Abstract
We conducted this systemic review and meta‐analysis in an attempt to evaluate the efficacy and safety of umifenovir in coronavirus disease 2019 (COVID‐19). We searched PubMed, Web of Science, Embase, Cochrane Library, China National Knowledge Infrastructure, and medRxiv database. We included both retrospective and prospective studies. The mean difference (MD) and risk ratio (RR) with 95% confidence intervals (CI) were applied to assess the effectiveness of umifenovir for COVID‐19. A total of 12 studies with 1052 patients were included in our final studies. Compared with control group, umifenovir was associated with higher negative rate of PCR on day 14 (RR:1.27; 95% CI: 1.04 to 1.55). However, umifenovir is not related to nucleus acid negative conversion time (MD: 0.09; 95% CI: −1.48 to 1.65), negative rate on day 7 (RR:1.09; 95% CI: 0.91 to 1.31), incidence of composite endpoint (RR:1.20; 95% CI: 0.61 to 2.37), rate of fever alleviation on day 7 (RR:1.00; 95% CI: 0.91 to 1.10), rate of cough alleviation on day 7 (RR:1.00; 95% CI: 0.85 to 1.18), or hospital length of stay (MD: 1.34; 95% CI: ‐2.08 to 4.76). Additionally, umifenovir was safe in COVID‐19 patients (RR for incidence of adverse events: 1.29; 95% CI: 0.57 to 2.92). The results of sensitivity analysis and subgroup analysis were similar to pooled results. There is no evidence to support the use of umifenovir for improving patient‐important outcomes in patients with COVID‐19.
Keywords: COVID‐19, efficacy, meta‐analysis, safety, umifenovir
Highlights
First meta‐analysis of efficacy and safety of umifenovir for COVID‐19.
Umifenovir is safe and related to higher negative rate of PCR on day 14 in COVID‐19.
The efficacy of umifenovir in COVID‐19 is limited.
Umifenovir could not improve patient‐important outcomes in COVID‐19.
1. BACKGROUND
The fast worldwide spread and outbreak of coronavirus disease 2019 (COVID‐19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, has leaded to over 6.4 million infected cases and 370 000 deaths so far. 1 The epidemiologic situation is still severe around the world now due to lack of vaccine and specific antiviral drugs against this highly infectious virus. This has caused anxiety and stress in the general population. 2 , 3
Based on previous treatments experience of severe acute respiratory syndrome (SARS) and Middle East Respiratory Syndrome (MERS), some potential antiviral drugs have been approved and used urgently in COVID‐19 patients, such as interferon, ribavirin, hydroxychloroquine, lopinavir and ritonavir, umifenovir, and so on. 4 , 5 However, the specific effectiveness of these drugs for COVID‐19 still remains unclear and controversial, especially considering that almost all recent multicenter, randomized, controlled trials (RCTs) failed to observed benefits of them. For instance, Cao et al 6 observed no benefit of lopinavir‐ritonavir treatment in adult patients with severe COVID‐19. Similarly, remdesivir was not associated with significant clinical benefits 7 and hydroxychloroquine did not result in a significantly higher probability of negative conversion. 8
Recently more and more researchers start focusing on possible efficacy of umifenovir in COVID‐19. Umifenovir is a broad‐spectrum antiviral agent which could effectively inhibit the fusion of virus with host cells and is already licensed for prophylaxis and treatment of influenza. 9 Previous research has elucidated that umifenovir is an efficient inhibitor of SARS‐CoV‐2 in vitro. 10 Nevertheless, little is known about the actual clinical efficacy of umifenovir in vivo due to lack of large‐scale RCTs. Some recent small‐scale clinical studies with limited sample size have drawn controversial conclusions about efficacy of umifenovir for COVID‐19. For example, Wang et al 11 found umifenovir treatment showed tendency to improve the discharging rate and decrease the mortality rate. However, Lian et al 12 demonstrated that umifenovir treatment is not associated with improved outcomes.
Given the emergency of COVID‐19 worldwide and uncertain effectiveness of umifenovir in patients, we conducted this systemic review and meta‐analysis in an attempt to evaluate the efficacy and safety of umifenovir in COVID‐19.
