Abstract
Objectives
A high frequency and a strong association of olfactory/gustatory impairment with COVID‐19 were reported. Its spontaneous evolution remains unknown. The aim of this study was to investigate the spontaneous evolution of olfactory disorders in COVID‐19 patients.
Study Design
Cross‐sectional study.
Methods
A total of 229 patients with laboratory‐confirmed COVID‐19 from March 1 through 31, 2020 in our institution were included. Among them, 140 patients (mean age, 38.5 years, 89 women) reported sudden olfactory/gustatory disorders during COVID‐19. All patients were interviewed by phone based on a questionnaire with 16 questions at time of survey. The primary end point was olfactory recovery rate at time of survey.
Results
The frequency of patients with olfactory disorders was higher before March 20, 2020 than since (70.3% vs. 53.9%, respectively) (P = .016). At time of survey (26 days of the mean time from anosmia onset), 95.71% reported to start an olfactory recovery. The mean time from olfactory loss onset to recovery onset was 11.6 days. Recovery started between the fourth and the fifteenth day after olfactory loss onset in 78.4% of patients. Complete olfactory recovery happened for 51.43% of patients. There was a significant relationship between the complete olfactory recovery and a short time from olfactory loss onset to recovery onset (P = .0004), absence of nasal obstruction (P = .023) and absence of sore/dry/tingling feeling in the nose (P = .007) in COVID‐19 patients.
Conclusion
Knowledge of spontaneous evolution of olfactory disorders allows reassuring patients and planning therapeutic strategies for persistent olfactory dysfunction after having definitely recovered from COVID‐19.
Level of Evidence
4 Laryngoscope, 130:2667–2673, 2020
Keywords: Olfactory disorders, olfactory dysfunction, COVID‐19, olfactory recovery, anosmia
INTRODUCTION
Many families of virus have been presented as causative agents of olfactory dysfunction during or after upper respiratory tract infection (URTI). The new coronavirus of current outbreaks (SARS‐CoV‐2) may also be a causative agent of olfactory impairment. Recently, a high frequency and a strong association of smell and taste impairment to COVID‐19 was reported. 1 , 2 , 3 , 4 , 5 Olfactory impairment/loss in patients with confirmed COVID‐19 was reported in 4.8% of 1099 patients in China, 6 in 19.4% of 320 patients 7 and in 34% of 59 hospitalized patients 8 in Italy, in 68% of 59 patients in United States, 3 and in 85.6% of 417 patients in Europe. 1 The frequency of taste disorders was similar to that of smell disorders.
It has been suggested that SARS‐CoV‐2 causes obstructive inflammation of olfactory clefts, 9 or targets and damages olfactory epithelium support and stem cells leading to olfactory disturbances in COVID‐19 patients. 10 On the other hand, some types of coronaviruses have been shown to propagate, after exposure to coronaviruses by inhalation, from the nasal epithelium past the cribriform plate to infect the olfactory bulb and downstream areas like the piriform cortex and the brain stem. 11 , 12 We do not know if SARS‐CoV‐2 is able to reach to the central olfactory system by this way.
Sudden olfactory loss without knowing the possibility of its recovery may lead to a very anxious condition and negatively impacts on patients' quality of life, particularly during current COVID‐19 pandemic. Knowing the spontaneous evolution of olfactory disorders and its possibility of recovery in COVID‐19 patients is very useful to inform and reassure patients. Moreover, it may contribute to understanding the mechanisms of olfactory dysfunctions in this condition from the time of symptom onset to its recovery. Finally, it may help clinicians to plan therapeutic strategies for persistent olfactory dysfunctions after having definitely recovered from COVID‐19 (systemic corticosteroid treatment, olfactory training, etc). The aim of this study was to investigate the spontaneous evolution of olfactory disorders in COVID‐19 patients.
