c). Numerous investigations have attempted to assess the relationship between air pollution and severity of COVID-19 around the world. Some studies show that people living in polluted areas are more vulnerable to SARS-CoV-2 infections and induced mortality as they are more prone to developing chronic respiratory conditions (Conticini et al., 2020; Murgante et al., 2020; Pansini and Fornacca, 2020; Zhu et al., 2020). Concentrations of PM exceeding limits may increase the susceptibility of COVID-19 infected individuals to respiratory complications while oxidant pollutants may impair the efficiency of lungs to clear the virus (Qu et al., 2020). Several studies have investigated exceedance of PM and its correlation with COVID-19 infectivity and lethality. Just small increases of long-term exposure to PM2.5 (1 μg/m3 increase) were found to be associated with an 8% increase in the COVID-19 death rate in the US (Wu et al., 2020c). One of the world's most hit countries with COVID-19 cases and fatalities is Italy and several studies have investigated the correlation between cases and air pollution. The regions of Lombardy and Emilia Romagna are one of Europe's most polluted areas and have exhibited the highest level of virus lethality in the world (Conticini et al., 2020). Some researchers reported correlation between COVID-19 incidents and air pollution (Coccia, 2020; Setti et al., 2020a), while others found that the correlation was not evident (Bontempi, 2020). For example, Setti et al. (2020a) reported that exceedance in daily PM10 beyond limits appeared to be a significant predictor of infection with COVID-19. Similarly, the number of infected people was higher in Italian cities with 100 days exceeding limits set for PM10 or ozone, having a low wind speed and lower temperature (Coccia, 2020). On the other hand, Bontempi (2020) reported that direct correlations between high concentrations of PM10 and diffusion of COVID-19 virus in Lombery and Piedmont, Italy were not evident. In particular, cities with highest PM10 pollution (Torino and Alessandria) had low infections cases, while Bergamo which exceeded PM10 concentration only few times, exhibited the highest infectious cases (Bontempi, 2020). Like PM, several studies have linked viral infectivity to exceedances in the other criteria pollutants. SARS-CoV-2 viral infections in China, USA, and Italy were found to be higher in areas afflicted by carbon monoxide (CO) and nitrogen dioxide (NO2), while higher mortality rates were found to be in areas correlated with high particulate matter (PM2.5), CO, and NO2 (Pansini and Fornacca, 2020). Zhu et al. (2020) observed positive associations of PM2.5, PM10, CO, NO2, and O3 with COVID-19 confirmed cases, and that short-term exposure to these pollutants is associated with increased risk of infection with COVID-19.
In terms of interpretation of how air pollution may enhance the spread of COVID-19, there are several points of view based on reasoning rather than on robust evidence. Several researchers view that the extent to which COVID-19 has spread in many countries cannot be solely explained by just exposure to droplets of respiratory secretions from an infected person or through fomite transmission from contact with contaminated objects. Setti et al. (2020b) postulated that through airborne transmission, PM could act as a droplet carrier, triggering the spread of the virus. However, the capability of this coronavirus to bind particulate matters remains to be established, but could be through adsorption (Fattorini and Regoli, 2020; Qu et al., 2020). Air borne transmission of SARS-CoV-2 has been postulated by Morawska and Cao (2020) as explanation to the trend in the increase of infections, and that air transmission should be seriously considered during the course of this pandemic. Under high PM concentration and atmospheric stability, viruses may create clusters with the PM reducing their diffusion coefficient and enhancing abundance into the atmosphere (Setti et al., 2020c). Setti et al. (2020c) presents the first evidence of SARS-CoV-2 RNA presence on outdoor PM through analysis of 34 PM10 samples of outdoor/airborne PM10 from an industrial site of Bergamo Province. However, correlation between the presence of virus on PM and COVID-19 outbreak progression has not been confirmed in that study, and links between air pollutants and the pandemic has not been validated (Setti et al., 2020c).