2. MATERIALS AND METHODS
2.1. Search strategy
A systematic and comprehensive search was conducted in six databases: PubMed, Web of Science, Embase, Cochrane Library, China National Knowledge Infrastructure, and medRxiv (https://www.medrxiv.org) from 1 December 2019 to 1 June 2020. Potentially relevant studies which reported the efficacy and safety of umifenovir in COVID‐19 were identified. There was no limitation in the publication language. Both Medical Subject Headings terms and free text words were used to increase sensitivity for search strategy. The following search terms were used: “COVID‐19” or “SARS‐CoV‐2” or “novel coronavirus pneumonia” or “novel coronavirus” and “arbidol” or “umifenovir” or “arbidol hydrochloride”. The listed references of relevant studies were also manually evaluated to insure a complete search.
All searched results were evaluated according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement. 13 At first the titles and abstracts were screened to identify related studies, and then full texts were evaluated carefully to determine included studies. The complete search and selection of related studies were performed by two independent researchers. Any disagreement was resolved through the third researcher and even team discussion until consensus was reached.
2.2. Inclusion and exclusion criteria
All retrospective, prospective observational studies and RCTs of adults investigating the effectiveness of umifenovir in COVID‐19 were considered for inclusion. The following inclusion criteria were used: (a) laboratory‐confirmed COVID‐19 adult patients (18 years or older); (b) patients were divided into umifenovir group in which the patients were all administrated with umifenovir, and control group in which none of patients was administrated with umifenovir; (c) outcomes included at least one of followings: polymerase chain reaction (PCR) negative conversion time and negative rate, rate of symptom alleviation, clinical progression, hospital length of stay (LOS) and adverse events; (d) with informed consent.
The following exclusion criteria were used: (a) studies with insufficient data; (b) commentary, editorials, expert opinions, case reports, letters and reviews; (c) non‐human studies.
2.3. Outcomes and data collection
The primary outcomes of our study included nucleus acid negative conversion time, negative rate of PCR on day 7, negative rate of PCR on day 14 and incidence of composite endpoint (admission to intensive care unit or mechanical ventilation or death). Nucleus acid negative conversion time was defined as the time of positive‐to‐negative conversion of SARS‐CoV‐2 nucleic acid. The negative conversion was confirmed when patients received at least two real time (RT)‐PCR tests taken at least 24‐hour apart and results were all negative. The negative rate on day 7 and day 14 were calculated from diagnosis with COVID‐19, admission into hospital or beginning of treatment, which depends on each individual study. The composite endpoint was used because the three individual components were all considered as serious outcomes of similar infectious diseases. 14
The secondary outcomes included rate of fever alleviation on day 7, rate of cough alleviation on day 7, hospital LOS and incidence of adverse events. Adverse events included digestive tract reactions (diarrhea, nausea, vomiting, etc), abnormal liver function tests or renal function tests, psychiatric symptom reactions (depression, acute confusion, etc).
Data were extracted by 2 investigators independently. Similarly, any disagreement was resolved through third investigator and team discussion until consensus was reached. Data were retrieved by using an Excel spreadsheet (Microsoft Corporation, Redmond, WA). The following information was extracted from each study: first author and publication year, study design, region, number of patients, specific therapies in umifenovir group and control group, key outcomes.
3. STATISTICAL ANALYSES
Data syntheses were completed by using Cochrane systematic review software Review Manager (RevMan; Version 5.2; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, 2014). The results were displayed in forest plots, and statistical significance was defined as P < .05. Based on the immunological study, the continuous variables were reported as mean and SD, 15 , 16 whereas dichotomous variables were reported as frequency and proportion. 17 Statistical heterogeneity among included studies was evaluated via Cochran Q test and Higgins I 2 statistic. 18 Significant heterogeneity was rendered as P < .1 or I 2 > 50%. The random‐effects model was used to calculate the pooled effects if significant heterogeneity was observed to generalize findings beyond the included studies by assuming that the selected studies are random samples from a larger population, 19 otherwise fixed‐effects model was used. Mean difference (MD) and 95% confidence interval (CI) were calculated for continuous data, with risk ratio (RR) and 95% CI for dichotomous data.
To investigate the potential sources of heterogeneity for primary outcomes, we performed sensitivity analysis to further verify conclusions of meta‐analysis by excluding some low‐quality and dubious studies, and subgroup analysis in terms of different sample size, study design, and administrations of antiviral drugs in control group.
3.1. Quality assessment
We used the Newcastle‐Ottawa quality assessment scale (NOS) to assess quality of the included studies. 20 The NOS consists of three parameters of quality: selection, comparability, and outcome. A score of 0 to 9 was allocated to each study. Generally, studies which earned 6 or higher points were regarded as high‐quality studies. Besides, the certainty of evidence at the outcome level was assessed using the GRADE approach. 21 Quality assessment was also conducted by two independent researchers.