MATERIALS AND METHODS
A total of 879 adult patients underwent COVID‐19 testing between March 1–31, 2020 in our tertiary hospital, University Hospital of Nancy, France. These patients were tested at the Infectious Disease Department because of flu symptoms. Most subjects were outpatients and some were hospitalized. Of those, a total of 261 consecutive adult patients were diagnosed COVID‐19 positive confirmed by a real‐time reverse transcriptase polymerase‐chain‐reaction (RT‐PCR) assay from nasopharyngeal swab specimens. A follow‐up phone call was done at the time of the survey. Patients with olfactory or gustatory dysfunctions before the pandemic (two patients), or patients hospitalized in the intensive care unit at time of survey (two patients), or patients with mental illness or unable to answer the questionnaire by telephone (two patients) were excluded. Five patients had died from COVID‐19 and 21 patients were unreachable despite at least three calls.
During the call, patients were asked if they had a sudden olfactory loss during their course of COVID‐19. If it was the case, an interview based on a questionnaire with 16 questions was done at the time of the survey. This questionnaire (French and English version in Appendix S1) was tested in 10 persons (pilot study) but it was not validated by any previous study. All symptoms were self‐reported and no objective measurements of olfactory or gustatory function or nasal obstruction were performed. Verbal consent was obtained from all patients who accepted to participate in this survey. This study was approved by the Institutional Review Board of University Hospital of Nancy, France.
These 16 questions had been chosen to characterize the timing of the occurrence of smell impairment and associated symptoms, the time from onset to recovery of olfactory loss. Sense of smell at the time of olfactory loss, and at the time of recovery or at the time of survey if there was no smell recovery were assessed using a subjective Olfaction Numerical Score (ONS) from 0 (no sense of smell) to 10 (normal sense of smell). Scores from 1 to 9 indicated progressively decreasing severity of smell impairment. Other symptoms were not investigated because they were reported in a large number of recent studies. The subjects were not asked to rate their olfactory or gustatory function prior to onset of their symptoms. No patient in this study received treatment for olfactory/gustatory loss.
Because the olfactory/gustatory loss was announced as a symptom of COVID‐19 in the mainstream media on March 20, 2020 in many countries and territories, we took into account the frequencies of olfactory disorders before and since March 20, 2020. Since this day, many patients were considered as COVID‐19 positive without laboratory confirmation when they had sudden olfactory disorders.
Statistical Analysis
Descriptive statistics for quantitative variables were expressed as mean ± standard deviation and for qualitative variables as percentages. Chi‐square or Fisher's exact tests were used for categorical variables. For continuous variables, the Wilcoxon‐Mann–Whitney test was used for the comparison of two groups and the Kruskal‐Wallis test for the comparison of three groups because of the non‐normal distribution of samples. Analyses were conducted using SAS v9.1 statistical software (SAS Inst., Cary, NC). A two‐tailed value of P < .05 was considered statistically significant.
RESULTS
A total of 229 patients (147 women and 82 men, mean age, 39.7 ± 13.7 years; age range, 18–89 years) were included into the study. Of those, 140 patients (61.14%) reported a sudden anosmia/dysgeusia during the course of COVID‐19. However, the frequency of self‐reported olfactory disorders in subjects with COVID‐19 positive was higher in patients who were tested before March 20, 2020 (71/101 patients; 70.3%) than in those tested since March 20, 2020 (69/128 patients; 53.9%) (P = .016).
There was no difference in term of sex and age between groups of patients with and without olfactory disorders (89 women and 51 men vs. 58 women and 31 men, P = .8; and 38.5 ± 13.4 years vs. 41.6 ± 14 years, P = .1, respectively). Five patients reported gustatory disorders without olfactory disorders. The mean interval from first non‐olfactory symptoms of COVID‐19 to survey date was 29.6 ± 7.3 days (range, 18–54 days).
Olfactory Disorders and Other Symptoms
Isolate olfactory/gustatory disorders were reported in five patients (3.57%). These symptoms appeared at the same time as other symptoms of COVID‐19 in 20 patients (14.29%) (Table I). For 109 patients with the onset of olfactory dysfunctions after other symptoms, the mean time from the onset of other symptoms to olfactory loss onset was 4.6 ± 2.7 days (range, 1–16 days). In contrast, olfactory impairment appeared from 1 up to 7 days before other symptoms in 6 patients (4.29%). Twenty‐nine patients (20.71%) reported nasal obstruction at the same time of occurrence of olfactory disorders. About 80% of patients had no nasal obstruction. Interestingly, 47 patients (33.57%) reported a sore/dry/tingling feeling in the nose. Of those, 38 had no nasal obstruction and nine with nasal obstruction. Gustatory disorders were reported in 124 patients (88.57%).