2.3. SARS-CoV-2 in solid waste
Few review articles were found on coronavirus or SARS-CoV-2 in municipal solid waste materials while no experimental research articles were found. The reason maybe that SARS-CoV-2 infected waste is typically generated from medical centers and hospitals which are then incinerated. However, with the worldwide lockdown and self-quarantine measures, solid waste containing viable SARS-CoV-2 are likely generated at the household level from COVID-19 infected individuals posing risk of infection to front line waste workers (Nghiem et al., 2020). In that regard, Mol and Caldas (2020) report that spread of the coronavirus may be increased by inadequate waste management through poor handling conditions particularly in developing countries with poor waste management strategies. Kharel (2020) reported that as many of the coronavirus patients in Nepal are asymptomatic, contaminated solid waste may result in transmission to waste collectors and pickers and then retransmission back to the community. Additionally, some of the waste materials are sold in informal markets to poor people, posing additional risk of infection (Kharel, 2020). In Nigeria, solid wastes are dumped in poorly managed dumpsites which are scavenged for recyclable materials and food for livestock, posing risk of infection with COVID-19 and exacerbating its spread within the community (Nzediegwu and Chang, 2020). Even in developed nations, the generation of glove and medical mask waste from households poses risk of infection and environmental pollution considering that the survival time of SARS-CoV-2 on hard surface and plastic is in the order of days (Nghiem et al., 2020; Saadat et al., 2020). There is not any published evidence for transmission of SARS-CoV-2 via solid waste route and the topic has not received attention from the scientific community. What is currently present in the literature relates to the fomite transmission route of SARS-CoV-2 rather than solid waste and management as plausible routes of transmission. In that regard, Aboubakr et al. (2020) presents the latest investigations on persistence of SARS-CoV-2 in non-porous surfaces (plastic, metal, glass, metals) and porous surfaces (paper, cardboard), which may assist in understanding the viral persistence of the same materials in solid waste. SARS-CoV-2 for example was reported to survive longer on surfaces of higher porosity such as surgical masks than on surfaces of lower porosity such as paper (Aboubakr et al., 2020), providing insight on viral persistence on gloves and mask waste generated from households during the lockdown period. SARS-CoV-2 was also found to be more stable on plastic and stainless steel than on copper and cardboard and despite its high stability in favorable environments, it is nevertheless susceptible to standard disinfection methods (Chin et al., 2020; van Doremalen et al., 2020; Kampf et al., 2020). Much of the information on SARS-CoV-2 persistence on solid surfaces is deduced from previous coronavirus studies (Aboubakr et al., 2020; Kampf et al., 2020). The limited available data on SARS-CoV-2 persistence on solid surfaces and solid waste materials calls for the need to elucidate solid waste and its management practices with COVID-19 spread.
2.4. SARS-CoV-2 in natural water
It is important to understand the fate of SARS-CoV-2 in the water environment to ensure public health protection measures are suitably set in place (Naddeo and Liu, 2020). However, there is currently limited data on the presence and viability of SARS-CoV-2 in water bodies (Cahill and Morris, 2020). Aboubakr et al. (2020) presented a review of studies on persistence of coronaviruses in chlorinated and dechlorinated water, which may help to understand the persistence of SARS-CoV-2 in waters. SARS-CoV-2 in the water environment may find its origin in the discharge of raw sewage into water bodies which is common practice in many parts of the world, lack of basic sanitation resulting in contamination of drinking water sources, accidental contamination by raw sewage, or where performance of sewage treatment plants is sub optimal (Guerrero-Latorre et al., 2020; Odih et al., 2020; Usman et al., 2020). Guerrero-Latorre et al., 2020 detected viral loads of SARS-CoV-2 from rivers in urban streams of Quito, Ecuador where wastewater is discharged into river streams. On the other hand, Haramoto et al. (2020) did not detect SARS-CoV-2 RNA in river water samples in Japan despite its detection in secondary treated wastewater. The risk of SARS-CoV-2 presence in sewage water cannot be neglected (WHO, 2020a), and contracting the virus via water sources is unknown and has not been investigated to date. The need to investigate is high particularly in developing countries with poor water and sewage infrastructure where the probability of faecal material reaching natural water bodies is high. There is also no evidence of potential aerosolized SARS-CoV-2 originating from wastewater used for irrigation (Usman et al., 2020). With research progress, the implications of research findings on SARS-CoV-2 in the water environment will assist public health officials in devising the proper mechanisms to protect the general public. There are a lot of aspects to be taken care of starting from the disinfection at the source, proper distribution, collection and safe practices for storage. Conventional treatment systems like chlorination and UV disinfection are expected to remediate COVID-19 presence in water systems similar to other coronaviruses (Pecson et al., 2020; WHO, 2020a).