4. RESULTS
A total of 290 records from all databases and other sources were identified. After duplicates removal, 137 records were screened for title and abstract. Then, 28 full‐text studies were further assessed for eligibility, and eventually 12 studies were enrolled in our final analysis, including 10 retrospective studies 11 , 12 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 and 2 randomized controlled trials 30 , 31 (Figure 1).
Figure 1.
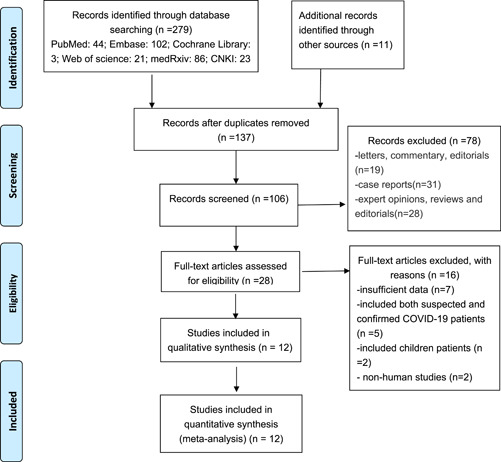
Study flow diagram
4.1. Study description
A total of 1052 patients were pooled from all included studies in our meta‐analysis. All studies were conducted in China. The sample size of each study varied from 32 to 236 patients. Among six included studies, the patients in control group were only administrated with standard care. And in the other six studies, control group were administrated with standard care plus other antiviral drugs, such as lopinavir/ritonavir, interferon, favipiravir, and so on. As for quality assessment, all eight studies earned at least six points. Details of characteristics of included studies are shown in Table 1.
Table 1.
Characteristics of included studies
| Reference | Study design | Region | No. of patients | Median age of patients, y | Severity of patients at admission | Intervention | Control | Outcomes | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Zhu et al 22 | Retrospective | Jiangsu, China | 50 | 26 | NA | Standard care plus arbidol (0.2 g tid) | Standard care plus lopinavir/ritonavir | Negative conversion time, negative rate on day 7 and day 14 | 7 |
| Chen et al 30 | Multicenter, open‐label, RCT | Hubei, China | 236 | NA | Not severe/critical patients | Standard care plus arbidol (0.2 g tid) | Standard care plus favipiravir | Rate of symptom alleviation on day 7, Incidence of composite endpoint and adverse events, | 8 |
| Wen et al 25 | Retrospective | Guangzhou, China | 94 | 54 | NA | Standard care plus arbidol (0.2 g tid) | Standard care | Negative rate of PCR on day 7 and day 14, Rate of symptom alleviation on day 7, Incidence of composite endpoint and adverse events | 7 |
| Chen et al 26 | Retrospective | Shanghai, China | 82 | 44 | NA | Standard care plus IFN‐α2b and arbidol (0.2 g tid) | Standard care plus IFN‐α2b | Negative rate on day 7, Incidence of composite endpoint and adverse events, | 8 |
| Li et al 31 | Exploratory RCT | Guangzhou, China | 52 | 50 | mild/moderate patients | Standard care plus arbidol (0.2 g tid) | Standard care | negative conversion time, Negative rate of PCR on day 7 and day 14, Rate of symptom alleviation on day 7 | 9 |
| Xu et al 24 | Multicenter retrospective cohort study | Hubei and Shenzhen, China | 141 | 51 | Not severe/critical patients (without ventilation) | Standard care plus inhaled IFN‐α2b (bid, 5 ×10*5 IU) and arbidol (0.2gtid) | Standard care plus inhaled IFN‐α2b (bid, 5 ×10*5 IU) | negative conversion time | 6 |
| Deng et al 28 | Retrospective cohort study | Guangdong Province, China | 33 | 41 | Not severe/critical patients (without ventilation) | Standard care plus lopinavir/ritonavir (0.4/0.1 g bid) and arbidol (0.2 g tid) | Standard care plus lopinavir/ritonavir (0.4/0.1 g bid) | Negative rate on day 7 and day 14 | 8 |
| Lan et al 29 | Retrospective observational study | Zhejiang, China. | 73 | 52 | non‐ICU patients | Standard care plus lopinavir/ritonavir (0.4/0.1 g bid) and arbidol (0.2 g tid) | Standard care plus lopinavir/ritonavir (0.4/0.1 g bid) | negative conversion time, Incidence of composite endpoint, Hospital length of stay | 7 |
| Liu et al 27 | Retrospective | Wuhan, China | 32 | 44 | NA | Standard care plus abidor (0.2 g tid) | Standard care | negative conversion time, Hospital length of stay, Incidence of composite endpoint | 6 |
| Lian et al 12 | Retrospective | Wuhan, China | 81 | 60 | non‐ICU patients | Standard care plus umifenovir (0.2 g tid) | Standard care | Hospital length of stay, negative conversion time | 8 |
| Wang et al 11 | Retrospective | Wuhan, China | 69 | 42 | NA | Standard care plus arbidol | Standard care | Incidence of composite endpoint | 6 |
| Chen et al 23 | Retrospective | Guangzhou, China | 109 | 48 | NA | Standard care plus arbidol | Standard care | negative conversion time, Negative rate of PCR on day 14, Hospital length of stay | 9 |
Abbreviations: ICU, intensive care unit; IFN: interferon; NA, not acquired; RCT, randomized controlled trial.