Table I.
Characteristics of Olfactory Disorders.
| Number of patients | Frequency (%) | |
|---|---|---|
| OD onset | ||
| Isolated OD | 5 | 3.57 |
| OD onset concomitant with other symptoms | 20 | 14.29 |
| OD onset after other symptoms | 109 | 77.86 |
| OD onset before other symptoms | 6 | 4.29 |
| Nasal Obstruction at OD onset | 29 | 20.71 |
| Sore/dry/tingling nasal cavity | 47 | 33.57 |
| Initial smell assessment | ||
| Complete smell loss (ONS = 0) | 90 | 64.29 |
| Profound smell loss (ONS = 1–3) | 31 | 22.14 |
| Moderate smell loss (ONS = 4–7) | 19 | 13.57 |
| Mild smell loss (ONS = 8–9) | 0 | 0 |
| Worsening of OD after onset | 12 | 22.22 |
| Permanent OD | 136 | 97.14 |
| Fluctuating OD | 4 | 2.86 |
| Parosmia | 21 | 15 |
| Phantosmia | 17 | 12.14 |
| Persistence of OD after other symptoms (n = 135) | ||
| No | 22 | 16.3 |
| Yes | 108 | 80 |
| OD and other symptoms still on‐going | 5 | 3.7 |
| Gustatory disorders | 124 | 88.57 |
| Gustatory disorders recovery (n = 124) | ||
| Unchanged | 6 | 4.84 |
| Partial | 38 | 30.65 |
| Total | 80 | 64.52 |
OD = olfactory disorders; ONS = olfaction numberical scale.
Time Between the Onset of Olfactory Disorders and the Evaluation
The mean time from onset of olfactory dysfunction to survey was 26 ± 7.6 days (range, 13–54 days). Figure 1 shows numbers of patients at the time of survey according to the time from the onset of olfactory disorders to the evaluation in each group.
Fig. 1.
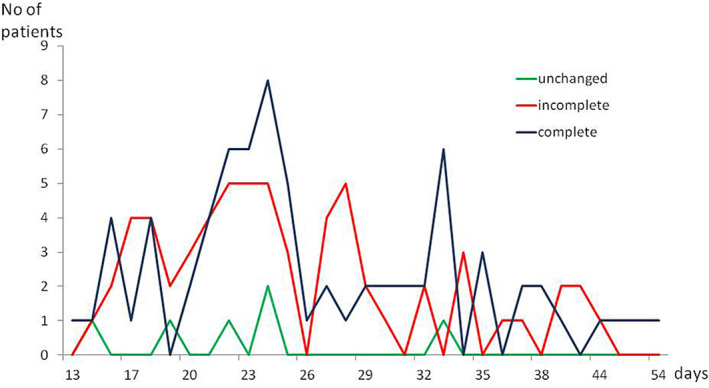
Numbers of patients at the time of survey according to the time from the onset the olfactory disorders to the evaluation in each group. For example, the time from the onset of olfactory disorders to the evaluation was 15 days (1 patient), 19 days (1 patient), 22 days (1 patient), 24 days (2 patients), and 34 days (1 patient) in the group without olfactory recovery.
Natural Evolution of Olfactory Disorders
At the time of survey, 134 patients (95.71%) reported to have an onset of olfactory recovery. The mean time from olfactory loss onset to olfactory recovery onset was 11.6 ± 6.2 days (range, 1–39 days; Fig. 2). Recovery of olfactory function was reported between the fourth and the sixteenth day after the onset of olfactory loss in 105/134 patients (78.4%) (Fig. 2). Among those, 72 patients (51.43%) had completely recovered their olfactory function (mean time from onset of olfactory dysfunction to survey: 26.8 ± 8.2 days [13–54 days]) and 62 patients (44.29%) had partially recovered their sense of smell (mean time from onset of olfactory dysfunction to survey: 25.3 ± 7.1 days [15–44 days]) (Table II). Six patients (4.29%) had not yet recovered their olfactory function (mean time from onset of olfactory dysfunction to survey: 22.8 ± 6 [15–33 days]) (Table II).