3. Wastewater surveillance for environmental detection of COVID-19 infections
Since wastewater contains viruses excreted from infected individuals in a given wastewater treatment plant catchment population, wastewater surveillance and quantification of SARS-CoV-2 can be an effective tool in estimating the number of infections in a community at an early stage of the outbreak (Ahmed et al., 2020; Mallapaty, 2020; Wu et al., 2020a). The early detection and determination of SARS-CoV-2 prevalence in a population can assist policy makers in formulating the appropriate mitigation policies and help to ensure healthcare institutions are not overwhelmed (Mao et al., 2020; Wu et al., 2020a). SARS-CoV-2 RNA is detected in wastewater using Real-Time Reverse-Transcriptase–Polymerase-Chain-Reaction Testing (RT-qPCR) (Lodder and Husman, 2020; Mao et al., 2020; Randazzo et al., 2020b). Wastewater RT-qPCR was reported to be a sensitive and reliable technique for early detection of SARS-CoV-2 outbreaks (Randazzo et al., 2020b). It is important to note that RNA detection in wastewater is not indicative of viable virus that is transmissible (Nghiem et al., 2020; WHO, 2020a, WHO, 2020b). Genetic material of SARS-CoV-2 has already been detected in wastewater samples from the Region of Valencia, Spain (Randazzo et al., 2020a), Amsterdam Airport Schiphol, Netherlands (Lodder and Husman, 2020), Australia (Ahmed et al., 2020), Massachusetts, USA (Wu et al., 2020a), France (Wurtzer et al., 2020), Milan, Italy (La Rosa et al., 2020; Rimoldi et al., 2020), Istanbul, Turkey (Kocamemi et al., 2020) and China (Wang et al., 2020d). The fact that wastewater samples at Amsterdam Airport Schiphol and in Tilburg, Netherlands tested positive for viral RNA by quantitative RT-PCR methodology only days after the confirmed cases of COVID-19 in the country shows the sensitivity of this surveillance system and how it can serve as an early warning tool (Lodder and Husman, 2020). Randazzo et al., 2020a, Randazzo et al., 2020b reported detection of SARS-CoV-2 RNA in wastewater when only 50–76 cases were reported in Valencia and 12–16 days before COVID-19 cases were declared in 3 out of 6 municipalities in Spain. However, there is a lack of standardized protocol for the detection of SARS-CoV-2 in wastewater with discrepancies among RT-qPCR N1, N2 and N3 assays. Massive population tests should be the first choice but wastewater monitorization may provide a reliable picture of the situation (Kitajima et al., 2020; Randazzo et al., 2020b).
Wastewater surveillance may serve as a complementary approach to clinical testing campaigns for assessing the prevalence of COVID-19 in a community (Wu et al., 2020a). One wastewater treatment plant can capture wastewater from more than a million residents, providing an estimate of the prevalence of COVID-19 within a large population (Mallapaty, 2020). Wastewater-based epidemiology (WBE) has some benefits over clinical testing particularly for poor nations with limited resources and may serve as an early detection tool, but cannot fully replace clinical testing which should be considered as the first choice (Hart and Halden, 2020; Randazzo et al., 2020b). Clinical screening comes with the challenges of undertaking a massive, time-consuming, and labor-intensive process constrained by availability of testing technologies (Mao et al., 2020). Wastewater surveillance on the other hand can account for individuals who have not been tested, or are asymptomatic, potentially symptomatic, presymptomatic, or only have mild symptoms (Lodder and Husman, 2020; Mallapaty, 2020). Additionally, different clinical diagnostic testing methods and assays makes country comparisons difficult (Kitajima et al., 2020). The importance of WBE is also highlighted by its ability to detect low levels of viruses, especially important at early stages of an outbreak or when infection levels are decreasing following intervention (Kitajima et al., 2020). Monitoring programs for coronavirus in wastewater treatment plants should be established to assess their fate during water and wastewater treatment (Naddeo and Liu, 2020).
Researchers can determine the abundance of COVID-19 in a population from wastewater samples by how much viral RNA is excreted in faeces and extrapolate the number of infected people from concentrations of viral RNA in wastewater (Mallapaty, 2020). SARS-CoV-2 RNA is first concentrated from wastewater, viral RNA copies are enumerated using RT-qPCR, and the estimated RNA copy numbers are then used to estimate the number of infected individuals in a catchment (Ahmed et al., 2020). There are limitations to wastewater-based epidemiology in establishing quantitative predictions from viral RNA resulting in severe over or underestimation of infected cases. These include the complexity of wastewater matrices, the dilute nature of biomarker in wastewater, inability to pinpoint specific locations, need for effective sampling techniques, need to develop effective virus concentration methods, and need for robust and sensitive RT-qPCR assays in such complex matrices (Ahmed et al., 2020; Hart and Halden, 2020; Mao et al., 2020).