Note: bid, twice a day; composite endpoint, admission to ICU or mechanical ventilation or death; tid, three times a day.
4.2. Outcomes
A total of 7 studies 12 , 22 , 23 , 24 , 27 , 29 , 31 reported nucleus acid negative conversion time (Figure 2). There was no significant difference of negative conversion time between umifenovir group and control group (MD: 0.09; 95% CI: −1.48 to 1.65; I 2 = 89%). There were six studies 12 , 22 , 25 , 26 , 28 , 31 and five studies 22 , 23 , 25 , 28 , 31 which reported negative rate of PCR on day 7 and day 14, respectively. Umifenovir was not associated with higher negative rate on day 7(RR:1.09; 95% CI: 0.91 to 1.31; I2 = 44%). However, umifenovir could increase negative rate of PCR on day 14 (RR:1.27; 95% CI: 1.04 to 1.55; I2 = 63%; Figure 3). The pooled results of 6 studies 11 , 25 , 26 , 27 , 29 , 30 revealed that umifenovir was not associated with incidence of composite endpoint (RR:1.20; 95% CI: 0.61 to 2.37; I 2 = 38%; Figure 4).
Figure 2.
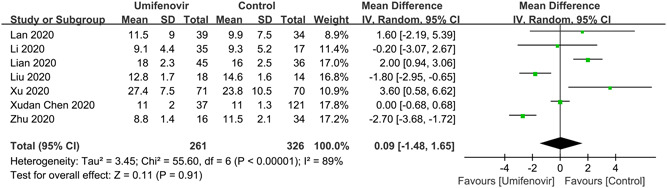
Forest plot for nucleus acid negative conversion time between umifenovir group and control group
Figure 3.

Forest plot for negative rate of polymerase chain reaction on day 7 and day 14 between umifenovir group and control group
Figure 4.
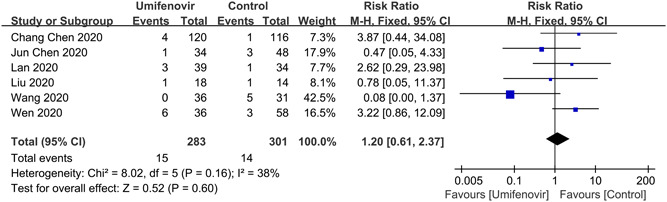
Forest plot for incidence of composite endpoint between umifenovir group and control group
Furthermore, our meta‐analysis showed that there was no significant association between umifenovir and secondary outcomes: rate of fever alleviation on day 7 (RR:1.00; 95% CI: 0.91 to 1.10; I 2 = 0%); rate of cough alleviation on day 7 (RR:1.00; 95% CI: 0.85 to 1.18; I 2 = 0%; Figure 5) and hospital LOS (MD: 1.34; 95% CI: −2.08 to 4.76; I 2 = 97%; Figure 6). Last but not the least, four studies 25 , 26 , 30 , 31 reported the safety of umifenovir. The pooled results showed that use of umifenovir was safe in COVID‐19 patients (RR for incidence of adverse events:1.29; 95% CI: 0.57 to 2.92; I 2 = 56%; Figure 7). According to the GRADE approach, these outcomes were all low certainty of evidence.
Figure 5.
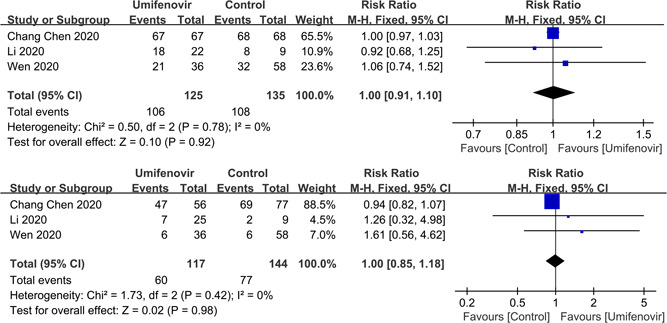
Forest plot for rate of fever alleviation and cough alleviation on day 7 between umifenovir group and control group
Figure 6.