Fig. 2.
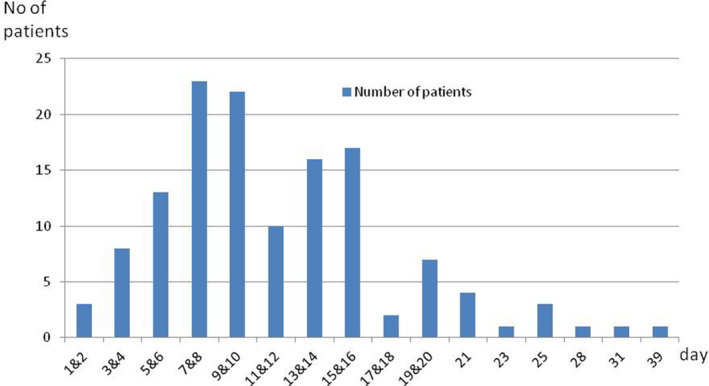
Numbers of patients according to the time from the onset of olfactory loss to the onset of olfactory recovery
Table II.
Relationships Between Characteristics of Olfactory Loss and the Chance of Olfactory Recovery as Well as the Degree of Olfactory Recovery.
| Recovery | Degree of olfactory recovery | ||||||
|---|---|---|---|---|---|---|---|
| No (n = 6) | Yes (n = 134) | P | Unchanged (n = 6) | Incomplete (n = 62) | Complete (n = 72) | P | |
| Age, years | 30.9 ± 7.4 | 38.9 ± 13.5 | 0.22 | 30.9 ± 7.4 | 37.7 ± 10.3 | 39.9 ± 15.7 | .5 |
| Time from smell loss to olfactory recovery onset, days | ‐ | 11.6 ± 6 | ‐ | 13.4 ± 6.4 | 10 ± 5.6 | .0004 | |
| Time from smell loss to survey, days | 22.8 ± 6 | 26.1 ± 7.6 | 0.3 | 22.8 ± 6 | 25.3 ± 7.1 | 26.8 ± 8.2 | .37 |
| Olfactory disorder onset | |||||||
| Isolated OD | 1 | 4 | 1 | 1 | 3 | ||
| OD concomitant with other symptoms | 1 | 19 | 0.06 | 1 | 10 | 9 | .21 |
| OD onset after other symptoms | 3 | 106 | 3 | 48 | 58 | ||
| OD onset before other symptoms | 1 | 5 | 1 | 3 | 2 | ||
| Nasal Obstruction at OD onset | |||||||
| No | 2 | 109 | 0.017 | 2 | 49 | 60 | .023 |
| Yes | 4 | 25 | 4 | 13 | 12 | ||
| Sore/dry/tingling nasal cavity | |||||||
| No | 4 | 89 | 0.99 | 4 | 33 | 56 | .007 |
| Yes | 2 | 45 | 2 | 29 | 16 | ||
| Permanent OD | |||||||
| Yes | 0 | 4 | 0.99 | 0 | 3 | 1 | .44 |
| No | 6 | 130 | 6 | 59 | 71 | ||
| Gustatory disorders | |||||||
| No | 0 | 16 | 0.99 | 0 | 5 | 11 | .39 |
| Yes | 6 | 118 | 6 | 57 | 61 | ||
| Gustatory disorders recovery | |||||||
| Unchanged | 3 | 3 | <0.0001 | 3 | 3 | 0 | < .0001 |
| Partial | 3 | 35 | 3 | 32 | 3 | ||
| Total | 0 | 80 | 0 | 22 | 58 | ||
OD = olfactory disorders.