Wu et al. (2020a) tested wastewater collected at a wastewater treatment plant in Massachusetts, confirmed the presence of SARS-CoV-2 using RT-qPCR, but found that viral titers were significantly higher than expected based on the clinically confirmed cases. On the other hand, Ahmed et al. (2020) detected SARS-CoV-2 RNA in untreated wastewater and estimated the number of infected individuals in reasonable agreement with clinical observations (Ahmed et al., 2020; Kitajima et al., 2020). The reasons for the discrepancy between confirmed cases and observed viral titers could be due to inaccurate assumptions of viral load in stool or loss assumptions in viral titer due to degradation, and presence of asymptomatic patients (Wu et al., 2020a). There is lack of a standardized protocol for the detection and quantification of SARS-CoV-2 in wastewater (Kitajima et al., 2020), where proper quantification of the scale of COVID-19 infection in a population from wastewater analysis requires overcoming challenges including: 1) determination of the quantities of viral RNA excreted in faeces, 2) ensuring samples are representative of population excretions over time periods, 3) ability of tests to detect viruses at low levels, 4) understanding the variability of viral shedding rates between people, 5) the establishment of quantitative predictions of the actual number of cases in a population from determined RNA concentrations, and 6) need to develop effective enveloped virus concentration methods (Ahmed et al., 2020; Mallapaty, 2020; Nghiem et al., 2020). Or et al. (2020) presented a preliminary a proof-of-concept for viral concentration method using polyethylene glycol or alum precipitation for the detection of SARS-CoV-2 RNA in sewage.
4. Strategies to combat future pandemics
Based on this presented review of COVID-19 infectivity and relation to environmental factors, strategies may be adopted to combat future pandemic by ability to assess its distribution and spread from environmental indicators.
4.1. Wastewater surveillance
There is need for development of a standardized protocol for quantification of SARS-CoV-2 in wastewater and overcoming existing challenges discussed previously (Kitajima et al., 2020). Wastewater surveillance may then serve as an important and quick tool to determining the spread of COVID-19 within a community and can assist policy makers in early detection of outbreak through analysis of SARS-CoV-2 RNA in wastewater samples. The information obtained can assist in identifying areas where transmission is occurring (Kitajima et al., 2020; Larsen et al., 2020), provide guidance on when to relax restrictions on population movement (Nghiem et al., 2020), assist in containing the pandemic once the curve is flattened, and help to prevent escalation where infections are minimal (Larsen et al., 2020).
4.2. Air quality indoors and outdoors
From our limited understanding of atmospheric pollution impact on COVID-19 spread and severity, integration of air quality protection and climate change mitigation within nations' sustainable development strategies may assist in curbing future viral outbreaks (Fattorini and Regoli, 2020; Pansini and Fornacca, 2020; Wu et al., 2020c). There is emphasized importance on continued enforcement of air pollution regulations to protect health during and after this pandemic as well as in the event of future outbreaks (Wu et al., 2020c). The pandemic is ongoing and the status of infections in the world is dynamically changing. Thus, the links between air pollution and COVID-19 infectivity and lethality are not established at this stage. Any future development in that regard may serve as a tool for policy makers in setting proactive strategies to face future pandemics. For example, if future robust evidence is made available linking air pollution to COVID-19 spread, then it may actually be plausible to predict pandemic impacts based on the number of days cities exceed pollutant limits (Coccia, 2020). Governments would be then able to prioritize regions with high pollutant concentrations and take proactive action to reduce atmospheric pollution. In terms of protection against spread of viral infectivity in indoor environments, attention should be given to building design that ensures increased ventilation rates, natural ventilation, proper air circulation, as well-engineered sanitary plumbing systems that minimize the potential for virus-laden aerosol to spread within the building (Gormley et al., 2020; Liu et al., 2020a; Morawska and Cao, 2020). As our understanding of aerosol transmission of SARS-CoV-2 is limited, further investigations are needed to devise methodologies for sampling and quantification of SARS-CoV-2 in aerosols (Liu et al., 2020a; Morawska and Cao, 2020).
4.3. Solid waste
Despite limited research on SARS-CoV-2 in municipal solid waste, the best waste management strategies should be implemented to limit workers' exposure to waste contaminated with viable virus, likely generated by households with symptomatic and non-symptomatic individuals. Practices may include separate collection services for infected households, delaying waste collection beyond the lifespan of the virus (72 h), and direct transport of collected waste to incinerators or landfills avoiding waste segregation at material recovery facilities (Nghiem et al., 2020).