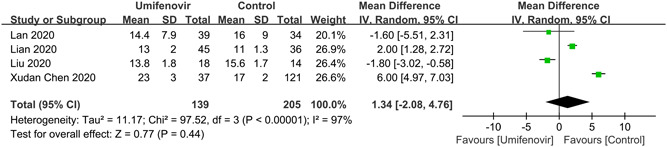
Forest plot for hospital length of stay between umifenovir group and control group
Figure 7.
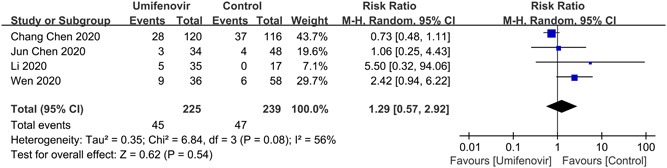
Forest plot for incidence of adverse events between umifenovir group and control group
4.3. Sensitivity analysis and subgroup analysis
Among these included studies, the study of Wen et al 25 and study of Chen et al 23 were both conducted in Guangzhou Eighth People's Hospital, China. It is possible that some data of confirmed COVID‐19 patients was duplicated. After team discussion, we decided to include both two studies in current meta‐analysis because their key outcomes were almost completely different. Besides, we noticed that the study of Wang et al 11 was mainly conducted to summary clinical features of 69 cases with COVID‐19 in Wuhan, China. As a result, the effectiveness of umifenovir was not a primary outcome, which means some bias might exist and affect the pooled results. As for four primary outcomes, the sensitivity analysis still had similar results with pooled results, after excluding the study of Wen et al and Wang et al (Table 2).
Table 2.
Sensitivity analysis and subgroup analysis of primary outcomes
| Analysis | No. of studies | Nucleus acid negative conversion time (MD)(95%CI) | P interaction | Negative rate of PCR on day 7 (RR)(95%CI) | Negative rate of PCR on day 14 (RR)(95%CI) | P interaction | Incidence of composite endpoint (RR)(95%CI) |
|---|---|---|---|---|---|---|---|
| Sensitivity analysis | 10 | 0.09(−1.48, 1.65) | 1.18(0.88, 1.57) | 1.35(1.03, 1.77) | 1.54(0.54, 4.4) | ||
| Sample size | .38 | .10 | |||||
| ≥100 | 3 | 1.49(−1.99, 4.96) | NA | 1.04(0.84, 1.29) | 3.87(0.44, 34.08) | ||
| <100 | 9 | −0.38(−2.61, 1.86) | 1.09(0.91, 1.31) | 1.37(1.07, 1.74) | 0.99(0.47, 2.06) | ||
| Study design | .84 | .64 | |||||
| RCT | 2 | −0.2(−3.07, 2.67) | 0.9(0.44, 1.84) | 1.2(0.9, 1.59) | 3.87(0.44, 34.08) | ||
| retrospective | 10 | 0.14(−1.57, 1.84) | 1.16(0.86, 1.56) | 1.31(1.02, 1.69) | 0.99(0.47, 2.06) | ||
| Antiviral drugs | .93 | .001 | |||||
| Control group without any antiviral drugs | 6 | 0.03(−1.59, 1.65) | 0.89(0.69, 1.15) | 1.10(0.96, 1.25) | 0.75(0.08, 7.43) | ||
| Control group with other antiviral drugs | 6 | 0.64(−3.82, 5.10) | 1.57(0.85, 2.90) | 1.75(1.35, 2.28) | 1.73(0.55, 5.47) |
Abbreviations: CI, confidence interval; MD, mean difference; NA, not acquired; RR, relative risk; RCT, randomized controlled trial.
We also performed subgroup analysis to explore the impacts of different sample size, study design, and administrations of antiviral drugs in control group on pooled results. We further conducted a test of interaction for the results with huge heterogeneity (negative conversion time and negative rate of PCR on day 14). As shown in table 2, the results of subgroup analysis were similar with pooled results. Generally, our conclusion is relatively stable and reliable.
4.4. Publication bias
Given that only under 10 studies were included in each outcome in our meta‐analysis, the approaches to evaluate publication bias might have limited efficacy. Therefore, publication bias was not assessed.