Table II shows relationships between characteristics of olfactory loss and the chance of olfactory recovery as well as the degree of olfactory recovery. The follow‐up was slightly longer, but not significantly, in the group with olfactory recovery than for the group without olfactory recovery (22.8 ± 6 vs 26.1 ± 7.6 days; P = .3). Interestingly, patients without nasal obstruction had more chance to recover their sense of smell than those with this symptom (P = .017). Regarding the degree of olfactory recovery, the time from smell loss onset to olfactory recovery onset was 10 ± 5.6 days in patients with complete olfactory recovery whereas this time was 13.4 ± 6.4 days in those with incomplete olfactory recovery (P = .0004). The time from smell loss onset to survey was 26.8 ± 8.2 days in patients with complete olfactory recovery and 25.3 ± 7.1 days in those with incomplete olfactory recovery (P = .37). Other factors such as nasal congestion or sore/dry/tingling feeling in the nose were significantly related to the degree of olfactory recovery (P = .023 and P = .007, respectively). It means that patients without those symptoms had more chance of complete recovery of their sense of smell. Finally, the recovery of olfactory function and taste was neither the same duration nor the same degree (P < .0001).
Table III shows spontaneous kinetic of olfactory recovery at each time point of survey. The percentage of complete smell recovery seemed to increase gradually as time goes on.
Table III.
Spontaneous Kinetic of Olfactory Recovery at Each Time Point of Survey.
| Degree of olfactory recovery | Day of follow‐up | |||||
|---|---|---|---|---|---|---|
| 14th (n = 139) | 17th (n = 130) | 21th (n = 109) | 25th (n = 63) | 28th (n = 48) | 35th (n = 19) | |
| Unchanged | 6 (4.32%) | 5 (3.85%) | 4 (3.67%) | 1 (1.59%) | 1 (2.08%) | 0 |
| Incomplete | 62 (44.60%) | 59 (45.38%) | 46 (42.20%) | 27 (42.86%) | 20 (41.67%) | 7 (36.84%) |
| Complete | 71 (51.08%) | 66 (50.77%) | 59 (54.13%) | 35 (55.56%) | 27 (56.25%) | 12 (63.16%) |
DISCUSSION
The findings of the present study can be summarized as follows: 1) about two thirds of patients reported a sudden olfactory/gustatory impairment; 2) olfactory/gustatory disorders can be isolated or associated with other symptoms; 3) 95% of patients recovered (incompletely to completely) their olfactory function at 1 month; 4) olfactory function was recovered between the fourth and the fifteenth day after the onset of olfactory loss in most patients; 5) half of patients completely recovered their olfactory function at 4 weeks; and 6) short time from smell loss to the onset of olfactory recovery, absence of nasal obstruction, and absence of sore/dry/tingling feeling in the nose were significantly related to the chance for complete olfactory recovery.
Recently, the olfactory loss outbreak during the COVID‐19 pandemic draws attention to many citizens and healthcare workers. Similarly to SARS‐CoV, causing severe acute respiratory syndrome active 2002–2004, and MERS‐CoV active 2012–present, SARS‐CoV‐2, belongs to the genus Betacoronavirus of the family Coronaviridae. However, olfactory disorders related to the first two viruses were extremely rare. One case of long‐lasting anosmia following SARS‐CoV infection was reported in 2006. 13 Unlike these viruses, SARS‐CoV‐2 causes a high rate of olfactory impairment during the course of the disease. Our findings showed a total rate of olfactory disorders at 61.14% of patients COVID‐19 positive confirmed by PCR. However, this rate was biased due to the potential effect of the mainstream media coverage of smell loss and COVID‐19 on March 20, 2020 in Europe and in the whole world regarding the strong relationship between the sudden olfactory disorders and COVID‐19. Since this day, many patients were considered to be COVID‐19 positive without laboratory confirmation when they had sudden olfactory disorders. Before March 20, 2020 the frequency of olfactory loss was reported in 70.3% of patients, whereas this frequency decreased to 53.9% of patients since that day. Our results were in line with findings of Menni et al. in which loss of smell and taste was self‐reported in two thirds of their large number of participants. 14 A higher rate (85.6%) was reported by Lechien et al. 1 ; however, it was probably due to a selection bias such as the inclusion of patients consulting their otorhinolaryngologists (maybe for sudden olfactory dysfunction), as well as self‐selected volunteers. In contrast, anosmia was reported only in 4.8% of patients in China. 6 This difference may be due to: 1) possible observation bias of Chinese publications related to lack of the investigation on olfactory function in COVID‐19 patients at the onset of the pandemic; 2) potential changes of the pathogenicity and virulence of SARS‐CoV‐2 during progression of the pandemic because Lei et al. found differences of epidemiological and clinical features of COVID‐19 patients in Wuhan and outside Wuhan 15 ; and 3) ethnic pattern. In fact, Cao et al. 16 demonstrated that there was a diversity of expression pattern of angiotensin converting enzyme 2 (ACE2), which is the receptor of SARS‐CoV‐2, in Asian and European populations. This may lead to different susceptibility or response to SARS‐CoV‐2 from different populations under similar conditions. However, most of current published articles regarding olfactory disorders related to COVID‐19 were based on self‐ratings, questionnaires, or screening tests until now. It was possible that olfactory disorders were not investigated in patients with SARS‐CoV in 2003 or MERS‐CoV.