5. Limitations
There are limitations to our current understanding of the environmental transmission of SARS-CoV-2 and links between environmental pollution and COVID-19 infectivity and spread. The research is moving at quick pace and the changing dynamics of infections worldwide presents its challenges to establishing robust evidence linking environmental conditions such as air pollution with COVID-19 infections. Experimental research on validation of faecal-oral and aerosol transmission of SARS-CoV-2 is also met with challenges, where robust evidence is yet to be presented through in-depth research efforts. Other areas of environmental research have not received attention such as COVID-19 transmission in solid waste. Several of the presented articles are pre-prints undergoing peer-review, which limits comparison of evidence with other published research. There needs to be caution in interpreting the existing findings and the results presented herein is a snapshot of the current understanding of the environmental related aspects of SARS-CoV-2.
6. Conclusion
Knowledge on SARS-CoV-2 within the environmental context maybe beneficial in establishing effective policies for mitigating the transmission of the disease and combating future pandemics. Early detection of COVID-19 and prevalence within a population can be achieved through surveillance of SARS-CoV-2 RNA in wastewaters, aiding decision makers in choosing the right times for tightening or relaxation of restrictions among other measures. Should future robust evidence be available on links between atmospheric pollution and COVID-19 spread, air quality maybe another tool of importance that can be employed to assess infectivity of COVID-19 and future infections. This may allow policy makers to establish proactive strategies to face future pandemics, prioritizing regions with high atmospheric pollution. Since air pollution mitigation actions during a viral outbreak may be of limited effectiveness, long-term strategies for air quality protection and regulatory enforcement are of major importance. Evidence is needed to validate transmission of SARS-CoV-2 via faecal-oral or aerosol pathways, which would immensely help our understanding on the infectivity and vast spread of the pandemic. Prevention of viral outbreak or spread within indoor environments such as hospitals or high-rise buildings due to circulation of virus-laden aerosol may be achieved by ensuring well-engineered sanitary plumbing systems and adequate air ventilation. Solid waste management and impact on COVID-19 spread has received little attention and there is need to assess the spread of the pandemic through waste materials specially in developing nations where inadequate practices may act to promote the spread of the pandemic within communities. Research is also needed to assess the impact of raw sewage discharge into water bodies, a topic that has received little attention. Overall, our understanding of the environment perspective of SARS-CoV-2 may help in detecting viral outbreak at early stages and assessing pandemic severity in effort to be equipped with the right tools to curb any future pandemic.
Declaration of competing interest
The authors for the submitted manuscript “SARS-CoV-2 in the environment: modes of transmission, early detection and potential role of pollution” certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Acknowledgments
This work was supported by the Division of Consultancy, Research and Innovation (CRI), Sharjah Environment Company – Bee'ah.
Editor: Ewa Korzeniewska
References
- Aboubakr H., Sharafeldin T.A., Goyal S.M. OSF Preprints. 2020. Stability of SARS-CoV2 and other coronaviruses in the environment and on common touch surfaces. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E.L., Turnham P., Griffin J.R., Clarke C.C. Consideration of the aerosol transmission for COVID-19 and public health. Risk Anal. 2020;40(5):902–907. doi: 10.1111/risa.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfinrud P., Stadnytskyi V., Bax C.E., Bax A. Visualizing speech-generated oral fluid droplets with laser light scattering. N. Engl. J. Med. 2020;382:2061–2063. doi: 10.1056/NEJMc2007800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi S., Bouvier N., Wexler A.S., Ristenpart W.D. The coronavirus pandemic and aerosols: does COVID-19 transmit via expiratory particles? Aerosol Sci. Technol. 2020;54(6):635–638. doi: 10.1080/02786826.2020.1749229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo D. An environmental and health perspective for COVID-19 outbreak: meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. Journal of Environmental Chemical Engineering. 2020 doi: 10.1016/j.jece.2020.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E. First data analysis about possible COVID-19 virus airborne diffusion due to air particulate matter (PM): the case of Lombardy (Italy) Environ. Res. 2020 doi: 10.1016/j.envres.2020.109639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill N., Morris D. Recreational waters–a potential transmission route for SARS-CoV-2 to humans? Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.140122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Travelers from countries with widespread sustained (ongoing) transmission arriving in the United States. 2020. https://www.cdc.gov/coronavirus/2019-ncov/travelers/after-travel-precautions.html