5. DISCUSSION
It is acknowledged most of COVID‐19 patients are now mainly receiving supportive and symptomatic therapies due to lack of clinical evidence for effective antiviral drugs against SARS‐coV‐2. Umifenovir has been recommended for the treatment of COVID‐19 in some countries currently. However, the clinical evidence is still limited.
To our knowledge, this is the first systematic review and meta‐analysis to assess exclusively efficacy and safety of umifenovir for COVID‐19. Liu et al 32 have ever conducted a systematic review of efficacy of umifenovir for COVID‐19 based on evidence in studies of SARS‐CoV‐2 and other acute viral infections. They included only four studies and concluded that there was limited evidence of uncertain effects of treatment using umifenovir in COVID‐19 patients. We included more studies and conducted further detailed meta‐analysis. Our main finding is that umifenovir is associated with higher negative rate of PCR on day 14 in COVID‐19 adult patients and this finding may be useful for countries with low socioeconomic status. 33 However, umifenovir is not associated with nucleus acid negative conversion time, negative rate of PCR on day 7, incidence of composite endpoint, rate of symptom alleviation on day 7, hospital LOS or incidence of adverse events. The reasons for increased PCR negative rate on day 14 are still unclear so far. According to previous reports, the median seroconversion time for antibodies, IgM and IgG were day‐11, day‐12, and day‐14, respectively 34 and the median duration of viral shedding was 20 days in clinical course of COVID‐19. 35 Therefore, it is possible that the effects of umifenovir on negative conversion rate are only observed since 2 or 3 weeks after onset.
Umifenovir is a small indole‐derivative molecule which can simultaneously block virus’ entry into target cells, inhibit synthesis of viral RNA, and stimulate immune via induction of serum interferon and activation of phagocytes. 9 Umifenovir has also direct and superior antiviral effects in early stage of viral replication in vitro for SARS. 36 Nevertheless, based on previous studies, the efficacy of umifenovir for COVID‐19 in vivo is unsatisfactory. One plausible explanation is that higher dose is needed to achieve equal suppression effect of SARS‐CoV‐2 in patients with that in vitro. For example, Sheahan et al 37 have elucidated remdesivir and IFN have superior antiviral activity against MERS‐CoV in vitro. However, this assumption needs to be verified in future studies.
In China, the standard of discharge includes improvement of clinical symptoms and CT, and at least two consecutive negative results of PCR. 38 Thus, the negative conversion time and negative rate were both defined as primary outcomes in meta‐analysis. Additionally, the composite endpoint which represents the clinical progression of illness in COVID‐19 patients was also considered as a primary outcome. Our preliminary meta‐analysis indicates that umifenovir has good safety and tolerability but limited efficacy. It should be noted that there were also some other outcome variables which we did not use in current meta‐analysis. For example, it is reported umifenovir was not superior compared with conventional supportive therapies in radiology improvement (via chest CT scores), clinical recovery rate on day 7, or cure rate. 12 , 29 , 30 , 31 Because of the overall results, and particularly the results in patient‐important outcomes, there is currently no place for the use of umifenovir in COVID patients.
There were also several similar original studies and reviews which arrived at similar conclusions. Peng et al 39 showed that umifenovir was not associated with hospital LOS (HR: 1.62; 95%CI: 0.37‐7.01) or time of SARS‐CoV‐2 RNA clearance (HR: 0.51; 95%CI: 0.11‐2.30) in multivariate analysis among children patients with COVID‐19. Zhong et al 40 have conducted a comprehensive meta‐analysis of therapies for SARS, MERS, and COVID‐19 and found umifenovir did not show a superior ability in virological eradication compared with control group (RR: 1.07; 95%: 0.83‐1.39).
Our study has several limitations. Firstly, most of included studies were retrospective cohort studies and all studies were conducted in China, which might lead to potential selection bias. It has to be acknowledged that the RCTs of umifenovir in COVID‐19 are still deficient so far. Therefore, we decided to include both observational studies and RCTs in this meta‐analysis in an attempt to provide a preliminary conclusion for the use of umifenovir in COVID‐19. Several ongoing clinical trials evaluating efficacy of umifenovir in COVID‐19 (NCT04260594, NCT04255017, NCT04252885) might clarify this issue in the future. Then, some outcomes included a small sample size of patients. Thirdly, the variations in population, severity of illness, timing of treatment, dosage, co‐treatments among included studies might lead to huge heterogeneity and influence our results. It should be noted that the antiviral drugs in the control group may also be contributing to heterogeneity, which was showed in the subgroup analysis for the results of negative rate of PCR on day 14. Nevertheless, we were not able to conduct further analysis because original individual data of patients’ age and severity were not available.