Mechanisms of olfactory disorders related to SARS‐CoV‐2 infection are still unknown, but it is probably the result of several patterns such as a nasal mucosal edema, olfactory epithelial damage (including neural and non‐neural epithelium), and even involvement of central olfactory pathways. It has been shown that ACE2 expression was found in the basal layer of the non‐keratinizing squamous epithelium in nasal and oral mucosa and the nasopharynx. 17 Sungnak et al. found that nasal epithelial cells, specifically goblet (secretory) cells and ciliated cells, display the highest ACE2 expression among human respiratory and gut epithelial cells. 18 They suggested that nasal goblet and ciliated cells had a particularly relevant role as early viral targets and potential reservoirs of SARS‐CoV‐2 infection. This is in line with the findings of Zou et al. who found higher viral loads detected in the nose than the throat soon after symptom onset. 19 Brann et al. 10 demonstrated that ACE2 and TMPRSS2 were not detected in mature olfactory sensory neurons but were detected in many sustentacular and olfactory stem cells in the human olfactory epithelium. They thought that SARS‐CoV‐2 did not directly enter olfactory sensory neurons, but instead could target olfactory epithelium support and stem cells causing cascading damages in the olfactory epithelium. Coronavirus infection of subsets of sustentacular cells may be sufficient to cause a disruption of olfactory function. 10 , 20 However, many viruses, including coronaviruses such as SARS‐CoV 11 or HCoV‐OC43, 12 have been shown to be able to infect the olfactory bulb and downstream areas such as the piriform cortex and the brain stem through the nasal epithelial pathway. So, SARS‐CoV‐2 may not be excluded from this pathway.
A post‐infectious olfactory impairment is typically associated with the common cold or influenza. 21 On the other hand, there is a close temporal connection between the subsiding of the URTI and the development of sudden olfactory disorders. 21 A post‐infectious olfactory loss may be incorrectly labeled as idiopathic olfactory loss in some patients because they may be unaware of the URTI episode. 22 Interestingly, isolate olfactory loss without other nasal or general symptoms was observed in 4% of our COVID‐19 patients. These cases could be incorrectly labeled as idiopathic if they were out of context of the COVID‐19 outbreak.
Most patients who suffered from acquired olfactory loss also complained of altered taste, describing food as bland because they often confuse loss of retronasal olfactory function (flavor) with taste. Gustatory dysfunction is often recovered when olfactory function comes back. For this reason, gustatory loss was self‐reported in most of COVID‐19 patients. However, without objective assessment of specific taste qualities (salty, sour, sweet, bitter, or umami/savory) in our study, we did not know if this was truly taste loss or instead a flavor perception change resulting from the olfactory loss.
The rates of spontaneous recovery of post‐infectious olfactory dysfunction at given points of follow‐up varied from 6% within 4 months of follow‐up 23 to 67% at 37 months of mean follow‐up. 24 However, the true rate of spontaneous recovery of olfactory function is still unknown because of: 1) lack of awareness of this condition among both patients and medical providers; 2) lack of epidemiological studies inventorying the rates of post‐infectious olfactory impairment and its spontaneous recovery in the short, middle, and long term; 3) selection bias as most patients with olfactory loss consulted many months, even many years after smell loss; and 4) methods assessing olfactory loss. In contrast, the evolution of olfactory disorders in most COVID‐19 patients can be followed‐up during the outbreak thanks to a database of tested patients.