- Chen L., Lou J., Bai Y., Wang M. COVID-19 disease with positive fecal and negative pharyngeal and sputum viral tests. Am. J. Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A., W.H., Chu J., T.S., Perera M., R.A., Hui K., P.Y., Yen H.-L., Chan M., C.W., Peiris M., Poon L., L.M. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020;1:e10. doi: 10.1016/S2666-5247(20)30003-3. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. CNR - National Research Council of Italy; 2020. Diffusion of COVID-19 Outbreaks: The Interaction between Air Pollution-to-Human and Human-to-Human Transmission Dynamics in Hinterland Regions with Cold Weather and Low Average Wind Speed. Working Paper CocciaLab N. 48/2020. [DOI] [Google Scholar]
- Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in northern Italy? Environ. Pollut. 2020 doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini D., Costabile F. Does air pollution influence COVID-19 outbreaks? Atmosphere. 2020 doi: 10.3390/atmos11040377. [DOI] [Google Scholar]
- Dutheil F., Baker J.S., Navel V. COVID-19 as a factor influencing air pollution? Environ. Pollut. 2020 doi: 10.1016/2Fj.envpol.2020.114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyoh C.E., Verla A.W., Qingyue W., Yadav D.K., Chowdhury A.H., Isiuku B.O., et al. Preprints 2020. 2020. Indirect exposure to novel coronavirus (SARS-CoV-2): An overview of current knowledge. 2020040460. [DOI] [Google Scholar]
- Fattorini D., Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ. Pollut. 2020 doi: 10.1016/j.envpol.2020.114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fears A.C., Klimstra W.B., Duprex P., Hartman A., Weaver S.C., Plante K.S., et al. Comparative dynamic aerosol efficiencies of three emergent coronaviruses and the unusual persistence of SARS-CoV-2 in aerosol suspensions. medRxiv. 2020 doi: 10.1101/2020.04.13.20063784. [DOI] [Google Scholar]
- Frontera A., Martin C., Vlachos K., Sgubin G. Regional air pollution persistence links to covid19 infection zoning. The Journal of Infection. 2020 doi: 10.1016/2Fj.jinf.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M., Aspray T., J., Kelly D., A. COVID-19: mitigating transmission via wastewater plumbing systems. Lancet Glob. Health. 2020;8(5):E643. doi: 10.1016/S2214-109X(20)30112-1. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A., Rodriguez-Gil C. Pathogen cross-transmission via building sanitary plumbing systems in a full scale pilot test-rig. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunig G., Durmus N., Marsh L. Preprints 2020. 2020. New coronavirus (COVID-19) pandemic: Complexities resulting in a tragedy. 2020040407. [Google Scholar]
- Guerrero-Latorre L., Ballesteros I., Villacres I., Granda M.G., Freire B., Rios-Touma B. First SARS-CoV-2 detection in river water: implications in low sanitation countries. medRxiv. 2020 doi: 10.1101/2020.06.14.20131201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food and Environmental Virology. 2009;1:10–14. doi: 10.1007/s12560-008-9001-6. [DOI] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. medRxiv. 2020 doi: 10.1101/2020.06.04.20122747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z.P., Dong Q.M., Song S.J., He L., Zhuang H. Detection for severe acute respiratory syndrome (SARS) coronavirus RNA in stool of SARS patients. Chinese Journal of Preventive Medicine. 2004;38(2):90–91. [PubMed] [Google Scholar]
- Heller L., Mota C.R., Greco D.B. COVID-19 faecal-oral transmission: are we asking the right questions? Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiseman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A., Singhal T., EL-Hasani S. Extent of infectious SARS-CoV-2 aerosolisation as a result of oesophagogastroduodenoscopy or colonoscopy. British J. Hosp. Med. 2020:1–7. doi: 10.12968/hmed.2020.0348. In this issue. [DOI] [PubMed] [Google Scholar]
- John D.E., Rose J.B. Review of factors affecting microbial survival in groundwater. Environmental Science & Technology. 2005;39(19):7345–7356. doi: 10.1021/es047995w. [DOI] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R.C., Cicinelli M.V., Gilbert S.S., Honavar S.G., Murthy G.S. COVID-19 pandemic: lessons learned and future directions. Indian J. Ophthalmol. 2020;68(5):703–710. doi: 10.4103/ijo.IJO_843_20. http://www.ijo.in/text.asp?2020/68/5/703/282901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharel T.P. Risk of COVID-19 for household waste workers in Nepal. International Journal of Multidisciplinary Sciences and Advanced Technology. 2020;1:116–123. [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerbaet C., Hamilton K., et al. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020 doi: 10.20944/preprints202004.0460.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocamemi B.A., Kurt H., Hacioglu S., Yarali C., Saatci A.M., Pakdemirli B. First data-set on SARS-CoV-2 detection for Istanbul wastewaters in Turkey. medRxiv. 2020 doi: 10.1101/2020.05.03.20089417. [DOI] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Ferraro G.B., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020 doi: 10.1101/2020.04.25.20079830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen D., Dinero R., Reddy E., Green H., Lane S., Shaw A., et al. A review of infectious disease surveillance to inform public health action against the novel coronavirus SARS-CoV-2. SocArXiv. 2020 doi: 10.31235/osf.io/uwdr6. [DOI] [Google Scholar]