Considering low quality and certainty of evidence and huge heterogeneity, it is difficult to draw a clear conclusion about the advantages of umifenovir for COVID‐19 up to now. However, to some degree, our conclusions might help physicians to comprehensively understand the mechanisms, effectiveness, indications of umifenovir in COVID‐19. More basic researchers are also needed to reveal the association of umifenovir, immunity of host and clearance of SARS‐coV‐2.
6. CONCLUSIONS
We found that umifenovir was safe and associated with higher negative rate of PCR on day 14 in laboratory‐confirmed COVID‐19 adult patients. However, it could not significantly shorten nucleus acid negative conversion time or hospital LOS, improve symptoms or decrease risk of disease progression. In conclusion, there is no evidence to support the use of umifenovir for improving patient‐important outcomes in patients with COVID‐19. Our conclusions need to be verified in future studies.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
DH, HY, TW, HY, RY, and ZL conceived the idea, designed, and supervised the study, drafted the manuscript, and had full access to all of the data and took responsibility for the integrity of the data. DH and HY collected data. DH and HY analyzed data and performed statistical analysis. All of the authors reviewed and approved the final version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by the Emergency Response Project for New Coronavirus of Science and Technology Department of Sichuan Provincial (2020YFS0005 and 2020YFS0009).
Huang D, Yu H, Wang T, Yang H, Yao R, Liang Z. Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID‐19): A systematic review and meta‐analysis. J Med Virol. 2021;93:481–490. 10.1002/jmv.26256
Dong Huang and He Yu contributed equally to this work.
Contributor Information
Rong Yao, Email: yaorong@wchscu.cn.
Zongan Liang, Email: liangza@scu.edu.cn.
REFERENCES
- 1. COVID‐19 Coronavirus pandemic. https://www.worldometers.info/coronavirus/. Accessed June 10, 2020.
- 2. Wang C, Pan R, Wan X, et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID‐19) epidemic among the general population in China. Int J Environ Res Public Health. 2020;17(5):1729. 10.3390/ijerph17051729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang C, Pan R, Wan X, et al. A longitudinal study on the mental health of general population during the COVID‐19 epidemic in China. Brain Behav Immun. 2020;87(20):40‐48. 10.1016/j.bbi.2020.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McKee DL, Sternberg A, Stange U, Laufer S, Naujokat C. Candidate drugs against SARS‐CoV‐2 and COVID‐19. Pharmacol Res. 2020;157:104859. 10.1016/j.phrs.2020.104859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Momattin H, Mohammed K, Zumla A, Memish ZA, Al‐Tawfiq JA. Therapeutic options for Middle East respiratory syndrome coronavirus (MERS‐CoV)–possible lessons from a systematic review of SARS‐CoV therapy. Int J Infect Dis. 2013;17(10):e792‐e798. 10.1016/j.ijid.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao B, Wang Y, Wen D, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe covid‐19. N Engl J Med. 2020;382(19):1787‐1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395(10236):1569‐1578. 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. 10.1136/bmj.m1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blaising J, Polyak SJ, Pécheur EI. Arbidol as a broad‐spectrum antiviral: an update. Antiviral Res. 2014;107:107‐194. 10.1016/j.antiviral.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang X, Cao R, Zhang H, et al. The anti‐influenza virus drug, arbidol is an efficient inhibitor of SARS‐CoV‐2 in vitro. Cell Discov. 2020;6:28. 10.1038/s41421-020-0169-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;ciaa272. 10.1093/cid/ciaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lian N, Xie H, Lin S, Huang J, Zhao J, Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020;S1198‐743X(20):30234‐2. 10.1016/j.cmi.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao HN, Lu HZ, Cao B, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med. 2013;368(24):2277‐2285. 10.1056/NEJMoa1305584 [DOI] [PubMed] [Google Scholar]