Adding to worries due to other disorders in those patients, the sudden olfactory/gustatory loss is another source of anxiety in this condition. Hence, knowledge of the spontaneous evolution of olfactory disorders during the course of COVID‐19 is important to reassure patients. Fortunately, most COVID‐19 patients with olfactory loss recovered their sense of smell about 2 to 4 weeks after the onset of this condition. This short time supports findings that the non‐neural olfactory epithelial cells are the potential target of SARS‐CoV‐2. 10 However, it is possible that stem cells such as horizontal basal cells can be infected. 10 In this case, more time is required to recover the olfactory function. If a large percentage of basal cells are damaged, the olfactory epithelium cannot effectively renew over time resulting in long‐lasting anosmia. 25
Interestingly, there was a strong relationship between the possibility of complete recovery of sense of smell and the short time from smell loss to the onset of olfactory recovery, absence of nasal obstruction, and absence of sore/dry/tingling feeling in the nose in COVID‐19 patients. In this condition, olfactory loss is probably due to obstructive inflammation of olfactory clefts as shown on imaging reported in one case of Eliezer et al. 9 Our study assessed at 26 ± 7.6 days from the onset of olfactory disorders in 140 COVID‐19 patients whereas the short‐term olfactory recovery was assessed in only 59/357 (16.5%) patients in the study of Lechien et al., in which the mean time between the onset of the infection and the evaluation was 9.2 ± 6.2 days with olfaction recovery rate at 44%. 1 We did not know why sore/dry/tingling feeling in the nose was a frequent complaint of COVID‐19 patients without other nasal symptoms. It could suggest that there were trigeminal effects of SARS‐CoV‐2 on nasal cavities in those subjects. By contrast, a reduction of chemesthesis (like burning, cooling, or tingling) in the mouth was reported in about 46% of subjects in Parma et al. 26
The limitations of our study are: 1) recall bias due to retrospective phone call; 2) lack of objective olfactory assessment because of the high risk of COVID‐19 exposure to healthcare workers; 3) short follow‐up; and 4) unknown prevalence of olfactory disorders in patients with serious conditions. In order to minimize the selection bias by self‐selected group using internet or the app such as online surveys, we tried to call all patients from the database of our hospital. However, 10% of our COVID‐19 patients did answer their phone. The other main limitation of our study is self‐report of all symptoms. It had been shown that olfactory self‐ratings were not reliable in normosmic subjects 27 , 28 but are reliable in severe hyposmic or anosmic subjects. 28 , 29 Therefore, the results of the present study should be interpreted with caution. In order to assess the olfactory recovery, we used a qualitative assessment (“Has your sense of smell started to recover?”) associated to within‐subject comparisons using numerical scale (from 0 to 10) to self‐rate patients' olfactory abilities for different time points. These two approaches were used to seek the coherence between answers in order to maximize a possible accuracy. However, we cannot know accuracy rates of our patients' self‐reports. We used the within‐subjects design by using numerical scale to assess olfactory function at the moment of olfactory loss and the survey time (eg, survey time minus onset of olfactory loss) to evaluate the degree of olfactory recovery. This method allows fine‐tuning the degree of olfactory. Other limitation is the upper anchor of rating scale that was considered as “normal sense of smell” without taking into account the (objective) individual olfactory status. Unfortunately, the olfactory status of our patients was not assessed (objectively and/or subjectively) before COVID‐19. However, we screened and excluded patients with recognized olfactory impairment before COVID‐19 (by asking patients during the phone call).
CONCLUSION
Olfactory and gustatory disorders were reported in two thirds of COVID‐19 patients. It can be an isolated symptom of COVID‐19. Knowledge of spontaneous evolution of olfactory disorders in COVID‐19 patients allows for reassuring patients and planning therapeutic strategies for persistent olfactory dysfunction after having definitely recovered from COVID‐19.
Supporting information
Appendix S1 Supplementary material
1) French version questionnaire
2) English version questionnaire.
Editor's Note: This Manuscript was accepted for publication on June 29, 2020.
The authors have no funding or conflicts of interest to disclose.