- Lau H., Khosrawipour V., Kocbach P., Mikolajczyk A., Schubert J., Bania J., Khosrawipour T. The positive impact of lockdown in Wuhan on containing the COVID-19 outbreak in China. Journal of Travel Medicine. 2020 doi: 10.1093/jtm/taaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Qian H., Hang J., Chen X., Hong L., Liang P., et al. Evidence for probable aerosol transmission of SARS-CoV-2 in a poorly ventilated restaurant. medRxiv. 2020 doi: 10.1101/2020.04.16.20067728. [DOI] [Google Scholar]
- Lin K., Marr L.C. Aerosolization of Ebola virus surrogates in wastewater systems. Environmental Science & Technology. 2017;51(5):2669–2675. doi: 10.1021/acs.est.6b04846. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., et al. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak. BioRxiv. 2020 doi: 10.1101/2020.03.08.982637. [DOI] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020 doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Lodder W., Husman A.M.D.R. SARS-CoV-2 in wastewater: potential health risk, but also data source. The Lancet Gastroenterology & Hepatology. 2020;5(6):533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. How sewage could reveal true scale of coronavirus outbreak. Nature. 2020;580(7802):176–177. doi: 10.1038/d41586-020-00973-x. [DOI] [PubMed] [Google Scholar]
- Mao K., Zhang H., Yang Z. Can a paper-based device trace COVID-19 sources with wastewater-based epidemiology? Environmental Science & Technology. 2020;54(7):3733–3735. doi: 10.1021/acs.est.0c01174. [DOI] [PubMed] [Google Scholar]
- Mol M.P.G., Caldas S. Can the human coronavirus epidemic also spread through solid waste? Waste Manag. Res. 2020;38(5):485–486. doi: 10.1177/2F0734242X20918312. [DOI] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020 doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgante B., Borruso G., Balletto G., Castiglia P., Dettori M. Preprints 2020. 2020. Why Italy first? Health, geographical and planning aspects of the Covid-19 outbreak. 2020050075. [DOI] [Google Scholar]
- Naddeo V., Liu H. Editorial perspectives: 2019 novel coronavirus (SARS-CoV 2): what is its fate in urban water cycle and how can the water research community respond? Environmental Science Water Research & Technology. 2020;6:1213–1216. doi: 10.1039/D0EW90015J. [DOI] [Google Scholar]
- Nghiem L., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Studies in Chemical and Environmental Engineering. 2020 doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzediegwu C., Chang S.X. Improper solid waste management increases potential for COVID-19 spread in developing countries. Resour. Conserv. Recycl. 2020 doi: 10.1016/2Fj.resconrec.2020.104947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odih E.E., Afolayan A.O., Akintayo I., Okeke I.N. Could water and sanitation shortfalls exacerbate SARS-CoV-2 transmission risks? The American Journal of Tropical Medicine and Hygiene. 2020 doi: 10.4269/ajtmh.20-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Or I.B., Yaniv K., Shagan M., Ozer E., Erster O., Mendelson E., et al. Regressing SARS-CoV-2 sewage measurements onto COVID-19 burden in the population: a proof-of-concept for quantitative environmental surveillance. medRxiv. 2020 doi: 10.1101/2020.04.26.20073569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansini R., .D., Fornacca Higher virulence of COVID-19 in the air-polluted regions of eight severely affected countries. medRxiv. 2020 doi: 10.1101/2020.04.30.20086496. [DOI] [Google Scholar]
- Pecson B., Gerrity D., Bibby K., Drewes J.E., Gerba C., Gersberg R., et al. Editorial perspectives: will SARS-CoV-2 reset public health requirements in the water industry? Integrating lessons of the past and emerging research. Environmental Science: Water Research & Technology. 2020 doi: 10.1039/D0EW90031A. [DOI] [Google Scholar]
- Peiris J.S.M., Chu C.M., Cheng V.C.C., Chan K.S., Hung I.F.N., Poon L.L., et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., Parneix P., Otter J., Pittet D. Putting some context to the aerosolization debate around SARS-CoV-2. The Journal of Hospital Infection. 2020 doi: 10.1016/2Fj.jhin.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu G., Li X., Hu L., Jiang G. An imperative need for research on the role of environmental factors in transmission of novel coronavirus (COVID-19) Environmental Science & Technology. 2020;54(7):3730–3732. doi: 10.1021/acs.est.0c01102. [DOI] [PubMed] [Google Scholar]