- 15. Ho RC, Thiaghu C, Ong H, et al. A meta‐analysis of serum and cerebrospinal fluid autoantibodies in neuropsychiatric systemic lupus erythematosus. Autoimmun Rev. 2016;15(2):124‐138. 10.1016/j.autrev.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 16. Ng A, Tam WW, Zhang MW, et al. IL‐1β, IL‐6, TNF‐ α and CRP in elderly patients with depression or Alzheimer's disease: systematic review and meta‐analysis. Sci Rep. 2018;8(1):12050. 10.1038/s41598-018-30487-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho RC, Ong H, Thiaghu C, Lu Y, Ho CS, Zhang MW. Genetic variants that are associated with neuropsychiatric systemic lupus erythematosus. J Rheumatol. 2016;43(3):541‐551. 10.3899/jrheum.150884 [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 19. Cheung MW, Ho RC, Lim Y, Mak A. Conducting a meta‐analysis: basics and good practices. Int J Rheum Dis. 2012;15(2):129‐135. 10.1111/j.1756-185X.2012.01712.x [DOI] [PubMed] [Google Scholar]
- 20. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 21. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924‐926. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu Z, Lu Z, Xu T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID‐19. J Infect. 2020;S0163‐4453(20):30188‐2. 10.1016/j.jinf.2020.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen X, Zhang Y, Zhu B, et al. Associations of clinical characteristics and antiviral drugs with viral RNA clearance in patients with COVID‐19 in Guangzhou, China: a retrospective cohort study. medRxiv. 2020. 10.1101/2020.04.09.20058941 [DOI] [Google Scholar]
- 24. Xu P, Huang J, Fan Z, et al. Arbidol/IFN‐α2b therapy for patients with corona virus disease 2019: a retrospective multicenter cohort study. Microbes Infect. 2020;S1286‐4579(20):30090‐30093. 10.1016/j.micinf.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wen CY, Xie ZW, Li YP, et al. Real‐world efficacy and safety of lopinavir/ritonavir and arbidol in treating with COVID‐19: an observational cohort study. Zhonghua Nei Ke Za Zhi. 2020;59(0):E012. 10.3760/cma.j.cn112138-20200227-00147 [DOI] [Google Scholar]
- 26. Chen J, Ling Y, Xi X, et al. Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia. J Infect Dis. 2020;02:86‐89. 10.3760/cma.j.cn311365-20200210-00050 [DOI] [Google Scholar]
- 27. Liu L, Yuan L, Feng Y, et al. Clinical study on combined scheme of Lianhuaqingwen capsules and abidole in the treatment for coronavirus disease 2019. Guangdong Med J, 1‐4, 10.13820/j.cnki.gdyx.20200913 [DOI] [Google Scholar]
- 28. Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. 2020;S0163‐4453(20):30113‐30114. 10.1016/j.jinf.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lan X, Shao C, Zeng X, Wu Z, Xu Y. Lopinavir‐ritonavir alone or combined with arbidol in the treatment of 73 hospitalized patients with COVID‐19: a pilot retrospective study. medRxiv. 2020. 10.1101/2020.04.25.20079079 [DOI] [PubMed] [Google Scholar]
- 30. Chen C, Zhang Y, Huang J, et al. Favipiravir versus arbidol for COVID‐19: a randomized clinical trial. medRxiv. 2020. 10.1101/2020.03.17.20037432 [DOI] [Google Scholar]
- 31. Li Y, Xie Z, Lin W, et al. An exploratory randomized controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID‐19 (ELACOI). medRxiv. 2020. 10.1101/2020.03.19.20038984 [DOI] [Google Scholar]
- 32. Liu W, Zhou P, Chen K, et al. Efficacy and safety of antiviral treatment for COVID‐19 from evidence in studies of SARSCoV‐2 and other acute viral infections: a systematic review and meta‐analysis. CMAJ. 2020. 10.1503/cmaj.200647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tran BX, Vu GT, Latkin CA, et al. Characterize health and economic vulnerabilities of workers to control the emergence of COVID‐19 in an industrial zone in Vietnam. Saf Sci. 2020;129:104811. 10.1016/j.ssci.2020.104811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020. 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khamitov RA, SIa L, Shchukina VN, Borisevich SV, Maksimov VA, Shuster AM. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr Virusol. 2008;53(4):9‐13. [PubMed] [Google Scholar]
- 37. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nat Commun. 2020;11(1):222. 10.1038/s41467-019-13940-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. China NHC New coronavirus pneumonia prevention and control program (7th Ed.). 2020. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. Accessed March 4, 2020 [Google Scholar]
- 39. Peng H, Gao P, Xu Q, et al. Coronavirus disease 2019 in children: characteristics, antimicrobial treatment, and outcomes. J Clin Virol. 2020;128:104425. 10.1016/j.jcv.2020.104425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhong H, Wang Y, Zhang ZL, et al. Efficacy and safety of current therapeutic options for COVID‐19 ‐ lessons to be learnt from SARS and MERS epidemic: a systematic review and meta‐analysis. Pharmacol Res. 2020;157:104872. 10.1016/j.phrs.2020.104872 [DOI] [PMC free article] [PubMed] [Google Scholar]