BIBLOGRAPHY
- 1. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol 2020;277:2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID‐19 pandemic. Rhinology 2020;58:295–298. [DOI] [PubMed] [Google Scholar]
- 3. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and Covid‐19 in patients presenting with influenza‐like symptoms. Int Forum Allergy Rhinol 2020;10:806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bagheri SH, Asghari A, Farhadi M, et al. Coincidence of COVID‐19 epidemic and olfactory dysfunction outbreak in Iran. Med J Islam Repub Iran. 2020;34:62. 10.34171/mjiri.34.62. [DOI] [PMC free article] [PubMed]
- 5. Moein ST, Hashemian SMR, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol 2020;1–7. 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID‐19 patients. Laryngoscope 2020;130:1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giacomelli A, Pezzati L, Conti F, et al. Self‐reported olfactory and taste disorders in SARS‐CoV‐2 patients: a cross‐sectional study. Clin Infect Dis 2020. 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eliezer M, Hautefort C, Hamel A‐L, et al. Sudden and complete olfactory loss function as a possible symptom of COVID‐19. JAMA Otolaryngol Head Neck Surg 2020;146(7):674–675. 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- 10. Brann D, Tsukahara T, Weinreb C, Logan DW, Datta SR. Non‐neural expression of SARS‐CoV‐2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID‐19 patients. bioRxiv 2020. 10.1101/2020.03.25.009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008;82:7264–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dubé M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ. Axonal transport enables neuron‐to‐neuron propagation of human coronavirus OC43. J Virol 2018;92:e00404–e00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hwang C‐S. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwan 2006;15:26–28. [PubMed] [Google Scholar]
- 14. Menni C, Valdes AM, Freidin MB, et al. Real‐time tracking of self‐reported symptoms to predict potential COVID‐19. Nat Med 2020;26:1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lei Z, Cao H, Jie Y, et al. A cross‐sectional comparison of epidemiological and clinical features of patients with coronavirus disease (COVID‐19) in Wuhan and outside Wuhan, China. Travel Med Infect Dis 2020;35:101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cao Y, Li L, Feng Z, et al. Comparative genetic analysis of the novel coronavirus (2019‐nCoV/SARS‐CoV‐2) receptor ACE2 in different populations. Cell Discov 2020;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sungnak W, Huang N, Bécavin C, et al. SARS‐CoV‐2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020;26:681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med 19 2020;382:1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fodoulian L, Tuberosa J, Rossier D, Landis BN, Carleton A, Rodriguez I. SARS‐CoV‐2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. bioRxiv 2020. 10.1101/2020.03.31.013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duncan HJ. Postviral olfactory loss. In: Seiden AM, ed. Taste and Smell Disorders. New York: Thieme; 1997:72–78. [Google Scholar]
- 22. Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol Suppl 2017;54:1–30. [DOI] [PubMed] [Google Scholar]
- 23. Hummel T, Rissom K, Reden J, Hähner A, Weidenbecher M, Hüttenbrink K‐B. Effects of olfactory training in patients with olfactory loss. Laryngoscope 2009;119:496–499. [DOI] [PubMed] [Google Scholar]
- 24. Duncan HJ, Seiden AM. Long‐term follow‐up of olfactory loss secondary to head trauma and upper respiratory tract infection. Arch Otolaryngol Head Neck Surg 1995;121:1183–1187. [DOI] [PubMed] [Google Scholar]
- 25. Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec 2002;269:33–49. [DOI] [PubMed] [Google Scholar]
- 26. Parma V, Ohla K, Veldhuizen MG, et al. More than smell. COVID‐19 is associated with severe impairment of smell, taste, and chemesthesis. medRxiv 2020. 10.1101/2020.05.04.20090902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Landis BN, Hummel T, Hugentobler M, Giger R, Lacroix J. Ratings of overall olfactory function. Chem Senses 2003;28:691–694. [DOI] [PubMed] [Google Scholar]
- 28. Nguyen DT, Nguyen‐Thi PL, Jankowski R. How does measured olfactory function correlate with self‐ratings of the sense of smell in patients with nasal polyposis? Laryngoscope 2012;122:947–952. [DOI] [PubMed] [Google Scholar]
- 29. Lötsch J, Hummel T. Clinical usefulness of self‐rated olfactory performance ‐ a data science‐based assessment of 6000 patients. Chem Senses 2019;44:357–364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplementary material
1) French version questionnaire
2) English version questionnaire.