- Randazzo W., Ferrando C.E., Sanjuan R., Domingo C.P., Sanchez G. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. medRxiv. 2020 doi: 10.1101/2020.04.23.20076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., et al. Presence and vitality of SARS-CoV-2 virus in wastewaters and rivers. medRxiv. 2020 doi: 10.1101/2020.05.01.20086009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat S., Rawtani D., Hussain C.M. Environmental perspective of COVID-19. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V., Morwitzer M.J., Creager H., Santarpia G.W., et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxIV. 2020 doi: 10.1101/2020.03.23.20039446. [DOI] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Perrone M.G., Piazzalunga A., et al. The potential role of particulate matter in the spreading of COVID-19 in northern Italy: first evidence-based research hypotheses. medRxiv. 2020 doi: 10.1101/2020.04.11.20061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., Gennaro G.D., Barbieri P., Perrone M.G., Borelli M., et al. SARS-Cov-2RNA found on particulate matter of Bergamo in northern Italy: first evidence. Environ. Res. 2020 doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., Gennaro G.D., Barbieri P., Perrone M.G., Borelli M., et al. Airborne transmission route of COVID-19: why 2 meters/6 feet of inter-personal distance could not be enough. Int. J. Environ. Res. Public Health. 2020 doi: 10.3390/ijerph17082932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Zhang M., Gao J., Zhang H., Kota S.H., et al. Effect of restricted emissions during COVID-19 on air quality in India. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zhu A., Li H., Zheng K., Zhuang Z., Chen Z., et al. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerging Microbes & Infections. 2020;9(1):991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabula J. Is SARS-CoV-2 transmitted by airborne route? 2020. https://www.psmid.org/wp-content/uploads/2020/04/Airborne-transmission-Abridged-v2-11Apr2020-JT-IGC.pdf
- Tran K., Cimon K., Severn M., Pessoa-Silva C., L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS ONE. 2012;7(4):e35797. doi: 10.1371/journal.pone.0035797. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usman M., Farooq M., Hanna K. Existence of SARS-CoV-2 in wastewater: implications for its environmental transmission in developing communities. Environ. Sci. Technol. 2020;54(13):7758–7759. doi: 10.1021/acs.est.0c02777. https://pubs.acs.org/action/showCitFormats?doi=10.1021/acs.est.0c02777&ref=pdf [DOI] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Jin M., Zhen B., Kong Q.X., Song N., et al. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126(1–2):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. J. Am. Med. Assoc. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yuan Y., Wang Q., Liu C., Zhi Q., Cao J. Changes in air quality related to the control of coronavirus in China: implications for traffic and industrial emissions. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.139133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Chen K., Zhu S., Wang P., Zhang H. Severe air pollution events not avoided by reduced anthropogenic activities during COVID-19 outbreak. Resour. Conserv. Recycl. 2020 doi: 10.1016/j.resconrec.2020.104814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y., et al. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the coronavirus disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2020. Interim Guidance. Water, Sanitation, Hygiene and Waste Management for the COVID-19 Virus: Technical Brief. (2020 Accessed date: 22 June 2020) [Google Scholar]
- WHO . 2020. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations: Scientific Brief. (No. WHO/2019-nCoV/Sci_Brief/Transmission_modes/2020.1) (Accessed date: 22 June 2020) [Google Scholar]
- WHO . World Health Organization; 2020. Coronavirus Disease (COVID-19) Situation Report −153.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200621-covid-19-sitrep-153.pdf?sfvrsn=c896464d_2 [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wong S.H., Lui R.N., Sung J.J. Covid?19 and the digestive system. J. Gastroenterol. Hepatol. 2020;35(5):744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Gu X., Lee W.L., Kauffman K., et al. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. medRxiv. 2020 doi: 10.1101/2020.04.05.20051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. The Lancet Gastroenterology & Hepatology. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Nethery R.C., Sabath B.M., Braun D., Dominici F. Exposure to air pollution and COVID-19 mortality in the United States: a nationwide cross-sectional study. medRxiv. 2020 doi: 10.1101/2020.04.05.20054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters. medRxiv. 2020 doi: 10.1101/2020.04.12.20062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li Y.X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environmental Science & Technology. 2016;50(10):5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen C., Zhu S., Shu C., Wang D., Song J., et al. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2(8):123–124. http://weekly.chinacdc.cn/en/article/doi/10.46234/ccdcw2020.033 [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Jingu X., Fengming H., Liqing C. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]